

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015812
ARTICLE
Preparation and Performance of a Fluorine-Free and Alkali-Free Liquid Accelerator for Shotcrete
1College of Material Science and Engineering, Guilin University of Technology, Guilin, 541004, China
2Guangxi Engineering and Technology Center for Utilization of Industrial Waste Residue in Building Materials, Guilin, 541004, China
3Guangxi Beibu Gulf Engineering Research Center for Green Marine Materials, Guilin, 541004, China
*Corresponding Author: Rongjin Liu. Email: liurj2008@sina.com
Received: 16 January 2021; Accepted: 25 February 2021
Abstract: Based on aluminum sulfate, a fluorine-free and alkali-free liquid accelerator (FF-AF-A) was prepared in this study. The setting time and compressive strength of three cement types with different FF-AF-A dosages were fully investigated. The compatibility of the FF-AF-A with the superplasticizers were also investigated, and the early hydration behavior and morphology of the hydration products of reference cement paste with the FF-AF-A were explored by hydration heat, X-ray diffractometry (XRD), and scanning electron microscopy (SEM). Test results indicated that adding the FF-AF-A at 8 wt% of the cement weight resulted in 2 min 35 s initial setting time and 6 min 30 s final setting time. The 1-day compressive strength of the cement mortar with 8 wt% of FF-AF-A reached 13.5 MPa, which represents an increase of 35% as compared to the strength of cement mortar without the FF-AF-A, and the 28-day compressive strength ratio was 119%. In addition, the FF-AF-A also showed good compatibility with different superplasticizer dosages. The results show that, when the FF-AF-A was added to the cement paste, it promoted the formation of ettringite crystals due to the aluminum ions (Al3+) and sulfate ions (SO42-) reacted with gypsum in the cement, as well as promoted the hydration of tricalcium aluminate (C3A) and tricalcium silicate (C3S) leading to the overall structure becomes more compact. As a consequence, the hydration heat rate of the cement sharply increased, the cement paste setting time is shortened, and the compressive strength of cement mortar is improved.
Keywords: Shotcrete; alkali-free liquid accelerator; setting time; compressive strength; mechanism
Shotcrete is widely used in the construction of mines, subways, automobile tunnels, and other underground systems, in addition to dams and other water structures, with a primary role as a reinforcement of the building surface [1,2]. Concrete accelerators are chemical admixtures that play an essential role in producing shotcrete, in which the admixtures accelerate the setting and hardening of the cement-based materials and improve the early strength development of the shotcrete by accelerating the hydration rate of the cement paste [3].
Different types of accelerators had different impacts on the setting and hardening, strength development, and durability of shotcrete. An et al. [4,5] found that the addition of aluminum sulfate introduced by alkali-free accelerator contributed to the rapid formation of ettringite crystals, as well as to early hydration of C3S. And the addition of sodium aluminate introduced by alkaline accelerator accelerated the consumption of gypsum and the transition from ettringite crystals to calcium mono sulfoaluminate hydrate (AFm), which suppressed the C3S further hydration. Zeng et al. [6] found that compared to the alkaline accelerator, the alkali-free accelerator has a higher later compressive strength of concrete. Zhang verified that the alkaline accelerator reduced the frost resistance and impermeability of concrete, which is not beneficial to the durability of concrete; while the alkali-free accelerator has little effect on the durability of shotcrete [7]. Thus, the properties of the accelerator determine to a large extent the quality of shotcrete [8]. In general, accelerators are divided into liquid and powder according to their morphological properties. However, a shotcrete with a powder accelerator has several disadvantages, including high rebound ratio, high dust pollution, and low final strength [9]. Therefore, the powder accelerators were gradually replaced by the liquid accelerators. The liquid accelerators are divided into alkaline and alkali-free depending on the different alkali contents [5]. The primary component of the alkaline liquid accelerator is sodium aluminate or sodium silicate. However, due to higher alkali contents, alkaline accelerator is not only hazardous to construction workers, but also induces alkali-aggregate reaction, which adversely affects the growth of strength and durability of concrete at a later age [10,11]. Compared to alkaline liquid accelerators, the advantages of the alkali-free liquid accelerators (dominated by aluminum sulfate) are low rebound ratio, low shrinkage, high final strength of hardened shotcrete, and more environmentally friendly. Therefore, the alkaline liquid accelerator is gradually replaced by the alkali-free liquid accelerator [12].
To further improve of the accelerator performance, it is often required the addition of other synergistic components, many scholars have done a great deal of research on it. A type of alkali-free liquid accelerator was synthetized [13], where the setting time can be shortened by adding large amounts as high as about 9% of triethanolamine (TEA). This is because TEA can chelate with the Al3+, which accelerates the hydration process of C3A and formation of the ettringite crystals [14]. Nevertheless, the TEA impact on the cement-based materials is closely associated with its content [15]. When the TEA substitution rate is too high, it is disadvantageous to the C3S hydration and to the early strength development of the cement [16,17]. Another type of synthetic alkali-free liquid accelerator consists mainly of aluminum sulfate and fluorine-containing compounds, since the fluoride ions (F-) can chelate with the Al3+ to form an fluorine-aluminum complex solution. The effects of different dosages of fluorine-aluminum complex solution on the performance of accelerator have been studied in the literature [18]. Previous studies suggest that fluorine-aluminum complex solution can effectively improve the stability of the accelerator and shortens the cement setting time. However, one drawback was noted as the early-age strength of the cement mortar was significantly reduced [18,19]. This is because the F- in the fluorine-aluminum complex solution, can damage the structure of the calcium silicate hydrates (C-S-H) gels and the overall structure of the cement mortar [20,21]. Ultimately, this will result in a decrease in the early-age strength of the cement mortar [17]. More importantly, excessive use of fluorine-containing compounds may cause harmful effects on the human body [19].
The problem of early strength reduction of concrete caused by high levels of TEA and F- content needs to be overcome, therefore, the preparation of an alkali-free liquid accelerator with no F-, low levels of TEA, excellent quick-setting effect, high early strength, and high later strength retention rate is important for the shotcrete industry. The main objective of this study is to develop a fluorine-free and alkali-free liquid accelerator, and investigate its impact on the cement paste such as setting time and compressive strength. Moreover, the compatibility of the FF-AF-A with different dosages of polycarboxylate superplasticizers will be studied, the early hydration behavior of cement with the FF-AF-A will be studied by using hydration heat, the morphological characteristics of hydration products at different age stages will be analyzed by XRD and SEM.
Reference cement and two types of Portland cement (Jidong cement and Conch cement) were used in this experiment. The reference cement was produced by China building materials academy, the Jidong cement was produced by Tangshan Jidong Cement Co., Ltd., Tangshan, China and the Conch cement was produced by Xing ’an Conch Cement Co., Ltd., Guilin, China; the main physical properties are shown in Tab. 1, and the chemical compositions of cement are shown in Tab. 2. The sand used in this experiment was ISO standard sand from Xiamen ISO Standard Sand Co., Ltd., Xiamen, China. Two types of polycarboxylate superplasticizers were used. The powder polycarboxylate superplasticizer was purchased from Shanghai Qinhe Chemical Co., Ltd., Shanghai, China the liquid polycarboxylate superplasticizer was purchased from Henan SiTong New Materials Co., Ltd., Nanyang, China and its solid content is 25%.
Aluminum sulfate (Al2(SO4)3·18H2O), magnesium sulfate (MgSO4, anhydrous), triethanolamine, and maleic acid were all analytical reagent. The amorphous Al2O3 gel was industrial grade reagent, Al2O3 content is greater than 50%.
Table 1: Physical properties of cement
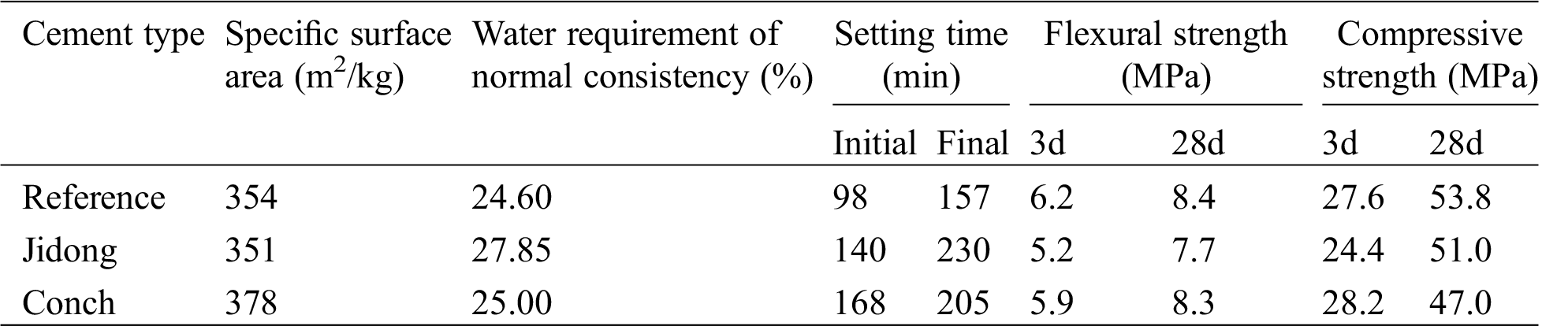
Table 2: Chemical compositions of cement/%
The preparation process of FF-AF-A was divided into four steps. First, aluminum sulfate was dissolved in water by stirring on magnetic stirrer at the rate of 150~200 rpm at 70°C~90°C. Second, the amorphous Al2O3 was added to the solution after the aluminum sulfate was dissolved completely. Third, the triethanolamine and the magnesium sulfate were mixed in the solution and stirred for 30-50 min to obtain the mother liquor of FF-AF-A. Finally, maleic acid was added and dissolved in the mother liquor. The proportions of mother liquor were 50% aluminum sulfate, 8% amorphous Al2O3, 3% triethanolamine, 4% magnesium sulfate, and 35% water, meanwhile, the content of maleic acid is 2% of the mother liquor. The FF-AF-A was prepared as shown in Fig. 1. The solid content of FF-AF-A was 48%.

Figure 1: The FF-AF-A
The test of setting time of cement paste was conducted according to the standard of GB/T 35159-2017 (Flash setting admixtures for shotcrete). The cement was weighted at 400 g, the water cement ratio (w/c) was 0.35 (including water in the FF-AF-A), and the FF-AF-A was added in different dosages by cement weight (%). The test of setting time ambient temperature was 20 ± 2°C.
The strength test of cement mortar was conducted according to the standard of JGJ/T70-2009 (Standard for test method of basic properties of construction mortar). Therefore, the mortar was prepared using 900 g cement, 1350 g standard sand, and 450 g of water (including water in the FF-AF-A). The mortar mixture was loaded into the 40 × 40 × 160 mm3 moulds and cured under the ambient temperature of 20 ± 2°C and a relative humidity of 95%.
The hydration heat and exothermic rate were characterized with the isothermal calorimeter (I-Cal 8000 HPC, Calmetrix Inc.,). Keep raw material temperature, instrument temperature and room temperature at 20 ± 2°C. The reference cement was weighted at 20 g, the water cement ratio (w/c) was 0.35, the FF-AF-A was added at 4 wt% and 8 wt% of the cement weight (%). When cement paste were mixed, added the FF-AF-A and stir quickly for 10 s, then place it in the sample cell.
The samples of reference cement pastes without and with 8 wt% FF-AF-A were hydrated at 7 min, 6 h, 24 h, and 28 days curing ages, then a few pieces of sample from the uncarbonized and unpolluted parts were immersed into ethanol for 24 h to terminate hydration, then sample were placed in a vacuum drying box and dried up to constant weight at 45°C. After cooling to room temperature, a small amount of the sample materials with 3 mm~5 mm diameter size were analysed by SEM (S-4800 type field emission scanning electron microcopy; Hitachi). The other samples were grinded and sieved to 74 µm, the powder samples were used for XRD (X’Pert PRO, PANalytical).
3.1 Effects of Different FF-AF-A Dosages and Cements on the Setting Time
The effect of the FF-AF-A on the setting time of different cement types was investigated, since the type of cement has a significant impact on the performance of shotcrete [22]. The effects of different the FF-AF-A dosages and cements on the setting time of the cement paste are shown in Fig. 2.
Test results indicated a gradual reduction in the initial and final setting times of the cement paste with increased FF-AF-A dosages. When the FF-AF-A dosage was 7 wt%, the initial and final setting times of reference cement pastes were 3 min 10 s and 9 min 35 s, respectively, which met the “Flash setting admixtures for shotcrete” requirements of the Chinese National Standard [GB/T 35159-2017]. This implies that the FF-AF-A has an excellent effect of accelerating setting. Moreover, the initial and final setting times of Conch cement and Jidong cement pastes with 7 wt% FF-AF-A were less than 5 and 12 min, respectively. This implies a very good compatibility between the FF-AF-A and different cement types.

Figure 2: Effect of the dosage of FF-AF-A on setting time of cement paste (a) Initial setting time (b) Final setting time
3.2 Effects of Different FF-AF-A Dosages and Cements on the Compressive Strength
Besides the setting time, the compressive strength of cement mortars with the accelerator is another significant criterion in developing and selecting the accelerator. The early strength of concrete mixed with alkaline accelerator usually increases rapidly, but the final strength loss rate is relatively large [23]. Therefore, it is necessary to test the effect of the FF-AF-A dosage on the early and final compressive strength of different cement types.
The effects of the FF-AF-A on the compressive strength of different types of cement at different dosages are shown in Fig. 3. It is evident from the Fig. 3 that the compressive strengths of various cement mortars with the FF-AF-A are improved compared to cement mortars without the FF-AF-A. At 8 wt% FF-AF-A dosage, the 1-day compressive strength of the reference cement mortar reached 13.5 MPa, and the 28-day compressive strength ratio was 119%. This illustrates that the FF-AF-A not only improves the early compressive strength of the cement mortars, but also contributes to the development of the final compressive strength. Meanwhile, FF-AF-A addition to Conch and Jidong cements yields a significant improvement in the 1-day compressive strength, with an increase of around 50% with 8 wt% and 7 wt% FF-AF-A dosage for Conch cement and Jidong cement, respectively. In addition, the 28 d compressive strength ratio of Conch cement and Jidong cement mortar with FF-AF-A were more than 100%. This also implies a very good compatibility between the FF-AF-A and different cement types.

Figure 3: Effect of the dosage of FF-AF-A on compressive strength of cement mortar (a) 1 d compressive strength (b) 28 d compressive strength ratio
3.3 Compatibility of FF-AF-A with Different Superplasticizer Dosages
Superplasticizer addition to the concrete mix enhances its fluidity, mechanical strength, and durability [24]. However, most superplasticizers contain some retarding ingredients, which have retarding effects on cement paste setting time; therefore, it is necessary to study the compatibility of the FF-AF-A with different dosages of superplasticizer.
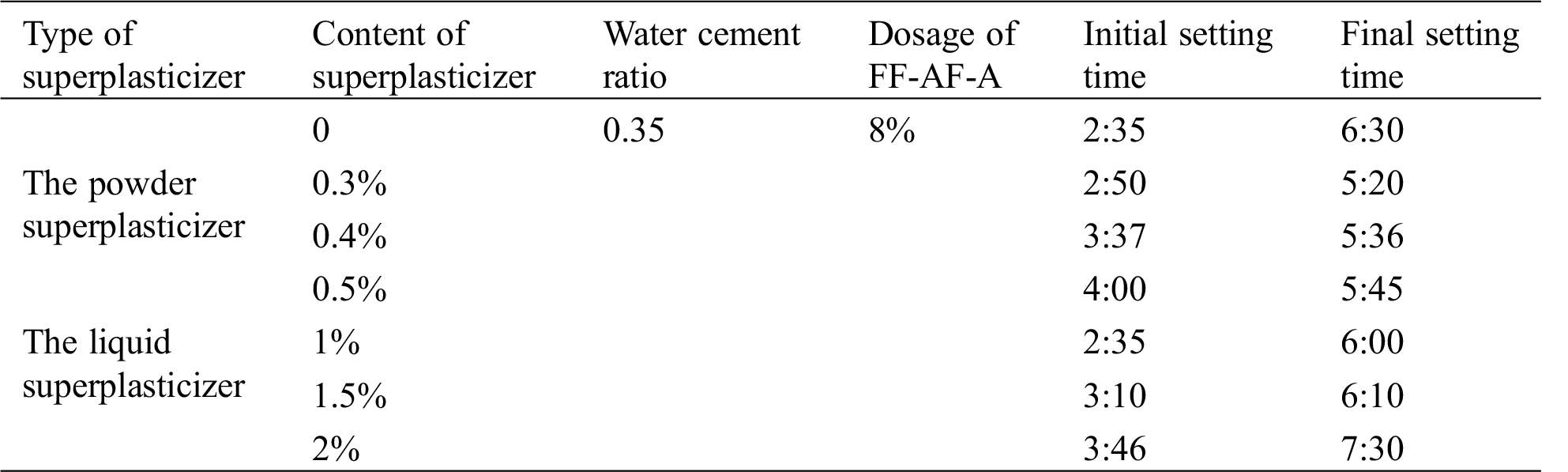
As shown in Tab. 3, with the increase of superplasticizer dosage, the initial setting times of the reference cement paste increased as well, while the range of increase was not very large. The final setting time of the reference cement paste decreased except with 2% the liquid superplasticizer content. This indicates that the FF-AF-A showed good compatibility with different superplasticizer dosages considering the cement paste setting time.
Table 3: The compatibility of the FF-AF-A with superplasticizer
The hydration exothermic rate and hydration heat of reference cement with different dosages of FF-AF-A were tested with the isothermal calorimeter, and the results are presented in Fig. 4. The hydration process of cement is divided into 5 periods, i.e., the earlier induction period (0–0.5h), the induction period (0.5–2.5), acceleration period (2.5–10.5), deceleration period (10.5–22.5), and stabilization period (22.5-) according to the change regulation of hydration heat release rate of cement [25].
From Fig. 4a, the addition of FF-AF-A noticeably increased the hydration exothermic rate in the earlier induction period of cement compared to that without the FF-AF-A, and the higher the FF-AF-A dosage, the higher the first exothermic peak. The first exothermic peak of the cement paste without FF-AF-A was only 24.37 mW/g within the earlier induction period of cement hydration reaction. When the FF-AF-A dosages increased to 4 wt% and 8 wt% of cement weight, the first exothermic peaks of hydration were 38.98 and 72.23 mW/g, respectively. This was due to FF-AF-A promoted the hydration of C3A. In addition, SO42- and Al3+ from the FF-AF-A also reacted with Ca2+ in the cement paste to produce the ettringite crystals. Meanwhile, the decrease in the concentration of Ca2+ in the cement paste, which in turn promoted the dissolution and hydration of other cement minerals. Therefore, the hydration exothermic rate is higher than the cement without FF-AF-A. This indicates that addition of the FF-AF-A could produce generous ettringite crystals, resulting in the rapid setting of cement paste and the initial setting state in approximately 3.0 min.
In addition, compared to cement paste without the FF-AF-A, the second higher exothermic peak of the cement pastes with the FF-AF-A was observed in advance, as shown in Fig. 4a, and the hydration exothermic rate was found to be higher. The second higher exothermic peak of the cement paste without FF-AF-A was in the range of 9.9–10.5 h, and the hydration exothermic rate was 3.06 mW/g. However, when the FF-AF-A is added at 4 wt% and 8 wt% of cement weight, the second exothermic peaks of hydration were in the range of 8.8 h and 8.1 h, respectively, and the hydration exothermic rate were 3.15 and 3.60 mW/g, respectively. This was because the second exothermic peak in the cement acceleration period was mainly caused by the C3S hydration [3]. The generation of ettringite crystals consumes a large amount of Ca(OH)2. The reduced concentration of Ca(OH)2 promoted the hydration of C3S and C2S and further promoted the setting and hardening of cement, which was beneficial to the development of mortar strength. Therefore, the accelerated pastes had higher early compressive strength. This indicates that the FF-AF-A promoted the hydration reaction of C3S, shortened the induction period of cement and the acceleration period of cement hydration ahead of schedule [4].
Fig. 4b indicates that the hydration heat of cement with the FF-AF-A clearly increased compared with the cement without the FF-AF-A within the first 0.5 h of cement hydration reaction. Fig. 4b also indicates that as the FF-AF-A dosage increases, the hydration heat of cement increases gradually. This was due to the fact that the FF-AF-A promoted the cement hydration reaction to produce the ettringite crystals, which leads to release of large amounts of hydration heat. Therefore, the higher the FF-AF-A dosage, the higher amount of ettringite crystals produced, and the higher amount of the hydration heat released. After 48 h of hydration, there was little or no difference in the hydration heat between the cement paste without FF-AF-A, cement paste with 4 wt% FF-AF-A, and cement paste with 8 wt% FF-AF-A. This was because the cement paste without FF-AF-A has also reached final setting and the hydration of cement is in a stable state, therefore, the hydration heat for three samples is not significantly different.

Figure 4: Effect of FF-AF-A on the hydration exothermic of cement pastes (a) Hydration exothermic rate of cement pastes (b) Hydration heat of cement pastes
To further illustrate the effect of the FF-AF-A on the hydration of cement, the structure of the reference cement without FF-AF-A and with 8 wt% FF-AF-A at different curing ages were analyzed by XRD, and the XRD patterns are shown in Fig. 5.
Fig. 5a shows the XRD pattern of the reference cement pastes without FF-AF-A at 7 min, 6 h, and 24 h curing ages. The hydration products were mainly unhydrated C3S, and C2S, as well as calcium hydroxide (Ca(OH)2) produced by the hydration of C3S. Ca(OH)2 was observed and ettringite crystals was not observed at the 7 min curing age. The XRD pattern at 6 h curing age showed the formation of ettringite crystals, however the peak of the ettringite crystals was relatively weak but the intensity of the peak of Ca(OH)2 became stronger. In particular, there was an increasing number of Ca(OH)2 over time. The XRD pattern at 24 h curing age showed no significant change in the characteristic peak of the ettringite crystals. This could be related to the slow hydration of cement which is caused by the C3A surface being covered with ettringite crystals.
Fig. 5b shows the XRD pattern of the reference cement pastes with 8 wt% FF-AF-A at 7 min, 6 h, and 24 h curing ages. The XRD pattern showed characteristic peak of ettringite crystals at 7 min, which implies that the hydration of cement generates a certain amount of ettringite crystals; thus, resulting in the setting and hardening of the cement paste. The XRD pattern at 6 h showed an increase in the intensity of the characteristic diffraction peak of ettringite crystals, which means that the ettringite crystals amount was increased over hydration time. The XRD pattern at 24 h showed that the intensities of the characteristic diffraction peak of ettringite crystals remain strong. While the characteristic diffraction peak of Ca(OH)2 also appeared, they were weak compared to the cement without FF-AF-A. This is due to the generation of ettringite crystals consumed a large amount of Ca(OH)2 produced by the hydration of C3S. The reduced concentration of Ca(OH)2 promoted the hydration of C3S and C2S and further promoted the setting and hardening of cement, which was beneficial to the development of mortar strength [17]. Meanwhile, the amount of ettringite in cement paste mixed with FF-AF-A was higher than that in cement paste without FF-AF-A. Therefore, the accelerated pastes had higher early compressive strength.
Fig. 6 shows the XRD pattern of the reference cement without FF-AF-A and with 8 wt% FF-AF-A at 28 d curing ages. The hydration products were mainly Ca(OH)2 and ettringite crystals. Compared with cement without FF-AF-A, the characteristic diffraction peak of ettringite crystals of cement with 8 wt% FF-AF-A was more noticeable, but the characteristic diffraction peak of Ca(OH)2 were weak. This is because most of the Ca2+ in the cement without FF-AF-A reacted to form Ca(OH)2 crystals, and only a small part of the Ca2+ formed ettringite crystals. However, FF-AF-A addition accelerated the hydration of cement and generated a large amount of ettringite crystals, and consumed a large amount of Ca2+; thus, the amount of Ca(OH)2 generated was small.
Overall, test results indicated that the addition of the FF-AF-A to cement paste accelerated the hydration process and changed the amount of cement hydration products, but it did not change the type of hydration products as shown in Figs. 5 and 6.
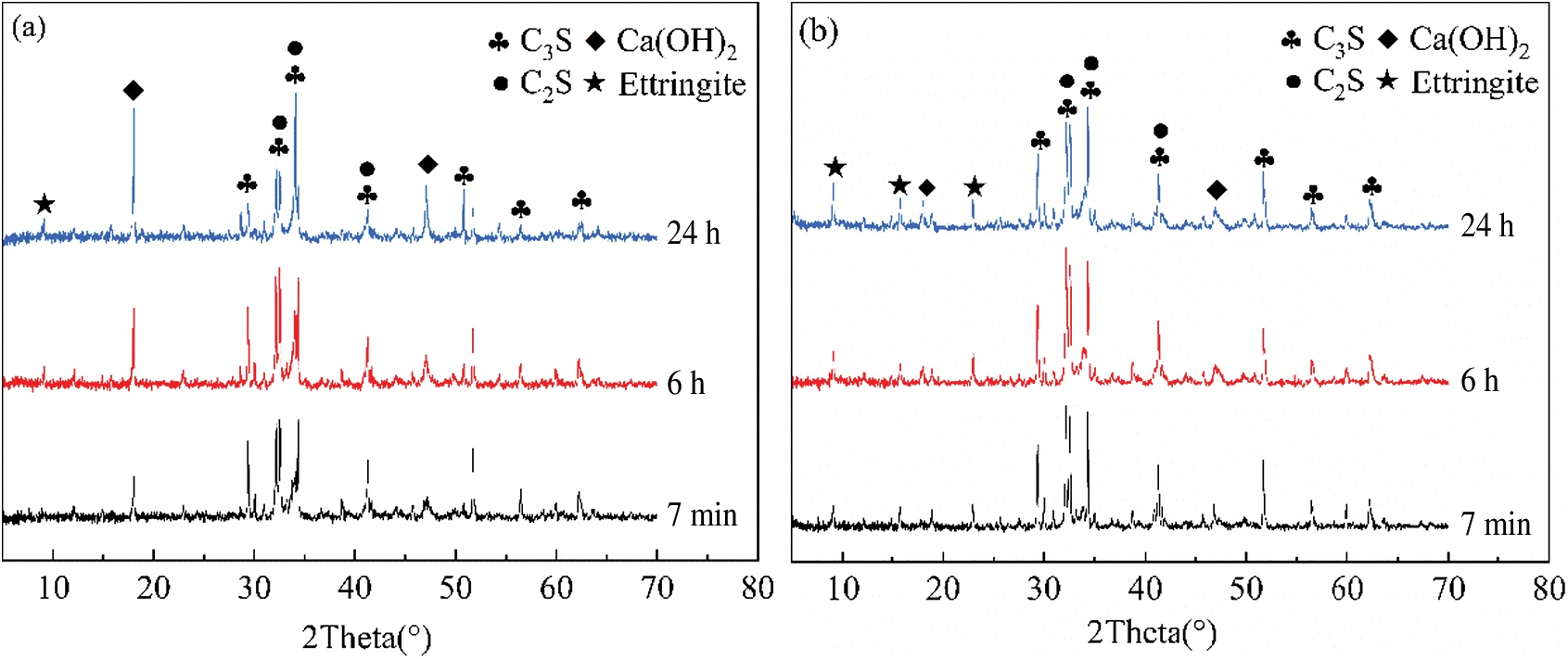
Figure 5: XRD pattern of early hydration process of cement paste: (a) Without FF-AF-A, (b) With 8% FF-AF-A
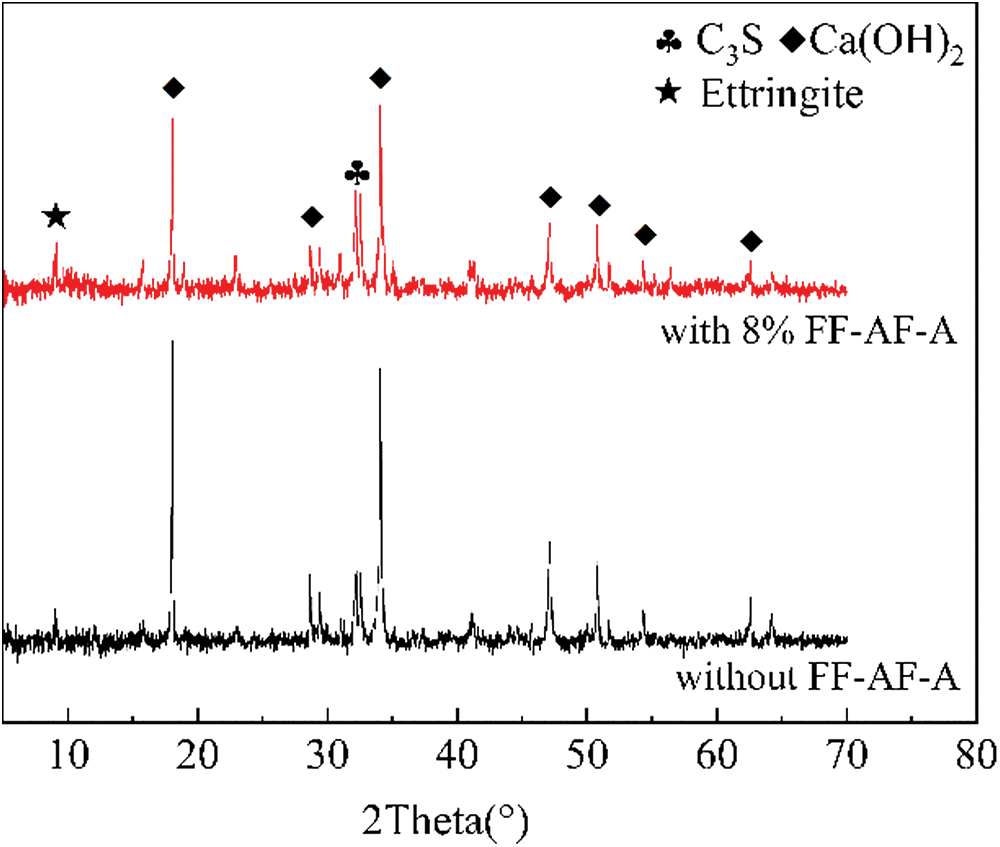
Figure 6: XRD pattern of 28 days of cement paste
In order to study the accelerating mechanism of the FF-AF-A, the microstructure SEM analysis was conducted on the hydration products of the reference cement with and without the FF-AF-A. Fig. 7 shows the SEM images of the hydration products of the reference cement paste at 7 min, 6 h, 24 h, and 28 d curing ages.
Figs. 7a–7d show the microstructure and morphology of the hydration products of the reference cement paste without the FF-AF-A. Ca(OH)2, C-S-H gels, and ettringite crystals were not visible at 7 min of cement hydration, and only some crystal whiskers were deposited on the surface of the cement particles, as shown in Fig. 7a. At the 6 h hydration time shown in Fig. 7b, it can be seen that the hydration products became denser, but there was no clear ettringite crystals production and at this time the structure of the cement paste is fluffy. At the 24 h hydration time shown in Fig. 7c, little needle-like ettringite crystals appeared on the surface of the cement particles to reduce the voids in the cement paste, but they did not form a relatively compact hydrated structure. Finally, at the 28 d hydration time shown in Fig. 7d, almost no ettringite crystals were discerned, and only a large number of Ca(OH)2 were stacked together running through the C-S-H gels.
Figs. 7e–7h show the microstructure and morphology of the hydration products of the reference cement paste with 8 wt% FF-AF-A. At the 7 min hydration time shown in Fig. 7e, a certain amount of short rod-like ettringite crystals were interdigitated, but the overall structure is not yet dense. This indicates that the FF-AF-A is used to shorten the cement setting time by accelerating the hydration reaction of C3A. At the 6 h hydration time shown in Fig. 7f, and when compared with the cement paste without FF-AF-A, it is clear that hydration products increased significantly, and not only ettringite crystals became longer and with higher quantities, but also a few petaloid Ca(OH)2 interpenetrating in the hydrogel also appeared. Cement particles were densely packed together without visible voids and the overall structure was more tightened. At the 24 h hydration time shown in Fig. 7g, a large number of rod-like ettringite crystals intricately filled with cement paste. Moreover, some C-S-H gels and hydration products grew on the surface of the cement particles, forming a very dense internal structure that substantially strengthens the early strength of the cement. Finally, at the 28 d hydration time shown in Fig. 7h, and in addition to the small amount of Ca(OH)2 that can be seen, a large number of rod-shaped ettringite crystals can be seen running through the C-S-H gels, which effectively ensures the final strength of the cement paste.
All things considered, test results demonstrate that the proposed FF-AF-A significantly increased Al3+ and SO42- contents, and produced a large number of ettringite crystals. More importantly, these needles and rod-like ettringite crystals copurified with other hydration products which resulted in forming a complex three-dimensional space structure, densified cement paste structure, shortened the cement paste setting time, and improved the compressive strength of the cement mortar.

Figure 7: SEM image of the cement paste. (a) Cement paste hydration for 7 min. (b) Cement paste hydration for 6 h. (c) Cement paste hydration for 24 h. (d) Cement paste hydration for 28 d. (e) Cement paste mixed with FF-AF-A 7 min. (f) Cement paste mixed with FF-AF-A 6 h. (g) Cement paste mixed with FF-AF-A 24 h. (h) Cement paste mixed with FF-AF-A 28 d
Based on the results of the study, the following conclusions are drawn:
1. A FF-AF-A for shotcrete was prepared. the addition of 8 wt% of the FF-AF-A to the reference cement paste resulted in 2 min 35 s and 6 min 30 s of initial setting time and final setting time, respectively. In addition, the FF-AF-A has shown good compatibility with different cement types and superplasticizer dosages.
2. The FF-AF-A efficiently increased the early and final compressive strength of the cement mortar. The 1-day compressive strength of reference cement mortar with 8 wt% FF-AF-A reached 13.5 MPa, which represents an increase of 35% as compared to the strength of reference cement mortar without FF-AF-A. The 28-day compressive strength ratio reached 119%.
3. The FF-AF-A accelerated the hydration reaction of the C3A through the reaction of Al3+ with Ca2+ and SO42- forming ettringite crystals, increased the early hydration exothermic rate and hydration heat of cement, and shortened the cement setting time. In addition, rod-like ettringite crystals formed the space network structure, densified cement paste structure, and improved the compressive strength of the cement mortar.
Funding Statement: The authors are grateful funding provided by National Key Research and Development Program of China (Project 2019YFC1906202), Guangxi Key Research and Development Plan (Guike AB19259008) and Major Science and Technology Special Project of Guangxi Province (Guike AA18242007-3).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, J., Niu, D., Zhang, Y. (2015). Mechanical properties, permeability and durability of accelerated shotcrete. Construction and Building Materials, 95(5), 312–328. DOI 10.1016/j.conbuildmat.2015.07.148. [Google Scholar] [CrossRef]
2. Liu, X., Ma, B., Tan, H., Gu, B., Zhang, T. et al. (2020). Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Construction and Building Materials, 232(2), 117179. DOI 10.1016/j.conbuildmat.2019.117179. [Google Scholar] [CrossRef]
3. Yang, R., He, T., Guan, M., Guo, X., Xu, Y. et al. (2020). Preparation and accelerating mechanism of aluminum sulfate-based alkali-free accelerating additive for sprayed concrete. Construction and Building Materials, 234(11), 117334. DOI 10.1016/j.conbuildmat.2019.117334. [Google Scholar] [CrossRef]
4. An, M., Zhang, G., Han, S. (2019). Research on the Influence mechanism of accelerator main components on cement hydration. Journal of Railway Engineering Society, 36(11), 74–81. DOI 10.3969/j.issn.1006-2106.2019.11.013. [Google Scholar] [CrossRef]
5. Salvador, R. P., Cavalaro, S. H. P., Segura, I., Figueiredo, A. D., Perez, J. (2016). Early age hydration of cement pastes with alkaline and alkali-free accelerators for sprayed concrete. Construction & Building Materials, 111, 386–398. DOI 10.1016/j.conbuildmat.2016.02.101. [Google Scholar] [CrossRef]
6. Zeng, L., Qiao, M., Wang, W., Zhao, S., Zhang, X. et al. (2020). Mechanical and interface properties of steel fiber reinforced shotcrete containing different setting accelerators. Journal of the Chinese Ceramic Society, 48(5), 659–664. [Google Scholar]
7. Zhang, J., Liu, J. (2012). A study of the effects of liquid setting accelerator on sprayed concrete durability. Modern Tunnelling Technology, 49(5), 169–174. DOI 10.3969/j.issn.1009-6582.2012.05.029. [Google Scholar] [CrossRef]
8. Qiu, Y., Ding, B., Gan, J., Guo, Z., Zheng, C. et al. (2017). Mechanism and preparation of liquid alkali-free liquid setting accelerator for shotcrete. IOP Conference Series: Materials Science and Engineering, 182(1), 012034. DOI 10.1088/1757-899X/182/1/012034. [Google Scholar] [CrossRef]
9. Sheng, Y., Xue, B., Li, H., Qiao, Y., Chen, H. et al. (2017). Preparation and performance of a new-type alkali-free liquid accelerator for shotcrete. Advances in Materials Science and Engineering, 2017(5), 1–9. DOI 10.1155/2017/1264590. [Google Scholar] [CrossRef]
10. Galan, I., Baldermann, A., Kusterle, W., Dietzel, M., Mittermayr, F. (2019). Durability of shotcrete for underground support-Review and update. Construction and Building Materials, 202, 465–493. DOI 10.1016/j.conbuildmat.2018.12.151. [Google Scholar] [CrossRef]
11. Won, J. P., Hwang, U. J., Kim, C. K., Lee, S. J. (2013). Mechanical performance of shotcrete made with a high-strength cement-based mineral accelerator. Construction and Building Materials, 49(6), 175–183. DOI 10.1016/j.conbuildmat.2013.08.014. [Google Scholar] [CrossRef]
12. Paglia, C., Wombacher, F., Böhni, H. (2003). The influence of alkali-free and alkaline shotcrete accelerators within cement systems: Influence of the temperature on the sulfate attack mechanisms and damage. Cement and Concrete Research, 33(3), 387–395. DOI 10.1016/S0008-8846(02)00967-5. [Google Scholar] [CrossRef]
13. Nie, Z., Ouyang, H., Ren, J., Wang, Y., Han, R. (2020). Study on reaction mechanism and cement adaptability of alkali-free liquid accelerator. Guangzhou Chemical Industry, 48(22), 80–82. DOI 10.3969/j.issn.1001-9677.2020.22.027. [Google Scholar] [CrossRef]
14. Kong, X., Lu, Z., Yan, J., Liu, H., Wang, D. (2013). Influence of triethanolamine on elemental concentrations in aqueous phase of hydrating cement pastes. Journal of the Chinese Ceramic Society, 41(7), 981–986. DOI 10.7521/j.issn.0454-5648.2013.07.16. [Google Scholar] [CrossRef]
15. Li, H., Xu, C., Dong, B., Chen, Q., Gu, L. et al. (2020). Differences between their influences of TEA and TEAHCl on the properties of cement paste. Construction and Building Materials, 239(3), 117797. DOI 10.1016/j.conbuildmat.2019.117797. [Google Scholar] [CrossRef]
16. Han, J., Wang, K., Shi, J., Wang, Y. (2015). Mechanism of triethanolamine on Portland cement hydration process and microstructure characteristics. Construction and Building Materials, 93(3), 457–462. DOI 10.1016/j.conbuildmat.2015.06.018. [Google Scholar] [CrossRef]
17. Wang, Z., Wang, J., Gan, J., Zhang, J. (2020). Current state of hydration reaction characteristics and setting/hardening mechanism of cement-accelerator-water system. Bulletin of the Chinese Ceramic Society, 39(10), 3045–3054. DOI 10.16552/j.cnki.issn1001-1625.20200610.001. [Google Scholar] [CrossRef]
18. Chen, Z., Wu, Y., Peng, P., Huang, Z., Chen, M. et al. (2020). Effect of fluoro-aluminum complexes on the properties of aluminum sulfate type accelerator. Materials Reports, 34, 178–180. http://www.mater-rep.com/EN/Y2020/V34/IZ1/178. [Google Scholar]
19. Yang, L., Tian, J., Yang, Y., Sun, L. (2017). State-of-art of research on liquid accelerators for shotcrete. Tunnel Construction, 37(5), 543–552. DOI 10.3973/j.issn.1672-741X.2017.05.004. [Google Scholar] [CrossRef]
20. Gu, C., Ren, H., Ren, Q., Zhang, B. (2019). Preparation and performance of alkali-free liquid accelerator with different Al-F complexes. New Building Materials, 46(6), 123–126. DOI 10.3969/j.issn.1001-702X.2019.06.031. [Google Scholar] [CrossRef]
21. Yu, S., Feng, E., Li, C., Huang, L., Li, S. (2019). Study on preparation and mechanism of low-sulfur alkali-free flash setting admixture. The World of Building Materials, 40(4), 1–5. DOI 10.3963/j.issn.1674-6066.2019.04.001. [Google Scholar] [CrossRef]
22. Salvado, R. P., Cavalaro, S. H. P., Rueda, A., Figueiredo, A. D. D. (2014). Effect of cement composition on the reactivity of alkali-free accelerating admixtures for shotcrete. International Symposium on Sprayed Concrete. Sandefjord, Norway. [Google Scholar]
23. Li, Y., Cai, B., Wu, Y., Chen, Z., Jin, C. (2020). Research progress of flash setting admixtures for shotcrete. Concrete, 9, 121–125. DOI 10.3969/j.issn.1002-3550.2020.09.029. [Google Scholar] [CrossRef]
24. Qian, X., Yu, C., Zhang, L., Qian, K., Fang, M. et al. (2020). Influence of superplasticizer type and dosage on early-age drying shrinkage of cement paste with consideration of pore size distribution and water loss. Journal of Wuhan University of Technology (Materials Science), 35(4), 758–767. DOI 10.1007/s11595-020-2318-1. [Google Scholar] [CrossRef]
25. Zeng, L., Qiao, M., Wang, W., Ran, Q., Hong, J. (2020). Effect of alkali-free accelerators on early age hydration of Portland cement. http://kns.cnki.net/kcms/detail/31.1764.TU.20191210.1413.004.html. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |