 | Fluid Dynamics & Materials Processing |  |
DOI: 10.32604/fdmp.2022.021662
ARTICLE
Influence of Urea Uneven Injection on the Performances of a Diesel Engine
School of Energy and Power Engineering, Shandong University, Jinan, 250061, China
*Corresponding Authors: Guoxiang Li. Email: liguox@sdu.edu.cn; Ke Sun. Email: sunkeke@sdu.edu.cn
Received: 26 January 2022; Accepted: 13 April 2022
Abstract: The influence of heterogeneous flow injection of urea at different velocities and temperatures on NOx conversion efficiency, ammonia storage and ammonia leakage is investigated experimentally. A diesel engine employing a selective catalytic reduction (SCR) technology is considered. It is found that for a fixed injection velocity, the degree of ammonia leakage changes depending on the temperature. The higher the temperature, the faster the catalytic reduction reaction and the smaller the degree of ammonia leakage. The temperature has a great influence on the catalytic reduction reaction rate. At an injection velocity of 10000/h, the average reaction rate at 420°C is 12 times higher than that at 180°C. The injection velocity has a weak influence on the reaction rate. When the injection velocity changes from 10000/h to 40000/h at the same temperature, the average reaction rate does not change appreciably. However, increasing the space velocity can accelerate the leakage of ammonia, thereby mitigating the benefits associated with the NOx conversion.
Keywords: Diesel engine; ammonia leak; conversion efficiency; the urea; flow
Compared with gasoline engines, diesel engines have higher thermal efficiency and lower
This paper is based on the research of matching vanadium VI diesel engine and the real case of SCR catalysts in ammonia storage and conversion efficiency. Ammonia leakage, according to the different operating conditions, formulate reasonable strategies of urea injection, making the whole machine have higher NOx conversion rate and lower
This test-bed mainly includes dynamometer, diesel engine, emission test system and SCR after treatment system, test using dynamometer for electrical dynamometer. The test bench arrangement is shown in Fig. 1. Parameters of the engine are shown in Table 1.

Figure 1: Engine test bench
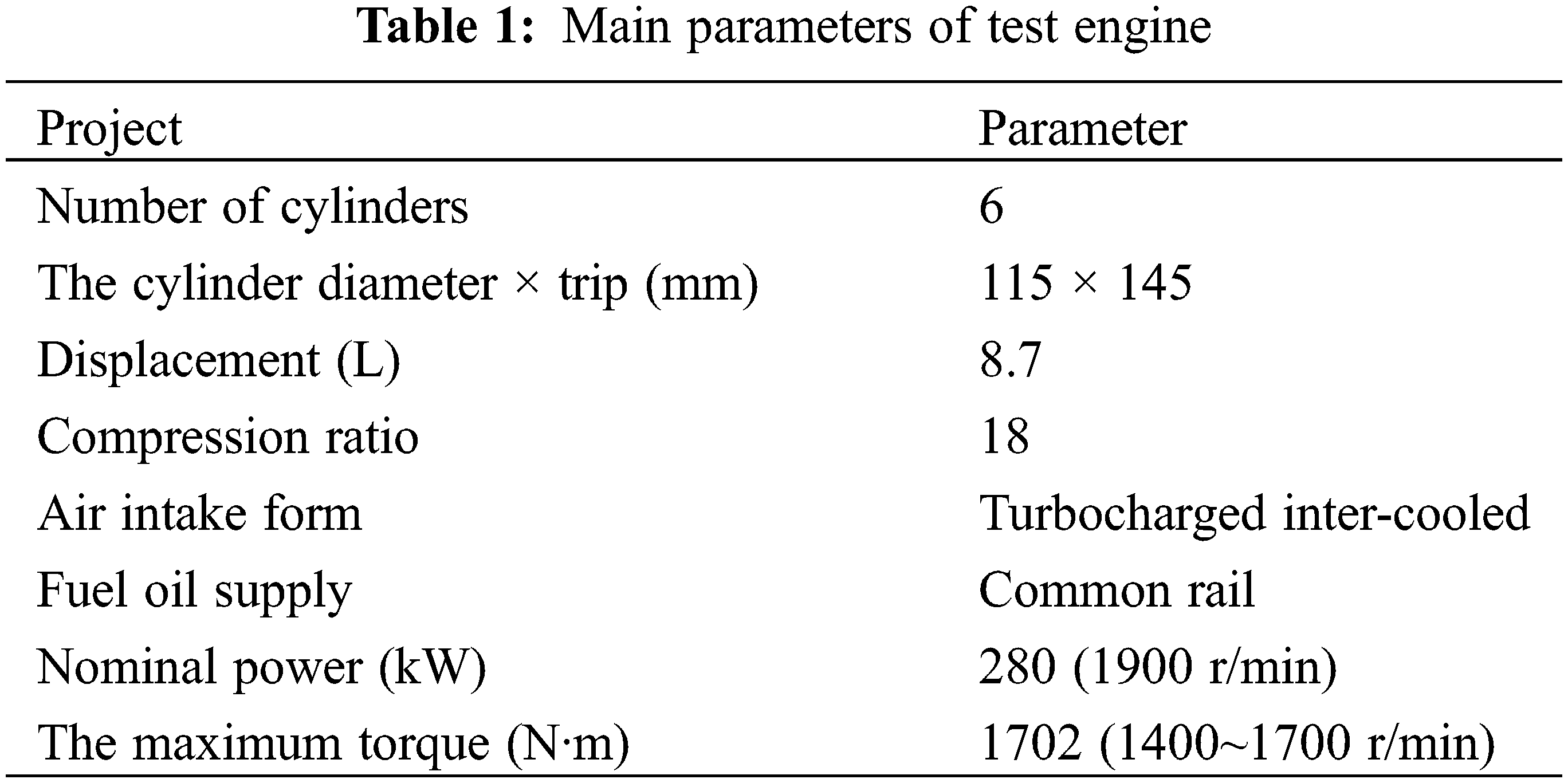
This test uses an integrated urea box and a 32.5% standard urea aqueous solution, it is in line with the implementation of GB/T 29518-2013. The test mainly used the AVL i60 emission meter to measure gas components such as NOx, and the ammonia analyzer to measure
Figure 2: Details of test section
The space velocity and the exhaust temperature of ammonia during storage and release were mainly studied experimentally, and the influence of ammonia storage on the conversion efficiency of NOx was analyzed. The space velocity is defined as the standard state of exhaust volume ratio and catalyst volume per hour.
It is necessary to test constant adjusting speed and torque to control space velocity and exhaust temperature respectively, and to provide enough ammonia to smoothly form ammonia storage [4]. Therefore, the ammonia-nitrogen ratio is 1.2:1 when spraying urea under all conditions in the experiment. During the test, the high temperature of waste gas was purged at 500°C first, and then the difference between the values of current NOx was about 20–30 PPM. When the peak value of NOx before and after the engine changes within 30 ppm and there is no leakage of
Continuously measure and record the volume concentration change process of NOx and
3.1 Ammonia Storage and NOx Conversion Efficiency
The saturated storage capacity of ammonia and its influence on the real-time conversion efficiency of NOx at different temperatures and space velocity were studied. The calculation method of ammonia storage capacity was from the initial urea injection until there was a leakage of ammonia with a volume concentration of 10 × 10−6, at which time the urea injection was stopped, and the storage capacity of ammonia in this process was the saturated storage capacity of ammonia.
As can be seen from Fig. 3, under the condition of space velocity of 20000/h, when the temperature is lower than 420°C, the saturated ammonia storage decreases linearly with the increase of temperature, with 22.52 g at 180°C and only 4.09 g at 420°C, which shows that the temperature has a great influence on the saturated ammonia storage.
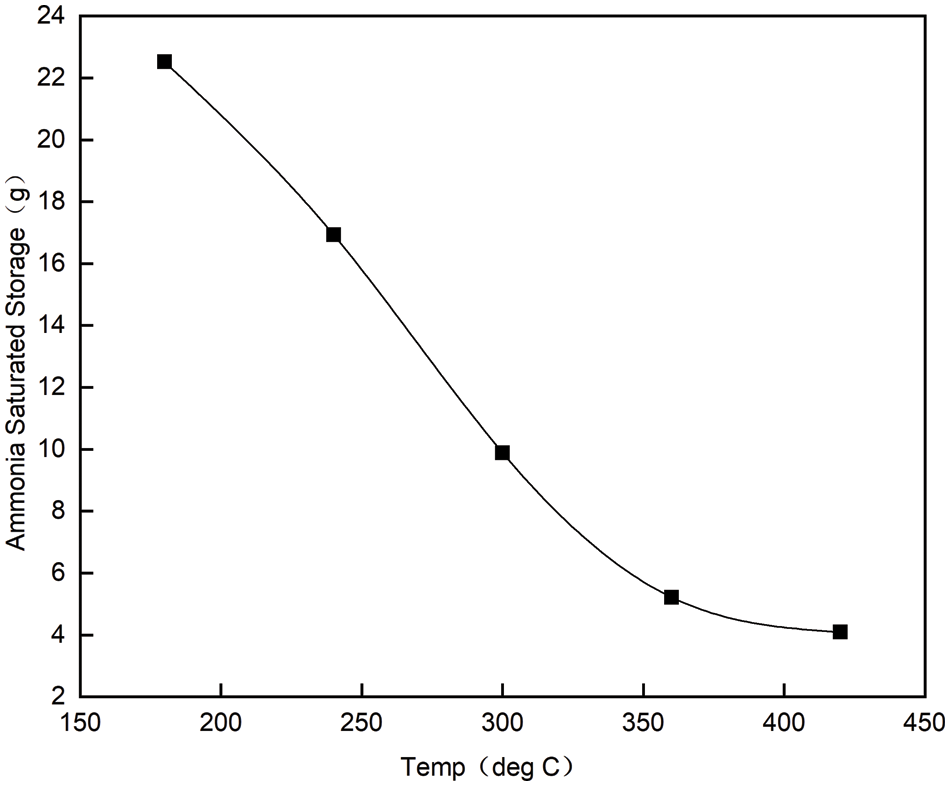
Figure 3: Saturated storage capacity of ammonia at space velocity of 20000/h
As can be seen from Fig. 4, when the temperature is maintained at 300°C, the saturated ammonia storage decreases slightly with the increase of space velocity, because increasing space velocity will cause ammonia leakage to occur more easily, thus reducing the final ammonia storage [5]. According to the changing trend of ammonia saturated storage when space velocity changes from 10000/h to 40000/h, it can be seen that space velocity has little influence on ammonia saturated storage.
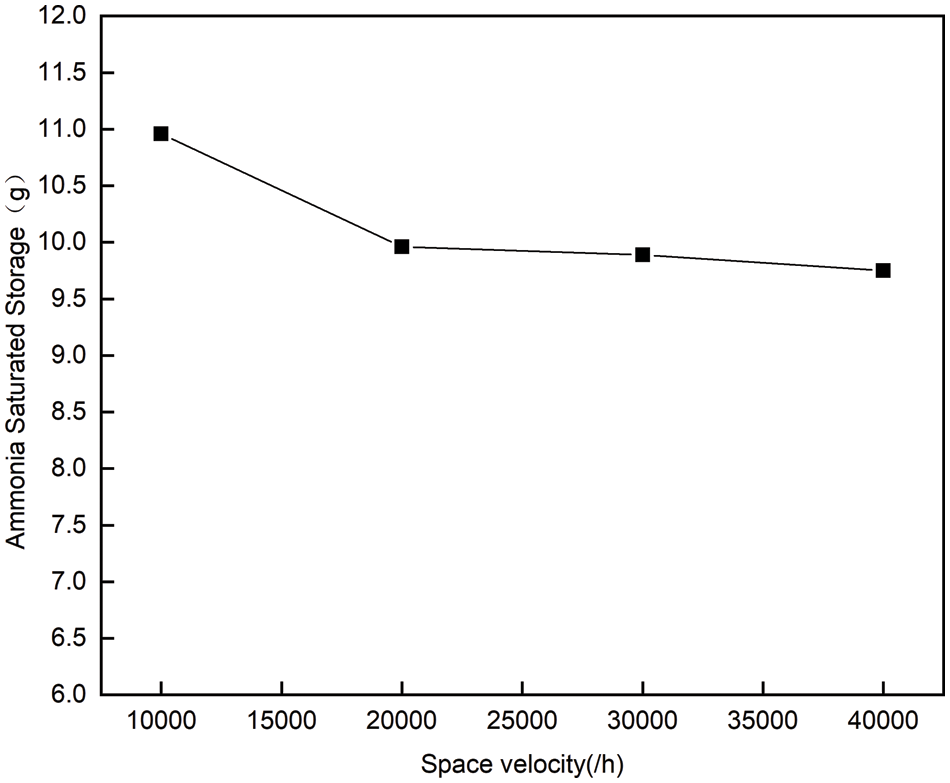
Figure 4: Saturated storage capacity of ammonia at 300°C
From Fig. 5, it can be concluded that when the temperature is controlled at 180°C, the conversion efficiency and ammonia storage capacity of NOx show a linear trend, which is due to the poor low-temperature activity of the catalyst, which leads to the slow reaction rate. When the storage amount of ammonia increases, there will be more ammonia to increase the reaction rate [6]. This shows that the conversion efficiency of NOx at low temperature is greatly influenced by ammonia storage.
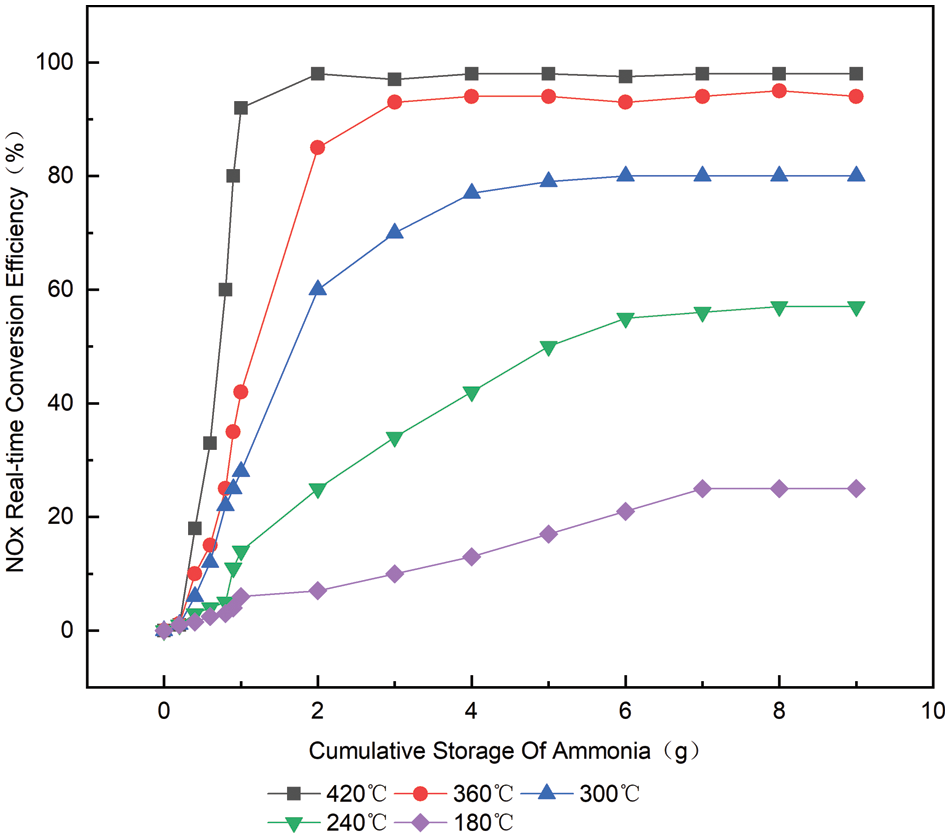
Figure 5: The relationship between the real-time conversion efficiency of
As the temperature rises, the activity of catalysts becomes stronger, then ammonia storage for NOx would also reduce the conversion efficiency of effects [7]. When the temperature is 420°C, the conversion efficiency of NOx reaches its peak after 7 s of urea injection, and the ammonia storage is only about 0.9 g at this time, which is basically unchanged from the conversion efficiency of NOx, and the ammonia leakage does not occur until ammonia storage reaches 1.59 g.
This is obtained from the curve of ammonia storage and the conversion efficiency of NOx in Fig. 5. Under the condition of low temperature and NOx, the conversion efficiency is affected by the large amount of ammonia storage. With the increase in ammonia storage, the conversion efficiency of NOx is significantly improved. As the temperature increases, the rate of catalytic reduction reaction accelerates, and the effect of ammonia storage on the NOx conversion efficiency gradually decreases. By 300°C, the effect of ammonia storage on the improvement of NOx conversion efficiency is already very limited. At high temperatures of 420°C, the amount of ammonia stored has little effect on NOx conversion efficiency [8,9].
3.2 The Influence of Temperature on the Reaction Rate
The average rate and reduction time of catalytic reduction reaction changes with the change of temperature under the same space velocity [10]. The time from the start of the reaction to 95% of the total steady-state decrease is called the decrease time, which reflects the main process of catalytic reduction. The reaction rate refers to the ratio of NOx reaction amount to the falling time. The total steady-state decline is defined as the volume concentration difference between the initial NOx and the ammonia leakage volume concentration of 10 × 10−6. Fig. 6 shows the change trend of volume concentration of 30000/h space velocity at 180°C, 240°C, 300°C, 360°C and 420°C, respectively, from the 40 s of urea injection to 10 × 10−6 NOx of ammonia leakage volume concentration. It can be seen from Fig. 6 that when the temperature is 180°C the concentration changes very gently. Because it is the catalytic reduction reaction rate that is slow at low temperature. As the temperature increases, the rate of catalytic reduction reaction also accelerates [11]. When the temperature is 180°C, 240°C, 300°C, 360°C and 420°C, the falling time are 450, 259, 197, 80 and 48 s, respectively.
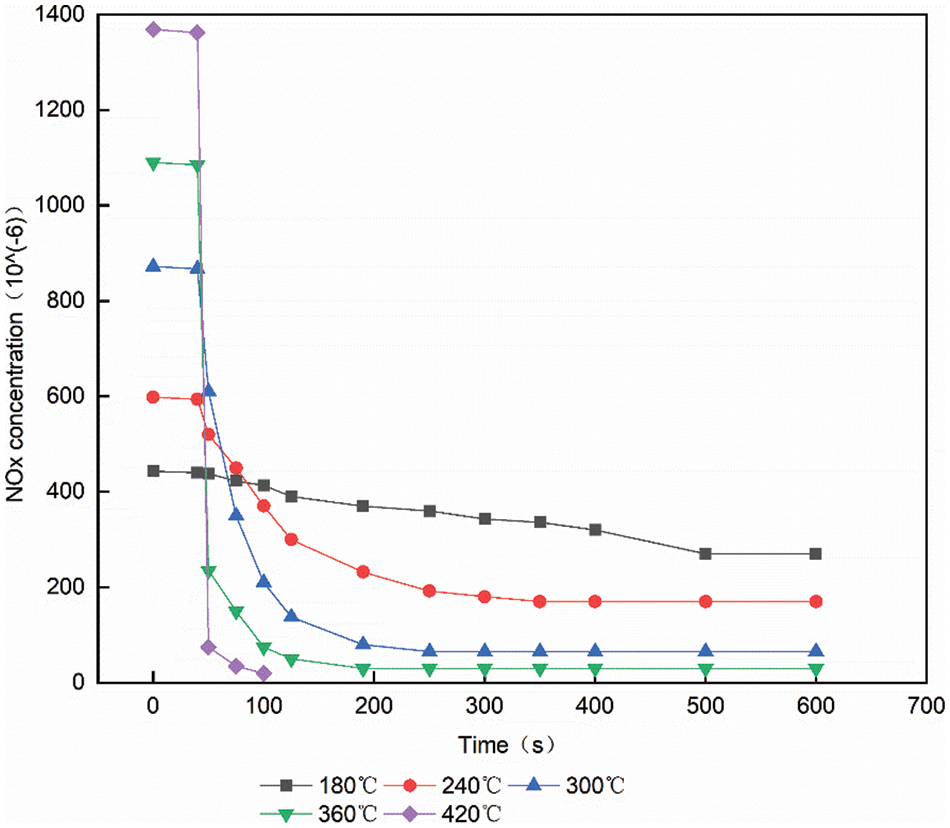
Figure 6: The volume concentration of the
The average response rate NOx at different temperatures is shown in Table 2 and Fig. 7. We can see that with the increase of temperature from 180°C to 420°C, the average reaction rate of NOx raised from 0.0004 to 0.0049 mol/s. Average indicated temperature on NOx has great influence on the reaction rate. The higher the temperature, the faster the reaction rate [12].
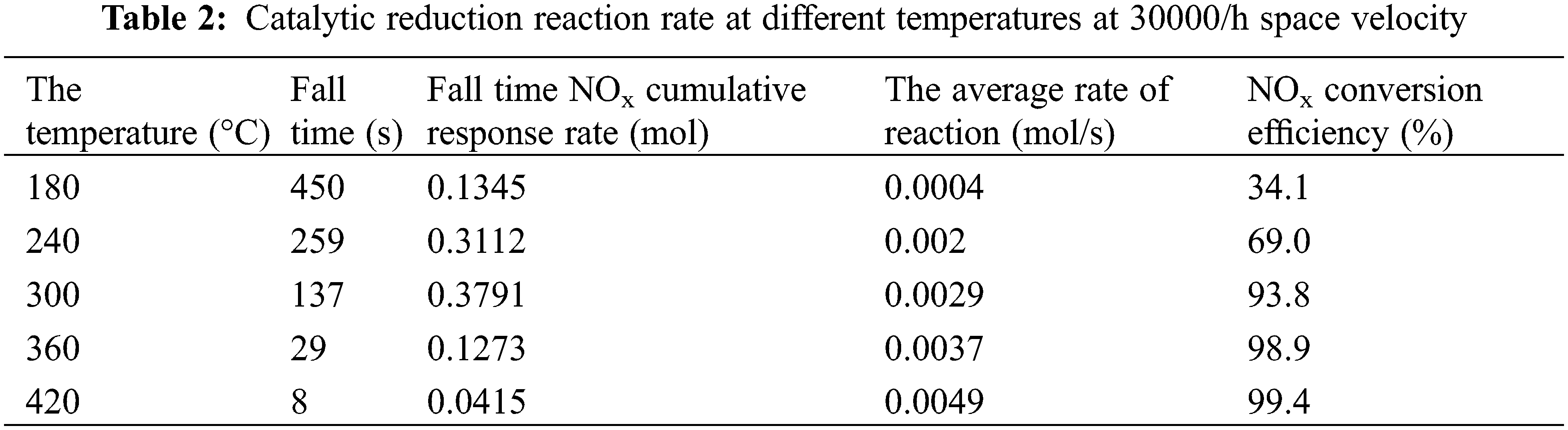
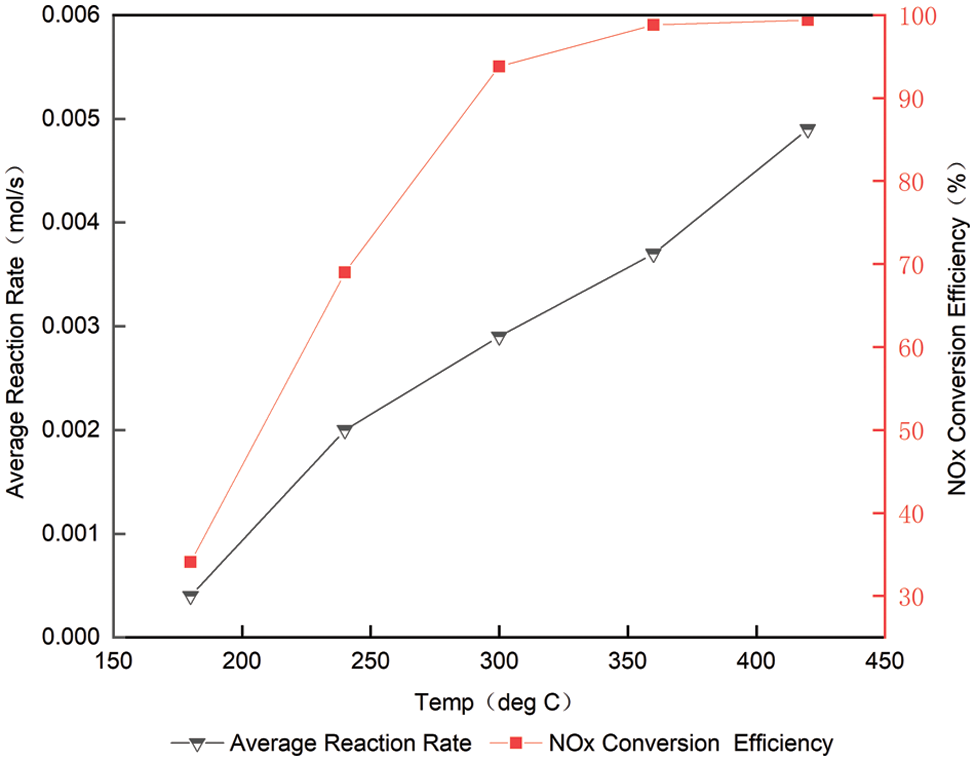
Figure 7: Average reaction rate at different temperatures at space velocity of 30000/h
3.3 Effect of Space Velocity on Reaction Rate
Experimental study on the constant temperature, catalytic reduction reaction rate and down time with space velocity changes. Fig. 8 shows the change trend of the volume concentration of NOx from urea injection to ammonia leakage volume concentration of 10 × 10−6 when the temperature is 300°C and the space velocity is 10000/h, 20000/h, 30000/h and 40000/h, respectively. As can be seen from Fig. 8, when the temperature remains unchanged, although the space velocity increases, the volume concentration of NOx does not have a great downward trend, and ammonia leakage will occur earlier. When the space velocity is 10000/h, there is 10 × 10−6 volume concentration of ammonia leakage after 210 s of urea injection, while when the space velocity is 40000/h, there is 10 × 10−6 volume concentration of ammonia leakage after 60 s of urea injection. It can be concluded that when other conditions are the same, the larger the space velocity, the easier ammonia leakage will occur [13,14]. The main reason is that
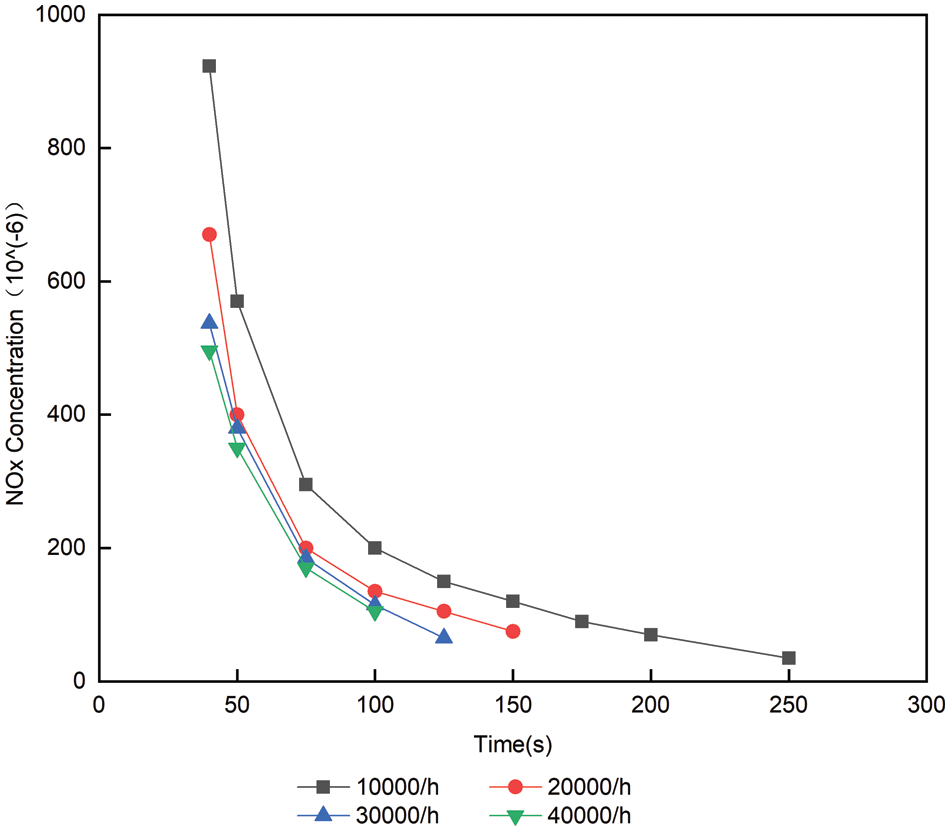
Figure 8: Volume concentration change of
When the temperature is 300°C, the average reaction rate and the total amount of NOx at the back end of SCR are shown in Table 3 and Fig. 9. Table 3 shows that the reaction rate is around 0.0030 mol/s at all space velocity, indicating that the main factors affecting the reaction rate is not space velocity, but temperature [15]. With the increase of space velocity, the cumulative reaction amount of NOx decreases slightly. It can be seen from Table 3 that when the space velocity increases from 10000/h to 4000/h, the conversion efficiency of NOx corresponding to the ammonia leakage volume concentration of 10 × 10−6 decreases by about 21% [16]. This is because increasing the space velocity will shorten the contact time between
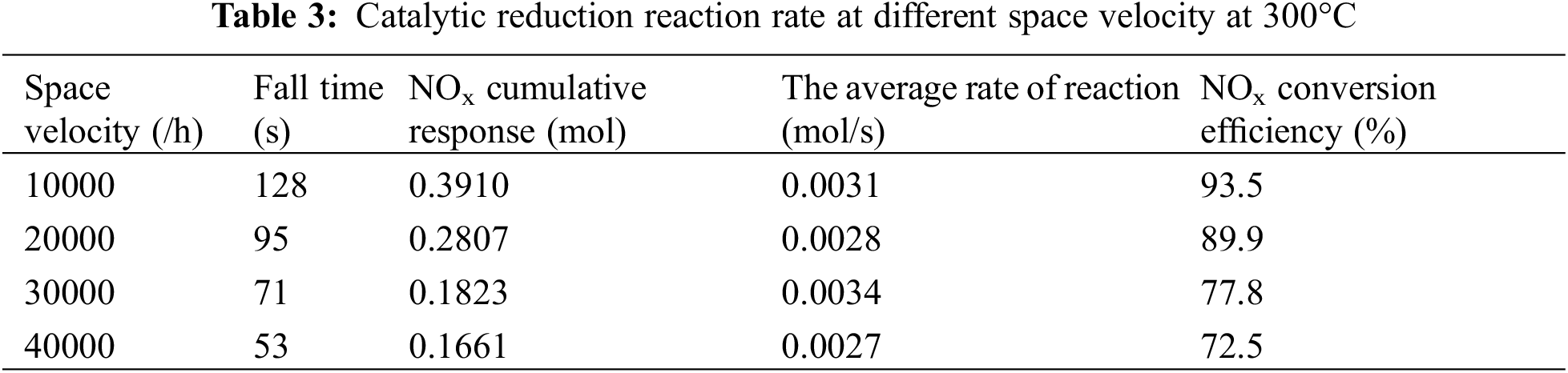
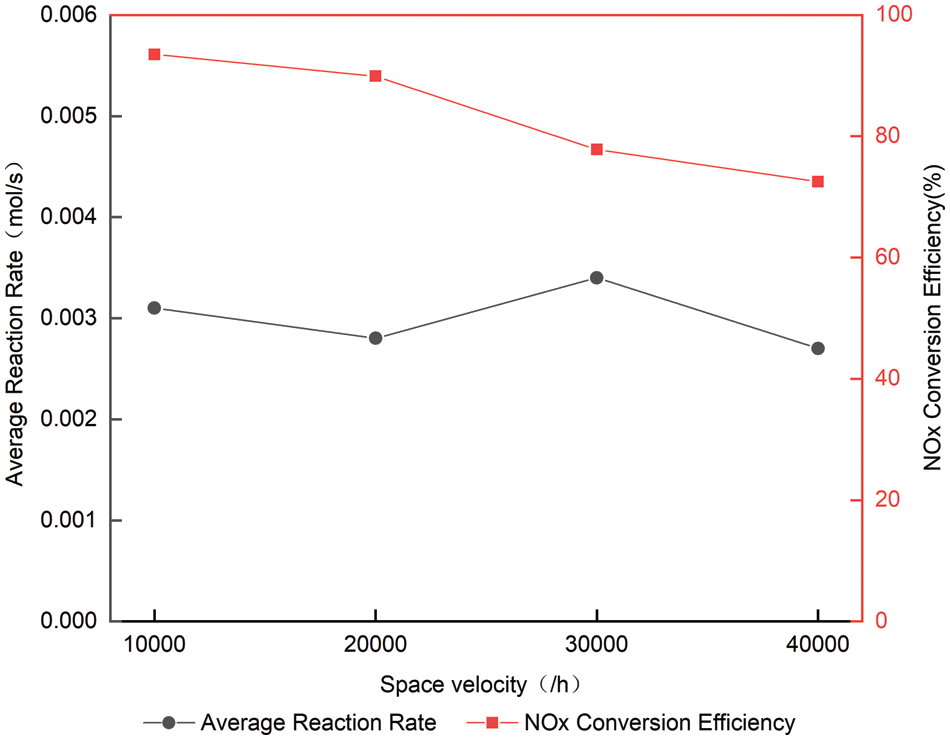
Figure 9: Average reaction rates at different space velocity at 300°C
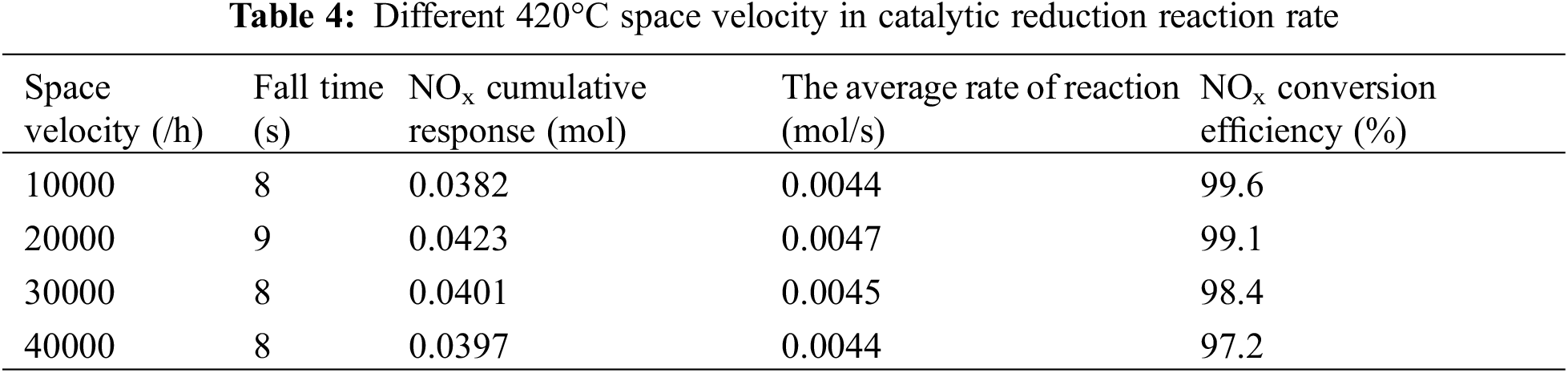
Figs. 10 and 11 and Table 4 compare the change process of the volume concentration of each space velocity NOx at 420°C and the average reaction rate of NOx, and urea injection starts from 38 s. As can be seen from Fig. 10, increasing the space velocity makes the ammonia leakage happen earlier, for the same reason as the analysis at 300°C. From Fig. 10, it can be concluded that the space velocity is increased at 420°C. Although the contact time between the catalyst and the reducing agent is shorter, the reaction rate is kept at about 0.0045 mol/s, which shows that the space velocity has little effect on the average reaction rate of NOx at high temperature, and the temperature at 420°C is the main factor to control the reaction rate [17,18]. The reason for this phenomenon is the same as that at 300°C. The higher the space velocity, the shorter the reaction time between
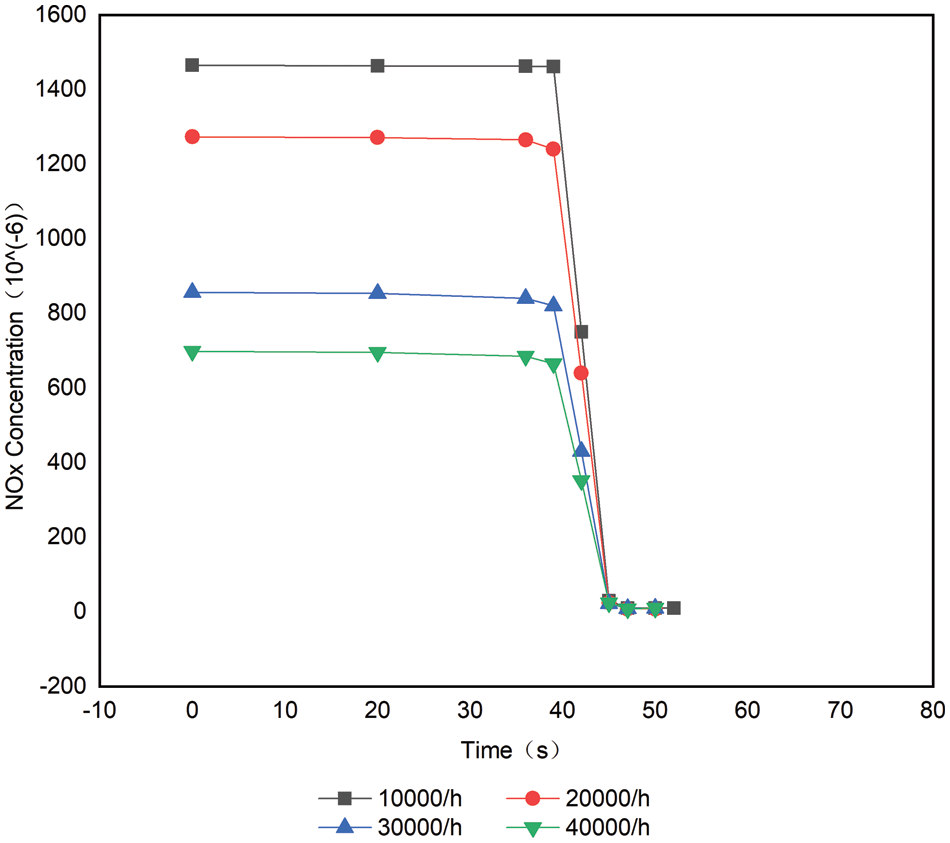
Figure 10: 420°C with different space velocity distribution at downstream
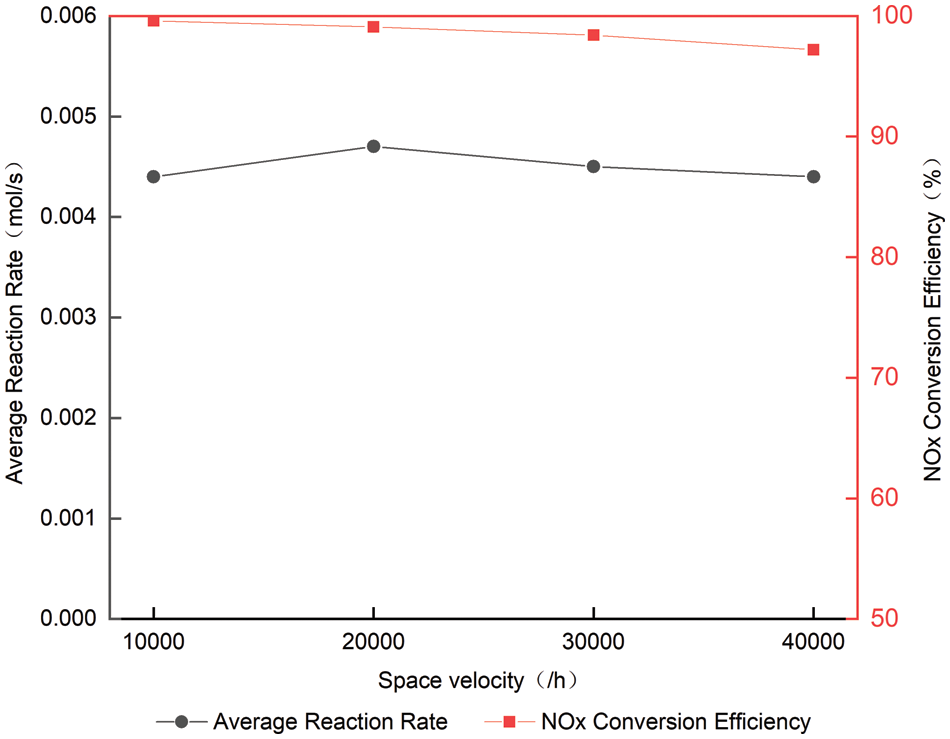
Figure 11: Average reaction rate at different space velocity at 420°C
1. The dynamic reaction process of SCR is quite different from the steady-state reaction process. When formulating the urea uniform flow injection strategy, the storage and release characteristics of ammonia in the catalyst and its influence on NOx conversion efficiency should be considered. In order to accurately control the urea injection quantity, it is necessary to study the dynamic reaction characteristics of the catalyst and establish relevant models.
2. The saturated ammonia storage capacity is greatly influenced by temperature, and when the space velocity is constant, the saturated ammonia storage capacity decreases sharply with the increase of temperature. However, space velocity has little effect on the saturated ammonia storage. When the temperature is constant, the saturated ammonia storage only slightly decreases with the increase of space velocity.
3. According to the relationship between ammonia storage and NOx conversion efficiency, increasing ammonia storage can be considered to improve NOx conversion efficiency at low temperature, but reasonable control of ammonia storage is needed, otherwise, the engine load will suddenly increase, the catalyst temperature will rise, and more ammonia stored before the ammonia storage capacity declines will be too late to consume, resulting in ammonia leakage.
Funding Statement: This work was supported by the Natural Science Foundation Project of Shandong Provincial (Grant No. ZR2019MEE041), and the open funds of National Engineering Laboratory of Mobile Source Emission Control Technology (Grant No. NELMS2019A01). The authors would like to thank the reviews whose constructive and detailed critique contributed to the quality of this paper.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Johnson, T., Joshi, A. (2018). Review of vehicle engine efficiency and emissions. SAE International Journal of Engines, 11(6), 1307–1330. DOI 10.4271/2018-01-0329. [Google Scholar] [CrossRef]
2. Mera, Z., Matzer, C., Hausberger, S., Fonseca, N. (2020). Performance of selective catalytic reduction (SCR) system in a diesel passenger car under real-world conditions. Applied Thermal Engineering, 181, 115983. DOI 10.1016/j.applthermaleng.2020.115983. [Google Scholar] [CrossRef]
3. Lin, Q., Chen, P. (2017). Model-based diagnostics of ammonia storage non-uniformity for a selective catalytic reduction system. 2017 American Control Conference (ACC), pp. 2594–2599. Washington State, USA. DOI 10.23919/ACC.2017.7963343. [Google Scholar] [CrossRef]
4. Sharp, C., Webb, C., Neely, G., Carter, M., Yoon, S. et al. (2017). Achieving ultra low NOx emissions levels with a 2017 heavy-duty on-highway TC diesel engine and an advanced technology emissions system-thermal management strategies. SAE International Journal of Engines, 10(4), 1697–1712. DOI 10.4271/2017-01-0954. [Google Scholar] [CrossRef]
5. Brack, W., Heine, B., Birkhold, F., Kruse, M., Schoch, G. et al. (2014). Kinetic modeling of urea decomposition based on systematic thermogravimetric analyses of urea and its most important by-products. Chemical Engineering Science, 106, 1–8. DOI 10.1016/j.ces.2013.11.013. [Google Scholar] [CrossRef]
6. Liu, S., Wang, B., Guo, Z., Wang, B., Zhang, Z. et al. (2023). Experimental investigation of urea injection strategy for close-coupled SCR aftertreatment system to meet ultra-low NOx emission regulation. Applied Thermal Engineering, 205(10), 117994. DOI 10.1016/j.applthermaleng.2021.117994. [Google Scholar] [CrossRef]
7. Gong, J., Narayanaswamy, K., Rutland, C. J. (2016). Heterogeneous ammonia storage model for NH3–SCR modeling. Industrial & Engineering Chemistry Research, 55(20), 5874–5884. DOI 10.1021/acs.iecr.6b01097. [Google Scholar] [CrossRef]
8. Ning, J., Yan, F. (2015). Robust nonlinear disturbance observer design for estimation of ammonia storage ratio in selective catalytic reduction systems. Journal of Dynamic Systems, Measurement, and Control, 137(12), 121012. DOI 10.1115/1.4031595. [Google Scholar] [CrossRef]
9. Feng, T., Lü, L. (2015). The characteristics of ammonia storage and the development of model-based control for diesel engine urea-SCR system. Journal of Industrial and Engineering Chemistry, 28, 97–109. DOI 10.1016/j.jiec.2015.02.004. [Google Scholar] [CrossRef]
10. Liu, B., Yao, D., Wu, F., Wei, L., Li, X. et al. (2019). Study on ammonia storage mechanism of Cu-SSZ-13 zeolite SCR catalyst for diesel engine. Journal of Chemical Engineering of Chinese Universities, 33(1), 103–109. DOI 10.3969/j.issn.1003-9015.2019.01.013. [Google Scholar] [CrossRef]
11. Hu, X., Wang, Y., Li, S., Sun, Q., Bai, S. et al. (2021). Assessment of the application of subcooled fluid boiling to diesel engines for heat transfer enhancement. Fluid Dynamics & Materials Processing, 17(6), 1049–1066. DOI 10.32604/fdmp.2021.016763. [Google Scholar] [CrossRef]
12. Brack, W., Heine, B., Birkhold, F., Kruse, M., Deutschmann, O. (2016). Formation of urea-based deposits in an exhaust system: Numerical predictions and experimental observations on a hot gas test bench. Emission Control Science and Technology, 2(3), 115–123. DOI 10.1007/s40825-016-0042-2. [Google Scholar] [CrossRef]
13. Han, L., Cai, S., Gao, M., Hasegawa, J., Wang, P. et al. (2019). Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chemical Reviews, 119(19), 10916–10976. DOI 10.1021/acs.chemrev.9b00202. [Google Scholar] [CrossRef]
14. Chen, P., Wang, J. (2015). Nonlinear model predictive control of integrated diesel engine and selective catalytic reduction system for simultaneous fuel economy improvement and emissions reductions. Journal of Dynamic Systems, 137(8), 2239. DOI 10.1115/1.4030252. [Google Scholar] [CrossRef]
15. Lin, Q., Chen, P. (2019). Estimation of ammonia storage nonuniformity for urea-based selective catalytic reduction systems. Journal of Dynamic Systems, Measurement, and Control, 141(4), 486. DOI 10.1115/1.4042143. [Google Scholar] [CrossRef]
16. Tan, L., Feng, P., Yang, S., Guo, Y., Liu, S. et al. (2018). CFD studies on effects of SCR mixers on the performance of urea conversion and mixing of the reducing agent. Chemical Engineering and Processing, 123(1), 82–88. DOI 10.1016/j.cep.2017.11.003. [Google Scholar] [CrossRef]
17. Sun, K., Li, D., Liu, H., Bai, S. (2020). Influence of diesel engine intake throttle and late post injection process on the rise of temperature in the diesel oxidation catalyst. Fluid Dynamics & Materials Processing, 16(3), 573–584. DOI 10.32604/fdmp.2020.09591. [Google Scholar] [CrossRef]
18. Zhu, J., Wang, X., Wang, G., Zhong, X., Li, Z. et al. (2022). Experimental analysis of the influence of exhaust thermal management on engine NOx emission. Fluid Dynamics & Materials Processing, 18(3), 701–711. DOI 10.32604/fdmp.2022.019311. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |