

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.019152
REVIEW
Pathways for Sustainable Utilization of Waste Chicken Eggshell
1Department of Mechanical and Industrial Engineering Technology, University of Johannesburg, Johannesburg, South Africa
2African Centre of Excellence, ACESPED University of Nigeria, Nsukka, Nigeria
3Faculty of Engineering and the Built Environment, University of Johannesburg, Johannesburg, South Africa
*Corresponding Author: Omojola Awogbemi. Email: oawogbemi@uj.ac.za
Received: 06 September 2021; Accepted: 04 November 2021
Abstract: Chicken eggshell is one of the most common wastes generated from households, restaurants and other food processing outlets. Waste Chicken Eggshells (WCES) also constitutes an environmental nuisance and ends up discarded at dumping site with no consideration of further usage. The main constituent of WCES is calcium carbonate from which calcium or calcium oxide can be extracted for various applications. This current effort reviews recently published literature on the diverse applications of WCES. The considered utilization avenues include catalysts for biofuel production, construction industry, wastewater purification, industrial sector, food industry, medical, and agricultural applications. The specific areas of application apart from the transesterification reactions include cement additives and replacement in concrete, asphalt binder, adsorbent of metals and dyes, production of hydroxyapatite, food supplement and fortification, dentistry, therapeutics, bone formation, drug delivery, poultry feeds as well as organic fertilizer. For most of the identified applications, the WCES is subjected to pretreatment and other modification techniques before utilization. The conversion of WCES to valuable products is a cost-effective, safe, environmentally friendly, non-toxic and viable means of waste disposal and utilization. More investigations are needed to further explore the benefits derivable from this bioresource.
Keywords: Waste chicken eggshells; catalysts; food supplements; medical applications; waste utilization; biofuel production
Nomenclature
| % | Percentage |
| rpm | revolution per minute |
| °C | Degree centigrade |
| wt% | Weight percent |
The utilization of food wastes for various applications has been recognized as one of the strategies for reducing the cost of production and the market price of some products. By definition, food wastes are any edible and allied inedible parts detached and discarded from the food value chain either at households, retail, or food service sectors. The wastes can either be processed, semi-processed, unprocessed, or raw food meant for human consumption. The edible parts of food are the parts that are meant for human consumption while the inedible parts, including bones, shells, peels, etc., are those that are discarded as wastes [1].
The Food and Agricultural Organization (FAO) estimated that the total edible food waste, globally, is 1.3 billion tonnes with a carbon footprint of 3.3 billion tonnes of CO2 equivalence per annum which is equivalent of about 1.4 hectares of land and 250 × 103 m3 of water annually [2]. Unconsumed foods have negative social, economic, and sanitary impacts and have been estimated to contribute 8–10% of greenhouse gas (GHG) emissions, globally [1]. The Sustainable Development Goals aim to reduce global food wastes by half by the year 2030 [3]. This is to prevent food losses and save humanity from hunger. Reducing food waste will guarantee more food gets to the consumers, reduce malnutrition and hunger, mitigate the emission of GHGs, improve food security, relieve pressures on land and water, enhance sanitation and a boost to effective waste management systems. Chicken eggshells are generated when raw or boiled chicken eggs are broken and used as foods in households, restaurants and other food processing outlets. Waste Chicken Eggshells (WCES) constitute an environmental nuisance when they are inappropriately discarded at dumping sites with no consideration of further usage.
The global production of chicken eggs increased from 73.9 million metric tonnes in 2016 to become 82.17 million metric tonnes in 2019 with China accounting for about 42.88% of the total egg production in 2019. China, the United States of America, India, Indonesia, and Brazil jointly produced 66.67% of the global egg production in 2019. Among African countries, Nigeria, South Africa, Morocco, Egypt, and Algeria top the egg production chart [4]. Fig. 1 shows the chicken egg production in some selected countries from 2013 to 2019.
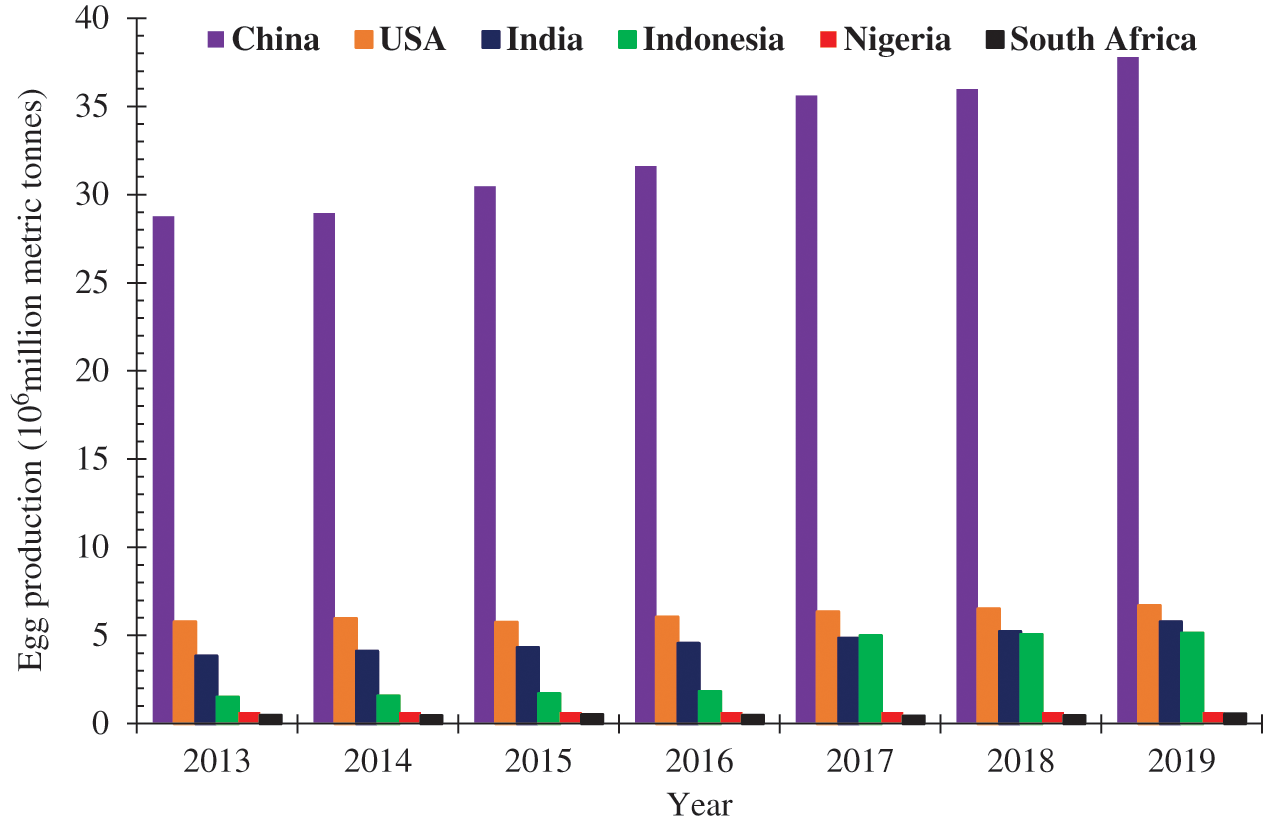
Figure 1: Chicken Egg production in some selected countries between 2013–2019 adapted from [4]
Characteristically, a fresh chicken egg is made up of three parts, i.e., the shell, egg white and egg yolk. Consumption of chicken eggs has continued to increase among various strata of the population due to its high protein content and many industrial applications. The risk of adults developing cardiovascular disease from the consumption of eggs is been overshadowed by the urge for essential nutrients contained in chicken eggs. Chicken eggs are rich in protein and essential amino acids needed for growth [5]. The many applications of chicken eggs in the food, biotechnology, and pharmaceutical industries have further increased its consumption among the population. WCES are the 15th source of food pollution and a major cause of environmental pollution. An estimated 250,000 tonnes of waste eggshell are generated and added to the landfill without any pretreatment. These waste eggshells are dumped in the landfills, they are non-biodegradable and provide a habitat for fungal growth, offensive odour and mosquito breeding thereby causing avoidable diseases [6,7]. An average chicken egg weighs about 60.9 g and consists of 11% shell, 63% albumen, and 27% yolk by weight [8]. Fig. 2 shows the structure and cross-section of a chicken egg. The eggshell, which has three layers, is a protective covering for the egg against harm and bacteria but allows the exchange of moisture and air.
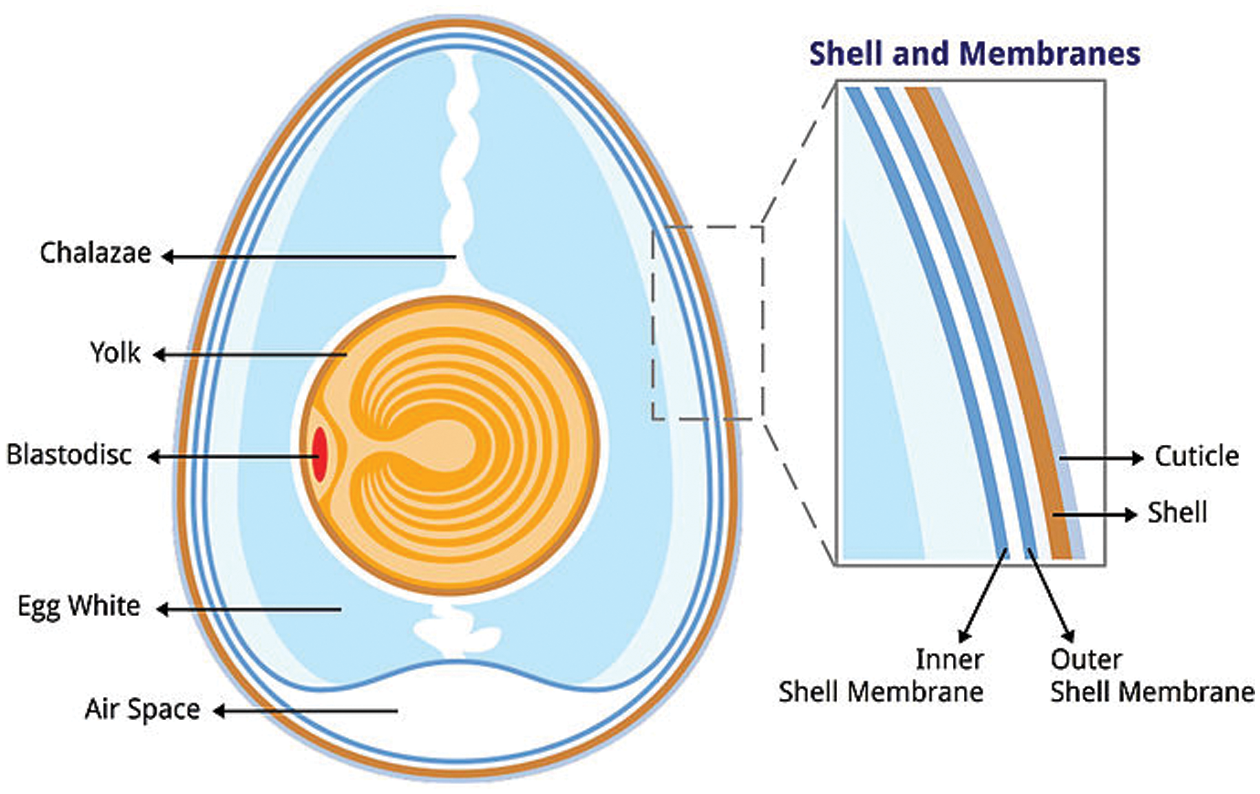
Figure 2: Structure and cross-sectional view of chicken eggshell [9]
Typically, chicken eggshell is chemically composed of 94% calcium carbonate (CaCO3), 1% magnesium carbonate, 1% calcium phosphate with the balance made up of water and other insoluble proteins [10,11]. The high percentage of calcium in chicken eggshells accounts for its high mechanical strength and makes it a valuable raw material for diverse applications. Waste eggshells are generated daily from the consumption of eggs in households and industrial concerns. With time, the increasing volume of waste eggshell generated and discarded indiscriminately has attracted the attention of environmentalists and researchers to investigate how the wastes can be converted into useful products. Traditionally, without any form of modification, WCES has been used to clean cooking pots, feed birds, make bone broth and used as fertilizer to grow vegetables. But much more than that, various investigations have been conducted on the use of eggshells as catalysts, in wastewater treatment and purification, concrete enhancement and environmental remediation.
Earlier, Hamideh et al. [7], Hamada et al. [12–15] reviewed the applications of eggshells in cement replacement in concrete, as a catalyst for biodiesel production, as adsorbent and wastewater decontamination, respectively. The various interventions on the use of WCES by Waheed et al. [11], Perez-Lopez et al. [16–18] are acknowledged. Despite all these completed studies on the application of WCES, the relevant question to ask, which serves as the motivation and basis for this work, is whether enough has been investigated and reported about this valuable biowaste? The current effort specifically focuses on the applications of WCES in diverse fields. The identified avenues of utilization for WCES are studied and interrogated using a compilation of the recent applications of WCES in various fields. Also, this contribution aims to shed more light on the recent efforts to reduce, reuse, and recycle WCES for catalytic, wastewater treatment, pharmaceutical, mechanochemistry, environmental remediation and material science applications. The current effort is limited to the applications of WCES as reported in the last five years.
3 Application of Waste Chicken Eggshells
Primarily, WCES is not produced to be used as food for human or animal consumption. As said earlier, the fact that an average chicken egg contains about 11% of the weight of WCES and is produced in large quantities makes researchers interested in how to put this important resource into use. Chicken eggshells are readily available for collection in large quantities from households, restaurants and food outlets, hatcheries, poultry farms, cake bakers, and other industries that use chicken eggs as raw materials. Calcium, the major constituent of chicken eggshells, has been found to possess abundant mechanical strength and is a major dietary requirement for adults. The presence of elements like boron, copper, iron, molybdenum, sulphur, silicon and zinc also makes chicken eggshells a potential raw material for many applications [11,19]. Many of the important applications of waste chicken eggshells are highlighted in this section.
The need to reduce the production cost and, ultimately, the market price of biodiesel has escalated the search for cheap and readily available materials with high calcium content to serve as a replacement for commercial calcium oxide (CaO). Catalytic synthesis of biodiesel is one of the approaches to meet the ever-increasing request for biodiesel. Biodiesel, which is generated through the transesterification of feedstocks such as edible oils, waste cooking oil (WCO), waste animal fats, recovered fats, etc. in the presence of catalysts and alcohol (methanol, ethanol) is a sustainable alternative for fossil-based diesel fuel. The continuous use of fossil-based diesel fuel in compression ignition (CI) engines has caused poor engine performance, increased emission of carbon dioxide (CO2) and other poisonous gases which negatively impact human health, exacerbate global warming and accelerate environmental degradation [20–22].
CaO is among the oxides of alkaline earth metals with high basicity which is utilized as a heterogeneous catalyst for commercial biodiesel synthesis. Advantages of CaO derived from WCES as a candidate for large-scale catalytic biodiesel production include its availability, low cost, low solubility in biodiesel, high biodiesel yield, low energy consumption, strong basicity, less water needed for washing, ease of separation, high reusability and less impact of the environment [21,23,24]. The few drawbacks in the application of waste chicken-derived CaO like its low surface area, leaching, and possible contamination when exposed to water have not diminished its acceptance for catalytic biodiesel production [25].
The preparation protocol for the conversion of WCES catalysts is as shown in Fig. 3. The discarded chicken eggshells are collected and washed with clean water to get rid of all the impurities adhering to the body of the shells, dried in the oven, pulverized into fine particles and sieved with the appropriate mesh sizes. The grinding is to boost the surface contact area and enhance the catalytic performance of the WCES material [26]. The powdered material, which consists of mainly CaCO3, can be used at this stage or subjected to further modification to improve its performance. Calcination is one of the modification techniques applied to heterogeneous catalysts to enhance their performance. During calcination, the WCES powder is heated to a temperature of about 800°C–900°C to thermally decompose CaCO3 to CaO and CO2 as shown in Eq. (1) [27,28].
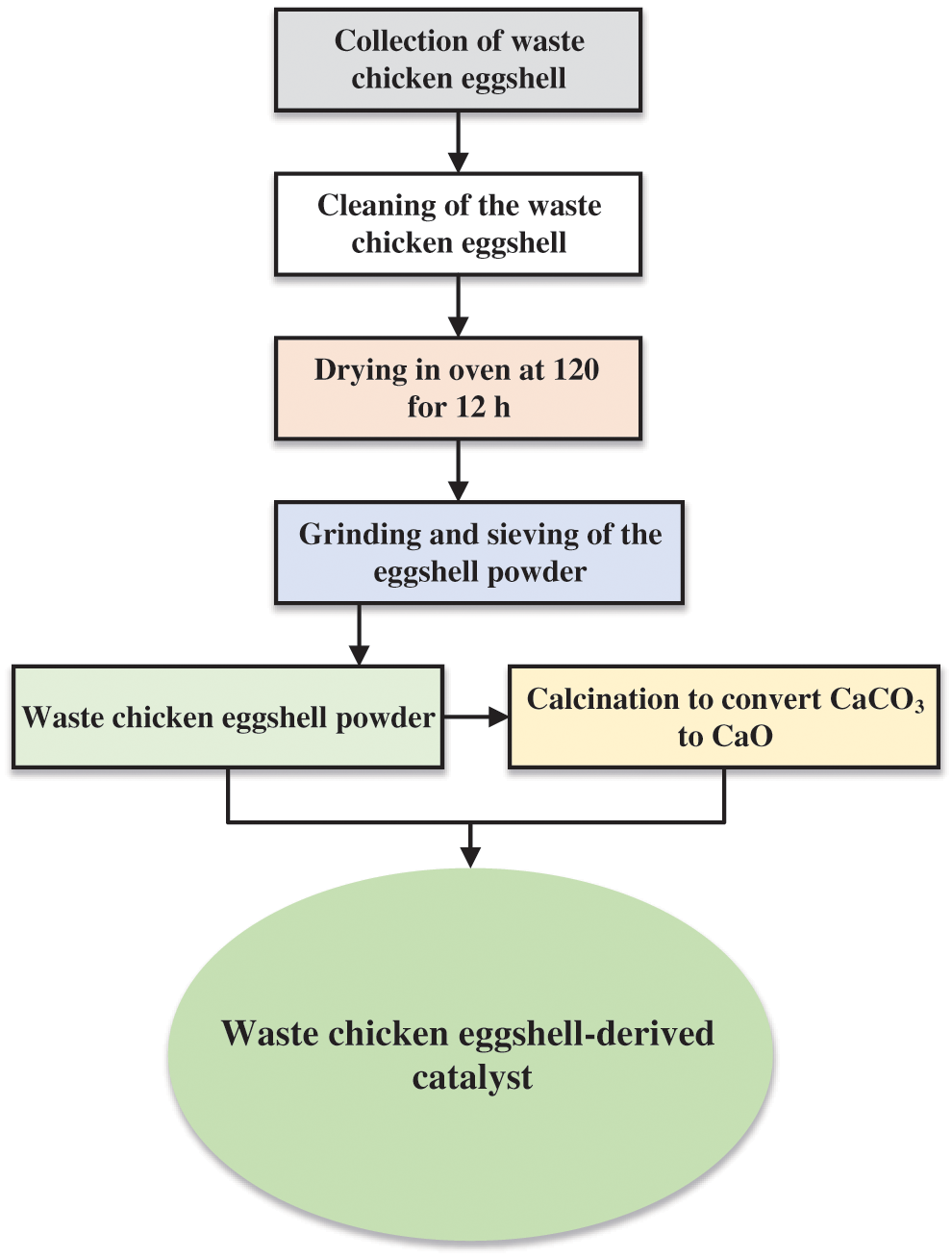
Figure 3: Flowchart for converting waste chicken eggshells to catalyst
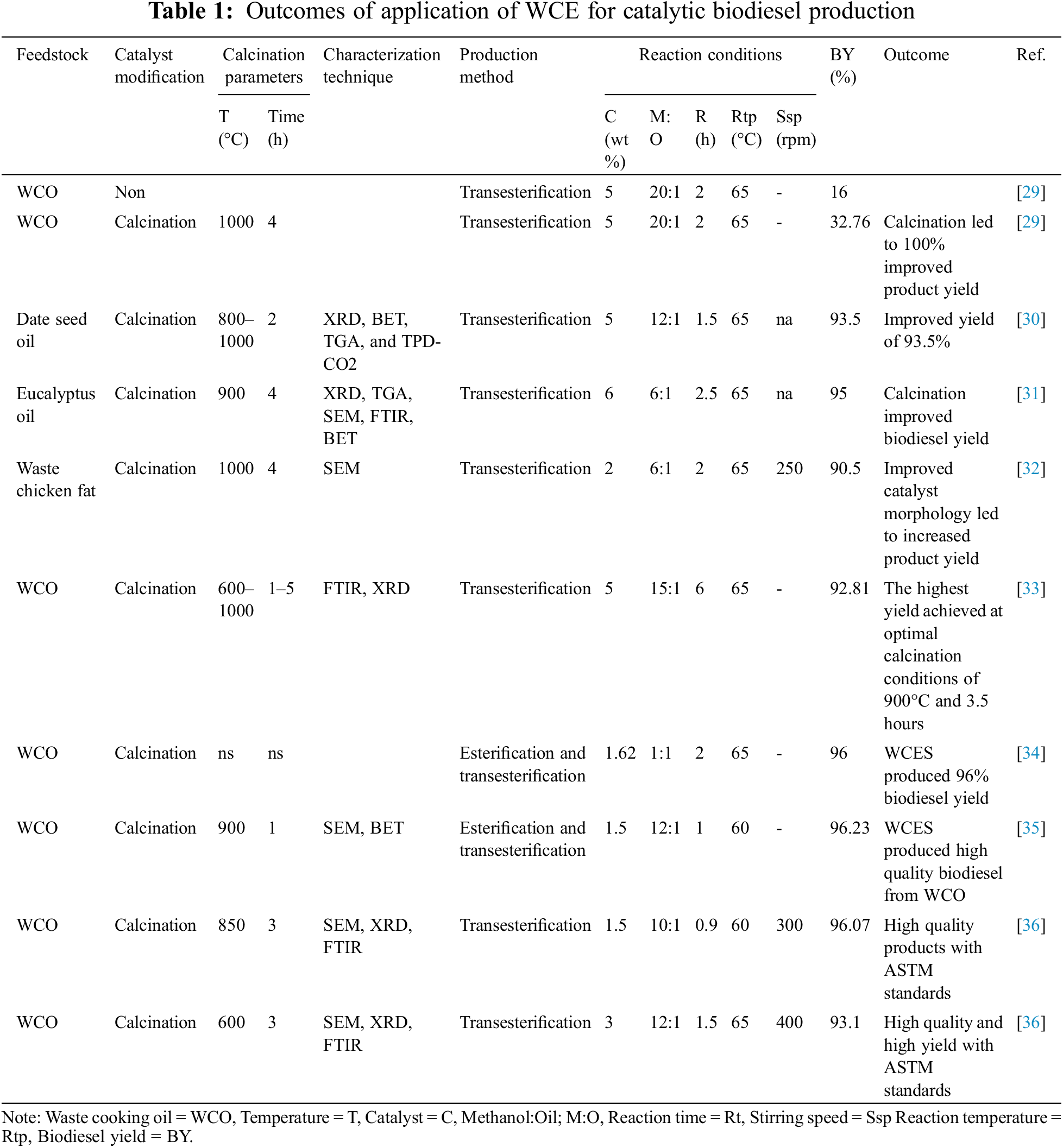
Recent researches on the deployment of CaO obtained from WCES as a catalyst for large-scale biodiesel production revealed chicken eggshells as a promising biowaste with catalytic applications. To verify the effect of calcination on the performance of catalyst derived from WCES, Kamaronzaman et al. [29] experimented with an uncalcined and calcined catalyst for the conversion of WCO into biodiesel and reported that calcined catalyst yielded 100% more than the uncalcined catalyst. This is in agreement with the submission of Farooq et al. [30] to the effect that calcination increases the active sites on the contact surface of the heterogeneous catalyst and consequently enhances its catalytic activity and performance.
Catalyst derived from WCES was used in the conversion of WCO and other feedstocks into biodiesel under mild temperatures resulting in high product yield [31,32]. These processes have proved to be cost-effective, easy to achieve, and commercially viable (Table 1). Similarly, Lani et al. [33–36] successfully employed CaO derived from WCE to synthesize biodiesel from WCO. They employed statistical and optimization techniques (Taguchi, response surface methodology, central composite design) and reported that the deployment of these techniques can boost the biodiesel yield by optimizing the production parameters.
Apart from the use of WCES for catalytic biodiesel production, the production of bioethanol is also catalyzed by CaO extracted from WCES. Liu et al. [37] and Hu et al. [38] utilized WCES in the hydrolysis of cellulose to bioethanol. The authors reported over 60% product yield under mild production conditions. Similarly, calcium nanoparticle derived from WCES has been used to improve anaerobic digestion of palm oil mill effluent into biogas. The WCES powder was modified by calcination and applied to improve catalytic biogas production [39]. Raheem et al. [40] extracted CaO from WCES for the catalytic gasification of lipid-extracted microalgae biomass. It was reported that the addition of the CaO derived from WCES enhanced the gasification reactions with evidence of increased H2 production and reduced generation of carbon monoxide (CO) and CO2.
Key conclusions:
• WCES can be converted into catalysts for biofuel production.
• Calcination improves the performance and catalytic activity of WCES in biodiesel synthesis.
• The economic and environmental benefits of utilization of WCES make it a viable alternative to commercial CaO.
Rapid population growth, rising urbanization, higher disposable income and increasing infrastructural development across nations have caused a steady rise in activities in the construction industries over the past few decades. Concrete, consisting of cement, fine or coarse aggregates and water, is the most frequently and extensively used composite material in the building and road construction industries. Cement is a binder and when used in conjunction with water, sets, hardens, adheres, and binds fine or coarse aggregates together. Global cement production has increased from 3.31 billion tons in 2010 to about 4.1 billion tonnes in 2020, with China contributing more than half to the total global cement production in 2020 (Fig. 4) [41,42]. A total of 4.42 billion tonnes is projected to be consumed, worldwide, in 2021, representing a growth rate of about 3% since 2018 [43].
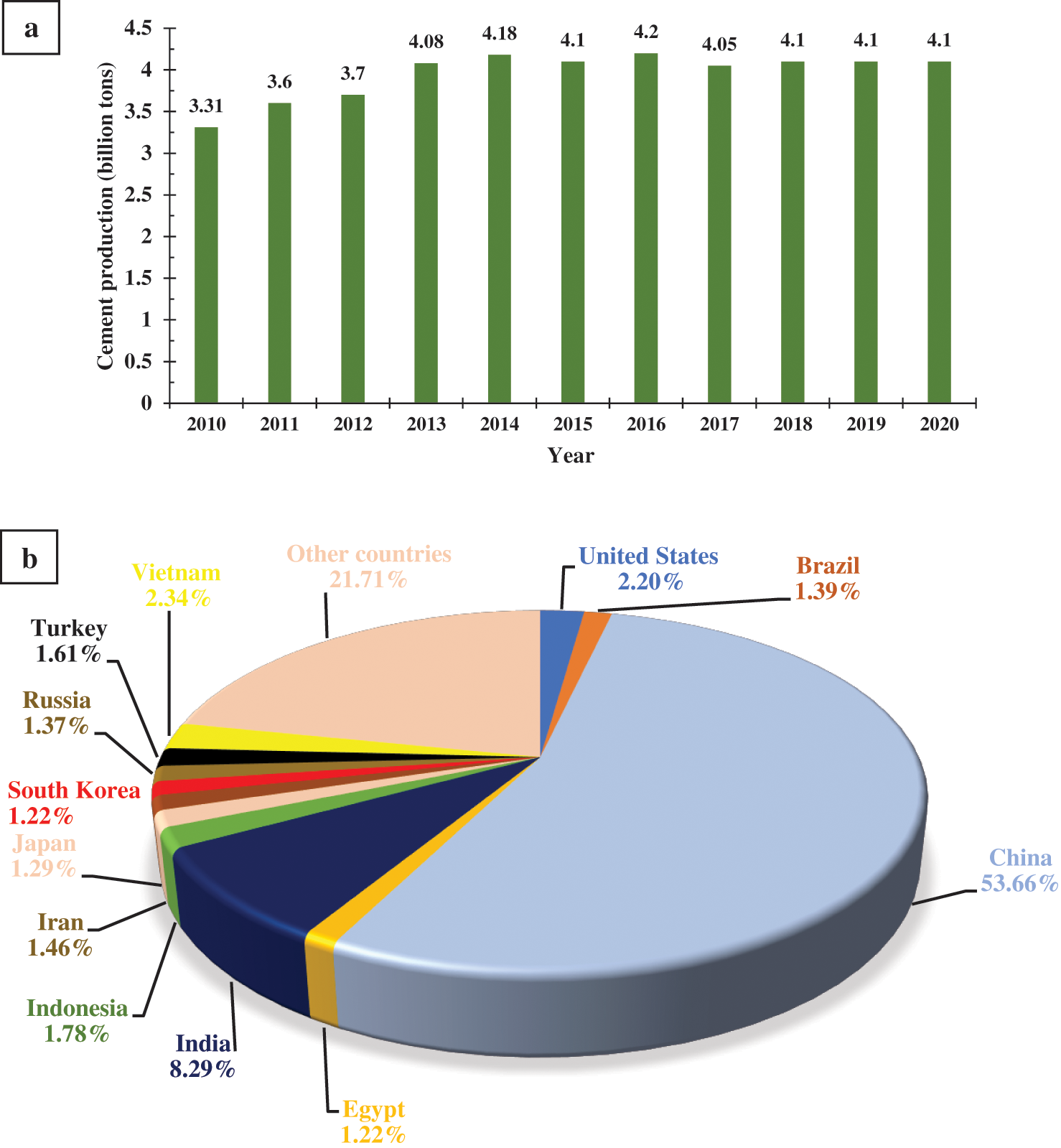
Figure 4: Cement production status (a) Global cement production from 2010 to 2010. (b) Country by country cement production in 2020. Compiled and developed from [41,42]
The overdependence on natural raw materials like limestone (CaCO3), bauxite, clay, or rock for cement production has led to increased cost of production, depletion of reservoirs of these materials, and exacerbated environmental degradation. The exploitation and extraction of limestone, silica, and alumina have continued to damage the landscape, disrupt the ecosystem, pollute the air, and contaminate aquatic and terrestrial habitats [44]. There have been concerns in the cement manufacturing industry about the increased energy consumption and emission of GHGs during cement production. For example, the cement industry consumes nearly 2% of the world’s primary energy and about 5% of the total industrial energy, globally [45]. Also, the cement industry contributed 3% to the global GHG emission in 2018. About 4050 metric tons of cement was produced in 2018 with 0.5 ton of CO2 emitted per ton of cement [46].
The majority of the energy consumption and emission occurs during the production of clinker, a component of cement, and thermal breakdown of CaCO3 to CaO. It is estimated that between 50% and 60% of CO2 emission is produced during the decomposition of CaCO3 to CaO. If there is no deliberate strategy implemented to arrest the current rate of emission, the CO2 emission from the cement industry is predicted to reach 2.34 billion tonnes by 2050 [45]. One of the strategies to stem the tide of high energy consumption and CO2 emissions from the cement production process without compromising production capacity is to find a sustainable replacement for natural CaCO3. WCES have been proposed as a viable option, to take advantage of their vast availability and abundant CaCO3 and CaO content, as aforementioned. Aside from the fact that CaCO3 occurs naturally in WCES, the conversion of WCES to CaO generates less CO2 than what happened during the production of clinker. Besides, lime, which constitute almost 60%–65% of cement, is found in abundance in WCES, about 63.8%–96.87% [27,47].
The preparation techniques for the conversion of WCES to CaO powder involve washing and drying to remove all the dirt on the body of the shells, crushing and sieving into fine particles, and high temperature (700°C–900°C) calcination to convert the CaCO3 to CaO [48]. Avenues for the utilization of WCES powder for construction applications include cement manufacturing, cement additive, concrete aggregate, and ceramic materials. The application of pulverized and calcinated WCES has been observed to reduce the production cost, improve the physical and mechanical properties, and lessen its environmental impact [49].
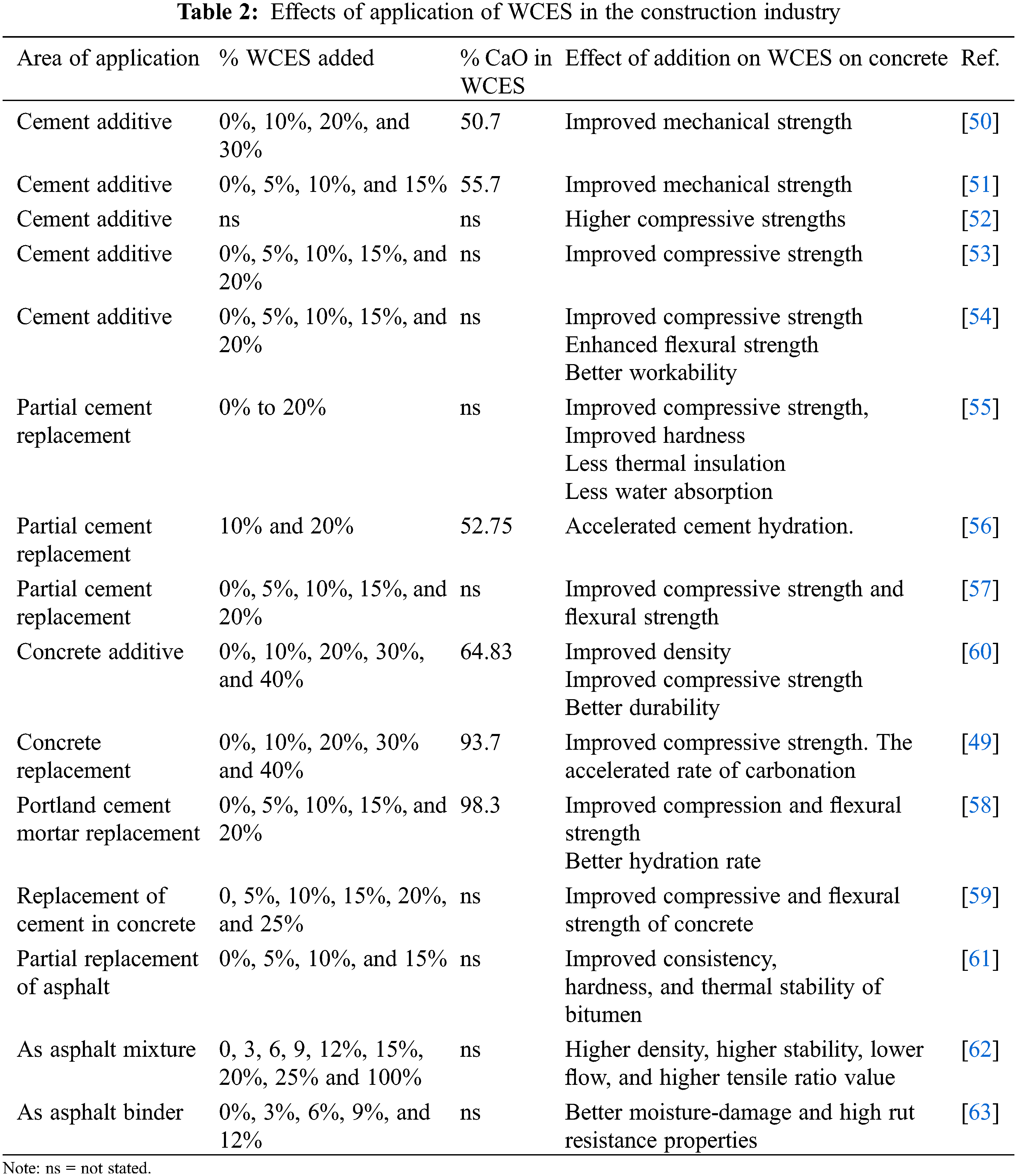
Documented research outcomes have shown that fragmentary replacement of Portland cement with calcinated WCES powder improves mechanical strength, compressive strength, flexural strength, and engenders better workability of the concrete [50–54]. In another research, Jaber et al. [55–57] compared the thermal insulation, hardness, water absorption, hydration, compressive strength and flexural strength of concrete prepared with WCES and that prepared with Portland cement. They reported an improvement in the tested properties of the concrete prepared with WCES. Outcomes of similar research conducted by Razali et al. [49], Pliya et al. [58,59] on the use of WCES as a partial replacement for fine aggregates and Portland cement were reported and well documented. Francis et al. [60] examined the impact of the addition of chicken eggshell powder on the compressed concrete and reported that addition of WCES led to improvement in the density, compressive strength, and durability of the concrete. The effect of the addition of various percentages of WCES to asphalt was investigated by Wang et al. [61–63] who revealed improved strength, hardness, and thermal stability of the bitumen. Table 2 is a compilation of the outcomes of some researches on the application of WCES as cement additive and partial concrete replacement.
Key conclusions:
• WCES is a viable resource that can be utilized in the construction industry to substitute limestone in cement production.
• CaO powder derived from WCES is a good replacement for Portland cement.
• Mechanical properties, strength, durability, thermal stability and functionality of concrete could be improved with the inclusion of fine aggregates of WCES.
• The addition of WCES powder advances the stability, density, and durability of asphalt.
• The utilization of WCES in the construction industry guarantees waste minimization, reduces landfill wastes, ensures conversion of waste to useful materials, and decelerates environmental degradation.
3.3 Wastewater Purification Applications
Though approximately 70% of the Earth’s crust is covered with water, lack of access to safe water is a major environmental problem, exacerbating malnutrition, and a leading cause of cholera, diarrhea, typhoid, dysentery, and other infectious diseases. In 2017, about 1.23 million deaths were recorded as a result of unsafe water sources globally [64]. Water resources have been under enormous stress due to industrialization, urbanization, rapid population growth and uncontrolled utilization for industrial and agricultural applications [65]. Accessibility to good water has been impaired over the last few decades partly due to the impact of pollution of ground and surface water by wastes from households and industries, alike. Untreated effluents that are toxic, acidic and carcinogenic generated from industries are frequently discharged into the environment and impairs terrestrial and aquatic life. Consumption of water contaminated with heavy metals like lead, chromium, copper, zinc, carbon dioxide, dye, etc., can have negative effects on humans. For example, the World Health Organization (WHO) has warned that exposure to lead can cause behaviour and learning difficulties in children; reduced development of the fetus and premature birth in pregnant women; and hypertension, renal impairment, and cardiovascular problems in adults [66]. With the rate of water pollution, contamination, and wastewater generation, water purification is the only respite to ensure healthy living and sustenance of life.
Various physical, chemical, and biological technologies have been deployed to decontaminate and purify wastewater but the adsorption method is more frequently applied due to its low cost, simplicity, flexibility, efficiency, and versatility in removing deadly contaminants. Available information from published literature lends credence to the utilization of activated charcoal and other biowastes to get rid of organic and inorganic pollutants from wastewater [67,68]. WCES is an emerging low-cost, environmentally friendly, and effective adsorbent with the potential to purify ground and wastewater. Also, CaO derived from WCES has low moisture content and its surface area can be increased by mechanical and thermal modification techniques. During the process, collected WCES is cleaned, oven-dried and crushed into fine particles, to increase the surface area. The WCES is later subjected to high-temperature calcination to convert the CaCO3 in the WCES to CaO. Though Mittal et al. [14], Carvalho et al. [15], and Tamjidi et al. [69] had reviewed the use of eggshells for water purification, the current intervention is focused specifically on the application of WCES for wastewater treatment and purification.
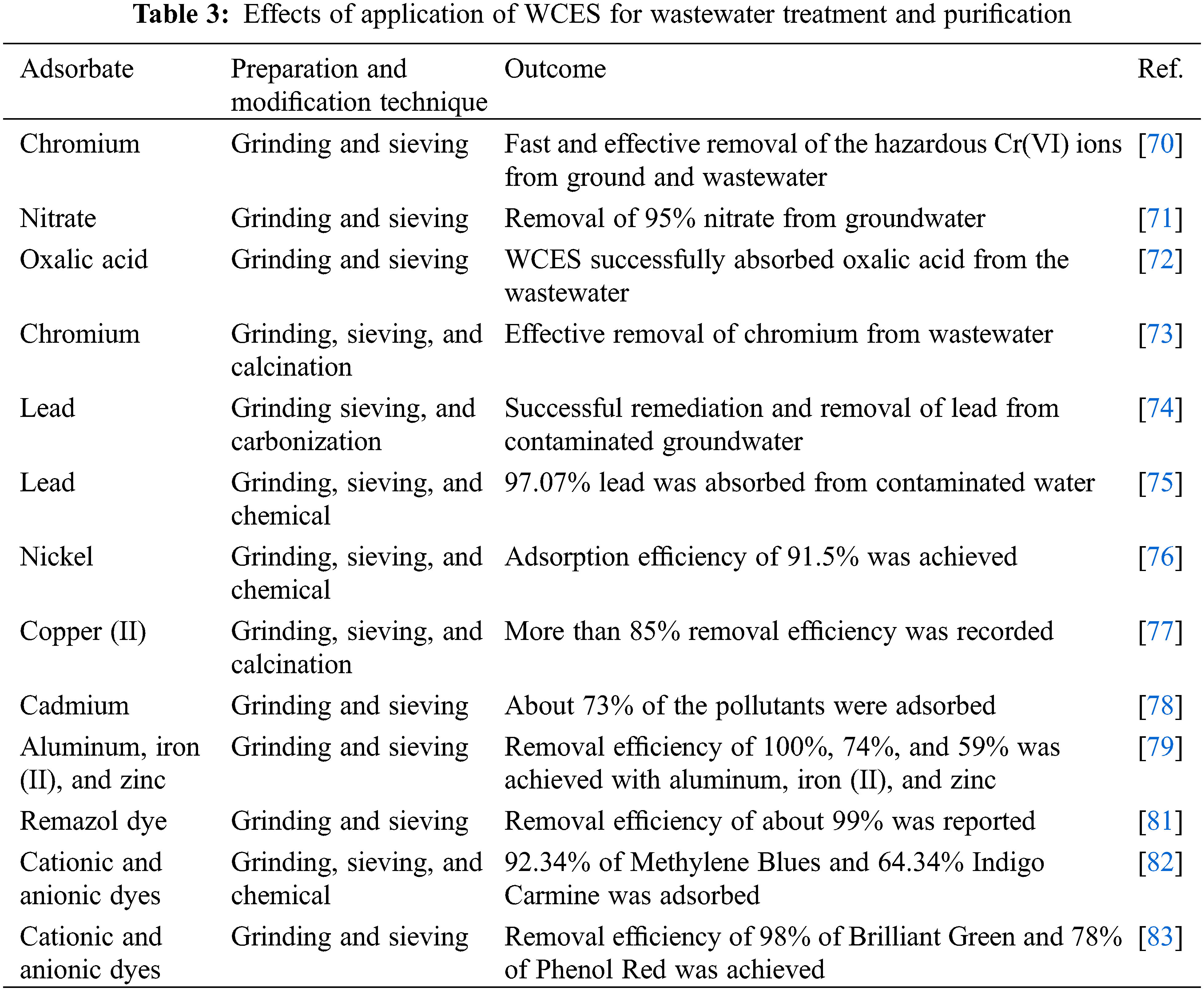
Daraei et al. [70–73] investigated the use of WCES powder for the removal of adsorbates like chromium, nitrate, oxalic acid, and heavy metals from ground and wastewater. They reported the efficacy and the applicability of WCES in removing the adsorbate. The application of WCES powder as an adsorbent for the elimination of lead has been well demonstrated by many researchers in recent years. Wang et al. [74] successfully used biochar developed from WCES powder low-cost adsorbent for the remediation of contaminated water while Jalu et al. [75] used CaO extracted from WCES as adsorbent for water decontamination. Their efforts were successful as up to 97% of the lead pollutant were removed. Other researchers also utilized WCES ground into fine powder for the adsorption of nickel [76], copper (II) [77], cadmium [78], and other metal ions [79] from wastewater. The authors reported high removal efficiency thereby reinforcing the performance of WCES powder as an economical, effective, and environmentally benign adsorbent for wastewater treatment.
WCES has also been demonstrated as an efficient and effective adsorbent for the removal of dyes from the ground and wastewaters. Wastewater from textile, food and beverages, cosmetics, pharmaceutical, etc., industries contain synthetic dyes that are difficult to remove. Water polluted with synthetic dyes is poisonous, mutagenic, carcinogenic and their ingestion can cause acute health and ecological worries [80]. Rápó et al. [81] explored the excellent adsorbent capability of WCES to remove Remazol dye from wastewater and reported that the toxic dye was oxidized to CO2 and water with a removal efficiency of about 99%. A similar approach was adopted by Hevira et al. [82] and Thakur et al. [83] when they extracted CaO from WCES for the elimination of cationic and anionic dyes. These investigations undoubtedly demonstrate WCES as a cost-effective, safe, non-toxic, and ecologically friendly adsorbent for the removal of dyes from wastewater. In all these cases, the adsorptive capability of WCES powder in the process mechanism was demonstrated with the effective absorber of the impurities and contaminants. Table 3 contains some of the applications of WCES for wastewater decontamination and purification.
Key conclusions:
• WCES is a resource for water decontamination, purification, and removal of adsorbates.
• No significant modification is required for the application of WCES as an adsorbent.
• Diverse adsorbates, including heavy metals and synthetic dyes, can be effectively adsorbed from ground and wastewater using CaO extracted from WCES.
• WCES is a low-cost, non-toxic, readily available and environmentally friendly adsorbent for effective ground and wastewater purification.
The scarcity and high cost of raw materials have led to the use of waste materials for various industrial applications. WCES is one of the viable resources that has been used frequently as raw materials for the production of various products. Due to the high CaCO3 content of WCES, which can easily be converted to CaO, industrialists have used WCES as a substitute for the production of ceramics, biphasic bone, polyvinyl chloride (PVC), lithium-sulfur (Li-S) batteries, plastic, and coating pigments for ink-jet printing paper. The process of extracting CaCO3 from WCES includes washing the collected chicken eggshells with chlorinated water, drying and crushing clean dried shells. The crushed shells are subjected to further milling and sieving until a fine powder WCES, rich in CaCO3, is achieved [84].
The WCES collected from various sources are subjected to mechanical or chemical modification techniques to improve their performance for a particular application. The mechanical modification method may include cleaning, removal of the membrane, drying, pulverization, sieving to convert the WCES into a fine powder. When WCES is pulverized into fine particles, it increases the surface area of the material and enhances its catalytic properties. The thermal treatment may include high-temperature calcination or carbonization in a furnace under air or nitrogen. The WCES powder is characterized by x-ray fluorescence (XRF), x-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), scanning electron microscope (SEM), x-ray diffraction (XRD), differential scanning calorimetry (DSC), Brunauer-Emmett-Teller (BET), inductively coupled plasma mass spectrometer (ICP-MS), etc., to ascertain the elemental composition, thermal, and spectroscopic behaviour. This is intended to validate the suitability and appropriateness of this resource for various industrial applications. Table 4 shows some of the industrial applications of WCES.
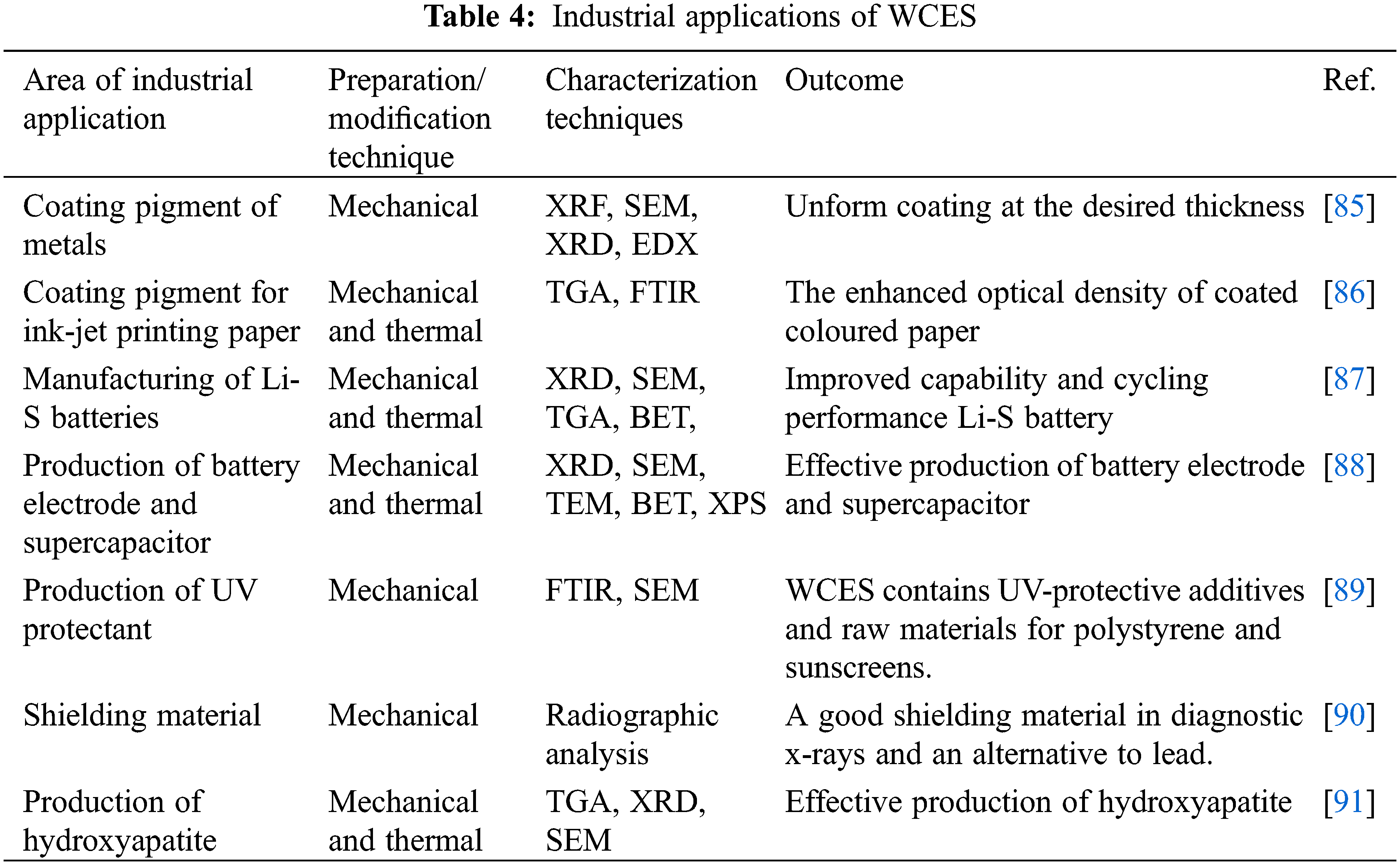
For example, CaCO3 extracted from WCES was demonstrated to be a suitable coating agent to prevent corrosion and protect welded carbon steel joints. Bakar et al. [85] adopted electrophoretic deposition for the coating of carbon steel with CaCO3 derived from WCES. Similarly, CaCO3 obtained from WCES was used as coating pigments for ink-jet printing paper. The outcome of the research showed an improved optical density of some coloured inks like cyan, magenta, and yellow of the coated paper [86]. In another application, the effect of N and S co-doped mesoporous carbon synthesized from WCES for the modification of the separator for high-performance Li-S batteries was investigated. The researchers explored the good surface area, mesoporous hollow structure, and the efficient electrochemical active sites of the WCES powder to improve the capability and cycling performance of the battery [87]. In the same vein, calcined WCES has been used for the low-cost production of a battery electrode and supercapacitor [88].
Fecheyr-Lippens et al. [89] explored WCES for the protection of two regularly used synthetic polymers (polystyrene and nylon) from ultraviolet (UV) degradation and reported that unprocessed WCES contains UV-protective additives for the protection of photodegradable materials. The outcome of the investigation further revealed WCES as a novel, cost-effective, durable, and ecologically benign protector of synthetic polymers from UV light and performed better than titanium dioxide (TiO2) and other inorganic additives. They concluded that WCES is an effective UV-protectant, a suitable replacement for TiO2, and sustainable raw material for the manufacturing of polystyrene and sunscreens. In another research, Alipio [90] investigated the potential of WCES for the manufacturing of shielding material against X-rays. The researcher concluded that WCES is a sustainable and low-cost alternative to lead as a shielding material against diagnostic X-rays. CaO derived from WCES has also been employed as a raw material for the synthesis of hydroxyapatite, a key constituent of human hard tissues like bones and teeth. The result of the research showed WCES as a cost-effective, sustainable, and readily available basic material for the production of inorganic biomaterials like hydroxyapatite [91]. These applications have made WCES a viable, low-cost, readily available, and environmentally friendly raw material for the production of useful products. It is also an avenue for waste minimization, landfill reduction, and waste management towards better sanitation strategies.
Key conclusions:
• WCES powder may be modified by mechanical or thermal techniques to improve its performance.
• Areas of application include metal and printing paper coating, production of UV protectants, Li-S batteries, battery electrodes, shielding material, hydroxyapatite, and energy storage.
3.5 Application as Food for Human Consumption
Chicken eggshells are among the top natural sources of calcium that are cheap, readily available, and accessible to rural households. Calcium is the most common mineral in the human body and an important component of nutrition. At every stage of human life, calcium is needed for strong bones and teeth, contributes to blood clotting, neurotransmission, helps in regulating blood pressure and proper functioning of the muscular tissues [92]. Hypocalcemia, a condition of calcium deficiency, can lead to muscle problems, extreme fatigue, osteopenia and osteoporosis, depression, nail and skin problem, and severe premenstrual syndrome. The daily calcium Recommended Intake (RI) for different age groups varies across various countries. For example, the average RI for calcium in some African countries is 238 mg/day in Uganda, 479 mg/day in South Africa, 636 mg/day in Nigeria and 760 mg/day in Cameroon. In Asia, the figure varies from 313 mg/day in Thailand to 794 mg/day in Singapore and among European countries, the figure varies from 728 mg/day in Belgium to 1,233 mg/day in Iceland [93]. Generally, the United States’ Office of Dietary Supplements, National Institutes of Health, has put the RI for calcium for different age groups to 200 mg/day for 0–6 months, 1,000–1,300 mg/day for adults, including pregnant and breastfeeding women [94].
Available information from previous researches put calcium content in WCES at between 35,000 mg/100 g and 40,000 mg/100 g [95]. Also, an average of one teaspoon of calcium powder, which is equivalent to about 2.21 g of calcium in the mode of CaCO3 and about 750–800 mg of calcium, can be extracted from a chicken eggshell to meet the daily RI for an adult. Calcium extracted from WCES is more readily absorbed by human cells with an absorbance rate of about 90% and better than the calcium gotten from limestone, coral shells, and other synthetic sources [96,97]. However, the CaCO3 derived from WCES is converted to calcium citrate (Ca(C6H6O7)), as shown in Eq. (2), by reacting with citric acid and used for food fortification [7,84]. Fig. 5 presents the process flowchart for the production of calcium citrate from WCES for food applications [84].
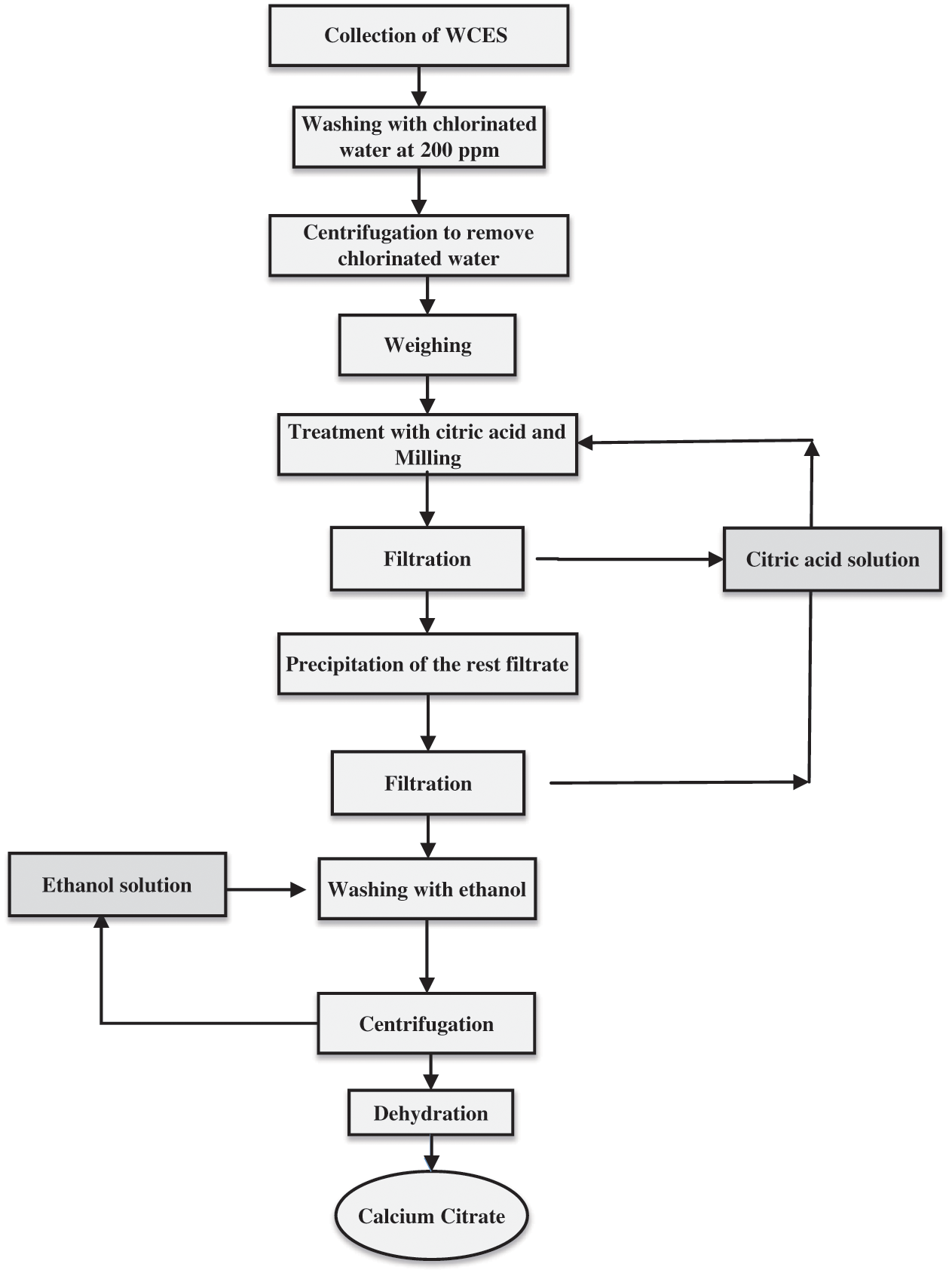
Figure 5: Flowchart for the preparation of calcium citrate for food production from WCES adapted from [80]
The process of extracting calcium from WCES includes washing the collected WCES under running water, soaking the clean shells in warm water to remove the membrane, boiling the clean shells at 100°C for about 10 min, drying the boiled shells in a clean oven at 90°C for 10 min and grinding of the oven-dried shells using mechanical grinder. Grinding and sieving operations of the WCES powder will continue until a uniform fine particle powder is achieved. The dry WCES powder is stored in a clean airtight glass container and used for human consumption.
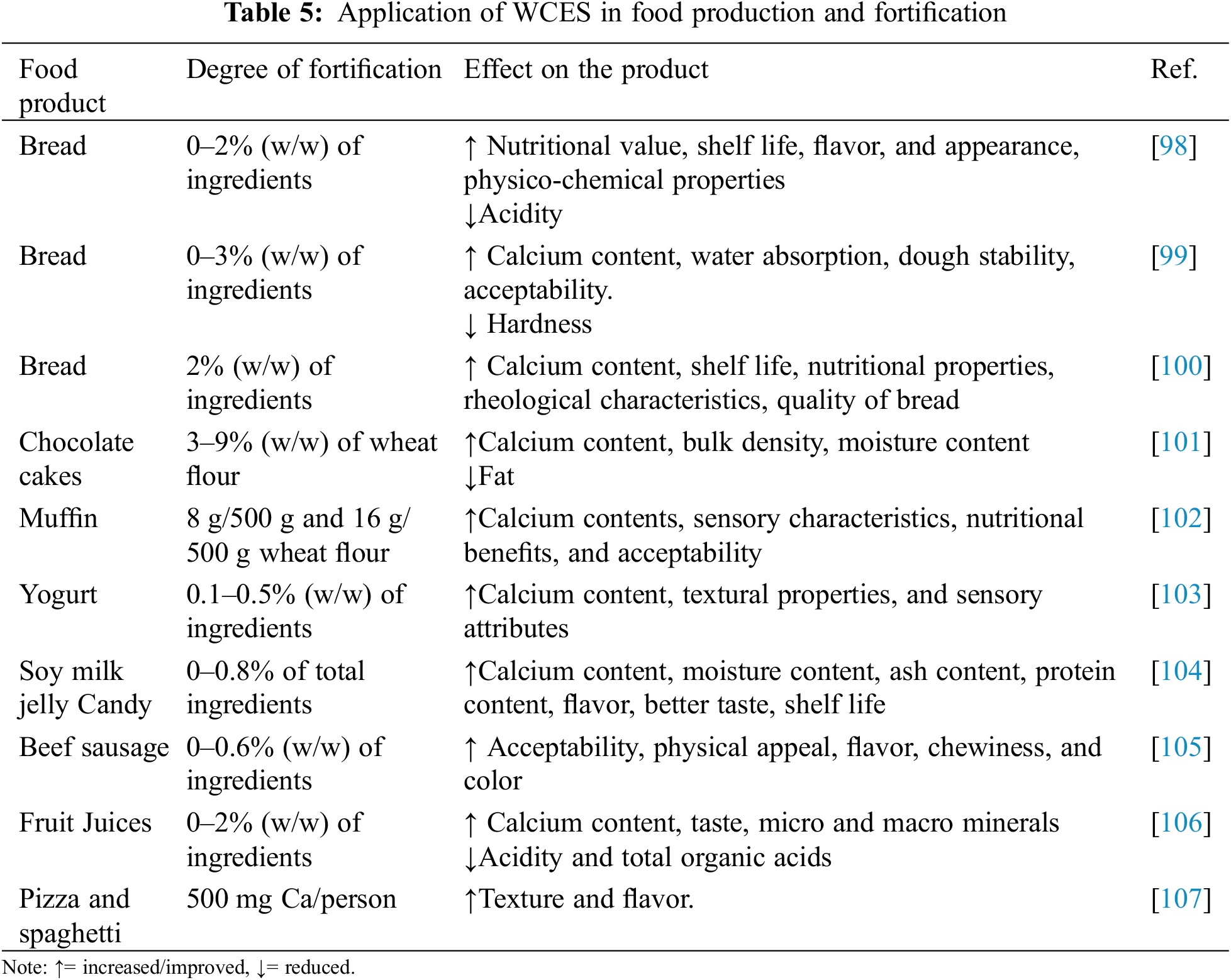
Many researchers have taken the advantage of the high concentration of calcium in WCES powder to fortify various food products with calcium. In separate researches, Platon et al. [98–100] added calcium powder derived from WCES for the production and fortification of bread and subjected the products to sensory evaluation, textural analysis, and nutritional properties analysis and compared with bread samples not fortified with WCES powder. The bread fortified with WCES powder was found to have more calcium content, better shelf life, physicochemical properties, more nutritional contents, more pleasant flavour, and better acceptability when compared with unfortified bread. Similar results were obtained when wheat flour was fortified with 3%, 6% and 9% WCES powder for the production of chocolate cakes. WCES powder supplementation with the recipe led to more nutritional contents and better physicochemical characteristics than unfortified cake. As the percentage of WCES powder supplementation increased, the calcium content, bulk density, moisture content, and ash content increased [101].
The pattern of results is not limited to bread and cakes alone. The fortification of muffins [102], yogurt [103], and soy milk jelly candy [104] with calcium derived from WCES powder improved the calcium content, protein content, shelf life, and general acceptability of the products. The impact of WCES-derived calcium supplementation of beef sausage [105], chokeberry and cranberry fruit juices [106], and pizza and spaghetti [107], showed enhanced nutritional contents, better sensory attributes, and wider acceptability by the consumers when compared with unfortified products. The consensus among investigators showed a positive and encouraging outlook for calcium supplementation and fortification to meet the nutritional need of the consumers at a lower cost, using natural supplements. Studies are ongoing on the suitability of chicken eggshell membrane for food fortification [96]. To meet the RI, particularly among populations with low calcium intake, calcium derived from WCES is used for food production and food fortification as shown in Table 5.
Key conclusions:
• The use of WCES powder for food production and fortification ensures waste recovery in the food sector, enhances food preservation and improves the nutritional enrichment of food with calcium.
• Food items fortified with calcium derived from WCES meet nutritional requirements, improved physicochemical properties, better sensory, and wider acceptability among the population.
• Food supplementation with calcium derived from WCES powder is a veritable and cost-effective way of achieving food security.
• Research gaps still exist in the deployment of chicken eggshells membrane powder for the fortification of diverse food products.
Apart from its utilization as food supplements, calcium has been extracted from WCES and used as a sustainable substitute for synthetic calcium for various dental, pharmaceutical, and other medical applications. Some of the factors advancing the medical application of WCES include its unrestricted availability, unhindered accessibility, biocompatibility, ease of application, inability to transmit disease, and ease of bone renewability [108]. This section will highlight the preparation of hydroxyapatite from WCES, applications of WCES in bone regeneration, teeth rehabilitation, as well as preventive and curative medicine.
Hydroxyapatite (Ca10(PO4)6(OH)2) is a useful inorganic biomaterial for various medical, adsorptions, and catalytic applications [109]. The medical applications of hydroxyapatite include efficient repair, regeneration, and replacements of skin, tissue, and gums, remediation of various orthopedic and dental defects as well as local drug delivery systems. However, from a cost, mechanical properties and environmental sustainability standpoint, the production of hydroxyapatite from waste materials will reduce pollution, ensure better sanitation and potentially reduce the cost of the product. In this wise, the use of food wastes, animal bones, fish scales, mollusc shells, seashells, oyster shells, and other biowastes raw materials for the production of hydroxyapatite has gained acceptance and wide patronage [110,111]. Synthesizing hydroxyapatite from WCES will reduce cost, serve as a viable way of disposing of chicken eggshell wastes and ensure good mechanical properties of the product. Besides, hydroxyapatite derived from WCES is highly effective, displayed greater bone formation and better orthopedic regenerative ability. Due to the high usability and many applications of hydroxyapatite, various techniques and approaches have been adopted for its production.
The use of hydrothermal, microwave irradiation, wet precipitation, sol-gel, solid-state reaction, mechanochemical, combustion, emulsion, pyrolysis, biomimetic, etc., and the peculiarities of each method, have been well reported in the literature [109,111–113]. Various characterization techniques, including TGA, DSC, FTIR, XRD, ICP-MS, SEM, TEM, and mechanical tests have been employed to test the suitability of different materials for the production of hydroxyapatite [111]. Eqs. (1), (3), and (4) show the chemical reactions for the synthesis of hydroxyapatite from WCES while Fig. 6 shows the flowchart of the production process [84].
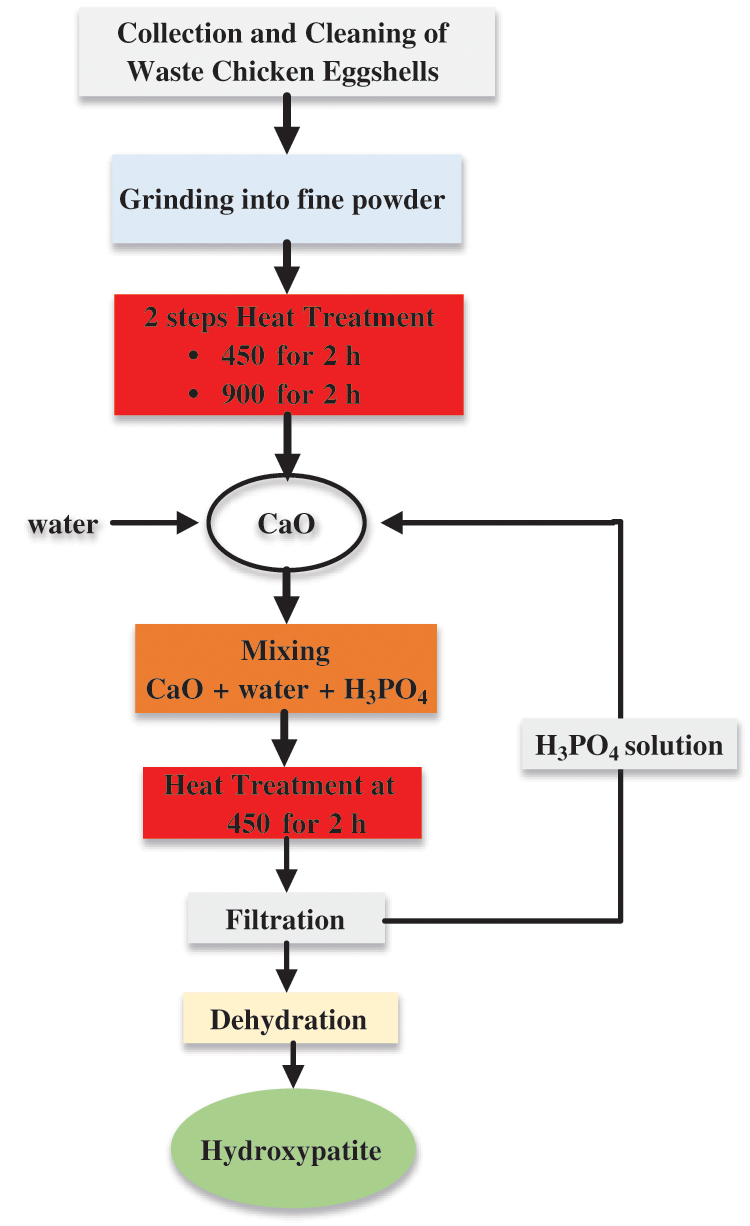
Figure 6: Flowchart for the synthesis of hydroxyapatite from WCES for medical applications adapted from [84]
The biomedical application of WCES was investigated by researchers when WCES powder was utilized as a low-cost abrasive material to reduce the surface roughness of artificial teeth, re-mineralize enamel surface lesion, and protect the enamel. They deployed various characterization techniques for the investigation and reported the capability and efficacy of calcium derived from WCES powder to reduce surface roughness, re-mineralize, and offer improved protective covering of the enamel, and ultimately leads to better oral health at a reduced cost [114–116] (Table 6). In a similar vein, CaCO3 from WCES powder has been used as bio-fillers to improve the mechanical properties of the denture to arrest the challenges associated with tooth loss which has been exacerbated by poor dental hygiene. In separate researches, Mohammed [117] and Kareem Burhan et al. [118] assessed the impacts of the addition of WCES powder as bio-composite on denture cleansers. They reinforced certain denture cleansers with different percentages of composites derived from WCES, tested the mechanical properties of the denture and compared their results with unreinforced cleansers. They reported that cleansers reinforced with WCES bio-composites showed improvement in the mechanical properties (ultimate tensile strength, impact, modulus of elasticity, elongation percentage at break, fracture toughness, impact strength, flexural strength) when compared with unreinforced cleansers.
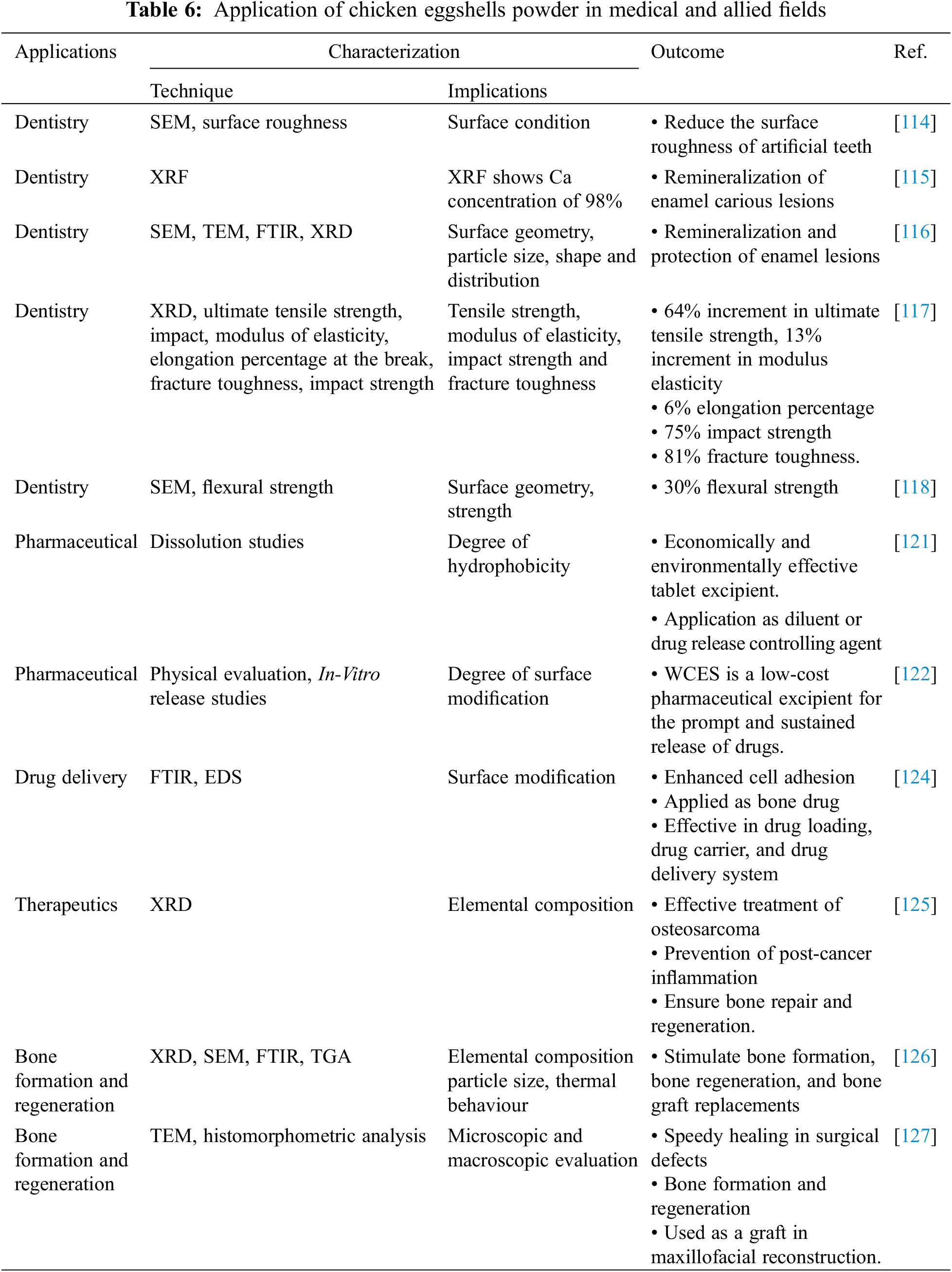
In the pharmaceutical industry, the application of WCES powder as a cheap pharmaceutical excipient has been established [119]. CaCO3 extracted from WCES was found to be within the required purity and pharmacopeial limits to be used as a cost-effective alternative pharmaceutical excipient and preparation of tablets containing CaCO3, Ca(C6H6O7), and calcium bis-glycinate [119,120]. Than et al. [121] and Habib et al. [122] undertook studies for the release of acetaminophen and aceclofenac tablets, respectively, by integrating WCES powder as a pharmaceutical excipient and compared the results with commercially available excipients. They both concluded that WCES powder is effective as tablet excipients and can be employed as a diluent or drug release controlling agent for prompt and sustained release of the drugs at a cheaper cost compared with the synthetic excipients. The application of CaCO3 derived from WCES for drug release and medications for the therapeutic intervention against cancer, inflammation, and bone regeneration has been investigated [123]. After their studies, Jayasree et al. [124] reported that the carbonated apatite nanocarrier system derived from WCES enhanced cell adhesion, useful as a bone drug, and was effective in drug loading, drug carrier, and drug delivery system. In a similar study, Verma et al. [125] confirmed the suitability of carbonated apatite nanocarriers derived from WCES for the treatment of osteosarcoma, prevention of post-cancer inflammation, and guarantee bone reparation and regeneration.
The field of tissue engineering has benefitted from the new knowledge on the application of WCES powder to heal bone defects caused by trauma, tumor resections, and congenital disease. Li et al. [126] developed calcium citrate from WCES and applied it to heal fractured bones of some patients. The authors corroborated the capability of nano-calcium citrate to promote new bone formation, bone regeneration, and bone graft replacements. A similar study by Salama et al. [127] further authenticated the application of WCES powder as a viable, safe, cheap, biodegradable, and biocompatible material with the capability for new bone formation and bone regeneration which can speed up recuperation in surgical defects and function as a graft in maxillofacial reconstruction. The utilization of WCES powder in the development of medications, dentistry, therapeutics, drug delivery, bone formation and regeneration and other medical applications is inexpensive, environmentally friendly, and a natural source of calcium. However, to avoid hypercalcemia and its attendant health consequences, the calcium concentration in the blood serum should be properly monitored. Going forward, the justification for the preference of amorphous CaCO3 over crystalline compounds in bone grafting and osteogenesis should be investigated.
Key conclusions:
• Hydroxyapatite derived from WCES powder is suitable for medical, dentistry, therapeutics, skin regeneration and repair, replacement of skin, tissue, and gums, remediation of orthopedic and dental defects as well as local drug delivery systems.
• Overconsumption of calcium from WCES should be avoided to guide against the over-concentration of calcium in the bloodstream.
• More researches are needed on the advantages of amorphous CaCO3 over the crystalline compound in bone grafting and osteogenesis.
One of the areas of applications of WCES is the agricultural sector for the production of fertilizers and poultry feeds. Eggshells collected from various sources can be converted to powder form and applied for agricultural and horticultural purposes. The processes for the preparation of WCES for use in the agricultural and horticultural sectors are similar to that of other aforementioned procedures which include washing of the collected WCES, drying and grinding into fine powder. In this case, there is no need for calcination as the CaCO3 is applied directly without decomposing it to CaO. The preference for CaCO3 derived from WCES over other natural sources of calcium is due to its purity and non-toxic nature. The application of crushed or powdered WCES contributes to plant vegetative growth and fruit yield more substantially than commercial fertilizer, in many cases. To enhance its solubility, WCES can be treated and dissolved into acetic acid to convert it into liquid as organic fertilizer. Besides, new methods for the conversion and application of CaCO3 derived from crushed WCES as organic fertilizers for various plants have been patented lately and documented in the literature [128]. The outcomes of the application of crushed WCES as organic fertilizer for cayenne pepper, cowpea, and sweet basil showed that the plants performed better in terms of growth, root length, and leaf quality more than the plants where commercial fertilizer was applied (Table 7). The authors recorded increased yield as a result of the application of WCES powder [129–131].
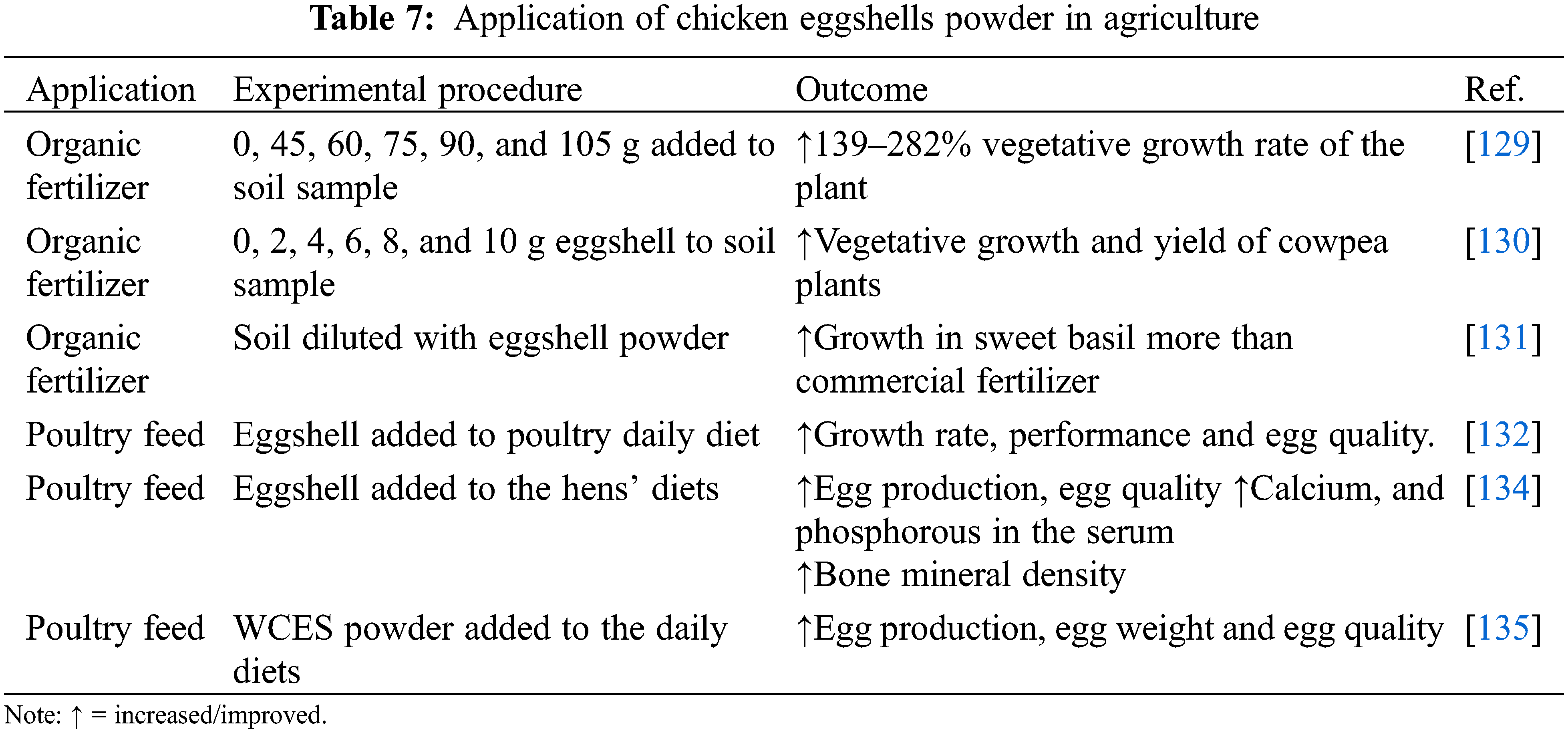
Chicken eggshells are also a source of proteins and minerals for animals. The Association of American Feed Control Officials (AAFCO) has approved the use of calcium from chicken eggshells to be included in the animal feed so that the animals can meet their daily calcium need. The enrichment of animal feeds with calcium derived from WCES helps in bone development and enhances the egg-laying capability of fowls due to the presence of phosphorous in the eggshell [96,132]. However, to eliminate any traces of contaminations by the microbial pathogens, the eggshells must be subjected to thermal treatment before pulverization to powdered form.
In an experiment, Okpanachi et al. [133] incorporated WCES into the diets of poultry layers and measured its effects on the performance, haematology, and quality of the egg. They reported that the birds witnessed improved growth rate, feed conversion rate, and better quality of the eggs. This was evident by the increased yolk width, albumen weight, and total egg weight. They attributed these improvements to the presence of calcium and phosphorous in the WCES used to fortify their feeds. Calcium extracted from WCES was added to the diets of old laying hens for seven weeks. The fowls recorded improved egg productivity, more calcium and phosphorous in the serum, better egg quality (egg weight, albumen height, yolk colour, shell strength, and shell thickness), and superior bone mineral density. The noticed improvements were traced to the addition of WCES to the hens’ diets [134]. A similar study by Neijat et al. [135] also recorded an improved egg quality, egg weight, and egg productivity of egg-laying hens fed with diets fortified with crushed WCES. This further confirms the utilization of crushed WCES to improve the nutrients, bone and eggshell strength, and overall egg productivity of hens at reduced cost thereby contributing to food security and a cleaner environment.
Key conclusions:
• Application of crushed WCES as organic fertilizer improves the vegetative growth and yield of plants.
• Crushed WCES subjected to high-temperature treatment is used in animal feeds production.
• WCES is a source of nutrients bone development, improved egg productivity and egg quality of egg-laying hens.
• The utilization of WCES in the agricultural sector will ensure improved soil fertility, better egg quality and productivity, contribute to food security and contribute to a cleaner environment.
4 Limitations and Prospects of Chicken Eggshell Applications
Though WCES has many advantages and applications, certain obvious limitations are militating against its utilization. The textural properties of WCES inhibit its preparation and manipulation processes thereby limiting its applications. The high impact strength and toughness of WCES prevent sufficient development of active surface area and pores. This condition reduces the specific surface area available for catalytic activity and impedes its utilization as a catalyst. One of the ways to counter this limitation is to subject the WCES powder to a well-controlled method of synthesis to ensure optimal specific surface area with substantively developed pores. This is one of the reasons why it is important to subject WCES to various modification techniques to adjust the specific surface area, make more pores and channels available, and proper positioning of the active catalytic surfaces [136].
The low toxicity and substantial efficacy of Ca derived from WCES are never in doubt and have made the resource a viable ingredient for various medical and pharmaceutical applications. However, investigations are continuing on the effectiveness of calcium-containing supplements for the management of osteoporosis and bone fracture. There is a likelihood of reappearance of cardiovascular and gastrointestinal cases as well as renal calculi when calcium derived from eggshells was used [137]. Also, ingestion of calcium supplements without corresponding normalizing calcium metabolism has been discovered to result in severe consequences in certain cases. More detailed investigations are still needed on the benefits of amorphous CaCO3 on bone grafting and osteogenesis. Some studies have shown the preference of crystalline CaCO3 over the amorphous compound. The application of more detailed structural characterization parameters on microscopic, mesoscopic, and molecular scales are recommended [138]. There are technological challenges in separating the eggshell membrane from the shell. This has resulted in the limitation in the investigation of the applications of eggshell membranes and the shell, separately. The deployment of cryo-grinding and homogenization is being proposed.
In terms of the physical consumption of WCES, it must be stressed that swallowing large fragments of eggshells might injure the throat and oesophagus. Chicken eggshells must be pulverized into a fine powder before consumption. Also, chicken eggshells may be contaminated with bacteria like Salmonella enteritidis. To incapacitate the bacteria, eggshells must be boiled before consumption. Chicken eggshells may contain some amount of aluminum, lead, cadmium, mercury and other toxic metals. The consumption of these metals impacts human health and predispose consumers to serious health challenges. These gravely limit the utilization of WCES for food fortification and supplements. The processing of WCES to utilizable form is not totally carbon neutral. The thermal decomposition of CaCO3 to extract CaO also yield CO2 and contributes to global emission.
Going forward, significant opportunities exist for the modification and deployment of chicken eggshells for various applications. There is increased interest in the use of tools of mechanochemistry to advance the application of WCES. The broad application of ball milling in WCES for the formation of nanophase, synthesis of bioceramics and production of composite materials need to be further investigated. A recent breakthrough by Sari et al. [139] where calcined WCES powder was deployed to catalyze the biogas production process needs to be further explored.
The increased development of the egg industry in recent decades due to population explosion and increased nutritional awareness has also given rise to the generation of more chicken eggshells. Waste chicken eggshell is a natural resource with significant hidden and untapped potentials. Bearing in mind that WCES is one of the most generated food wastes and the 15th leading cause of environmental pollution, the utilization for myriads of applications will ensure the sustainable development of our planet. This review showcases the utilization of chicken eggshells in biodiesel synthesis, construction industry, wastewater purification, soil remediation, food supplements, and fortification, as well as therapeutics and pharmaceutical applications. The fact that a substantial number of publications on the utilization of waste chicken eggshells were published in the last few years indicates the level of interest the subject has attracted among various researchers globally.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. United Nations Environment Programme (2021). Food Waste Index Report 2021. Nairobi. https://www.unep.org/resources/report/unep-food-waste-index-report-2021. [Google Scholar]
2. FAO (2013). Food wastage: Key facts and figures. http://www.fao.org/news/story/en/item/196402/. [Google Scholar]
3. FAO (2016). Sustainable development goals. http://www.fao.org/sustainable-development-goals/indicators/1231/en/. [Google Scholar]
4. Production of Eggs Primary. https://knoema.com/atlas/topics/Agriculture/Live-Stock-Production-Production-Quantity/Production-of-eggs-primary. [Google Scholar]
5. Godos, J., Micek, A., Brzostek, T., Toledo, E., Iacoviello, L. et al. (2021). Egg consumption and cardiovascular risk: A dose–response meta-analysis of prospective cohort studies. European Journal of Nutrition, 60(4), 1833–1862. DOI 10.1007/s00394-020-02345-7. [Google Scholar] [CrossRef]
6. Ajala, E., Eletta, O., Oyeniyi, S. (2018). Characterization and evaluation of chicken eggshell for use as a bio-resource. Arid Zone Journal of Engineering, Technology and Environment, 14(1), 26–40. [Google Scholar]
7. Faridi, H., Arabhosseini, A. (2018). Application of eggshell wastes as valuable and utilizable products: A review. Research in Agricultural Engineering, 64(2), 104–114. DOI 10.17221/6/2017-RAE. [Google Scholar] [CrossRef]
8. Laca, A., Laca, A., Díaz, M. (2017). Eggshell waste as catalyst: A review. Journal of Environmental Management, 197, 351–359. DOI 10.1016/j.jenvman.2017.03.088. [Google Scholar] [CrossRef]
9. Eggshell. https://www.wikiwand.com/en/Eggshell. [Google Scholar]
10. Abdulrahman, I., Tijani, H. I., Mohammed, B. A., Saidu, H., Yusuf, H. et al. (2014). From garbage to biomaterials: An overview on egg shell based hydroxyapatite. Journal of Materials, 2014, 1–6. DOI 10.1155/2014/802467. [Google Scholar] [CrossRef]
11. Waheed, M., Butt, M. S., Shehzad, A., Adzahan, N. M., Shabbir, M. A. et al. (2019). Eggshell calcium: A cheap alternative to expensive supplements. Trends in Food Science & Technology, 91, 219–230. DOI 10.1016/j.tifs.2019.07.021. [Google Scholar] [CrossRef]
12. Hamada, H. M., Tayeh, B. A., Al-Attar, A., Yahaya, F. M., Muthusamy, K. et al. (2020). The present state of the use of eggshell powder in concrete: A review. Journal of Building Engineering, 32, 101583. DOI 10.1016/j.jobe.2020.101583. [Google Scholar] [CrossRef]
13. Tan, Y. H., Abdullah, M. O., Nolasco-Hipolito, C., Taufiq-Yap, Y. H. (2015). Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Applied Energy, 160, 58–70. DOI 10.1016/j.apenergy.2015.09.023. [Google Scholar] [CrossRef]
14. Mittal, A., Teotia, M., Soni, R. K., Mittal, J. (2016). Applications of egg shell and egg shell membrane as adsorbents: A review. Journal of Molecular Liquids, 223, 376–387. DOI 10.1016/j.molliq.2016.08.065. [Google Scholar] [CrossRef]
15. Carvalho, J., Araújo, J., Castro, F. (2011). Alternative low-cost adsorbent for water and wastewater decontamination derived from eggshell waste: An overview. Waste and Biomass Valorization, 2(2), 157–167. DOI 10.1007/s12649-010-9058-y. [Google Scholar] [CrossRef]
16. Lima, D. S., Perez-Lopez, O. W. (2020). Oxidative coupling of methane to light olefins using waste eggshell as catalyst. Inorganic Chemistry Communications, 116, 107928. DOI 10.1016/j.inoche.2020.107928S. [Google Scholar] [CrossRef]
17. Park, S., Choi, K. S., Lee, D., Kim, D., Lim, K. T. et al. (2016). Eggshell membrane: Review and impact on engineering. Biosystems Engineering, 151, 446–463. DOI 10.1016/j.biosystemseng.2016.10.014. [Google Scholar] [CrossRef]
18. Kulshreshtha, G., Ahmed, T. A., Wu, L., Diep, T., Hincke, M. T. (2020). A novel eco-friendly green approach to produce particalized eggshell membrane (PEM) for skin health applications. Biomaterials Science, 8(19), 5346–5361. DOI 10.1039/D0BM01110J. [Google Scholar] [CrossRef]
19. Sembiring, H. B., Pasaribu, N., Sitepu, J. (2021). Calcium carbonate from chicken eggshells as adsorbents. AIP Conference Proceedings, 2342(1), 070005. DOI 10.1063/5.0046388. [Google Scholar] [CrossRef]
20. Health Effects Institute (2020). State of Global Air 2020. https://www.stateofglobalair.org. [Google Scholar]
21. Soria-Figueroa, E., Mena-Cervantes, V. Y., García-Solares, M., Hernández-Altamirano, R., Vazquez-Arenas, J. (2020). Statistical optimization of biodiesel production from waste cooking oil using CaO as catalyst in a robinson-mahoney type reactor. Fuel, 282, 118853. DOI 10.1016/j.fuel.2020.118853. [Google Scholar] [CrossRef]
22. Kumar, C. B., Lata, D. B., Mahto, D. (2021). Effect of addition of di-tert butyl peroxide (DTBP) on performance and exhaust emissions of dual fuel diesel engine with hydrogen as a secondary fuel. International Journal of Hydrogen Energy, 46(14), 9595–9612. DOI 10.1016/j.ijhydene.2020.12.129. [Google Scholar] [CrossRef]
23. Awogbemi, O., Inambao, F. L., Onuh, E. I. (2018). Development and characterization of chicken eggshell waste as potential catalyst for biodiesel production. International Journal of Mechanical Engineering and Technology, 9(12), 1329–1346. [Google Scholar]
24. Awogbemi, O., Kallon, D. V. V., Aigbodion, V. S. (2021). Trends in the development and utilization of agricultural wastes as heterogeneous catalyst for biodiesel production. Journal of the Energy Institute, 98, 244–258. DOI 10.1016/j.joei.2021.06.017. [Google Scholar] [CrossRef]
25. Lani, N. S., Ngadi, N., Inuwa, I. M. (2020). New route for the synthesis of silica-supported calcium oxide catalyst in biodiesel production. Renewable Energy, 156, 1266–1277. DOI 10.1016/j.renene.2019.10.132. [Google Scholar] [CrossRef]
26. Ajala, E. O., Ajala, M. A., Odetoye, T. E., Aderibigbe, F. A., Osanyinpeju, H. O. et al. (2020). Thermal modification of chicken eggshell as heterogeneous catalyst for palm kernel biodiesel production in an optimization process. Biomass Conversion and Biorefinery, 11, 2599–26151. DOI 10.1007/s13399-020-00636-x. [Google Scholar] [CrossRef]
27. Awogbemi, O., Inambao, F., Onuh, E. I. (2020). Modification and characterization of chicken eggshell for possible catalytic applications. Heliyon, 6(10), e05283. DOI 10.1016/j.heliyon.2020.e05283. [Google Scholar] [CrossRef]
28. Awogbemi, O., Onuh, E. I., Komolafe, C. A. (2019). Thermal degradation and spectroscopic study of neat palm oil, waste palm oil, and waste palm oil methyl ester. IOP Conference Series: Earth and Environmental Science, 331, 012032. DOI 10.1088/1755-1315/331/1/012032. [Google Scholar] [CrossRef]
29. Farid Fitri Kamaronzaman, M., Kahar, H., Hassan, N., Farhan Hanafi, M., Sapawe, N. (2020). Optimization of biodiesel production from waste cooking oil using eggshell catalyst. Materials Today: Proceedings, 31, 324–328. DOI 10.1016/j.matpr.2020.06.080. [Google Scholar] [CrossRef]
30. Farooq, M., Ramli, A., Naeem, A., Mahmood, T., Ahmad, S. et al. (2018). Biodiesel production from date seed oil (Phoenix dactylifera L.) via egg shell derived heterogeneous catalyst. Chemical Engineering Research and Design, 132, 644–651. DOI 10.1016/j.cherd.2018.02.002. [Google Scholar] [CrossRef]
31. Rahman, W. U., Fatima, A., Anwer, A. H., Athar, M., Khan, M. Z. et al. (2019). Biodiesel synthesis from eucalyptus oil by utilizing waste egg shell derived calcium based metal oxide catalyst. Process Safety and Environmental Protection, 122, 313–319. DOI 10.1016/j.psep.2018.12.015. [Google Scholar] [CrossRef]
32. Odetoye, T. E., Agu, J. O., Ajala, E. O. (2021). Biodiesel production from poultry wastes: Waste chicken fat and eggshell. Journal of Environmental Chemical Engineering, 9(4), 105654. DOI 10.1016/j.jece.2021.105654. [Google Scholar] [CrossRef]
33. Lani, N., Ngadi, N., Jusoh, M., Mohamad, Z., Zakaria, Z. (2019). Outstanding performance of waste chicken eggshell derived CaO as a green catalyst in biodiesel production: Optimization of calcination conditions. Journal of Physics: Conference Series, 1349(1), 012051. DOI 10.1088/1742-6596/1349/1/012051. [Google Scholar] [CrossRef]
34. Tan, Y. H., Abdullah, M. O., Nolasco-Hipolito, C., Ahmad Zauzi, N. S. (2017). Application of RSM and taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renewable Energy, 114, 437–447. DOI 10.1016/j.renene.2017.07.024. [Google Scholar] [CrossRef]
35. Santya, G., Maheswaran, T., Yee, K. F. (2019). Optimization of biodiesel production from high free fatty acid river catfish oil (Pangasius hypothalamus) and waste cooking oil catalyzed by waste chicken egg shells derived catalyst. SN Applied Sciences, 1(2), 152. DOI 10.1007/s42452-018-0155-z. [Google Scholar] [CrossRef]
36. Gupta, A. R., Rathod, V. K. (2018). Waste cooking oil and waste chicken eggshells derived solid base catalyst for the biodiesel production: Optimization and kinetics. Waste Management, 79, 169–178. DOI 10.1016/j.wasman.2018.07.022. [Google Scholar] [CrossRef]
37. Liu, M., Jia, S., Gong, Y., Song, C., Guo, X. (2013). Effective hydrolysis of cellulose into glucose over sulfonated sugar-derived carbon in an ionic liquid. Industrial & Engineering Chemistry Research, 52(24), 8167–8173. DOI 10.1021/ie400571e. [Google Scholar] [CrossRef]
38. Hu, S., Smith, T. J., Lou, W., Zong, M. (2014). Efficient hydrolysis of cellulose over a novel sucralose-derived solid acid with cellulose-binding and catalytic sites. Journal of Agricultural and Food Chemistry, 62(8), 1905–1911. DOI 10.1021/jf405712b. [Google Scholar] [CrossRef]
39. Sari, Y. W., Listiani, E., Putri, S. Y., Abidin, Z. (2020). Prospective of eggshell nanocalcium in improving biogas production from palm oil mill effluent. Waste and Biomass Valorization, 11(9), 4631–4638. DOI 10.1007/s12649-019-00786-8. [Google Scholar] [CrossRef]
40. Raheem, A., Liu, H., Ji, G., Zhao, M. (2019). Gasification of lipid-extracted microalgae biomass promoted by waste eggshell as CaO catalyst. Algal Research, 42, 101601. DOI 10.1016/j.algal.2019.101601. [Google Scholar] [CrossRef]
41. Global Cement Production 1995–2020. https://www.statista.com/statistics/1087115/global-cement-production-volume/. [Google Scholar]
42. Cement Statistics and Information. https://www.usgs.gov/centers/nmic/cement-statistics-and-information. [Google Scholar]
43. Global cement market (Production, consumption, imports & exportsInsight, trends and forecast 2019–2021. https://www.researchandmarkets.com/reports/4871690/global-cement-market-production-consumption. [Google Scholar]
44. Soltanzadeh, F., Emam-Jomeh, M., Edalat-Behbahani, A., Soltan-Zadeh, Z. (2018). Development and characterization of blended cements containing seashell powder. Construction and Building Materials, 161, 292–304. DOI 10.1016/j.conbuildmat.2017.11.111. [Google Scholar] [CrossRef]
45. Benhelal, E., Shamsaei, E., Rashid, M. I. (2021). Challenges against CO2 abatement strategies in cement industry: A review. Journal of Environmental Sciences, 104, 84–101. DOI 10.1016/j.jes.2020.11.020. [Google Scholar] [CrossRef]
46. Cement. https://www.iea.org/reports/cement. [Google Scholar]
47. Zanelato, C. B., Pires, A. F., da Silva, S. N., Galdino, A. G. S. (2020). Development of biphasic bone cement obtained from chicken eggshell. Journal of Materials Research and Technology, 9(4), 7297–7304. DOI 10.1016/j.jmrt.2020.05.053. [Google Scholar] [CrossRef]
48. Hart, A. (2020). Mini-review of waste shell-derived materials’ applications. Waste Management & Research, 38(5), 514–527. DOI 10.1177/0734242X19897812. [Google Scholar] [CrossRef]
49. Razali, N., Azizan, M. A., Pa’ee, K. F., Razali, N., Jumadi, N. (2020). Preliminary studies on calcinated chicken eggshells as fine aggregates replacement in conventional concrete. Materials Today: Proceedings, 31, 354–359. DOI 10.1016/j.matpr.2020.06.232. [Google Scholar] [CrossRef]
50. Amaral, M. C., Siqueira, F. B., Destefani, A. Z., Holanda, J. N. F. (2013). Soil–cement bricks incorporated with eggshell waste. Proceedings of the Institution of Civil Engineers-Waste and Resource Management, 166(3), 137–141. DOI 10.1680/warm.12.00024. [Google Scholar] [CrossRef]
51. Parthasarathi, N., Prakash, M., Satyanarayanan, K. (2017). Experimental study on partial replacement of cement with egg shell powder and silica fume. Rasayan Journal of Chemistry, 10(2), 442–449. DOI 10.7324/RJC.2017.1021689. [Google Scholar] [CrossRef]
52. Bandhavya, G., Sandeep, K., Bindhushree, G. (2017). An experimental study on partial replacement of cement with egg shell powder in concrete. International Research Journal of Engineering and Technology, 4(6), 2318–2323. [Google Scholar]
53. Balouch, N., Rashid, K., Javed, S., Ahmad, T. (2017). Experimental study on compressive strength of concrete by partial replacement of cement with eggshell powder. Technical Journal of University of Engineering and Technology, 22, 21–27. [Google Scholar]
54. Ing, D. S., Choo, C. S. (2014). Eggshell powder: Potential filler in concrete. Proceedings of 8th Malaysian Technical Universities Conference on Engineering and Technology. [Google Scholar]
55. Jaber, H. A., Mahdi, R. S., Hassan, A. K. (2020). Influence of eggshell powder on the portland cement mortar properties. Materials Today: Proceedings, 20, 391–396. DOI 10.1016/j.matpr.2019.09.153. [Google Scholar] [CrossRef]
56. Shiferaw, N., Habte, L., Thenepalli, T., Ahn, J. W. (2019). Effect of eggshell powder on the hydration of cement paste. Materials, 12(15), 2483. DOI 10.3390/ma12152483. [Google Scholar] [CrossRef]
57. Yu, T. Y., Ing, D. S., Choo, C. S., Azed, M. A. (2017). Natural lime treated as partial cement replacement to produce concrete. International Journal on Advanced Science Engineering Information Technology, 7, 1798–1804. [Google Scholar]
58. Pliya, P., Cree, D. (2015). Limestone derived eggshell powder as a replacement in portland cement mortar. Construction and Building Materials, 95, 1–9. DOI 10.1016/j.conbuildmat.2015.07.103/. [Google Scholar] [CrossRef]
59. Vasudevan, G., Wei, S. C. (2020). Utilisation of eggshell powder (ESP) as partial replacement of cement incorporating superplasticizer. IOP Conference Series: Materials Science and Engineering, 840(1), 012016. DOI 10.1088/1757-899X/840/1/012016. [Google Scholar] [CrossRef]
60. Francis, A., Yalley, P., Arkoh, K. (2016). Improving compressed laterite bricks using powdered eggshells. International Journal of Engineering and Science, 5(4), 65–70. [Google Scholar]
61. Wang, X., Ji, G., Zhang, Y., Guo, Y., Zhao, J. (2021). Research on high-and low-temperature characteristics of bitumen blended with waste eggshell powder. Materials, 14(8), 2020. DOI 10.3390/ma14082020. [Google Scholar] [CrossRef]
62. Masued, G. G. (2019). Investigating the ability of using eggshell powder as a filler in Hot Mix asphalt mixture. IOP Conference Series: Materials Science and Engineering, 518, 022047. DOI 10.1088/1757-899X/518/2/022047. [Google Scholar] [CrossRef]
63. Huang, J., Shiva Kumar, G., Ren, J., Sun, Y., Li, Y. et al. (2021). Towards the potential usage of eggshell powder as bio-modifier for asphalt binder and mixture: Workability and mechanical properties. International Journal of Pavement Engineering, 1–13. DOI 10.1080/10298436.2021.1905809. [Google Scholar] [CrossRef]
64. Clean Water. https://ourworldindata.org/water-access. [Google Scholar]
65. Azizullah, A., Khattak, M. N. K., Richter, P., Häder, D. P. (2011). Water pollution in Pakistan and its impact on public health—A review. Environment International, 37(2), 479–497. DOI 10.1016/j.envint.2010.10.007. [Google Scholar] [CrossRef]
66. Lead Poisoning and Health. https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health. [Google Scholar]
67. Abbaszadeh, S., Wan Alwi, S. R., Webb, C., Ghasemi, N., Muhamad, I. I. (2016). Treatment of lead-contaminated water using activated carbon adsorbent from locally available papaya peel biowaste. Journal of Cleaner Production, 118, 210–222. DOI 10.1016/j.jclepro.2016.01.054. [Google Scholar] [CrossRef]
68. Wong, S., Ngadi, N., Inuwa, I. M., Hassan, O. (2018). Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. Journal of Cleaner Production, 175, 361–375. DOI 10.1016/j.jclepro.2017.12.059. [Google Scholar] [CrossRef]
69. Tamjidi, S., Ameri, A. (2020). A review of the application of sea material shells as low cost and effective bio-adsorbent for removal of heavy metals from wastewater. Environmental Science and Pollution Research, 27(25), 31105–31119. DOI 10.1007/s11356-020-09655-7. [Google Scholar] [CrossRef]
70. Daraei, H., Mittal, A., Noorisepehr, M., Mittal, J. (2015). Separation of chromium from water samples using eggshell powder as a low-cost sorbent: Kinetic and thermodynamic studies. Desalination and Water Treatment, 53(1), 214–220. DOI 10.1080/19443994.2013.837011. [Google Scholar] [CrossRef]
71. Jendia, A. H., Hamzah, S., Abuhabib, A., El-Ashgar, N. M. (2020). Removal of nitrate from groundwater by eggshell biowaste. Water Supply, 20(7), 2514–2529. DOI 10.2166/ws.2020.151. [Google Scholar] [CrossRef]
72. Ikram, M., Rehman, A. U., Ali, S., Ali, S., Ul, S. et al. (2016). The adsorptive potential of chicken egg shells for the removal of oxalic acid from wastewater. Journal of Biomedical Engineering and Informatics, 2(2), 118–131. 10.5430/jbei.v2n2p118. [Google Scholar] [CrossRef]
73. Renu, M. A., Singh, K., Upadhyaya, S., Dohare, R. (2017). Removal of heavy metals from wastewater using modified agricultural adsorbents. Materials Today: Proceedings, 4(9), 10534–10538. DOI 10.1016/j.matpr.2017.06.415. [Google Scholar] [CrossRef]
74. Wang, H., Gao, B., Fang, J., Ok, Y. S., Xue, Y. et al. (2018). Engineered biochar derived from eggshell-treated biomass for removal of aqueous lead. Ecological Engineering, 121, 124–129. DOI 10.1016/j.ecoleng.2017.06.029. [Google Scholar] [CrossRef]
75. Jalu, R. G., Chamada, T. A., Kasirajan, D. R. (2021). Calcium oxide nanoparticles synthesis from hen eggshells for removal of lead (Pb(II)) from aqueous solution. Environmental Challenges, 100193. DOI 10.1016/j.envc.2021.100193. [Google Scholar] [CrossRef]
76. de Angelis, G., Medeghini, L., Conte, A. M., Mignardi, S. (2017). Recycling of eggshell waste into low-cost adsorbent for Ni removal from wastewater. Journal of Cleaner Production, 164, 1497–1506. DOI 10.1016/j.jclepro.2017.07.085. [Google Scholar] [CrossRef]
77. Madiabu, M. J., Untung, J., Solihat, I., Ichzan, A. M. (2021). Equilibrium and kinetic study of removal copper (II) from aqueous solution using chicken eggshells: Low cost sorbent. Molekul, 16(1), 28–37. 10.20884/1.jm.2021.16.1.658. [Google Scholar] [CrossRef]
78. Tizo, M. S., Blanco, L. A. V., Cagas, A. C. Q., Dela Cruz, B. R. B., Encoy, J. C. et al. (2018). Efficiency of calcium carbonate from eggshells as an adsorbent for cadmium removal in aqueous solution. Sustainable Environment Research, 28(6), 326–332. DOI 10.1016/j.serj.2018.09.002. [Google Scholar] [CrossRef]
79. Pettinato, M., Chakraborty, S., Arafat, H. A., Calabro, V. (2015). Eggshell: A green adsorbent for heavy metal removal in an MBR system. Ecotoxicology and Environmental Safety, 121, 57–62. DOI 10.1016/j.ecoenv.2015.05.046. [Google Scholar] [CrossRef]
80. Piaskowski, K., Świderska-Dąbrowska, R., Zarzycki, P. K. (2018). Dye removal from water and wastewater using various physical, chemical, and biological processes. Journal of AOAC International, 101(5), 1371–1384. DOI 10.5740/jaoacint.18-0051. [Google Scholar] [CrossRef]
81. Rápó, E., Aradi, L. E., Szabó, Á., Posta, K., Szép, R. et al. (2020). Adsorption of remazol brilliant violet-5R textile dye from aqueous solutions by using eggshell waste biosorbent. Scientific Reports, 10(1), 1–12. DOI 10.1038/s41598-020-65334-0. [Google Scholar] [CrossRef]
82. Hevira, L., Rahmi, A., Zein, R., Zilfa, Z., Rahmayeni, R. (2020). The fast and of low-cost-adsorbent to the removal of cationic and anionic dye using chicken eggshell with its membrane. Mediterranean Journal of Chemistry, 10(3), 294–301. DOI 10.13171/mjc02003261271lh. [Google Scholar] [CrossRef]
83. Thakur, S., Singh, S., Pal, B. (2021). Superior adsorptive removal of brilliant green and phenol red dyes mixture by CaO nanoparticles extracted from egg shells. Journal of Nanostructure in Chemistry, 1–15. DOI 10.1007/s40097-021-00412-x. [Google Scholar] [CrossRef]
84. Oliveira, D. A., Benelli, P., Amante, E. R. (2013). A literature review on adding value to solid residues: Egg shells. Journal of Cleaner Production, 46, 42–47. DOI 10.1016/j.jclepro.2012.09.045. [Google Scholar] [CrossRef]
85. Bakar, T. A. A., Rosly, M. F., Jafar, N. S. A. M. (2017). Eggshell coated grey cast iron for corrosion applications. Jurnal Teknologi, 79(7–4). DOI 10.11113/jt.v79.12258. [Google Scholar] [CrossRef]
86. Yoo, S., Hsieh, J. S., Zou, P., Kokoszka, J. (2009). Utilization of calcium carbonate particles from eggshell waste as coating pigments for ink-jet printing paper. Bioresource Technology, 100(24), 6416–6421. DOI 10.1016/j.biortech.2009.06.112. [Google Scholar] [CrossRef]
87. Yuan, X., Wu, L., He, X., Zeinu, K., Huang, L. et al. (2017). Separator modified with N, S co-doped mesoporous carbon using egg shell as template for high performance lithium-sulfur batteries. Chemical Engineering Journal, 320, 178–188. DOI 10.1016/j.cej.2017.03.022. [Google Scholar] [CrossRef]
88. Minakshi, M., Higley, S., Baur, C., Mitchell, D. R., Jones, R. T. et al. (2019). Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Advances, 9(46), 26981–26995. DOI 10.1039/C9RA04289J. [Google Scholar] [CrossRef]
89. Fecheyr-Lippens, D., Nallapaneni, A., Shawkey, M. D. (2017). Exploring the use of unprocessed waste chicken eggshells for UV-protective applications. Sustainability, 9(2), 232–243. DOI 10.3390/su9020232. [Google Scholar] [CrossRef]
90. Alipio, M. M. (2019). Eggshells as alternative shielding material against diagnostic X-rays. CMU Journal of Science, 23(2), 40–45. DOI 10.52751/VXNF1485. [Google Scholar] [CrossRef]
91. Ćurković, L., Žmak, I., Kurajica, S., Tonković, M., Šokčević, Z. et al. (2017). From eggshells biowaste to hydroxyapatite biomaterial. Biomaterial. Material Science and Engineering Technology, 48(8), 797–802. DOI 10.1002/mawe.201700052. [Google Scholar] [CrossRef]
92. New review: Comparison global calcium intake. https://www.frieslandcampinainstitute.com/news/new-review-comparison-global-calcium-intake. [Google Scholar]
93. Balk, E., Adam, G., Langberg, V., Earley, A., Clark, P. et al. (2017). Global dietary calcium intake among adults: A systematic review. Osteoporosis International, 28(12), 3315–3324. DOI 10.1007/s00198-017-4230-x. [Google Scholar] [CrossRef]
94. Calcium (2021). https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/. [Google Scholar]
95. Arnold, M., Rajagukguk, Y. V., Gramza-Michałowska, A. (2021). Functional food for elderly high in antioxidant and chicken eggshell calcium to reduce the risk of osteoporosis—A narrative review. Foods, 10(3), 656. DOI 10.3390/foods10030656. [Google Scholar] [CrossRef]
96. Aditya, S., Stephen, J., Radhakrishnan, M. (2021). Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review. Trends in Food Science & Technology, 115, 422–432. DOI 10.1016/j.tifs.2021.06.047. [Google Scholar] [CrossRef]
97. King’Ori, A. (2011). A review of the uses of poultry eggshells and shell membranes. International Journal of Poultry Science, 10(11), 908–912. DOI 10.3923/ijps.2011.908.912. [Google Scholar] [CrossRef]
98. Platon, N., Arus, V. A., Georgescu, A., Nistor, I. D., Barsan, N. (2020). White bread fortified with calcium from eggshell powder. Revista de Chimie, 71, 299–306. DOI 10.37358/Rev.Chim.1949. [Google Scholar] [CrossRef]
99. Khan, F. A., Ameer, K., Qaiser, M. A., Pasha, I., Mahmood, Q. et al. (2020). Development and analysis of bread fortified with calcium extracted from chicken eggshells of Pakistani market. Food Science and Technology, 41, 14–20. DOI 10.1590/fst.07220. [Google Scholar] [CrossRef]
100. Alsuhaibani, A. M. A. (2018). Rheological and nutritional properties and sensory evaluation of bread fortified with natural sources of calcium. Journal of Food Quality, 2018, 8308361. DOI 10.1155/2018/8308361. [Google Scholar] [CrossRef]
101. Ray, S., Barman, A. K., Roy, P. K., Singh, B. K. (2017). Chicken eggshell powder as dietary calcium source in chocolate cakes. The Pharma Innovation, 6(9), 1–4. [Google Scholar]
102. Afzal, F., Mueen-ud-Din, G., Nadeem, M., Murtaza, M. A., Mahmood, S. (2020). Effect of eggshell powder fortification on the physicochemical and organoleptic characteristics of muffins. Pure and Applied Biology, 9(2), 1488–1496. 10.19045/bspab.2020.90154. [Google Scholar] [CrossRef]
103. El-Shibiny, S., Abd El-Gawad, M. A. M., Assem, F. M., El-Sayed, S. M. (2018). The use of nano-sized eggshell powder for calcium fortification of cow? s and buffalo? s milk yogurts. Acta Scientiarum Polonorum Technologia Alimentaria, 17(1), 37–49. 10.17306/J.AFS.2018.054. [Google Scholar] [CrossRef]
104. Novelina, N., Anggraini, T., Putri, L. N. (2020). Characteristics of jelly candy made from soybean milk and addition of eggshell powder. Asian Journal of Applied Research for Community Development and Empowerment, 4(1), 39–43. DOI 10.29165/ajarcde.v4i1.37. [Google Scholar] [CrossRef]
105. Prasetyo, B., Prayitno, A. H. (2021). The sensory characteristics of fortified beef sausage with duck eggshell nano-calcium. IOP Conference Series: Earth and Environmental Science, 672(1), 012042. DOI 10.1088/1755-1315/672/1/012042. [Google Scholar] [CrossRef]
106. Lachowicz, S., Oszmiański, J., Wilczyńska, M., Zaguła, G., Saletnik, B. et al. (2020). Impact mineralization of chokeberry and cranberry fruit juices using a new functional additive on the protection of bioactive compounds and antioxidative properties. Molecules, 25(3), 659. DOI 10.3390/molecules25030659. [Google Scholar] [CrossRef]
107. Brun, L. R., Lupo, M., Delorenzi, D. A., di Loreto, V. E., Rigalli, A. (2013). Chicken eggshell as suitable calcium source at home. International Journal of Food Sciences and Nutrition, 64(6), 740–743. DOI 10.3109/09637486.2013.787399. [Google Scholar] [CrossRef]
108. Onwubu, S. C., Singh, S., Vahed, A., Kanny, K. (2018). Eggshells: From waste to medical applications. In: Ahmed, S., Kanchi, S., Kumar, G. (Eds.Handbook of biopolymers. pp. 263–286. Singapore: Pan Stanford Publishing. [Google Scholar]
109. Biedrzycka, A., Skwarek, E., Hanna, U. M. (2021). Hydroxyapatite with magnetic core: Synthesis methods, properties, adsorption and medical applications. Advances in Colloid and Interface Science, 291, 102401. DOI 10.1016/j.cis.2021.102401. [Google Scholar] [CrossRef]
110. Bee, S. L., Hamid, Z. A. A. (2020). Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceramics International, 46(11), 17149–17175. DOI 10.1016/j.ceramint.2020.04.103. [Google Scholar] [CrossRef]
111. Sharifianjazi, F., Esmaeilkhanian, A., Moradi, M., Pakseresht, A., Asl, M. S. et al. (2021). Biocompatibility and mechanical properties of pigeon bone waste extracted natural nano-hydroxyapatite for bone tissue engineering. Materials Science and Engineering, 264, 114950. DOI 10.1016/j.mseb.2020.114950. [Google Scholar] [CrossRef]
112. Szcześ, A., Hołysz, L., Chibowski, E. (2017). Synthesis of hydroxyapatite for biomedical applications. Advances in Colloid and Interface Science, 249, 321–330. DOI 10.1016/j.cis.2017.04.007. [Google Scholar] [CrossRef]
113. Goh, K. W., Wong, Y. H., Ramesh, S., Chandran, H., Krishnasamy, S. et al. (2021). Effect of pH on the properties of eggshell-derived hydroxyapatite bioceramic synthesized by wet chemical method assisted by microwave irradiation. Ceramics International, 47(7), 8879–8887. DOI 10.1016/j.ceramint.2020.12.009. [Google Scholar] [CrossRef]
114. Onwubu, S. C., Vahed, A., Singh, S., Kanny, K. M. (2017). Reducing the surface roughness of dental acrylic resins by using an eggshell abrasive material. The Journal of Prosthetic Dentistry, 117(2), 310–314. DOI 10.1016/j.prosdent.2016.06.024. [Google Scholar] [CrossRef]
115. Mony, B., Ebenezar, A. R., Ghani, M. F., Narayanan, A. (2015). Effect of chicken egg shell powder solution on early enamel carious lesions: An in vitro preliminary study. Journal of Clinical and Diagnostic Research, 9(3), ZC30–ZC32. DOI 10.7860/JCDR/2015/11404.5656. [Google Scholar] [CrossRef]
116. Onwubu, S. C., Mdluli, P. S., Singh, S., Madikizela, L., Ngombane, Y. (2019). Characterization and in vitro evaluation of an acid resistant nanosized dental eggshell-titanium dioxide material. Advanced Powder Technology, 30(4), 766–773. DOI 10.1016/j.apt.2019.01.005. [Google Scholar] [CrossRef]
117. Mohammed, R. A. (2020). PMMA–eggshell composite preparation and studying mechanical properties as a dental material. Journal of Engineering and Sustainable Development, 24(2), 30–47. DOI 10.31272/jeasd.24.2.3. [Google Scholar] [CrossRef]
118. Kareemburhan, S., Kareem, N. E., Abed, M. M. (2020). Evaluation effect of certain denture cleansers on flexural strength of sustainable PMMA biocomposite. Test Engineering and Mechanics, 1, 11020–11027. [Google Scholar]
119. Rasheed, S. P., Shivashankar, M., Dev, S., Azeem, A. K. (2019). Treatment of biowaste to pharmaceutical excipient. Materials Today: Proceedings, 15, 316–322. DOI 10.1016/j.matpr.2019.05.011. [Google Scholar] [CrossRef]
120. Siemiradzka, W., Dolinska, B., Ryszka, F. (2018). New sources of calcium (chicken eggshells, chelates)-preparation of raw material and tablets. Current Pharmaceutical Biotechnology, 19(7), 566–572. DOI 10.2174/1389201019666180723103853. [Google Scholar] [CrossRef]
121. Than, M., Lawanprasert, P., Jateleela, S. (2012). Utilization of eggshell powder as excipient in fast and sustained release Acetaminophen tablets. Mahidol University Journal of Pharmaceutical Sciences, 39(3–4), 32–38. [Google Scholar]
122. Habib, M. A., Mahmud, M. F., Islam, M. T., Hasan, M., Ahamed, S. K. et al. (2015). New approach for sustained release dosage form design: Aceclofenac. Mahidol University Journal of Pharmaceutical Sciences, 42, 93–100. [Google Scholar]
123. Lesnierowski, G., Stangierski, J. (2018). What’s new in chicken egg research and technology for human health promotion?–A review. Trends in Food Science & Technology, 71, 46–51. DOI 10.1016/j.tifs.2017.10.022. [Google Scholar] [CrossRef]
124. Jayasree, R., Madhumathi, K., Rana, D., Ramalingam, M., Nankar, R. P. et al. (2018). Development of egg shell derived carbonated apatite nanocarrier system for drug delivery. Journal of Nanoscience and Nanotechnology, 18(4), 2318–2324. DOI 10.1166/jnn.2018.14377. [Google Scholar] [CrossRef]
125. Verma, A. H., Kumar, T., Madhumathi, K., Rubaiya, Y., Ramalingan, M. et al. (2019). Curcumin releasing eggshell derived carbonated apatite nanocarriers for combined anti-cancer, anti-inflammatory and bone regenerative therapy. Journal of Nanoscience and Nanotechnology, 19(11), 6872–6880. DOI 10.1166/jnn.2019.16640. [Google Scholar] [CrossRef]
126. Li, J., Liu, Y., Gao, Y., Zhong, L., Zou, Q. et al. (2016). Preparation and properties of calcium citrate nanosheets for bone graft substitute. Bioengineered, 7(5), 376–381. DOI 10.1080/21655979.2016.1226656. [Google Scholar] [CrossRef]
127. Salama, R., Khashaba, M., El Rouby, D. (2019). Histomorphometric evaluation of a nano-sized eggshell-containing supplement as a natural alloplast: An animal study. The Saudi Dental Journal, 31(3), 375–381. DOI 10.1016/j.sdentj.2019.03.011. [Google Scholar] [CrossRef]
128. Ahmed, T. A., Wu, L., Younes, M., Hincke, M. (2021). Biotechnological applications of eggshell: Recent advances. Frontiers in Bioengineering and Biotechnology, 9, 548–567. DOI 10.3389/fbioe.2021.675364. [Google Scholar] [CrossRef]
129. Anugrah, R. D., Rafvenia, M., Safahi, L. (2021). The effect of eggshell organic fertilizer on vegetative growth of cayenne pepper (Capsicum frutescens L). IOP Conference Series: Earth and Environmental Science, 755(1), 012001. DOI 10.1088/1755-1315/755/1/012001. [Google Scholar] [CrossRef]
130. Radha, T., Karthikeyan, G. (2019). Hen eggshell waste as fertilizer for the growth of phaseolus vulgaris (Cow Pea seeds). Research Journal of Life Sciences, Bioinformatics, Pharmaceutical and Chemical Sciences, 5, 398–406. DOI 10.26479/2019.0501.35. [Google Scholar] [CrossRef]
131. Wijaya, V. T., Teo, S. S. (2019). Evaluation of eggshell as organic fertilizer on sweet basil. International Journal of Sustainable Agricultural Research, 6(2), 79–86. DOI 10.18488/journal.70.2019.62.79.86. [Google Scholar] [CrossRef]
132. Quina, M. J., Soares, M. A. R., Quinta-Ferreira, R. (2017). Applications of industrial eggshell as a valuable anthropogenic resource. Resources, Conservation and Recycling, 123, 176–186. DOI 10.1016/j.resconrec.2016.09.027. [Google Scholar] [CrossRef]
133. Okpanachi, U., Yusuf, K. A., Ikubaje, M. K., Okpanachi, G. C. A. (2021). Effects of egg shell meal on the performance and haematology of layers and their egg quality. African Journal of Science, Technology, Innovation and Development, 13(1), 89–96. DOI 10.1080/20421338.2020.1838111. [Google Scholar] [CrossRef]
134. Lee, W. D., Kothari, D., Niu, K. M., Lim, J. M., Park, D. H. et al. (2021). Superiority of coarse eggshell as a calcium source over limestone, cockle shell, oyster shell, and fine eggshell in old laying hens. Scientific Reports, 11(1), 1–10. DOI 10.1038/s41598-021-92589-y. [Google Scholar] [CrossRef]
135. Neijat, M., House, J. D., Guenter, W., Kebreab, E. (2011). Calcium and phosphorus dynamics in commercial laying hens housed in conventional or enriched cage systems. Poultry Science, 90(10), 2383–2396. DOI 10.3382/ps.2011-01401. [Google Scholar] [CrossRef]
136. Pavlović, S. M., Marinković, D. M., Kostić, M. D., Janković-Častvan, I. M., Mojović, L. V. et al. (2020). A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel, 267, 117171. DOI 10.1016/j.fuel.2020.117171. [Google Scholar] [CrossRef]
137. Reid, I. R., Bolland, M. J. (2020). Calcium and/or vitamin D supplementation for the prevention of fragility fractures: Who needs it? Nutrients, 12(4), 1011. DOI 10.3390/nu12041011. [Google Scholar] [CrossRef]
138. Baláž, M., Boldyreva, E. V., Rybin, D., Pavlović, S., Rodríguez-Padrón, D. et al. (2020). State-of-the-art of eggshell waste in materials science: Recent advances in catalysis, pharmaceutical applications and mechanochemistry. Frontiers in Bioengineering and Biotechnology, 8, 1522. DOI 10.3389/fbioe.2020.612567. [Google Scholar] [CrossRef]
139. Sari, Y. W., Listiani, E., Putri, S. Y., Abidin, Z. (2019). Prospective of eggshell nanocalcium in improving biogas production from palm oil mill effluent. Waste and Biomass Valorization, 11, 4631–4638. DOI 10.1007/s12649-019-00786-8. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |