
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015645
ARTICLE
Synthesis and Characterization of Green Potassium Nanoparticles from Sideroxylon Capiri and Evaluation of Their Potential Antimicrobial
1Tecnológico Nacional de México, Instituto Tecnológico de Tuxtla Gutiérrez, Tuxtla, Gutiérrez, C.P. 03940, México
2Instituto de Ciencias Agrícolas de la Universidad Autónoma de Baja California (ICA-UABC), Ejido Nuevo León, C.P. 21705, México
3Instituto de Ingeniería de la Universidad Autónoma de Baja California, Mexicali, 21100, México
*Corresponding Author: Daniel Gonzalez-Mendoza. Email: danielg@uabc.edu.mx
Received: 01 January 2021; Accepted: 19 February 2021
Abstract: In the present study, the green synthesis of potassium nanoparticles (K-NPs) was assessed using aqueous extract of Sideroxylon capiri. The potassium nanoparticles were analyzed by UV-visible spectroscopic techniques, X-ray spectrometers of energy dispersive (SEM-EDS) and dynamic light scattering. The results showed high values at 3.5 keV confirming the formation of potassium nanoparticles and the SEM analysis showed an agglomerated particles size between 360 to 200 nm with a spherical morphology. The K-NPs showed an effective antibacterial activity against the test organisms mainly with Bacillus cereus, Enterobacter aerogenes, Fusarium solani and Botrytis cinerea. However further studies about nanotoxicity of K-NPs are needed to confirm their potential in the control of the pathogen microorganisms under field conditions.
Keywords: Antimicrobial effect; green synthesis; Sideroxylon capiri; biocontrol
The bionanotechnology offers tools to improve the productivity of field across of the incorporation of biological molecules in agronomy sciences. Green synthesis has great relevance due at elimination of toxic chemicals and the use of biological compounds from plants for reduction and capping of metallic nanoparticles [1]. Diverse studies have shown that green nanoparticles can be used as nanopesticides, nanoherbicides or nanofertilizer to increase the productivity of crops and the protection against several insect pest and microbial diseases [2,3]. Different nanoparticles from different sources metallic (e.g., Cu, Ag, Au, etc.) are considered as the most promising due to their properties such as antibacterial, antifungal, and antiviral activities which can be incorporated into agroindustry [4]. However, Ag-nanoparticles in organisms can caused stress oxidative into cells and interfere with various metabolic processes [5,6]. Similar results reported by Mortezaee et al. [7] mention that most metallic nanoparticles can directly produce free radicals through the release of metal ions and through interactions with water molecules. Therefore, the use of metals with minor toxicity in the green synthesis of nanoparticles are necessary to help overcome resistant microorganisms. In this sense, some studies showed that potassium chloride has an antimicrobial effect on pathogenic microorganisms (e.g., Staphylococcus aureus, Listeria monocytogenes and Escherichia coli) without affecting probiotic bacteria [8,9]. Dong et al. [10] and Sidorov et al. [11] mention the simple preparation of potassium nanoparticles using different physic methods (anti-solvent precipitation and electron bombardment). However, studies about the use of plants such as Sideroxylon capiri (tempisque) for the synthesis of potassium nanoparticles as a green chemistry method are scarce. Sideroxylon capiri is a tree native to Mexico, which leaves, and fruits are used as a food condiment and the rural population in traditional medicine as an antiseptic for cleaning wounds. Currently, S. capiri have been the focus of scientific interest mainly because of their significant content of total phenols, flavonoids, antioxidant activity in fruits and to treat kidney diseases [12].Therefore in this study we report the first synthesis of potassium nanoparticles by Sideroxylon capiri extracts and the evaluation of their bactericidal and antifungal activities against Bacillus cereus, Enterobacter aerogenes, Fusarium solani and Botrytis cinerea causing of spoilage of fresh fruits and vegetables.
2.1 Preparation of Plant Material
The research was carried out in the biotechnology laboratory of the Institute of Agricultural Sciences of the Autonomous University of Baja California, Mexico, in the period from January to July 2019. The leaves were collected at the property called Rancho “El Capricho,” municipality of Suchiapa, Chiapas (16° 13′ N, 93° ′ W). Samples of fresh and healthy leaves of Sideroxylon capiri were collected from a native population in Chiapas, Mexico. Afterwards fresh leaves were pulverized in an analytical mill, tamized (mesh 24), and 10 g were mixed with 100 mL distilled water. Then mixture was kept in agitation to 2.5 g for 24 h at constant temperature (60°C). To potassium nanoparticles (K-NPs) synthesis, 10 mL of aqueous extract of S. capiri was mixed with 40 mL of 10 mM solution of KCl in 100 mL Erlenmeyer flask and heated at 60°C for 30 min. The absorbance spectra of samples obtain were recorded at wavelengths in the range from 200 to 500 nm with 5 nm increments using a UV VIS Spectrophotometer, (DR6000™ USA). The process of bioproduction of K-NPs, was determinate by the color change in the reaction mixture (metal solution + aqueous extract of S. capiri) at pH 5.0. Afterwards, the K-NPs were centrifugate at 11200 g for 10 min and washed with sterile distilled water. Finally, the samples were transferred to freeze dryer and the powder obtain was used in microbiological assays.
2.2 Green Potassium Nanoparticles Synthesis and Characterization
Scanning electron microscope (SEM) of nanoparticles was performed using a JEOL 6010 equipped with secondary and backscattered electron detectors and EDX detectors was employed. The EDS analysis, of K-NPs were realized, according to Abdelmoteleb et al. [13]. On the other hand, to identify the molecules in S. capiri leaf extracts responsible for reducing and capping the fourier transform infrared spectral measurements (FTIR) analysis was realized. For characterization of size and zeta potential of K-NPs in solution, the dynamic light scattering (DLS) was used and the information obtain was analyzing according to Ruiz-Romero et al. [5].
50 µL of Bacillus cereus and Enterobacter aerogenes, were inoculated on the surface of nutrient agar plates. Subsequently, 20 μl of K-NPs solution with 100 mg/mL were prepared in distilled water and was added to a 5 mm sterile filter paper discs and allowed to dry. K-NPs containing discs were placed in plate and another paper discs were embedded with only leaf extract (control). The plates were incubated at 30 ± 2°C for 24. After this period, it was possible to observe inhibition zone, which appeared as a clear area around the disks. To determine the antifungal potential of K-NPs, 100 mg/mL was incorporated into potato dextrose agar medium (PDA) and then 5 mm-diameter-PDA plugs with pathogen mycelium (Fusarium solani, Fusarium oxisporum and Botritys cinerea) were transferred to the center of the PDA + K-NPs Petri dish. The PDA without any nanoparticles served as the positive control (only leaf extract), negative control (discs with only water) and control absolute (KCl solution), respectively. Three replications were maintained for each treatment and all the inoculated Petri dishes were incubated at 28 ± 1°C. The radial growth of the test fungus was measured in all the treatments after three days and compared with the negative control. The percent inhibition of fungal growth was estimated according to Abdelmoteleb and Gonzalez-Mendoza [14].
One analysis of variance (ANOVA) was determined using Statistica software version 9.0 and the Tukeý test (p ≤ 0.05) was calculated.
K-NPs of clear color showed a peak of absorption at 270 nm (Figs. 1a, 1b). According to previous studies, the nanoparticles have different absorption peaks, silver, and copper nanoparticles, are in the range of 450–560 nm [15,16]. In this sense our studies confirmed that the synthesized K-NPs from S. capiri are formed in the range of 270–280 nm.
3.2 Infrared Spectra (FT-IR) of S. capiri and K-NPs Analysis
The FTIR analysis of S. capiri and K-NPs are show in Fig. 2. The bands observed in aqueous extract of S. capiri showed differences in the bands 3296, 2932, 1638 and 1356 cm−1 (Fig. 2A). Our results showed phenolic and hydroxyl groups (3296 cm−1). The results to 2932 cm−1 can be related with the valence oscillations of CH and N-H bonds within the benzene ring. For aromatic amines, one band was observed at 1638 cm−1 and the bands close to 1356 cm−1 can be probably related to C-O of phenolic OH and nanoparticles formation (Fig. 2B) [5,17,18].
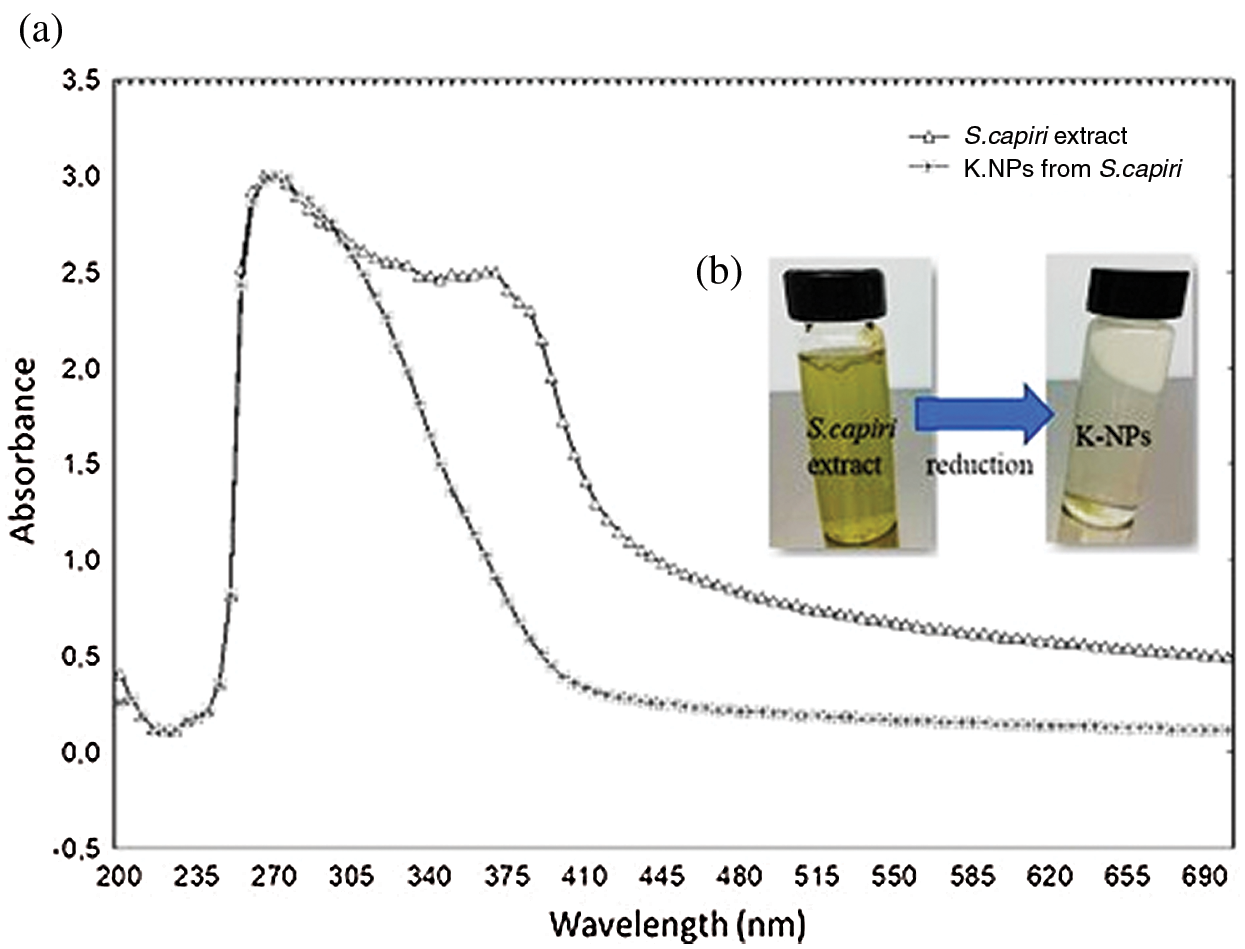
Figure 1: UV-Vis absortion spectrum of K- nanoparticles from S.capiri extract (a) and green synthesis of potassium-nanoparticles using extracts of S. capiri (b)
3.3 Scanning Electron Microscope (SEM) and EDX Analysis
The SEM analysis for K-NPs from S. capiri is present in Fig. 3A. Our results showed that the morphology of the synthesized K-NPs is near to be spherical in shape with size of 360 to 200 nm. In the Fig. 3B, show the presence of pure K (9%) followed by peaks Cl (5.33%), C (70.21%) and O (20.38%) atoms on the surface of potassium nanoparticles which might have come from the plant leaf extract according to EDX analysis [2,19]. According to the EDX result, the green synthesized K-NPs also produce a strong signal at 3.5 keV as shown in Fig. 3B, which confirms the existence of K and the organic components which are present along with the NPs [20].
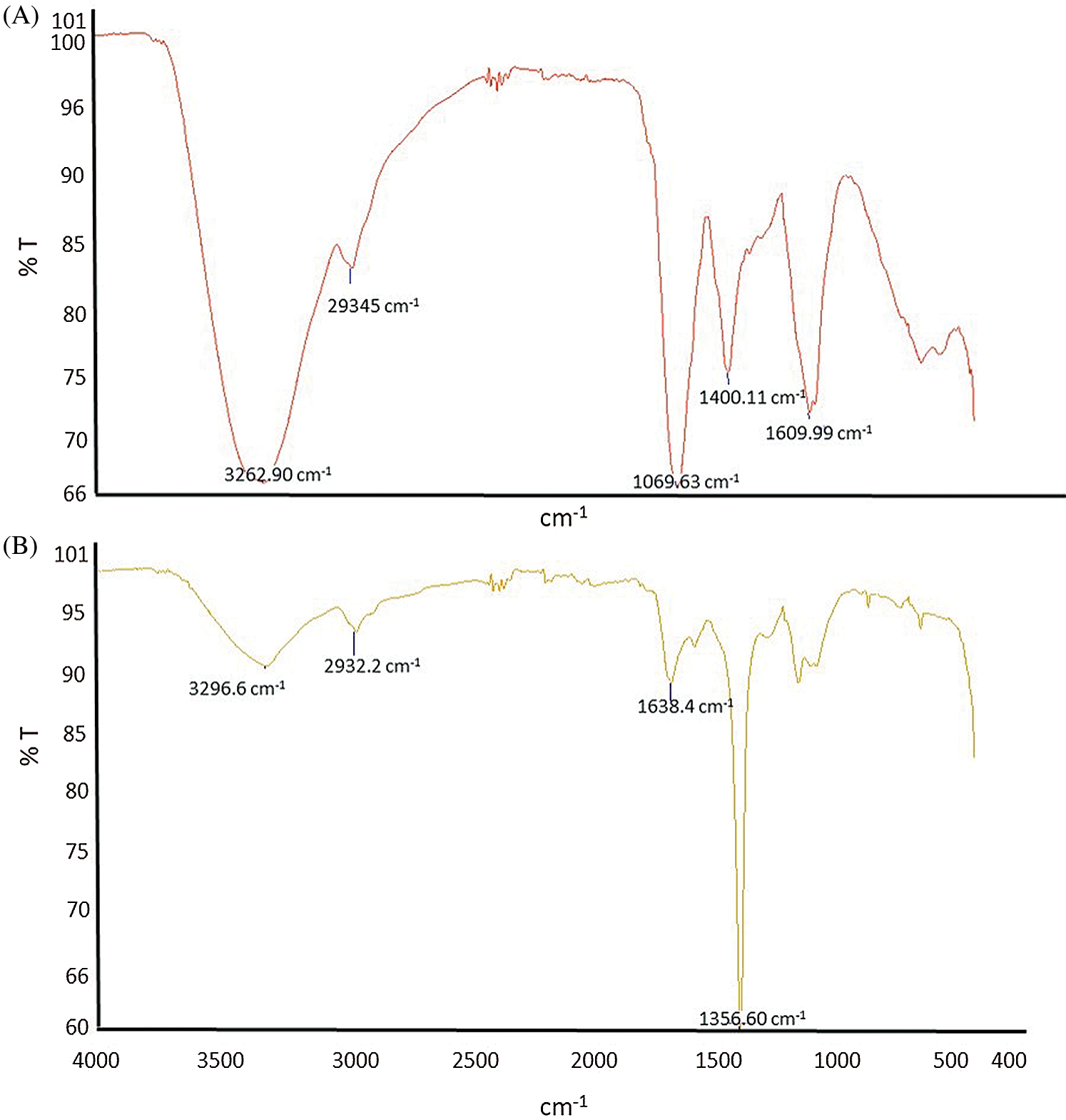
Figure 2: FTIR spectrum of aqueous extract (A) and K-NPs (B), extracted from Sideroxylon capiri.
The average hydrodynamic size of the K-NPs was determined by DLS analysis as shown in Fig. 4. The results showed a major particle size distribution peak at 24 nm. On the other hand, zeta potential (ZP) is an important property of nanoparticles because this governs the physical stability of these [21,22]. The ZP values are typically in the range of +100 to −100 mV and their magnitude is a prediction of the colloidal stability [22]. In this sense, the positive value of zeta potential (156.8 mV) for K-NPs obtained indicative a long term stability of the colloids. In reference to our studies Yu et al. [23] showed that the positive surface charge in SeNPs could be attributed to the adsorption of bioactive components (e.g., chitosan). A possible explanation for the positive value of zeta potential is the adsorption of protonated biomolecules, according to Keijok et al. [24], who observed that zeta potential of AuNPs synthesized by traditional sodium citrate chemistry and compared to green synthesis under optimal conditions with Coffea arabica showed a changed from a negative value for AuNPs with citrate to a positive value for AuNPs with Coffea arabica.
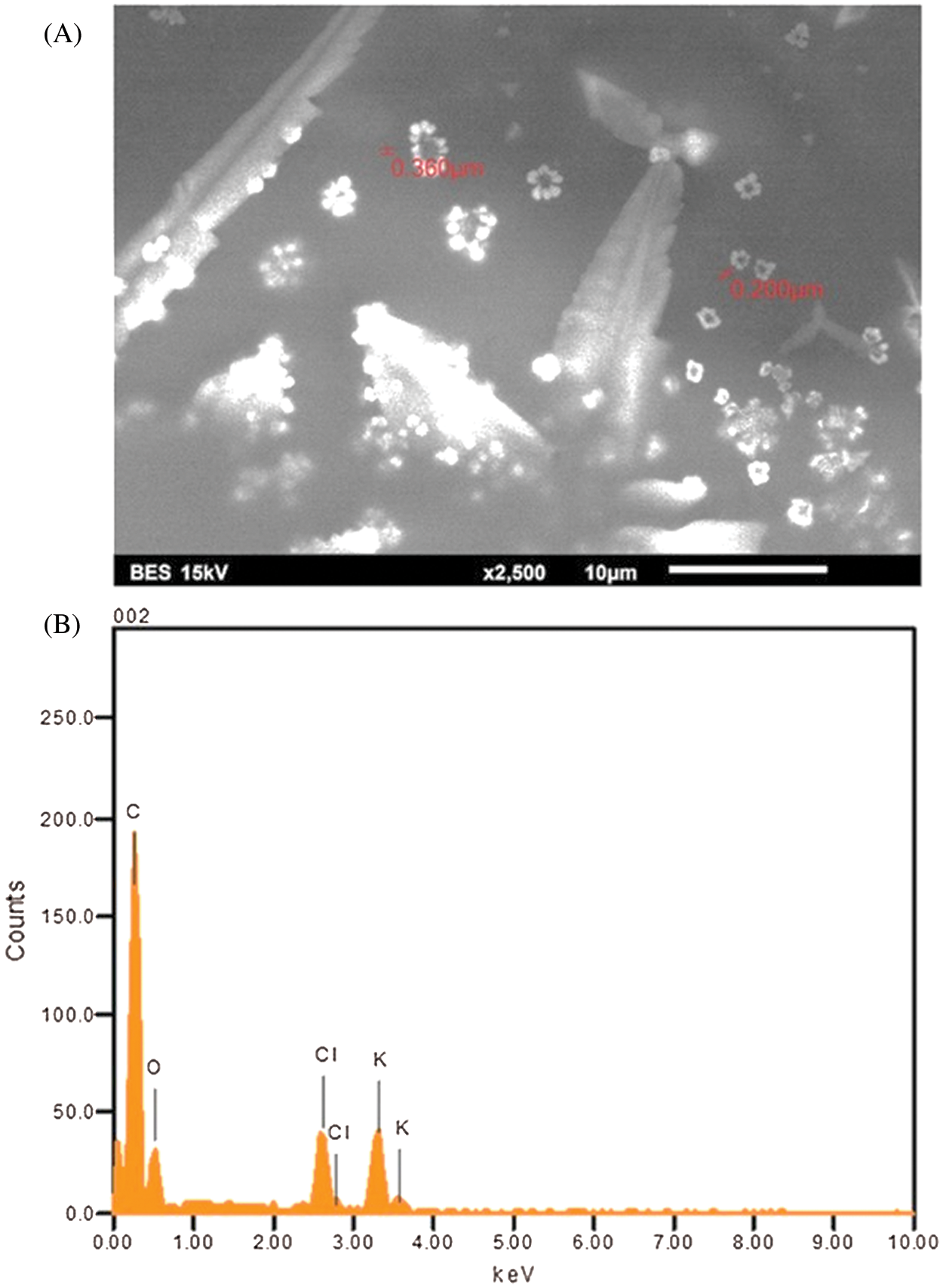
Figure 3: Scanning electron microscopy (A) and Energy dispersive X-ray spectrometer image of potassium nanoparticles produced from Sideroxylon capiri (B)
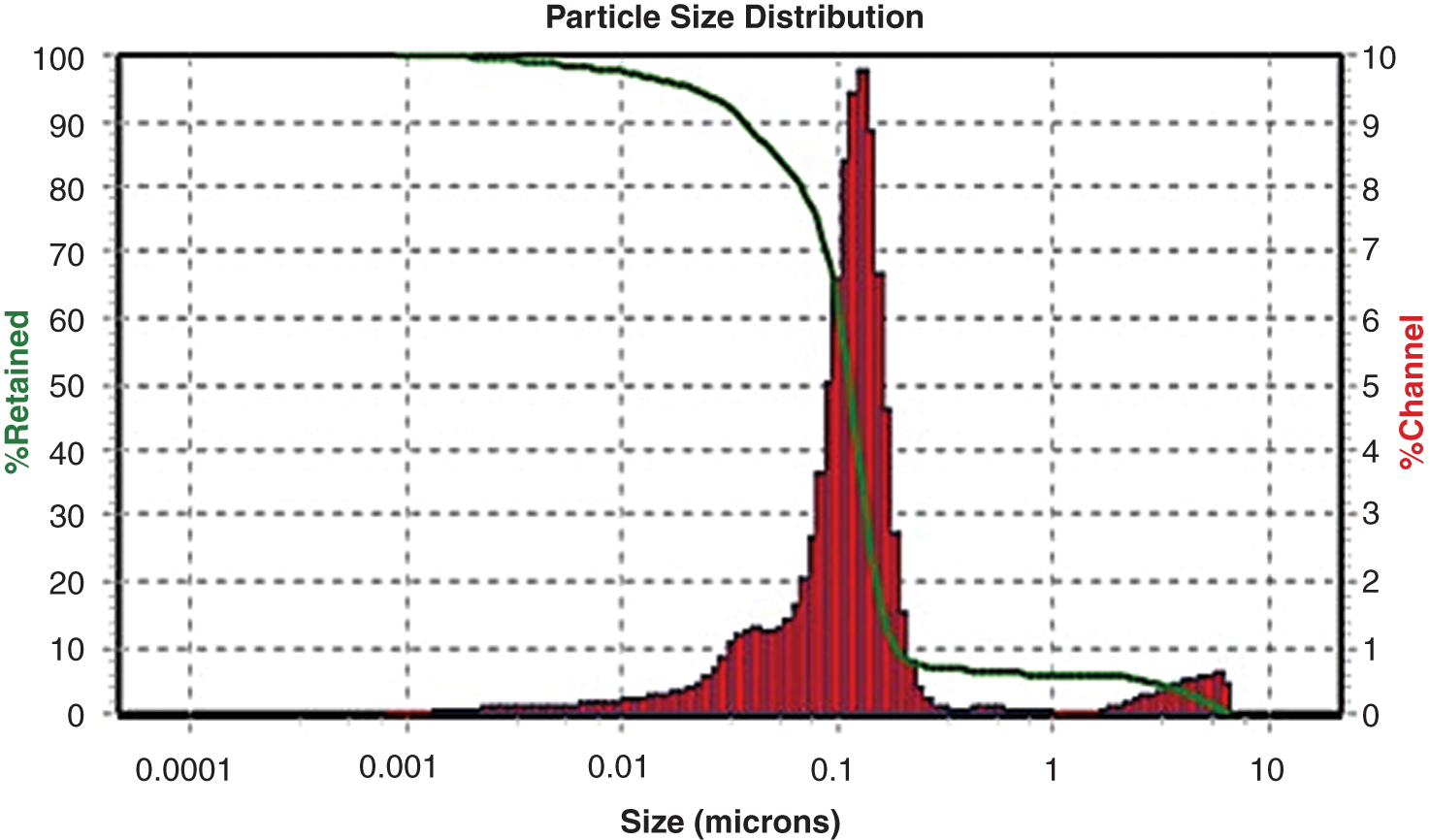
Figure 4: Particle size distribution of K-NPs from Sideroxylon capiri using dynamic light scattering measurements (DLS)
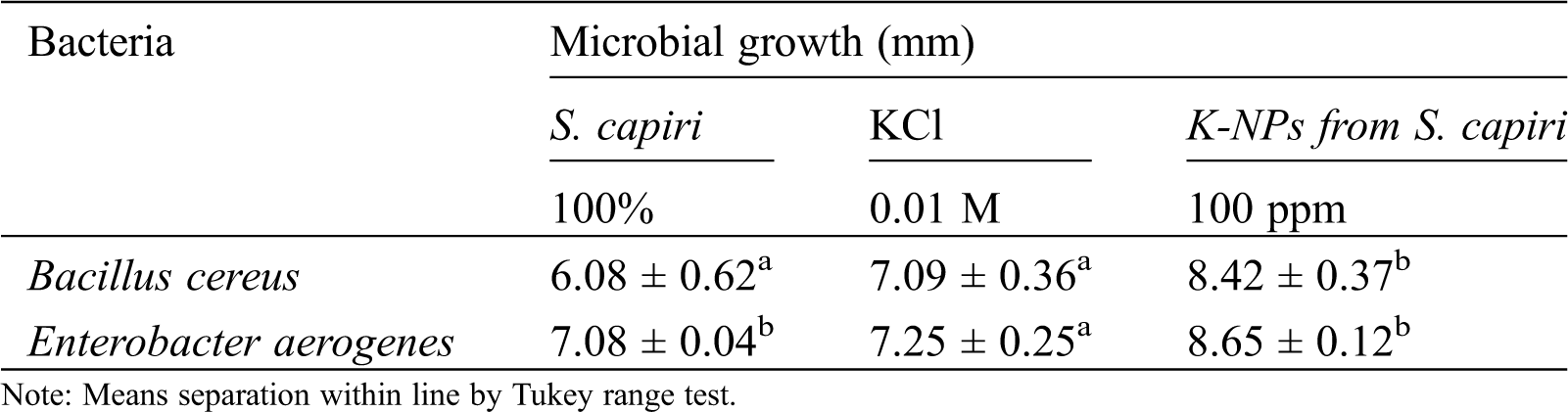
3.5 Antimicrobial Activities of Synthesized K-NPs
Tab. 1, show that both microorganisms tested were inhibited by action of K-NPs compared with only aqueous extract from S. capiri and KCl (10 mM). The inhibition can be results of toxic effects in dissolution of the outer membrane of bacterial surface by action of oxidative stress induced by reactive oxygen species (ROS) from metal/oxide nanoparticles [24].
Table 1: Effect of K-NPs from Sideroxylon capiri in microbial growth
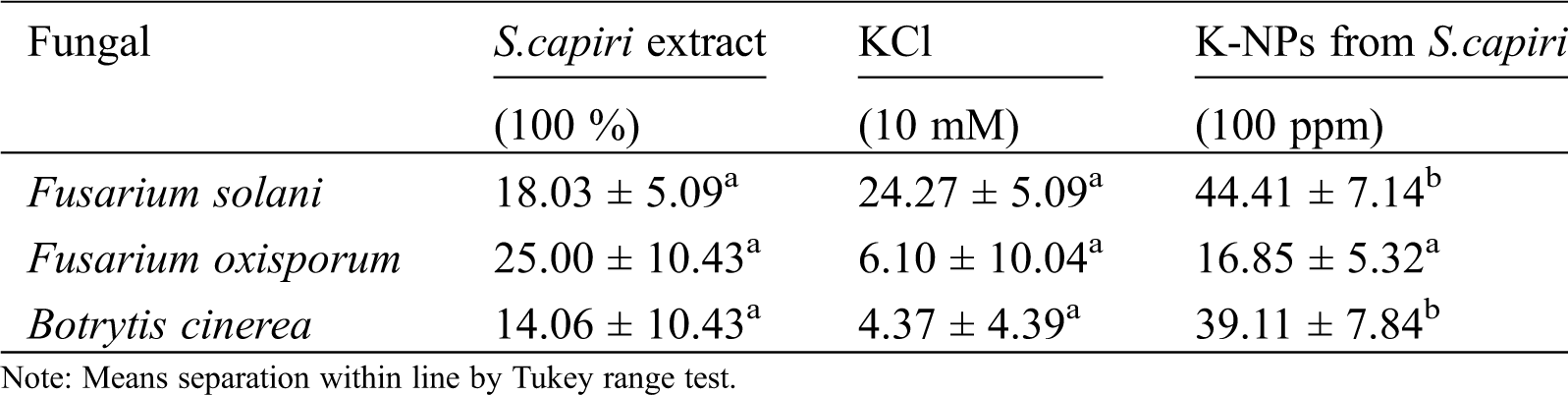
The Tab. 2, showed that mycelial growth inhibition was statistical distinct in plant extract and K-NPs of S. capiri. The K-NPs present showed a superior mycelial growth inhibition of F.solani and B.cinerea compared with only the aqueous extracts (Tab. 2). In contrast S.capiri extract showed higher inhibitory effect against F. oxisporum compared with K-NPs (Tab. 2). The exact mechanisms of metallic nanoparticles in the inhibition of phytopathogenic fungi are not yet fully explored. However certain authors reported that metallic nanoparticles can induce morphological abnormalities in the fungal mycelia and host cell contents have been linked with disorganization and deformities in mycelia hypha, and conidial structures [18]. Today synthesizing green K-NPs using aqueous extract plant has been poorly explored although has been recognized the need of further studies to confirm their potential of K-NPs from S. capiri, native plant from southwest Mexican, in the control of the pathogen microorganisms with agronomic relevance.
Table 2: Mycelial growth inhibition by K-NPs from Sideroxylon capiri
In the present study our results showed the biosynthesis of potassium nanoparticles using the aqueous extract of Sideroxylon capiri. These nanoparticles showed promising bioactive potential in the control of bacterial (Klebsiella pneumoniae, and Enterobacter aerogenes) and pathogenic fungus (Fusarium solani and Botrytis cinerea). Our study reports the feasibility of Sideroxylon capiri leaves as reducing agent for the formation of potassium nanoparticles. However, studies about the influence of reaction medium pH in the colloidal stability of the K-nanoparticles are needed to optimize the green process. Further studies about nanotoxicity of K-NPs are needed to confirm their potential in the control of the other fungal pathogens under laboratory conditions, previous studies in field.
Funding Statement: This research was funded by Universidad Autonoma de Baja California and Instituto Tecnologico de Tuxtla Gutierrez, Mexíco.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Singh, A., Gautam, P. K., Verma, A., Vishal, S., Pingali, M. et al. (2020). Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnology Reports, 25(1), e00427. DOI 10.1016/j.btre.2020.e00427. [Google Scholar] [CrossRef]
2. Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. et al. (2020). Nanotechnology in agriculture: current status, challenges and future opportunities. Science of the Total Environment, 721, 137778. DOI 10.1016/j.scitotenv.2020.137778. [Google Scholar] [CrossRef]
3. Shang, Y., Hasan, M. K., Ahammed, G. J., Li, M., Yin, H. et al. (2019). Applications of nanotechnology in plant growth and crop protection: a review. Molecules, 24(14), 2558. DOI 10.3390/molecules24142558. [Google Scholar] [CrossRef]
4. McClements, D. J. (2020). Nanotechnology approaches for improving the healthiness and sustainability of the modern food supply. American Chemical Society-Omega, 5, 29623–29630. [Google Scholar]
5. Ruiz-Romero, P., Valdez-Salas, B., González-Mendoza, D., Mendez-Trujillo, V. (2018). Antifungal effects of silver phytonanoparticles from Yucca shilerifera against strawberry soil-borne pathogens: Fusarium solani and Macrophomina phaseolina. Mycobiology, 46(1), 47–51. DOI 10.1080/12298093.2018.1454011. [Google Scholar] [CrossRef]
6. Bahadar, H., Maqbool, F., Niaz, K., Abdollahi, M. (2016). Toxicity of nanoparticles and an overview of current experimental models. Iran Biomedical Journal, 20, 1–11. [Google Scholar]
7. Mortezaee, K., Najafi, M., Samadian, H., Barabadi, H., Azarnezhad, A. et al. (2019). Redox interactions and genotoxicity of metal-based nanoparticles: a comprehensive review. Chemico-Biological Interactions, 312(1 Supplement), 108814. DOI 10.1016/j.cbi.2019.108814. [Google Scholar] [CrossRef]
8. Bidlas, E., Lambert, R. J. W. (2008). Comparing the antimicrobial effectiveness of NaCl and KCl with a view to salt/sodium replacement. International Journal of Food Microbiology, 124(1), 98–102. DOI 10.1016/j.ijfoodmicro.2008.02.031. [Google Scholar] [CrossRef]
9. Boziaris, I. S., Skandamis, P. N., Anastasiadi, M., Nychas, G. J. E. (2007). Effect of NaCl and KCl on fate and growth/no growth interfaces of Listeria monocytogenes Scott A at different pH and nisin concentrations. Journal of Applied Microbiology, 102(3), 796–805. DOI 10.1111/j.1365-2672.2006.03117.x. [Google Scholar] [CrossRef]
10. Dong, Y., Bian, X., Fu, Y., Shao, Q., Jiang, J. (2018). Simple preparation of potassium sulfate nanoparticles. Crystal Engineering Communications, 20(47), 7713–7718. DOI 10.1039/C8CE01373J. [Google Scholar] [CrossRef]
11. Sidorov, A. I., Yurina, U. V., Podsvirov, O. A. (2019). Formation of sodium and potassium nanoparticles upon local electron bombardment of alkali-halide crystals. Technical Physics, 64(7), 1017–1023. DOI 10.1134/S1063784219070223. [Google Scholar] [CrossRef]
12. Robles-García, M. A., Aguilar, A. J., Gutiérrez-Lomelí, M., Rodríguez-Félix, F., Morales del Rio, A. et al. (2016). Identificación cualitativa de metabolitos secundarios y determinación de la citotoxicidad de extractos de tempisque (Sideroxylum capiri pittier). Biotecnia, 18(3), 3–8. DOI 10.18633/biotecnia.v18i3.328. [Google Scholar] [CrossRef]
13. Abdelmoteleb, A., Valdez-Salas, B., Ceceña-Duran, C., Tzintzun-Camacho, O., Gutierrez-Miceli, F. et al. (2017). Silver nanoparticles from Prosopis glandulosa and their potential application as biocontrol of Acinetobacter calcoaceticus and Bacillus cereus. Chemical Speciation and Bioavailability, 29(1), 1–5. DOI 10.1080/09542299.2016.1252693. [Google Scholar] [CrossRef]
14. Abdelmoteleb, A., Gonzalez-Mendoza, D. (2020). A novel streptomyces rhizobacteria from desert soil with diverse anti-fungal properties. Rhizosphere, 16(12), 100243. DOI 10.1016/j.rhisph.2020.100243. [Google Scholar] [CrossRef]
15. Gonzalez-Mendoza, D., Valdez-Salas, B., Bernardo-Mazariegos, E., Tzintzun-Camacho, E., Gutierrez-Miceli, F. et al. (2019). Influence of monometallic and bimetallic phytonanoparticles on physiological status of mezquite. Open Life Science, 14(1), 62–68. DOI 10.1515/biol-2019-0008. [Google Scholar] [CrossRef]
16. Bernardo-Mazariegos, E., Valdez-Salas, B., Gonzalez-Mendoza, D., Abdelmoteleb, A., Tzintzun-Camacho, O. et al. (2019). Silver nanoparticles from Justicia spicigera and their antimicrobial potentialities in the biocontrol of foodborne bacteria and phytopathogenic fungi. Revista Argentina de Microbiologia, 51(2), 103–109. DOI 10.1016/j.ram.2018.05.002. [Google Scholar] [CrossRef]
17. González-Mendoza, D., Valdez-Salas, B., Carrillo-Beltran, M., Castro-Lopez, S., Mendez-Trujillo, V. et al. (2018). Antimicrobial effects of silver-phyconanoparticles from Sargassun vulgare against spoilage of fresh vegetables caused by Bacillus cereus, Fusarium solani and Alternaria alternate. Internatioal Journal Agriculture and Biology, 20, 1230–1234. [Google Scholar]
18. Mendez-Trujillo, V., Valdez-Salas, B., Carrillo-Beltran, M., Curiel-Alvarez, M. A., Tzintzun-Camach, O. (2019). Green synthesis of bimetallic nanoparticles from Prosopis juliflora (Sw) DC., and its effect against cotton mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Phyton-International Journal of Experimental Botany, 88, 269–275.1. [Google Scholar]
19. Leon-Jimenez, E., Valdez-Salas, B., Gonzalez-Mendoza, D., Tzintun-Camacho, O. (2019). Synthesis and insecticide activity of Cu-nanoparticles from Prosopis juliflora (Sw) DC and Pluchea sericea (Nutt.) on Phenacoccussolenopsis Tinsley (Hemiptera, Pseudococcidae). Revista de la Sociedad Entomologica Argentina, 78(2), 12–21. DOI 10.25085/rsea.780202. [Google Scholar] [CrossRef]
20. Escárcega-González, C. E., Garza-Cervantes, J. A., Vázquez-Rodríguez, A., Montelongo-Peralta, L. Z., Treviño-Gonzalez, M. T. et al. (2018). In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. International Journal of Nanomedicine, 13, 2349–2363. DOI 10.2147/IJN.S160605. [Google Scholar] [CrossRef]
21. Akhlesh, K., Thareja, J. S. (2019). In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 524–539. DOI 10.1080/21691401.2018.1561457. [Google Scholar] [CrossRef]
22. Lin, P. C., Lin, S., Wang, P. C., Sridhar, R. (2014). Techniques for physicochemical characterization of nanomaterials. Biotechnology Advances, 32(4), 711–726. DOI 10.1016/j.biotechadv.2013.11.006. [Google Scholar] [CrossRef]
23. Yu, B., Zhang, Y., Zheng, W., Fan, C., Chen, T. (2012). Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorganic Chemistry, 51(16), 8956–8963. DOI 10.1021/ic301050v. [Google Scholar] [CrossRef]
24. Keijok, W. J., Pereira, R. H. A., Alvarez, L. A. C., Ribeiro-Prado, A., da Silva, A. R. et al. (2019). Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Scientific Report, 9(1), 515. DOI 10.1038/s41598-019-52496-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |