

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.022995
ARTICLE
Sono-Transesterification of Kapok Seed Oil with CaO:BaO-(x:y)/Active Natural Zeolite Catalyst
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Malang, Malang, 65145, Indonesia
*Corresponding Author: Sumari Sumari. Email: sumari.fmipa@um.ac.id
Received: 04 April 2022; Accepted: 13 May 2022
Abstract: Kapok seed oil is an environmentally friendly biodiesel feedstock. The problem of the best catalyst for transesterification of Kapok seed oil still continues today. The research developed a base-heterogeneous catalyst with the effect of ultrasonic waves during transesterification. So, the study aims: (1) to determine the type of natural zeolite used, (2) to determine the characteristics of the synthesized CaO:BaO/ANZ catalyst, and (3) to determine the effectiveness of the CaO:BaO/ANZ catalyst in the biodiesel production process through sono-transesterification of kapok seed oil. The research stages consisted of: (1) activation of natural zeolite and its characterization, (2) preparation of CaO:BaO/ANZ catalyst, (3) characterization of CaO:BaO/ANZ catalyst, (4) esterification and utilization of catalyst in the transesterification, (5) analysis of transesterified products. The results showed that the natural zeolite type from the southern area of Malang is mordenite (MOR) type. Then, after the natural zeolite was impregnated, the obtained catalyst compositions were CaO:BaO-9:9/ANZ, CaO:BaO-14:9/ANZ, and CaO:BaO-4:11/ANZ with a surface area of 129.7; 124.6; 128.5 m2/g, and acidity of 1.037; 0.254; and 0.685 mmol/g, respectively. The best catalyst for transesterification of kapok seed oil was CaO:BaO-9:9/ANZ, which resulted in a biodiesel yield of 73.6% with a density of 889.6 kg/m3 and kinematic viscosity of 2.987 cSt. The characteristics of the produced biodiesel meet requirement of SNI 7182:2015.
Keywords: Natural zeolite; heterogeneous catalyst; biodiesel; kapok seed oil; transesterification; ultrasonication
Nomenclature
| NZ | Natural Zeolite |
| ANZ | Activated Natural Zeolite |
Petroleum fuel has a very high use, while the source of petroleum fuel was running low currently [1]. To prevent environmental destruction shortly, one potential solution is the development of environmentally friendly biodiesel fuel. Many countries mandate the utilization of biodiesel synthesized from various raw materials that are abundant and renewable. It has less emission, is biodegradable, and has small toxicity. Furthermore, its production helps rural economies [2]. So, biodiesel is a good alternative diesel fuel [3].
Kapok seed oil is a renewable resource. The basic potential of the kapok seed oil is its abundant availability which is widely growing in tropical countries. Kapok seed oil does not compete with daily food needs [4]. The old tree can produce several hundred pods that contain seeds as an oil source. The oil has a saturated fatty acid content of 71.95% which is higher than coconut oil [5]. Therefore, Kapok seed oil has great potential to be used as a raw material in the synthesis of biodiesel.
The most common method for biodiesel synthesis is the transesterification reaction [6]. Transesterification enables the use of various raw materials of triglycerides to produce biodiesel with standard quality. The process of biodiesel synthesis via transesterification of kapok seed oil can be supported using a catalyst [7]. The presence of the catalyst in the reaction will cause more efficiency in synthesis. Natural zeolite is often used as a catalyst because of its large pore size [8]. Natural zeolite is an aluminosilicate rock that is abundant in nature and also in Malang [9,10]. The property of zeolite can be increased by modification such as impregnation to improve the performance of zeolite as a catalyst. The presence of catalyst influences the yield of biodiesel and the composition and properties of biodiesel [11].
Impregnation using transition metals will produce acidic heterogeneous catalysts while impregnation with alkali and alkaline earth metal oxides will produce alkaline heterogeneous catalysts [12]. Base-heterogeneous catalysts are generally metal oxides and buffered metal oxides. The metals used in this impregnation method are metals from Group 2 (alkaline earth) [13,14]. Alkaline oxides, especially alkaline earth, are bases that have good catalytic properties [15]. In the previous study, CaO was impregnated with natural zeolite successfully [16]. The optimum conditions of transesterification of waste cooking oil using CaO/Natural Zeolite were mole ratio of oil:methanol about 1:15, 5 h reaction, 5% wt catalyst, and 60°C temperature that it yielded about 88.34% biodiesel [17]. On the other hand, BaO/Zeolite Y has been used for transesterification of castor oil in conditions of 65°C temperature, a mole ratio of oil:methanol 1:9, 2% wt catalyst for 2 h which it yielded 94% biodiesel [13]. So, BaO can be used to improve the catalytic activity. In this study, metal oxides of CaO and BaO were used for impregnation into natural zeolite to obtain a base-heterogeneous catalyst. The catalyst will be utilized in the transesterification in the synthesis of biodiesel from Kapok seed oil. The active sites of a catalyst will increase the selectivity of the catalyst [18].
Base-heterogeneous catalyst CaO–SrO has been investigated in palm oil transesterification reaction [19]. The results of the study indicated that the conversion of vegetable oil into the obtained methyl ester is 93.215% with a reaction rate constant, k = 1.00 · 10−2 min−1 obtained at the reactant ratio 1:6, the ratio of the catalyst 50:50 and the total catalyst 12% wt of reactants. Then, the CaO-ZnO catalyst has been investigated in the synthesis of methyl esters from Off Grade Crude Palm Oil (CPO) with the highest yield of 84.74% [20]. Then, research on CaO–MgO catalyst in the transesterification of kapok seed oil resulted in the largest yield percentage of 59.58% [21]. From a previous study, alkaline earth oxide catalysts need to be developed as transesterification catalysts. Research by Santoso reported that ultrasonic-assisted transesterification (as known as sono-transesterification) of Off Grade CPO obtained 88.06% yield of biodiesel with K2O/Al2O3 35% wt catalyst [22]. The types of oxides showed different catalyst activities and the ultrasonic gave an increase to the yield. The ultrasound effect can assist the miscibility of oil and methanol and decrease the amount of catalyst for the reaction [23].
In this study, therefore, the use of CaO:BaO catalyst impregnated on natural zeolite was a unique combination of base-heterogeneous catalyst. The study was carried out to determine the activity of the alkaline earth oxide catalyst in the sono-transesterification process. This study will describe the catalytic activity of CaO:BaO/ANZ that has been synthesized. Instrumentation analysis was carried out to confirm the synthesized material & biodiesel.
The tools used in this study were mortar and pestle, 100 mesh sieve, analytical balance, oven, furnace, porcelain dish, measuring flask, dropper, Erlenmeyer, beaker, measuring cup, desiccator, spatula, stirring rod, spray bottle, and pole. stative, ring, funnel, aluminum foil, stopwatch, thermometer, universal indicator, reflux kit, ultrasonic cleaner (Branson 42 kHz), water bath shaker, Oswald viscosimeter, hot plate. X-Ray Diffraction (XRD) (Brand: PanAnalytical, Type: Expert Pro), X-Ray Fluorescence (XRF) (Brand: PANanalytical, Type: Manipal 4), gas chromatography-mass spectrometry (GC-MS) (Brand: Shimadzu, Type: GCMS QP2010 Plus). The materials used in this study were natural zeolite from the southern region of Malang, Kapok Seed Oil, Ba(OH)2 pa, Ca(OH)2 pa, HF 40%, HCl 32%, 1 M NH4Cl, ammonia, H2SO4 97%, anhydrous MgSO4, methanol, distilled water, 1% methylene blue, and demineralized water.
2.1 Activation of Natural Zeolite
Natural zeolite was crushed and sieved through a 100 mesh sieve, washed with distilled water and dried in an oven at 150°C, and calcined. Next, the zeolite was immersed in 1% HF solution (1:2) for 30 min (300 rpm), neutralized with distilled water, dried at 150°C for 2 h, and then calcined. Furthermore, the zeolite was soaked with 6 M HCl (1:2), refluxed at 60°C for 5 h, neutralized with distilled water, dried at 150°C for 2 h, and then calcined. Furthermore, the zeolite was stirred in 1 M NH4Cl solution (1:2) for 2 h (300 rpm), neutralized with distilled water, and dried at 150°C. The zeolite was then calcined in a furnace at 500°C for 4 h.
2.2 Impregnation of Natural Zeolite
A total of 15 grams of active zeolite was added to a 5% Ba(OH)2 solution, the mixture was shaken for 24 h, washed until neutral, dried at 150°C, then calcined at 500°C for 4 h. As a result, BaO/ANZ was obtained. Next, the material was put into a 5% Ca(OH)2 solution, stirred for 24 h, washed until neutral, dried at 150°C, and calcined at 500°C for 4 h. At this stage, CaO:BaO/ANZ is obtained. The other impregnation was conducted with 5% Ba(OH)2 and 7% Ca(OH)2, and the last impregnation was 7% Ba(OH)2 and 5% Ca(OH)2. Catalysts were characterized by analysis of XRD, XRF, surface area test [24], and catalyst acidity test [25].
Kapok seed oil was weighed as much as 20 grams, then heated at 40°C using a hotplate. It is then put in an Erlenmeyer containing a mixture of methanol and catalyst which has been heated at a temperature of 40°C in an ultrasonic chamber. The ultrasonic process was carried out by keeping the water temperature at 65°C for 15 min. It is then transferred into a separatory funnel for washing using warm water. Then anhydrous MgSO4 was added to absorb the remaining water. Then the biodiesel product was evaporated in an oven at 70°C for 2 h to reduce the water content in the biodiesel. Furthermore, the biodiesel products were characterized the yield, density, and viscosity. The compound composed of triglycerides was analyzed by GC-MS.
3.1 Characterization of Natural Zeolite
Natural Zeolite (NZ) with a size of 100 mesh was washed and then dried in the oven at 150°C for 2 h. The NZ was activated using HF for desilication purposes. The second activation used 6 M HCl, and a dealumination reaction occurs. On the activation using 6 M HCl, some of the impurity oxides, especially free alumina and the other metal oxides, dissolved. It was indicated by a change in the color of the natural zeolite material from brown to white, and the washing water as a filtrate is yellow. Furthermore, the last activation using 1 M NH4Cl which is intended to remove interfering metal cations through ion exchange, and NH4+ cations are bound in the zeolite framework. Heating treatment after the ion exchange process, the NH4+ ions will turn into NH3 gas and form H-Natural Zeolite. The final result, Natural H-Zeolite (ANZ), is opaque white which indicates that the impurities in the zeolite framework have been reduced, as shown in Fig. 1b.

Figure 1: Photoshoot of (a) Net Natural Zeolite (NZ) and (b) Activated Natural Zeolite (ANZ)
The next step was the characterization of NZ and ANZ using XRD and XRF. Based on the XRD diffractograms obtained indicated that the zeolite used in this study was a mordenite type of zeolite as shown in Fig. 2. Data of XRF-He characterization were to determine the percentage of oxide composition in Natural Zeolite and obtained the data written in Table 1.
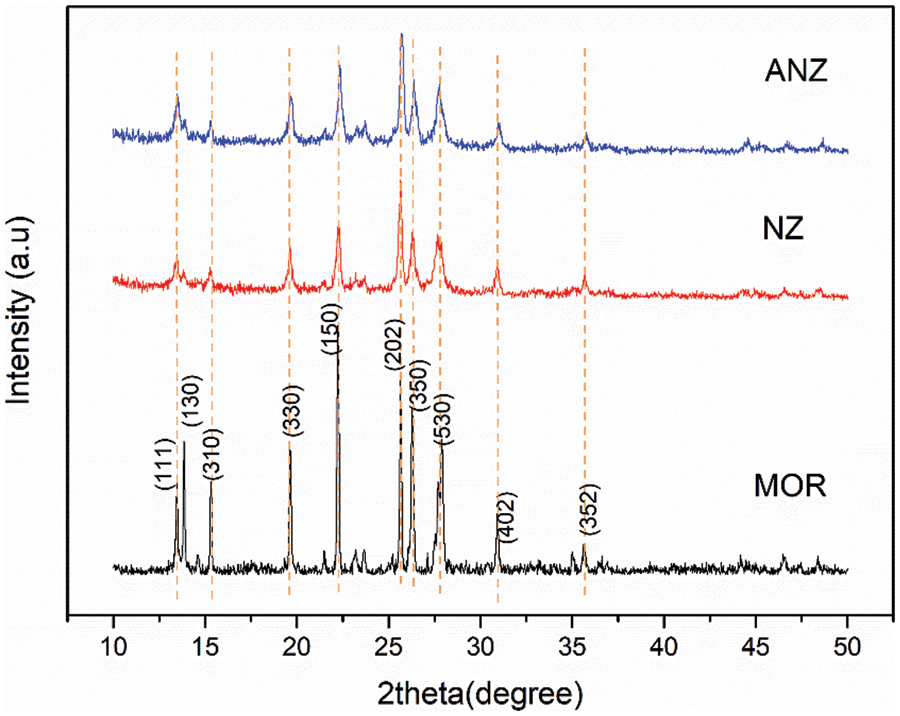
Figure 2: XRD of Mordenite (MOR) standard, Natural Zeolite (NZ), and Activated Natural Zeolite (ANZ)
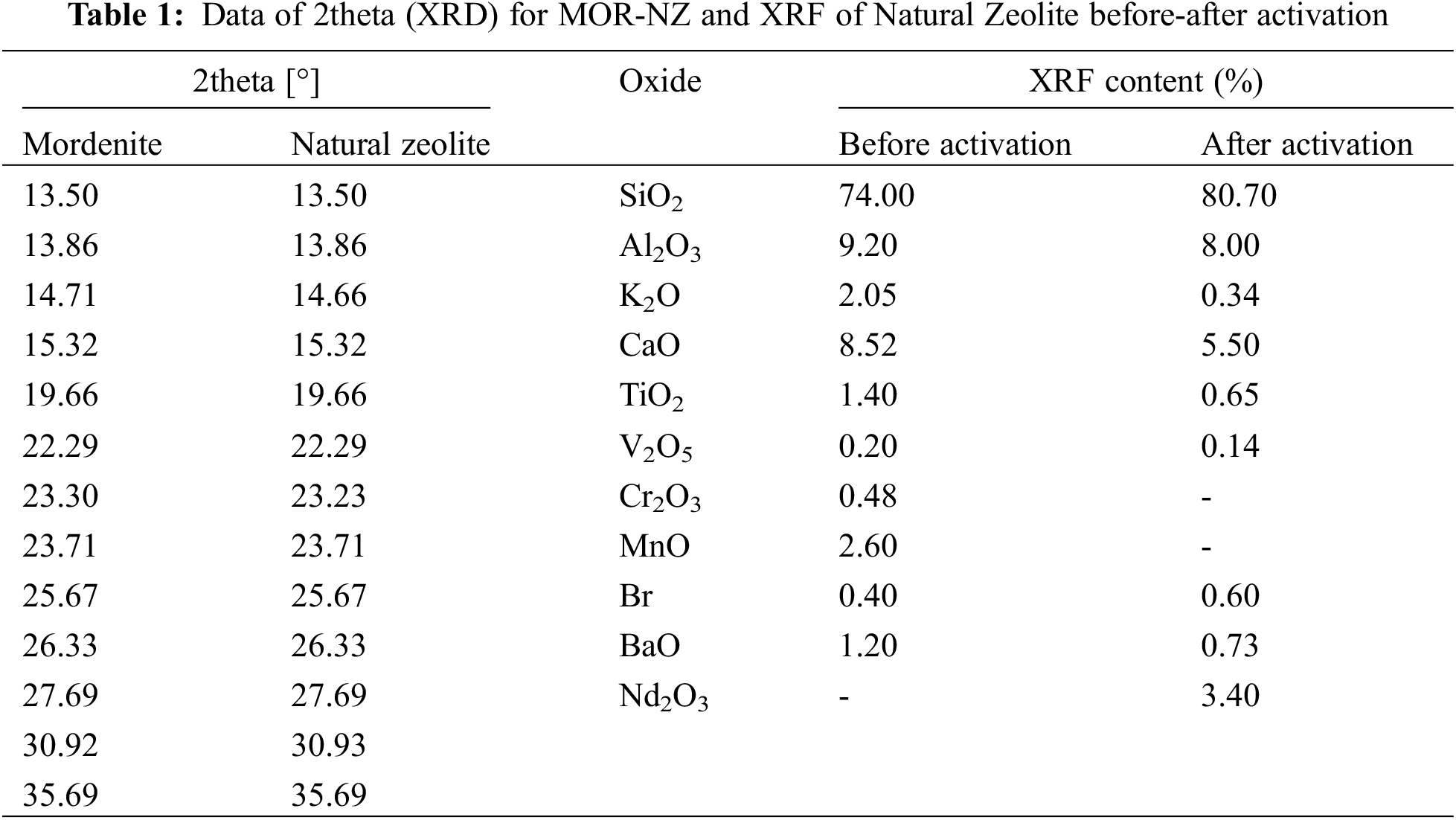
Determination of NZ zeolite type was done through identification of 2θ peaks produced which was then compared with JCPDS No-6239 data as shown in Fig. 2 and Table 1. The XRD results showed sharp peaks on the ANZ diffractogram as shown in Fig. 2. It appears that there is a resemblance between NZ/ANZ and MOR diffractogram. It also shows that ANZ has a lower crystallinity than MOR crystallinity, but ANZ has higher crystallinity than NZ. The higher the sharp peak of the pattern, the higher the crystallinity [26]. The XRF results in Table 1 show that the number of impurities in NZ has been reduced after activation. There are two kinds of CaO in zeolite:free CaO and CaO bonded in the framework of Zeolite. So, activation of NZ becomes ANZ was a success to eliminate free CaO, but CaO bonded in the framework of Zeolite remain.
3.2 Characterization of CaO:BaO(x:y)/Active Natural Zeolite
Impregnation of CaO and BaO oxides in ANZ was carried out by a wet process has been carried out. In this study, 5% wt Ba(OH)2 was impregnated into ANZ-produced catalysts of BaO-9/ANZ (two samples) and 7% wt Ba(OH)2 was impregnated into ANZ resulting in BaO-11/ANZ. Each BaO-9/ANZ which was impregnated with 5% and 7% Ca(OH)2 obtained catalysts of CaO:BaO-9:9/ANZ and CaO:BaO–14:9/ANZ. While BaO-11/ANZ was impregnated with 5% Ca (OH)2 yielded CaO:BaO-4:11/ANZ. The results of the XRF analysis of the catalyst can be seen in Table 3. While the XRD diffractogram results of the catalysts can be seen in Fig. 3 and Table 2.
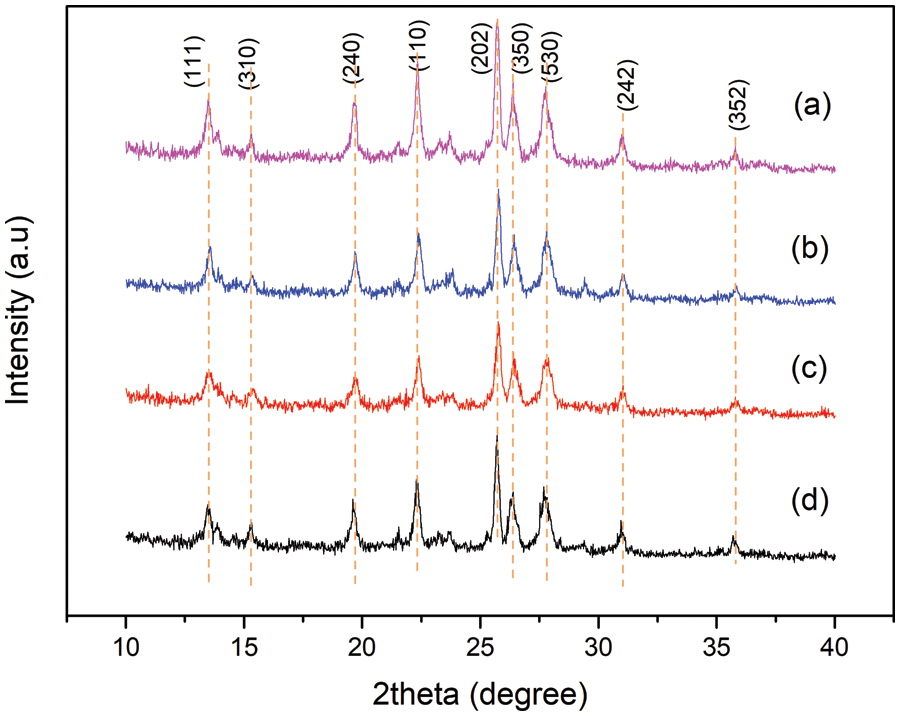
Figure 3: XRD of (a) ANZ, (b) CaO:BaO-14:9/ANZ, (c) CaO: BaO-4:11/ANZ, (d) CaO:BaO-9:9/ANZ
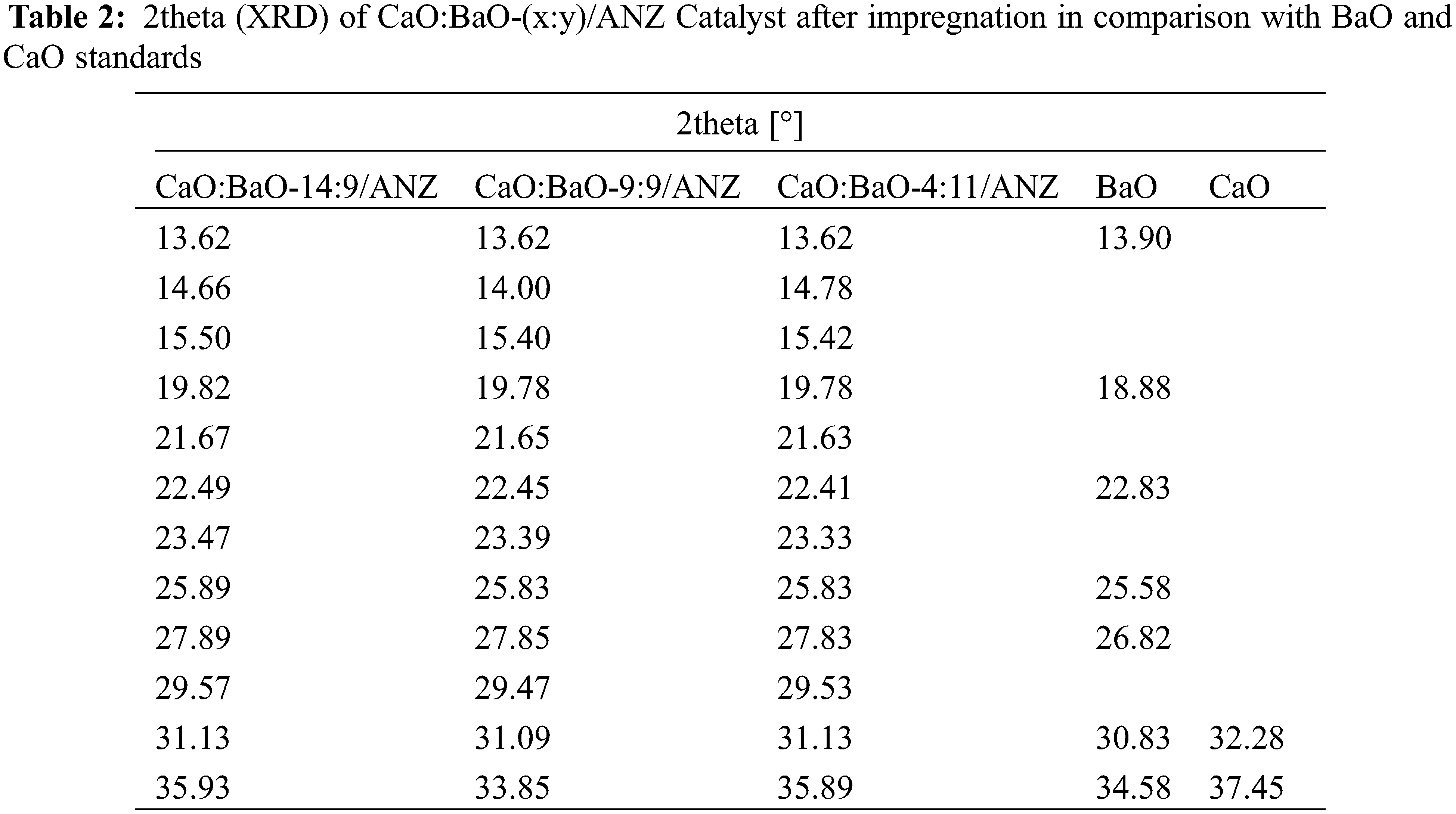
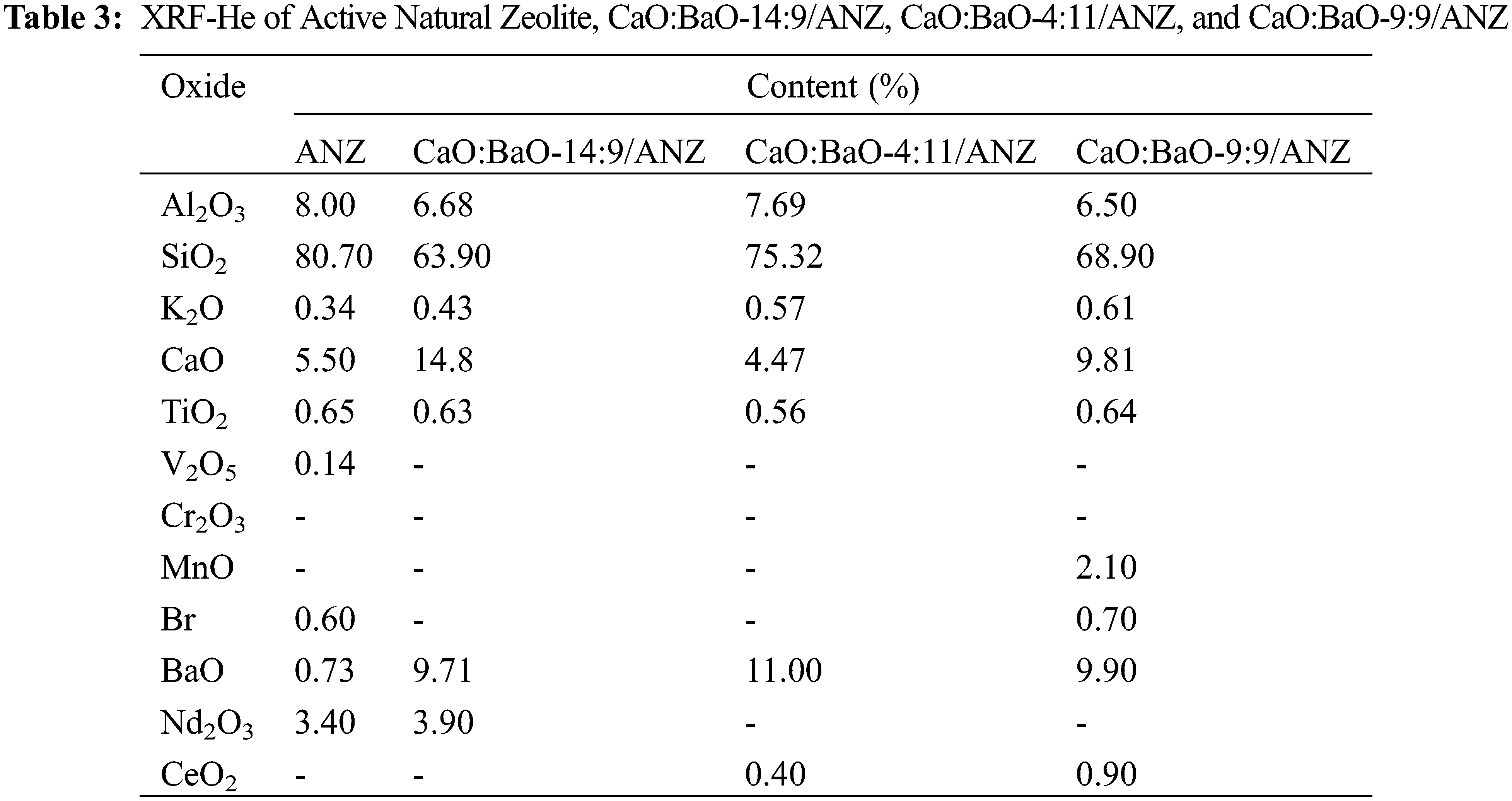
The impregnation of ANZ with CaO and BaO showed the same XRD diffractogram pattern with ANZ beginning, even though calcination was done. This indicates that the impregnation of CaO and BaO did not change the crystal structure of ANZ [16]. The previous researcher, Wu [27] explained that the oxides of Ca and Ba can be evenly distributed on the surface of the zeolite so that the diffractogram pattern of CaO and BaO cannot be seen clearly. There is a slight change in peaks of diffractogram in the range of 2theta of 20–24 degree that is a decrease in intensity.
Table 3 showed that CaO and BaO levels increase with the increasing percentage of Ca(OH)2 and Ba(OH)2 impregnated. It is following the results of previous studies on natural zeolite impregnation using metal-oxide [28]. During calcination, the calcium and barium hydroxide on the surface of the zeolite will be converted to CaO and BaO [16]. The formation of Oxides on the surface of natural zeolite because of calcination brings a change in the composition of Al and Si in the zeolite framework. The composition of Si/Al in the zeolite decreased because of the addition of both metal oxides in the total constituent compounds. The decreased level of Si and Al will be followed by the change in the hygroscopic properties of the zeolite [29]. This phenomenon is caused by a decrease in the active site [AlO4]– in the zeolite, which will eventually lead to a decrease in the amount of water in the zeolite. The CaO level in the CaO:BaO-4:11/ANZ is 4.47 which is lower than that in the ANZ. This anomaly can be explained based on the procedure that Ba(OH)2 solution was added first before the Ca(OH)2 solution. In the addition Ba (OH)2 more concentrated can replace calcium ions in the zeolite framework because of ion exchange happened. On the other hand, the barium oxide bound first with the greater concentration will inhibit the binding of calcium oxide added in the next step.
The acidity test of NZ, ANZ, and ANZ catalyst impregnated with oxides of CaO and BaO is shown in Fig. 4. It was found that there is an increase in the acidity level of NZ after activation treatment. The starting material of NZ has a lot of impurities (Table 1) which causes a lot of blocked acid sites. The activation process becomes ANZ making the acidity value increase drastically but it goes down because of the addition of Calcium and Barium oxides. It is due to the increasing number of CaO and BaO that occupies the pores of ANZ that causes the level of basicity increases or the acidity level decreases.
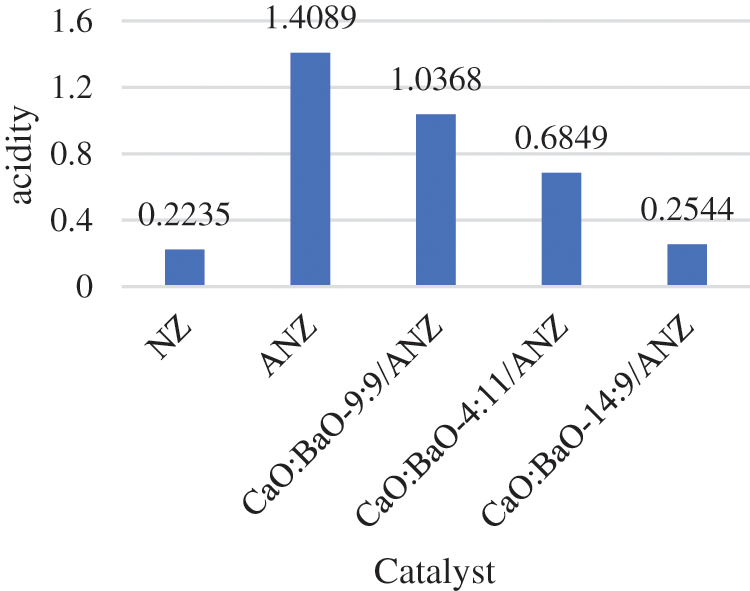
Figure 4: Acidity test of NZ, ANZ, CaO:BaO-14:9/ANZ, CaO:BaO-4:11/ANZ, and CaO:BaO-9:9/ANZ
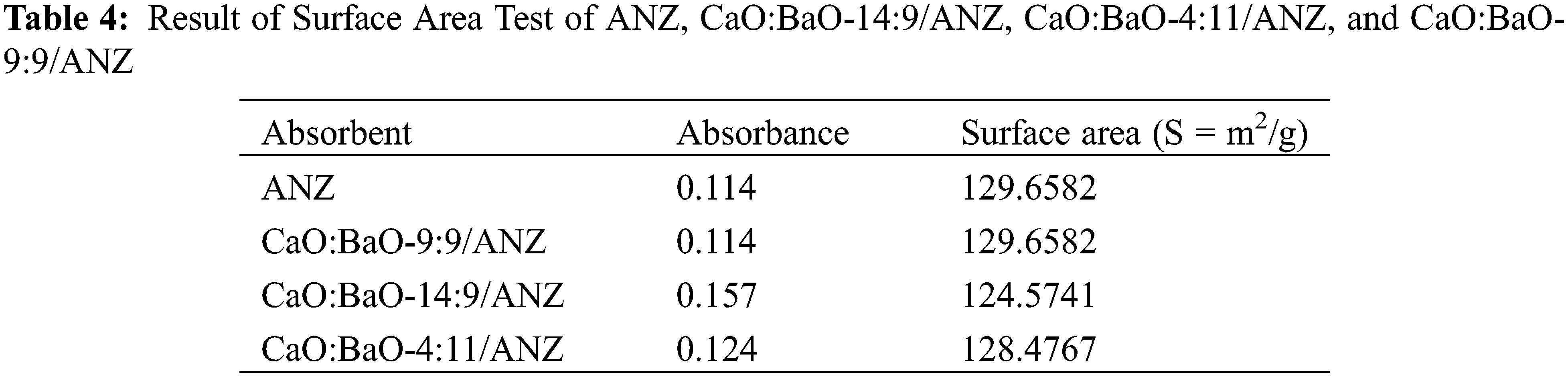
The surface area test showed a decrease in ANZ surface area after impregnation with CaO and BaO as shown in Table 4. In general, the impregnation of calcium and barium oxides into natural zeolites makes their surface area decrease along with the number of metal oxides. It is due to the filling of the pores by metal oxide particles [27]. Another study explains the partial closure of the natural zeolite network by oxide particles which eventually form aggregates [30].
The transesterification process was carried out with the assistance of ultrasonic. It is considered that trans-esterification using ultrasonic waves can produce greater conversion [31]. The frequency of ultrasonic waves used was 42 kHz with a temperature of 65°C for 15 min. The biodiesel obtained sono-transesterification process was washed with warm water to dissolve the remaining glycerol and methanol. The anhydrous MgSO4 was added to the biodiesel to bind the remaining washing water. The product was also heated for 3 h at 70°C to eliminate the water content in the biodiesel. At the next stage, each biodiesel obtained was measured for the density and the viscosity and compared with the biodiesel quality standard (SNI 7182: 2015) namely the density (40°C) of 850–890 kg/m3 and viscosity of 2.3–6.0 cSt. Based on the SNI quality standards, biodiesel products that meet the required quality standards are biodiesel produced through a transesterification process using CaO:BaO-9:9/ANZ and CaO:BaO-14:9/ANZ catalysts as shown in Table 5. The biodiesel yield was tested using GC-MS to identify the content of the biodiesel constituent compounds the chromatogram obtained is shown in Fig. 5 and the yield of each constituent compound is shown in Table 6.
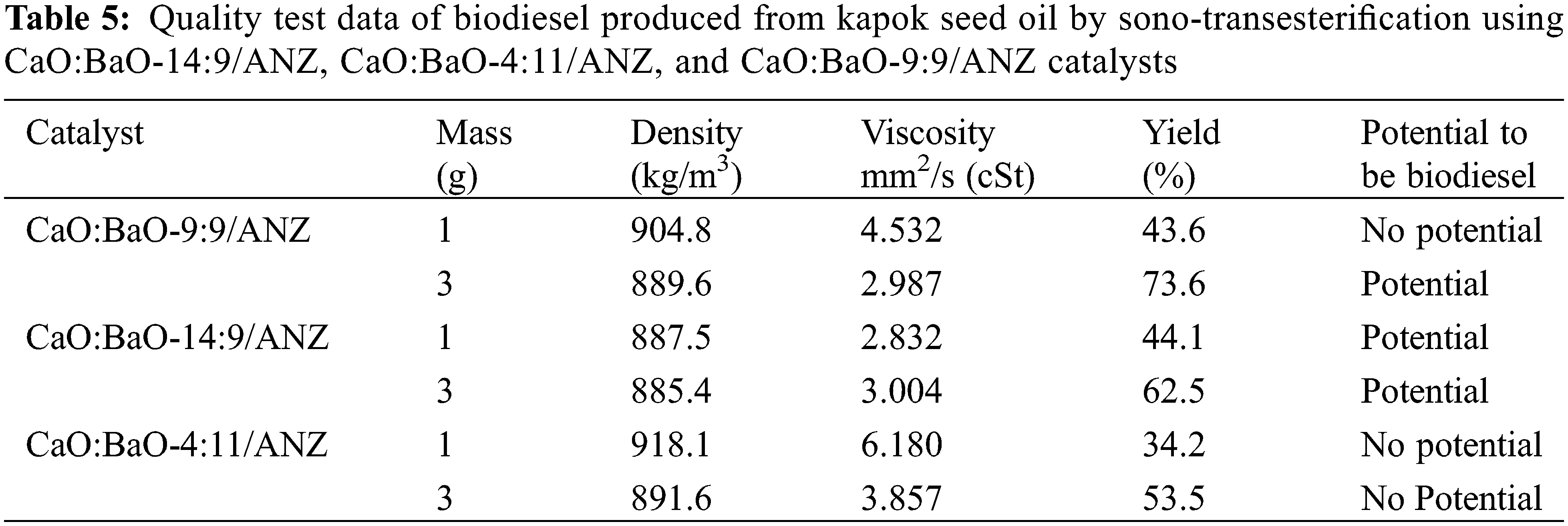
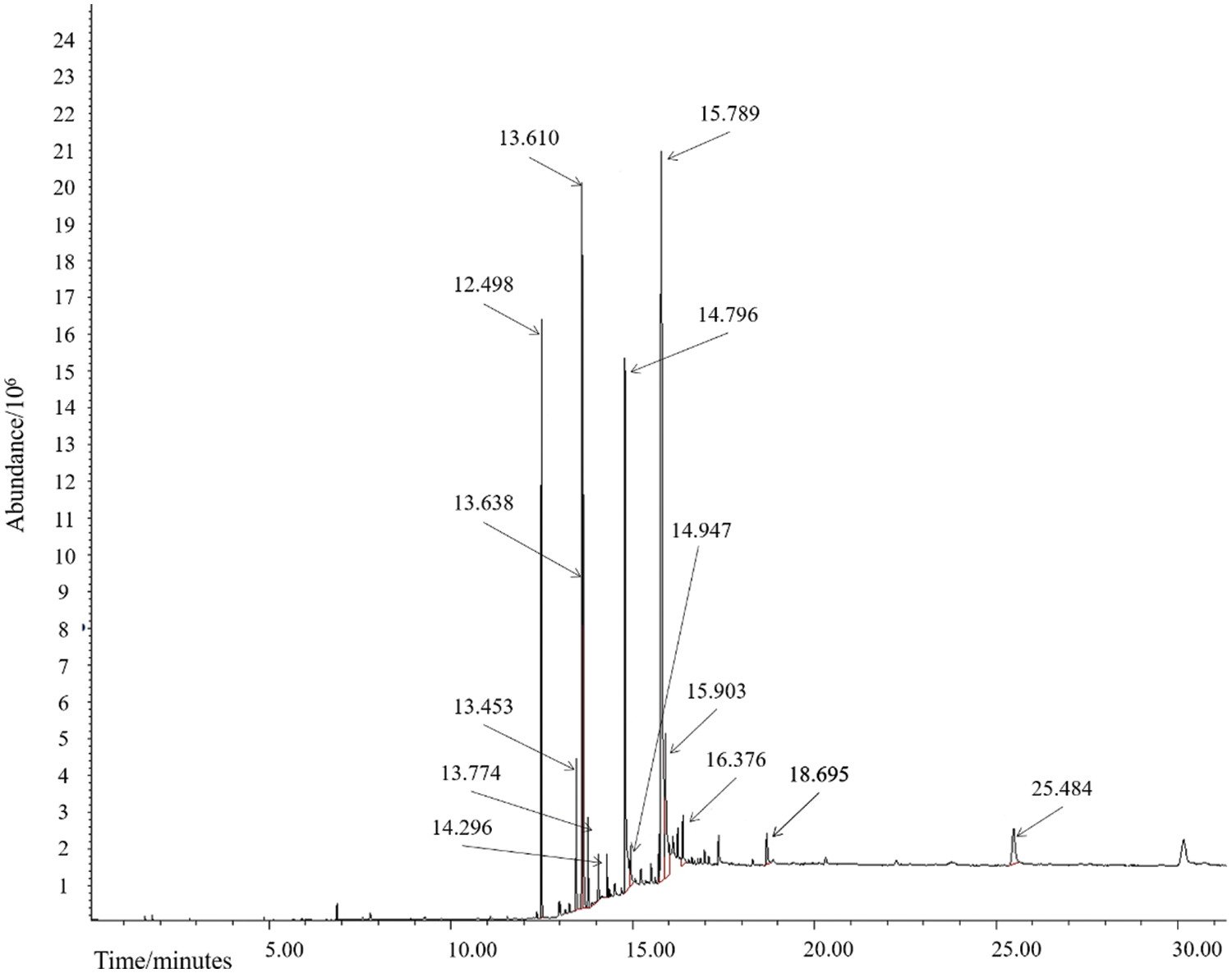
Figure 5: GC-MS Chromatogram of the biodiesel produced from kapok seed oil that was reacted at 65°C for 15 min with ultrasonic wave assistance
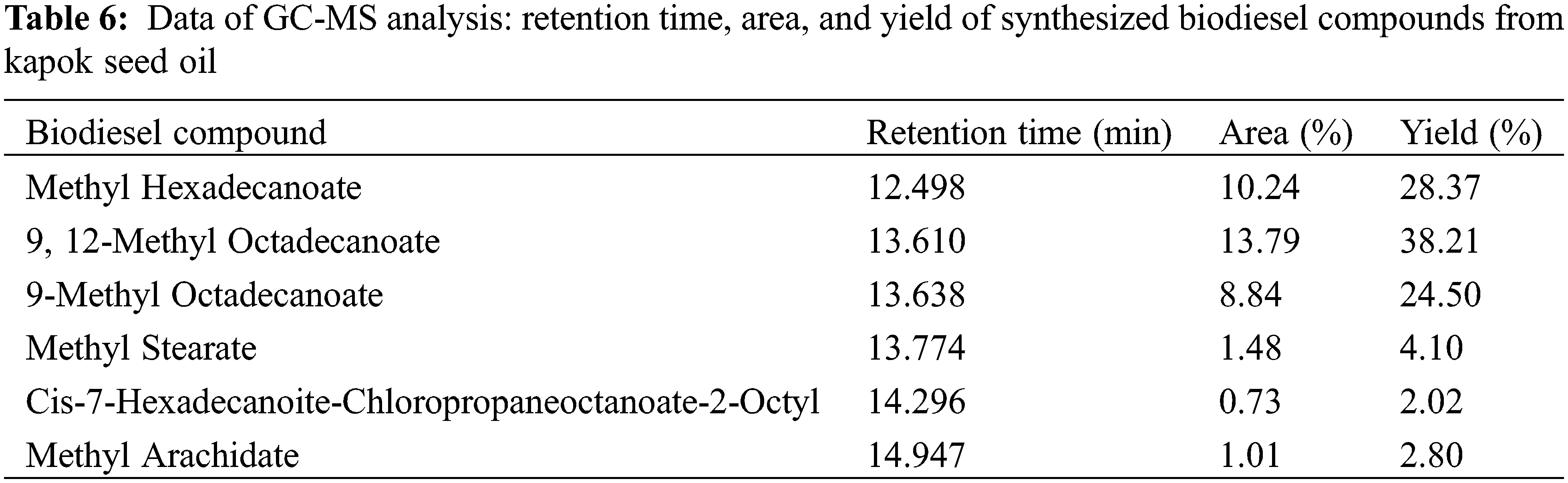
Fig. 5 shows that there are six chromatogram peaks as the result of GC-MS analysis of the biodiesel yielded from the transesterification of kapok seed oil. The retention time, area, and yield of each component composed of the biodiesel are written in Table 6. From these data, it can be stated that methyl esters have been obtained which means that biodiesel has been formed from the trans-esterification process of kapok seed oil using ANZ catalyst impregnated with CaO and BaO. The highest yield obtained from this study is 73.6% lower than that of the previous study (88.34%) [17]. Therefore, CaO-BaO-(x:y)/ANZ catalysts need to be developed into an intensification process technique [32].
The natural zeolite used in this study is a type of mordenite zeolite. Natural Zeolite (NZ) was successfully activated to produce Active Natural Zeolite (ANZ) with better crystallinity than NZ. CaO:BaO-9:9/ANZ, CaO:BaO-14:9/ANZ, and CaO:BaO-4:11/ANZ catalysts were successfully synthesized using ANZ with CaO and BaO supported by a decrease in surface area and acidity value of ANZ. The synthesis of biodiesel was successfully carried out using a catalyst CaO:BaO-9:9/ANZ resulting in % a yield of 73.63% and CaO:BaO-14:9/ANZ produced % a yield of 62.49% at reaction conditions of 65°C in 15 min ultrasonic wave effect. The presence of alkaline earth metal oxide in the zeolite will affect the basicity, the more the oxide loading, the higher the basicity. The practical implications are: (1) zeolite matrix is slightly acid, so it needs to add the certain oxide into zeolite for increasing the basicity, (2) more basicity of catalyst, higher catalytic activity to produce biodiesel. The limitation study is: (1) the study only for a combination of CaO and BaO impregnated into the natural zeolite. The future study suggests the combination of alkaline metal and alkaline earth metal oxides. The potential technology for the improvement of research and development was machine learning [33].
Acknowledgement: The authors would like to thank the Chancellor and Chair of LP2M UM, who provided PNBP research funds.
Funding Statement: The funding that has supported the work, namely: PNBP Universitas Negeri Malang to Dr. Sumari, M. Si.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Perea-Moreno, M. A., Samerón-Manzano, E., Perea-Moreno, A. J. (2019). Biomass as renewable energy: Worldwide research trends. Sustainability, 11(3), 863. DOI 10.3390/su11030863. [Google Scholar] [CrossRef]
2. Gebremariam, S. N., Marchetti, J. M. (2017). Biodiesel production technologies: Review. AIMS Energy, 5(3), 425–457. DOI 10.3934/energy.2017.3.425. [Google Scholar] [CrossRef]
3. Sumari, S., Santoso, A., Asrori, M. R. (2021). A review: Synthesis of biodiesel from low/off grade crude palm oil on pretreatment, transesterification, and characteristics. Orbital, 13(4), 385–391. DOI 10.17807/orbital.v13i4.1632. [Google Scholar] [CrossRef]
4. Santoso, A., Sumari, S., Zakiyya, U. U., Nur, A. T. (2019). Methyl ester synthesis of crude palm oil off grade using the K2O/Al2O3 catalyst and its potential as biodiesel. IOP Conference Series: Materials Science and Engineering, 515(1), 012042. DOI 10.1088/1757-899X/515/1/012042. [Google Scholar] [CrossRef]
5. Frederic, N. P., Ikhsan, H. D. (2013). Pembuatan biodiesel dari minyak biji kapok dengan proses esterifikasi transesterifikasi. Jurnal Teknologi Kimia Dan Industry, 2(2), 262–266. [Google Scholar]
6. Avhad, M. R., Marchetti, J. M. (2015). A review on recent advancement in catalytic materials for biodiesel production. Renewable and Sustainable Energy Reviews, 50, 696–718. DOI 10.1016/j.rser.2015.05.038. [Google Scholar] [CrossRef]
7. Santoso, A., Rizky, M., Sumari, S., Wijaya, A. R., Retnosari, R. et al. (2021). Pengaruh jenis alkohol pada sintesis alkil ester dari CPO melalui reaksi transesterifikasi menggunakan katalis heterogen CaO-MgO. Jurnal Rekayasa Bahan Alam Dan Energi Berkelanjutan, 5(1), 1–9. [Google Scholar]
8. Atikah, W. S. (2017). Karakterisasi zeolit alam gunung kidul teraktivasi sebagai media adsorben pewarna tekstil. Arena Tekstil, 32(1), 17–24. DOI 10.31266/at.v32i1.2650. [Google Scholar] [CrossRef]
9. Król, M. (2020). Natural vs. synthetic zeolites. Crystals, 10(7), 622. DOI 10.3390/cryst10070622. [Google Scholar] [CrossRef]
10. Santoso, A., Putri, D. E. K., Rusdi, M., Sumari, S., Wijaya, A. R. et al. (2021). The effect of basic catalyst concentration on tobacco oil transesterification (voor-oogst) using ultra-sonic wave and its potential as renewable energy. AIP Conference Proceedings, 2330(1), 070008. DOI 10.1063/5.0043406. [Google Scholar] [CrossRef]
11. Aderibigbe, F. A., Mustapha, S. I., Adewoye, T. L., Mohammed, I. A., Gbadegesin, A. B. et al. (2020). Qualitative role of heterogeneous catalysts in biodiesel production from Jatropha curcas oil. Biofuel Research Journal, 7(2), 1159–1169. DOI 10.18331/BRJ2020.7.2.4. [Google Scholar] [CrossRef]
12. Sánchez-Faba, E. M., Ferrero, G. O., Dias, J. M., Eimer, G. A. (2019). Alternative raw materials to produce biodiesel through alkaline heterogeneous catalysis. Catalysts, 9(8), 690. DOI 10.3390/catal9080690. [Google Scholar] [CrossRef]
13. Anwaristiawan, D., Harjito, W. N. (2018). Modifikasi katalis BaO/Zeolit Y pada reaksi transesterifikasi minyak biji jarak (Jatropha curcas L. ) menjadi biodiesel. Indonesian Journal of Chemical Science, 7(3), 292–298. [Google Scholar]
14. Lawan, I., Garba, Z. N., Zhou, W., Zhang, M., Yuan, Z. (2020). Synergies between the microwave reactor and CaO/Zeolite catalyst in waste lard biodiesel production. Renewable Energy, 145, 2550–2560. DOI 10.1016/j.renene.2019.08.008. [Google Scholar] [CrossRef]
15. Mierczynski, P., Ciesielski, R., Kedziora, A., Maniukiewicz, W., Shtyka, O. et al. (2015). Biodiesel production on MgO, CaO, SrO and BaO oxides Supported on (SrO)(Al(2O3) mixed oxide. Catalysis Letters, 145(5), 1196–1205. DOI 10.1007/s10562-015-1503-x. [Google Scholar] [CrossRef]
16. Wisnu, I. M., Putra, A. (2017). Pembuatan dan karakterisasi katalis CaO/Zeolit alam. Jurnal Media Sains, 1(1), 12–18. [Google Scholar]
17. Putra, I. M. W. A. (2017). Production of biodiesel from waste cooking oil by transesterification reaction using CaO/Natural Zeolite catalysts. Cakra Kimia (Indonesian E-Journal of Applied Chemistry), 5(2), 51–57. [Google Scholar]
18. Fattahi, N., Triantafyllidis, K., Luque, R., Ramazani, A. (2019). Zeolite-based catalysts: A valuable approach toward ester bond formation. Catalysts, 9(9), 758. DOI 10.3390/catal9090758. [Google Scholar] [CrossRef]
19. Sari, T. I., Said, M., Summa, A., Sari, A. K. (2011). Katalis basa heterogen campuran CaO dan SrO pada reaksi tranesterifikasi minyak kelapa sawit. Prosiding Seminar Nasional AVoER Ke, vol. 3, pp. 26–27. Palembang, Indonesia. [Google Scholar]
20. Santoso, A., Hanindita, C. F. A., Sumari, S., Rachman, I. B. (2019). Synthesis of biodiesel from low-quality crude palm oil with heterogeneous catalyst Cao-ZnO. IOP Conference Series: Materials Science and Engineering, 515, 12082. DOI 10.1088/1757-899x/515/1/012082. [Google Scholar] [CrossRef]
21. Agoes, S., Suryandari, A. S., Soe’eib, S., Asri, N. P., (2019). Biodiesel production from kapok seed oil using bimetallic oxide MgO/CaO as catalyst in continuous fixed bed reactor. Proceedings of the 2nd International Conference on Quran and Hadith Studies Information Technology and Media, pp. 1–8, Bandung, Indonesia. DOI 10.4108/eai.2-10-2018.2295462. [Google Scholar] [CrossRef]
22. Santoso, A., Wijaya, A. R., Purwaningtyas, C. F., Sukarianingsih, D., Retnosari, R. et al. (2020). The effect of K2O concentration in K2O/Al2O3 catalyst on methyl ester (biodiesel) synthesis from CPO off grade with ultrasonic wave. IOP Conference Series: Materials Science and Engineering, 833, 012043. DOI 10.1088/1757-899X/833/1/012043. [Google Scholar] [CrossRef]
23. Cella, R., Stefani, H. A. (2018). Ultrasonic reactions. In: Green techniques for organic synthesis and medicinal chemistry. USA: John Wiley & Sons, Ltd. DOI 10.1002/9781119288152.ch14. [Google Scholar] [CrossRef]
24. Hakiki, M., Makiyi, M., Nuryoto, N., Rahmayetty, R., Kustiningsih, I. et al. (2021). Pengaruh lokasi zeolit alam bayah terhadap adsorpsi amonium: Studi kinetika dan kesetimbangan. Jurnal Teknologi Lingkungan, 22(1), 18–28. DOI 10.29122/jtl.v22i1.4403. [Google Scholar] [CrossRef]
25. Lestari, S., Sundaryono, A., Elvia, R. (2019). Preparasi dan karakterisasi katalis Mo-Ni/hz dengan metode impregnasi untuk cracking katalitik minyak limbah cair pengolahan kelapa sawit menjadi bahan bakar nabati. Alotrop: Jurnal Pendidikan Dan Ilmu Kimia, 3(1), 91–97. DOI 10.33369/atp.v3i1.9047. [Google Scholar] [CrossRef]
26. Trisunaryanti, W., Triyono, T., Armunanto, R., Hastuti, L. P., Ristiana, D. D. et al. (2018). Hydrocracking of α-cellulose using Co, Ni, and Pd supported on mordenite catalysts. Indonesian Journal of Chemistry, 18(1), 166–172. DOI 10.22146/ijc.26491. [Google Scholar] [CrossRef]
27. Wu, H., Zhang, J., Wei, Q., Zheng, J., Zhang, J. (2013). Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts. Fuel Processing Technology, 109, 13–18. DOI 10.1016/j.fuproc.2012.09.032. [Google Scholar] [CrossRef]
28. Arni, S., Sumari, S., Santoso, A., Tamara, T. (2020). The effect of aging and crystallization time on the synthesis and characteristics of zeolite-Y from malang-quartzite silica. IOP Conference Series: Materials Science and Engineering, 833(1), 012060. DOI 10.1088/1757-899X/833/1/012060. [Google Scholar] [CrossRef]
29. Amri, S., Utomo, M. P. (2017). Preparasi dan karakterisasi komposit ZnO-zeolit untuk fotodegradasi zat warna Congo red. Jurnal Kimia Dasar, 6(2), 29–36. [Google Scholar]
30. Luz-Martínez, S., Romero, R., López, J. C., Romero, A., Sánchez-Mendieta, V. et al. (2011). Preparation and characterization of CaO nanoparticles/NaX zeolite zatalysts for the transesterification of sunflower oil. Industrial and Engineering Chemistry Research, 50(5), 2665–2670. DOI 10.1021/ie1006867. [Google Scholar] [CrossRef]
31. Putri, S. K., Suprapto, S. R. (2013). Studi proses pembuatan biodiesel dari minyak kelapa (coconut oil) dengan bantuan gelombang ultrasonik. Jurnal Rekayasa Proses, 6(1), 20–25. DOI 10.22146/jrekpros.2453. [Google Scholar] [CrossRef]
32. Patil, A. D., Baral, S. S. (2021). Process intensification of thumba methyl ester (biodiesel) production using hydrodynamic cavitation. Chemical Engineering Research and Design, 171, 277–292. DOI 10.1016/j.cherd.2021.05.007. [Google Scholar] [CrossRef]
33. Aghbashlo, M., Peng, W., Tabatabaei, M., Kalogirou, S. A., Soltanian, S. et al. (2021). Machine learning technology in biodiesel research: A review. Progress in Energy and Combustion Science, 85, 100904. DOI 10.1016/j.pecs.2021.100904. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |