

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.020001
ARTICLE
The Fabrication of Water-Soluble Chitosan Capsule Shell Modified by Alginate and Gembili Starch (Dioscorea esculenta L)
Chemistry Department, Faculty of Science and Analytical Data, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia
*Corresponding Author: Yatim Lailun Ni’mah. Email: yatimnikmah@gmail.com
Received: 28 October 2021; Accepted: 07 January 2022
Abstract: Capsule shells have been successfully fabricated from water-soluble chitosan (WSC) with the addition of alginate and Gembili starch. WSC was synthesized from crab shell chitosan by depolymerization reaction. The capsule shells were made with the composition of WSC: Alginate, 2:1, 3:1 and 4:1 (w/w) with and without the addition of Gembili starch. Gembili starch was added with a ratio of Alginate: Starch, 1:1 (w/w). The capsule shell properties were evaluated according to Indonesian Pharmacopoeia standard. The solubility test showed that the capsule shells were comply with the standard. The highest degrees of swelling in water and HCl 0.1 N solution were 491.93% and 410.51%, respectively. The highest degradation percentages in water and HCl 0.1 N solution were 57.80% and 21.44%, respectively. The observation of physical appearance indicated that the capsule shell with WSC: Alginate: Starch in ratio of 3:1:1 has appearance close to commercial capsule shell.
Keywords: Capsule shell; water soluble chitosan; capsule shell degradation; capsule shell solubility
The capsule is a drug delivery system that is used in the distribution process of drugs to achieve a therapeutic effect in the human body. Drug carrier material was used to increase the efficiency of treatment, protect the drug from degradative changes in the digestive system (in the stomach and intestines), prevent drug accumulation in the blood plasma, and reduce the side effects of the drug [1]. The capsule has many advantages compared to other oral preparations, one of which is that the combination of ingredients can be varied according to patient need and dosage.
There are two types of capsule shells, namely hard and soft capsule shells. The most common and widely used capsule shell is the hard capsule shell because the hard capsule shell has better properties, i.e., odorless [2] has a bland taste, is easy to swallow, and can dissolve in a suitable time [3].
The most common material that used in the manufacture of the capsule in the pharmaceutical industry is gelatin [4]. Generally, gelatin on the market was produced from the skin and bones of cows or pork. Based on data from Gelatin manufactures of Europe 2005, the largest production of gelatin in the world was came from pork skin, i.e., 44.50% (136,000 tons), the second was from cowhide, i.e., 27.60% (84,000 tons) and the remaining 1.30% (4,000 tons) was from fish and goat bones [5]. A large amount of gelatin made from raw pork skin has caused some people, especially Muslims, worried about the halal requirement of the products [6]. Gelatin raw material also has a risk of viral contamination that causes bovine spongiform encephalopathy (BSE), foot and mouth disease (FMD), and swine influenza [7]. Based on this, research to find the halal alternative of gelatin sources needs to be carried out.
Previous research has been carried out to find alternative materials to replace gelatin using natural polymers, namely chitosan [8]. Chitosan has been studied to be applied as a base material in making hard capsule shells [9]. Chitosan is a deacetylation product of chitin that is categorized as safe material to be used in the food ingredient. Chitosan has low toxicity, anti-bacterial, anti-cancer, biocompatible, and biodegradable properties [10]. The studies indicated that chitosan capsules have low solubility in HCl and do not dissolve in water. This is because chitosan has a high molecular weight, so a method to increase the solubility of chitosan in water was developed. One of the methods that can be used to increase the solubility of chitosan in water is depolymerization. Depolymerization is a reaction to break down the polymer chain so that the product has low molecular weight and can be dissolved in water. The chitosan that was able to be dissolved in water was called water-soluble chitosan (WSC) [11].
The synthesize of WSC from shrimp shell waste has been carried out by chitin deacetylation and depolymerization using H2O2 [12]. Several researches have been carried out in utilizing chitin from various marine sources, i.e., squid cartilage [13], shrimp shells [14], clamshells, crab shells [15], vaname shrimp shells [16] and axle shells to be synthesized into water-soluble chitosan by deacetylation process [13].
Alginate has also been used as a material in capsule shells for drug delivery systems [17]. Alginate was widely studied and used in medicine as a drug release material, stabilizer, and gelling agent because of its low toxicity, bio-compatibility, and inexpensive [18].
Starch can be used as capsule raw material, additive, thickening agent, gelling agent, filming agent, and stabilizing agent. Usually, starch comes from natural ingredients, one of which is the Gembili tuber (Dioscorea esculenta L). Gembili tubers have a high starch yield at around 21.44% so they have the potential to be developed as a source of starch for excipients in pharmaceutical formulations [19].
Based on this background, the research to fabricate capsule shells from WSC with the addition of alginate and starch, especially Gembili starch, was conducted. The product characterization including FTIR, swelling test, degradation test, solubility test, and drug release test to determine the quality of the capsule shell was carried out.
The materials used in this study were commercial crab shell chitosan (Chimultiguna brand), aquademin, CH3COOH (Merck, 100%), H2O2 (Merck, 30%), NaOH (Merck, 99.9%), C2H5OH pa, Na alginate, HCl (Merck, 37%), Decolsin capsules (PT Medifarma Laboratories), CaCO3 (SAP chemicals 99.9%), Gembili starch, blue textile dye, olive oil. All materials were purchased from the local chemical store in Surabaya, Indonesia.
Gembili tubers were peeled, washed, cut, and mashed to produce a fine pulp. Water was added to make soft porridge then filtered using cloth. The filtrate obtained was settled for 24 h. The clear liquid obtained was decanted. The starch precipitate was washed using aquademin then dried at 60°C for 24 h. The dry starch was sieved using 100 mesh [20].
2.2.2 Synthesis of Water-Soluble Chitosan
Crab shell chitosan was dissolved in acetic acid 2% solution with a ratio of 1:30 (w/v). The solution was heated at 40°C. H2O2 30% was added to the chitosan solution with a ratio of 1:1 (w/v) and reacted for 4 h. The solution was neutralized using NaOH 10%. The mixture was filtered and the filtrate obtained was mixed with absolute ethanol twice the volume of the filtrate. The solution was incubated in the refrigerator for 24 h. After incubation, the solution was filtered to obtain water-soluble chitosan (WSC). The WSC was heated at 60°C to evaporate ethanol. FTIR analysis was carried out to characterize WSC obtained [19].
2.2.3 The Fabrication of WSC: Alginate Capsule Shell
Water-soluble chitosan (WSC) was dissolved in aquademin and stirred at 60°C for ±4 h. Sodium alginate of 2.5 gr was added to the WSC solution and stirred for another 2.5 h. Aquademin was added to the WSC mixture to make 100 mL WSC: Alginate gel product. WSC: Alginate gel was filtered and sonicated. The capsule was fabricated by dipping a pen into a gel solution for ±15 s. The casted capsule was removed from the pen and dried at 60°C in an oven. The dyeing and drying processes were repeated 3 times [17].
2.2.4 The Fabrication of WSC: Alginate: Starch Capsules Shell
The fabrication of WSC: Alginate: Starch was carried out as in 2.2.3. Gembili starch of 2.5 gr was dissolved in 6 mL of water and stirred for ±30 min to obtain a homogeneous solution. The Gembili starch solution was added to WSC: Alginate mixture and diluted to 100 mL using aquademin so that WSC: Alginate: Starch gel solution was obtained. WSC: Alginate: Starch gel was filtered and sonicated. The capsule shell fabrication was carried out as in 2.2.3. In this study, WSC was denoted as W, alginate as A, and starch as P.
2.2.5 Characterization of FTIR (Fourier Transform Infrared) Chitosan, Water-Soluble Chitosan/WSC) and Capsule Shell
FTIR characterization was carried out at wavenumbers of 400–4000 cm−1 with a resolution of 4 cm−1. The sample was mixed with KBr powder in the ratio of 1:10 and homogenized using mortar and pestle. The sample was pressed using a hydraulic pump to form a thin disk and measured using an FTIR spectrophotometer [3].
2.2.6 Characteristics of Capsule Shell
Swelling Test
WSC: Alginate and WSC: Alginate: Starch capsule shells were dried and weighed. The capsule shell was put into beakers containing 50 mL of aquademin and HCl 0.1 N for 10, 20, 30, and 40 min at 37°C. Every 10 min the wet capsule was weighed. The degree of swelling was calculated according to Eq. (1) [3].
Degradation Test
WSC: Alginate and WSC: Alginate: Starch capsule shells were dried and weighed. The capsule shell was put into aquademin and 50 mL of HCl 0.1 N for 10, 20, and 30 min at 37°C. Every 10 min, the capsule shell was removed from the water and HCl 0.1 N and dried in the oven. The dried capsules were weighed. The percent of degradation was calculated according to Eq. (2) [17].
Solubility Test
WSC: Alginate and WSC: Alginate: Starch capsule shells were put into beakers containing 50 mL of aquademin and HCl 0.1 N and stirred at 100 rpm at 37°C. The time required for the capsule shells to dissolve completely in water and HCl 0.1 N solution was recorded [3].
3.1 Synthesis of Water-Soluble Chitosan (WSC)
Chitosan is a polymer with a high molecular weight so that it is not dissolved in water. Chitosan can be converted into a smaller molecular weight polymer to make it dissolve in water. One of the methods to increase the chitosan solubility is depolymerization. H2O2 was one of the reagents that can be used to depolymerize chitosan to form water-soluble chitosan (WSC) [11]. The synthesis of WSC was carried out by adding CH3COOH 2% solution into chitosan at 40°C. The ratio of chitosan: CH3COOH 2% was 1:30 (w/v). The addition of CH3COOH was to dissolve chitosan to form a thick brownish-yellow gel. The carboxyl group (-COOH) in acetic acid interact with the amine group in chitosan. The amine group was released as protonated chitosan, a cationic amino group (-NH+) [21]. The solubility of WSC in water at 40°C was better than at room temperature [15]. The next step was the addition of H2O2 30% at 40°C for 4 h. The ratio of chitosan: H2O2 was 1:1 (w/v). H2O2 was used for depolymerization because it is environmentally friendly and the byproduct was not dangerous. Chitosan depolymerization using H2O2 was relatively slow [22].
The R–NH2 group in chitosan reacts with the H+ ion to form R–NH+ which causes a decrease in the concentration of the H+. The release of H+ ions produces the formation of Hydroperoxyl anions (HOO-) which are very unstable so that they will decompose into hydroxyl radicals (HO•) which are highly reactive Eqs. (3)–(7). The hydroxyl radical reacts very quickly through the decomposition of the H atom which binds to the C atom according to Eq. (8) which causes the β-1,4 glycosidic bond to break from the chitosan so that water-soluble chitosan was formed [23].
NaOH 10% (w/w) was added to neutralize solution pH and to stop the depolymerization reaction. The chitosan solution was filtered and the filtrate obtained was mixed with ethanol as much as twice the volume of the filtrate. The ethanol was added to precipitate water-soluble chitosan (WSC). The WSC obtained was incubated in the refrigerator for 24 h. The WSC obtained from this study was a yellowish-white solid as shown in Fig. 1.

Figure 1: Water-soluble chitosan (WSC) solids
The water-soluble chitosan (WSC) obtained in this study was in accordance with the WSC obtained in the previous study [3].
3.2 Synthesis of WSC: Alginate Capsule Shell
The fabrication of WSC: Alginate capsule shells were carried out by heating the WSC at 60°C to evaporate the ethanol residue. The dry WSC was dissolved in aquademin and stirred at 60°C for ±4 h. At this stage, a pale yellow WSC solution was obtained. The WSC solution was mixed with sodium alginate as much as 2.5 gr, then closed tightly using aluminum foil and stirred for ±2.5 h. The alginate used in this study was food-grade alginate that is free from cellulose and bleach so that it is safe to be used as a gelling agent for capsule shells. Aquademin was added to the alginate and WSC mixture to obtain a 100 mL gel solution. The gel solution was filtered and sonicated. Filtration was carried out to remove WSC and starch that was not dissolved. The sonicator was used to remove bubbles trapped in the gel solution during the heating and stirring process.
The molding of the capsule shell was carried out by dipping a pen in the gel solution for ±15 s. The dipping pen was lubricated with olive oil to facilitate the removal of the dry capsule shell after molding. The capsule shell obtained was dried in an oven at 60°C to dry. The process of dyeing and drying was repeated 3 times to obtain a capsule shell with suitable thickness and uniformity. The dried capsule shell was cut according to the capsule shell specifications.
The physical properties of the capsule shell obtained were shown in Table 1. The image of WSC: Alginate from this study and commercial capsule shell were shown in Figs. 2a–2d.

3.3 Synthesis of WSC: Alginate: Starch Capsules Shell
The fabrication of WSC: Alginate capsule shell with the addition of Gembili starch with Alginate: Starch ratio of 1:1 was carried out. The starch was used as a thickening, gelling, filming, and stabilizing agent [19]. The ratios of WSC: Alginate: Starch were 2:1:1, 3:1:1, 4:1:1 (w/w). The variations were carried out to optimize the capsule shells obtained according to their physicochemical properties [3]. The evaluation criteria of capsule shells were based on physical appearance from Fig. 2. The physical appearance comparison of the capsule shell obtained from this study compared to the commercial one were shown in Table 2. The images of WSC: Alginate: Starch capsule shell and a commercial capsule shell were shown in Figs. 3a–3d.
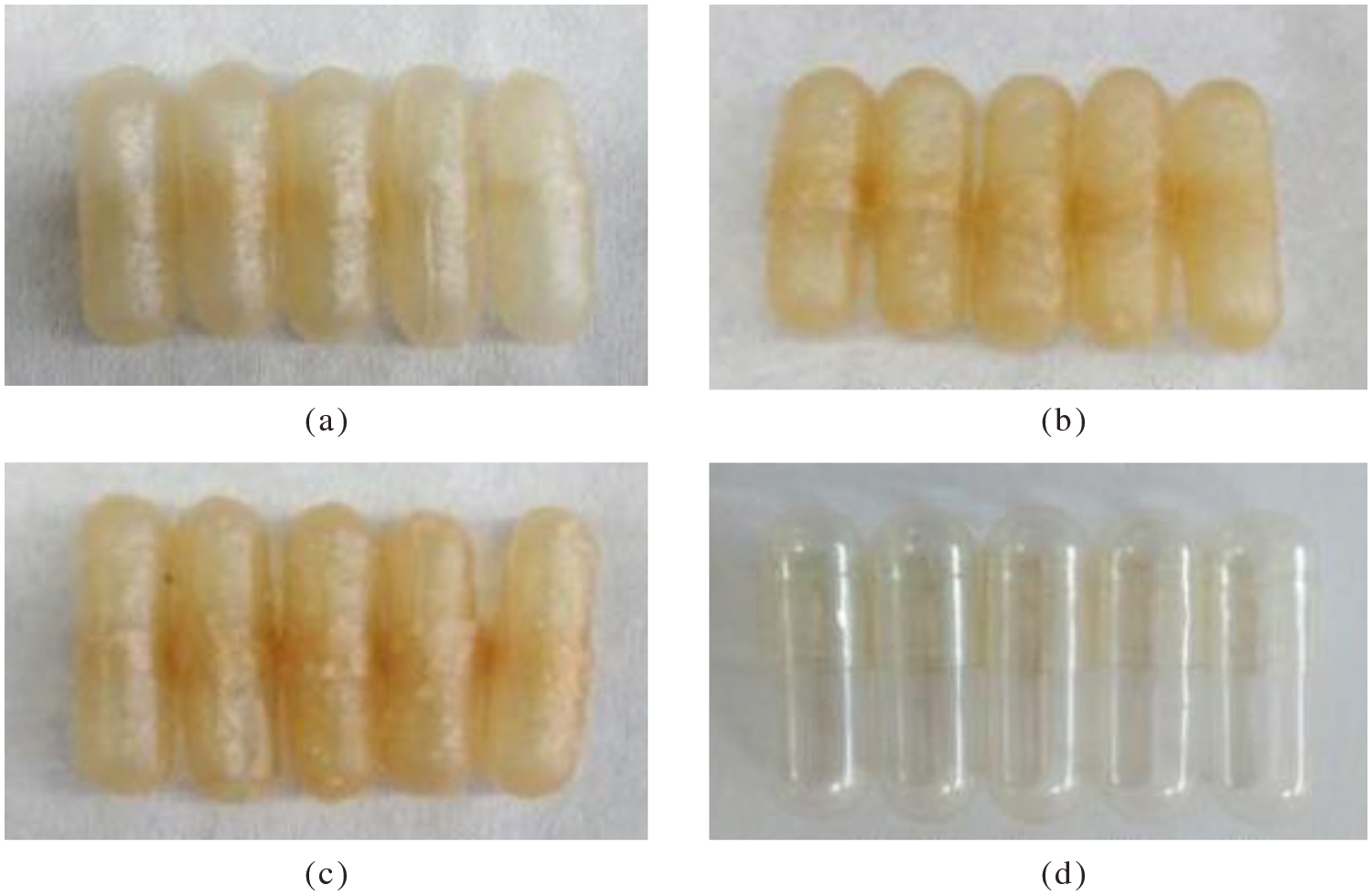
Figure 2: WSC: Alginate (a) W:A (2:1), (b) W:A (3:1), (c) W:A (4:1) and (d) commercial capsule shells
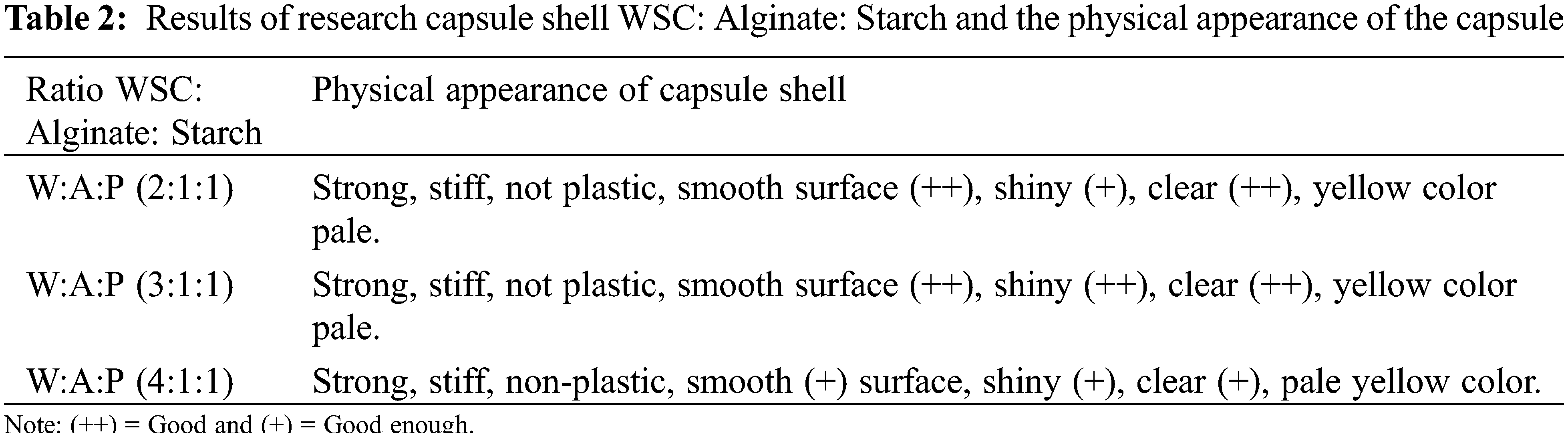
Figure 3: WSC: Alginate: Starch (a) W:A:P (2:1:1), (b) W:A:P (3:1:1), (c) W:A:P (4:1:1) and (d) Commercial capsule shells
3.4 Characterization of WSC: Alginate and WSC: Alginate: Starch Capsule Shell
The swelling test was conducted to determine the amount of liquid that was absorbed into the capsule matrix and to determine the resistance of the capsule to a liquid. Water and HCl 0.1 N solution were used as swelling medium because water represents the liquid in the mouth and HCl 0.1 N solution represents the liquid in the stomach. The swelling test results of WSC: Alginate and WSC: Alginate: Starch capsule shell were shown in Figs. 4 and 5.
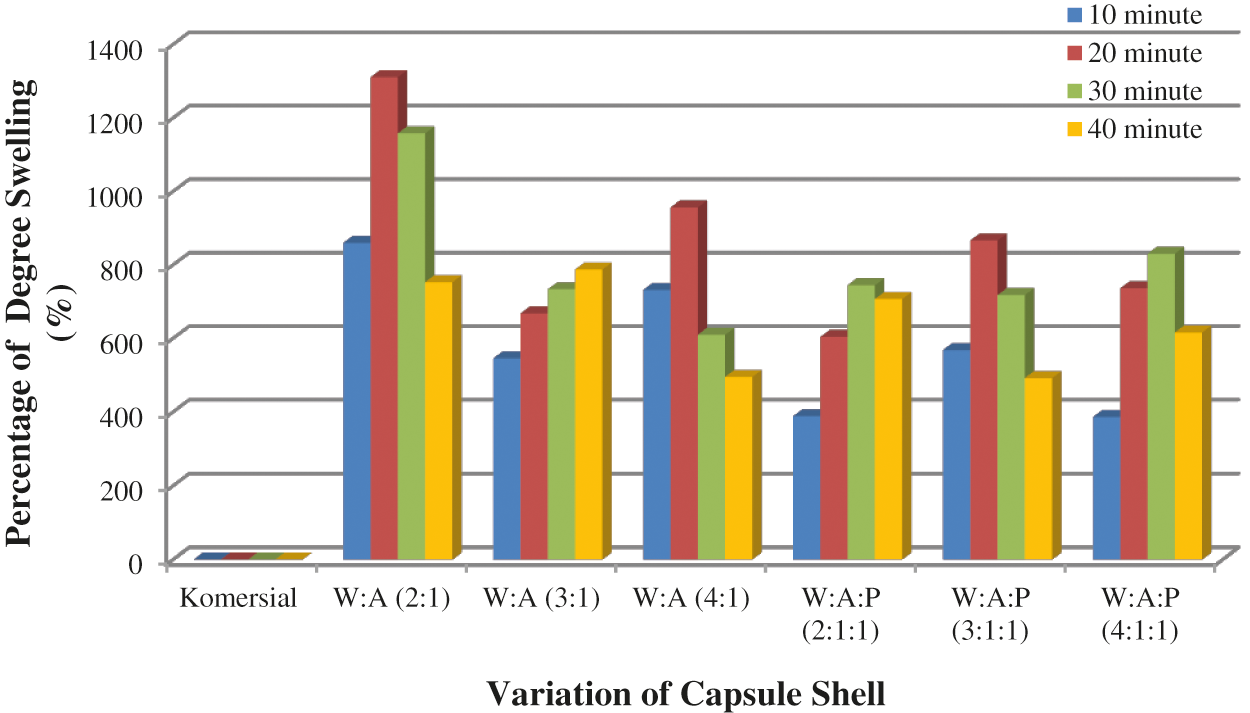
Figure 4: WSC: Alginate and WSC: Alginate: Starch capsule shell swelling test results in the water
The swelling test in water indicates that the commercial capsule shell has reached the maximum swelling degree in 10 min as it was completely dissolved at about 10 min. WSC: Alginate with the composition (W: A, 4:1) has the maximum swelling degree which was 494.86%. The WSC: Alginate: Starch (W:A:P, 3:1:1) capsule shell’s degree of swelling was 491.93%.
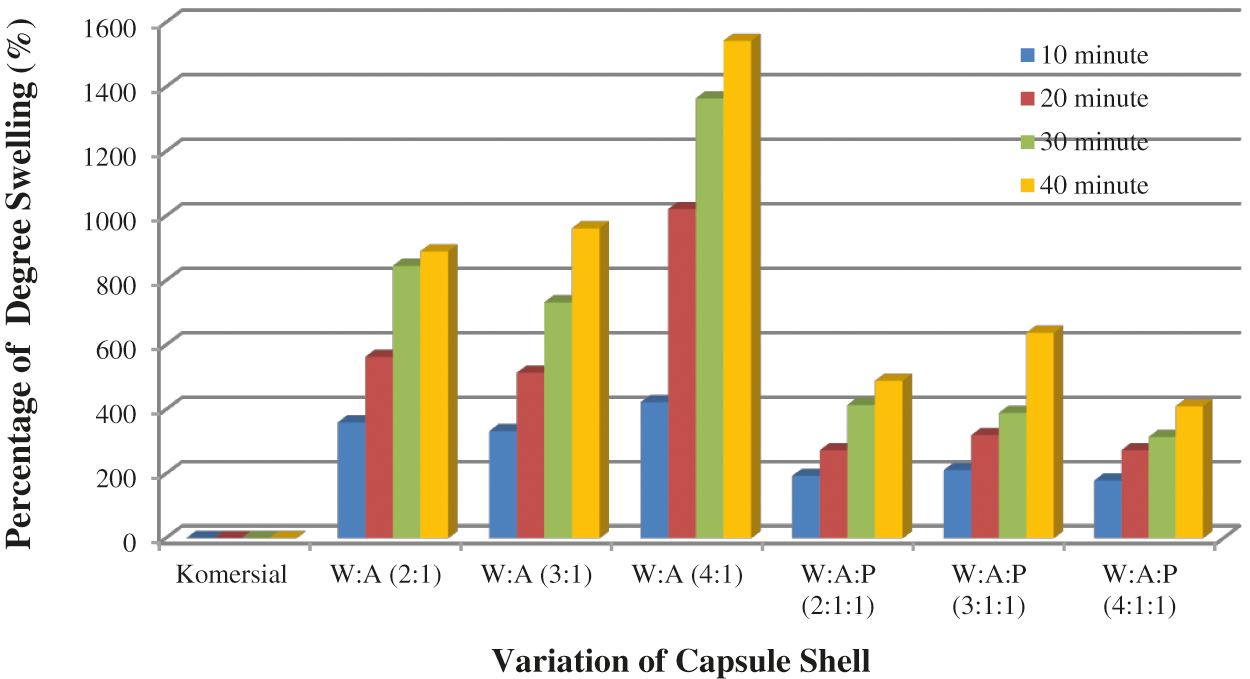
Figure 5: WSC: Alginate and WSC: Alginate: Starch capsule shell swelling test results in 0.1 N HCl
The swelling test data in HCl 0.1 N indicates that the commercial capsule shells in HCl 0.1 N have completely dissolved after being immersed for 10 min. WSC: Alginate (W:A, 2:1) capsule shells degree of swelling was 890.86%. WSC: Alginate: Starch (W:A:P, 4:1:1) capsule shells best degree of swelling was 410.51%. The swelling degree value of the capsule shell depends on the application of the capsule shell. Capsule shells with a swelling degree in the range of 100% can be applied to release the drug in the stomach. Capsule with the degree of swelling >100% can be applied to encapsulate drugs that are released in the intestine [2].
The capsule shell degradation test was carried out to determine the mass lost within a certain time. The degradation test was carried out by immersing the capsule shells in water and HCl 0.1 N solution for 30 min with 10-minute intervals of dry mass weighting [3]. The degradation test of the WSC: Alginate and WSC:Alginate: Starch capsule shells were shown in Figs. 6 and 7.
The degradation test in water, in this study, indicates that the commercial capsule shell in water in the first 20 min has reached the maximum degree of degradation because at about 20 min the capsule shell has completely dissolved. WSC: Alginate capsule shell with the best degree of degradation was WSC: Alginate (W:A) with a composition of 4:1, 65.01%. WSC: Alginate: Starch capsule shell best degree of degradation was WSC: Alginate: Starch (W:A:P) with a composition 2:1:1 which was 57.80%.
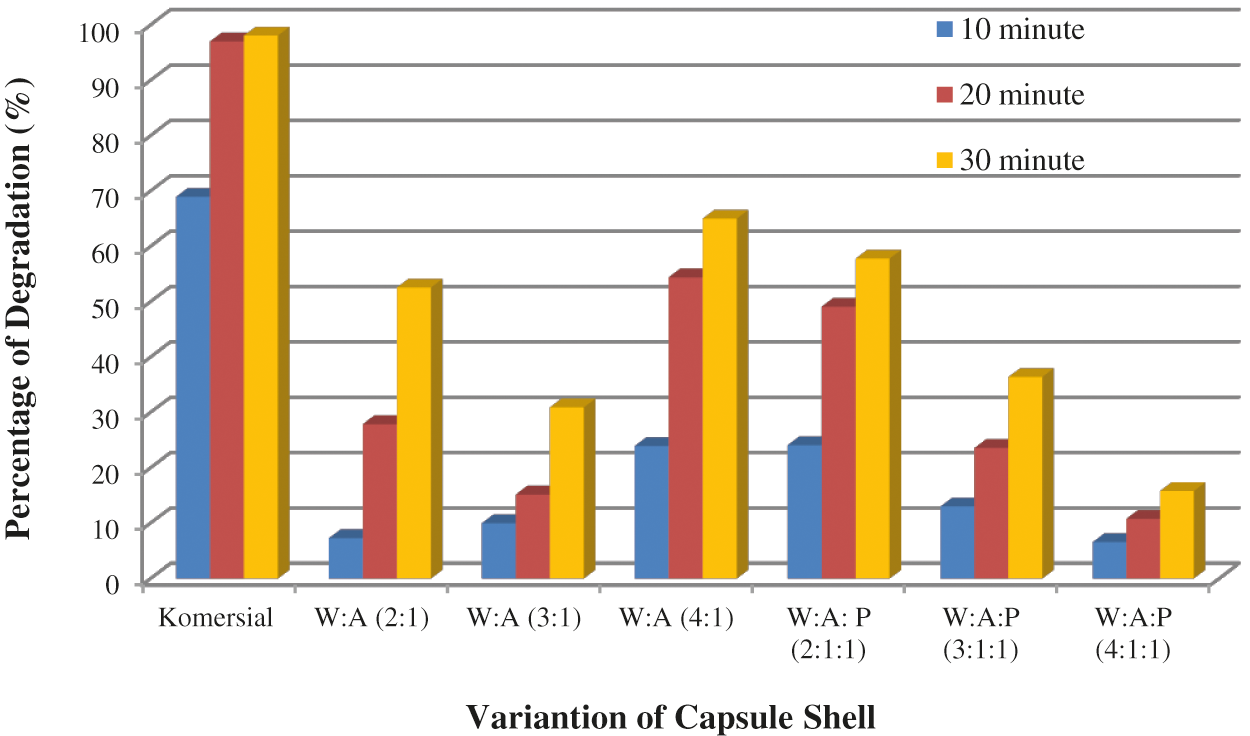
Figure 6: The results of the WSC capsule shell degradation test: Alginate and WSC capsule shell: Alginate: Starch in water
The degradation test in HCl 0.1 N showed that the commercial capsule shell in HCl 0.1 N in the first 20 min had reached the maximum degree of degradation because before 20 min, the capsule shell had completely dissolved. WSC: Alginate capsule shell’s best degree of degradation was WSC: Alginate with a composition (W:A, 4:1) that was 30.02%. WSC: Alginate: Starch capsule shell with the best degree of degradation was WSC: Alginate: Starch with a composition of (W:A:P, 3:1:1) that was 21.44%.
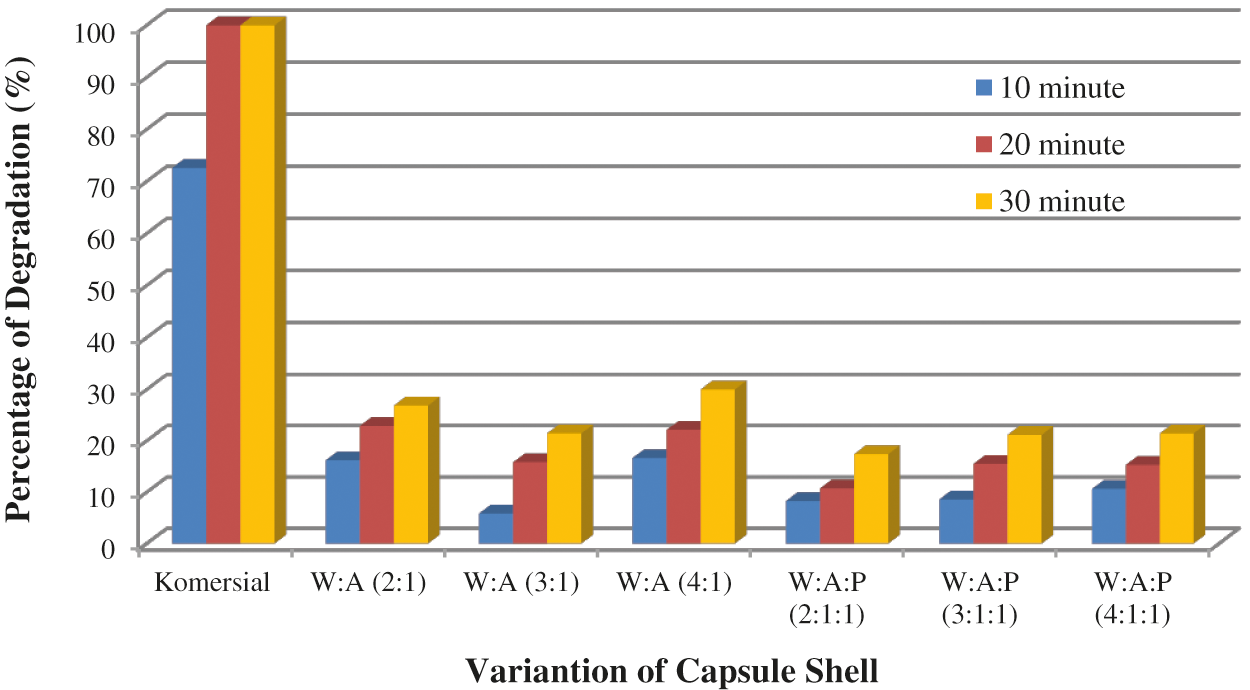
Figure 7: The WSC: Alginate and WSC: Alginate: Starch capsule shell degradation test in HCl 0.1 N
The solubility test was conducted to determine the time required for the capsule shell to be completely dissolved. The solubility test carried out in the water and HCl 0.1 N medium was shown in Table 3.
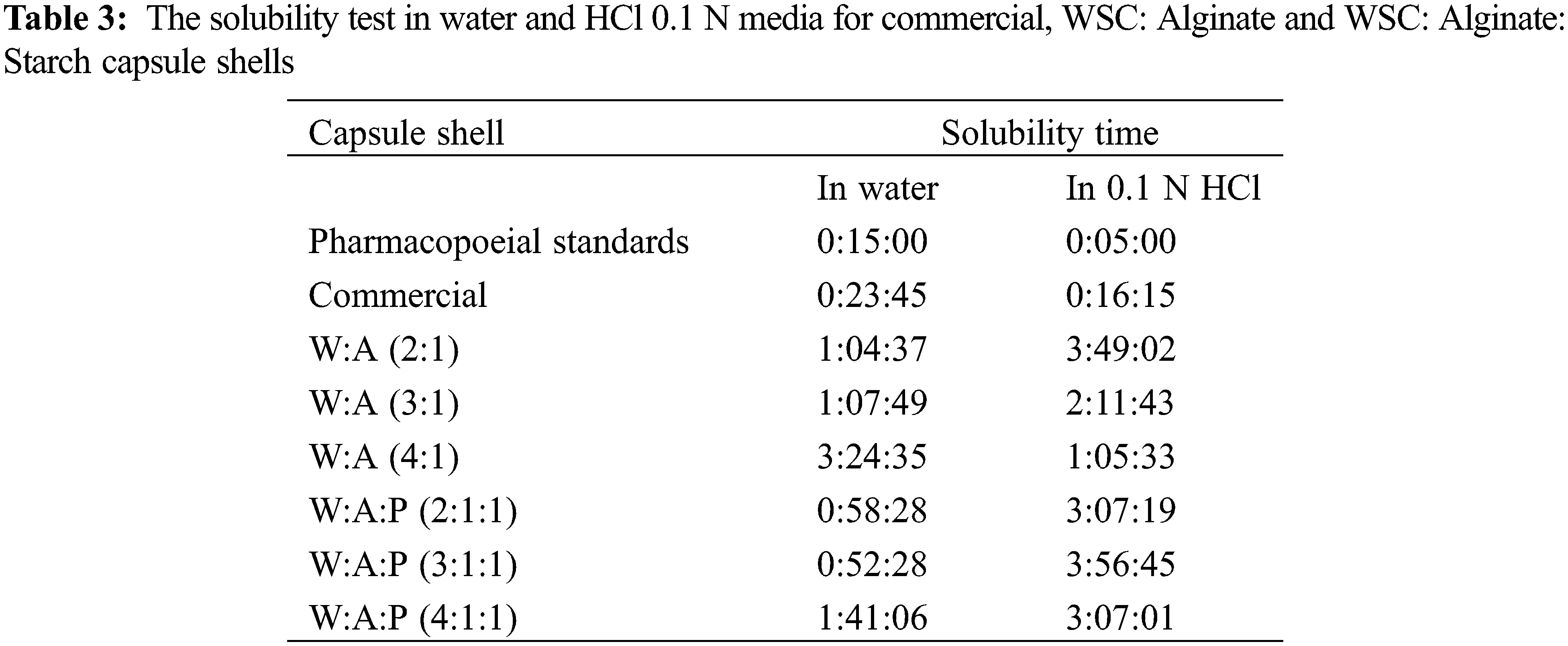
The solubility standard for capsule shells in water is at least 15 min, whereas in HCl 0.1 N is 5 min [24]. The capsule shell with the composition of WSC: Alginate: Starch was thicker than WSC: Alginate, so it has a longer solubility time. The solubility test in water showed that the capsule shell of WSC: Alginate (2:1) had the fastest solubility, which was 1 h 4 min 37 s. WSC: Alginate: Starch (3:1:1) capsule shell has the fastest solubility which was 52 min 28 s.
The solubility test in HCl 0.1 N showed that the WSC: Alginate (4:1) had the fastest solubility time of 1 h 5 min 33 s. WSC: Alginate: Starch (4:1:1) has the fastest solubility time of 3 h 7 min 1 s. The difference in the solubility time between the variations in the capsule shell was due to the different thicknesses between the capsule shells.
3.4.4 Fourier Transform Infrared (FTIR) Characterization
FTIR analysis was applied to identify functional groups of the compound. The FTIR spectra of crab chitosan, WSC, WSC capsule shell: Alginate, and WSC capsule shell: Alginate: Starch was shown in Fig. 8. The FTIR of chitosan showed a strong vibration at a wavenumber of 3285.50 cm−1 that originating from the hydroxyl group (O–H), a peak at a wavenumber of 2873.81 cm−1 was caused by the stretching vibration of CH (–CH2–), the peak at a wavenumber of 1636.75 cm−1 was caused by the bending vibration of the NH group (R–NH2) and the peak at a wavenumber of 1376.82 cm−1 was caused by the bending vibration of CH (–CH2–). Other than that, there was also absorption caused by stretching vibration C–O (–C–O–C) at the wavenumber 1027.26 cm−1.
FTIR spectra of the WSC at a wavenumber of 3277.12 cm−1 indicated the presence of stretching vibration of O–H, peak at a wavenumber of 2875.81 cm−1 indicated the presence of stretching vibration of CH (–CH2–), peak at a wavenumber of 155.59 cm−1 indicates a bending vibration of NH (R–NH2) and the peak at a wavenumber of 1376.75 cm−1 indicates a bending vibration of CH (–CH2–). In addition, there was also absorption at the wavenumber of 1023.26 cm−1 that was caused by the stretching vibration of C–O (–C–O–C).
FTIR spectra of WSC: Alginate (2:1) capsule shells generally have the same pattern as FTIR from WSC. FTIR Spectra of WSC: Alginate has a peak at a wavenumber of 3257 cm−1 which was the stretching vibration of O–H, peak at a wavenumber of 2919 cm−1 came from the vibration of C–H sp3, peak at a wavenumber of 1581 cm−1 was the peak of NH (R–NH2) bending vibration. However, in the FTIR spectra of the WSC-alginate capsule shell, there was a decrease in the intensity of the NH (R–NH2) group and there was a new absorption at wave number 1412 cm−1 which was the peak of NH group (R–NH+) vibrations. This peak was appear due to the protonation of the NH2 group to become NH+ during WSC and alginate mixing.
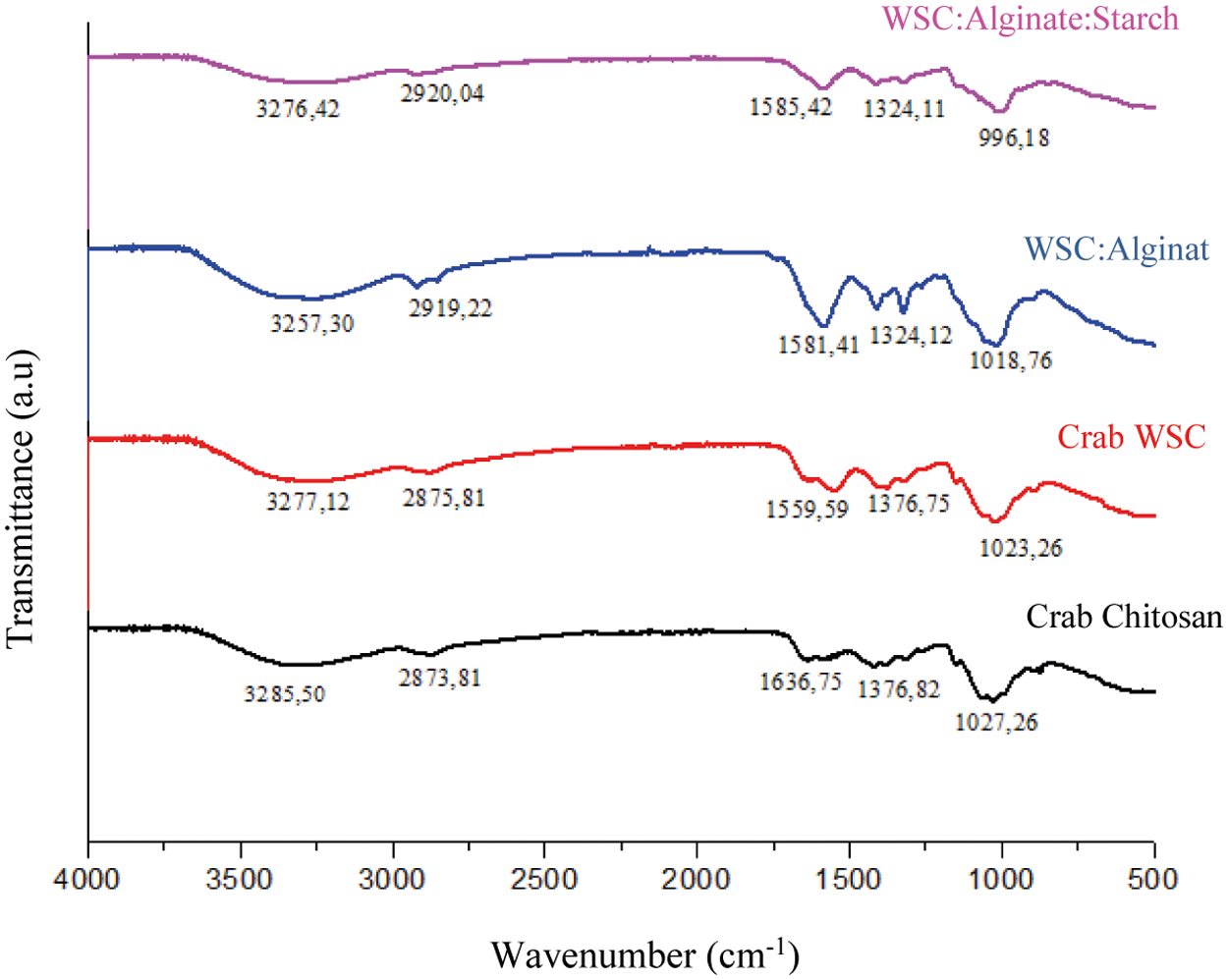
Figure 8: FTIR test results of small chitosan, crab WSC, WSC capsule shell: Alginate, and WSC capsule shell: Alginate: Gembili starch
FTIR spectra of WSC: Alginate: Starch (2:1:1) capsule shell generally have the same pattern as FTIR from chitosan, WSC, and WSC: Alginate. WSC: Alginate: Starch FTIR spectra has a strong vibration at a wavenumber of 3276 cm−1 originating from the hydroxyl group (O–H) vibration, the peak at a wavenumber of 2920 cm−1 was caused by stretching vibrations of CH (–CH2–), The peak at a wavenumber of 1585 cm−1 was caused by the bending vibration of the NH group (R–NH2) and the peak at a wavenumber of 1324 cm−1 was caused by the bending vibration of CH (–CH2–).
Water-soluble chitosan (WSC) from crab shell that combined with alginate and Gembili starch has been successfully fabricated as capsule shell. WSC: Alginate capsule shell with the addition of Gembili starch has a stronger, stiffer, smoother, shiny, and clear capsule shell surface than WSC: Alginate capsule shell. FTIR spectra of the capsule shell showed vibrations that come from the molecules that make up chitosan, alginate, and starch. The best degrees of swelling in water and HCl 0.1 N solution were 491.93% and 410.51%, respectively. The best degradation in water and HCl 0.1 N solution were 57.80% and 21.44%, respectively. The observation of capsule shell physical appearance shows that WSC: Alginate: Starch 3: 1: 1 capsule shell was closer to the properties of the commercial capsule shell.
Acknowledgement: The authors gratefully acknowledge financial support from the Institut Teknologi Sepuluh Nopember for this work, under the project scheme of the Publication Writing and IPR Incentive Program (PPHKI).
Funding Statement: This research received no external funding.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Shargel, L., Wu-Pong, S., Yu, A. B. C. (1988). Biofarmasetika dan farmakokinetika terapan. Indonesia: Airlangga University Press. [Google Scholar]
2. Darmokoesoemo, H., Pudjiastuti, P., Rahmatullah, B., Kusuma, H. S. (2017). Retraction notice to “Novel drug delivery carrier from alginate-carrageenan and glycerol as plasticizer”. Results in Physics, 19, 2979–2989. DOI 10.1016/j.rinp.2020.103539. [Google Scholar] [CrossRef]
3. Ni’mah, Y. L., Pertiwi, A., Harmami, H., Ulfin, I., Fadlan, A. (2020). Synthesis of capsule from crab water-soluble chitosan and alginate. AIP Conference Proceedings, 2229(1), 030008. DOI 10.1063/5.0002652. [Google Scholar] [CrossRef]
4. Suptijah, P., Suseno, S. H., Kurniawati, K. (2012). Aplikasi karagenan sebagai cangkang kapsul keras alternatif pengganti kapsul gelatin. Jurnal Pengolahan Hasil Perikanan Indonesia, 15(3), 223–231. DOI 10.17844/jphpi.v15i3.21434. [Google Scholar] [CrossRef]
5. Harianto, H., Tazwir, T., Peranginangin, R. (2008). Studi teknik pengeringan gelatin ikan dengan alat pengering kabinet. Jurnal Pascapanen dan Bioteknologi Kelautan dan Perikanan, 3(1), 89–96. DOI 10.15578/jpbkp.v3i1.13. [Google Scholar] [CrossRef]
6. Jannah, A. (2008). Gelatin: Tinjauan kehalalan dan alternatif produksi. Malang: UIN-Maliki Press. [Google Scholar]
7. Eveline, Santoso, J., Widjaja, I. (2011). Kajian konsentrasi dan rasio gelatin dari kulit ikan patin dan kappa karagenan pada pembuatan jeli. Jurnal Pengolahan Hasil Perikanan Indonesia, 14(2), 98–105. DOI 10.17844/jphpi.v14i2.5318. [Google Scholar] [CrossRef]
8. Feng, Q., Zeng, G., Yang, P., Wang, C., Cai, J. (2005). Self-assembly and characterization of polyelectrolyte complex films of hyaluronic acid/chitosan. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 257, 85–88. DOI 10.1016/j.colsurfa.2004.10.099. [Google Scholar] [CrossRef]
9. Yoshizawa, T., Shin-ya, Y., Hong, K. J., Kajiuchi, T. (2005). pH− and temperature-sensitive release behaviors from polyelectrolyte complex films composed of chitosan and PAOMA copolymer. European Journal of Pharmaceuticts and Biopharmaceutics, 59(2), 307–313. DOI 10.1016/j.ejpb.2004.08.002. [Google Scholar] [CrossRef]
10. Alami, R., Permatasari, L. (2016). Industry pharmaceuticals: Chitosan as an alternative replacement gelatin capsules on shell. Jurnal of Medical and Bioengineering, 5(1), 67–71. DOI 10.12720/jomb.5.1.67-71. [Google Scholar] [CrossRef]
11. Du, Y., Zhao, Y., Dai, S., Yang, B. (2009). Preparation of water-soluble chitosan from shrimp shell and its antibacterial activity. Innovative Food Science & Emerging Technologies, 10(1), 103–107. DOI 10.1016/j.ifset.2008.07.004. [Google Scholar] [CrossRef]
12. Ni’mah, Y. L., Harmami, H., Ulfin, I., Suprapto, S., Saleh, C. W. (2019). Water-soluble chitosan preparation from marine sources. Malaysian Journal of Fundamental and Applied Sciences, 15(2), 159–163. DOI 10.11113/mjfas.v15n2.971. [Google Scholar] [CrossRef]
13. Yusharani, M. S., Ni’mah, Y. L. (2019). Synthesis of water-soluble chitosan from squid pens waste as raw material for capsule shell: Temperature deacetylation and reaction time. IOP Conference Series: Material Science Engineering, 509, 012070. DOI 10.1088/1757-899X/509/1/012070. [Google Scholar] [CrossRef]
14. Lestari, P. I., Ulfin, I., Harmami, H., Suprapto, S., Ni’mah, Y. L. (2018). Water-soluble chitosan from waste swimming crab shell (Portunus pelagicus). AIP Conference Proceedings, 2049(1), 020085. DOI 10.1063/1.5082490. [Google Scholar] [CrossRef]
15. Pambudi, G. B. R., Ulfin, I., Harmami, H., Suprapto, S., Kurniawan, F. et al. (2018). Synthesis of water-soluble chitosan from crab shells (Scylla serrata) waste. AIP Conference Proceedings, 2049(1), 020086. DOI 10.1063/1.5082491. [Google Scholar] [CrossRef]
16. Harmami, H., Ulfin, I., Sakinah, A. H., Ni’mah, Y. L. (2019). Water-soluble chitosan from shrimp and mussel shells as corrosion inhibitor on tinplate in 2% NaCl. Malaysian Journal of Fundamental and Applied Sciences, 15(2), 212–217. DOI 10.11113/mjfas.v15n2.972. [Google Scholar] [CrossRef]
17. Tiany, H. K., Ulfin, I., Harmami, H., Ni’mah, Y. L. (2019). Synthesis of halal membrane capsule from water-soluble chitosan by adding sodium lauryl ether sulphate. IOP Conference Series: Material Science Engineering, 509, 012050. DOI 10.1088/1757-899X/509/1/012050. [Google Scholar] [CrossRef]
18. Lee, K. Y., Mooney, D. J. (2012). Alginate: Properties and biomedical applications. Progress in Polymer Science, 37(1), 106–126. DOI 10.1016/j.progpolymsci.2011.06.003. [Google Scholar] [CrossRef]
19. Richana, N., Sunarti, T. C. (2019). Karakterisasi sifat fisikokimia tepung umbi dan tepung pati dari umbi ganyong, suweg, ubikelapa dan gembili. Jurnal Penelitian Pascapanen Pertanian, 1(1), 29–37. DOI 10.21082/jpasca.v1n1.2004.29-37. [Google Scholar] [CrossRef]
20. Syofyan, S., Yelni, E. A., Azhar, R. (2013). Penggunaan kombinasi pati bengkuang–avicel PH101 sebagai bahan pengisi co-process tablet isoniazid cetak langsung. Jurnal Farmasi Higea, 5(1), 42–50. DOI 10.52689/higea.v5i1.75. [Google Scholar] [CrossRef]
21. Bourtoom, T., Chinnan, M. S. (2008). Preparation and properties of rice starch-chitosan blend biodegradable film. LWT-Food Science and Technology, 41(9), 1633–1641. DOI 10.1016/j.lwt.2007.10.014. [Google Scholar] [CrossRef]
22. Tanasale, M. F. J. D. P., Telussa, I., Sekewael, S. J. (2016). Ekstraksi dan karakterisasi kitosan dari kulit udang windu (penaeus monodon) serta proses depolimerisasi kitosan dengan hidrogen peroksida berdasarkan variasi suhu pemanasan. Indonesian Journal of Chemical Research, 3(2), 308–318. [Google Scholar]
23. Qin, C., Du, Y., Xiao, L. (2002). Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polymer Degradation and Stability, 76(2), 211–218. DOI 10.1016/S0141-3910(02)00016-2. [Google Scholar] [CrossRef]
24. Departemen Kesehatan Republik Indonesia (1995). Farmakope Indonesia Edisi IV. Jakarta: Departemen Kesehatan Republik Indonesia. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |