

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.022393
REVIEW
Phytoremediation of Rare Tailings-Contaminated Soil
State Key Laboratory for Nuclear Resources and Environment, East China University of Technology, Nanchang, 330013, China
*Corresponding Author: Zhirong Liu. Email: zhrliu@ecit.cn
Received: 08 March 2022; Accepted: 18 April 2022
Abstract: In order to achieve the goal of circular economy and sustainable development of ecological environment, it is important to separate and recover associated elements from rare mineral resources. Compared with traditional physical and chemical remediation methods of contaminated soil, phytoremediation is regarded as the most promising green in-situ restoration technology. The purpose of this review is to effectively alleviate the environmental problems caused by rare tailings contaminated soil through phytoremediation and realize the recovery of uranium-thorium, rare earth elements (REEs) and tantalum-niobium. This review took rare tailings with uranium-thorium, REEs, tantalum-niobium in China as the research object, then the background, significance, mechanisms and application strategies of phytoremediation were elaborated. In addition, the cases of species with tolerance to uranium-thorium, tantalum-niobium as well as REEs and their remediation mechanisms were summarized, respectively. Particularly, the typical plants represented by Brassica juncea, Sunflower, Phytolacca americana, Dicranopteris dichotoma, Salix spp., etc., were very effective in the remediation of rare tailings. The influence factors of phytoremediation efficiency of tailings contaminated soil were discussed. Two main factors were the mobility of heavy metals in soil (external cause) and the enrichment ability of species (internal cause). Since the traditional phytoremediation also had some limitations, in view of this, the work discussed some auxiliary methods (such as chelating agents or microbial assisted restoration) to improve the efficiency of phytoremediation. Finally, the future development of phytoremediation and potential application directions were explored.
Keywords: Rare tailings; uranium-thorium; rare earth elements; tantalum-niobium; phytoremediation
Many high-tech industries require rare heavy metals, such as uranium (U), thorium (Th), rare earth elements (REEs) [1], tantalum (Ta) and niobium (Nb) have been identified as mineral resources of high strategic importance, and their production and demand are increasing [2]. Due to the chemical similarity with REEs, uranium and thorium often occur in rare earth minerals by lattice substitution. Therefore, uranium and thorium elements are present in the residues produced during the processing of rare earth tailings [3,4]. Tantalum and niobium are transition metals with similar physical and chemical properties. Since the contraction of lanthanides, tantalum and niobium are related to each other, which almost always occur in pairs. Furthermore, they form tantalates and niobates that contain large amounts of REEs, uranium and thorium [5]. Tantalum and niobium are also often considered critical materials for modern society.
Among the mineral resources in China, uranium-thorium, tantalum-niobium and rare earth ores are often found in natural environments in an associative and symbiotic relationship [6]. A large contribution to ore-associated REEs is made by uranium-thorium minerals, additionally, tantalum and niobium are also important elements that are closely associated with uranium-thorium minerals. Many minerals such as tantalum-niobium ore, biotite and monazite often contain certain amounts of radionuclides (especially uranium and thorium), along with associated REEs [7]. China is rich in reserves of uranium-thorium minerals, tantalum-niobium ores and rare earth resources. However, these rare heavy metal tailings have complex composition, low content of useful components, and are difficult to recover and extract of heavy metals [8–10]. Additionally, many mines do not handle the waste properly after processing, leaving a great number of abandoned tailings, which not only makes the utilization rate of tailings low, but also endangers the ecological environment [6]. Therefore, it is important to recover and separate rare heavy metals from rare tailings resources.
In recent years, with the rapid development of economy, people have neglected the harm caused by the heavy metals remaining in tailings to the biological environment of mining area while exploiting the mineral resources. The risk of heavy metal contamination in mine waste cannot be ignored, especially when the tailings are affected by human activities [11]. After the smelting and processing of tantalum-niobium ore or monazite, the uranium-thorium and REEs in ore are largely retained in the tailings and slag. These toxic heavy metals are spread through water, soil, atmosphere and other media, remain in the wastewater or soil, through the biological chain and biological cycle eventually enter our bodies, threatening human health. Since uranium and thorium are radioactive, the extraction of radioactive components from mine solid waste is essential for the sustainable development of the environment and our human health. If uranium-thorium, tantalum-niobium and REEs in rare tailings can be recovered and reused in a suitable way, it can rationally dispose of waste resources, also can provide a new way for the source of nuclear fuel in China. In order to improve the ecological environment of mining areas and maximize the utilization of tailings resources, researchers have carried out a lot of research on tailings remediation technology [12]. At present, the commonly used methods include physical remediation, chemical remediation and biological remediation [13]. Among them, the phytoremediation has been widely used as a sustainable restoration method to improve soil quality and mitigate metal toxicity [14–16].
Phytoremediation is an economical and solar-powered remediation technology, which is particularly effective for shallow heavy metal contaminated soil by introducing tolerant plants to repair mine tailings [12]. The purpose of this review is to use phytoremediation to effectively alleviate the environmental problems caused by rare tailings contaminated soil and realize the extraction or recovery of rare critical metals (uranium-thorium, REEs and tantalum-niobium). This is of great relevance to the protection of the environment and human health around rare tailings areas. To this end, this review took rare tailings with uranium-thorium, REEs, tantalum-niobium in China as the research object, followed existing theories and cases, and discussed the research progress of phytoremediation of rare tailings. This work reviewed the background, significance, methods, mechanisms and specific applications of phytoremediation in recent years. In addition, we particularly summarized the cases of species with tolerance to uranium-thorium, tantalum-niobium as well as REEs and their remediation techniques, then explained the factors influencing phytoremediation of rare tailings. Since traditional phytoremediation also has some limitations, in view of this, the review discussed some auxiliary methods to form an effective combination with phytoremediation to improve the efficiency of restoration. Finally, the future development of phytoremediation and potential application directions were explored.
2 The Necessity of Phytoremediation
The number of articles on phytoremediation in the last decade (2012–2022) are organized, and the data are shown in Fig. 1 (data from Google Scholar, accessed 25 February 2022). We can find that phytoremediation techniques have been studied for a long time, and the research on phytoremediation of heavy metals accounts for a relatively large proportion. The overall increasing trend in the number of studies on all topics in the table year by year shows that phytoremediation method gains more and more attention and becomes a hot topic for academic research. Therefore, phytoremediation of rare tailings is necessary.
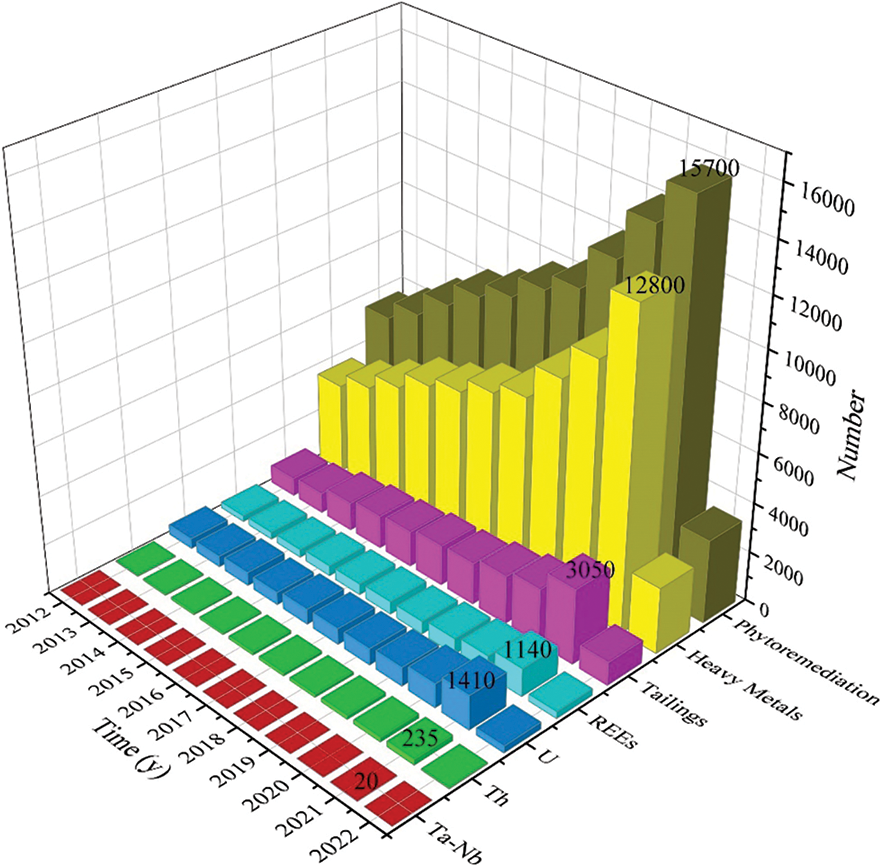
Figure 1: Number of phytoremediation articles within 2012–2022 [17]
Mine tailings are currently considered as a wide range of environmental pollutants, so it is necessary to adopt appropriate remediation methods to treat them [12]. Traditional methods for removing heavy metal ions from soil include adsorption, soil replacement, electrokinetic remediation and so on (Fig. 2). However, these physical and chemical remediation methods have serious limitations in practice because of low efficiency, expensive, disruption of soil structure, and easy secondary pollution [18–20]. In contrast, phytoremediation, as an economical efficient, and environmental in situ remediation technology for heavy metal contaminated soil, is considered to be the most promising green restoration method at present [18–21]. The key is to find specific plants that are adapted to the environment of the contaminated area, and have a high tolerance or accumulation capacity for heavy metal elements [22].
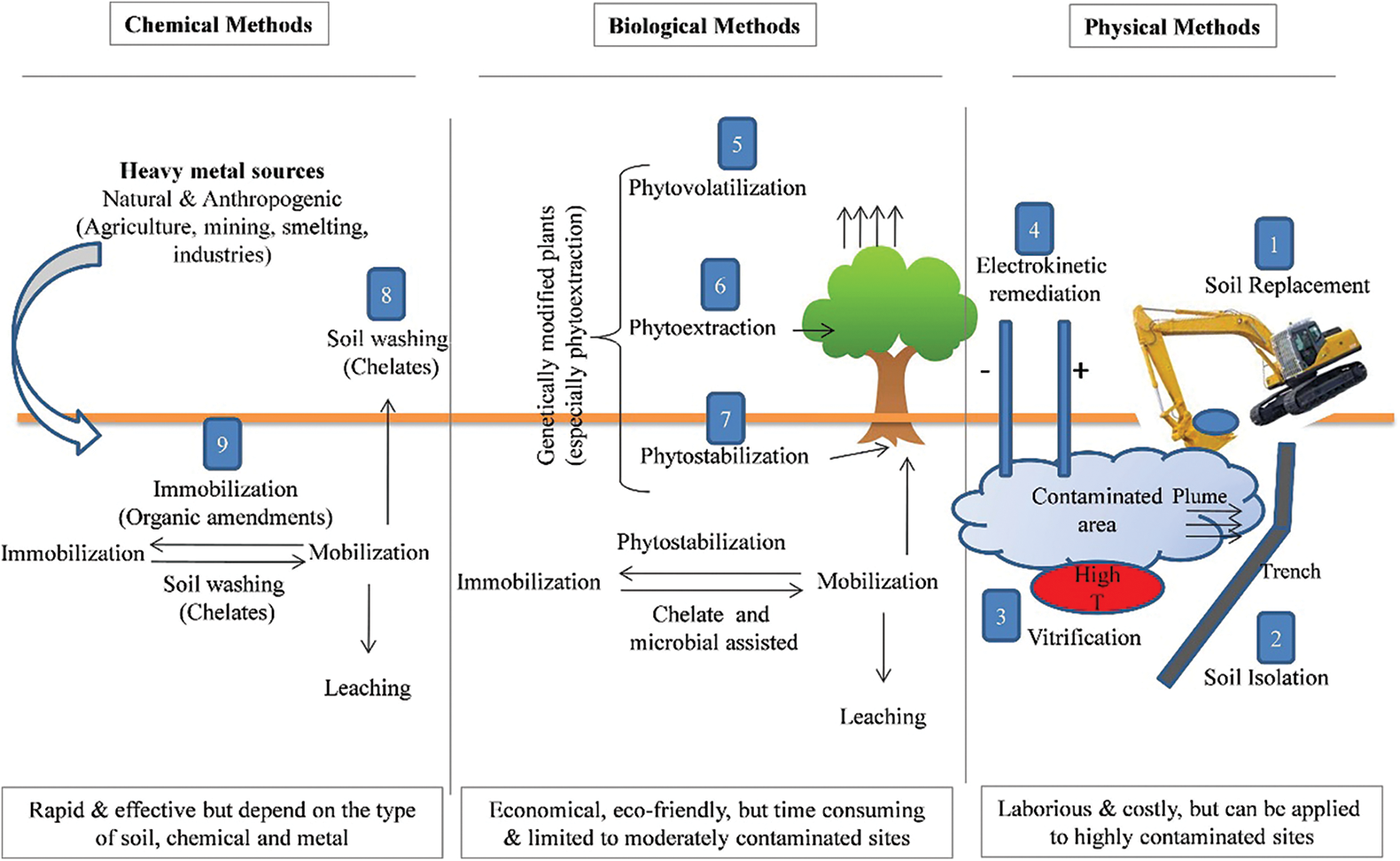
Figure 2: Classification and comparison of remediation methods for heavy metal contaminated soils [23]
3 The Methods of Phytoremediation
Currently, phytoextraction is one of the most promising and discussed remediation methods among all phytoremediation methods, and there is an important concept in phytoextraction: hyperaccumulator [12]. In nature, some plants are super-enriched for one or several heavy metal elements and can transfer them from the soil to the above-ground section through roots, thus reducing the content of heavy metal pollutants in the soil, these plants are called hyperaccumulator plants [24]. Hyperaccumulator plants can uptake metals into their above-ground biomass and absorb more than 1% of their dry weight, these plants can also be called hyperaccumulators, indicators and accumulators [24,25]. The selection of suitable hyperaccumulator plants for remediation of heavy metals contaminated soil is the key to the application of phytoremediation technology.
Most of the hyperaccumulators are very special and can absorb metals from their roots, accumulating 100–1000 times more pollutants than normal plants [26]. Species are considered hyperaccumulators if the dry weight of most metals absorbed is greater than 1000 mg/kg [27]. Hyperaccumulator plants emphasize the fact that plants should be highly tolerant of the heavy metal elements to survive and reproduce successfully [28]. Hyperaccumulator plants carry out normal metabolic activities and physiological functions without showing any obvious symptoms of stress when the growing environment is exposed to excessive metal concentrations [29]. Root systems have a greater relationship with plant mass, and species with higher transpiration are also characteristics of hyperaccumulator plants [30]. In conclusion, hyperaccumulator plants exhibit the following main characteristics: (i) high accumulation; (ii) large translocation (translocation factor > 1); (iii) very high tolerance (no toxicity symptoms during growth); (iv) rapid growth cycle; and (v) large above-ground biomass [31,32].
Typically, hyperaccumulator plants take up large amounts of one or more heavy metals and other pollutants from the soil. Variant Sedum alfredii Hance is a typical example that can act as a hyperaccumulator for the simultaneous removal of uranium and thorium from root system [33]. As of March 2020, the Global Hyperaccumulator Database has reported 759 species (82 families) of heavy metal hyperaccumulators around the world [34]. Most of these plants have been identified as hyperaccumulators in nickel, zinc, cadmium, copper and so on [34,35]. Compared with these common elements, research on hyperaccumulator plants of important rare heavy metal elements such as uranium-thorium, tantalum-niobium, and REEs has lagged behind. We hold the opinion that the core principle of phytoremediation of rare tailings is to explore the strong tolerance and super-enrichment ability of certain special species to heavy metals such as uranium-thorium, REEs, tantalum-niobium, etc. Furthermore, to remediate the contaminated soil by removing toxic heavy metal elements from the external environment through hyperaccumulator plants. Therefore, phytoremediation of rare tailings using hyperaccumulators is one of the main purposes of this review. It should be noted that there are differences in the repair efficiency of hyperaccumulators. Two main factors are the mobility of heavy metals in soil [36] (external cause) and the enrichment ability of species [37] (internal cause). Furthermore, Stojanović et al. [27] believed that the level of uptake of heavy metals by hyperaccumulators is not only related to the type of substrate (soil or tailings), plant species and organs, but also to the cultivar. So the factors influencing the phytoremediation of heavy metal elements are also within the scope of our study.
3.2 Addition of Chelating Agents
The addition of biodegradable physicochemical factors such as chelating agents and micronutrients can increase the uptake of heavy metals by plants [38]. The use of chelating agents as nutrients can alter the coordination structure and presence mode of heavy metal elements, because chelating agents have oxygen-containing groups that can complex with metal ions, thus enhancing the migration of heavy metals. Nutrients are very effective in the mobilization of heavy metals by plants. By applying chelates to the soil, the uptake of many metal compounds by plants is increased, but at the same time the effects on other phytoremediation functions must be taken into account [30]. The chelate induction can enhance heavy metal elements uptake and transport. Khalid et al. believe the utilization of chelating agents to treat contaminated soil can increase the efficiency of phytoremediation by 200 times, moreover, it can reduce the time required for remediation [23]. The addition of chemical amendments to the soil can reduce the biological effectiveness and biotoxicity of inherent heavy metals, then provide a better environment for plants [39].
For example, additives such as SDS (Sodium Dodecyl Sulfate), EDTA (Ethylene Diamine Tetraacetic Acid), and EGTA (Ethylene Glycol Tetraacetic Acid) can improve phytoremediation [40]. Chen et al. [41] studied biodegradable chelators to promote the remediation of cadmium and uranium in sunflower. Phytoremediation effect of CA (Citric Acid), OA (Oxalic Acid), and EDDS (Ethylene Diamine Disuccinic Acid) on uranium contaminated soil was also investigated, the result showed that the chelating agents maximized the uptake of uranium and facilitated the phytoremediation of uranium-contaminated soil by hyperaccumulator plants [42]. Similarly, phosphate strongly inhibits the accumulation of uranium and thorium in roots, while CA enhances their transport from roots to stems [43,44]. Therefore, we can know that chelating agents such as OA and inorganic phosphates can be used as auxiliary tools for phytoremediation to help modify the plants to improve the remediation effect.
3.3 Microbial Assisted and Electrokinetic-Enhanced Phytoremediation
Many microorganisms have the special potential to promote phytoremediation. The use of microorganisms such as specific degrading bacteria, endophytic bacteria and plant growth promoting bacteria in association with phytoremediation can enhance the degradation of heavy metals in soil by plants [45]. Bacterial genera Mycobacterium, Alcaligenes, Pseudomonas, and Bacillus have been used widely in phytoremediation [46–48]. The metabolites (siderophores, organic acids, etc.) produced by microorganisms have been proposed to be involved in plant rhizosphere geochemical processes [49]. Endophytic bacteria with plant growth-promoting activity can facilitate plant adaptation to heavy metal polluted soil environment [50]. Rhizosphere microorganisms can promote plant growth and improve the tolerance of species to heavy metals by altering the mobility of heavy metal ions in contaminated soil [51]. During phytoremediation, plant growth–promoting bacteria tends to be more competitive in the process of plant-microbe interactions [52]. Plant growth-promoting bacteria enriches in rhizosphere soil, providing a new idea for phytoremediation of rare tailings. In addition to the above, the application of transgenic plants with restorative properties can also increase the content of antioxidant enzymes and metal detoxifiers, which increasing the ability of the species to enrich heavy metals [23].
Electrokinetic-enhanced phytoremediation is also widely used in the restoration of soil contaminated by heavy metals such as uranium. Electric fields can enhance the removal of heavy metals from soil by increasing the bioavailability through desorption and transport of contaminants [53]. Phytoremediation also helps to restore soil properties and improve soil structure damaged by electric remediation [54,55]. Experiments showed that the use of direct current electric fields in the vicinity of ryegrass had a positive effect on germination rate and plant growth [56]. The accumulation of heavy metals in the ryegrass rhizosphere was greatly increased by the optimization of electrochemical and biological processes in soil under the polarity reversal direct current field [57]. Li et al. [58] found that the potential feasibility of electrokinetic-enhanced phytoremediation of uranium polluted soils by sunflower and Indian mustard. The coupled phytoremediation–electrokinetic technology improves biomass, enhances uptake of heavy metals by plants, and facilitates the transfer of toxic elements from the roots to aboveground parts [53]. Furthermore, soil electrical conductivity may also be an important factor in the efficiency of phytoremediation enhanced by electric field [57].
Phytoremediation technology is a current hot topic of academic research which has been discussed by numerous experts and scholars. Some specific methods on phytoremediation have been elaborated before. In this part, we summarize the latest mechanism of phytoremediation based on previous literature research results to understand more deeply the way of phytoremediation of rare tailings. Phytoremediation is a universally accepted and sustainable bioremediation method that uses green plants to remove heavy metal contaminants from soil [59]. Phytoremediation includes several technologies and applications that vary greatly in the processes and mechanisms by which heavy metals are fixed, removed, or degraded by plants. Depending on the different uptake mechanisms, phytoremediation is mainly classified as phytoextraction, phytostabilization, phytovolatilization, and rhizofiltration [23].
Firstly, the most important mechanism is phytoextraction. Phytoextraction is the uptake of pollutants from soil or water by plant roots and their transport to accumulate in above-ground biomass (branches or leaves) [36]. Generally, plants used for phytoextraction should have the ability to produce high biomass or accumulate contaminants [60–62]. The phytoextraction strategy is based on three types: (i) hyperaccumulator plants selected from natural populations; (ii) plants characterized by high growth rates and high biomass yields; (iii) plants obtained through genetic engineering strategies with high heavy metal tolerance and improved phytoextraction efficiency [26,62]. Mahar et al. [63] considered that phytoextraction allows for large-scale cleanup of soils with uneven contamination patterns. Because natural processes are used to treat metal-contaminated sites in situ without any excavation or physical removal, phytoextraction has long-term effects, however, storage, handling, and placement of contaminated plant biomass may affect the outcome of phytoremediation [23]. For example, Salix spp. shows great potential for phytoextraction because it can accumulate heavy metals, and has the ability to recover from heavy metal toxicity [64].
Phytostabilization is different from phytoextraction, phytoextraction is the extraction of heavy metals from contaminated soil and their transfer to the harvestable above-ground parts of plants, while phytostabilization immobilizes heavy metals in the plant root zone [65]. Phytostabilization does not reduce contaminant concentrations, but contaminants remain in place, so the contaminated area needs to be monitored regularly to ensure optimal stabilization conditions are maintained [23]. Phytostabilization involves the use of plants to absorb or immobilize high levels of contamination in the soil [30]. The aim of this technique is to reduce the mobility and biological effectiveness of metal contaminants in the soil-plant system, thereby limiting heavy metal ion entry into the food chain [30,66]. The task of phytostabilization is not to remediate contaminated soil, but to reduce contamination of media and passivate sequestration of metal contaminants, so various organic and inorganic soil amendments can promote absorption of heavy metals [63].
The next step is phytovolatilization. Phytovolatilization is the process of absorbing pollutants from the soil, transporting them through the xylem, converting them into less toxic volatile substances, and releasing them into the atmosphere [38]. However, because phytovolatilization just transfers pollutants from the soil to the atmosphere and redeposits them there without fully immobilizing, phytovolatilization has some limitations [23].
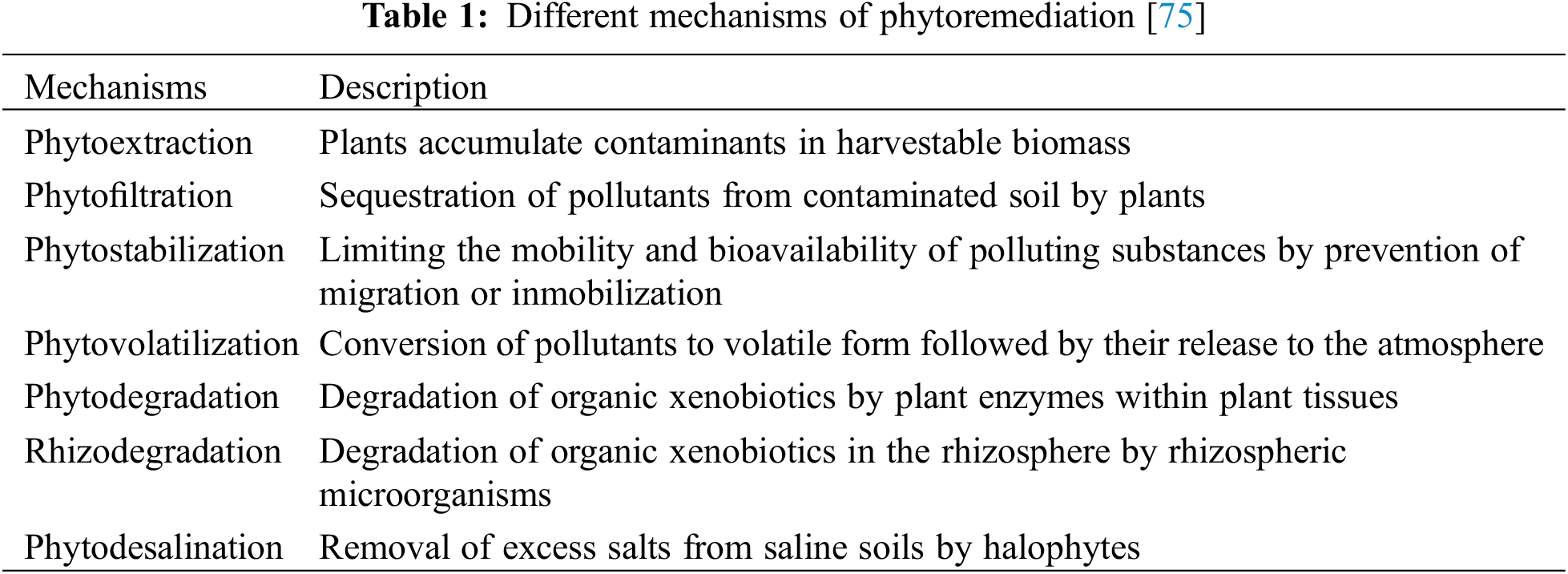
Finally, there is the rhizofiltration process. In rhizofiltration, we can use plants to extract impurities from contaminated soil and water environments, then plants transfer them through the root system [67,68]. Some plants are highly tolerant to heavy metals in the inter-root soil and can significantly reduce the concentration of heavy metals [59,69]. The uptake or the precipitation of heavy metals by the root system limit the transport of contaminants in the soil, in which the species used to achieve remediation change the soil chemical properties, thus facilitating the uptake and precipitation processes of heavy metals in the soil [70,71]. Plants that accumulate large amounts of heavy metals in roots with minimal impact on growth are desirable characteristics for phytoremediation, as this indicates that they are tolerant [72]. It should be noted that plants used for rhizosphere filtration should be harvested and treated only when root sorption is at its maximum [73,74]. Various phytoremediation mechanisms are described in Table 1.
4 Phytoremediation of Different Heavy Metals
4.1.1 Significance of Phytoremediation of Uranium and Thorium
Uranium and thorium often coexist, which inevitably leads to the joint pollution of uranium and thorium in the environment [76]. Due to the high toxicity of radionuclides to human health and ecosystems, methods for efficient removal and recovery of uranium and thorium from tailings are of great interest [77]. Phytoremediation is increasingly regarded as an important tool for in-situ remediation of uranium-thorium contaminated soils because of its high efficiency and environmental friendliness in terms of its ability to absorb and accumulate heavy metals [78,79]. This method is currently one of the most promising technologies for large-scale treatment of radionuclide contaminated soil shown in Fig. 3 [80].
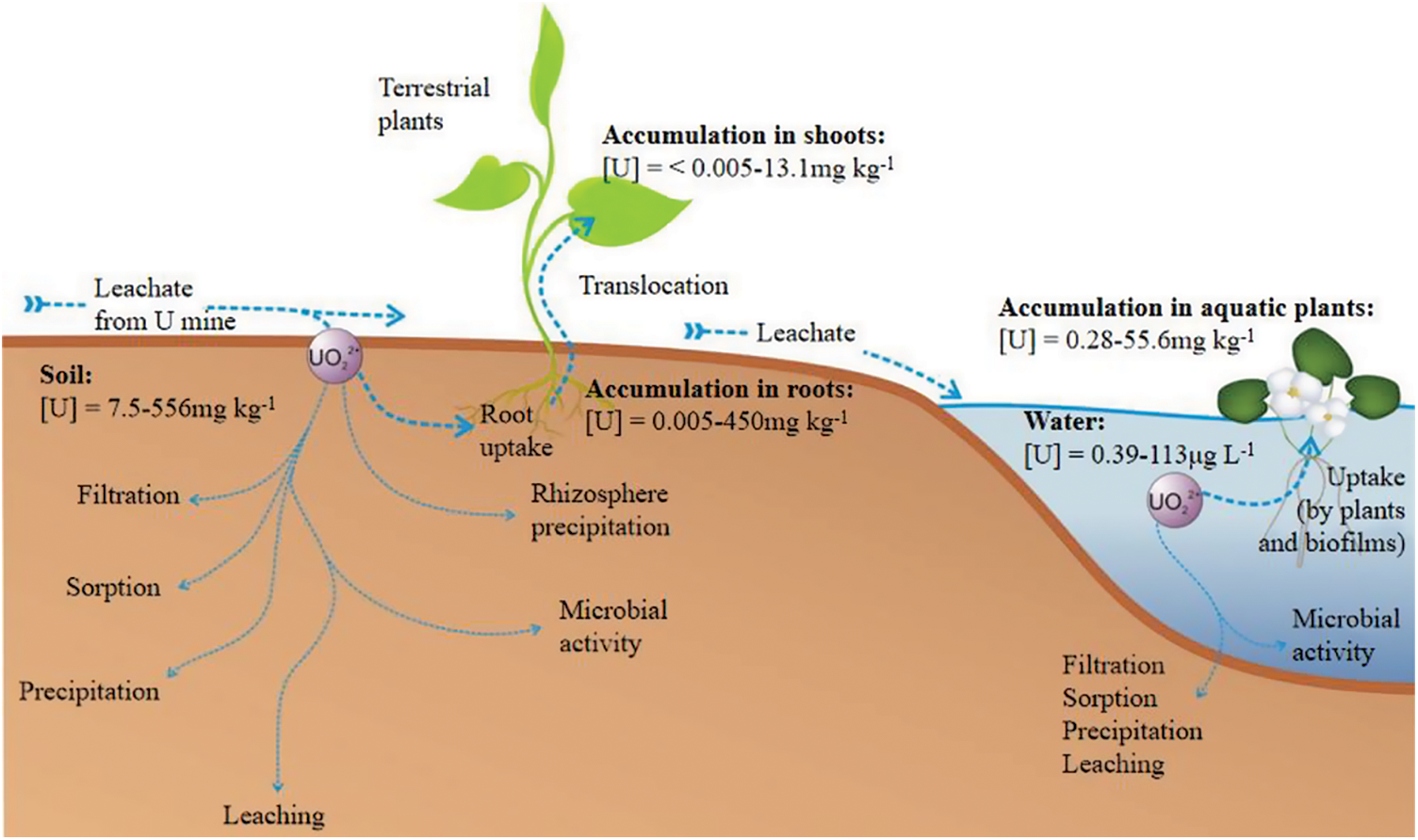
Figure 3: Land plants accumulate uranium [83]
Under natural conditions, uranium and thorium are released into the soil through weathering, erosion, and deposition in the surface environment, and secondary waste from the phytoremediation process may produce leachate containing heavy metals, which are harmful to the environment [81]. Specific plants cultivated for remediation of radionuclide contaminated sites need to meet two key factors: (i) the ability to absorb radioactive material to relatively high levels (ii) without affecting growth or high biomass production [81]. Since uranium and thorium are not essential elements for plants, their uptake process may be a passive uptake mechanism, and it has been hypothesized that radionuclide absorption is the main enrichment mechanism [82].
4.1.2 Plants Capable of Accumulating Uranium and Thorium
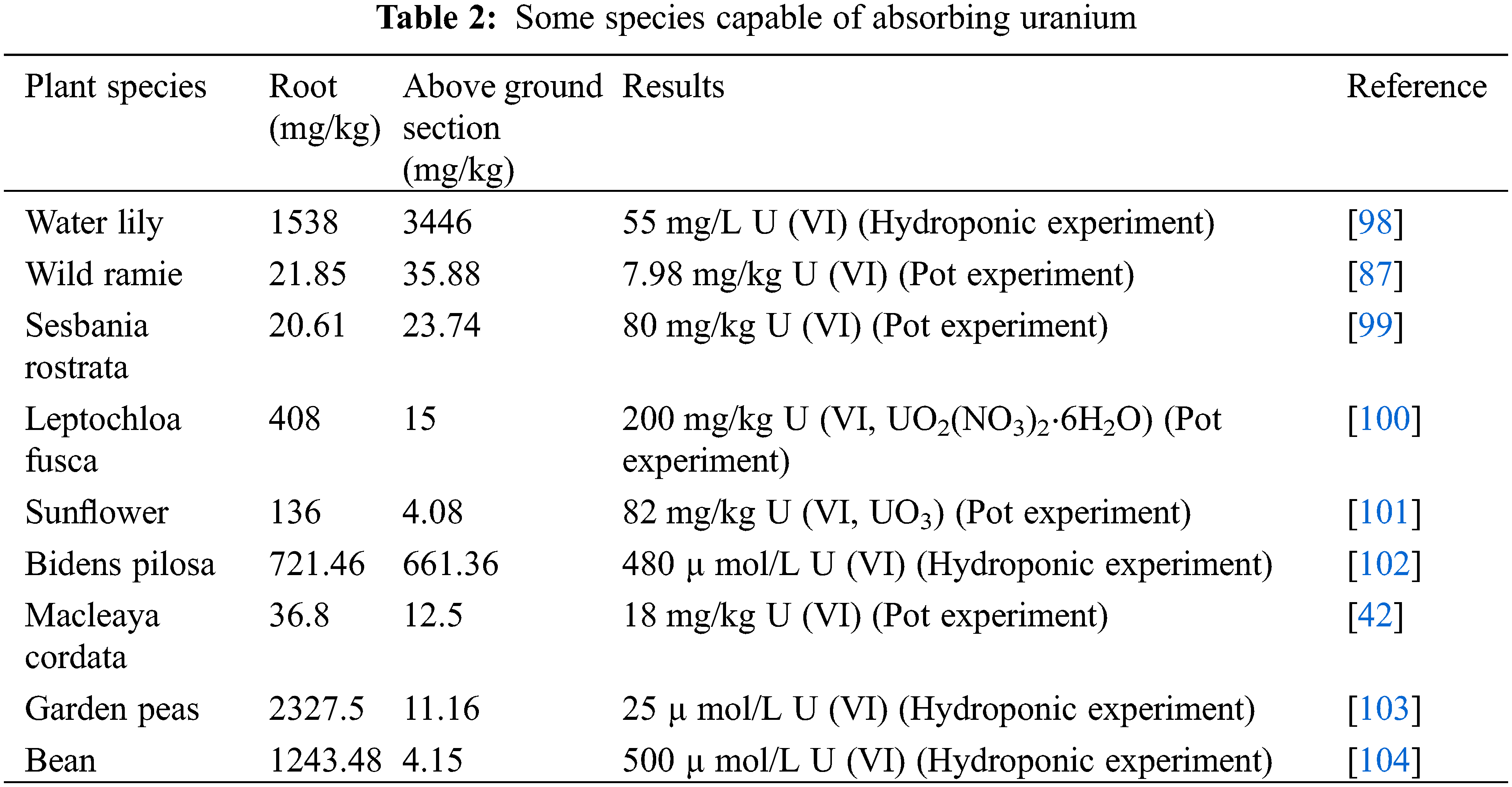
The ability of hyperaccumulators to accumulate and translocate heavy metals varies depending on plant species and heavy metals, and even within the same plant, the absorption effect ratio of uranium and thorium varies greatly under different conditions [84]. Therefore, the selection of suitable plant species is of great importance for soils contaminated with heavy metals [85]. There are three indicator factors to evaluate the phytoremediation effect, which are transfer factor [86] (TF = target element concentration in the plant above ground/target element concentration in the plant roots); bioconcentration factor [86] (BF = target element concentration in the plant/target element concentration in the soil) and phytoremediation factor [86] (PF = target element concentration in plant above ground × plant above ground biomass/target element concentration in soil). For example, the Boehmeria nivea accumulates large amounts of uranium in the aboveground, and it was found the BF > 4, TF > 1.5, which suggests that Boehmeria nivea may be a potential hyperaccumulator of uranium [87].
Helianthus annuus L. [88], Brassica juncea [89], and Arabidopsis thaliana [90] can also enrich uranium from the soil. In uranium-contaminated soils, Brassica juncea has a significant increase in above-ground uranium concentration and a strong extraction, accumulation and tolerance capacity for uranium, making it a better candidate plant for remediation of soils [89,91]. Qi et al. [89] investigated the effect of a low molecular weight organic acid mixture on uranium, and the results showed that the mixture acid promoted uranium uptake by Brassica juncea roots and root-to-ground translocation in uranium-contaminated soils. By releasing uranium from the soil into solution, the mixed acid increased the exchangeable uranium content of the contaminated soil, and reduced the uranium content of carbonate and Fe-Mn oxides, which favored the accumulation of uranium in mustard [89]. As for Arabidopsis thaliana, different pH levels affect the uptake and translocation of uranium by Arabidopsis thaliana, high absorption and low translocation at low pH, low absorption but higher translocation at high pH [90]. Sunflower (Helianthus annuus L.) contained more than 15,000 mg/kg dry weight of uranium and was considered by the researchers to be a typical hyperaccumulator [27]. The translocation factors show that uranium is mainly enriched in the root of sunflower, and the migration of uranium to the aboveground is limited, thus achieving the mitigation of the damage to the aboveground [92].
The vetiver grass plays an important role in the immobilization of uranium in soil by preventing the contamination of soil with harmful heavy metals through the absorption of uranium [93]. Sha et al. [94] found that Salix spp. could also accumulate large amounts of uranium in the above-ground portion after remediation treatment of contaminated soil, which significantly remediated the uranium-contaminated soil. The accumulation of uranium in plant tissues showed the following trend: root > leave > stem > flower/fruit, which confirms the unique efficiency of roots in accumulating radionuclides from the soil [83]. Vicia fabaL. is considered to be an excellent specie because of its ability to exhibit high root accumulation and low translocation [95,96]. Moreover, the Vicia fabaL. roots are well developed and tolerant to uranium, and can obtain uranium from contaminated sites and accumulate in the cell walls of the roots [95,97]. By analyzing uranium tailings samples, Yan et al. [86] concluded that the concentrations of 238U, 232Th in the samples exceeded the natural radionuclide content of Chinese soils, and used pokeberry as a candidate specie for phytoremediation of radionuclide-contaminated soils based on TF, BF, and PF. However, since the pokeberry is an invasive plant in China, we need to study it in depth in order to carry out phytoremediation in a rational manner.
In addition to the plants mentioned above, some other species capable of absorbing uranium are shown in Table 2 (under pot experiments and hydroponic experiments), and most of the plants in the Table 2 had greater root accumulation than above ground, showing a strong root accumulation capacity of uranium.
Understanding the phytotoxic mechanism of thorium is important to improve the accumulation of thorium in plants. Fu et al. [105] analyzed the toxic response of Vicia faba seedlings to thorium, they found that thorium is mainly distributed in cell walls and present in roots as residues, and that high concentrations of thorium cause abnormal root growth. In Nicotiana glutinosa L., the content of thorium in the roots was higher than that in the aboveground, and the transfer to the aboveground was greatly restricted [106]. Tobacco plants also have a good potential for thorium accumulation, because the transport of heavy metals to the upper part of the plant is very limited, the highest content of thorium is found in the roots of tobacco plants [107]. The uptake and distribution of thorium can be improved by changing growing conditions. The absorption of thorium is greatly increased when the environmental medium is free of phosphate ions, in addition, the presence of high concentrations of organic acids (such as CA, OA or tartaric) increases the accumulation of thorium in plant tissues [107].
Phragmites australies has a strong absorption capacity for thorium and uranium [108]. M. floridulus and rice flat sedge are superior in terms of concentration as well as TF, BF and PF, and have better potential for treating thorium-contaminated soils in southern uranium tailings [109]. By comparing with the world average, it is known that both Fagonia boveana and Zilla spinosa accumulate uranium and thorium, and their enrichment capacity is many times higher than the average of other plants, which can be used for exploration and phytoremediation of radioactive elements [110]. The mixed culture of Avena sativa L. with Lupinus albus L. increases the concentration of REEs and uranium-thorium in the soil, therefore, mixed culture may be a promising method for phytoremediation [111]. The concentration of heavy metal elements has an important effect on the uptake of uranium-thorium in plants. The variant Sedum alfredii Hance can be used as a specie for the simultaneous removal of uranium and thorium from plant roots [33]. Huang et al. [112] conducted experiments on the perennial variant Sedum alfredii Hance and found that low concentrations of thorium stimulated plant growth and thus uranium uptake, but as thorium concentration increased, variant Sedum alfredii Hance inhibited the uptake of uranium. Similarly, Brassica juncea var. foliosa is also a promising thorium plant extract specie due to its high biomass, fast growth rate and excellence tolerance to thorium, in addition to its ability to absorb uranium. The low concentrations of thorium promoted plant growth at the whole plant level, while high concentrations of thorium cause damage to organelles and limit plant growth [113].
4.1.3 Other Phytoremediation Methods for Uranium and Thorium
Ancillary measures such as the application of chelating agents [79], plant growth promoting bacteria [78] and plant growth regulators [114] are also important methods to improve the efficiency of plant extraction of uranium and thorium. Some complexing agents in the environment can form complexes with heavy metals, thus affecting the bioavailability, which is important for the uptake of uranium and thorium by plants [43,44]. Low molecular weight organic acids are bioactive compounds in plant root secretions that significantly enhance the uptake of radionuclides by plants in the soil [99]. Application of chelating agents such as EDDS can enhance the bio-availability of uranium-contaminated soil and promote the uptake of uranium in the soil-plant system [41,79]. Phosphate strongly inhibits the accumulation of uranium and thorium in plant roots, while CA enhances their transport from roots to stems [43,44]. In addition to these, the combination of multiple species close to their natural environment is more effective than the usual single species culture selection [82].
In general, the uptake of radionuclides by plants depends on soil type, soil pH, plant species, root development, target element concentration, organic matter content and so on [109]. The content of uranium and thorium in plant species varies widely, mainly due to differences in the enrichment characteristics of the plants themselves and the concentration of the elements in the tailings [37]. The capacity of plants to absorb uranium and thorium is inseparable from plant species and cultivars, and the accumulation capacity of different types of species varies [27,115]. In addition, soil pH has a strong influence on transport of heavy metal elements and ion exchange reactions, which in turn affects the absorption of uranium-thorium [116]. Uranium and thorium uptake by plants is dependent on pH because it affects the chemical form of elements, the bioavailability and the physiological properties of plants [83]. The potential of a plant for phytoremediation also depends on the ability of radionuclides to translocate and accumulate, which is closely related to its chemical properties [86]. Likewise, carbonates, phosphates, and organic acids play an important role in the accumulation of thorium, the presence of carbonate or phosphate anions inhibit the absorption of thorium, and the acids promote the accumulation of thorium above ground but hinder the uptake of thorium in roots [44]. It should be noted that the many influencing factors mentioned here are only relative to the overall situation, not absolute, and still need to be analyzed according to specific plants, environments, and elements.
4.2.1 Hyperaccumulation Plants of REEs
The widespread mining of ion adsorption-type rare earth deposits in China has led to large tailings ponds [117]. It is a feasible method to extract REEs by plants in situ phytoremediation, therefore, the selection of suitable species is important for tailings restoration and soil improvement.
Among the plants found to be super-enriched in REEs, most of them are ferns. It is one of the few published flowering species with high rare earth concentration. Phytolacca americana L. has a fast growth rate and high biomass, and its accumulation of REEs in roots and leaves is as high as 386 and 1040 mg/kg, respectively, much higher than normal plants, and it grows naturally as an exotic weed in rare earth mining sites in China [118]. Phytolacca americana L. has a certain potential in phytoextraction of REEs, and can accumulate many heavy metals such as aluminum, manganese, iron and zinc at the same time in addition to REEs [119]. The content of REEs in the leaves is positively correlated with the content of Al, Fe and Zn, which indicating that the coexistence of REEs with these elements. However, the content of light REEs was negatively correlated with the content of P, which indicating that the uptake of REEs and phosphorus was competitive. In terms of plant modification, the extraction of REEs from plants modified with organic materials and biochar was explored. Phytolacca americana L. treated with both modified materials significantly improved soil properties, particularly, biochar at low doses was an effective way to enhance the extraction efficiency of REEs by phosphorus [120]. Liu et al. [121] revealed the effect of organic acids on the uptake and transfer mechanism of REEs in root systems, providing a basis for improving the phytoextraction efficiency.
Two rare earth hyperaccumulator species, Dicranopteris dichotoma (or Dicranopteris linearis) and Pronephrium simplex in Table 3, both preferentially accumulate light REEs, are found in the rare earth mines [28]. Dicranopteris dichotoma has been found to have the ability to remove REEs from contaminated soil and is widely grown as a part of the remediation process of soil contaminated with REEs [122]. As an effective remediation phytoextractor of REEs, Dicranopteris dichotoma extracts more light rare earths than heavy rare earths, and it can be used for phytoextraction and phytostabilization [123]. Pronephrium simplex was also confirmed to be a rare earth hyperaccumulator collective in both outdoor and indoor experiments [28].
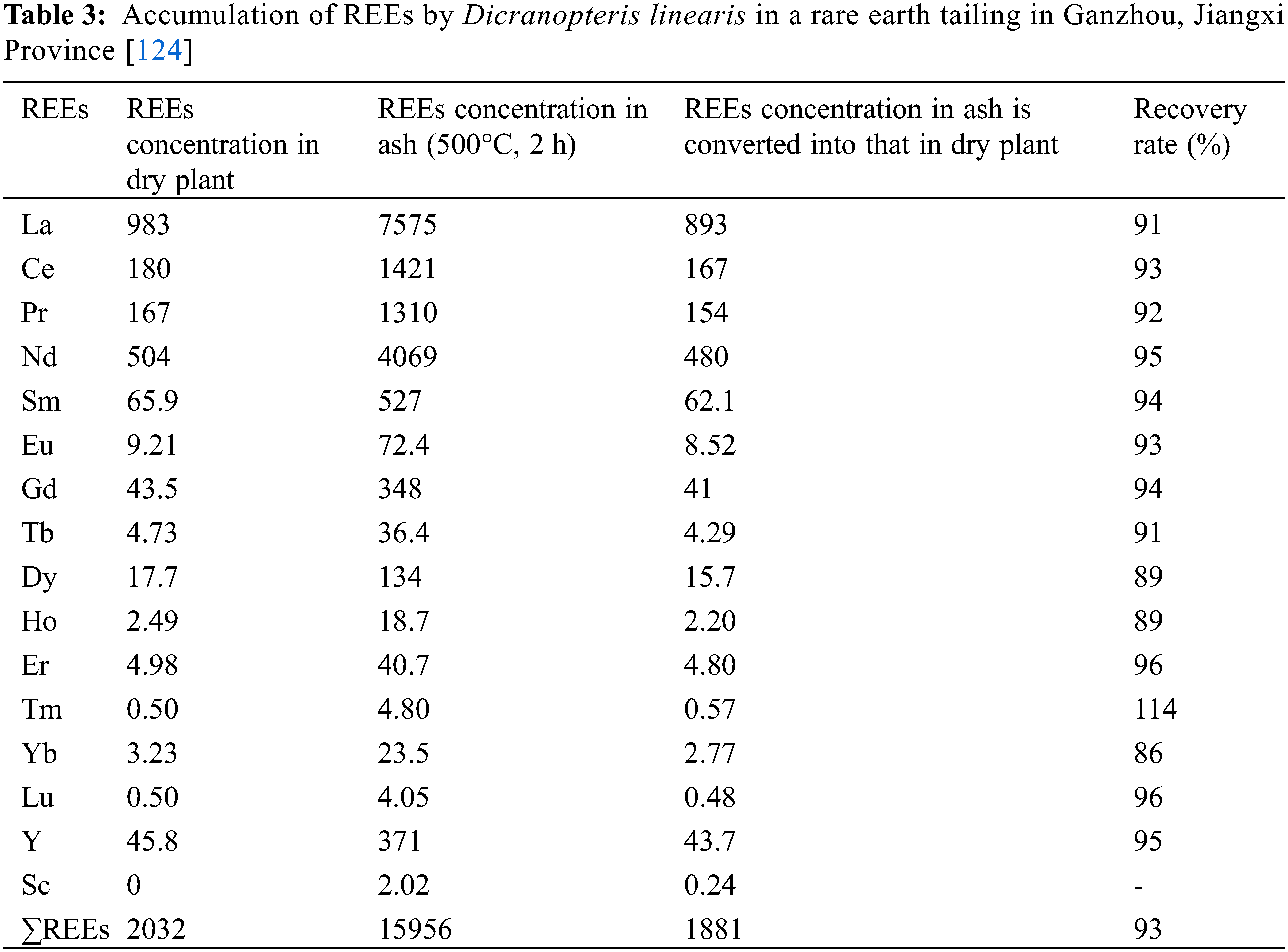
Among the recorded REEs hyper-enriched plants, Dicranopteris pedata is very typical for the extraction of REEs with high efficiency [125]. Furthermore, Salix spp. is also a suitable material for rare earth mine site remediation, and the uptake and accumulation pattern of REEs is mainly shown as root > stem > leaf, expect for La, Salix spp. accumulates small amounts of Y, Nd, Ce, Tb, and Dy [126]. Mikołajczak et al. [127] studied the phytoremediation potential of five herbaceous plants (Papaver rhoeas L., Taraxacum officinale, Achillea millefolium L., Tripleurospermum inodorum and Artemisia vulgaris L.) for REEs and observed a significant accumulation of REEs in plant biomass.
Any species selected for phytoextraction should be resistant to the presence of REEs in the contaminated soil environment. Taraxacum officinaleas and Pharagmites australis have been used for the remediation of REEs, which can be recovered from plants using various extraction methods, and the extracted REEs can be used as fertilizers [128]. Pachystroma longifolium, Solanum lycocarpum, and Citrus spp. have a high accumulation of REEs in the aboveground part [129–131]. The ramie planted in mine sites can be used not only for vegetation restoration and phytostabilization of ion adsorbed rare earth tailings, but also for biomass production with a lower capacity to accumulate REEs [132].
About the modification of phytoremediation, using sludge biochar to amend Alfalfa can promote the growth of Alfalfa, change its root morphology, improve soil properties and microbial diversity, thus accelerating the restoration process [133]. The combination of organic modification and ramie cultivation for phytostabilization is a promising strategy for remediation of contaminated soil, this method improves soil quality and reduces the extractable concentration of toxic elements [117]. Plants have a good symbiotic relationship with arbuscular mycorrhizal fungi in REEs tailings, which can alleviate the toxic effects of REEs and heavy metals on plants, enhance the ability of plants to remediate the ecosystem [134].
In general, the factors influencing the phytoremediation of REEs are similar to those of uranium-thorium, but there are some special situations. Plant species, the morphology and migration processes of REEs in soil, and soil properties are the main factors affecting the distribution patterns of REEs [135]. Both the concentration and bioavailability of REEs are directly related to plant biomass, root growth, and plant extraction of the target elements [136]. The chemical characteristics of the soil environment in which the REEs are present also affect their transport, and cation exchange helps to regulate the transport of REEs in the particular environment [137].
There is no doubt that ion-adsorption REEs mine tailings soils without phytoremediation are more contaminated than those with phytoremediation. The soil pH affects the action and effectiveness of heavy metals in soil for plants. When the pH in tailings soil is low, the toxicity of REEs will be further enhanced due to the increase of free ions in organisms, which will be harmful to the soil environment [138]. The addition of organic fertilizers and the planting of plants can increase the nutrient content of the soil and reduce the effectiveness of REEs, thus improving the soil quality of tailings [52].
Tantalum and niobium are stable transition metals which coexist in nature with very similar physicochemical characteristics [139]. In rare tailings, tantalum and niobium are often found in association with thorium, uranium and REEs [140,141]. The production of niobium and tantalum from ores requires the separation and purification of associated elements. Beneficiation and extraction of tantalum and niobium from tantalum-niobium tailings becomes challenging in the presence of associated elements such as rare earths, thorium and uranium [142]. While the best phytoremediation method needs to be site-specific, some specific plants have shown a superior ability to accumulate tantalum and niobium in their tissues that deserves our attention.
Various plants also respond differently to the uptake of different metals, and the accumulation and translocation capacity of plants varies depending on the plant species and the metals. Due to the low solubility of tantalum-niobium and the poor stability of organic complexes, tantalum-niobium is less mobile in soil [143]. Alekseenko et al. [144] studied the content of several chemical elements in the mine soil and found the highest concentration of Ba, Nb, Sc, Sr and Zn accumulation in regional woody plants, it also showed that the characteristics of different deposits have an important influence on phytoremediation. A large amount of heavy metal elements such as tantalum, niobium and thorium were found in a tailings pond, and the suitability of the hyperaccumulator plant Salix spp. for remediation was evaluated in detail, thus we can cultivate Salix spp. for restoration of contaminated soil [145]. Cunha et al. [146] collected fern samples at the mine, where the plants were enriched in Y, Zr, Nb, Pb, Sn, and Th, and there were inconsistent variations in the biosorption coefficients of low mobility elements such as Nb, and Ta.
In addition to mining areas, phytoremediation in other environments is also useful for tantalum-niobium extraction. Mangroves can absorb tantalum and niobium from the substrate through phytoextraction processes, and the increased metal content in the growing environment facilitates the extraction of these metals by the plants [5]. Furthermore, the total concentration of tantalum-niobium in plants was also found to be related to the degree of enrichment in the sediment, and more tantalum-niobium was accumulated in the root system compared to other tissues [5].
However, there is a large lag in the identification of tantalum and niobium hyperaccumulator plants compared to hyperaccumulators of other elements. Based on the paragenetic relationship between minerals, phytoremediation of rare tailings can be carried out at the level of associated elements such as uranium-thorium, rare earths, etc., which are able to inform the research of this article.
The purpose of this review is to use phytoremediation to effectively alleviate the environmental problems caused by rare tailings contaminated soil and realize the extraction or recovery of uranium-thorium, REEs and tantalum-niobium. This is of great relevance to the protection of the environment and human health around rare tailings areas. In this review, we elaborated the background, significance, mechanisms and application strategies of phytoremediation. In addition, the repair methods such as hyperaccumulator plants, use of chelating agents, microbial assisted and electrokinetic-enhanced phytoremediation were described. After reviewing previous studies, we found that many species had strong phytoremediation ability and could enrich rare heavy metals such as uranium, thorium, REEs, tantalum and niobium from the soil environment through different plant tissues. Particularly, the typical plants represented by Brassica juncea, Sunflower, Phytolacca americana, Dicranopteris dichotoma, Salix spp., etc., were very effective in the remediation of rare tailings. Most plants showed strong root accumulation of rare heavy metals than above ground. Moreover, organic acids, inorganic salts and other chelating agents could be used as auxiliary tools for phytoremediation of rare tailings, which increased the accumulation of rare heavy metals in plant organs. Microorganisms in the root system could also facilitate the transport of metal ions and improve the restoration efficiency. We should be aware that there are differences in the repair efficiency of hyperaccumulators. The influence factors of phytoremediation efficiency of tailings contaminated soil were discussed. Two main factors were the mobility of heavy metals in soil (external cause) and the enrichment ability of species (internal cause). Furthermore, the absorption of rare heavy metals by plants also depended on the influence of soil type, soil pH, plant species, concentration of target elements, organic matter content and so on, which need to be considered comprehensively when repairing rare tailings.
Since phytoremediation has disadvantages such as slow growth rate of hyperaccumulation plants, low biomass and long cycle time for soil remediation, it is difficult to meet the requirements of rapid remediation of contaminated soil. Therefore, there is still much room for development of phytoremediation strategy using special species to purify toxic metals in tailings. The key to phytoremediation technology is the assessment of the unique tolerance of specific plants to the target elements. Future research in phytoremediation should continue to search for hyperaccumulators with high biomass and high ability to enrich tantalum-niobium, uranium-thorium, and REEs, especially hyper-accumulating species for tantalum and niobium elements.
Specifically, the directions for future research are as follows:
a) In practical application, phytoremediation technology is only suitable for shallow soil with medium and low pollution concentration, and the remediation scope is limited by the depth of plant roots. Therefore, it is also a new direction to use physical or chemical methods to pretreat contaminated soil before phytoremediation, and to develop super-enriched plants with strong root system while applying various technologies together.
b) Pay attention to the problem of secondary pollution. After the hyperaccumulators transfer the heavy metal elements in rare tailings from roots to the aboveground, the plants should be recovered and reprocessed in time. This is to prevent the toxic elements enriched by plant decay from re-migrating to the biological chain and causing secondary pollution.
c) Enhancement of exploitation of woody plants. At present, most of the hyperaccumulation plants discovered are herbaceous plants, and there are fewer species of woody plants. Herbaceous plants have short growth cycles and small biomass, and their absorption of heavy metals in rare tailings is less than that of woody plants. Moreover, woody plants are ecologically important for vegetation restoration and landscape beautification of mining areas. Thus, in the future, we should increase the exploration and research on hyperaccumulated woody plants with large biomass and high tolerance ability.
d) Pay attention to the plant domestication. We need to strengthen the research on genetic recombination in phytoremediation and to enhance collaboration in the fields of genetic engineering, geochemistry, microbiology, and agricultural engineering. By integrating different expertise, we can think about how to improve the utilization of phytoremediation by inducing plant modification through human factors.
e) Phytoremediation is a process for specific elements in specific locations. Therefore, different regional environmental conditions (soil types, climate conditions, Ph conditions, biodiversity, etc.) need to be considered before application in situ to select the best remediation method.
Funding Statement: This work was supported by the National Natural Science Fund Program (21866006, 11875105); General Project of Jiangxi Province Key Research and Development Program (20203BBFL63070, 20192BBG70062).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Dushyantha, N., Batapola, N., Ilankoon, I. M. S. K., Rohitha, S., Premasiri, R. et al. (2020). The story of rare earth elements (REEsOccurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geology Reviews, 122, 103521. DOI 10.1016/j.oregeorev.2020.103521. [Google Scholar] [CrossRef]
2. Schulz, K. J., DeYoung, J. H., Seal, R. R., Bradley, D. C. (2017). Critical mineral resources of the United States—An introduction. Critical Mineral Resources of the United States: Economic and Environmental Geology and Prospects for Future Supply, Reston, Virginia: US. Geological Survey. [Google Scholar]
3. Whitty-Léveillé, L., Reynier, N., Larivière, D. (2018). Rapid and selective leaching of actinides and rare earth elements from rare earth-bearing minerals and ores. Hydrometallurgy, 177, 187–196. DOI 10.1016/j.hydromet.2018.03.015. [Google Scholar] [CrossRef]
4. Amaral, J. C., Sá, M. L., Morais, C. A. (2018). Recovery of uranium, thorium and rare earth from industrial residues. Hydrometallurgy, 181, 148–155. DOI 10.1016/j.hydromet.2018.09.009. [Google Scholar] [CrossRef]
5. Ray, R., Dutta, B., Mandal, S., González, A., Pokrovsky, O. et al. (2020). Bioaccumulation of vanadium (Vniobium (Nb) and tantalum (Ta) in diverse mangroves of the Indian sundarbans. Plant and Soil, 448(1), 553–564. DOI 10.1007/s11104-020-04450-2. [Google Scholar] [CrossRef]
6. Zhu, Z., Pranolo, Y., Cheng, C. (2015). Separation of uranium and thorium from rare earths for rare earth production–A review. Minerals Engineering, 77, 185–196. DOI 10.1016/j.mineng.2015.03.012. [Google Scholar] [CrossRef]
7. Ibrahim, M. E., Orabi, A. H., Falila, N. I., Ismaiel, D. A., Salem, H. M. (2020). Processing of the mineralized black mica for the recovery of uranium, rare earth elements, niobium, and tantalum. Hydrometallurgy, 197, 105474. DOI 10.1016/j.hydromet.2020.105474. [Google Scholar] [CrossRef]
8. Cui, C., Wang, B., Zhao, Y., Wang, Q., Sun, Z. (2019). China’s regional sustainability assessment on mineral resources: Results from an improved analytic hierarchy process-based normal cloud model. Journal of Cleaner Production, 210, 105–120. DOI 10.1016/j.jclepro.2018.10.324. [Google Scholar] [CrossRef]
9. Wu, B., Shang, H., Wen, J. (2015). Sulfuric acid leaching of low-grade refractory tantalum–niobium and associated rare earths minerals in Panxi area of China. Rare Metals, 34(3), 202–206. DOI 10.1007/s12598-014-0436-7. [Google Scholar] [CrossRef]
10. Ding, Y., Wang, J., Wang, G., Xue, Q. (2012). Innovative methodology for separating of rare earth and iron from bayan Obo complex iron Ore. ISIJ International, 52(10), 1772–1777. DOI 10.2355/isijinternational.52.1772. [Google Scholar] [CrossRef]
11. Li, Z., Hadioui, M., Wilkinson, K. J. (2019). Conditions affecting the release of thorium and uranium from the tailings of a niobium mine. Environmental Pollution, 247, 206–215. DOI 10.1016/j.envpol.2018.12.042. [Google Scholar] [CrossRef]
12. Wang, L., Ji, B., Hu, Y., Liu, R., Sun, W. (2017). A review on in situ phytoremediation of mine tailings. Chemosphere, 184, 594–600. DOI 10.1016/j.chemosphere.2017.06.025. [Google Scholar] [CrossRef]
13. Kumar Yadav, K., Gupta, N., Kumar, A., Reece, L. M., Singh, N. et al. (2018). Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecological Engineering, 120, 274–298. DOI 10.1016/j.ecoleng.2018.05.039. [Google Scholar] [CrossRef]
14. Zhou, L. Y., Li, Z. L., Liu, W., Liu, S. H., Zhang, L. M. et al. (2015). Restoration of rare earth mine areas: Organic amendments and phytoremediation. Environmental Science and Pollution Research, 22(21), 17151–17160. DOI 10.1007/s11356-015-4875-y. [Google Scholar] [CrossRef]
15. Liu, S. H., Liu, W., Yang, M. X., Zhou, L. Y., Liang, H. (2016). The genetic diversity of soil bacteria affected by phytoremediation in a typical barren rare earth mined site of South China. SpringerPlus, 5(1), 1–12. DOI 10.1186/s40064-016-2814-0. [Google Scholar] [CrossRef]
16. Wei, Z. W., Hao, Z. K., Li, X. H., Guan, Z. B., Cai, Y. J. et al. (2019). The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Science of the Total Environment, 670(1), 950–960. DOI 10.1016/j.scitotenv.2019.03.118. [Google Scholar] [CrossRef]
17. Google Scholar (2022). https://scholar.google.com/. Accessed 25 February 2022. [Google Scholar]
18. Wang, Y., Huang, L., Wang, Z., Wang, L., Han, Y. et al. (2019). Application of polypyrrole flexible electrode for electrokinetic remediation of Cr(VI)-contaminated soil in a main-auxiliary electrode system. Chemical Engineering Journal, 373, 131–139. DOI 10.1016/j.cej.2019.05.016. [Google Scholar] [CrossRef]
19. Yu, Y., Liu, M., Yang, J. (2018). Characteristics of vanadium adsorption on and desorption from humic acid. Chemistry and Ecology, 34(6), 548–564. DOI 10.1080/02757540.2018.1452915. [Google Scholar] [CrossRef]
20. Patel, A. K., Das, N., Goswami, R., Kumar, M. (2019). Arsenic mobility and potential co-leaching of fluoride from the sediments of three tributaries of the upper Brahmaputra floodplain, Lakhimpur, Assam, India. Journal of Geochemical Exploration, 203, 45–58. DOI 10.1016/j.gexplo.2019.04.004. [Google Scholar] [CrossRef]
21. Ali, H., Khan, E., Sajad, M. A. (2013). Phytoremediation of heavy metals—Concepts and applications. Chemosphere, 91(7), 869–881. DOI 10.1016/j.chemosphere.2013.01.075. [Google Scholar] [CrossRef]
22. Fernández, S., Poschenrieder, C., Marcenò, C., Gallego, J. R., Jiménez-Gámez, D. et al. (2017). Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, North of Spain. Journal of Geochemical Exploration, 174, 10–20. DOI 10.1016/j.gexplo.2016.05.015. [Google Scholar] [CrossRef]
23. Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I. et al. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 182, 247–268. DOI 10.1016/j.gexplo.2016.11.021. [Google Scholar] [CrossRef]
24. Baker, A. J., McGrath, S., Reeves, R. D., Smith, J. (2000). Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In: Phytoremediation of contaminated soil and water. Boca Raton, Florida: CRC Press. [Google Scholar]
25. Burges, A., Alkorta, I., Epelde, L., Garbisu, C. (2018). From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. International Journal of Phytoremediation, 20(4), 384–397. DOI 10.1080/15226514.2017.1365340. [Google Scholar] [CrossRef]
26. Yan, A., Wang, Y., Tan, S. N., Mohd Yusof, M. L., Ghosh, S. et al. (2020). Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Frontiers in Plant Science, 11, 359. DOI 10.3389/fpls.2020.00359. [Google Scholar] [CrossRef]
27. Stojanović, M., Pezo, L., Lačnjevac,Č, Mihajlović, M., Petrović, J. et al. (2016). Biometric approach in selecting plants for phytoaccumulation of uranium. International Journal of Phytoremediation, 18(5), 527–533. DOI 10.1080/15226514.2015.1115966. [Google Scholar] [CrossRef]
28. Li, J., Gurajala, H. K., Wu, L., van der Ent, A., Qiu, R. et al. (2018). Hyperaccumulator plants from China: A synthesis of the current state of knowledge. Environmental Science & Technology, 52(21), 11980–11994. DOI 10.1021/acs.est.8b01060. [Google Scholar] [CrossRef]
29. Viehweger, K. (2014). How plants cope with heavy metals. Botanical Studies, 55(1), 1–12. DOI 10.1186/1999-3110-55-35. [Google Scholar] [CrossRef]
30. Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J. et al. (2014). Remediation of heavy metal(loid)s contaminated soils–to mobilize or to immobilize? Journal of Hazardous Materials, 266, 141–166. DOI 10.1016/j.jhazmat.2013.12.018. [Google Scholar] [CrossRef]
31. Liu, S., Ali, S., Yang, R., Tao, J., Ren, B. (2019). A newly discovered Cd-hyperaccumulator Lantana camara L. Journal of Hazardous Materials, 371, 233–242. DOI 10.1016/j.jhazmat.2019.03.016. [Google Scholar] [CrossRef]
32. Ge, J., Wang, H., Lin, J., Tian, S., Zhao, J. et al. (2020). Nickel tolerance, translocation and accumulation in a Cd/Zn co-hyperaccumulator plant Sedum alfredii. Journal of Hazardous Materials, 398, 123074. DOI 10.1016/j.jhazmat.2020.123074. [Google Scholar] [CrossRef]
33. Huang, Z. L., Tang, S. Q., Zhang, L., Ma, L. J., Ding, S. D. et al. (2017). Interaction between U and Th on their uptake, distribution, and toxicity in V S. alfredii based on the phytoremediation of U and Th. Environmental Science and Pollution Research, 24(3), 2996–3005. DOI 10.1007/s11356-016-8037-7. [Google Scholar] [CrossRef]
34. Manara, A., Fasani, E., Furini, A., DalCorso, G. (2020). Evolution of the metal hyperaccumulation and hypertolerance traits. Plant, Cell & Environment, 43(12), 2969–2986. DOI 10.1111/pce.13821. [Google Scholar] [CrossRef]
35. Roger, D. R., Alan, J. M. B., Tanguy, J., Peter, D. E., Guillaume, E. et al. (2018). A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytologist, 218(2), 407–411. DOI 10.1111/nph.14907. [Google Scholar] [CrossRef]
36. Ehsan, S., Ali, S., Noureen, S., Mahmood, K., Farid, M. et al. (2014). Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicology and Environmental Safety, 106, 164–172. DOI 10.1016/j.ecoenv.2014.03.007. [Google Scholar] [CrossRef]
37. Li, G., Hu, N., Ding, D., Zheng, J., Liu, Y. et al. (2011). Screening of plant species for phytoremediation of uranium, thorium, barium, nickel, strontium and lead contaminated soils from a uranium mill tailings repository in South China. Bulletin of Environmental Contamination and Toxicology, 86(6), 646–652. DOI 10.1007/s00128-011-0291-2. [Google Scholar] [CrossRef]
38. Awa, S. H., Hadibarata, T. (2020). Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water, Air, & Soil Pollution, 231(2), 1–15. DOI 10.1007/s11270-020-4426-0. [Google Scholar] [CrossRef]
39. Chaney, R. L., Baklanov, I. A. (2017). Phytoremediation and phytomining: Status and promise. Advances in Botanical Research, 83, 189–221. USA: Academic Press. [Google Scholar]
40. Hasan, M. M., Uddin, M. N., Ara-Sharmeen, I. F., Alharby, H., Alzahrani, Y. et al. (2019). Assisting phytoremediation of heavy metals using chemical amendments. Plants, 8(9), 295. DOI 10.3390/plants8090295. [Google Scholar] [CrossRef]
41. Chen, L., Yang, J., Wang, D. (2020). Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. Journal of Cleaner Production, 263, 121491. DOI 10.1016/j.jclepro.2020.121491. [Google Scholar] [CrossRef]
42. Hu, N., Lang, T., Ding, D., Hu, J., Li, C. et al. (2019). Enhancement of repeated applications of chelates on phytoremediation of uranium contaminated soil by Macleaya cordata. Journal of Environmental Radioactivity, 199, 58–65. DOI 10.1016/j.jenvrad.2018.12.023. [Google Scholar] [CrossRef]
43. Du, L., Feng, X., Huang, Z., Liu, B., Jin, Y. et al. (2016). The effect of U speciation in cultivation solution on the uptake of U by variant Sedum alfredii. Environmental Science and Pollution Research, 23(10), 9964–9971. DOI 10.1007/s11356-016-6226-z. [Google Scholar] [CrossRef]
44. Wang, D., Zhou, S., Liu, L., Du, L., Wang, J. et al. (2015). The influence of different hydroponic conditions on thorium uptake by Brassica juncea var. foliosa. Environmental Science and Pollution Research, 22(9), 6941–6949. DOI 10.1007/s11356-014-3914-4. [Google Scholar] [CrossRef]
45. Yang, Y., Liu, Y., Li, Z., Wang, Z., Li, C. et al. (2020). Significance of soil microbe in microbial-assisted phytoremediation: An effective way to enhance phytoremediation of contaminated soil. International Journal of Environmental Science and Technology, 17(4), 2477–2484. DOI 10.1007/s13762-020-02668-2. [Google Scholar] [CrossRef]
46. Cui, G., Chien, M. F., Suto, K., Inoue, C. (2017). Analysis of stable 1,2-dichlorobenzene-degrading enrichments and two newly isolated degrading strains, Acidovorax sp. sk40 and Ralstonia sp. sk41. Applied Microbiology and Biotechnology, 101(17), 6821–6828. DOI 10.1007/s00253-017-8406-2. [Google Scholar] [CrossRef]
47. Vergani, L., Mapelli, F., Zanardini, E., Terzaghi, E., di Guardo, A. et al. (2017). Phyto-rhizoremediation of polychlorinated biphenyl contaminated soils: An outlook on plant-microbe beneficial interactions. Science of the Total Environment, 575, 1395–1406. DOI 10.1016/j.scitotenv.2016.09.218. [Google Scholar] [CrossRef]
48. Sun, Y., Qiu, J., Chen, D., Ye, J., Chen, J. (2016). Characterization of the novel dimethyl sulfide-degrading bacterium alcaligenes sp. SY1 and its biochemical degradation pathway. Journal of Hazardous Materials, 304, 543–552. DOI 10.1016/j.jhazmat.2015.11.006. [Google Scholar] [CrossRef]
49. Rajkumar, M., Sandhya, S., Prasad, M. N., Freitas, H. (2012). Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnology Advances, 30(6), 1562–1574. DOI 10.1016/j.biotechadv.2012.04.011. [Google Scholar] [CrossRef]
50. Afzal, M., Khan, Q. M., Sessitsch, A. (2014). Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere, 117, 232–242. DOI 10.1016/j.chemosphere.2014.06.078. [Google Scholar] [CrossRef]
51. Hou, D., Wang, K., Liu, T., Wang, H., Lin, Z. et al. (2017). Unique rhizosphere micro-characteristics facilitate phytoextraction of multiple metals in soil by the hyperaccumulating plant Sedum alfredii. Environmental Science & Technology, 51(10), 5675–5684. DOI 10.1021/acs.est.6b06531. [Google Scholar] [CrossRef]
52. Liu, Y., Zhong, X., Huot, H., Liu, W., Liu, C. et al. (2020). Reclamation with organic amendments and plants remodels the diversity and structure of bacterial community in ion-adsorption rare earth element mine tailings. Journal of Soils and Sediments, 20(10), 3669–3680. DOI 10.1007/s11368-020-02704-1. [Google Scholar] [CrossRef]
53. Cameselle, C., Chirakkara, R. A., Reddy, K. R. (2013). Electrokinetic-enhanced phytoremediation of soils: Status and opportunities. Chemosphere, 93(4), 626–636. DOI 10.1016/j.chemosphere.2013.06.029. [Google Scholar] [CrossRef]
54. Cang, L., Zhou, D., Wang, Q., Fan, G. (2012). Impact of electrokinetic-assisted phytoremediation of heavy metal contaminated soil on its physicochemical properties, enzymatic and microbial activities. Electrochimica Acta, 86, 41–48. DOI 10.1016/j.electacta.2012.04.112. [Google Scholar] [CrossRef]
55. Chirakkara, R. A., Reddy, K. R., Cameselle, C. (2015). Electrokinetic amendment in phytoremediation of mixed contaminated soil. Electrochimica Acta, 181, 179–191. DOI 10.1016/j.electacta.2015.01.025. [Google Scholar] [CrossRef]
56. Acosta-Santoyo, G., Cameselle, C., Bustos, E. (2017). Electrokinetic–enhanced ryegrass cultures in soils polluted with organic and inorganic compounds. Environmental Research, 158, 118–125. DOI 10.1016/j.envres.2017.06.004. [Google Scholar] [CrossRef]
57. Yuan, L., Guo, P., Guo, S., Wang, J., Huang, Y. (2021). Influence of electrical fields enhanced phytoremediation of multi-metal contaminated soil on soil parameters and plants uptake in different soil sections. Environmental Research, 198, 111290. DOI 10.1016/j.envres.2021.111290. [Google Scholar] [CrossRef]
58. Li, J., Zhang, J., Larson, S. L., Ballard, J. H., Guo, K. et al. (2019). Electrokinetic-enhanced phytoremediation of uranium-contaminated soil using sunflower and Indian mustard. International Journal of Phytoremediation, 21(12), 1197–1204. DOI 10.1080/15226514.2019.1612847. [Google Scholar] [CrossRef]
59. Yan, X., An, J., Yin, Y., Gao, C., Wang, B. et al. (2022). Heavy metals uptake and translocation of typical wetland plants and their ecological effects on the coastal soil of a contaminated bay in Northeast China. Science of the Total Environment, 803, 149871. DOI 10.1016/j.scitotenv.2021.149871. [Google Scholar] [CrossRef]
60. Lei, M., Wan, X., Guo, G., Yang, J., Chen, T. (2018). Phytoextraction of arsenic-contaminated soil with pteris vittata in Henan Province, China: Comprehensive evaluation of remediation efficiency correcting for atmospheric depositions. Environmental Science and Pollution Research, 25(1), 124–131. DOI 10.1007/s11356-016-8184-x. [Google Scholar] [CrossRef]
61. Sterckeman, T., Gossiaux, L., Guimont, S., Sirguey, C. (2019). How could phytoextraction reduce Cd content in soils under annual crops? Simulations in the French context. Science of the Total Environment, 654, 751–762. DOI 10.1016/j.scitotenv.2018.11.173. [Google Scholar] [CrossRef]
62. Suman, J., Uhlik, O., Viktorova, J., Macek, T. (2018). Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Frontiers in Plant Science, 4,1476. DOI 10.3389/fpls.2018.01476. [Google Scholar] [CrossRef]
63. Mahar, A., Wang, P., Ali, A., Awasthi, M. K., Lahori, A. H. et al. (2016). Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicology and Environmental Safety, 126, 111–121. DOI 10.1016/j.ecoenv.2015.12.023. [Google Scholar] [CrossRef]
64. Thomas, G., Sheridan, C., Holm, P. E. (2021). A critical review of phytoremediation for acid mine drainage-impacted environments. Science of the Total Environment, 811,152230. DOI 10.1016/j.scitotenv.2021.152230. [Google Scholar] [CrossRef]
65. El Aafi, N., Saidi, N., Maltouf, A. F., Perez-Palacios, P., Dary, M. et al. (2015). Prospecting metal-tolerant rhizobia for phytoremediation of mining soils from Morocco using Anthyllis vulneraria L. Environmental Science and Pollution Research, 22(6), 4500–4512. DOI 10.1007/s11356-014-3596-y. [Google Scholar] [CrossRef]
66. Ashraf, S., Ali, Q., Zahir, Z. A., Ashraf, S., Asghar, H. N. (2019). Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicology and Environmental Safety, 174, 714–727. DOI 10.1016/j.ecoenv.2019.02.068. [Google Scholar] [CrossRef]
67. Mohsin, M., Kuittinen, S., Salam, M. M. A., Peräniemi, S., Laine, S. et al. (2019). Chelate-assisted phytoextraction: Growth and ecophysiological responses by Salix schwerinii E.L wolf grown in artificially polluted soils. Journal of Geochemical Exploration, 205, 106335. DOI 10.1016/j.gexplo.2019.106335. [Google Scholar] [CrossRef]
68. Li, X., Xiao, J., Salam, M. M. A., Ma, C., Chen, G. (2021). Impacts of bamboo biochar on the phytoremediation potential of Salix psammophila grown in multi-metals contaminated soil. International Journal of Phytoremediation, 23(4), 387–399. DOI 10.1080/15226514.2020.1816893. [Google Scholar] [CrossRef]
69. Lakkireddy, K., Kües, U. (2017). Bulk isolation of basidiospores from wild mushrooms by electrostatic attraction with low risk of microbial contaminations. AMB Express, 7(1), 28. DOI 10.1186/s13568-017-0326-0. [Google Scholar] [CrossRef]
70. Briskine, R. V., Paape, T., Shimizu-Inatsugi, R., Nishiyama, T., Akama, S. et al. (2017). Genome assembly and annotation of Arabidopsis halleri, a model for heavy metal hyperaccumulation and evolutionary ecology. Molecular Ecology Resources, 17(5), 1025–1036. DOI 10.1111/1755-0998.12604. [Google Scholar] [CrossRef]
71. García-Sánchez, M., Košnář, Z., Mercl, F., Aranda, E., Tlustoš, P. (2018). A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicology and Environmental Safety, 147, 165–174. DOI 10.1016/j.ecoenv.2017.08.012. [Google Scholar] [CrossRef]
72. Covre, W. P., da Silveira Pereira, W. V., Gonçalves, D. A. M., Teixeira, O. M. M., do Amarante, C. B. et al. (2020). Phytoremediation potential of Khaya ivorensis and Cedrela fissilis in copper contaminated soil. Journal of Environmental Management, 268, 110733. DOI 10.1016/j.jenvman.2020.110733. [Google Scholar] [CrossRef]
73. Cristaldi, A., Conti, G. O., Jho, E. H., Zuccarello, P., Grasso, A. et al. (2017). Phytoremediation of contaminated soils by heavy metals and PAHs. A Brief Review. Environmental Technology & Innovation, 8, 309–326. DOI 10.1016/j.eti.2017.08.002. [Google Scholar] [CrossRef]
74. Pérez-Palacios, P., Agostini, E., Ibáñez, S. G., Talano, M. A., Rodríguez-Llorente, I. D. et al. (2017). Removal of copper from aqueous solutions by rhizofiltration using genetically modified hairy roots expressing a bacterial Cu-binding protein. Environmental Technology, 38(22), 2877–2888. DOI 10.1080/09593330.2017.1281350. [Google Scholar] [CrossRef]
75. Paz-Ferreiro, J., Lu, H., Fu, S., Méndez, A., Gascó, G. (2014). Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth, 5(1), 65–75. DOI 10.5194/se-5-65-2014. [Google Scholar] [CrossRef]
76. Nasab, M. E. (2014). Solvent extraction separation of uranium (VI) and thorium (IV) with neutral organophosphorus and amine ligands. Fuel, 116, 595–600. DOI 10.1016/j.fuel.2013.08.043. [Google Scholar] [CrossRef]
77. Song, W., Wang, X., Wang, Q., Shao, D., Wang, X. (2015). Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Physical Chemistry Chemical Physics, 17(1), 398–406. DOI 10.1039/C4CP04289A. [Google Scholar] [CrossRef]
78. Qi, X., Hao, X., Chen, X., Xiao, S., Chen, S. et al. (2019). Integrated phytoremediation system for uranium-contaminated soils by adding a plant growth promoting bacterial mixture and mowing grass. Journal of Soils and Sediments, 19(4), 1799–1808. DOI 10.1007/s11368-018-2182-1. [Google Scholar] [CrossRef]
79. Hu, N., Chen, S., Lang, T., Zhang, H., Chen, W. et al. (2021). A novel method for determining the adequate dose of a chelating agent for phytoremediation of radionulides contaminated soils by M. cordata. Journal of Environmental Radioactivity, 227, 106468. DOI 10.1016/j.jenvrad.2020.106468. [Google Scholar] [CrossRef]
80. Sharma, S., Singh, B., Manchanda, V. (2015). Phytoremediation: Role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environmental Science and Pollution Research, 22(2), 946–962. DOI 10.1007/s11356-014-3635-8. [Google Scholar] [CrossRef]
81. Gupta, D., Chatterjee, S., Datta, S., Voronina, A., Walther, C. (2016). Radionuclides: Accumulation and transport in plants. Reviews of Environmental Contamination and Toxicology, Cham: Springer. [Google Scholar]
82. Pratas, J., Paulo, C., Favas, P. J., Venkatachalam, P. (2014). Potential of aquatic plants for phytofiltration of uranium-contaminated waters in laboratory conditions. Ecological Engineering, 69, 170–176. DOI 10.1016/j.ecoleng.2014.03.046. [Google Scholar] [CrossRef]
83. Favas, P. J., Pratas, J., Mitra, S., Sarkar, S. K., Venkatachalam, P. (2016). Biogeochemistry of uranium in the soil-plant and water-plant systems in an old uranium mine. Science of the Total Environment, 568, 350–368. DOI 10.1016/j.scitotenv.2016.06.024. [Google Scholar] [CrossRef]
84. Jha, V., Tripathi, R., Sethy, N., Sahoo, S. (2016). Uptake of uranium by aquatic plants growing in fresh water ecosystem around uranium mill tailings pond at Jaduguda, India. Science of the Total Environment, 539, 175–184. DOI 10.1016/j.scitotenv.2015.08.120. [Google Scholar] [CrossRef]
85. Zhang, L., Liu, W., Liu, S., Zhang, P., Ye, C. et al. (2020). Revegetation of a barren rare earth mine using native plant species in reciprocal plantation: Effect of phytoremediation on soil microbiological communities. Environmental Science and Pollution Research, 27(2), 2107–2119. DOI 10.1007/s11356-019-06645-2. [Google Scholar] [CrossRef]
86. Yan, X., Luo, X. (2016). Uptake of uranium, thorium, radium and potassium by four kinds of dominant plants grown in uranium mill tailing soils from the southern part of China. Radioprotection, 51(2), 141–144. DOI 10.1051/radiopro/2015031. [Google Scholar] [CrossRef]
87. Wang, W., Luo, X., Liu, L., Zhang, Y., Zhao, H. (2018). Ramie (Boehmeria nivea)’s uranium bioconcentration and tolerance attributes. Journal of Environmental Radioactivity, 184, 152–157. DOI 10.1016/j.jenvrad.2018.01.016. [Google Scholar] [CrossRef]
88. Laurette, J., Larue, C., Llorens, I., Jaillard, D., Jouneau, P. H. et al. (2012). Speciation of uranium in plants upon root accumulation and root-to-shoot translocation: A XAS and TEM study. Environmental and Experimental Botany, 77, 87–95. DOI 10.1016/j.envexpbot.2011.11.005. [Google Scholar] [CrossRef]
89. Qi, F., Zha, Z., Du, L., Feng, X., Wang, D. et al. (2014). Impact of mixed low-molecular-weight organic acids on uranium accumulation and distribution in a variant of mustard (Brassica juncea var. tumida). Journal of Radioanalytical and Nuclear Chemistry, 302(1), 149–159. DOI 10.1007/s10967-014-3279-7. [Google Scholar] [CrossRef]
90. Saenen, E., Horemans, N., Vanhoudt, N., Vandenhove, H., Biermans, G. et al. (2013). Effects of pH on uranium uptake and oxidative stress responses induced in Arabidopsis thaliana. Environmental Toxicology and Chemistry, 32(9), 2125–2133. DOI 10.1002/etc.2290. [Google Scholar] [CrossRef]
91. Chen, L., Long, C., Wang, D., Yang, J. (2020). Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere, 242, 125112. DOI 10.1016/j.chemosphere.2019.125112. [Google Scholar] [CrossRef]
92. Alsabbagh, A. H., Abuqudaira, T. M. (2017). Phytoremediation of Jordanian uranium-rich soil using sunflower. Water, Air, & Soil Pollution, 228(6), 1–9. DOI 10.1007/s11270-017-3396-3. [Google Scholar] [CrossRef]
93. Banerjee, R., Goswami, P., Mukherjee, A. (2018). Stabilization of iron ore mine spoil dump sites with vetiver system. In: Bio-geotechnologies for mine site rehabilitation, pp. 393–413. Netherlands: Elsevier. [Google Scholar]
94. Sha, Y., Hu, N., Wang, Y., Chen, S., Zou, C. et al. (2019). Enhanced phytoremediation of uranium contaminated soil by artificially constructed plant community plots. Journal of Environmental Radioactivity, 208, 106036. DOI 10.1016/j.jenvrad.2019.106036. [Google Scholar] [CrossRef]
95. Lai, J., Liu, Z., Luo, X. (2020). A metabolomic, transcriptomic profiling, and mineral nutrient metabolism study of the phytotoxicity mechanism of uranium. Journal of Hazardous Materials, 386, 121437. DOI 10.1016/j.jhazmat.2019.121437. [Google Scholar] [CrossRef]
96. Chen, X., Wu, G., Ma, Q., Lai, J., Luo, X. et al. (2020). Cytotoxic and genotoxic evaluation and the toxicological mechanism of uranium in Vicia faba root. Environmental and Experimental Botany, 179, 104227. DOI 10.1016/j.envexpbot.2020.104227. [Google Scholar] [CrossRef]
97. Malaviya, P., Singh, A. (2012). Phytoremediation strategies for remediation of uranium-contaminated environments: A review. Critical Reviews in Environmental Science and Technology, 42(24), 2575–2647. DOI 10.1080/10643389.2011.592761. [Google Scholar] [CrossRef]
98. Li, C., Wang, M., Luo, X., Liang, L., Han, X. et al. (2019). Accumulation and effects of uranium on aquatic macrophyte Nymphaea tetragona Georgi: Potential application to phytoremediation and environmental monitoring. Journal of Environmental Radioactivity, 198, 43–49. DOI 10.1016/j.jenvrad.2018.12.018. [Google Scholar] [CrossRef]
99. Ren, C., Kong, C., Wang, S., Xie, Z. (2019). Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere, 217, 773–779. DOI 10.1016/j.chemosphere.2018.11.085. [Google Scholar] [CrossRef]
100. Ahsan, M. T., Najam-ul-Haq, M., Idrees, M., Ullah, I., Afzal, M. (2017). Bacterial endophytes enhance phytostabilization in soils contaminated with uranium and lead. International Journal of Phytoremediation, 19(10), 937–946. DOI 10.1080/15226514.2017.1303813. [Google Scholar] [CrossRef]
101. Meng, F., Jin, D., Guo, K., Larson, S. L., Ballard, J. H. et al. (2018). Influences of U sources and forms on its bioaccumulation in Indian mustard and sunflower. Water, Air, & Soil Pollution, 229(11), 1–11. DOI 10.1007/s11270-018-4023-7. [Google Scholar] [CrossRef]
102. Imran, M., Hu, S., Luo, X., Cao, Y., Samo, N. (2019). Phytoremediation through Bidens pilosa L., a nonhazardous approach for uranium remediation of contaminated water. International Journal of Phytoremediation, 21(8), 752–759. DOI 10.1080/15226514.2018.1556594. [Google Scholar] [CrossRef]
103. Gupta, D. K., Vuković, A., Semenishchev, V. S., Inouhe, M., Walther, C. (2020). Uranium accumulation and its phytotoxicity symptoms in Pisum sativum L. Environmental Science and Pollution Research, 27(3), 3513–3522. DOI 10.1007/s11356-019-07068-9. [Google Scholar] [CrossRef]
104. Yang, M., Jawitz, J. W., Lee, M. (2015). Uranium and cesium accumulation in bean (Phaseolus vulgaris L. var. vulgaris) and its potential for uranium rhizofiltration. Journal of Environmental Radioactivity, 140, 42–49. DOI 10.1016/j.jenvrad.2014.10.015. [Google Scholar] [CrossRef]
105. Fu, Q., Lai, J., Li, C., Ji, X., Luo, X. (2022). Phytotoxicity mechanism of the natural radionuclide thorium in Vicia faba. Journal of Hazardous Materials, 424, 127718. DOI 10.1016/j.jhazmat.2021.127718. [Google Scholar] [CrossRef]
106. Soudek, P., Hrdinová, A., Valseca, I. R., Lhotáková, Z., Mihaljevič, M. et al. (2019). Thorium as an environment stressor for growth of Nicotiana glutinosa plants. Environmental and Experimental Botany, 164, 84–100. DOI 10.1016/j.envexpbot.2019.03.027. [Google Scholar] [CrossRef]
107. Soudek, P., Kufner, D., Petrová, Š, Mihaljevič, M., Vaněk, T. (2013). Composition of hydroponic medium affects thorium uptake by tobacco plants. Chemosphere, 92(9), 1090–1098. DOI 10.1016/j.chemosphere.2013.01.046. [Google Scholar] [CrossRef]
108. Jagetiya, B., Sharma, A., Soni, A., Khatik, U. K. (2014). Phytoremediation of radionuclides: A report on the state of the art. In: Radionuclide contamination and remediation through plants. Cham: Springer. [Google Scholar]
109. Yan, X. (2016). Uptake of radionuclide thorium by twelve native plants grown in uranium mill tailings soils from South part of China. Nuclear Engineering and Design, 304, 80–83. DOI 10.1016/j.nucengdes.2016.04.019. [Google Scholar] [CrossRef]
110. Ebyan, O. A. M. (2019). Distribution and bioaccumulation of uranium and thorium in natural soil and wild plants of wadi El-missikat, central eastern desert, Egypt. Arab Journal of Nuclear Sciences and Applications, 52(4), 159–166. DOI 10.21608/ajnsa.2019.13252.1218. [Google Scholar] [CrossRef]
111. Wiche, O., Székely, B., Kummer, N. A., Moschner, C., Heilmeier, H. (2016). Effects of intercropping of oat (Avena sativa L.) with white lupin (Lupinus albus L.) on the mobility of target elements for phytoremediation and phytomining in soil solution. International Journal of Phytoremediation, 18(9), 900–907. DOI 10.1080/15226514.2016.1156635. [Google Scholar] [CrossRef]
112. Huang, R., Lu, Y., Yang, H., Huang, W., Chen, K. (2016). Effects of arbuscular mycorrhizal fungi on caesium accumulation and the ascorbate-glutathione cycle of Sorghum halepense. ScienceAsia, 42(5), 323–331. DOI 10.2306/scienceasia1513-1874.2016.42.323. [Google Scholar] [CrossRef]
113. Zhou, S., Kai, H., Zha, Z., Fang, Z., Wang, D. et al. (2016). Subcellular distribution and chemical forms of thorium in Brassica juncea var. foliosa. Journal of Environmental Radioactivity, 157, 60–66. DOI 10.1016/j.jenvrad.2016.03.003. [Google Scholar] [CrossRef]
114. Chen, L., Hu, W. F., Long, C., Wang, D. (2021). Exogenous plant growth regulator alleviate the adverse effects of U and Cd stress in sunflower (Helianthus annuus L.) and improve the efficacy of U and Cd remediation. Chemosphere, 262, 127809. DOI 10.1016/j.chemosphere.2020.127809. [Google Scholar] [CrossRef]
115. Chen, L., Liu, J., Zhang, W., Zhou, J., Luo, D. et al. (2021). Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. Journal of Hazardous Materials, 413, 125319. DOI 10.1016/j.jhazmat.2021.125319. [Google Scholar] [CrossRef]
116. Yan, X., Luo, X. (2015). Radionuclides distribution, properties, and microbial diversity of soils in uranium mill tailings from Southeastern China. Journal of Environmental Radioactivity, 139, 85–90. DOI 10.1016/j.jenvrad.2014.09.019. [Google Scholar] [CrossRef]
117. Liu, C., Liu, W., Huot, H., Guo, M., Zhu, S. et al. (2022). Biogeochemical cycles of nutrients, rare earth elements (REEs) and Al in soil-plant system in ion-adsorption REE mine tailings remediated with amendment and ramie (Boehmeria nivea L.). Science of the Total Environment, 809, 152075. DOI 10.1016/j.scitotenv.2021.152075. [Google Scholar] [CrossRef]
118. Yuan, M., Liu, C., Liu, W., Guo, M., Morel, J. L. et al. (2018). Accumulation and fractionation of rare earth elements (REEs) in the naturally grown Phytolacca americana L. in Southern China. International Journal of Phytoremediation, 20(5), 415–423. DOI 10.1080/15226514.2017.1365336. [Google Scholar] [CrossRef]
119. Liu, C., Liu, W., van der Ent, A., Morel, J. L., Zheng, H. et al. (2021). Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolacca americana in response to soil properties. Chemosphere, 282, 131096. DOI 10.1016/j.chemosphere.2021.131096. [Google Scholar] [CrossRef]
120. Liu, W., Chen, Y., Huot, H., Liu, C., Guo, M. et al. (2020). Phytoextraction of rare earth elements from ion-adsorption mine tailings by Phytolacca americana: Effects of organic material and biochar amendment. Journal of Cleaner Production, 275, 122959. DOI 10.1016/j.jclepro.2020.122959. [Google Scholar] [CrossRef]
121. Liu, C., Sun, D., Zheng, H., Wang, G., Liu, W. et al. (2022). The limited exclusion and efficient translocation mediated by organic acids contribute to rare earth element hyperaccumulation in phytolacca americana. Science of the Total Environment, 805, 150335. DOI 10.1016/j.scitotenv.2021.150335. [Google Scholar] [CrossRef]
122. Qin, B., Liu, W., He, E., Li, Y., Liu, C. et al. (2019). Vacuum pyrolysis method for reclamation of rare earth elements from hyperaccumulator dicranopteris dichotoma grown in contaminated soil. Journal of Cleaner Production, 229, 480–488. DOI 10.1016/j.jclepro.2019.05.031. [Google Scholar] [CrossRef]
123. Chen, Z., Chen, Z. (2020). Clipping strategy to assist phytoremediation by hyperaccumulator dicranopteris dichotoma at rare earth mines. International Journal of Phytoremediation, 22(10), 1038–1047. DOI 10.1080/15226514.2020.1725870. [Google Scholar] [CrossRef]
124. Liu, C., Yuan, M., Liu, W., Guo, M., Zheng, H. et al. (2021). Element case studies: Rare earth elements. In: Agromining: Farming for metals. Cham: Springer. [Google Scholar]
125. Chen, H., Chen, H., Chen, Z. (2021). A review of in situ phytoextraction of rare earth elements from contaminated soils. International Journal of Phytoremediation, 24, 1–10. DOI 10.1080/15226514.2021.1957770. [Google Scholar] [CrossRef]
126. Mohsin, M., Salam, M. M. A., Nawrot, N., Kaipiainen, E., Lane, D. J. et al. (2022). Phytoextraction and recovery of rare earth elements using willow (Salix spp.). Science of the Total Environment, 809, 152209. DOI 10.1016/j.scitotenv.2021.152209. [Google Scholar] [CrossRef]
127. Mikołajczak, P., Borowiak, K., Niedzielski, P. (2017). Phytoextraction of rare earth elements in herbaceous plant species growing close to roads. Environmental Science and Pollution Research, 24(16), 14091–14103. DOI 10.1007/s11356-017-8944-2. [Google Scholar] [CrossRef]
128. Avishek, K., Hazra, M. (2022). Plant based removal and recovery of rare earth elements. In: Environmental technologies to treat rare earth elements pollution: Principles and engineering. London: IWA Publishing. [Google Scholar]
129. de Carvalho, F. H., Barbosa de Souza Jr, P. F., da Silva, L. A., de França, E. J., Araújo, E. S. et al. (2019). Study of ashes from Pachystroma longifolium leaf as stabilizer for PVC exposed to γ-lrradiation. Macromolecular Symposia, 383(1), 1800021. DOI 10.1002/masy.201800021. [Google Scholar] [CrossRef]
130. Kovaříková, M., Tomášková, I., Soudek, P. (2019). Rare earth elements in plants. Biologia Plantarum, 63(1), 20–32. DOI 10.32615/bp.2019.003. [Google Scholar] [CrossRef]
131. Turra, C., de Nadai Fernandes, E. A., Bacchi, M. A., Sarriés, G. A., Reyes, A. E. L. (2019). Uptake of rare earth elements by citrus plants from phosphate fertilizers. Plant and Soil, 437(1), 291–299. DOI 10.1007/s11104-019-03979-1. [Google Scholar] [CrossRef]
132. Segovia, C., Hedri, M., Huot, H., Zigler-Devin, I., Liu, C. et al. (2021). Industrial ramie growing on reclaimed ion-adsorption rare earth elements mine tailings in Southern China: Defibration and fibers quality. Waste and Biomass Valorization, 12(11), 6255–6260. DOI 10.1007/s12649-021-01442-w. [Google Scholar] [CrossRef]
133. Luo, C., Deng, Y., Inubushi, K., Liang, J., Zhu, S. et al. (2018). Sludge biochar amendment and Alfalfa revegetation improve soil physicochemical properties and increase diversity of soil microbes in soils from a rare earth element mining wasteland. International Journal of Environmental Research and Public Health, 15(5), 965. DOI 10.3390/ijerph15050965. [Google Scholar] [CrossRef]
134. Guo, W., Zhao, R., Zhao, W., Fu, R., Guo, J. et al. (2013). Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. Applied Soil Ecology, 72, 85–92. DOI 10.1016/j.apsoil.2013.06.001. [Google Scholar] [CrossRef]
135. Wen, B., Yuan, D., Shan, X., Li, F., Zhang, S. (2001). The influence of rare earth element fertilizer application on the distribution and bioaccumulation of rare earth elements in plants under field conditions. Chemical Speciation & Bioavailability, 13(2), 39–48. DOI 10.3184/095422901783726825. [Google Scholar] [CrossRef]
136. Zhang, C., Li, Q., Zhang, M., Zhang, N., Li, M. (2013). Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharmaceutica Sinica B, 3(1), 20–24. DOI 10.1016/j.apsb.2012.12.005. [Google Scholar] [CrossRef]
137. Dinali, G. S., Root, R. A., Amistadi, M. K., Chorover, J., Lopes, G. et al. (2019). Rare earth elements (REY) sorption on soils of contrasting mineralogy and texture. Environment International, 128, 279–291. DOI 10.1016/j.envint.2019.04.022. [Google Scholar] [CrossRef]
138. Zhao, C., Shi, X., Xie, S., Liu, W., He, E. et al. (2019). Ecological risk assessment of neodymium and yttrium on rare earth element mine sites in Ganzhou, China. Bulletin of Environmental Contamination and Toxicology, 103(4), 565–570. DOI 10.1007/s00128-019-02690-2. [Google Scholar] [CrossRef]
139. Carvalho, F. P., Tufa, M. B., Oliveira, J. M., Malta, M. (2021). Radionuclides and radiation exposure in tantalite mining, Ethiopia. Archives of Environmental Contamination and Toxicology, 81(4), 648–659. DOI 10.1007/s00244-021-00858-8. [Google Scholar] [CrossRef]
140. Dampare, S., Nyarko, B., Osae, S., Akaho, E., Asiedu, D. et al. (2005). Simultaneous determination of tantalum, niobium, thorium and uranium in placer columbite-tantalite deposits from the Akim Oda District of Ghana by epithermal instrumental neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry, 265(1), 53–59. DOI 10.1007/s10967-005-0860-0. [Google Scholar] [CrossRef]
141. Zglinicki, K., Szamałek, K., Wołkowicz, S. (2021). Critical minerals from post-processing tailing. A case study from Bangka Island, Indonesia. Minerals, 11(4), 352. DOI 10.3390/min11040352. [Google Scholar] [CrossRef]
142. Cheru, M. S., Albertodel Rosario, A. V., Yimam, A., Tadesse, B., Berhe, G. G. (2019). Hydrometallurgical removal of uranium and thorium from Ethiopian tantalite ore. Physicochemical Problems of Mineral Processing, 55, 448–457. DOI 10.5277/PPMP18153. [Google Scholar] [CrossRef]
143. Gakwerere, F. (2012). An investigation of the level of selected trace metals in plant species within the vicinity of tantalum mining area in Gatumba, Ngororero district, Rwanda (Ph.D. Thesis). University of South Africa, Pretoria. [Google Scholar]
144. Alekseenko, V., Shvydkaya, N., Bech, J., Puzanov, A., Nastavkin, A. (2021). Trace element accumulation by soils and plants in the North Caucasian geochemical province. Journal of Mining Institute, 247, 141–153. DOI 10.31897/PMI.2021.1.15. [Google Scholar] [CrossRef]
145. Krasavtseva, E., Maksimova, V., Gorbacheva, T. (2021). Assessment of the ground and plant chemistry in an area affected by rare metal ore concentration waste storage. Research Square, 1–10. DOI 10.21203/rs.3.rs-406947/v1. [Google Scholar] [CrossRef]
146. Cunha, L., Nardi, M. C., Pereira, L., Neto, V., Vedana, A., L. (2014). Evaluation of biological absorption coefficient of trace elements in plants from the pitinga mine district, Amazonian Region. Revista do Instituto Geológico, 35(1), 19–29. DOI 10.5935/0100-929X.20140002. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |