

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014131
ARTICLE
Synthetic Process of Bio-Based Phenol Formaldehyde Adhesive Derived from Demethylated Wheat Straw Alkali Lignin and Its Curing Behavior
1Faculty of Materials Science & Engineering, Changzhou University, Changzhou, 213164, China
2Jiangsu Key Laboratory of Environmentally Friendly Polymeric Materials, School of Materials Science and Engineering, Changzhou University, Changzhou, 213164, China
3Organization Department of the Party Committee, Changshu Institute of Technology, Changshu, 215500, China
4School of Petrochemical Engineering, Changzhou University, Changzhou, 213164, China
*Corresponding Author: Jinchun Li. Email: Lijinchun88@163.com
Received: 03 September 2020; Accepted: 06 October 2020
Abstract: Lignin is a natural biopolymer with a complex three-dimensional network, commercially obtained from waste liquid of paper pulp and bioethanol production, and could be a candidate for preparation of environment-friendly bio-based polyphenol material. In the present work, the demethylated wheat straw alkali lignin (D-Lig), prepared by demethylation of wheat straw alkali lignin (Lig) using an in-situ generated Lewis acid, was used to synthesize bio-based phenol formaldehyde resin adhesive (D-LPF) applied in plywood. Effects of synthetic process’s factors, including lignin substitution for phenol, NaOH concentration and molar ratio of formaldehyde to phenol, on the bonding strength and free formaldehyde content of D-LPF were investigated in detail, and the optimum synthetic process of D-LPF was obtained as following: Lignin substitution for phenol 60%, NaOH concentration 5.0% and molar ratio of formaldehyde to phenol 2.0, and under the optimum reaction condition, the D-LPF presented lower free formaldehyde content (0.18%) and higher bonding strength (2.19 MPa), which was better than those of containing-lignin phenol formaldehyde resin adhesive (LPF). Additionally, the curing behavior of the adhesive was studied by differential scanning calorimetry (DSC) combined with gel time. It can be obtained that D-LPF resin adhesive had the shortest gel time, and fastest curing rate, compared with those of PF and L-PF resin adhesives. The curing kinetics data was fitted well by Kissinger model using non-isothermal DSC method, and the average activation energy value was 85.3 kJ/mol, slightly higher than that of commercial PF resin, while lower than that of LPF (90.2 kJ/mol). Finally, based on the analytical results of high temperature fourier transform infrared spectroscopy (FTIR), a possible curing mechanism of D-LPF was proposed.
Keywords: Lignin; demethylation; phenol-formaldehyde resin; biobased adhesive; synthetic process; curing behavior
In recent years, sustainability of polymer materials has drawn more and more attention due to the depletion of petroleum on which most of monomers or chemicals are based. Petrochemical-based adhesives are required in many wood processing industries such as particleboard, wood panels, fiber boards and plywood etc. Among these various adhesives, phenol-formaldehyde (PF) adhesive has been extensively used due to its excellent properties in high bonding strength, water resistance, thermal stability and chemical stability [1,2]. Generally, PF adhesive is synthesized by reaction between phenol and formaldehyde under basic or acid conditions. The increasing depletion of petroleum leads to higher cost of original material to prepare PF adhesives, which makes a restriction for the application of PF adhesives in wood industries [3]. Therefore, many investigations have been carried out in searching for renewable natural resources and low-cost substitutes for phenol in the preparation of PF adhesives. Tannin, lignin and bagasse have been successfully applied in phenol formaldehyde adhesives [4,5]. However, it was reported that compared with commercial PF adhesives, most of tannin-phenol-formaldehyde adhesives or phenol-urea-formaldehyde adhesives have weaker bonding strength, higher curing temperature and longer curing time, which is unbeneficial to their practical application [6].
Lignin, as an abundant, renewable, inexpensive and readily available natural source, was a macromolecular with three-dimensional network structure, and consists of three phenylpropanoid units, namely p-hydroxyphenyl propane, guaiacylpropane and syringylpropane units [7,8]. Furthermore, lignin is one of the most abundant organic polymeric materials on the earth, secondary to cellulose. Lignin is a kind of polyphenol with similar structure to phenol, and it is considered as the most promising substitute for phenol targeting adhesive applications [9]. The reaction between lignin and formaldehyde proceeds similarly to that of phenol and formaldehyde, including methylolation and methylol condensation reactions [10,11]. Gravitis et al. [12] reported a kind of PF adhesive prepared by mixing 10 wt% of lignin with commercial phenol-formaldehyde resin to be used as a binder to manufacture plywood, and it was found that the introduction of lignin into the adhesive has little negative influence on the plywood’s mechanical property. Moreover, Qiao et al. [13] obtained soda lignin derived from cornstalk residues from bioethanol production, and the lignin took the place of 20 wt% of phenol to modify phenol-formaldehyde adhesive, whose bonding strength descended slightly as well as little higher free formaldehyde content compared with unmodified PF adhesive.
Although lignin could be directly used in the PF adhesive either as filler or as a substitute for phenol, it is not commercially attractive due to its lower reactivity causing longer curing time, higher curing temperature and lower bonding strength [14–16]. Therefore, it is necessary to modify lignin to enhance reactive sites and active functional groups. Many efforts have been done to raise the reactivity of lignin, such as methylolation [17], phenolation [18], demethylation [19], reduction [20], and oxidation [21]. Khan et al. [22] prepared methylolated lignin to synthesize lignin-based PF adhesives, and found that dry bonding strength of the adhesive with up to 50% substitution of lignin for phenol was 1.54 MPa, higher than that of unmodified lignin-based PF adhesive. In addition, phenolated steam explosion lignin (SEL) was used to prepare PF adhesive, and under the optimum synthetic process of the adhesive, the properties of adhesive with replacement percentage of SEL for phenol up to 70 wt% and its corresponding plywoods met the Chinese National Standard (GB/T 9846-2004) for first grade plywood [23]. Moreover, Wu et al. [24] prepared demethylated wheat straw soda lignin by using sulfur at 225–235°C under high pressure, and lignin’s reactivity in phenol formaldehyde adhesives was enhanced. Among the above methods, demethylation is an effective one to increase the reactivity of lignin [25,26]. Recently, Wang et al. [27] prepared demethylated lignin using Halogen Acids (namely, hydrochloric acid and hydrobromic acid) as catalyst, respectively, and it was found that demethylation with HI has the higher degree, and the lignin after demethylation can take the place up to 50% of phenol in PF resins under the condition of meeting performance standards for exterior-grade plywood panels.
Generally, the properties of the cured resins rely on the curing extent as well as its composition. Therefore, it is of importance to investigate the curing process of phenolic resins to provide much more valuable information for their application. Differential scanning calorimetry (DSC) technique has been frequently used to research the curing behavior of thermoset polymer [28]. Several models, such as Borchardt [29], Paysepara [30] and Ozawa [31] models, have been applied to fit the data from DSC to obtain the related dynamic parameters. Among those, the Kissinger iso-conversional method is widely used, and it is a very simple one based on the relation between exothermic peak temperature (Tp) and heating rate (β) [32,33]. It is reported by Liu et al. [34] that the curing reaction kinetics data of lignin formaldehyde resin can be fitted well by Kissinger method, and the related kinetic parameters were obtained.
In this research, wheat straw alkali lignin (Lig) as a by-product of pulp industry was demethylated by in-situ generated Lewis acid (hydriodic acid) with catalyst iodocylclohexane in N,N′-dimethyl formamide (DMF), and the demethylated lignin was applied to replace part of phenol to synthesize bio-based PF resin adhesives (D-LPF). Effects of different factors, including substitution of lignin for phenol, molar ratio of formaldehyde to phenol and catalyst concentration, on the properties of D-LPF were investigated in order to optimize the synthetic process of D-LPF. Moreover, the curing behavior of adhesives was studied by differential scanning calorimetry (DSC), and the Kissinger model was used to fit non-isothermal DSC data. Finally, a possible curing mechanism of D-LPF was proposed.
Wheat straw alkali lignin with hydroxyl group content 6.65 wt%, and weight average molecular weight 6000 g/mol accompanied with polydispersity index 1.3, was provided by Shandong Quanlin Paper Co., Ltd., China, and its density was 1.30 g/cm3. Both N,N- dimethyl formamide and ethyl ether were AR grade, and obtained from Strong Functional Chemical Co., Ltd., China. Iodocyclohexane was supplied by Tokyo Chemical Plant Type Club. The other chemicals were purchased from Medicine Group Chemical Reagent Co., Ltd., China.
2.2 Preparation of Demethylated Wheat Straw Alkali Lignin
Demethylated wheat straw alkali lignin (D-Lig) was prepared according to the previous work [35]. Wheat straw alkali lignin(Lig) and N,N-dimethyl formamide accompanied with iodocyclohexane were charged into a 50 mL three-neck round bottom flask. The mixture was agitated at 145°C under nitrogen atmosphere for 3 h before cooling down to room temperature to finish the reaction, and then diluted with dichloromethane. The collected solution was dried a vacuum oven to obtain the crude product, and then washed twice with deionized water in 100 mL per time. Finally, D-WSAL was obtained by centrifugation (5000 rpm) and dried under vacuum at 80°C before being used.
2.3 Synthesis of Resin Adhesives
The self-made phenol formaldehyde (PF) resin adhesive and phenol formaldehyde resin adhesives containing lignin or demethylated lignin were prepared in a 100 mL four-neck round bottom flask.
2.3.1 Preparation of PF Resin Adhesive
PF resin adhesive was prepared following the approach described in the reference [36]. Briefly, 3.60 g phenol, 13.50 g formaldehyde, 0.75 g NaOH and 2.70 g distilled water were added into the flask and stirred at 60°C for 1 h. Then the reaction temperature was gradually increased to 90°C in 1 h before 6.75 g formaldehyde was added into the solution for further 2 h reaction.
2.3.2 Preparation of LPF and D-LPF Resin Adhesives
LPF and D-LPF resin adhesives were prepared following the similar procedure as PF adhesive, with the only difference being that 60 wt% of phenol was replaced with Lig and D-Lig, respectively.
2.4 Synthetic Process of Plywood
The prepared resin adhesives mentioned above were used to prepare lap-shear specimens for testing according to the process reported by Liu et al. [23]. In order to obtain three –layer bonded samples, standard single-layer pine veneers with size of 100 mm × 25 mm × 4.0 mm were kept 5 wt% moisture content, and covered with 125 g/m2 adhesive, and the middle layer was a core layer. And then the specimens were pressed at the temperature of 150°C under the pressure of 1.0 MPa for 6 minutes. The bonded wood samples were kept at room temperature for 24 h before measurement.
2.5 Characterization and Measurement
FTIR spectra of samples were recorded by an fourier infrared spectrometer from American Nicolet Co., Ltd., USA in the range of wavenumber from 4000 to 400 cm−1 with a spectral resolution of 4 cm−1.
2.5.2 Properties Testing Resin Adhesives
Resin adhesives’ properties were tested according to GB/T 14074-2006 of China. For free phenol content, firstly distilled outphenol in resin adhesive using steam distillation, and then titrated it by saturated bromine aqueous solution. And for free formaldehyde content, determined by hydroxylamine hydrochloride. Finally, gel time was measured as followed: Placing 1 g sample in a test tube with 12 mm diameter, and placing it in an oil bath at 150°C, meanwhile starting time, up to the initial occurrence of gelation, the time was over, and the time interval was referred as gel time.
2.5.3 Mechanical Properties of Lap-Shear Specimens
Mechanical properties, including dry and wet bonding strength, were tested according to GB/T 17657-2013 of China using electronic universal testing machine (Shanghai Yi Huan Instrument Technology Co., Ltd., China). Specimens were treated according to the following procedure: (1) Firstly, immersed in boiling water for 4 h, and then dried at 63°C for 20 h, and finally immersed in boiling water for 4 h again before cooling for 10 minutes. Samples were tested under the conditions that a constant loading speed was 10 MPa/min, and maximum load was recorded with an accuracy of 10 N. Two samples were determined in parallel.
Curing behavior of the PF, LPF and D-LPF resin adhesives were tested by the Differential Scanning Calorimeter (DSC, Mettler Toledo, Stare System). The pans utilized were pressure-proof medium (ME-26929) and the weight of a sample was 10 mg. The pans, with a volume of 120 μl, can withstand a vapour pressure up to 10 MPa. Iso-conversional method was conducted under nitrogen atmosphere with the temperature scanning from 40 to 200°C, the heating rates 5, 10, 15 and 20 °C/min respectively, accompanied with the 50 mL/min of N2 flow.
In general, the kinetic model of thermoset polymers is based on a single-step kinetic equation that relates to the curing evolution (dα/dT) at a constant temperature with some function of the reactant concentration, f(α), through a rate constant k. The rate equation can be expressed as follows:

where A is the pre-exponential factor, E is the activation energy which is independent of conversion, R is the gas constant, and α is the curing degree. In this way, Kissinger proposed the following model [37]:

where β is the heating rate, and expressed as dT/dt. By taking the logarithm of the above Kissinger equation, Kissinger equation was deduced as follows:

where Tp is the peak temperature. Eα can be obtained by plotting of ln(β/Tp2) vs. 1/Tp using the DSC data at different heating rates.
2.5.6 High Temperature Fourier Transform Infrared Spectroscopy (FTIR)
High temperature fourier transform infrared spectroscopy was used to characterize the structure change of D-LPF resin adhesive during the curing process at different temperatures. The solution composed of a small amount of phenolic resin and ethanol was coated on the sodium bromide crystal, and then the crystal was dried in a vacuum oven at 40°C. The sample was placed into the sample holder, preheated for 15 min at 110°C, and then heated at 150°C for curing for 40 min before measured with FTIR.
FTIR spectra of lignin(Lig) and demethylated lignin(D-Lig) shown in Fig. 1, and the characteristic peaks were assigned and shown in Tab. 1. It can be seen that C-O stretching absorption peaks of syringyl(S) and guaiacy(G) structures, and C-H bending of aromatic ring respectively appeared at 1372, 1264, and 843 cm−1 in Lig, corresponding to [38]. The key difference of Lig and D-Lig lies in relative peak area of phenolic hydroxyl and methoxyl groups, in which D-Lig showed more phenolic hydroxyl groups, and had lower mehoxyl content. Fourier transform infrared spectroscopy semi-quantitative method was an effective way to measurethe relative content of typical groups [39]. After demethylation of Lig, its phenolic hydroxyl group content increased to 7.21 from 1.01, with over 6 fold increase; while methoxyl group content decreased to 0.25 from 0.82 with less than 3 fold reduction. the content of phenolic hydroxyl group obviously increased more than the reduction value of the methoxy group, which might be attributed to ether bond’s hydrolysis in part during the process of demethylation. It can be seen that lignin was effectively demethylated.
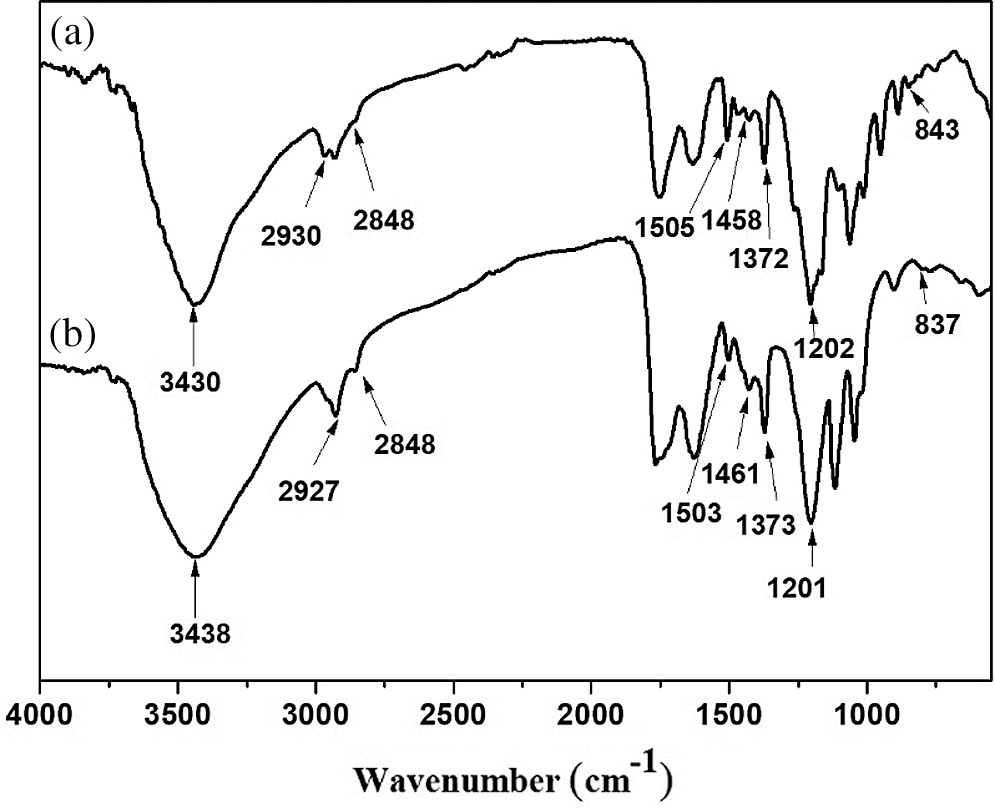
Figure 1: FTIR spectra of Lig (a) and D-Lig (b)
Table 1: Assignments of characteristic absorption peaks from the FTIR spectra of WSAL and D-WSAL
3.2 Process Optimization for Adhesive Preparation
D-Lig synthesized above was applied to prepare bio-based phenol formaldehyde adhesives. Various factors, including substitution of lignin for phenol, molar ratio of formaldehyde to phenol and catalyst concentration were investigated to understand their effect on the adhesive properties, thus the optimum preparation process was obtained for making the adhesives with good properties.
3.2.1 Lignin Substitution for Phenol
Fig. 2 showed the effect of lignin substitution for phenol on the physico-mechanical properties of adhesives. With the increase of lignin substitution for phenol from 0 to 60%, the bonding strength decreased from 2.42 to 1.88 MPa and the free formaldehyde content increased from 0.03 to 0.33%. This may be ascribed to lower reactivity of demethylated lignin with formaldehyde. While, lignin’s larger molecular chain could cause the increase of resin’s cross-linking density, which benefits to the enhancement of bonding strength. Therefore, the descent trend of adhesive strength is not obvious before lignin substitution of phenol reaching 60%. Thereafter, with the increasing of lignin substitution up to 70%, the bonding strength of the adhesive decreased rapidly to 1.38 MPa. Meanwhile the free formaldehyde content increased to 0.61%. Addition of much more lignin caused high viscosity of the system leading to incomplete reaction. From a comprehensive point that D-LPF resin adhesive had both higher bonding strength and lower free formaldehyde content, 60 wt% of lignin substitution for phenol is chosen for the adhesive preparation.
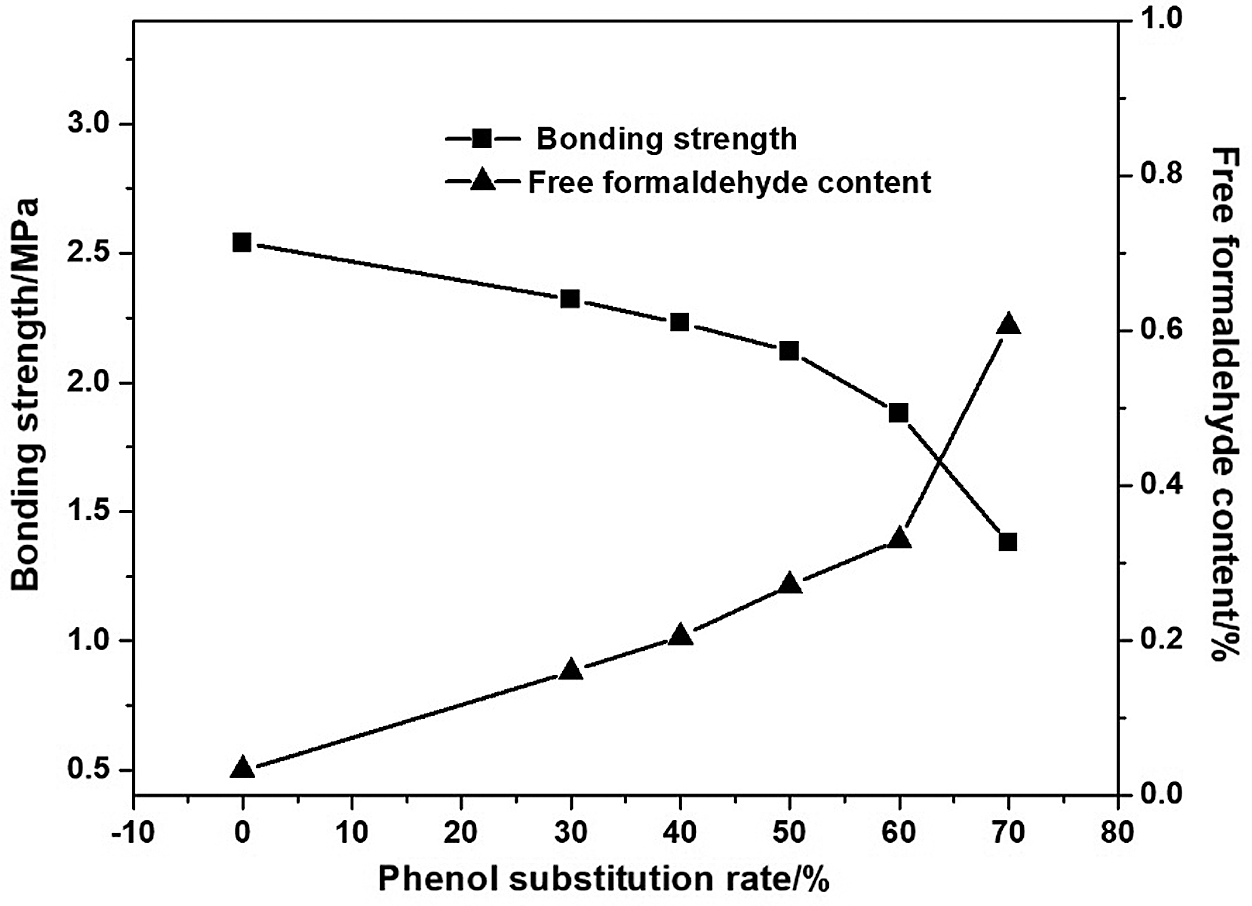
Figure 2: Effect of lignin substitution for phenol on bonding strength and free formaldehyde content of adhesives
Effect of catalyst (NaOH) concentration on the adhesive properties is given in Fig. 3. It is observed that the free formaldehyde content of LPF adhesive reduced drastically with the increasing of the NaOH concentration; meanwhile the adhesive strength increased and reached the peak (2.11 MPa) at with the free formaldehyde content reduced to 0.22%. Catalyst could enhance the solubility of lignin in water, which led to the decrease of the system’s viscosity, and thus the reaction can be carried out thoroughly. Above 5 wt% NaOH, the bonding strength of LPF dropped to 1.98 MPa. This may be due to the Cannizzaro reaction of the formaldehyde in the system to produce methanol under alkaline condition, which weakens the reaction effectiveness of formaldehyde with lignin and phenol [40]; furthermore, the higher pH in the reaction system caused hydrolysis of lignin. Both of the above reasons resulted in the decrease of the adhesives’ bonding strength. Therefore, 5 wt% NaOH was used in the preparation of LPF adhesive.
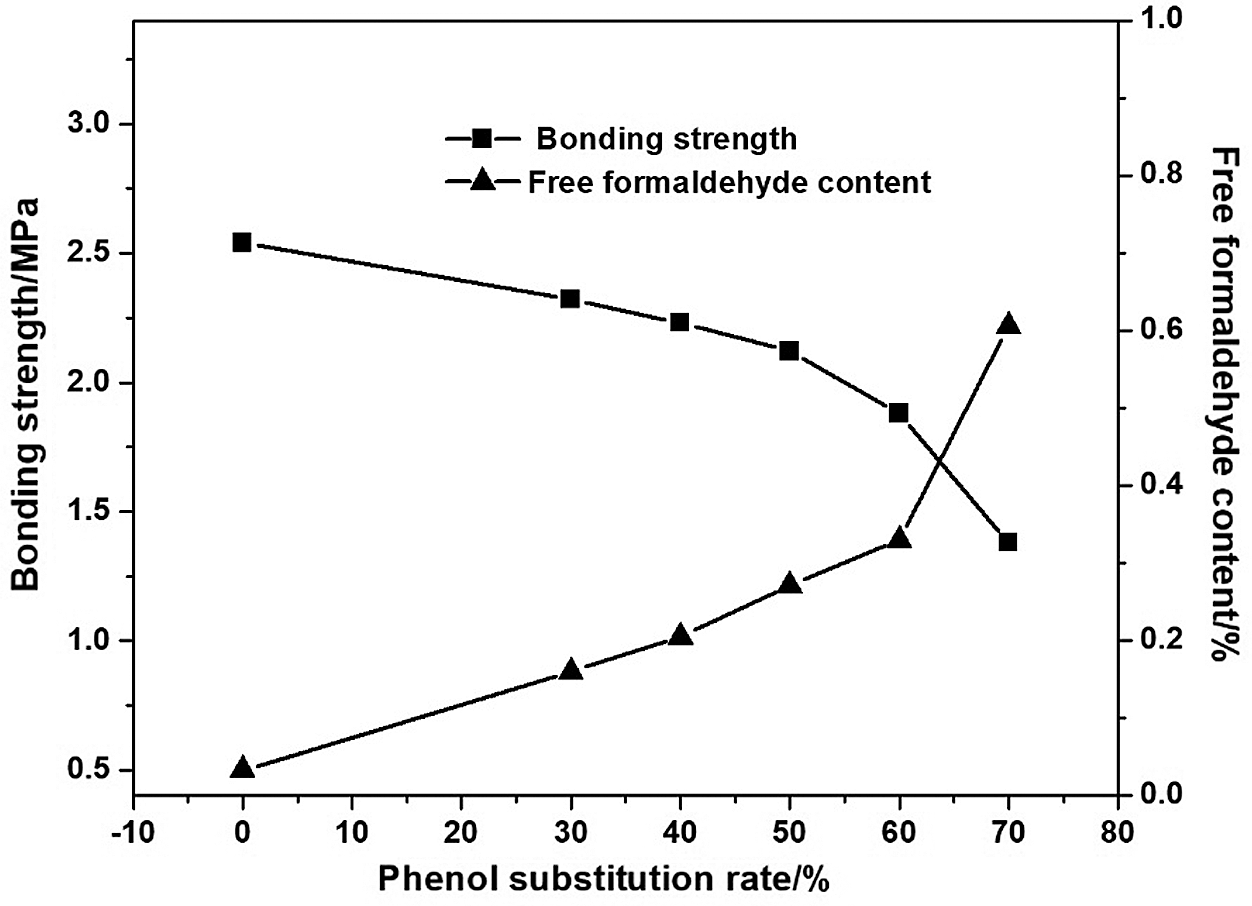
Figure 3: Effect of NaOH concentration on the bonding strength and free formaldehyde content of adhesives
3.2.3 Molar Ratio of Formaldehyde to Phenol
Effect of molar ratio of formaldehyde to phenol on adhesive properties is given in Fig. 4. It can be observed that with the increase of the molar ratio, the bonding strength rose up to 2.2 MPa the maximum at the molar ratio 2.0, and then decreased rapidly. This may be due to the hydrogen bond formation between excessive formaldehyde molecules, which hindered the cross-linking reaction, and led to the decrease of bonding strength. Furthermore, the free formaldehyde content was raised with the increase of the molar ratio of formaldehyde to phenol. It may be ascribed to the existence of excessive formaldehyde in the adhesive after the gelation. Based on the above consideration, the molar ratio of formaldehyde/phenol is chosen as 2.0 for the preparation of LPF adhesive.
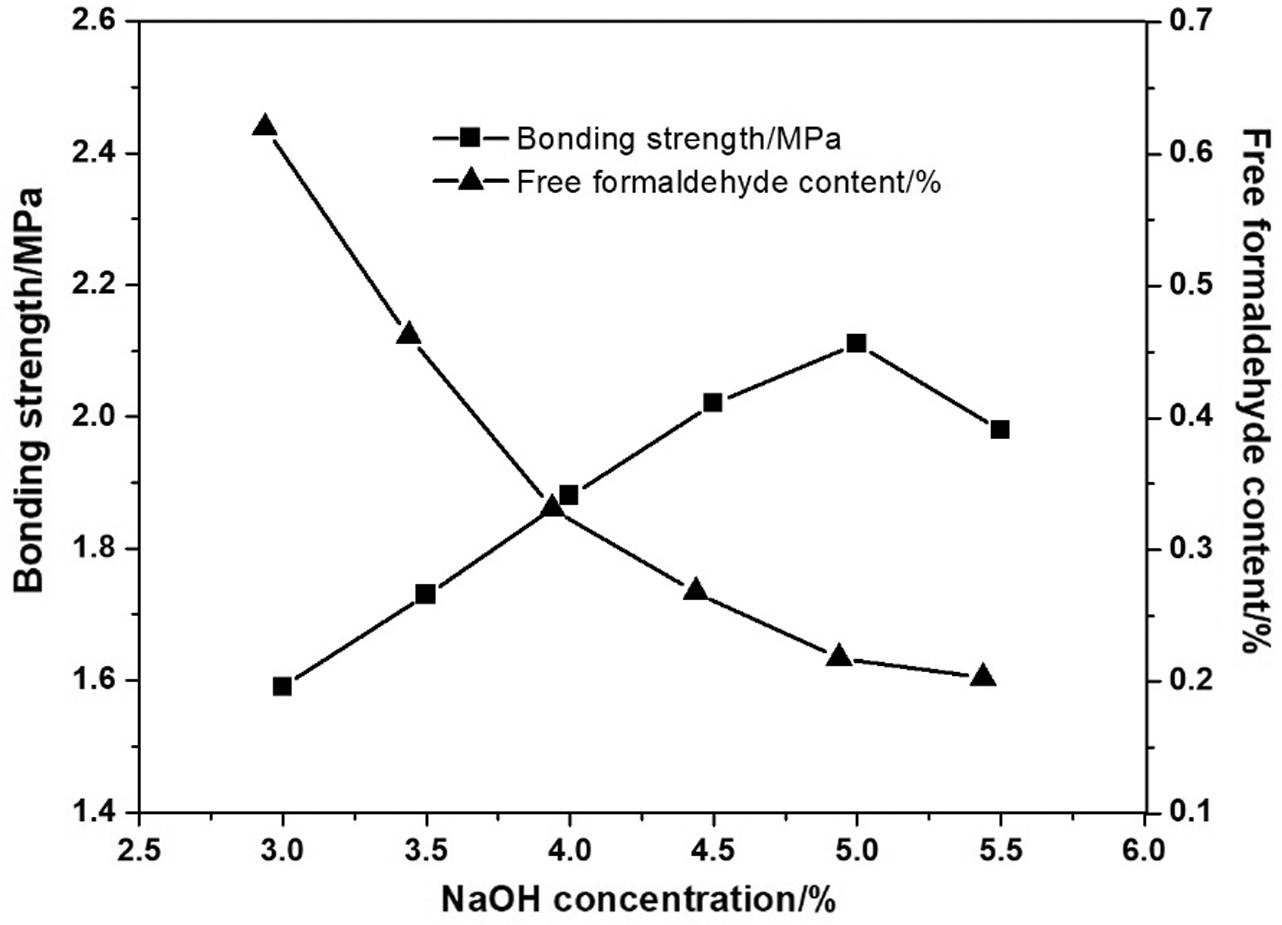
Figure 4: Effect of molar ratio of formaldehyde to phenol on the properties of adhesives
3.3 D-LPF Resin Adhesives’ Performance
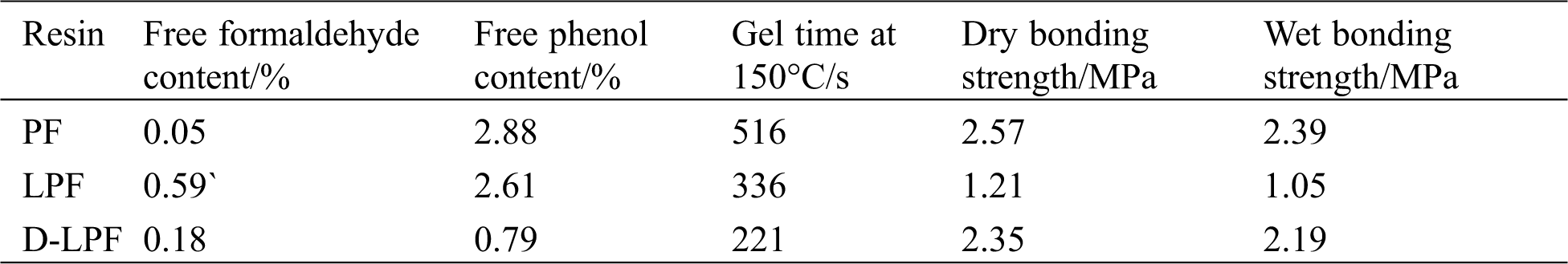
Demethylated lignin-containing PF resin adhesive (D-LPF) was prepared following the optimized synthetic process. Homemade PF and lignin-containing PF (LPF) with the same molar of phenol substitution were also prepared for comparison of the adhesive properties (Tab. 2). The results showed that D-LPF resin adhesive had lowerfree formaldehyde content and free phenol content, namely 0.18% and 0.79%, respectively, which were significantly lower than those of LPF resin adhesive. Furthermore, D-LPF showed higher dry, increasing to 2.35 MPa from 1.21 MPa, and higher wet bonding strength enhanced to 2.19 MPa from 1.05 MPa. Compared with PF resin adhesive, D-LPF resin adhesive presented a little high free formaldehyde content increasing from 0.05 to 0.59%, while its free phenol content reduced from to 0.79% from 2.88%, meanwhile both the dry bonding strength and wet bonding strength provided little change. Additionally, the D-LPF resin adhesive’s gel time shortened in contrast to that of LPF. This may be caused by D-Lig having higher reactivity and lower molecular weight than Lig. However, the gel time of both LPF and D-LPF resin adhesives was shorter than that of PF resin adhesive, which is in accordance with the results reported in literature [41]. It could be interpreted by the incomplete curing reaction caused by higher viscosity of LPF or D-LPF adhesive. Although the bonding strength of both LPF and D-LPF resin adhesives was less than that of PF resin adhesive, they still met the requirement of GB/T 17657-2013 of China for I grade plywood in which the bonding strength should be no less than 0.8 MPa.
Table 2: Properties of PF, LPF and D-LPF resin adhesives
3.4 Curing Behavior of D-LPF Resin Adhesives
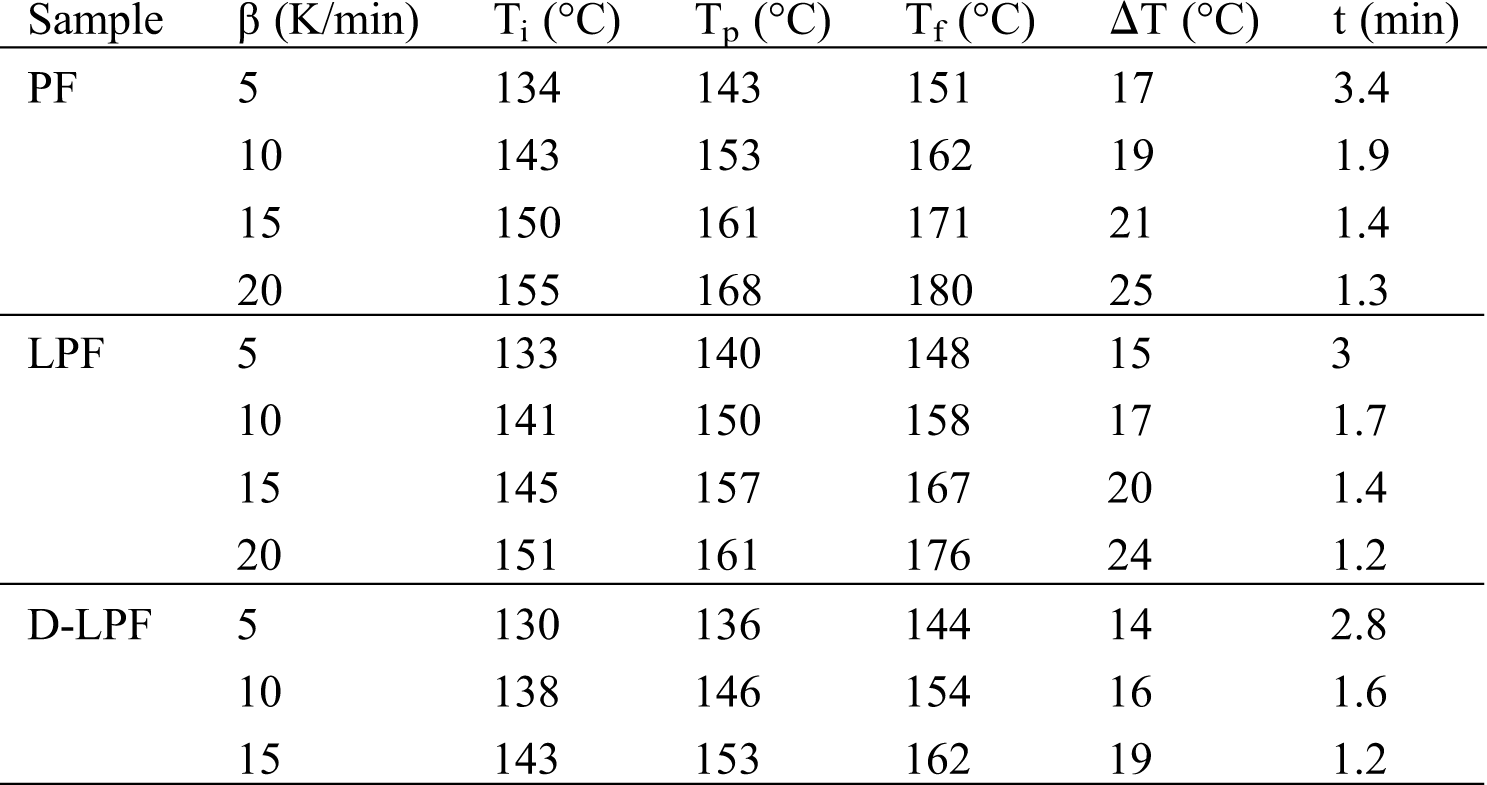
Fig. 5 showed the DSC curves of the D-LPF resin adhesives accompanied with PF and LPF resin adhesives at different heating rates. There was only one peak in the DSC curves, which was caused by the condensation reaction between the phenol and formaldehyde. The curing reaction of phenolic resin was generally based on the condensation reaction between two different functional groups [42]. Firstly, the condensation reaction occurred between hydroxymethyl group and ortho- and para-free positions of phenol (or/and lignin in the case of lignin–based phenolic resin). Secondly, the condensation reaction took place among methylol phenols, i.e., phenol and/or methylol phenol, and thus the desired resols were obtained. Moreover, with the increase of heating rate, the exothermic peak gradually moved toward high temperature, which was also reported by other literature [43]. Additionally, the curing temperature at various heating rates was obtained (Fig. 5) and listed in Tab. 3. The results suggested that with the increase of heating rate, the curing temperature, which was the temperature required for the curing of the adhesive, and corresponding to the peak temperature (Tp) from the starting temperature (Ti) to the final temperature (Tf) obtained by the DSC curing curves, significantly increased. While the reaction times of all three adhesives significantly decreased. In addition, at the same heating rate, the curing time of D-LPF was the shortest, while that of PF was the longest, which is consistent with the results of gel time mentioned above.
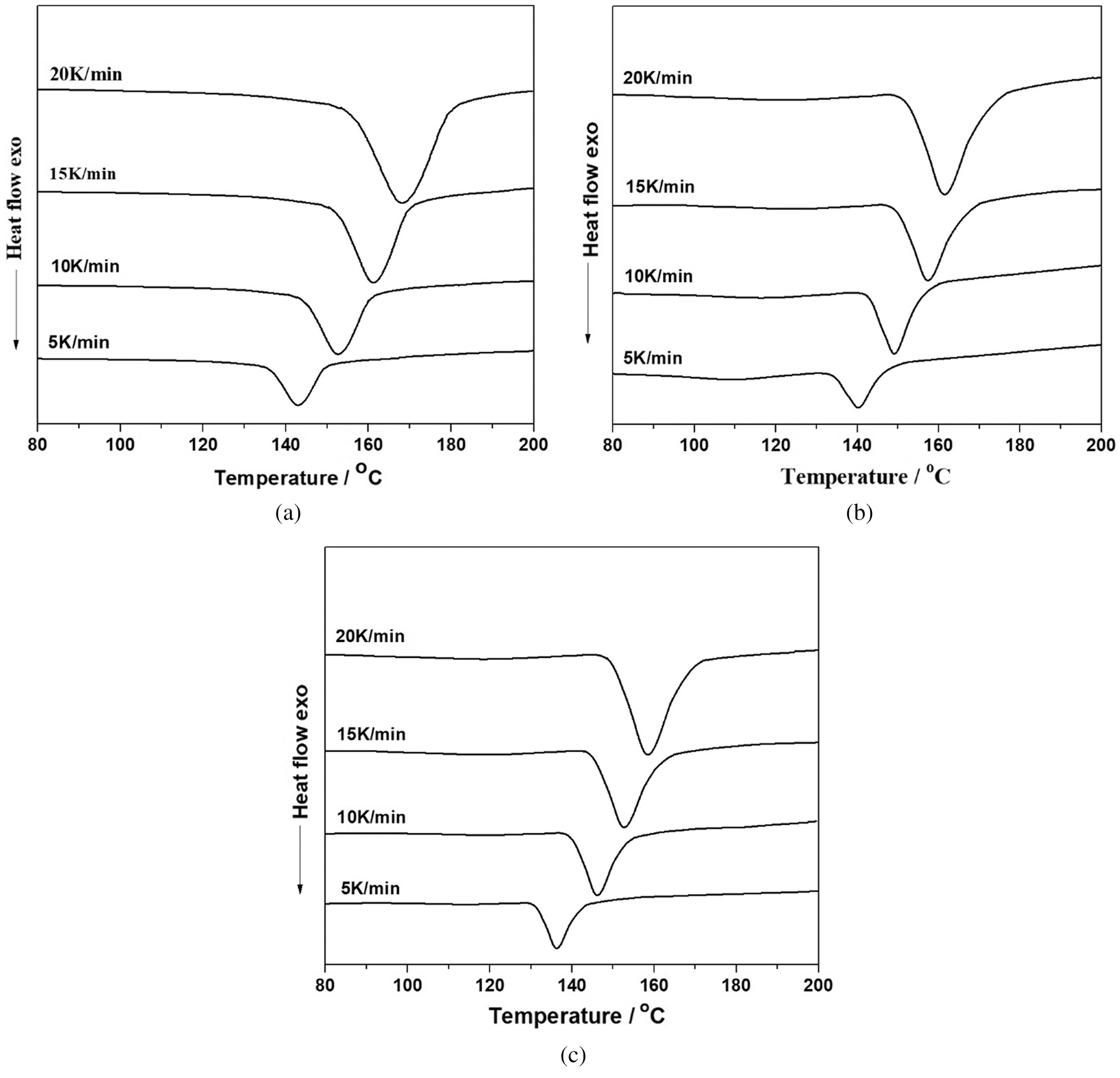
Figure 5: Curing behavior of PF (a), LPF (b) and D-LPF (c) at various heating rates as temperature scanning from 40 to 190°C
Table 3: Curing kinetic parameters of adhesives at various heating rates
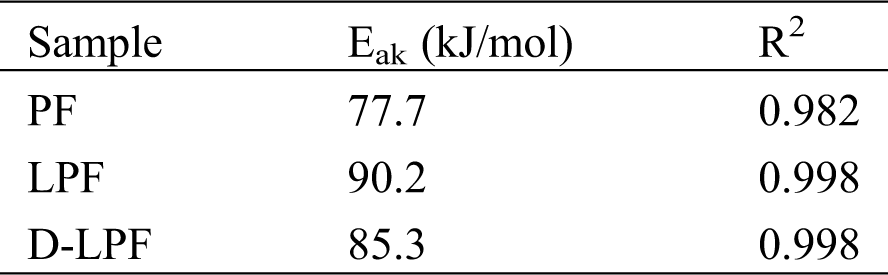
Kissinger model presented in Eq. (3) was used to fit the above kinetic data, and the plots of ln(β/Tp2) versus 1/Tp for the three resole resins were shown in Fig. 6. The activation energy (Eak) and the correlation coefficients (R2) were obtained by the fitting equation as shown in Tab. 4. The correlation coefficients were beyond 0.98, suggesting that the curing kinetic data were fitted well by Kissinger’s iso-conversional method. Moreover, it can be observed that the activation energy of PF resin was 77.7 kJ/mol, which was consistent with reported results [37]. While the activation energy of PF adhesive with lignin increased significantly to 90.2 kJ/mol, revealing decrease of the curing reactivity. Furthermore, when the lignin was replaced by demethylated lignin in PF adhesive, the activation energy was 85.3 kJ/mol, which was a bit higher than that of PF resin, and lower than that of LPF. Therefore, it can be inferred that the curing reactivity of D-LPF was enhanced after the lignin was demethylated. Generally, curing reactivity determines the curing degree which is directly related to the bonding strength. Therefore, it can be prefaced that the bonding strength of the three adhesives were followed as: PF > D-LPF > LPF, which is in consistence with above results.
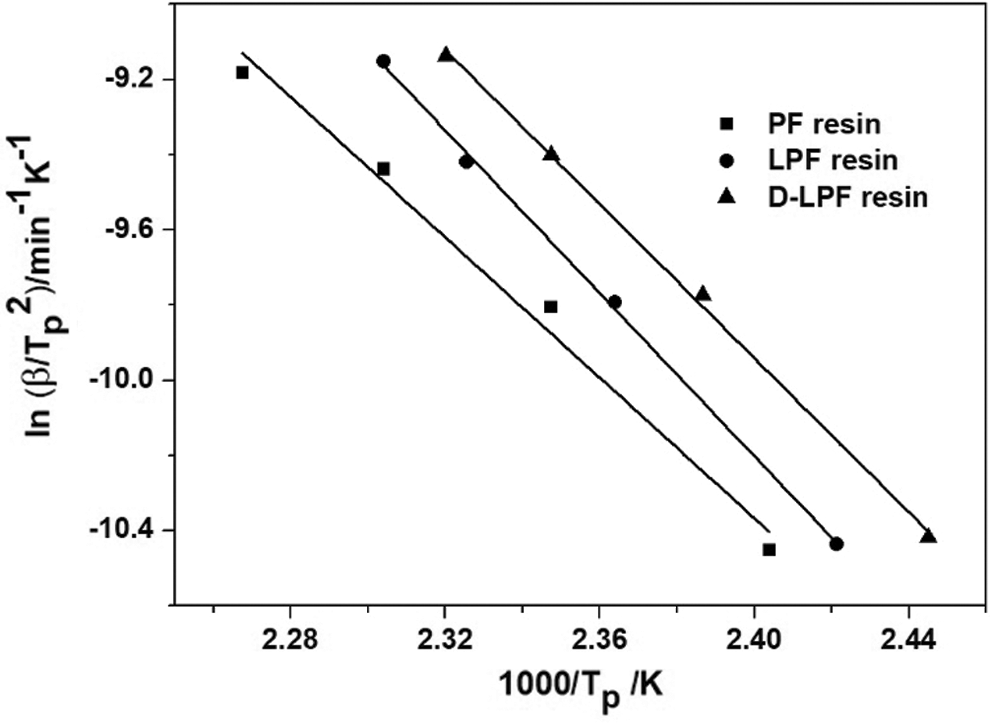
Figure 6: Plot of ln (β/Tp2) versus 1000/Tp for three adhesives
Table 4: Activity energy (Eak) and correlation coefficients (R2) of the curing processes of the three adhesives by using Kissinger’s iso-conversional method
3.5 Possible Curing Mechanism of D-LPF
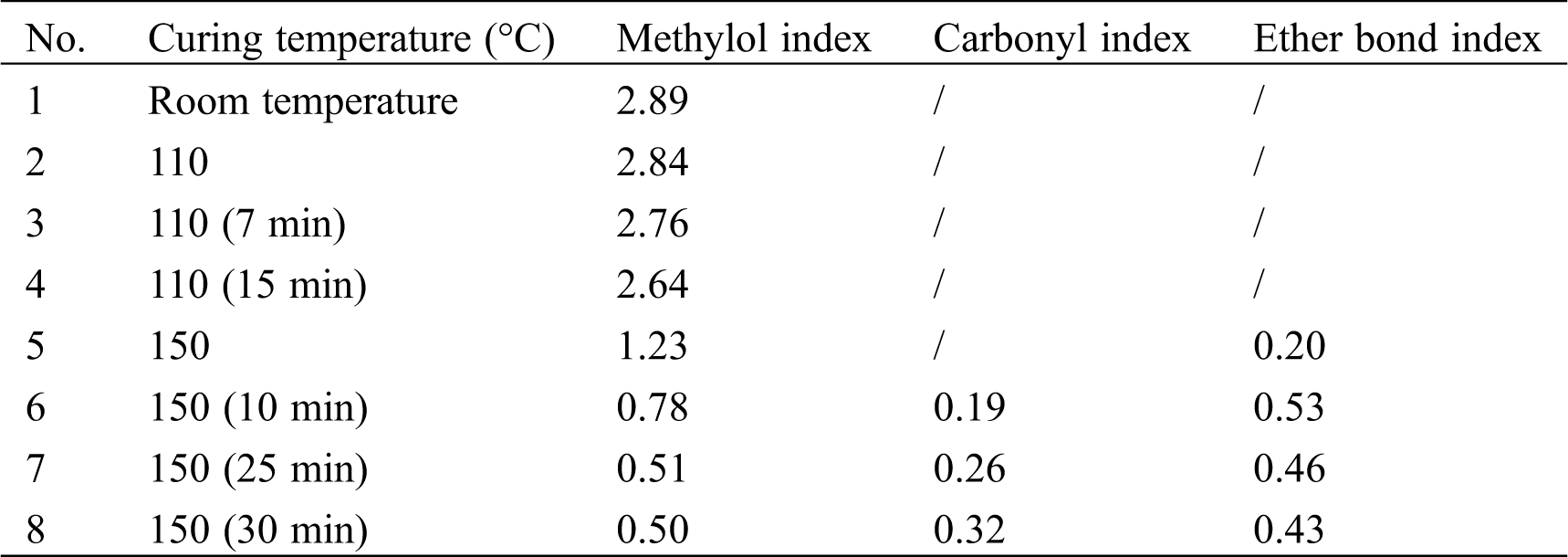
The high temperature infrared spectra of LPF prepared under optimized conditions were recorded and presented in Fig. 7. The unreacted hydroxyl methyl and newly generated carbonyl groups can be quantitatively characterized based on the change of hydroxyl methyl index and carbonyl index during the LPF curing process [44]. The hydroxyl methyl index is defined as the ratio of the absorption peak heights of hydroxyl methyl (1024 cm−1) to benzene ring (1604 cm−1), the methylene ether index defined as the ratio of the absorption peak heights of methylene ether bond (1045 cm−1) to benzene ring and the carbonyl index is defined as the ratio of the absorption peak heights of carbonyl bond (1646 cm−1) to benzene ring (Tab. 5). The results showed that the hydroxyl methyl index decreased with the increase of temperature from room temperature to 150°C, and at 150°C the index dropped quickly. At the beginning of the resin curing process, neither carbonyl absorption peak nor ether absorption peaks were observed in IR spectra. Subsequently, when the curing system was heated up to 150°C, methylene ether absorption peak appeared (1045 cm−1) [45], which may be due to the occurrence of condensation reaction between hydroxyl methyl and phenol. With the curing process going on, the stretching vibration absorption peak (1646 cm−1) of carbonyl group appeared, which could be attributed to the generation of carbonyl caused by the oxidation of methylene ether, and therefore the carbonyl index rose with the extension of the curing time at 150°C. 1. at 25°C; 2–4. at 110°C for 0 min, 7 min and 15 min, respectively; 5–8. at 150°C for 0 min, 10 min, 25 min and 40 min, respectively.
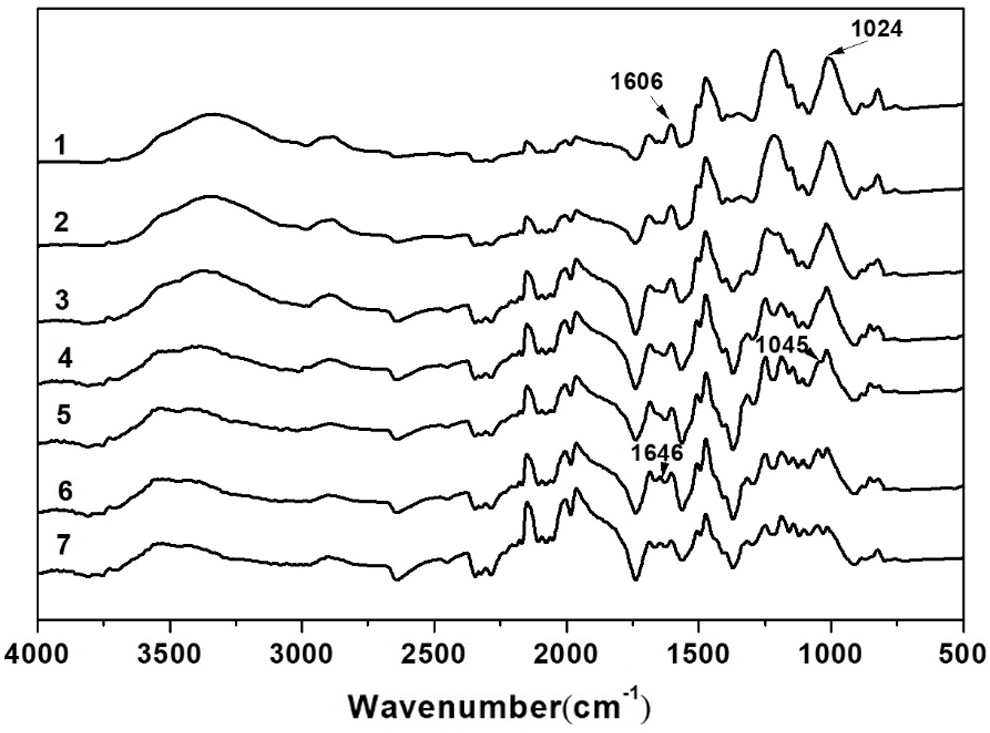
Figure 7: IR spectra of D-LPF during curing process under different conditions
Table 5: Chemical group indexes obtained from IR spectra of D-LPF during curing process
Demethylated WSAL (D-WSAL) was successfully synthesized through the reaction of WSAL with the in situ generated Lewis acid. The D-WSAL was used to partly substitute phenol in the preparation of PF adhesive to make D-LPF, and the optimum synthetic conditions were obtained. Compared with LPF, the D-LPF adhesive had shorter gel time, lower free formaldehyde content and free phenol content, but higher bonding strength. Additionally, all the lignin adhesives met the requirement of the Chinese National Standard (GB/T 17657-2013) for I grade plywood. Based on the DSC analysis, D-LPF adhesive had the shortest gel time and fastest curing rate, compared with those of PF and LPF adhesives. Moreover, the curing kinetic process could be fitted well by iso-conversional Kissinger model, and the average activation energy of curing D-LPF resin was slightly higher than that of commercial PF resin, while lower than that of LPF adhesive. Thus, the D-LPF adhesive has great potential to be applied in the wood industry.
Funding Statement: This work was supported by the National Natural Science Foundation of China (51473024) and by University Science Research General Project of Jiangsu Province (16KJD430001).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hirano, K., Asami, M. (2013). Phenolic resins−100 years of progress and their future. Reactive and Functional Polymers, 73(2), 256–269. DOI 10.1016/j.reactfunctpolym.2012.07.003. [Google Scholar] [CrossRef]
2. Zhang, W., Ma, Y., Xu, Y., Wang, C., Chu, F. (2013). Lignocellulosic ethanol residue-based lignin–phenol–formaldehyde resin adhesive. International Journal of Adhesion & adhesives, 40, 11–18. DOI 10.1016/j.ijadhadh.2012.08.004. [Google Scholar] [CrossRef]
3. Domínguez, J., Oliet, M., Alonso, M., Rojo, E., Rodríguez, F. (2013). Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin. Industrial Crops and Products, 42, 308–314. DOI 10.1016/j.indcrop.2012.06.004. [Google Scholar] [CrossRef]
4. Thebault, M., Kutuzova, L., Jury, S., Eicher, I., Zikulnig−Rusch, E. M. et al. (2020). Effect of phenolation, lignin-type and degree of substitution on the properties of lignin-modified phenol-formaldehyde impregnation resins: Molecular weight distribution, wetting behavior, rheological properties and thermal curing profiles. Journal of Renewable Materials, 8(6), 603–630. DOI 10.32604/jrm.2020.09616. [Google Scholar] [CrossRef]
5. Anris, S. P. E., Athomo, A. B. B., Safou-Tchiama, R., Leroyer, L., Vidal, M. et al. (2020). Development of green adhesives for fiberboard manufacturing, using okoume bark tannins and hexamine - characterization by(1)H NMR, TMA, TGA and DSC analysis. Journal of Adhesion Science and Technology, 256, 1–14. DOI 10.1080/01694243.2020.1808356. [Google Scholar] [CrossRef]
6. Jin, Y., Cheng, X., Zheng, Z. (2010). Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresource Technology, 101(6), 2046–2048. DOI 10.1016/j.biortech.2009.09.085. [Google Scholar] [CrossRef]
7. Anderson, E. M., Stone, M. L., Katahira, R., Reed, M., Muchero, W. et al. (2019). Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nature Communications, 10, 2033–2042. [Google Scholar]
8. Wang, Z., Ganewatta, M. S., Tang, C. (2020). Sustainable polymers from biomass: Bridging chemistry with materials and processing. Progress in Polymer Science, 101(101197), 1–14. DOI 10.1016/j.progpolymsci.2019.101197. [Google Scholar] [CrossRef]
9. Luo, H., Abu-Omar, M. M. (2017). Chemicals from lignin. Encyclopedia of Sustainable Technologies, 3, 573–585. [Google Scholar]
10. Effendi, A., Gerhauser, H., Brindgwater, A. (2008). Production of renewable phenolic resins by thermochemical conversion of biomass: A review. Renewable & Sustainable Energy Review, 12, 2092–2016. [Google Scholar]
11. Tofanica, B. (2012). Cereal straw as a resource for sustainable biomaterials and biofuels-chemistry, extractives, lignins, hemicelluloses and cellulose. Industrial Crops and Products, 40, 33–34. DOI 10.1016/j.indcrop.2012.02.026. [Google Scholar] [CrossRef]
12. Gravitis, J., Abolins, J., Tupciauskas, R., Vēveris, A. (2010). Lignin from steam-exploded wood as binder in wood composites. Journal of Environmental Engineering and Landscape Management, 18(2), 75–84. DOI 10.3846/jeelm.2010.09. [Google Scholar] [CrossRef]
13. Qiao, W., Li, S., Guo, G., Han, S., Ren, S. (2015). Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin. Journal of Industrial and Engineering Chemistry, 21, 1417–1422. DOI 10.1016/j.jiec.2014.06.016. [Google Scholar] [CrossRef]
14. Buranov, A., Mazza, G. (2008). Lignin in straw of herbaceous crops. Industrial Crops and Products, 28(3), 237–259. DOI 10.1016/j.indcrop.2008.03.008. [Google Scholar] [CrossRef]
15. Sahoo, S., Seydibeyoğlu, M., Mohanty, A., Misra, M. (2011). Characterization of industrial lignins for their utilization in future value added applications. Biomass and Bioenergy, 35(10), 4230–4237. DOI 10.1016/j.biombioe.2011.07.009. [Google Scholar] [CrossRef]
16. Kalami, S., Arefmanesh, M., Master, E., Nejad, M. (2017). Replacing 100% of phenol in phenolic adhesive formulations with lignin. Journal of Applied Polymer Science, 45124, 1–9. [Google Scholar]
17. Wang, G., Chen, H. (2014). Carbohydrate elimination of alkaline-extracted lignin liquor by steam explosion and its methylolation for substitution of phenolic adhesive. Industrial Crops and Products, 53, 93–101. DOI 10.1016/j.indcrop.2013.12.020. [Google Scholar] [CrossRef]
18. Gong, X., Liu, T., Yu, S., Meng, Y., Lu, J. et al. (2020). The preparation and performance of a novel lignin-based adhesive without formaldehyde. Industrial Crops and Products, 153, 112593. DOI 10.1016/j.indcrop.2020.112593. [Google Scholar] [CrossRef]
19. Ibrahim, V., Mendoza, L., Mamo, G., Hatti-Kaul, R. (2011). Blue laccase from Galerina sp.: Properties and potential for Kraft lignin demethylation. Process Biochemistry, 46(1), 379–384. DOI 10.1016/j.procbio.2010.07.013. [Google Scholar] [CrossRef]
20. Li, X., Bonawitz, N., Weng, J., Chapple, C. (2010). The growth reduction associated with repressed lignin biosynthesis in Arabidopsis thaliana is independent of flavonoids. Plant Cell, 22(5), 1620–1632. DOI 10.1105/tpc.110.074161. [Google Scholar] [CrossRef]
21. Raj, A., Devendra, L., Sukumaran, R. (2020). Comparative evaluation of laccase mediated oxidized and unoxidized lignin of sugarcane bagasse for the synthesis of lignin-based formaldehyde resin. Industrial Crops and Products, 150, 112385. DOI 10.1016/j.indcrop.2020.112385. [Google Scholar] [CrossRef]
22. Khan, M., Ashraf, S., Malhotra, V. (2004). Development and characterization of a wood adhesive using bagasse lignin. International Journal of Adhesion and Adhesives, 24(6), 485–493. DOI 10.1016/j.ijadhadh.2004.01.003. [Google Scholar] [CrossRef]
23. Liu, G., Qiu, X., Xing, D. (2007). Phenolation modification of wheat straw soda lignin and its utilization in preparation of lignin-based phenolic formaldehyde resins adhesive. Journal of Chemical Engineering of Chinese Universities, 21(4), 678–684. [Google Scholar]
24. Wu, S., Zhang, H. (2011). Characteristics of demethylated wheat straw soda lignin and its utilization in lignin-based phenolic formaldehyde resins. Cellulose Chemistry & Technology, 35, 253–262. [Google Scholar]
25. Makara, G., Klubek, K., Anderson, W. (2002). An efficient synthesis of 5,7-Dimethoxy-4-methylphthalide, a key intermediate in the synthesis of mycophenolic acid. Journal of Organic Chemistry, 60(17), 5717–5718. DOI 10.1021/jo00122a071. [Google Scholar] [CrossRef]
26. Chung, H., Washburn, N. (2012). Improved lignin polyurethane properties with lewis acid treatment. ACS Applied Materials & Interfaces, 4(6), 2840–2846. DOI 10.1021/am300425x. [Google Scholar] [CrossRef]
27. Wang, H., Eberhardt, T., Wang, C., Gao, S., Pan, H. (2019). Demethylation of alkali lignin with halogen acids and its application to phenolic resins. Polymers, 11(1771), 1–16. [Google Scholar]
28. Alonso, M., Oliet, M., Garcia, J., Rodriguez, F., Echeverria, J. (2006). Transformation of dynamic DSC results into isothermal data for the curing kinetics study of the resol resins. Journal of Thermal Analysis and Calorimetry, 86(3), 797–802. DOI 10.1007/s10973-005-7277-4. [Google Scholar] [CrossRef]
29. Borchardt, H., Daniels, F. (1956). The application of differential thermal analysis to the study of reaction kinetics. Journal of the American Chemical Society, 79(1), 41–46. DOI 10.1021/ja01558a009. [Google Scholar] [CrossRef]
30. Paysepara, H., Hua, Y., Feng, S., Yuan, Z., Shui, H. et al. (2020). Bio-phenol formaldehyde (BPF) resoles prepared using phenolic extracts from the biocrude oils derived from hydrothermal liquefaction of hydrolysis lignin. Reactive & Functional Polymers, 146, 1–8. [Google Scholar]
31. Ozawa, T. (1965). A new method of analyzing thermogravimetric data. Bulletin of the Chemical Society of Japan, 38(11), 1881–1886. DOI 10.1246/bcsj.38.1881. [Google Scholar] [CrossRef]
32. Burnham, A. (2000). Computational aspects of kinetic analysis. Part D. The ICTAC kinetics project-multi-thermal-history model-fitting methods and their relation to isoconversional methods. Thermochimica Acta, 355(1–2), 165–170. DOI 10.1016/S0040-6031(00)00446-9. [Google Scholar] [CrossRef]
33. Roduit, B. (2000). Computational aspects of kinetic analysis. Part E. The ICTAC Kinetics Project-numerical techniques and kinetics of solid state processes. Thermochimica Acta, 355(1–2), 171–180. DOI 10.1016/S0040-6031(00)00447-0. [Google Scholar] [CrossRef]
34. Liu, G., Ge, H. (2011). Kinetics and mechanism of curing reaction of LPF resin. Guangzhou Chemical Industy, 39, 66–68. [Google Scholar]
35. Song, Y., Wang, Z., Yan, N., Zhang, R., Li, J. (2016). Demethylation of wheat straw alkali lignin for application in phenol formaldehyde adhesives. Polymers, 8(6), 209–211. DOI 10.3390/polym8060209. [Google Scholar] [CrossRef]
36. Tabarsa, T., Jahanshahi, S., Ashori, A. (2011). Mechanical and physical properties of heat straw boards bonded with a tannin modified phenol–formaldehyde adhesive. Composites Part B: Engineering, 42(2), 176–180. DOI 10.1016/j.compositesb.2010.09.012. [Google Scholar] [CrossRef]
37. Alonso, M., Oliet, M., Garcia, J., Rodriguez, F., Echeverria, J. (2006). Gelation and isoconversional kinetic analysis of lignin-phenol-formaldehyde resol resins cure. Chemical Engineering Journal, 122(3), 159–166. DOI 10.1016/j.cej.2006.06.008. [Google Scholar] [CrossRef]
38. Monteil−Rivera, F., Phuong, M., Ye, M., Halasz, A. (2013). Isolation and characterization of herbaceous lignins for applications in biomaterials. Industrial Crops and Products, 41, 356–364. DOI 10.1016/j.indcrop.2012.04.049. [Google Scholar] [CrossRef]
39. Qiu, X. Q., Yang, D. J., Zhou, H. F. (2013). Study on the activity of the activated alkali lignin by laccase. Acta Polymeric Sinica, 2, 232–240. [Google Scholar]
40. Osetrov, A., Ugryumov, S. (2016). Assessment of activation energy of modified phenol-formaldehyde resin. Polymer Science Series D, 9(1), 31–32. DOI 10.1134/S1995421216010160. [Google Scholar] [CrossRef]
41. Zhao, M., Jing, J., Zhu, Y. (2016). Preparation and performance of lignin-phenol-formaldehyde adhesives. International Journal of Adhesion and Adhesives, 64, 163–167. DOI 10.1016/j.ijadhadh.2015.10.010. [Google Scholar] [CrossRef]
42. Lee, W., Chang, K., Tseng, I. (2012). Properties of phenol-formaldehyde resins prepared from phenol–liquefied lignin. Journal of Applied Polymer Science, 124, 4782–4788. [Google Scholar]
43. Patachia, S., Vasile, C. (2013). Thermal behavior of some wood species treated with ionic liquid. Industrial Crops and Products, 44, 511–519. DOI 10.1016/j.indcrop.2012.10.003. [Google Scholar] [CrossRef]
44. Ji, K., Deng, W., Zhang, Y., You, Y., Mao, R. et al. (2003). Study on the characterization on the curing process of the new phenolic resin. Engineering Plastics Application, 31, 44–47. [Google Scholar]
45. Man, Y., Huang, Z. (1981). Study of curing process of resole type phenol formaldehyde resins by infrared spectroscopy. Polymer Communications, 1, 403–408. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |