

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.021706
ARTICLE
Cellulose Fibre from Schinus molle and Its Characterization
1Laboratory for the Application of Materials to the Environment, Water, and Energy (LR21ES15), Faculty of Sciences of Gafsa, University of Gafsa, Gafsa, 2112, Tunisia
2Faculty of Sciences of Gafsa, University of Gafsa, Gafsa, 2112, Tunisia
3Laboratory of Environmental Chemistry and Cleaner Process (LR21ES04), University of Monastir, Monastir, 5019, Tunisia
4Higher Institute of Technological Studies (ISET) of Ksar-Hellal, Ksar-Hellal, 5070, Tunisia
5University of Grenoble Alpes, CNRS, Grenoble INP, LGP2, Grenoble, F-38000, France
6Organic Chemistry Laboratory (LR17ES08), Faculty of Sciences of Sfax, University of Sfax, Sfax, 3029, Tunisia
*Corresponding Author: Mohamed N. Belgacem. Email: naceur.belgacem@pagora.grenoble-inp.fr
Received: 30 January 2022; Accepted: 28 March 2022
Abstract: The exploitation of biomass represents a major environmental challenge related to the protection of the environment and the progressive exhaust of fossil resources. In this perspective, the main objective of this work is the extraction and the characterization of natural lignocellulosic fibers from the Schinus molle. The cellulose fibre extraction was investigated employing conditions of alkali treatment. After the alkaline steps, a bleaching treatment was done and let to a yield about 45% pure cellulose. The identification of the chemical composition of Schinus molle reveals that this raw material contains a high level of biopolymers with a cellulose rate of 53.2%. Extracted cellulose fibers have been characterized by several techniques such as scanning electron microscopy, Fourier transform infrared, X-ray diffraction, Morfi, and by the determination of their degree of polymerization. FT-IR results confirm the purity of the cellulosic fibers, and XRD analysis reveals that the crystallinity increases after the delignification and bleaching treatments.
Keywords: Schinus molle; cellulose; alkaline treatment; morphological studies
The search for alternative sources of energy and raw materials is becoming more intensive. Therefore, the objective of a large number of publications as well as the new trends of our manufacturing is the valorization of biomass. Regarding this background, innovation is accelerating, particularly in the field of materials. Extensive academic and industrial research efforts are now focusing on creating products made from renewable, sustainable and environment friends’ resources to substitute conventional materials [1–6]. Thus, strong interest is being shown in cellulosic fibres with the aim of replacing petroleum products in order to meet industrial needs. Because of that, natural lignocellulosic materials which are made up of different components, until now, have been relatively more and/or less valorized. There has been the advantage of sustainable development, excellent biocompatibility, untoxic and biodegradable compared to synthetic polymers, also, there has been paid to natural polymers for environmental preservation [4,7]. Nowadays, the natural polymers derived from natural resources present a key challenge in terms of environmental protection and the gradual exhaustion of the world’s fossil reserves. There has been rapidly growing for several applications such as, automotive industries, pharmaceuticals, agriculture.
Cellulose is a semi-crystalline biopolymer that is very abundant renewable polymer on earth [8]. It represents more than 50% of the biomass, and the major substance which makes up the cell walls of plants, constituting about one half to one-third of all plant tissues constantly renewed by photosynthesis [9,10]. Generally, the most plant materials are made up of about 40–55 wt% of cellulose, 15–35 wt% of lignin and 25–40 wt% of hemicelluloses, and the composition varies depending on their organic source [8]. Within the natural plant cell wall, the crystalline cellulose is linked to lignin and hemicelluloses, thereby making it difficult to obtain pure cellulose [11]. The structure of cellulose derives from the dimerization of the β-glucose monomer, which subsequently gives rise to the repeating unit known as cellobiose. In 1920, Staudinger [12] proposed a structure of cellulose as a linear polysaccharide of anhydro glucose units that are linked with carbon atom one and four by β-glycosidic bond. This is verified by the existence of three hydroxyl groups (OH) with different acidity/reactivity. Secondary OH positioned at C–2, and C–3 position, while the primary OH located at the C–6 position. It can be also confirmed by the formation of various intermolecular and intramolecular hydrogen bonds [9,10]. As we know that the cellulose is intimately attached to hemicellulose and lignin, consequently severe extraction conditions are required to isolate the cellulose. Several treatment and standard methods have been studied to extract the cellulose. They are carried out by considering two parameters: the decreasing of lignin and hemicellulose content. Conversely, the crystallinity of the cellulose is increasing. Such techniques use both chemical and physicochemical methods, among which are pulping process [9,13], steam explosion [14], alkaline treatment [15–17], etc.
Due to its abundance, renewability and interesting properties (low density, high strength, high tensile modulus, ecology, durability…), cellulose has many applications in various fields: pharmaceuticals, chemical feedstocks, paper manufacturing and liquid fuel production [18–23]. In this regard, a number of researchers have been conducted to study the use of lignocellulosic fibers. Tunisia has the advantage of a varied and rich flora that produces a variety of natural matters like essential oils, organic and wide-ranging aromas, also, as fabrication of paper, bio-composites and cellulose derivatives [24–26]. In this context, Schinus molle is a plant widely distributed in Tunisian. This species has the potential to be utilized as alternative, renewable, and sustainable source of cellulosic wood fiber. Schinus molle (Schinus molle L.; Anacardiaceae) is a fast-growing evergreen tree (up to 10 m in tall) [27], with perennial foliage that loses one third of its leaves per year. Schinus molle is a native to southern Brazil, Uruguay, Bolivia, Peru, and central Argentina and has been cultivated throughout Europe. It has also been introduced to parts of the United States, Africa, Asia and Europe, at which point, it has been categorized as an invasive plant [28]. This species is characterized by stress resistance, tolerance to poor soils [29]. This better adaptation to various climates and soils allows this plant to be used as a highly efficient source of biomass production. Consequently, a number of applications for this plant are used, such as culinary, ornamental and medicinal species due to its biological effects, namely anti-inflammatory, antioxidant, antidiabetic, anti-ulcer, antibacterial, anti-tumoral, anti-depreѕѕant [30–32].
The objective of this study is to investigate the viability of cellulose isolated from the Schinus molle as an abundant, renewable, and low-cost source. This paper describes the investigation of possible valorisation of lignocellulosic residue which, to the best our knowledge, is reported for the first time. The new cellulose fibres allow envisaging the valorisation of such crops as raw material for cellulose derivatives or as lignocellulosic fibres for papermaking and/or fibre-reinforced composite materials. For this purpose, cellulose was extracted from the Schinus molle under several alkaline processing to obtain a material with high pulping yield. Several techniques were employed to characterize the cellulose from Schinus molle such as, XRD, TEM, FTIR, and Morfi. Also, the obtained cellulose was characterized by the determination of the degree of polymerization.
2.1 Raw Material and Chemicals
During this work, Schinus molle was chosen as a raw material (R-SM). This plant was collected from Gafsa Region (Tunisia) in February 2019. The material was then washed to remove sand and moisture, following by drying under natural condition: average humidity 12.93% and average temperature 25°C. The raw material was shopped into small sizes of 5 × 5 × 5 for facilitating the fiber extraction, followed by grinding and screening (Fig. 1).
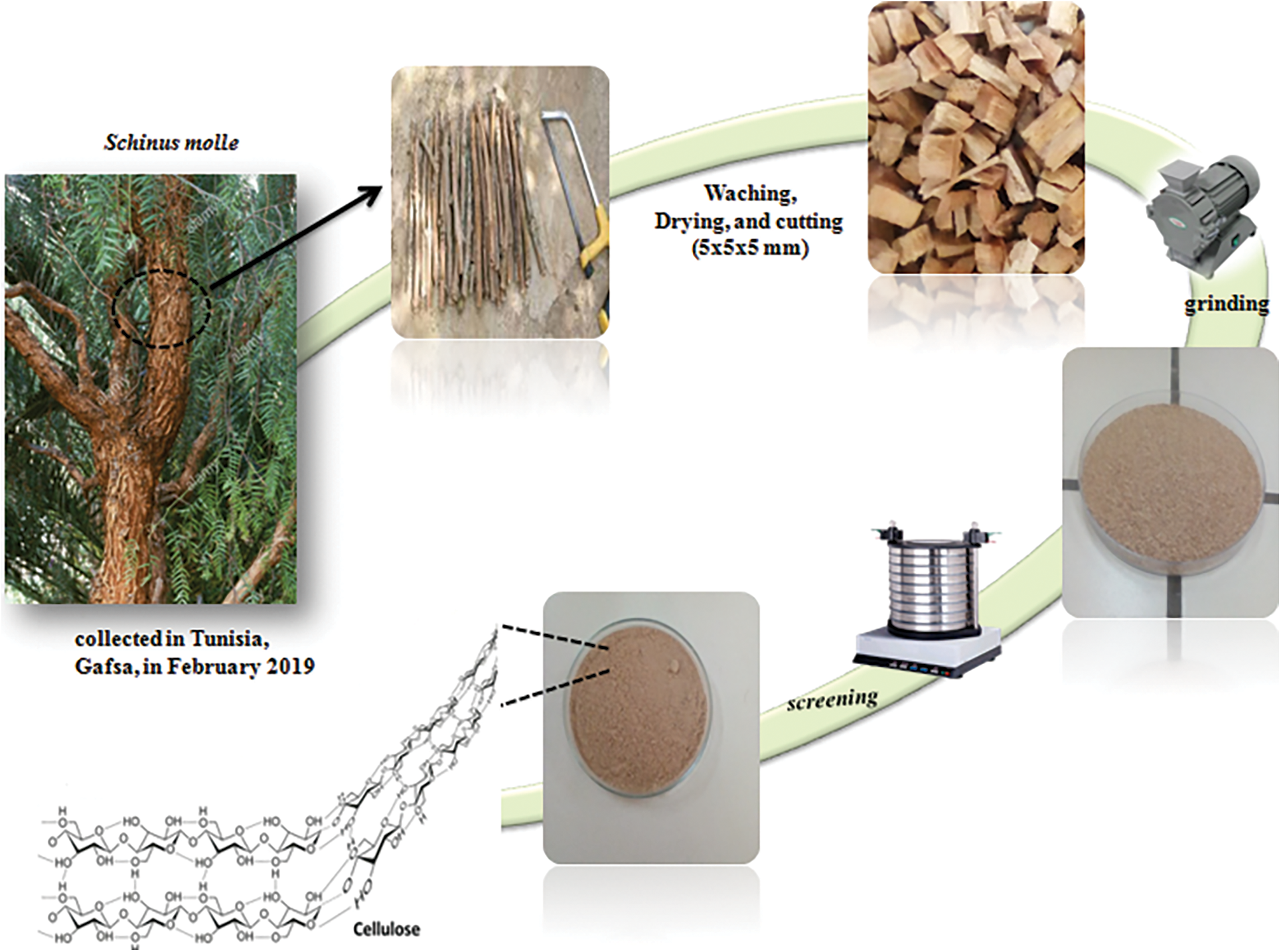
Figure 1: Different steps of Schinus molle preparation
The following were used as bleaching agents: Sodium chlorite, acetic acid, and sodium hydroxide. All these chemicals were purchased from Sigma Aldrich and utilized without further purification.
2.2 Extraction of Cellulose Fiber from Schinus molle
Two steps are needed to accomplish the preparation of cellulose from Schinus molle, namely, delignification and bleaching. The most part of lignin and hemicellulose was eliminated by treating dry fibers with 10% sodium hydroxide solution at 160°C for 2 h.
The obtained fibers were then washed with water. The last step consisted in removing hemicellulose from the isolated fibers was carried out. This stage consisted in obtaining fibers with a high level of purity [33]. The obtained fibers were mixed in sodium chlorite solution (1.7%) and tampon acetate. The reaction was done in water at 80°C, with a mechanical stirring of the suspension. After 2 h, a filtration and washing with distilled water was carried out until the suspension had a pH equal to that of the distilled water. The treatment was repeated twice times.
Chemical composition of Schinus molle was quantified utilizing standardized methods and common procedures. The extracts were obtained according to common TAPPI methods, in particular, extracts in cold and hot water (T207 cm-08), extracts in 1% sodium hydroxide solution (T212 om-07), and extracts in ethanol-toluene system (T224 om-07). Then, lignin and cellulose contents were determined in accordance with TAPPI methods T222 om-06 and T203 cm-99, respectively. Holocellulose amount was assessed using the methodology of Wise et al. [34]. Finally, ash content has been determined applying the TAPPI standard method T221 om-07, involving calcinations of the organic matter under 600°C during 8 h. Ash obtained was subjected to an analysis of the mineral fraction which was carried out at the “Creallins groupe”.
The morphology of the surfaces of the raw material and the cellulosic materials from the Schinus molle were visualized by SEM device. The analysis was carried out using the JOEL JSM-IT 100, which features an acceleration voltage of 0.5–30 kW and a resolution of 4.5 nm (the fibre surfaces were coated with gold in order to make them conductive before SEM analysis).
The morphological analysis was also assessed by Morfi. The Morfi is a laboratory analyzer developed by Grenoble INP Pagora and the Technical Paper Center (CTP). This technique is applied to investigate the principal morphological parameters of fibres suspended in water; it allows the following quantities to be obtained: fiber length, width, number of bends and fiber curvature, linear mass, percentage area or length of fine elements.
The infrared spectrums were obtained in absorbance mode using a spectrometer Ѕhimadzu 8400-Ѕ. The detection range was between 600 and 4000 сm−1. The samples were grinded in the presence of KBr anhydrous at a rate of 1% by weight, and then packaged in the form of tablets.
The crystallinity of raw material (R-SM), holocellulose (H-SM), and delignificated-bleached fibre (F-SM) was investigated by X-ray diffractometer (D8-Advance Bruker AXS GmbH) at ambient temperature (≈23°C) with a monochromatic Cu Kα radiation source in step-scan mode with an angle 2θ (5–60°), for a current of 4 mA and a scan time of 5 min. the approach of Segal et al. [35] was used to calculate the crystallinity index the cellulose was used, which gives the ratio between peak heights (I002-IAm) and total intensity I002. The crystallinity index is given by the following empirical Eq. (1):
where, IAm: the intensity relative to the amorphous phase, and I002: the maximum of the peak relative to the crystalline zone.
2.3.4 Degree of Polymerization
To determine the degree of polymerization, the method used consists in measuring at 25°C the flow time of cellulose solution dissolved in cupriethylene diamine (CED). The protocol used (NFT 12–005 standard) is to determine the intrinsic viscosity [ɳ] (cm3/g). The average degree of polymerization (DPV) is obtained by Mark-Houwink’s Eq. (2):
K and α are constant corresponding to the couple CED/cellulose solution at 25°C: K = 7.510–3 m2/S2 and α = 1.
3.1 Chemical Composition of Schinus molle
The chemical composition of the lignocellulosic fibres of the Schinus molle was determined by applying standardized methods. The results obtained are shown in Table 1.
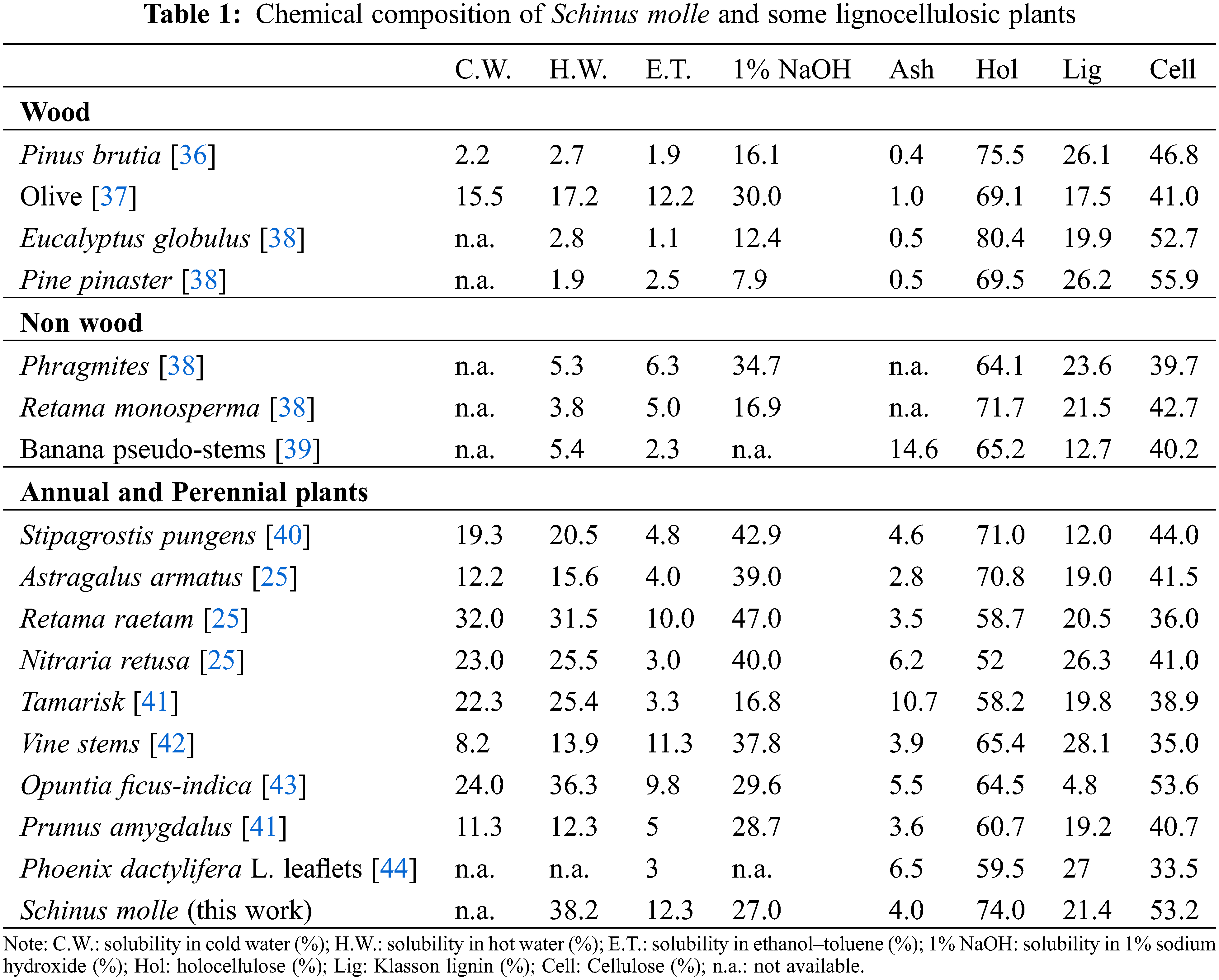
Schinus molle has a relatively high rate in extractible in hot water (i.e., 38.2%) and in a 1% NaOH solution (i.e., 27%) as well as in the ethanol/toluene system have a yield of about 12.3%. Such this behavior was observed largely in the common some annual plants like Vine stems [42], Opuntia ficus-indica [43], Stripagrostis pugens [40], Retama raetam and Nitraria retusa [26]. Lignocellulosic fibres of the Schinus molle have a Klason lignin content of 21.4%, which is comparable in general with some wood and non-wood annual plants for example Eucalyptus globules, Phragmites, and Retama monosperma [38], Retama raetam [25], Tamarisk and Prunus amygdalus [41]. Nevertheless, it is still higher than that of annual plants such as Opuntia ficus-indica [43], Astragalus armatus [25], Stipagrostis pungens [40].
The investigated plant has a holocellulose rate of 74%, which is comparable to that for wood and non-wood, whereas it seems be higher than for many annual and perennial plants (Table 1). The amount of α-cellulose (53.2%) was similar to Opuntia ficus-indica [43]. The Schinus molle has a higher proportion of α-cellulose than the different lignocellulosic fibres especially in the case of annual plants. Thus, the amount of the ash was found about 4% which is similar to some annual and non-wood plants but slightly higher than that of wood sources. The detailed characterisation of the lignin and holocellulose was not yet established and will be the matter of investigation in the next studies of our group. Since the significant amount of ash for this raw material, their chemical composition was determined. As a result, the ash is mainly composed of Ca, Cl, K and Na. A very low absolute quantity of silicon (15 ppm w/w on o.d. raw material) was found. This result considered as positive factor such the high amount of ashes cannot be considered as problematic, in terms of delignification and papermaking treatments, since the silicon-based salts are negligible. This new lignocellulosic residue can be considered as potential sources of fibers for textile and papermaking industries, as well as for innovative materials such as cellulose fibre-based composites.
3.2 Morphological Analysis of Schinus molle
Morphological characterization of the dried and grinded Schinus molle is performed by scanning electron microscopy. The SEM micrographs (Fig. 2) show the morphology, dimension, and organization of lignocellulosic fibres [45].
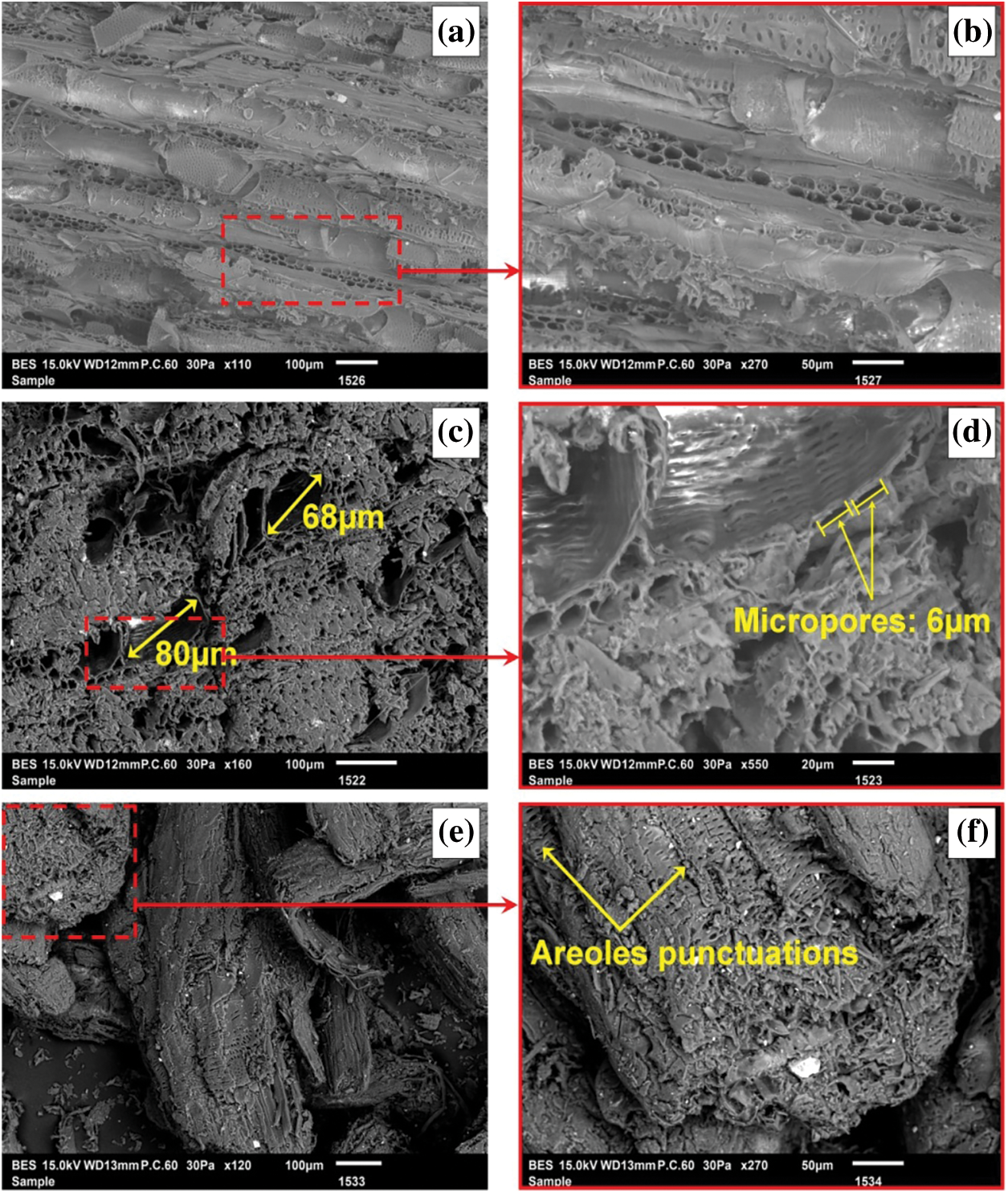
Figure 2: SEM microphotographs of lignocellulosic fibres obtained from Schinus molle in different shapes: radial section (a, b), cross-section (c, d), ground fibre (e, f)
Figs. 2a, 2b display the SEM image of the radial cross-section of Schinus molle cutters represent clearly the presence of the interconnected horizontal canals. These canals have tubular structures composed by cells secreting resin [46]. Such this structure is clearly visible after enlargement (Fig. 2b). The presence of tracheids renders the material accessible to solvents/reagents and sap by passing from one cell to another through punctuation under the effect of osmotic pressure, which is why a fine porous structure is observed between the tubular canals [47].
The cross-section (Figs. 2c, 2d) reveals the existence of libero ligneous vessels and main conductive vessels with diameters of 68 and 80 μm surrounded by a variable number of secondary canals of about 6 μm in diameter (Figs. 2c, 2d). The principal vessels have large punctuations (Fig. 2d) which are responsible for the exchange of substances with adjacent cells, thus implying an adsorption phenomenon [47]. Both radial and cross-sectional structures obtained for Schinus molle are similar to those observed for lignocellulosic wood fibres [48,49].
3.3 Pulping and Characterization of Different Materials from Schinus molle
Soda pulp preparation from Schinus molle has given a yield of about a 45% after delignification stage. This value is similar to that obtained from wood plants such as Prunus amygdalus (45.2%) [41], Stipagrostis pungens (43%) [40], and found to be higher than those obtained for some others annual plants such as Astragalus armatus, Retama raetam, Pituranthos chloranthus and Nitraria retusa (30%–35%) [25]. The amount of soda pulping yield decreases to 40% after bleaching process indicates the complete removal of non-cellulosic components. In the following, we are focused to characterize the different state of Schinus molle.
To investigate the chemical structure changes in untreated and post-treatment fibres namely: raw material from Schinus molle (R-SM), holocellulose from Schinus molle (H-SM) and cellulose fibres from Schinus molle (F-SM), FT-IR spectroscopy was done, and spectra are presented in Fig. 3.
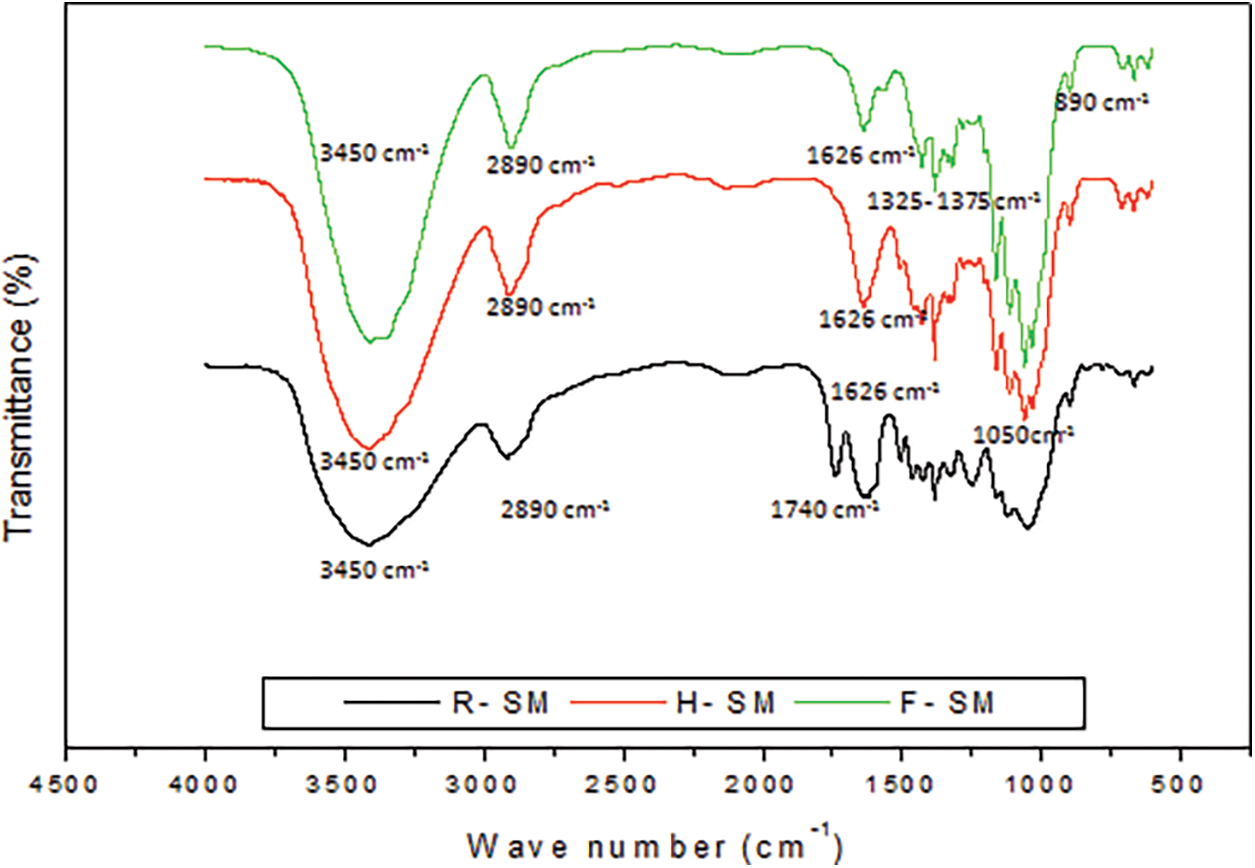
Figure 3: Infrared spectrum of R-SM, H-SM and F-SM
The adsorption bands in the region of 3350 and 2950 cm−1, represent the stretching vibration of the intermolecular and intramolecular OH band, which may be related to hydroxyl group exposure of cellulose molecules, which allows absorption of water and moisture by hydrogen bonds [9,50,51]. The band at 1740 cm−1 found in the spectrum of the R-SM and H-SM correspond to the C = O stretching of the carbonyl group, probably attributed to the hemicellulose and/or lignin molecule. Disappearances of this peak in the spectra of F-SM indicated that the both of the hemicellulose and lignin were removed after the chemical treatment of the fibre [52], this can be related also to the solubilization of hemicellulose and the depolymerization of lignin. The band at 2895 cm−1 is related to CH2 groups [50,51], as well as its intensity increased with the treatments. As different treatments are applied, the percentage of pure cellulose is increased, resulting in an increase in the intensity of the CH2 group in the β-glucosides rings of the cellulose molecules.
The presence of cellulose is also confirmed by the increase of the band located in the range of 1050 cm−1, indicating a relative increase for cellulose compared to R-SM and H-SM samples, thereby indicating the effectiveness of this method to remove hemicellulose, lignin and other fibrous compounds. The absorption bands observed around 1325 and 1375 cm−1, respectively, attributed to the deformation, vibration of the OH group and the elongation vibration of the –CH band with a band around 1028 cm−1 relative to the –C3–O deformation vibration of cellulose. Additionally, an absorption band at 890 cm−1, which corresponds to the deformation vibration of the glycosidic bonds [52,53] between the units of β-glucose of C1–O–C4 cellulose [43], which is the fingerprint of the cellulose [54].
Morphological behavior of delignificated-bleached fibre was studied out by using scanning electron microscopy (SEM) in order to get an idea concerning the fibrillary aspect and individualization of the cellulosic fibers (Fig. 4).
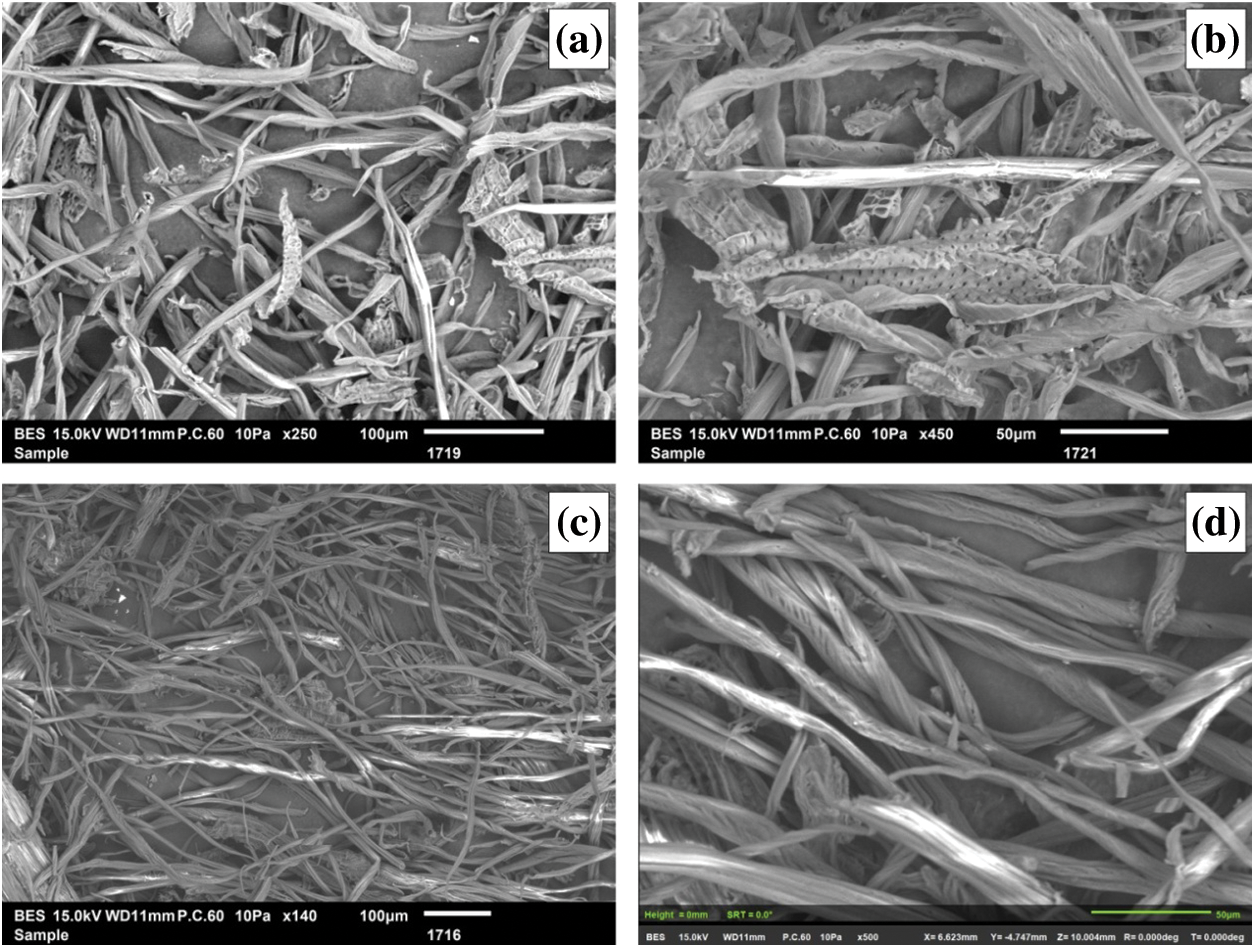
Figure 4: SEM microphotographs of F-SM [100 μm and 50 μm]
Figs. 4c and 4d show clearly the defibrillation of F-SM occurring alkali treatment, which prove the removal of the non-cellulosic fibers like lignin, hemicellulose [45]. The preservation of the structure of the parenchymal cells with punctuation distributed over its entire surface also shown in Figs. 4a, 4b. The analysis by SEM reveals also the presence of capillarity phenomenon, which is explained by the swelling of the fibers following the absorption of water. This phenomenon is based on the evacuation of the included air in the fibers [48].
3.3.3 X-Ray Diffraction Analysis (XRD)
All patterns presented two strong and intense peaks in the 2θ range studied (Fig. 5), one at 16° and the other at 22°. The typical cellulose I structure was observed in the diffraction pattern for each starting material. Thus, strong crystalline peaks at 16° and 22° corresponding, respectively, to the (110) and (002) planes of crystals and weak crystalline peak at 34.8° assigned to the (004) plane of the crystals are observed in Fig. 5 [55–57]. Other peaks were also noted indicating the presence of silica (SiO2) and calcium oxalate (CaC2O4.H2O) crystals [48].
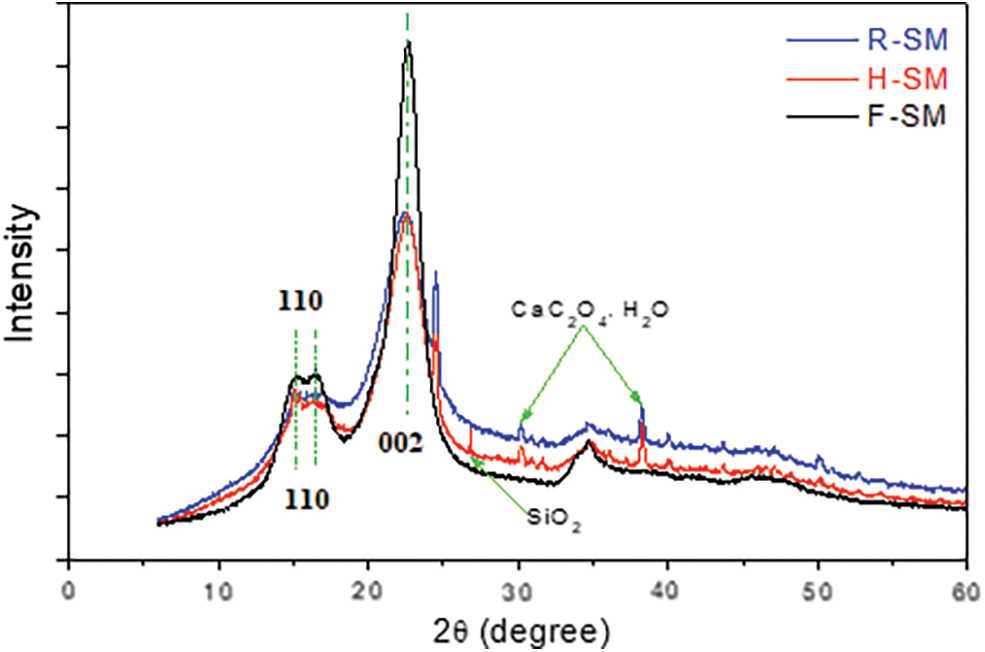
Figure 5: X-ray diffraction pattern of R-SM, H-SM, and F-SM
The crystallinity index of F-SM (i.e., 70%) was found to be higher than that of R-SM (i.e., 52.5%) and H-SM (i.e., 54.5%) because of the elimination of the hemicellulose and lignin using the alkali and bleaching treatment steps. It can be also explained by the ability to solubilize the amorphous region with the alkaline process [50,58,59]. Such as this result was largely observed as previously reported by several research [52,56,60], where they noticed that the crystallinity index increases after chemical treatment of F-SM.
3.3.4 Measuring Cellulosic Fibre Sizes
The length of the H-SM and F-SM are 618 and 415 μm, respectively, and their widths are 33.1 and 29.8 μm, respectively (Table 2).
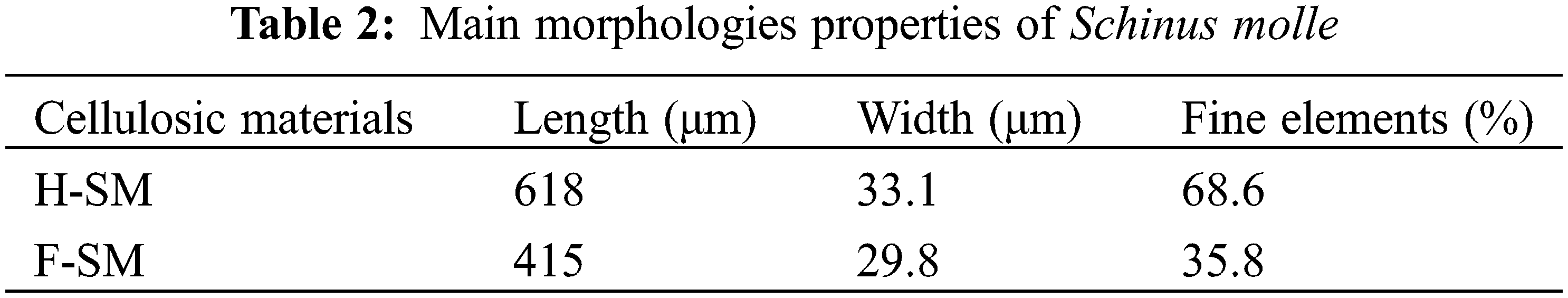
Observing the morphology, it can be noticed that the F-SM has the shortest fibers (371 μm) compared to the cellulosic fibers of some annual plants: Salix spp. (420 μm) [61], non-wood type Ipomoea carnea (600 μm) [62], and other wood plants like Cynara cardunculus (993 μm) and Eucalyptus globulus (763 μm) [63]. This can be explained by the destruction of the fibers under the effect of alkaline treatment. In addition, the width of the Schinus molle fibers is greater than other fibers from vine stems (24 μm) [42], and Pituranthos chloranthus (22.9 μm) [25]. This could be due to the swelling of the fibres by the hydration effect during the delignification process [48]. The lignocellulosic fibres have a fine element content of 28.5%, which is comparable to than observed for Pituranthos chloranthus [25], and higher than that of Astragalus armadas (17.1%) [25] and Stipagorstis pugens (24.3%) [40]. This high content is associated with the presence of elements soluble in hot water, such as sugars and the destruction of fibers during the delignification process [48].
3.3.5 Determination of Degree of Polymerization (DPv)
The degree of polymerization (DPv) of F-SM is in the range of 650. This value is low compared to those of bleached wood fibers (800–1200) and some non-wood sources (800–1000) [25,43]. In contrast, this value is higher than that observed for Tamarisk Sp (386), Prunus amygdalus (405) [41] and other annual plants such as Opuntia ficus-indica (500) [43].
Schinus molle is an interesting alternative source for producing cellulose fibers which can be utilized in several applications, namely in the textile and papermaking industries, as well as for innovative materials such as cellulose fiber-based composites, thus preserving the forest resource and meeting the increasing demand in pulps. The extraction of cellulose from Schinus molle was performed by a basic process using a sodium hydroxide solution for the delignification step followed by a bleaching step involving by a sodium chlorite solution. The yield extracted cellulose is about 45% after the delignification and bleaching step. The pattern of cellulose showed the elimination of non-cellulosic components such as hemicellulose, lignin… through the loss of the peak at 1740 cm−1 indicating the C = O stretching vibration of the lignin carbonyl unit. The chemical composition, morphological investigation, and FTIR analyses confirmed the removal of non-cellulosic materials and proved the feasibility to extract the cellulose from the Schinus molle. As perspectives to this work, our upcoming publications will be devoted to studying the isolation of nanofibres of cellulose from the Schinus molle and their performance in nano paper applications.
Acknowledgement: This work was financially supported by the Tunisian Ministry of Higher Education and the “PHC Utique” Program of the French Ministry of Foreign Affairs and the Tunisian Ministry of Higher Education and Scientific Research (CMCU Project No. 18G1132) as well as to CMPTM 17TM22 for the financial support.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Nie, S., Liu, X., Wu, Z., Zhan, L., Yin, G. et al. (2014). Kinetics study of oxidation of the lignin model compounds by chlorine dioxide. Chemical Engineering Journal, 241, 410–417. DOI 10.1016/j.cej.2013.10.068. [Google Scholar] [CrossRef]
2. Yao, S., Nie, S., Zhu, H., Wang, S., Song, X. et al. (2017). Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Industrial Crops and Products, 96, 178–185. DOI 10.1016/j.indcrop.2016.11.046. [Google Scholar] [CrossRef]
3. Senthilkumar, K., Saba, N., Rajini, N., Chandrasekar, M., Jawaid, M. et al. (2018). Mechanical properties evaluation of sisal fibre reinforced polymer composites: A review. Construction and Building Materials, 174, 713–729. DOI 10.1016/j.conbuildmat.2018.04.143. [Google Scholar] [CrossRef]
4. Safri, S. N. A., Sultan, M. T. H., Jawaid, M., Jayakrishna, K. (2018). Impact behaviour of hybrid composites for structural applications: A review. Composites Part B: Engineering, 133, 112–121. DOI 10.32604/jrm.2020.08724. [Google Scholar] [CrossRef]
5. Gheith, M. H., Abdelaziz, M., Ghori, W., Saba, N., Asim, M. et al. (2019). Flexural, thermal and dynamic mechanical properties of date palm fibres reinforced epoxy composites. Journal of Materials Research and Technology, 8, 853–860. DOI 10.1016/j.jmrt.2018.06.013. [Google Scholar] [CrossRef]
6. Asim, M., Jawaid, M., Khan, A., Asiri, A. M., Malik, M. A. (2020). Effects of date palm fibres loading on mechanical and thermal properties of date palm reinforced phenolic composites. Journal of Materials Research and Technology, 9, 3614–3621. DOI 10.1016/j.jmrt.2020.01.099. [Google Scholar] [CrossRef]
7. Khalil, A., Bhat, H. P. S., IreanaYusra, A. F. (2012). Green composites from sustainable cellulose nanofibrils: A review. Carbohydrate Polymers, 87(2), 963–979. DOI 10.1016/j.carbpol.2011.08.078. [Google Scholar] [CrossRef]
8. Dorrestijn, E., Laarhoven, L. J. J., Arends, I. W. C. E., Mulder, P. (2000). The occurrence and reactivity of phenoxyl linkages in lignin and low rank coal. Journal Analyticaland Applied Pyrolysis, 54(1–2), 153–192. DOI 10.1016/S0165-2370(99)00082-0. [Google Scholar] [CrossRef]
9. Mandal, A., Chakrabarty, D. (2011). Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydrate Polymers, 86(3), 1291–1299. DOI 10.1016/j.carbpol.2011.06.030. [Google Scholar] [CrossRef]
10. Sun, X. F., Xu, F., Sun, R. C., Fowler, P., Baird, M. S. (2005). Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydrate Polymers, 340(1), 97–106. DOI 10.1016/j.carres.2004.10.022. [Google Scholar] [CrossRef]
11. Rachtanapun, P., Luankamin, S., Tanprasert, K., Suriyaterm, R. (2012). Carboxymethyl cellulose film from durian rind. LWT-Food Science and Technology, 48(1), 52–58. DOI 10.1016/j.lwt.2012.02.029. [Google Scholar] [CrossRef]
12. Staudinger, H. (1920). Überpolymerisation. Berichte Der Deutschen Chemischen Gesellschaft, 53, 1073–1085. DOI 10.1002/cber.19200530627. [Google Scholar] [CrossRef]
13. Alotabi, M. D., Alshammari, B. A., Saba, N., Alothman, O. Y., Kian, L. K. et al. (2020). Microcrystalline cellulose from fruit bunch stalk of date palm: Isolation and characterization. Journal Polymers and the Environment, 28(6), 1766–1775. DOI 10.1007/s10924-020-01725-8. [Google Scholar] [CrossRef]
14. Wang, K., Jiang, J. X., Xu, F., Sun, R. C. (2009). Influence of steaming explosion time on the physic-chemical properties of cellulose from Lespedeza stalks (Lespedeza crytobotrya). Bioresource Technology, 100(21), 5288–5294. DOI 10.1016/j.biortech.2009.05.019. [Google Scholar] [CrossRef]
15. Siqueira, G., Bras, J., Dufresne, A. (2010). Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers, 2, 728–765. DOI 10.3390/polym2040728. [Google Scholar] [CrossRef]
16. Vilaseca, F., Del, R. R., Serrat, R., Alba, J., Mutje, P. et al. (2018). Macro and micro-mechanics behavior of stiffness in alkaline treated hemp core fibers polypropylene-based composites. Composites Part B: Engineering, 144, 118–125. DOI 10.1016/j.compositesb.2018.02.029. [Google Scholar] [CrossRef]
17. Asim, M., Jawaid, M., Fouad, H., Alothman, O. Y. (2021). Effect of surface modified date palm fibre loading on mechanical, thermal properties of date palm reinforced phenolic composites. Composite Structure, 267, 113913. DOI 10.1016/j.compstruct.2021.113913. [Google Scholar] [CrossRef]
18. Cheng, H., Li, L. J., Wang, B., Feng, X., Mao, Z. et al. (2020). Multifaceted applications of cellulosic porous materials in environment, energy, and health. Progress in Polymer Science, 106, 101253. DOI 10.1016/j.progpolymsci.2020.101253. [Google Scholar] [CrossRef]
19. Liu, K., Du, H., Zheng, T., Liu, H., Zhang, M. et al. (2021). Recent advances in cellulose and its derivatives for oilfield applications. Carbohydrate Polymers, 259, 117740. DOI 10.1016/j.carbpol.2021.117740. [Google Scholar] [CrossRef]
20. Thakur, V. K., Thakur, M. K., Gupta, R. K. (2013). Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydrate Polymers, 98, 820–828. DOI 10.1016/j.carbpol.2013.06.072. [Google Scholar] [CrossRef]
21. Odesanya, K. O., Ahmad, R., Jawaid, M., Bingol, S., Adebayo, G. O. et al. (2021). Natural fibre-reinforced composite for ballistic applications: A review. Journal Polymer and Environment, 29, 3795–3812.DOI 10.1007/s10924-021-02169-4. [Google Scholar] [CrossRef]
22. Chee, S. S., Jawaid, M., Sultan, M. T. H., Alothman, O. Y., Abdullah, L. C. (2019). Thermomechanical and dynamic mechanical properties of bamboo/woven kenaf mat reinforecd epoxy hybrid composites. Composites Part B: Engineering, 163, 165–174. DOI 10.1016/j.compositesb.2018.11.039. [Google Scholar] [CrossRef]
23. Siakeng, R., Jawaid, M., Asim, M., Siengchin, S. (2020). Accelerated weathering and soil burial effect on biodegradability, colour and texture of coir/Pineapple leaf fibres/PLA biocomposites. Polymers, 12, 458. DOI 10.3390/polym12020458. [Google Scholar] [CrossRef]
24. Elhleli, H., Mannai, F., Khiari, R., Moussaoui, Y. (2021). The use of mucilage extracted from Opuntia ficus indica as a microencapsulating shell. Journal of the Serbian Chemical Society, 86, 25–38. DOI 10.2298/JSC200229033E. [Google Scholar] [CrossRef]
25. Ferhi, F., Satyajit, D., Elaloui, E., Moussaoui, Y., Yanez, J. G. (2014). Chemical characterization and suitability for papermaking applications studied on four species naturally growing in Tunisia. Industrial Crops and Products, 61, 180–185. DOI 10.1016/j.indcrop.2014.07.001. [Google Scholar] [CrossRef]
26. Mannai, F., Elhleli, H., Dufresne, A., Elaloui, E., Moussaoui, Y. (2020). Opuntia (Cactaceae) fibrous network-reinforced composites: Thermal, viscoelastic. Interfacial Adhesion and Biodegradation Behavior. Fibers and Polymers, 21, 2353–2363.DOI 10.1007/s12221-020-9675-4. [Google Scholar] [CrossRef]
27. Mejía-Díaz, L. A., Rutiaga-Quiñones, J. G. (2008). Chemical composition of Schinus molle L. wood and kraft pulping process. Revista Mexicana of Chemical Engineering, 7, 145–149. [Google Scholar]
28. Bañuelas, D. C., Questad, E. J., Bobich, E. G. (2019). Interactions between the invasive Schinus molle (Peruvian pepper tree) with six plant species commonly found in Southern California nature reserves. Urban for Urban Greening, 43, 126348. DOI 10.1016/j.ufug.2019.05.010. [Google Scholar] [CrossRef]
29. Olafsson, K., Jaroszewski, J. W., Smitt, U. W., Nyman, U. (1997). Isolation of angiotensin converting enzyme (ACE) inhibiting triterpenes from Schinus molle. Planta Medica, 63, 352–355. DOI 10.1055/s-2006-957699. [Google Scholar] [CrossRef]
30. Deveci, O., Sukan, A., Tuzun, N., Kocabas, E. E. H. (2010). Chemical composition, repellent and antimicrobial activity of Schinus molle L. Journal of Medicinal Plants Research, 4, 2211–2216. DOI 10.5897/JMPR10.326. [Google Scholar] [CrossRef]
31. Feriani, A., Tir, M., Hamed, M., Sila, A., Nahdi, S. et al. (2020). Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammatory and anti-nociceptive properties. International Journal of Biological Macromolecules, 165, 2576–2587. DOI 10.1016/j.ijbiomac.2020.10.123. [Google Scholar] [CrossRef]
32. Machado, D. G., Kaster, M. P., Binfaré, R. W., Dias, M., Santos, A. R. et al. (2007). Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: Evidence for the involvement of the monoaminergic system. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31, 421–428. DOI 10.1016/j.pnpbp.2006.11.004. [Google Scholar] [CrossRef]
33. Dufresne, A. (2012). Nanocellulose from nature to high performance tailored materials. Berlin, Boston:De Gruyter. DOI 10.1515/9783110254600. [Google Scholar] [CrossRef]
34. Wise, L., Murphy, E., Addieco, M. A. A. (1946). Chlorite holocellulose: Its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade Journal, 122, 35–43. [Google Scholar]
35. Segal, L., Creely, J. J., Martin, A. E., Conrad, C. M. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal, 29, 786–794. DOI 10.1177/004051755902901003. [Google Scholar] [CrossRef]
36. Copur, Y., Tozluoglu, A. (2008). A comparison of kraft, PS, kraft-AQ and kraft-naBH4 pulps of brutia pine. Bioresource Technology, 99, 909–913. DOI 10.1016/j.biortech.2007.04.015. [Google Scholar] [CrossRef]
37. Jimenez, L., Sanchez, I., Lopez, F. (1992). Olive wood as a raw material for paper manufacture. TAPPI Journal, 11, 89–91. [Google Scholar]
38. Jimenez, L., Rodriguez, A., Perez, A., Moral, A., Serrano, L. (2008). Alternative raw materials and pulping process using clean technologies. Industrial Crops and Products, 28, 11–16. DOI 10.1016/j.indcrop.2007.12.005. [Google Scholar] [CrossRef]
39. Cordeiro, N., Belgacem, M. N., Torres, I., Moura, J. C. V. (2004). Chemical composition and pulping of banana pseudo-stems. Industrial Crops and Products, 19, 147–154. DOI 10.1016/j.indcrop.2003.09.001. [Google Scholar] [CrossRef]
40. Ferhi, F., Das, S., Moussaoui, Y., Elaloui, E., Yanez, J. G. (2014). Paper from Stipagorstis pungens. Industrial Crops and Products, 59, 109–114. DOI 10.1016/j.indcrop.2014.05.015. [Google Scholar] [CrossRef]
41. Mechi, N., Khiari, R., Elaloui, E., Belgacem, M. N. (2016). Preparation of paper sheet from cellulosic fibers obtained from Prunus amygdalus and Tamarisksp. Cellulose Chemistry and Technology, 7, 863–872. DOI 10.1016/j.powtec.2017.02.055. [Google Scholar] [CrossRef]
42. Mansouri, S., Khiari, R., Bendouissa, N., Saadallah, S., Mauret, E. et al. (2012). Chemical composition and pulp characterization of Tunisian vine stems. Industrial Crops and Products, 36, 22–27. DOI 10.1016/j.indcrop.2011.07.036. [Google Scholar] [CrossRef]
43. Mannai, F., Ammar, M., Yanez, J. G., Elaloui, E., Moussaoui, Y. (2016). Cellulose fiber from Tunisian Barbary Fig “Opuntia ficus-indica” for papermaking. Cellulose, 23, 2061–2072. DOI 10.1007/s10570-016-0899-9. [Google Scholar] [CrossRef]
44. Bendahou, A., Dufrense, A., Kaddamia, H., Habibi, Y. (2007). Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydrate Polymers, 68, 601–608. DOI 10.1016/j.carbpol.2006.10.016. [Google Scholar] [CrossRef]
45. Chandra, C. S. J., George, N., Narayanankutty, S. K. (2016). Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydrate Polymers, 142, 158–166. DOI 10.1016/j.carbpol.2016.01.015. [Google Scholar] [CrossRef]
46. Lin, J., Hu, Y., He, Y., Ceulmans, R. (2001). Systematic survey of resin canals in pinaceae. Belgian Journal of Botany, 135(1–2), 3–14. [Google Scholar]
47. Benyoucef, S., Harrache, D. (2015). Caractérisation de la microstructure de sciure de bois de pin sylvestre “Pinussylvestris’’ microstructure characterization of Scots pine “Pinussylvestris” sawdust. Journal of Materials and Environmental Science, 6(3), 765–772. [Google Scholar]
48. Mannai, F., Ammar, M., Yanez, J. G., Elaloui, E., Moussaoui, Y. (2018). Alkaline delignification of cactus fibers for pulp and papermaking applications. Journal of Polymers and Environment, 26, 798–806. DOI 10.1007/s10924-017-0968-7. [Google Scholar] [CrossRef]
49. Cown, D. J., Donaldson, L. A., Downes, G. M. (2011). A review of resin features in radiata pine. New Zealand Journal of Forestry Science, 41, 41–60. [Google Scholar]
50. Pereira, P. H. F., Ornaghi Junior, H. L., Coutinho, L. V., Duchemin, B., Cioffi, M. O. H. (2020). Obtaining cellulose nanocrystals from pineapple crown fibers by free-chlorite hydrolysis with sulfuric acid: Physical, chemical and structural characterization. Cellulose, 27, 5745–5756. DOI 10.1007/s10570-020-03179-6. [Google Scholar] [CrossRef]
51. Xie, J., Hse, C. Y., de Hoop, C. F., Hu, T., Qi, J. et al. (2016). Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydrate Polymers, 151, 725–734. DOI 10.1016/j.carbpol.2016.06.011. [Google Scholar] [CrossRef]
52. Dahlem, M. A., Borsoi, C., Hansen, B., Catto, A. L. (2019). Evaluation of different methods for extraction of nanocellulose from yerba mate residues. Carbohydrate Polymers, 218, 78–86. DOI 10.1016/j.carbpol.2019.04.064. [Google Scholar] [CrossRef]
53. Phanthong, P., Guan, G., Ma, Y., Hao, X., Abudula, A. (2016). Effect of ball milling on the production of nanocellulose using mild acid hydrolysis method. Journal of the Taiwan Institute of Chemical Engineers, 60, 617–622. DOI 10.1016/j.jtice.2015.11.001. [Google Scholar] [CrossRef]
54. Sun, Y., Lin, L., Deng, H., Li, J., He, B. et al. (2008). Structural changes of bamboo cellulose in formic acid. BioResources, 3, 297–315. [Google Scholar]
55. Kouadri, I., Satha, H. (2018). Extraction and characterization of cellulose and cellulose nanofibers from citrulluscolocynthis seeds. Industrial Crops and Products, 124, 787–796. DOI 10.1016/j.indcrop.2018.08.051. [Google Scholar] [CrossRef]
56. Melikoğlu, A. Y., Bilek, S. E., Cesur, S. (2019). Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydrate Polymers, 215, 330–337. DOI 10.1016/j.carbpol.2019.03.103. [Google Scholar] [CrossRef]
57. Yang, Y., Yang, J., Cao, J., Wang, Z. (2018). Pretreatment with concurrent UV photocatalysis and alkaline H2O2 enhanced the enzymatic hydrolysis of sisal waste. Bioresource Technology, 267, 517–523. DOI 10.1016/j.biortech.2018.07.038. [Google Scholar] [CrossRef]
58. Asad, M., Saba, N., Asiri, A. M., Jawaid, M., Indarti, E. et al. (2018). Preparation and characterization of nanocomposite films from oil palm pulp nanocellulose/poly (Vinyl alcohol) by casting method. Carbohydrate Polymers, 191, 103–111. DOI 10.1016/j.carbpol.2018.03.015. [Google Scholar] [CrossRef]
59. Cherian, B. M., Pothan, L. A., Nguyen-Chung, T., Mennig, G., Kottaisamy, M. et al. (2008). A novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. Journal of Agricultural Food Chemistry, 56, 5617–5627. DOI 10.1021/jf8003674. [Google Scholar] [CrossRef]
60. Adel, A. M., El-Wahab, Z. H. A., Ibrahim, A. A., Al-Shemy, M. T. (2010). Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. acid catalyzed hydrolysis. Bioresource Technology, 101, 4446–4455. DOI 10.1016/j.biortech.2010.01.047. [Google Scholar] [CrossRef]
61. Ai, J., Tschirner, U. (2010). Fiber length and pulping characteristics of switchgrass, alfalfa stems, hybrid poplar and willow biomasses. Bioresource Technology, 101, 215–221. DOI 10.1016/j.biortech.2009.07.090. [Google Scholar] [CrossRef]
62. Dutt, D., Upadhyaya, J. S., Tyagi, C. H., Kumar, A., Lal, M. (2008). Studies on Ipomeacarnea and Cannabis sativa as an alternative pulp blend for softwood: An optimization of kraft delignification process. Industrial Crops and Products, 28, 128–136. DOI 10.1016/j.indcrop.2008.02.001. [Google Scholar] [CrossRef]
63. Abrantes, S., Amaral, M. E., Costa, A. P., Duarte, A. P. (2007). Cynara cardunculus L. alkaline pulps: Alternatives fibers for paper and paperboard production. Bioresource Technology, 98, 2873–2878. DOI 10.1016/j.biortech.2006.09.052. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |