DOI:10.32604/iasc.2023.025819

| Intelligent Automation & Soft Computing DOI:10.32604/iasc.2023.025819 |  |
| Article |
A Novel Technique for Detecting Various Thyroid Diseases Using Deep Learning
1Department of Information Technology, Sri Sai Ram Engineering College, Chennai, 600044, India
2College of Computer and Information Sciences (CCIS), Majmaah University, Majmaah, 11952, Kingdom of Saudi Arabia
3Department of Computer Science and Engineering, Sri Sai Ram Engineering College, Chennai, 602109, India
4Associate Solutions Engineer, Pegasystems, Chennai, 600044, India
5Systems Engineer, Infosys Ltd., Chennai, 600119, India
*Corresponding Author: Soma Prathibha. Email: somaprathi25@gmail.com
Received: 06 December 2021; Accepted: 12 February 2022
Abstract: Thyroid disease is a medical condition caused due to the excess release of thyroid hormone. It is released by the thyroid gland which is in front of the neck just below the larynx. Medical pictures such as X-rays and CT scans can, however, be used to diagnose it. In this proposed model, Deep Learning technology is used to detect thyroid diseases. A Convolution Neural Network (CNN) based modified ResNet architecture is employed to detect five different types of thyroid diseases namely 1. Hypothyroid 2. Hyperthyroid 3. Thyroid cancer 4. Thyroiditis 5. Thyroid nodules. In the proposed work, the training method is enhanced using dual optimizers for better accuracy and results. Keras, a Python library that is high level runs as the main part of the Tensor Flow framework. It is used in the proposed work to implement deep learning techniques. The comparative analysis of the proposed model and the existing work helps to show that there is a great improvement in the performance metrics in classifying the type of thyroid disease. By applying Adam and SGD (Stochastic Gradient Descent) optimizers in the training phase of the proposed model it was identified that these increase the operational efficiency of the modified ResNet model. After retraining the model with SGD, the modified ResNet provides more accuracy of about 97% whereas the basic ResNet architecture attains 94% accuracy. A web-based framework is also developed which yields the type of thyroid disease as the output for a given input scanned image of the system.
Keywords: Thyroid; deep learning; convolution neural network; modified ResNet; dual optimizer
According to studies, people who suffer from thyroid diseases adds up to 42 million people in India, from which the majority are female patients who suffer from hypothyroidism [1]. It is found that the affected patient ratio of women and men is up to 8:1. This clearly shows that the production of thyroid hormones is improper in females and requires quick action. Discussing the types of thyroid diseases, the most frequent cases are either secretion of too many hormones or way fewer hormones, namely hypothyroidism and hyperthyroidism. This type is found in most cases. Other varieties of disease are caused due to lumps or enlargement of the thyroid gland, known as thyroid nodules goitre. This gland plays an important role in controlling many vital functions of our body and is also responsible for all metabolic functions. Significantly, early detection of thyroid diseases is a primary factor that will reflect in the reduction of new cases. Thyroid detection is more error-prone than other diseases. Errors can occur when the scan images are manually interpreted which is time-consuming and also the accuracy is not guaranteed. Hence, it is not only important to create an efficient system for the issue but also necessary as the thyroid cases have increased from 6.8 percent to 10.7 percent in recent times. Initial diagnosis of thyroid diseases is highly recommended to save the lives of many people. The age-old method of detecting thyroid is error-prone and has its downsides and can also put people’s lives at stake. This process is cumbersome in an era of modern science and technology where the advancement in the medical field is so vast to ignore. Thus, making use of the technology at hand we have devised a very novel, deep learning architecture, to efficiently detect and identify the existence of five distinct types of thyroid problems. This way we can detect the presence of thyroid in the preliminary stage cost-effectively and smartly without letting it aggravate and prevent any threats thus saving any kind of errors that might happen in manual detection.
Significance to its importance, early detection of thyroid diseases is a primary factor that will reflect in the reduction of new cases. The fact that thyroid detection is different from various other diseases. It is prone to many malfunctions like the manual interpretation of scan images, demands high time consumption, and lacks accuracy. Hence, it is not only important to derive an efficient system for the issue but also necessary as the thyroid cases have increased from 6.8 percent to 10.7 percent in recent times. Thus, motive of the proposed system is to articulate a system that is highly profound to detect and identify the presence of thyroid diseases without any kind of havoc. In the existing system, prediction of thyroid diseases has been done using machine learning algorithms where the accuracy is driven in low rates compared to deep learning networks. The fact that the branch of neural networks under deep learning solve problems from end to end unlike machine learning techniques, that needs to be broken down into pieces and then solved.
The main contribution of the proposed work are i). Identify different types of thyroid diseases from the ultrasound images accurately using dual optimizers SGD and Adam on CNN with modified ResNet architecture ii). Evaluate the performance of the proposed modified ResNet architecture using SGD and Adam Optimizer iii). Compare the performance of Modified ResNet architecture with various iv). Develop a web-based framework using React JS to facilitate the uploading of thyroid images of the patient and finding the type of thyroid disease for the patient.
Deep Neural Networks (DNN)
Deep neural networks belong in Artificial intelligence, i.e., we can say DNN is its subset and it also is one of the fields of machine learning. Here, nothing is programmed explicitly. Numerous nonlinear processing units of the machine learning classes are used to perform feature extraction as well as transformation. The input by each one of the successive layers is the output from each preceding layer. Deep learning models are capable of focusing on the precise features themselves. It requires small guidance from the programmer’s side. It is very helpful in solving problems involving dimensionality. When we have a big number of inputs and outputs, deep learning methods are applied.
Deep Neural Networks are made up of neural network topologies that play a vital part in deep learning approaches. There are just 2–3 hidden layers in traditional neural networks, however, Deep Neural Networks might have over 150. It learns directly from the data, and deep learning models are trained without the need for manual feature extraction.
For larger datasets, deep learning expects to give higher accuracy and learns the features so precisely that outperforms any traditional method. Since deep learning comes with feature engineering, it gives much-expected results than machine learning. In [2] authors have discussed various deep learning algorithms using medical image segmentation. Deep learning has been used to address various real time problems. In [3] authors proposed a combined approach involving image processing and deep learning algorithms to detect the cracks present in building structures such as bridges and other massive structures
1.1 Convolution Neural Networks (CNN)
Deep Neural Networks of this sort are the most well-known. A CNN convolution learns the characteristics of the input data. It employs two-dimensional convolutional layers, making it ideal for processing 2D data such as pictures. In [4] authors have proposed a CNN based model for analysis/detection of COVID-19, to assist the medical practitioners to expedite the diagnostic process amongst high workload conditions. It does away with the necessity for manual feature extraction, removing the need to identify the features that are utilized to classify images. Among the neural networks employed in image processing, the Deep Convolutional Neural Network is important [5]. For automatic learning, CNN has numerous feature extraction steps. The image classification pipeline used by Deep CNN is depicted in the diagram below. The input image is first passed, after which the convolution layers are used to extract the input image’s features, and the pooling layers are used to lower the spatial resolution of the features. In general, there are two types of layer pooling: average and maximal pooling. Fully linked layers are a stack of convolution and pooling layers that execute high-level reasoning, analysing the output of all previous layers. ResNet architecture of CNN is used in the proposed system. Fig. 1 depicts the image classification pipeline in CNN. The following section provides a functional overview of the many layers in CNN.

Figure 1: Image classification pipeline in CNN
Input Layers: Inputs given to the nodes are classified into two layers namely the Image input layer and the Sequence Input layer. The images are added to the network with the input layer and it applies data normalization. The data is put to the network with a sequence input layer. Learnable Layers: Using a two-dimensional convolutional layer, the input is filtered using sliding filters horizontally and vertically and also by carrying out computations on input weights. With the Fully Connected Layer, the input is multiplied by a weight matrix. The bias vector and the weight matrix are then added. These two layers learn all of the image’s characteristics. Activation Layer: The activation layer is performed right before the learnable layer. RELU (Rectified Linear Unit) in the activation helps to do the threshold operations to each element of the input. Here the values that are negative are made to zero so as to discard the uncleared spots in the input. Normalization and Dropout Layers: Batch Normalization normalizes each and every input channel across the mini-batch. The first layer normalizes each channel’s activations by doing computations with a mini-batch mean value. After completing computations input shifting and scaling using learnable scale factor is done. The purpose of this layer is to help in training and improve network sensitivity. Input element needs to be initialized to zero in drop out layer randomly with a preset probability value. Pooling Layers: One of the most critical steps in model training is the pooling layer. Down sampling is performed by the Average Pooling 2d Layer. By splitting the input and finding the average values of each zone, rectangular pooling regions are created. Down sampling is also performed by MaxPooling2dLayer by finding the maximum of rectangular sections obtained from the input.
Output Layers: The two most notable functions in the output layer are the SoftMax layer and the classification layer, which is named after the loss function. This software is used to train the multiclass classification network. Finally, it gathers all of the learned models into one location, where they are assessed for accuracy.
There are various techniques used in the detection of thyroid diseases. In this section, a comprehensive literature survey about the various works carried out for classifying thyroid disease is presented.
The input image features like shape, image edge, the composition of the image are some of the major factors that can depict the probability of thyroid cancer occurrence. In [6] authors have used the DENSENET algorithm with the Augmentation technique on SPECT, Ultrasound, and CT images for diagnosis of thyroid disease. In [7] authors use a dataset of 1200 Ultrasound images and apply CNN and faster R-CNN for the classification of thyroid granules. In [8] authors have carried out review work on evaluating thyroid order using different machine learning algorithms on medical images comprised of ultrasound and CT scan images. Conic Section Function neural network algorithm on a data set with different image types USG, SPECT images, and planar scintigraphy to detect thyroid nodules is used by the researchers [9].
To identify a disease accurately recognition of patterns is vital in disease diagnosis. These machine learning models can deduce the output primarily based on the fed inputs which are interlinked to the previous values. Numerous classification algorithms are used for validating the problem statements. It narrates the algorithms used for image classification and datasets that are used in several research works [10]. KNN based thyroid classification is applied for evaluating the thyroid classification problem [11]. In [12] authors have used different machine learning algorithms for predicting the hyperthyroid and hypothyroid. The authors have used both the UCI machine learning repository and a local dataset. In their work, it was reported that the decision tree model gives better performance. In [13] authors have used two stage deep learning algorithm to predict thyroid malignancy from dataset consisting of cytopathology Whole Side Images (WSI). In [14] authors have used ensemble modeling for thyroid classification problems in women. Authors have used a dataset of 12000 instances and it was observed that Ensemble II gives high accuracy over Ensemble I. In [15] authors have applied object detection model T1-RADS for identifying the genetic risk in thyroid disease.
Recently, few research works have applied deep learning algorithms for the problem of thyroid detection and classification because of their capability of self-learning feature extraction within the scope of incremental learning. In [16], a comparison study of machine learning algorithms with faster R-CNN using RESNET architecture is implemented for the identification of thyroid nodules. In [17], thyroid papillary cancer identification using modified faster R-CNN is proposed. Recently, authors have [18] applied Hidden Markov Model for detection and segmentation of thyroid region from ultrasound images. In [19], authors used ANN to classify thyroid nodules using Region of Interest (RoI) from blocks for texture calculation and for segmentation they use Active Contour without Edge (ACwE). Unlike existing research work, in the proposed work a comprehensive model is used to classify all the types of thyroid diseases using modified ResNet architecture in CNN.
In some of the existing works, online transfer learning for differential diagnosis of benign and malignant thyroid nodules from ultrasound images are carried out, however most of the existing works implement detection of single thyroid disease where as in the proposed system detection of five different types of thyroid disease using CNN with modified RESNET architecture. One of the main disadvantages of transfer learning is only works if the initial and target problems of both models are similar enough. Also performance may be poor if the first round of training data required for the new task is too far from the data of the old task. Our main function here is to predict the presence of thyroid disease, which requires capturing spatial features of the image and locating them accurately. This is best achieved using convolution neural networks as artificial neural networks have major drawbacks like it loses the spatial feature of the image and fails to capture the sequential information of input data that is required for processing. While recurrent neural networks cannot be used as it is capable of processing only text and audio data that will not be necessary as everything here is an image. With the help of convolution neural networks along with the slight modifications in ResNet architecture, we can classify the types of thyroid disease from an ultrasound image. This architecture carries out both feature extraction and classification of medical datasets and also updates the model with high accuracy using dual optimizers. Hence, by applying novel deep learning architecture, prediction of the presence of thyroid disease will be identified at an early stage without any kind of malfunctions or delay.
Convolution Neural Network is one of the emerging deep learning algorithms which has proved to be providing greater performance in various areas associated with object detection, computer vision, and image processing. Many of the research works show AI and deep learning-based techniques show accurate disease predictions in medical imaging-based diagnosis. Recently, we can see many improvements in the network architecture of CNN which along with the technological advances are used to solve many interesting problems in medical imaging. In the proposed system, modified ResNet architecture is applied in the classification of all types of thyroid disease from X-ray images. In ResNet architecture problems from the gradient vanishing is minimized by using residual learning block. In this work, we also implement dual optimizers using Adam and SGD in the training process to improve the accuracy of the proposed model.
In the proposed system firstly, the datasets i.e., X-ray images of various thyroid diseases like a. Hyperthyroid b. Hypothyroid c. Thyroid nodules d. Thyroid cancer e. Thyroiditis f. Normal Thyroid is collected and labelled separately. Next, the medical images undergo augmentation to expand the training dataset for better performance. In the proposed work, the Image Data Generator of Keras is used to augment the images. It is then followed by preprocessing, training, and evaluation of the training dataset with the testing dataset. The block diagram in Fig. 2 shows the overall procedure of the novel architecture development in the detection of thyroid diseases.

Figure 2: Block diagram for the proposed system
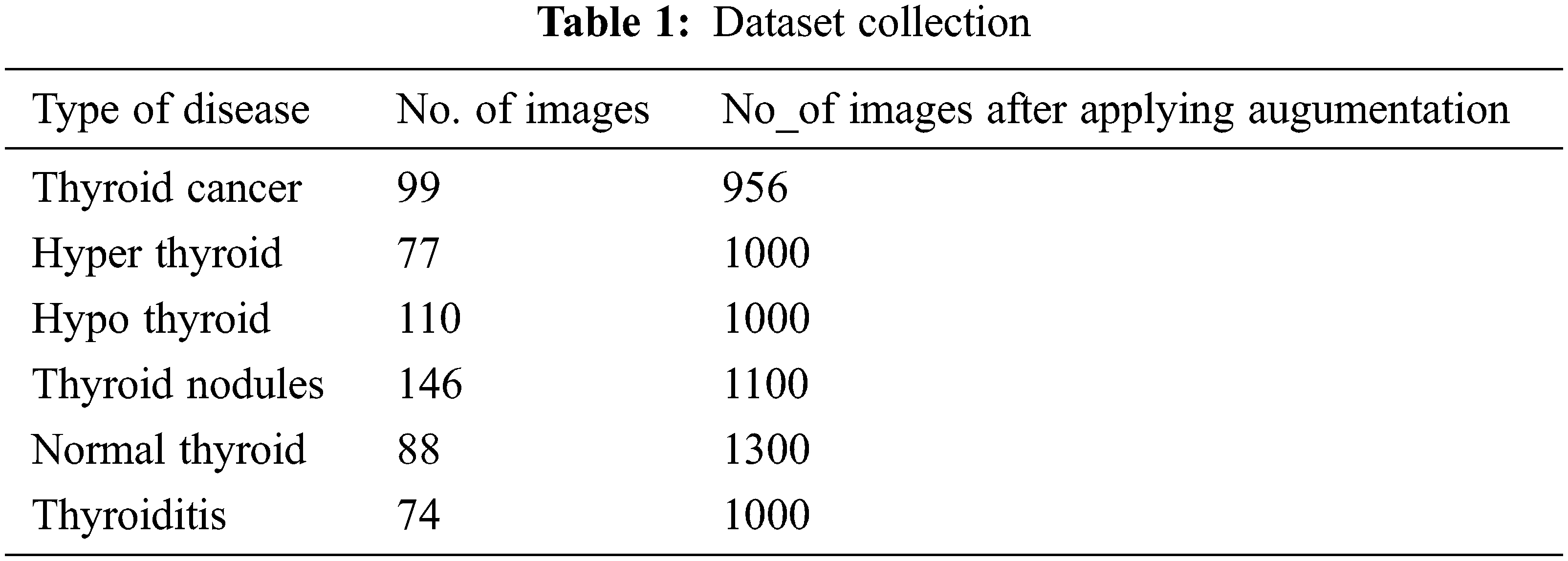
Most of the existing works have evaluated the machine learning or deep learning models using X-ray or CT scan datasets from the local hospitals. Since data has become such a valuable commodity in the deep learning era, many start-ups have started to offer their image annotation services where they will gather and label the data. One such platform the datasets gathered for the proposed model evaluation is from Kaggle [20–23]. New Thyroid dataset and thyroid dataset which contains ultrasound images for hyper and hypothyroid diseases available at UCI repository are also used for the performance evaluation of the proposed system. The number of datasets collected for the evaluation of the proposed system is described in Tab. 1:
The collected datasets have been labelled in accordance with the disease and the sample images from each disease are shown in Fig. 3.

Figure 3: Sample datasets
The performance of deep learning neural networks often improves with the amount of data available. In [24] augmentation techniques are developed for gray scale images and authors demonstrate that the generated augmented data set is highly effective for CNN algorithms used in machine vision. The size of the dataset is one of the important attributes for the quality performance that can be expected from the deep learning algorithms. In this work, the size of the dataset is increased by using the Image Data Generator class from Keras to perform data augmentation. It uses the classical operations rotation, shearing, cropping, flipping, etc., for increasing the size of the data set. In this work, flipping and rotation have been used to increase the size of the dataset. In the initial step, flipping is applied for each class of the thyroid dataset to double the number of images. After this operation, the resultant data set has 956 Thyroid cancer images, 1000 hyperthyroid images, 1000 hypothyroid images, 1100 thyroid nodules, 1000 Thyroiditis images, and 1300 normal thyroid images. In the next phase, the flipped thyroid classes of images are rotated by 45 degrees, 90 degrees, 180 degrees, and 270 degrees. Fig. 4, illustrates a set of augmentation operations of datasets applied to the initial dataset used in the proposed system.

Figure 4: Transformation of images after augmentation
All the collected datasets have different sizes and unclear images. To convert all the datasets into the same sizes and to remove obscure data, data pre-processing has been done. Pre-processing techniques used in the proposed system are simple pre-processing, aspect-aware pre-processing, and image to array pre-processing. Simple pre-processing removes unclear or unwanted images from the dataset. Since the datasets are collected in various forms, aspect-aware pre-processing helps convert the datasets into the same sizes and gives a high resolution to the image. Image to array pre-processing reduces the image weight by converting the RGB value into a matrix ranging from 0 to 1. Incorporating all these preprocessing techniques into the datasets, finally, the image obtained after the preprocessing is depicted in Fig. 5.

Figure 5: Image preprocessing
3.3 Modified ResNet Architecture
The ResNet network architecture is made out of residual blocks and was proposed in 2015 by Microsoft. The residual block helps learn the model much deeper through the activation function. The activation function ‘a’ is given where w and b are weight and bias respectively in the Eq. (1).
The advantage of ResNet is that it can use more layers to improve accuracy, unlike other Deep Neural Networks architecture. In other neural networks architectures, if more layers are added, it results in a Vanishing/Exploding gradient that avoids us learning deeper into the network which will not boost the accuracy due to the reduction of weight (vanishing gradient), and also increases error rates. To avoid such conditions, ResNet uses the skip connection technique where it skips training of some activation layers and feeds the output to the upcoming layers as shown in Fig. 6. This skip connection technique is used in residual blocks which allows us to train many deeper neural networks. Therefore in the proposed work, ResNet is used for providing better accuracy

Figure 6: Skip connection in residual block
In Fig. 7, the input of the neural network block is x, and I(x) is the true distribution. The residual is calculated based on the difference between output and input, it is calculated using in Eq. (2)
rearranging it we get,

Figure 7: Comparison of precision, recall, and F1-scoreoutcomes
3.4 Enhancement in the Training Using Dual Optimizer
In the proposed system the training is enhanced in ResNet architecture by carrying out batch normalization, activation and Conv2D occurs three times in the residual module where for each time the Conv2D extracts the feature of the image by (1, 1), (3, 3), and (1, 1) respectively. For the first time, Conv2D has already extracted every pixel of the image and also covered every edge of the image by using the (1, 1) matrix. So for the third time instead of extracting the feature by (1, 1), the proposed system extraction of the feature is modified by (3, 3) which helps the model to learn more and give better accuracy. Optimization is one of the important processes in training. Also to improve the accuracy of the proposed system, Stochastic Gradient Descent, and Adam optimization techniques [25] are used in the proposed system. The training is first done using Adam which is the combination of AdaGrad and RMSProp which in turn are the extensions of Stochastic Gradient.
where =
Eq. (4) is the SGD equation to update parameters in neural networks where it reduces the parameter variance which in turn leads to good accuracy.
where θ-is a parameter,η-learning rate,
4 Result Discussions and Outcomes
In this section, the details of the results of a comparative study of the proposed CNN with the Modified ResNet Architecture and the CNN with Naive ResNet Architecture is discussed. The proposed system is validated by conducting experiments implemented using TensorFlow, a deep learning framework performed on a workstation having specifications of 8 GB RAM and an Intel (R) Core (TM) i7 CPU. After collecting various medical images, we augmented the datasets and pre-processed them accordingly. Later the processed images were trained using the ResNet deep neural network in CNN with Adam and SGD as optimizers. In the following section, results of the comparison of Naive ResNet Architecture and proposed modified ResNet Architecture with dual optimizers are discussed. The original data set was increased and there were around 2000 images in the resultant dataset after applying augmentation and pre-processing.
For any effective deep learning model, the prominent process is evaluating the proposed model, certain key classification metrics like Precision, Accuracy, F1-score, Recall, have been computed for the purpose of evaluation of the proposed work. It is based on the totals of the True Positive, False Positive, True Negative, and False Negative. The equations for evaluating these metrics are given below.
Accuracy is the overall percentage obtained for the accurate prediction of the model. It is the most important metric among all and it also determines whether the model is successful or not, it is calculated as follows
A Recall is a sensitive rate, in this work it depicts that out of all thyroid affected patients it tells how many it has correctly identified.
Precision is the measure of the model confidence and it specifies patients the model correctly identifies having thyroid disease
The harmonic mean of the proposed model’s accuracy and recall is F1-score. Higher the classification metrics, the higher the performance of the proposed model.
4.2 Experiment1: Proposed Model Evaluation Using Optimizer I
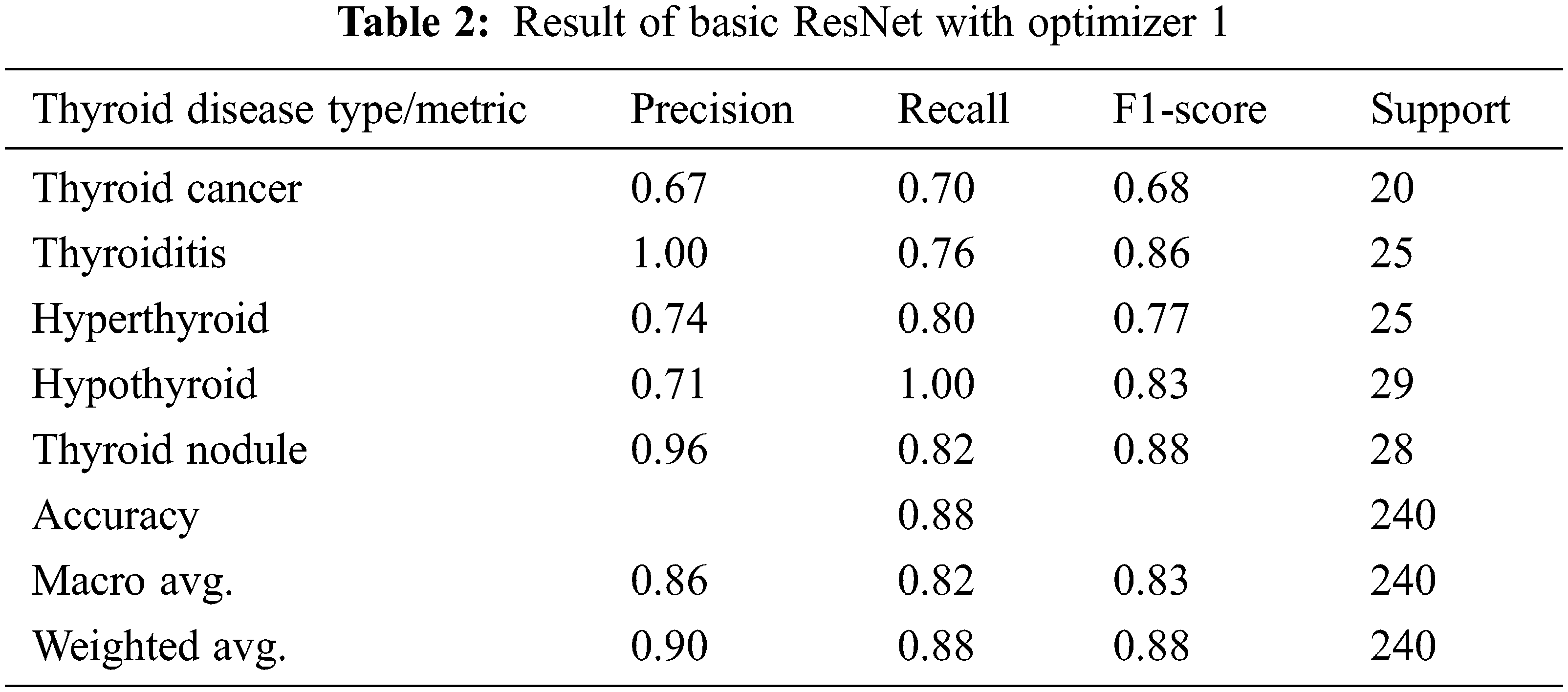
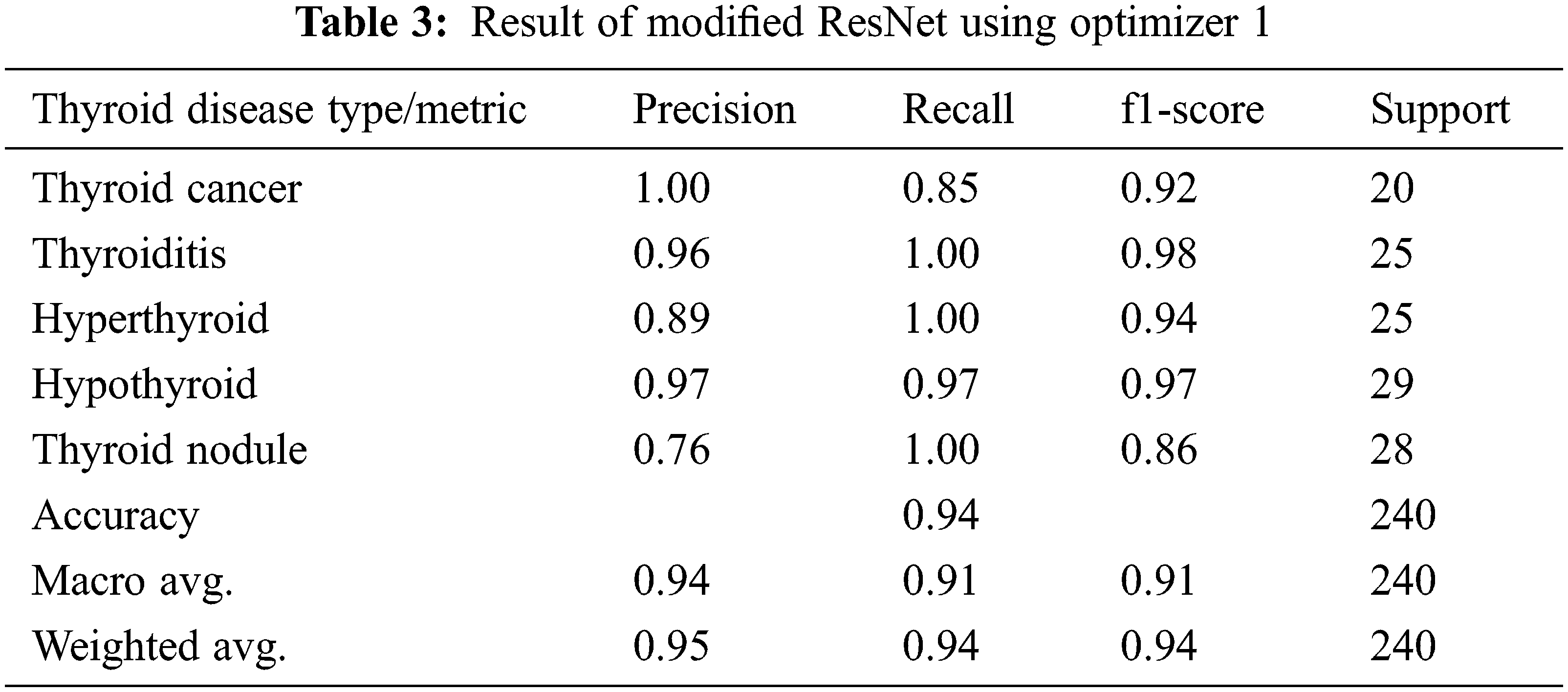
In the first experiment, we have applied an Adam optimization to the modified ResNet-50 architecture to improve the accuracy, and this is the first level of optimization in the proposed system. In the first experiment optimizer, I is applied for the basic ResNet Architecture and the results of this experiment are given in Tab. 2. It is evident from the table that there is an overall accuracy of 88% given by the model. In the next experiment proposed modified ResNet Architecture is combined with the optimizer, I, and the outcomes of the experiment mentioned above are given in Tab. 3. Results clearly show that optimizer I is more effective in modified ResNet compared to the basic ResNet model.
4.3 Experiment2: Proposed Model Evaluation Using Optimizer II
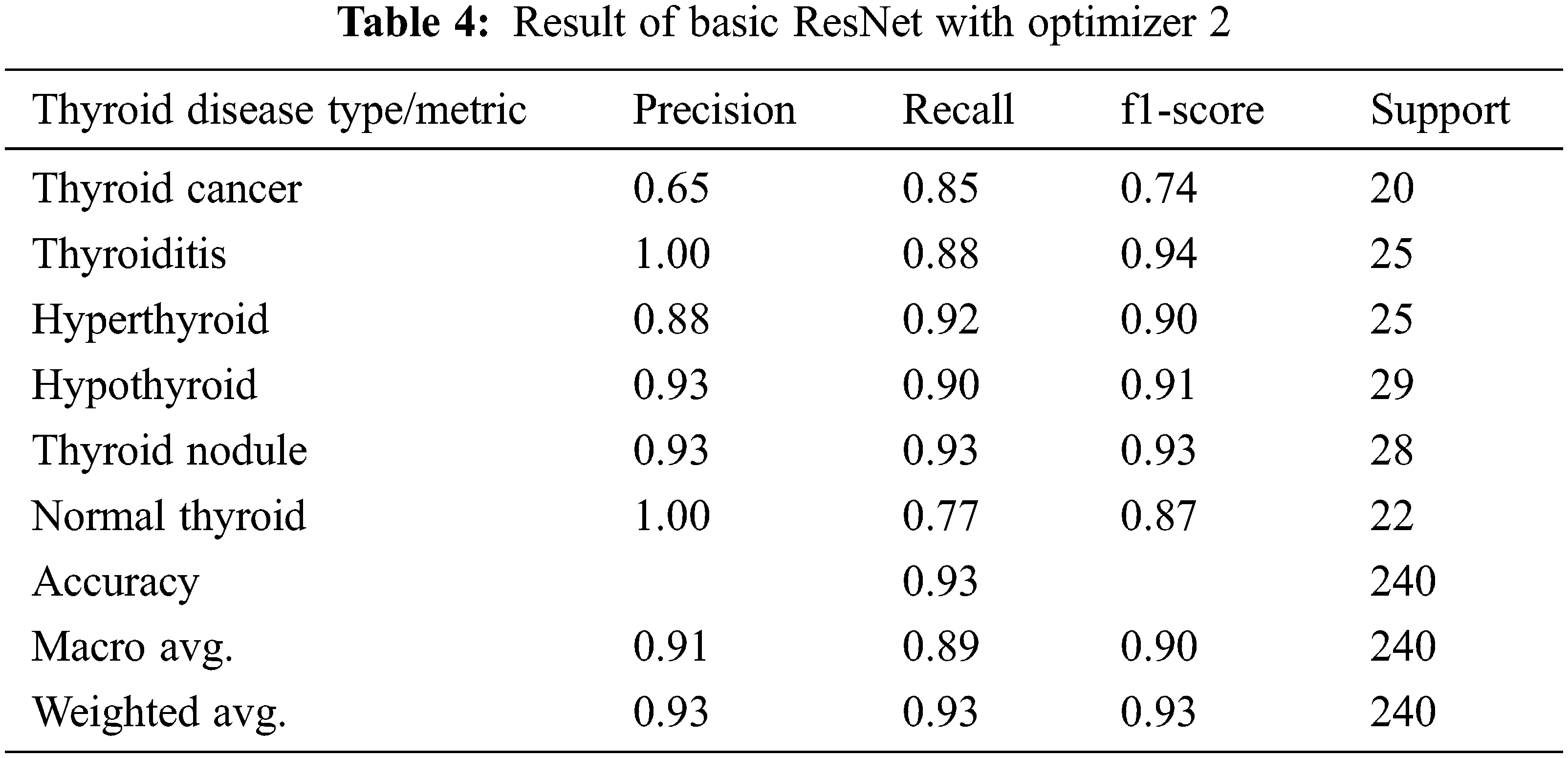
In Experiment 2, Adam Optimizer with SGD is further used to increase the precision of the model we have proposed to classify the thyroid type of patient and this is considered as level 2 of optimization in this work. In Tab. 4 experimental results of applying basic ResNet with the Optimizer, II is given and the results show that it gives an overall accuracy of 93%, whereas the modified ResNet with Optimizer II is able to give the highest accuracy of 97% and these results are given in Tab. 5. Results clearly indicate that the proposed model gives better accuracy results with both the optimizers over basic Resnet Architecture.

The variation in the values of performance metrics based on different optimizers used in both basic and modified ResNet architecture is plotted as a graph for easy understanding. In the Fig. 7, the precision metric depicts that how precise the model is out of those predicted positive and then the actual positive value predicts how many positive values occurred.
From Fig. 7, it is evident that the fourth classifier Modified ResN + 111et with dual optimizer provides the highest precision of 98% with almost 8% improvement over the basic ResNet classifier with optimizer 1. It can be seen from the figure that dual optimizers during training give better results in identifying accurate true positives.
Another statistic that illustrates the correctness of the suggested model is recall, which shows that thyroid illness classification is right by indicating the number of true positives vs. the number of true positives and false negatives. It basically displays how many positive values the model has labelled. As shown in Fig. 8, the number of genuine positives from the Naive RestNet with optimizer 1 is the lowest, with an 87 percent recall value, and there is a considerable rise in recall values when the dual optimizer is used.

Figure 8: Comparison of accuracy for adam and SGD optimizers
F1-score is another crucial performance parameter that is used to evaluate the efficiency of the model proposed. The harmonic mean of precision and recall is represented by it. F1 score has very significant importance in evaluating the proposed model as the cost of false positives and false negatives have a severe effect. We can see from the above graph that, the dual optimizer-based modified ResNet architecture gives better performance for F1-score over other optimizers. In Fig. 8 comparison of accuracy, recall, and F1-score for all the four models are shown. It can be seen from the figure that the CNN model with modified ResNet architecture using optimizers in training gives better performance over the basic ResNet architecture.
4.4 Experiment 3: Comparison of Adam and SGD Optimizers
In this to analyze the performance of the optimizers in the experiments were conducted. Now coming to the overall accuracy obtained, the modified ResNet gives a tremendous change in terms of accuracy over basic Resnet. As in Fig. 8, the variation on Adam optimizing the basic Resnet gives lower accuracy than the modified ResNet. After retraining the model with SGD, the modified Resnet gives more accuracy of about 97% whereas the basic architecture attains 94%. It can be noticed that SGD optimizer results in the high accuracy compared to Adam optimizer.
The training loss occurs when the model is being trained and occurs when the model is unable to learn the features. The validation loss, on the other hand, fails to evaluate the projected result. The training accuracy and validation accuracy both determine how much the model has learned and how accurate it is in producing correct results. The model’s loss and accuracy data are saved for each epoch. The graph in Fig. 9 shows the number of epochs against training loss vs. validation loss and training accuracy against validation accuracy.

Figure 9: Training loss and accuracy
4.5 Experiment 4: Comparative Analysis of Different CNN Network Architecture
Comparison on other Deep Neural Networks such as VGG16, VGG19, InceptionV3, and Exception over the proposed system has been implemented. The proposed system gave a good accuracy when compared to the other Deep Neural Networks. The comparison is illustrated in Tab. 6.

As shown in the above table, we can find that the performance of ResNet50 network architecture is superior to InceptionV3, VGG16, VGG19, and Exception. This indicates that the proposed classifier based on the Resnet architecture has resolved the Thyroid disease classification more effectively than the other four classifiers.
This work investigates the methods to improve the CNN-based deep learning algorithm with ResNet-50 architecture. This helps discern the existence of thyroid maladies and helps people take preventive steps to prevent the infliction of the disease. To greatly boost the accuracy of the suggested model for complete categorization of thyroid disease, two optimizers, Adam and Stochastic Descent Gradient algorithm, are proposed. Experiment results indicate that proposed dual optimizer model gives accuracy of 97% which is better than existing algorithm. Also, proposed model outperforms the existing systems in other metrics F1-Score and Recall. In the future, new ways for recognizing more types of cancer regions in the context will be researched. In addition, more research will be conducted to determine how to create a comprehensive and realistic diagnostic report. The results of the investigation on the applicability of several enhanced augmentation approaches with real-time datasets will be examined in the future. The research can also be extended to classify thyroid nodule detection in ultrasound videos and additionally the proposed system can be enhanced to track and observe the changes in the surrounding tissues.
Funding Statement: Dr. Deepak Dahiya would like to thank Deanship of Scientific Re-search at Majmaah University for supporting his work under Project No. (R-2022-45)
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. S. Bagcchi, “Hypothyroidism in India: More to be done,” The Lancet Diabetes & Endocrinology, vol. 2, no. 10, pp. 778, 2014. [Google Scholar]
2. K. A. Davamani, C. R. Robin, S. Amudha and L. J. Anbarasi, “Biomedical Image Segmentation by Deep Learning Methods,” in The Computational Analysis and Deep Learning for Medical Care, 1sted, Scrievener Publishing LLC, Beverly, USA, pp. 131–154, 2021. [Google Scholar]
3. A. Reghukumar and L. J. Anbarasi, “Crack detection in concrete structures using image processing and deep learning,” in Advances in Electrical and Computer Technologies, Lecture Notes in Electrical Engineering, vol. 711, Singapore: Springer, 2021. [Google Scholar]
4. S. Sanket, M. Vergin Raja Sarobin, L. Jani Anbarasi, J. Thakor, U. Singh et al., “Detection of novel coronavirus from chest X-rays using deep convolutional neural networks,” in Multimedia Tools & Applications, Switzerland AG, Springer Nature, pp. 1–26, 2021. [Google Scholar]
5. Y. LeCun, Y. Bengio and G. Hinton, “Deep learning,” Nature, vol. 521, no. 7553, pp. 436–444, 2015. [Google Scholar]
6. L. Ma, C. Ma, Y. Liu and X. Wang, “Thyroid diagnosis from SPECT images using convolutional neural network with optimization,” Computational Intelligence & Neuroscience, vol. 2019, Article ID 6212759, pp. 11, 2019. [Google Scholar]
7. H. Li, J. Weng, Y. Shi, W. Gu, Y. Mao et al., “An improved deep learning approach for detection ff thyroid papillary cancer in ultrasound images,” Scientific Reports, vol. 8, no. 1, pp. 1–12, 2018. [Google Scholar]
8. X. Li, S. Zhang, Q. Zhang, X. Wei, Y. Pan et al., “Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: A retrospective, multicohort, diagnostic study,” The Lancet Oncology, vol. 20, no. 2, pp. 193–201, 2019. [Google Scholar]
9. D. T. Nguyen, J. K. Kang, T. D. Pham, G. Batchuluun and K. R. Park, “Ultrasound image-based diagnosis of malignant thyroid nodule using artificial intelligence,” Sensors, vol. 20, no. 7, pp. 1822, 2020. [Google Scholar]
10. A. Sheeja Agustin and S. S. Babu, “A review of thyroid disorder detection, segmentation and classification on medical images,” International Journal of Engineering and Advanced Technology (IJEAT), vol. 32, pp. 2249–8958, 2013. [Google Scholar]
11. G. Chaubey, D. Bisen, S. Arjaria and V. Yadav, “Thyroid disease prediction using machine learning approaches,” National Academy Science Letters, vol. 44, no. 3, pp. 233–238, 2021. [Google Scholar]
12. I. Ioniţă and L. Ioniţă, “Prediction of thyroid disease using data mining techniques,” BRAIN. Broad Research in Artificial Intelligence and Neuroscience, vol. 7, no. 3, pp. 115–124, 2016. [Google Scholar]
13. D. Dov, S. Z. Kovalsky, S. Assaad, J. Cohen, D. E. Range et al., “Weakly supervised instance learning for thyroid malignancy prediction from whole slide cytopathology images,” Medical Image Analysis, vol. 67, pp. 101814, 2021. [Google Scholar]
14. D. C. Yadav and S. Pal, “To generate an ensemble model for women thyroid prediction using data mining techniques,” Asian Pacific Journal of Cancer Prevention: APJCP, vol. 20, no. 4, pp. 1275, 2019. [Google Scholar]
15. S. Wang, J. Xu, A. Tahmasebi, K. Daniels, J. B. Liu et al., “Incorporation of a machine learning algorithm with object detection within the thyroid imaging reporting and data system improves the diagnosis of genetic risk,” Frontiers in Oncology, vol. 10, pp. 2481, 2020. [Google Scholar]
16. X. Yu, H. Wang and L. Ma, “Detection of thyroid nodules with ultrasound images based on deep learning,” Current Medical Imaging, vol. 16, no. 2, pp. 174–180, 2020. [Google Scholar]
17. H. Li, J. Weng, Y. Shi, W. Gu, Y. Mao et al., “An improved deep learning approach for detection of thyroid papillary cancer in ultrasound images,” Scientific Reports, vol. 8, no. 1, pp. 1–12, 2018. [Google Scholar]
18. B. Shankarlal, P. D. Sathya and V. P. Sakthivel, “Screening thyroid tumor in ultrasound thyroid images using hidden markov model,” in Proc: Third Int. Conf. on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, pp. 1420–1425, 2021. [Google Scholar]
19. X. A. Kesarkar and K. V. Kulhalli, “Thyroid nodule detection using artificial neural network,” in Proc: Int. Conf. on Artificial Intelligence and Smart Systems (ICAIS), Coimbatore, India, pp. 11–15, 2021. [Google Scholar]
20. https://www.kaggle.com/officialdataset/thyroid-hyper. [Google Scholar]
21. https://www.kaggle.com/officialdataset/thyroid-nodule. [Google Scholar]
22. https://www.kaggle.com/officialdataset/thyroid-ditis. [Google Scholar]
23. https://www.kaggle.com/officialdataset/thyroid-cancer. [Google Scholar]
24. J. Wang and S. Lee, “Data augmentation methods applying grayscale images for convolutional neural networks in machine vision,” Applied Sciences, vol. 11, no. 15, pp. 6721, 2021. [Google Scholar]
25. H. Robbins and S. Monro, “A stochastic approximation method,” The Annals of Mathematical Statistics, vol. 22, no. 3, pp. 400–407, 1951. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |