

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.020691
ARTICLE
Catalytic Pyrolysis of Soybean Oil with CaO/Bio-Char Based Catalyst to Produce High Quality Biofuel
Biomass Group, College of Engineering, Nanjing Agricultural University, Nanjing, 210031, China
*Corresponding Author: Zhen Fang. Email: zhenfang@njau.edu.cn
Received: 07 December 2021; Accepted: 25 February 2022
Abstract: In this paper, CaO/bio-char was synthesized by directly co-pyrolysis of Ca(OH)2 and rice straw, and used as catalyst to catalytic pyrolysis of soybean oil to produce high quality biofuel. In this co-pyrolysis process, CaO particles has been successfully embedded on the bio-char surface. During the catalytic pyrolysis process, CaO/bio-char showed a good catalytic performance on the deoxygenation of soybean oil. Pyrolysis temperature affected the pyrolysis reactions and pyrolytic products distributions dramatically, higher pyrolysis temperature lead to seriously cracking reactions, lower bio-oil yield and higher gases yield, and lower pyrolysis temperature lead to higher bio-oil yield with higher oxygenated compounds content and lower hydrocarbons contents, the suitable pyrolysis temperature was around 650°C. Under the optimal conditions (650°C with WHSV at 6.4 h−1 and carrier gas flow rate at 100 ml/min), the selectivity (%) of hydrocarbons in the bio-oil was more than 90%. CaO/bio-char catalyst still shows good catalytic deoxygenation activity after 4 cycles. 1 g of CaO/bio-char catalyst can catalyze pyrolysis of 32 g of soybean oil to produce high-quality liquid fuel. Bio-char based catalyst has been proved to be a promising catalyst for catalytic conversion of triglyceride-based lipids into high quality liquid biofuel.
Keywords: Carbonization; CaO/bio-char; catalytic pyrolysis; deoxygenation; high quality biofuel
Abbreviation
| A&E | Acid and esters |
| AL | Alcohols |
| E | Ethers |
| HC | Hydrocarbons |
| (K) | Ketones and aldehydes |
| N | N-contained chemicals |
| WHSV | Weight hourly space velocity |
| SEM | Scanning electron microscope |
| EDS | Energy dispersive spectrometer |
| GC-MS | Gas chromatography-mass spectrometer |
With the depletion of fossil resources and more serious environment pollution, the search for renewable fuels has become urgent [1,2]. Lipids are common renewable resources that can be obtained from plants and animals, about 4 to 8 million tons of non-edible lipids were produced in China per year [3,4]. The main component of lipids is triglyceride. The advantages of lipids (e.g., clean, renewable, availability, biodegradability and CO2 neutrality) show great potential for producing bio-fuel [5,6].
Pyrolysis is a technology that thermal decomposition of biomass under inert atmosphere to produce gas, liquid and solid products, which has great potential for the sustainable production of renewable biofuels, carbon dioxide emission reduction and carbon neutrality [7–10]. Pyrolysis technology has been widely used in biofuel production due to its advantages of low cost, high compatibility with existing refinery facilities and fuel standards [11,12]. However, pyrolytic bio-oil still has disadvantages of high oxygen content, high acid value, poor stability, difficulty in storage at low temperature and low calorific value, which limits its wide utilization [13,14]. To solve the above problems, many researchers put forward catalytic pyrolysis technology, which can improve the quality of pyrolytic bio-oil to meet the requirements of fuel standards through introducing catalysts in the pyrolysis process [15,16].
The quality and yield of pyrolytic bio-oil are closely related to the type and amount of catalyst, suitable catalyst is vital for the catalytic pyrolysis process. Many kinds of catalysts (e.g., metal salts, metal oxides, micropore and mesoporous material, etc.) had been used to catalytic upgrading of lipids into high quality bio-fuel. Abdelfattah et al. used different metal salts (NaOH, KOH, and Na2CO3) as catalysts in the slow pyrolysis process of raw castor oil to produce biofuel, and found the above metal salts showed good catalytic deoxygenation performance [17]. Wako et al. [18] used ZrO2 to catalytic cracking of waste cooking oil to produce hydrocarbon-rich bio-fuel and the higher calorific value (HCV) of bio-fuel reached 40.8 MJ/kg. They also found the higher HCV were corresponding to the cracking reactions and active sites of ZrO2 catalyst. Wang et al. [19] also used CaO to catalytic pyrolysis of waste clay oil to produce high quality bio-fuel. de Morais Araújo et al. [20] used AlMCM-41(Si/Al = 50) as catalyst to catalytic pyrolysis of sunflower oil to produce biofuel, and found the acid sites of AlMCM-41 were essential to the deoxygenation of bio-oil.
Bio-char is a kind of stable carbon-rich product from biomass (e.g., crop straw, wood, etc.) by pyrolysis at high temperature under inert atmosphere, and has many advantages of rich stable carbon, rich microporous structure, rich functional groups, and rich nutrient elements [21–23]. Bio-char is not only widely used as biological fertilizer, adsorption material, supercapacitor material, also used as catalyst and catalyst support for catalytic biomass conversion [24,25]. Suganuma et al. [26] reported that bio-char based solid acid materials could catalytic hydrolysis of biomass and showed good performance. Kastner et al. [27] used bio-char bearing SO3H groups as catalyst to catalytic esterification of free fatty acids into green biodiesel. Ye et al. [28] used activated carbon-based solid acid catalyst (ACSA) to catalytic pyrolysis of cellulose and biomass to selectively produce levoglucosenone, and found that the ACSA catalyst showed the same catalytic performance as other solid catalysts (e.g., SO42−/ZrO2). Hu et al. [29] synthesized char supported Ni catalyst to catalytic cracking of biomass to produce aromatic-rich bio-oil. Wang et al. [30] also used Fe/char as catalyst to catalytic steam reforming of bio-oil and showed good catalytic performance. Cao et al. [31] synthesized Seaweed-derived bio-char and used it as heterogeneous catalyst for effectively converting macroalgae into acid-free bio-oil via catalytic pyrolysis process.
Herein, CaO/bio-char synthesized by directly co-pyrolysis of Ca(OH)2 and rice straw, was used to catalytic pyrolysis of soybean oil to produce high quality biofuel. The catalyst was characterized by scanning electron microscope coupling with-energy dispersive spectrometer and mapping (SEM-EDS-mapping) and N2-adsorption/desorption. Besides, the pyrolysis parameters, such as pyrolytic temperature, weight hourly space velocity (WHSV), and contacting time between pyrolysis vapors and catalysts, were investigated systematically. The main compositions of bio-oil were analyzed by gas chromatography-mass spectrometer (GC-MS). The stability of CaO/bio-char catalyst was also investigated.
The rice straw was obtained from Changzhou (Jiangsu Province). Before the carbonization process, rice straw should be crushed into 40–60 mesh pieces and dried at 60°C until constant weight. The soybean oil (Golden dragon fish) was obtained from National Golden Dragon Fish Co., Ltd., Shanghai. Methanol (≥99.5%), Ca(OH)2 (AR) and quartz sand (∼40 meshes) were purchased from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). Air, N2 (99.999%), Ar (99.999%), He (99.999%) were purchased from Nanjing Special Gases Factory (Nanjing, Jiangsu Province, China). The standard gas for gas calibration was purchased from Dalian Special Gases Co., Ltd. (Dalian, China). All the raw materials, chemicals and gases were used without further purification.
In this study, the CaO/bio-char catalyst was prepared via in situ co-pyrolysis method. Before the pyrolysis process, 0.5 g of Ca(OH)2 and 9.5 g of rice straw were added into a 100 g of water in round-bottom flask and stirred at room temperature for 2 h. Then, the mixture was and dried at 105°C to remove the water. After drying, the powder was grounded to 60 meshes. The powder was added into a quartz tube heated by a furnace slowly at room temperature under N2 atmosphere. After feeding, the temperature was raised to 700°C at a heating rate of 5 °C/min. The temperature was then kept at 700°C for 2 h before cooling to room temperature. The catalyst was stored in a glovebox before use.
2.3 Catalytic Pyrolysis Experiments and Product Analysis
The procedures of experiment and products analysis in this study were similar to those we reported before [32,33]. The experiment of catalytic pyrolysis of soybean oil over CaO/bio-char was carried out in a quartz tube reactor heated by an electric furnace. Due to the small particle size of CaO/bio-char catalyst, the reactor tube was easily blocked. Herein, the quartz sand was used as the catalyst bed dispersant in this study. In each run, 0.3 g of CaO/bio-char catalyst and 2 g of quartz sand (∼40 meshes) were mixed uniformly and fixed in the quartz tube, then was heated to a desired temperature, and soybean oil was subsequently fed batch by a peristaltic pump at a certain rate and purged with carrier gas. The volatile oil products were trapped in a cold trap by liquid nitrogen and analyzed by GC-MS (Agilent 7890-5977B, Santa Clara, CA) equipped with an HP-5 MS capillary column (30 m × 0.25 mm × 0.25 mm), the non-condensable gas products were collected with a gas bag and analyzed by GC (GC-2014C, Shimadzu, Kyoto).
The yields of bio-char, bio-oil and gases were calculated based on Eqs. (1)–(3), respectively.
2.4 Catalyst Characterizations
The surface morphology, energy dispersive spectrometer (EDS) and mapping of CaO/bio-char were investigated by scanning electron microscopy (SEM) (Nova NanoSEM NPE 218, FEI, USA). The surface morphology of CaO/bio-char was investigated by SEM on an S4800 instrument (Hitachi, Tokyo). The nitrogen adsorption/desorption isotherms of the catalyst was also measured by Autosorb-iQ (Quantachrome, Boynton Beach, FL). The surface area and total volume were determined by the Barrett-Emmet-Taller (BET) method and Barrett-Joyner-Halenda (BJH) method, respectively.
3.1 Characterizations of CaO/Bio-Char Catalyst
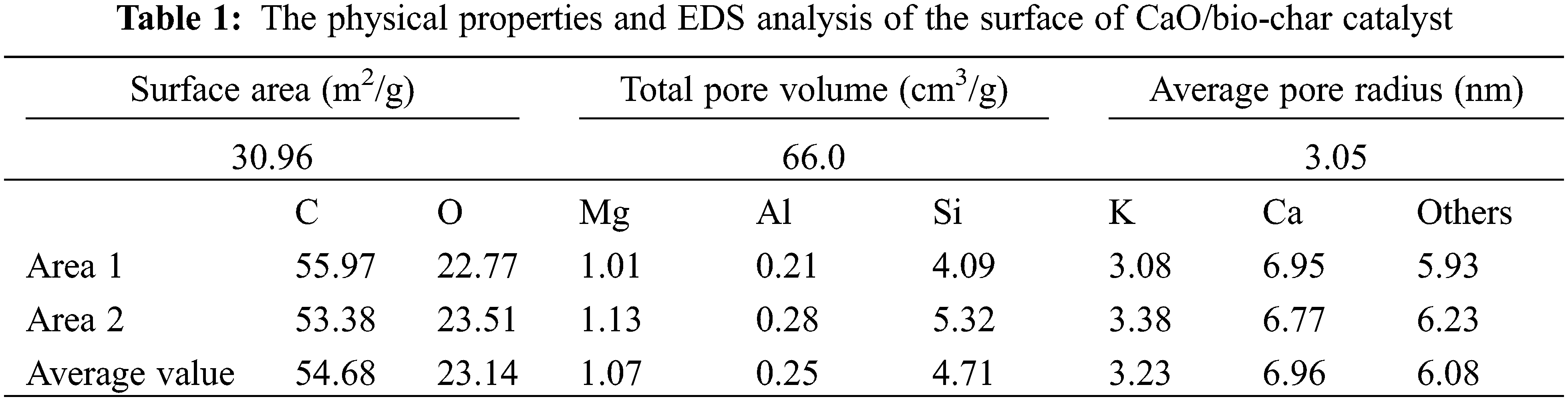
The prepared CaO/bio-char catalyst was characterized with SEM-EDS-mapping and N2-adsorption/desorption. From Fig. 1, CaO/bio-char has rough surface, irregular structure and pore structure. Besides, some tiny particles are dispersed uniformly on the surface, which may be the Ca-based particles formed during the carbonization process and the elemental mapping of CaO/bio-char catalyst in Fig. 2 also indicates that CaO particles has been successfully embedded on the bio-char. In addition, the surface elemental contents are also quantified through the EDS analysis and shown in Table 1. The contents of C, O and Ca were 54.68%, 23.14% and 6.96%, respectively. Meanwhile, small amounts of Mg, Al, Si and K are also analyzed, the contents of them are 1.07%, 0.21%, 4.09% and 3.08%, respectively. The porosity properties of CaO/bio-char are also shown in Table 1, the BET surface area and pore volume of CaO/bio-char are about 30.96 m2/g and 66.0 cm3/g, respectively.
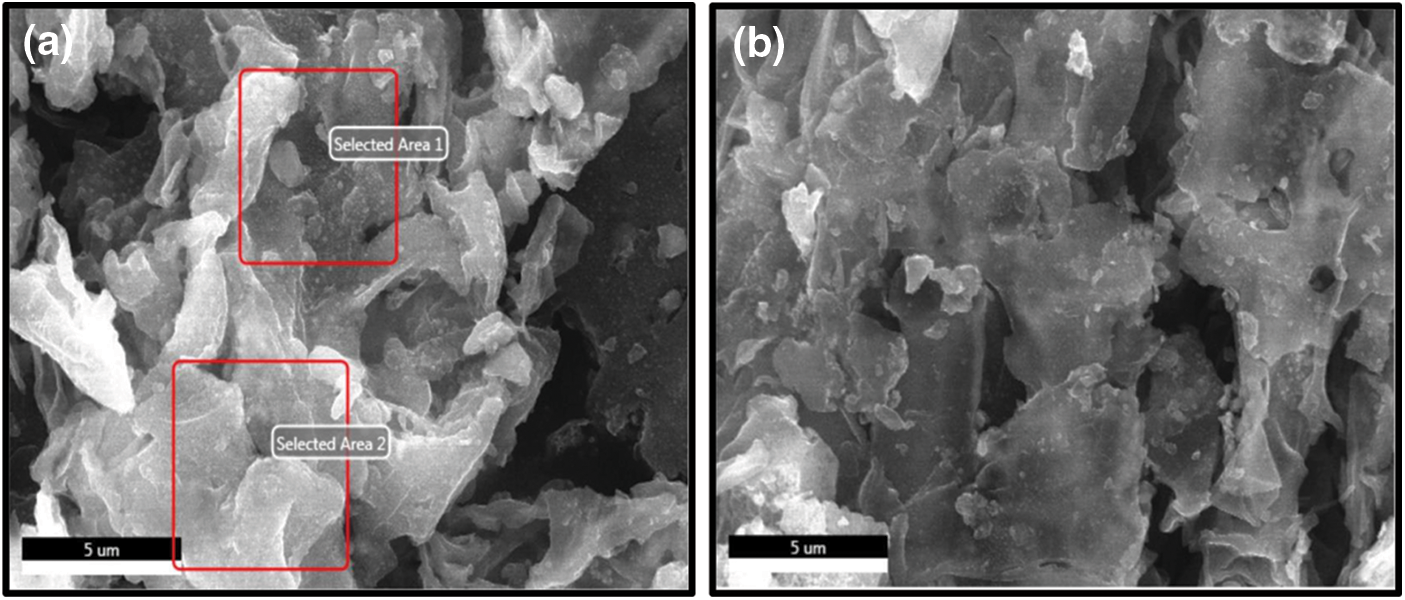
Figure 1: The SEM-EDS (a), SEM (b) of CaO/bio-char catalyst
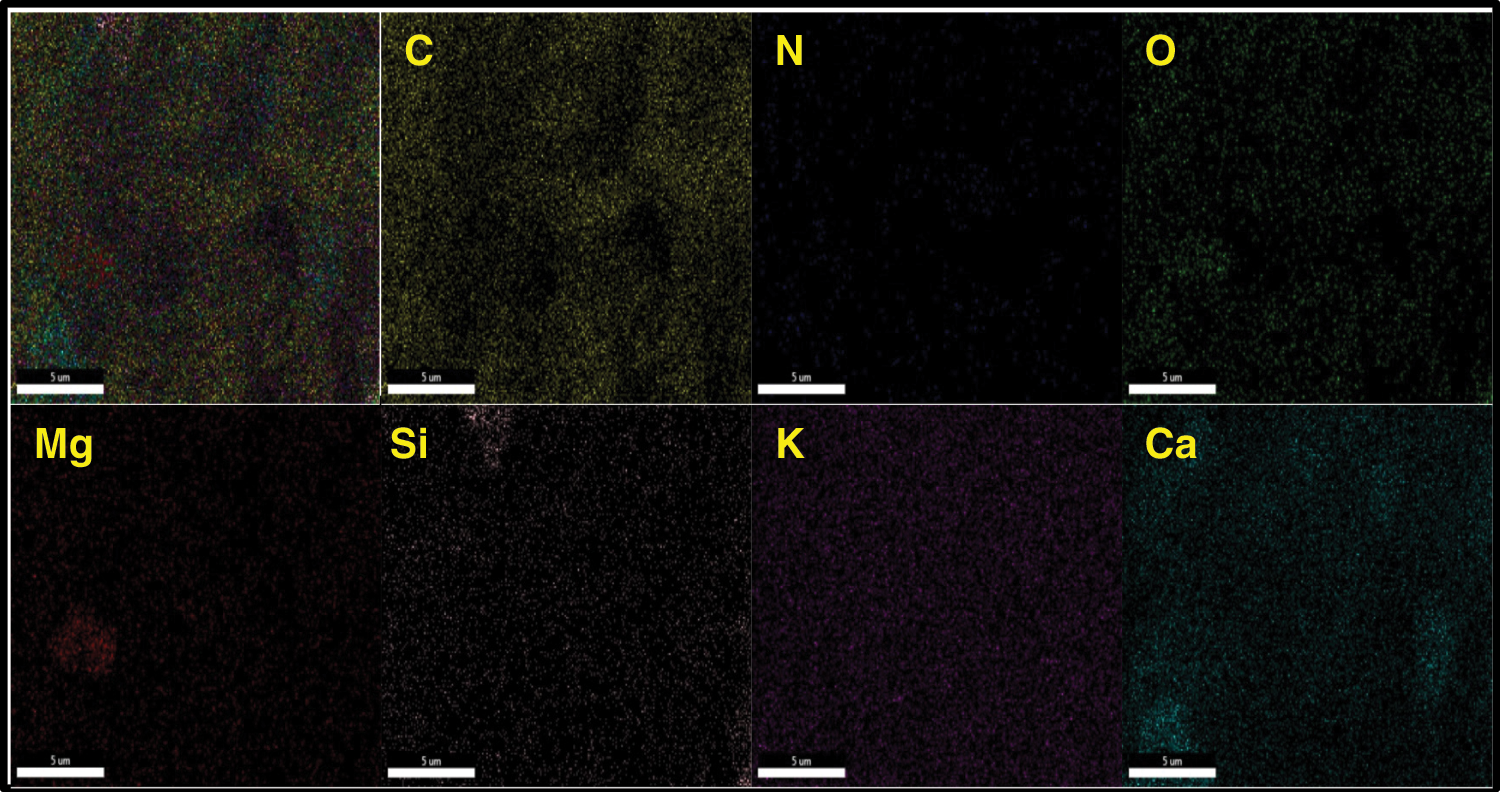
Figure 2: The SEM-mapping of CaO/bio-char catalyst
3.2 The Catalytic Performance of CaO/Bio-Char Catalyst
To investigate the catalytic performance of CaO/bio-char catalyst, the experiments of direct pyrolysis of soybean oil, direct pyrolysis of soybean oil over quartz sand and catalytic pyrolysis of soybean oil with CaO/bio-char were carried out firstly. Fig. 3 shows (a) GC-MS spectra of bio-oils, (b) overall yields, (c) chemical compositions of bio-oils, and (d) chemical compositions of gases of different pyrolysis experiments. Quartz sand, similar to the quartz wool, is usually used as the catalyst dispersant to ensure continuous reaction [34,35]. Direct pyrolysis of soybean oil and direct pyrolysis of soybean oil over quartz sand were carried out firstly to ensure the effect of quartz sand on the soybean oil pyrolysis behavior. Fig. 3a shows that the GC-MS spectra of direct pyrolysis of soybean oil and direct pyrolysis of soybean oil over quartz sand were similar. The detailed compositions of detected compounds and their relative peak area (%) in the bio-oil under different pyrolysis conditions are shown in Tables S1–S3 in the supplementary information. Furthermore, the overall yields (Fig. 3b) and peak areas (%) of different chemical classes in the bio-oils (Fig. 3c) of pyrolysis over quartz sand were also similar to those of direct pyrolysis of soybean oil. The above results indicated that the catalytic effect of quartz sand was little on the soybean oil pyrolysis behavior.
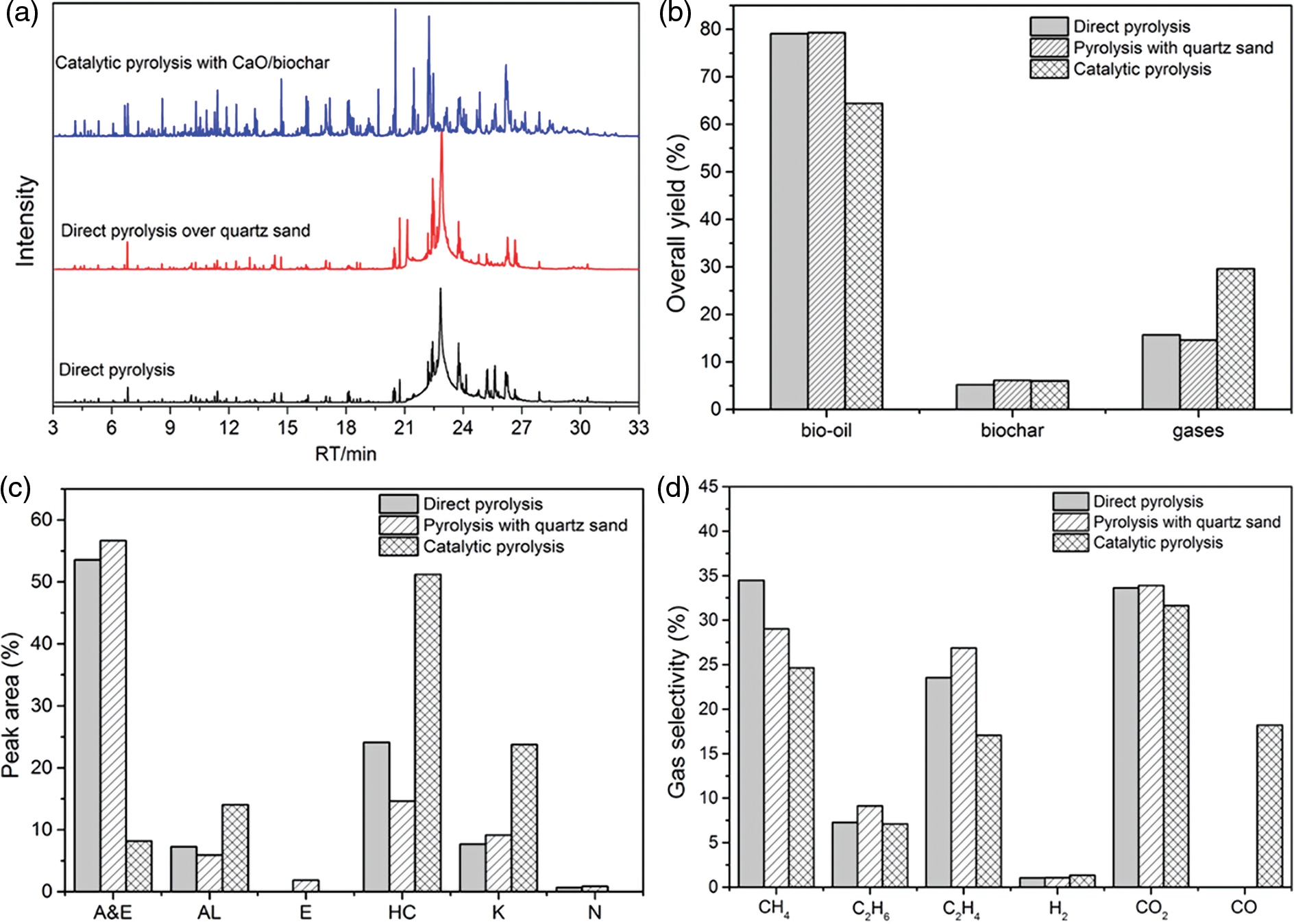
Figure 3: Yields and product distributions of direct pyrolysis and catalytic pyrolysis (a) GC-MS spectra of bio-oils, (b) overall yields, (c) chemical compositions of bio-oils, and (d) chemical compositions of gases
Fig. 3a also shows that typical GC-MS spectra of bio-oil obtained via catalytic pyrolysis with CaO/bio-char was different to that of direct pyrolysis, many compounds with short retention time appeared in the GC-MS spectra of catalytic bio-oil, which indicated that CaO/bio-char had great influence on the soybean oil pyrolysis behavior. Furthermore, as shown in Fig. 3b, lower yield of bio-oil and higher yield of gas products were obtained during the catalytic pyrolysis process. The bio-oil yield decreased from 79.1% to 64.4%, and the gases yield increased from 15.7% to 29.5%. The above results indicated that CaO/bio-char could promote the soybean oil cracking to form more gas compounds with small molecular. Fig. 3c shows the relative peak area (%) of different chemical classes of bio-oils. As shown in Fig. 3c, the main organic components of bio-oils could be classified into six groups, i.e., acid and esters (A&E), alcohols (AL), ethers (E), hydrocarbons (HC), ketones and aldehydes (K), and N-contained chemicals (N) based on the chemical structures of main components. The relative peak areas (%) of different chemical classes of catalytic bio-oil were also different from those of direct pyrolytic bio-oil, especially for aid and esters, alcohols, hydrocarbons, ketones and aldehydes, and N-contained chemicals. Higher relative peak areas (%) of hydrocarbons, alcohols and ketones and aldehydes, and lower relative peak areas (%) of acids & esters were detecting in the catalytic bio-oil. The peak area (%) of alcohols, hydrocarbons and ketones increased from 7.26%, 24.09% and 7.68% to 14.05%, 51.20% and 23.75%, respectively. Meanwhile, the acid and ester decreased from 53.55% to 8.19%. The above results indicated that indicated that CaO/bio-char could also effectively promote the transformation of acid esters into hydrocarbons, alcohols, ketones and aldehydes via during the pyrolysis process, in addition to the promoting cracking reaction. Alcohols were probably generated from ketenes via hydrogenation process [36,37]. The catalytic performance could be similar to the metal oxides based catalyst, such as NiAl-LDO/MCM-4, Fe3O4 nanoparticles [38–40]. Fig. 3d shows that a bit higher H2 selectivity could be obtained via the catalytic pyrolysis process, which could indicate the higher relative peak area (%) of alcohols detected in the catalytic bio-oil. Ketones were mainly generated from acids and esters via alkali-catalyzed decarboxylation into ketones reactions [41–43]. CaO/bio-char is a kind of alkali catalyst, which could promote the reaction of decarboxylation to ketone. The hydrocarbons were mainly generated from the acids and esters via the catalytic decarboxylation and decarboxylation reactions. In addition, higher selectivity of CO was detected in the catalytic gases products, and the selectivity of CO2 and CO in the catalytic gases reached 49.85%, which indicated that CaO/bio-char is an effective deoxidation catalyst. Therefore, the above results showed that CaO/bio-char showed good potential to catalyze deoxygenation of soybean bio-oil to produce high quality bio-fuel via pyrolysis process.
3.3 Effect of Pyrolysis Conditions on the High Quality Bio-Oil Production
The pyrolysis process is a process in which substances decompose when heated. Therefore, pyrolysis temperature is an extremely important factor in the pyrolysis process, which can affect the pyrolysis reactions and pyrolytic products distributions dramatically. Herein, different pyrolysis temperatures in the range of 500°C to 700°C increases by 50°C were selected to investigate their influence on the overall yields and chemical compositions of bio-oils by fixing the catalyst dosage (0.3 g of CaO/bio-char catalyst mixed with 2 g of quartz sand), WHSV (5.3 h−1) and N2 flow rate (100 ml/min) (Fig. 4). Fig. 4a showed that pyrolysis temperature had a great influence on the pyrolytic product distributions. With the pyrolysis temperature increasing from 500°C to 700°C, the bio-oil yield decreased from 65.5% to 37.8%. Compared to the effect on the bio-oil yield, the pyrolysis temperature had a opposite effect on the yield of gas products, the gases yield increased from 28.8% to 59.4% with the pyrolysis temperature increasing from 500°C to 700°C. Higher pyrolysis temperature would lead to more seriously cracking reactions to form small molecular gas products, rather than to form the liquid products.
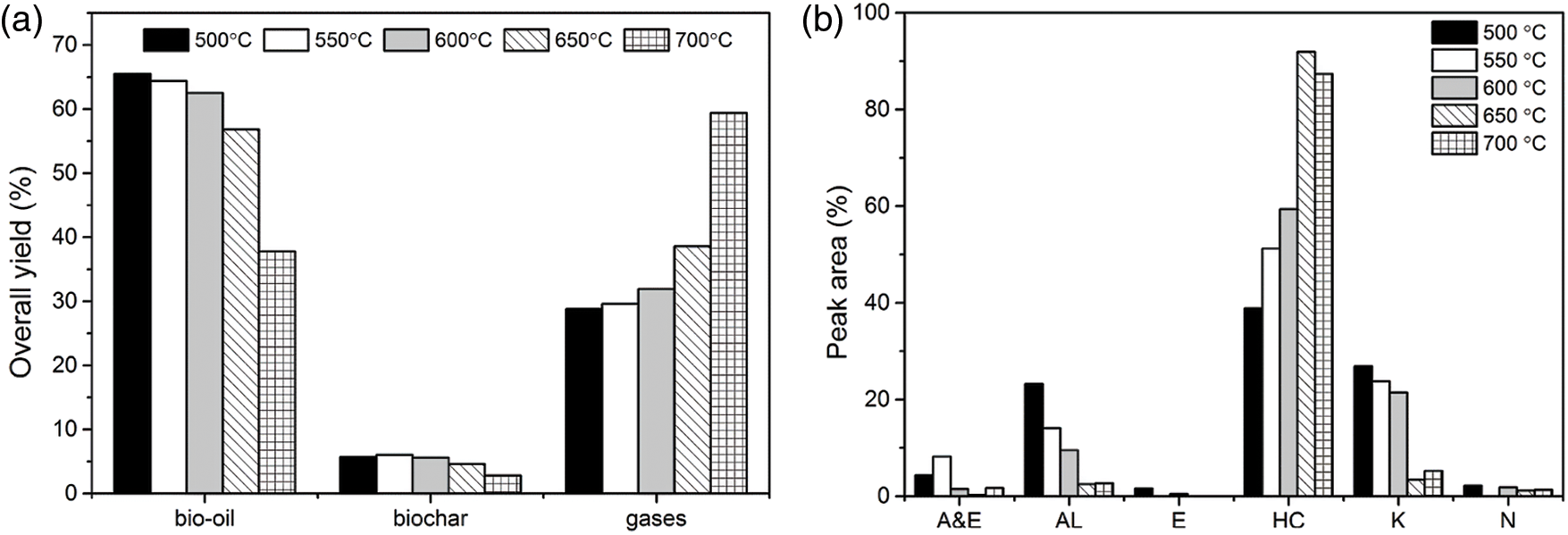
Figure 4: Effect of pyrolysis temperature on yields and products of catalytic pyrolysis of soybean oil (a) overall yields of products, (b) chemical compositions of bio-oils
Moreover, the effect of pyrolysis temperature in the range of 500°C to 700°C on the distribution of liquid products were also analyzed by GC/MS and classified into six groups in Fig. 4b. When the pyrolysis temperature was at 500°C, the selectivity of A&E was less than 10%, it indicated that CaO/bio-char showed good catalytic performance on the decarboxylation of triglyceride during the pyrolysis process. While, the selectivity of alcohols and ketones was around 50%, it indicated that the deoxygenation efficiency was not high at lower temperature. Increasing pyrolysis temperature could increase the deoxygenation efficiency, more hydrocarbons were produced. When the pyrolysis temperature was at 650°C, the selectivity of hydrocarbons in the liquid products was more than 90%. A&E and alcohols are almost undetectable in the liquid products. Therefore, the deoxygenation effect of CaO/bio-char catalyst was also affected by pyrolysis temperature greatly, higher pyrolysis temperature promoted the deoxygenation reaction during the catalytic pyrolysis. Therefore, the optimal pyrolysis temperature for producing high quality bio-fuel was determined at 650°C through comprehensively consider of the bio-oil properties, yield.
Besides the pyrolysis temperature, the effect of WHSV (mass of soybean oil feeding rate/CaO/bio-char dosage) was investigated at 650°C with 0.3 g of CaO/bio-char catalyst (mixed with 2 g of quartz sand) by changing the soybean oil feeding rate from 1.6 to 3.75 g/h (WHSV from 3.2 to 7.5 h−1). Fig. 5 shows the effect of WHSV on (a) product distributions, (b) chemical compositions of bio-oils. It is shown in Fig. 5a that increasing the WHSV could increase the yield of bio-oil and decrease the yield of gases. The effect of WHSV on the bio-char production was not obviously, and the yield of bio-char was around 5% in the range of 3.2 to 7.5 h−1. When WHSV increased to 7.5 h−1, the bio-oil yield was up to 60.8%, which was much higher as that of 3.2 h−1 (41.5%). In addition, the yield of gases also decreased from 54.3% to 35.5%. The above results indicated that increasing soybean oil feeding rate could inhibit the seriously cracking reactions during the pyrolysis process.
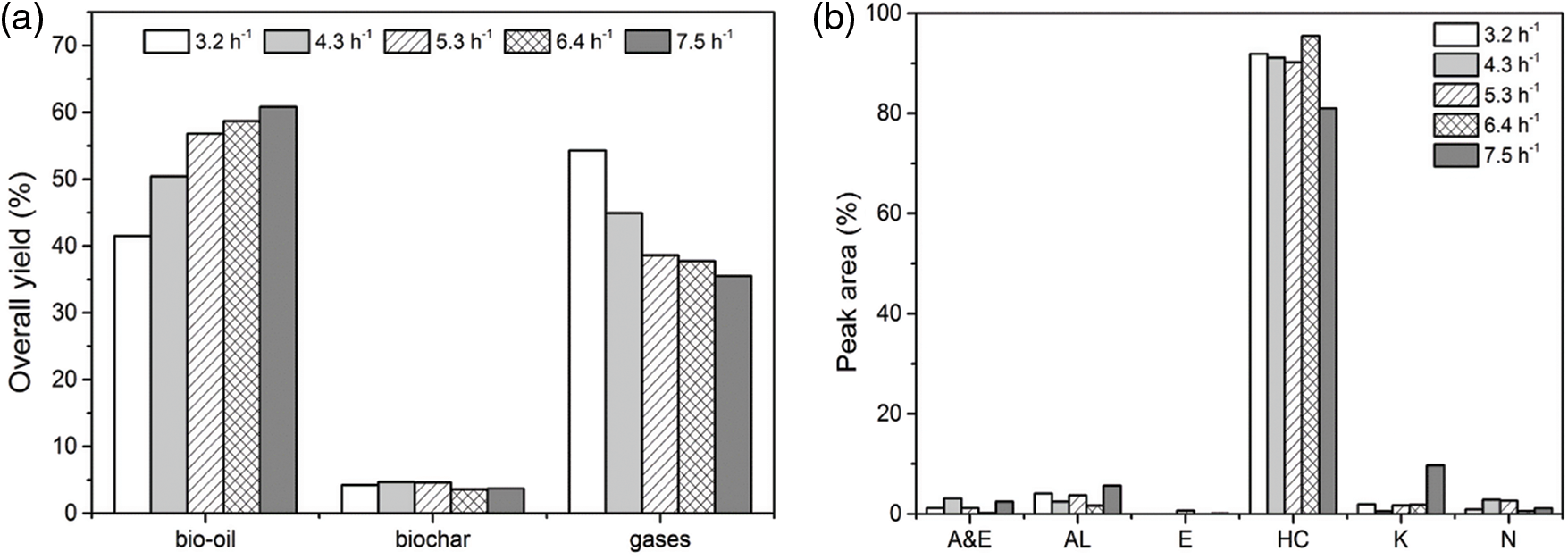
Figure 5: Effect of WHSV on yields and products of catalytic pyrolysis of soybean oil (a) overall yields of products, (b) chemical compositions of bio-oils
In addition, effect of WHSV on the quality of pyrolytic bio-oils was also analyzed and shown in Fig. 4b. From Fig. 5b, it shows that WHSV had an effect on the chemical compositions. When the WHSV was kept in the range of 3.2 to 6.4 h−1, WHSV had a slight influence on the bio-oil compositions, and CaO/bio-char could keep a good catalytic performance on the deoxygenation reactions. The selectivity of Hydrocarbons was around 90%. While when the WHSV increased to with increasing to 7.5 h−1, the selectivity of HCs decreased to 81% and selectivity of Alcohols and Ketones had a slight increasing. Therefore, the optimal WHSV for this catalytic process could be at 6.4 h−1.
3.3.3 Effect of Carrier Gas Flow Rate
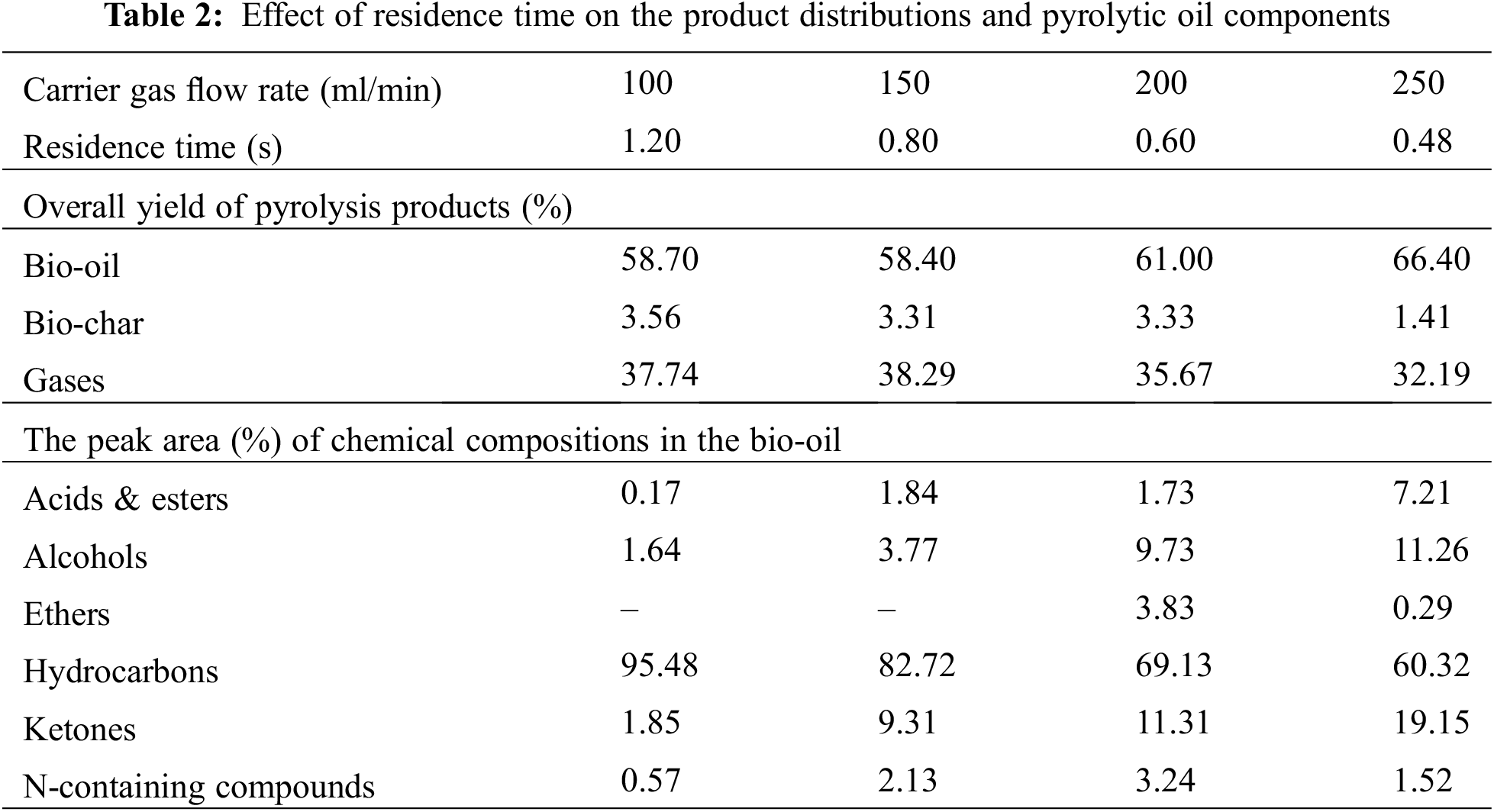
The contacting between the pyrolytic vapor and the catalyst also influenced the product distributions during the catalytic pyrolysis process. Herein, the effect of contacting time between the pyrolytic soybean oil vapors and CaO/bio-char catalyst was investigated by changing the carrier gas flow rate (from 100 to 250 ml/min of N2). Lower carrier gas flow rate could cause higher contacting time between pyrolysis vapors and catalyst. Table 2 shows the effect of carrier gas flow rate on the product distributions and chemical compositions of liquid products. Higher carrier gas and short residence time could cause higher bio-oil yield. When the carrier gas flow rate was at 250 ml/min (contacting time was about 0.48 s), the bio-oil yield increased to 66.4%, which was higher than that at 100 ml/min (58.3%). Moreover, the yields of bio-char and gases also decreased slightly with the carrier gas flow rate increasing and contacting time decreasing. However, the oil components also changed dramatically with the contacting time changing. From Table 2, it can be seen that the selectivity of hydrocarbons decreased obviously and the selectivity of oxygenated compounds (including Acid & esters, alcohols and ketones) increased a lot. Above results indicated that higher carrier gas flow rate would lead to too short contacting time between pyrolysis vapors and CaO/bio-char catalyst, resulting in the worse deoxygenation efficiency and high content of oxygenated compounds in the liquid products. Therefore, the optimal condition for producing high quality bio-fuel from soybean oil via catalytic pyrolysis with CaO/bio-char was at 650°C with carrier gas flow rate at 100 ml/min.
The stability of the CaO/bio-char catalyst was also investigated via conducting pyrolysis experiments at optimal conditions for 4 cycles. For each cycle, the used catalyst was calcined at 700°C for 2 h under N2 atmosphere to stabilize the amorphous carbon on the surface area. Fig. 6 shows the overall yields of bio-oil, gases, bio-char and the detailed chemical compositions of bio-oils of each catalyst recycle. From Fig. 6, the yield of bio-oil increased with 4 cycles, while the yield of gas products decreased. The bio-char yield kept stable. For the chemical compositions, the selectivity of hydrocarbons just show a slight decrease with the cycle number increasing, was still around 90%. The above results indicated that the catalytic deoxygenation activity of CaO/bio-char catalyst had a good stability after 4 cycles, it indicated that 1 g of CaO/bio-char catalyst can catalyze pyrolysis of 32 g of soybean oil to produce high-quality liquid fuel.
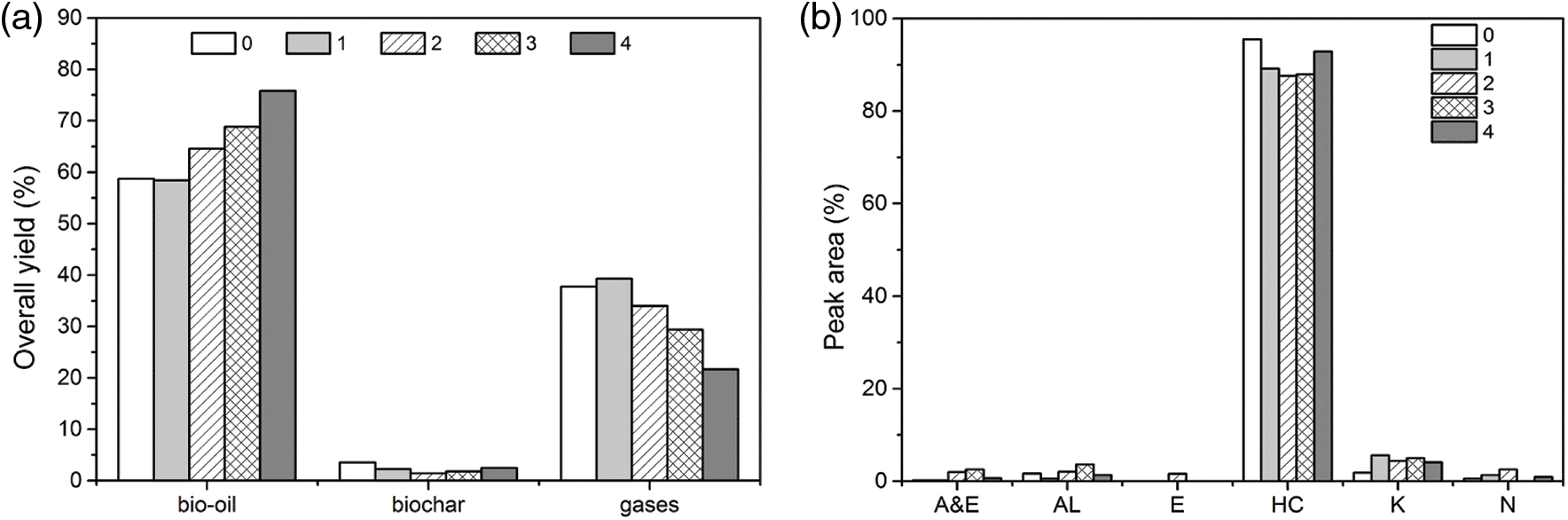
Figure 6: Catalyst cycles (a) Overall yield and (b) Chemical compositions of bio-oils
Herein, CaO/bio-char was synthesized by directly co-pyrolysis of Ca(OH)2 and rice straw at 700°C and kept 2 h, and which was used as catalyst to catalytic pyrolysis of soybean oil to produce high quality biofuel and showed good catalytic performance on the deoxygenation of soybean oil into hydrocarbon-based high quality biofuel. Pyrolysis temperature affected the pyrolysis reactions and pyrolytic products distributions dramatically, higher pyrolysis temperature lead to seriously cracking reactions, lower bio-oil yield and higher gases yield, and lower pyrolysis temperature lead to higher bio-oil yield with higher oxygenated compounds content and lower hydrocarbons contents, the suitable pyrolysis temperature was around 650°C. For WHSV, increasing the WHSV could increase the yield of bio-oil and had a slight influence on the quality of bio-oil, the suitable WHSV was around 6.4 h−1. Carrier gas flow rate determines the contacting time between the pyrolytic soybean oil vapors and CaO/bio-char catalyst, higher carrier gas flow rate would lead to short contacting time and resulting in the worse deoxygenation efficiency and high content of oxygenated compounds, the suitable carrier gas flow rate was at about 100 ml/min and contacting time between pyrolysis vapors with catalyst was about 1.2 s. Therefore, the optimal conditions were 650°C with WHSV at 6.4 h−1 and carrier gas flow rate at 100 ml/min. Meanwhile, the selectivity (%) of hydrocarbons in the bio-oil was more than 90%. For catalyst stability tests, CaO/bio-char catalyst showed good catalytic deoxygenation activity after 4 cycles. 1 g of CaO/bio-char catalyst can catalyze pyrolysis of 32 g of soybean oil to produce high-quality liquid fuel. Bio-char based catalyst has been proved to be a promising catalyst for catalytic conversion of triglyceride-based lipids into high quality liquid biofuel.
Acknowledgement: The authors are grateful to the financial supports from the Natural Science Foundation of China, Natural Science Foundation of Jiangsu Province, China Postdoctoral Science Foundation, and “Innovation & Entrepreneurship Talents” Introduction Plan of Jiangsu Province.
Funding Statement: The paper was supported by the Natural Science Foundation of China (No. 51906112), Natural Science Foundation of Jiangsu Province (No. BK20180548), China Postdoctoral Science Foundation (2019M651852), and “Innovation & Entrepreneurship Talents” Introduction Plan of Jiangsu Province.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, S., Dai, G., Yang, H., Luo, Z. (2017). Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Progress in Energy & Combustion Science, 62, 33–86. DOI 10.1016/j.pecs.2017.05.004. [Google Scholar] [CrossRef]
2. Xu, Y., Li, G., Sun, Z. (2016). Development of biodiesel industry in China: Upon the terms of production and consumption. Renewable and Sustainable Energy Reviews, 54, 318–330. DOI 10.1016/j.rser.2015.10.035. [Google Scholar] [CrossRef]
3. Xu, J., Jiang, J., Zhao, J. (2016). Thermochemical conversion of triglycerides for production of drop-in liquid fuels. Renewable and Sustainable Energy Reviews, 58, 331–340. DOI 10.1016/j.rser.2015.12.315. [Google Scholar] [CrossRef]
4. Lam, S. S., Liew, R. K., Jusoh, A., Chong, C. T., Ani, F. N. et al. (2016). Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renewable and Sustainable Energy Reviews, 53, 741–753. DOI 10.1016/j.rser.2015.09.005. [Google Scholar] [CrossRef]
5. Viswanathan, K., Wang, S. (2021). Experimental investigation on the application of preheated fish oil ethyl ester as a fuel in diesel engine. Fuel, 285, 119244. DOI 10.1016/j.fuel.2020.119244. [Google Scholar] [CrossRef]
6. Smith, B., Greenwell, H. C., Whiting, A. (2009). Catalytic upgrading of tri-glycerides and fatty acids to transport biofuels. Energy & Environmental Science, 2(3), 262–271. DOI 10.1039/B814123A. [Google Scholar] [CrossRef]
7. Xu, L., Jiang, L., Zhang, H., Fang, Z., Smith, R. L. (2020). Introduction to pyrolysis as a thermo-chemical conversion technology. In: Production of biofuels and chemicals with pyrolysis, vol. 10, pp. 3–30. Singapore: Springer. [Google Scholar]
8. Hoang, A., Ong, H., Fattah, I. M. R., Chong, C., Cheng, C. et al. (2021). Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Processing Technology, 223, 106997. DOI 10.1016/j.fuproc.2021.106997. [Google Scholar] [CrossRef]
9. Hoang, A., Nizetic, S., Ong, H., Mofijur, M., Ahmed, S. et al. (2021). Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere, 281, 130878. DOI 10.1016/j.chemosphere.2021.130878. [Google Scholar] [CrossRef]
10. Hoang, A., Nguyen, T., Nguyen, H. (2020). Scrap tire pyrolysis as a potential strategy for waste management pathway: A review. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. DOI 10.1080/15567036.2020.1745336. [Google Scholar] [CrossRef]
11. Wang, S., Zhao, S., Cheng, X., Qian, L., Barati, B. et al. (2021). Study on two-step hydrothermal liquefaction of macroalgae for improving bio-oil. Bioresource Technology, 319, 124176. DOI 10.1016/j.biortech.2020.124176. [Google Scholar] [CrossRef]
12. Vassilev, S. V., Vassileva, C. G., Vassilev, V. S. (2015). Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel, 158, 330–350. DOI 10.1016/j.fuel.2015.05.050. [Google Scholar] [CrossRef]
13. Wei, M., Marrakchi, F., Yuan, C., Cheng, X., Jiang, D. et al. (2022). Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. Journal of Hazardous Materials, 425, 127887. DOI 10.1016/j.jhazmat.2021.127887. [Google Scholar] [CrossRef]
14. Vispute, T. P., Zhang, H., Sanna, A., Xiao, R., Huber, G. W. (2010). Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science, 330(6008), 1222–1227. DOI 10.1126/science.1194218. [Google Scholar] [CrossRef]
15. Carlson, T. R., Tompsett, G. A., Conner, W. C., Huber, G. W. (2009). Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks. Topics in Catalysis, 52(3), 241. DOI 10.1007/s11244-008-9160-6. [Google Scholar] [CrossRef]
16. Liu, C., Wang, H., Karim, A. M., Sun, J., Wang, Y. (2014). Catalytic fast pyrolysis of lignocellulosic biomass. Chemical Society Reviews, 43(22), 7594–7623. DOI 10.1039/C3CS60414D. [Google Scholar] [CrossRef]
17. Abdelfattah, M. S. H., Abu-Elyazeed, O. S. M., Abdelazeem, M. A. (2018). On biodiesels from castor raw oil using catalytic pyrolysis. Energy, 143, 950–960. DOI 10.1016/j.energy.2017.09.095. [Google Scholar] [CrossRef]
18. Wako, F. M., Reshad, A. S., Bhalerao, M. S., Goud, V. V. (2018). Catalytic cracking of waste cooking oil for biofuel production using zirconium oxide catalyst. Industrial Crops and Products, 118, 282–289. DOI 10.1016/j.indcrop.2018.03.057. [Google Scholar] [CrossRef]
19. Wang, S., Yuan, C., Esakkimuthu, S., Xu, L., Cao, B. et al. (2019). Catalytic pyrolysis of waste clay oil to produce high quality biofuel. Journal of Analytical and Applied Pyrolysis, 141, 104633. DOI 10.1016/j.jaap.2019.104633. [Google Scholar] [CrossRef]
20. de Morais Araújo, A. M., de Oliveira Lima, R., Gondim, A. D., Diniz, J., di Souza, L. et al. (2017). Thermal and catalytic pyrolysis of sunflower oil using AlMCM-41. Renewable Energy, 101, 900–906. DOI 10.1016/j.renene.2016.09.058. [Google Scholar] [CrossRef]
21. Sohi, S. P., Krull, E., Lopez-Capel, E., Bol, R. (2010). A review of biochar and its use and function in soil. Advances in Agronomy, 105, 47–82. DOI 10.1016/S0065-2113(10)05002-9. [Google Scholar] [CrossRef]
22. Wang, J., Wang, S. (2019). Preparation, modification and environmental application of biochar: A review. Journal of Cleaner Production, 227, 1002–1022. DOI 10.1016/j.jclepro.2019.04.282. [Google Scholar] [CrossRef]
23. Cha, J. S., Park, S. H., Jung, S. C., Ryu, C., Jeon, J. K. et al. (2016). Production and utilization of biochar: A review. Journal of Industrial and Engineering Chemistry, 40, 1–15. DOI 10.1016/j.jiec.2016.06.002. [Google Scholar] [CrossRef]
24. Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N. et al. (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, 19–33. DOI 10.1016/j.chemosphere.2013.10.071. [Google Scholar] [CrossRef]
25. Lee, J., Kim, K. H., Kwon, E. E. (2017). Biochar as a catalyst. Renewable and Sustainable Energy Reviews, 77, 70–79. DOI 10.1016/j.rser.2017.04.002. [Google Scholar] [CrossRef]
26. Suganuma, S., Nakajima, K., Kitano, M., Yamaguchi, D., Kato, H. et al. (2008). Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. Journal of the American Chemical Society, 130(38), 12787–12793. DOI 10.1021/ja803983h. [Google Scholar] [CrossRef]
27. Kastner, J. R., Miller, J., Geller, D. P., Locklin, J., Keith, L. H. et al. (2012). Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catalysis Today, 190(1), 122–132. DOI 10.1016/j.cattod.2012.02.006. [Google Scholar] [CrossRef]
28. Ye, X. N., Lu, Q., Wang, X., Guo, H. Q., Cui, M. S. et al. (2017). Catalytic fast pyrolysis of cellulose and biomass to selectively produce levoglucosenone using activated carbon catalyst. ACS Sustainable Chemistry & Engineering, 5(11), 10815–10825. DOI 10.1021/acssuschemeng.7b02762. [Google Scholar] [CrossRef]
29. Hu, M., Laghari, M., Cui, B., Xiao, B., Zhang, B. et al. (2018). Catalytic cracking of biomass tar over char supported nickel catalyst. Energy, 145, 228–237. DOI 10.1016/j.energy.2017.12.096. [Google Scholar] [CrossRef]
30. Wang, S., Shan, R., Lu, T., Zhang, Y., Yuan, H. et al. (2020). Pyrolysis char derived from waste peat for catalytic reforming of tar model compound. Applied Energy, 263, 114565. DOI 10.1016/j.apenergy.2020.114565. [Google Scholar] [CrossRef]
31. Cao, B., Yuan, J., Jiang, D., Wang, S., Barati, B. et al. (2021). Seaweed-derived biochar with multiple active sites as a heterogeneous catalyst for converting macroalgae into acid-free bio-oil containing abundant ester and sugar substances. Fuel, 285, 119164. DOI 10.1016/j.fuel.2020.119164. [Google Scholar] [CrossRef]
32. Xu, L., Chen, S., Song, H., Liu, Y., Shi, C. et al. (2020). Comprehensively utilization of spent bleaching clay for producing high quality bio-fuel via fast pyrolysis process. Energy, 190, 116371. DOI 10.1016/j.energy.2019.116371. [Google Scholar] [CrossRef]
33. Xu, L., Zhong, Q. Q., Dong, Q., Zhang, L. Y., Fang, Z. (2019). Co-production of phenolic oil and CaO/char deoxidation catalyst via catalytic fast pyrolysis of phenol-formaldehyde resin with Ca(OH)2. Journal of Analytical and Applied Pyrolysis, 142, 104663. DOI 10.1016/j.jaap.2019.104663. [Google Scholar] [CrossRef]
34. Zhang, T., Ai, H., Liu, Q. (2019). La2O3-promoted Ni/Al2O3 catalyst for CO methanation: Enhanced catalytic activity and stability. Energy Technology, 7(10), 1900531. DOI 10.1002/ente.201900531. [Google Scholar] [CrossRef]
35. Meng, J., Zhao, Z., Wang, X., Zheng, A., Zhang, D. et al. (2018). Comparative study on phenol and naphthalene steam reforming over Ni-Fe alloy catalysts supported on olivine synthesized by different methods. Energy Conversion and Management, 168, 60–73. DOI 10.1016/j.enconman.2018.04.112. [Google Scholar] [CrossRef]
36. Barati, B., Zeng, K., Baeyens, J., Wang, S., Addy, M. et al. (2021). Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass and Bioenergy, 145, 105927. DOI 10.1016/j.biombioe.2020.105927. [Google Scholar] [CrossRef]
37. Wang, S., Zhao, S., Uzoejinwa, B., Zheng, A., Wang, Q. et al. (2020). A State-of-the-art review on dual purpose seaweeds utilization for wastewater treatment and crude bio-oil production. Energy Conversion and Management, 222, 113253. DOI 10.1016/j.enconman.2020.113253. [Google Scholar] [CrossRef]
38. Wang, Z., Xiong, Y. (2020). Simultaneous improvement in qualities of bio-oil and syngas from catalytic pyrolysis of rice husk by demineralization. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. DOI 10.1080/15567036.2020.1824038. [Google Scholar] [CrossRef]
39. Imran Din, M., Sadaf, S., Hussain, Z., Khalid, R. (2020). Assembly of superparamagnetic iron oxide nanoparticles (Fe3O4-Nps) for catalytic pyrolysis of corn cob biomass. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. DOI 10.1080/15567036.2020.1767235. [Google Scholar] [CrossRef]
40. Xu, W., Gao, L., Yang, H., Xiao, G., Ding, K. et al. (2020). Catalytic pyrolysis of distilled lemon grass over Ni-Al based oxides supported on MCM-41. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. DOI 10.1080/15567036.2020.1814905. [Google Scholar] [CrossRef]
41. Lakshmikandan, M., Murugesan, A. G., Wang, S., Abomohra, A., Anjelin Jovita, P. et al. (2020). Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. Journal of Cleaner Production, 247, 119398. DOI 10.1016/j.jclepro.2019.119398. [Google Scholar] [CrossRef]
42. Wang, S., Zhao, S., Cheng, X., Qian, L., Barati, B. et al. (2021). Study on two-step hydrothermal liquefaction of macroalgae for improving bio-oil. Bioresource Technology, 319, 124176. DOI 10.1016/j.biortech.2020.124176. [Google Scholar] [CrossRef]
43. Xu, S., Cao, B., Uzoejinwa, B., Odey, E., Wang, S. et al. (2020). Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Safety and Environmental Protection, 137, 34–48. DOI 10.1016/j.psep.2020.02.001. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |