

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.018183
REVIEW
Potential Economic Value of Chitin and Its Derivatives as Major Biomaterials of Seafood Waste, with Particular Reference to Southeast Asia
1Institute of Research Management and Services, Research and Innovation Management Complex, University of Malaya, Kuala Lumpur, 50603, Malaysia
2Department of Restorative Dentistry, Faculty of Dentistry, MAHSA University, Saujana Putra Campus, Jenjarum, 42610, Malaysia
3Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur, 50603, Malaysia
4Department of Oral Biology & Biomedical Sciences, Faculty of Dentistry, MAHSA University, Saujana Putra Campus, Jenjarum, 42610, Malaysia
*Corresponding Author: Fong Fong Liew. Email: ffliew@mahsa.edu.my
Received: 06 July 2021; Accepted: 08 September 2021
Abstract: With a growing population, changes in consumerism behavior and trends in consumption in Indo-Pacific Asia, our seafood processing and consumption practices produce a large volume of waste products. There are several advantages in regulating and sustaining shellfish processing industries. The major advantage of waste management is that it leads to better conservation of natural resources in the long run. Shrimp shell waste contains useful biomaterials, which are still untapped due to inadequate waste disposal and solid waste management. Chitin, the major component of shell waste, can be extracted either chemically or biologically. The chemical extraction approaches, which use acids and alkali, could be an environmental burden. On the other hand, biological methods can be eco-friendly alternatives for shell waste management. In this review, recent trends in management of shellfish waste as sources of chitin, conversion of chitin into chitosan, economic aspects of waste treatment and application of chitosan will be discussed.
Keywords: Seafood waste; waste management; chitin; chitosan; economic value; Southeast Asian fisheries
Asia and the Pacific play a critical role in agricultural productivity, and this area is very crucial for fishing and aquaculture activities. In addition, aquaculture is particularly highly diversified and contributes to the economic well-being of Southeast Asia [1,2]. In the year 2018, aquaculture production in South Asia was 13 million tons [3]. The overall inland and marine captures were 49.2 million tons in the year 2016, which increased to 49.9 million tons in 2018 [4]. The Southeast Asia region covers a large expanse of marine water and does not have any high sea areas. The South China Sea has a total area of approximately 3.3 million km2. Many Southeast Asia’s developing countries depend more on coastal fish farming than on marine resources, particularly the Philippines and Vietnam, which are frequently exposed to typhoons [5]. Contiguous to the South China Sea are the Malacca Straits, the Java, Flores, Banda, Ceram, Molucca, Celebes and Sulu seas, and the Philippine Sea [6]. This semi-closed sea and its contiguous waters provide primary sources of food and protein for people, as well as sources of income and livelihoods for many people by generating several billion dollars in gross domestic product (GDP) for the region [1,7].
It is anticipated that total fish consumption and demand will grow with rapid population growth, which is estimated to grow at 2.3% annually between the years 2010 and 2030. This is likely to have an impact on both capture and aquaculture production, resulting in voluminous amounts of seafood discards. The global fishing and aquaculture sector, which has been growing since the 1970s, produces 6 to 8 million tons of waste each year [8]. Crustacean production in Asia was 14,111 thousand tons, while mollusk production was 31,671 tons [1,9].
How can the fishery waste be beneficially used? Till to date, some companies are producing pet food, fishmeal or fertilizer from seafood waste [10], even now a lot of seafood waste is being discarded as landfill. Although the fishing and aquaculture sector can be a tight margin sector, this would boost profitability through the creative recycling strategy which can be achieved to minimize waste. The objective of this article is to discuss the sustainable use of seafood shell resources, the recovery process of valuable ingredients, particularly chitin, and improve current practice or policy in waste management. The study also discusses the relationships and applications of shell waste, including chitin, calcium, protein, and carotenoids. In addition, current insights on the preparation of chitin nanomaterials are discussed along with their practical applications. Subsequently, the promising applications of chitin-based nanotechnology, economic value, and the use of chitosan in the area are also pointed out.
2 Southeast Asia: Potential Seafood Waste from Seafood Consumption
Fishery activity can be viewed as a multi-gear and multi-species related process. Both inland and marine water fishery operations are carried out in a traditional way and carried out by small scale fishermen and family scale ventures. Only a small proportion of Southeast Asia’s fishery industry has transitioned from small-scale capture fisheries processing, mostly marketed domestically, to larger-scale export-oriented fisheries in Asia, particularly in Southeast Asia, where despite being the largest producers of seafood goods, fishing families in many parts of rural areas remain socially and economically impoverished. It is only with technical assistance, increased investment from the private sector, and demand from both local and foreign markets, that the aquaculture industry will be able to further contribute to the improvement of the economy of the region. Information related to the fishing community of the Southeast Asia region, their employment, financial status, fishing production, and consumption is limited. Of the 11 countries that comprise the Southeast Asia region, data from ten countries is available from the Our World in Data (OWID) data base [11–13]. Moreover, the relative sluggishness of their economic activity is projected from year 2030 to year 2050 as a result of many major constraints, including fish farming in areas of nearly maximum capacity, limited access to quality freshwater, quality of fish seeds and feeds, competitiveness in land use, volatility in fish prices, challenges to regional governance and regulation, and degradation of the environment [14]. In conjunction with global total fishery production, a better understanding of different patterns in meat, fish, and seafood consumption habits is crucial in investigating the sustainability of food security, as well as the impact of food supply chains at local, national, and global levels [15].
As depicted in Fig. 1, the carbon footprint in Southeast Asia could be quite different from that of other global regions. Part of this variation is due to income differences, the presence of vegetarians or the religious prohibition on eating beef. Fish and shrimp comprise a significant portion of the Southeast Asian population’s diet (40% to 50%). As a result, it contributes to more seafood waste production on an annual basis. With reference to data from the Food and Agriculture Organization (FAO), Malaysia is one of the top fish-consuming countries in Asia, with annual consumption exceeding 40 kg/capita since 1980, nearly double the amount of Thailand, Vietnam, Indonesia or Laos, as depicted in Fig. 2.
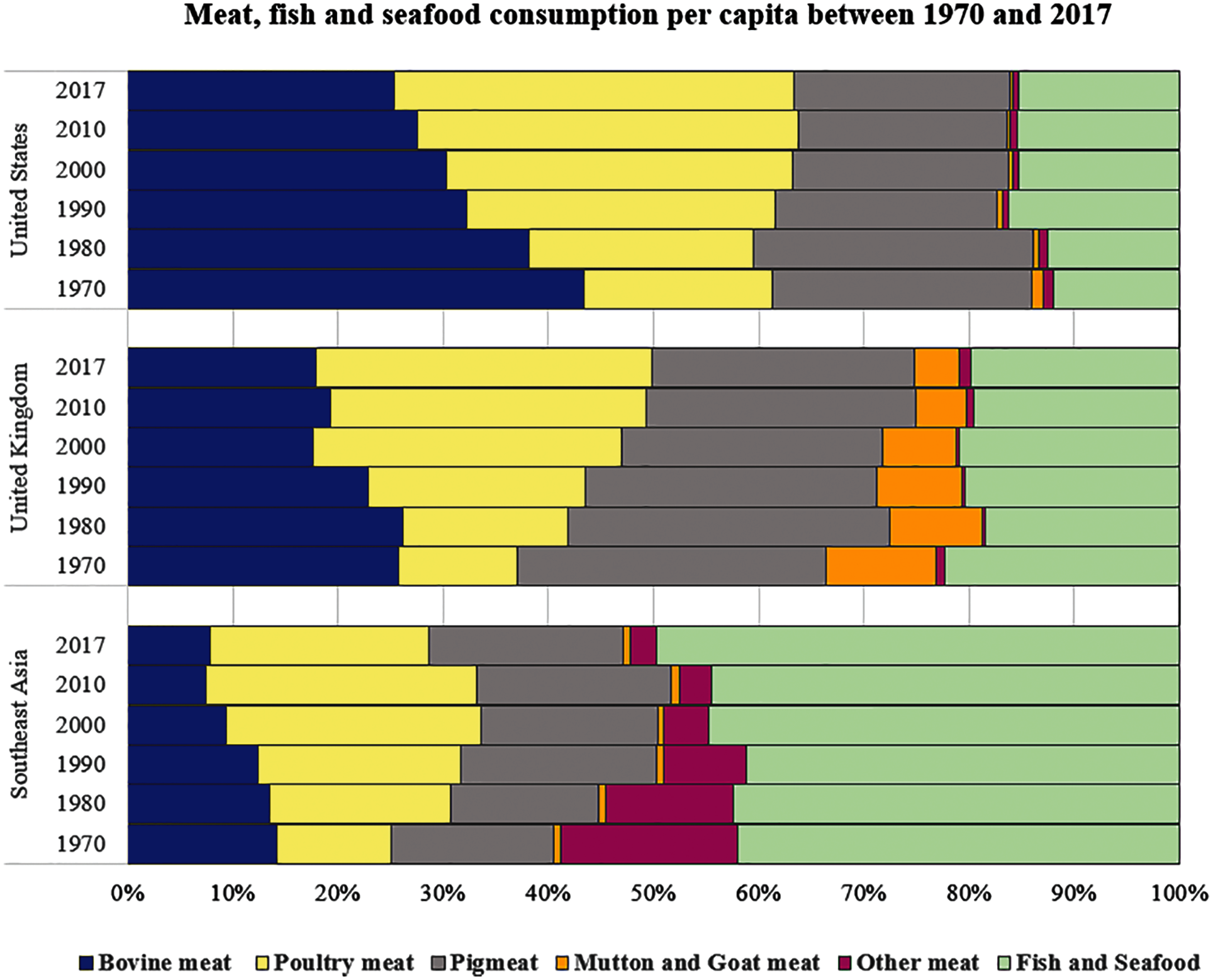
Figure 1: The pattern and amount of meat, fish, and seafood consumption per capita between 1970 and 2017. Fish and seafood are major sources of protein for the population in Southeast Asia [11,12]
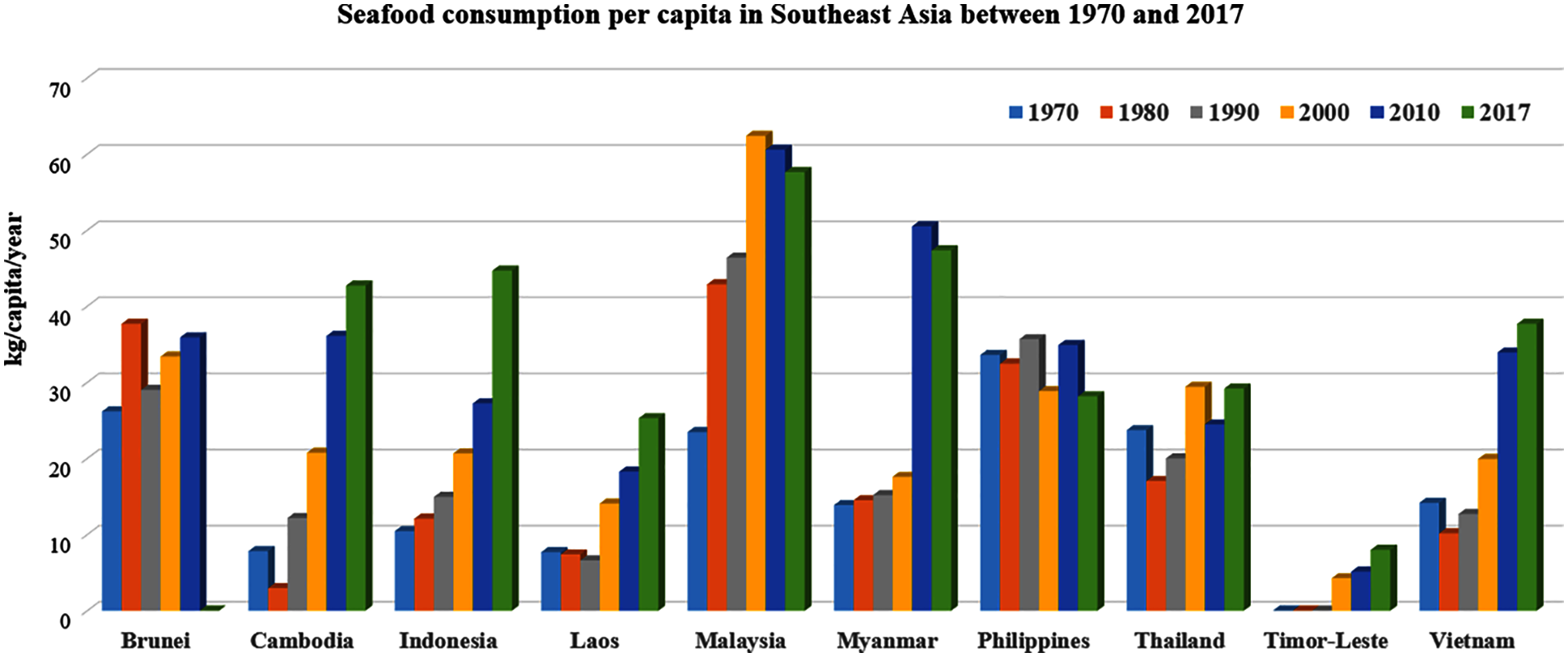
Figure 2: The amount of seafood consumed per capita between 1970 and 2017 in Southeast Asia. Seafood consumption in Malaysia is the highest in Southeast Asia. In general, seafood consumption has increased from the year 1970 to 2017, except in Brunei and the Philippines [11]
The majority of overall fish demand is assigned to domestic consumption, supplemented by exports and non-food use. With increased fish consumption, the overall production of fish and seafood from Southeast Asia has quadrupled in the last 60 years. The inland and marine capture in Southeast Asia during the period 1960 to 2018 has been provided by the FAO, as shown in Fig. 3. Thailand has shown a reduction in fish capture since 2000, whereas the Philippines has shown a tendency to reduce since the 2010s. In Malaysia and Myanmar, fish production has increased steadily since the 1960s, while overfishing in Indonesia could be a concern in Southeast Asia. Increased fishing activity is indicative of humanity’s growing footprint in the world’s oceans. Over fishing can have a detrimental effect on the fish population.
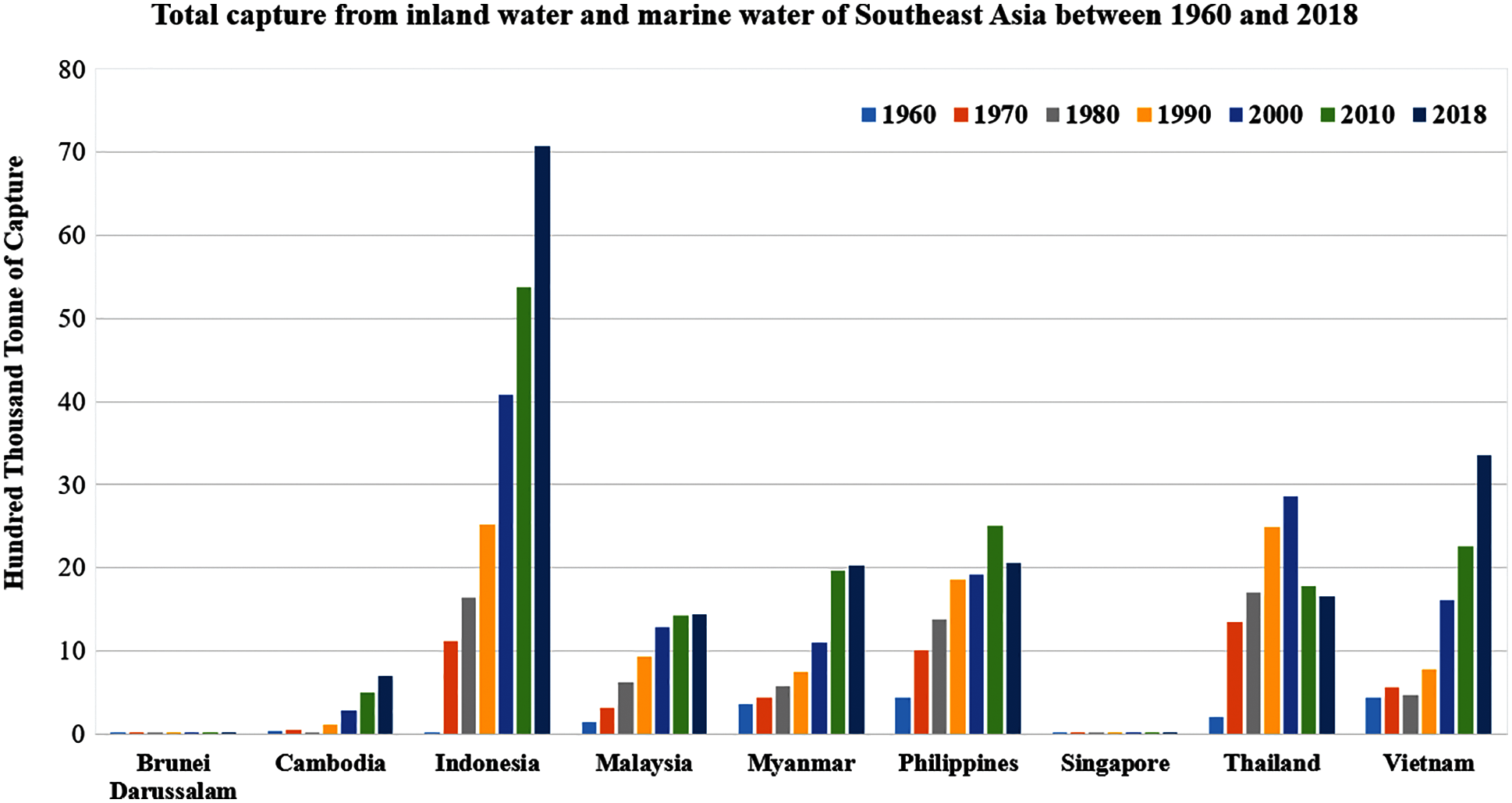
Figure 3: Total capture from inland water and marine water in Southeast Asia between 1960 and 2018. The overall production of crustaceans, diadromous fishes, freshwater fishes and mollusks from capture has been increased since the 1960s in Southeast Asian countries [11,12]
The exponential growth in seafood production results in approximately 35% to 45% waste, including shell waste, at a volume of about 1 million tons [16]. A large part of this waste is being discharged into the coastal zone, which causes serious environmental pollution. Nevertheless, if properly treated, at least some of the waste can also be a source of interesting raw materials that can have diverse applications [17,18], including composting and recycling into animal feed and organic compost [10,19]. It was also reported that around 35% of food is lost worldwide as a result of spillage and deterioration during storage, processing, distribution, and waste at the consumption point. Food supply chains in developing nations must be reinforced by among other things, enabling small farmers to organize, diversify, and scale up their production and sales [20]. Bioconversion alternatives, in conjunction with a bio-refinery strategy, have the potential to provide environmentally friendly and cost-effective processes that can support sustainable seafood production [21].
In Southeast Asia, total seafood capture and aquaculture production are 183 and 128 hundred thousand tons respectively. Meanwhile, in 2017, fish and seafood consumption was 320 kg/capita/year [3]. In this context, tons of chitosan are potentially produced annually, especially from shrimp, fish scales, and crab shell residues. Indeed, increased fishing, aquaculture and seafood consumption can provide large quantities of chitin and chitosan as biowaste [22] and can be extracted commercially as competent polymers. However, current waste management in Southeast Asia is insufficient, and has negative environmental and human health consequences. The contamination of landfills in Southeast Asia is getting serious. The rate of waste generation is associated with the different levels of income and the degree of urbanization as well. Many landfill sites are unsanitary as a result of land acquisition issues, poor collection and disposal fees, and an insufficient number of landfill sites. While there are a range of laws relating to waste management, however people do not honor the waste management laws in most countries. In some underdeveloped countries, the laws on waste management are especially lacking. Most ASEAN countries are uncertain about the institutional structure for waste management. Moreover, they lack of resources, especially funding, technology, waste management, capabilities, and skills [8,23,24]. Therefore, it is important to properly monitor the production, storage, collection, transfer, transportation, transformation, and disposal of shellfish waste. Strict rules on the handling of waste shells should be applied.
Crustacean shells are typically made up of 20% to 50% calcium and magnesium carbonate, 20% to 40% protein, and 15% to 40% chitin [25]. Chitin and its main derivative, chitosan, are promising biomaterials, that are natural aminopolysaccharide polymers. These materials are crucial in strengthening and protecting marine crustaceans, mollusks, insects, shrimp, and fishes [26]. Chitin is closely related to ingredients like protein, calcium carbonate, and lipids. Protein can be composted and used as animal feed, whereas calcium carbonate is used as a nutritional supplement, an antacid, and in industries such as plastics and construction [27].
It is estimated that Southeast Asia will generate one quarter of the world’s seafood by 2030, and that aquaculture, fisheries and shrimp waste will produce biological waste, and cause a range of environmental problems. Many shrimp species in Southeast Asia are prone to diseases. Currently, only a small number of the Penaeidae family are farmed. Among shrimp species cultured in Southeast Asia, the genera Metapenaeus (14 species) and Parapenaeopsis (9 species) are the common predominant species [28,29]. The members of the Penaeidae are all marine, although they are mostly found in coastal and swampy areas where the water is typically of a high salinity brackish type. However, for the genus Metapenaeus, they breed fairly far off at sea. Generally, the larval stages shift towards the coastal areas in order to grow up and return to the offshore grounds at maturity to breed [30]. On the other hand, the members of the Caridea are primarily freshwater, in particular the members of the Macrobrachium genus. There are some exceptions, Macrobrachium equidens, Macrobrachium rosenbergii, Caridina propinqua, Palaemon styliferus, and Palaemon tenuipes are also found in brackish water [31,32].
The output of shrimp waste from the processing industries has greatly increased in recent years as shrimp farms and catches have grown. Indeed, 40% of the shrimp are edible, and the remainder is discarded, accounting for 45% to 50% of the wet weight [33,34]. Shrimp shells are the most common commercial source of chitin, which is used to make chitosan, chito-oligosaccharide, and glucosamine [35,36]. Generally, chitin encrusted with calcium carbonate, collagen, astaxanthin, and lipid residues make up about 20% to 40% of shrimp biowaste [37,38]. Astaxanthin is in high demand for food, feed, nutraceutical, and medicinal applications. This has prompted major efforts to increase the supply of astaxanthin from biological products or biowaste rather than synthetic sources. Therefore, recently, the production of astaxanthin from natural sources has emerged as one of the most exciting biotechnological practices [39].
Decaying crab waste can release ammonia gas and nitrates, and can potentially contaminate ground water and freshwater wells that provide drinking water. Waste recycling could reduce the risk of crab shell deterioration in the environment [40,41].
Advances in commercialization of the source of chitin could reduce the overall cost of bioremediation technology. It is able to adsorb metal ions and also hydrophobic organic molecules, making it valuable in wastewater treatment and other industrial applications [42]. Volatile fatty acids, alcohols, and ammonia have been shown to be involved in chitin fermentation [43]. Chitin from crab waste contains nitrogen and is commonly used in pharmaceutical, carbon dioxide or textile industries, as opposed to many other biomass forms [8], and is used for emulsifying food additives. It is also marketed as an anti-inflammatory supplement to reduce cholesterol, encourage weight loss, and regulate blood pressure. Chitosan can be used to manufacture biodegradable plastic [44].
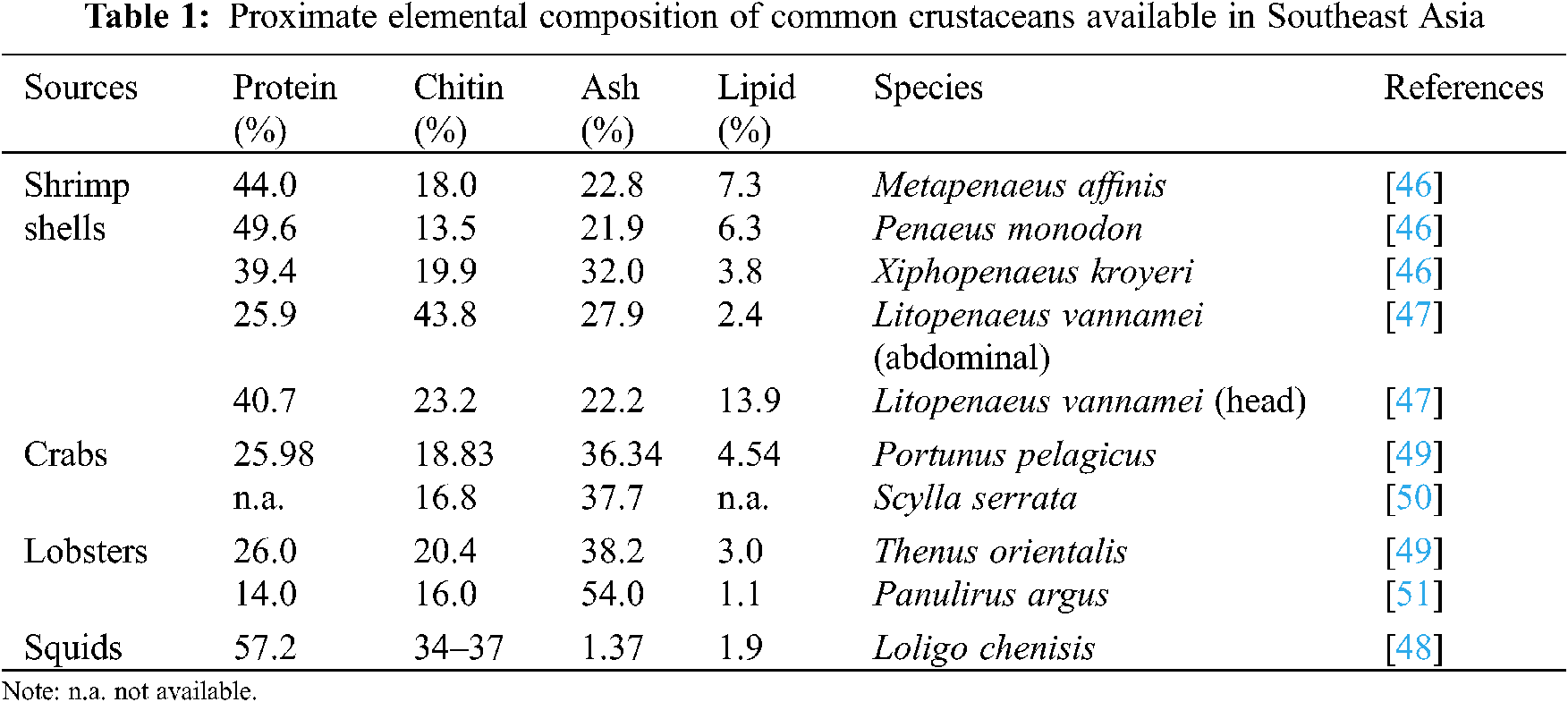
Indeed, better collaboration and investment by entrepreneurs and environmental officials are helpful to mitigate waste disposal problems [45]. Chitin and its deacetylated form chitosan are biomaterials of great importance with various roles in different fields. A description of the amount of chitin from different waste resources from Southeast Asia is summarized in Table 1. The percentage of chitin from shrimp shell waste is between 13.5% to 43.8% [46,47], 34% to 37% chitin available from squid shell waste [48]. Recoverable amounts of chitin from crabs and lobsters are between 16% to 20% [49–51]. A summary of the application of organic compounds from shrimp shells and crab shells is tabulated in Table 2.
4 Characteristic of Chitin and Chitosan
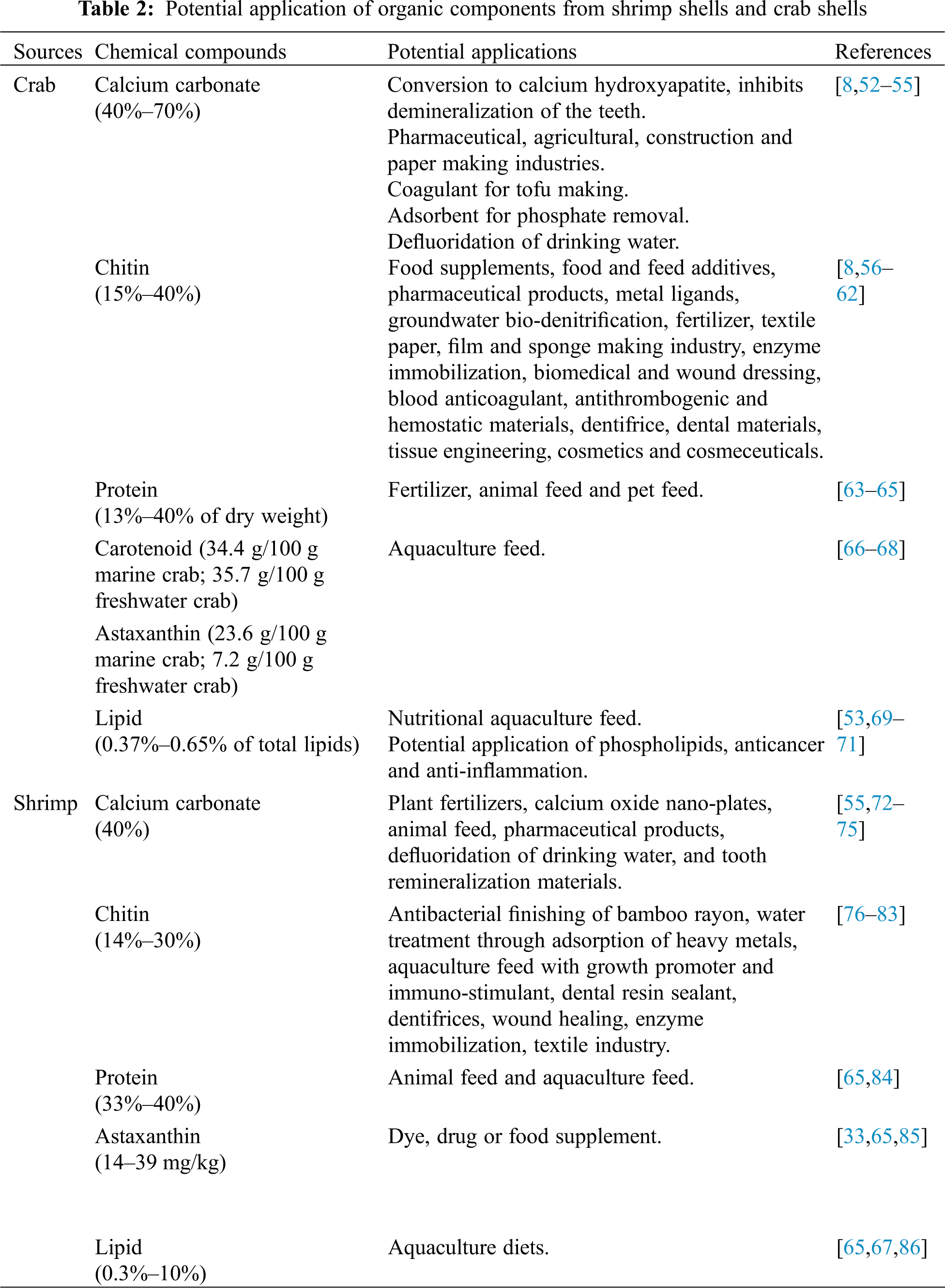
Chitin is a highly insoluble linear polysaccharide (C8H13O5N)n, with repetition units of N-acetyl-glucosamine (GlcNAc), which is a monosaccharide derivative of glucose, as depicted in Fig. 4c. These units form covalent β-1,4 linkages. This natural polysaccharide was first discovered in 1884 [87], the second most abundant natural polymer, only second to cellulose [35]. These polymer molecules are versatile and can form solid structures on their own or combine with other components such as calcium carbonate and metal silicates to make even stronger substances [88]. Chitin has its own unique assembling manner naturally. It can be assembled in at least three different manners, and these are allomorphs named as α-, β-, or γ-chitin. α-Chitin is composed of anti-parallel chains of GlcNAc. β-Chitin, is a less common allomorph that contains chains aligned in a parallel manner, which results in weaker intermolecular interactions as compared to the α-Chitin. Furthermore, γ- has two chains that run parallel to each other and a third chain that runs antiparallel to the two [35,89,90]. Other than the chain assemble manner, the physicochemical properties such as X-ray diffraction and thermal properties of the α-, β-, and γ- chitin are very different. Jang et al. [91] reported that α-chitin has 4 crystalline reflections, β-chitin has 2 crystalline reflections, and γ-chitin shows a combination of the characteristics of α- and β-chitin.
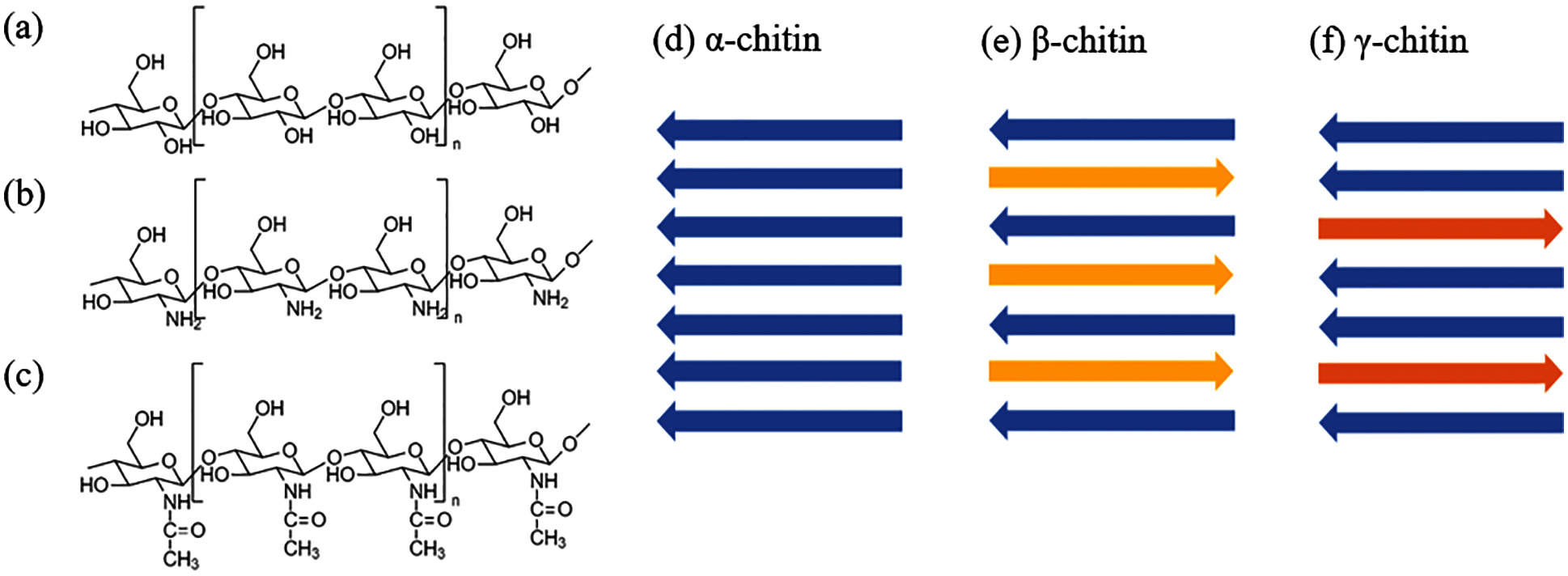
Figure 4: The molecular structure of (a) cellulose, (b) chitosan and (c) chitin (n = repeating unit), and different chitin conformations (d) α-chitin, (e) β-chitin and (f) γ-chitin
Chitosan is a type of N-deacetylated chitin that is made up of a random distribution of β-(1–4)-linked D-glucosamine and N-acetyl-D-glucosamine. Chitin deacetylation can be accomplished in alkaline circumstances by utilizing a higher percentage of alkaline solution or enzymatic treatment with chitin deacetylase [90]. Chitosan deacetylation typically ranges from 50% to 98%, with commercially available chitosan deacetylated at an average of 80% [90,92,93]. Chitosan has certain limitations in its pure form, some chemical modifications can improve its chemical properties. On the other hand, chitosan can be soluble in acidic aqueous solutions depending on the degree of deacetylation [35,87]. Water solubility increases with the increasing degree of deacetylation [90].
Chitin and chitosan have various applications and advantages, as depicted in Fig. 5. Biodegradability, nontoxicity, biocompatibility [94,95], and mucoadhesive properties [96] are the most desirable properties of chitosan. Chitosan in various forms can be used, including powder [97], flakes [98], fibers [99], composites [100], films or membranes [101]. Chitosan can be converted into nanoparticles or nanofibers [102,103]. Chitin is commercially important in comparison with synthetically replaced cellulose (1.25%) due to its high concentration of nitrogen, which is 6.89% [104]. Chitin is a valuable agent for chelating. Chitosan is nonetheless more suited for several fields, including farming, waste water therapy, dental science, cosmetics, pharmaceutics, and the food industry, compared with the chitin sector [88,105,106]. Chitin derivatives are interesting biopolymers that can increase the viscosity of vegetable oil for bio-lubricant production [107]. The physical-chemical features of shrimp shell chitin and its function in the development of arthropod exoskeletons are being investigated to explore chitin asthma and allergy [108]. Chitin is used as a substrate for chitin deacetylase [109].
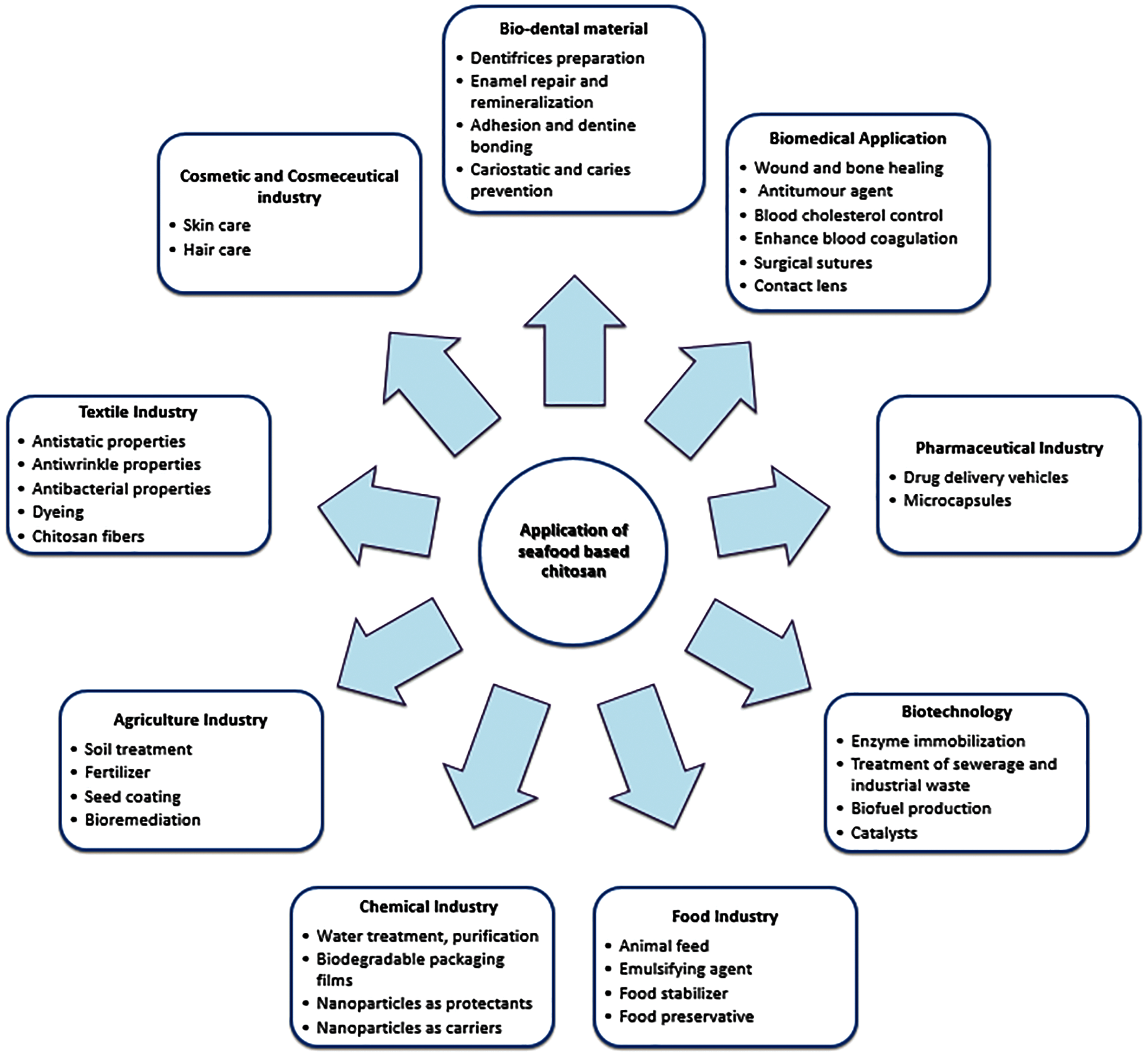
Figure 5: Applications of seafood based chitosan in various industries [61,110–112]
5 Techno-Economic of Chitosan Production
The processing and generation of crustacean waste in Southeast Asia are rising at a fast rate. Chitosan from both crustacean and fish wastes are obtainable by different techniques, and is based on the composition of the source or organisms. These nutraceutical compounds are: chitin, chitosan, glucosamine, astaxanthin, and shrimp carotenoprotein. For the time being, the majority of these approaches rely on chemical and physical agents for mineral, protein, and pigment removal, as well as deacetylation [113,114]. The first step in conventional separation of chitin from waste is mineral removal from the fundamental structure of the exoskeleton. The following step is the removal of proteins associated with all components. The order of deproteinization and demineralization by mineral acid is known to be interchangeable. The third step is the removal of pigments such as carotenoid [115].
The objective of demineralization is to remove calcium carbonate, calcium phosphate, and other minerals by using dilute acids at room temperature, with reaction times varying from 1 h to 24 h [116,117]. Higher acid and basic concentrations are the first way of obtaining chitosan. The demineralization could take place under 10% hydrochloric acid (HCl), room temperature, a solid/solvent ratio of 1:10 (w/v), and within 22 h of stirring conditions. At 70°C, at a solid/solvent ratio of 1:15 (w/v), the desalted shell would be decomposed into 10% sodium hydroxide (NaOH) over 24 h [116,117]. In general, chemical extraction has an inevitably adverse effect on the molecular weight and degree of acetylation. This is most likely due to the rupture of the chitosan polymeric chain in the presence of a concentrated hot NaOH reaction over a long incubation time. The length of the chitosan molecule determines its viscosity. Therefore, in order to yield very viscous chitosan in a short incubation period, increase the reaction temperature by 10°C or increase the incubation time by one hour [118].
Lertsutthiwong et al. [119] extracted chitin from shrimp through chemical treatment. In the first attempt, deproteinization treatment with NaOH (1%, 2%, and 4%) was performed and followed by demineralization using 4% HCl, whereas in the second attempt, demineralization was performed and followed by deproteinization. The result revealed that, if deproteinization precedes demineralization, chitin becomes whiter with an increased concentration of NaOH. With the usage of 4% HCl, the process of decalcification would be adequate to extract minerals within 2 h. Indeed, prolonged decalcification time would not cause much difference in the content of ash, but it might contribute to polymer degradation. Once chemical treatment begins with 4% HCl demineralization for either 2 h or 12 h, colored matter and protein will remain bound to the solid matrix. These products should be separated during subsequent deproteinization. An increase in the proportion of NaOH would enhance the removal of protein and colored materials. The yield of chitin for most conditions was 20% to 27% [119].
When deproteinization is first performed, the defensive protein coating is removed and the unprotected chitin is exposed to HCl. This causes effective demineralization as well as increased hydrolysis and probable material loss in the solid chitin fraction. On the other hand, if demineralization is achieved first, chitin is covered by adhering collagen, resulting in less backbone chain hydrolysis and a higher yield of chitin [119]. Therefore, demineralization should also be followed by deproteinization for the development of high viscosity chitosan. Over the years, many methods for producing chitosan from various seafood wastes have been produced and proposed by several researchers. All of these formed the basis for the development of the chitosan industry by chemical process. However, the majority of the treatments indicated have been carried out at high pressure temperatures above 100°C.
Generally, proteins are removed with mild alkaline solutions (1% to 10%) ranging from room temperature to boiling temperature, reaction time varies from 0.5 h to 24 h. An increase in the sample-to-solution ratio above 1:4 (w/v) only has a small impact on chitosan quality [120]. In the year 2005, Naznin [121] performed both demineralization and deproteinization with different concentrations of NaCl (10% to 50%) and NaOH (0.5, 1.0, 1.5, and 2.0 N) at room temperature. The best combination was overnight demineralization with 30% HCl followed by deproteinization with 1.5 N NaOH. Puvvada et al. [122] deproteinized samples by boiling them in 2% and 4% NaOH for 24 h, followed by demineralization in 1% NaCl. The chitosan yield was found to be 35.49%. Hossain et al. [123] demonstrated that 3% HCI and 4% NaOH at ambient temperatures (28 ± 2°C) were appropriate for demineralization and deproteinization. In order to avoid undesired modification of native chitins, ethylenediamine-tetra-acetic acid (EDTA) can be added for demineralization [124,125]. In general, the yield of chitosan is promising with chemical approaches, but a major drawback is that a great deal of water is used to extract the target component and to neutralize acid.
Recently, Alshehri et al. [126] applied some other steps in the production of chitosan. Along with the protein removal procedure, the researchers utilized oven-dried shrimp shells and boiled them for 1 h in a solution containing 2% and 4% w/v NaOH. The treated shrimp shells were pulverized to produce sizes ranging from 0.5 mm to 5.0 mm. The mineral removal protocol was accompanied by 1% HCl and 4 parts of samples for 24 h. The dried shrimp shell powder was treated with 50 ml of 2% NaOH solution for about 1 h to decompose albumin into water-soluble fractions. During the deacetylation process, the obtained chitin was boiled in 50% NaOH for 2 h at 100°C. The sample was repeatedly washed with 50% NaOH. Eventually, the sample was dried in a hot air oven at 110°C for 6 h to obtain a milky white powder.
For the bleaching procedure, the collected precipitate will be treated for 1 h with 1% potassium permanganate solution and then with 1% oxalic acid solution for another 1 h. Finally, at the boiling temperature, the sample is treated with an alkaline solution. The deacetylation (DDA) and molecular weight of chitosan are important for assessing the nature of the physical and biological properties of chitosan. Chitin deacetylation allows 40% to 45% NaOH at a temperature of 60°C to 130°C to remove acetyl from chitin for 12 h to manufacture chitosan [35,127]. From the literature, two main methods have been proposed, including heterogeneous and homogeneous settings. Chitosan formed by N-deacetylation of chitin under uneven conditions has a normal distribution of GlcNAc blocks, whereas chitosan prepared under homogeneous conditions has coalescence of both GlcNAc and GlcN random (Berneuil) [128]. An important property used to characterize chitosan is its solubility. According to this parameter, chitosan is sufficiently deacetylated with chitin to form a dilute hydrochloric acid soluble salt (DDA 80% to 85% or more), with an abundance of amino groups in the molecule, and can be considered as cationic polyelectrolytes [129].
Another green chemical method that has recently emerged is the selective extraction of chitosan via deep eutectic solvent (DES). DES is the greener alternative to conventional ionic liquids, fluids that can be self-associated with hydrogen bonding interactions in order to form eutectics with lower melting points compared with each individual component. DES is the appropriate mixture of hydrogen bond accepter and donor. Ionic liquids are a singular form of liquid consisting of a very large organic cation and inorganic or an organic anion that melt below 100°C. The advantages of ionic liquids, as indicated by DES, are generally safe for the environment, including low toxicity, low vapor pressure, chemical and thermal resilience, non-flammable, potential high solubility, fusion, and low adaptability [130,131]. In addition, treatments are less expensive and use less water. This approach also shows great potential to overcome the difficulties and processing capabilities of difficult-to-dissolve bio-fertilizers such as cellulose, lignin, and starch [132].
This approach for chitosan production, including the application of ionic liquids, has been noticed as early as 2001. The design of DES was first presented by Abbott et al. [133], and DES was classified into four groups, type I being a combination of organic salts and metal salts, type II being a combination of organic salts and metal hydrate, type III is a mixture of organic salts and a compound that is a hydrogen bond donor, and type IV is a combination of a metal chloride and a compound that is a hydrogen bond donor [134]. As per depicted in Table 3, potential chemical combinations in the proper ratio enable chitosan extraction from seafood waste. When blended in the correct ratio, many primary metabolites, such as sucrose, glucose, mannose, fructose, citric acid, malonic acid, maleic acid, and natural organic acids from plants, will change their state from solid to liquid. This discovery resulted in a new class of DES called natural deep eutectic solvents (NADES). Nevertheless, modern ionic liquids and deep eutectic solvents do have drawbacks to be introduced to the current chemical industry. Operating temperatures above 90°C increase maintenance costs, and there is a risk of human and environmental pollution due to the lack of solvent recycling [135,136].
The green chemistry process was further improved by Feng et al. [137], they transformed shrimp shells into o-attached chitin directly using NADES, however the working temperature was above 90°C. Meanwhile, the one pot process of decalcifying, deproteinization, and acylation is able to remove the minerals and proteins simultaneously. In 2020, Bradić et al. [136] demonstrated chitin extraction by using NADES at a lower temperature of 60°C to 90°C. In addition, these same NADES can be recycled without loss in the capacity to break the shrimp body. The transformation from traditional chemical extraction to a green chemical approach provides a simple, efficient, and environmentally friendly approach to producing value-added chitin derivatives from seafood waste.
5.1 Importance of Biological Extraction
Common chemical extraction methods for chitin often require varying combinations of demineralization, deproteinization, decoloration, and deacetylation procedures. The use of strong acid and alkali solutions at high temperatures or pressures with lengthy incubation is the major drawback of these chemical processes. The entire process consumes a lot of energy as well as a lot of acid or base. Furthermore, chemical processing can harm the physicochemical characteristics of chitin and result in chemical-containing effluent discharge. Despite the use of deep eutectic solvents, which may be an alternative green chemistry application, the use of urea, thiourea, choline chloride, and other chemical compounds may have a negative impact on the environment, low toxicity, and limited biodegradability. Therefore, the expenses, the use of electricity, and higher risk in the production and purification process could restrict industrial chitosan extraction [139].
Despite the fact that the biological extraction process is now limited to laboratory experiments, it has the potential to replace chemical techniques that have a number of commercial limitations. Further advancements in minimizing chitin degradation and lowering contaminants to acceptable levels may boost the biological extraction advantage of chitin. In order to overcome these concerns, a biotechnology approach has been proposed, the use of proteolytic enzymes in digesting proteins or the use of microorganism-mediated fermentation [140,141]. The focus of chitosan manufacture by biological extraction is on the discovery of novel and improved production processes. Enzymatic conversion, chitosan biological activity networks, and chitosan physicochemical properties after microorganism-mediated fermentation [142].
5.1.1 Chitin Enzymatic Deproteinization
Many literature recommends the replacement of chemical deproteinization with enzymatic methods or microbial proteases. Various proteolytic enzymes, including alkalase, pancreatin, papain, pepsin, and trypsin, were utilized to extract protein from chitin. Enzyme detection and operational state optimization are vital, and 5% to 10% of the residual protein remaining coupled to purified chitin may be a less efficient enzyme process. The final, isolated chitin is next treated with moderate alkaline treatment for a brief duration, to increase its purity and retain the structure of chitin [87]. The order of demineralization and deproteinization in biological extraction is mostly irrelevant to the yield and uniformity of chitin purification. Minerals can obstruct proteolytic enzymes from accessing the substrate. As a result, demineralization will be conducted first to increase tissue permeability, limit the interaction of potential enzyme inhibitors, and promote proteolytic enzyme activity.
Younes et al. [87] improved enzyme deproteinization before demineralization. Several microbial proteases were compared in this study based on their efficacy in shrimp shell deproteinization. Bacillus mojavensis A21, Bacillus subtilis A26, Aspergillus clavatus ES1, Bacillus licheniformis NH1, Bacillus licheniformis MP1, and Vibrio metschnikovii J1 were applied for enzymatic deproteinization. Using an enzyme to substrate (E:S) ratio of 7.75 U/mg, 60°C, and 6 h of incubation, Bacillus mojavensis A21 protease can remove up to 77.3% of shell proteins. Chitin samples were then characterized and compared to the samples prepared by chemical deproteinization [143]. Hamdi et al. [144] isolated chitin from Portunus segnis blue crab and Penaeus kerathurus shrimp using chemical demineralization and enzymatic deproteinization approaches. For blue crab shells, the percentage of deproteinization was about 85% and for shrimp was about 91%, with an E:S ratio of 5 U/mg after 3 h of incubation at 50°C. The use of enzymatic deproteinization also limits the cost of commercially available enzymes. Due to the current availability of coexisting proteases, crude extracted proteases derived predominantly from bacteria from fish viscera are less expensive and more effective [87]. Recently, da Silva Lucas et al. [145] isolated chitin and chitosan from the exoskeletons of edible insects. Enzymatic hydrolysis began with the addition of the enzyme alcalase in a 2% (w/w; E:S) proportion, followed by 8 h of deacetylation with 40% NaOH at 90°C with continuous mechanical agitation.
5.1.2 Extraction by Fermentation
Another cost-effective method for chitin extraction from seafood waste involves two separate fermentation processes, one with and one without lactic acid bacteria. This method consists of fermenting chitin sources in a controlled fermentation phase with microorganisms. The process can be accomplished by the addition of selected microbe strains, one- and two-stage fermentation, co-fermentation or subsequent fermentation [146]. Lactic acid bacteria fermentation is a revolutionary approach to enzymatic chitin extraction and can be combined with chemical treatments that reduce the amount of acid and alkali required. The bacteria strains ferment waste materials and produce organic acids in situ. Lactobacillus sp. strains, particularly Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus helveticus, are the most commonly utilized bacterial strains for fermentation. In situ, the bacteria strains ferment waste materials and create organic acids [147,148].
During the fermentation process, two phases will be formed. Liquid fraction rich in proteins, minerals and pigments, and a solid phase containing rudimentary chitin. The action of lactic acid will facilitate the precipitation of chitin and the formation of calcium lactate after chemical reaction with calcium carbonate. Lactic acid obtained by glucose conversion concurrently helps to lower the pH level and thus activates proteases. This method was also utilized to extract additional components from silage shrimp waste, such as carotenoids [147,148]. The shrimp and crab shells contain higher amount of calcium, in general Lactobacillus plantarum is efficiently used to remove calcium after deproteinization [149]. Bacillus subtilis [150], Pseudomonas aeruginosa [151], and Serratia marcescens [152,153] also been reported as effective types of bacterial strains for protein digestion.
Khanafari et al. [154] reported a comparative analysis of the extraction of chitin and chitosan from shrimp waste by chemical and microbial methods. The biochemical process of extraction involved deproteinization with 2% w/v NaOH solution (30:1 v/w, 90°C, 2 h), separation of alkali-insoluble fraction by centrifugation (4000 rpm, 15 min), extraction of chitosan from the fraction under reflux (10% v/v acetic acid 40:1 v/w, 60°C, 6 h), followed by separation of crude chitin by centrifugation (4000 rpm, 15 min), and precipitation of chitosan from the extract at pH 9, which adjusted with a 4 M NaOH solution. The yield of chitin and chitosan by alkali-acid treatment were 510 mg/g and 410 mg/g, respectively. On the other hand, biological extraction of chitin by using Lactobacillus plantarum (PTTC 1058), Lactobacillus acidophilus (PTTC 1643), and Lactobacillus rhamnosus (PTCC 1637) provides high reproducibility in lesser time, decreased solvent consumption, lower energy intake, and is environmentally friendly since no waste is produced. The Lactobacillus plantarum (PTTC 1058) was reported as an attractive source of recovery for chitin and chitosan, chitin and chitosan yields were 700 mg/g and 420 mg/g, respectively [154]. The findings showed that the microbial approach was superior to the chemical method.
Duan et al. [155] investigated the fermentation of shrimp waste on a laboratory and pilot scale with Lactobacillus acidophilus for 48 h of fermentation. In pilot fermentation, the pH was 3.99 and 3.86, respectively, after 12 h and 24 h of fermentation. The mineral and protein content were 0.98% and 8.44% respectively after 48 h of fermentation. The remainder of fermented shrimp waste consists of less than 1% minerals and is rapidly turned into chitin with a simple bleaching process. Recent advancements in fermentation developments have led to the practical deployment of diverse manufacturing procedures to replace old chemical synthesis methods. In industrial applications, these natural processes may be exploited to generate the final product in a regulated manner. For the processing of bulk chemicals and enzymes, solid state fermentation offers several advantages. Lactobacillus sp. and Clostridium sp. are two popular bacterial strains utilized for ensilation [148]. The ensilation can be used in addition to organic acids and Lactobacillus sp. treatment. Shrimp waste ensilation can be accomplished biologically or chemically. The ensilation of crustacean shells can be accomplished through in situ processing of low-cost organic acids, the production of lactic acid by bacteria, which results in the liquefaction of semi-solid waste, a low pH, and the activation of proteases. The process provides numerous advantages as a method of preservation and allows for the recovery of value-added by-products such as chitin, proteins, and pigments to a broader market [84,156,157].
Pseudomonas sp., Bacillus sp., and Aspergillus sp. have been considered for chitin recovery. Sedaghat et al. [158] reported the recovery of chitin from shrimp shell waste by utilizing protease secreted by Pseudomonas aeruginosa. The influence of three parameters on the demineralization and deproteinization of shrimp shell waste was investigated, including glucose concentrations, inoculation levels, and fermentation period. According to the findings of this study, the ideal conditions for chitin extraction are 20% glucose, 20% inoculum, and 6 days of fermentation, which resulted in 82% and 94% demineralization and deprotenization, respectively. Bacillus pumilus A1, Bacillus mojavensis A21, Bacillus licheniformis RP1, Bacillus cereus SV1, Bacillus amyloliquefaciens An6, and Bacillus subtilis A26 were examined by Ghorbel-Bellaaj et al. [159] for fermentation of shrimp waste. The data demonstrated that all Bacillus strains could deproteinate shrimp waste. The highest degree of deproteinization was attained by Bacillus cereus SV1. Teng et al. [160] conducted an interesting study on the continuous generation of chitin from shrimp shells, engaging Aspergillus niger for fermentation. The fungal proteolytic enzymes deproteinate and demineralize shrimp shells, releasing amino acids that provide nitrogen for fungal growth.
During bacteria fermentation, both demineralization and deproteinization occur mostly at the same time, lactic acid bacteria produce lactic acid. Microorganisms mediated by deproteinization and demineralization can produce a fraction of liquor rich in proteins, minerals, and carotenoids, particularly astaxanthin and a chitin-rich solid fraction. This fraction may be used as feed for animals [84]. The biologically isolated chitin from shrimp head debris was co-fermented with the protease-producing bacteria Bacillus licheniformis 21,886 and the acid-producing bacteria Gluconobacter oxydans in a series of steps. The deproteinization and demineralization efficiencies were 87% and 93.5%, respectively, when waste was initially co-fermented, at a chitin value of 90.8% [161].
Zhang et al. [162] conducted a large-scale, three-step fermentation of Serratia marcescens, Lactobacillus plantarum, and Rhizopus japonicus M193 for chitin extract. For the processing of chitosan in normal consistency, 1 M NaOH and 1 M HCl were used to extract fermented residues at room temperature for 24 h and the resulting chitosan solids were dissolved in 2% acetic acid at 30°C for 16 h and centrifuged at 12,000 rpm for 20 min, followed by precipitation by 40% NaOH up to pH 9.0 and then separated by centrifugation at 12,000 rpm for 20 min. The efficiency of both deproteinization and demineralization utilizing Lactobacillus plantarum could be achieved during the first two days. On the second day, the pH of the slurry would drop even further. The formation of lactic acid is inextricably connected to continuous demineralization. Simultaneous enzymatic hydrolysis and fermentation was also reported by Dun et al. [163], chitin extraction from crayfish shell waste powder was investigated by using Bacillus coagulans LA204, and proteinase K. This one pot reaction was performed at 50°C with 5% (w/v) shell waste, 5% (w/v) glucose, 1000 U proteinase kg−1 shell waste, and 10% inoculation of Bacillus coagulans LA204.
In particular, a single-step extraction of chitin may be used by co-culture of lactic acid-producing bacteria and proteases. The solubilized proteins and minerals derived from the fermentation procedure can be processed into animal feed. The optimized green techniques have many beneficial effects and efficiency on recycling seafood waste, water usage could be further reduced to 50% in comparison to conventional methods. Another advantage of applying microorganism in chitosan extraction from seafood waste is the reduction of acidic and alkaline solutions usage. The production costs would be further reduced. The seafood waste as raw materials, implying a new component in the crustacean production chain that includes the fishing, aquaculture, and processing industries. Biologic fermentation technologies have been envisioned as one of the most ecologically sustainable, safe, technologically scalable, and commercially feasible alternative techniques [154].
5.1.3 Biological Deacetylation
The deacetylation procedure is a hydrolysis of acetamide groups in chitin using a strong NaOH solution at high temperatures (100°C or higher) that results in chitosan production. Chemical deacetylation is widely used since it is affordable and allows for mass manufacturing, but it also has drawbacks, such as higher energy consumption and higher air pollutants owing to alkaline conditions. An alternate enzymatic technique employing chitin deacetylases has been investigated to circumvent these constraints in the manufacture of chitosan. The use of chitin deacetylases to convert chitin to chitosan allows for the synthesis of innovative, well-defined chitosan in a controlled, non-degradable manner. This approach is very useful for producing chitosan oligomers [87,146]. Using bacterial, fungal, and viral chitin deacetylases, they created various potential partly acetylated chitosan tetramers with a certain degree of acetylation and acetylation pattern. The availability of these completely developed tetramers expands the scope of research into chitosan structure-function relationships [164].
Due to their huge molecular weight, low solubility, and high viscosity of chitosan solutions, chitin and chitosan have restricted biological uses. Chito-oligosaccharide, which is formed when chitin and chitosan are broken down, is thought to be a superior substitute [142]. Chitin deacetylase (EC 3.5.1.41) catalyzes the hydrolysis of N-acetamido bonds in chitin to produce chitosan [165]. The Mucor rouxii fungal extract’s deacetylase action was the first active enzyme discovered and partially purified in the mid-1970s. Many more chitin deacetylases and chito-oligosaccharide deacetylases (EC 3.5.1.105) were discovered and isolated from a diverse range of organisms, including archaea, marine bacteria, fungi, and insects [166,167]. The enzymes are either excreted in the periplasmic zone or in the culture media, exhibiting outstanding thermal stability at an optimal temperature of 50°C and show a very high specificity for the N-acetyl-d-glucosamine polymers associated with β-(1, 4). The performance of enzymes is pH-dependent. The pH of enzymes affects their performance. It is critical to highlight that the chitin deacetylases generated by Colletotrichum lindemuthianum and Aspergillus nidulans are not inhibited by acetate and are hence suitable for future biotechnological applications. The comparison of chemical and biological methods in chitosan preparation from seafood waste is summarized as depicted in Fig. 6.
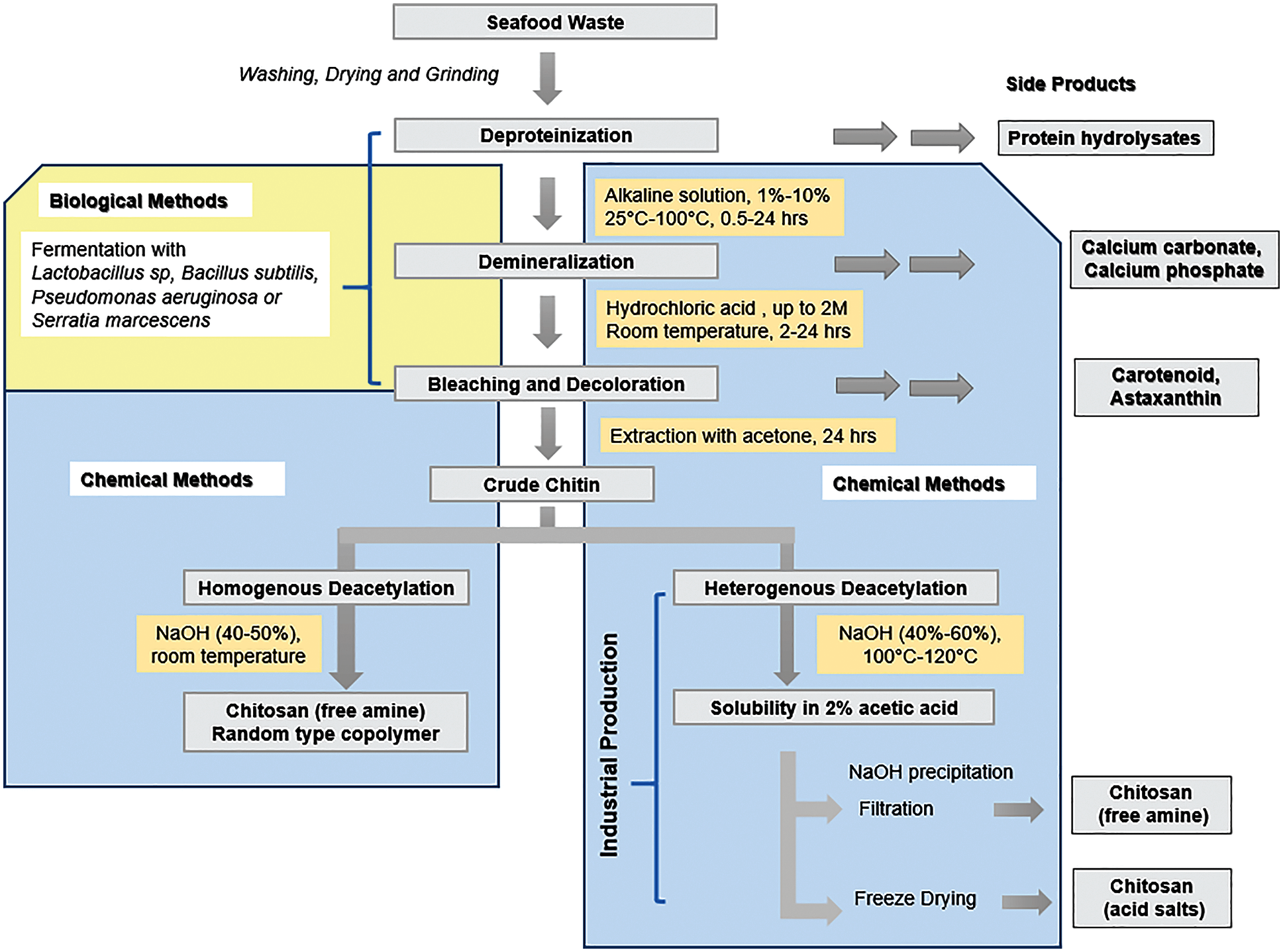
Figure 6: Comparison biological and chemical methodology for chitosan production
5.2 Bioeconomy of Chitin and Chitosan
Chitosan research started a long time ago. The PubMed database recorded the first publication related to chitosan in the year 1953. Since then, the number of research publications exploring the potential of chitosan in different applications, and ways of increasing its production has increased, as shown in Fig. 7. One of the key elements fueling the growing interest in chitosan research is the abundant supply of raw materials. The global demand for chitosan-based biomaterials is currently dominated by North America and Europe, where the products are increasingly used for biomedical and agricultural industries. Asia Pacific is fast turning into another major user and manufacturer of chitosan-based biomaterials, boosting the demand for chitosan-based biomaterials in the region.
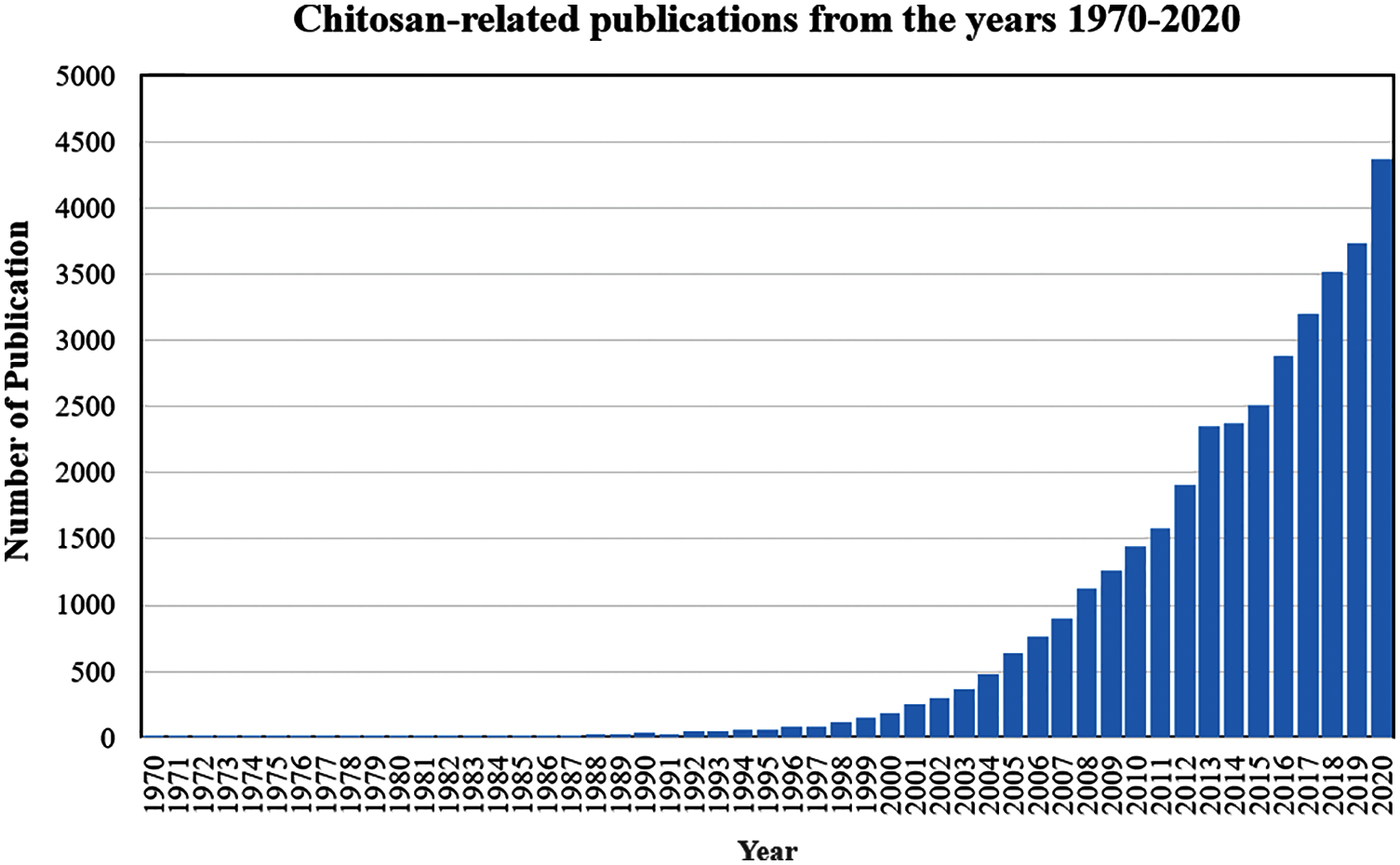
Figure 7: Chitosan-related publications from the years 1970 to 2020. The first paper on chitosan was published in the year 1953, and since then, growing research interest in chitosan has resulted in an exponential increase in the number of publications
The global demand for chitosan derivatives is expected to grow at a CAGR (compound annual growth rate) of around 6.3% over the next 5 years. As of the year 2015, the global chitosan market size was reported at USD 1,205 million. This amount has increased by more than 300%, reaching USD 5.71 billion in 2018, and is expected to grow at a CAGR of 20.8% through 2025. The expected growth is mainly due to the increasing application of chitosan in various industries, such as agriculture, water treatment industry, medical, dental, cosmetic, pharmaceutical, and food [138,168,169].
Applications of chitosan are in diverse areas. Chitosan is ideal for agricultural application with its biodegradable and non-toxic characteristics. It is essential for improving germination, leaf formation, seed harvesting, soil moisture retention, and increasing crop production. The demand for chitosan-based biomaterials is also driven by the growth of the water treatment industry. As the population of countries such as China, India, and Bangladesh in the Asia-Pacific region grows, the demand for clean drinking water has increased. Countries like India and China are facing the problem of water pollution. It is estimated that this situation will increase the demand for chitosan-based biomaterials in the region by improving the consumption of water treatment compounds. The promising potential of chitosan in the field of biological applications is encouraging research activities. Chitosan-based biomaterials are used in a wide range of medical industry applications such as orthopedics and periodontal applications, cancer detection, regenerative medicine, and medication delivery, as depicted in Fig. 5 [8,56–62,170–172].
The unique features of chitosan, such as biocompatibility, biodegradability, non-toxic properties, bio-adhesion, and muco-adhesiveness, are important in the medical field. The need for chitosan-based biomedical applications is expected to grow as new technologies emerge. It is expected that ongoing research activities will focus on discovering new applications of chitosan and developing existing technologies, which will further contribute to the growth of the chitosan market [94,96].
6 Current Research Trends on Chitosan
Chitosan, an economical multifunctional polymer with several desirable physicochemical and biological characteristics, obtainable from seafood waste, has been investigated for use in agriculture, pharmacy, and biomedicine. In the past two years, research has focused on exploring the potential of chitosan application, especially within the areas of the food industry [173–175], health care [176–178], and environmental application [179,180]. Through its numerous functional groups, chitosan allows for diverse forms of interaction, such as ionic hydrogen bonding, which is one of the reasons for its appeal in a variety of applications [181,182]. This study presented tremendous potential for researchers to develop an efficient and environmentally friendly methodology for chitosan extraction and production in innovative ways.
The increasing demand of consumers for food quality and safety has led to increasing research activities in the areas of food production and packaging. Amongst the many factors contributing to food quality and safety, microorganism contamination has drawn much attention. In the past, chemical food preservatives such as sodium nitrite, potassium sorbate, and sodium benzoate were used in the food industry to prevent microbial growth [183]. Over time, the need to explore alternatives to these preservatives has grown due to the increasing concerns about the safety of these chemical preservatives for long-term consumption. The alternative will need to at least fulfill the criteria of cost effectiveness and food safety, in addition to high antimicrobial activity. Chitosan, as well as chitosan film coating, enables it to increase the shelf life of food. Therefore, it is the most recent targeted material for this purpose [173–175,184]. Edible film with a visible colorimetric indicator, which changes color when the pH value changes due to rotting, is a promising direction for food quality management [185]. Another contemporary development in extending food shelf life is the development of biodegradable polymer packaging materials. Polylactic acid with chitosan nanoparticles that are used as plasticizers with polyvinyl alcohol and polyethylene glycol as the link agents has a substantial improvement in tensile strength and antimicrobial action against aerobic microorganisms. An amphiphilic nanoparticle made from a polylactic acid filler with chitosan grafting is a more flexible material and greatly improves the characteristics of the polylactic acid fillers [186].
Chitosan is one of the popular materials for the development of therapeutic and diagnostic applications and also in the area of nanomedicine [176–178] to improve drug delivery efficiency and drug therapeutic index [187,188]. Nanoparticle products for the diagnosis of cancer imaging as well as for pharmaco-therapeutic treatment have been established, and the fabrication of nanoparticle systems by applying chitosan has been explored. Today, the use of chitosan in medicinal treatments as nanocarriers and chitosan-based nanoformulations is still under investigation [189,190].
Chitosan is also playing a very important role in the area of sensor development, especially in the topic of biological evaluation. The versatility of chitosan in forming various forms of structures such as films, microgel/hydrogel and nanocomposites, and its adhesive properties are the main reasons for its being a popular candidate for sensor development [191,192]. These chitosan nanocomposite-based biosensors have shown high sensitivity, selectivity, and stability for detecting a variety of targets, ranging from biomolecules, DNA, and microorganisms. It expands possibilities in the field of biosensor research [193].
The combination of the biological and chemical properties of chitosan allows for a wide range of applications. The versatility of structural formations has enabled application not only for water treatment but also for air and soil treatments [179,180,182]. Research has proven that chitosan-based adsorbents work well for the removal of organic and inorganic pollutants. Therefore, chitosan modification by various physical and chemical processes has emerged as one of the promising strategies for the removal of contaminants from aqueous medium [194,195].
The potential of chitosan has never been exhausted, and it still has a lot of room to grow. All of this was made possible by the enormous possibilities provided by the combination of its chemical and biological characteristics. The possibility of chitosan applications, therefore, will only be limited by the creativity of the research.
Southeast Asia, with its high consumption of fish and seafood per capita, produces tons of fishery and crustacean waste. These fishery and crustacean waste share common chemical compounds such as calcium carbonate, chitin, protein, and lipids. Therefore, seafood waste extractable compounds carry huge economic potential, generally in the area of agriculture and pharmaceuticals. The economic value of these compounds has driven the increasing trend in the number of research on these compounds, especially chitin and its derivative, chitosan. The global market for chitosan increased more than 300% from the year of 2015 to 2018 and is projected to continue at a CARG value of 20.8% until 2025.
Research activities on chitin and chitosan encompass different areas. Some researchers focus on process improvement to meet current needs. In recent years, ensuring environmental safety in preparation and purification of chitin and chitosan has become one of the hot factors to be considered in research. Cost-effective and time saving efforts have always been the factors to be considered in designing the methodology by the researchers. Exploring the potential uses of chitin and chitosan is another popular area of research. Potential innovative applications of chitin and chitosan in the field of biomedical and pharmaceutical remains as one of the popular areas of research. To date, the potential of chitin and chitosan remains wide open. Interest in exploring the potential of these compounds for diverse applications can be reflected in the numbers of publications and filed/granted patents, as pointed out in this article. Researchers that continuously contributed their efforts to chitin and chitosan research often shared common goals. These efforts, in turn, help turn seafood waste into wealth by generating novel materials for human wellbeing.
Acknowledgement: The authors are grateful for the support provided by Research Management Centre (RMC), MAHSA University.
Funding Statement: This review paper was supported by Research No. RP170-05/19, MAHSA Research Grant, MAHSA University.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Béné, C., Arthur, R., Norbury, H., Allison, E. H., Beveridge, M. et al. (2016). Contribution of fisheries and aquaculture to food security and poverty reduction: Assessing the current evidence. World Development, 79, 177–196. DOI 10.1016/j.worlddev.2015.11.007. [Google Scholar] [CrossRef]
2. Beveridge, M. C. M., Thilsted, S. H., Phillips, M. J., Metian, M., Troell, M. et al. (2013). Meeting the food and nutrition needs of the poor: The role of fish and the opportunities and challenges emerging from the rise of aquaculture. Journal of Fish Biology, 83, 1067–1084. DOI 10.1111/jfb.12187. [Google Scholar] [CrossRef]
3. FAO. (2020). The state of world fisheries and aquaculture 2020: Sustainability in Action. Sustainability in action. Rome. [Google Scholar]
4. FAO. (2020). FishStatJ—Software for fishery and aquaculture statistical time series. http://www.fao.org/fishery/statistics/software/fishstatj/en. [Google Scholar]
5. Morton, B., Blackmore, G. (2001). South China Sea. Marine Pollution Bulletin, 42(12), 1236–1263. DOI 10.1016/S0025-326X(01)00240-5. [Google Scholar] [CrossRef]
6. Wyrtki, K. (1961). Chapter 1 introduction. In: Wyrtki, K. (Ed.Physical oceanography of the Southeast Asian Waters. pp. 3–5. University of California, Scripps Institution of Oceanography. [Google Scholar]
7. Williams, M., Choo, P. (2003). Fisheries production in Asia: Its role in food security and nutrition. NAGA. WorldFish Center Quarterly, 26(2), 11–16. [Google Scholar]
8. Yan, N., Chen, X. (2015). Sustainability: Don’t waste seafood waste. Nature News, 524(7564), 155. DOI 10.1038/524155a. [Google Scholar] [CrossRef]
9. World Bank. (2013). Fish to 2030: Prospects for fisheries and aquaculture. World Bank Report Number 83177-GLB. Washington, D.C.: The World Bank. [Google Scholar]
10. Brack, W. M., Slater, B. J., Car, B. F. (2010). Chitin and chitosan from marine organisms. In: Kim, S. K. (Ed.Chitin, chitosan, oligosaccharides and their derivatives: Biological activities and applications. pp. 11–24. Boca Raton:CRC Press. [Google Scholar]
11. FAO. (2018). The state of world fisheries and aquaculture 2018: Meeting the sustainable development goals. Rome. [Google Scholar]
12. Menasveta, D. (2000). The sustainable contribution of fisheries to food security in Southeast Asia. Sustainable Contribution of Fisheries to Food Security. http://www.fao.org/3/x6956e/x6956e07.htm. [Google Scholar]
13. Sanchez-Sabate, R., Sabaté, J. (2019). Consumer attitudes towards environmental concerns of meat consumption: A systematic review. International Journal of Environmental Research and Public Health, 16(7), 1220. DOI 10.3390/ijerph16071220. [Google Scholar] [CrossRef]
14. Chan, C. Y., Tran, N., Dao, D. C., Sulser, T. B., Philips, M. J. et al. (2017). Fish to 2050 in the ASEAN region. WorldFish and Washington DC, USA: International Food Policy Research Institute (IFPRI). http://ebrary.ifpri.org/cdm/ref/collection/p15738coll2/id/131069. [Google Scholar]
15. Kearney, J. (2010). Food consumption trends and drivers. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1554), 2793–2807. DOI 10.1098/rstb.2010.0149. [Google Scholar] [CrossRef]
16. Suryawanshi, N., Jujjavarapu, S., Ayothiraman, S. (2019). Marine shell industrial wastes—An abundant source of chitin and its derivatives: Constituents, pretreatment, fermentation, and pleiotropic applications—A revisit. International Journal of Environmental Science and Technology, 1–22. DOI 10.1007/s13762-018-02204-3. [Google Scholar] [CrossRef]
17. Nisha, S., Seenivasan, A., Vasanth, D. (2016). Chitin and its derivatives: Structure, production, and their applications. International Conference on Signal Processing, Communication, Power and Embedded System, IEEE, pp. 127–130. Paralakhemundi, Odisha, India. [Google Scholar]
18. Mao, X., Guo, N., Sun, J., Xue, C. (2017). Comprehensive utilization of shrimp waste based on biotechnological methods: A review. Journal of Cleaner Production, 143, 814–823. DOI 10.1016/j.jclepro.2016.12.042. [Google Scholar] [CrossRef]
19. Schmitz, C., González Auza, L., Koberidze, D., Rasche, S., Fischer, R. et al. (2019). Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Marine Drugs, 17(8), 452. DOI 10.3390/md17080452. [Google Scholar] [CrossRef]
20. Gustavsson, J., Cederberg, C., Sonesson, U., van Otterdijk, R., Meybeck, A. (2011). Global food losses and food waste: Extent causes and prevention. Rome: Food and Agriculture Organization. [Google Scholar]
21. Venugopal, V. (2021). Valorization of seafood processing discards: Bioconversion and bio-refinery approaches. Frontiers in Sustainable Food Systems, 5, 132. DOI 10.3389/fsufs.2021.611835. [Google Scholar] [CrossRef]
22. Kim, S. K., Venkatesan, J. (2013). Introduction to marine biomaterials. In: Kim, S. K. (Ed.Marine biomaterials: Characterization, isolation and applications, 1st edition, pp. 3–16. Boca Raton: CRC Press. [Google Scholar]
23. Ngoc, U. N., Schnitzer, H. (2009). Sustainable solutions for solid waste management in Southeast Asian countries. Waste Management, 29(6), 1982–1995. DOI 10.1016/j.wasman.2008.08.031. [Google Scholar] [CrossRef]
24. Eng, C. T., Paw, J. N., Guarin, F. Y. (1989). The environmental impact of aquaculture and the effects of pollution on coastal aquaculture development in Southeast Asia. Marine Pollution Bulletin, 20(7), 335–343. DOI 10.1016/0025-326X(89)90157-4. [Google Scholar] [CrossRef]
25. Khoushab, F., Yamabhai, M. (2010). Chitin research revisited. Marine Drugs, 8(7), 1988–2012. DOI 10.3390/md8071988. [Google Scholar] [CrossRef]
26. Azuma, K., Izumi, R., Osaki, T., Ifuku, S., Morimoto, M. et al. (2015). Chitin, chitosan, and its derivatives for wound healing: Old and new materials. Journal of Functional Biomaterials, 6, 104–142. DOI 10.3390/jfb6010104. [Google Scholar] [CrossRef]
27. Hülsey, M. J. (2018). Shell biorefinery: A comprehensive introduction. Green Energy & Environment, 3(4), 318–327. DOI 10.1016/j.gee.2018.07.007. [Google Scholar] [CrossRef]
28. Kow, T. A. (1967). Unit stocks of shrimps and prawns in the ipfc region and unit fisheries exploiting them. FAO Fisheries Report, 2, 205–217. [Google Scholar]
29. Holthuis, L. B. (1965). List of species of shrimps and prawns of economic value. FAO Fisheries Technical Paper, 52, 1–21. [Google Scholar]
30. Achuthankutty, C., Nair, S. S. (1980). Mangrove swamps as fry source for shrimp culture-A case study. Mahasagar, 13(3), 269–276. [Google Scholar]
31. Wowor, D., Choy, S. C. (2001). The freshwater prawns of the genus Macrobrachium Bate. 1868 (Crustacea: Decapoda: Palaemonidae) from Brunei Darussalam. Raffles Bulletin of Zoology, 49(2), 269–290. [Google Scholar]
32. Radhakrishnan, E., Deshmukh, V., Maheswarudu, G., Josileen, J., Dineshbabu, A. P. et al. (2012). Prawn fauna (Crustacea: Decapoda) of India—An annotated checklist of the penaeoid, sergestoid. Stenopodid and Caridean prawns. Journal of the Marine Biological Association of India, 54(1), 50–72. DOI 10.6024/jmbai.2012.54.1.01697-08. [Google Scholar] [CrossRef]
33. Kandra, P., Challa, M. M., Jyothi, H. K. P. (2012). Efficient use of shrimp waste: Present and future trends. Applied Microbiology and Biotechnology, 93(1), 17–29. DOI 10.1007/s00253-011-3651-2. [Google Scholar] [CrossRef]
34. Zhu, P., Gu, Z., Hong, S., Lian, H. (2017). One-pot production of chitin with high purity from lobster shells using choline chloride-malonic acid deep eutectic solvent. Carbohydrate Polymers, 177, 217–223. DOI 10.1016/j.carbpol.2017.09.001. [Google Scholar] [CrossRef]
35. Elieh-Ali-Komi, D., Hamblin, M. R. (2016). Chitin and chitosan: Production and application of versatile biomedical nanomaterials. International Journal of Advanced Research, 4(3), 411. [Google Scholar]
36. Hayes, M. (2012). Chitin, chitosan and their derivatives from marine rest raw materials: Potential food and pharmaceutical applications. In: Hayes, M. (Ed.Marine bioactive compounds. pp. 115–128. Boston, Massachusetts: Springer. DOI 10.1007/978-1-4614-1247-2_4. [Google Scholar] [CrossRef]
37. Xu, Y., Gallert, C., Winter, J. (2008). Chitin purification from shrimp wastes by microbial deproteination and decalcification. Applied Microbiology and Biotechnology, 79(4), 687–697. DOI 10.1007/s00253-008-1471-9. [Google Scholar] [CrossRef]
38. Chang, K. L. B., Tsai, G. (1997). Response surface optimization and kinetics of isolating chitin from pink shrimp (Solenocera melantho) shell waste. Journal of Agricultural and Food Chemistry, 45(5), 1900–1904. DOI 10.1021/jf9606870. [Google Scholar] [CrossRef]
39. Ambati, R. R., Phang, S. M., Ravi, S., Aswathanarayana, R. G. (2014). Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications–A review. Marine Drugs, 12(1), 128–152. DOI 10.3390/md12010128. [Google Scholar] [CrossRef]
40. Romano, N., Zeng, C. (2013). Toxic effects of ammonia, nitrite, and nitrate to decapod crustaceans: A review on factors influencing their toxicity, physiological consequences, and coping mechanisms. Reviews in Fisheries Science, 21(1), 1–21. DOI 10.1080/10641262.2012.753404. [Google Scholar] [CrossRef]
41. Camargo, J. A., Alonso, Á. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environment International, 32(6), 831–849. DOI 10.1016/j.envint.2006.05.002. [Google Scholar] [CrossRef]
42. Yadav, M., Goswami, P., Paritosh, K., Kumar, M., Pareek, N. et al. (2019). Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresources and Bioprocessing, 6(8), 1–20. DOI 10.1186/s40643-019-0243-y. [Google Scholar] [CrossRef]
43. Rittmann, B. E., McCarty, P. L. (2001). Correction: Chapter 1 basic microbiology. In: Rittmann, B. E., McCarty, P. L. (Eds.Environmental biotechnology: Principles and applications, 2nd edition, pp. 1–120. New York, Chicago, San Francisco, Athens, London, Madrid, Mexico City, Milan, New Delhi, Singapore, Sydney, Toronto: McGraw-Hill Education. [Google Scholar]
44. Morin-Crini, N., Lichtfouse, E., Torri, G., Crini, G. (2019). Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environmental Chemistry Letters, 17(4), 1667–1692. DOI 10.1007/s10311-019-00904-x. [Google Scholar] [CrossRef]
45. Wang, C. H., Doan, C. T., Nguyen, V. B., Nguyen, A. D., Wang, A. L. (2019). Reclamation of fishery processing waste: A mini-review. Molecules, 24 (12), 2234. DOI 10.3390/molecules24122234. [Google Scholar] [CrossRef]
46. Ngoan, L., Lindberg, J., Ogle, B., Thomke, S. (2000). Anatomical proportions and chemical and amino acid composition of common shrimp species in central Vietnam. Asian-Australasian Journal of Animal Sciences, 13(10), 1422–1428. DOI 10.5713/ajas.2000.1422. [Google Scholar] [CrossRef]
47. Ploydee, E., Chaiyanan, S. (2014). Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. International Journal of Polymer Science, 2014, 1–8. DOI 10.1155/2014/162173. [Google Scholar] [CrossRef]
48. Cuong, H. N., Minh, N. C., van Hoa, N., Trang, T. S. (2016). Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). International Journal of Biological Macromolecules, 93, 442–447. DOI 10.1016/j.ijbiomac.2016.08.085. [Google Scholar] [CrossRef]
49. Toliba, A. O., Rabie, M., El-Araby, G. M. (2014). Extending the shelf-life of cold stored strawberry by chitosan and carnauba coatings. Zagazig Journal of Agricultural Research, 41(5), 1067–1076. [Google Scholar]
50. Narudin, N. A. H., Mahadi, A. H., Kusrini, E., Usman, A. (2020). Chitin, chitosan, and submicron-sized chitosan particles prepared from Scylla serrata shells. Material International, 2(2), 139–149. DOI 10.33263/Materials22.139149. [Google Scholar] [CrossRef]
51. Ramírez, M. Á., González, P., Fagundo, J. R., Suarez, M., Melian, C. et al. (2017). Chitin preparation by demineralizing deproteinized lobster shells with CO2 and a cationite. Journal of Renewable Materials, 5(1), 30–37. DOI 10.7569/JRM.2016.634121. [Google Scholar] [CrossRef]
52. Raya, I., Mayasari, E., Yahya, A., Latunra, A. I. (2015). Shynthesis and characterizations of calcium hydroxyapatite derived from crabs shells (Portunus pelagicus) and its potency in safeguard against to dental demineralizations. International Journal of Biomaterials, 2015, 1–8. DOI 10.1155/2015/469176. [Google Scholar] [CrossRef]
53. Jun, J. Y., Jung, M. J., Jeong, I. H., Kim, G. W., Sim, J. M. et al. (2019). Effects of crab shell extract as a coagulant on the textural and sensorial properties of tofu (soybean curd). Food Science & Nutrition, 7(2), 547–553. DOI 10.1002/fsn3.837. [Google Scholar] [CrossRef]
54. Jeon, D. J., Yeom, S. H. (2009). Recycling wasted biomaterial, crab shells, as an adsorbent for the removal of high concentration of phosphate. Bioresource Technology, 100(9), 2646–2649. DOI 10.1016/j.biortech.2008.11.035. [Google Scholar] [CrossRef]
55. Wagutu, A. W., Machunda, R., Jande, Y. A. C. (2018). Crustacean derived calcium phosphate systems: Application in defluoridation of drinking water in East African rift valley. Journal of Hazardous Materials, 347, 95–105. DOI 10.1016/j.jhazmat.2017.12.049. [Google Scholar] [CrossRef]
56. Olorunsola, E. O., Olayemi, O. J., Majekodunmi, S. O., Etukudo, U. B. (2015). Extraction and physicochemical characterization of a potential multifunctional pharma-excipient from crab shell wastes. African Journal of Biotechnology, 14(40), 2856–2861. DOI 10.5897/AJB2015.14819. [Google Scholar] [CrossRef]
57. Pandharipande, S., Bhagat, P. H. (2016). Synthesis of chitin from crab shells and its utilization in preparation of nanostructured film. Synthesis, 5(5). [Google Scholar]
58. Robinson-Lora, M. A., Brennan, R. A. (2009). The use of crab-shell chitin for biological denitrification: Batch and column tests. Bioresource Technology, 100(2), 534–541. DOI 10.1016/j.biortech.2008.06.052. [Google Scholar] [CrossRef]
59. Hirano, S. (1996). Chitin biotechnology applications. Biotechnology Annual Review, 2, 237–258. DOI 10.1016/S1387-2656(08)70012-7. [Google Scholar] [CrossRef]
60. Yang, T. L. (2011). Chitin-based materials in tissue engineering: Applications in soft tissue and epithelial organ. International Journal of Molecular Sciences, 12(3), 1936–1963. DOI 10.3390/ijms12031936. [Google Scholar] [CrossRef]
61. Aranaz, I., Acosta, N., Civera, C., Elorza, B., Mingo, J. et al. (2018). Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers, 10(2), 213. DOI 10.3390/polym10020213. [Google Scholar] [CrossRef]
62. Wieckiewicz, M., Boening, K. W., Grychowska, N., Paradowska-Stolarz, A. (2017). Clinical application of chitosan in dental specialities. Mini Reviews in Medicinal Chemistry, 17(5), 401–409. DOI 10.2174/1389557516666160418123054. [Google Scholar] [CrossRef]
63. Özoğul, Y. (2000). The possibility of using crustacean waste products (CWP) on rainbow trout (Oncorhynchus mykiss) feeding. Turkish Journal of Biology, 24(4), 845–854. [Google Scholar]
64. Pires, C., Marques, A., Carvalho, M., Batista, I. (2017). Chemical characterization of Cancer pagurus, Maja squinado, Necora puber and Carcinus maenas shells. Poultry, Fisheries and Wildlife Sciences, 5(1). DOI 10.4172/2375-446X.1000181. [Google Scholar] [CrossRef]
65. Rødde, R. H., Einbu, A., Vårum, K. M. (2008). A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydrate Polymers, 71(3), 388–393. DOI 10.1016/j.carbpol.2007.06.006. [Google Scholar] [CrossRef]
66. Kouchi, H., Nasab, M., Shabanpour, B. (2012). Extraction of carotenoids from crustacean waste using organic solvents. The 1st International and The 4th National Congress on Recycling of Organic Waste in Agriculture. pp. 26–27. Isfahan. http://conference.khuisf.ac.ir/DorsaPax/userfiles/file/pazhohesh/crowa91/31.pdf. [Google Scholar]
67. Guillou, A., Khalil, M., Adambounou, L. (1995). Effects of silage preservation on astaxanthin forms and fatty acid profiles of processed shrimp (Pandalus borealis) waste. Aquaculture, 130(4), 351–360. DOI 10.1016/0044-8486(94)00324-H. [Google Scholar] [CrossRef]
68. Pangantihon-Kühlmann, M. P., Millamena, O., Chern, Y. (1998). Effect of dietary astaxanthin and vitamin a on the reproductive performance of Penaeus monodon broodstock. Aquatic Living Resources, 11(6), 403–409. DOI 10.1016/S0990-7440(99)80006-0. [Google Scholar] [CrossRef]
69. Naczk, M., Williams, J., Brennan, K., Liyanapathirana, C., Shahidi, F. (2004). Compositional characteristics of green crab (Carcinus maenas). Food Chemistry, 88(3), 429–434. DOI 10.1016/j.foodchem.2004.01.056. [Google Scholar] [CrossRef]
70. Copeman, L. A., Stoner, A. W., Ottmar, M. L., Daly, B., Parrish, C. C. et al. (2012). Total lipids, lipid classes, and fatty acids of newly settled red king crab (Paralithodes camtschaticusComparison of hatchery-cultured and wild crabs. Journal of Shellfish Research, 31(1), 153–165. DOI 10.2983/035.031.0119. [Google Scholar] [CrossRef]
71. Nguyen, T. P. L., Nguyen, V. T. A., Do, T. T. T., Nguyen Quang, T., Pham, Q. L. et al. (2020). Fatty acid composition, phospholipid molecules, and bioactivities of lipids of the mud crab Scylla paramamosain. Journal of Chemistry, 2020, 1–9. DOI 10.2983/035.031.0119. [Google Scholar] [CrossRef]
72. Øvsthus, I., Breland, T. A., Hagen, S. F., Brandt, K., Wold, A. B. et al. (2015). Effects of organic and waste-derived fertilizers on yield, nitrogen and glucosinolate contents, and sensory quality of broccoli (Brassica oleracea L. var. italica). Journal of Agricultural and Food Chemistry, 63(50), 10757–10767. DOI 10.1021/acs.jafc.5b04631. [Google Scholar] [CrossRef]
73. Gedda, G., Pandey, S., Lin, Y. C., Wu, H. F. (2015). Antibacterial effect of calcium oxide nano-plates fabricated from shrimp shells. Green Chemistry, 17(6), 3276–3280. DOI 10.1039/C5GC00615E. [Google Scholar] [CrossRef]
74. Deng, J. J., Mao, H. H., Fang, W., Li, Z. Q., Shi, D. et al. (2020). Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. Journal of Cleaner Production, 271, 122655. DOI 10.1016/j.jclepro.2020.122655. [Google Scholar] [CrossRef]
75. Asmawati, A., Thalib, B., Thalib, A. M., Reni, D., Hasyim, R. (2018). Comparison of blood clam (Anadara granosa) shell paste, shrimp (Litopenaeus vannamei) shell paste and casein phosphopeptide-amorphus calcium phosphate (CPP-aCP) paste as teeth remineralization material. Journal of Dentomaxillofacial Science, 3(3), 162–165. DOI 10.15562/jdmfs.v3i3.834. [Google Scholar] [CrossRef]
76. Teli, M., Sheikh, J. (2012). Extraction of chitosan from shrimp shells waste and application in antibacterial finishing of bamboo rayon. International Journal of Biological Macromolecules, 50(5), 1195–1200. DOI 10.1016/j.ijbiomac.2012.04.003. [Google Scholar] [CrossRef]
77. Ali, M. E. A., Aboelfadl, M. M. S., Selim, A. M., Khalil, H. F., Elkady, G. M. (2018). Chitosan nanoparticles extracted from shrimp shells, application for removal of Fe(II) and Mn(II) from aqueous phases. Separation Science and Technology, 53(18), 2870–2881. DOI 10.1080/01496395.2018.1489845. [Google Scholar] [CrossRef]
78. Rochana, W., Niroshan, W., Tiruchenduran, S., Sulaiman, M. A., Mahesh, D. (2019). Effects of chitosan on growth, immune responses and survival of juvenile tiger shrimp (Penaeus monodon fabricius, 1798). International Journal of Fisheries and Aquatic Studies, 7(4), 129–133. [Google Scholar]
79. Mahapoka, E., Arirachakaran, P., Watthanaphanit, A., Rujiravanit, R., Poolthong, S. (2012). Chitosan whiskers from shrimp shells incorporated into dimethacrylate-based dental resin sealant. Dental Materials Journal, 31(2), 273–279. DOI 10.4012/dmj.2011-071. [Google Scholar] [CrossRef]
80. Achmad, H., Ramadhany, Y. F. (2017). Effectiveness of chitosan tooth paste from white shrimp (Litopenaeus vannamei) to reduce number of Streptococcus mutans in the case of early childhood caries. Journal of International Dental and Medical Research, 10(2), 358. DOI 10.2991/idsm-17.2018.11. [Google Scholar] [CrossRef]
81. Gani, A., Hamrun, N., Adam, A. M., Pakki, E., Achmad, H. et al. (2020). The effect of white shrimp head chitosan gel (Litopenaeus vannamei) on inhibitory strength of periodontopathogenic bacteria and accelerating wound healing (in vitro, histological, and clinical tests). Systematic Reviews in Pharmacy, 11(4), 258–267. [Google Scholar]
82. Juang, R. S., Wu, F. C., Tseng, R. L. (2001). Solute adsorption and enzyme immobilization on chitosan beads prepared from shrimp shell wastes. Bioresource Technology, 80(3), 187–193. DOI 10.1016/S0960-8524(01)00090-6. [Google Scholar] [CrossRef]
83. Butola, B. (2020). A synergistic combination of shrimp shell derived chitosan polysaccharide with Citrus sinensis peel extract for the development of colourful and bioactive cellulosic textile. International Journal of Biological Macromolecules, 158, 94–103. DOI 10.1016/j.ijbiomac.2020.04.209. [Google Scholar] [CrossRef]
84. Shahidi, F., Synowiecki, J. (1991). Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. Journal of Agricultural and Food Chemistry, 39(8), 1527–1532. DOI 10.1021/jf00008a032. [Google Scholar] [CrossRef]
85. Lim, K. C., Yusoff, F. M., Shariff, M., Kamarudin, M. S. (2018). Astaxanthin as feed supplement in aquatic animals. Reviews in Aquaculture, 10(3), 738–773. DOI 10.1111/raq.12200. [Google Scholar] [CrossRef]
86. Synowiecki, J., Al-Khateeb, N. A. A. Q. (2000). The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chemistry, 68(2), 147–152. DOI 10.1016/S0308-8146(99)00165-X. [Google Scholar] [CrossRef]
87. Younes, I., Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs, 13(3), 1133–1174. DOI 10.3390/md13031133. [Google Scholar] [CrossRef]
88. Rashid, I., Daraghmeh, N., Al-Remawi, M., Leharne, S. A., Chowdhry, B. Z. et al. (2009). Characterization of chitin–metal silicates as binding superdisintegrants. Journal of Pharmaceutical Sciences, 98(12), 4887–4901. DOI 10.1002/jps.21781. [Google Scholar] [CrossRef]
89. Alvarez, F. J. (2014). The effect of chitin size, shape, source and purification method on immune recognition. Molecules, 19, 4433–4451. DOI 10.3390/molecules19044433. [Google Scholar] [CrossRef]
90. Rinaudo, M. (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31(7), 603–632. DOI 10.1016/j.progpolymsci.2006.06.001. [Google Scholar] [CrossRef]
91. Jang, M. K., Kong, B. G., Jeong, Y. I., Lee, C. H., Nah, J. W. (2004). Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. Journal of Polymer Science Part A: Polymer Chemistry, 42(14), 3423–3432. DOI 10.1002/pola.20176. [Google Scholar] [CrossRef]
92. Dufresne, A., Cavaillé, J. Y., Dupeyre, D., Garcia-Ramirez, M., Romero, J. (1999). Morphology, phase continuity and mechanical behaviour of polyamide 6/chitosan blends. Polymer, 40(7), 1657–1666. DOI 10.1016/S0032-3861(98)00335-8. [Google Scholar] [CrossRef]
93. Baldrick, P. (2010). The safety of chitosan as a pharmaceutical excipient. Regulatory Toxicology and Pharmacology, 56(3), 290–299. DOI 10.1016/j.yrtph.2009.09.015. [Google Scholar] [CrossRef]
94. Aranaz, I., Harris, R., Heras, A. (2010). Chitosan amphiphilic derivatives. Chemistry and Applications. Current Organic Chemistry, 14(3), 308–330. DOI 10.2174/138527210790231919. [Google Scholar] [CrossRef]
95. Adamo, F., Isabella, O. (2003). The role of chitosan in drug delivery: Current and potential applications. American Journal of Drug Delivery, 1, 43–59. DOI 10.2165/00137696-200301010-00004. [Google Scholar] [CrossRef]
96. Karn, P. R., Vanić, Z., Pepić, I., Škalko-Basnet, N. (2011). Mucoadhesive liposomal delivery systems: The choice of coating material. Drug Development and Andustrial Pharmacy, 37(4), 482–488. DOI 10.3109/03639045.2010.523425. [Google Scholar] [CrossRef]
97. Sadeghi-Kiakhani, M., Arami, M., Gharanjig, K. (2013). Preparation of chitosan-ethyl acrylate as a biopolymer adsorbent for basic dyes removal from colored solutions. Journal of Environmental Chemical Engineering, 1(30), 406–415. DOI 10.1016/j.jece.2013.06.001. [Google Scholar] [CrossRef]
98. Adarsh, K., Madhu, G. (2014). A comparative study on metal adsorption properties of different forms of chitosan. International Journal of Innovative Science Engineering and Technology, 3, 9609–9617. [Google Scholar]
99. Pillai, C., Paul, W., Sharma, C. P. (2009). Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Progress in Polymer Science, 34(7), 641–678. DOI 10.1016/j.progpolymsci.2009.04.001. [Google Scholar] [CrossRef]
100. Ngah, W. W., Ab Ghani, S., Kamari, A. (2005). Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresource Technology, 96(5), 443–450. DOI 10.1016/j.biortech.2004.05.022. [Google Scholar] [CrossRef]
101. Qiao, C., Ma, X., Wang, X., Liu, L. (2021). Structure and properties of chitosan films: Effect of the type of solvent acid. LWT, 135, 109984. DOI 10.1016/j.lwt.2020.109984. [Google Scholar] [CrossRef]
102. Dehaghi, S. M., Rahmanifar, B., Moradi, A. M., Azar, P. A. (2014). Removal of permethrin pesticide from water by chitosan–zinc oxide nanoparticles composite as an adsorbent. Journal of Saudi Chemical Society, 18(4), 348–355. DOI 10.1016/j.jscs.2014.01.004. [Google Scholar] [CrossRef]
103. Qian, L., Zhang, H. (2010). Green synthesis of chitosan-based nanofibers and their applications. Green Chemistry, 12(7), 1207–1214. DOI 10.1039/B927125B. [Google Scholar] [CrossRef]
104. Islam, S., Khan, M., Alam, A. N. (2016). Production of chitin and chitosan from shrimp shell wastes. Journal of the Bangladesh Agricultural University, 14(2), 253–259. [Google Scholar]
105. Reddy, N., Yang, Y. (2015). Introduction to chitin, chitosan, and alginate fibers. In: Reddy, N., Yang, Y. (Eds.Innovative Biofibers from Renewable Resources. pp. 93–94. Berlin, Heidelberg: Springer. [Google Scholar]
106. Renault, F., Sancey, B., Badot, P. M., Crini, G. (2009). Chitosan for coagulation/flocculation processes–An eco-friendly approach. European Polymer Journal, 45(5), 1337–1348. DOI 10.1016/j.eurpolymj.2008.12.027. [Google Scholar] [CrossRef]
107. Sánchez, R., Stringari, G., Franco, J. M., Valencia, C., Gallegos, C. (2011). Use of chitin, chitosan and acylated derivatives as thickener agents of vegetable oils for bio-lubricant applications. Carbohydrate Polymers, 85(3), 705–714. DOI 10.1016/j.carbpol.2011.03.049. [Google Scholar] [CrossRef]
108. Brinchmann, B. C., Bayat, M., Brogger, T., Muttuvelu, D. V., Tjonneland, A. et al. (2011). A possible role of chitin in the pathogenesis of asthma and allergy. Annals of Agricultural and Environmental Medicine, 18(1), 7–12. [Google Scholar]
109. Zhao, Y., Park, R. D., Muzzarelli, R. A. (2010). Chitin deacetylases: Properties and applications. Marine Drugs, 8(1), 24–46. DOI 10.3390/md8010024. [Google Scholar] [CrossRef]
110. Worrall, E. A., Hamid, A., Mody, K. T., Mitter, N., Pappu, H. R. (2018). Nanotechnology for plant disease management. Agronomy, 8(12), 285. DOI 10.3390/agronomy8120285. [Google Scholar] [CrossRef]
111. Dodane, V., Vilivalam, V. D. (1998). Pharmaceutical applications of chitosan. Pharmaceutical Science & Technology Today, 1(6), 246–253. DOI 10.1016/j.cis.2018.11.008. [Google Scholar] [CrossRef]
112. Pal, P., Pal, A., Nakashima, K., Yadav, B. K. (2020). Applications of chitosan in environmental remediation: A review. Chemosphere, 266, 128934. DOI 10.1016/j.chemosphere.2020.128934. [Google Scholar] [CrossRef]
113. Ahing, F. A., Wid, N. (2016). Extraction and characterization of chitosan from shrimp shell waste in Sabah. Transactions on Science and Technology, 3(1–2), 227–237. [Google Scholar]
114. Nwe, N., Furuike, T., Tamura, H., (2011). Chitosan from aquatic and terrestrial organisms and microorganisms: production, properties and applications. In: Johnson, B. M., Berkel, Z. E. (Eds.Biodegradable materials. pp. 29–50. New York: Nova Science Publishers Inc. [Google Scholar]
115. Zainol Abidin, N. A., Kormin, F., Zainol Abidin, N. A., Mohamed Anuar, N. A. F., Abu Bakar, M. F. (2020). The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. International Journal of Molecular Sciences, 21(14), 4978. DOI 10.3390/ijms21144978. [Google Scholar] [CrossRef]
116. No, H. K., Meyers, S. P., Lee, K. S. (1989). Isolation and characterization of chitin from crawfish shell waste. Journal of Agricultural and Food Chemistry, 37(3), 575–579. DOI 10.1021/jf00087a001. [Google Scholar] [CrossRef]
117. Nessa, F., Masum, S. M., Asaduzzaman, M., Roy, S. K., Hossain, M. M. et al. (2010). A process for the preparation of chitin and chitosan from prawn shell waste. Bangladesh Journal of Scientific and Industrial Research, 45(4), 323–330. DOI 10.3329/bjsir.v45i4.7330. [Google Scholar] [CrossRef]
118. Bajaj, M., Winter, J., Gallert, C. (2011). Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochemical Engineering Journal, 56 (1–2), 51–62. DOI 10.1016/j.bej.2011.05.006. [Google Scholar] [CrossRef]
119. Lertsutthiwong, P., How, N. C., Chandrkrachang, S., Stevens, W. F. (2002). Effect of chemical treatment on the characteristics of shrimp chitosan. Journal of Metals, Materials and Minerals, 12(1), 11–18. [Google Scholar]
120. Synowiecki, J., Al-Khateeb, N. A. (2003). Production, properties, and some new applications of chitin and its derivatives. Critical Reviews in Food Science and Nutrition, 43(2), 145–171. DOI 10.1080/10408690390826473. [Google Scholar] [CrossRef]
121. Naznin, R. (2005). Extraction of chitin and chitosan from shrimp (Metapenaeus monoceros) shell by chemical method. Pakistan Journal of Biological Science, 8(7), 1051–1054. DOI 10.3923/pjbs.2005.1051.1054. [Google Scholar] [CrossRef]
122. Puvvada, Y. S., Vankayalapati, S., Sukhavasi, S. (2012). Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. International Current Pharmaceutical Journal, 1(9), 258–263. DOI 10.3329/icpj.v1i9.11616. [Google Scholar] [CrossRef]
123. Hossain, M., Iqbal, A. (2014). Production and characterization of chitosan from shrimp waste. Journal of the Bangladesh Agricultural University, 12(1), 153–160. DOI 10.3329/jbau.v12i1.21405. [Google Scholar] [CrossRef]
124. Austin, P., Brine, C., Castle, J., Zikakis, J. P. (1981). Chitin: New facets of research. Science, 212(4496), 749–753. DOI 10.1126/science.7221561. [Google Scholar] [CrossRef]
125. Roberts, G. (1997). Chitosan production routes and their role in determining the structure and properties of the product. Advances in Chitin Science, 2, 22–31. [Google Scholar]
126. Alshehri, M. A., Trivedi, S., Panneerselvam, C. (2020). Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance. Green Processing and Synthesis, 9(1), 675–684. DOI 10.1515/gps-2020-0062. [Google Scholar] [CrossRef]
127. Yuan, Y., Chesnutt, B. M., Haggard, W. O., Bumgardner, J. D. (2011). Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials, 4(8), 1399–1416. DOI 10.3390/ma4081399. [Google Scholar] [CrossRef]
128. Aiba, S. I. (1993). Studies on chitosan: 6. Relationship between N-acetyl group distribution pattern and chitinase digestibility of partially N-acetylated chitosans. International Journal of Biological Macromolecules, 15(4), 241–245. DOI 10.1016/0141-8130(93)90044-M. [Google Scholar] [CrossRef]
129. Orrego, C. E., Salgado, N. (2014). Green logistics in chitin/chitosan industry. In: Cardona Alzate, C. A., Sarache Castro, W. A. (Eds.Green Supply Chains: Applications in Agroindustries, pp. 27–43. Manizales, Colombia: Universidad Nacional de Colombia. [Google Scholar]
130. Carriazo, D., Serrano, M. C., Gutiérrez, M. C., Ferrer, M. L., del Monte, F. (2012). Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chemical Society Reviews, 41(14), 4996–5014. DOI 10.1039/C2CS15353J. [Google Scholar] [CrossRef]
131. Paiva, A., Craveiro, R., Aroso, I., Martins, M., Reis, R. L. et al. (2014). Natural deep eutectic solvents–solvents for the 21st Century. ACS Sustainable Chemistry & Engineering, 2(5), 1063–1071. DOI 10.1021/sc500096j. [Google Scholar] [CrossRef]
132. Lynam, J. G., Kumar, N., Wong, M. J. (2017). Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresource Technology, 238, 684–689. DOI 10.1016/j.biortech.2017.04.079. [Google Scholar] [CrossRef]
133. Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., Rasheed, R. K. (2004). Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. Journal of the American Chemical Society, 126(29), 9142–9147. DOI 10.1021/ja048266j. [Google Scholar] [CrossRef]
134. Smith, E. L., Abbott, A. P., Ryder, K. S. (2014). Deep eutectic solvents (DESs) and their applications. Chemical Reviews, 114(21), 11060–11082. DOI 10.1021/cr300162p. [Google Scholar] [CrossRef]
135. Choi, Y. H., Verpoorte, R. (2019). Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Current Opinion in Food Science, 26, 87–93. DOI 10.1016/j.cofs.2019.04.003. [Google Scholar] [CrossRef]
136. Bradić, B., Novak, U., Likozar, B. (2020). Crustacean shell bio-refining to chitin by natural deep eutectic solvents. Green Processing and Synthesis, 9(1), 13–25. DOI 10.1515/gps-2020-0002. [Google Scholar] [CrossRef]
137. Feng, M., Lu, X., Zhang, J., Li, Y., Shi, C. et al. (2019). Direct conversion of shrimp shells to O-acylated chitin with antibacterial and anti-tumor effects by natural deep eutectic solvents. Green Chemistry, 21(1), 87–98. DOI 10.1039/C8GC02506A. [Google Scholar] [CrossRef]
138. Zhang, H., Mardyani, S., Chan, W. C., Kumacheva, E. (2006). Design of biocompatible chitosan microgels for targeted pH-mediated intracellular release of cancer therapeutics. Biomacromolecules, 7(5), 1568–1572. DOI 10.1021/bm050912z. [Google Scholar] [CrossRef]
139. Kudłak, B., Owczarek, K., Namieśnik, J. (2015). Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environmental Science and Pollution Research, 22(16), 11975–11992. DOI 10.1007/s11356-015-4794-y. [Google Scholar] [CrossRef]
140. Pighinelli, L., Broquá, J., Zanin, B., Zanin, B. G., Flach, A. M. et al. (2019). Methods of chitin production a short review. American Journal of Biomedical Science and Research, 3, 307–314. DOI 10.34297/AJBSR.2019.03.000682. [Google Scholar] [CrossRef]
141. Mohan, K., Ganesan, A. R., Muralisankar, T., Jayakumar, R., Sathishkumar, P. et al. (2020). Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends in Food Science & Technology, 105, 17–42. DOI 10.1016/j.tifs.2020.08.016. [Google Scholar] [CrossRef]
142. Kaczmarek, M. B., Struszczyk-Swita, K., Li, X., Szczęsna-Antczak, M., Daroch, M. (2019). Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Frontiers in Bioengineering and Biotechnology, 7, 243. DOI 10.3389/fbioe.2019.00243. [Google Scholar] [CrossRef]
143. Younes, I., Ghorbel-Bellaaj, O., Nasri, R., Chaabouni, M., Rinaudo, M. et al. (2012). Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochemistry, 47(12), 2032–2039. DOI 10.1016/j.procbio.2012.07.017. [Google Scholar] [CrossRef]
144. Hamdi, M., Hammami, A., Hajji, S., Jridi, M., Nasri, M. et al. (2017). Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. International Journal of Biological Macromolecules, 101, 455–463. DOI 10.1016/j.ijbiomac.2017.02.103. [Google Scholar] [CrossRef]
145. Lucas, D. S., Oreste, A. J., Costa, E. Q., López, H. L. G., Saad, H. M. C. D. M. et al. (2021). Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chemistry, 343, 128550. DOI 10.1016/j.foodchem.2020.128550. [Google Scholar] [CrossRef]
146. Casadidio, C., Peregrina, D. V., Gigliobianco, M. R., Deng, S., Censi, R. et al. (2019). Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Marine Drugs, 17(6), 369. DOI 10.3390/md17060369. [Google Scholar] [CrossRef]
147. Healy, M., Romo, C., Bustos, R. (1994). Bioconversion of marine crustacean shell waste. Resources, Conservation and Recycling, 11(1–4), 139–147. DOI 10.1016/0921-3449(94)90085-X. [Google Scholar] [CrossRef]
148. Raimbault, M. (1998). General and microbiological aspects of solid substrate fermentation. Electronic Journal of Biotechnology, 1(3), 26–27. DOI 10.2225/vol1-issue3-fulltext-9. [Google Scholar] [CrossRef]
149. Zhang, H., Jin, Y., Deng, Y., Wang, D., Zhao, Y. (2012). Production of chitin from shrimp shell powders using Serratia marcescens b742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydrate Research, 362, 13–20. DOI 10.1016/j.carres.2012.09.011. [Google Scholar] [CrossRef]
150. Gamal, R. F., El-Tayeb, T. S., Raffat, E. I., Ibrahim, H. M., Bashandy, A. S. (2016). Optimization of chitin yield from shrimp shell waste by Bacillus subtilis and impact of gamma irradiation on production of low molecular weight chitosan. International Journal of Biological Macromolecules, 91, 598–608. DOI 10.1016/j.ijbiomac.2016.06.008. [Google Scholar] [CrossRef]
151. Sedaghat, F., Yousefzadi, M., Toiserkani, H., Najafipour, S. (2016). Chitin from Penaeus merguiensis via microbial fermentation processing and antioxidant activity. International Journal of Biological Macromolecules, 82, 279–283. DOI 10.1016/j.ijbiomac.2015.10.070. [Google Scholar] [CrossRef]
152. Jo, G., Jung, W., Kuk, J., Oh, K. T., Kim, Y. J. et al. (2008). Screening of protease-producing Serratia marcescens FS-3 and its application to deproteinization of crab shell waste for chitin extraction. Carbohydrate Polymers, 74(3), 504–508. DOI 10.1016/j.carbpol.2008.04.019. [Google Scholar] [CrossRef]
153. Jung, W., Jo, G., Kuk, J., Kim, Y. J., Oh, K. T. et al. (2007). Production of chitin from red crab shell waste by successive fermentation with Lactobacillus paracasei KCTC-3074 and Serratia marcescens FS-3. Carbohydrate Polymers, 68(4), 746–750. DOI 10.1016/j.carbpol.2006.08.011. [Google Scholar] [CrossRef]
154. Khanafari, A., Marandi, R., Sanatei, S. (2008). Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Journal of Environmental Health Science & Engineering, 5(1), 1–24. [Google Scholar]
155. Duan, S., Li, L., Zhuang, Z., Wu, W., Hong, S. et al. (2012). Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydrate Polymers, 89(4), 1283–1288. DOI 10.1016/j.carbpol.2012.04.051. [Google Scholar] [CrossRef]
156. de Lurdes, M., Dapkevičius, E., Batista, I., Rombouts, F. M., Houben, J. H. (1998). Lipid and protein changes during the ensilage of blue whiting (Micromesistius poutassou Risso) by acid and biological methods. Food Chemistry, 63(1), 97–102. DOI 10.1016/S0308-81469700156-8. [Google Scholar] [CrossRef]
157. Hall, G., De Silva, S. (1994). Shrimp waste ensilation. Infofish International, 2, 27–30. [Google Scholar]
158. Sedaghat, F., Yousefzadi, M., Toiserkani, H., Najafipour, S. (2017). Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. International Journal of Biological Macromolecules, 104, 883–888. DOI 10.1016/j.ijbiomac.2017.06.099. [Google Scholar] [CrossRef]
159. Ghorbel-Bellaaj, O., Younes, I., Maâlej, H., Hajji, S., Nasri, M. (2012). Chitin extraction from shrimp shell waste using Bacillus bacteria. International Journal of Biological Macromolecules, 51(5), 1196–1201. DOI 10.1016/j.ijbiomac.2012.08.034. [Google Scholar] [CrossRef]
160. Teng, W. L., Khor, E., Tan, T. K., Lim, L. Y., Tan, S. C. (2001). Concurrent production of chitin from shrimp shells and fungi. Carbohydrate Research, 332(3), 305–316. DOI 10.1016/S0008-6215(01)00084-2. [Google Scholar] [CrossRef]
161. Liu, P., Liu, S., Guo, N., Mao, X., Lin, H. et al. (2014). Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochemical Engineering Journal, 91, 10–15. DOI 10.1016/j.bej.2014.07.004. [Google Scholar] [CrossRef]
162. Zhang, H., Yun, S., Song, L., Zhang, Y., Zhao, Y. (2017). The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. International Journal of Biological Macromolecules, 96, 334–339. DOI 10.1016/j.ijbiomac.2016.12.017. [Google Scholar] [CrossRef]
163. Dun, Y., Li, Y., Xu, J., Hu, Y., Zhang, C. et al. (2019). Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. International Journal of Biological Macromolecules, 23, 420–426. DOI 10.1016/j.ijbiomac.2018.11.088. [Google Scholar] [CrossRef]
164. Hembach, L., Cord-Landwehr, S., Moerschbacher, B. M. (2017). Enzymatic production of all fourteen partially acetylated chitosan tetramers using different chitin deacetylases acting in forward or reverse mode. Scientific Reports, 7(1), 1–11. DOI 10.1038/s41598-017-17950-6. [Google Scholar] [CrossRef]
165. Lombard, V., Golaconda Ramulu, H., Drula, E., Coutinho, P. M., Henrissat, B. (2014). The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Research, 42(D1), D490–D495. DOI 10.1093/nar/gkt1178. [Google Scholar] [CrossRef]
166. Araki, Y., Ito, E. (1975). A pathway of chitosan formation in Mucor rouxii: Enzymatic deacetylation of chitin. European Journal of Biochemistry, 55(1), 71–78. DOI 10.1111/j.1432-1033.1975.tb02139.x. [Google Scholar] [CrossRef]
167. Elnashar, M. M. M. (2011). Biotechnology of biopolymers. Rejika, Croatia: InTech. DOI 10.5772/683. [Google Scholar] [CrossRef]
168. Honarkar, H., Barikani, M. (2009). Applications of biopolymers I: Chitosan. Monatshefte für Chemie-Chemical Monthly, 140(12), 1403–1420. DOI 10.1007/s00706-009-0197-4. [Google Scholar] [CrossRef]
169. Share & trends analysis report by application (Pharmaceutical & biomedical, water treatment, cosmetics, food & beverageby region (APAC, North America, Europe, MEAand segment forecasts, 2020–2027, Grand View Research. (2020). Research and markets. https://www.grandviewresearch.com/industry-analysis/global-chitosan-market. [Google Scholar]
170. Kara, A., Tamburaci, S., Tihminlioglu, F., Havitcioglu, H. (2019). Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules, 130, 266–279. DOI 10.1016/j.ijbiomac.2019.02.067. [Google Scholar] [CrossRef]
171. Terada, M., Izumi, K., Ohnuki, H., Saito, T., Kato, H. et al. (2012). Construction and characterization of a tissue-engineered oral mucosa equivalent based on a chitosan-fish scale collagen composite. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 100(7), 1792–1802. DOI 10.1002/jbm.b.32746. [Google Scholar] [CrossRef]
172. Tran, T. D. M., Nguyen, C. T., Do, T. Q., Bach, G. L., Trinh, N. H. et al. (2020). Preparation and characterization of chitosan/fish scale collagen/lovastatin nanocomposites. Journal of Polymers and the Environment, 28(11), 2851–2863. DOI 10.1007/s10924-020-01819-3. [Google Scholar] [CrossRef]
173. Bi, J., Tian, C., Zhang, G. L., Hao, H., Hou, H. M. (2021). Novel procyanidins-loaded chitosan-graft-polyvinyl alcohol film with sustained antibacterial activity for food packaging. Food Chemistry, 365, 130534. DOI 10.1016/j.foodchem.2021.130534. [Google Scholar] [CrossRef]
174. Ni, Y., Nie, H., Wang, J., Lin, J., Wang, Q. et al. (2022). Enhanced functional properties of chitosan films incorporated with curcumin-loaded hollow graphitic carbon nitride nanoparticles for bananas preservation. Food Chemistry, 366, 130539. DOI 10.1016/j.foodchem.2021.130539. [Google Scholar] [CrossRef]
175. Huang, P., Huang, C., Ma, X., Gao, C., Sun, F. et al. (2021). Effect of pH on the mechanical, interfacial, and emulsification properties of chitosan microgels. Food Hydrocolloids, 121, 106972. DOI 10.1016/j.foodhyd.2021.106972. [Google Scholar] [CrossRef]
176. Dan, S., Sharma, D., Rastogi, K., Shaloo, Ojha, H. et al. (2022). Therapeutic and diagnostic applications of nanocomposites in the treatment Alzheimer’s disease studies. Biointerface Research in Applied Chemistry, 12(1), 940–960. DOI 10.33263/BRIAC121.940960. [Google Scholar] [CrossRef]
177. Dahri, M., Akbarialiabad, H., Jahromi, A. M., Maleki, R. (2021). Loading and release of cancer chemotherapy drugs utilizing simultaneous temperature and pH-responsive nanohybrid. BMC Pharmacology and Toxicology, 22(1), 1–10. DOI 10.1186/s40360-021-00508-8. [Google Scholar] [CrossRef]
178. Taherian, A., Esfandiari, N., Rouhani, S. (2021). Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnology, 12(1), 1–20. DOI 10.1186/s12645-021-00086-8. [Google Scholar] [CrossRef]
179. Wang, Y., Han, Z., Li, A., Cui, C. (2021). Enhanced electrokinetic remediation of heavy metals contaminated soil by biodegradable complexing agents. Environmental Pollution, 283, 117111. DOI 10.1016/j.envpol.2021.117111. [Google Scholar] [CrossRef]
180. Mishra, D., Yadav, R., Singh, R. P., Taneja, A., Tiwari, R. et al. (2021). The incorporation of lemongrass oil into chitosan-nanocellulose composite for bioaerosol reduction in indoor air. Environmental Pollution, 285, 117407. DOI 10.1016/j.envpol.2021.117407. [Google Scholar] [CrossRef]
181. Zhang, D., Wang, Y., Wang, X., Chen, B., Wang, Y. et al. (2021). Physical and chemical synergistic strategy: A facile approach to fabricate monovalent ion permselective membranes. Chemical Engineering Science, 245, 116873. DOI 10.1016/j.ces.2021.116873. [Google Scholar] [CrossRef]
182. Cho, C. W., Lim, C. R., Cho, B. G., Mun, S. B., Choi, J. W. et al. (2021). Development of prediction models for adsorption properties of chitin and chitosan for micropollutants. Chemical Engineering Journal, 426, 131341. DOI 10.1016/j.cej.2021.131341. [Google Scholar] [CrossRef]
183. MacDonald, R., Reitmeier, C. (2017). Food Processing. In: MacDonald, R., Reitmeier, C. (Eds.Understanding food systems: Agriculture, food science, and nutrition in the United States, pp. 179–223. USA: Elsevier Science Publishing Co, Inc. [Google Scholar]
184. Abdel-Naeem, H. H., Sallam, K. I., Malak, N. M. (2021). Improvement of the microbial quality, antioxidant activity, phenolic and flavonoid contents, and shelf life of smoked herring (Clupea harengus) during frozen storage by using chitosan edible coating. Food Control, 130, 108317. DOI 10.1016/j.foodcont.2021.108317. [Google Scholar] [CrossRef]
185. Ma, Q., Liang, T., Cao, L., Wang, L. (2018). Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. International Journal of Biological Macromolecules, 108, 576–584. DOI 10.1016/j.ijbiomac.2017.12.049. [Google Scholar] [CrossRef]
186. Fathima, P. E., Panda, S. K., Ashraf, P. M., Varghese, T. O., Bindu, J. (2018). Polylactic acid/chitosan films for packaging of Indian white prawn (Fenneropenaeus indicus). International Journal of Biological Macromolecules, 117, 1002–1010. DOI 10.1016/j.ijbiomac.2018.05.214. [Google Scholar] [CrossRef]
187. Shi, J., Kantoff, P. W., Wooster, R., Farokhzad, O. C. (2017). Cancer nanomedicine: Progress, challenges and opportunities. Nature Reviews Cancer, 17(1), 20–37. DOI 10.1038/nrc.2016.108. [Google Scholar] [CrossRef]
188. Esfandiari, N., Arzanani, M. K., Koohi-Habibi, M. (2018). The study of toxicity and pathogenicity risk of potato virus X/Herceptin nanoparticles as agents for cancer therapy. Cancer Nanotechnology, 9(1), 1–13. DOI 10.1186/s12645-018-0036-6. [Google Scholar] [CrossRef]
189. Garg, U., Chauhan, S., Nagaich, U., Jain, N. (2019). Current advances in chitosan nanoparticles based drug delivery and targeting. Advanced Pharmaceutical Bulletin, 9(2), 195–204. DOI 10.15171/apb.2019.023. [Google Scholar] [CrossRef]
190. Saeed, R. M., Dmour, I., Taha, M. O. (2020). Stable chitosan-based nanoparticles using polyphosphoric acid or hexametaphosphate for tandem ionotropic/covalent crosslinking and subsequent investigation as novel vehicles for drug delivery. Frontiers in Bioengineering and Biotechnology, 8, 4. DOI 10.3389/fbioe.2020.00004. [Google Scholar] [CrossRef]
191. Nasrollahpour, H., Isildak, I., Rashidi, M. R., Hashemi, E. A., Naseri, A. et al. (2021). Ultrasensitive bioassaying of HER-2 protein for diagnosis of breast cancer using reduced graphene oxide/chitosan as nanobiocompatible platform. Cancer Nanotechnology, 12(1), 1–16. DOI 10.1186/s12645-021-00082-y. [Google Scholar] [CrossRef]
192. Jia, Z., Müller, M., Le Gall, T., Riool, M., Müller, M. et al. (2021). Multiplexed detection and differentiation of bacterial enzymes and bacteria by color-encoded sensor hydrogels. Bioactive Materials, 6(12), 4286–4300. DOI 10.1016/j.bioactmat.2021.04.022. [Google Scholar] [CrossRef]
193. Jiang, Y., Wu, J. (2019). Recent development in chitosan nanocomposites for surface-based biosensor applications. Electrophoresis, 40(16–17), 2084–2097. DOI 10.1002/elps.201900066. [Google Scholar] [CrossRef]
194. da Silva Alves, D. C., Healy, B., Pinto, L. A., Cadaval, T. R., Breslin, C. B. (2021). Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules, 26(3), 594. DOI 10.3390/molecules26030594. [Google Scholar] [CrossRef]
195. Wang, J., Zhuang, S. (2017). Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Critical Reviews in Environmental Science and Technology, 47(23), 2331–2386. DOI 10.1080/10643389.2017.1421845. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |