

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.019590
ARTICLE
Effect of MMT on Flame Retardancy of PLA/IFR/LDH Composites
State Key Laboratory of Environment-Friendly Energy Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang, 621010, China
*Corresponding Authors: Lin Chen. Email: chenlin101101@aliyun.com; Ying Xiong. Email: xiongying@swust.edu.cn
Received: 30 September 2021; Accepted: 05 January 2022
Abstract: Nano filler synergistic intumescent flame retardant (IFR) system is an effective way to improve the flame retardant properties of polymer. In this study, the effects of montmorillonite (MMT) on the flame retardant properties of polylactic acid/layered double hydroxides (PLA/LDH) and PLA/IFR/LDH were investigated. The results show that both LDH and LDH/IFR can reduce the peak heat release rate (HRR) of PLA and prolong the combustion time of PLA; When a proportionate MMT is introduced into PLA/LDH and PLA/IFR/LDH systems, respectively, MMT will not only affect the degradation process of PLA composites during combustion, but also the PLA composites can form a more stable carbon layer during combustion, which could decrease the peak HRR and prolong combustion time of PLA composite. Furthermore, when 0.5 wt% MMT is incorporated, the peak HRR of LDH/IFR/LDH composite is reduced by 16.17% and the combustion time is prolonged by about 70 s. This study can provide an opportunity to further optimize the properties of intumescent flame retardant polymer.
Keywords: Polylactic acid; montmorillonite; layered double hydroxides; intumescent flame retardant; synergistic effect
Polylactic acid (PLA), as a nonpetroleum based environmentally friendly biodegradable plastic, is a non-toxic, nonirritating thermoplastic polyester. It has the advantages of good transparency and high strength [1,2]. However, PLA is flammable and accompanied by serious dripping, limiting its application in automotive, construction, electric and electric vehicles and other fields requiring high flame retardant performance [3,4]. Therefore, improving the flame retardant properties of PLA composites is an inevitable choice to promote the further promotion of applications of PLA composites [5–7].
Intumescent flame retardant (IFR) is the most promising halogen-free flame retardant, which has the advantages of halogen-free environmental protection, low toxicity and low corrosion. It plays a flame retardant role by forming a dense and continuous expanded carbon layer to block the entry of oxygen and heat [8–10]. Generally speaking, IFR mainly includes acid source, gas source and carbon source. However, the flame retardant efficiency of IFR is low, and a good flame retardant performance in polymers with IFR can be achieved only with a higher dosage. Because of the shortcomings of IFR, researchers focused on improving the flame retardant efficiency of IFR and reducing its addition. Adding some components to IFR can improve the flame retardant efficiency of IFR, and the total heat release and heat release rate of the composite system decreased significantly during combustion. When the two flame retardants are used in combination, the flame retardant effect of the flame retardant material is greater than the sum of the flame retardant effects when they are used alone in the material, and it is said that the two flame retardants have a synergistic effect [11,12]. In addition, for IFR, such synergists mainly include zeolite [13], MMT [14], LDH [15], sepiolite [16], halloysite [17], metal oxide and its salts [18,19], metal hydroxide [20] and other substances. According to the main flame retardant elements, it can be divided into aluminum containing synergist, phosphorus containing synergist and silicon containing synergist.
Based on the above studies, the main researchers used a single synergistic to improve the flame retardant performance of IFR, and did not further optimize the performance after synergistic modification. The research shows that both MMT and LDH can improve the flame retardant performance of IFR. At the same time, LDH and MMT have relevant laminate charges [21], introducing them into PLA at the same time maybe form a more stable barrier layer. Therefore, this study explores the changes of flame retardant performance of LDH synergistic intumescent flame retardant PLA systems under the action of different MMT, and it lays a foundation for the further development of efficient intumescent flame retardant systems.
PLA (REVODE101) was purchased from Zhejiang Haizheng Biomaterials Co., Ltd., China. Pentaerythritol phosphate ester (PEPA) was provided by Hubei Jinleda Chemical Co., Ltd., China. Organically modified montmorillonite (MMT) was produced by Sichuan Santai Fine Bentonite Co., Ltd., China. Sodium hydroxide (NaOH), magnesium nitrate (Mg(NO3)2⋅6H2O), aluminum nitrate (Al(NO3)3⋅9H2O), melamine (MA) were obtained from Chengdu Jinshan Chemical Reagent Co., Ltd., China.
2.2 Preparation of MgAl LDH Modified by Sodium Dodecyl Sulfate and MMT Modified by Cetyl Trimethyl Ammonium Bromide
Firstly, Mg (NO3)2⋅6H2O (0.18 mol) and Al (NO3)3⋅9H2O (0.06 mol) were dissolved in deionized water and recorded as solution I. Secondly, a certain amount of sodium dodecyl sulfate (SDS) was dissolved in deionized water and added to solution I with mixing evenly, and it is recorded as solution II. And the pH value of solution II was adjusted to 10 by NaOH solution; Thirdly, the mixed solution was stirred at 70°C for 12 h under a nitrogen atmosphere [22]. Finally, the precipitate was filtered and washed with deionized water many times, and the obtained product was dried at 60°C for 24 h, then the modified MgAl LDH (OLDH) was prepared.
Firstly, 25 g of Na-MMT was dispersed in 250 g of deionized water under vigorous stirring for 0.5 h, and the solution remained still for 1 h. Secondly, removed supernatant and bottom sediment, so purified Na-MMT solution was obtained. Finally, 15 g of CTAB was dispersed into purified Na-MMT solution and heated to around 70°C under continuous stirring for 2 h [23,24]. Subsequently, the organic clay (MMT-CTAB) was handled by vacuum filtration and washed several times with deionized water until no halide anions were detected. The product (OMMT) was freeze-drying for further use.
2.3 Preparation of PLA Composites
PLA, MMT, LDH, PEPA were dried at 80°C overnight before use. PLA composites with the desired amount of MMT, LDH and PEPA were melted compound using a two-roll mill at 160°C for 10 min. Adjusting the mass ratios of PLA/LDH/MMT to 99.5/0.5/0, 99.25/0.5/0.25, 99/0.5/0.5, 98.5/0.5/1, 97.5/0.5/2 were designated as PLAx (x = 1, 2, 3, 4, and 5), respectively. The mass ratios of PLA/PEPA+MA/MMT/LDH to 75/25/0/0, 74.5/25/0.5/0, 74.25/25/0.5/0.25, 74/25/0.5/0.5, 73.5/25/0.5/1, 72.5/25/0.5/2 were designated as PLAx (x = 6, 7, 8, 9, and 10), and the mass ratio between PEPA and MA was 4/1.
2.4 Testing and Characterization
X-ray diffraction (XRD) characterization was carried out on a panalytical x’pert Pro X-ray diffractometer equipped with a copper anode (Cu Kα radiation, λ = 1.54187 Å). The X-ray source was operated at 40 kV and 40 mA.
The morphology of all electrocatalysts was investigated by field emission scanning electron microscope (FESEM, UItra55, Carl zeissNTS GmbH, Germany) and transmission electron microscope (TEM, Libra200, Carl zeiss irts Co., Germany).
Q5000IR (TA instrument, USA) thermal analyzer was used for thermogravimetric analysis (TGA). Nitrogen atmosphere mode was used with a temperature range from room temperature to 500°C with a heating rate of 20 °C/min.
Cone calorimeter 6180 (Siemens analyzer) was used to test the combustion properties of composites according to ISO5660-1 standard. The sample size was 100 mm × 100 mm × 3 mm, and the radiation flux was 35 kW/m2.
The X-ray photoelectron spectroscopy (XPS) was carried out with a VG Escalab Mark II spectrometer (Thermo-VG Scientific Ltd., UK), using Al Kα excitation radiation (hv = 1486.6 eV).
3.1 Characterization of OLDH, OMMT and PLA Composites
The XRD of LDH and MMT before and after modification was shown in Fig. 1a, and the interlayer spacing of OLDH and OMMT was larger than that of LDH and MMT, respectively, indicating that SDS and CTAB were intercalated into the layers of LDH and MMT, the interlayer spacing of MMT and LDHT was expanded from 0.76 to 2.61 nm, and from 0.81 to 3.29 nm, respectively, which was beneficial to the uniform dispersion of LDH and MMT in PLA. Figs. 1b and 1c were the TEM analysis of PLA/OLDH and PLA/OMMT, respectively, which showed that OMMT and OLDH were well dispersed in PLA. When they were placed in PLA simultaneously, they could promote better dispersion of each other in PLA (Fig. 1d). The contact interface of nanoparticles in PLA4 was more blurred than that of PLA/OLDH and PLA/OMMT, which indicated that the OLDH and OMMT and PLA matrix had good interface compatibility. At the same time, SEM analysis showed (Figs. 1e and 1f) that OLDH and OMMT do not reunite in PLA, and the dispersion was excellent.
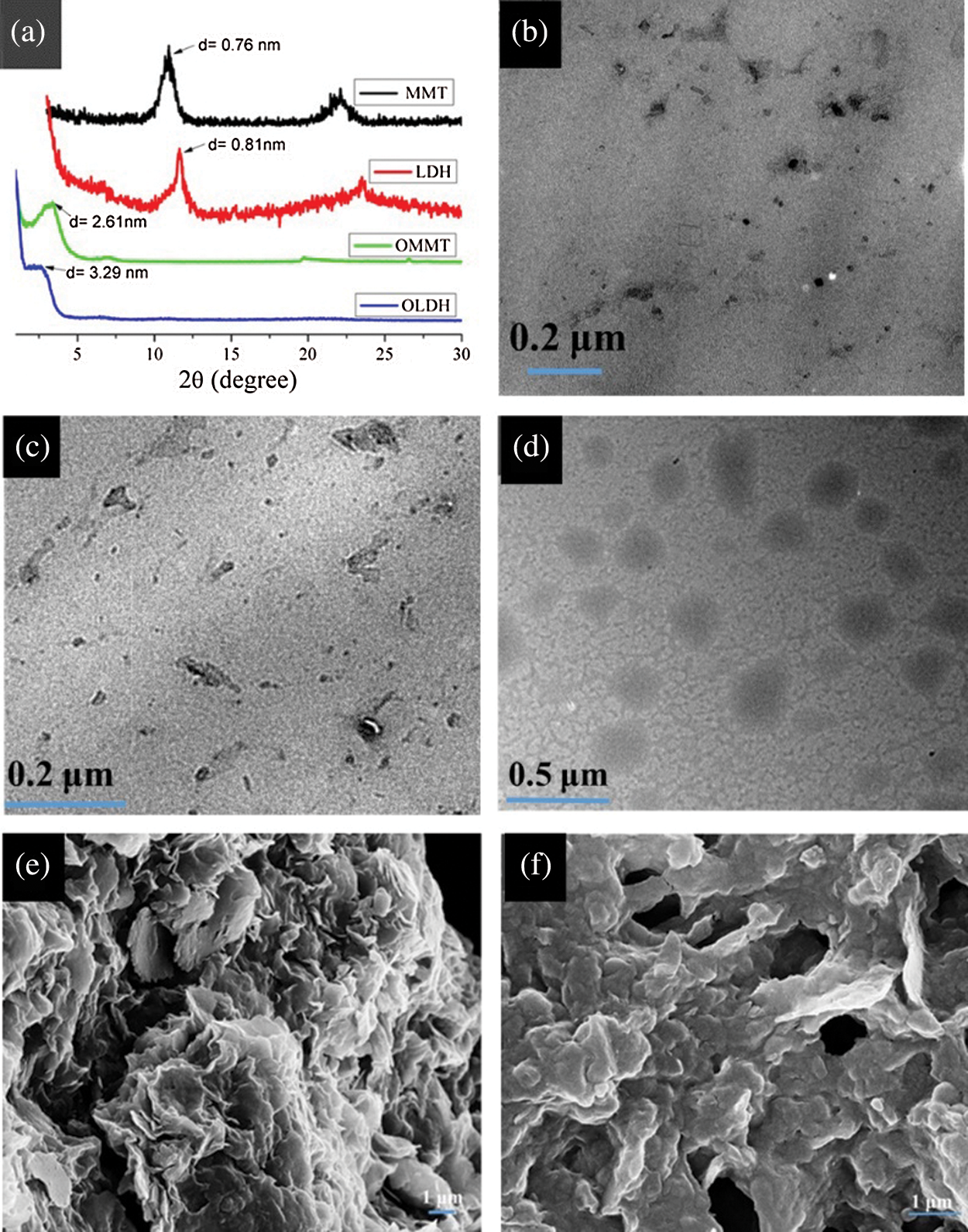
Figure 1: (a) XRD spectra of LDH and MMT before and after modification. (b–d) TEM images of PLA/OLDH, PLA/OMMT, PLA4, respectively. (e, f) SEM of PLA1 and PLA4, respectively
3.2 Thermal Degradation Behavior of PLA Composites
Fig. 2a showed the effect of MMT on the thermal stability of PLA/LDH composites. With the addition of a small amount of LDH, the thermal stability of PLA decreased. However, when MMT was added to the PLA/LDH composite system, the thermal stability of the composites increased firstly and then decreased with the increase of MMT addition, indicating that MMT could improve the thermal stability of the PLA/LDH composite system at an appropriate proportion. Interestingly, the thermal stability of the composites was lower than that of PLA, indicating that the addition of LDH and MMT promoted the early thermal degradation of PLA. However, when IFR was introduced into PLA/LDH composites, the charring properties of the composites were significantly improved, and when the content of MMT was 1 wt%, the composites showed excellent charring properties. The results showed that MMT had synergistic forming charring ability when with an appropriate ratio for PLA/LDH/IFR, which could protect matrix more efficiently. In all, when intumescent flame retardant was added to PLA/LDH system, the thermal degradation of PLA would be accelerated for forming a more stable intumescent char layer. Furthermore, MMT can make the surface of intumescent char layer form a more stable ceramic structure, so that the char layer could better protect the matrix.
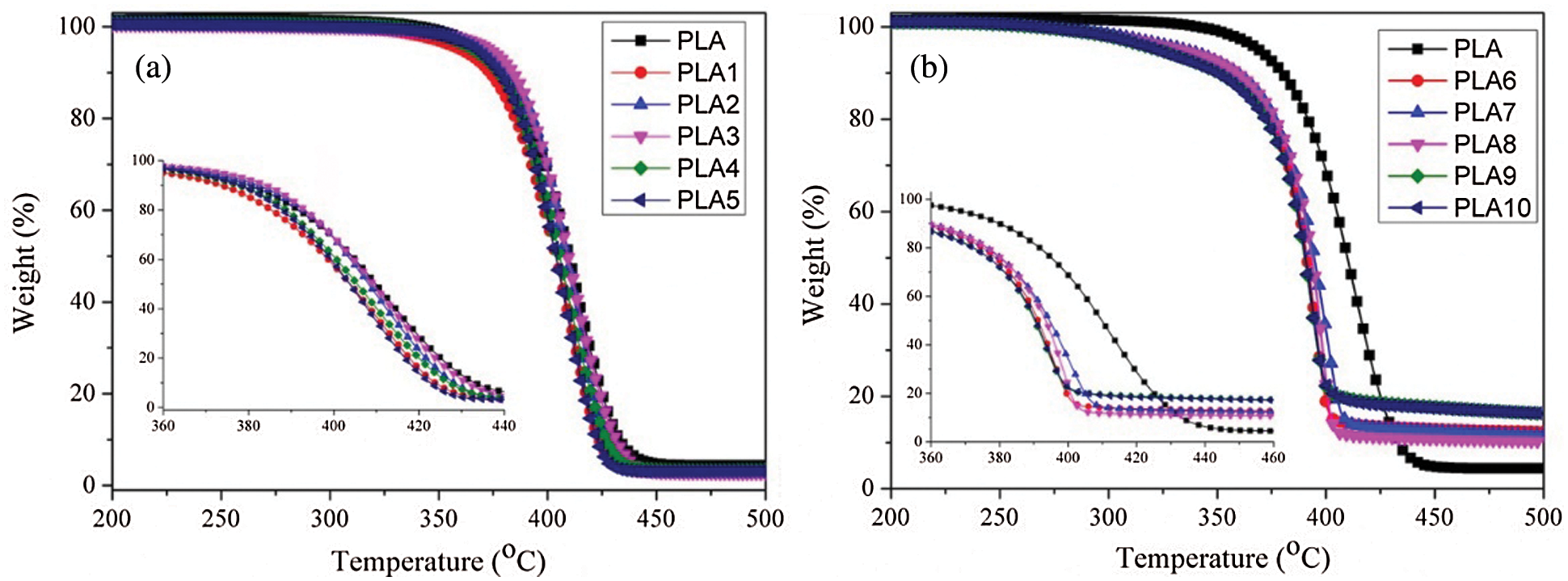
Figure 2: Effect of MMT on the thermal stability of PLA/LDH (a) and PLA/LDH/IFR (b), respectively
3.3 Combustion Properties of PLA Composites
Cone calorimetry is one of the most effective ways to analyze the combustion properties of various polymer materials [25,26]. The effect of MMT on the combustion parameters of PLA/LDH composites and PLA/IFR/LDH composites was evaluated by cone calorimetry.
The heat release rate (HRR) is a very critical parameter, and can be used to express the intensity of a fire. From Fig. 3a, 0.5 wt% LDH showed an efficient flame retardancy, and MMT could affect the combustion property of PLA/LDH composite. When there was an appropriate ratio between LDH and MMT, composites showed lower peak HRR and longer combustion time than that of pure PLA.
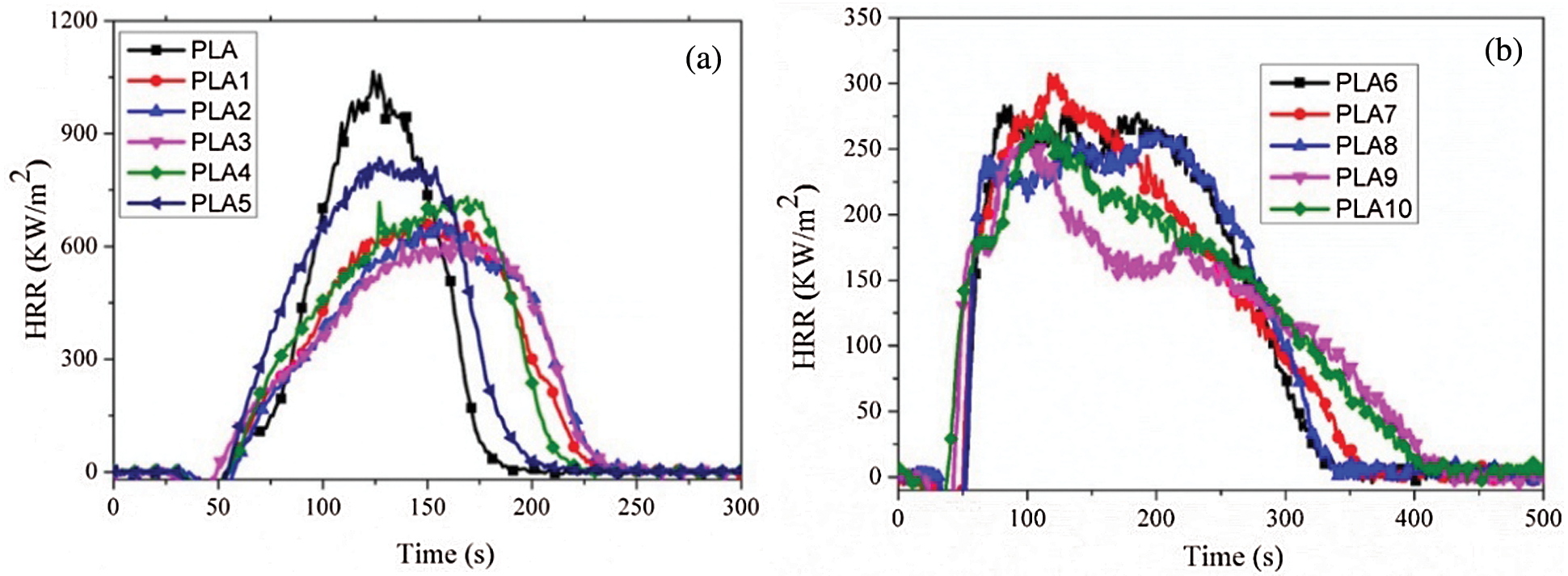
Figure 3: HRR curves of PLA composites
The char residues of PLA1 and PLA4 showed in Figs. 4a and 4b, respectively, PLA4 showed a more and dense charring than that of PLA1, this phenomenon indicated that the solid char was formed by the introduction of LDH for PLA, and MMT could improve the formation of char, which could decrease the peak HRR and prolong the combustion time.
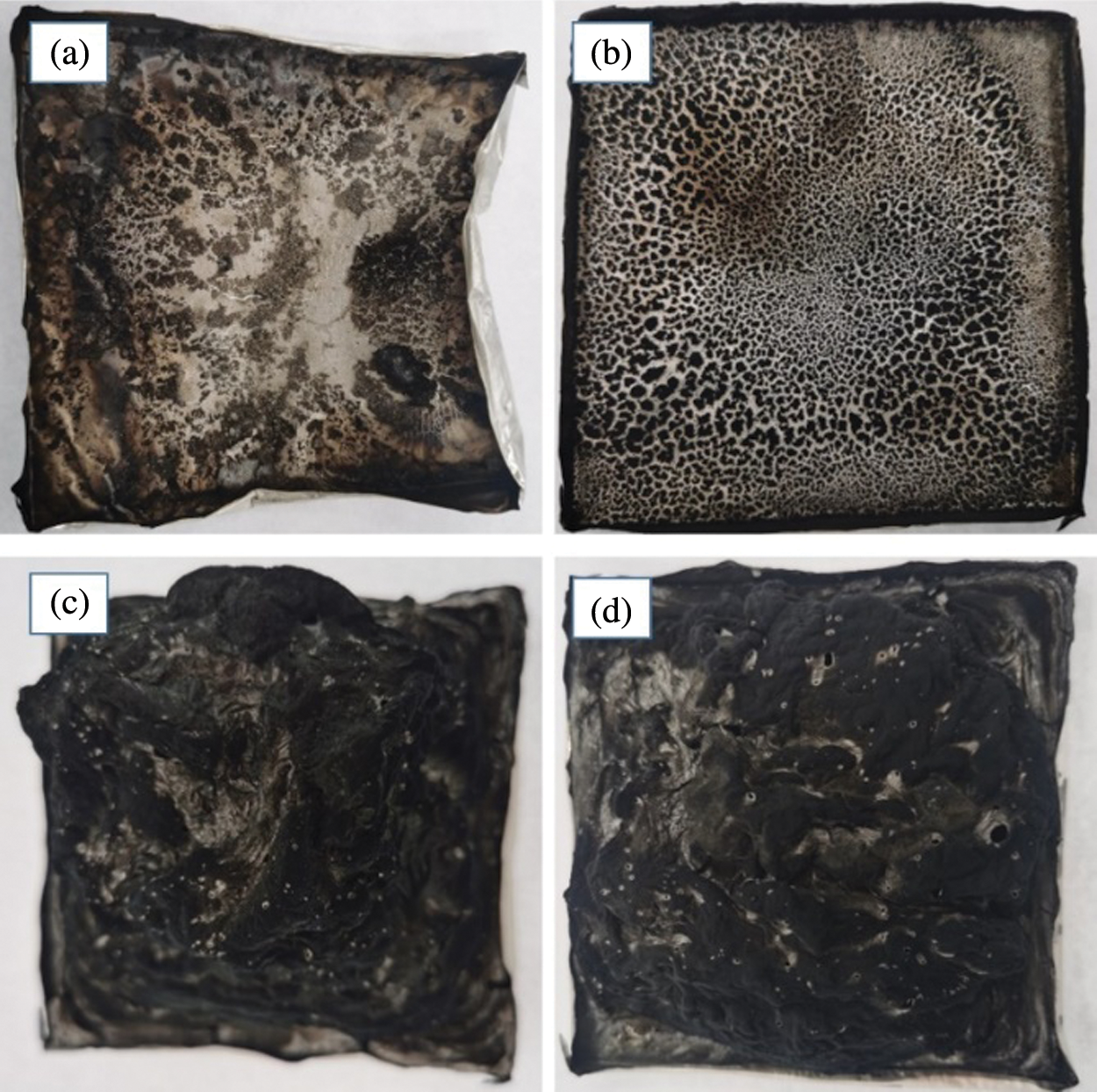
Figure 4: Digital photographs of (a) PLA1, (b) PLA4, (c) PLA6, (d) PLA9
When 25 wt% IFR was introduced into PLA/LDH system (Fig. 3b), the peak HRR of PLA1 (672.2 kW/m2) decreased to 306.8 kW/m2 (PLA6), and the flame time increased. Based on PLA/LDH/IFR system, MMT could improve the flame retardant effectiveness of LDH/IFR in PLA. Notably, the result of PLA9 showed a lower peak HRR (257.2 kW/m2), and the flame time was longer than that of other PLA composites, at the same time, the char of PLA9 (Fig. 4c) was denser than that of PLA6 (Fig. 4d). To further investigate the effect of MMT on the carbonization performance of PLA/LDH/IFR, XPS was used to analyze the structure of the char residue of PLA6 and PAL9, respectively (Fig. 5). The analysis results revealed that the position and relative strength of XPS component of the carbon layer were almost unchanged before and after the addition of MMT, indicating that MMT did not affect the chemical process of carbon layer formation, the main reason for the improvement of flame retardant performance could be that MMT interpenetrated in the intumescent carbon layer, and formed a more stable ceramic structure, so that the char layer could better protect the matrix.
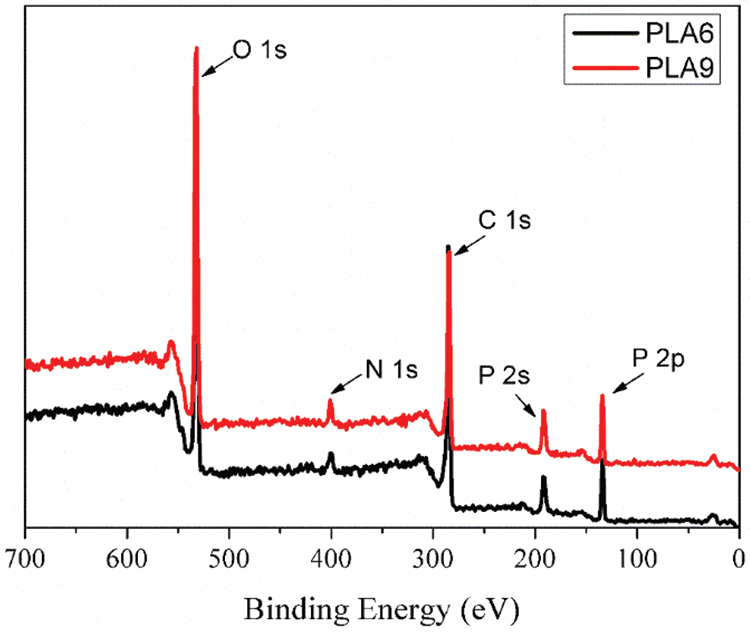
Figure 5: XPS of char residues of PLA6 and PLA9
To further investigate the synergistic mechanism between MMT and LDH/IFR in PLA, the morphologies of surface char residues of PLA6 and PLA9 were performed by SEM, as shown in Fig. 6, the char residues of PLA6 holed numerous “popcorn-like” structures (Fig. 6a); however, PLA9 showed a smooth char surface with few micro-size particles. This was due to the reinforced charring of MMT in the condensed phase, and the char residues of PLA9 more effectively inhibited the production of volatile small molecules and transfer of heat than that of PLA6, so that matrix material was better protected. The results meant that MMT could show a synergistic effect with LDH/IFR in flame retardant PLA.
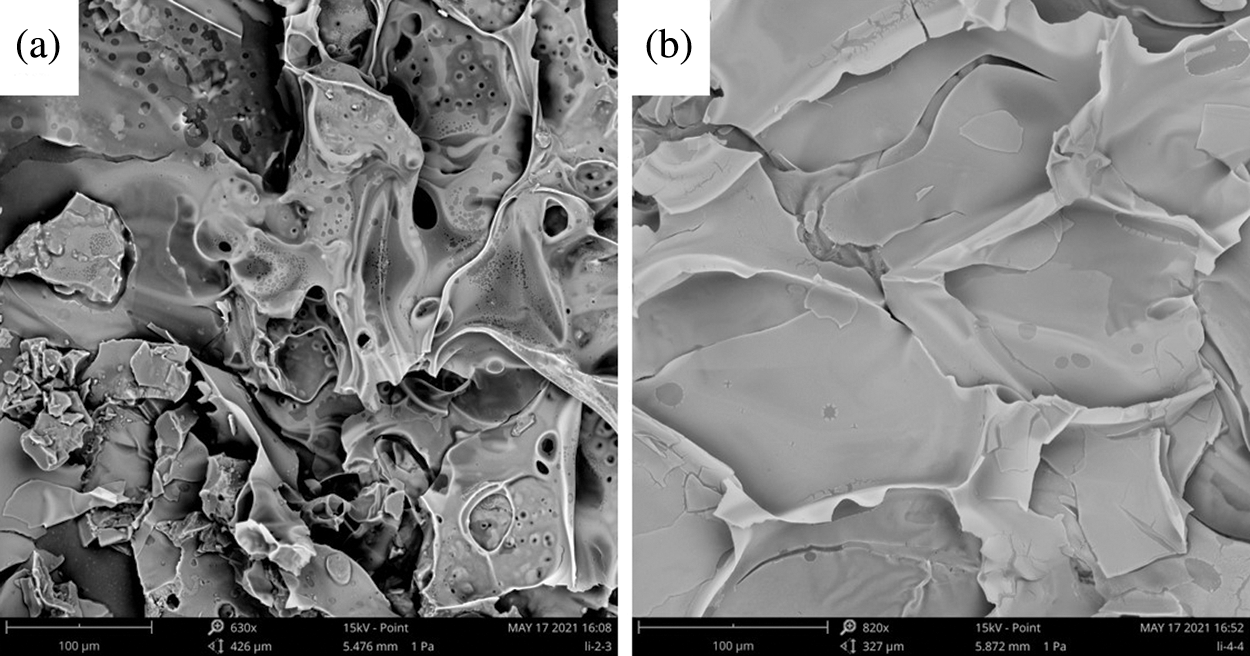
Figure 6: SEM images of surface char residues of PLA6 and PLA9
CO and CO2 are the main culprits, causing fatalities in a fire scenario because of their asphyxiant characteristics. Therefore, it is significant to research the emission evolution of CO and CO2. The CO and CO2 production profiles were presented in Figs. 7 and 8. From Figs. 7a and 7b, it was found that the addition of LDH and MMT could significantly reduce the peak of CO production rate (COPR) and prolong the release time, indicating that the addition of nanoparticles went against the pyrolysis of PLA, reduced the peak COPR and prolonged the release time. When IFR was added to PLA/LDH system, the peak COPR of PLA6 was greatly increased, and the release time was prolonged. The results showed that the addition of IFR could form a more stable char layer and prevent the contact between oxygen and combustible gases. However, when MMT was introduced into PLA/LDH/FR system with an appropriate proportion, MMT could reduce the peak COPR and prolong the release time of PLA composites. Combining with HRR analysis, MMT promoted the formation of a more stable carbon layer in PLA/LDH/IFR system, which hindered the pyrolysis of matrix materials. From the analysis data of CO total yield (COTY), LDH and MMT could reduce the COTY release of PLA; while with the addition of IFR, the COTY release increased significantly, and the appropriate content of MMT could reduce the total COTY of PLA/LDH/IFR system. Similarly, the addition of LDH and MMT could reduce the peak CO2 release and prolong the release time, but with the addition of IFR, the peak of CO2 production rate (CO2PR) of the PLA6 decreased significantly. It was worth noting that when 1 wt% MMT was added to the PLA/LDH/IFR system, the composite showed the lowest peak CO2PR and the longest release time. Through the analysis of the CO2 total yield (CO2TY), it could be seen that LDH and MMT promote PLA combustion, CO2TY increased, but the introduction of IFR could greatly reduce the CO2TY, which indicated that MMT and LDH/IFR showed a synergistic effect in reducing CO2TY in PLA composites.
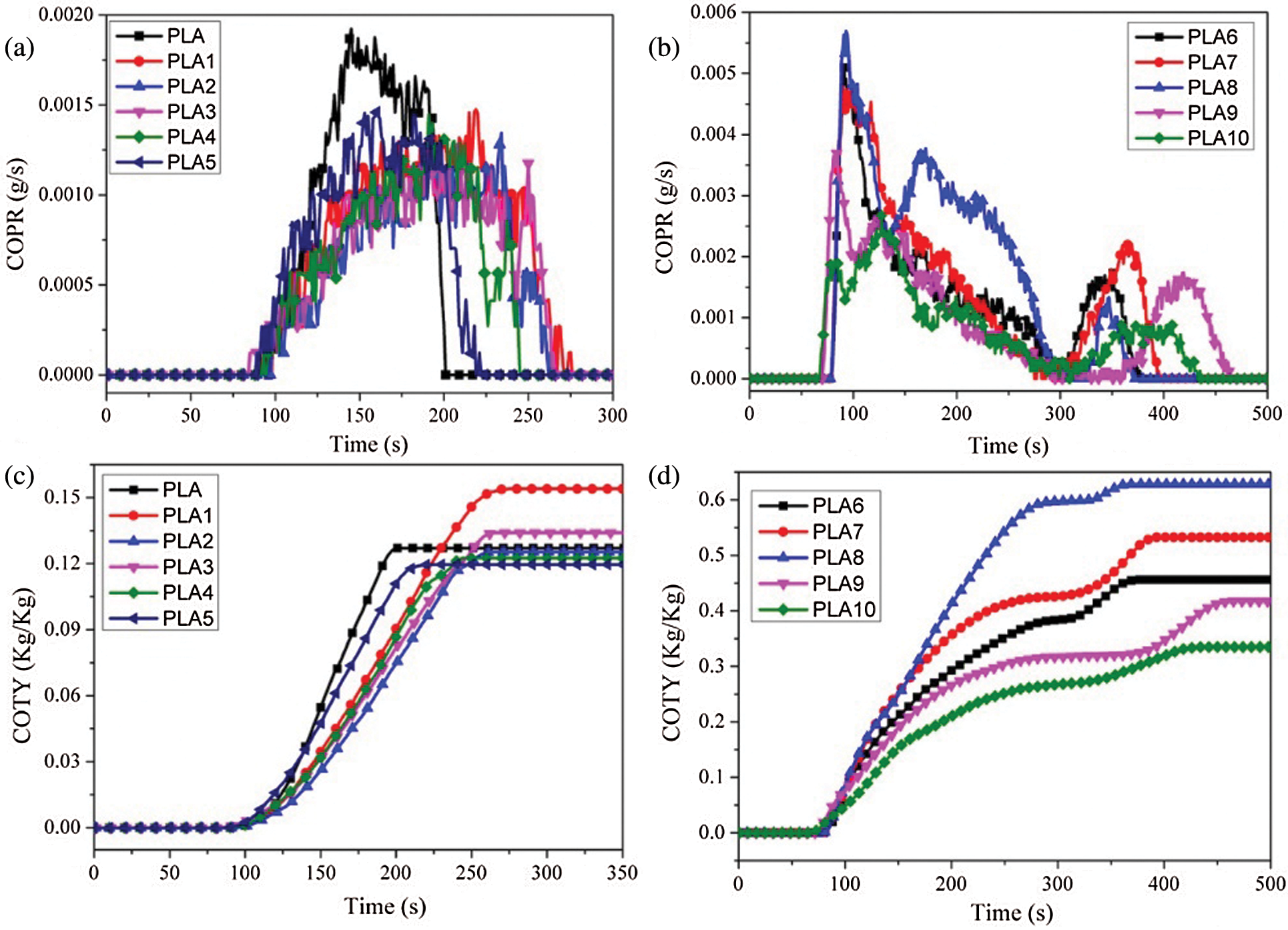
Figure 7: (a, b) COPR, (c, d) COTY curves of PLA composites
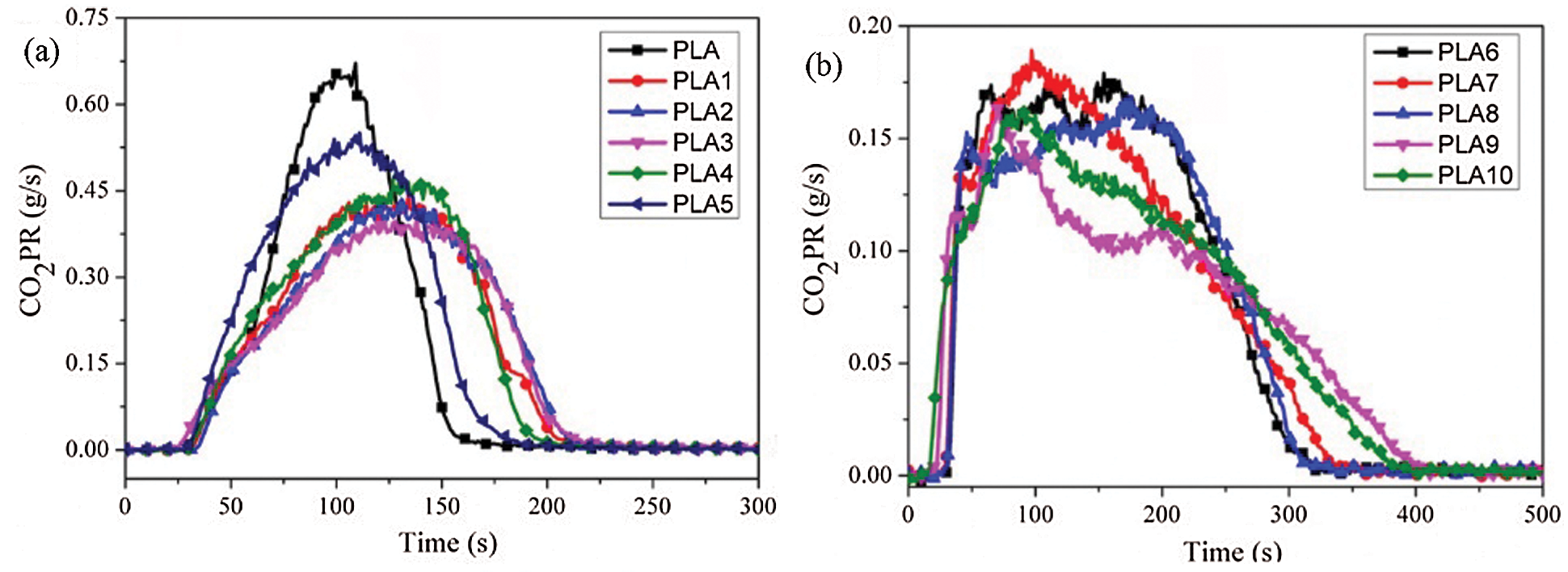
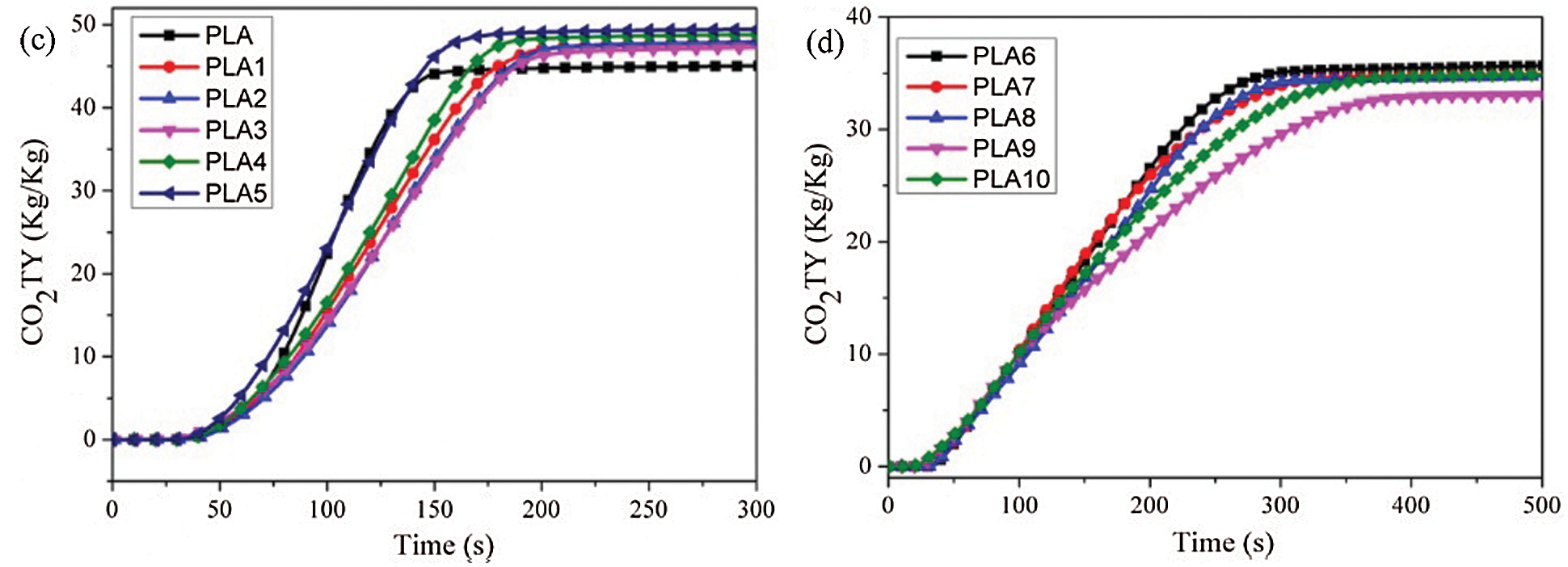
Figure 8: (a, b) CO2PR, (c, d) CO2TY curves of PLA composites
The mass loss rate reflects the pyrolysis rate and pyrolysis behavior of the material under a certain fire intensity. Fig. 9 showed the mass loss curve rate of PLA composites. From Fig. 9a, when PLA was ignited, LDH could effectively reduce the mass loss rate of PLA, and the addition of an appropriate amount of MMT could improve the thermal stability of PLA/LDH. When IFR was introduced into PLA/LDH system (Fig. 9b), the mass loss rate of PLA composites decreased significantly, and PLA9 showed the best performance, but the carbon formation of the composites did not change significantly. The results indicated that the addition of MMT did not change the pyrolysis process of PLA/LDH/IFR composite, but physically strengthened the protective effect of the carbon layer on the matrix. The total heat release rate (THR) was another critical parameter used to evaluate the flame intensity. The slope of THR represents the flame propagation rate. From Fig. 9c, with the addition of LDH, the flame propagation rate decreased, but the combustion time increased; The addition of an appropriate mass of MMT could further reduce the flame propagation rate of the PLA composites, indicating that LDH could prevent PLA combustion and owned a synergistic effect with MMT. When IFR was introduced into PLA/LDH system (Fig. 9d), the flame propagation rate and THR of PLA composites decreased significantly, and 1 wt% MMT was introduced into PLA6, and the composites showed the least THR, indicating that MTT owns synergistic effect with IFR/LDH to reduce the combustion performance in PLA composites.
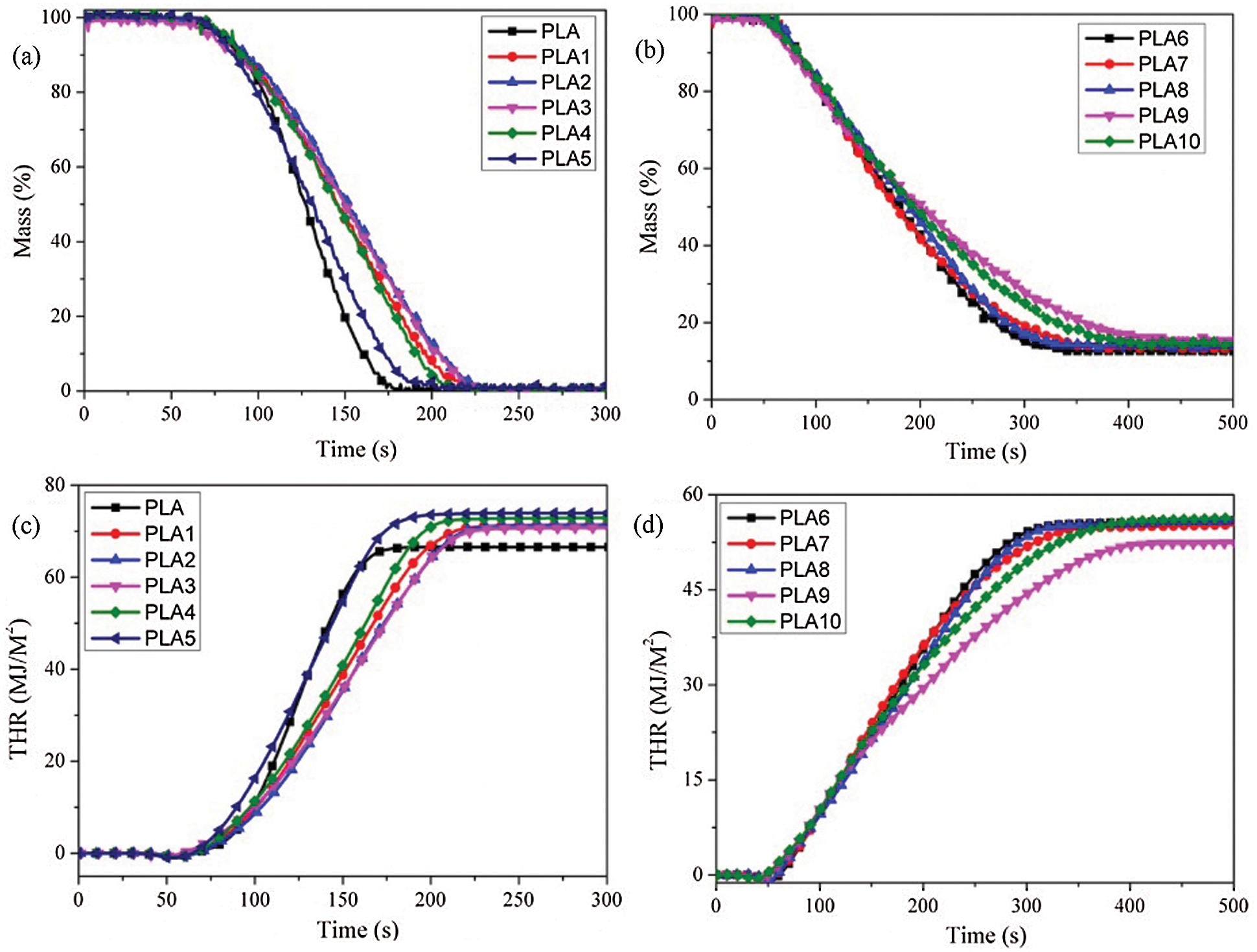
Figure 9: (a, b) the mass loss rate and (c, d) total heat release curves of PLA composites
This study investigated the effect of MMT on the flame retardant properties of PLA/LDH and PLA/IFR/LDH composites. The results indicated that LDH and MMT were well dispersed in PLA; MMT could improve the stability of char for PLA/LDH or PLA/IFR/LDH composites during combustion, but did not change the carbon formation process of polymers; During the combustion of PLA composites, MMT could cooperate with LDH or IFR/LDH system to reduce the peak HRR, prolong the combustion time and reduce the fire risk of PLA composites. The above research work can provide a new strategy for the development of multifunctional synergistic intumescent flame retardant PLA composites.
Funding Statement: The study was financially supported Project of State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology (20FKSY15, 19FKSY0110), Longshan Academic Talent Research Supporting Program of SWUST (17LZX636, 18LZX629).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hamad, K., Kaseem, M., Ayyoob, M., Joo, J., Deri, F. (2018). Polylactic acid blends: The future of green, light and tough. Progress in Polymer Science, 85, 83–127. DOI 10.1016/j.progpolymsci.2018.07.001. [Google Scholar] [CrossRef]
2. Prambauer, M., Wendeler, C., Weitzenboeck, J., Burgstaller, C. (2019). Biodegradable geotextiles–An overview of existing and potential materials. Geotextiles and Geomembranes, 47(1), 48–59. DOI 10.1016/j.geotexmem.2018.09.006. [Google Scholar] [CrossRef]
3. Chow, W. S., Teoh, E. L., Karger-Kocsis, J. (2018). Flame retarded poly(lactic acidA review. Express Polymer Letters, 12(5), 396–417. DOI 10.3144/expresspolymlett.2018.34. [Google Scholar] [CrossRef]
4. Tawiah, B., Yu, B., Fei, B. (2018). Advances in flame retardant poly(lactic acid). Polymers, 10(8), 876. DOI 10.3390/polym10080876. [Google Scholar] [CrossRef]
5. Li, W., Zhang, L., Chai, W., Yin, N., Semple, K. et al. (2021). Enhancement of flame retardancy and mechanical properties of polylactic acid with a biodegradable fire-retardant filler system based on bamboo charcoal. Polymers, 13(13), 2167. DOI 10.3390/polym13132167. [Google Scholar] [CrossRef]
6. Liu, Y., Liu, Y., Yang, R. (2021). Polylactic acid flame-retarded by nano-compound of form II ammonium polyphosphate with montmorillonite. Journal of Fire Sciences, 39(6), 495–511. DOI 10.1177/07349041211025456. [Google Scholar] [CrossRef]
7. Zhan, Y., Wu, X., Wang, S., Yuan, B., Fang, Q. et al. (2021). Synthesis of a bio-based flame retardant via a facile strategy and its synergistic effect with ammonium polyphosphate on the flame retardancy of polylactic acid. Polymer Degradation and Stability, 191, 109681. DOI 10.1016/j.polymdegradstab.2021.109684. [Google Scholar] [CrossRef]
8. Chen, K., Yang, D., Shi, Y., Feng, Y., Fu, L. et al. (2021). Synergistic function of N-P-Cu containing supermolecular assembly networks in intumescent flame retardant thermoplastic polyurethane. Polymers for Advanced Technologies, 32(11), 4450–4463. DOI 10.1002/pat.5448. [Google Scholar] [CrossRef]
9. Chen, L., Song, L., Lv, P., Jie, G., Tai, Q. et al. (2011). A new intumescent flame retardant containing phosphorus and nitrogen: Preparation, thermal properties and application to UV curable coating. Progress in Organic Coatings, 70(1), 59–66. DOI 10.1016/j.porgcoat.2010.10.002. [Google Scholar] [CrossRef]
10. Nie, S., Zhang, C., Peng, C., Wang, D. Y., Ding, D. et al. (2015). Study of the synergistic effect of nanoporous nickel phosphates on novel intumescent flame retardant polypropylene composites. Journal of Spectroscopy, 2015, 1–7. DOI 10.1155/2015/289298. [Google Scholar] [CrossRef]
11. Yang, H., Yu, B., Xu, X., Bourbigot, S., Wang, H. et al. (2020). Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chemistry, 22(7), 2129–2161. DOI 10.1039/D0GC00449A. [Google Scholar] [CrossRef]
12. Cai, W., Li, Z., Mu, X., He, L., Zhou, X. et al. (2021). Barrier function of graphene for suppressing the smoke toxicity of polymer/black phosphorous nanocomposites with mechanism change. Journal of Hazardous Materials, 404, 124106. DOI 10.1016/j.jhazmat.2020.124106. [Google Scholar] [CrossRef]
13. Wang, G., Xu, W., Chen, R., Li, W., Liu, Y. et al. (2020). Synergistic effect between zeolitic imidazolate framework-8 and expandable graphite to improve the flame retardancy and smoke suppression of polyurethane elastomer. Journal of Applied Polymer Science, 137(1), 48048. DOI 10.1002/app.48048. [Google Scholar] [CrossRef]
14. Yue, X., Li, C., Li, Y. (2021). Using colloidal lignin intercalated montmorillonite nanosheets as synergistic and reinforced agent for flame-retardant poly(butylene succinate) composites. Polymers for Advanced Technologies, 32(6), 2552–2565. DOI 10.1002/pat.5287. [Google Scholar] [CrossRef]
15. Zhang, D., Ma, Z., Zhang, Z., Ning, H., Wang, Y. (2021). Graphene oxide nanosheets decorated with mgAlCr-layered double hydroxide nanosheets as flame retardant coatings for steel. ACS Applied Nano Materials, 4(8), 8241–8250. DOI 10.1021/acsanm.1c01431. [Google Scholar] [CrossRef]
16. Li, X., Liang, D., Hu, Z., He, J., Bian, X. et al. (2021). Synergistic effects of polyoxometalate-based ionic liquid-doped sepiolite in intumescent flame-retardant high-density polyethylene. Polymers for Advanced Technologies, 32(5), 2240–2251. DOI 10.1002/pat.5258. [Google Scholar] [CrossRef]
17. Wang, Y., Liu, C., Lai, J., Lu, C., Wu, X. et al. (2020). Soy protein and halloysite nanotubes-assisted preparation of environmentally friendly intumescent flame retardant for poly(butylene succinate). Polymer Testing, 81, 106174. DOI 10.1016/j.polymertesting.2019.106174. [Google Scholar] [CrossRef]
18. Chen, Y., Xu, L., Wu, X., Xu, B. (2019). The influence of nano ZnO coated by phosphazene/triazine bi-group molecular on the flame retardant property and mechanical property of intumescent flame retardant poly(lactic acid) composites. Thermochimica Acta, 679, 178336. DOI 10.1016/j.tca.2019.178336. [Google Scholar] [CrossRef]
19. Xu, Z., Jia, H., Yan, L., Chu, Z., Zhou, H. (2021). Synergistic effects of organically modified montmorillonite in combination with metal oxides on the fire safety enhancement of intumescent flame-retarded epoxy resins. Journal of Vinyl and Additive Technology, 27(1), 161–173. DOI 10.1002/vnl.21793. [Google Scholar] [CrossRef]
20. Wang, W., Peng, Y., Zammarano, M., Zhang, W., Li, J. (2017). Effect of ammonium polyphosphate to aluminum hydroxide mass ratio on the properties of wood-flour/polypropylene composites. Polymers, 9(11), 615. DOI 10.3390/polym9110615. [Google Scholar] [CrossRef]
21. Hu, Z., Zhang, P., Xie, R., Li, M., Lu, Z. et al. (2018). Controlled synthesis of train-structured montmorillonite/layered double hydroxide nanocomposites by regulating the hydrolysis of polylactic acid. Journal of Materials Science, 53(23), 15859–15870. DOI 10.1007/s10853-018-2758-6. [Google Scholar] [CrossRef]
22. Costa, F. R., Leuteritz, A., Wagenknecht, U., Landwehr, M., Jehnichen, D. et al. (2009). Alkyl sulfonate modified LDH: Effect of alkyl chain length on intercalation behavior, particle morphology and thermal stability. Applied Clay Science, 44(1–2), 7–14. DOI 10.1016/j.clay.2008.12.020. [Google Scholar] [CrossRef]
23. Akinwunmi, B., Sun, L., Hirvi, J. T., Kasa, S., Pakkanen, T. (2019). Influence of temperature on the swelling pressure of bentonite clay. Chemical Physics, 516, 177–181. DOI 10.1016/j.chemphys.2018.09.009. [Google Scholar] [CrossRef]
24. Zheng, Y., Zaoui, A. (2013). Temperature effects on the diffusion of water and monovalent counterions in the hydrated montmorillonite. Physica A: Statistical Mechanics and its Applications, 392(23), 5994–6001. DOI 10.1016/j.physa.2013.07.019. [Google Scholar] [CrossRef]
25. Mensah, R., Xu, Q., Asante-Okyere, S., Jin, C., Bentum-Micah, G. (2019). Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments. Journal of Thermal Analysis and Calorimetry, 136(2), 589–599. DOI 10.1007/s10973-018-7661-5. [Google Scholar] [CrossRef]
26. Xie, T., Wei, R., Wang, Z., Wang, J. (2020). Comparative analysis of thermal oxidative decomposition and fire characteristics for different straw powders via thermogravimetry and cone calorimetry. Process Safety and Environmental Protection, 134, 121–130. DOI 10.1016/j.psep.2019.11.028. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |