 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2022.024889
REVIEW
Hydrochar Pelletization towards Solid Biofuel from Biowaste Hydrothermal Carbonization
Faculty of Geosciences and Environmental Engineering, Southwest Jiao Tong University, Chengdu, 611756, China
*Corresponding Authors: Tengfei Wang. Email: wtfgxp@163.com; Junmin Chen. Email: cjm@swjtu.edu.cn
Received: 12 June 2022; Accepted: 22 June 2022
Abstract: Hydrothermal carbonization is highly applicable to high moisture biomass upgrading due to fact that moisture involved can be directly used as reaction media under subcritical-water region. With this, value-added utilization of hydrochar as solid fuel with high carbon and energy density is one of the important pathways for biomass conversion. In this review, the dewatering properties of hydrochar after the hydrothermal carbonization of biowaste, coalification degree with elemental composition and evolution, pelletization of hydrochar to enhance the mechanical properties and density, coupled with the combustion properties of hydrochar biofuel were discussed with various biomass and carbonization parameters. Potential applications for the co-combustion with coal, cleaner properties and energy balance for biowaste hydrothermal carbonization were presented as well as the challenges.
Keywords: Biomass; hydrothermal carbonization; hydrochar; pelletization
Biomass represents the non-fossil energy and biodegradable organic material derived from plants, agriculture and forestry waste [1]. In addition to traditional lignocellulosic materials, scientists focus more on biowaste valorization like sludge and microalgae in recent years due to the access to recover carbon sources and the reduction of environment burden simultaneously [2]. Generally, bottleneck of biomass resource utilization is the high moisture, low mass and carbon density, and low energy content. Considering the high cost of separation and treatment of water involved in biomass, new techniques are required to develop to meet reaction with both biowaste and water, and hydrothermal carbonization (HTC), liquefication (HTL), and gasification (HG) have got enough attention to replace the traditional pyrolysis pathways [3]. Compared with traditional thermal conversion pathways like high temperature or low temperature pyrolysis, microwave pyrolysis, flash pyrolysis, HTC conversion pathways have showed several advantages like the flexible regulation, high efficiency, and short cycle in biomass pretreatment, upgrading, and materials preparation [4,5]. Another difference is that solid-liquid separation is required for the hydrochar and liquid phase, and this is also problem for industrialization application of HTC.
Under high temperature and pressure, water can be used as reactants, catalyst, and energy of the media during subcritical region. As shown in Fig. 1, the dielectric constant water decreased significantly below the critical point [6]. Thus, varying hydrothermal parameters or severity can efficiently convert biomass into desired mass yield of hydrochar, biocrude-oil and gas products. Especially, a series of reactions such as hydrolysis, dehydration, ploymerization reaction is the domain reaction for biomass carbonization [7,8]. For HTC of biowaste, feedstock undergos hydrolysis, dehydration, decarboxylation reaction, after which intermediate products such as HMF undergo re-dehydration or isomerization, and the ploymerization of these chemicals formed typical hydrochar with core-shell structure [9]. The carbonization mechanism is basically concluded from the chemical structure and surface characteristics until now which is generally regarded as the mimic process of natural coalification [9]. One of the most typical tendency is that the dehydration and decarboxylation reaction can significantly reduce the O and H content in hydrochar, and aromatic structure dominates at high hydrothermal severity [10]. The increased carbon density and unique structure stimulate researchers to apply hydrochar as solid biofuel or carbon materials. However, HHVs depended much on feedstock and hydrothermal parameters, and low energy content can not support a stable combustion and co-combustion with coal [11]. In addition, calorific value increases to some extent after HTC, and part of nitrogen, sulfur, chlorine can be removed by water-washing release or cracked in liquid phase, so cleaner performance of hydrochar can be achieved [12,13]. The differences between hydrochar and coal are the energy density, physical-chemical structure, and fixed carbon content, so pelletization process is expected to enhance the physical properties and energy density of hydrochar [14]. However, since works related to hydrochar as pellet fuel is still in initial stage, investigation on energy consumption and pelletization mechanism involved in pellet structure are facing challenges [15,16,17].

Figure 1: Temperature range of subcritical and supercritical water properties for hydrothermal conversion of biomass
In this review, we focused on the hydrothermal carbonization of biomass for solid fuel pelletization, and the fuel properties for dewaterability, element composition and evolution tendency, and corresponding pelletization process and combustion characteristics were discussed. Additionally, future perspectives and challenges were presented about HTC and hydrochar fuel applications.
2 Unique Properties of Hydrochar towards Solid Biofuel
2.1 The Dewatering Properties of Hydrochar
Direct combustion of biomass requires more energy consumption for moisture drying and pollutant gas like SO2 and NOx can be released by reaction between water volatilization during combustion [18]. Therefore, hydrothermal carbonization shows advantage for water removal due to the dehydration reaction and removal of hydroxyl groups. Moisture in the biomass is generally divided into free water and bound water [19,20]. Former is easy to be physically removed by heating, while the combined water is aroused by hydroxyl and carboxyl groups in organic matter to form hydrogen bonds, which is difficult to be removed directly [21]. As shown in Fig. 2, the physical structure of biomass is cracked in subcritical water by hydrolysis reaction, and a series of small molecular substances are formed, and the channel in hydrochar became bigger, which resulted in high diffusivity. After HTC, hydrophilic functional groups like hydroxyl groups on hydrochar surface can be significantly weakened compared to feedstock and it can be decreased with the increase of carbonization degree. At the same time, solid-phase condensation reaction occurs during hydrothermal carbonization, since hydrochar is composed of components and spherical structures formed by solid-solid pyrolysis, its pore size is more abundant than that of feedstock, so HTC can be used as one pathway to release water. Coupled with the increased hydrophobic properties, more bound water is converted into free form that can be easily removed by physical separation. Additionally, water produced can not only serve as a solvent, but also participates in hydrothermal carbonization as catalyst and medium [22]. Typical application of dewatering is the hydrothermal conversion of sludge, which showed that hydrothermal carbonization can hydrolyze the viscous organic matter in sludge and destroy its colloidal structure, and release macromolecular organic matter from cell, thereby enhancing the dewatering performance of sludge [23]. The dewatering performance depended much on the feedstock and HTC parameters, as presented in Table 1. For instance, in Peng et al.’s work [24], sludge with woody biomass was mixed as feedstock for HTC, and the dewatering performance is followed by the biomass species, mixer ratio and carbonization temperature (Fig. 3).
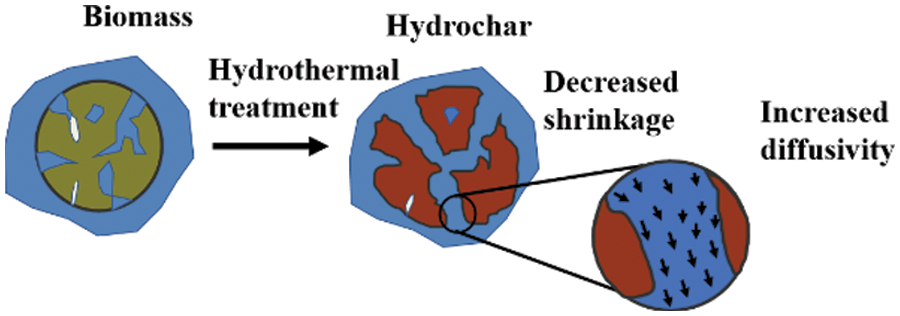
Figure 2: Schematic diagram of channel in hydrochar after hydrothermal carbonization of biomass
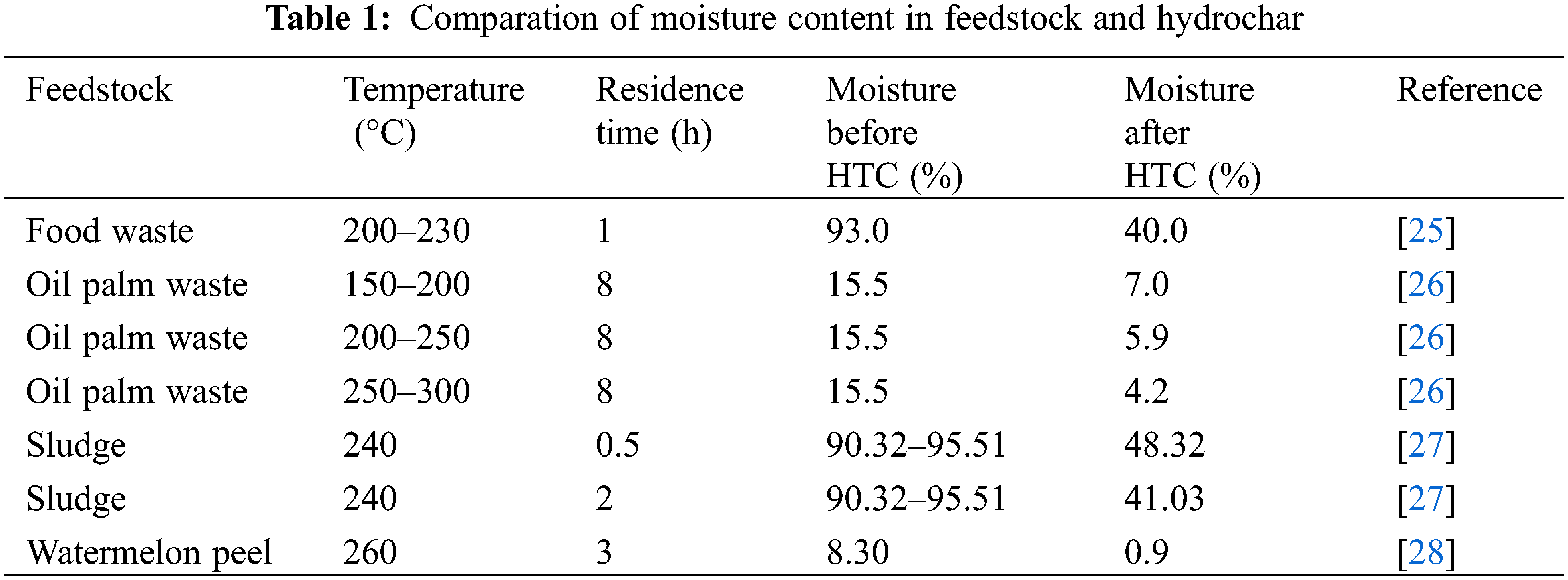

Figure 3: Tendency of the moisture ratio in sludge and lignocellulose mixture derived hydrochar with time of mechanical dewatering and thermal drying [24]
2.2 Elemental Composition and Coalification of Hydrochar
For mimic the coalification process of biomass hydrothermal carbonization, researchers used elemental composition to trace the atomic ratio change including C, H, N, O, and the information can provide details about the degree of dehydration and decarboxylation [29]. Thus, calorific value can be evaluated for hydrochar, and the value ranged from 15–35 MJ/kg generally, which is severally depended on feedstock and hydrothermal severity, since oxygen content and ash have obvious effect on the fuel quality [30]. Even this, the coalification degree of hydrochar is lower compared to typical coal like anthracite and bituminous coal, and in most works hydrochar quality is close to the lignite [31]. As indicated by Table 2, both hydrothermal parameters and feedstocks are dominant factors during HTC. Food waste and woody biomass hydrochar have relatively higher C content than others due to the high sugers which can he easily carbonized. With temperature and residence time increased, both O/C and H/C decreased, along with higher HH Vs. Sludge and MSW derived hydrochar has low HHVs due to the high ash content. Even this, hydrochar fuel with dehydrogenation and deoxygenation is essential when high quality fuel like coal is prepared, but the balance of oxygen should be considered when oxygen-rich fuels is prepared.

The evolution of the elements is highly related to the carbonization mechanism. Feedstocks firstly hydrolysis with temperature increased, and the degradation of hemicellulose is favored than that of lignin and cellulose. After the broken of the body, two pathways of the compositions in liquid products are generally accepted. One is the pyrolysis-like process, which means a directly solid-solid conversion from biomass to hydrochar during HTC, and another is the ploymerization of the chemicals like HMF and the derives to form secondary hydrochar [9]. The pathways depend on parameters. From the view of basic structure of carbon, with the increase of carbonization degree of hydrochar, furan structure transitions to aromatic-rich structure, and carbon density, calorific value of hydrochar increases accordingly [9]. For example, Wang et al. [41] used food waste as feedstock for HTC, and It can be clearly seen that the increase of temperature increased the aromatic structure in hydrochar, and the furan-atomic ratio decreased, which is the directly evidence of the loss of H and O at high hydrothermal severity [42]. Therefore, the atomic number ratio of O/C and H/C also decreases, and calorific value of hydrochar also increased accordingly, which is the most powerful evidence to simulate coalification in the natural process, and is widely used to evaluate the degree of coalification of hydrothermal coals compared with lignite, bituminous and anthracite coal [43]. In addition to reaction temperature, residence time is another factor for varying the elemental composition. However, when reaction time is not enough to support the ploymerization of hydrolyzed intermediates like furans and the derived chemicals in liquid phase, low yield with high oxygen content is obtained [7]. Nevertheless, long residence time will also require high energy consumption. Therefore, reasonable control of residence time is critical for industrial application of hydrothermal carbonization technology.
As observed from Table 2, the elemental composition and calorific value of hydrochar not only depend on the parameters of hydrothermal carbonization, but also can be affected by composition of feedstock. For example, biowaste contained high organic content is prefer to be carbonized than that of high-ash species. In our previous work [41], it suggested that carbonization degree of N-containing biowaste defers from that of N-free biowaste, since Mallard reaction happened and N can be incorporated into hydrochar, which resulted in high N ratio and low C ratio in hydrochar [44]. Until now, the conversion of biomass for solid fuel preparation still faces challenges on fuel upgrading by varying elemental content, and balance of energy consumption of hydrothermal carbonization, which is also the bottleneck for the sustainable development of its industrial application in future.
2.3 Pelletization of Hydrochar as Solid Biofuel
Pelletization of hydrochar is essential for solid biofuel preparation and shows great advantage than biomass pelletization like low ash and high mass density, proper moisture, and the enhanced mechanical properties [45]. However, mechanical properties of hydrochar pellets depend on temperature, pressure, particle size, moisture content, feedstock and binders [46]. Generally, A wide range of temperatures from room condition to 140°C in pelletization process is conducted, and physical modification after pelletization can help the hydrochar particles densification by formation ordered structure. After pelletization, the energy density, mass density and compressive strength have been improved to varying degrees, and the detail pelletization process is depicted in Fig. 4.
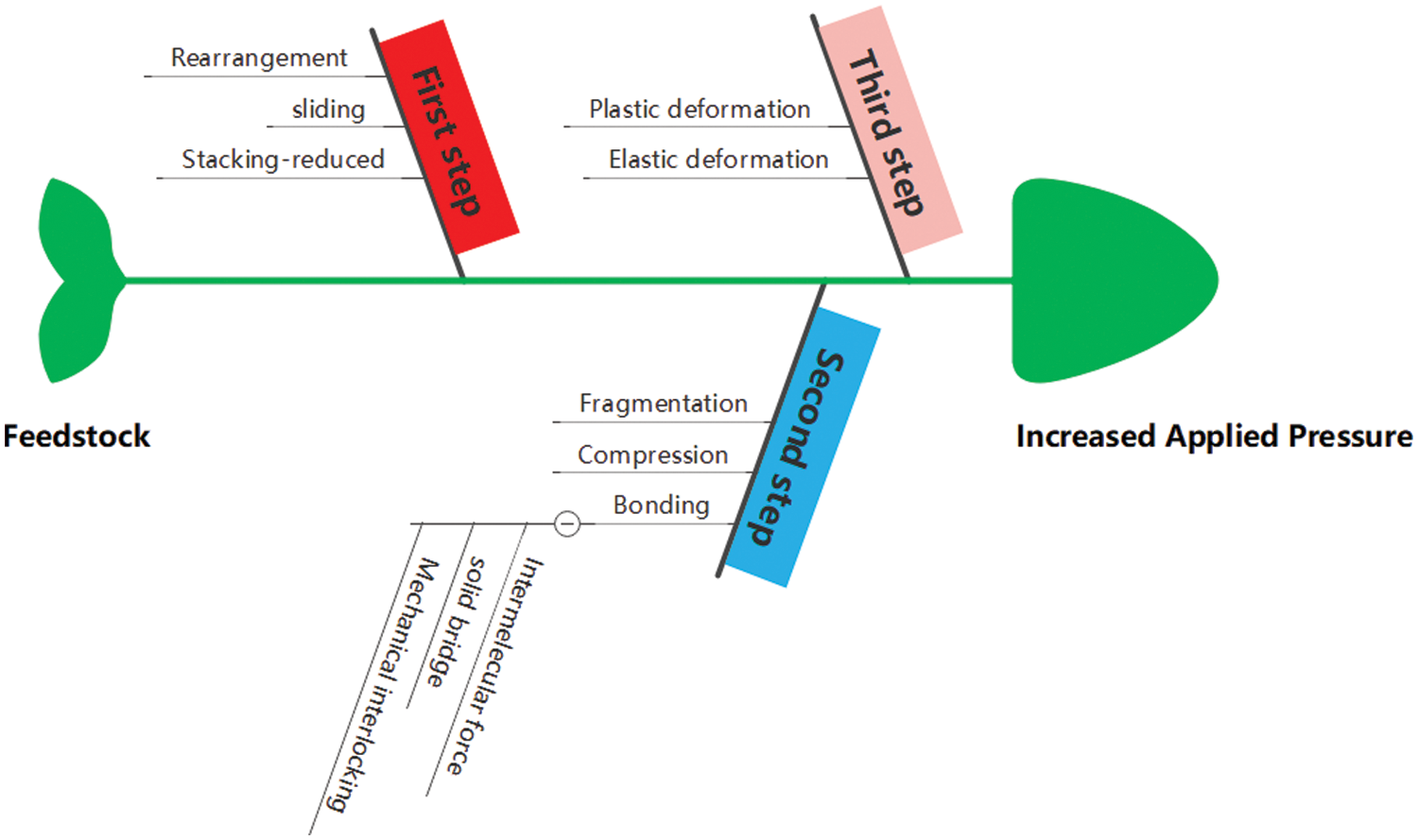
Figure 4: Detail pelletization process of feedstock and changes involved in pellet
In the absence of solid bridges as studied, particles are close enough to bind forces such as intermolecular (valence, hydrogen, and van der Waals), electrostatic, and can make solid particles stick to each other attached. Previous works sudied adhesion theories and proposed a microscopic explanation for particle bonding, and driving force for adhesion from the electronic interaction between molecules, and maximum gravitational force is close to the minimum potential energy by forming chemical bonds [47]. Due to the action of high pressure and high temperature, solid bridges can be formed by molecular diffusion from one particle to another. Solid bridges can be crystallization of certain components, chemical reactions, hardening of binders (such as molasses and tar), solidification of molten components, or linkages between particles, as shown in Fig. 5 [45,48,49]. For lignocellulose derived hydrochar, solid bridges formed from carbonized cellulose is critical considering the content and thermal stable structure than that of hemicellulose. Thus, temperature should be well controlled during HTC to make sure that cellulose and lignin maintains the main physical properties. Meanwhile, the HTC temperature may also have obvious effect on the energy consumption during pelletization of hydrochar. In Wang et al.’s work [22], energy consumption of hydrochar pelletizaiton increased when hydrochar is prepared from 220°C to 260°C, while the high food waste ratio in woody waste blend can decrease approximately 15–20 J/g for the compression process. This suggested that the properties of hydrochar is dominant for the pelletization. Liu et al. [50] reported that liquid bridge can be formed by the local melting of binding components at particle junctions under high densification pressure, and the gaps between hydrochar particles can be reduced. In some works, binders like starch, molasses, and calcium oxide were used to enhance the solid or liquid bridge structure in hydrochar pellet. Binder typically softens at the glass transition temperature or melts at the melting temperature to provide a liquid bridge connecting adjacent particles. For example, at glass transition temperature, lignin softens and flows under pressure, contacting surrounding particles and forming covalent bonds [51].

Figure 5: The surface and structure of typical pellet: (a) pelletization with the natural binders and forms coating and melting structure in corn stover pellet; (b) hydrochar with molasses as binder forming solid bridge structure between particles [16,47]
The role of moisture during pelletization is important, since moisture can act as both a binder and a lubricant, so energy consumption of friction can be reduced [52]. Additionally, under the action of heat and pressure, water-soluble components in biomass, such as starch, protein, sugar, soda ash and salt, can undergo starch gelatinization, protein denaturation or salt dissolution in the presence of moisture, thereby improving the degree of binding [16,45]. Water can also form a thin film around the particles, and Van der Waals forces can be further promoted for the binding, as contact area between particles increases. Generally, water content is added with 5%–15% mass ratio during pelletization which is used for lubrication, while high water content can cause pellet fracture due to the higher hydrophobic properties of hydrochar than coal. In addition to feedstock, the pelletization process is related to the holding time, relaxation time, pressure, and compression rate, and systematically optimize these parameters in works is critical to obtain pellet with excellent durability and strength. The energy consumption should also be concerned at the same time [53].
2.4 Combustion Properties of Hydrochar Biofuel
An intuitive advantage is that hydrochar has better combustion performance after HTC. Compared to biomass, a higher ignition point for hydrochar can be achieved, which means a save environment for the hydrochar storage is excepted [38]. It reported that the ignition temperature shifts to a high temperature range around 100°C [54]. As a result, the temperature at maximum peak moves into the high temperature range for hydrochar combustion. In addition, the combustion rate was largely improved, representing about 2~3 times higher than that of biowaste, regardless of hydrothermal severity. Since volatile content is removed during the HTC stage, more fixed carbon can be recovered in hydrochar. For instance, lignin mainly degrades at temperature above 260°C, and the high carbon density and matrix makes it thermal stable [55]. With this regard, HTC parameters have significant influence on the combustion properties of hydrochar. Chen et al. [43] showed HTC temperature had a significant impact on combustion behavior and activation energy of hydrochars. The first combustion decomposition peak of hydrochars were much higher than feedstock, leading to a better combustion performance [5].
Additionally, application of binders also optimizes the combustion properties of hydrochar. For example, Wang et al. [15] studied the effect of four organic binders (protein, starch, lignin and molasses) on woody biomass pellets. Regarding combustion performance, a reduction in ignition temperature was observed for all binder hydrochar. Thus, ratio of fixed carbon and volatile content in hydrochar is highly related to combustion properties. Considering these, co-combustion of hydrochar with coals is investigated in many works towards industrial application. Low ignition temperature and high combustion reactivity of hydrochar make the co-combustion attractive. However, binders will affect the burnout temperature of hydrochar pellet, and this depends on binder species. For example, molasses enhances the combustion process can delay combustion with a higher final temperature. Thus, binder should be used based on the combustion requirement. Muthuraman et al. [56] compared the co-combustion characteristics of Indian coal with MSW derived hydrochar and stated that the devolatilization properties of coal could be improved by blending hydrochar, and the ignition temperature was significantly reduced, which became more prominent with increasing the blending ratio of hydrochar resulting from its high volatile matter content. Additionally, single biomass hydrothermal carbonization may not be able to achieve satisfactory combustion results. Mixture of different biomass for HTC can often improve the combustion performance of hydrochar. For example, Lang et al. [57] found that the combustion weight loss in the second stage was significantly higher than that of pig manure due to the presence of more volatile substances in straw, and the complete combustion time was shorter. However, pig manure has a higher combustion temperature with metal oxides and inorganic minerals. After mixing the two biomasses for HTC, the combustion performance and stability of the mixture were improved.
Meanwhile, hydrochar has showed the cleaner combustion properties than that of feedstock. As it proved, inorganic elements can be released into liquid product and low ash can be achieved [58]. As for N, S, and Cl, these elements also undergo same trend but the forms in hydrochar which is directly to gas release during combustion. In He et al.’s work [59], denitrification is considered to be main reaction happened in HTC of sludge, and it showed the nitrogen was mainly released in liquid products as ammonia nitrogen, while nitrogen in hydrochar changed to stable form like pyridines-N. For sulfur, many works have showed that more inorganic forms of S in hydrochar was observed compared to feedstock, which will also reduce the SO2 during combustion [2]. Since Cl has smaller atomic radius than that of N and S, most works reported that approximately 90% can be released into liquid phase, and washing process can also help for the reduction of Cl in hydrochar [11]. Based on these, HTC has showed the advantage for cleaner biofuel production, but the transformation and balance of these elements in HTC products still need further works.
3 Conclusion and Future Perspectives
In this paper, hydrothermal carbonization of biowaste for solid biofuel was reviewed about the dewatering properties, elemental composition, pelletization and combustion. The investigation on hydorchar as biofuel is limited to the optimization of hydrothermal carbonization parameters, the control of physical characteristics of fuel after pelletization, and the study of optimal combustion characteristics. Therefore, various balance controls like mass and energy balance need to be noted, which are common to researchers working on hydrothermal carbonization technologies. For preparation of solid fuel from hydrochar, future works should also pay attention to the goal of high calorific value, easy pelletization, stability of combustion, cleaner production of hydrochar. A simple balance for hydrochar fuel production is presented in Fig. 6 and future challenges and possible research hotspots are as follows:

Figure 6: A simple balance for pelletization of hydrochar as solid biofuel production
(1)Instu-analysis of the carbonization process of biomass with time and temperature should be cared instead of solo analysis of the hydrochar;
(2)The mechanism involved in hydrochar pelletization like solid-physics and models should be got more attention because this benefits the decrease of energy consumption during pelletization;
(3)The inter-effect of various feedstock derived hydrochar during pelletization should be investigated in future with the binders since single biowaste may not conducive to pelletization with high mechanical strength;
(4)Recycle of liquid products for HTC is essential, and it should also be considered for recycle optimization and coalification of hydrochar.
Funding Statement: This work is supported by the Fundamental Research Funds for the Central Universities of Southwest Jiaotong University; supported by Sichuan Science and Technology Program (2021YFS0284).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Liu, Z., Wang, Z., Chen, H., Cai, T., Liu, Z. et al. (2021). Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environmental Pollution, 268(3), 115910. DOI 10.1016/j.envpol.2020.115910. [Google Scholar] [CrossRef]
2. Zhuang, X., Zhan, H., Huang, Y., Song, Y., Yin, X. et al. (2018). Denitrification and desulphurization of industrial biowastes via hydrothermal modification. Bioresource Technology, 254(2), 121–129. DOI 10.1016/j.biortech.2018.01.061. [Google Scholar] [CrossRef]
3. Watson, J., Si, B., Wang, Z., Wang, T., Valentine, A. et al. (2021). Towards transportation fuel production from food waste: Potential of biocrude oil distillates for gasoline, diesel, and jet fuel. Fuel, 301(9–10), 121028. DOI 10.1016/j.fuel.2021.121028. [Google Scholar] [CrossRef]
4. Wang, L., Chang, Y., Zhang, X., Yang, F., Li, Y. et al. (2020). Hydrothermal co-carbonization of sewage sludge and high concentration phenolic wastewater for production of solid biofuel with increased calorific value. Journal of Cleaner Production, 255(4), 120317. DOI 10.1016/j.jclepro.2020.120317. [Google Scholar] [CrossRef]
5. Nicolae, S. A., Au, H., Modugno, P., Luo, H., Szego, A. E. et al. (2020). Recent advances in hydrothermal carbonisation: from tailored carbon materials and biochemicals to applications and bioenergy. Green Chemistry, 22(15), 4747–4800. DOI 10.1039/D0GC00998A. [Google Scholar] [CrossRef]
6. He, C., Chen, C. L., Giannis, A., Yang, Y., Wang, J. Y. et al. (2014). Hydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: A review. Renewable and Sustainable Energy Reviews, 39, 1127–1142. DOI 10.1016/j.rser.2014.07.141. [Google Scholar] [CrossRef]
7. Wang, T., Zhai, Y., Zhu, Y., Li, C., Zeng, G. et al. (2018). A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renewable and Sustainable Energy Reviews, 90(1), 223–247. DOI 10.1016/j.rser.2018.03.071. [Google Scholar] [CrossRef]
8. Sasaki, M., Fang, Z., Fukushima, Y., Adschiri, T., Arai, K. et al. (2000). Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Industrial & Engineering Chemistry Research, 39(8), 2883–2890. DOI 10.1021/ie990690j. [Google Scholar] [CrossRef]
9. Falco, C., Sevilla, M., White, R. J., Rothe, R., Titirici, M. M. et al. (2012). Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem, 5(9), 1834–1840. DOI 10.1002/cssc.201200022. [Google Scholar] [CrossRef]
10. Heidari, M., Dutta, A., Acharya, B., Mahmud, S. (2019). A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. Journal of the Energy Institute, 92(6), 1779–1799. DOI 10.1016/j.joei.2018.12.003. [Google Scholar] [CrossRef]
11. Prawisudha, P., Namioka, T., Yoshikawa, K. (2012). Coal alternative fuel production from municipal solid wastes employing hydrothermal treatment. Applied Energy, 90(1), 298–304. DOI 10.1016/j.apenergy.2011.03.021. [Google Scholar] [CrossRef]
12. Małgorzata, W., Maciej, Ś., Bogusław, L. (2021). Hydrothermal co-carbonization of sewage sludge and fuel additives: Combustion performance of hydrochar. Renewable Energy, 178(4), 1046–1056. DOI 10.1016/j.renene.2021.06.101. [Google Scholar] [CrossRef]
13. Ma, D., Zhang, G., Zhao, P., Areeprasert, C., Shen, Y. et al. (2015). Hydrothermal treatment of antibiotic mycelial dreg: More understing from fuel characteristics. Chemical Engineering Journal, 273, 147–155. DOI 10.1016/j.cej.2015.01.041. [Google Scholar] [CrossRef]
14. Liu, Z., Guo, Y., Balasubramanian, R., Hoekman, S. K. (2016). Mechanical stability and combustion characteristics of hydrochar/lignite blend pellets. Fuel, 164, 59–65. DOI 10.1016/j.fuel.2015.10.004. [Google Scholar] [CrossRef]
15. Wang, Z., Zhai, Y., Wang, T., Wang, B., Peng, C. et al. (2020). Pelletizing of hydrochar biofuels with organic binders. Fuel, 280, 118659. DOI 10.1016/j.fuel.2020.118659. [Google Scholar] [CrossRef]
16. Zhai, Y., Wang, T., Zhu, Y., Peng, C., Wang, B. et al. (2018). Production of fuel pellets via hydrothermal carbonization of food waste using molasses as a binder. Waste Management, 77, 185–194. DOI 10.1016/j.wasman.2018.05.022. [Google Scholar] [CrossRef]
17. Leng, L., Yang, L., Leng, S., Zhang, W., Zhou, Y. et al. (2021). A review on nitrogen transformation in hydrochar during hydrothermal carbonization of biomass containing nitrogen. Science of the Total Environment, 756, 143679. DOI 10.1016/j.scitotenv.2020.143679. [Google Scholar] [CrossRef]
18. Akarsu, K., Duman, G., Yilmazer, A., Keskin, T., Azbar, N. et al. (2019). Sustainable valorization of food wastes into solid fuel by hydrothermal carbonization. Bioresource Technology, 292, 121959. DOI 10.1016/j.biortech.2019.121959. [Google Scholar] [CrossRef]
19. Wang, B., Zhai, Y., Wang, T., Li, S., Peng, C. et al. (2019). Fabrication of bean dreg-derived carbon with high adsorption for methylene blue: Effect of hydrothermal pretreatment and pyrolysis process. Bioresource Technology, 274, 525–532. DOI 10.1016/j.biortech.2018.12.022. [Google Scholar] [CrossRef]
20. Wang, L., Li, A. (2015). Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Research, 68(2), 291–303. DOI 10.1016/j.watres.2014.10.016. [Google Scholar] [CrossRef]
21. Qiao, W., Wang, W., Wan, X., Xia, Z., Deng, Z. et al. (2010). Improve sludge dewatering performance by hydrothermal treatment. Journal of Residuals Science & Technology, 7(1), 7–11. [Google Scholar]
22. Wang, T., Zhai, Y., Li, H., Zhu, Y., Li, S. et al. (2018). Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresource Technology, 267, 371–377. DOI 10.1016/j.biortech.2018.07.059. [Google Scholar] [CrossRef]
23. Wang, L., Li, A., Chang, Y. (2016). Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Heavy metals, volatile organic compounds and combustion characteristics of hydrochar. Chemical Engineering Journal, 297, 1–10. DOI 10.1016/j.cej.2016.03.131. [Google Scholar] [CrossRef]
24. Zhai, Y., Peng, C., Xu, B., Wang, T., Li, C. et al. (2017). Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy, 127(11), 167–174. DOI 10.1016/j.energy.2017.03.116. [Google Scholar] [CrossRef]
25. Mannarino, G., Sarrion, A., Diaz, E., Gori, R., de la Rubia, M. A. et al. (2022). Improved energy recovery from food waste through hydrothermal carbonization and anaerobic digestion. Waste Management, 142(8), 9–18. DOI 10.1016/j.wasman.2022.02.003. [Google Scholar] [CrossRef]
26. Yek, P. N. Y., Liew, R. K., Wan Mahari, W. A., Peng, W., Sonne, C. et al. (2022). Production of value-added hydrochar from single-mode microwave hydrothermal carbonization of oil palm waste for de-chlorination of domestic water. Journal of Analytical and Applied Pyrolysis, 833, 154968. [Google Scholar]
27. Gao, N., Li, Z., Quan, C., Miskolczi, N., Egedy, A. (2019). A new method combining hydrothermal carbonization and mechanical compression in-situ for sewage sludge dewatering: Bench-scale verification. Journal of Analytical and Applied Pyrolysis, 139(7), 187–195. DOI 10.1016/j.jaap.2019.02.003. [Google Scholar] [CrossRef]
28. Fakudze, S., Wei, Y., Zhou, P., Han, J., Chen, J. (2022). Synergistic effects of process-generated organic acids during co-hydrothermal carbonization of watermelon peel and high-sulfur coal. Journal of Environmental Chemical Engineering, 10(3), 107519. DOI 10.1016/j.jece.2022.107519. [Google Scholar] [CrossRef]
29. Kambo, H. S., Dutta, A. (2015). A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renewable and Sustainable Energy Reviews, 45(2), 359–378. DOI 10.1016/j.rser.2015.01.050. [Google Scholar] [CrossRef]
30. Watson, J., Wang, T., Si, B., Chen, W. T., Aierzhati, A. et al. (2020). Valorization of hydrothermal liquefaction aqueous phase: Pathways towards commercial viability. Progress in Energy and Combustion Science, 77, 100819. DOI 10.1016/j.pecs.2019.100819. [Google Scholar] [CrossRef]
31. Liu, Z., Quek, A., Kent Hoekman, S., Balasubramanian, R. (2013). Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel, 103, 943–949. DOI 10.1016/j.fuel.2012.07.069. [Google Scholar] [CrossRef]
32. Castro, J. S., Assemany, P. P., Carneiro, A. C. O., Ferreira, J., de Jesus Junior, M. M. et al. (2021). Hydrothermal carbonization of microalgae biomass produced in agro-industrial effluent: Products, characterization and applications. Science of the Total Environment, 768, 144480. DOI 10.1016/j.scitotenv.2020.144480. [Google Scholar] [CrossRef]
33. Yu, Y., Lau, A., Sokhansanj, S. (2022). Hydrothermal carbonization and pelletization of moistened wheat straw. Renewable Energy, 190, 1018–1028. DOI 10.1016/j.renene.2022.03.152. [Google Scholar] [CrossRef]
34. Aragon-Briceño, C., Pożarlik, A., Bramer, E., Brem, G., Wang, S. et al. (2022). Integration of hydrothermal carbonization treatment for water and energy recovery from organic fraction of municipal solid waste digestate. Renewable Energy, 184(1), 577–591. DOI 10.1016/j.renene.2021.11.106. [Google Scholar] [CrossRef]
35. Liang, W., Wang, G., Xu, R., Ning, X., Zhang, J. et al. (2022). Hydrothermal carbonization of forest waste into solid fuel: Mechanism and combustion behavior. Energy, 246(4), 123343. DOI 10.1016/j.energy.2022.123343. [Google Scholar] [CrossRef]
36. Wilk, M., Magdziarz, A., Kalemba-Rec, I., Szymańska-Chargot, M. (2020). Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy, 202, 117717. DOI 10.1016/j.energy.2020.117717. [Google Scholar] [CrossRef]
37. Sharma, H. B., Dubey, B. K. (2020). Co-hydrothermal carbonization of food waste with yard waste for solid biofuel production: Hydrochar characterization and its pelletization. Waste Management, 118(8), 521–533. DOI 10.1016/j.wasman.2020.09.009. [Google Scholar] [CrossRef]
38. Zhan, H., Zhuang, X., Zhang, S., Chang, G., Wang, X. et al. (2022). Evaluation on the enhanced solid biofuel from co-hydrothermal carbonization of pharmaceutical biowastes with lignite. Fuel, 318(3), 123626. DOI 10.1016/j.fuel.2022.123626. [Google Scholar] [CrossRef]
39. Abdullah, I., Ahmad, N., Hussain, M., Ahmed, A., Ahmed, U. et al. (2022). Conversion of biomass blends (walnut shell and pearl millet) for the production of solid biofuel via torrefaction under different conditions. Chemosphere, 295, 133894. DOI 10.1016/j.chemosphere.2022.133894. [Google Scholar] [CrossRef]
40. Silva, R. D. V. K., Lei, Z., Shimizu, K., Zhang, Z. (2020). Hydrothermal treatment of sewage sludge to produce solid biofuel: Focus on fuel characteristics. Bioresource Technology Reports, 11(3), 100453. DOI 10.1016/j.biteb.2020.100453. [Google Scholar] [CrossRef]
41. Wang, T., Zhai, Y., Zhu, Y., Peng, C., Xu, B. et al. (2018). Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresource Technology, 247, 182–189. DOI 10.1016/j.biortech.2017.09.076. [Google Scholar] [CrossRef]
42. Sevilla, M., Fuertes, A. B. (2009). The production of carbon materials by hydrothermal carbonization of cellulose. Carbon, 47(9), 2281–2289. DOI 10.1016/j.carbon.2009.04.026. [Google Scholar] [CrossRef]
43. Chen, W. H., Lin, B. J., Lin, Y. Y., Chu, Y. S., Ubando, A. T. et al. (2021). Progress in biomass torrefaction: Principles, applications and challenges. Progress in Energy and Combustion Science, 82, 100887. DOI 10.1016/j.pecs.2020.100887. [Google Scholar] [CrossRef]
44. Falco, C., Sevilla, M., White, R. J., Rothe, R., Titirici, M. M. (2012). Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem, 5(9), 1834–1840. DOI 10.1002/cssc.201200022. [Google Scholar] [CrossRef]
45. Kaliyan, N., Vance Morey, R. (2009). Factors affecting strength and durability of densified biomass products. Biomass and Bioenergy, 33(3), 337–359. DOI 10.1016/j.biombioe.2008.08.005. [Google Scholar] [CrossRef]
46. Li, H., Liu, X., Legros, R., Bi, X. T., Jim Lim, C. et al. (2012). Pelletization of torrefied sawdust and properties of torrefied pellets. Applied Energy, 93, 680–685. DOI 10.1016/j.apenergy.2012.01.002. [Google Scholar] [CrossRef]
47. Kaliyan, N., Morey, R. V. (2010). Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn stover and switchgrass. Bioresource Technology, 101(3), 1082–1090. DOI 10.1016/j.biortech.2009.08.064. [Google Scholar] [CrossRef]
48. Mišljenović, N., Čolović, R., Vukmirović, Đ., Brlek, T., Bringas, C. S. (2016). The effects of sugar beet molasses on wheat straw pelleting and pellet quality. A comparative study of pelleting by using a single pellet press and a pilot-scale pellet press. Fuel Processing Technology, 144(2), 220–229. DOI 10.1016/j.fuproc.2016.01.001. [Google Scholar] [CrossRef]
49. Hu, Q., Shao, J., Yang, H., Yao, D., Wang, X. et al. (2015). Effects of binders on the properties of bio-char pellets. Applied Energy, 157(Supplement 1), 508–516. DOI 10.1016/j.apenergy.2015.05.019. [Google Scholar] [CrossRef]
50. Liu, Z., Quek, A., Balasubramanian, R. (2014). Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Applied Energy, 113, 1315–1322. DOI 10.1016/j.apenergy.2013.08.087. [Google Scholar] [CrossRef]
51. Shailendrasingh, P. R., Sachin, V. J., Bhaskar, N. T. (2020). Methods to improve properties of fuel pellets obtained from different biomass sources: Effect of biomass blends and binders. Fuel Processing Technology, 199, 106255. DOI 10.1016/j.fuproc.2019.106255. [Google Scholar] [CrossRef]
52. Kambo H. S., Dutta A. (2014). Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Applied Energy, 135, 182–191. DOI 10.1016/j.apenergy.2014.08.094. [Google Scholar] [CrossRef]
53. Reza, M. T., Lynam, J. G., Vasquez, V. R., Coronella, C. J. (2012). Pelletization of biochar from hydrothermally carbonized wood. Environmental Progress & Sustainable Energy, 31(2), 225–234. DOI 10.1002/ep.11615. [Google Scholar] [CrossRef]
54. Liu Z., Balasubramanian R. (2014). Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTPA comparative evaluation. Applied Energy, 114, 857–864. DOI 10.1016/j.apenergy.2013.06.027. [Google Scholar] [CrossRef]
55. Zhao, P., Shen, Y., Ge, S., Chen, Z., Yoshikawa, K. (2014). Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Applied Energy, 131, 345–367. DOI 10.1016/j.apenergy.2014.06.038. [Google Scholar] [CrossRef]
56. Muthuraman, M., Namioka, T., Yoshikawa, K. (2010). A comparison of co-combustion characteristics of coal with wood and hydrothermally treated municipal solid waste. Bioresource Technology, 101(7), 2477–2482. DOI 10.1016/j.biortech.2009.11.060. [Google Scholar] [CrossRef]
57. Lang, Q., Guo, Y., Zheng, Q., Liu, Z., Gai, C. (2018). Co-hydrothermal carbonization of lignocellulosic biomass and swine manure: Hydrochar properties and heavy metal transformation behavior. Bioresource Technology, 266, 242–248. DOI 10.1016/j.biortech.2018.06.084. [Google Scholar] [CrossRef]
58. Zhao, P., Lin, C., Li, Y., Zhang, J., Huang, N. et al. (2022). Combustion and slagging characteristics of hydrochar derived from the co-hydrothermal carbonization of PVC and alkali coal. Energy, 244(7), 122653. DOI 10.1016/j.energy.2021.122653. [Google Scholar] [CrossRef]
59. He, C., Wang, K., Yang, Y., Amaniampong, P. N., Wang, J. Y. (2015). Effective nitrogen removal and recovery from dewatered sewage sludge using a novel integrated system of accelerated hydrothermal deamination and air stripping. Environmental Science and Technology, 49(11), 6872–6880. DOI 10.1021/acs.est.5b00652. [Google Scholar] [CrossRef]
