
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015143
ARTICLE
Effect of the Proportion of Bamboo Scraps on the Properties of Bamboo Scraps/Magnesium Oxychloride Composites
College of Materials Science and Engineering, Central South University of Forestry and Technology, Changsha, 410004, China
*Corresponding Author: Yingfeng Zuo. Email: zuoyf1986@163.com
Received: 25 November 2020; Accepted: 16 December 2020
Abstract: This study was designed to solve the problem of large waste volume from bamboo processing residues in recent years. Using magnesium oxychloride (MO) cementitious material as the main material and bamboo residue (BR) as the reinforcing material, a BR/MO composite material was prepared. The effects of BR amount on the molding properties, mechanical strength, and water resistance of BR/MO composites were examined and discussed. Scanning electron microscopy (SEM), X-ray diffractometry (XRD), and thermogravimetric analysis were used to characterize composite microscopic morphology, crystalline structure, and heat resistance. The results showed that, when the BR content was 1.00% (by wt), the flowability of MO paste was beneficial to composite molding. Composite mechanical properties and water resistance were greatly affected by BR addition. When the BR content was 1.00%, composite compressive and bending strengths and softening coefficient all reached maximum values. Meanwhile, increases in water absorption by 24 h and decreases of contact angle were small. These results suggested that, when the BR content was 1.00%, composite mechanical properties and water resistance were the best and the mechanical strength also improved with extended composite storage time. SEM analysis indicated that BR played the role of a reinforcing phase in MO matrices. However, when the BR content exceeded 1.00%, interfacial bonding between BR and MO became less. XRD analysis showed that, with 1.00% BR content, composites showed more 5-phase crystals with high strength. This further explained the reason why this composite’s mechanical properties were the best and the heat resistance not deteriorated due to BR, which was easily decomposed.
Keywords: Bamboo scraps; magnesium oxychloride cementation; mechanical properties; water resistance; interface bonding; crystal structure
China has a vast area of bamboo forests and abundant bamboo resources. New bamboo products have been developed, such as bamboo plywood, laminated timber, reconstituted timber, and winding composite pressure pipe, and the bamboo industry plays a very important role in the national economy [1]. Although researchers continue to improve bamboo processing and application technology, the utilization rate of bamboo remains not ideal [2,3]. Bamboo residues produced in the process of bamboo felling and processing have not been rationally used, which not only causes a serious waste of resources but also causes serious environmental pollution. Therefore, how to use these bamboo residues in high quality and efficiency strategies has become an urgent problem in the field of comprehensive bamboo utilization. At the same time, the efficient use of bamboo residues can increase bamboo added value, which would meet the requirements of green and sustainable development in China.
The most commonly used and most effective method used in research to prepare bamboo scraps wood-based panels is by mixing bamboo residues and adhesives and hot pressing [4]. However, traditional bamboo scraps boards mostly use aldehyde-containing adhesives, such as urea-formaldehyde and phenol-formaldehyde resins [5], which not only release formaldehyde gas harmful to the human body during production and use, but also affect its application areas due to product performance, color, and other issues. In view of this, the use of abundant, safe, nontoxic, and inexpensive inorganic cementing materials as binders to produce bamboo scrap refuse (BR)/inorganic composites has become the focus of many studies [6]. Among the commonly-used inorganic cementitious materials, magnesium oxychloride (MO) cementitious materials have been most studied and their application widespread due to their huge raw material reserves and excellent performance [7]. MO cementitious material has the advantages of green environmental protection, low alkalinity, good heat insulation performance, low corrosion of plant fibers, and good composite properties [8]. However, MO cementitious materials have defects, such as poor water resistance, high brittleness, easy moisture absorption, and easy cracking [9]. In response to these drawbacks, Wang et al. [10] and Wang et al. [11] have used straw and corn fibers to enhance and modify MO inorganic adhesive, demonstrating that plant fibers can indeed enhance the mechanical properties of MO cementitious materials and water resistance. Therefore, bamboo scrap refuse (BR)/MO composites produced by mixing MO cementitious materials and bamboo residues have received increasing attention [12]. This development can not only improve the utilization rate of bamboo resources, but also provide people with green and healthy building materials, thus achieving two goals. However, BR/MO composites have problems, such as poor interface compatibility between BR and MO cementitious material, which affects composite mechanical properties. In this study, the influence of BR content on the properties of BR/MO composites was discussed and the mechanism of performance improvement discussed. The overall purpose was to prepare BR/MO composites with excellent performance and provide technical guidance and theoretical support for product applications.
Light/y-burnt magnesia, industrial grade, 200 mesh fineness, 9% sieve residue rate, containing (by % wt) 86.59 MgO, 5.43 SiO2, 1.48 CaO, 0.62 Al2O3, 0.34 Fe2O3, and 5.54 loss on ignition was obtained from Shenyang Teok Chemical Co., Ltd (Shenyang, China). Magnesium chloride hexahydrate, industrial grade, and magnesium sulfate heptahydrate, industrial grade was obtained from Shandong Weifang Jiuzhuo Chemical Co., Ltd. (Shandong, China). Bamboo scraps, including bamboo green and bamboo yellow, 40–80 mesh, was obtained from Hunan Taohuajiang Bamboo Technology Co., Ltd. (Yiyang, China).
2.2 BR/MO Composite Material Preparation
The preparation process of BR/MO composite material is shown in Fig. 1. Magnesium chloride hexahydrate, magnesium sulfate heptahydrate, and ultrapure water made brine were mixed to molar ratio of MgO, MgCl2·6H2O, MgSO4·7H2O, and water in the entire system at 6/1/1/17, respectively. Then, dried BR and magnesium oxide added slowly to the brine and well mixed. The homogenized slurry was then poured into a mold and placed in a constant temperature and relative humidity box (25°C and ~60% R.H.) for curing. After hydration and curing for 24 h, samples were demolded and held a stable R.H. for 3 d after curing to obtain the finished composite. Because BR was loose and had a low density, its volume is relatively large. Therefore, the ratio of BR to MO slurry was treated by volume (Fig. 2).
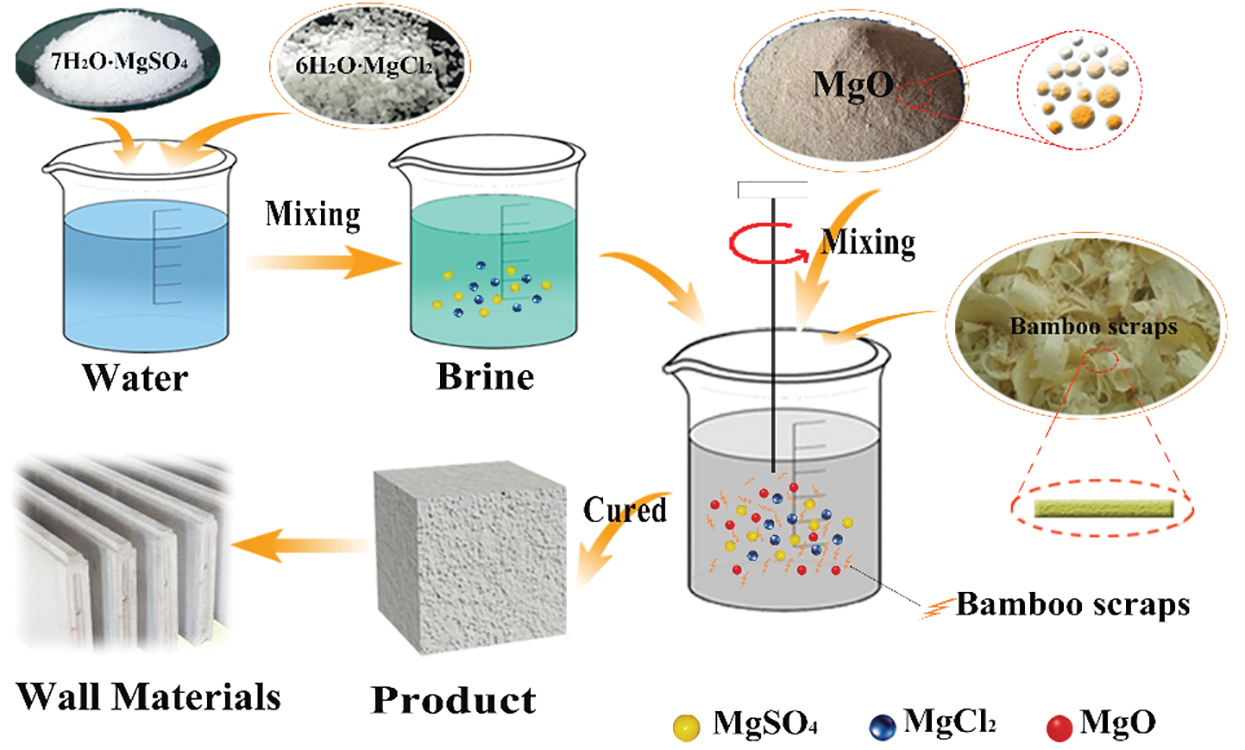
Figure 1: Composite material preparation process
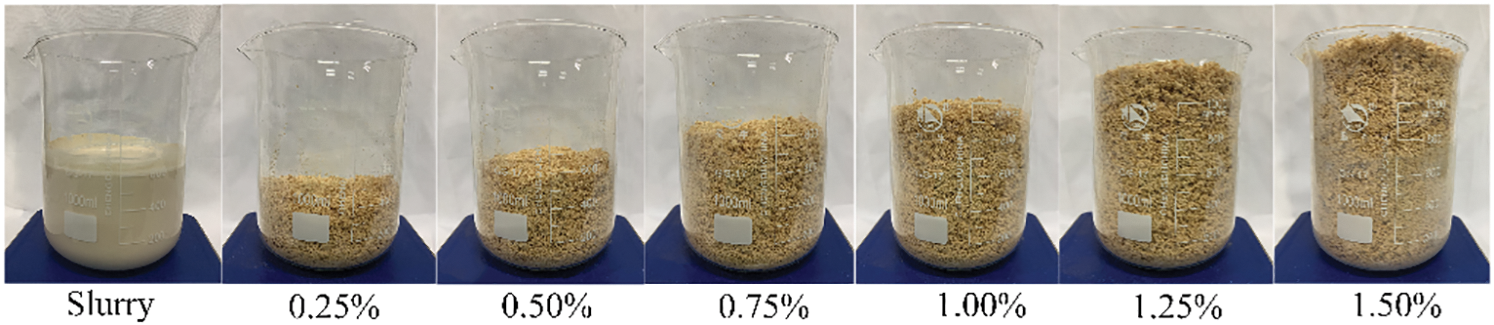
Figure 2: Photos of MO slurry and BR for experiments
2.3 Performance Testing and Characterization
2.3.1 Slurry Viscosity Measurement
Viscosity was measured using a microcomputer-based digital display viscometer (Shanghai Jinghai Instrument Co., Ltd., Shanghai, China). Conditions included a 2# rotor, 12 rev, and sample volume at ~50 mL.
2.3.2 Compressive Strength Test
Referring to Chinese Standard GB/T 5486-2008, test specimens were of 40 × 40 × 40 mm3. After a specimen was dried to absolute dryness, it was pressurized with a universal testing machine at a speed of 20 mm/min and the pressure recorded at specimen destruction.
Referring again to GB/T5486-2008, specimens were 160 × 40 × 40 mm3. After drying to absolute dryness, a universal testing machine was used to apply pressure at a constant rate of 10 mm/min and the pressure at specimen breaking recorded.
2.3.4 Water Absorption Rate Measurement
Referring again to GB/T5486-2008, specimens were of 40 × 40 × 40 mm3, an absolutely dry compression test piece was weighed (M0, g), soaked in water at 20 ± 5°C for 24 h, removed, and wiped dry with a towel, and weighed again (Mg, g). The water absorption rate of a specimen was as follows:
2.3.5 Scanning Electron Microscope (SEM) Analysis
A Quanta 450 scanning electron microscope (SEM; FEI Co., Hillsboro, OR, USA), at an accelerating voltage 20 kV, was used to obtain and observe SEM images of gold-sputtered specimen fragments.
2.3.6 Thermogravimetric Analysis (TGA)
This analysis was performed on the STA 449 F3 simultaneous thermal analyzer system (NETZSCH Co., Germany) from rm temperature to 600°C, heating rate of 10 °C/min, nitrogen flow rate at 30 mL/min, and sample amount at ~5 mg.
2.3.7 X-ray Diffraction Analysis
An Xd-2 X-ray diffractometer (Beijing General Instrument Co., Ltd., Beijing, China) was used to analyze for composite crystal phase structure and crystal type. The test voltage was 40 kV, current at 35 mA, scanning speed at 8°/min, and scan range of 50°–70°.
The data in this research was statistically evaluated using the Minitab Version 15 statistical software package. It reported with the mean and standard deviation of the number of replicates. A single-factor analysis of variance was used to determine the significance difference between the mean value according to the minimum significance difference criterion of 95% confidence level (p < 0.05).
3.1 Mechanical Property Analysis
BR is a hydrophilic material and contains a large number of hydrophilic groups. BR addition affected the viscosity of MO and affected the molding performance of BR/MO composites, thereby affecting the strength of the composites. The viscosities of BR/MO slurry prepared with different BR content were measured (Fig. 3a). When the BR content did not exceed 1.00% (by wt), increased BR gradually led to increased slurry viscosity. On one hand, hydrophilic groups in BR absorbed moisture from the MO slurry, which made the slurry thicker and, on the other hand, after BR was mixed with MO, entanglement occurred in the slurry, which decreased slurry fluidity. At 1.00%-point, slurry viscosity was improved, which improved composite molding performance. However, when the BR content exceeded 1.00%, slurry viscosity rapidly increased, resulting in a sharp drop in slurry fluidity. This caused a large amount of BR to aggregate and unevenly distribute, and the composite molding performance was reduced, which also affected composite mechanical properties.
The compressive and bending strengths of the MO without BR was low while these strengths in BR/MO composites significantly increased (Figs. 3b and 3c). On one hand, these results were mainly due to BR being a tough material (Fig. 3d). When tough BR was added to form a composite in an appropriate amount, there were interfacial bonding and mechanical meshing forces between BR and the MO matrix, which made the composite form a mechanical interlocking chaotic support system [13]. When the composite was subjected to external forces, BR in the system transmitted and absorbed a certain amount of energy, which increased the compressive failure load. On the other hand, lignin and semifibers in BR were hydrolyzed into sugar acids and monosaccharides in the MO alkaline environment and resulting decomposition products had a protective effect on composite crystals (Fig. 6c). At the same time, BR addition increased slurry viscosity, which was then more conducive to composite molding, thus significantly improving composite toughness and strength (Fig. 3a). However, when BR content increased to 1.00%, composite compressive and bending strengths decreased with increased BR. On one hand, slurry fluidity deteriorated, which made BR dispersibility in the MO system worse and the BR distribution not uniform, resulting in void defects in the composite, which led to a decrease in composite compressive strength [14]. On the other hand, excessive BR addition made the negative effects of debonding at interfaces between BR and the MO matrix in the composite system greater than the positive enhancement effect, resulting in decreased compressive strength. Composite compressive and bending strengths after storage for 7 and 28 d were similar to the strength measured at 3 d and increased with prolonged storage time (Figs. 3b and 3c). This showed that the BR/MO composites had a long-term hydration process, which was thus suitable for long-term use, and the mechanical properties did not decrease with time. The above analysis showed that the addition of a certain amount of BR significantly improved composite bending strength, which confirmed that BR increased the toughness of brittle MO materials.
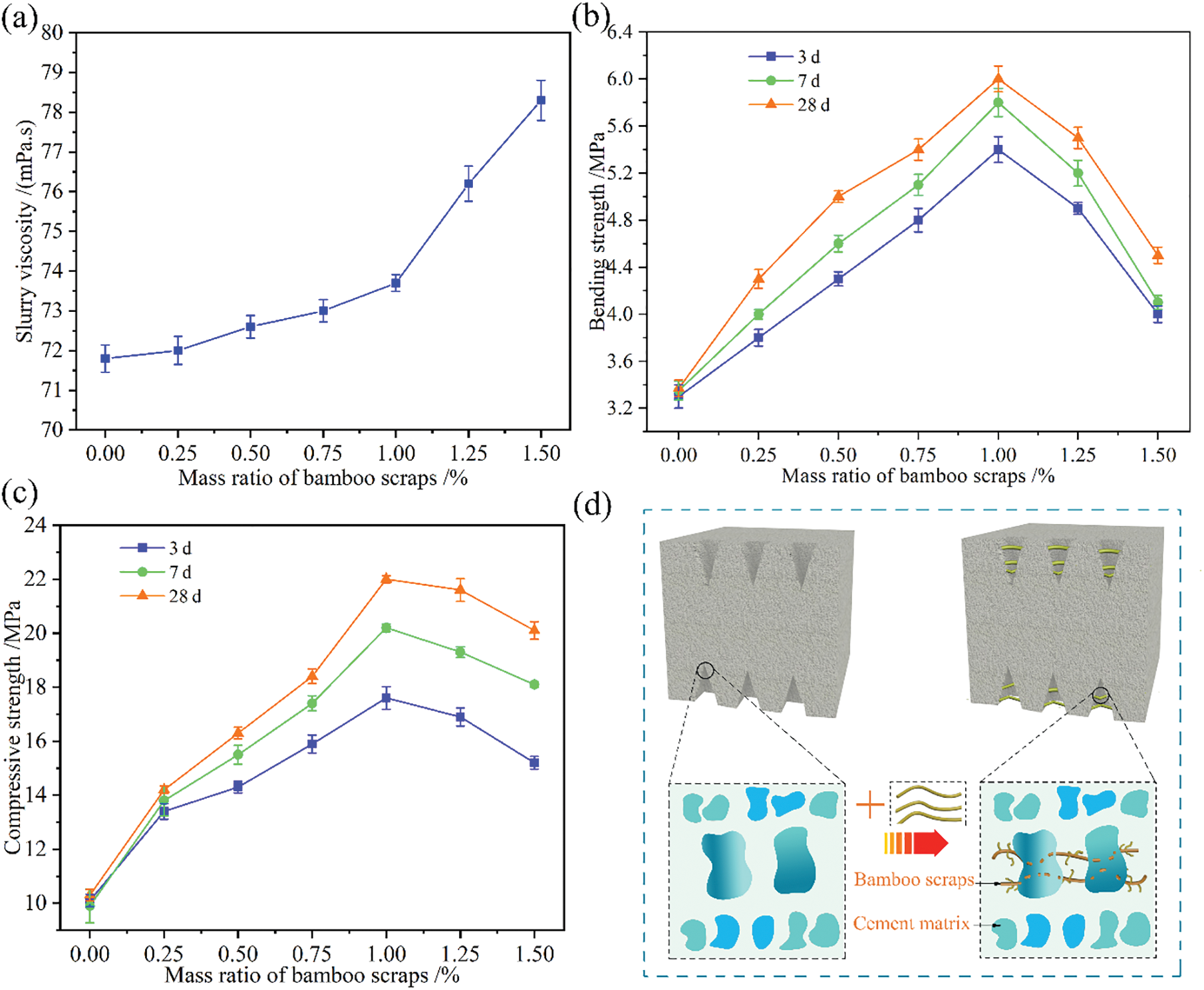
Figure 3: Mechanical properties of BR/MO composites. slurry viscosity with different BR content (a), bending strength of composites with different BR content (b), compressive strength of composites with different amount of bamboo scraps (c), and BR mechanism (d)
BR is a hydrophilic material and its addition directly affected water absorption by BR/MO composites, then affecting composite water resistance. The water absorption and contact angle of composites prepared with different BR content were assessed (Figs. 4a and 4b). As a general trend, the more BR added, the higher the composite water absorption and the smaller the contact angle. This was because there was a large number of hydrophilic hydroxyl groups on BR, such that the more BR added, the more water-absorbing hydroxyl groups present. When the BR content increased from 0.00% to 1.00%, increases in composite water absorption were small and decreases in surface contact angle small. This was because, with less added BR, MO cementitious material wrapped BR better and water molecules had difficulty entering the composite and H-bonding with BR. However, when the BR content exceeded 1.00%, composite water absorption rate rapidly increased and reduction in surface contact significantly increased. As the BR content increased, slurry fluidity became lower and BR uniformity in the MO system also lower (Fig. 3a). At the same time, as BR increased, the ability of MO material to wrap BR deteriorated. These two reasons led to more hydrophilic BR being exposed, which significantly increasing composite water absorption rate material and reducing the surface contact angle.
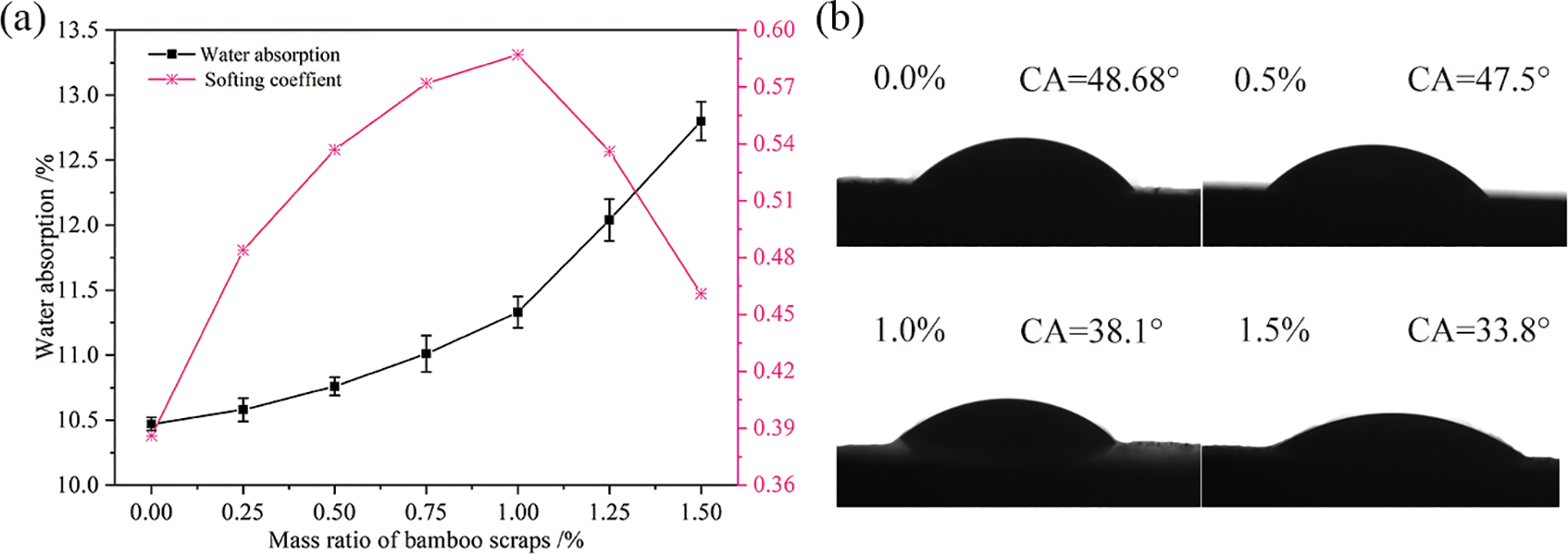
Figure 4: Water resistance. Water absorption and softening coefficient of composites with different amount of bamboo scraps (a) and contact angle of composites with different BR content (b)
After the composite absorbed water, its strength was reduced to a certain extent and thus it was necessary to investigate the strength retention rate after water absorption, that is to measure the softening coefficient. Here, the composite water resistance was further verified by examining the softening coefficient of the BR/MO composites prepared with different BR content (Fig. 4a). The softening coefficient of MO was only 0.386 when without BR, because MO material had poor adhesion after water molecule erosion and there were many pores in the MO matrix, which easily allowed water molecule invasion and hydrolysis from ion migration [15]. When the BR content was <1.00%, the composite softening coefficient gradually increased. First, one reason for this was that BR addition made the composite form a more chaotic support system and BR could have absorbed and transmitted a certain amount of energy. Thus, increasing BR content increased the composite strength retention rate after water absorption. Second, there was a large number of H-bonds in BR that could have absorbed some of the invading water molecules, thus reducing resulting erosion of MO. Third, BR produced hydrolysates, which protected the 5-phase crystallization and prevented it from decomposing under water molecule erosion (Fig. 6c). However, when BR content exceeded 1.00%, the composite softening coefficient rapidly decreased. On one hand, excessive BR addition caused BR accumulation in the MO system, resulting in uneven dispersion, easy production of bubbles and cracks, and likely entrance of water molecules into the composite, resulting in reduced water resistance. On the other hand, if the BR content was too great, the number of hydrophilic groups in the composite increased, resulting in a significant increase in composite water absorption. The present analytical results of water absorption, contact angle, and softening coefficient all showed that BR was the reinforcing phase in the composite, which was beneficial for improving composite strength. However, BR is also a hydrophilic material, which caused the composite to absorb water, thus changing the mechanical properties.
3.3 Interface Topography Analysis
BR played a supporting role in this composite system and MO a gelling role, but there were cracks and air bubbles at junction of BR and MO, thereby affecting various performances. Therefore, the influence mechanism of BR on the properties of BR/MO composite was explored by SEM to observe morphological characteristics of surfaces and interfaces in BR/MO composites (Fig. 5).
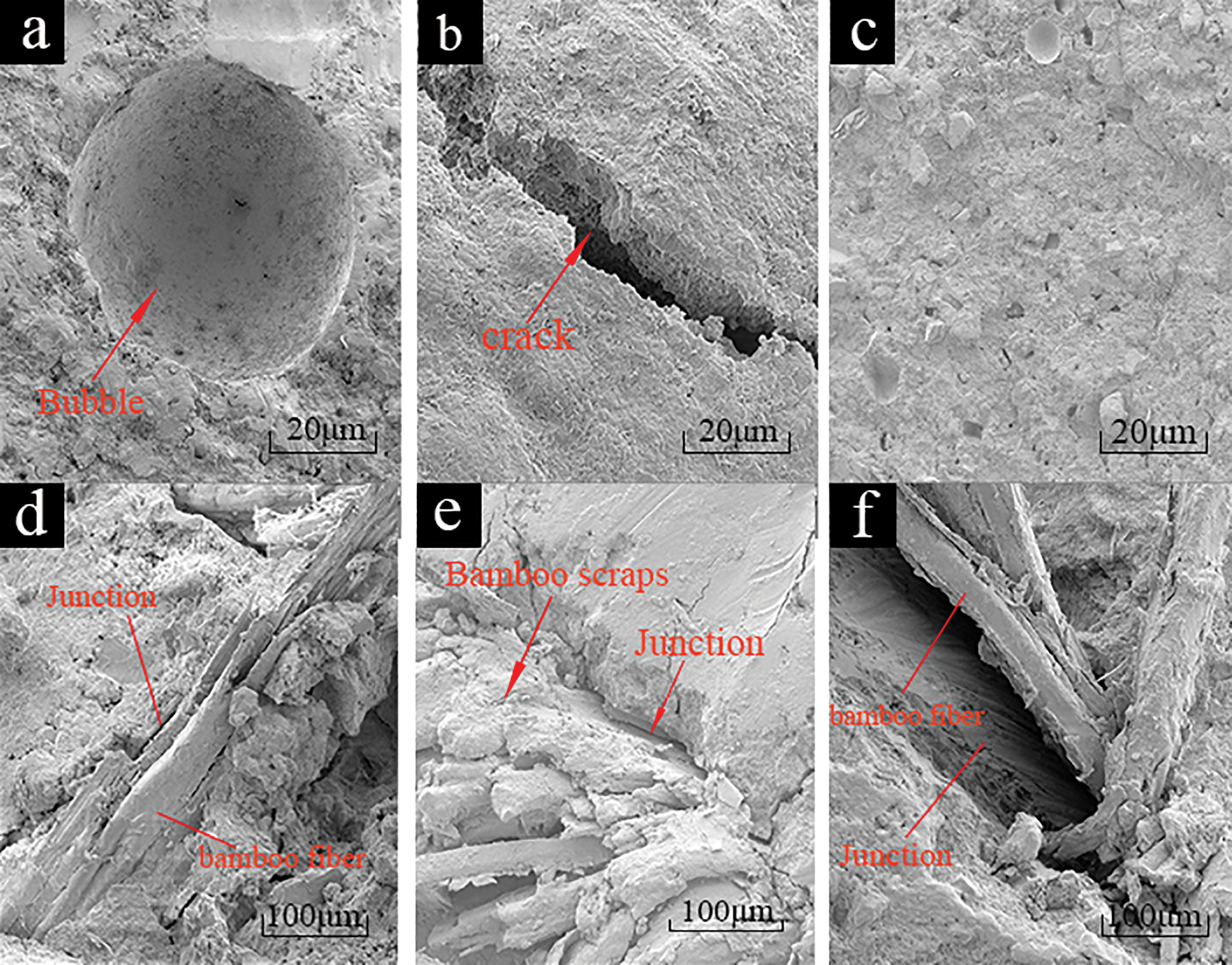
Figure 5: Electron micrograph of a composite surface. Surface of MO without BR (a and b); surface of composite with 1.00% BR), with interface between BR and MO (c); bonding interface between BR and MO in composite with 0.50% content (d); bonding interface between BR and MO in composite with 1.00% BR (e); and bonding interface between BR and MO in composite with 1.5% BR (f)
There were more bubbles and cracks observed in MO without BR (Figs. 5a and 5b). This was because MO slurry without BR had a low viscosity and large slurry water content, which caused a large number of bubbles to be generated by water evaporation in MO during the molding process. At the same time, MO was a brittle inorganic material, which was prone to cracks during the molding process. Bubbles and cracks in the composite with 1.00% BR were significantly reduced (Fig. 5c). BR contains hydrophilic hydroxyl groups, which can absorb slurry water, thereby, here, reducing bubbles generated by water evaporation in the composite. At the same time, BR played a reinforcing role in the composite system, which effectively reduced MO cracking and improved molding stability. There were smaller cracks at the junction of BR and MO in composite with 0.50% BR, but MO next to BR showed certain cracks and a few holes (Fig. 5d). With composite BR content at 1.00%, cracks and holes at the bonding interfaces between BR and MP matrix were smaller, which showed that composite with 1.00% BR had better molding stability (Fig. 5e). When the BR content was increased to 1.50% large cracks occurred at bonding interfaces between BR and MO in the composite (Fig. 5f). On one hand, when BR content was too large, BR in the composite aggregated and caused bubbles, with interface matrix collapse at gathering places. On the other hand, there was a stratum corneum on BR surfaces and interfaces between organic BR and inorganic MO were incompatible [16]. When the BR was too much, BR and the MO matrix exhibited a clear phase separation. This showed that, when BR content was too much, interface compatibility between BR and MO matrix was poor, which seriously affected composite strength and water resistance.
3.4 Crystal Structure Analysis
The effects of BR content on the crystalline structure of BR/MO composite were explored by performing XRD analyses on these composites (Fig. 6a). In composites, 5Mg (OH)2·MgCl2·8H2O (5 phase) and 3 Mg(OH)2·MgCl2·8H2O (3 phase) were the main crystal phases, but 3 and 5 phases can be interconverted [17]. Among these, needle-like 5-phase is the strength phase of MO. The more 5-phase present, the better the material strength [18]. Here, when the BR content increased from 0.00% to 1.00%, the 3-phase crystallization of the composite at 2θ angles of 19, 37, and 58° weakened and at 2θ angles of 32.5°, 43°, and 62.5°, 5-phase crystal enhanced (Fig. 6a). This showed that BR addition was beneficial to the nucleation of 5-phase crystallization, which was then beneficial to the conversion of 3-phase crystallization to 5-phase. This also explained that, when BR increases from 0.00% to 1.00%, composite mechanical properties gradually increased. This was because lignin, hemicellulose, and other components in BR were hydrolyzed into monosaccharides and sugar acids under the alkaline conditions of MO cementitious material (Fig. 6c). The 5-phase crystals in MO were wrapped on BR, which was conducive to the existence of 5-phase crystals [19]. However, when the BR content was increased to 1.50%, 3-phase crystallization in the composite at 2θ angles of 19°, 37°, and 58° was enhanced and 5-phase crystallization at 2θ angles of 24°, 43°, and 62.5° weakened. This was because with too much BR, water and sugar exudates retarded the cement, which was not conducive to the existence of 5-phase crystallization. The above analysis showed that BR addition changed composite crystalline structure. Addition of a proper amount of BR was conducive to MO nucleation, which increased the 5-phase crystallization of the composite.
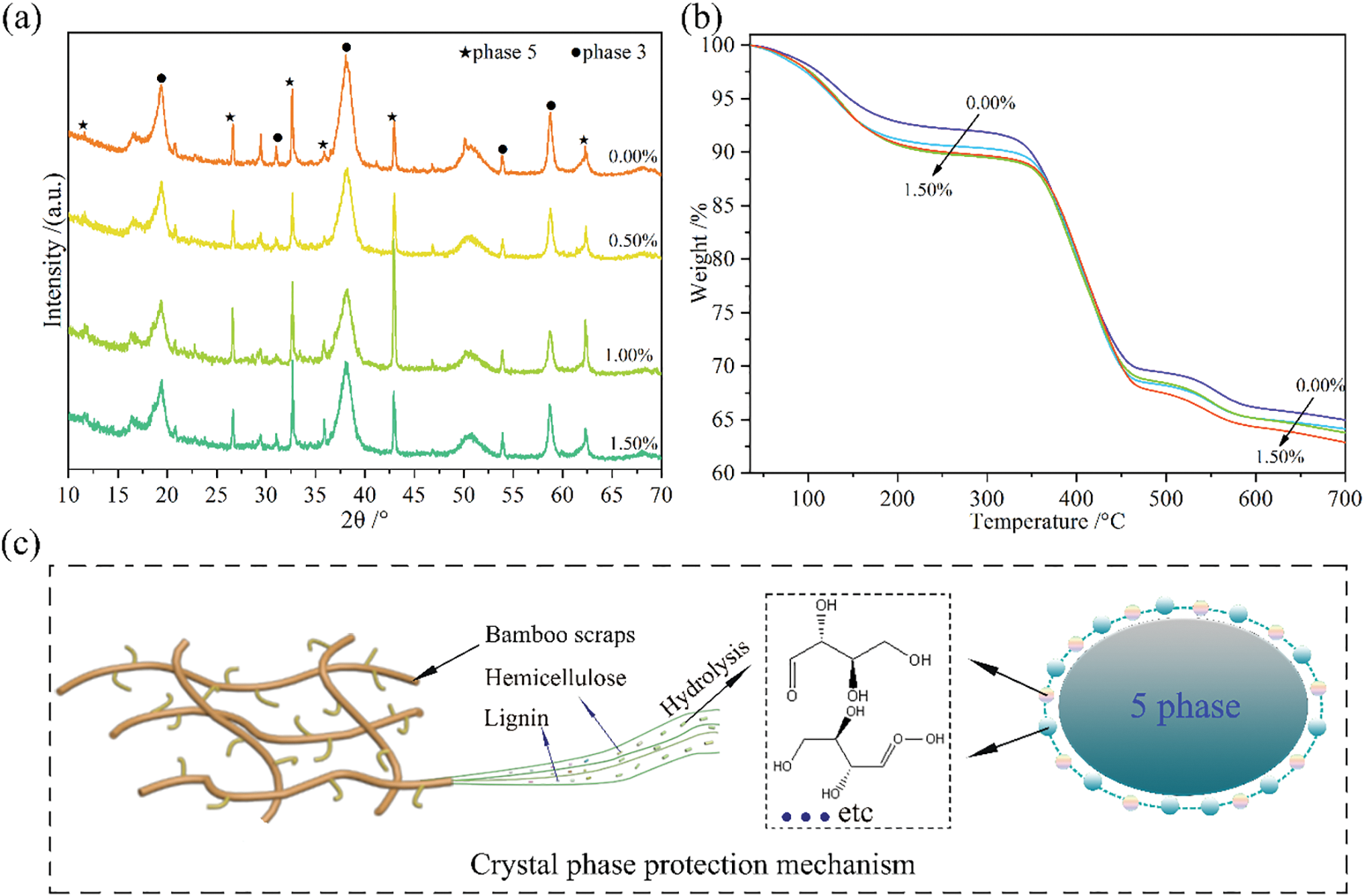
Figure 6: Material characterization. XRD diffraction pattern of BR/MO composites (a), TGA curves of composites (b), and crystal protection mechanism (c)
3.5 Thermal Performance Analysis
The heat resistance of BR/MO composite is an important indicator for evaluating the usable temperature range of the product, reflecting its thermal stability. At the same time, changes in composite crystalline structure inevitably affects its thermal decomposition, such that it is very important to analyze composite thermal stability. Here, the thermal stability of BR/MO composites was explored by thermogravimetric analysis to characterize composites prepared with different BR content. The resulting TGA curve showed that the thermal weight loss curve of the composite could be divided into three stages, with room temperature to ~250°C the first stage of weight loss (Fig. 6b). This stage was mainly evaporation of free water in the composite and thermal decomposition of hemicellulose and magnesium chloride in BR. Temperatures of 250°C–480°C was the second weight loss stage, including the decomposition of crystal water in MO and pyrolysis of cellulose and lignin in BR. Above 480°C, the third weight loss stage included complete decomposition of BR and complete expulsion of the remaining crystal water in MO. There was a significant difference in the thermal decomposition weight loss rate of composites in the first and third stages, with both showing a trend in which increased BR and composite thermal weight loss rate increased. This was mainly due to the fact that the thermal decomposition rate of organic BR was significantly higher than that of inorganic MO, and BR addition, with faster thermal decomposition, resulted in greater thermal decomposition of the composites. However, the thermal weight loss rate of composites when BR increased from 0.00% to 0.05% was significantly greater than that of BR from 0.05% to 1.50%. This was affected by changes in composite crystalline structure. After BR addition, 5-phase crystals with better heat resistance in MO increased, which yielded the composite thermal decomposition rate slower.
When the BR content was 1.00% (by wt), MO slurry fluidity was conducive to composite molding, the mechanical properties and water resistance of composites were better, and the mechanical strength improved as composites were stored longer. SEM analysis showed that BR played the role of a reinforcing phase in the MO matrix but, when the BR content exceeded 1.00%, interfacial bonding between BR and the MO matrix decreased. This explained that, with changes in BR content, composite mechanical properties first increased and then decreased. XRD analysis showed that, when BR was 1.0%, there were more 5-phase crystals in the composites. This further explained the reason for this BR content exhibiting the best composite mechanical properties and, at the same time, composite heat resistance did not deteriorate due to this BR content, which was easily decomposed by heat.
Funding Statement: This work has been supported by the National Natural Science Foundation of China (31971743), Forestry Science and Technology Innovation Outstanding Youth Scientific Research Project of Hunan Province, China (XLK201945), Natural Science Foundation of Hunan Province, China (2019JJ40540), Hunan Provincial Technical Innovation Platform and Talent Program in Science and Technology, China (2019RS2040) and National College Students Innovation and Entrepreneurship Training Program in China (S202010538013).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Li, H. T., Xuan, Y. W., Xu, B., Li, S. H. (2020). Bamboo application in civil engineering field. Journal of Forestry Engineering, 5(6), 1–10. DOI 10.13360/j.issn.2096-1359.202003001. [Google Scholar] [CrossRef]
2. Qian, S. P., Wang, H., Zarei, E., Sheng, K. C. (2015). Effect of hydrothermal pretreatment on the properties of moso bamboo particles reinforced polyvinyl chloride composites. Composites Part B: Engineering, 82, 23–29. DOI 10.1016/j.compositesb.2015.08.007. [Google Scholar] [CrossRef]
3. Lou, Z. C., Yuan, T. C., Wang, Q. Y. (2021). Fabrication of crack-free flattened bamboo and its macro-/micro- morphological and mechanical properties. Journal of Renewable Materials. DOI 10.32604/jrm.2021.014285. [Google Scholar] [CrossRef]
4. Widyorini, R., Umemura, K., Isnan, R., Putra, D. R., Awaludin, A. (2016). Manufacture and properties of citric acid-bonded particleboard made from bamboo materials. European Journal of Wood and Wood Products, 74(1), 57–65. DOI 10.1007/s00107-015-0967-0. [Google Scholar] [CrossRef]
5. Biswas, D., Bose, S. K., Hossain, M. M. (2011). Physical and mechanical properties of urea formaldehyde-bonded particleboard made from bamboo waste. International Journal of Adhesion and Adhesives, 31(2), 84–87. DOI 10.1016/j.ijadhadh.2010.11.006. [Google Scholar] [CrossRef]
6. Zhang, Y. F., Zheng, J., Guo, H. L., Li, Y. W., Lu, M. G. (2015). Urea formaldehyde resin with low formaldehyde content modified by phenol formaldehyde intermediates and properties of its bamboo particleboards. Journal of Applied Polymer Science, 132(27), 42280. DOI 10.1002/app.42280. [Google Scholar] [CrossRef]
7. Li, Y., Yu, H. F., Zheng, L. N., Wen, J., Wu, C. Y. (2013). Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Construction and Building Materials, 38, 1–7. DOI 10.1016/j.conbuildmat.2012.06.016. [Google Scholar] [CrossRef]
8. Zuo, Y. F., Xiao, J. H., Wang, J., Liu, W. J., Li, X. G. et al. (2018). Preparation and characterization of fire retardant straw/magnesium cement composites with an organic-inorganic network structure. Construction and Building Materials, 171, 404–413. DOI 10.1016/j.conbuildmat.2018.03.111. [Google Scholar] [CrossRef]
9. Tang, S. W., Hu, Y., Ren, W., Yu, P., Huang, Q. et al. (2019). Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the micro-scale. Construction and Building Materials, 204, 684–690. DOI 10.1016/j.conbuildmat.2019.01.232. [Google Scholar] [CrossRef]
10. Wang, J., Zuo, Y. F., Xiao, J. H., Li, P., Wu, Y. Q. (2019). Construction of compatible interface of straw/magnesia lightweight materials by alkali treatment. Construction and Building Materials, 228, 116712. DOI 10.1016/j.conbuildmat.2019.116712. [Google Scholar] [CrossRef]
11. Wang, F. G., Xu, X. D., Zhou, B. M., Zhong, S. Y. (2019). Preparation of straw-magnesium oxychloride cement composites. Journal of Building Materials, 22(1), 135–141. DOI 10.3969/j.issn.1007-9629.2019.01.020. [Google Scholar] [CrossRef]
12. He, P. P., Hossain, M. U., Poon, C. S., Tsang, D. C. W. (2019). Mechanical, durability and environmental aspects of magnesium oxychloride cement boards incorporating waste wood. Journal of Cleaner Production, 207, 391–399. DOI 10.1016/j.jclepro.2018.10.015. [Google Scholar] [CrossRef]
13. Filho, R. D. T., Scrivener, K., England, G. L., Ghavami, K. (2000). Durability of alkali-sensitive sisal and coconut fibres in cement mortar composites. Cement and Concrete Composites, 22(2), 127–143. DOI 10.1016/S0958-9465(99)00039-6. [Google Scholar] [CrossRef]
14. Wang, J., Xiao, J. H., Zuo, Y. F., Guan, P. F., Wu, Y. Q. (2018). Preparation and properties of straw/magnesium oxychloride cement inorganic light mass composites. Acta Materiae Compositae Sinica, 35(11), 3162–3171. DOI 10.13801/j.cnki.fhclxb.20180205.002. [Google Scholar] [CrossRef]
15. Zuo, Y. F., Wang, J., Xiao, J. H., Li, W. H., Wu, Y. Q. (2018). Modulation of strength and pore structure of magnesium inorganic foaming material. Journal of Building Materials, 21(3), 394–401. DOI 10.3969/j.issn.1007-9629.2018.03.008. [Google Scholar] [CrossRef]
16. Gong, W., Yu, H. F., Ma, H. Y., Qiao, H. X., Chen, G. F. (2019). Study on corrosion and anticorrosion of rebar in magnesium oxychloride cement concrete. Emerging Materials Research, 8(1), 94–104. DOI 10.1680/jemmr.18.00012. [Google Scholar] [CrossRef]
17. Wang, D. X., Di, S. J., Gao, X. Y., Wang, R. H., Chen, Z. G. (2020). Strength properties and associated mechanisms of magnesium oxychloride cement-solidified urban river sludge. Construction and Building Materials, 250, 118933. DOI 10.1016/j.conbuildmat.2020.118933. [Google Scholar] [CrossRef]
18. Ye, Q. Q., Han, Y. F., Zhang, S. F., Gao, Q., Zhang, W. (2020). Bioinspired and biomineralized magnesium oxychloride cement with enhanced compressive strength and water resistance. Journal of Hazardous Materials, 383, 121099. DOI 10.1016/j.jhazmat.2019.121099. [Google Scholar] [CrossRef]
19. Chen, X. Y., Zhang, T. T., Bi, W. L., Cheeseman, C. (2019). Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Construction and Building Materials, 213, 528–536. DOI 10.1016/j.conbuildmat.2019.04.086. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |