 Journal of Renewable Materials
Journal of Renewable Materials
 Journal of Renewable Materials Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014374
ARTICLE
Advances in the Structural Composition of Biomass: Fundamental and Bioenergy Applications
1Department of Chemical Engineering and Technology, Indian Institute of Technology (BHU), Varanasi, 221005, India
2Department of Chemical Engineering, Madan Mohan Malaviya University of Technology, Gorakhpur, 273010, India
3Department of Environmental Studies, Satyawati College, University of Delhi, Delhi, 110052, India
4Department of Basic Medical Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
5Departentof Biotechnology, Faculty of Biosciences, Integral University, Lucknow, 226026, India
6Biorefining and Advanced Materials Research Center, Scotland’s Rural College (SRUC), Edinburgh, EH9 3JG, UK
*Corresponding Authors: Neha Srivastava. Email: sri.neha10may@gmail.com; Vijay Kumar Thakur. Email: Vijay.Thakur@sruc.ac.uk
Received: 22 September 2020; Accepted: 09 November 2020
Abstract: Increased environmental pollution due to the organic wastes over the world is one of the most burning issues. These organic wastes lie under the category of biodegradable waste and can be effectively degraded from their complex compound into simple one by the action of microbes or other living organisms. Moreover, lignocellulosic biomass is a major part of the biodegradable waste and belongs to the group of renewable energy source, which can be very effective for bioenergy production. Biomasses are made up of different compounds such as cellulose, hemicelluloses, lignin and protein. Apart from these components, based on the structural analysis biomass also consist of bioactive substances such as carotenoids, flavonoids, lignin and antioxidants. This review explores a complete overview of the classification, component and the structure of the biomass. Moreover, it discusses how biomasses can play the key role of substrate in many sectors such as industrial bioenergy production including gaseous and liquid biofuels.
Keywords: Biomass; classification; composition; bioactive substance; biofuels
The continuous increase in environmental pollution is damaging the earth’s atmosphere and severely affecting the life of our contemporary society [1]. These environmental contaminations include heavy and toxic metals, residue and disposal from the various industries [2]. Environmental pollution is one of the most serious issues, which is very common everywhere either in the developed countries such as USA, China, Russia or in the developing countries like India, Afghanistan, Argentina, etc. [3]. In addition, the continuous decrease is being observed in the quality of the environment everywhere, resulting in loss of biological diversity, vegetation, increased in toxic substance in the atmospheric air and therefore causing serious damage directly or indirectly in every sector [4−6]. Further, in general, environmental wastes are divided into two categories as per the feasibility of degradation regarded as biodegradable and non-biodegradable [7−9]. Biodegradable wastes are degraded by different microorganisms from their large complex formed to simple compounds/molecules and produce water and carbon dioxide as a byproduct during the process. Moreover, biodegradation can be carried out through various approaches such as aerobic digestion, composting, anaerobic digestion or some natural phenomena [10,11]. Biodegradable waste includes different types of biomasses such as food waste, animal waste, kitchen waste, and slaughterhouse waste [12]. On the other hand, non-biodegradable wastes include chemicals and harmful materials which cannot be degraded by the natural means. Metals, plastics, chemicals, water bottles glasses and many synthetic polymers are the example of non-biodegradable wastes [13].
Biomasses are biodegradable wastes and known as the organic matter which is produced from either plant or animal and belongs to the group of renewable energy sources. Biomasses utilize energy directly or indirectly from the main source of energy, i.e., the sun. Plants utilize light energy generated from the sun through the process of photosynthesis for their growth and when they are burned release heat, light and chemical energy [14]. Therefore, these biomasses have been utilized as the source of energy from the past several hundreds of years. These biomasses are easily available everywhere and act as an alternative when there is a shortage of conventional source of energy [15]. Biomass also helps in reducing the greenhouse gases from the atmosphere and produces pure oxygen.
One the basis of the structural components, biomass can be made up with a combination of many compounds such as lipids, cellulose, sugar, hemicelluloses, starches hydrocarbons, water and many other compounds [Fig. 1] [16]. Biomass also contains some bioactive compounds such as carotenoids, flavonoids, lignans and antioxidants [17]. Extraction of these bioactive compounds is dependent upon the sustainability of both, the environment as well as economic of reutilization and purification of these compounds. Biomasses are being applied in many areas like in industrial sector where they are used as the source of energy for running boiler and heaters, at the domestic level used for cooking and as the source of light, whereas in the agricultural sector it is used as manure [18]. However, currently, several applications of biomasses are going on with the implementation of innovative ideas e.g., as the chief raw material for the biogas production, the substrate for the biofuels production, and as the carrier in biofertilizers for carrying microbes to the plants [19]. There are numerous advantages of using biomasses as a substrate in the processes such as energy production. Biomasses do not release any kind of harmful gases and do not pollute the environment by any means, therefore if they are properly managed, can perform the role of sustainable energy sources [20]. Application of biomasses for the biofuels production offers plenty of benefits in terms of cost, availability as the raw materials, and environment management. [21]. Since most of the lignocellulosic biomasses such as rice husk/rice straws, wheat straws, maize, switchgrass, soybeans and plant wastes are sustainable, these wastes are unlikely to run out anytime soon permits the application of in productive nature and these types of crops are continuously planted [22]. Increase in demand for fuels results growth and developments in industries related to biofuel production which will increase employment as well as will help in reframing the economy of the country. Application of biofuels will reduce the requirement of fossil fuels and many experts’ beliefs that dependency of different countries will shift from fossil fuel to biofuels within in some upcoming years. [23−26]. This review focuses on a detailed discussion about the classification of biomass-based on its component and their structural analysis. Also, how a molecule like mannans, xylans, arabinogalactans and galactans varies from each other based on the structure has been discussed. Moreover, the various processes involved in the production of biofuels and types of liquid and gaseous biofuels using biomasses is briefly explained.
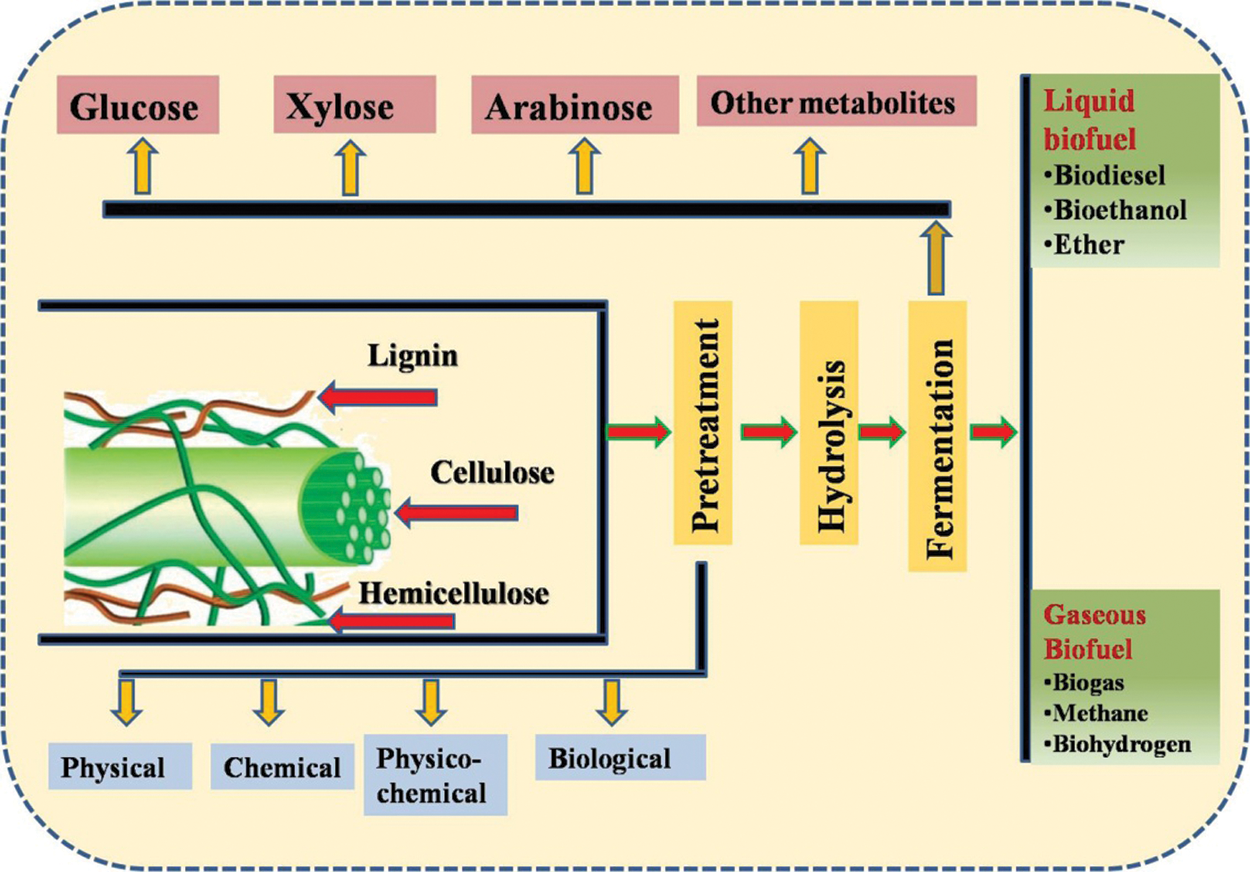
Figure 1: An overview of the processes involved in biofuels production [27]
2 Classification of the Composition of Lignocellulosic Biomass
Lignocellulosic biomass is the combination of the many chemical and biological compounds which are bounded with each other to form the lignocellulosic structure [28]. These compounds may include cellulose, hemicelluloses, lignin, fat, starch, water-soluble sugar, amino acids and some other complex compounds [29,30] [Fig. 2]. Polysaccharides with higher molecular weight are about 60% to 80% of the total biomass constituents which along with lignin forms a complete biomass structure.
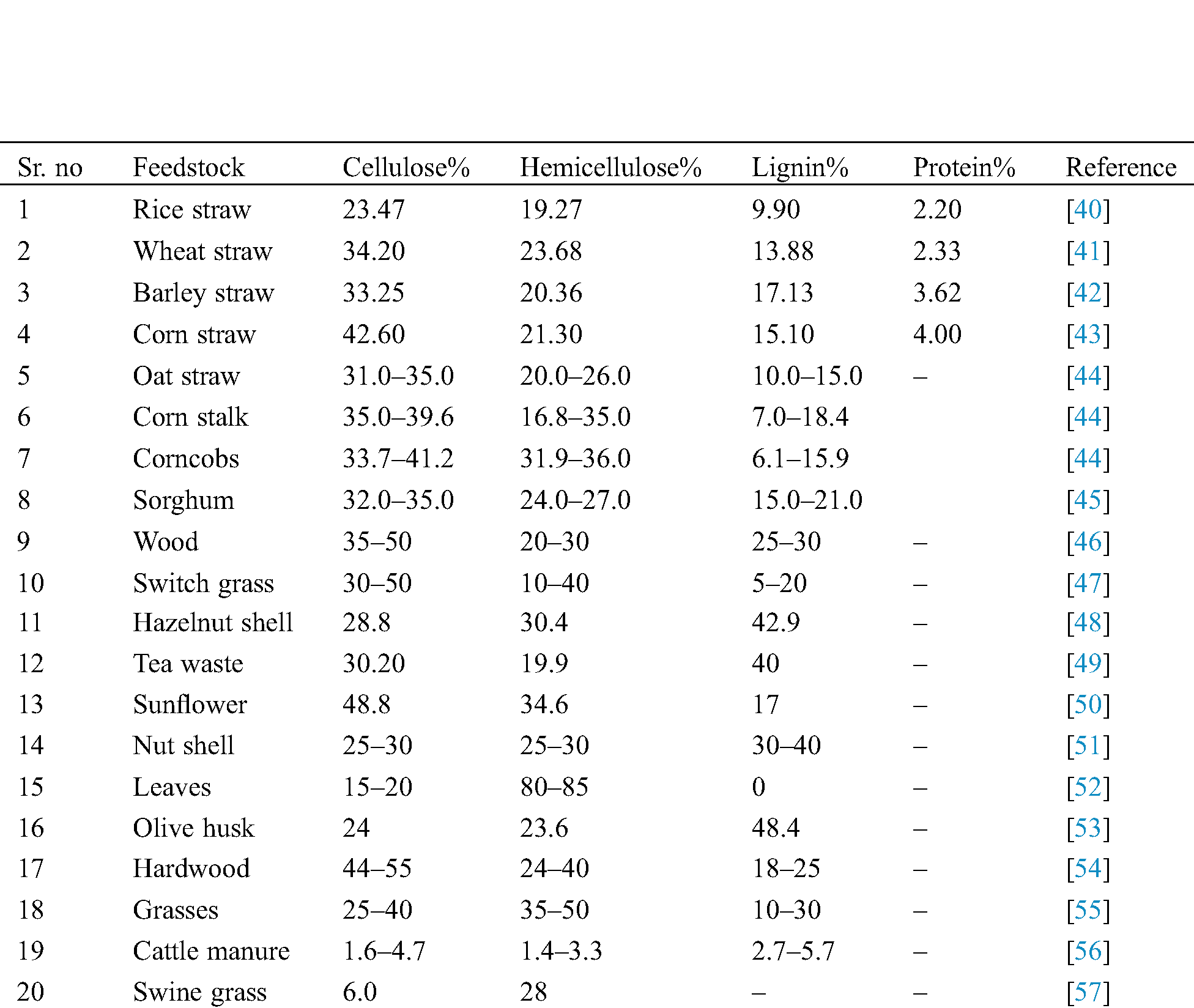
Three major sections consist in lignocellulosic biomasses are lignin (C81H92O28) (outer surface), hemicelluloses [(C5H8O4)m] (below lignin) and cellulose [(C6H10O5)n] (core) in which cellulose is having ~40% composition and act as a major source for the production of biofuels [31]. Further, lignocellulosic biomass such as waste from agriculture sector like wheat straw, wheat bran, rice straw, corn stover and sugarcane bagasse etc. contains biomolecules like lignin, hemicelluloses and cellulose [32]. Compositions of different types of agricultural biomasses utilized as a substrate for biofuels production have been summarized in Tab. 1. Further, the composition of these biomolecules varies in the different substrate, e.g., in case of wheat straw the concentration of cellulose varies from 33% to 40%, hemicelluloses from 20% to 25%, and lignin from 15% to 20% (w/w). Celluloses are homopolysaccharide having long polymeric chain containing (1,4)-d-glucopyranoseas a unit molecule which is linked with each other through β-1,4 glycosidic bond [33]. Hemicelluloses are heteropolysaccharide molecules made up of different molecules of sugar such as mannose, glucose, xylose and these are interconnected with each other using β-1,4 and β-1,6 glycosidic bonds. Apart from these, lignin is also biomolecules which have 3-C chains which are interconnected with each other by the help of the ring structure of phenyl propane [34]. Proteins are also available in the biomass structure which is about 15% of the total lignocellulosic biomass composition, playing a role of byproduct being produced after the pretreatment process [35].
In a study, Raud et al. [36] experimented enhancing the yield of ethanol following the pretreatment of barley straw method. In this method, nitrogen gas was used at high pressure (1 to 60 bar) and elevated temperature (25°C to 175°C) to degrade the protective layer of lignin and thus exposed cellulose and hemicelluloses for more efficient hydrolysis by the microbes. This pretreatment could increase the yield of glucose by 115% as compared to barley straw without pretreatment blank sample as well as the production of ethanol was also increased by 117 g/Kg of biomass [36]. Salapa et al. [37] experimented the pretreatment by using a different solvent like ethanol, butanol, methanol, acetone, etc. It was found that the pretreatment done in the presence of ethanol at 180°C for 40 min increases the exposure of cellulose by 89% and the yield of ethanol by 67%. Moreover, it was found that the utilization of diethylene as a solvent for the pretreatment at 160°C for 40 min increases the production of ethanol by 65% [37]. Zheng et al. [38] performed an experiment of the pretreatment of wheat straw for increasing the conversion of cellulose molecules. In this experiment, three different methods were used including hot water pretreatment, sulfuric acid (H2SO4) treatment and sodium hydroxide (NaOH) pretreatment. The highest cellulose conversion of 87% was found by using 4% NaOH at 121°C. In addition, it was observed that NaOH deals with the removal of lignin whereas H2SO4 deals with the removal of hemicelluloses [38].
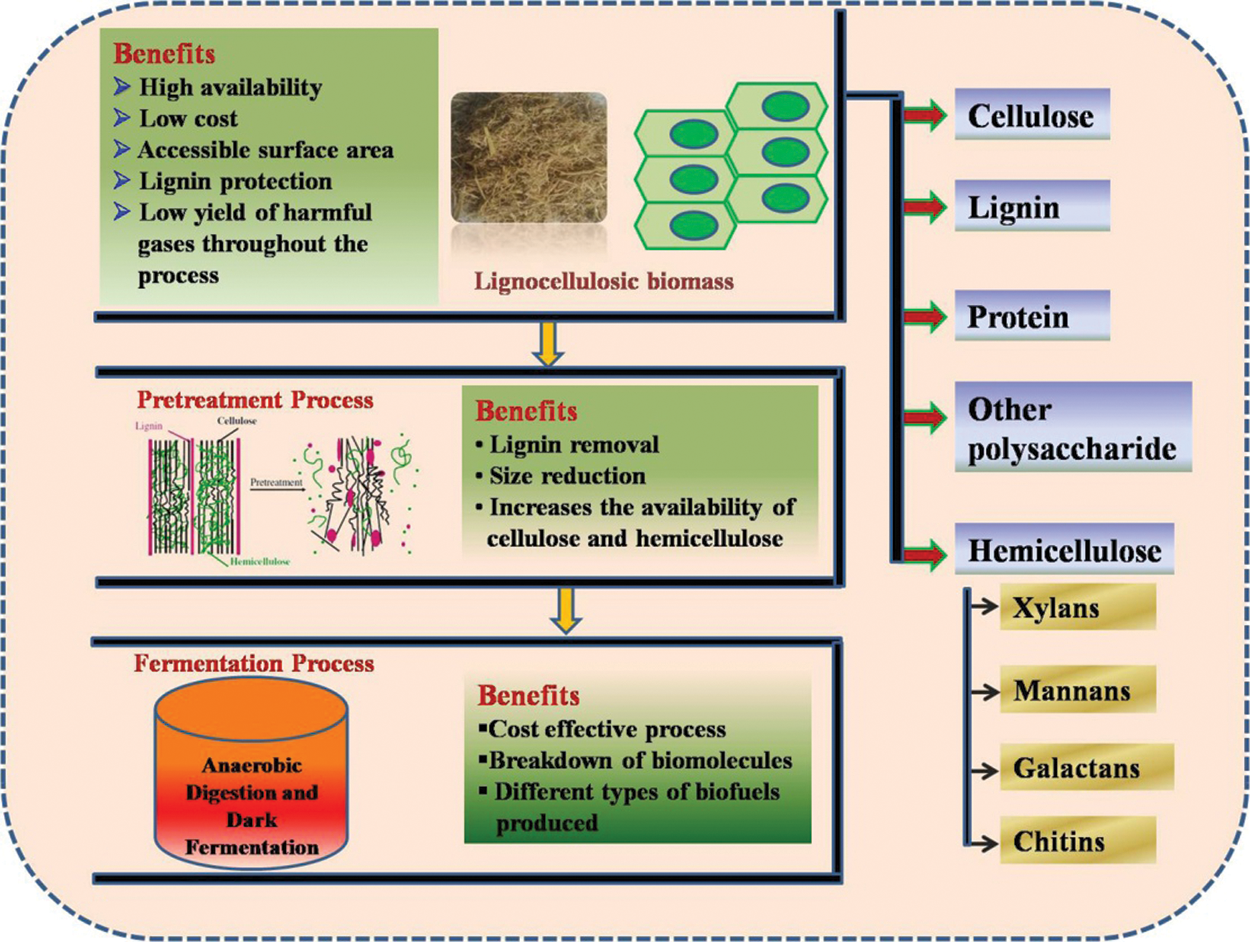
Figure 2: Structural analysis of biomass and the effect of the different process on biomass [39]
Table 1: Composition of agricultural biomass used as a substrate
Cellulose is a polymeric molecule consisting of D-glucose with a long and linear chain and having high molecular weight. These cellulosic molecules contain about five thousand to ten thousand monomer units, that are available only in plants and treated as the most abundant molecules of polysaccharide found in the environment. Further, it is estimated that about 40% to 50% of the total carbon on the planet is available in the form of a cellulose molecule [24,58]. A cellulose molecule is linear in nature and rotation in the molecule get reserved due to the presence of hydrogen bond between every unit which results from its ribbon-like structure [25,59]. This ribbon-like structure contains hydrophobic group at the surface whereas the hydrophilic group is arranged laterally. This results in the development of a cluster of the polymer as well as a fractal-like feature because of such type of specific arrangement within the ribbon-like chain. Further, any kind of translational motion is not possible because of the induced forces within the molecule but these forces increase elasticity as well as flexibility [60]. This resistive nature of long-chain fiber molecule provides strength as well as great mechanical resistance to the plant. The same effects are also viewed within an animal cell which prevents them from getting ruptured by high intercellular pressure developed within the membrane [61]. Amorphous and crystalline regions are present within the structure of the plant cell alternatively which show high resistive nature towards cellulase enzymes. An amorphous section is always at risk of getting attacked by the cellulase enzyme which results in degradation of a glucose molecule. The major application of this cellulosic component can be of great interest for the production of biofuel as the implementation of these compounds has many advantages as compared to other sources [62]. Moreover, this approach does not use food and grains materials but utilize agricultural wastes which are the by-products of the cultivation process.
The second most common compound in lignocellulosic biomass is hemicellulose which is about 20% to 30% of the total biomass composition. Hemicellulose is also a chain polymer which is similar to the cellulose molecule but it differs based on the molecular structure. One of the most common differences between cellulose and hemicellulose is that the cellulose is having a linear chain whereas hemicellulose is having a branched-chain molecular structure [63]. This molecule includes 500 to 3000 monomeric unit of glucose having five and six carbon atoms, attached to form a branch chain polymer. This heterogeneously branched-chain is the major part of a plant cell which is directly connected with the surface of the ribbon-like structure of the cellulose molecules [64]. As per the variation in types of plant, structure, as well as the content of the hemicellulose molecules is located at a different position in the structure. In this molecule, different types of sugar units along with various substituents are arranged in different ratios which results in the formation of branched structure [65]. This hemicellulose which is a complex molecule can be degraded by the biological, physico-chemical, physical and chemical technique into smaller compounds. In the physical technique, hemicelluloses are treated thermally between 180°C to 350°C in which many gases, ketones, coal etc. are being released [66]. The amorphous nature makes hemicellulose water-soluble as well as increases its reactive nature when they are hydrolyzed. It develops a connection between the cellulose molecules and plays a very important role in woods of binding this molecular chain of different cellulose molecules. Apart from cellulose hemicelluloses are amorphous and show adhesive nature towards properties like dehydration. Different molecules such as mannans, xylans, arabinogalactans and galactans are combined to form hemicelluloses. These molecules vary from each other based on arrangement and linkage between them as shown in Fig. 3 [67].
Mannans are the main components of hemicelluloses generally available across the cell walls of the plants larger in size. Hemicellulose gets bounded with cellulose in the wood with the help of mannans as it shows a great affinity towards it. Mannans are characterized into four different kinds which include galacto-glucomannans, linear-mannans, galactomannans and gluco-mannans [68] Mannans accept the molecular structure having linear structure along with a backbone and depending upon the number of mannose, glucose ormannans unit attached by β-(1–4) glycoside bond. Linear mannans have 1,4-linked β-D-mannopyranosyl unit as well as it contains much sugar in small amount especially in form of galactose [69]. These mannans have high demands in food and dairy industries where it is used for different activities. Mannans are generally used for coating purposes, production of gels, moisture absorbents, for improving textures, for stabilizing and for modifying the viscosity of the liquid. Mannanases helps in the degradation of mannans molecules, makes it available for different purposes like fuel production, production of fruit juice as well as reduction of viscosity in the coffee extract [70].
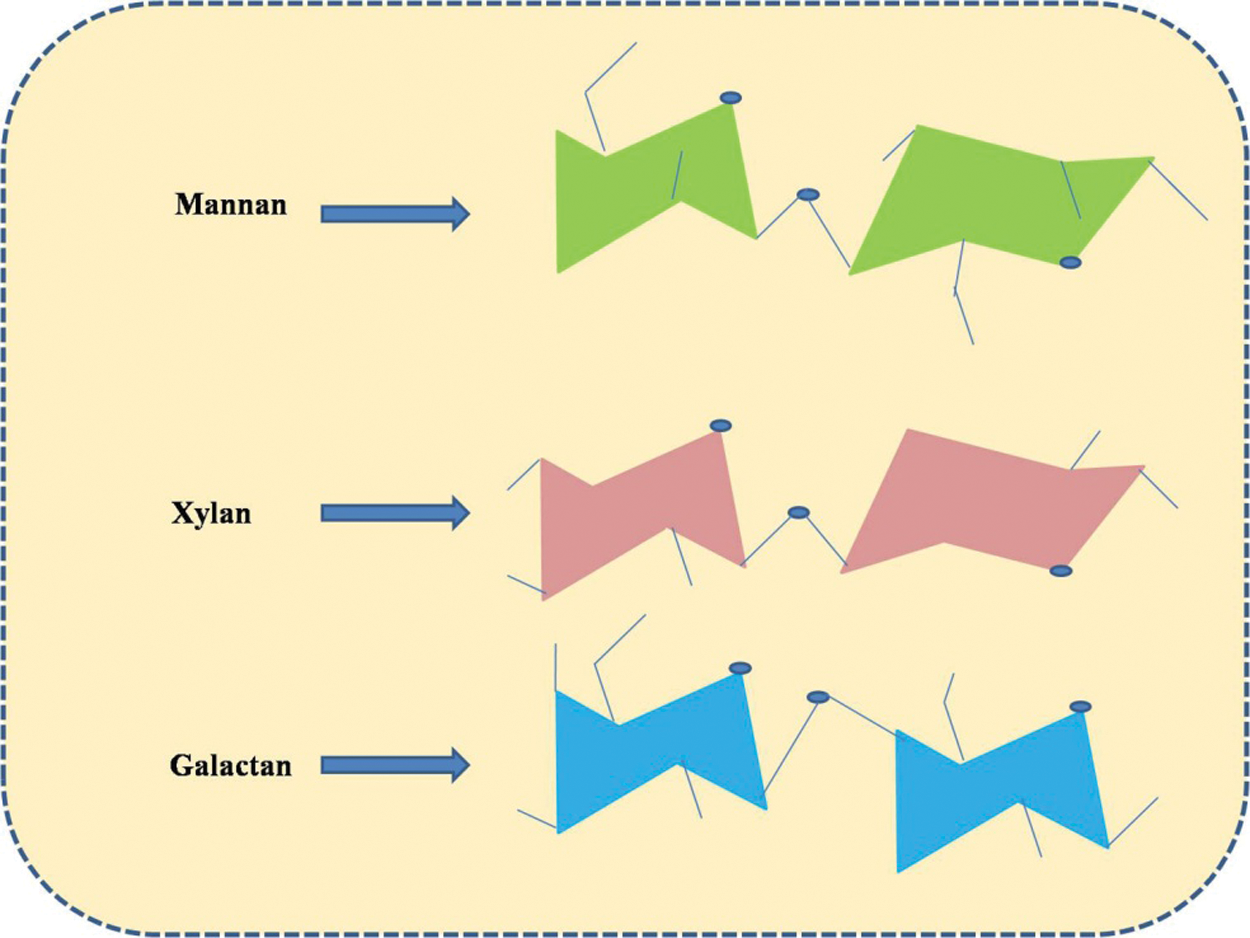
Figure 3: Arrangements and linkage of Xylan, Mannan and Galactan
Xylans belong to polysaccharides polymer composed of many monomeric units of xylems. In the structure of xylans, the primary chains contain D-β-xylopyranose of which the monomeric unit is attached using 1,4 bond [71]. The straight polymeric chain contains many other small chains which contain mannose, rhamnose, xylose, arabinose or 4-o-methylglucuronic acid. Xylans are water-soluble and it can be increased by reducing the degree of polymerization in the molecules [72]. Based on the structure, xylans are a very common type of hemicelluloses found in the hardwood of the plant and as a major part of the residual crops. Xylans plays a very important role in the daily life of every living being, whereas the quality of cereal flours, as well as the dough hardness, is directly affected by the presence of xylans. It also plays a significant role in medical sectors such as sweetener for diabetic patients and help to reduce dental cavities. Application of Xylanase enzyme is to degrade xylan by producing Xylo-oligosaccharides by which production of biofuel can be increased [71−73].
Galactans exists in the form of arabinogalactans, appears generally in larch trees and not commonly found in all kind of plants as compared to polysaccharides molecules in the groups. The structure of galactans includes a long polymeric chain of galactose which is attached by 1, 3 and 1, 6 bonds [74]. This polymeric chain includes long straight chain in which 4-α-galactopyranosyl and 3-β-D-galactopyranosyl have attached alternatively with each other. These molecules are polysaccharide structure found in algae, seeds and some kinds of buds and flowers. Some of the most popular types of galactans include isolated galactans from yellow lupin seeds, larch, algae and other types of seeds [75,76]. Galactans have a very wide range of application in different industries, as it helps in texture development in cheese, stabilization of viscosity in dairy products as well as toothpaste. Apart from all these it also plays a significant role in the pharmaceutical industries as a stabilizing, thickening as well as gelling agent [75−77].
2.2.4 Chitin and Peptidoglycan
Chitin is a type of hemicellulose having long polysaccharide chain made up of many monomeric units of N-acetylglucosamine which are attached by the help of β-1, 4 bonds [78]. This linkage is similar to the bonds found in the cellulose molecule which attach many glucose units. On comparing the structure of chitin with cellulose there exist acetylamine group at C2 position in case of chitin molecule whereas cellulose molecule has a hydroxyl group at this position [79]. The rigidity in the cell is due to the availability of peptidoglycan polymer which also prepares a thin layer on the cell wall of bacteria [80]. This long polymeric chain consists of many N-acetylmuramic and N-acetylglucosamine units which are linked together to form peptidoglycan polymer commonly known as glycan
Lignin is defined as the protective cover for lignocellulosic biomass which helps in binding, cementing and arranging together with the fibres which increase the resistive and compactness nature of the woods. It prevents cellulose as well as hemicellulose from getting effected by foreign microbes or activities [81]. Therefore, to extract the cellulose and hemicelluloses it is essential to remove the lignin from the lignocellulosic biomass. The lignin molecules contain three different aromatic structure of hydroxycinnamyl alcohol p- sinapyl, coumaryl, and coniferyl alcohols which vary based on the degree of the methoxylation. The removal of lignin is done with the help of pretreatment in which lignocellulosic biomasses have to pass through several steps like boiling, heating, pressurizing and biological degradation [82]. Generally, pretreatment can be done either by biological, physical or chemical techniques, and every technique has both benefits as well as side effects. In physical technique, pretreatment can be performed by using steps like grinding, milling or pressurizing, etc. The main side effects of the physical pretreatment techniques are high operation cost as well as high consumption of energies [83−85]. In the chemical technique of pretreatment, solubilization of lignin and digestion of celluloses are increased. This technique involves steps like a steam explosion, oxidation, ozonolysis, alkalis, and implementation of acids and ionic liquid during the pretreatment. These steps increase the efficiency of the pretreatment processes but its harmful impact on the environment reduces its practical demand [86]. Similarly, in the biological technique of pretreatment, the major role is played by different fungi such as white rot or brown rot which breaks the structure of lignin. These steps reduce the cost, increase the energy output as well as reduce the involvement of chemicals. But, one the major side effect of this process is that it utilizes more time and the hydrolysis steps are very slow as compared to other pretreatment techniques [87]. Therefore, chemical and physical techniques are mostly preferred. Yan et al. [88] performed a pretreatment experiment of grass waste by using dilute NaOH along with H2O2 under the mild climatic conditions. It was observed that the high recovery of holocellulose could be achieved which was about 73.8%, whereas about 73.2% of lignin got removed following this technique [88]. Huang et al. [89] performed a modification in the traditional pretreatment technique of alkaline H2O2. In this technique, ethanol was added in the system which increases the removal of lignin from 74.9% to 80.0% at 100°C. Along with this, some amount of carbohydrates also gets dissolved in the process which was further recovered in which hemicelluloses were ~67.6% and glucan was ~83.3% [89]. Sheng et al. [90] performed an experiment in which the effect of ascorbic acid was observed on wheat straw, corn stover as well as a corncob. It was found that the application of these weak diluted acid can be improved the hydrolysis by 12.47%, 18.78% and 13.57% [90].
Along with lignin, hemicelluloses and cellulose, protein molecules are about 15% of the total lignocellulosic biomass which is produced as a byproduct in the process of pretreatment. Crops such as sunflowers, soybean, palm and jatropha seeds are the main substrate for vegetable oil production which includes protein content in high concentration which is about 0.4 to 0.6 mass fractions [76]. Proteins are available in a different amount in different types of biomasses which varies from the concentrations of ~3.3% to 15%. Protein extractions through dry lignocellulosic feedstock have received much attention of researchers for producing biofuels [91]. Proteins are the complex biomolecules made up of monomeric unit known as amino acids, are attached using peptides bonds and result in a large polypeptide chain. The smaller chains containing less than 20 to 30 residues are hardly counted in the groups of proteins they are generally considered as peptides or oligopeptides. These large polypeptide molecules get degraded by the process known as proteolysis which is finally converted into the amino acids. These slow reactions are catalysed by the microorganisms such as protease, peptidase and proteinase [92]. These proteases are generally of two types which are exopeptidase and endopeptidases. Exopeptidases break amino acids from carbon and nitrogen terminals whereas endopeptidases degrade the internal linkages.
The reserve polysaccharides is also a long polymeric chain similar to skeletal polysaccharides which do not differ based on the monomeric units, but the position of linkage and attachment which differ them from each other. This variation of the basis of a structure affects the nature of flexibility between different glycosidic linkages [93]. This reserve polymer found to be more flexible as compared with fibrous polymers because of low torsional rotation as well as a minimum hindrance. These polymers have 1,6 glycosidic linkage which provides it with the nature of extreme flexibility [94]. One of the most common natures of these long-chain polysaccharides is its extreme branching. Reserve polysaccharides are being prepared using different cells in the plant during many stages of physical development. This growth especially occurs during the photosynthesis which later digested for providing carbohydrate for the metabolism of the cells [95]. These reserve polysaccharides are utilized for a short duration in the process of cell metabolism and then finally gets stored in the form of colloidal or solid-state. These reserve polysaccharides are deposited generally in plastids, cell vacuoles or in the region of the cell wall [96]. Starch is only the one type of polysaccharide which is known to be found in plastids, and this plastid contains a different section of the cell in which starch is being prepared for the growth of the plants which are larger higher plant.
Biomasses can be classified into different categories based on their scope and purposes [Fig. 4]. There are no any such specific rules or way of the classifications of biomasses; therefore, depending upon the quality, composition, application and nature of their existence we can classify biomasses into different groups [97]. Depending upon the products, origin as well as the function of the biomass, generally biomasses can be classified into two different ways. Classification can be based on the types of biomass exists in the surrounding and also based on its application as the substrate [98]. One of the most popular characterizations of biomass into different categories are biomass in form of woods, herbaceous biomass, waste from animals and humans, aquatic biomass [99].
Wood biomass generally contains many different components in which major section is of carbohydrates and lignin. This group includes different type of biomass such as roots residues, trees, leaves, barks and woody shrubs found above as well as below the ground [100]. Such biomass can be transformed into the different form of energy by the help of numerous process of conversion such as combustion/gasification which directly converts it in the form of energy and light [101]. We can obtain such types of biomasses for the production of energy through sources like agricultural and urban wastes, wastes generated after the consumption of woods, residuals of non-merchant timbers and the byproduct produced during the processes [102]. This type of biomasses is now a day most beneficial renewable sources of energy being used around the world. The biomass which does not have any stems having woods and die back when the season of growth terminates are classified as herbaceous biomass [103]. This biomass includes seeds and the grains crops produced from food processing industries as well as the byproducts released in the process such as straws and husk. Herbaceous biomass is classified into two different categories which are energy crops and agricultural byproducts [104]. Crops which are exploited in sectors of bioenergy are commonly known as energy crops whereas the agricultural byproducts are the residues of the food industries, farms and foods. Some of them are also used to feed the animals, source for light and kitchen purposes [105]. Due to the lack of monitoring about its availability and its potential as a source of energy, it is not utilized properly everywhere.
Waste from animals and humans include meat, bones, human dung and different types of animal manures. Earlier these wastes are collected and sold as fertilizers or simply applied in the agricultural lands [106]. But the implementation of different rules and regulations by the governments have resulted in control of environmental pollution, heath and odour related problems which finally plays an important role in waste management. For the conversion of these types of biomass to useful products, anaerobic degradation of these wastes is the best technique used so far. The energy produced from these wastes includes biofuels and biogases which are used to produce electricity which can be further used in different ways whereas biogas can be directly used in cooking in remote areas [107,108]. The aquatic biomass contains different types of aquatic plants and microorganisms such as microalgae and macroalgae. Microalgae are the type of multi-cellular microbes which are classified into different categories such as diatoms, golden and green algae [109]. Diatoms are unicellular brown algae. These microbes are very small having a size of few micrometres. Golden algae are same as diatoms or brown algae and produce carbohydrates and oils. Whereas green algae are generally found in freshwater resources and mostly produces starch, even though oils are also one of the products that can be obtained from these algae [110−111].
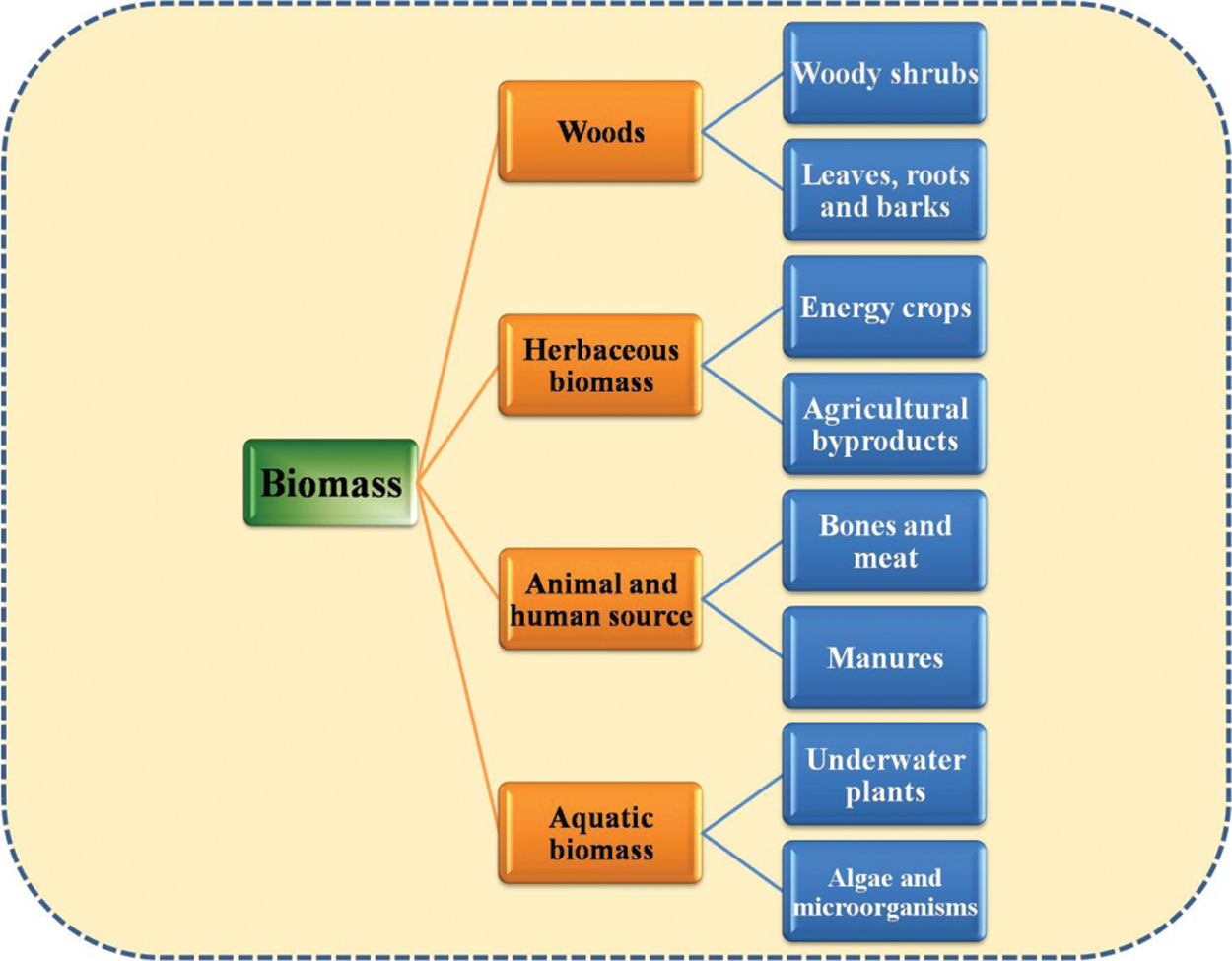
Figure 4: Classification of biomass
To control the environmental pollution every sector must focus on the 3R principle which explains about Reduce, Reuse and Recycle instead of burning or disposing of. These steps not only help to control all kind of pollutions but also play an important role in the reduction of cost, its availability as well as renewability [30,112]. Biomass can be used in different industries to fulfill the waste management hierarchy which includes energy, foods, fertilizers and many more. As the production of biomass is increasing day by day, it is required to develop more suitable and practical approaches for waste management which can help to sustain the environment [113]. Some beneficial application of biomass includes application in the energy sector, for biofertilizers production, for water treatment, in cement industries, for production of thermal insulators as well as for construction purposes.
4.1 Application of Biomass for Biofuel Production
Biomass is one of the best and versatile substrates which can be used for the production of energy [102]. The major factor responsible for the energy production through the biomass is the availability of organic matter which includes wood chips, rice hulls, sugarcane, timbers, trees, leaves and peanut shells etc. There is a wide range of biomasses, being implemented for the production of energy at every level from the small scale of their use in homes for cooking purposes to large scale e.g., for boilers and powerhouses in different industries [114]. Biofuel is a type of renewable source of energy, being produced from biomass has been of high demand at the global level in recent years. Biofuel is one of the best sources of energy which is renewable as well as reduces air pollution, greenhouse gases and also the dependency on carbon-based energy sources [115].
There are mainly two types of biofuels being used nowadays are ethanol and biodiesel [116]. Developed countries like the UK and USA are working on the production of bioethanol using marine yeast and seawater as a media. In the year 2019, the United States had produced the maximum amount of ethanol which is about 15.8 billion gallons; Brazil was in the second position with 8.6 billion gallons. In this list, India is at the fifth position with 530 million gallons of production in the year 2019 [117]. Biodiesel is generally used in normal diesel engines alone or as a blend with petrodiesel. Different countries utilize different feedstock as per their availability, e.g., different parts of Europe utilizes sunflower and rapeseeds as a substrate, soybean is commonly used in the United States, canola oil is used in Canada whereas in tropical countries palm oils are mainly used [115−117]. The basic difference between the ethanol and biodiesel ethanol is a type of alcohol whereas biodiesel is a type of oil Ethanol is an alcohol produced through the fermentation technique and can be used as a substitute or along with gasoline, while biodiesel is generated by extracting naturally occurring oils from different plants and seeds by the process known as the transesterification [118]. The production of biofuel is focused not only to fulfil the requirements of energy production at the decentralized level but also to fulfil the requirements of transport [118−120]. This generates the interest of regional groups as well as involves the lands of regional communities for the production of these biofuels.
4.2 Bioprocessing Involved in Biofuel Production
Bioprocessing is the application of living organisms and their constituents, generally based on enzymes following different industrial processing and the consequent product which provides the opportunity to consume less energy and less water and therefore results in less effluent issue [119]. In this context, biofuels are non-toxic, completely combustible and eco-friendly which makes it as an alternative to fossil fuels. There are varieties of activities involved in the production of biofuels which is generally classified into two different processes known as the upstream and downstream processes [120].
The upstream processes include storage of liquid materials, inhibitory chemicals and particulate removal from the product, purification and sterilization etc. The upstream process includes the development of a microbial strain distinguished by the capacity to synthesize the required commercial value of a specific product [121]. The strain is then subjected to the enhanced protocol to optimize the strain’s ability to produce the product at an economical level. Downstream processing includes cell isolation from the fermentation broth, purification and extraction of desired product and removal or recycling of waste. In this processing, numerous steps that accompany the fermentation processes include suitable methods for extracting, purifying and characterizing the fermentation substance being sought [122]. A vast array of downstream processing can be implemented such as centrifugation, filtration as well as chromatography. Such approaches differ from the chemical and physical characteristics of the final product as well as the target grade [123].
The separation of the cell is the first step involved in the upstream process as mentioned above in which growth of the microbes and cells also take place. The upstream process involves the development of media, inoculums growth as well as development of that inoculums by the help of genetic engineering [124]. This engineering follows the procedure of genetics as well as focus on the kinetics involved in the growth of the cell along with the process. The development of the product will be improved by following the proper steps and this process terminates when the harvesting process of the cell gets finished [125]. The downstream process starts after the completion of the upstream process, which involves different steps such as ultra-centrifugation or centrifugation which are used to separate biomass. After the completion of these processes, to release the developed product cell disruption occurs [126]. The separations of the liquid medium and solid medium are obtained using processes like filtration or centrifugation. Metabolites purification occurs before concentrating the broth which causes removal of water as well as metabolite polishing. In the end, the produced are transferred to the market in sealed packed manure after the process of the formulation [127]. After the bioprocessing different types of biofuels are obtained either in the form of gaseous phase (biogas, biohydrogen or methane) or liquid biofuel (biodiesel, bioethanol or ether) as shown in Fig. 5.
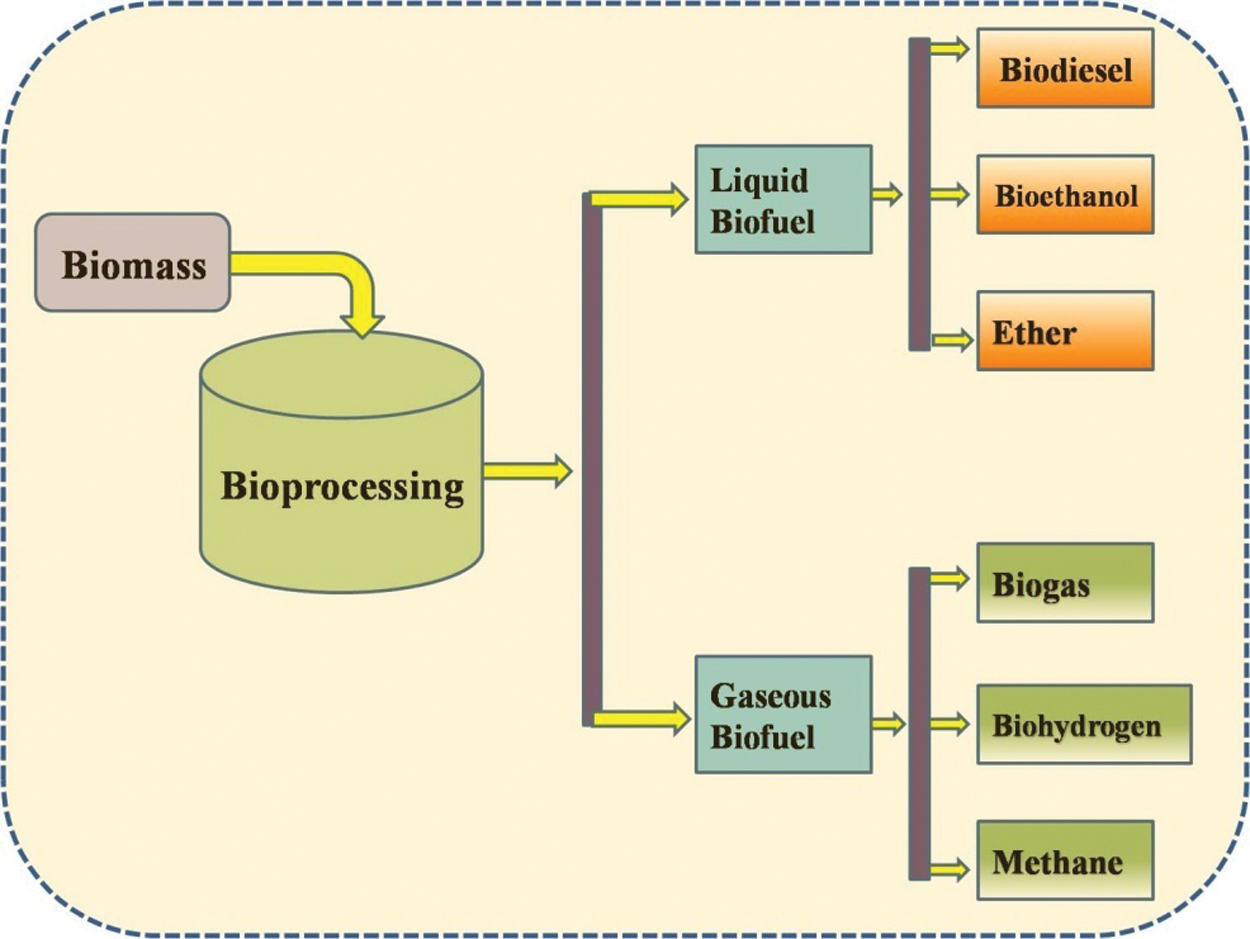
Figure 5: Classification of biofuels based on its phase and existence
There are different types of liquid biofuels produced by using a variety of biomass as a substrate such as rice husk, wheat straws, maize straws etc. These liquid biofuels include biodiesel, bioethanol and ethers, etc. [128]. One of the best products of liquid biofuels is the biodiesel which is generally produced from two different sources including collected waste of vegetable oils as well as fats of animals and oil from rich nuts and seeds. The process of transesterification is involved to produce methyl ester (biodiesel) using the feedstock [129]. These types of biodiesels can be used in compression-ignition engines generally with 5% blending whereas it can be used at 100% in specially developed engines. The economy of biofuels is low because of the low heating value of biofuels but with a certain blend level, about 20% can increase the efficiency of the combustion [130]. This can increase the fuel economy without making an impact on or in the performance of the vehicle.
Bioethanol is the type of biofuels which generally produced from the fermentation of different crops rich in sugar or by different steps of hydrolysis of the starch crops. The produced bioethanol can be blended with the conventional petrol having 5% additive and can be applied in different spark plugged ignition engines without any variation in its design [131]. The generated product has a high octane number but results in some problem in the performance of vehicle which includes the sensitivity of water and increase in vapour pressure. But the higher blending about 10% can increase the octane number and the volumetric efficiency of the vehicle gets increase [132]. This can increase the chances of compression ratio without causing the knocking during the combustion of fuel. At the high percentage of blending such as E85, AFR will decrease because the ethanol which was added has around 3.5% of oxygen in it [133].
Ethyl tertiary butyl ether is a type of biofuels can be produced from bioethanol by making some amendment in the steps of production. These steps are additional processes in which the materials get reacted with isobutylene to convert it into the ester [134]. The produced product can be blended up to 15% in conventional petrol because it is less volatile then bioethanol. This nature of ethyl tertiary butyl ether makes it a valuable product having a high octane number. Ethyl tertiary butyl ether can replace this biobutanol because it helps to solve the problems which generally occur during the application of biobutanol [112]. However, there are many possibilities of water pollution caused by the application of ethyl tertiary butyl ether. After the modification and amendments in the technique, this biofuels can be used in different ways because of its several benefits [135].
Gaseous biofuels is one of the emerging biofuels using lignocellulosic biomass and household wastes for the production purpose. These types of gaseous biofuels include biogas, biohydrogen and methane [136]. Biogas is a type of gaseous biofuel generated by anaerobic degradation of organic waste like biomass, cow dung, agricultural residue, green waste, sugar cane and cassava [137]. The production of biogas is classified into different steps which occur in the anaerobic reactor. These steps are as follow; pre-treatment, methanogenesis, acidogenesis hydrolysis and acetogenesis. In this process, the main role is being performed by the microorganism which is classified into two different groups based on the generated products during the process [138]. The two main groups of these microbes are the methane-producing bacteria (methanogens-Methanoculleus, Methanosarcinales, Methanobacteriales) and acid-producing bacterial (acidogenic-Moorellathermoacetica, Clostridium formiaceticum, Acetobacterwoodii, Clostridiumtermoautotrophicum) [139]. Methanogenesis is one the critical step in the process of the acidogenesis because about 70% of methane used in anaerobic digestion are produced in this step only. Whereas, during acetogenesis ethanol, VFAs (volatile fatty acids) with more than two carbons get converted by acetate-forming bacteria into carbon dioxide and hydrogen (main product) and acetate [140]. In these step, only methanogens convert hydrogen (oxidizing) and carbon dioxide (reducing) to methane whereas acetoclastic methanogens convert acetate to methane. Therefore, the produced biogas contains 1%–5% other gases, including hydrogen, carbon dioxide (35% to 40%) and methane (55% to 60%) [141].
Among different types of renewable source of energy, biohydrogen production is treated as the major alternatives which can replace the application of fossil fuel. There is no production of carbon dioxide during the combustion of biohydrogen. Biohydrogen is one of the most powerful fuels which can be used for running heavy types of equipment like vehicles motors. Along with these, it also used as major fuel in aerospace crafts, and production of heat energy does not release any kind of greenhouse gases [142]. Hydrogen produces 2.74 times more energy than any kind of other hydrocarbons having the energy amount of 121 Kj/gm [143]. The produced biohydrogen can be used at different sources such as fuel cell or for direct combustion. Due to the vast application of hydrogen and increased in demands, it has forced researchers to find an alternative and cost-effective techniques for biohydrogen production. Based on the hydrogen evolving process the system can be divided into four categories: (i) Biophotolysis; (ii) Photo-fermentation; (iii) Dark fermentation; (iv) Electro-fermentation [144].
Biophotolysis process is also known as the water-splitting photosynthesis because it uses only water, sunlight and the microorganisms which include green algae and cyanobacteria. Bio photolysis can be further divided into two types; direct process and indirect process [142]. In the direct process photon from the light energy arbitrate water-splitting are transported as an electron carrier and reduces hydrogenase enzyme which led to the formation of hydrogen. On the other hand, in case of in-indirect process carbohydrates are reduced to form hydrogen via photo-synthesis which changes light energy into the chemical energy [145]. The photo-fermentation process utilizes light energy and biomass to produce hydrogen and carbon dioxide in almost stoichiometric ratio. Theoretically, the complete degradation of biomass takes place during the process of photo fermentation. In this process degradation of organic acids e.g., lactic and butyric take place to biohydrogen and carbon dioxide with the help of photosynthetic bacteria under the anaerobic as well as the anoxic environment and it involves the use of nitrogenase without ammonium ions [146]. Dark fermentation is the most widely used process for the production of biohydrogen as the rate involved in this process is higher than the photo-fermentation and photolysis but the yield of hydrogen on the substrate is generally low due to the production of many byproducts. Dark fermentation is the processes of conversion of the organic molecules into biohydrogen using bacteria using various enzymes in anaerobic conditions [147]. The electro-fermentation process is also named as microbial electrolysis cells or bio catalyzed electrolysis cells. This process uses a variety of substrate for the production of hydrogen with the help of external potential apart from the potential generated by the microorganisms [148,149].
This review explored a detail structural overview of the lignocellulosic biomass and its composition, classification as well as its utilization for bioenergy application. Biomass is made up of different biological as well as chemical compounds which can be efficiently converted into the value-added product. These biochemical compounds include cellulose, hemicelluloses, lignin, fat, starch, water-soluble sugar, amino acids and some other complex compounds. Moreover, mannans, xylans, arabinogalactans and galactans are combined to form the hemicelluloses structure. Biomasses can be categorized into different section as per their applications and the future prospective. However, there are no any specific rule or way of the classifications of the biomasses, and therefore, depending upon the quality, composition, application and nature of the existence we can classify biomasses into different groups. Biomass can be classified into four different categories like biomass in form of woods, herbaceous biomass, waste from animals and humans and aquatic biomass. By exploring the importance of lignocellulosic biomass and its application over the other existing biomasses may have potential in terms of environmental impact, in the energy sector, bioprocessing involved in the biofuel production, liquid as well as the gaseous biofuels produced from the lignocellulosic biomass.
Highlights
1. Explores the advancement of structural component for biomass for biofuels application.
2. Detailed structure exposure of biomass for maximum utilization of application purpose.
3. Discusses the advantages of lignocellulosic biomass for the biofuels production application.
4. Discusses existing in the utilization of biomass for its value addition
5. Focuses on a sustainable solution for future scale-up studies for biomass to the biofuels production process.
Acknowledgement: Authors N. S. thankfully acknowledges to Department of Chemical Engineering and Technology IIT (BHU) Varanasi. Author M. S. acknowledges the Science and Engineering Research Board for SERB Research Scientist award and also to DST for DST INSPIRE Faculty award [IFA-13-MS-02].
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Pappu, A., Pickering, K. L., Thakur, V. K. (2019). Manufacturing and characterization of sustainable hybrid composites using sisal and hemp fibres as reinforcement of poly (lactic acid) via injection moulding. Industrial Crops and Products, 137, 260–269. DOI 10.1016/j.indcrop.2019.05.040. [Google Scholar] [CrossRef]
2. Ates, B., Koytepe, S., Ulu, A., Gurses, C., Thakur, V. K. (2020). Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chemical Reviews, 120(17), 9304–9362. DOI 10.1021/acs.chemrev.9b00553. [Google Scholar] [CrossRef]
3. Lee, N., George, C. (2013). Environmental assessment in developing and transitional countries: Principles, methods and practice. John Wiley & Sons. [Google Scholar]
4. Sharma, B., Thakur, S., Mamba, G., Prateek, P. Gupta, R. K. et al. (2020). Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. Journal of Environmental Chemical Engineering, 104608. [Google Scholar]
5. Bajpai, P. (2016). Pretreatment of lignocellulosic biomass for biofuel production, pp. 87. Singapore: Springer.
6. Chaudhary, J., Thakur, S., Mamba, G., Prateek, Gupta, R. K. (2020). Hydrogel of gelatin in the presence of graphite for the adsorption of dye: Towards the concept for water purification. Journal of Environmental Chemical Engineering, 104762. DOI 10.1016/j.jece.2020.104762. [Google Scholar] [CrossRef]
7. Wróblewska–Krepsztul, J., Rydzkowski, T., Borowski, G., Szczypiński, M. Klepka, T. et al. (2018). Recent progress in biodegradable polymers and nanocomposite−based packaging materials for sustainable environment. International Journal of Polymer Analysis and Characterization, 23(4), 383–395. DOI 10.1080/1023666X.2018.1455382. [Google Scholar] [CrossRef]
8. Thakur, V. K., Voicu, S. I. (2016). Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydrate Polymers, 146, 148–165. DOI 10.1016/j.carbpol.2016.03.030.
9. Thakur, S., Sharma, B., Verma, A., Chaudhary, J., Tamulevicius, S. et al. (2018). Recent progress in sodium alginate based sustainable hydrogels for environmental applications. Journal of Cleaner Production, 198, 143–159. DOI 10.1016/j.jclepro.2018.06.259. [Google Scholar] [CrossRef]
10. Yellishetty, M., Mudd, G. M., Ranjith, P. G., Tharumarajah, A. (2011). Environmental life−cycle comparisons of steel production and recycling: Sustainability issues, problems and prospects. Environmental Science & Policy, 14(6), 650–663. DOI 10.1016/j.envsci.2011.04.008. [Google Scholar] [CrossRef]
11. Srivastava, V., Goel, G., Thakur, V. K., Singh, R. P., Ferreira de Araujo, A. S. et al. (2020). Analysis and advanced characterization of municipal solid waste vermicompost maturity for a green environment. Journal of Environmental Management, 255, 109914. DOI 10.1016/j.jenvman.2019.109914. [Google Scholar] [CrossRef]
12. Katinas, V., Marčiukaitis, M., Perednis, E., Dzenajavičienė, E. F. (2019). Analysis of biodegradable waste use for energy generation in Lithuania. Renewable and Sustainable Energy Reviews, 101, 559–567. DOI 10.1016/j.rser.2018.11.022. [Google Scholar] [CrossRef]
13. Agarwal, R., Prakash, A. A. (2018). Reuse & recycle of non−biodegradable waste as construction materials. International Journal of Engineering Research, 7(3), 235–238. DOI 10.5958/2319-6890.2018.00066.1. [Google Scholar] [CrossRef]
14. Grippi, D., Clemente, R., Bernal, M. P. (2020). Chemical and bioenergetic characterization of biofuels from plant biomass: Perspectives for southern europe. Applied Sciences, 10(10), 3571. DOI 10.3390/app10103571. [Google Scholar] [CrossRef]
15. Siwal, S. S., Zhang, Q., Sun, C., Thakur, S., Gupta, V. K. et al. (2020). Energy production from steam gasification processes and parameters that contemplate in biomass gasifier–A review. Bioresource Technology, 297, 122481. DOI 10.1016/j.biortech.2019.122481. [Google Scholar] [CrossRef]
16. Domingues, R. R., Trugilho, P. F., Silva, C. A., Melo, I. C. N. D., Melo, L. C. et al. (2017). Properties of biochar derived from wood and high−nutrient biomasses with the aim of agronomic and environmental benefits. PLoS One, 12(5), e0176884. DOI 10.1371/journal.pone.0176884. [Google Scholar] [CrossRef]
17. Choe, E., Min, D. B. (2009). Mechanisms of antioxidants in the oxidation of foods. Comprehensive Reviews in Food Science and Food Safety, 8(4), 345–358. DOI 10.1111/j.1541-4337.2009.00085.x. [Google Scholar] [CrossRef]
18. Padam, B. S., Tin, H. S., Chye, F. Y., Abdullah, M. I. (2014). Banana by–products: An under–utilized renewable food biomass with great potential. Journal of Food Science and Technology, 51(12), 3527–3545. DOI 10.1007/s13197-012-0861-2. [Google Scholar] [CrossRef]
19. De Corato, U., De Bari, I., Viola, E., Pugliese, M. (2018). Assessing the main opportunities of integrated biorefining from agro–bioenergy co/by–products and agroindustrial residues into high–value added products associated to some emerging markets: A review. Renewable and Sustainable Energy Reviews, 88, 326–346. DOI 10.1016/j.rser.2018.02.041. [Google Scholar] [CrossRef]
20. Rawat, I., Kumar, R. R., Mutanda, T., Bux, F. (2011). Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Applied Energy, 88(10), 3411–3424. DOI 10.1016/j.apenergy.2010.11.025. [Google Scholar] [CrossRef]
21. Larnaudie, V., Bule, M., San, K. Y., Vadlani, P. V., Mosby, J. et al. (2020). Life cycle environmental and cost evaluation of renewable diesel production. Fuel, 279, 118429. DOI 10.1016/j.fuel.2020.118429. [Google Scholar] [CrossRef]
22. Dharmaraja, J., Shobana, S., Arvindnarayan, S., Vadivel, M., Atabani, A. E. et al. (2020). Biobutanol from lignocellulosic biomass: Bioprocess strategies. Lignocellulosic biomass to liquid biofuels, pp. 169–193. Academic Press. [Google Scholar]
23. Yusoff, M. N. A. M., Zulkifli, N. W. M., Sukiman, N. L., Chyuan, O. H., Hassan, M. H. et al. (2020). Sustainability of Palm Biodiesel in Transportation: A review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenergy Research, 1–18. [Google Scholar]
24. Lizundia, E., Puglia, D., Nguyen, T. D., Armentano, I. (2020). Cellulose nanocrystal based multifunctional nanohybrids. Progress in Materials Science, 112, 100668. DOI 10.1016/j.pmatsci.2020.100668. [Google Scholar] [CrossRef]
25. Sharif, F., Muhammad, N., Zafar, T. (2020). Cellulose based biomaterials: Benefits and challenges. In: Biofibers and biopolymers for biocomposites, pp. 229–246. Cham: Springer. [Google Scholar]
26. Bamdad, H., Hawboldt, K., MacQuarrie, S. (2018). A review on common adsorbents for acid gases removal: focus on biochar. Renewable and Sustainable Energy Reviews, 81, 1705–1720. DOI 10.1016/j.rser.2017.05.261. [Google Scholar] [CrossRef]
27. Alonso, D. M., Wettstein, S. G., Dumesic, J. A. (2012). Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chemical Society Reviews, 41(24), 8075–8098. DOI 10.1039/c2cs35188a. [Google Scholar] [CrossRef]
28. Thakur, V. K., Thakur, M. K. (2015). Recent advances in green hydrogels from lignin: A review. International Journal of Biological Macromolecules, 72, 834–847. DOI 10.1016/j.ijbiomac.2014.09.044. [Google Scholar] [CrossRef]
29. Chen, H. (2014). Chemical composition and structure of natural lignocellulose. Biotechnology of Lignocellulose, pp. 25–71. Dordrecht: Springer. [Google Scholar]
30. Naito, S., Maeda, N., Fujimoto, A. (2020). U.S. patent application No. 16/627,888. [Google Scholar]
31. Woiciechowski, A. L., Neto, C. J. D., de Souza Vandenberghe, L. P., de Carvalho Neto, D. P., Sydney, A. C. N. et al. (2020). Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance–Conventional processing and recent advances. Bioresource Technology, 304, 122848. DOI 10.1016/j.biortech.2020.122848. [Google Scholar] [CrossRef]
32. Trache, D., Thakur, V. K., Boukherroub, R. (2020). Cellulose nanocrystals/graphene hybrids—A promising new class of materials for advanced applications. Nanomaterials, 10(8), 1523. DOI 10.3390/nano10081523. [Google Scholar] [CrossRef]
33. Platnieks, O., Barkane, A., Ijudina, N., Gaidukova, G., Thakur, V. K. et al. (2020). Sustainable tetra pak recycled cellulose/Poly (Butylene succinate) based woody−like composites for a circular economy. Journal of Cleaner Production, 270, 122321. DOI 10.1016/j.jclepro.2020.122321. [Google Scholar] [CrossRef]
34. Deng, W., Zhang, H., Xue, L., Zhang, Q., Wang, Y. (2015). Selective activation of the C–O bonds in lignocellulosic biomass for the efficient production of chemicals. Chinese Journal of Catalysis, 36(9), 1440–1460. DOI 10.1016/S1872-2067(15)60923-8. [Google Scholar] [CrossRef]
35. Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J. et al. (2008). Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure, 1617(1), 1–16. [Google Scholar]
36. Raud, M., Olt, J., Kikas, T. (2016). N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass and Bioenergy, 90, 1–6. DOI 10.1016/j.biombioe.2016.03.034. [Google Scholar] [CrossRef]
37. Salapa, I., Katsimpouras, C., Topakas, E., Sidiras, D. (2017). Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass and Bioenergy, 100, 10–16. DOI 10.1016/j.biombioe.2017.03.011. [Google Scholar] [CrossRef]
38. Zheng, Q., Zhou, T., Wang, Y., Cao, X., Wu, S. et al. (2018). Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Scientific Reports, 8(1), 1–9. DOI 10.1038/s41598-017-17765-5. [Google Scholar] [CrossRef]
39. Liu, Z., Fei, B. (2013). Characteristics of moso bamboo with chemical pretreatment. Sustainable Degradation of Lignocellulosic Biomass–Techniques, Applications and Commercialization. Anuj K. Chandel and Silvio Silvério da Silva, IntechOpen, 10.5772/55379. [Google Scholar] [CrossRef]
40. Sravan, J. S., Butti, S. K., Sarkar, O., Krishna, K. V., Mohan, S. V. (2018). Electrofermentation of food waste-Regulating acidogenesis towards enhanced volatile fatty acids production. Chemical Engineering Journal, 334, 1709–1718. DOI 10.1016/j.cej.2017.11.005. [Google Scholar] [CrossRef]
41. Miranda Neto, M. (2018). Desenvolvimento de processo hidrotérmico e enzimático para a obtenção de açúcares redutores a partir da palha de arroz−BRS AG (Master’s Thesis). 2018. http://repositorio.furg.br/handle/1/7908. [Google Scholar]
42. Kaushik, A., Singh, M. (2011). Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization. Carbohydrate Research, 346(1), 76–85. DOI 10.1016/j.carres.2010.10.020. [Google Scholar] [CrossRef]
43. García–Aparicio, M. P., Ballesteros, M., Manzanares, P., Ballesteros, I., González, A. et al. (2007). Xylanase contribution to the efficiency of cellulose enzymatic hydrolysis of barley straw. Applied biochemistry and biotecnology, pp. 353–365. Humana Press. [Google Scholar]
44. Zhang, P., Dong, S. J., Ma, H. H., Zhang, B. X., Wang, Y. F. et al. (2015). Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Industrial Crops and Products, 76, 688–696. DOI 10.1016/j.indcrop.2015.07.037. [Google Scholar] [CrossRef]
45. Passoth, V., Sandgren, M. (2019). Biofuel production from straw hydrolysates: Current achievements and perspectives. Applied Microbiology and Biotechnology, 103(13), 5105–5116. DOI 10.1007/s00253-019-09863-3. [Google Scholar] [CrossRef]
46. Zhu, J. Y., Sabo, R., Luo, X. (2011). Integrated production of nano–fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chemistry, 13(5), 1339–1344. DOI 10.1039/c1gc15103g. [Google Scholar] [CrossRef]
47. David, K., Ragauskas, A. J. (2010). Switchgrass as an energy crop for biofuel production: A review of its ligno−cellulosic chemical properties. Energy & Environmental Science, 3(9), 1182–1190. DOI 10.1039/b926617h. [Google Scholar] [CrossRef]
48. Uyan, M., Alptekin, F. M., Cebi, D., Celiktas, M. S. (2020). Bioconversion of hazelnut shell using near critical water pretreatment for second generation biofuel production. Fuel, 273, 117641. DOI 10.1016/j.fuel.2020.117641. [Google Scholar] [CrossRef]
49. Kim, J. H., Jung, S., Park, Y. K., Kwon, E. E. (2020). CO2−cofed catalytic pyrolysis of tea waste over Ni/SiO2 for the enhanced formation of syngas. Journal of Hazardous Materials, 122637. [Google Scholar]
50. Yigezu, Z. D., Muthukumar, K. (2015). Biofuel production by catalytic cracking of sunflower oil using vanadium pentoxide. Journal of Analytical and Applied Pyrolysis, 112, 341–347. DOI 10.1016/j.jaap.2015.01.002. [Google Scholar] [CrossRef]
51. Sanjeeva, S. K., Pinto, M. P., Narayanan, M. M., Kini, G. M., Nair, C. B. et al. (2014). Distilled technical cashew nut shell liquid (DT−CNSL) as an effective biofuel and additive to stabilize triglyceride biofuels in diesel. Renewable Energy, 71, 81–88. DOI 10.1016/j.renene.2014.05.024. [Google Scholar] [CrossRef]
52. Martins, M. T. B., de Souza, W. R., da Cunha, B. A. D. B., Basso, M. F., de Oliveira, N. G. et al. (2016). Characterization of sugarcane (Saccharum spp.) leaf senescence: Implications for biofuel production. Biotechnology for Biofuels, 9(1), S9. DOI 10.1186/s13068-016-0568-0. [Google Scholar] [CrossRef]
53. Messineo, A., Volpe, R., Asdrubali, F. (2012). Evaluation of net energy obtainable from combustion of stabilised olive mill by−products. Energies, 5(5), 1384–1397. DOI 10.3390/en5051384. [Google Scholar] [CrossRef]
54. Santos, R. B., Lee, J. M., Jameel, H., Chang, H. M., Lucia, L. A. (2012). Effects of hardwood structural and chemical characteristics on enzymatic hydrolysis for biofuel production. Bioresource Technology, 110, 232–238. DOI 10.1016/j.biortech.2012.01.085. [Google Scholar] [CrossRef]
55. Weijde, T. V. D., Alvim Kamei, C. L., Torres, A. F., Vermerris, W., Dolstra, O. et al. (2013). The potential of C4 grasses for cellulosic biofuel production. Frontiers in Plant Science, 4, 107. [Google Scholar]
56. Westerholm, M., Hansson, M., Schnürer, A. (2012). Improved biogas production from whole stillage by co−digestion with cattle manure. Bioresource Technology, 114, 314–319. DOI 10.1016/j.biortech.2012.03.005. [Google Scholar] [CrossRef]
57. Xie, S., Lawlor, P. G., Frost, J. P., Hu, Z., Zhan, X. (2011). Effect of pig manure to grass silage ratio on methane production in batch anaerobic co−digestion of concentrated pig manure and grass silage. Bioresource Technology, 102(10), 5728–5733. DOI 10.1016/j.biortech.2011.03.009. [Google Scholar] [CrossRef]
58. Singha, A. S., Shama, A., Thakur, V. K. (2008). X-Ray diffraction, morphological, and thermal studies on methylmethacrylate graft copolymerized saccharum ciliare fiber. International Journal of Polymer Analysis and Characterization, 13(6), 447–462. DOI 10.1080/10236660802399747. [Google Scholar] [CrossRef]
59. Singha, A. S., Thakur, V. K. (2018). Synthesis, characterisation and analysis of hibiscus Sabdariffa Fibre reinforced polymer matrix based composites. Polymers and Polymer Composites, 17(3), 189–194. DOI 10.1177/096739110901700308. [Google Scholar] [CrossRef]
60. Trache, D., Hussin, M. H., Haafiz, M. K. M., Thakur, V. K. (2017). Recent progress in cellulose nanocrystals: Sources and production. Nanoscale, 9(5), 1763–1786. DOI 10.1039/C6NR09494E. [Google Scholar] [CrossRef]
61. Dolan, G. (2017). Bio-Tribology of plant cell walls: Measuring the interactive forces between cell wall components (Ph.D. Thesis). School of Chemical Engineering, The University of Queensland, 10.14264/uql.2017.714, [Google Scholar] [CrossRef]
62. Kont, R. (2017). The acquisition of cellulose chain by a processive cellobiohydrolase (Doctoral Dissertation). [Google Scholar]
63. Singha, A. S., Thakur, V. K. (2008). Fabrication of Hibiscus sabdariffa fibre reinforced polymer composites. Iranian Polymer Journal, 17, 541–553. [Google Scholar]
64. Chaabane, F. B., Marchal, R. (2013). Upgrading the hemicellulosic fraction of biomass into biofuel. Oil & Gas Science and Technology–Revue d’IFP Energies nouvelles, 68(4), 663–680. DOI 10.2516/ogst/2012093. [Google Scholar] [CrossRef]
65. Rafiqul, I. S. M., Sakinah, A. M. M., Zularisam, A. W. (2017). Hydrolysis of lignocellulosic biomass for recovering hemicellulose: State of the art. Waste Biomass Management–A Holistic Approach, pp. 73–106.Cham: Springer. [Google Scholar]
66. Yilgor, N., Unsal, O., Kartal, S. N. (2001). Physical, mechanical, and chemical properties of steamed beech wood. Forest Products Journal, 51(11), 89. [Google Scholar]
67. Wu, S., Shen, D., Hu, J., Zhang, H., Xiao, R. (2016). Cellulose–hemicellulose interactions during fast pyrolysis with different temperatures and mixing methods. Biomass and Bioenergy, 95, 55–63. DOI 10.1016/j.biombioe.2016.09.015. [Google Scholar] [CrossRef]
68. Ebringerová, A. (2005). Structural diversity and application potential of hemicelluloses. Macromolecular Symposia, 232(1), 1–12. DOI 10.1002/masy.200551401. [Google Scholar] [CrossRef]
69. Naidjonoka, P., Hernandez, M. A., Pálsson, G. K., Heinrich, F., Stålbrand, H. (2020). On the interaction of softwood hemicellulose with cellulose surfaces in relation to molecular structure and physicochemical properties of hemicellulose. Soft Matter, 16(30), 7063–7076. DOI 10.1039/D0SM00264J. [Google Scholar] [CrossRef]
70. Singh, S., Singh, G., Arya, S. K. (2018). Mannans: An overview of properties and application in food products. International Journal of Biological Macromolecules, 119, 79–95. DOI 10.1016/j.ijbiomac.2018.07.130. [Google Scholar] [CrossRef]
71. Shen, D. K., Gu, S., Bridgwater, A. V. (2010). Study on the pyrolytic behaviour of xylan–based hemicellulose using TG-FTIR and Py-GC–FTIR. Journal of Analytical and Applied Pyrolysis, 87(2), 199–206. DOI 10.1016/j.jaap.2009.12.001. [Google Scholar] [CrossRef]
72. Chen, W. H., Kuo, P. C. (2011). Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy, 36(11), 6451–6460. DOI 10.1016/j.energy.2011.09.022. [Google Scholar] [CrossRef]
73. Motta, F. L., Andrade, C. C. P., Santana, M. H. A. (2013). A review of xylanase production by the fermentation of xylan: Classification, characterization and applications. Sustainable degradation of lignocellulosic biomass–techniques, applications and commercialization, vol. 1. [Google Scholar]
74. Kacurakova, M., Capek, P., Sasinkova, V., Wellner, N., Ebringerova, A. (2000). FT−IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydrate Polymers, 43(2), 195–203. DOI 10.1016/S0144-8617(00)00151-X. [Google Scholar] [CrossRef]
75. Laine, C. (2005). Structures of hemicelluloses and pectins in wood and pulp. (Dissertation for the degree of Doctor of Science in Technology). Helsinki University of Technology, [Google Scholar]
76. Li, R., Wu, G. (2020). Preparation of polysaccharide−based hydrogels via radiation technique. Hydrogels based on natural polymers, pp. 119–148. Elsevier. [Google Scholar]
77. Cunha, A. G., Gandini, A. (2010). Turning polysaccharides into hydrophobic materials: A critical review. Part 1. Cellulose. Cellulose, 17(5), 875–889. DOI 10.1007/s10570-010-9434-6. [Google Scholar] [CrossRef]
78. Ashrafizadeh, M., Ahmadi, Z., Mohamadi, N., Zarrabi, A., Abasi, S. et al. (2020). Chitosan–based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. International Journal of Biological Macromolecules, 145, 282–300. DOI 10.1016/j.ijbiomac.2019.12.145. [Google Scholar] [CrossRef]
79. Thakur, V. K., Thakur, M. K. (2014). Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustainable Chemistry and Engineering, 2(12), 2637–2652. DOI 10.1021/sc500634p. [Google Scholar] [CrossRef]
80. Farkas, C., Rezessy–Szabó, J. M., Gupta, V. K., Bujna, E. Csernus, O. et al. (2020). Application of chitosan−based particles for deinking of printed paper and its bioethanol fermentation. Fuel, 280, 118570. DOI 10.1016/j.fuel.2020.118570. [Google Scholar] [CrossRef]
81. He, J., Huang, C., Lai, C., Huang, C., Li, M. et al. (2020). The effect of lignin degradation products on the generation of pseudo–lignin during dilute acid pretreatment. Industrial Crops and Products, 146, 112205. DOI 10.1016/j.indcrop.2020.112205. [Google Scholar] [CrossRef]
82. Tan, B., Yin, R., Yang, W., Zhang, J., Xu, Z. et al. (2020). Soil fauna show different degradation patterns of lignin and cellulose along an elevational gradient. Applied Soil Ecology, 155, 103673. DOI 10.1016/j.apsoil.2020.103673. [Google Scholar] [CrossRef]
83. Khan, M. U., Ahring, B. K. (2019). Lignin degradation under anaerobic digestion: Influence of lignin modifications−A review. Biomass and Bioenergy, 128, 105325. DOI 10.1016/j.biombioe.2019.105325. [Google Scholar] [CrossRef]
84. Zheng, Y., Guo, M., Zhou, Q., Liu, H. (2019). Effect of lignin degradation product sinapyl alcohol on laccase catalysis during lignin degradation. Industrial Crops and Products, 139, 111544. DOI 10.1016/j.indcrop.2019.111544.
85. Wang, N., Zhang, C., Zhu, W., Weng, Y. (2020). Improving interfacial adhesion of PLA/Lignin composites by one–step solvent–free modification method. Journal of Renewable Materials, 8(9), 1139–1147. DOI 10.32604/jrm.2020.09961. [Google Scholar] [CrossRef]
86. Thébault, M., Li, Y., Beuc, C., Frömel–Frybort, S. Zikulnig–Rusch, E. M. et al. (2020). Impregnated paper–based decorative laminates prepared from Lignin–substituted phenolic resins. Journal of Renewable Materials, 8(10), 1181–1198. [Google Scholar]
87. Cheng, C., Sun, W., Hu, B., Tao, G., Peng, C. et al. (2020). Analysis of the mechanism and effectiveness of Lignin in improving the high–temperature thermal stability of Asphalt. Journal of Renewable Materials, 8(10), 1243–1255. [Google Scholar]
88. Yan, X., Cheng, J. R., Wang, Y. T., Zhu, M. J. (2020). Enhanced lignin removal and enzymolysis efficiency of grass waste by hydrogen peroxide synergized dilute alkali pretreatment. Bioresource Technology, 301, 122756. DOI 10.1016/j.biortech.2020.122756. [Google Scholar] [CrossRef]
89. Huang, C., Fang, G., Yu, L., Zhou, Y., Meng, X. et al. (2020). Maximizing enzymatic hydrolysis efficiency of bamboo with a mild ethanol–assistant alkaline peroxide pretreatment. Bioresource Technology, 299, 122568. DOI 10.1016/j.biortech.2019.122568. [Google Scholar] [CrossRef]
90. Sheng, Y., Tan, X., Gu, Y., Zhou, X., Tu, M. et al. (2021). Effect of ascorbic acid assisted dilute acid pretreatment on lignin removal and enzyme digestibility of agricultural residues. Renewable Energy, 163, 732–739. DOI 10.1016/j.renene.2020.08.135. [Google Scholar] [CrossRef]
91. Harris, P. V., Welner, D., McFarland, K. C., Re, E., Navarro Poulsen, J. C. et al. (2010). Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry, 49(15), 3305–3316. DOI 10.1021/bi100009p. [Google Scholar] [CrossRef]
92. Luo, X., Liu, J., Zheng, P., Li, M., Zhou, Y. et al. (2019). Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnology for Biofuels, 12(1), 1036. DOI 10.1186/s13068-018-1346-y. [Google Scholar] [CrossRef]
93. Fu, H., Yang, S. T., Wang, M., Wang, J., Tang, I. C. (2017). Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co–utilization. Bioresource Technology, 234, 389–396. DOI 10.1016/j.biortech.2017.03.073. [Google Scholar] [CrossRef]
94. Foster, C. E., Martin, T. M., Pauly, M. (2010). Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: Carbohydrates. JoVE (Journal of Visualized Experiments), 37(37), e1837. [Google Scholar]
95. Artzi, L., Bayer, E. A., Moraïs, S. (2017). Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nature Reviews Microbiology, 15(2), 83–95. DOI 10.1038/nrmicro.2016.164. [Google Scholar] [CrossRef]
96. Bonechi, C., Consumi, M., Donati, A., Leone, G., Magnani, A. et al. (2017). Biomass: An overview. Bioenergy systems for the future, pp. 3–42. Woodhead Publishing. [Google Scholar]
97. Frandsen, K. E., Simmons, T. J., Dupree, P., Poulsen, J. C. N., Hemsworth, G. R. et al. (2016). The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nature Chemical Biology, 12(4), 298–303. DOI 10.1038/nchembio.2029. [Google Scholar] [CrossRef]
98. Silva, F. C., Cruz, N. C., Tarelho, L. A., Rodrigues, S. M. (2019). Use of biomass ash–based materials as soil fertilisers: Critical review of the existing regulatory framework. Journal of Cleaner Production, 214, 112–124. DOI 10.1016/j.jclepro.2018.12.268. [Google Scholar] [CrossRef]
99. Tumuluru, J. S., Wright, C. T., Boardman, R. D., Yancey, N. A., Sokhansanj, S. (2011). A review on biomass classification and composition, co–firing issues and pretreatment methods. 2011 Louisville, Kentucky. American Society of Agricultural and Biological Engineers. [Google Scholar]
100. Vassilev, S. V., Vassileva, C. G., Vassilev, V. S. (2015). Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel, 158, 330–350. DOI 10.1016/j.fuel.2015.05.050. [Google Scholar] [CrossRef]
101. Toklu, E. (2017). Biomass energy potential and utilization in Turkey. Renewable Energy, 107, 235–244. DOI 10.1016/j.renene.2017.02.008. [Google Scholar] [CrossRef]
102. Magdziarz, A., Wilk, M., Straka, R. (2017). Combustion process of torrefied wood biomass. Journal of Thermal Analysis and Calorimetry, 127(2), 1339–1349. DOI 10.1007/s10973-016-5731-0. [Google Scholar] [CrossRef]
103. Theapparat, Y., Chandumpai, A., Faroongsarng, D. (2018). Physicochemistry and utilization of wood vinegar from carbonization of tropical biomass waste. Tropical forests–New edition. IntechOpen. [Google Scholar]
104. Fernández, M. J., Mediavilla, I., Barro, R., Borjabad, E., Ramos, R. et al. (2019). Sintering reduction of herbaceous biomass when blended with woody biomass: Predictive and combustion tests. Fuel, 239, 1115–1124. DOI 10.1016/j.fuel.2018.11.115. [Google Scholar] [CrossRef]
105. Smit, A., Huijgen, W. (2017). Effective fractionation of lignocellulose in herbaceous biomass and hardwood using a mild acetone organosolv process. Green Chemistry, 19(22), 5505–5514. DOI 10.1039/C7GC02379K. [Google Scholar] [CrossRef]
106. Hillerton, J. E., Irvine, C. R., Bryan, M. A., Scott, D., Merchant, S. C. (2017). Use of antimicrobials for animals in New Zealand, and in comparison with other countries. New Zealand Veterinary Journal, 65(2), 71–77. DOI 10.1080/00480169.2016.1171736. [Google Scholar] [CrossRef]
107. Peterson, C. A., Brown, R. C. (2020). Oxidation kinetics of biochar from woody and herbaceous biomass. Chemical Engineering Journal, 401, 126043. DOI 10.1016/j.cej.2020.126043. [Google Scholar] [CrossRef]
108. Gorji, M. Y., Sartipipour, M., Tari, M. (2017). Indigenous energy production based on biomass in Rural Areas (Case Study: Tinouj Village, Qom, Iran), 35, 15–28. [Google Scholar]
109. Tenorio, A. T., Kyriakopoulou, K. E., Suarez–Garcia, E., van den Berg, C., van den Goot, A. J. (2018). Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass–Consequences for industrial use. Trends in Food Science & Technology, 71, 235–245. DOI 10.1016/j.pngs.2017.11.010. [Google Scholar] [CrossRef]
110. Zehnsdorf, A., Moeller, L., Stabenau, N., Bauer, A., Wedwitschka, H. et al. (2018). Biomass potential analysis of aquatic biomass and challenges for its use as a nonconventional substrate in anaerobic digestion plants. Engineering in Life Sciences, 18(7), 492–497. DOI 10.1002/elsc.201800032. [Google Scholar] [CrossRef]
111. Kumar, A., Adamopoulos, S., Jones, D., Amiandamhen, S. O. (2020). Forest biomass availability and utilization potential in Sweden: A review. Waste and Biomass Valorization, 11(3), 315. DOI 10.1007/s12649-020-00947-0. [Google Scholar] [CrossRef]
112. Pahla, G., Ntuli, F., Muzenda, E. (2018). Torrefaction of landfill food waste for possible application in biomass co–firing. Waste Management, 71, 512–520. DOI 10.1016/j.wasman.2017.10.035. [Google Scholar] [CrossRef]
113. Zhang, X., Che, Q., Cui, X., Wei, Z., Zhang, X. et al. (2018). Application of biomass pyrolytic polygeneration by a moving bed: Characteristics of products and energy efficiency analysis. Bioresource Technology, 254, 130–138. DOI 10.1016/j.biortech.2018.01.083. [Google Scholar] [CrossRef]
114. Faaij, A. P. (2018). Securing sustainable resource availability of biomass for energy applications in europe: Review of recent literature. Hg. v. University of Groningen. [Google Scholar]
115. Eiben, C. B., Tian, T., Thompson, M. G., Mendez–Perez, D. Kaplan, N. et al. (2020). Adenosine triphosphate and carbon efficient route to second generation biofuel isopentanol. ACS Synthetic Biology, 9(3), 468–474. DOI 10.1021/acssynbio.9b00402. [Google Scholar] [CrossRef]
116. Aparicio, E., Rodríguez–Jasso, R. M., Lara, A., Loredo–Treviño, A. Aguilar, C. N. et al. (2020). Biofuels production of third generation biorefinery from macroalgal biomass in the Mexican context: An overview. Sustainable seaweed technologies, pp. 393–446. Elsevier. [Google Scholar]
117. Yesilyurt, M. K., Cesur, C., Aslan, V., Yilbasi, Z. (2020). The production of biodiesel from safflower (Carthamus tinctorius L.) oil as a potential feedstock and its usage in compression ignition engine: A comprehensive review. Renewable and Sustainable Energy Reviews, 119, 109574. DOI 10.1016/j.rser.2019.109574. [Google Scholar] [CrossRef]
118. Dong, T., Knoshaug, E. P., Pienkos, P. T., Laurens, L. M. (2016). Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Applied Energy, 177, 879–895. DOI 10.1016/j.apenergy.2016.06.002. [Google Scholar] [CrossRef]
119. Alam, M. A., Wang, Z., Yuan, Z. (2017). Generation and harvesting of microalgae biomass for biofuel production. Prospects and challenges in algal biotechnology, pp. 89–111. Singapore: Springer. [Google Scholar]
120. Jiang, Y., Dong, W., Xin, F., Jiang, M. (2020). Designing synthetic microbial consortia for biofuel production. Trends in biotechnology, 38(8), 828–831. [Google Scholar]
121. Mbaneme–Smith, V., Chinn, M. S. (2015). Consolidated bioprocessing for biofuel production: recent advances. Energy and Emission Control Technologies, 3, 23. [Google Scholar]
122. John, J., Kaimal, K. S., Smith, M. L., Rahman, P. K., Chellam, P. V. (2020). Advances in upstream and downstream strategies of pectinase bioprocessing: A review. International Journal of Biological Macromolecules. DOI 10.1016/j.ijbiomac.2020.06.224. [Google Scholar] [CrossRef]
123. Madhavan, A., Jose, A. A., Parameswaran, B., Sindhu, R., Sukumaran, R. K. et al. (2017). Synthetic biology and metabolic engineering approaches and its impact on non–conventional yeast and biofuel production. Frontiers in Energy Research, 5, 861. DOI 10.3389/fenrg.2017.00008. [Google Scholar] [CrossRef]
124. Behera, B., Acharya, A., Gargey, I. A., Aly, N., Balasubramanian, P. (2019). Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresource Technology Reports, 5, 297–316. DOI 10.1016/j.biteb.2018.08.001. [Google Scholar] [CrossRef]
125. Lari, Z., Ahmadzadeh, H., Hosseini, M. (2019). Cell wall disruption: A critical upstream process for biofuel production. Advances in feedstock conversion technologies for alternative fuels and bioproducts, pp. 21–35. Woodhead Publishing. [Google Scholar]
126. Gunukula, S., Daigneault, A., Boateng, A. A., Mullen, C. A., DeSisto, W. J. et al. (2019). Influence of upstream, distributed biomass–densifying technologies on the economics of biofuel production. Fuel, 249, 326–333. DOI 10.1016/j.fuel.2019.03.079. [Google Scholar] [CrossRef]
127. Yew, G. Y., Lee, S. Y., Show, P. L., Tao, Y., Law, C. L. et al. (2019). Recent advances in algae biodiesel production: From upstream cultivation to downstream processing. Bioresource Technology Reports, 7, 100227. DOI 10.1016/j.biteb.2019.100227. [Google Scholar] [CrossRef]
128. Bharathiraja, B., Jayamuthunagai, J., Sudharsanaa, T., Bharghavi, A., Praveenkumar, R. et al. (2017). Biobutanol-An impending biofuel for future: A review on upstream and downstream processing tecniques. Renewable and Sustainable Energy Reviews, 68, 788–807. DOI 10.1016/j.rser.2016.10.017. [Google Scholar] [CrossRef]
129. Soo, V. W., McAnulty, M. J., Tripathi, A., Zhu, F., Zhang, L. et al. (2016). Reversing methanogenesis to capture methane for liquid biofuel precursors. Microbial Cell Factories, 15(1), 621. DOI 10.1186/s12934-015-0397-z. [Google Scholar] [CrossRef]
130. Chen, J., Li, J., Dong, W., Zhang, X., Tyagi, R. D. et al. (2018). The potential of microalgae in biodiesel production. Renewable and Sustainable Energy Reviews, 90, 336–346. DOI 10.1016/j.rser.2018.03.073. [Google Scholar] [CrossRef]
131. Veljković, V. B., Biberdžić, M. O., Banković-Ilić, I. B., Djalović, I. G. Tasić, M. B. et al. (2018). Biodiesel production from corn oil: A review. Renewable and Sustainable Energy Reviews, 91, 531–548. DOI 10.1016/j.rser.2018.04.024. [Google Scholar] [CrossRef]
132. Aditiya, H. B., Mahlia, T. M. I., Chong, W. T., Nur, H., Sebayang, A. H. (2016). Second generation bioethanol production: A critical review. Renewable and Sustainable Energy Reviews, 66, 631–653. DOI 10.1016/j.rser.2016.07.015. [Google Scholar] [CrossRef]
133. Azhar, S. H. M., Abdulla, R., Jambo, S. A., Marbawi, H., Gansau, J. A. et al. (2017). Yeasts in sustainable bioethanol production: A review. Biochemistry and Biophysics Reports, 10, 52–61. DOI 10.1016/j.bbrep.2017.03.003. [Google Scholar] [CrossRef]
134. Zabed, H., Sahu, J. N., Suely, A., Boyce, A. N., Faruq, G. (2017). Bioethanol production from renewable sources: Current perspectives and technological progress. Renewable and Sustainable Energy Reviews, 71, 475–501. DOI 10.1016/j.rser.2016.12.076. [Google Scholar] [CrossRef]
135. Mufrodi, Z., Aditya, W. (2019). Process design study of methyl tertiary–butyl ether production using reactive distillation. 2019 Ahmad Dahlan International Conference Series on Engineering and Science (ADICS–ES 2019). Atlantis Press. [Google Scholar]
136. Weng, Z., Shi, Y., Suda, M., Yanagiba, Y., Kawamoto, T. et al. (2018). Inhalation exposure to low levels of ethyl tertiary butyl ether: Its genetic effects were significantly modified by ALDH2 activity. Environmental and Molecular Mutagenesis, 60(2), 145–153. DOI 10.1002/em.22256. [Google Scholar] [CrossRef]
137. Wirth, R., Lakatos, G., Böjti, T., Maróti, G., Bagi, Z. et al. (2018). Anaerobic gaseous biofuel production using microalgal biomass-A review. Anaerobe, 52, 1–8. DOI 10.1016/j.anaerobe.2018.05.008. [Google Scholar] [CrossRef]
138. Achinas, S., Achinas, V., Euverink, G. J. W. (2017). A technological overview of biogas production from biowaste. Engineering, 3(3), 299–307. DOI 10.1016/J.ENG.2017.03.002. [Google Scholar] [CrossRef]
139. Patinvoh, R. J., Osadolor, O. A., Chandolias, K., Horváth, I. S., Taherzadeh, M. J. (2017). Innovative pretreatment strategies for biogas production. Bioresource Technology, 224, 13–24. DOI 10.1016/j.biortech.2016.11.083. [Google Scholar] [CrossRef]
140. Ali, S. S., Al–Tohamy, R., Manni, A., Luz, F. C., Elsamahy, T. et al. (2019). Enhanced digestion of bio–pretreated sawdust using a novel bacterial consortium: Microbial community structure and methane–producing pathways. Fuel, 254, 115604. DOI 10.1016/j.fuel.2019.06.012. [Google Scholar] [CrossRef]
141. Lyu, Z., Shao, N., Akinyemi, T., Whitman, W. B. (2018). Methanogenesis. Current Biology, 28(13), R727–R732. DOI 10.1016/j.cub.2018.05.021. [Google Scholar] [CrossRef]
142. Hagos, K., Zong, J., Li, D., Liu, C., Lu, X. (2017). Anaerobic co–digestion process for biogas production: Progress, challenges and perspectives. Renewable and Sustainable Energy Reviews, 76, 1485–1496. DOI 10.1016/j.rser.2016.11.184. [Google Scholar] [CrossRef]
143. Nagarajan, D., Lee, D. J., Kondo, A., Chang, J. S. (2017). Recent insights into biohydrogen production by microalgae–From biophotolysis to dark fermentation. Bioresource Technology, 227, 373–387. DOI 10.1016/j.biortech.2016.12.104. [Google Scholar] [CrossRef]
144. Argun, H., Gokfiliz, P., Karapinar, I. (2017). Biohydrogen production potential of different biomass sources. Biohydrogen production: Sustainability of current technology and future perspective, pp. 11–48. New Delhi: Springer. [Google Scholar]
145. M’Arimi, M. M., Kiprop, A. K., Ramkat, R. C., Kiriamiti, H. K. (2020). Progress in applications of advanced oxidation processes for promotion of biohydrogen production by fermentation processes. Biomass Conversion and Biorefinery, 1–25. [Google Scholar]
146. Sengmee, D., Cheirsilp, B., Suksaroge, T. T., Prasertsan, P. (2017). Biophotolysis–based hydrogen and lipid production by oleaginous microalgae using crude glycerol as exogenous carbon source. International Journal of Hydrogen Energy, 42(4), 1970–1976. [Google Scholar]
147. Budiman, P. M., Wu, T. Y. (2018). Role of chemicals addition in affecting biohydrogen production through photofermentation. Energy Conversion and Management, 165, 509–527. DOI 10.1016/j.enconman.2018.01.058. [Google Scholar] [CrossRef]
148. Lu, J., Chen, C., Huang, C., Leu, S., Lee, D. (2020). Dark fermentation production of volatile fatty acids from glucose with biochar amended biological consortium. Bioresource Technology, 303, 122921. DOI 10.1016/j.biortech.2020.122921. [Google Scholar] [CrossRef]
149. Bhagchandanii, D. D., Babu, R. P., Sonawane, J. M., Khanna, M., Pandit, S. et al. (2020). A comprehensive understanding of electro–fermentation. Fermentation, 6(3), 92. DOI 10.3390/fermentation6030092. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |