 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.023314
REVIEW
A Review of Soy-Tannin Gelling for Resins Applications
LERMAB-ENSTIB, University of Lorraine, 27 rue Philippe, Seguin, Epinal, 88000, France
*Corresponding Author: Antonio Pizzi. Email: antonio.pizzi@univ-lorraine.fr
Received: 19 April 2022; Accepted: 14 June 2022
Abstract: Soy flour (SF), soy protein and soy protein isolates (SPI) have been the focus of increasing research on their application as new materials for a variety of applications, mainly for wood adhesives and other resins. Tannins too have been the focus of increasing research for similar applications. While both materials are classed as non-toxic and have achieved interesting results the majority of the numerous and rather inventive approaches have still relied on some sort of hardeners or cross-linkers to bring either of them or even their combination to achieve acceptable results. The paper after a presentation of the two materials and their characteristics concentrates on the formation of gels, gelling and even hardening in the case of soy-tannin combined resins. The chapter than finishes with details of the formation of resins giving suitable wood adhesive of acceptable performance by the covalent coreaction of soy protein and tannin without any other hardener, thus totally bio-sourced, non-toxic and environment friendly as a base of further advances to expect in future by these two materials combination.
Keywords: Soy; tannin; soy-tannin coreaction; gelling; hardening; wood
Vegetable tannins have been used for centuries for leather tanning, even millennia, by using barks or woods containing tannin. World War II marked the zenith of the tannin extraction industry for leather tanning [1]. After the war synthetic materials progressively substituted leather in the shoe soles industry. The crisis that ensued led to numerous tannin extraction factories closing down [2]. Tannins resurgence started in the early 1970’s at first with their development as bio-based adhesives. Now a continuous growth for different tannin applications for new renewable materials is in full swing.
Tannin extracts appearance is rather variable. They can be white or off-white amorphous powders, or pastes, or almost black to brown to reddish powders when spray-dried. Their taste is rather astringent. They are found in many higher plants roots, barks, wood, leaves and seeds. Their role in the plant is mainly defensive by complexing proteins irreversibly, thus protecting the plant against insects, fungi, herbivores and bacterial attacks and other generalized infections. Considerable research work is going on at present on their pharmacological and medical effects on human and farm animal health. Two main classes of tannins exist: (i) hydrolysable tannins with two subgroups, namely ellagi-tannins and gallo-tannins, and (ii) condensed polyflavonoid tannins, these not hydrolysis sensitive [2].
Hydrolysable tannins, are derivatives of gallic acid, being classed according to the hydrolysis products obtained in gallo-tannins (composed of glucose and gallic acid) and ellagi-tannins (composed of glucose and biaryl units of gallic acid). They are also often mistakenly referred to by the general and undefined, mistaken term “tannic acid” without that this terms explains either the real structure of the tannin used or from which plant does such a “tannic acid” originate.
1.2.1 Gallo-Tannins and “Tannic Acid”
“Tannic acid”, a misnomer for hydrolysable tannins, is a well-known substance cited in the literature. Tannic acid is in reality a mixture of rather similar compounds, such as tri-(digalloyl)-di-(galloyl)-glucose, tetra-(digalloyl)-glucose and penta-(digalloyl)-glucose, etc. [3–6].
A D-glucose-derived polyol residue occurs in most plants-isolated gallo-tannins, although a large number of different types of polyol can occur. Two more classes exist, gallo-tannins such as for tara gallo-tannins (composed of glucose and quinic and gallic acid) and caffe-tannins (quinic acid esterified with caffeic acids plus glucose compounds) [3,4] (Fig. 1).
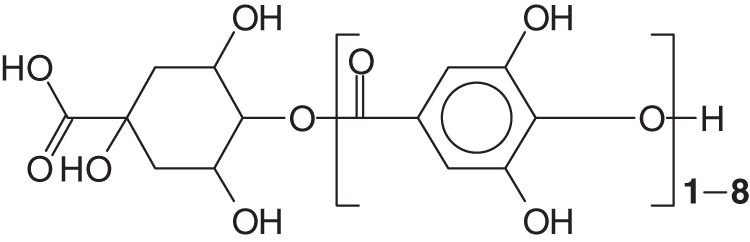
Figure 1: Structure example of tara tannin [3,4] and caffeic acid-tannins (quinic acid esterified with caffeic acids plus glucose compounds)
The name of ellagi-tannin derives from the presence of ellagic acid (Fig. 2) in these materials. Ellagic acid is by definition a diester of gallic acid of formula
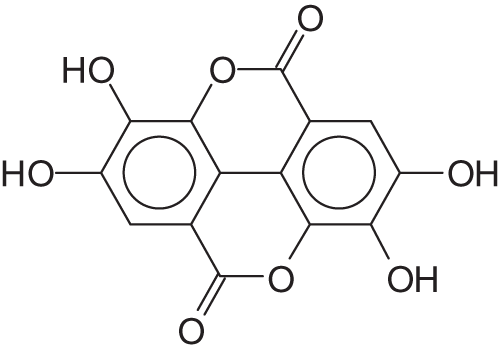
Figure 2: Ellagic acid
Ellagi-tannins, differently from gallo-tannins, comprise additional binding motifs arising from additional galloyl fragments of gallic acid moieties oxidative coupling reactions [6]. Thus, the ellagi-tannins biosynthesis is an oxidative enzymatic progression of gallo-tannins. An example of ellagi-tannin is the tannin of chestnut wood (Fig. 3).
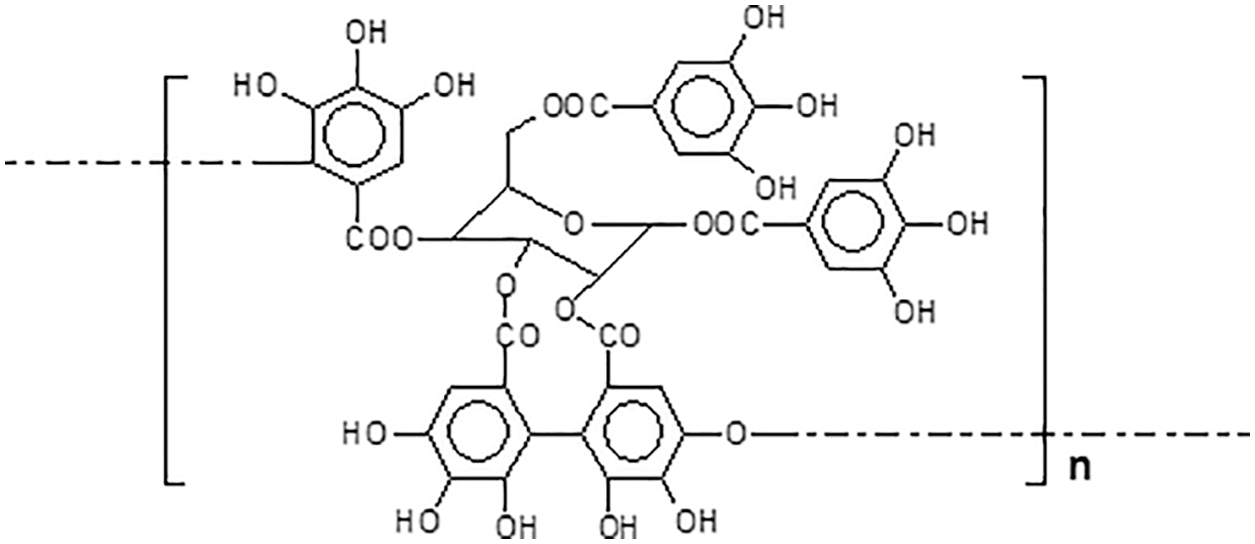
Figure 3: Schematic representation of the repeating unit of the ellagi-tannin of chestnut wood
Condensed tannins are linked flavonoid units oligomers having different degrees of condensation. Commercial tannin extracts contain their precursor flavonoid units, namely flavan-3-ols and flavan-3,4-diols, and other flavonoid analogs [7–9], carbohydrates such as monosaccharides and hydrocolloid gums, these being fragments of hemicelluloses, and traces of amino-and imino-acids [2]. Simple carbohydrates (hexoses, pentoses and disaccharides), complex glucuronates (hydrocolloid gums) and oligomers from hydrolysed hemicelluloses are also present, and often in noticeable proportions. Some flavonoid units in tannins may sometime be attached to carbohydrate chains of variable degree of polymerization [10,11] (Fig. 4). The viscosity of tannin extract solutions can be decreased or increased by the presence of all these different materials when these are in proportions high enough.
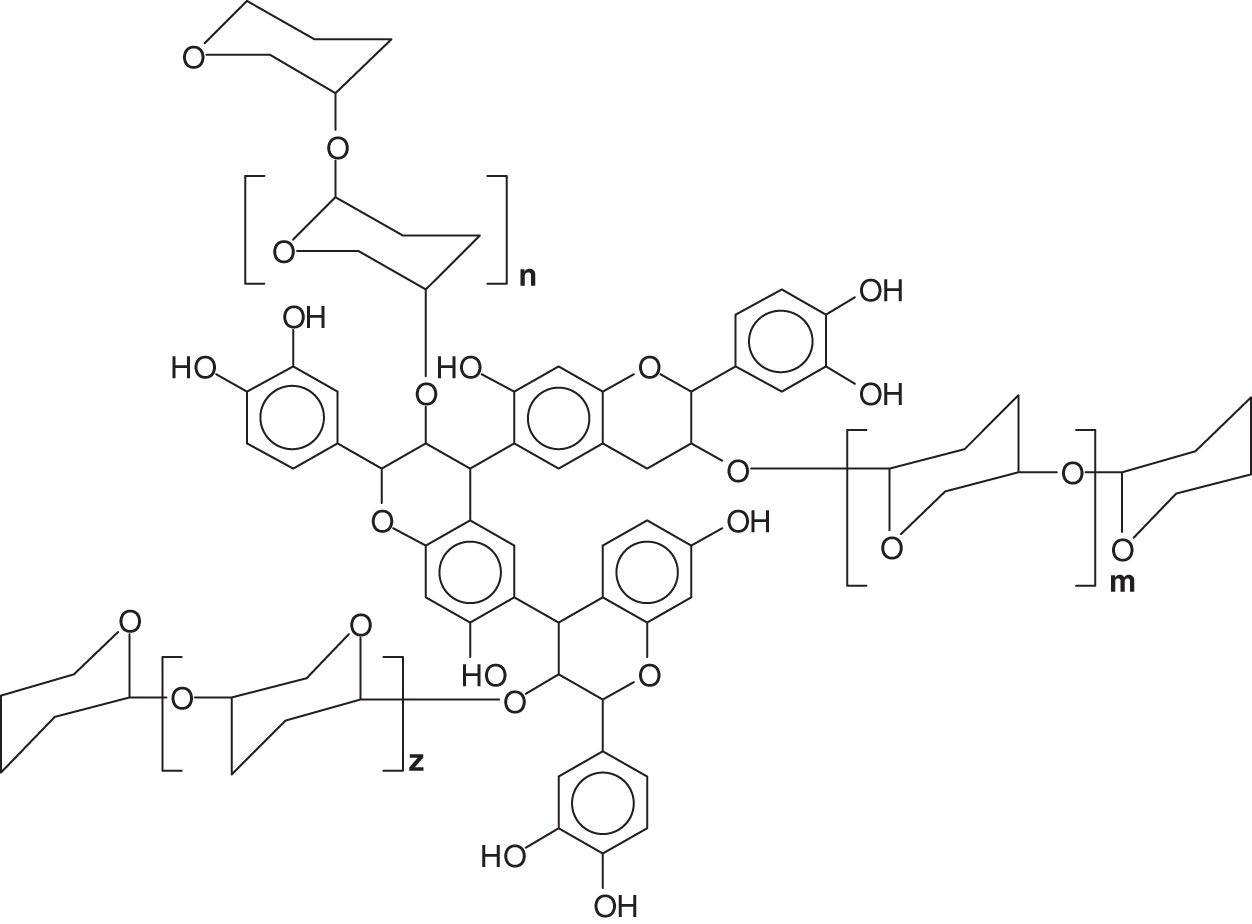
Figure 4: Polyflavonoids and carbohydrates linkages
Note: the –OH groups on the carbohydrates are not shown for visual ease of the structure.
Monoflavonoids, and diflavonoids are classed as “phenolic non-tannins”, are extensively studied in commercial tannin extracts as they are simpler reflections of higher oligomers. They comprise flavan-3,4-diols, flavan-3-ols, dihydroflavonoids (flavonols), flavanones, chalcones and coumaran-3-ols, and their dimers [6,7,10,11]. Typical are those of the black mimosa bark extract (Acacia mearnsii, formerly mollissima, de Wildt) where the four possible combinations of resorcinol and phloroglucinol (A-rings) with catechol and pyrogallol (B-rings) coexist (Fig. 5). The percentage of monoflavonoids in the tannin extract is as low as 3%–5% of its total phenolics [7]. Flavan-3,4-diols and some flavan-3-ols are the only monomer units participating to mimosa tannin formation.
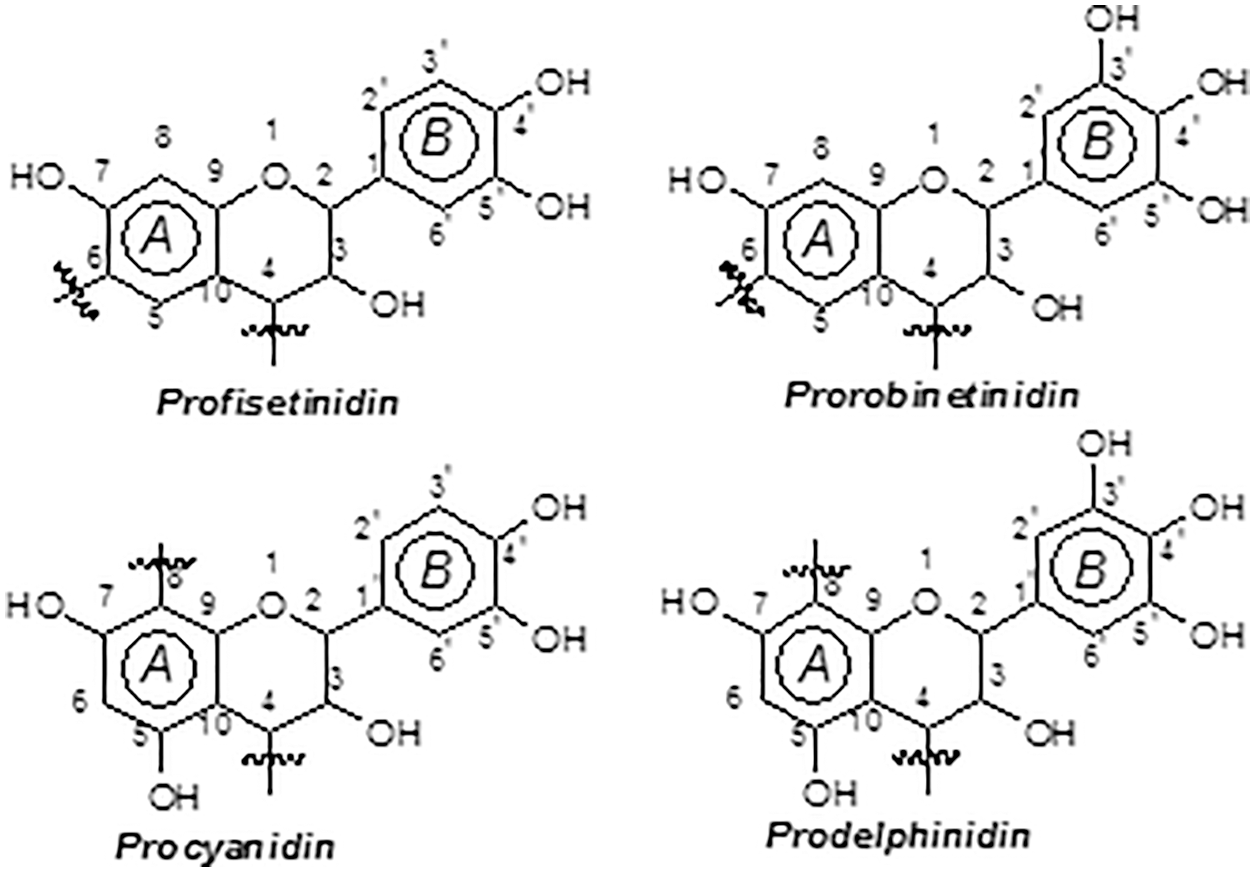
Figure 5: The four repeating flavonoid units in condensed tannins
The four combinations of resorcinol and phloroglucinol A-ring types with catechol and pyrogallol B-ring types are the four pattern in mimosa bark tannin extract which are present. The pattern based on resorcinol A-ring and pyrogallol B-ring (robinetinidin) is the principal polyphenolic one for mimosa tannin and constitutes approximately 70% of the total phenolics of this extract. A secondary pattern based on resorcinol A-rings and catechol B-rings (fisetinidin) constitutes roughly 25% of total phenolics. Two minority combinations constituted of phloroglucinol (A-ring)-pyrogallol (B-ring) (gallocatechin/delphinidin) and phloroglucinol (A-ring)-catechol (B-ring) flavonoids (catechin, epicatechin) in of A-and B-rings are complete the two predominant patterns. The total phenolics of commercial mimosa bark extract that these four patterns represent are in the 65%–84% range of total extract solids. The rest are the “non-tannins”.
This “non-tannins” definition originates in the leather trade, as only any polyphenolic oligomer higher of, and comprising a trimer is considered a “tannin”. Non-tannins percentages are quite variable with the type of tannin extraction. Carbohydrates, hydrocolloid gums, amino and imino acid fractions constitute the non-phenolic non-tannins [2,12].
1.3.1 Condensed Flavonoid Tannins Reactions
Condensed flavonoid tannins can undergo several reactions impacting on their capability to adapt to different applications. The main tannin chemistry reactions are extensively presented in all the literature reviews dedicated to such a subject. For more complete and in depth information the reader is advised to consult these reviews [2,13,14]. Common tannin reactions are briefly described hereunder:
1. Hydrolysis and acid or alkaline condensation [2,13,15,16–18]: This reaction produces “phlobaphenes” (Fig. 6) (also called “tanners red”) [19] which are solid precipitates both unreactive and insoluble;
2. Sulphitation: The oldest tannin and leather chemistry reaction. It improves tannins solubility and decreases its viscosity in water [2,20]. However a sulphite excess can be counterproductive in certain applications [21];
3. Catechinic acid rearrangement: A damaging rearrangement occurring with ease easily when using flavonoid models in solution [10,22] but not in the real colloidal tannin extract situation [17,23–27];
4. Catalytic tannin self-condensation: Self-condensing and hardening of polyflavonoid tannins occurs when small quantities (2%–3%) of certain Lewis acids catalyze such a reaction. Among these silica smoke, nanosilica and silicates at high pH, and also boric acid and aluminun trioxide are the clearer examples [28]. This reaction is exothermic and so rapid that a 40%−50% solids content, pH 12 tannin extract water solution will gel and harden in 20–30 min at 25°C. At a higher concentrations of catalyst the reaction will be even faster due to its higher exothermicity.
5. Metals complexation: Tannin metal ion coordination complexes are well known to form through the ortho-diphenol hydroxyl groups on the tannin B-rings [28]. This characteristic is used on water solutions to eliminate or decrease toxic metals concentration [29–31], in mining as for example to separate from its copper matrix rare metals such as germanium, for metal surfaces paint primers and to prepare ferric tannates black inks (Fig. 7);
6. Reactions of tannins with formaldehyde and other aldehydes: Due to their phenolic nature, tannins undergo the same alkali-or acid-catalysed reaction with formaldehyde than phenols. This reaction has acquired increased importance with the use of these tannins as wood adhesives and flame resistant rigid foams.
7. Reactivity and orientation of electrophilic substitutions of flavonoids such as reaction with aldehydes: The most reactive flavonoid sites are on the A-ring C8 site when this site is free (Fig. 8) [2,15]. The second still very reactive site is on the A-ring C6, althought its reactivity is lower than that of the C8 site, due to the lower steric hindrance of the latter [2,15]. In general only these two A-ring sites are involved in the reaction. The particularly low reactivity of the B-ring is well known but at pHs as high as 10 or higher some substitution can also occur on the B-ring 6’ site (Fig. 6), thus also contributing to cross-linking [32,33]. Thus, for procyanidin and delphinidin tannins (predominant phloroglucinol A-rings), such as pine bark tannins, the sites reactivity sequence is C8 > C6 > C6’, when these are free. For profisetinidin and prorobinetinidin tannins (predominant resorcinol A-rings) the sites reactivity sequence is instead C6 > C8 > C6’ due to the C6 site in Fig. 8 being more accessible and presenting a lower steric hindrance [2,32].
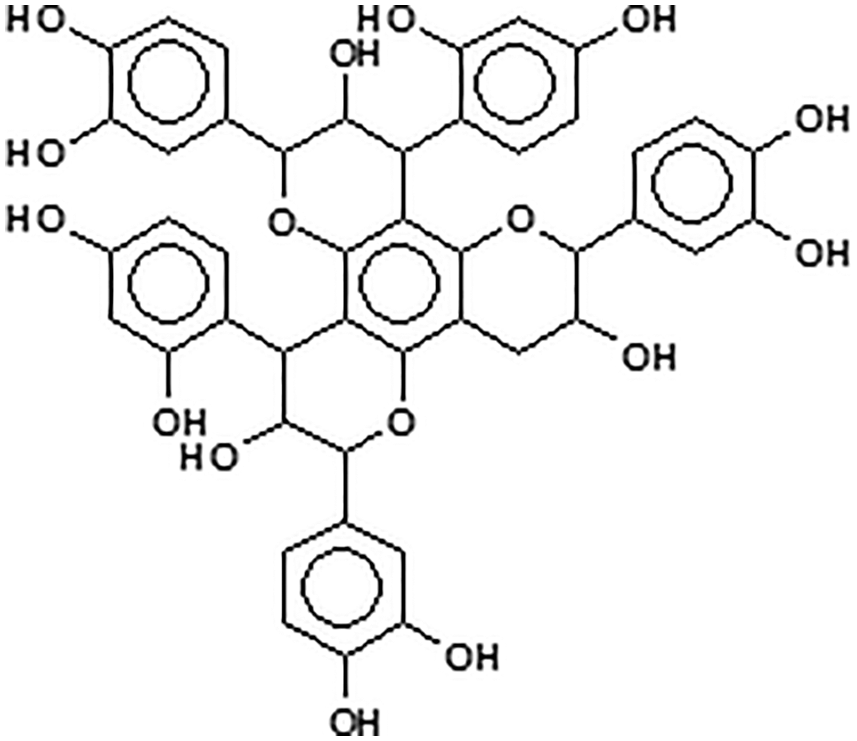
Figure 6: Chemical structure of phlobaphenes
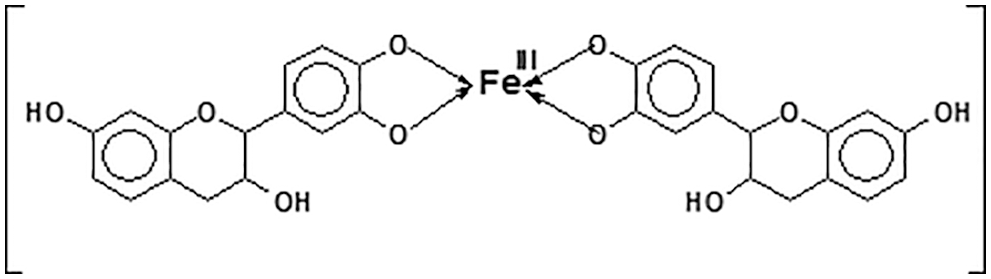
Figure 7: Ferric tannate
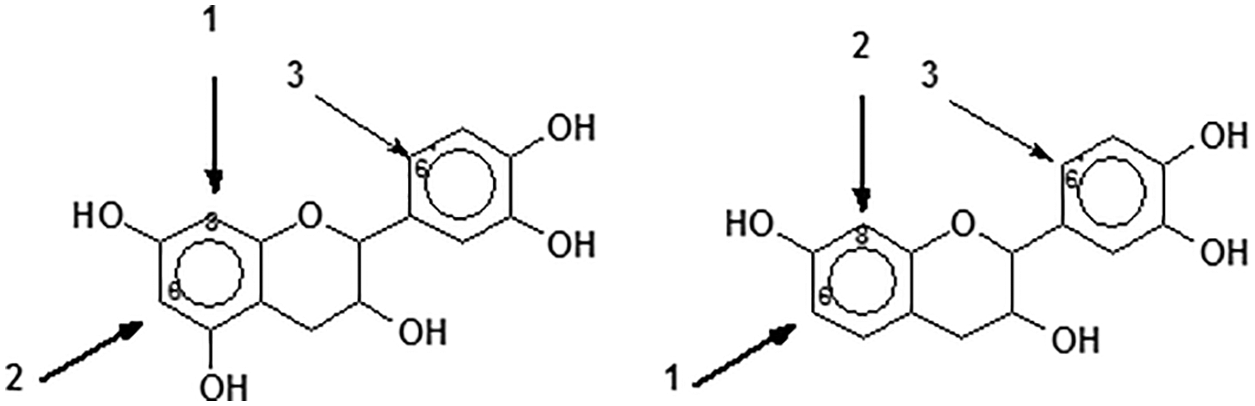
Figure 8: Reactive sites of flavonoid units
The gel time at 100°C (Tgel) is a useful parameter to determine the reactivity at different pHs and roughly distinguish the type of tannin. The condensed tannins Tgel curve for reaction with aldehydes is always bell-shaped. Normally, the longest Tgel is around pH 4–5, while the shortest ones are at lower and higher pHs, depending on the type of tannin [2,34,35].
While the literature on the tridimensional structure of flavonoid monomers is abundant, only one molecular mechanics work on the topic of the tridimensional structure of condensed tannins is published [36]. In this the applicability of these materials correlation to their 3D structure is presented. The helical structure of some condensed tannins oligomers presents the B-rings outwards (Fig. 9) which renders the hydroxyl groups of the B-rings particularly available to facilitate adherence to a cellulose substrate, metallic coordination complexes formation [29–31], and others [37–39].
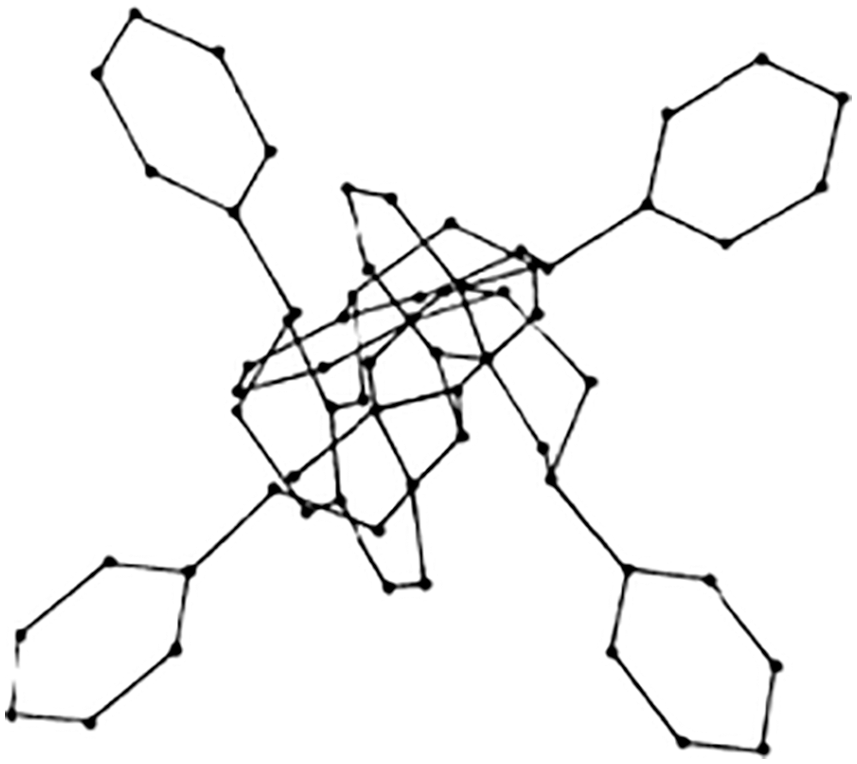
Figure 9: Example of a 4,8-linked tetraflavonoid oligomer tridimensional structure
The presence of higher molecular mass tannin structures and noticeable proportions of carbohydrates oligomers (fragments of hemicelluloses) in a tannin extract at a 40%–50% concentration in water renders such solutions in a colloidal state as shown by their zeta-potentials [40], and confirmed by 13C NMR.
A countercurrent hot water extraction (at 70°C–90°C), with no pressure, of comminuted bark or wood is the traditional industrial extraction method for commercial condensed tannins. Small addition percentages of sodium sulfite or metabisulfite alone, or sometimes even accompanied by traces of sodium bicarbonate, are used to increase the solubility of high molecular weight tannin oligomers. Tannin sulphitation can (or not) occur during or after their extraction to improve the extract water solubility [2,13]. After concentration to around 35% solids the solution is spray-dried to obtain a stable, easily soluble fine tannin powder [19].
This extraction process as described has been in industrial operation from the early 20th century for mimosa (Acacia mearnsii) and Quebracho (Schinopsis sp.) tannin extracts, these two being the two largest sources of commercial condensed tannin extracts in the world. For these two tannins the percentage extraction yield is of 28% to 33% by weight of the original bark or wood. This renders extraction very profitable. The higher molecular weight fractions of procyanidin or prodelphinidin tannins and their internal rearrangements lower their extraction yields to the 12%−15% range. Adding urea as a small percentage in the extraction leads to higher yields in the 18%–25% range, increasing the tannin extract economic interest [19]. Organic solvents extraction gives better yields, but it is both too expensive and unacceptable for the relatively simple plant extraction technology. However, when the tannins produced are earmarked for use as food additives or for pharmaceuticals, thus to be used pure, an organic solvent based second extraction of the tannin extract itself is needed to remove the carbohydrates and traces of other materials from the extract.
Tannins do also autocondense [41–44] and form gels, with formaldehyde or other aldehydes [45–50], but in respect of this review their main gelling reactions are by reaction with silica and silicates, first found and applied for wood adhesives [41–44] and afterwards for other applications [46,47,50] or by reaction with ammonia [51–53]. These two approaches are of interest in some fields, the first approach including also soy-tannins gelling for wood adhesives.
2 Soy Raw Material Source and Preparation
Soybeans have since 5000 years been a very important agricultural crop due to their high content of both triglyceride oil and edible proteins [54]. After oil removal the residue can be used for food [55]. When instead, soybean meal is to be used for adhesives, the solid residue is heated at a temperature lower than 70°C to preserve the proteins solubility in alkali [55]. The protein content of oil-free soybean meal is in 35% to 55% range worldwide, while a protein content of 44%–52% range is usual for industrial grades. Carbohydrates and ashes constitute around 30% and 5%–6% respectively of soy meal [56] with around 10% moisture content. Adhesive grade ‘‘untoasted’’ soybean meal is generally the one performing best, but it needs grounding or milling to a really very fine flour [55,57] at least 40%, and preferably 60%–80%, passing through a 46-mm (325-mesh) screen.
In traditional soy adhesives several points of interest must be considered. Soybean flour will not disperse in water to yield adhesive but will just wet and swell in water. To treat with an alkaline solution material is needed. Almost any organic or inorganic alkali will disperse wetted soybean flour to some degree. But optimum bonding needs for the soy flour to be dispersed with several percent of sodium hydroxide or trisodium phosphate or other strong alkali [58]. This is done to break the internal hydrogen bonds interactions within the spatial tertiary structure of the protein, to thus unfold it to render available all its complex polar structure for wood adhesion. However, this strong alkaline treatment, although needed to develop adhesion, does subject the structure of the protein to progressive degradation by acid hydrolysis. A alkali-dispersed soybean adhesive decreases slowly in viscosity and loses adhesive capability within a 6–12 h storage time, showing thus a limit in useful life. The applied films of such alkaline soy adhesives are almost colorless, but nonetheless they produce on curing a brown-reddish stain on the wood surface as the alkali burns the cellulose of the wood substrate [55]. Thus a lower strength alkali must be used if a colorless glue line is required [55,59], but this at the expenses of a lower adhesive bond strength.
Soy protein isolates (SPI) are generally obtained from an alkaline extract of defatted and de-hulled soybeans by isoelectric precipitation. They are constituted to a level of about 90% protein, mainly glycinin and β-conglycinin [55]. Thus, from the defatted soy flour the protein is first solubilized in water, then precipitated separated and dried. There have been numerous research work on the use of both soy flour and SPI by different, very original and imaginative approaches, and positive modifications for wood adhesives. The literature on the subject is very extensive [60–105]. A considerable amount of this research has not only been focused to improve bonding strength but mainly to improve water and moisture resistance of hardened soy adhesives.
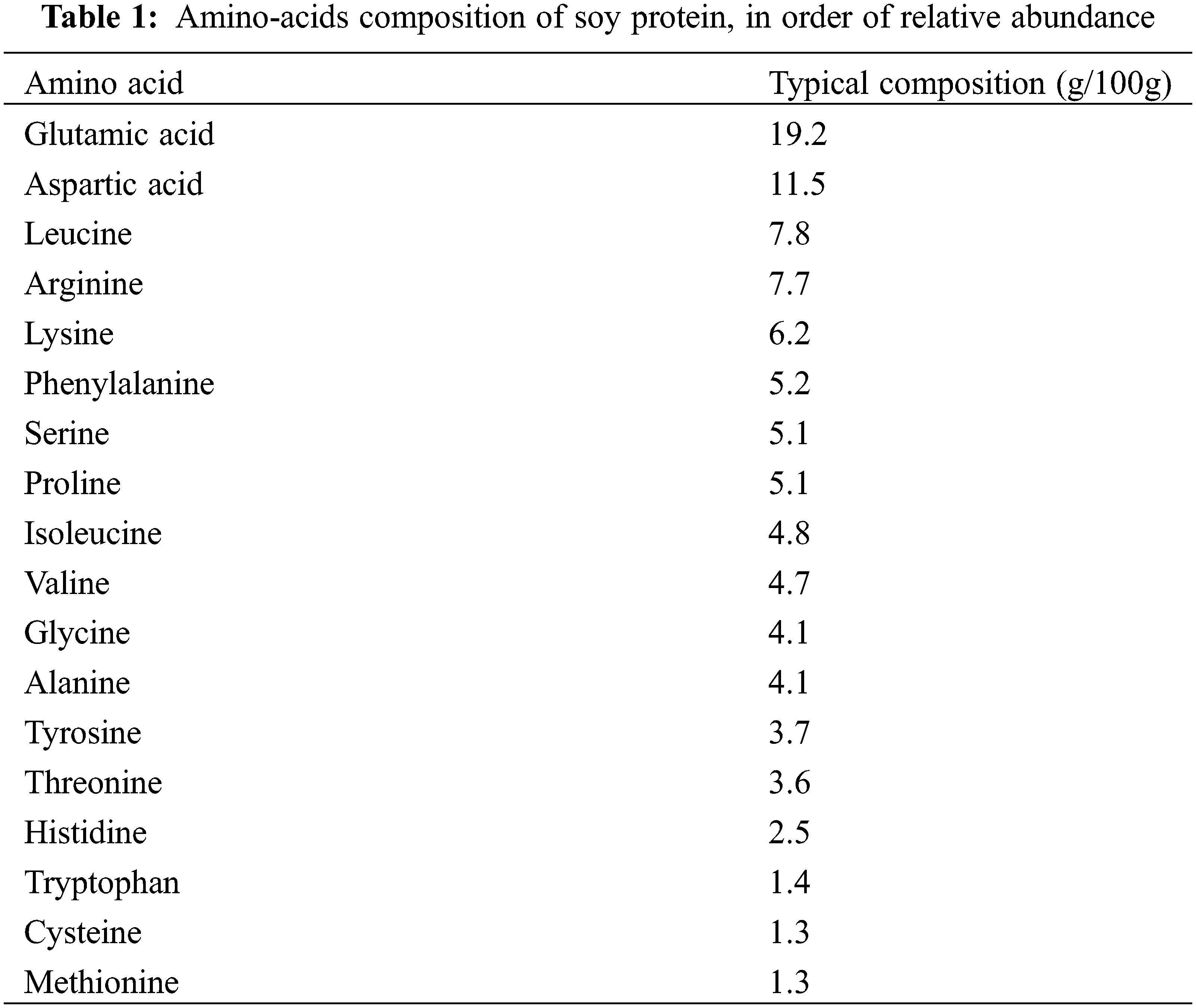
A determinant parameter when the focus of interest is soy proteins gelling with tannins is the amino acids composition of the soy protein. This is shown in Table 1. The dominant amino-acids in the soy proteins peptide chains are glutamic acid, aspartic acid, arginine and lysine. All these 4 amino acids present either a carboxylic acid function or an amino group on their side chain and that does not participate to the skeletal peptide bonds of the protein and thus free to react with other compounds. They constitute among them alone 44.6% of the amino acids content of soy protein. A fifth amino acid also presents a free amino group capable of reaction, this being tryptophan, although adding is feebler proportion to the other 4 brings to the considerable 46% proportion the amino acids content of soy protein that can cross-link with another material.
3 Tannin-Soy-Formaldehyde Gels, Gelification and Hardening
Organic gels of tannin and soy protein and its hydrolysates are known and their technology has been published in the food and nutritional industry. They are based on the age old secondary forces interaction between the two materials as used in the manufacture of leather. Their development is aimed at a variety of different nutritional uses but definitely not for adhesives or other solids materials [106–109]. Namely, they remain to the stage of rather soft gels, for example to be used as fat replacers for meat products [110], stabilizing proteins Pickering emulsions with tannins [111], to minimize or remove tannin astringency in certain fruits [112], or even for materials nano-encapsulation using soy protein hydrolysates–tannic acid complexes obtained by photocatalysis [113], and many other similar uses. The focus of this review is instead on solid materials application, thus rather different then the food and nutritional applications described.
Tannin only organic gels can be prepared. Apart the tannin-formaldehyde or tannin-aldehyde gels tannins do also form gels by autocondensation [41–44] and form gels, with formaldehyde or other aldehydes [45–50], their gelling also being catalyzed by reaction with silica and silicates, first found and applied for wood adhesives [41–44] and afterwards also for other applications [46,47,50,114] or by reaction with ammonia [51–53]. These approaches are of interest in some fields, the first approach including also soy-tannins gelling for wood adhesives, or alternatively in combination with another biomaterial, such as lignin [115] or soy flour protein [47]. Soy-tannin-formaldehyde has been proposed as a wood particleboard binder [116]. However, diluted resins may be used for preparing gels. Denatured soy protein reacted with formaldehyde and crosslinked with a condensed tannin gels were prepared at a number of pHs, cured at 85°C, and supercritical CO2 dried, forming in this manner one of the “greenest” organic aerogels [47]. The authors proposed for these tannin-soy-formaldehyde gel systems a reaction mechanism (Fig. 10) [47], by FTIR, 13C-NMR and XPS analyses. Under the particular specific conditions used the method employed was by denaturing first the soy protein to expose the protein skeletal amide groups (N), then reacting it with formaldehyde to form hydroxymethyl groups on the N atoms, followed then by copolymerizing with the tannin (T). The authors indicated that crosslinking occurred by formation of methylene bridges connecting soy and tannin (N–CH2–T) or soy to soy (N–CH2–N) and tannin to tannin (T–CH2–T) (Fig. 7b). The highest Tgel for tannin-soy-formaldehyde gels was at pH 6 and 85°C [47] due to the shift of system reactivity when comparing it to the pH 4–5 and 50°C for tannin-resorcinol-formaldehyde gels [35].
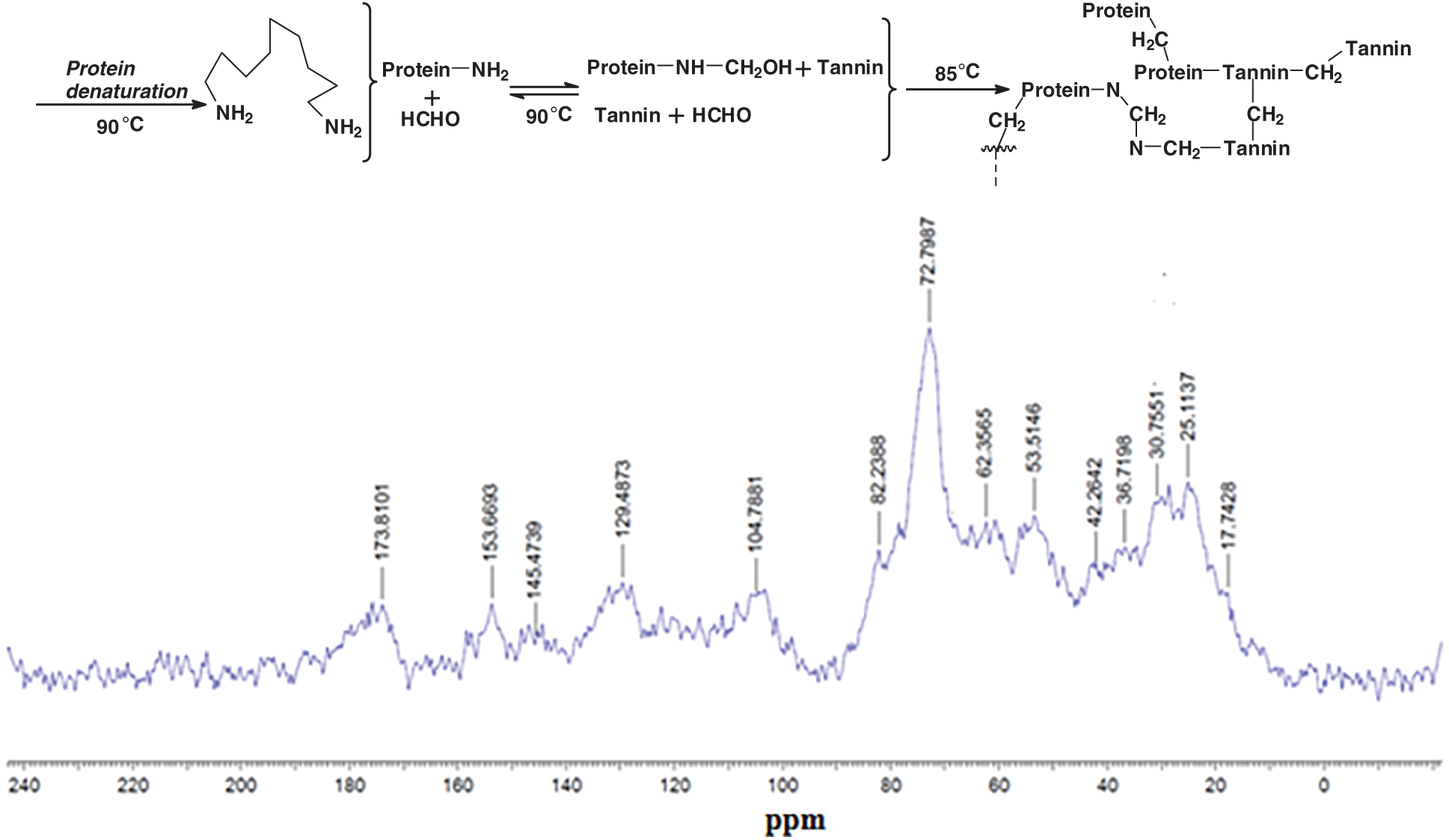
Figure 10: Suggestion of crosslinking mechanism for a soy-tannin-formaldehyde gel system (top) and 13C NMR spectra at pH 6 gel (bottom) [35]
Formaldehyde-free tannin gels are well known and present also an interesting effect. In effect, tannins gel very fast, at room temperature, in really short times. In depth studies on this gelling reaction and its mechanism, and the subsequent hardening, has been carried out [28,42–44] and is caused by the exothermal auto-condensation of tannins catalyzed by certain Lewis acid, such as silica smoke, nano-silica, silicates such as waterglass, boric acid and aluminum trioxide [28,42–44]. The time of gelling varies according to the amount and type of Lewis acid added, from as short as a couple of minutes to as long as 30 min, gelling being also promoted by the strong exothermal reaction generated. Tannins can also produce formaldehyde-free gels by reacting with ammonia at a higher concentration. This causes substitution with amine groups of all or the majority of the tannin hydroxyl groups, followed by crosslinking by spontaneous oligomerization of the aminated tannin through –N = bridges [51–53]. Such hydrogels present a porous texture depending on the relative proportions of the materials used. They can be freeze-dried, dried under normal conditions, or or dried under supercritical conditions. This approach has been used to pyrolyse the hydrogels, leading to carbon xerogels, cryogels or aerogels all particularly rich in N [117,118].
However, the mechanism of formaldehyde based gels explained above [47] is only part of the story. First of all bridges of the type Protein-CH2-N-CH2-N-CH2-Tannin are an incorrect interpretation in Fig. 7, the -N-CH2-N-bridge of the shoulder on the side of the big 72 ppm peak being a Protein-CH2-N-CH2-Protein bridge type only. The peak at 53 ppm is correctly assigned to a Protein-N-CH2-Tannin. The peaks at 42, 32 and 30 ppm are also correctly assigned to Tannin-CH2-Tannin-methylene bridges between different tannin sites. A direct tannin-protein linkage without presence of formaldehyde as indicated in the original article of Fig. 10 [47] is not noticeable by 13C nuclear magnetic resonance (13C NMR), being this masked, if it even occurs, by the bridges formed by the formaldehyde. In effect other pointed studies under different conditions [99] have not been able to observe with 13C NMR the presence or not of such direct linkages under different conditions when masked by formaldehyde or other formaldehyde-yielding compounds are used. A different techniques was needed then to be used. In materials where gelling must eventually lead to hardening such as in wood adhesives the mechanism has always been thought to be rather different. A first indication of how different, was obtained from a series of several publications in which soy protein isolate was reacted with both condensed tannins and with hydrolysable tannins [119–121].
In the first two of these [119,120] “tannic acid”, thus a commercial chestnut hydrolysable tannin, was employed for preparing adhesives from soy-flour to bond fiberboards and plywood. Differential scanning calorimetry (DSC) in the 25°C–220°C range, thermogravimetric analysis (TGA) and its temperature dependent derivate (DTG) for different soy adhesives were measured between 40°C and 300°C. Fourier Transform Infrared (FTIR) analysis indicated that tannic acid addition to a soy-adhesive decreased both its viscosity and pH. Thus, tannic acid appeared to improve the adhesion performance of soy flour resins. The DSC analysis indicated that both soy binders’ glass transition temperature and temperature of denaturation decreased by tannic acid addition. TGA and DTG indicated that soy flour thermal degradation appeared to start above 146°C. By FTIR spectroscopy it was determined that tannic acid and the amino acid of soy protein in soy flour appeared to react well (Fig. 11). Moreover, the plywood bonded with the tannic acid modification of soy adhesives showed good water resistance by both delamination and shear strength tests. Fiberboards properties such as Modulus of Rupture (MOR), Modulus of Elasticity (MOE), Internal Bond (IB) strength, and water resistance were improved, by the tannic acid modification of soy adhesives.
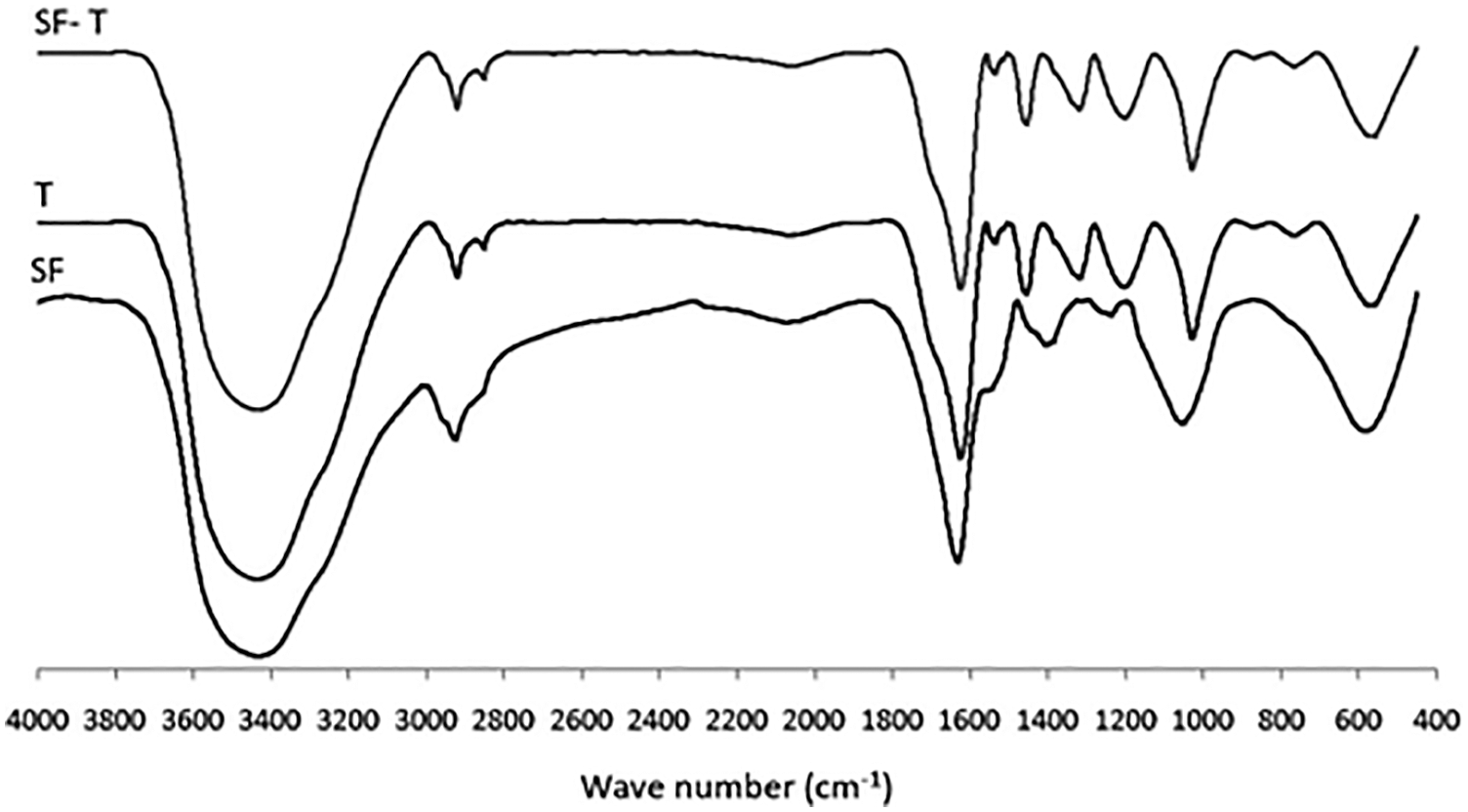
Figure 11: FTIR spectra for tannic acid (T), soy flour (S) and tannic-acid-soy flour (ST)
In the approach taken in this work urea was also used to decrease and control the viscosity of the soy-tannin combination to allow its application as a wood adhesive. The spectra of soy flour-urea and soy flour-urea-tannic acid are shown in Figs. 11 and 12. These show peak intensity changes due to the reaction of the tannic acid with the soy protein amino acids and that the addition to soy flour-urea of tannic acid increases the intensity of the 3465, 2959, 2926, 1243 and 1054 cm−1 peaks. The C = O stretching peak at 1641 cm−1 of the amide II also decreases in intensity by the addition of tannic acid. Such a reaction looks like occurring according to two mechanisms: (i) by the tannic acid phenolic –OH groups reacting with the side chains amine groups of amino acids possessing a side chain amine group, even with amino groups that are converted eventually in amide groups once they are included in the skeletal structure of the protein. Soy protein contains a remarkably high proportion of amino acids possessing side chain amine groups. Fig. 12 shows that chemical bonds form between tannic acid and amino acids, but again at this stage cross-liking was dominated by the methylene groups formed by formaldehyde with all the other three materials, namely soy, tannin and urea. (ii) By numerous tannin-protein secondary forces interaction: this is the same mass of interactions in tanning proteins to produce leather and why proteins precipitate from solution by tannins addition. The tannin and the protein are almost irreversibly linked by millions of these secondary forces. A 3-stage model of the tannins and proteins interaction has been proposed by Charlton et al. in 2002 [122]: In its initial phase, the tannin aromatic ring planar surface and hydrophobic sites of proteins such as pyrrolidine rings of prolyl residues interact to form hydrophobic associations. At the same time, the hydroxyl groups of the tannin complexing the H-acceptor sites (carbonyl and –NH2 groups) of proteins are helped in the complexes stabilization by the hydrogen bonds between the two (Fig. 13). Then afterwards, the self-association of the protein-tannin complexes occurs by further hydrogen bonding. This results in larger but soluble tannin-protein complexes then aggregating. In the end the aggregated complexes become sufficiently large to form insoluble precipitates.
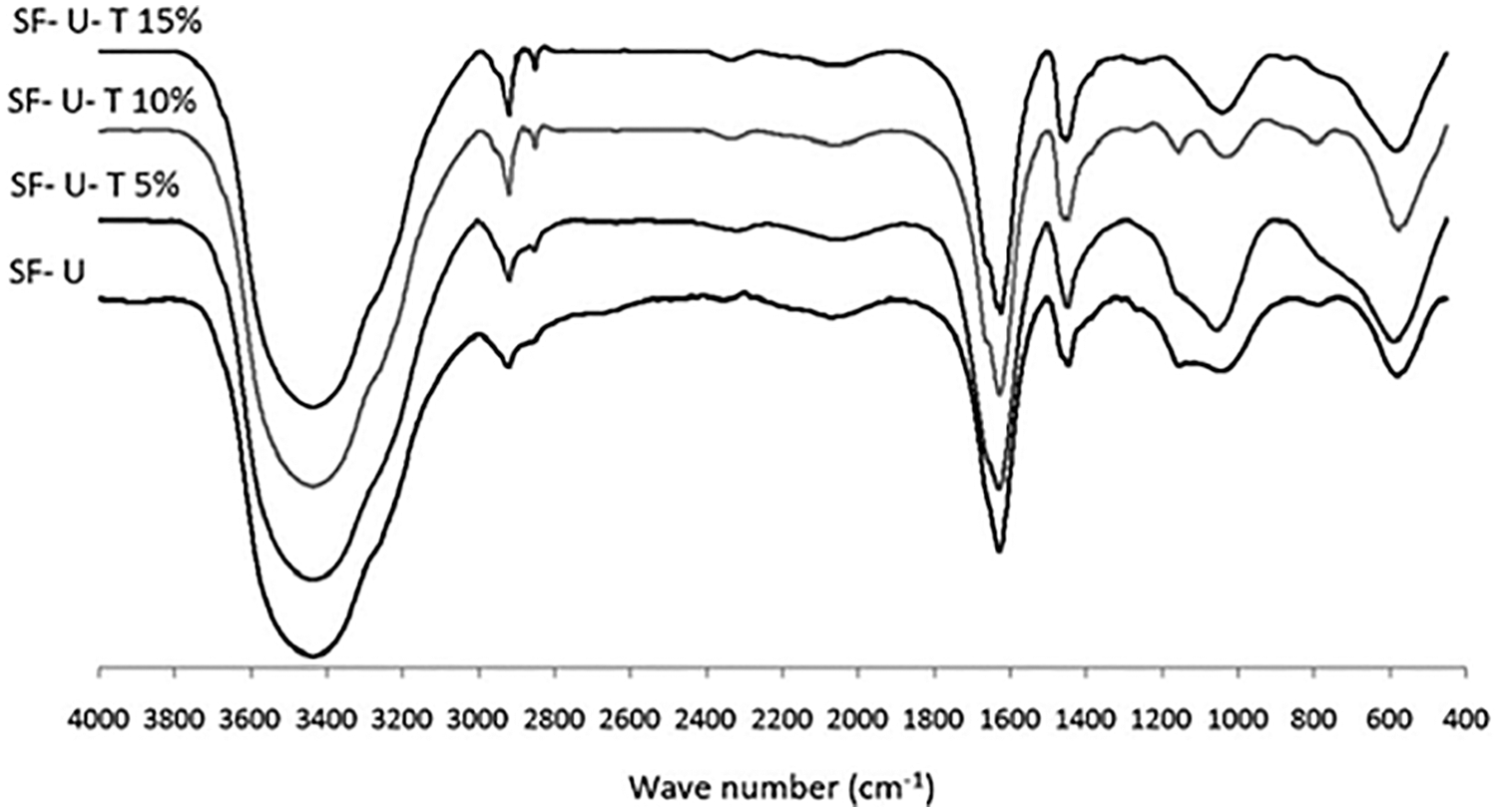
Figure 12: FTIR spectra of Soy flour-urea and soy flour-urea-tannic acid (5, 10 and 15 wt%) adhesives; SF: Soy flour, U: Urea, T: Tannic acid
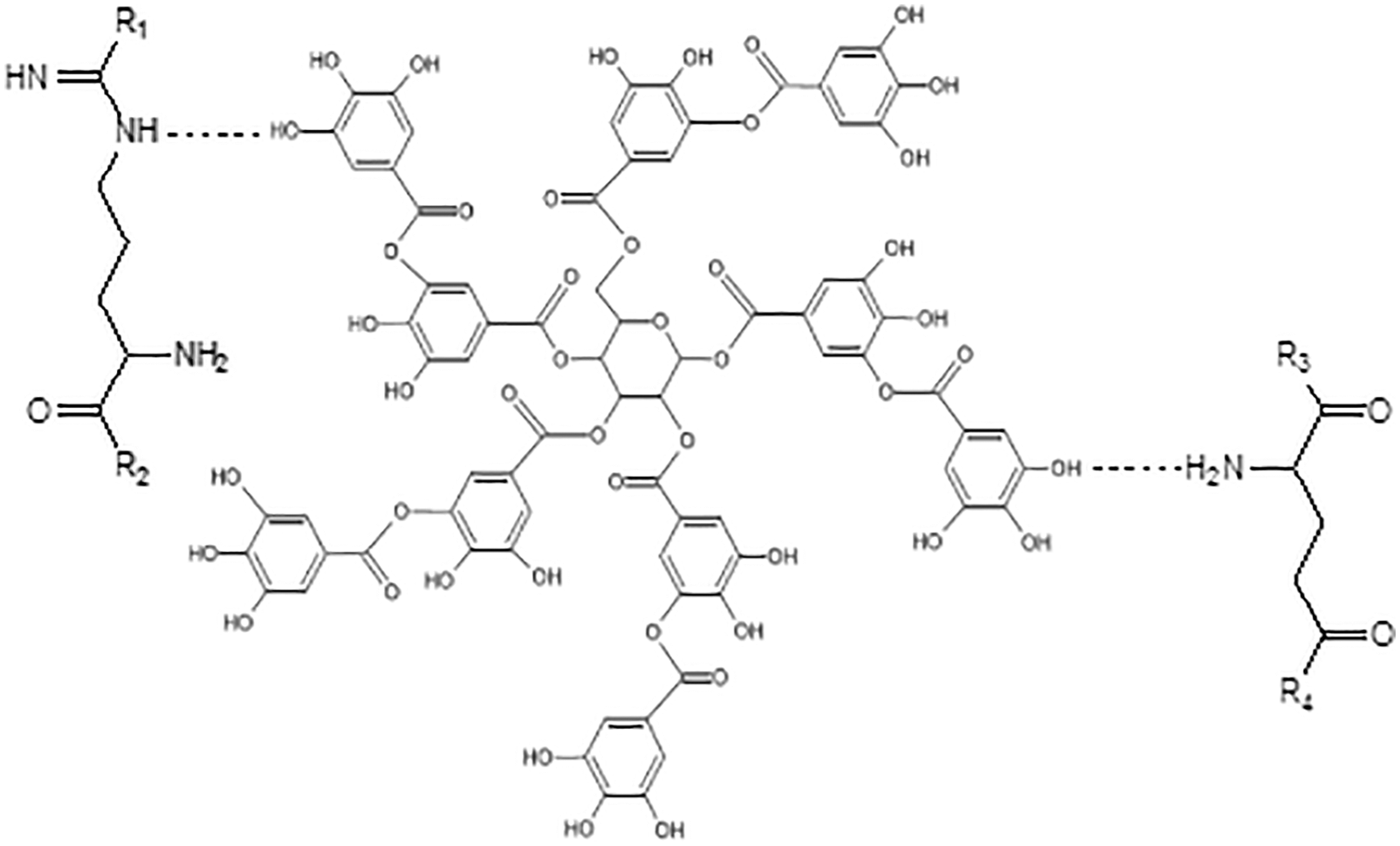
Figure 13: Possible hydrogen bonds between tannic acid and amino acid of polypeptide chains
The urea addition to the soy resin adhesive is one reason for the lowering of the MOE at higher tannin proportions. Urea is known to contribute to partially block some flavonoid tannins rearrangements and condensation reactions [19], mimosa tannin included. The urea effect on a flavonoid tannin self-condensation reaction is shown in Fig. 14. Urea has shown in previous work to be a blocking agent of tannin self-condensation, since with tannin model compounds it has successfully decreased self-condensation, such as also m-phenylenediamine and phloroglucinol are able to do [19].
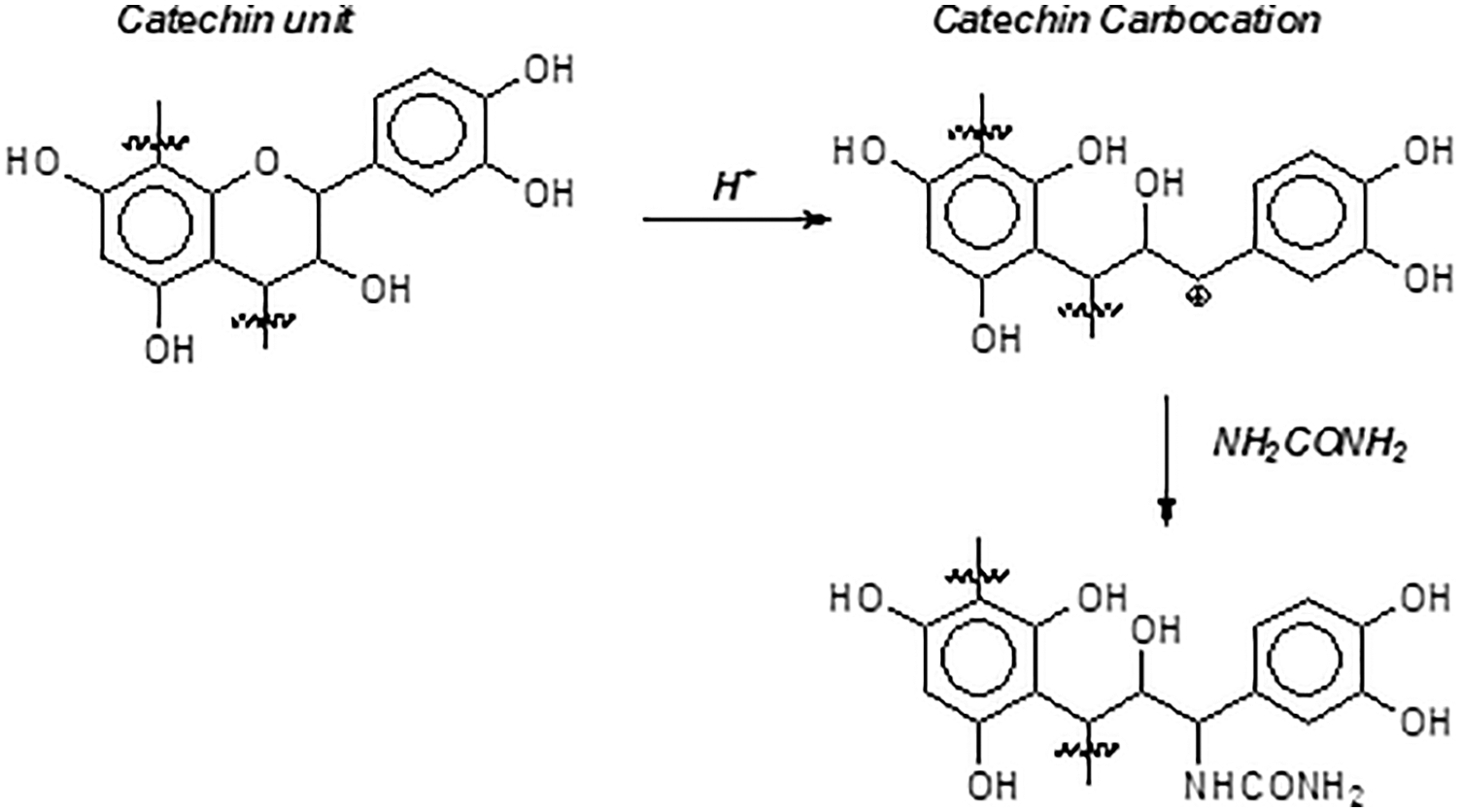
Figure 14: Effect of urea on catechin unit in procyanidin/delphinidin type polyflavonoid tannins condensation reaction
The use of tannins in combination with soy flour to make wood particleboard has also been investigated. Thus, the feasibility of using three types of commercial tannins, namely two flavonoid condensed types (quebracho, mimosa) and one hydrolysable type (chestnut tannins) as cross-linking materials for soy adhesives has been investigated. The chemical bond formation and adhesion behaviors of tannin-modified soy adhesives were also analyzed by Matrix Assisted Laser Desorption Ionization Time-of-Flight (MALDI-ToF) mass spectrometry and thermomechanical analysis (TMA), indeed indicating the formation of tannin with amino acids chemical bonds. The TMA analysis showed that The MOE of soy adhesive increased by addition of tannins to its formulation. Also the presence of covalent bonds formation was confirmed by MALDI-ToF mass spectrometry. The improvement of the mechanical resistance of soy bonded joints by addition of tannin had already been found one year before, but due to the lack of the adequate analytical technique the formation of these bonds and their potentiality was not noticed, being drowned in the formaldehyde-based cross-linking, the improvement being logically ascribed to the presence of formaldehyde cross-linking between the two biomaterials [99].
The results indicated that new compounds did form by reactions of different hydrolysable and condensed polyflavonoid tannin extracts with soy protein amino acids. The interaction between tannin constituents and amino acids occurs by reaction of the carboxyl group (−COOH) of gallic acid of chestnut tannin with the amino groups of amino acids side chains but not with the amide groups in the peptide skeletal chains of the protein. It must be pointed out that only single, unbound amino acid amino groups react but that these amino group once transformed in amide groups in the protein peptide skeletal chain cannot react anymore, at least not easily. Thus, the reaction is then limited only to amino acids in the chains that possess a side chain with an unbound amino or carboxylic acid group. The hydroxyl groups (−OH) on the phenolic rings of quebracho and mimosa tannins also react with amino acid functional groups. As the analysis were carried out at ambient temperature two types of linkages were revealed, namely ionic linkages, but also covalent linkages between the two materials. Fig. 15 shows also cross-linking between peptide chains by ionic and covalent bond formation between tannin extracts and amino acids. The difference between the two forms of each compound were determined by MALDI ToF indicating that in this particular case this analysis technique allowed to distinguish fine differences in the structure of the simpler reaction products [120].
Figure 15: Ionic and covalent bond formations between tannin and soy protein constituents
It must be pointed out that while this reactions clearly appeared to occur between soy protein hydrolysates and tannin these were anyhow partially masked by the domination exercised in hardening by the effect of the reactions of hardeners such as formaldehyde and hexamine. As these were considered essential to obtain a good adhesive. Thus, species where an ionic bond is formed between lysine and gallic acid from the hydrolysable tannin such as species I.
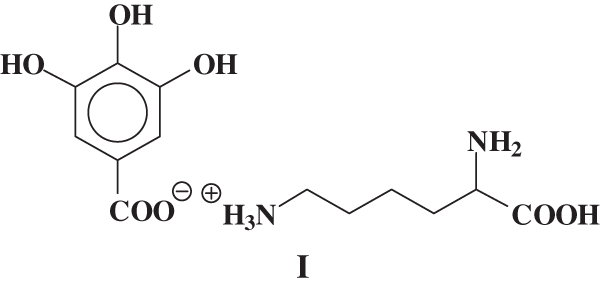
In MALDI-ToF analysis a peak at 169 + 147 = 316 Da is observed, and the equivalent peak if one –OH or –NH2 is protonated is found at 317 or 318 Da. The same effect if Na+ is linked to the species. The second possible reaction: is leading to the formation of a covalent bond. Thus a compound of formula II:
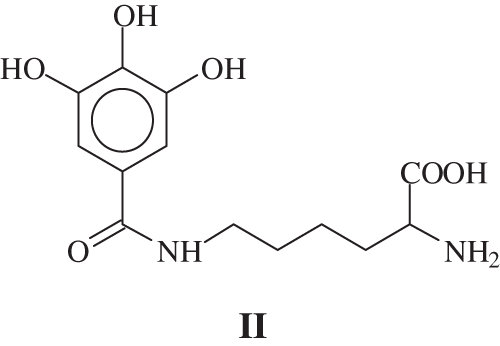
is obtained, that would give 146 + 170 + 23 − 18 = 321 Da if Na+ is considered or would give 298 Da if the compounds has not Na+. These are also been found.
Again in these approaches gelling and hardening was predominated through reactions of the two chemical species with formaldehyde or hexamine, this partially masking the contribution of the direct reaction of tannin and soy.
Finally in a third phase [121] hexamethylenetetramine and glyoxal, two different hardener types, were used with two different commercial tannins, chestnut (a hydrolysable tannin) and mimosa (a condensed flavonoid tannin). The two different hardeners, namely hexamine (with tannins a non-formaldehyde yielding compound) [122–125] and glyoxal, were used to eliminate any formaldehyde while still hardening and upgrading soy resins for particleboard bonding. A number of soy resin formulations have been tested by DSC (differential scanning calorimetry) in the 25°C to 200°C range, and by TMA in the 25°C to 250°C range. The physical properties of different adhesive formulations such as viscosity, solid contents, acidity and density were also measured. One-layer laboratory particleboards bonded with the experimental adhesive formulations were prepared and tested. Based on the results obtained, tannins appear to decrease effectively soy-based adhesives viscosity. The DSC indicated that tannin appears to change the thermal properties of soy flour, decreasing its glass transition temperature Tg and its temperature of denaturation Td. The TMA also indicated that chestnut tannin extract reacts well with soy flour and improves its adhesion properties, and that both types of tannin decreased both the gel time and gel temperature of the adhesive. Furthermore, the IB strength of particleboards bonded with soy adhesives modified with a tannin are higher than for particleboard bonded with unmodified soy adhesive. Equally, marked decreases in water absorption and thickness swelling were recorded for the particleboards bonded with soy adhesives modified by an added tannin, hence a further improvement. In a subsequent paper [126], different amounts of soy flour (SF) and soy protein isolate (SPI) were used with the three tannins. While in this paper the contribution of the direct reaction of a tannin with soy protein hydrolysate or the protein in soy flour or both was demonstrated, the use of an added hexamine as hardener still did not demonstrate that such a reaction could be used alone to bond successfully wood without the addition of an added hardener.
At this stage a breakthrough on the pure reaction of tannins with soy proteins occurred [127], coming from a different direction, namely the work on the reaction of a flavonoid tannin with collagen, to explain some behavior of vegetable tannin in leather tanning and its durability or cracking at higher temperature. Collagen powder hydrolysates were reacted with a solution of commercial mimosa bark tannin extract. The mixture was prepared at ambient temperature and at 80°C to determine what reactions, if any, did occur between the collagen protein through its amino acids and the polyphenolic condensed tannin. The reaction products obtained were analyzed by MALDI ToF mass spectrometry. Reactions between the two materials did appear to occur, with the formation of a relatively small proportion of covalent and ionic linkages at ambient temperature but a considerable proportion of covalent linkages tannin-protein amino acids and the disappearance of ionic bonds. The linkages between the two materials appeared to be by esterification by the –COOH groups of glutamic and aspartic acid of the aliphatic alcohol-OH on the C3 site of the heterocycle of the tannin flavonoid units (III) and by amination of the phenolic –OHs of the tannin by the amino groups of the non-skeletal side chains of arginine (IV). The proportion of covalent linkages increases markedly and predominate with increasing temperatures. This tightening of the tannin-protein covalent network formed was investigated to understand if it was an additional contributing factor both to leather wear resistance and performance as well to leather shrinking when this is subjected to excessive temperatures.
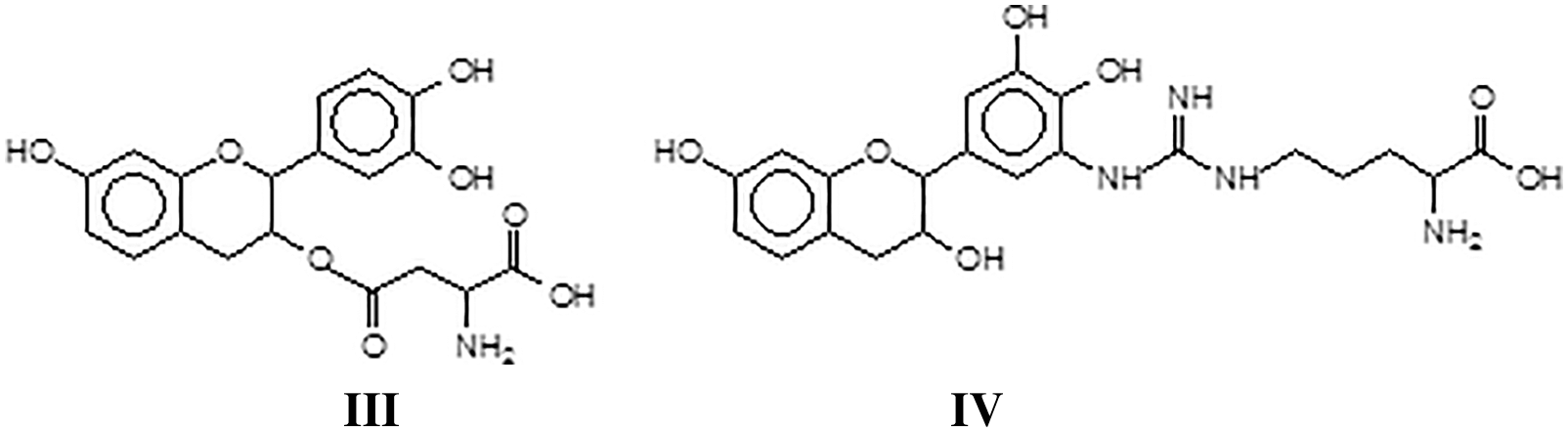
A sequence of aspartic acid-gallocatechin dimer-arginine-leucin-aspartic acid-glycin-glutammic acid (V).
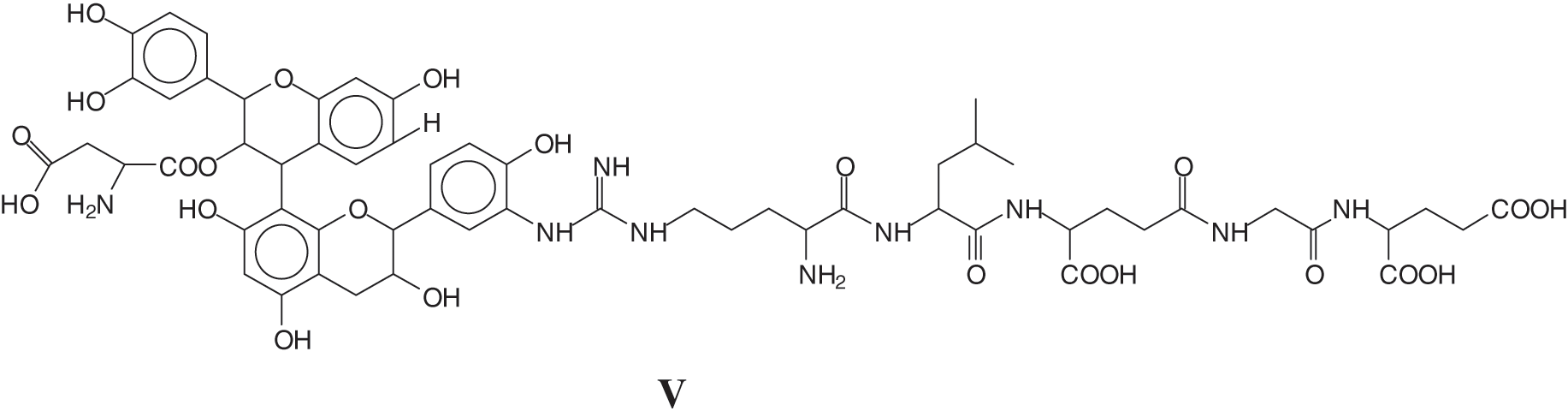
and/or
A sequence of aspartic acid-catechin-arginine-leucine-aspartic acid-glycine-glutamic acid-catechin (VI).
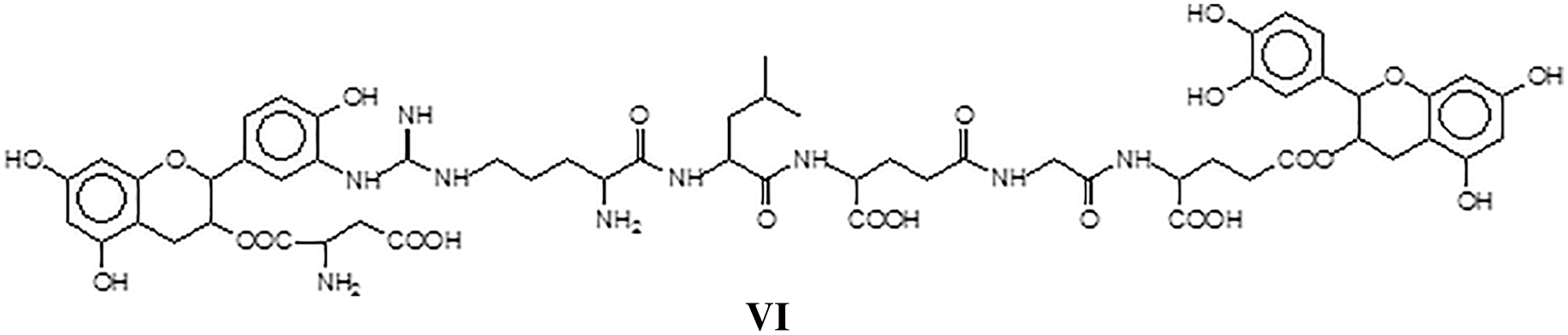
The residual groups in the above amino acids sequence can also react, forming species of higher molecular weight indicating that extensive covalent cross-linking can in reality occur between tannin and protein as for example in structure VII assigned to the peak at 1805 Da, this being an example of how the body of the protein is cross-linked by the tannin
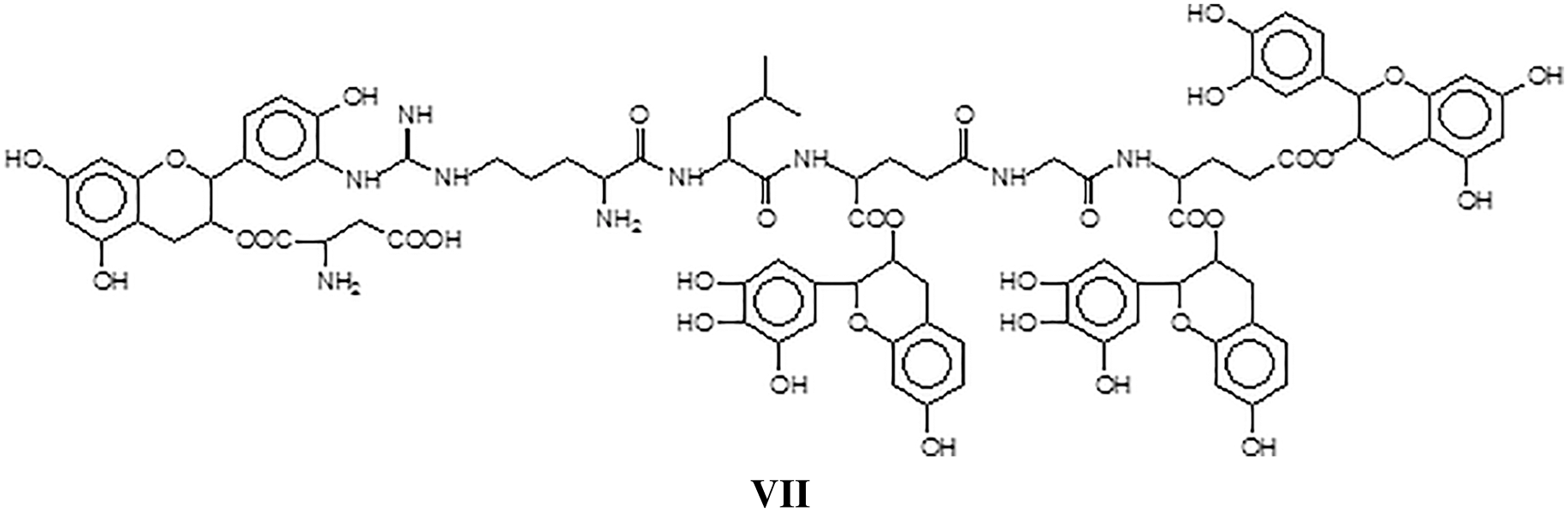
and/or structure VIII
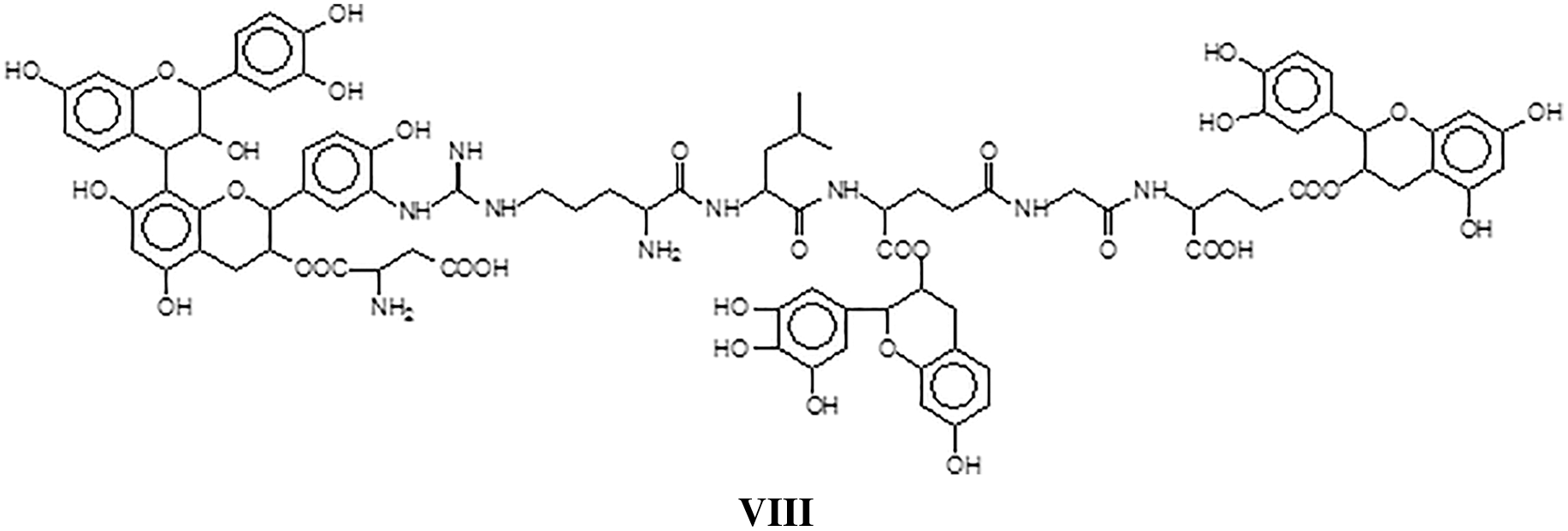
All the above indicated that in the tanning of hides with vegetal tannins a certain number of covalent and ionic bonds do occur between tannin and protein contributing to leather solidity and wear resistance [127]. The proportion of these linkages increases with increasing temperatures of tanning, and in particular the proportion of tannin-protein covalent linkages does increase until these become the only one presents [127]. While such covalent cross-linking may well be a contributing factor to the stability and performance of leather, when this becomes excessive as when leather is subjected to higher temperatures, may well be also a strong additional, contributing factor to leather shrinking. This work however brought the realization that such mechanism may well be active for other proteins too, and after all for soy protein for wood adhesives [127].
For this reason a study of concept was established on the initial application for totally bio adhesives for wood based only on the covalent reaction between SPI and a commercial flavonoid tannin, namely quebracho tannin, without any added hardener [128]. The adhesive was composed exclusively of the two vegetable biomaterials mentioned, thus is totally environment friendly and non-toxic as tannin has been classified as being not at all toxic by the European Commission REACH program [129]. The pre-reaction between the two yielded the best plywood bonding results when limited to a temperature of 40°C, final cross-linking being achieved during the plywood higher temperature hot pressing procedure, as for any other thermosetting adhesive (Fig. 16) [128]. Pre-reaction at higher temperature, as used in the leather work [127] namely 60°C and 80°C, did not work successfully for wood adhesives because its use achieved extensive premature cross-linking that made it lose any activity to cross-link further when hot pressed for bonding plywood (Fig. 16). The reaction was followed by thermomechanical analysis, by MALDI ToF mass spectrometry, and by plywood shear strength testing dry, after 24 h cold water soak and 1 h in boiling water. The adhesive of this approach lends itself to be further reinforced by the multitude of different original approaches on soy resins and adhesives already developed by several other research groups [60–105].
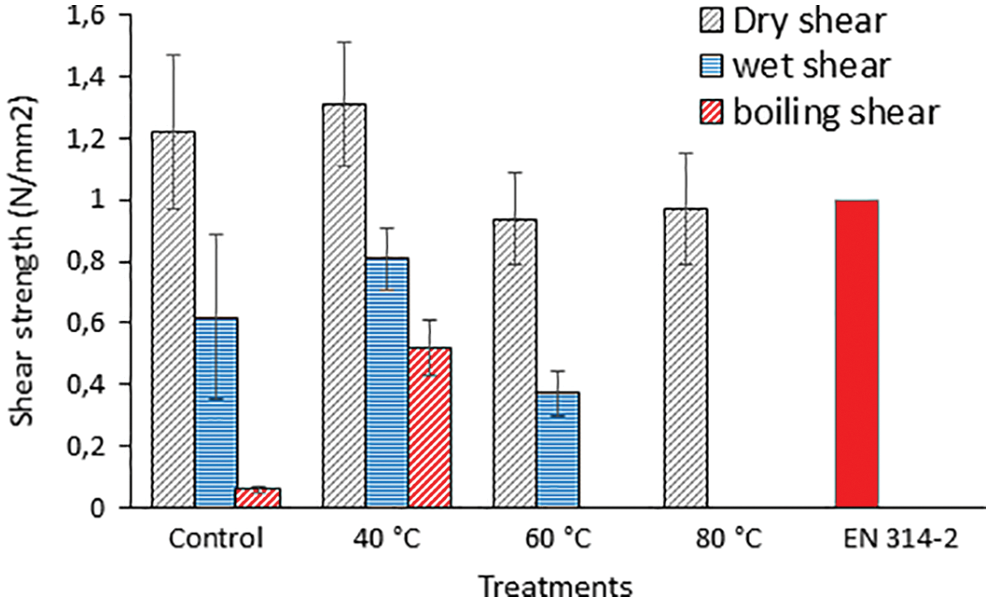
Figure 16: Comparison with a soy only control and with the relative European Norm of plywood shear strengths dry, after 24 h cold water soaking an 3 h in boiling water for the soy-tannin resins pre-reacted at 40°C, 60°C and 80°C
The MALDI ToF analysis showed on top of similar species already identified for the tannin-collagen work [127] also tannin-soy protein isolate species by direct reaction of the flavonoid tannin with short peptide sequences of the protein [128]. Examples of these are the species at 1465 Da of a sequence of glutamic acid-glycine-aspartic acid-robinetenidin-catechin-arginine-leucin-aspartic acid-glycin-glutamic acid, where the peptide sequence is linked to a flavonoid dimer composed of robinetinidin and catechin (IX)
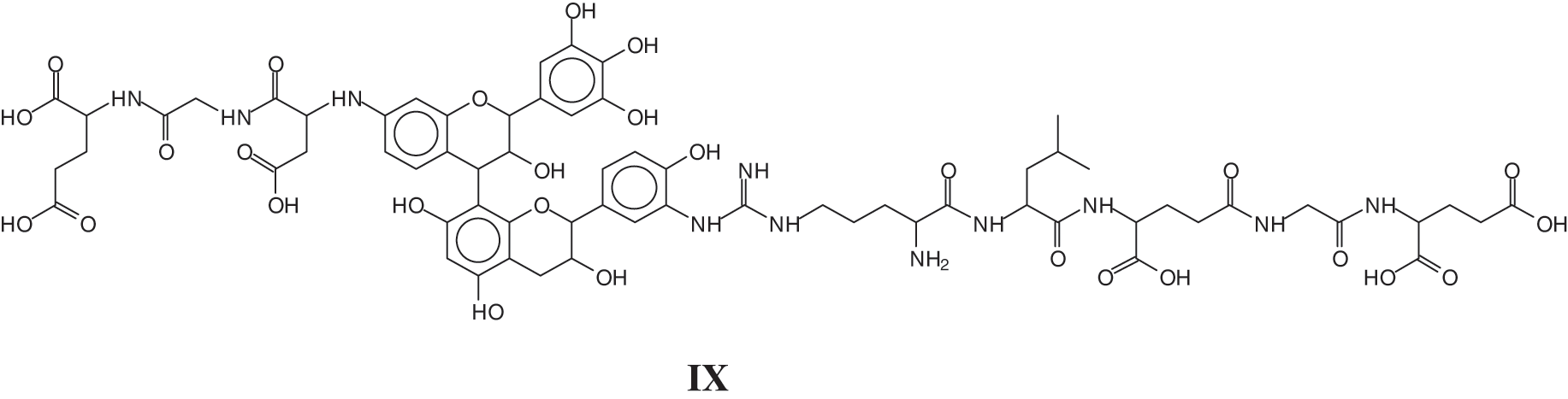
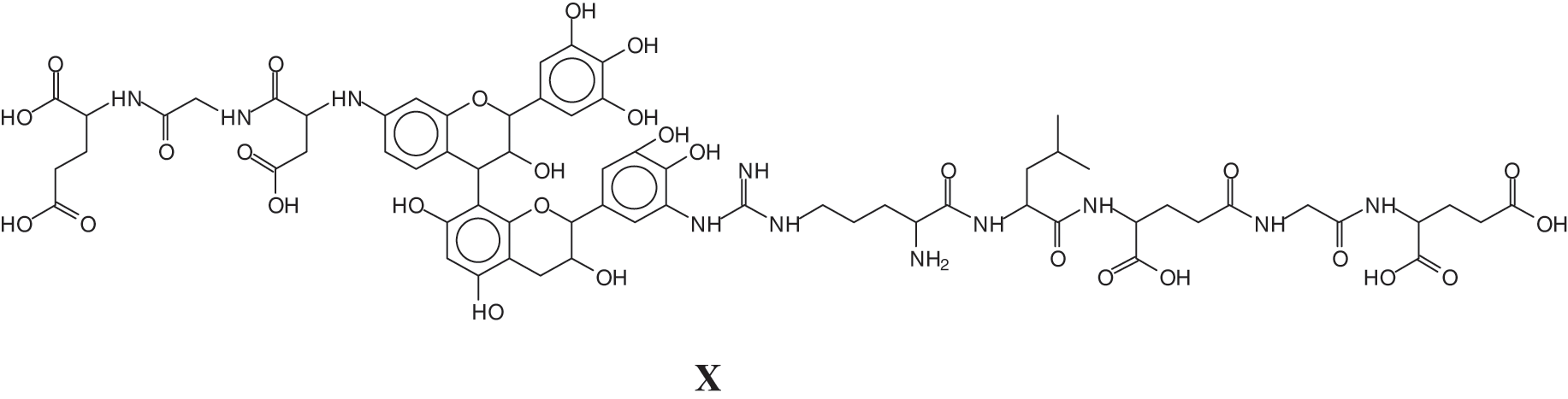
And a species at 1481 Da of a sequence of glutamic acid-glycine-aspartic acid-robinetenidin-gallocatechin--arginine-leucin-aspartic acid-glycin-glutamic acid, where the peptide sequence is linked to a flavonoid dimer composed of robinetinidin and gallocatechin (X).
Transferring what found from wood adhesives and leather under the conditions exposed above on the covalent reaction of tannin and soy protein also the general reaction of a tannin as exposed at beginning of this review will apply. Thus, for gels of several different types, like for example aerogels, not only it is possible as said to obtain a tannin-gel by addition of silica smoke or silicates, but also to obtain the same type of gels with a soy-tannin coreacted material, thus a soy protein-tannin gel by addition of silica smoke, silicates, boric acid and aluminum trioxide [28,41–44] without any formaldehyde. Equally, such a result possibly opens the way to the use of other natural polyphenolic materials, such as lignosulphonates, alkali lignin and organosolv lignin, to form gels of different types, and thermosetting resins and adhesives by reaction with soy protein and soy flour. But this is left to the innumerable research groups concentrating on soy adhesives.
Gels of soy protein and soy flour with tannin are an interesting area of investigation. Soy is a food crop extremely abundant, non-toxic and not expensive while tannin is classed as non-toxic by the European Commision Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) ruling. Numerous works in this area exist and are all based on a number of different approaches. One can distinguish (i) gels by condensation of tannin and soy through methylene linkages generated by reaction of the two biosourced, renewable materials with formaldehyde or other aldehyde. (ii) Gels obtained by autocondensation of the tannin once this is linked by secondary forces to the protein, and (iii) and gels formed by the covalent condensation without added hardeners of the tannin directly with the protein non-skeletal amino et carboxylic acid groups not belonging to the peptide links of the protein. It is this last reaction, already successful in producing plywood adhesives without any other material, but only by tannin reacted covalently with either SPI or Soy flour, that shows potential to even further improve tannin-soy adhesives and resins for a number of application.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declares that they have no conflicts of interest to report regarding the present study.
References
1. Caller, L. (1989). Le fabbriche italiane di estratto di castagno. San Michele Mondovi (CNItaly: Silva Chimica. [Google Scholar]
2. Pizzi, A. (1983). Tannin-based wood adhesives. In: Pizzi, A. (Ed.Wood adhesives chemistry and technology, vol. 1, pp. 178–246. New York, NY, USA: Marcel Dekker. [Google Scholar]
3. Giovando, S., Pizzi, A., Pasch, H., Pretorius, N. (2013). Structure and oligomers distribution of commercial Tara (Caesalpina spinosa) hydrolysable tannin. ProLigno, 9, 22–31. [Google Scholar]
4. Radebe, N., Rode, K., Pizzi, A., Giovando, S., Pasch, H. (2013). MALDI-TOF-CID for the microstructure elucidation of polymeric hydrolysable tannins. Journal of Applied Polymer Science, 128(1), 97–107. [Google Scholar]
5. Pizzi, A., Pasch, H., Rode, K., Giovando, S. (2009). Polymer structure of commercial hydrolisable tannins by MALDI-TOF mass spectrometry. Journal of Applied Polymer Science, 113(6), 3847–3859. [Google Scholar]
6. Pasch, H., Pizzi, A. (2002). On the macromolecular structure of chestnut ellagitannins by MALDI-TOF mass spectrometry. Journal of Applied Polymer Science, 85(2), 429–437. [Google Scholar]
7. Drewes, S. E., Roux, D. G. (1963). Condensed tannins. 15. Interrelationships of flavonoid components in wattle-bark extract. Biochemistry Journal, 87(1), 167–172. [Google Scholar]
8. Roux, D. G., Paulus, E. (1961). Condensed tannins. 8. The isolation and distribution of interrelated heartwood components of Schinopsis spp. Biochemistry Journal, 78(4), 785–789. [Google Scholar]
9. Saayman, H. M., Roux, D. G. (1965). The origins of tannins and flavonoids in black-wattle barks and heartwoods, and their associated ‘non-tannin’ components. Biochemistry Journal, 97(3), 794–801. [Google Scholar]
10. Drovou, S., Pizzi, A., Lacoste, C., Zhang, J., Abdulla, S. et al. (2015). Flavonoid tannins linked to long carbohydrate chains-MALDI-TOF analysis of the tannin extract of the African locust bean shells. Industrial Crops and Products, 67, 25–32. [Google Scholar]
11. Abdalla, S., Pizzi, A., Ayed, N., Charrier, F., Bahabri, F. et al. (2014). MALDI-TOF and 13C NMR analysis of Tunisian Zizyphus jujuba root bark tannins. Industrial Crops and Products, 59, 277–281. [Google Scholar]
12. Roux, D. G. (1965). Modern applications of mimosa extract. Grahamstown, South Africa: Leather Industries Research Institute. [Google Scholar]
13. Pizzi, A. (1994). Advanced wood adhesives technology. New York: M. Dekker. [Google Scholar]
14. Pizzi, A. (2003). Natural phenolic adhesives 1: Tannin. In: Pizzi, A., Mittal, K. L. (Eds.Handbook of adhesive technology. New York: Marcel Dekker. [Google Scholar]
15. Roux, D. G., Ferreira, D., Hundt, H. K. L., Malan, E. (1975). Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Applied Polymer Symposia, 28, 335–353. [Google Scholar]
16. Christiansen, A. W., Gillespie, R. H. (1986). Wood Adhesives in 1985: Status and needs. In: Forest products laboratory. Madison, Wis: FPRS. [Google Scholar]
17. Pizzi, A., Stephanou, A. (1993). Comparative and differential behaviour of pine vs. pecan nut tannin adhesives for particleboard. Holzforschung und Holzverwertung, 45, 30–33. [Google Scholar]
18. McGraw, G. W., Rials, T. G., Steynberg, J. P., Hemingway, R. W. (1992). Chemistry of pecan tannins and analysis of cure of pecan tannin-based cold-setting adhesives with a DMA ‘Micro-Beam’ test. In: Hemingway, R. W., Laks, P. E. (Eds.Plant polyphenols, vol. 59. Boston, MA: Basic Life Sciences, Springer. [Google Scholar]
19. Sealy-Fisher, V. J., Pizzi, A. (1992). Increased pine tannins extraction and wood adhesives development by phlobaphenes minimization. Holz als Roh und Werkstoff, 50(5), 212–220. DOI 10.1007/BF02663290. [Google Scholar] [CrossRef]
20. Roux, D. G. (1965Wattle tannin and mimosa extract. Grahamstown, South Africa: Leather Ind. Reaserch Inst. [Google Scholar]
21. Pizzi, A. (1979). Sulphited tannins for exterior wood adhesives. Colloid and Polymer Science, 257(1), 37–40. DOI 10.1007/BF01539014. [Google Scholar] [CrossRef]
22. Ohara, S., Hemingway, R. W. (1991). Condensed tannins: The formation of a diarylpropanol-catechinic acid dimer from base-catalyzed reactions of (+)-catechin. Journal of Wood Chemistry and Technology, 11(2), 195–208. DOI 10.1080/02773819108050270. [Google Scholar] [CrossRef]
23. Pizzi, A. (1982). Pine tannin adhesives for particleboard. Holz als Roh und Werkstoff, 40(8), 293–301. DOI 10.1007/BF02610623. [Google Scholar] [CrossRef]
24. Pizzi, A., von Leyser, E. P., Valenzuela, J., Clark, J. G. (1993). The chemistry and development of pine tannin adhesives for exterior particleboard. Holzforschung, 47(2), 168–174. DOI 10.1515/hfsg.1993.47.2.168. [Google Scholar] [CrossRef]
25. Valenzuela, J., von Leyser, E., Pizzi, A., Westermeyer, C., Gorrini, B. (2012). Industrial production of pine tannin-bonded particleboard and MDF. European Journal of Wood and Wood Products, 70(5), 735–740. DOI 10.1007/s00107-012-0610-2. [Google Scholar] [CrossRef]
26. Pizzi, A., Valenezuela, J., Westermeyer, C. (1994). Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard. Holz als Roh und Werkstoff, 52(5), 311–315. DOI 10.1007/BF02621421. [Google Scholar] [CrossRef]
27. Pizzi, A., Stephanou, A. (1994). Fast vs. slow-reacting non-modified tannin extracts for exterior particleboard adhesives. Holz als Roh und Werkstoff, 52(4), 218–222. DOI 10.1007/BF02619095. [Google Scholar] [CrossRef]
28. Meikleham, N., Pizzi, A., Stephanou, A. (1994). Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates. I. 13C-NMR, 29Si-NMR, X-ray, and polarimetry studies and mechanism. Journal of Applied Polymer Science, 54(12), 1827–1845. [Google Scholar]
29. Slabbert, N. (1992). Complexation of condensed tannins with metal ions. In: Hemingway, R. E., Laks, P. E. (Eds.Plant polyphenols: Biogenesis, chemical properties, and significance. New York: Plenum Press. [Google Scholar]
30. Tondi, G., Oo, C. W., Pizzi, A., Trosa, A., Thevenon, M. F. (2009). Metal adsorption of tannin based rigid foams. Industrial Crops and Products, 29(2–3), 336–340. DOI 10.1016/j.indcrop.2008.06.006. [Google Scholar] [CrossRef]
31. Oo, C. W., Kassim, M. J., Pizzi, A. (2009). Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II). Industrial Crops and Products, 30(1), 152–161. DOI 10.1016/j.indcrop.2009.03.002. [Google Scholar] [CrossRef]
32. Pizzi, A., Scharfetter, H. O. (1978). The chemistry and development of tannin-based adhesives for exterior plywood. Journal of Applied Polymer Science, 22(6), 1745–1761. DOI 10.1002/app.1978.070220623. [Google Scholar] [CrossRef]
33. Hillis, W. E., Urbach, G. (2007). The reaction of (+)-catechin with formaldehyde. Journal of Applied Chemistry, 9(9), 474–482. DOI 10.1002/jctb.5010090904. [Google Scholar] [CrossRef]
34. Mitsunaga, T., Doi, T., Kondo, Y., Abe, I. (1998). Color development of proanthocyanidins in vanillin-hydrochloric acid reaction. Journal of Wood Science, 44(2), 125–130. DOI 10.1007/BF00526257. [Google Scholar] [CrossRef]
35. Szczurek, A., Amaral-Labat, G., Fierro, V., Pizzi, A., Masson, E. et al. (2011). The use of tannin to prepare carbon gels. Part I: Carbon aerogels. Carbon, 49(8), 2773–2784. [Google Scholar]
36. Pizzi, A., Cameron, F. A., Eaton, N. J. (1985). The tridimensional structure of polyflavonoid tannins by conformational analysis. Journal of Macromolecular Science: Part A–Chemistry, 22(4), 515–540. DOI 10.1080/00222338508056617. [Google Scholar] [CrossRef]
37. Thébault, M., Pizzi, A., Essawy, H. A., Barhoum, A., van Assche, G. (2015). Isocyanate free condensed tannin-based polyurethanes. European Polymer Journal, 67(3), 513–526. DOI 10.1016/j.eurpolymj.2014.10.022. [Google Scholar] [CrossRef]
38. Pizzi, A. (1979). Tannin-based polyurethane adhesives. Journal of Applied Polymer Science, 23(6), 1889–1891. DOI 10.1002/app.1979.070230631. [Google Scholar] [CrossRef]
39. Pizzi, A. (1978). Tannin-formaldehyde exterior wood adhesives through flavonoid B-ring cross linking. Journal of Applied Polymer Science, 22(8), 2397–2399. DOI 10.1002/app.1978.070220831. [Google Scholar] [CrossRef]
40. Pizzi, A., Stephanou, A. (1994). A 13C NMR study of polyflavonoid tannin adhesive intermediates. II. Colloidal state reactions. Journal of Applied Polymer Science, 51(13), 2125–2130. [Google Scholar]
41. Pizzi, A., Meikleham, N., Dombo, B., Roll, W. (1995). Autocondensation-based, zero-emission, tannin adhesives for particleboard. Holz als Roh und Werkstoff, 53, 201–204. [Google Scholar]
42. Garcia, R., Pizzi, A. (1998). Polycondensation and autocondensation networks in polyflavonoid tannins. I. Final networks. Journal of Applied Polymer Science, 70(6), 1083–1091. [Google Scholar]
43. Garcia, R., Pizzi, A. (1998). Polycondensation and autocondensation networks in polyflavonoid tannins. II. Polycondensation versus autocondensation. Journal of Applied Polymer Science, 70(6), 1093–1110. [Google Scholar]
44. Garcia, R., Pizzi, A. (1998). Cross-linked and entanglement networks in thermomechanical analysis of polycondensation resins. Journal of Applied Polymer Science, 70(6), 1111–1116. [Google Scholar]
45. Brinker, C. J., Scherer, G. W. (1990). Sol–gel science. The physics and chemistry of Sol–gel processing. San Diego: Academic Press. [Google Scholar]
46. Szczurek, A., Amaral-Labat, G., Fierro, V., Pizzi, A., Celzard, A. (2011). The use of tannin to prepare carbon gels. Part II. Carbon cryogels. Carbon, 49(8), 2785–2794. DOI 10.1016/j.carbon.2011.03.005. [Google Scholar] [CrossRef]
47. Amaral-Labat, G., Grishechko, L., Szczurek, A., Fierro, V., Pizzi, A. et al. (2012). Highly mesoporous organic aerogels derived from soy and tannin. Green Chemistry, 14(11), 3099–3106. DOI 10.1039/c2gc36263e. [Google Scholar] [CrossRef]
48. Hench, L. L., West, J. K. (1990). The sol-gel process. Chemical Reviews, 90(1), 33–72. DOI 10.1021/cr00099a003. [Google Scholar] [CrossRef]
49. Pekala, R. W., Schaefer, D. W. (1993). Structure of organic aerogels. 1. Morphology and scaling. Macromolecules, 26(20), 5487–5493. DOI 10.1021/ma00072a029. [Google Scholar] [CrossRef]
50. Amaral-Labat, G., Szczurek, A., Fierro, V., Pizzi, A., Celzard, A. (2013). Systematic studies of tannin-formaldehyde aerogels: Preparation and properties. Science and Technology of Advanced Materials, 14(1), 015001. DOI 10.1088/1468-6996/14/1/015001. [Google Scholar] [CrossRef]
51. Braghiroli, F., Fierro, V., Pizzi, A., Rode, K., Radke, W. et al. (2013). Condensation reaction of flavonoid tannins with ammonia. Industrial Crops and Products, 44(4), 330–335. DOI 10.1016/j.indcrop.2012.11.024. [Google Scholar] [CrossRef]
52. Braghiroli, F., Fierro, V., Izquierdo, M. T., Parmentier, J., Pizzi, A. et al. (2015). High surface-high N-content carbons prepared by hydrothermal treatment of aminated tannin. Industrial Crops and Products, 66, 282–290. DOI 10.1016/j.indcrop.2014.11.022. [Google Scholar] [CrossRef]
53. Braghiroli, F., Amaral-Labat, G., Boss, A. F. N., Lacoste, C., Pizzi, A. (2019). Tannin gels and their carbon derivatives: A review. Biomolecules, 9(10), 587. DOI 10.3390/biom9100587. [Google Scholar] [CrossRef]
54. Pen Ts’ao Kong Mu, The Records of Chinese Emperor Shen-Nung, 2838 B.C. [Google Scholar]
55. Lambuth, A. L. (2003). Protein adhesives for wood. In: Pizzi, A., Mittal, K. L. (Eds.Handbook of adhesive technology, 2nd editionpp. 457–478. New York: Marcel Dekker. [Google Scholar]
56. Burnett, R. S. (1951). Soybeans and soybean products. New York: Wiley-Interscience. [Google Scholar]
57. Davidson, G. (1929). Process of preparing substances composed in part of protein-containing cells for the production of adhesives. US patent 1724695. [Google Scholar]
58. Brother, G. H., Smith, A. K., Circle, S. J. (1940). Soybean protein. Washington DC: U.S. Department of Agriculture, Bureau of Agricultural Chemistry. [Google Scholar]
59. Davidson, G., Laucks, I. F. (1931). Process of making a water resistant double decomposition adhesive and to the product thereof. US patent 1813387. [Google Scholar]
60. Wescott, J. M., Frihart, C. R., Traska, A. E. (2006). High-soy-containing water-durable adhesives. Journal of Adhesion Science and Technology, 20(8), 859–873. DOI 10.1163/156856106777638734. [Google Scholar] [CrossRef]
61. Wu, Z., Liang, J., Lei, H., Zhang, B., Xi, X. et al. (2021). Study on the soy protein-based adhesive cross-linked by glyoxal. Journal of Renewable Materials, 9(2), 205–208. DOI 10.32604/jrm.2021.013655. [Google Scholar] [CrossRef]
62. Frihart, C. R., Lorenz, L. (2019). Specific oxidants improve the wood bonding strength of soy and other plant flours. Journal of Polymer Science, Part A: Polymer Chemistry, 57(9), 1017–1023. DOI 10.1002/pola.29357. [Google Scholar] [CrossRef]
63. Cavins, J. F., Wolek, Y. F. K., Inglett, G. E., Cowan, C. (1972). Amino acid analysis of soybean meal: Interlaboratory study, pp. 686. Peoria, IL, USA: USDA Northern Marketing and Nutrition Research Division, Agricultural Research Service, US Department of Agriculture. [Google Scholar]
64. Frihart, C. R., Pizzi, A., Xi, X., Lorenz, L. (2019). Reactions of Soy flour and Soy protein by non-volatile aldehydes generation by specific oxidation. Polymers, 11(9), 1478. DOI 10.3390/polym11091478. [Google Scholar] [CrossRef]
65. Chen, N., Lin, Q., Zheng, P., Rao, J., Zeng, Q. et al. (2019). A sustainable bio-based adhesive derived from defatted soy flour and epichlorohydrin. Wood Science and Technology, 53(4), 801–817. DOI 10.1007/s00226-019-01102-2. [Google Scholar] [CrossRef]
66. Gu, W., Li, F., Liu, X., Gao, Q., Gong, S. et al. (2020). Borate chemistry inspired by cell walls converts soy protein into high-strength, antibacterial, flame-retardant adhesive. Green Chemistry, 22(4), 1319–1328. DOI 10.1039/C9GC03875B. [Google Scholar] [CrossRef]
67. Zhou, Y., Wu, T., Zeng, G., Cai, Y., Luo, J. et al. (2021). A tough, anti-mildew and anti-counterfeiting soybean protein adhesive enhanced by gecko-inspired functional fiber and bio-based epoxide. Journal of Cleaner Production, 323(6), 129194. DOI 10.1016/j.jclepro.2021.129194. [Google Scholar] [CrossRef]
68. Zhao, S., Wang, W., Zhi, L., Li, L., Li, J. et al. (2019). Core-shell nanohybrid elastomer based on co-deposition strategy to improve performance of soy protein adhesive. ACS Applied Material Interfaces, 11(35), 32414–32442. DOI 10.1021/acsami.9b11385. [Google Scholar] [CrossRef]
69. Zhang, Y., Zhang, M., Chen, M., Luo, J., Li, X. et al. (2018). Preparation and characterization of a soy protein-based high-performance adhesive with a hyperbranched cross-linked structure. Chemical Engineering Journal, 354(15), 1032–1041. DOI 10.1016/j.cej.2018.08.072. [Google Scholar] [CrossRef]
70. Pang, H., Wang, Y., Chang, Z., Xia, C., Han, C. et al. (2021). Soy meal adhesive with high strength and water resistance via carboxymethylated wood fiber-induced crosslinking. Cellulose, 28(6), 3569–3584. DOI 10.1007/s10570-021-03732-x. [Google Scholar] [CrossRef]
71. Xu, C., Xu, Y., Chen, M., Zhang, Y., Li, J. et al. (2020). Soy protein adhesive with bio-based epoxidized daidzein for high strength and mildew resistance. Chemical Engineering Journal, 390(15), 124622. DOI 10.1016/j.cej.2020.124622. [Google Scholar] [CrossRef]
72. Jin, S., Li, K., Gao, Q., Zhang, W., Chen, H. et al. (2020). Multiple crosslinking strategy to achieve high bonding strength and antibacterial properties of double-network soy adhesive. Journal of Cleaner Production, 254(1), 120143. DOI 10.1016/j.jclepro.2020.120143. [Google Scholar] [CrossRef]
73. Xi, X., Pizzi, A., Gerardin, C., Chen, X., Amirou, S. (2020). Soy protein isolate-based polyamides as wood adhesives. Wood Science and Technology, 54(1), 89–102. DOI 10.1007/s00226-019-01141-9. [Google Scholar] [CrossRef]
74. Luo, J., Luo, J., Bai, Y., Gao, Q., Li, J. (2016). A high performance soy protein-based bio-adhesive enhanced with a melamine/epichlorohydrin prepolymer and its application on plywood. RSC Advances, 6(72), 67669–67676. DOI 10.1039/C6RA15597A. [Google Scholar] [CrossRef]
75. Liu, K., Wang, K., Gao, Q., Zhang, W., Zhou, W. et al. (2019). Bioinspired design by gecko structure and mussel chemistry for bio-based adhesive system through incorporating natural fibers. Journal of Cleaner Production, 236(1), 117591. DOI 10.1016/j.jclepro.2019.07.066. [Google Scholar] [CrossRef]
76. Gu, W., Liu, X., Ye, Q., Gao, Q., Gong, S. et al. (2020). Bio-inspired co-deposition strategy of aramid fibers to improve performance of soy protein isolate-based adhesive. Industrial Crops and Products, 150(6), 112424. DOI 10.1016/j.indcrop.2020.112424. [Google Scholar] [CrossRef]
77. Yang, G., Sui, N., Yang, B. (2010). Effects of ultrasonic on properties of modified soybean protein-based adhesives for duplex paper. Advanced Materials Research, 152–153, 1866–1872. DOI 10.4028/www.scientific.net/AMR.152-153.1866. [Google Scholar] [CrossRef]
78. Zheng, P., Chen, N., Mahfuzul Islam, S. M., Ju, L. K., Liu, J. et al. (2019). Delopment of self-cross-linked soy adhesive by enzyme complex from aspergillus niger for production of all-biomass composite materials. ACS Sustainable Chemistry and Engineering, 7(4), 3909–3916. DOI 10.1021/acssuschemeng.8b04993. [Google Scholar] [CrossRef]
79. Pang, H., Zhao, S., Wang, Z., Zhang, W., Zhang, S. J. et al. (2020). Development of soy protein-based adhesive with high water resistance and bonding strength by waterborne epoxy crosslinking strategy. International Journal of Adhesion and Adhesives, 100(25), 102600. DOI 10.1016/j.ijadhadh.2020.102600. [Google Scholar] [CrossRef]
80. Ma, C., Pang, H., Shen, Y., Liang, Z., Li, J. et al. (2021). Plant polyphenol-inspired crosslinking strategy toward high bonding strength and mildew resistance for soy protein adhesives. Macromolecular Materials and Engineering, 306(12), 2100543. DOI 10.1002/mame.202100543. [Google Scholar] [CrossRef]
81. Xu, Y., Han, Y., Chen, M., Luo, J., Shi, S. Q. et al. (2021). Constructing a triple network structure to prepare strong, tough, and mildew resistant soy protein adhesive. Composites Part B: Engineering, 211(15), 108677. DOI 10.1016/j.compositesb.2021.108677. [Google Scholar] [CrossRef]
82. Li, K., Jin, S., Li, X., Li, J., Shi, S. Q. et al. (2021). Bioinspired interface engineering of soybean meal-based adhesive incorporated with biomineralized cellulose nanofibrils and a functional aminoclay. Chemical Engineering Journal, 421(1), 129820. DOI 10.1016/j.cej.2021.129820. [Google Scholar] [CrossRef]
83. Li, W., Chen, M., Li, Y., Sun, J., Liu, Y. et al. (2020). Improving mildew resistance of soy meal by Nano-Ag/TiO2, Zinc Pyrithione and 4-Cumylphenol. Polymers, 12(1), 169. [Google Scholar]
84. Li, K., Jin, S., Wei, Y., Li, X., Li, J. et al. (2021). Bioinspired hyperbranched protein adhesive based on boronic acid-functionalized cellulose nanofibril and water-soluble polyester. Composites Part B: Engineering, 219(15), 108943. [Google Scholar]
85. Chen, F., Zhang, J. (2010). Effects of plasticization and shear stress on phase structure development and properties of soy protein blends. ACS Applied Material Interfaces, 2(11), 3324–3332. [Google Scholar]
86. Luo, J., Zhou, Y., Gao, Q., Li, J., Yan, N. (2020). From wastes to functions: A new soybean meal and bark-based adhesive. ACS Sustainable Chemistry and Engineering, 8(29), 10767–10773. [Google Scholar]
87. Zeng, Y., Yang, W., Xu, P., Cai, X., Dong, W. et al. (2022). The bonding strength, water resistance and flame retardancy of soy protein-based adhesive by incorporating tailor-made core-shell nanohybrid compounds. Chemical Engineering Journal, 428(15), 132390. [Google Scholar]
88. Xu, Y., Han, Y., Li, Y., Li, J., Li, J. et al. (2022). Preparation of a strong, mildew-resistant, and flame retardant biomimetic multifunctional soy protein adhesive via the construction of an organic-inorganic hybrid multiple-bonding structure. Chemical Engineering Journal, 437(1), 135437. [Google Scholar]
89. Wang, Z., Zhao, S., Pang, H., Zhang, W., Zhang, S. et al. (2019). Developing eco-friendly high-strength soy adhesives with improved ductility through multiphase core-shell hyperbranched polysiloxane. ACS Sustainable Chemistry and Engineering, 7(8), 7784–7794. [Google Scholar]
90. Wang, Z., Kang, H., Liu, H., Zhang, S., Xia, C. et al. (2020). Dual-network nanocross-linking strategy to improve bulk mechanical and water-resistant adhesion properties of biobased wood adhesives. ACS Sustainable Chemistry and Engineering, 8(44), 16430–16440. [Google Scholar]
91. Zeng, Y., Yang, W., Xu, P., Ma, P. (2022). A water-resistance soy protein-based adhesive for various substrates application by incorporating tailor-made hydrophobic nanocrystalline cellulose. Composites Communications, 236, 101151. [Google Scholar]
92. Li, J., Zhang, F., Lyu, Y., Jiang, S., Li, X. et al. (2022). Acacia mangium tannin functionalized graphene nanoplatelets produced via ball-milling for sustainable soy protein-based film. Industrial Crops and Products, 177, 114478. [Google Scholar]
93. Zeng, Y., Yang, W., Xu, P., Cai, X., Dong, W. et al. (2022). The bonding strength, water resistance and flame retardancy of soy protein-based adhesive by incorporating tailor-made core-shell nanohybrid compounds. Chemical Engineering Journal, 428(15), 132390. DOI 10.1016/j.cej.2021.132390. [Google Scholar] [CrossRef]
94. Kan, Y., Sun, B., Kan, H., Bai, Y., Gao, Z. (2021). Preparation and characterization of a melamine-urea-glyoxal resin and its modified soybean adhesive. International Journal of Adhesion and Adhesives, 111, 102986. DOI 10.1016/j.ijadhadh.2021.102986. [Google Scholar] [CrossRef]
95. Sun, Z., Sun, B., Bai, Y., Gao, Z. (2021). Economical improvement on the performances of a soybean flour-based adhesive for wood composites via montmorillonite hybridization. Composites Part B: Engineering, 217(15), 108920. DOI 10.1016/j.compositesb.2021.108920. [Google Scholar] [CrossRef]
96. Zhu, X., Song, C., Sun, X., Wang, D., Cai, D. et al. (2021). Improved water resistance of TA-modified soy adhesive: Effect of complexation. International Journal of Adhesion and Adhesives, 108, 102858. DOI 10.1016/j.ijadhadh.2021.102858. [Google Scholar] [CrossRef]
97. Arias, A., González-García, S., Feijoo, G., Moreira, M. T. (2021). Environmental benefits of soy-based bio-adhesives as an alternative to formaldehyde-based options. Environmental Science and Pollution Research, 28(23), 29781–29794. DOI 10.1007/s11356-021-12766-4. [Google Scholar] [CrossRef]
98. Zeng, Y., Xu, P., Yang, W., Chu, H., Wang, W. et al. (2021). Soy protein-based adhesive with superior bonding strength and water resistance by designing densely crosslinking networks. European Polymer Journal, 142(5), 110128. DOI 10.1016/j.eurpolymj.2020.110128. [Google Scholar] [CrossRef]
99. Liu, C., Zhang, Y., Li, X., Luo, J., Gao, Q. et al. (2017). Green bio-thermoset resins derived from soy protein isolate and condensed tannins. Industrial Crops and Products, 108(1), 63–71. DOI 10.1016/j.indcrop.2017.06.026. [Google Scholar] [CrossRef]
100. Chen, X., Pizzi, A., Xi, X., Zhou, X., Fredon, E. et al. (2021). Soy protein isolate non-isocyanates polyurethanes (NIPU) wood adhesives. Journal of Renewable Materials, 9(6), 1045–1057. DOI 10.32604/jrm.2021.015066. [Google Scholar] [CrossRef]
101. Nanda, P. K., Rao, K. K., Nayak, P. L. (2007). Biodegradable polymers. XI. Spectral, thermal, morphological and biodegradability properties of environment-friendly green plastics of soy protein modified with thiosemicarbazide. Journal of Applied Polymer Science, 103(5), 3134–3142. DOI 10.1002/(ISSN)1097-4628. [Google Scholar] [CrossRef]
102. Schwarzkopf, M., Huang, J., Li, K. (2010). A formaldehyde-free soy-based adhesive for making oriented strandboard. Journal of Adhesion, 86(3), 352–364. DOI 10.1080/00218460903482549. [Google Scholar] [CrossRef]
103. Luo, J., Li, L., Luo, J., Li, X., Li, K. et al. (2017). A high solid content bioadhesive derived from soybean meal and egg white: Preparation and properties. Journal of Polymers and the Environment, 25(3), 948–959. DOI 10.1007/s10924-016-0875-3. [Google Scholar] [CrossRef]
104. Mokhtari, C., Malek, F., Halila, S., Khiari, R. (2021). New biobased polyurethane materials from modified vegetable oil. Journal of Renewable Materials, 9(7), 1213–1223. DOI 10.32604/jrm.2021.015475. [Google Scholar] [CrossRef]
105. Mucci, V. L., Hormaizgui, M. E. V., Aranguren, M. I. (2020). Plant oil-based waterborne polyurethanes: A brief review. Journal of Renewable Materials, 8(6), 579–601. DOI 10.32604/jrm.2020.09455. [Google Scholar] [CrossRef]
106. L’hocine, L., Boye, J. I., Arcand, Y. (2006). Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. Food Science, 17(3), C137–C145. [Google Scholar]
107. Wagner, J. R., Sorgentini, D. A., Añón, M. C. (2000). Commercial and laboratory-prepared soy protein isolates. Journal of Agricultural and Food Chemisry, 48(8), 3159–3165. DOI 10.1021/jf990823b. [Google Scholar] [CrossRef]
108. Cao, N., Fu, Y., Jh, J. (2007). Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocolloids, 21(7), 1153–1162. DOI 10.1016/j.foodhyd.2006.09.001. [Google Scholar] [CrossRef]
109. Guo, Y., Bao, Y., Sun, K., Chang, C., Liu, W. (2021). Effects of covalent interactions and gel characteristics on soy protein-tannic acid conjugates prepared under alkaline conditions. Food Hydrocolloids, 112(8), 106293. DOI 10.1016/j.foodhyd.2020.106293. [Google Scholar] [CrossRef]
110. Freire, M., Cofrades, S., Pérez-Jiménez, J., Gómez-Estaca, J., Jiménez-Colmenero, F. et al. (2018). Emulsion gels containing n-3 fatty acids and condensed tannins designed as functional fat replacers. Food Research International, 113(4), 465–473. DOI 10.1016/j.foodres.2018.07.041. [Google Scholar] [CrossRef]
111. do Prado Silva, T., Munari Benetti, J. V., Alexandrino, T. T. D. B., Assis, O. B. G., de Ruiter, J. et al. (2021). Whey protein isolate microgel properties tuned by crosslinking with organic acids to achieve stabilization of pickering emulsions. Foods, 10(6), 1296. DOI 10.3390/foods10061296. [Google Scholar] [CrossRef]
112. Tsurunaga, Y. (2018). Formation of a protein-tannin complex to remove astringency during processing of Western-style persimmon jelly. Acta Horticulturae, 28(1195), 177–182. DOI 10.17660/ActaHortic.2018.1195.28. [Google Scholar] [CrossRef]
113. Jin, B., Zhou, X., Zhou, S., Liu, Y., Zheng, Z. et al. (2019). Nano-encapsulation of curcumin using soy protein hydrolysates–tannic acid complexes regulated by photocatalysis: A study on the storage stability and in vitro release. Journal of Microencapsulation, 36(4), 385–398. DOI 10.1080/02652048.2019.1637473. [Google Scholar] [CrossRef]
114. Szczurek, A., Fierro, V., Medjahdi, G., Celzard, A. (2019). Carbon aerogels prepared by autocondensation of flavonoid tannin. Carbon Resources Conversion, 2(1), 72–84. DOI 10.1016/j.crcon.2019.02.001. [Google Scholar] [CrossRef]
115. Grishechko, L. I., Amaral-Labat, G., Szczurek, A., Fierro, V., Kuznetsov, B. N. et al. (2013). New tannin-lignin aerogels. Industrial Crops and Products, 41, 347–355. DOI 10.1016/j.indcrop.2012.04.052. [Google Scholar] [CrossRef]
116. Amaral-Labat, G. A., Pizzi, A., Gonçalves, A. R., Celzard, A., Rigolet, S. et al. (2008). Environment-friendly soy flour-based resins without formaldehyde. Journal of Applied Polymer Science, 108(1), 624–632. DOI 10.1002/(ISSN)1097-4628. [Google Scholar] [CrossRef]
117. Braghiroli, F. L., Fierro, V., Szczurek, A., Stein, N., Parmentier, J. et al. (2015). Hydrothermally treated aminated tannin as precursor of N-doped carbon gels for supercapacitors. Carbon, 90(3), 63–74. DOI 10.1016/j.carbon.2015.03.038. [Google Scholar] [CrossRef]
118. Braghiroli, F. L., Fierro, V., Szczurek, A., Stein, N., Parmentier, J. et al. (2015). Electrochemical performances of hydrothermal tannin-based carbons doped with nitrogen. Industrial Crops and Products, 70, 332–340. DOI 10.1016/j.indcrop.2015.03.046. [Google Scholar] [CrossRef]
119. Ghahri, S., Mohebby, B., Pizzi, A., Mirshokraie, A., Mansouri, H. M. (2018). Improving water resistance of soy-based adhesive by vegetable tannin. Journal of Polymers and the Environment, 26(5), 1881–1890. DOI 10.1007/s10924-017-1090-6. [Google Scholar] [CrossRef]
120. Ghahri, S., Pizzi, A., Mohebby, B., Mirshokraie, A., Mansouri, H. M. (2018). Soy-based, tannin-modified plywood adhesives. Journal of Adhesion, 94(3), 218–237. DOI 10.1080/00218464.2016.1258310. [Google Scholar] [CrossRef]
121. Ghahri, S., Pizzi, A. (2018). Improving soy-based adhesives for wood particleboard by tannins addition. Wood Science and Technology, 52(1), 261–279. DOI 10.1007/s00226-017-0957-y. [Google Scholar] [CrossRef]
122. Charlton, A. J., Baxter, N. J., Lokmankhan, M., Moir, A. J., Haslam, E. et al. (2002). Polyphenol/peptide binding and precipitation. Journal of Agricultural and Food Chemistry, 50(6), 1593–1601. DOI 10.1021/jf010897z. [Google Scholar] [CrossRef]
123. Kamoun, C., Pizzi, A. (2000). Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 1: Hexamine decomposition and reactive intermediates. Holzforschung Holzverwertung, 52(1), 16–19. [Google Scholar]
124. Kamoun, C., Pizzi, A. (2000). Mechanism of hexamine as a non-aldehyde polycondensation hardener, Part 2: Hexamine recomposition and reactive intermediates. Holzforschung Holzverwertung, 52(3), 66–67. [Google Scholar]
125. Kamoun, C., Pizzi, A., Zanetti, M. (2003). Upgrading of MUF resins by buffering additives–Part 1: Hexamine sulphate effect and its limits. Journal of Applied Polymer Science, 90(1), 203–214. DOI 10.1002/(ISSN)1097-4628. [Google Scholar] [CrossRef]
126. Ghahri, S., Chen, X., Pizzi, A., Hajihassani, R., Papadopoulos, A. N. (2021). Natural tannins as new cross-linking materials for soy-based adhesives. Polymers, 13(4), 595. DOI 10.3390/polym13040595. [Google Scholar] [CrossRef]
127. Pizzi, A. (2021). Covalent and ionic bonding between tannin and collagen in leather making and shrinking: A MALDI-ToF study. Journal of Renewable Materials, 9(8), 1345–1364. DOI 10.32604/jrm.2021.015663. [Google Scholar] [CrossRef]
128. Ghahri, S., Pizzi, A., Hajihassani, R. A. (2022). A study of concept to prepare totally biosourced wood adhesives from only soy protein and tannin. Polymers, 14(6), 1150. DOI 10.3390/polym14061150. [Google Scholar] [CrossRef]
129. Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) (2006). European commission ruling EC 1907/2006; L396, pp. 1–849. Brussels, Belgium: European Parliament and Council. [Google Scholar]
