
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.016092
ARTICLE
Study of Burning Behaviors and Fire Risk of Flame Retardant Plywood by Cone Calorimeter and TG Test
1College of Forestry, Guizhou University, Guiyang, 550025, China
2Yunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming, 650224, China
3ENSTIB-LERMAB, University of Lorraine, Epinal, 88051, France
4IUT-LERMAB, University of Lorraine, Epinal, 88000, France
*Corresponding Authors: Zhigang Wu. Email: wzhigang9@163.com; Bengang Zhang. Email: zhangbengang390@gmail.com
Received: 05 February 2021; Accepted: 11 March 2021
Abstract: A flame retardant composition was prepared by using phosphoguanidine, guanidine sulfamate, disodium octaborate tetrahydrate and dodecyl dimethyl benzyl ammonium chloride. Veneers were immersed in such flame retardant mixture to prepare plywood. The combustion characteristics and thermal stability of plywood were assessed using a cone calorimeter and TG. Results showed that: (1) High concentration and loading of flame retardant were beneficial for the fire resistance of the plywood. (2) The limiting oxygen index (LOI) and residual mass of plywood processed using the flame retardant was increased by 87.52% and 58.66% compared to those of the untreated plywood, while the average heat release rate (av-HRR), total heat release (THR), effective heat of combustion (EHC), total smoke release (TSR), CO yield (COY), CO2 yield (CO2Y) and oxygen consumption were decreased by 44.3%, 82.9%, 47.0%, 86.0%, 89.9%, 50.1% and 83.1%, respectively. (3) Treated plywood which had a low fire growth index (FGI) displayed a later combustion heat release rate peak and slower flame spread than observed for the untreated material. Combustion of treated plywood displayed a higher fire performance index (FPI), indicating a longer time to ignition. This suggests that burning structures from this material would be subject to a longer time for escape from the structure and would present lower fire risk than similar structures containing treated plywood. (4) TG results demonstrated that the presence of the flame retardant can decrease the pyrolysis temperature for hemicellulose and cellulose, change the decomposition and reaction progress for plywood degradation and promote dehydration carbonization and accelerated charformation. Moreover, the formed char was more stable than that combustion of untreated plywood. (5) The flame retardant contains nitrogen (N), phosphorus (P), boron (B), chlorine (Cl) and guanidine (Gu) compounds. The adhesive also contains N and P compounds. These substances display flame resistance and supplement each other to generate flame retardance than any one used alone. By changing the thermolysis and thermal decomposition processes, the heat release and smoke release from plywood, undergoing combustion was reduced. This controlled generation of combustible substances and promoted dehydration and carbonization to form char. As a result, the flame resistance of plywood was improved significantly. The probability of smoke asphyxia or poisoning death of those trapped in structures containing treated plywood during fire accidents can be decreased dramatically.
Keywords: Flame retardant; plywood; cone calorimeter; burning behaviors; fire risk
Nomenclature
| LOI: | limiting oxygen index |
| HRR: | heat release rate |
| av-HRR: | average heat release rate |
| EHC: | effective heat of combustion |
| Av-EHC: | average effective heat of combustion |
| TSR: | total smoke release |
| COY: | CO yield |
| CO2Y: | CO2 yield |
| FPI: | fire performance index |
| FGI: | fire growth index |
| TG: | thermogravimetric |
Plywood, fiberboard and particleboard are the main products of the wood-based panel industry. Plywood has the longest development history and displays many excellent properties, such as high strength, easy processing and maintenance of the patterns and textures of natural timbers. Moreover, plywood is subject to relatively lower energy requirements and greenhouse gas emissions during consumption compared to that for fiberboard and particleboard. As a result, plywood has occupied a dominant role in the wood-based panel industry and has been widely applied in indoor decoration, furniture, aspects of the building industry, and so on [1–5]. The demands for plywood have been strongly increasing. However, plywood is a combustible material. It is not only combustible, but also can release abundant heat during combustion, which results in accelerated flame spread. Therefore, flame retardant treatment for plywood is an effective way to decrease fire hazards using associated with its use [6–9].
Gay-Lussac processed timbers using ammonium phosphate, ammonium chloride and borax This represents the first example of the fire retarding treatment of wood. This approach has been tested over time and is still used today [10]. With the continuous development of fire retardant technology, compounds containing many elements have been applied to enhance fire resistance of wood. Among such compounds, these containing N, P, B, Si, Mg, Al and Br have been utilized as flame retardant. Because of non-toxicity, smoke inhibition, low cost, inducement of good flame resistance and susceptibility to simple processing, flame retardants containing P, N and B have been of major interest as flame retardants for wood [11–13]. CunninghamiaLanceolata immersed in boric acid solution has been used for the treatments of wood. This treatment decreased reaction rate of pyrolysis and the treated specimen displayed good flame resistance [14]. Douglas fir treated with boric acid and borax, displayed lower combustion and mass loss during combustion than did untreated material [15]. Low pressure processing of timbers using aqueous 5% diammonium hydrogen phosphate and ammonium sulfate generated material with longer TTI than that for control timbers. Combustion was accompanied by lower heat release and volatile combustible product yield than observed for control timbers [16]. A P-N-B FRW flame retardant prepared by Northeast Forestry University not only displayed good fire resistanceand the level of fire retardance for timbers was increased to B1 level, but also exhibited corrosion resistance and insect control [17]. Eucalyptus immersed in ammonium polyphosphate, boric acid and borax solution, generated material with decreased THR and TSR for combustion compared to that for untreated material. Ammonium polyphosphate, boric acid and borax may have cooperative effects on retardation and smoke inhibition [18].
Guanidine compounds may surve as high-efficiency and low-toxicity bactericides.These include phosphoguanidine, guanidine sulfate and guanidine sulfamate. This compounds have been utilized in several areas, such as medical treatment and public health, agricultural product protection and foods. Moreover, guanidine compounds which contain P and C have some flame retardant properties. A flame additive retardant based on phosphoguanidine induces good fire resistance. A flame retardant based on guanidine sulfamate imparts excellent combustion inhibition [19–21].
In this study, a flame retardant composition was prepared using phosphoguanidine, guanidine sulfamate, disodium octaborate tetrahydrate and dodecyl dimethyl benzyl ammonium chloride. Combustion characteristics including HRR, mass loss rate, oxygen content, CO2 content and thermal stability of plywood treated with the prepared flame retardant formulation were evaluated using cone calorimetoy and TG [22,23]. The results may be used to evaluate the combustion behavior of treated plywood in a real fire environment.
Phosphoguanidine (with a purity of 98%), disodium octaborate tetrahydrate (with a purity of 99.5%), guanidine sulfamate (with a purity of 99%) and dodecyldimethylbenzylammoniumchloride (with a purity of 99%) were purchased from Sinopharm Chemical Reagent Co., Ltd., China). All other chemicals mentioned in this work were reagent grade. Powdery urea formaldehyde resin (C360), which was used by mixing with water (mass ratio of resin power to water was 100:80) and then adding 0.5% ammonium chloride for the preparation of plywoods, was purchased from Malaysia. Pinus massoniana wood with a size of 2200 mm (length) × 130 mm (width) × 2.5 mm (thickness) and moisture content 10%–14% was purchased from Rongjiang Guizhou, China, and after drying, knotless, and normally grown sapwood (without reaction wood, decay or insect or fungal damages) materials were selected.
2.2 Preparation of Flame Retardant
Flame retardants FR1 and FR2 were selected based on pre-experiments. The concentration of FR1 was 12%, which was composed of 5% phosphoguanidine, 5% guanidine sulfamate, 1% disodium octaborate tetrahydrate and 1% dodecyldimethylbenzylammoniumchloride. The concentration of FR2 was 14%, which was composed of 5% phosphoguanidine, 5% guanidine sulfamate, 3% disodium octaborate tetrahydrate and 1% dodecyldimethylbenzylammoniumchloride. Distilled water was the solvent.
2.3 Preparation of Flame Retardant Veneer
Pinus massoniana veneers were dried in an oven (101-1AB electric blast drying oven) at 60 ± 5°C until reaching constant weights and then moved out and stored in glass drier to cool down to the room temperature, weighted and then put in vacuum chamber. The flame retardant was poured into the chamber until 5 cm higher than the surface of the piles of wood samples under vacuum conditions (–0.09 MPa, 60 min). Next, the samples were taken out and the surface liquids of each wood sample was removed gently by a piece of filter paper. The wood samples were put in indoor environment for about one week and then dried in an oven at 90 ± 5°C until the moisture content at 5%~9%.
2.4 Preparation of Five-Layer Plywood
The flame retardant veneers with a double-sided adhesive loading of 220 g/m2 were rested at room temperature for 15–20 min. The assembled veneers were then exposed to single-layer hot press unit (XLB type) at Shanghai Rubber Machinery Plant and pressed with a pressure of 1.5 MPa at 100°C for 15 min to obtain a plywood panel. FR1-plywood and FR2-plywood were gotten, respectively.
2.5 Limiting Oxygen Index (LOI)
With references to GB/T 2406.2-2009 Oxygen index test of combustion behaviors of plastics, oxygen index was tested by a TTech-GBT2406-2 intelligent oxygen index analyzer.
Cone calorimeter tests were performed according to the procedures indicated in the ISO 5660-1-2015 standard using a Fire Testing Technology cone calorimeter FTT2000 (Fire Testing Technology, Ltd., UK). The plywood panel was conditioned in the laboratory at 20 ± 2°C and relative humidity of 65 ± 5% for 1 day and then cutting into specimens with dimensions of 100 mm (length) × 100 mm (width) × 10 mm (thickness) prior to testing. The fire scenario was comprised of four steps: ignition, growth, fully developed, and decay. The tests were conducted with 50 kW/m2 of heat flux which corresponded to the fully developed step.
According to the results of cone calorimeter tests, the heat release rate (HRR), total heat release (THR), effective heat of combustion (EHC), total smoke release (TSR), CO yield (COY), CO2 yield (CO2Y) and total oxygen consumption (TOC) were gotten, and fire performance index (FPI) and fire growth index (FGI) were also calculated.
2.7 Thermogravimetric Analysis (TG)
A thermogravimetric (TG) analyzer (NETZSCH; Germany) was used for evaluating the thermal resistance of the samples under nitrogen atmosphere at a heating rate 10 °C/min from room temperature up to 700°C.
3.1 The Limiting Oxygen Index (LOI) Analysis
LOI of plywood is shown in Fig. 1. Flame resistance of timbers is generally measured by LOI and it refers to the lowest oxygen concentration needed for maintaining combustion for timbers. The higher LOI implies the greater difficulties in combustion; otherwise, the lower LOI indicates that the timbers are easier for combustion. LOI of the untreated plywood was 26.3 vol%, indicating that the plywood was combustible. LOIs of FR1-plywood and FR2-plywood were increased by 73.6% and 87.5% compared with that of the untreated plywood to 45.6 vol% and 49.3 vol%. This proved that the flame resistance of plywood after flame retardant treatment was improved significantly and the plywood almost reached the flame retardant level. The LOI of FR2-plywood was higher than that of FR1-plywood, which was related with the high concentration of FR2 and high loading.
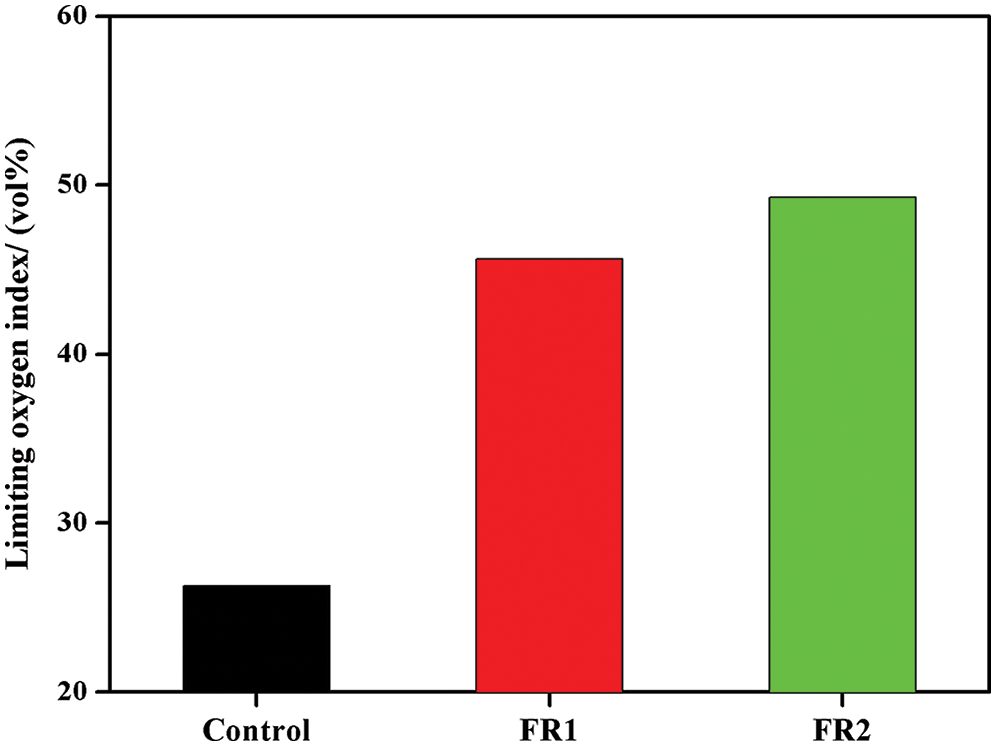
Figure 1: Limiting oxygen index of plywoods
3.2 Heat Release Rate (HRR) Analysis
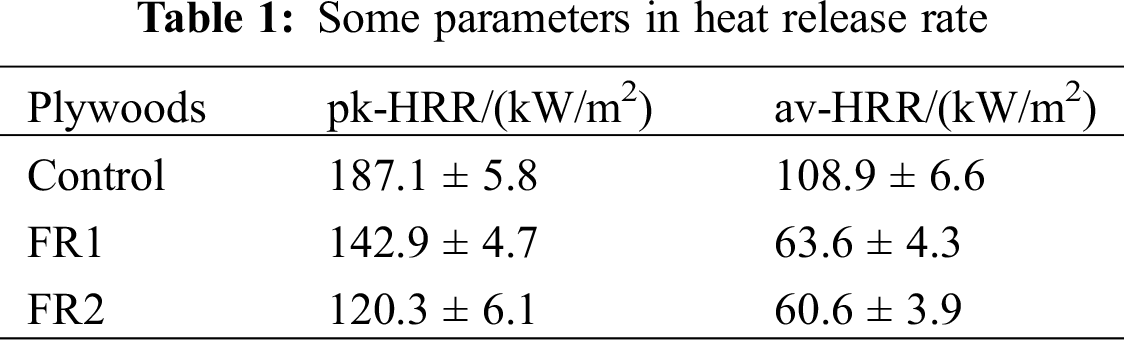
HRR, pk-HRR and av-HRR are commonly used to evaluate combustion performances of materials. All of these indexes can reflect degrees of material combustion and output of volatile combustible products, and they can be used to evaluate the flame spreading trend and degree of fire hazards. The higher values of HRR, pk-HRR and av-HRR indicate the higher heat release of materials from combustion and the fire hazards are the larger. Heat release rate and parameters of plywood at combustion are shown in Fig. 2 and Tab. 1.
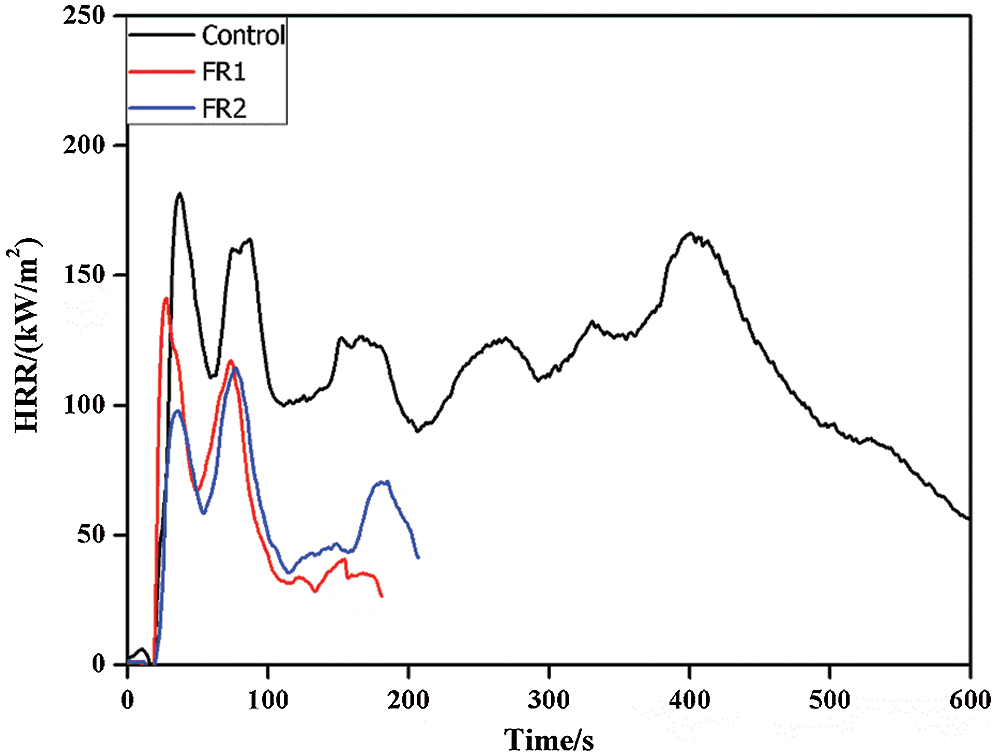
Figure 2: Heat release rate of plywoods
Plywood was burned under a cone calorimeter. The heat source was mainly from the radiation heat of the cone heater. Radiation heat of flame did not act on the wood directly. Instead, they supported continuous pyrolysis of timbers through the solid-phase conduction of heat on the surface carbon layer, and the carbon layer had certain fire retardation. Therefore, plywood had two peaks of combustion. The first peak was the carbonization of wood, in which there was a very serious pyrolysis of plywood and a lot of combustible gases were generated. The HRR reached the peak due to the flame combustion of combustible gasses. When the carbonization stage reached the extreme, the internal stress of the carbonization layer increased with the deepening of the carbonization layer. The carbonization layer developed fracturing phenomenon when the internal stress reached the extreme. Under this circumstance, the combustible gases in plywood were volatilized onto surface and HRR reached the second peak.
FR1-plywood and FR2-plywood both were quenched at about 200 s and stopped releasing heat (the FR2-plywood was about 60 s earlier than the FR1-plywood). On contrary, the untreated plywood continued to burn until complete carbonization. This was because flame retardant formed a continuous and dense carbon layer upon the contact of heat released from the combustion, which isolated oxygen and heat transmission in the air. Moreover, phosphoguanidine and guanidine sulfamate were decomposed during combustion and released a lot of inflammable gases to dilute combustible gases, which stopped rapid spreading of flames and accelerated char-forming, thus quenching the fire early. The combustion of plywood was quenched earlier when there were the higher concentration of flame retardant and the higher loading capacity.
pk-HRR of the untreated plywood was 187.13 kW/m2. In contrast, pk-HRR of FR-1 plywood and FR2-plywood were decreased by 20.3% and 35.7%, valuing 142.9 and 120.3 kW/m2. The av-HRR of the untreated plywood is108.9 kW/m2, which was 41.6% and 44.3% higher than those of FR1-plywood and FR2-plywood (63.6 kW/m2 and 60.6 kW/m2, respectively). HRR, pk-HRR and av-HRR of plywood after flame retardant treatment were lower than those of the untreated plywood, especially for the FR2-plywood. To sum up, the flame retardant significantly decreased heat release during combustion of plywood. Moreover, heat release of plywood was negatively related with concentration of flame retardant and loading.
3.3 Total Heat Release (THR) Analysis
THR refers to the total heat release of materials per unit area throughout the process from ignition to quenching. The higher value of THR indicates the higher fire hazards. The combustibility and flame resistance of materials can be evaluated better by combining HRR and THR. THR results during combustion of plywood are shown in Fig. 3. According to the HRR results, THR curves also could be divided into two stages, namely, carbonization stage and combustion stage. The THR curve in the carbonization stage was relatively steep, whereas the THR curve in the combustion stage was relatively stable. From the ignition to 60 s, plywood experienced strong thermolysis and generated a lot of combustible gases, thus resulting in the high growth rate of THR. Subsequently, the production of combustible gases of the untreated plywood was decreased. The combustible gases on the surface had been basically burned at 400 s. Later, the combustion heat after 400 s were mainly produced by the volatilization and combustion of combustible gases from inside of carbonized plywood to surface. The heat release in the carbonization stage accounted for 70% of total heat. Therefore, the heat released from combustion of plywood mainly came from this stage.
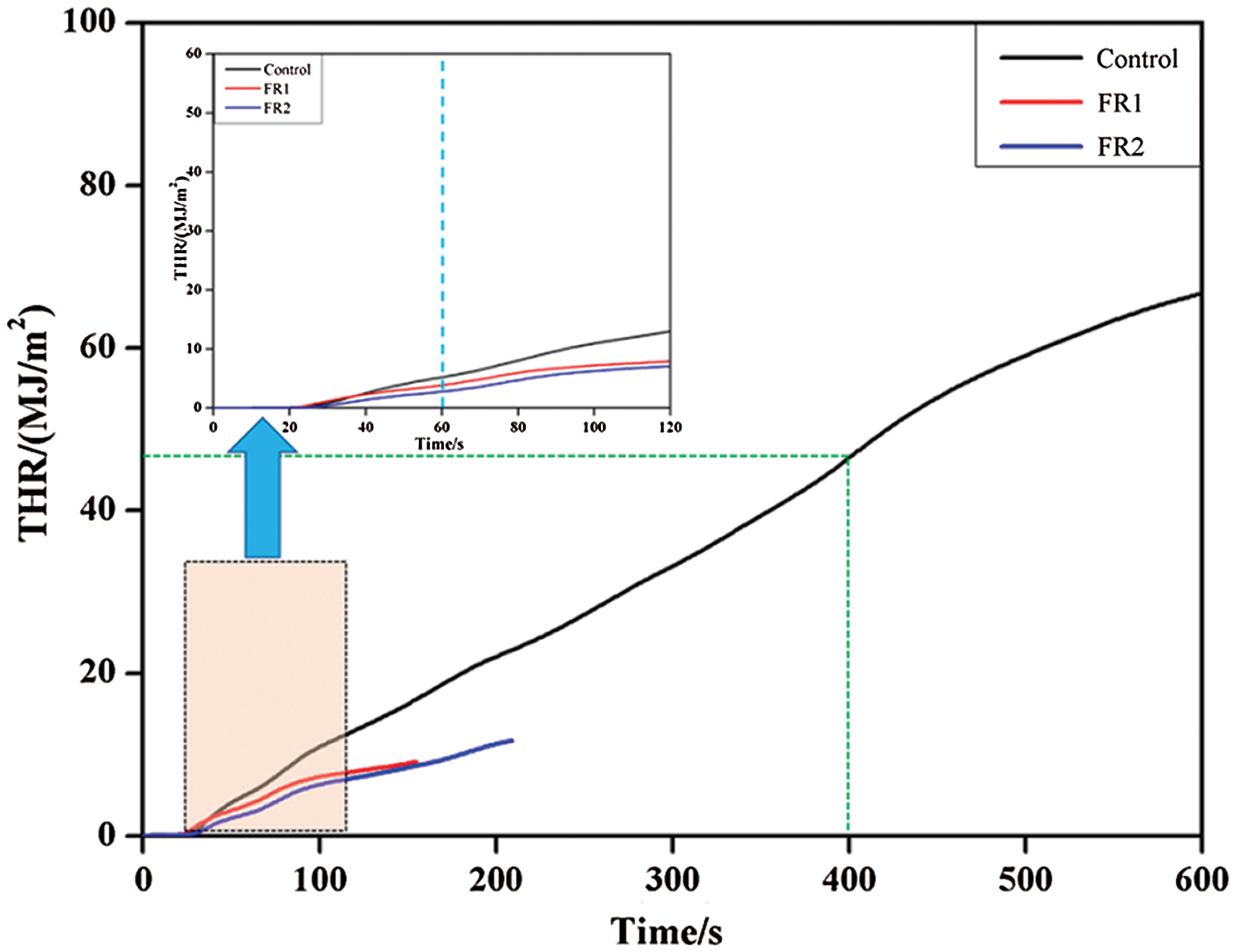
Figure 3: Total heat release of plywoods
The FR1-plywood and FR2-plywood stopped combustion in the carbonization stage. Moreover, the combustion time of FR1-plywood was nearly 60 s shorter than that of FR2-plywood. This was because that the boric acid content in FR2 was higher than that of FR1 and a dense carbon layer was formed in a relatively shorter time. The volatilization of internal combustible gases was decelerated and a non-flame combustion for about 60s was continued. In the whole combustion period, the THR of the untreated plywood was 69.2 MJ/m2, while THR of FR1-plywood and FR2-plywood were decreased by 86.2% and 82.9% to 9.6 kW/m2 and 11.9 kW/m2. Combining with results of HRR and THR, the plywood processed by the flame retardant not only had low heat release rate and low fire growth rate, but also showed evident quenching functions and decreased the fire hazards.
3.4 Effective Heat Combustion (EHC) Analysis
EHC refers to the heat release and mass loss ratio at a moment and it reflects the combustion degree of volatile gases in the meteorological flame. In other words, EHC refers to the quantity of effective combustion components in the gas phase. The EHC curves of plywood also presented the similar variation law with HRR and it could be divided into the carbonization stage and combustion stage. In the carbonization stage, plywood was completely carbonized. In this stage, combustion was mainly the thermal oxidation of carbon substances and further oxidization of products CO. The combustion heat per unit mass of carbon was high and the carbon layer was easy to be cracked and layered, accompanied with great instantaneous mass loss or heat release. So, the EHC curves in this stage fluctuated greatly and the numerical value was relatively high (Tab. 2 and Fig. 4).
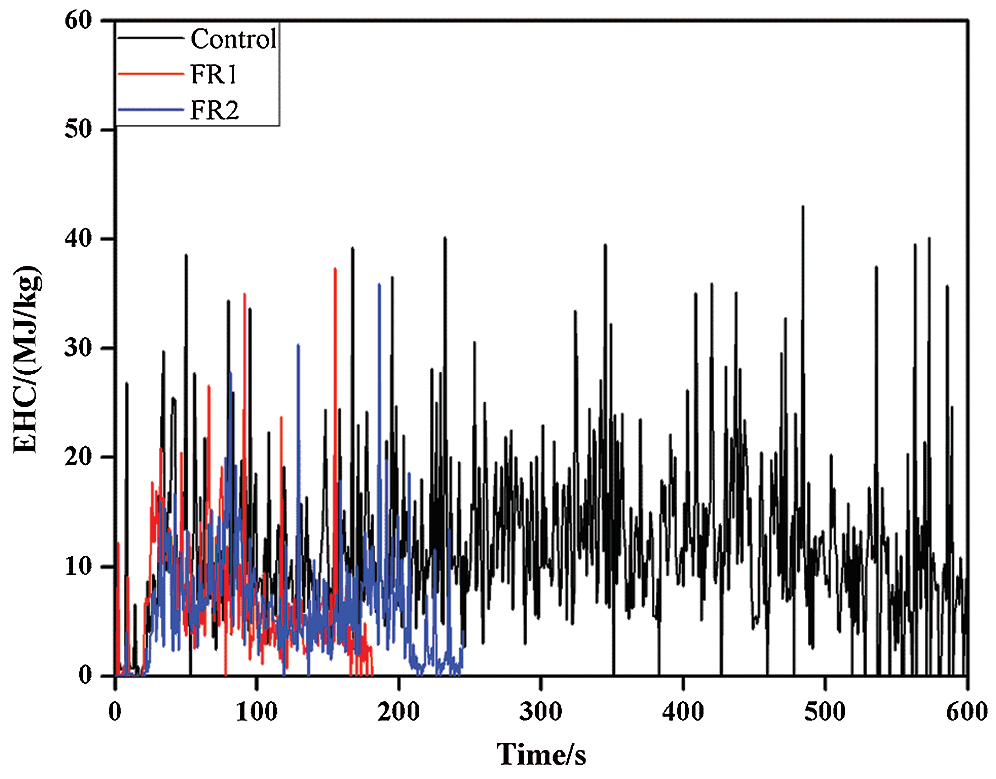
Figure 4: Effective heat combustion of plywoods
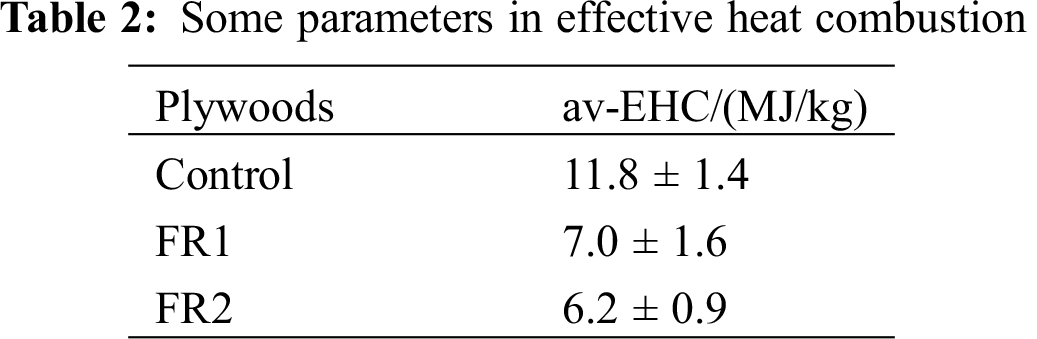
The average EHC (av-EHC) of the untreated plywood was 11.8 MJ/kg, while the av-EHC of FR1-plywood and FR2-plywood were decreased by 40.7% and 47.0% to 7.0 MJ/kg and 6.2 MJ/kg, respectively. This demonstrated that FR1 and FR2 inhibited the pyrolytic reaction of combustible volatile substances during heating of plywood to different extents, thus decreasing the concentration of combustible substances in volatile products. As a result, EHC decreased. In addition, phosphoguanidine and guanidine sulfamate in flame retardant released inflammable gases during the combustion process under heat, which further diluted the concentration of combustible gases. Moreover, phosphoguanidine formed phosphoric acids during the pyrolysis, thus further forming a sticky membrane layer of phosphoric acid and polyphosphoric acid on plywood surface. On one hand, it hindered the transmission of heat into plywood. On the other hand, it isolated oxygen and hindered flame propagation and spreading effectively. As a result, the flame resistance of plywood was improved.
The residual mass after combustion of plywood is shown in Fig. 5. The residual mass of the untreated plywood after combustion was 18.2%. The residual masses of FR1-plywood and FR2-plywood were 77.5% and 76.8%, which were 59.4% and 58.7% higher than that of the untreated plywood. In other words, flame retardant inhibited combustions of plywood effectively and plywood was difficult to have pyrolysis and had stronger char-forming growth rate during combustion. Therefore, plywoods processed using FR1 and FR2 could maintain high strength for a long time during the fire accidents, and the probability of death of those trapped in structures containing flame retardant treated plywood during fire accidents can be decreased dramatically, indicating that the safety was improved significantly.
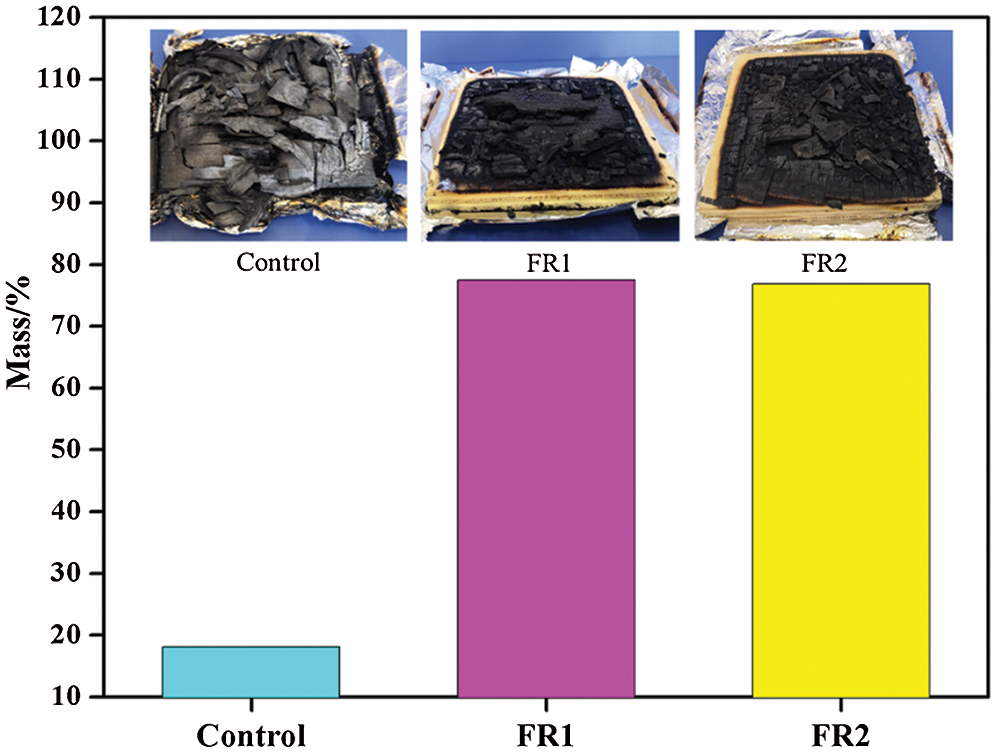
Figure 5: The residual mass plywoods after combustion
3.6 Smoking Properties Analysis
TSR, COY, CO2Y and oxygen consumption of plywood during the combustion are shown in Fig. 6. The TSR of the untreated plywood was very high, reaching 112.1 m2/m2. Fire accidents of plywood may cause serious consequences. The visibility of combustion environment dropped quickly and the smoke concentration increased quickly, which made people lost and fail to see the sign of exit. As a result, the speeds of evacuating, escaping and fighting decreased significantly. People would be poisoned more seriously if they stayed in such environment for a longer time, thus causing greater casualties. The borax substances contained in FR1 and FR2 had the stronger smoke inhibition effect. TSR of FR1-plywood and FR2-plywood were 38.4 m2/m2 and 15.8 m2/m2, which were 65.7% and 86.0% lower compared to that of the untreated plywood.
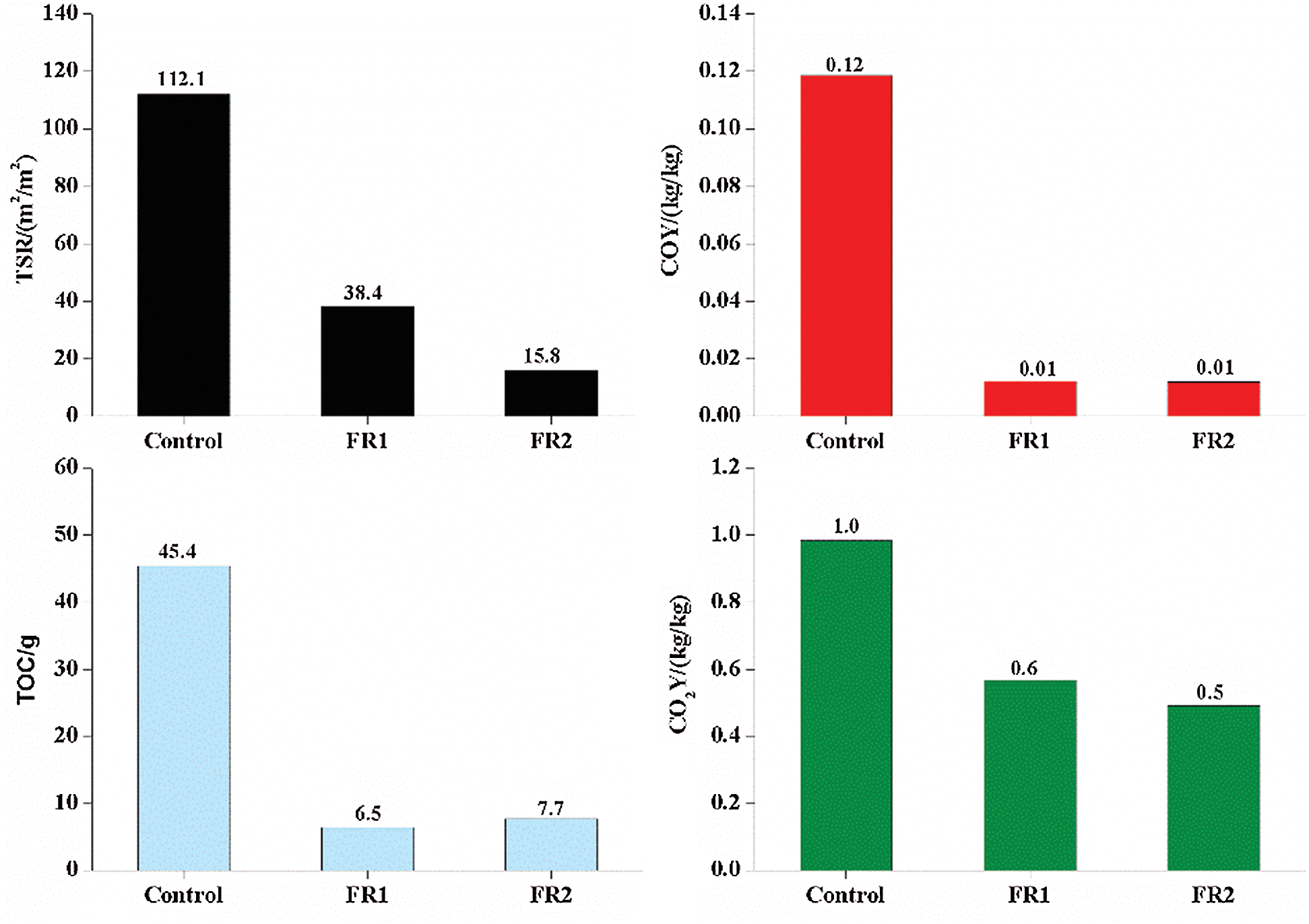
Figure 6: Smoking properties of plywoods
CO is the main poison gas which is generated from the combustion of wood materials and it is one of the primary toxicants that cause deaths. The combustion process of plywood was also the process of oxygen consumption. In early combustion, flame combustion took the dominant role and it required participation of oxygen. In this stage, it was mainly aerobic combustion and it would consume abundant external oxygen, accompanied with carbonization of plywood. In the combustion process, combustible gases were generated. With the gradual increase of combustible gases, these combustible gases diffused to surrounding air of plywood and decreased the oxygen concentration in air. Therefore, there was insufficient oxygen needed for complete combustion of timbers and plywood might have anaerobic combustion to generate toxic gases like CO. Plywood might develop fracturing phenomenon after reaching the carbonization to some extent, thus diffusing air and combustible gases in plywood. In this stage, oxygen concentration increased accordingly, while the consumed oxygen concentration and internally diffused oxygen concentration reached a balance state. With the intensification of reaction, oxygen continued to decrease and the secondary anaerobic combustion occured to produce CO again.
CO2Y represents the mass yield of CO2 per unit mass of plywood. The CO2Y increases, while COY decreases, indicating that the plywood is burned more completely and the toxicity of smoke is the weaker. CO2Y and COY of the untreated plywood were 1.0 kg/kg and 0.12 kg/kg, respectively. CO2Y and COY of the FR1-plywood were 0.6 kg/kg and 0.01 kg/kg, respectively. CO2Y and COY of the FR2-plywood were 0.5 kg/kg and 0.01 kg/kg, respectively. CO2Y and COY were decreased after flame retardant treatment. In particular, COY decreased more significantly. It indicated that the part of plywood after flame retardant treatment involved in the combustion was burned fully and the produced smoke had low toxicity, which won more time for escaping to some extent.
3.7 Evaluation of Fire Hazard of Plywoods
The potential fire hazard of plywood is evaluated comprehensively using FPI and FGI. FPI is the ratio between TTI and pk-HRR: FPI = TTI/pkHRR. It reflects the combustion tendency of plywood and has some practical significance whether materials are easy to develop flashover after ignition. The smaller FPI indicates the smaller fire hazard. FGI is the ratio between pk-HRR and the time of arrival: FGI = pkHRR/T. Given the larger FGI, it takes the shorter time to reach a high pk-HRR and the fire hazard are the higher. The FPI and FGI results of plywoods are shown in Fig. 7. FR2-plywood showed high FPI (0.2 s·m2·kW-1) and low FGI (1.6 kW·m-2·s-1), indicating the higher safety hazards.
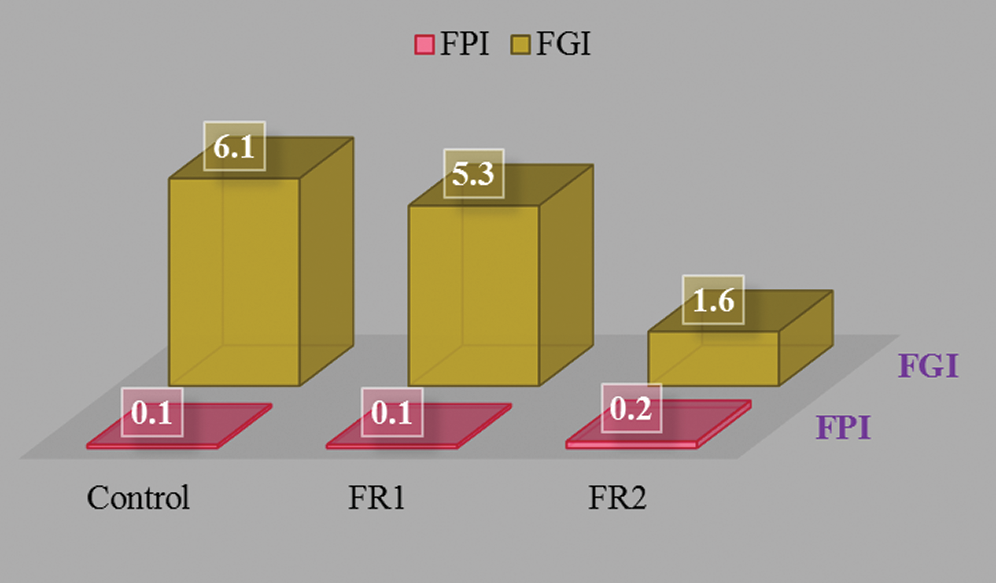
Figure 7: FPI and FGI of plywoods
The TG curves of plywood when the temperature rise rate is 10 K/min are shown in Fig. 8 and Tab. 3. Combining with HRR and TG-DTG curve features, thermolysis of plywood generally can be divided into four stages [24]. Stage 1: 30~150°C. This was the endothermic reaction. In this stage, mass loss rates of the untreated plywood, FR1-plywood and FR2-plywood were 3.6%, 3.8% and 3.9%, showing a small difference. Moreover, thermolysis rate of plywood was very slow. The evaporation of water in plywood took the dominant role, while the chemical composition of wood remained basically constant. Stage 2: 150~287°C. This was also an endothermic reaction. In this stage, mass loss rates of the untreated plywood, FR1-plywood and FR2-plywood were 16.6%, 23.0% and 17.6%, also showing a small difference. Moreover, thermolysis of plywood was relatively evident and hemicellulose was mainly decomposed into CO2, CO and few acetic acid. Stage 3: 287~45°C. This stage was a sharp decomposition of plywood and a lot of heat was released. It was the most dangerous heat release stage at combustion. In this stage, mass loss rates of the untreated plywood, FR1-plywood and FR2-plywood were 56.1%, 38.9% and 44.5%, respectively. A lot of decomposition products were formed and the liquid products contained acetic acids, methyl alcohol and wood tar. Among the produced gaseous products, there was a small yield of CO2, while the production of combustible gases like CO, CH4 and ethylene were increased. Char and nondecomposable ashes were left. Stage 4: 450~700°C. In this stage, the residual carbon surface and oxygen reacted to form solid-phase combustion with the increase of temperature and mass tended to be stable.
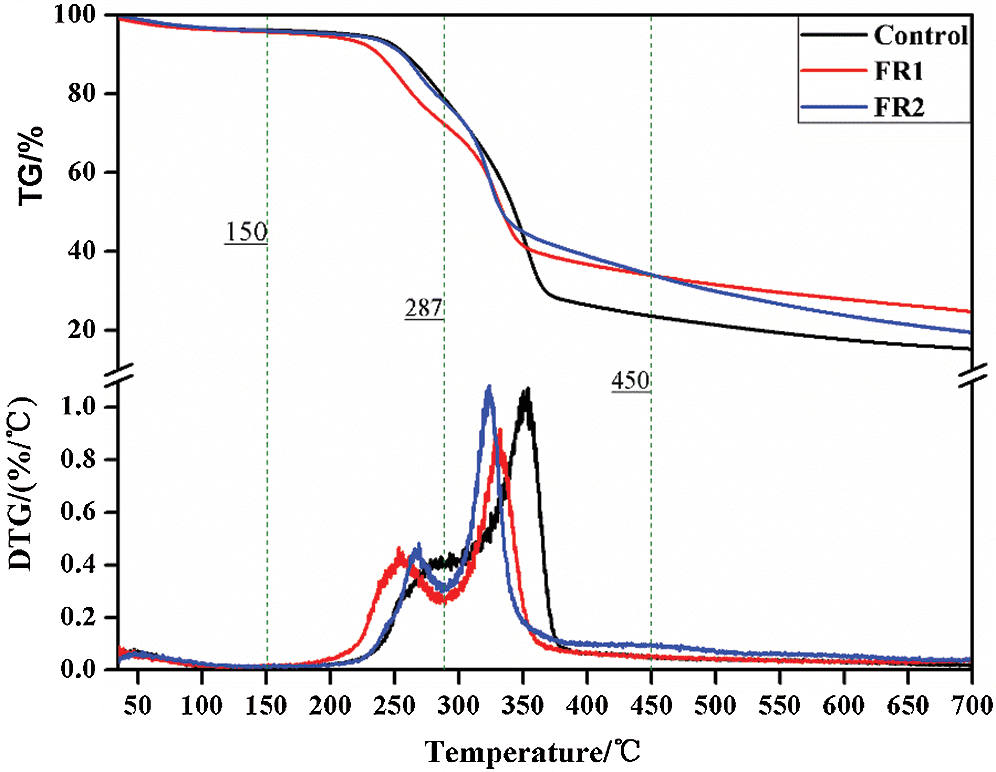
Figure 8: TG-DTG curves of plywoods
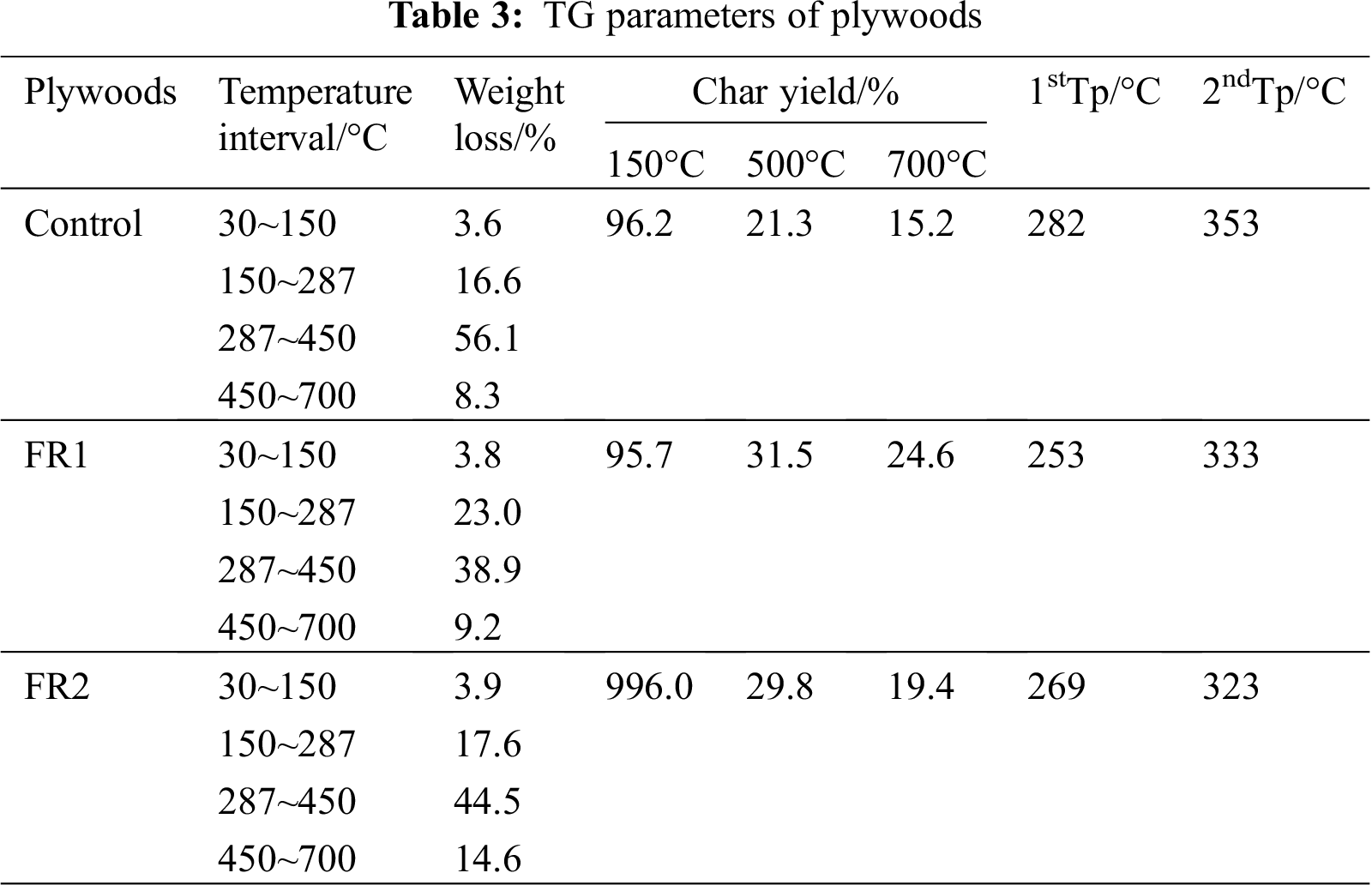
The temperatures for pyrolysis peaks of hemicellulose and cellulose of the untreated plywood were 282°C and 353°C, respectively. These two peaks of FR1-plywood were 253°C and 333°C, and the two peaks of FR2-plywood were 269°C and 323°C, respectively. This indicated that flame retardant decreased the pyrolysis temperature of plywood, and promote generation of combustible substances under a low ignition temperature. Before 352°C, the mass loss of the plywood treated using flame retardant was higher than that of the unprocessed plywood, but the opposite phenomenon was observed after 352°C. This indicated that flame retardant catalyzed the decomposition of wood and changed decomposition and reaction process of plywood, so that plywood developed toward the direction of generating more char and more stable char. As a result, flame retardant could promote dehydration, carbonization of timbers and accelerate char formation. It performed well in inhibiting flame spreading and heat production.
3.9 Analysis of Fire Hazard and Combustion Behavior of Plywood
It can be seen from Fig. 9 that fire hazards are mainly determined by the heat effect and smoke effect of plywood combustion. Heat effect referred to the heat damages to the human and environment which were caused by spreading of heat from combustion to the surrounding environment in the radiation, convection and conduction. Smoke effect referred to the damages to the human and environment caused by the smoke and poisonous gases from combustion of materials. Fire hazards of materials were actually the comprehensive performances of potential heat risks and smoke risks of materials. Therefore, a good flame retardant shall be able to decrease heat release and smoke release of plywood simultaneously.
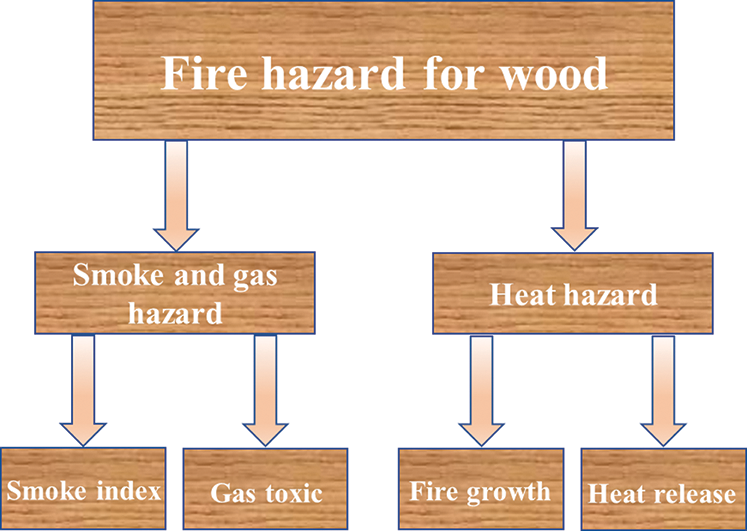
Figure 9: The analysis diagram of fire hazard of plywood
In the combustion test of a cone calorimeter, ignition occurred in the pyrolysis gases above the plywood and then flame diffused onto the whole surface. This process was a gas-phase ignition process of solid polymers and it belonged to a forced ignition. The plywood after ignition continued to pyrolysis under the effect of external heat flux and radiation heat of flames. The combustion thermal decomposition layer extended continuously toward the inside of plywood, while flame was always burning on the surface [25]. The flame combustion was maintained by the pyrolysis gases which were volatilized continuously from the inside. Due to the isolation of carbon layer on the surface, heat did not act on wood directly, but supporting continuous pyrolysis of timbers through solid-phase conduction of heat on the surface carbon layer. Due to the existence of carbon layer, the combustion process of plywood became more complicated. Some oxygen diffused onto the surface of carbon layer and the carbon layer could be oxidized, which was a heat release process and provided some heat to the timbers during pyrolysis. During the combustion process, the pyrolysis part and the unaffected part of plywood were on one interface which advanced continuously toward the unaffected timbers (Fig. 10).
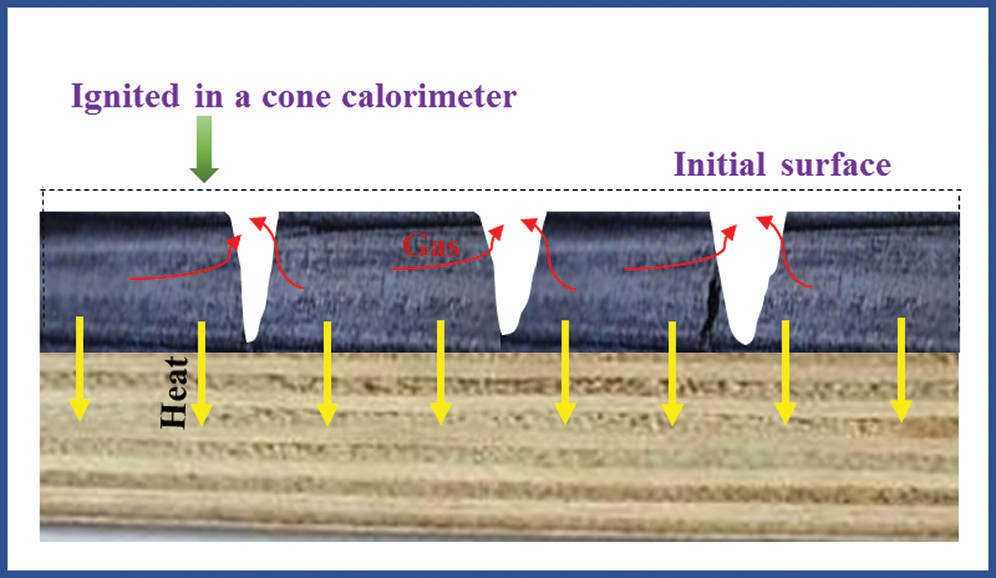
Figure 10: The analysis of plywood burning behavior
FR1 and FR2 contained N, P, B, Cl and guanidine compounds and they played different fire retardation effect during the combustion of plywood. Phosphorous compounds generated metaphosphoric acids in the thermal decomposition, which could accelerate dehydration during pyrolysis of plywood and promote carbonization. Free radicals like PO· and HPO· captured active H· or OH· in the gas phases and the fire retardation effect was achieved by the combination of free radicals. Boron compounds could not only adsorb heat quickly under a high temperature, lower the timber temperature and delay combustion of ignition, but also forming a fusion melting substance to cover the surface of plywood, isolating propagation of oxygen and heat, promoting the generation of carbon and decreasing formation of volatile substances. A reasonable mixing ratio of borax and boric acid might control the flame surface combustion of timbers completely. After heat absorption, N-containing compounds could be decomposed and noninflammable gases (e.g., nitrogen and ammonia gas) were produced. On one hand, these noninflammable gases could decrease concentration of oxygen and combustible gases surrounding the timber. On the other hand, the produced nitric oxides could also capture free radicals in timbers, inhibit the chain reaction and thereby inhibited combustion of plywood [26,27]. Cl-containing compounds dissociated Cl atoms under flame state and captured hydrogen from the combustible gases to produce HCl. The HCl could inactivate the active free radicals which maintained the combustion of plywood. As a result, the reaction chain transmission stopped and combustion was inhibited. In addition, the halogen steam with a high density formed a covering layer on the surface of combustible substances, thus realizing the effect of fire retardation. Guanidine compound could decrease pyrolysis activation energy of timbers and promote generation of combustible substances in the range of low ignition temperature [26–30]. Consequently, pyrolysis temperature of plywood decreased and combustible substances were formed and released under a low temperature, and finally diffused under the premise of non-ignition.
In addition, there were cured adhesive in plywood and a certain amount of nitrogen and chlorine compounds in adhesive and curing agent. They could also develop some fire retardation effect. In a word, components of flame retardant were not isolated. Instead, they supplemented each other and formed synergistic effect. The production of flammable substances could be controlled and the dehydration and carbonization of timbers were promoted by changing the thermolysis and thermal decomposition of plywood, thus improving flame resistance of plywood significantly.
A flame retardant composition was prepared by using phosphoguanidine, guanidine sulfamate, disodium octaborate tetrahydrate and dodecyl dimethyl benzyl ammonium chloride. Veneers were immersed in such flame retardant to prepare plywood. The combustion characteristics and thermal stability of plywood were tested by a cone calorimeter and TG. Results showed that:
1. Higher concentration and loading of flame retardant were beneficial for the fire resistance of the plywood. The LOI and residual mass of plywood processed using the flame retardant was increased by 87.5% and 58.7% compared to those of the untreated plywood, while the av-HRR, THR, EHC, TSR, COY, CO2Y and oxygen consumption were decreased by 44.4%, 82.9%, 47.0%, 86.0%, 89.9%, 50.1% and 83.1%, respectively.
2. Treated plywood which had a low fire growth index (FGI) displayed a later combustion heat release rate peak and slower flame spread than observed for the untreated material. Combustion of treated plywood displayed a higher fire performance index (FPI), indicating a longer time to ignition. This suggests that burning structures from this material would be subject to a longer time for escape from the structure and would present lower fire risk than similar structures containing treated plywood.
3. The flame retardant can decrease the pyrolysis temperature for hemicellulose and cellulose, change the decomposition and reaction progress for plywood degradation and promote dehydration carbonization and accelerated charformation. Moreover, the formed char was more stable than that combustion of untreated plywood.
4. The flame retardant contained N, P, B, Cl and Gu compounds. The adhesive also contains N and P compounds. These substances display flame resistance and supplement each other to generate flame retardance than any one used alone. By changing the thermolysis and thermal decomposition processes, the heat release and smoke release from plywood, undergoing combustion was reduced. This controlled generation of combustible substances and promoted dehydration and carbonization to form char. As a result, the flame resistance of plywood was improved significantly. The probability of smoke asphyxia or poisoning death of those trapped in structures containing treated plywood during fire accidents can be decreased dramatically.
Funding Statement: This work was supported by Science-technology Support Foundation of Guizhou Province of China (Nos. [2019]2308, [2020]1Y125, NY [2015]3027, and ZK [2021]162), National Natural Science Foundation of China (No. 31800481), Forestry Department Foundation of Guizhou Province of China (No. [2018]13), Cultivation Project of Guizhou University of China (No. [2019]37).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Li, W., van den Bulcke, J., De Windt, I., Dhaene, J., van Acker, J. (2016). Moisture behavior and structural changes of plywood during outdoor exposure. European Journal of Wood and Wood Products, 74(2), 211–221. DOI 10.1007/s00107-015-0992-z. [Google Scholar] [CrossRef]
2. Gao, Q., Liu, Z., Li, J. (2020). Research progress of soy protein adhesive for wood-based composites. Journal of Forestry Engineering, 5(2), 1–11. [Google Scholar]
3. Lu, J., Jiang, P., Chen, Z., Li, L. (2020). Characteristic analysis of flame retardant particleboard using three methods of combustion performance evaluation. Journal of Forestry Engineering, 5(5), 28–34. DOI 10.13360/j.issn.2096-1359.201911023. [Google Scholar] [CrossRef]
4. Tang, Q., Lu, X., Guo, W., Fang, L. (2020). Research on the combustion characteristics of high density fiberboard treated with MPP and AP. Journal of Forestry Engineering, 5(6), 29–35. DOI 10.13360/j.issn.2096-1359.201912022. [Google Scholar] [CrossRef]
5. Dieste, A., Krause, A., Bollmus, S., Holger Militz, H. (2008). Physical and mechanical properties of plywood produced with 1.3-dimethylol-4.5-dihydroxyethyleneurea (DMDHEU)-modified veneers of Betula sp. and Fagus sylvatica. Holz Roh Werkst, 66(4), 281–287. DOI 10.1007/s00107-008-0247-3. [Google Scholar] [CrossRef]
6. Cao, J. Z. (2019). A review on wood protectant dispersion systems and their liquid penetration. Journal of Forestry Engineering, 4(3), 1–9. DOI 10.13360/j.issn.2096-1359.2019.03.001. [Google Scholar] [CrossRef]
7. Yu, L., Tian, M., Li, L., Wu, Z., Chen, S. et al. (2020). Study of nano colloidal silica sol based protectant on the prevention of masson pine. Wood Research, 65(5), 797–808. DOI 10.37763/wr.1336-4561/65.5.797808. [Google Scholar] [CrossRef]
8. Nicholas, D. D., Siau, J. F. (1973). Factors influencing the treatability of wood. Wood deterioration and its prevention by preservative treatment. New York: Syracuse University Press. [Google Scholar]
9. Chen, X., Li, J., Gao, M., Yue, L., Zhou, X. (2019). Fire protection properties of wood in waterborne epoxy coatings containing functionalized graphene oxide. Journal of Wood Chemistry and Echnology, 39(5), 313–328. DOI 10.1080/02773813.2019.1601740. [Google Scholar] [CrossRef]
10. Cao, Y., Wang, X., Li, Y., Shen, D., Dai, Y. P. et al. (2020). Effect of high temperature oil heat treatment on the starch content and mold-resistant property of bamboo. Journal of Forestry Engineering, 5(2), 109–115. DOI 10.13360/j.issn.2096-1359.201905016. [Google Scholar] [CrossRef]
11. Pan, J., Mu, J., Wu, Z., Zhang, X. (2014). Effect of nitrogen-phosphorus fire retardant blended with Mg(OH)2/Al(OH)3 and nano-SiO2 on fire-retardant behaviour and hygroscopicity of poplar. Fire and Materials, 38(8), 817–826. [Google Scholar]
12. Chu, D., Mu, J., Zhang, L. (2017). Promotion effect of NP fire retardant pre-treatment on heat-treated poplar wood. Part 2: Hygroscopicity, leaching resistance, and thermal stability. Holzforschung, 71(3), 217–223. DOI 10.1515/hf-2016-0213. [Google Scholar] [CrossRef]
13. Wang, T., Liu, T., Ma, T., Li, L., Wang, Q. et al. (2018). Study on degradation of phosphorus and nitrogen composite UV-cured flame retardant coating on wood surface. Progress in Organic Coatings, 124(42095), 240–248. DOI 10.1016/j.porgcoat.2018.08.017. [Google Scholar] [CrossRef]
14. Biasi, C. D., Branca, C., Galgano, A. (2007). Flame retarding of wood by impregnation with boric acid pyrolysis products and char oxidationrates. Polymer Degradation and Stability, 92(5), 752–764. DOI 10.1016/j.polymdegradstab.2007.02.007. [Google Scholar] [CrossRef]
15. Baysal, E., Altinok, M., Colak, M., Ozaki, K., Toker, H. (2007). Fire resistance of Douglasfir (Pseudotsugamenzieesi) treated with borates and natural extractives. Bioresource Technology, 98(5), 1101–1105. DOI 10.1016/j.biortech.2006.04.023. [Google Scholar] [CrossRef]
16. Branca, C., Biasi, C. D. (2007). Oxidation characteristics of chars generated from wood impregnated with (NH4)2HPO4 and (NH4)2SO4. Thermochimica Acta, 456(2), 120–127. DOI 10.1016/j.tca.2007.02.009. [Google Scholar] [CrossRef]
17. Wang, S., Wang, F., Wang, Q., Guo, C. (2014). Synergistic effect of flame retardant FRW mixed with ammonium polyphosphate. China Wood Industry, 28(3), 17–21. DOI 10.19455/j.mcgy.2014.03.006. [Google Scholar] [CrossRef]
18. Yang, J., Zhu, X., Tian, C., Yao, C., Wu, Y. (2014). Properties of eucalyptus plywood treated with boron-nitrogen and phosphorus flame retardants. China Forest Products Industry, 41(5), 17–20. DOI 10.19531/j.issn1001-5299.2014.05.007. [Google Scholar] [CrossRef]
19. Gao, M., Ling, B., Yang, S., Zhao, M. (2005). Flame retardance of wood treated with guanidine compounds characterized by thermal degradation behavior. Journal of Analytical and Applied Pyrolysis, 73(1), 151–156. DOI 10.1016/j.jaap.2005.01.006. [Google Scholar] [CrossRef]
20. Wang, N., Liu, Y., Liu, Y., Wang, Q. (2017). Properties and mechanisms of different guanidine flame retardant wood pulp paper. Journal of Analytical and Applied Pyrolysis, 128, 224–231. DOI 10.1016/j.jaap.2017.10.007. [Google Scholar] [CrossRef]
21. Xiao, Z., Liu, S., Zhang, Z., Mai, C., Xie, Y. et al. (2018). Fire retardancy of an aqueous, intumescent, and translucent wood varnish based on guanylurea phosphate and melamine-urea-formaldehyde resin. Progress in Organic Coatings, 121, 64–72. DOI 10.1016/j.porgcoat.2018.04.015. [Google Scholar] [CrossRef]
22. Fateh, T., Rogaume, T., Luche, J., Richard, F., Jabouille, F. (2014). Characterization of the thermal decomposition of two kinds of plywood with a cone calorimeter-FTIR apparatus. Journal of Analytical and Applied Pyrolysis, 107, 87–100. DOI 10.1016/j.jaap.2014.02.008. [Google Scholar] [CrossRef]
23. Kim, J., Lee, J. H., Kim, S. (2012). Estimating the fire behavior of wood flooring using a cone calorimeter. Journal of Thermal Analysis and Calorimetry, 110(2), 677–683. DOI 10.1007/s10973-011-1902-1. [Google Scholar] [CrossRef]
24. Kim, H. J. (2018). Study on the internal temperature of flame resistant treated wood exposed to a standard fire. Fire Science and Engineering, 32(3), 14–18. DOI 10.7731/KIFSE.2018.32.3.014. [Google Scholar] [CrossRef]
25. Lowden, L. A., Hull, T. R. (2013). Flammability behaviour of wood and a review of the methods for its reduction. Fire Science Reviews, 2(1), 4. DOI 10.1186/2193-0414-2-4. [Google Scholar] [CrossRef]
26. Yan, L., Xu, Z. F., Liu, D. L. (2019). Synthesis and application of novel magnesium phosphate ester flame retardants for transparent intumescent fire-retardant coatings applied on wood substrates. Progress in Organic Coatings, 129, 327–337. DOI 10.1016/j.porgcoat.2019.01.013. [Google Scholar] [CrossRef]
27. Palanti, S., Feci, E., Predieri, G., Vignali, F. (2012). A wood treatment based on siloxanes and boric acid against fungal decay andcoleopter Hylotrupesbajulus. International Biodeterioration & Biodegradation, 75, 49–54. DOI 10.1016/j.ibiod.2012.07.019. [Google Scholar] [CrossRef]
28. Ren, Y. L., Wang, Y. L., Wang, L. J., Liu, T. T. (2015). Evaluation of intumescent fire retardants and synergistic agents for use in wood flour/recycled polypropylene composites. Construction and Building Materials, 76, 273–278. DOI 10.1016/j.conbuildmat.2014.12.004. [Google Scholar] [CrossRef]
29. Giúdice, C. A., Benı́tezb, J. C. (2001). Zinc borates as flame-retardant pigments in chlorine-containing coatings. Progress in Organic Coatings, 42(1–2), 82–88. DOI 10.1016/S0300-9440(01)00159-X. [Google Scholar] [CrossRef]
30. Tondi, G., Wieland, S., Wimmer, T., Thevenon, M. F., Pizzi, A. et al. (2012). Tannin-boron preservatives for wood buildings: mechanical and fire properties. European Journal of Wood and Wood Products, 70(5), 689–696. DOI 10.1007/s00107-012-0603-1. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |