

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.020489
ARTICLE
Characterization of Carboxymethyl Cellulose Made from Bamboo Harvesting Residues
School of Forestry and Landscape Architecture, Anhui Agricultural University, Hefei, 230036, China
*Corresponding Author: Shuangyan Zhang. Email: zsyhj_2006@163.com
Received: 26 November 2021; Accepted: 19 January 2022
Abstract: Bamboo harvesting residues are wastes by-products of bamboo industries that contain holocellulose for about 63.14% to 70.71%, which often be discarded, incinerated or buried. In this study, carboxymethyl cellulose was prepared from bamboo harvesting residues (bamboo-branch and bamboo-tip) as raw materials. The chemical composition of bamboo harvesting residues, the viscosity and degree of substitution of carboxymethyl cellulose were determined. Carboxymethyl cellulose obtained was further characterized and compared by means of FTIR, SEM, XRD and TG. Results showed that under the optimized identical conditions, the viscosity and degree of substitution of carboxymethyl cellulose from bamboo-branch and bamboo-tip were 6.0 and 78.9 mPa·s, 0.75 and 0.89, respectively. Carboxymethyl cellulose obtained from bamboo-tip displayed a lower crystallinity and a better thermal stability as compared to synthetic carboxymethyl cellulose obtained from bamboo-branch and bamboo-culm.
Keywords: Bamboo harvesting residues; bamboo-branch; bamboo-tip; carboxymethyl cellulose; characterization
Carboxymethyl cellulose (CMC) is one of the most important cellulose derivatives, a linear polysaccharide of anhydro-glucose [1]. It is produced by reacting alkali cellulose with monochloroacetic acid (MCA) or its sodium salt (NaMCA). Due to its characteristics such as renewable, biocompatible, biodegradable, availability, non-toxic, low-cost synthesis process, hydrophilicity, and likewise many other properties, CMC now has a wide of applications in various fields, for example, paper, textile, adhesives, food, biomedical, construction, agriculture and wastewater treatment [2,3]. Initially, the production of CMC was from wood-based biomaterials, such as spruce wood [4]. However, the use of wood as source of CMC is considered less effective because wood is often used as household raw material and has long growing time [5]. Therefore, a number of alternative abundant and underutilized cheaper sources have been introduced by many researchers in the literature as effectual alternative candidates, including some plant-based precursors and some waste materials [6–9]. Among them, bamboo, an inexpensive, fast-growing, naturally available, sustainable resource, has gained tremendous attention of researchers to be applied for the production of CMC.
Bamboo is a perennial grass of Bambusoideae, widely distributed in tropical and subtropical regions, and it is one of the most valuable biomaterials in the world today. At present, the area of bamboo forest in the world has reached 22 million hectares, accounting for about 1% of the forest area. The annual output of bamboo is 15 million to 20 million tons [10]. China has the largest area, species, and output bamboo resources in the world. According to the 9th National Forest Resources Inventory Report, the bamboo forest area in China is 6.41 billion hectares, of which the moso bamboo forest area accounts for about 73% [11]. As a kind of biological material with numerous advantages including sustainability and biodegradability, appropriate processing with physical, chemical, and mechanical treatments, bamboo can be commonly used as material for making biodegradable agriculture mulching, furniture, paper, natural fiber, activated carbon, and granulated fuel [12–14]. Unfortunately, nowadays, the processing utilization rate of bamboo in China is only 35%–40% [15]. The harvesting and processing of bamboo resources generate a large amount of bamboo residues wastes such as bamboo tips, branches, leaves, shavings, bamboo yellow, bamboo blue and so on, but excluding culms, which are often not reasonably utilized in industrial applications. With the rapid development of bamboo industry, various bamboo residues wastes have gradually increased, and the residues of bamboo harvesting and processing have reached about 28.17 million tons in China [16]. Currently, these wastes are incinerated, buried, or used as the boiler burning material directly, which not only pollute the environment, but also waste resources. Therefore, considering the environmental protection and sustainable development issues, how to make full and efficient utilization of these wastes to achieve “zero” surplus of resources is an urgent problem for comprehensive utilization of bamboo.
Bamboo harvesting residues (e.g., branches, tips, leaves, roots, shavings, etc.) are wastes by-products of bamboo industry. It is an abundant lignocellulose by-products in China. Bamboo tips (Zhejiang Province, China) contain 73.06% holocellulose, 24.73% lignin, 1.22% ash and extractive substances [16]. Bamboo shavings contain 33%–45% cellulose, along with lignin, protein, hemicellulose, and pectin, as well as some other minor extracts [6]. Compared to bamboo culms, bamboo harvesting residues are more highly available at a negligible cost, or sometimes even free of charge. Hence, bamboo harvesting residues may be used as a kind of low-cost, plentiful materials for CMC production. The synthesis of CMC from bamboo harvesting residues source has already been reported. For instance, Chen et al. [17] synthesized CMC from pretreated bamboo shaving with a degree of substitution ≥0.8, and viscosity of 1% CMC aqueous solution was above 260 mPa⋅s. In this study, under the conventional method, CMCs were mainly made with bamboo tips and branches obtained from bamboo harvesting residues. Then the characterization of bamboo harvesting residues and CMCs obtained were performed and compared. The objective of present study is to introduce bamboo harvesting residues as precursor to produce CMC. This will not only be helpful to recycle and reuse this waste for useful material production, but also to increase the income of bamboo farmers.
Moso bamboo harvesting residues, three years old, mainly including bamboo branches and bamboo tips were obtained from Jinzhai County, Anhui Province, China. The collected bamboo branches were removed bamboo leaves. Bamboo tips (wall thickness <2 mm) were cut from top position of each bamboo. Then the samples were ground to powder with a mill and the size of bamboo powder used in the test were about 250–425 μm and 180–250 μm (Fig. 1). Finally, the bamboo powder samples were dried at 105°C until a constant weight before the experiments.

Figure 1: Preparation process of bamboo samples
CMC was prepared from bamboo harvesting residues, according to the conventional method, that is the alkylation-etherification process, as reported before [1,17,18]. Firstly, 10 g of the screened bamboo powder (size 180–250 μm) was added to a conical flask containing 300 mL of distilled water, which was done to remove impurities present in bamboo powder like starch. The mixture was then heated in a in a boiling water bath for 2 h. After that, the residue was filtered and dried at 60°C in an oven. Secondly, 300 mL of 15% NaOH (w/v) solution was mixed with 10 g of residue sample from last step, which was done to remove hemicellulose and part of lignin. Then, the mixture was stirred for 3 h at 80°C. After that, the residue was washed several times until the drain become neutral and subsequently filtered and dried in the oven at 60°C. Thirdly, 10 g sample from second step was added into a conical flask containing of 300 mL distilled water, which was prepared by adding 3 g of sodium chlorite (80%, w/w) and 2 mL of acetic acid into the conical flask. The mixture then placed into the water bath at 75°C, which was followed by adding sodium chlorite (3 g) and acetic acid (2 mL) every 1 h for 4 h. Afterwards, the residue was filtered, washed and dried in an oven at 60°C. Fourthly, 4 g sample from third step was added into 20 mL of 15% NaOH and 80 mL of 100% ethanol solution, which was then stirred for 1 h at 30°C. After that, the temperature was increased to 65°C, which was followed by adding 5 g of monochloroacetic acid. The reactant was then stirred for 3 h. Finally, the reactant was filtrated and the residue was then washed washed with 90% acetic acid and 80% ethanol. The powder obtained after drying was CMC (Fig. 2).

Figure 2: The process for preparing CMC from different parts of bamboo powder
Note: Bamboo culms were cut from 1.5 m height position of each bamboo.
Traditional wet chemical analysis was performed to investigate the chemical composition of bamboo harvesting residues (size 250–425 μm). The contents of ash, benzene-ethanol extractives, 1% NaOH solubility, lignin, holocellulose and alpha-cellulose were determined according to the GB/T 742-2008 [19], GB/T 2677.6-1994 [20], GB/T 2677.5-1993 [21], GB/T 2677.8-1994 [22], GB/T 2677.10-1995 [23] and GB/T 744-1989 [24] standard. The hemicellulose content was calculated by subtracting the alpha-cellulose content from the holocellulose content.
2.4 Degree of Substitution (DS) of CMC
The degree of substitution (DS) of CMC was estimated using the ashing method according Wang et al. [25], it was calculated as follows:
where DS is the degree of substitution. CB is the milliequivalent of carboxymethyl per gram of sample. V1 is the volume of the sulfuric acid standard titration solution. c1 is the actual concentration of sulfuric acid standard titration solution. V2 is the volume of sodium hydroxide standard titration solution. c2 is the actual concentration of sodium hydroxide standard titration solution. m is the mass of CMC sample. 0.162 is the molecular weight of the anhydrous glucose unit of cellulose. 0.080 is the net increment in the anhydrous glucose unit for every substituted carboxymethyl group.
The viscosity of the CMC samples was measured at 20 g/L CMC concentration using a standard method GB 1904-2005 [26].
2.6.1 Fourier Transform Infrared Spectroscopy Analysis (FTIR)
FTIR spectroscopy was conducted on samples using a Fourier transform infrared spectroscopy (Nicolet 6670, USA), in which samples were palletized with KBr powder. The scanning scale was from 4000 to 400 cm−1.
2.6.2 Scanning Electron Microscope Analysis (SEM)
The morphological images of samples were observed by S-4800 scanning electron microscope (SEM) produced by Hitachi, Japanese. The magnification was 100 times.
2.6.3 X-Ray Diffraction Analysis (XRD)
XRD tests of samples were obtained on an X-ray diffractometer (XD-3, China) with a Co target (λ = 0.154 nm), and the data was collected with a 2θ scanning range from 10° to 40°. The crystallinity index (CrI) was calculated according to the formula proposed by Segal et al. [27].
where CrI is the crystallinity index. I002 is the maximum intensity of (002) lattice diffraction angle. Iam is the intensity of the baseline at 2θ about 18°.
2.6.4 Thermogravimetric Analysis (TG)
Thermogravimetric analysis was observed though a thermogravimetic analyzer (TGA 209, Netzsch, Germany). The samples of about 3.0–5.0 mg were heated from 30°C up to 600°C at a heating rate of 10 °C/min under nitrogen atmosphere (60 mL/min).
3.1 Chemical Composition of Bamboo Harvesting Residues
The chemical composition of bamboo harvesting residues, including bamboo-branch and bamboo-tip were presented in Table 1. Cellulose, hemicellulose and lignin were the main compounds, followed by extractives, the smallest component was ash in the bamboo harvesting residues. As shown in Table 1, bamboo-tip had higher cellulose content (43.75%) than that of both bamboo-branch (43.48%) and bamboo-culm (43.00%). This difference was also observed for lignin content, which was lower for bamboo-tip (24.35%) than that of bamboo-branch (24.51%) and bamboo-culm (24.44%) (Table 1). Lower lignin content and higher cellulose content in lignocellulosic materials are desirable in the cellulose derivative production, which may increase the products yield and quality of the derivative [28]. Therefore, bamboo-tip may be considered more suitable for obtaining cellulose derivatives, as compared with bamboo-branch and bamboo-culm.
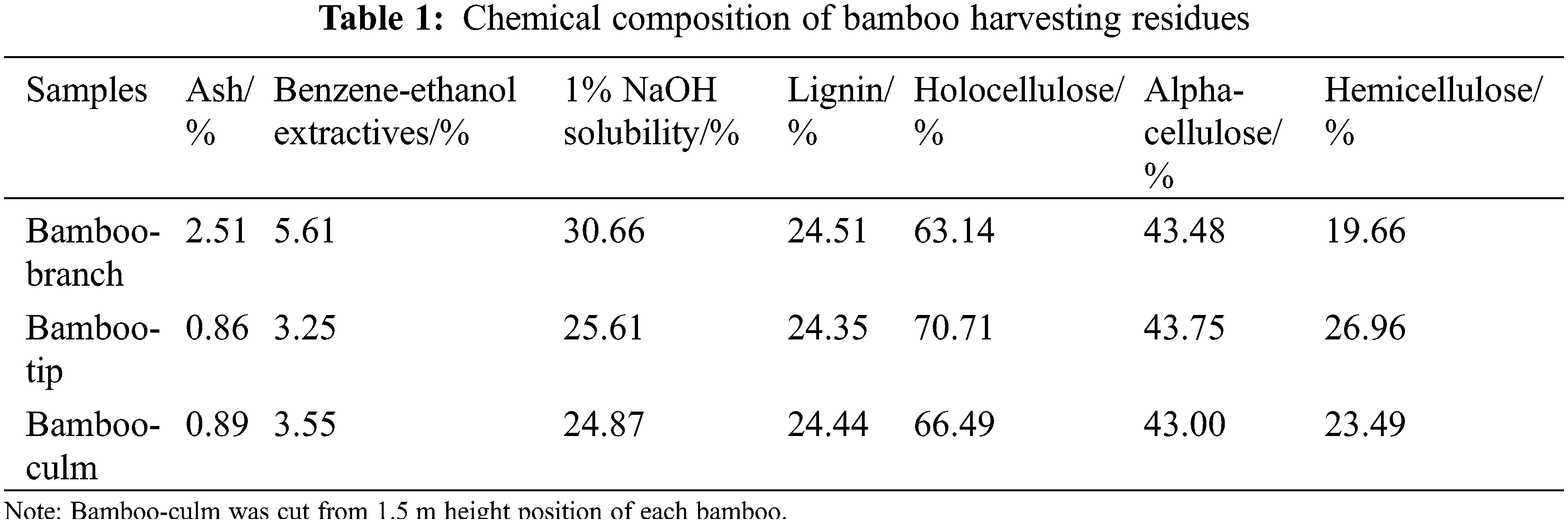
Bamboo-branch was characterized for a relative high content of ash and extractives. In our study, the bamboo-branch had the highest ash content (2.51%), which was nearly three times that of bamboo-tip (0.86%) and bamboo-culm (0.89%), respectively. Amount of extractives in bamboo-tip was similar to bamboo-culm, but less than that of bambo-branch, which implied high natural durability of bamboo-branch [29].
The main step in the process of synthesized CMC is the formation of alkali cellulose. Small oligosaccharides and other low molar mass components can be extracted from cellulose by alkali treatment. These alkali extractives will be also etherified, yielding low molar mass derivatives that may affect the properties of the cellulose derivative. Table 1 showes the solubility of samples in 1% NaOH. For bamboo harvesting residues, the amount of alkali soluble material was from 25.61% to 30.66%.
The DS is the replacement of hydroxyl group of cellulose with carboxymethyl group during the carboxymethylation. It has a major influence on the properties and the potential uses of CMC [30]. The DS of synthesized CMCs from bamboo harvesting residues was met with the commercially available CMC grades (0.6–1.25) [31]. As shown in Table 2, the CMC derived from bamboo-tip had a DS of 0.89, which is much higher than that of bamboo-branch (0.75) and bamboo-culm (0.73). These data were related to the chemical composition of bamboo harvesting residues that bamboo-tip has higher alpha-cellulose content (Table 1). When the DS is below 0.4, the CMC is swellable but insoluble, while above this value, CMC is fully soluble with its hydro affinity increasing with increasing DS [32]. Therefore, CMCs made from bamboo harvesting residues were fully soluble in water, obviously.
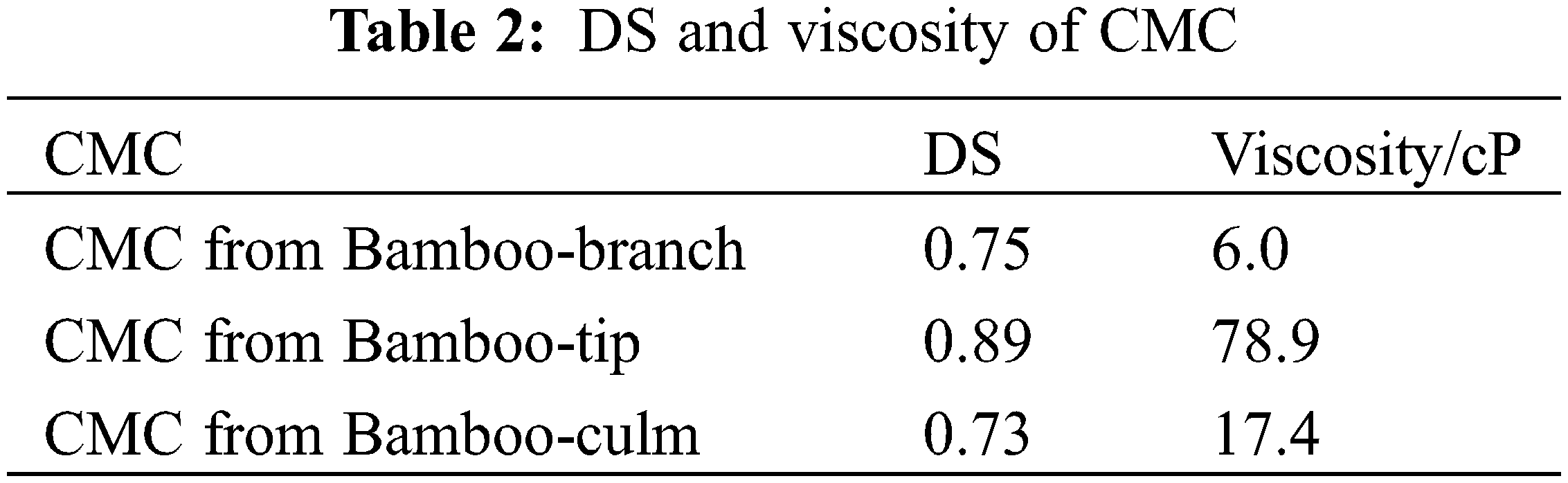
Viscosity of CMC is an important parameter for the industrial use. It provides information for flow characteristics of the fluid flow involved in processing operations and products using different concentrations of CMC [32]. The viscosity of CMCs from bamboo harvesting residues were in the range of 6.0–78.9 cP (Table 2). This might be due to the value of DS. The highest value of viscosity for CMC from bamboo-tip was only 78.9 cP.
The FTIR spectra of bamboo harvesting residues and CMCs were shown in Fig. 3. As shown in Fig. 3a, the peaks at the wave numbers of 3410 and 2905 cm−1 were attributed to -OH and -CH groups stretching vibration [33]. The spectra for bamboo harvesting residues showed unconjugated C=O stretching of carboxyl and acetyl from hemicellulose at 1734 cm−1 [34]. The peaks at 1510 and 1455 cm−1 were attributed to aromatic skeleton and CH3 deformation in lignin (Fig. 3a), respectively [35]. The peaks at 1734, 1510, and 1455 cm−1 disappeared for CMCs (Fig. 3a), which confirmed the efficient removal of lignin and hemicellulose from bamboo. The peaks at 1610 and 1422 cm−1 were appeared in the FTIR spectra of CMCs (Fig. 3b). According to Adinugraha et al. [36] the stretching vibration of carboxyl groups and carboxyl groups as salts have wave numbers about 1600–1640 cm−1 and 1400–1450 cm−1, respectively. This indicated that the hydroxyl groups in the cellulose were replaced with carboxyl group when carboxymethylation reaction occur. Therefore, CMCs from bamboo harvesting residues were synthesized successfully.
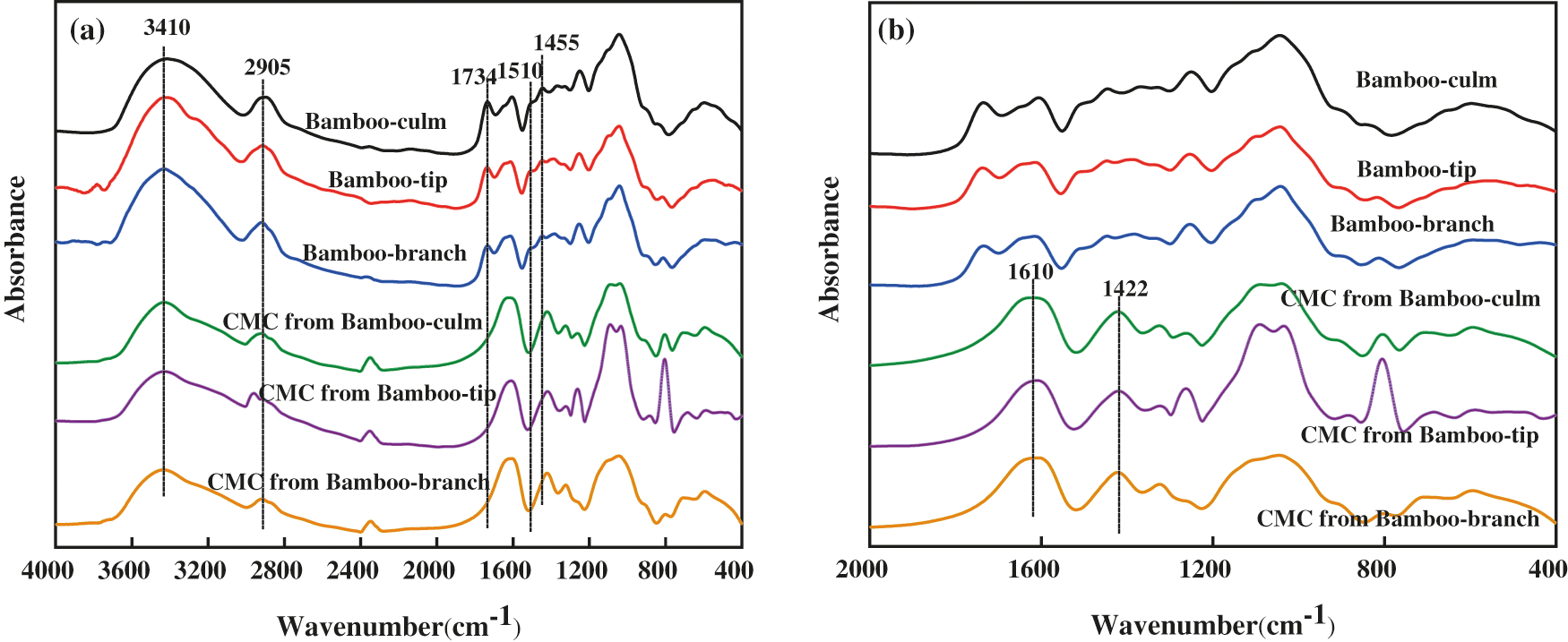
Figure 3: The FTIR spectra of the bamboo-tip, bamboo-branch, bamboo-culm, CMC from bamboo-tip, CMC from bamboo-branch and CMC from bamboo-culm, (a) The region in the range of 400–4000 cm−1, (b) The region in the range of 400–2000 cm−1
SEM images of bamboo harvesting residues and CMCs were exhibited in Fig. 4. As shown in Fig. 4, fibers from bamboo harvesting residues showed smooth surface, compact structure, and large volume, which were similar to that from bamboo-culm. However, SEM images of CMCs showed rod-like and ribbon shapes with a thin and short fibers. The surface of CMCs appeared rough on which obvious cracks and grooves can be observed. These changes for CMCs were mainly due to the destruction of hydrogen bonding between cellulose by the alkalization and etherification process [37].

Figure 4: SEM images of the bamboo-tip, bamboo-branch, bamboo-culm, CMC from bamboo-tip, CMC from bamboo-branch and CMC from bamboo-culm
The XRD diffractograms of bamboo harvesting residues and CMCs were displayed in Fig. 5. As shown in Fig. 5, the bamboo harvesting residues displayed the typical diffraction pattern of cellulose I with peaks at 2θ = 16° (101), 22° (002) and 34.7° (040), was similar to bamboo-culm [34,38]. The crystallinity index of bamboo-tip and bamboo-branch were 47.12% and 46.49%, respectively. This was consistent with the previous obtained chemical composition analysis results.

Figure 5: The XRD patterns of the bamboo-tip, bamboo-branch, bamboo-culm, CMC from bamboo-tip, CMC from bamboo-branch and CMC from bamboo-culm
For the CMCs, the peak at 16° disappeared completely and a peak at around 21° was shown instead (Fig. 5), indicating that the hydrogen bonds between cellulose were weakened and the crystal structures were destroyed [39]. The crystallinity index of CMCs from bamboo-tip and bamboo-branch were 39.98% and 45.09%, respectively, which were lower than those of bamboo harvesting residues (Fig. 6). This phenomenon was supposed to be the cleavage of the broadening hydrogen bonds due to carboxymethyl substitution at the hydroxyl groups of cellulose [36]. The crystallinity of CMC from bamboo-tip was lower than that from bamboo-branch and bamboo-culm. This might be due to the DS of CMC, that is, the higher the DS of CMC resulted in the decrease of the crystallinity [40].

Figure 6: The crystallinity of the bamboo-tip, bamboo-branch, bamboo-culm, CMC from bamboo-tip, CMC from bamboo-branch and CMC from bamboo-culm
The differences in thermal degradation, characterised by peak temperature and weight loss for bamboo harvesting residues and CMCs were shown in Fig. 7 and Table 3. The degradation process of bamboo harvesting residues and CMCs were comprised of three distinct stages, which were found differences significantly. For bamboo harvesting residues, the three stages degradation process were similar to bamboo-culm. They were observed about at 30°C–101°C, 101°C–371°C, and 371°C–600°C, respectively, which were mainly attributed to the evaporation of adsorbed water and degradation of impurities, the decomposition of cellulose, hemicellulose and partial lignin, and the decomposition of residual lignin, respectively [41]. For CMCs, the total degradation curves shifted to the lower temperature (Fig. 7), which had the more char yields. The three stages degradation process were mainly due to the evaporation of adsorbed water and degradation of impurities, the decarboxylation of CMC with the release of carbon dioxide and carbon monoxide, and the CMC backbone broken down [18,42].
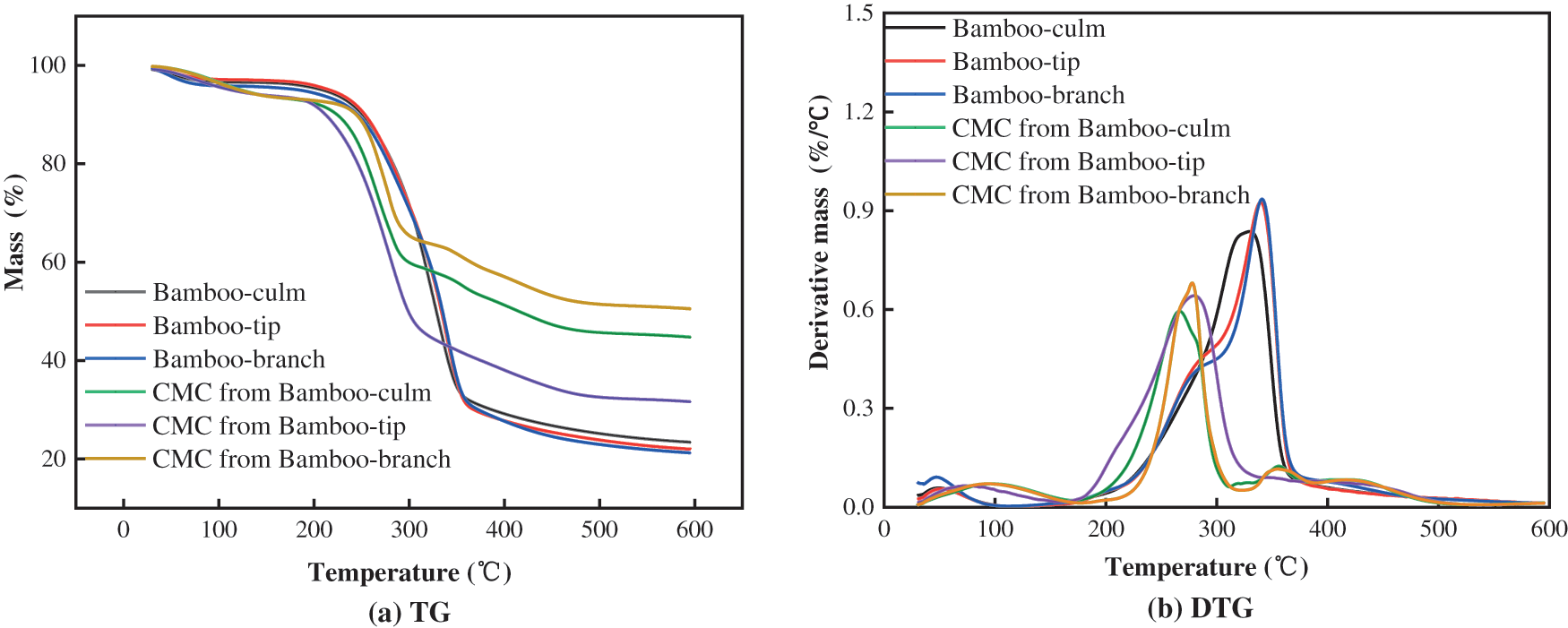
Figure 7: TG (a) and DTG (b) curves of the bamboo-tip, bamboo-branch, bamboo-culm, CMC from bamboo-tip, CMC from bamboo-branch and CMC from bamboo-culm
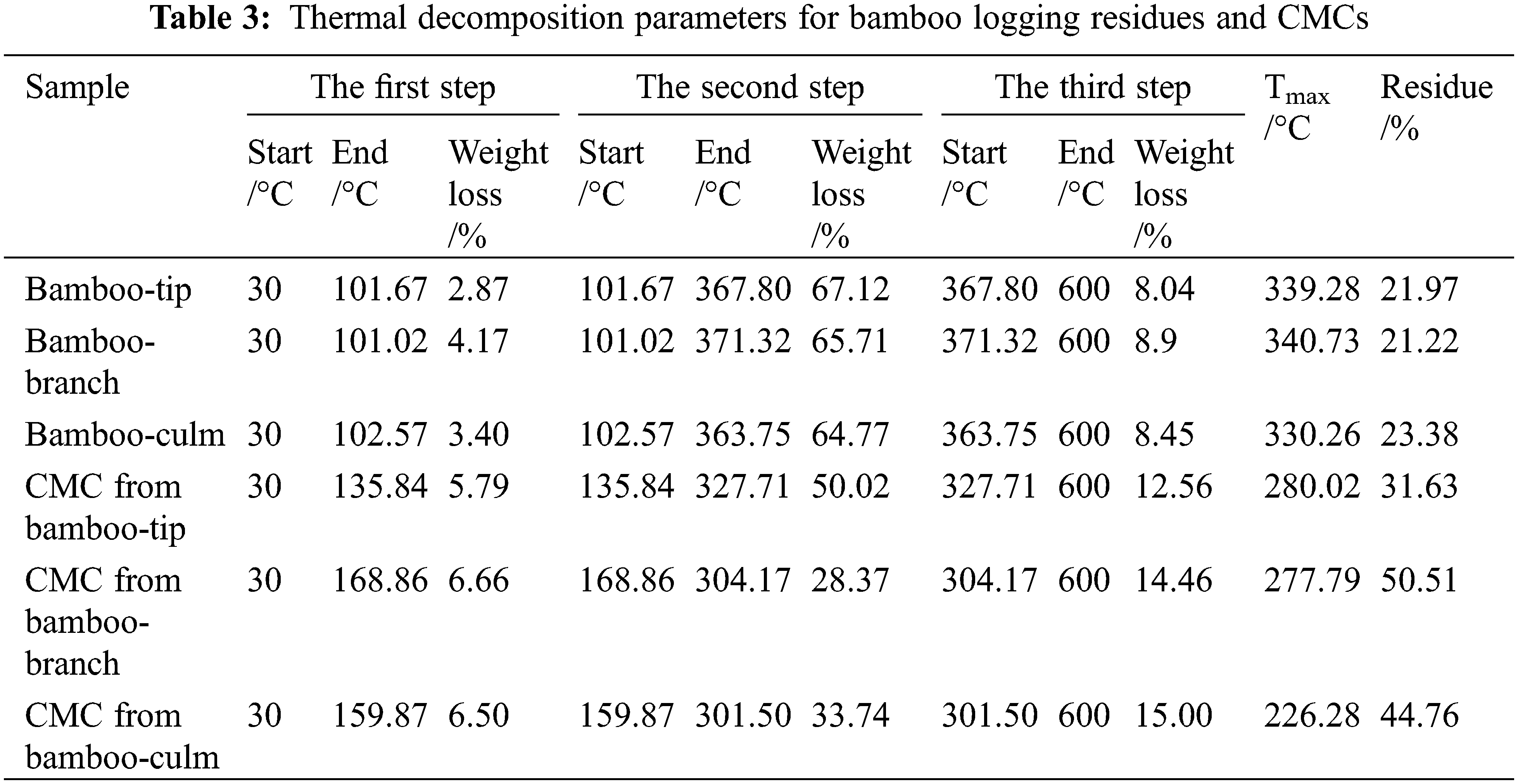
As shown in Fig. 7b, the major degradation peak temperature at 339°C–340°C for bamboo harvesting residues, which also was similar to bamboo-culm. However, for CMCs, the major degradation peak was lower than that for bamboo harvesting residues, which led to a poor thermal stability. This could be associated with the reduction in crystallinity upon carboxymethylation. During the preparation of CMC, carboxymethylation destroyed the the crystal structure of cellulose and made the internal space of CMC become loose [43]. Moreover, CMC from bamboo-tip had the highest the major degradation peak temperature, suggesting that CMC from bamboo-tip had the best thermal stability. Some studies had confirmed that the DS of the samples play a decisive role in their thermal stability [30]. Compared with other CMC prepared with bamboo, the DS of CMCs from bamboo harvesting residues was the highest, 0.89 (Table 2). As shown in Table 3, the final residual weight for CMC was higher than bamboo harvesting residues. This might be related to a large number of sodium ions in CMCs [44].
In this study, lab-made CMC can be successfully synthesized from bamboo harvesting residues using optimized conventional method. Simultaneously, the characterization of bamboo harvesting residues and lab-made CMC obtained from bamboo harvesting residues were performed and analyzed. The following conclusions can be drawn:
1. Cellulose, hemicellulose and lignin were the main chemical compounds of bamboo harvesting residues, followed by extractives and ash. The chemical composition of bamboo-tip presented a high content of cellulose and a lower content of lignin, while bamboo-branch had a relative high content of ash and extractives.
2. CMC made from bamboo-tip had the highest degree of substitution. The viscosity of CMC obtained from bamboo harvesting residues was in the range of 6.0–78.9 cP at 25°C.
3. The CMC obtained from bamboo harvesting residues showed similarity of the characteristics to the reference on infrared spectra, SEM, XRD diffractogram and TG analysis. The morphology of CMCs obtained showed ribbon and rod-like shapes with a thin and short fibers. The crystal structure of CMCs changed and the crystallinity reduced. CMCs exhibited a low major degradation temperature and a high residual weight. Moreover, the thermal stability of CMC made from bamboo-tip was the best. This research could contribute to provide reference information for CMC products prepared from bamboo harvesting residues wastes and a value-added application way for bamboo wastes.
Acknowledgement: The authors greatly acknowledge the collaboration of Jinzhai Bamboo industry alliance for supplying the material of bamboo harvesting residues.
Funding Statement: This work is supported by the National Forestry and Grassland Administration/Beijing Open Fund of Key Laboratory of Bamboo and Rattan Science and Technology (Grant No. ICBR-2020-10) and University Scientific Research Project of Anhui Province (Grant No. KJ2020A0130).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Saifur Rahman, M., Saif Hasan, M., Nitai, A. S., Nam, S., Karmakar, A. K. et al. (2021). Recent developments of carboxymethyl cellulose. Polymers, 13(8), 1345. DOI 10.3390/polym13081345. [Google Scholar] [CrossRef]
2. Rania, B., Hend, A. E., Sherif, E., Mohamed, M., Hanan, E. et al. (2021). Spectroscopic and thermal analyses for the effect of acetic acid on the plasticized sodium carboxymethyl cellulose. Journal of Molecular Structure, 1224, 129013. DOI 10.1016/j.molstruc.2020.129013. [Google Scholar] [CrossRef]
3. Harish, K., Ankur, G., Sushil, K., Jin-Won, P. (2019). Development of silver nanoparticles-loaded CMC hydrogel using bamboo as a raw material for special medical applications. Chemical Papers, 73(4), 953–964. DOI 10.1007/s11696-018-0650-0. [Google Scholar] [CrossRef]
4. Revol, J. F., Goring, D. A. I. (1981). On the mechanism of the mercerization of cellulose in wood. Journal of Applied Polymer Science, 26(4), 1275–1282. DOI 10.1002/app.1981.070260419. [Google Scholar] [CrossRef]
5. Suryadi, H., Sutriyo, S. S., Fauziah, G. (2019). Characterization sodium carboxymethyl cellulose from alpha cellulose betung bamboo (Dendrocalamus asper). Pharmacognosy Journal, 11(5), 894–900. DOI 10.5530/pj.2019.11.143. [Google Scholar] [CrossRef]
6. Singh, R. K., Singh, A. K. (2013). Optimization of reaction conditions for preparing carboxymethyl cellulose from corn cobic agricultural waste. Waste and Biomass Valorization, 4(1), 129–137. DOI 10.1007/s12649-012-9123-9. [Google Scholar] [CrossRef]
7. Rodsamran, P., Sothornvit, R. (2020). Carboxymethyl cellulose from rice stubble waste. Songklanakarin Journal of Science and Technology, 42(2), 454–460. DOI 10.14456/sjst-psu.2020.59. [Google Scholar] [CrossRef]
8. Joshi, G., Naithani, S., Varshney, V. K., Bisht, S. S., Rana, V. et al. (2015). Synthesis and characterization of carboxymethyl cellulose from office waste paper: A greener approach towards waste management. Waste Management, 38, 33–40. DOI 10.1016/j.wasman.2014.11.015. [Google Scholar] [CrossRef]
9. Bidgoli, H., Zamani, A., Jeihanipour, A., Taherzadeh, M. J. (2014). Preparation of carboxymethyl cellulose superabsorbents from waste textiles. Fibers and Polymers, 15(3), 431–436. DOI 10.1007/s12221-014-0431-5. [Google Scholar] [CrossRef]
10. Zhang, N., Xu, H., Yang, J., Xie, J. C., Wei, M. et al. (2020). Effects of liquid hot water combined with 1, 4-butanediol on chemical composition and structure of moso bamboo. Applied Biochemistry and Biotechnology, 190(4), 1177–1186. DOI 10.1007/s12010-019-03173-0. [Google Scholar] [CrossRef]
11. Wang, Q. Y., Wu, X. W., Yuan, C. L., Lou, Z. C., Li, Y. J. (2020). Effect of saturated steam heat treatment on physical and chemical properties of bamboo. Molecules, 25(8), 1999. DOI 10.3390/molecules25081999. [Google Scholar] [CrossRef]
12. Chen, J. P., Guagliano, M., Shi, M. H., Jiang, X. S., Zhou, H. P. (2021). A comprehensive overview of bamboo scrimber and its new development in China. European Journal of Wood and Wood Products, 79(2), 363–379. DOI 10.1007/s00107-020-01622-w. [Google Scholar] [CrossRef]
13. Sharma, B., Shah, D. U., Beaugrand, J., Janeček, E. R., Scherman, O. A. et al. (2018). Chemical composition of processed bamboo for structural applications. Cellulose, 25(6), 3255–3266. DOI 10.1007/s10570-018-1789-0. [Google Scholar] [CrossRef]
14. Xu, Y. M., Li, Q., Ma, L. P. (2021). Bamboo-derived carboxymethyl cellulose for liquid film as renewable and biodegradable agriculture mulching. International Journal of Biological Macromolecules, 192, 611–617. DOI 10.1016/j.ijbiomac.2021.09.152. [Google Scholar] [CrossRef]
15. Gu, X. R., Deng, X. M., Liu, Y. N., Zeng, Q. P., Wu, X. L. et al. (2016). Review on comprehensive utilization of bamboo residues. Transactions of the Chinese Society of Agricultural Engineering, 32(1), 236–242. DOI 10.11975/j.issn.1002-6819.2016.01.033. [Google Scholar] [CrossRef]
16. Zheng, L., Wu, Y. Q., Zuo, Y. F., Jiang, X. S., Zhou, H. P. (2021). Research status and prospects of residual use of bamboo residues. World Forestry Research, 34(3), 82–88. DOI 10.13348/j.cnki.sjlyyj.2021.0002.y. [Google Scholar] [CrossRef]
17. Chen, W. Q., Lou, D. P. (2014). Synthesis of sodium carboxymethyl cellulose based on pretreated bamboo shaving. Advanced Materials Research, 997, 169–172. DOI 10.4028/www.scientific.net/AMR.997.169. [Google Scholar] [CrossRef]
18. Obele, C. M., Ibenta, M. E., Chukwuneke, J. L., Nwanonenyi, S. C. (2021). Carboxymethyl cellulose and cellulose nanocrystals from cassava stem as thickeners in reactive printing of cotton. Cellulose, 28(4), 2615–2633. DOI 10.1007/s10570-021-03694-0. [Google Scholar] [CrossRef]
19. National Standard of the People’s Republic of China (2008). GB/T 742-2008. Fibrous raw material, pulp, paper, and board–Determination of ash. China. [Google Scholar]
20. National Standard of the People’s Republic of China (1994). GB/T 2677.6-1994. Fibrous raw material–Determination of solvent extractives. China. [Google Scholar]
21. National Standard of the People’s Republic of China (1993). GB/T 2677.5-1993. Fibrous raw material–Determination of one percent sodium hydroxide solubility. China. [Google Scholar]
22. National Standard of the People’s Republic of China (1994). GB/T 2677.8-1994. Fibrous raw material–Determination of acid-insoluble lignin. China. [Google Scholar]
23. National Standard of the People’s Republic of China (1995). GB/T 2677.10-1995. Fibrous raw material–Determination of holocellulose. China. [Google Scholar]
24. National Standard of the People’s Republic of China (1989). GB/T 744-1989. Pulp–Determination of α-cellulose. China. [Google Scholar]
25. Wang, X., Zhai, Y., Zhan, W. (2015). Determination of the degree of substitution of carboxymethyl cellulose sodium. Journal of Food Safety and Quality, 6(8), 3145–3148. DOI 10.19812/j.cnki.jfsq11-5956/ts.2015.08.054. [Google Scholar] [CrossRef]
26. National Standard of the People’s Republic of China (2005). GB 1904-2005. Food additive-sodium carboxymethyl cellulose. China. [Google Scholar]
27. Segal, L., Creely, J. J., Martin, A. E., Conrad, C. M. (1959). An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal, 29(10), 786–794. DOI 10.1177/004051755902901003. [Google Scholar] [CrossRef]
28. Barba, C., Montané, D., Rinaudo, M., Farriol, X. (2002). Synthesis and characterization of carboxymethylcelluloses (CMC) from non-wood fibers I. Accessibility of cellulose fibers and CMC synthesis. Cellulose, 9(3–4), 319–326. DOI 10.1023/A:1021184509189. [Google Scholar] [CrossRef]
29. Ruiz-Aquino, F., Ruiz-Ángel, S., Feria-Reyes, R., Santiago-García, W., Suárez-Mota, M. E. et al. (2019). Wood chemical composition of five tree species from Oaxaca, Mexico. BioResources, 14(4), 9829–9839. DOI 10.15376/biores.14.4.9826-9839. [Google Scholar] [CrossRef]
30. Casaburi, A., Rojo, U. M., Cerrutti, P., Vázquez, A., Foresti, M. L. (2018). Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocolloids, 75, 147–156. DOI 10.1016/j.foodhyd.2017.09.002. [Google Scholar] [CrossRef]
31. Ferro, M., Castiglione, F., Panzeri, W., Dispenza, R., Santini, L. et al. (2017). Non-destructive and direct determination of the degree of substitution of carboxymethyl cellulose by HR-MAS 13C NMR spectroscopy. Carbohydrate Polymers, 169, 16–22. DOI 10.1016/j.carbpol.2017.03.097. [Google Scholar] [CrossRef]
32. Bono, A., Ying, P. H., Yan, F. Y., Muei, C. L., Sarbatly, R. et al. (2009). Synthesis and characterization of carboxymethyl cellulose from palm kernel cake. Advances in Natural and Applied Sciences, 3(1), 5–11. [Google Scholar]
33. Ni, H. Y., Li, Y. G., Fu, S. W. (2018). Morphological structure and properties of bamboo shell fiber. Journal of Natural Fibers, 15(4), 586–595. DOI 10.1080/15440478.2017.1349710. [Google Scholar] [CrossRef]
34. Li, M. Y., Fang, B. S., Zhao, Y., Tong, T., Hou, X. H. et al. (2014). Investigation into the deterioration process of archaeological bamboo strips of China from four different periods by chemical and anatomical analysis. Polymer Degradation and Stability, 109(1), 71–78. DOI 10.1016/j.polymdegradstab.2014.06.022. [Google Scholar] [CrossRef]
35. Tomak, E. D., Topaloglu, E., Gumuskaya, E., Yildiz, U. C., Ay, N. (2013). An FT-IR study of the changes in chemical composition of bamboo degraded by brown-rot fungi. International Biodeterioration & Biodegradation, 85, 131–138. DOI 10.1016/j.ibiod.2013.05.029. [Google Scholar] [CrossRef]
36. Adinugraha, M. P., Marseno, D. W., Haryadi (2006). Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydrate Polymers, 62(2), 164–169. DOI 10.1016/j.carbpol.2005.07.019. [Google Scholar] [CrossRef]
37. Yue, L. N., Zheng, Y. D., Xie, Y. J., Liu, S. M., Guo, S. L. et al. (2016). Preparation of a carboxymethylated bacterial cellulose/polyaniline composite gel membrane and its characterization. RSC Advances, 6(73), 68599–68605. DOI 10.1039/c6ra07646g. [Google Scholar] [CrossRef]
38. Chen, Q. Y., Wei, L., Miao, Q. X., Luo, X. L., Ma, X. J. et al. (2016). Physical and chemical properties of different parts of green bamboo. Transactions of China Pulp and Paper, 31(3), 1–6. DOI 10.11981/j.issn.1000-6842.2016.03.1. [Google Scholar] [CrossRef]
39. Chen, J., Li, H., Fang, C. Q., Cheng, Y. L., Tan, T. T. et al. (2020). Synthesis and structure of carboxymethylcellulose with a high degree of substitution derived from waste disposable paper cups. Carbohydrate Polymers, 237, 116040. DOI 10.1016/j.carbpol.2020.116040. [Google Scholar] [CrossRef]
40. Lin, X. Q., Qu, T. Z., Qu, S. Q. (1990). Kinetics of the carboxymethylation of cellulose in the isopropyl alcohol system. Acta Polymerica, 41(4), 220–222. DOI 10.1002/actp.1990.010410406. [Google Scholar] [CrossRef]
41. Feng, Z. X., Zhang, T., Yang, J. F., Ni, L. M., Gao, Q. et al. (2021). Effect of natural stacking pretreatment on pyrolysis characteristics of moso bamboo and Chinese fir blends. Journal of Thermal Analysis & Calorimetry, 145(1), 119–127. DOI 10.1007/s10973-020-09686-9. [Google Scholar] [CrossRef]
42. Priya, G., Narendrakumar, U., Manjubala, I. (2019). Thermal behavior of carboxymethyl cellulose in the presence of polycarboxylic acid crosslinkers. Journal of Thermal Analysis and Calorimetry, 138(1), 89–95. DOI 10.1007/s10973-019-08171-2. [Google Scholar] [CrossRef]
43. Mhenni, M. F., Moussa, A., Belgacem, M. N., Moussa, I., Khiari, R. (2019). Preparation and characterization of carboxymethyl cellulose with a high degree of substitution from agricultural wastes. Fibers and Polymers, 20(5), 933–943. DOI 10.1007/s12221-019-8665-x. [Google Scholar] [CrossRef]
44. Kurdtabar, M., Baghestani, G., Bardajee, G. R. (2018). Development of a novel thermo-responsive hydrogel-coated gold nanorods as a drug delivery system. Gold Bulletin, 52(1), 9–17. DOI 10.1007/s13404-018-0248-x. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |