 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2023.022535
ARTICLE
Synthesis and Antioxidant Activity of (E) ω-Formylcamphene-Based Thiazole Hydrazone Derivatives
1College of Forestry, Jiangxi Agricultural University, East China Woody Fragrance and Flavor Engineering Research Center of National Forestry and Grassland Administration, Camphor Engineering Research Center of NFGA, Nanchang, 330045, China
2College of Science, Jiangxi Agricultural University, Nanchang, 330045, China
3College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang, 330027, China
*Corresponding Authors: Shangxing Chen. Email: csxing@126.com; Ji Zhang. Email: ex1990111@163.com
Received: 15 March 2022; Accepted: 29 April 2022
Abstract: (E) ω-formylcamphene was synthesized from α-pinene, the main component of turpentine, and then reacted with thiosemicarbazide to obtain (E) ω-formylcamphene thiosemicarbazide 3, which was reacted with 14 α-bromoacetophenone compounds to obtain 14 (E) ω-formylcamphene thiazole hydrazone compounds 5a–5n; the yields were all above 80%. The structures of the target compounds were characterized by IR, 1H-NMR, 13C-NMR, and HR-MS analyses. Then, 500, 250, 125, 62.5, and 31.25 mg/L drug solutions were prepared. Free radical scavenging experiments of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and 2, 2-bis (3-ethyl-benzothiazole-6-sulfonic acid) diammonium salt (ABTS) were carried out with Trolox and L-ascorbic acid as the control samples. The scavenging rates of 14 compounds for DPPH and ABTS free radicals were obtained; the IC50 values of scavenging free radicals were fitted using SPSS software. The results show that 14 (E) ω-formylcamphene-based thiazole hydrazone compounds exhibited good scavenging effects on the two free radicals, especially when the concentration of the drug solution was 125 and 62.5 mg/L; most compounds exceeded the scavenging efficiency of Trolox and L-ascorbic acid.
Keywords: (E) ω-formylcamphene; thiosemicarbazone; thiazohydrazone; structural analysis; antioxidant activity
In a biological system, oxidative stress is a state of imbalance between the oxidation and antioxidation in the body, and a negative effect produced by free radicals in the body, which is considered to be an important factor leading to aging [1,2] and disease [3,4]. A large number of reactive oxygen species (ROS) generated by oxidative stress will destroy the antioxidant defense system. Free radicals such as superoxide anion radical, hydroxyl radical, and hydrogen peroxide radical can cause oxidative damage to nucleic acids, proteins, and biofilms, leading to hypertension, diabetes, heart disease, and Alzheimer’s disease [5–7]. Antioxidants can interact with free radicals to prevent the destruction of reactive oxygen species; therefore, many studies have been conducted on the design and development of new and effective antioxidants [8,9]. In recent years, the research and development of antioxidants has become an important research topic worldwide. However, synthetic chemical antioxidants have some potential health hazards; therefore, the antioxidants prepared by the modification of natural products have attracted much attention [10–12].
Camphene, a dicyclic monoterpene compound, exists in a variety of natural volatile oils, such as camphor oil, citronella oil, turpentine oil, and cedar oil [13], and it can be prepared from the main component of turpentine α-pinene [14]. It is a renewable raw material in the fine chemical industry and can participate in many chemical reactions [15]. (E) ω-formylcamphene is synthesized by the Vilsmeier–Haack formylation reaction of camphene, and a series of camphene derivatives such as ω-acyl camphene, internal isocamphene alkyl methanol and alkyl ethers, alcohol acetate, and isocamphene alkyl ketone oxime can be synthesized from (E) ω-formylcamphene [16–19]. Some of these derivatives exhibit good biological activities. Thiazole is a five-atom heterocyclic compound and a very important scaffold in the pharmaceutical chemistry [20,21]. Many compounds with this structure often have antioxidant [22], antibacterial [23,24], antitumor [25], and other biological activities. The derivatives of thiazolylhydrazone compounds contain two active groups of thiazolyl ring and hydrazone (R–C=N–N–R), which may produce new compounds with higher biological activities. Therefore, they have received much attention in medicine, pesticides, and materials [26].
In this study, (E) ω-formylcamphene was prepared by the Vilsmeier-Hack formylation reaction of camphene with thiosemicarbazide to obtain (E) ω-formylcamphene thiosemicarbazide derivatives. DPPH and ABTS methods were used to determine the free radical scavenging capacity of the compounds, and the antioxidant activity of the derivatives was analyzed to provide a reference for the development of new antioxidants.
All the chemicals are commercially available (Aladdin Biochemical Technology Co., Ltd., Shanghai, China). All the solvents were distilled and dried according to standard procedures. Melting points were determined using a WRS-2 melting point apparatus (Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China). FT-IR spectra of the compounds were recorded using a Nicolet IS10 FT-IR spectrometer. Proton nuclear magnetic resonance spectra (1H NMR and 13C NMR) were obtained at 400 MHz using a Bruker DPX 400 spectrometer (Bruker, Germany). Spectra were recorded in CDCl3 and DMSO solutions, and TMS was used as the internal standard. ESI-MS were recorded on a Hybrid Quadrupole-TOF Mass Spectrometer. Multifunctional Enzyme Marker (SpectraMax M2, Meigu Molecular Instruments Co., Ltd., Shanghai, China) was selected to measure the activities during the experiment.
2.2.1 Formylcamphene 2 was Synthesized by Vilsmeier–Haack Formylation Reaction
In a three-necked flask, DMF (90–100 mL) was added at low temperature and then POCl3 (74 mL) was dropped through a drip funnel under stirring conditions to maintain the temperature below 10°C. After dropping, the temperature was increased to 60°C–70°C, and 105 g camphene dissolved in 1, 2-dichloroethane solution (40 mL) was slowly dropped into the bottle through the drop funnel under stirring conditions. The mixture was stirred at 80°C–85°C for additional 8 h under condensation and circumfluence conditions. At the end of the reaction, 200–300 g cold water was added and cooled to separate the organic layer. Then saturated NaOH was added to neutralize the water layer to pH 7–8. The organic layer was extracted with toluene, and washed with saturated salt water (3 ×, 100 mL). (E) ω-formylcamphene 2 was obtained by vacuum distillation.
2.2.2 Synthesis of (E) ω-Formylcamphene Thiosemicarbazone 3
In a ground Erlenmeyer flask, a solution of thiosemicarbazide (0.05 mol) was prepared in an ethanol aqueous solution (60 mL, VETOH:VH2O = 1:1). The resulting solution was stirred for 15 min at 50°C until the solid was dissolved. Then, the corresponding formylcamphene 2 (0.05 mol) was added slowly, and the mixture was stirred at 40°C–45°C for additional 24 h under condensation and circumfluence conditions. The reaction mixture was cooled and stand for 1–2 h. The reaction mixture was filtered to obtain a solid and washed with petroleum ether (3 ×, 100 mL). The crude product was recrystallized from ethanol to afford compound 3.
2.2.3 Synthesis of (E) ω-Formylcamphene-Based Thiazole Hydrazone Derivatives 5a–5n
In a ground Erlenmeyer flask, the appropriate (E) ω-formylcamphene-based thiosemicarbazone 3 (0.05 mol) and substituted 2-bromoacetophenone 4 (0.05 mol) were added to an absolute alcohol solution (60 mL). The resulting mixture was stirred at room temperature for 5–6 h. The reaction mixture was filtered to obtain a solid. The resulting solid was washed with petroleum ether (3 ×, 100 mL), filtered, and dried under vacuum to afford (E) ω-formylcamphene-based thiazole hydrazone derivatives 5.
The characterization data of the compounds (3, 5a–5n) are shown in Table 1.

2.3.1 DPPH Radical Scavenging Activity
The DPPH assays of compounds 5a–5n were performed using a method reported previously by Moussa et al. [27] with some modifications. DPPH• has an intense violet color with a maximum absorbance at 517 nm, but turns colorless as unpaired electrons are scavenged by antioxidants. Ethanol was used as the solvent to dissolve compound 5, and the final concentration was 500 μM concentration. Then, compound 5 was diluted twice to prepare five groups of concentrations. Reaction mixtures containing 50 μL of sample and 200 μL of 100 μM DPPH• (prepared in ethanol) were incubated in a dark place at 37°C for 30 min. The absorbance was measured at 517 nm (A1), and the percentage inhibition was calculated against a control. A blank sample containing 200 μL of ethanol in the DPPH• solution was prepared, and its absorbance was measured (A0). This assay uses Trolox and L-ascorbic acid as positive controls. The experiment was carried out in triplicate. The activity was determined using a microplate reader and analyzed using SPSS software to obtain IC50. Radical scavenging activity was calculated using the following formula:
2.3.2 ABTS Radical Scavenging Activity
The ABTS+ radical scavenging assays of compounds 5a–5n were performed using a previously reported method by Wołosiak et al. [28] with some modifications. ABTS was dissolved in deionized water to a concentration of 7 mM. ABTS radical cation (ABTS+•) was produced by reacting ABTS solution with 1.4 mM potassium persulfate and allowing the mixture to stand in the dark at 4°C for 12–16 h before use. For the study, the ABTS+• solution was diluted in ethanol to an absorbance of 0.70 ± 0.02 at 734 nm to form the test reagent. Ethanol was used as the solvent to dissolve compound 5, and the final concentration was 500 μM concentration. Then, compound 5 was diluted twice to prepare five groups of concentrations. Reaction mixtures containing 50 μL of sample and 200 μL of reagent were incubated at room temperature for 30 min, and the absorbance reading was taken after the initial mixing (A1). A sample blank reading using ethanol was also taken (A0). All the solutions were used on the day of preparation, and all determinations were carried out in triplicate. This assay used Trolox and L-ascorbic acid as positive controls. The percentage of inhibition of ABTS+• was calculated using Eq. (1). The activity was determined using a microplate reader and analyzed using SPSS software to obtain IC50.
3.1 Structural Characterizations
The synthetic route of (E) ω-formylcamphene-based thiazole hydrazone derivatives is shown in Fig. 1. In this experiment, (E) ω-formylcamphene 2 obtained by the Vilsmeier–Haack formylation reaction of camphene 1 was used as the raw material, and (E) ω-formylcamphene-based thiosemicarbazone 3 was obtained by condensation reaction. Then, the condensation of compound 3 with differently substituted 2-bromoacetophenone 4 afforded the target (E) ω-formylcamphene-based thiazole hydrazone derivatives 5a–5n.
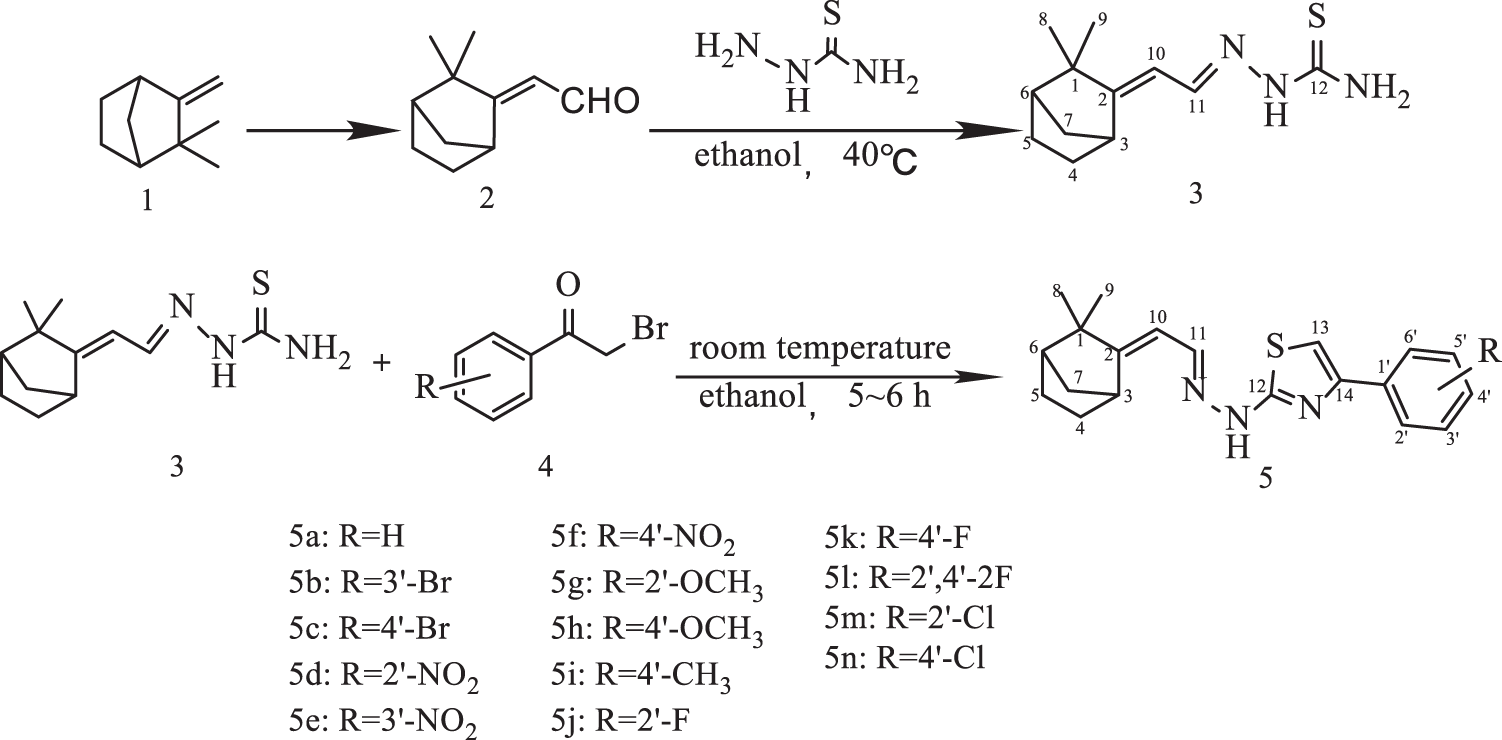
Figure 1: Synthesis of (E) ω-formylcamphene-based thiazole hydrazone derivatives
The structure of synthesized compounds (5a–5n) was determined by IR, 1H NMR, 13C NMR, and HR-MS analyses. IR spectrum of compound 3 showed a strong vibration absorption peak at 3425, 3257, and 3131 cm−1, which can be attributed to N-H and NH2 groups. In the IR spectra of 14 compounds 5a–5n, only one absorption peak was observed in the range of 3362–3432 cm−1, which is the vibrational absorption of NH bond in these compounds, and the peak for the absorption of NH2 group completely disappeared. All the 15 compounds have C=N structure with strong absorption peaks near 1620 cm−1 and weak absorption peaks from 3020 cm−1 to 3150 cm−1. They are the vibrational absorption of 10-CH, 11-CH, 13-CH, and benzene ring CH. Because a nitro (NO2) group is present on the benzene ring of 5d, 5e, and 5f molecules, two absorption peaks at 1526 and 1345 cm−1 appeared on the IR spectrum; 5g and 5h showed absorption peaks at 1250 and 1020 cm−1, which are the characteristic peaks of methoxy (OCH3) group attached to a benzene ring; 13 compounds 5b–5n showed a clear absorption peak in the range of 740–850 cm−1, indicating the presence of one (or two) substituent(s) on the benzene ring of thiazole hydrazone compounds. In the 1H NMR spectra, each compound showed the signals for 10-CH, 11-CH, and NH groups (5.8, 8.2, 12–13 ppm), two single-peak absorption signals for 8-CH3 and 9-CH3 (1.05–1.20 ppm), and proton signals of benzene ring at 6.9–8.6 ppm. The displacement values were different with different substituents on the ring. In the 13C NHR spectra, different carbon atoms in each compound showed different absorption signals. In the HR-MS spectra, all compounds showed M+H and M–H values, consistent with the molecular formula.
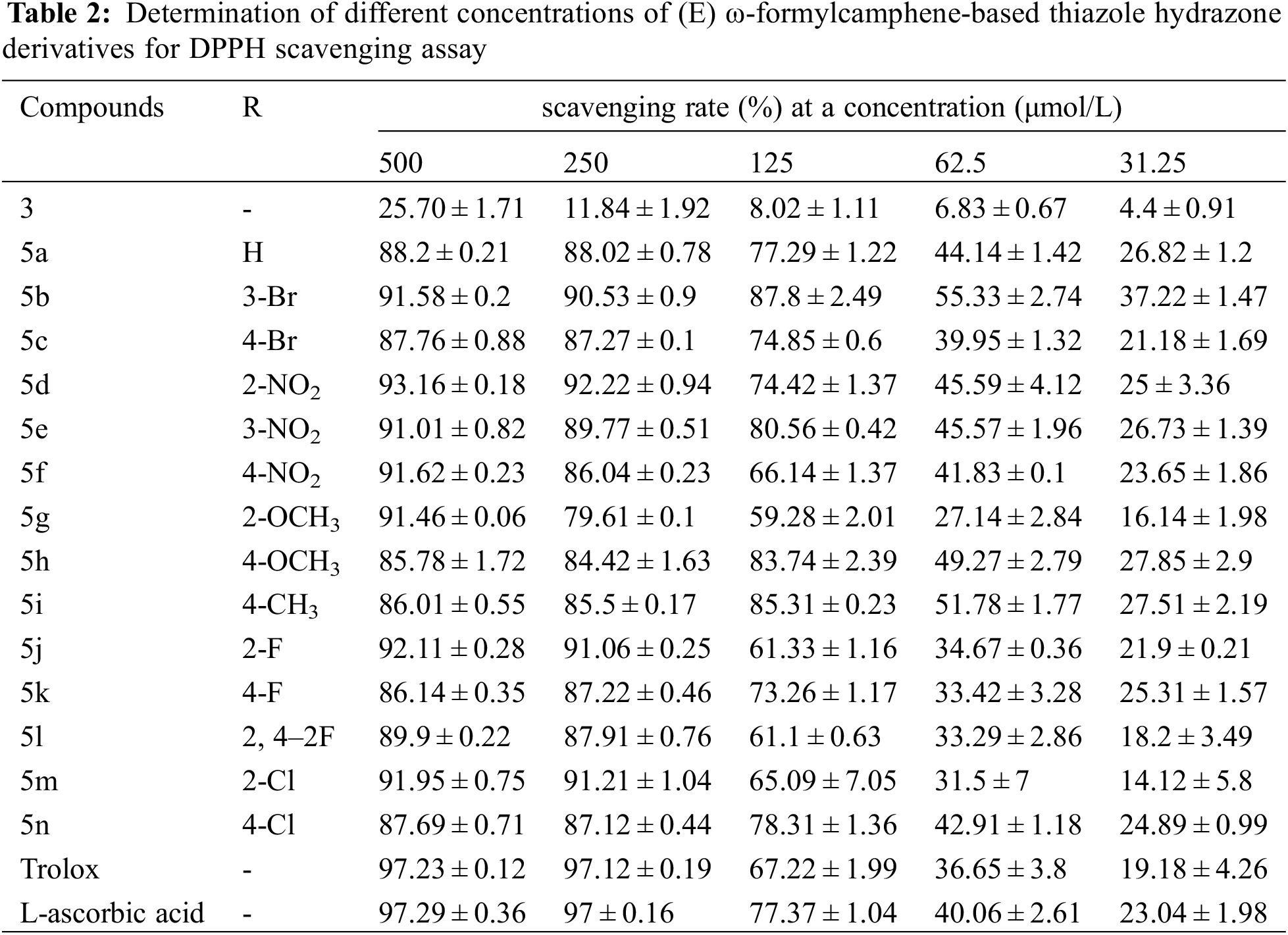
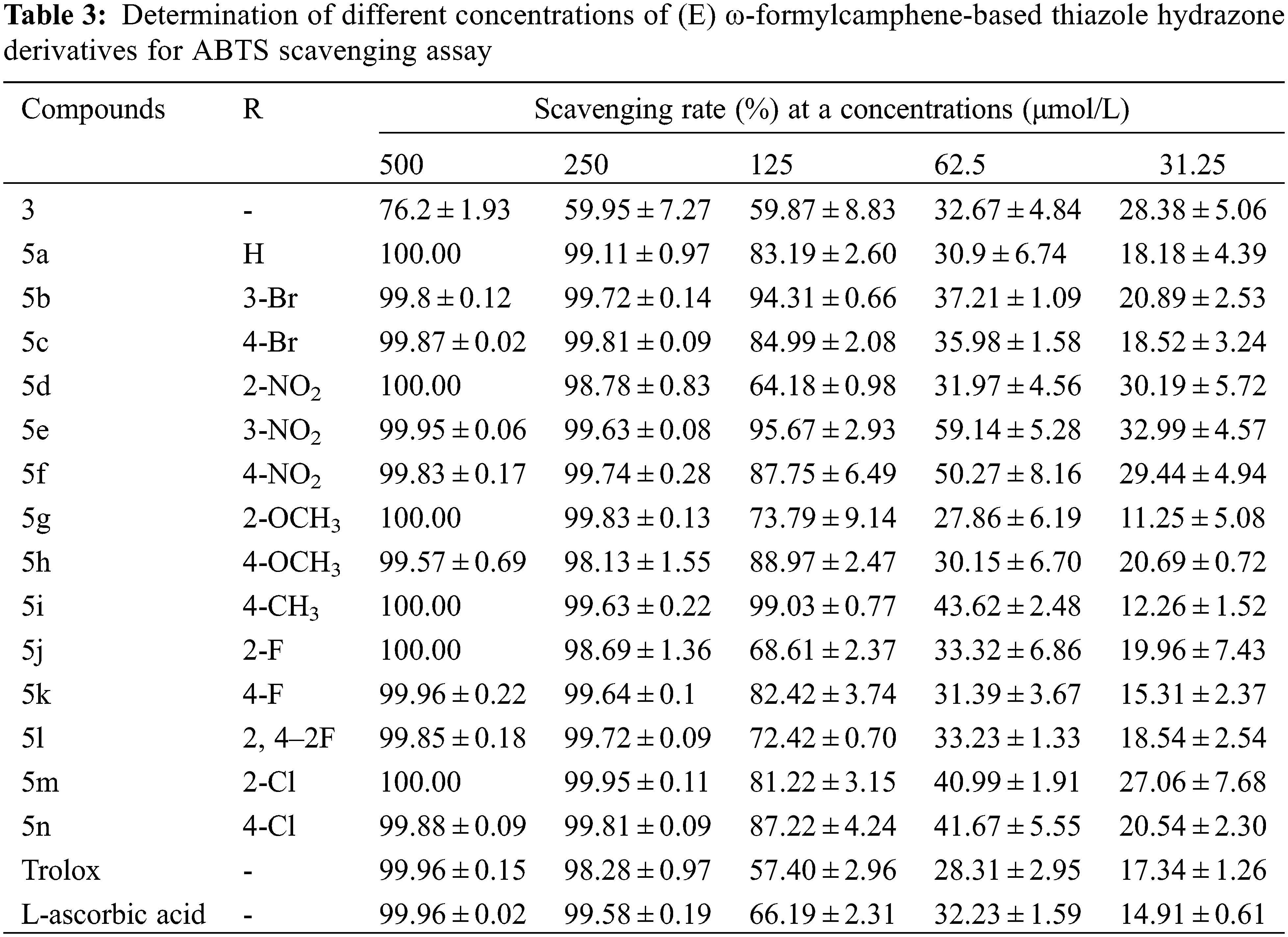
The scavenging rates of compounds 3, 5a–5n and the control samples Trolox and L-ascorbic acid on DPPH and ABTS free radicals at five different concentrations are shown in Tables 2 and 3.
3.2.1 DPPH Radical Scavenging Activity
Table 2 shows that the DPPH radical scavenging rates of (E) ω-formylcamphene-based thiazole hydrazone compounds at the concentrations of 500 mg/L and 250 mg/L are slightly lower than those of the control samples, but both were above 85% (except 5g and 5h: 79.6% and 84.4%). When the drug concentration decreased to 125 mg/L, the scavenging rates of 5b, 5e, 5 h, and 5i were still above 80%, higher than L-ascorbic acid (77.3%). The scavenging rates of 5a, 5c, 5d, 5k, and 5n were also over 73%, close to L-ascorbic acid and higher than Trolox (67%). At a concentration of 62.5 mg/L, the DPPH free radical scavenging rates of eight compounds (5a, 5b, 5d, 5e, 5f, 5 h, 5i, and 5n) were still above 40%, higher than those of L-ascorbic acid (40%) and Trolox (36.6%). These results indicate that the synthesized thiazolylhydrazone compounds exhibited good scavenging effect on DPPH free radical.
3.2.2 ABTS Radical Scavenging Activity
Table 3 shows that when the concentration of the drug solution was 500 and 250 mg/L, the scavenging rates of 14 thiazolylhydrazone compounds against ABTS free radicals were all above 98%, most of them were higher than 99.6%. Among them, the scavenging rates of 5a, 5d, 5 g, 5i, 5j, and 5 m at 500 mg/L were as high as 100%, better than those of the two control samples. At a concentration of 125 mg/L, the scavenging rates of 5b, 5i, and 5e were still over 94%, and the scavenging rates of 5a, 5e, 5f, 5 h, 5k, 5 m and 5n were still above 80%. The scavenging rates of the remaining four compounds were higher than those of two control samples (66% and 57.4%).
According to the free radical scavenging rates listed in Tables 2 and 3, the IC50 values of the two free radical scavenging effects of the compounds fitted using SPSS software are shown in Table 4. The IC50 values of 5a, 5b, 5d, 5e, 5 h, and 5i were less than 65.2 μmol/L in the DPPH radical scavenging test, better than L-ascorbic acid (66.27 μmol/L). Also, 5e, 5f, 5k, and 5n were superior to Trolox (75.74 μmol/L). Among the ABTS radical scavenging activities, compounds 5e (45.8 μmol/L) and 5f (52.82 μmol/L) had the lowest IC50 values, while compounds 5b, 5iand 5 m had lower IC50 values than 61 μmol/L. Moreover, other compounds were also better than Trolox (86.21 μmol/L) and L-ascorbic acid (79.70 μmol/L).
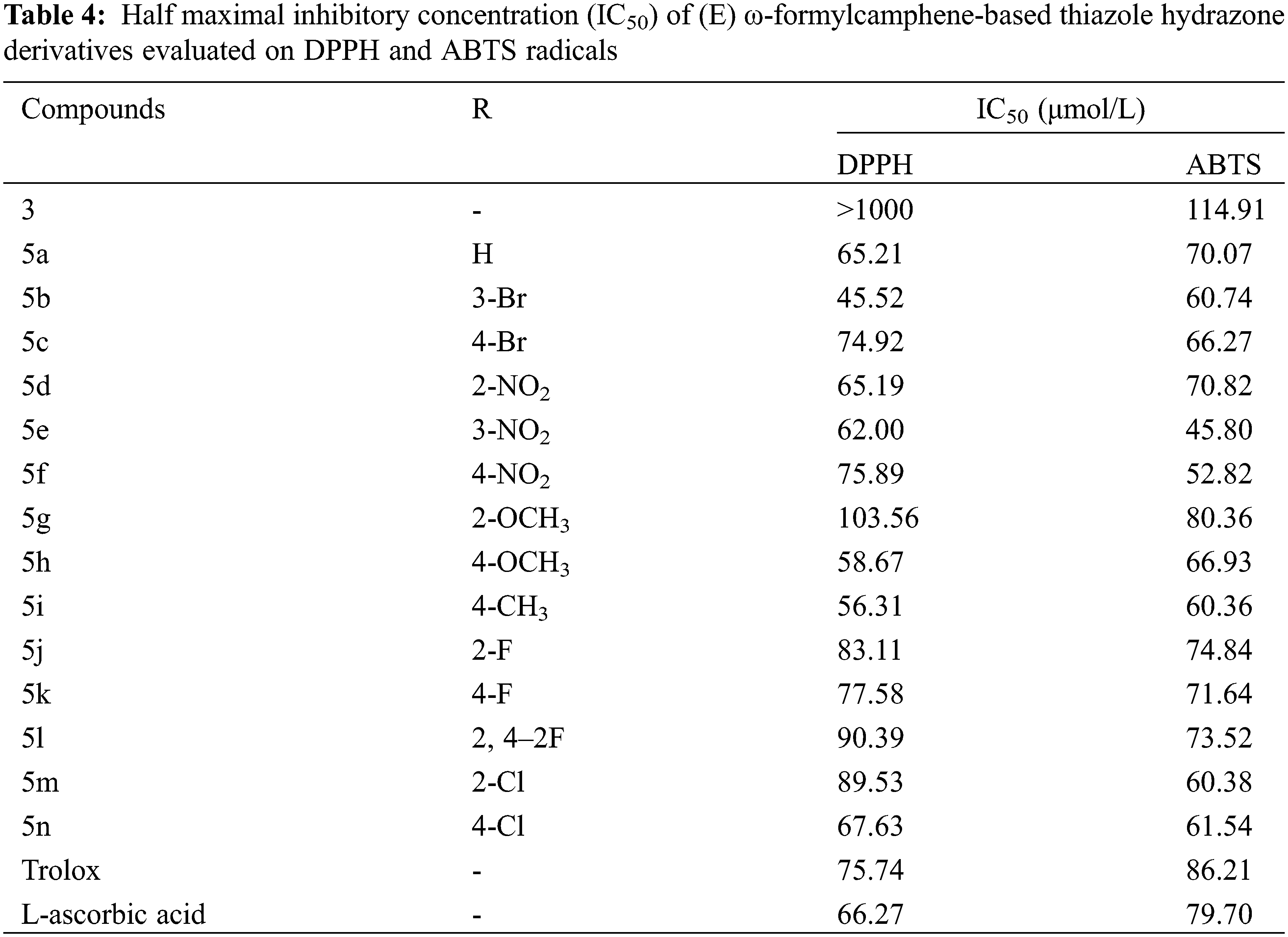
The results of antioxidant activity test show that the thiazole hydrazone derivatives that were synthesized by the blend of thiazolyl ring and hydrazone exerted better antioxidant activity on DPPH and ABTS free radical scavenging. All thiazole hydrazone derivatives have stronger antioxidant activity than (E) ω-formylcamphene thiosemicarbazone 3. Some derivatives with different substituents exhibited moderate-to-significant antioxidant activity. For DPPH free radical, the four most potent compounds in the increasing order were 5e < 5h < 5i < 5b. For ABTS free radical, the four most potent compounds in the increasing order were 5m < 5i < 5f < 5e. This result was similar to our previous research, which demonstrated that thiazolyl ring structure and hydrazone structure are key structures for exerting antioxidant activity [29,30], and the substituted aromatic groups can capture free radicals through the degradation of potential aromatic structures [31].
In this study, 14 (E) ω-formylcamphene thiazolylhydrazone compounds were synthesized by the condensation of (E) ω-formylcamphene with thiosemicarbazide and reaction with 14 α-bromoacetophenones. The structures of all compounds were characterized by IR, 1H NMR, 13C NMR, and HR-MS analyses. The scavenging rates of 14 compounds against two free radicals were determined by DPPH and ABTS free radical scavenging tests, and the IC50 values for scavenging the two free radicals were fitted using SPSS software. The results of antioxidant activity tests show that 14 compounds had good scavenging effects on the two free radicals; especially at the concentration of 125 and 62.5 mg/L, the scavenging rate and IC50 values were better than the positive controls Trolox and L-ascorbic acid. In addition, the new compounds exhibited excellent antioxidant activity in vitro. At the same time, from the experimental results, it is very meaningful to synthesize (E) ω-formylcamphene thiazole hydrazone derivatives from (E) ω-formylcamphene thiosemicarbazide.
Acknowledgement: This work is supported by the National Natural Science Foundation (No. 31960295), Jiangxi Province Academic and Technical Leaders Training Program Leading Talents Project (20204BCJ22022), Special Funding for Major Scientific and Technological Research and Development in Jiangxi Province (20203ABC28W016).
Funding Statement: This research was funded by the National Natural Science Foundation (No. 31960295), Jiangxi Province Academic and Technical Leaders Training Program Leading Talents Project (20204BCJ22022), Special Funding for Major Scientific and Technological Research and Development in Jiangxi Province (20203ABC28W016).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study
References
1. Ndlovu, G., Fouche, G., Tselanyane, M., Cordier, W., Steenkamp, V. et al. (2013). In vitro determination of the anti-aging potential of four southern african medicinal plants. BMC Complementary and Alternative Medicine, 13, 2–7. DOI 10.1186/1472-6882-13-304. [Google Scholar] [CrossRef]
2. Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L. et al. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 2018(13), 757–772. DOI 10.2147/cia.s158513. [Google Scholar] [CrossRef]
3. Basu, S. (2010). Fatty acid oxidation and isoprostanes: Oxidative strain and oxidative stress. Prostaglandins Leukot Essent Fatty Acids, 82(4–6), 219–225. DOI 10.1016/j.plefa.2010.02.031. [Google Scholar] [CrossRef]
4. Zhao, M. J., Yuan, S., Zi, H., Gu, J. M., Zeng, X. T. (2021). Oxidative stress links aging-associated cardiovascular diseases and prostatic diseases. Oxidative Medicine and, Cellular Longevity, 2021, 1–12. DOI 10.1155/2021/5896136. [Google Scholar] [CrossRef]
5. Tan, W., Li, Q., Li, W., Dong, F., Guo, Z. Y. et al. (2016). Synthesis and antioxidant property of novel 1, 2, 3-triazole-linked starch derivatives via ‘click chemistry’. International Journal of Biological Macromolecules, 82, 404–410. DOI 10.1016/j.ijbiomac.2015.10.007. [Google Scholar] [CrossRef]
6. Yuan, T., Yang, T., Chen, H., Fu, D., Hu, Y. et al. (2019). New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biology, 20, 247–260. DOI 10.1016/j.redox.2018.09.025. [Google Scholar] [CrossRef]
7. Nascimento, V., Ferreira, N. L., Canto, R. F., Schott, K. L., Waczuk, E. P. et al. (2014). Synthesis and biological evaluation of new nitrogen-containing diselenides. European Journal of Medicinal Chemistry, 87, 131–139. DOI 10.1016/j.ejmech.2014.09.022. [Google Scholar] [CrossRef]
8. Brewer, M. S. (2011). Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Comprehensive Reviews in Food Science and Food Safety, 10(4), 221–247. DOI 10.1111/j.1541-4337.2011.00156.x. [Google Scholar] [CrossRef]
9. Liu, Z. Q. (2019). Bridging free radical chemistry with drug discovery: A promising way for finding novel drugs efficiently. European Journal of Medicinal Chemistry, 189, 112020. DOI 10.1016/j.ejmech.2019.112020. [Google Scholar] [CrossRef]
10. Vuong, T. V. (2021). Natural products and their derivatives with antibacterial, antioxidant and anticancer activities. Antibiotics, 10(1), 70. DOI 10.3390/antibiotics10010070. [Google Scholar] [CrossRef]
11. Gonzalez-Baro, A. C., Izquierdo, D., Heras, A., Colina, A. (2020). UV/vis spectroelectrochemistry of o-vanillin: Study of the antioxidant properties. Journal of Electroanalytical Chemistry, 859, 113844. DOI 10.1016/j.jelechem.2020.113844. [Google Scholar] [CrossRef]
12. Kim, M. R. (2021). Antioxidants of natural products. Antioxidants, 10(4), 612. DOI 10.3390/antiox10040612. [Google Scholar] [CrossRef]
13. Cutillas, A. B., Carrasco, A., Martinez-Gutierrez, R., Tomas, V., Tudela, J. et al. (2018). Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosystems, 152(6), 1282–1292. DOI 10.1080/11263504.2018.1445129. [Google Scholar] [CrossRef]
14. Yi, F. P., Mao, H. F. (2016). Synthetic spice technology (second edition). Beijing: China Light Industry Press. [Google Scholar]
15. Naoufal, E. H., Tariq, A., Naoual, E. M., Aicha, E. B., Nasreddine, E. O. et al. (2021). In vitro and in vivo biological investigations of camphene and its mechanism insights: A review. Food Reviews International, 1–28. DOI 10.1080/87559129.2021.1936007. [Google Scholar] [CrossRef]
16. Xiao, Z. Q., He, Y. H. (1993). Reaction research and application in synthesis of camphenal (IIICatalytic hydrogenation. Journal of Jiangxi Normal University (Natural Science Edition), 17(3), 11–18. [Google Scholar]
17. Weng, Y. H., Xiao, Z. Q., Chen, J. Z., Fan, G. R., Wang, P. et al. (2015). Research progress on synthesis of camphene derivatives. Guangzhou Chemical Industry, 43(21), 16–21. DOI 10.3969/j.issn.1001-9677.2015.21.005. [Google Scholar] [CrossRef]
18. Yang, L. J., Liu, H. C., Xia, D. S., Wang, S. F. (2020). Antioxidant properties of camphene-based thiosemicarbazones: Experimental and theoretical evaluation. Molecules, 25(5), 1192. DOI 10.3390/molecules25051192. [Google Scholar] [CrossRef]
19. Feng, X. Z., Xiao, Z. Q., Wang, Z. D., Fan, G. R., Chen, J. Z. (2020). The synthesis and antibacterial activity of E-type camphene aldoxime and E-type camphene nitrile. Journal of Jiangxi Normal University (Natural Science Edition), 44(2), 4. [Google Scholar]
20. Popat, M. J., Srinivas, K., Atam, B. T., Sheshanath, V. B., Rajendra, P. P. (2021). A review on biological and medicinal significance of thiazoles. Phosphorus, Sulfur, and Silicon and the Related Elements, 196(10), 879–895. DOI 10.1080/10426507.2021.1945601. [Google Scholar] [CrossRef]
21. Swarnagowri, N., Santhosh, L. G. (2019). A review on recent synthetic strategies and pharmacological importance of 1,3-thiazole derivatives. Mini-Reviews in Medicinal Chemistry, 19(3), 215–238. DOI 10.2174/1389557518666180816112151. [Google Scholar] [CrossRef]
22. Md., S. S., Mohammad, M. R., Md., D. I., Abdullah, A., Junaid, U. A. et al. (2022). Synthesis, antimicrobial and antioxidant evaluation with in silico studies of new thiazole schiff base derivatives. Journal of Molecular Structure, 1248, 131465. DOI 10.1016/j.molstruc.2021.131465. [Google Scholar] [CrossRef]
23. Abdel-Galil, E., Girges, M. M., Said, G. E. (2020). Synthesis and biological studies of some new thiazoles, hydrazones and azine derivatives based on 3, 5-diphenylcyclohex-2-en-1-one. ChemistrySelect, 5(10), 3075–3079. DOI 10.1002/slct.201904770. [Google Scholar] [CrossRef]
24. Adole, V. A., More, R. A., Jagdale, B. S., Pawar, T. B., Chobe, S. S. (2020). Efficient synthesis, antibacterial, antifungal, antioxidant and cytotoxicity study of 2-(2-hydrazineyl) thiazole derivatives. ChemistrySelect, 5(9), 2778–2786. DOI 10.1002/slct.201904609. [Google Scholar] [CrossRef]
25. Mohareb, R., Khalil, E., Mayhoub, A., Abdallah, A. (2019). Novel synthesis of pyran, thiophene, and pyridine derivatives incorporating thiazole ring and their antitumor evaluation. Journal of Heterocyclic Chemistry, 57(3), 1330–1343. DOI 10.1002/jhet.3870. [Google Scholar] [CrossRef]
26. Chen, Z., Duan, W., Lin, G., Zhang, R., Luo, M. et al. (2017). Synthesis and antifungal activity of novel myrtenal-based thiazole-hydrazone compounds. Scientia Silvae Sinicae, 53(12), 93–101. DOI 10.11707/j.1001-7488.20171210. [Google Scholar] [CrossRef]
27. Moussa, I., Khiari, R., Moussa, A., Mortha, G., Mhenni, M. F. (2019). Structural characterization and antioxidant activity of lignin extracted from Ficus carica L. Journal of Renewable Materials, 7(4), 345–354. DOI 10.32604/jrm.2019.04011. [Google Scholar] [CrossRef]
28. Wołosiak, R., Drużyńska, B., Derewiaka, D., Piecyk, M., Majewska, E. et al. (2021). Verification of the conditions for determination of antioxidant activity by ABTS and DPPH assays—A practical approach. Molecules, 27(1), 50. DOI 10.3390/molecules27010050. [Google Scholar] [CrossRef]
29. Shih, M. H., Ke, F. Y. (2004). Synthesis and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorganic & Medicinal Chemistry, 12(17), 4633–4643. DOI 10.1016/j.bmc.2004.06.033. [Google Scholar] [CrossRef]
30. Shih, M. H., Su, Y. S., Wu, C. L. (2007). Synthesis of aromatic substituted hydrazino-thiazole derivatives to clarify structural characterization and antioxidant activity between 3-arylsydnonyl and aryl substituted hydrazino-thiazoles. Chemical & Pharmaceutical Bulletin, 55(8), 1126. DOI 10.1248/cpb.55.1126. [Google Scholar] [CrossRef]
31. Mitra, I., Saha, A., Roy, K. (2013). Predictive chemometric modeling of DPPH free radical-scavenging activity of azole derivatives using 2D-and 3D-quantitative structure-activity relationship tools. Future Medicinal Chemistry, 5(3), 261–280. DOI 10.4155/FMC.12.207. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |