

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.014216
ARTICLE
Preparation and Properties of Bio-Based Flame Retardant Polyvinyl Alcohol
1Country National and Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, Hunan Normal University, Changsha, 410081, China
2Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol Fiber Material, Huaihua University, Huaihua, 418000, China
*Corresponding Authors: Hongwei Lin. Email: Linhongwei1968@163.com; Shengpei Su. Email: shengpei_academi@sohu.com
Received: 10 September 2020; Accepted: 19 October 2020
Abstract: Polyvinyl alcohol (PVA) has been widely used in the fields of medical, food and packaging due to its excellent biocompatibility, good fiber-forming and film-forming properties. However, the high flammability of PVA has greatly limited its wider applications. The flame-retardant PVA was prepared by melt blending of a bio-based flame retardant (prepared from lignin, phosphoric acid and carbamide) with thermoplastic PVA (TPVA). The chemical structure, morphology, thermal properties, mechanical properties, fire property and fluidity of this flame retardant PVA were investigated by Fourier transform infrared spectrometer(FTIR), field emission scanning electron microscope(SEM), thermogravimetric analyzer(TGA), impact tester, universal testing machine, horizontal-vertical burning tester, limiting oxygen indexer(LOI) and melt flow rate meter(MFR). The results showed that the prepared flame retardant had good compatibility with the PVA substrate; The impact strength, melt flow rate, fire property and char residue of this PVA material increased with the content of bio-based flame retardant. When the content of flame retardant was of 20%, the five indices including impact strength, melt flow rate, UL-94 level, LOI and char residual were 11.3 KJ/m2, 21.2 g/10 min, V-0 UL-94 level, 33.1%, and 19.2%, respectively. This research can promote the high-value utilization of lignin and the application of PVA in the fields of fire protection.
Keywords: Bio-based flame retardant; lignin; ammonium polyphosphate; polyvinyl alcohol
Biodegradable polymer materials have attracted increasing research attention. Polyvinyl alcohol (PVA) is a biodegradable polymer material with good chemical stability and biocompatibility. Due to its good fiber-forming and film-forming properties, PVA has been widely used in chemical, pharmaceutical, medical, food and packaging fields [1–3]. However, the high flammability of PVA has greatly limited its wide applications. Therefore, various kinds of flame retardants are added to PVA to increase its flame retardancy [4–6].
According to the interaction between PVA and flame retardant, the prepared flame-retardant PVA can be classified into two types. One is prepared based on the reactivity of hydroxyl group in PVA molecular chain [7–11]. The other one is prepared through the addition of inorganic flame retardant [12–15], P-based flame retardant [16–18], N-based flame retardant [19–21], or intumescent flame retardant [22–25] to PVA. The former one suffers from the problems of complicated preparation process and high cost, while the latter one has the disadvantage of poor compatibility leading to poor mechanical properties of composites with high loading of additives [26]. Therefore, it is necessary to design a flame-retardant PVA that achieves a balance among flame retardancy, processability and mechanical properties.
Lignin is a natural renewable resource [27]. Industrial lignin is an inexpensive by-product from pulp industry. However, industrial lignins are often directly discharged into the environment or burned, which not only means a waste of resources, but also causes pollution to the environment [28,29]. However, lignin has good charcoal properties, flame retardancy, compatibility with polymer and reactivity, which are attributed to the polar or reactive groups such as hydroxyl groups, ether bonds and unsaturated double bonds in its chemical structure [30]. Adding lignin into PVA has become an effective method to develop environmentally friendly and flame-retardant polymer materials and will expand the application of PVA and lignin. At present, the method for preparing the PVA/lignin composites material is mainly based on solution method, which has high energy consumption and low production efficiency [31–34]. There is no report on the preparation of lignin-based flame retardant/PVA composites by melt blending.
In this study, a bio-based flame retardant prepared by chemical reaction using lignin, phosphoric acid and urea will be introduced. Then, a flame-retardant PVA composite will be prepared by melt blending this bio-based flame retardant with TPVA. To evaluate this bio-based flame retardant and the PVA composites made from, structure and properties including chemical structure of bio-based flame retardant, morphology, thermal properties, mechanical properties,fire property and fluidity of the PVA composites will be tested.
Thermoplastic polyvinyl alcohol (TPVA) was obtained by treating PVA according to known procedure [35]. Acidic-aqueous lignin was obtained from Anhui Geyi Circular Economy Industrial Park Co., Ltd., Phosphoric acid (analytical purity) and carbamide (analytical purity) were purchased from Chengdu Jinshan Chemical Reagent Co., Ltd., Chengdu, China. Dimethicone-500 (Defoamer, analytical purity) was purchased from Tianjin Guangfu Fine Chemical Research Institute.
2.2.1 Preparation of Ammonium Polyphosphate and Lignin-Based Flame Retardant
Phosphoric acid and carbamide were added to a round bottom flask at a mass ratio of 1:1, and then the temperature was raised to 100°C at a heating rate of 10°C/min. After bubbles were generated, the reaction was continued for 20 min, and then the prepolymer was poured into a porcelain bowl under stirring conditions. The prepolymer was further polymerized and solidified in an oven at 230°C for 2 h. It was then cooled, pulverized, washed to remove unreacted materials and dried to obtain a ammonium polyphosphate (APP) with a yield of 51.34%. In addition, phosphoric acid and carbamide were added to another round bottom flask, and then the temperature was raised to 100°C at a heating rate of 10°C/min. After the carbamide was completely melted, lignin and a small amount of dimethyl silicone oil (the mass ratio of phosphoric acid, carbamide, lignin and dimethyl silicone oil was 1:1:0.3:0.01) were added to the flask. After bubbles were generated, the steps were the same as those given above for the preparation of APP, and finally a lignin-based flame retardant (L-APP) was obtained with a yield of 54.42% [36].
2.2.2 Preparation of Flame-Retardant PVA
First, the TPVA and L-APP were uniformly mixed according to the formula listed in Tab. 1 and the mixture was made to pass through a twin-screw extruder (MTS-20B, Nanjing Jieente Electromechanical Co., Ltd., Nanjing, China). Then, the injection molding machine (ZH-88D, China Changsha Haibo Electromechanical Equipment Co., Ltd.,, Changsha, China) was used to prepare the flame-retardant PVA samples. Extruder temperatures were set as follows: 1-zone 130°C, 2-zone 160°C, 3-zone 180°C, 4-zone 200°C, 5-zone 205°C, 6-zone 205°C, and extrusion die of 200°C. Injection molding machine temperatures were set as follows: 1-zone 200°C, 2-zone 210°C, 3-zone 215°C, and 4-zone 215°C.
Table 1: Formula for preparing the samples
2.3 Performance Testing and Structural Characterization
The sample was characterized by FTIR (IR Prestige-21 type, Shimadzu Corporation, Japan) and the scan wave number was in the range of 4000–500 cm−1. All samples were pressed into tablets with potassium bromide.
MFR was measured in accordance with GB/T 3682-2000 using a XNR-400A MFR meter (China Zhangjiagang Longhua Machinery Manufacturing Co., Ltd.) with a testing temperature of 230°C and a load mass of 5 kg.
Mechanical properties were obtained accordance with GB/T 1040-2006 using WDW-30 universal testing machine (China Jinan Huaheng Experimental Equipment Co., Ltd., Jinan, China) with a stretching speed of 20 mm/min was used.
Impact performance was obtained in accordance with GB/T 1843-2008 using XBL-22 impact tester (China Shenzhen Kaiqiangli Experimental Instrument Co., Ltd., Shenzhen, China) with V-notched sample and pendulum impact energy of 5.5 J was used.
Vertical burning test was performed in accordance with GB 8624-2008 on a CZF-5 horizontal-vertical burning tester (Nanjing Yulei Instrument Equipment Co., Ltd., Nanjing, China) and the sample size was 120 mm × 10 mm × 4 mm.
The LOI was measured in accordance with GB/T 2406.2-2009 using a ZR-01 type LOI meter (Qingdao Shanfang Instrument Co., Ltd., China), and the sample size was 80 mm × 10 mm × 4 mm.
Zeiss-SIGMAHD SEM (Zhe Zeiss, Germany) was used in the experiment. The voltage was set at 5.0 kV and the surface of the sample was sprayed with gold before scanning.
The instrument used was a Pyris Diamond type TG analyzer (PE Company, USA). The temperature range was set at 30–800°C, with a heating rate of 20°C/min and air atmosphere.
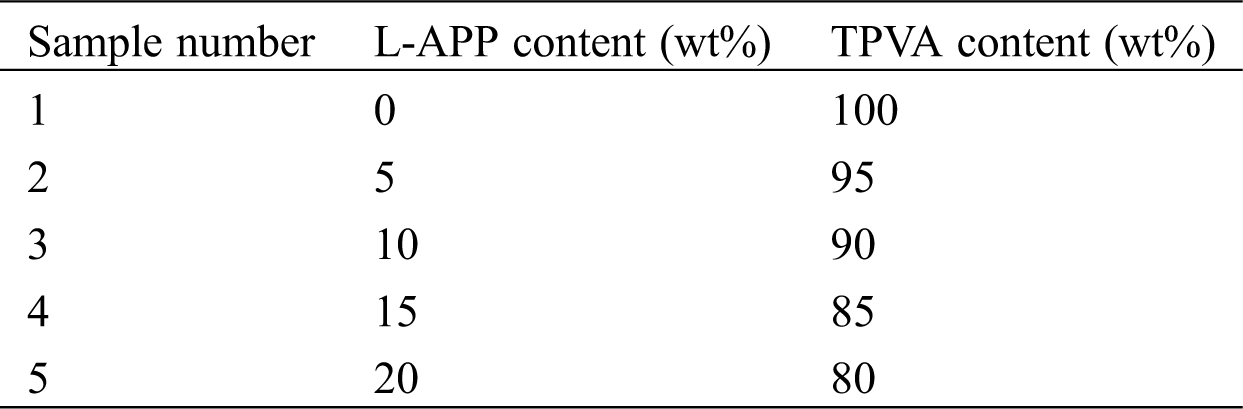
In order to prove that lignin reacted with phosphoric acid and carbamide, the FTIR spectra of lignin, APP and L-APP were obtained and present in Fig. 1.
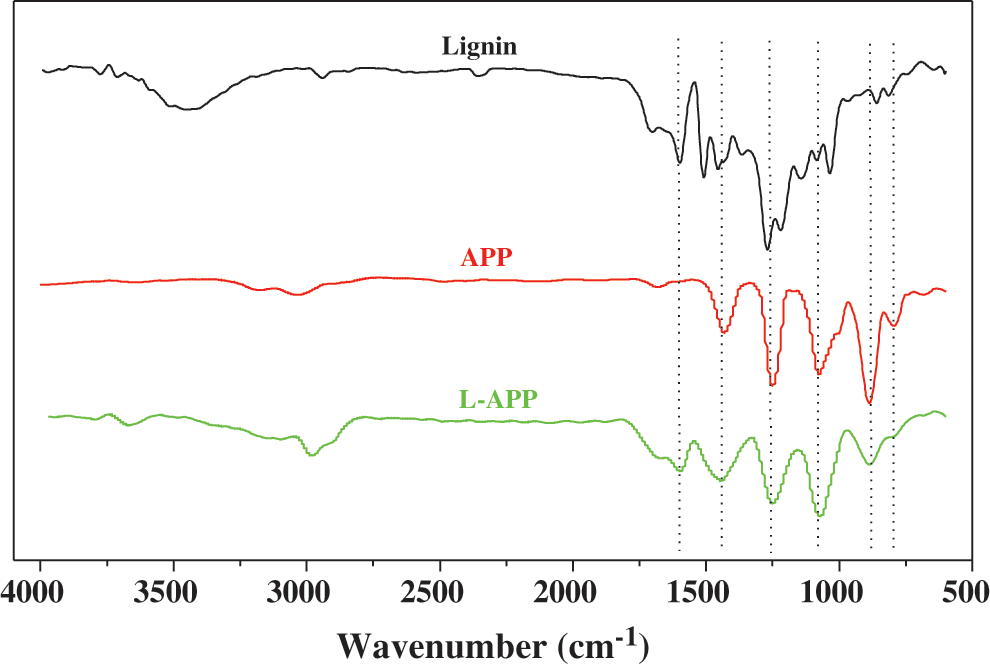
Figure 1: FTIR spectra of lignin, APP and L-APP
In the FTIR spectrum of lignin, the band at 3420 cm−1 is attributed to -OH stretching vibration while the bands at 1597, 1508 and 1430 cm−1 are attributed to aromatic nucleus vibration [37]. In the infrared spectrum of APP, double bands between 2800 and 3300 cm−1 are attributed to N-H stretching vibration; band at 1257 cm−1 is attributed to P = O stretching vibration; bands at 1078 and 800 cm−1 are attributed to P-O-P symmetric stretching vibration; and band at 884 cm−1 is attributed to P-O asymmetric stretching vibration. The infrared spectra of L-APP shows a new aromatic nucleus vibration band at 1600 cm−1. Moreover, the intensity of the vibration peaks at 800 cm−1 and 884 cm−1 (P-O-P and P-O characteristic peaks) were significantly weakened, and the intensity of the vibration peak near 1070 cm−1 was increased, which could prove that there were P-O-C characteristic groups in L-APP [38]. This indicates that lignin has reacted with phosphoric acid and carbamide and a new bio-based flame retardant L-APP has been formed.
To investigate the interfaces between PVA and APP as well as between PVA and L-APP in the composites, the impact fracture surfaces of TPVA, L-APP/TPVA and APP/TPVA composites, and L-APP, pentaerythritol, mannitol were observed (Fig. 2).
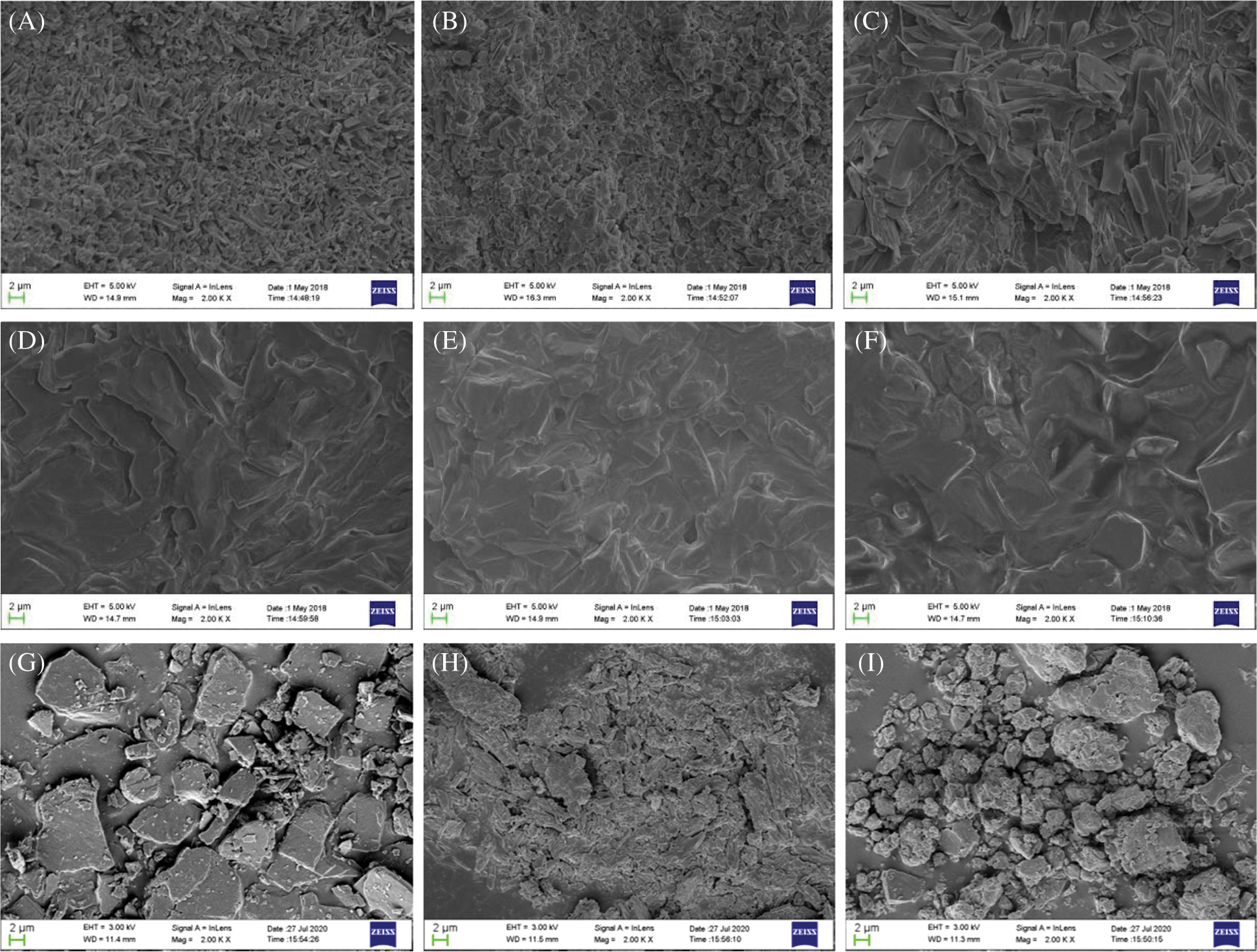
Figure 2: SEM images obtained from: A-TPVA; B-flame-retardant PVA with 5% L-APP, C-flame-retardant PVA with 10% L-APP, D-flame-retardant PVA with 15% L-APP, E-flame-retardant PVA with 20% L-APP, F-flame-retardant PVA with 20% APP, G-mannitol, H-pentaerythritol, I-L-APP (X2000)
Fig. 2A is a photograph of the TPVA impact fracture surface. In conjunction with Figs. 2G and 2H, it can be seen that the sheet-like objects on the impact fracture surface were a mixture of pentaerythritol and mannitol [35]. It can be seen from Fig. 2I that L-APP is also a sheet structure. As shown in Figs. 2B–2E, the volume of the flakes on the impact fracture surface increased as the content of L-APP. Comparing Figs. 2E and 2F, it was found that the volume of the sheet-like objects on the impact fracture surface of the sample containing 20% APP is larger, which indicates that the agglomeration of APP in PVA is more serious. This indicated that the addition of lignin containing a large amount of hydroxyl groups greatly improved the compatibility between L-APP and PVA.
MFR is a key indicator for the processing of TPVA. Flame-retardant PVA materials containing different contents of L-APP were prepared and their MFR were measured (Fig. 3).
Figure 3: Effect of L-APP content on the MFR of samples
The MFR of flame-retardant PVA with L-APP is higher than that of TPVA (Fig. 3). The addition of L-APP enhanced the separation of molecules in the PVA substrate.1 [39], In other words, L-APP has a plasticizing effect on PVA. Overall, the MFR of flame-retardant PVA material increased first and then decreased with the increase of L-APP content. When L-APP content was within a certain range, it played a plasticizing role and the relative motion between PVA molecules gradually became easier. Once the content of L-APP exceeds a certain value, the hydrogen bond formed between the hydrogen-bonded L-APP molecule and the PVA molecule begins to take effect, making the molecular movement of PVA more and more difficult [40,41].
Mechanical properties are the most important properties of polymers. In this experiment, the tensile strength and impact performance of TPVA and flame-retardant PVA materials containing different amounts of L-APP were tested (Figs. 4 and 5).
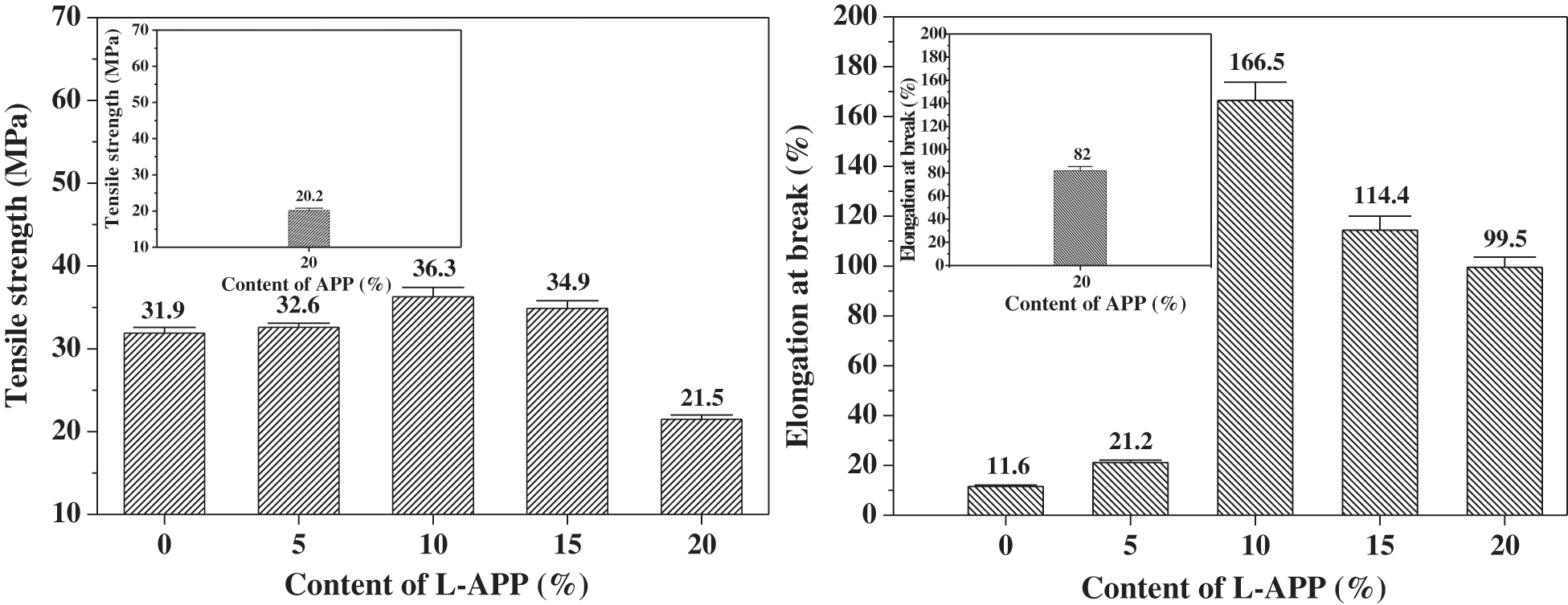
Figure 4: Effect of L-APP and APP content on the tensile strength and elongation at break of flame retardant PVA composites
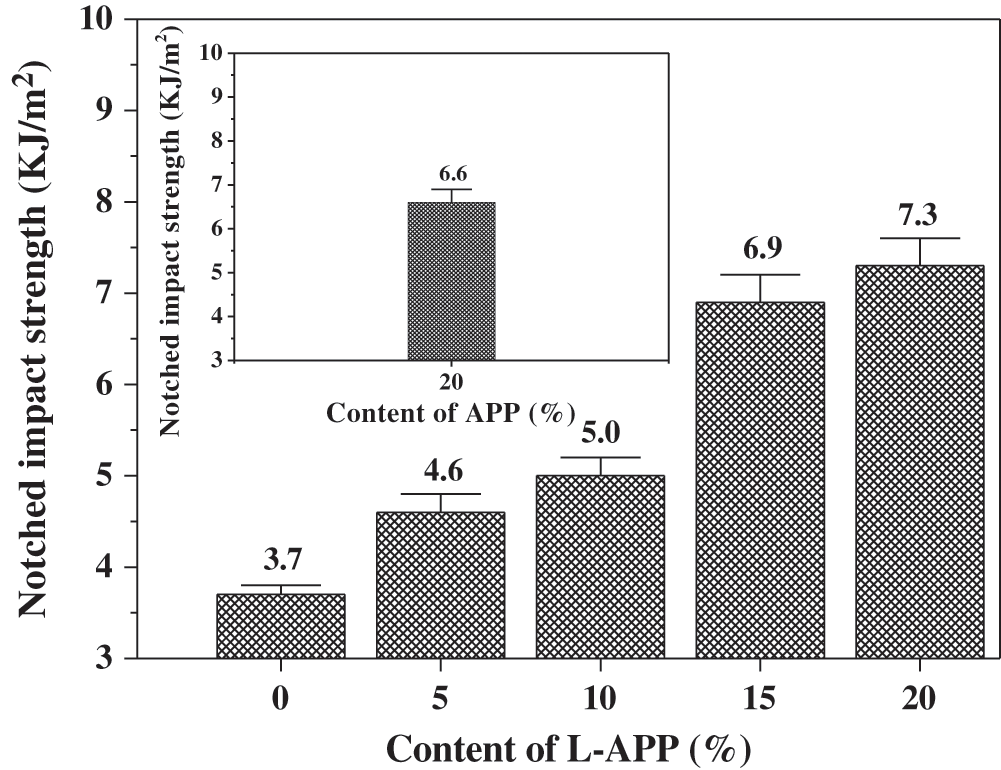
Figure 5: Effect of L-APP and APP content on the notched impact strength of samples
The tensile strength and elongation at break of flame-retardant PVA material both increased first and then decreased with the content of L-APP (Fig. 4). When the loading of L-APP was of 10%, the tensile strength and elongation at break reached their maximum values of 36.3 MPa and 166.0%, respectively. Those values increased by 14% and 1410% compared with those of TPVA, respectively.
L-APP contains a large amount of -OH, which enable it to be well dispersed in the PVA matrix. Strong hydrogen bonds can be formed between L-APP and PVA. This enables even stress distribution when the material is subjected to external forces. Overall, the strong interfacial hydrogen bonds improved the tensile strength of the material. Besides, the bonding between L-APP and PVA and its steric hindrance effect reduced the number of hydrogen bonds formed between PVA molecules. This is conducive to increasing the elongation at break of flame-retardant materials. When the L-APP content exceeded 10 wt%, however, the number of newly formed hydrogen bonds between L-APP and PVA was smaller than the number of destroyed hydrogen bonds between PVA molecules, thus the tensile properties decreased [7,42].
In Fig. 5, the notched impact strength of flame-retardant PVA material enhanced with the increase of L-APP content. The bonding between L-APP and PVA and its steric hindrance effect reduced the number of hydrogen bonds formed between PVA molecules [35,43]. This helped to increase the flexibility of PVA molecules and the impact strength of flame-retardant materials.
In addition, it can be seen from Figs. 4 and 5 that the tensile strength, elongation at break and impact strength of the sample containing 20% APP were lower than those of the sample containing the same content of L-APP. This is due to the worse compatibility of APP and PVA, and the more serious aggregation of APP in the PVA substrate.
3.5 Flame Retardant Performance
In order to simulate ignition in the atmosphere, the sample was ignited and burned in a mixture of oxygen and nitrogen at different concentrations. This enables to determine the minimum oxygen concentration (i.e., LOI) required to maintain the combustion of material. TPVA and flame-retardant PVA were tested for LOI and the results are shown in Fig. 6.
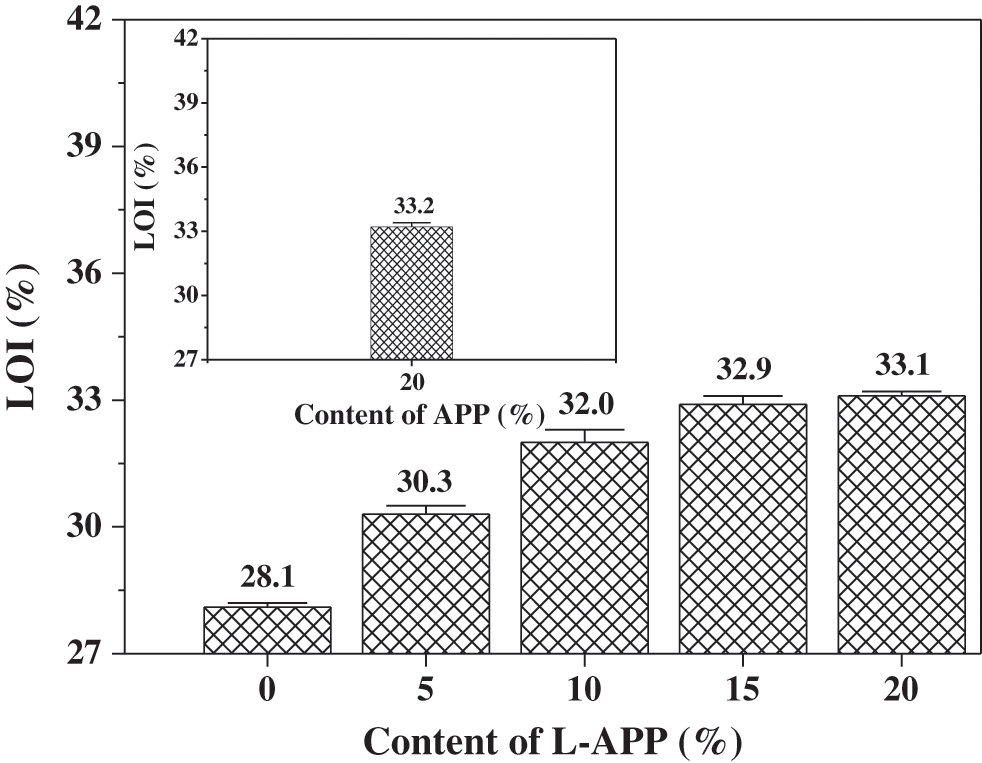
Figure 6: Effect of L-APP and APP content on LOI of flame retardant PVA composites
The LOI of the TPVA was 28.1%, indicating a certain degree of flame retardancy. The TPVA material contains a large amount of hydroxyl groups. During combustion, the PVA molecular chains underwent dehydration before breaking, and a large amount of carbides and water vapor were generated. The carbonized components no longer generated flammable gases and the presence of water lowered the combustion temperature and inhibited the combustion process. Experimental data indicated that the LOI of TPVA material was further improved by adding L-APP. When the content of L-APP reached 20%, the LOI of the material was as high as 33.1%, which is equivalent to the material containing 20% APP. This is due to the synergistic flame retardant effects of phosphorus and nitrogen in L-APP as well as the char-formation effect of lignin, which enhanced insulation shield effect to heat flux, oxygen and combustible pyrolysis products [44,45].
Vertical burning test was performed to investigate the flammability, and burning rate and flame spread of pure PVA and flame-retardant PVA was taken to determine their fire danger levels. The test results are shown in Tab. 2.
Table 2: UL-94 test results of PVA composites
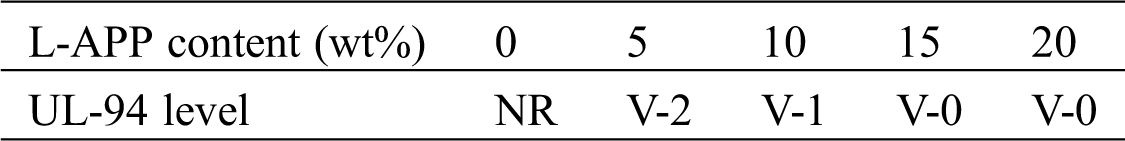
The total burning time of TPVA was 375 s, there were drips during the burning process, and the cotton underneath catches fire. When the L-APP content was 5%, the total burning time of flame-retardant PVA material was 224 s, a small amount of dripping occurred during the burning process, and the ignited cotton underneath was quickly extinguished. The material only reached V-2 UL-94 level of fire retardancy at this loading level. As the L-APP content reached 15%, the total burning time of flame-retardant PVA material was 42 s and no drips were produced during combustion. The material reached V-0 UL-94 level of flame retardancy. The burning time of flame-retardant PVA materials decreased with the increase of L-APP content, indicating increasing flame retardancy of material.
The carbon layer formed during combustion can insulate heat and combustible gas, and thus prevent the combustion of the polymer. SEM was performed here to investigate the morphology of the carbon layer of TPVA and flame-retardant PVA composites after LOI measurement (Fig. 7).
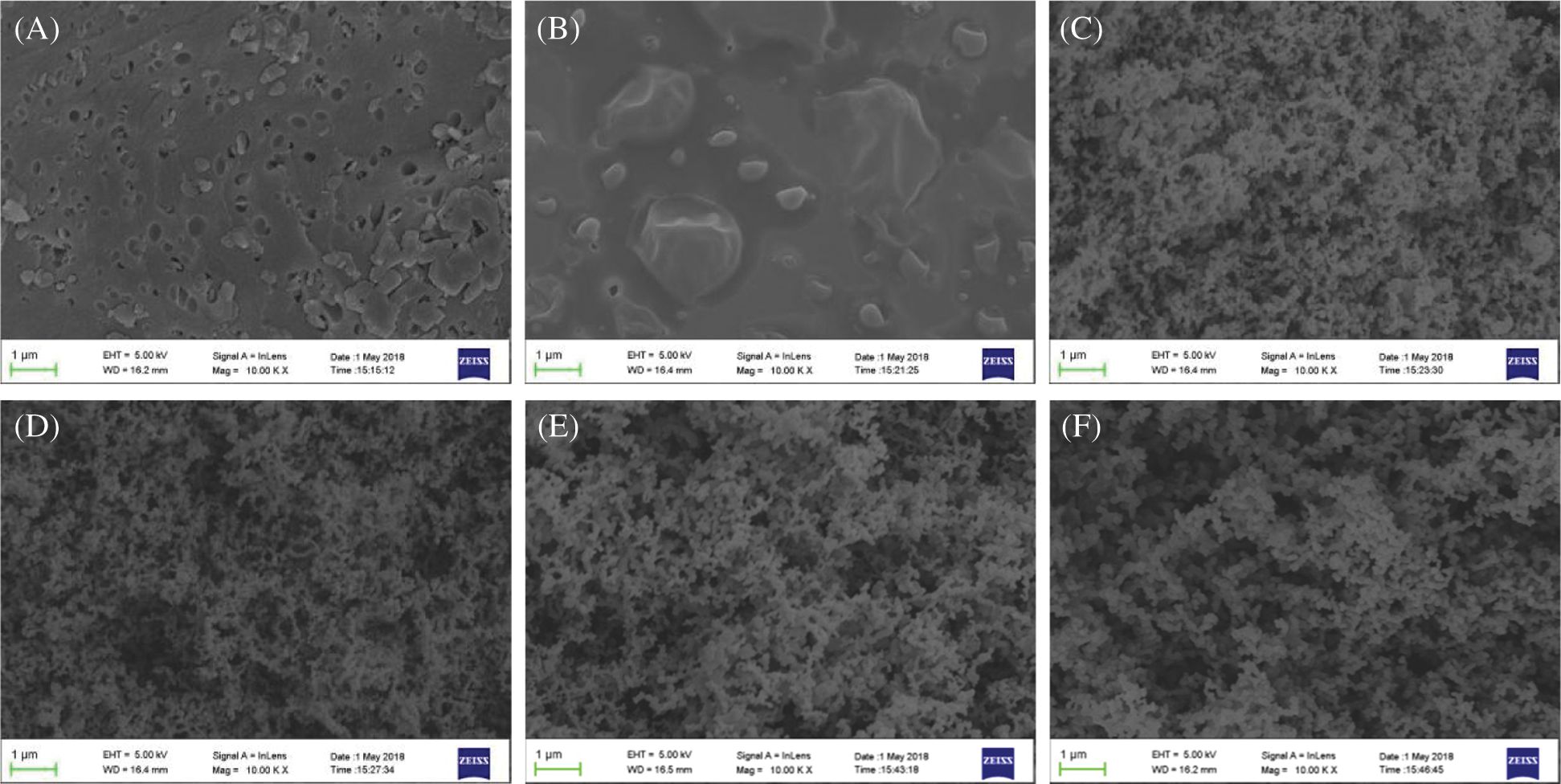
Figure 7: SEM images of residues obtained from: A-pure PVA, B-flame-retardant PVA at a loading of 5% L-APP, C-flame-retardant PVA at a loading of 10% L-APP, D-flame-retardant PVA at a loading of 15% L-APP, E-flame-retardant PVA at a loading of 20% L-APP, E-flame-retardant PVA at a loading of 20% APP(X10000)
The carbon layer of TPVA is discontinuous and filled with pores (Fig. 7). After 5% of L-APP was added, the carbon layer becomes continuous and there are tablets formed. This means that there are acid sources or carbon-forming substances in L-APP, and the charcoal effect is good. As shown in Figs. 7C and 7F, When the content of L-APP reached 10%, the carbon layer shows a honeycomb structure, and many air bubbles are uniformly distributed in the carbon layer, which is similar to APP. This indicates that there are many gas sources in L-APP and the expansion and foaming effect is good.
Thermogravimetric method was used to investigate the thermal degradation of PVA composites. The test results are shown in Fig. 8.
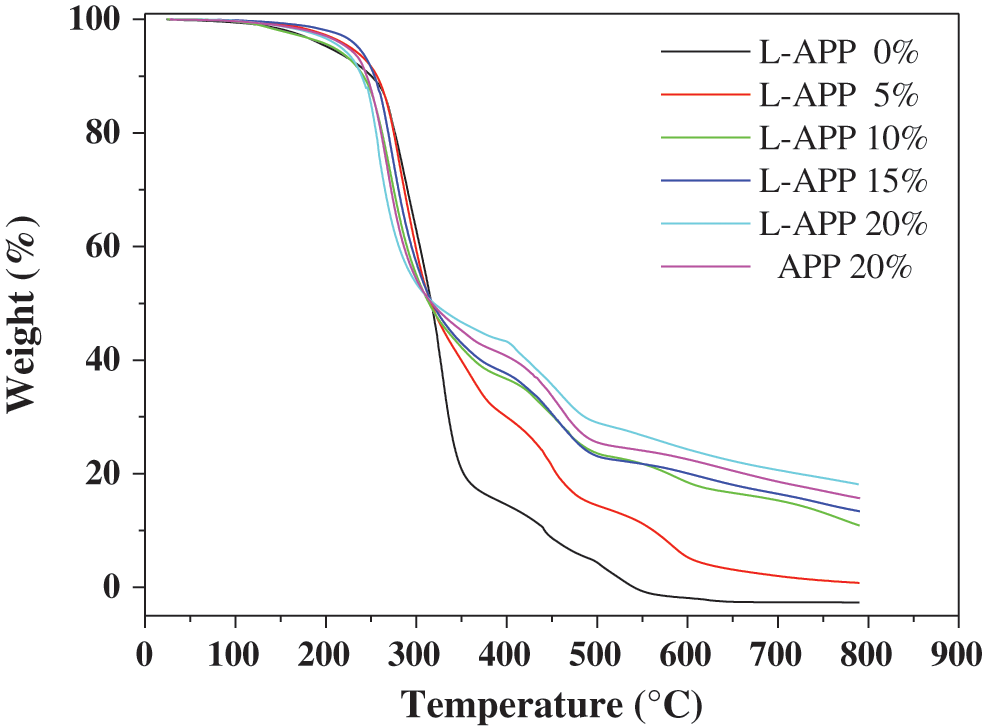
Figure 8: TGA curves of PVA and flame-retardant PVA
In Fig. 8, the weight loss of pure PVA can be divided into four stages. First, the sample underwent a slight weight loss from room temperature to 230°C, which is mainly due to the decomposition of auxiliary agent contained in the TPVA. Second, when temperature increased from 230 to 350°C, dramatic weight loss occurred and the weight loss ratio reached 81%. This is due to the decomposition of non-crystalline region of PVA. Third, as the temperature rose from 350 to 550°C, the weight loss became slower and is due to the decomposition of crystalline region of PVA. Finally, the weight of the sample remained almost stable as the temperature exceeded 500°C [46].
The weight loss of flame-retardant PVA with 5% L-APP can be roughly divided into five stages. First, the slight weight loss from room temperature to 230°C is mainly due to the decomposition of additives. Second, the dramatic weight loss (67%) in 240–375°C is mainly due to the decomposition of non-crystalline region of PVA and L-APP branches. The relatively slow weight loss in 375–600°C is mainly attributed to the decomposition of crystalline region of PVA and most of L-APP. The slow weight loss in 600–760°C is mainly caused by the decomposition of remaining L-APP. After 760°C, the weight of the sample remained almost unchanged. With increase in L-APP content, the weight loss trend of flame-retardant TPVA is similar to that of flame-retardant PVA with 5% L-APP. However, the temperatures corresponding to the latter four stages of weight loss gradually increased with increase in L-APP content. This means that adding L-APP can improve the thermal stability of PVA.
Also note that the residual carbon weight of flame-retardant PVA was greater than that of pure PVA. As the L-APP content increased, the residual carbon weight of flame-retardant PVA also increased. When the L-APP content was 20%, the weight of the residual carbon of the flame retardant TPVA was 18.1% by weight, but the value of the sample containing 20% APP was only 15.7%. This further shows that the char-forming ability of L-APP is better than that of APP, and the flame retardancy of flame-retardant PVA increases as the content of L-APP.
Infrared analysis showed that chemical reaction occurred among lignin, phosphoric acid and carbamide. The compatibility of TPVA and L-APP is better than that of APP. Compared with APP/TPVA composite materials, L-APP/TPVA composite materials had the same excellent flame retardant performance and better mechanical properties and carbon forming ability. Furthermore, the processing fluidity, tensile strength, elongation at break, impact strength and thermal stability of TPVA were found to be improved with the addition of L-APP. When the L-APP content reaches 15%, the LOI, UL-94, tensile strength, elongation at break, impact strength and MFI of the flame retardant PVA were 32.9%, V-0, 34.9 MPa, 114.0%, 6.9 KJ/m2, 18.5 g/10 min, respectively. Expermental data indicated that PVA composites with fire retardancy and high performance of mechanical properties was successfully prepared. This work can promote the high-value utilization of lignin and the application of PVA in the fields of fire protection.
Funding Statement: This work was financially supported by the following funds: National Natural Science Foundation of China (51803055), Hunan Provincial Natural Foundation of China (2019JJ50472), Scientific Research Fund of Hunan Provincial Education Department of China (18C0979, 19A391), Opening Fund of National & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources (KF201802), Hunan Province Key Field R&D Program Project (2019GK2246), Key Scientific Research Project of Huaihua University (HHUY2019-04), Hunan Provincial Key Research and Development Program (2018GK2062) and Science and Technology Plan Project of Huaihua City (2020R3101).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Shu, Y., Luo, Q. L., Zhao, X. P., Ouyang, Y. J., Su, S. P. (2019). Preparation and properties of functionalized lignin-modified polyvinyl alcohol. International Polymer Processing, 5(5), 551–556. DOI 10.3139/217.3841. [Google Scholar] [CrossRef]
2. Cui, X. M. (2009). Progress of modification and application of retardant ammonium polyphosphate. World Plastics, 27(10), 38–41. [Google Scholar]
3. Yang, J. Y., Bi, Y. T., Wu, J. X., Sun, X. L. (2015). Research advances in chemical modification of lignin. Journal of Shanghai Institute of Technology (Natural Science), 15(1), 29–39. [Google Scholar]
4. Zhou, M., Zeng, S. H., Li, X., Wang, K. Zheng, S. et al. (2019). Flame-retardant phosphorus-containing polyvinyl alcohol film with high transparency and flexibility. Chemistry & Bioengineering, 36(3), 7–12. [Google Scholar]
5. Shen, P., Zhao, H. B., Huang, W., Chen, H. B. (2016). Poly(vinyl alcohol)/clay aerogel composites with enhanced flame retardancy. RSC Advances, 6(111), 109809–109814. DOI 10.1039/C6RA21689G. [Google Scholar] [CrossRef]
6. Goudarzi, M., Ghanbari, D., Salavati-Niasari, M. (2015). Room temperature preparation of aluminum hydroxide nanoparticles and flame retardant poly vinyl alcohol nanocomposite. Journal of Nanostructures, 5(2), 110–115. [Google Scholar]
7. Zhang, X. Y., Zhu, P., Sui, S. Y., Liu, J., Bian, X. Y. (2018). Preparation and properties of polyvinyl alcohol fiber modified with pyrovatex CP. Synthetic Fiber in China, 47(1), 17–20. [Google Scholar]
8. Peng, S., Zhou, M., Liu, F. Y., Zhang, C., Liu, X. Q. (2017). Flame-retardant polyvinyl alcohol membrane with high transparency based on a reactive phosphorus-containing compound. Royal Society Open Science, 4(8), 170512. DOI 10.1098/rsos.170512. [Google Scholar] [CrossRef]
9. Ma, Z. P., Wang, C. X., Jia, G. P., Wang, A. M., Wang, J. (2017). A study on preparation and performance of fiame-retardant PVA fiber. China Textile Leader, 9, 44–47. [Google Scholar]
10. Wu, N. J., Niu, F. K., Lang, W. C., Xia, M. F. (2019). Highly efficient flame-retardant and low-smoke-toxicity poly(vinyl alcohol)/alginate/montmorillonite composite aerogels by two-step crosslinking strategy. Carbohydrate Polymers, 221, 221–230. DOI 10.1016/j.carbpol.2019.06.007. [Google Scholar] [CrossRef]
11. Xing, C. Y., Zeng, S. L., Qi, S. K., Jiang, M. J., Xu, L. (2020). Poly (vinyl alcohol)/β-cyclodextrin composite fiber with good flame retardant and super-smoke suppression properties. Polymers, 12(5), 1078. DOI 10.3390/polym12051078. [Google Scholar] [CrossRef]
12. Kang, A. H., Shang, K., Ye, D. D., Wang, Y. T., Wang, H. et al. (2017). Rejuvenated fly ash in poly(vinyl alcohol)-based composite aerogels with high fire safety and smoke suppression. Chemical Engineering Journal, 327, 992–999. DOI 10.1016/j.cej.2017.06.158. [Google Scholar] [CrossRef]
13. Shabanian, M., Khoobi, M., Hemati, F., Khonakdar, H. A., Faghihi, K. (2015). Effects of polyethyleneimine-functionalized MCM-41 on flame retardancy and thermal stability of polyvinyl alcohol. Particuology, 19(2), 14–21. DOI 10.1016/j.partic.2014.04.004. [Google Scholar] [CrossRef]
14. Jin, X. X., Wang, J. F., Dai, L. Z., Liu, X. Y., Li, L. et al. (2020). Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chemical Engineering Journal, 380, 122475. DOI 10.1016/j.cej.2019.122475. [Google Scholar] [CrossRef]
15. Guo, W. W., Liu, J. J., Zhang, P., Song, L., Wang, X. et al. (2018). Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Composites Science and Technology, 158, 128–136. DOI 10.1016/j.compscitech.2018.01.020. [Google Scholar] [CrossRef]
16. Xu, L. F., Lei, C. H., Xu, R. J., Zhang, X. Q., Zhang, F. (2016). Hybridization of α-zirconium phosphate with hexachlorocyclotriphosphazene and its application in the flame retardant poly(vinyl alcohol) composites. Polymer Degradation & Stability, 133, 378–388. DOI 10.1016/j.polymdegradstab.2016.09.025. [Google Scholar] [CrossRef]
17. Xie, W., Han, Z. Q., Zhang, Z. D., Liu, Y., Wang, Q. (2019). Hydrogen bond complexasion to prepare guanidine phosphate flame retardant poly(vinyl alcohol) membrane with high transparency. Composites Part B: Engineering, 176, 107265. DOI 10.1016/j.compositesb.2019.107265. [Google Scholar] [CrossRef]
18. Luo, Y., Xie, D. L., Chen, Y. F., Han, T., Chen, R. J. et al. (2019). Synergistic effect of ammonium polyphosphate and α-zirconium phosphate in flame-retardant poly(vinyl alcohol) aerogels. Polymer Degradation and Stability, 170, 109019. DOI 10.1016/j.polymdegradstab.2019.109019. [Google Scholar] [CrossRef]
19. Zhou, Y. S., Yu, J. R., Shen, Y., Wang, Y., Zhu, J. et al. (2017). Structures and properties of benzoguanamine modified melamine formaldehyde/polyvinyl alcohol fibers. Polymeric Materials Science and Engineering, 33(2), 50–55. [Google Scholar]
20. Ji, S. S., Zhou, W. L., Liu, P. Q., Jiang, M. J., Xu, J. J. (2016). Microencapsulation of melamine pyrophosphate with silicon dioxide for flame retardant modification of polyvinyl alcohol. Journal of Chemical Engineering of Chinese Universities, 30(3), 693–699. [Google Scholar]
21. Guo, D., Wang, Q., Bai, S. (2013). Poly(vinyl alcohol)/melamine phosphate composites prepared through thermal processing: Thermal stability and flame retardancy. Polymers for Advanced Technologies, 24(3), 339–347. DOI 10.1002/pat.3089. [Google Scholar] [CrossRef]
22. Hu, S., Song, L., Pan, H., Hu, Y. (2013). Effect of a novel chitosan-based flame retardant on thermal and flammability properties of polyvinyl alcohol. Journal of Thermal Analysis and Calorimetry, 112(2), 859–864. DOI 10.1007/s10973-012-2686-7. [Google Scholar] [CrossRef]
23. Liu, P. J., Chen, W. H., Liu, Y., Bai, S. B., Wang, Q. (2014). Thermal melt processing to prepare halogen-free flame retardant poly(vinyl alcohol). Polymer Degradation and Stability, 109, 261–269. DOI 10.1016/j.polymdegradstab.2014.07.021. [Google Scholar] [CrossRef]
24. Gong, G. Z., Feng, D., Du, X. H., Wang, X. H., Wang, S. F. (2020). Preparation and properties of citrus peel cellulose/ammonium polyphosphate/melamine/polyvinyl alcohol composite membrane. China Plastics Industry, 48(6), 172–177. [Google Scholar]
25. Zhang, Q. R., Wang, X. Y., Tao, X. J., Li, Z., Li, X. et al. (2019). Polyvinyl alcohol composite aerogel with remarkable flame retardancy, chemical durability and self-cleaning property. Composites Communications, 15, 96–102. DOI 10.1016/j.coco.2019.07.003. [Google Scholar] [CrossRef]
26. Guo, Z. Y., Zhang, P., Cao, Y. X., Wang, W. J. (2018). Research progress in halogen-free flame retarded polyvinyl alcohol. Chinese Polymer Bulletin, 31(7), 50–57. [Google Scholar]
27. Chan, W. M., Leong, Y. T., Chew, I. M. L. (2020). Multiple-criteria evaluation of centralized chilled water hub powered by industrial waste heat and renewable energy. Journal of Cleaner Production, 247, 119150. DOI 10.1016/j.jclepro.2019.119570. [Google Scholar] [CrossRef]
28. Wei, W. B., Song, W., Zhang, S. B. (2018). Study on lignin modified HAp/PVA biocomposite. China Forest Products Industry, 45(6), 8–11. [Google Scholar]
29. Zhao, C. X., Chen, A. H., Jiang, E. C., Qin, L. Y. (2017). Pyrolysis of industrial waste lignin: Analysis of product yields and character. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 39(5), 458–464. DOI 10.1080/15567036.2016.1217293. [Google Scholar] [CrossRef]
30. Shu, Y., Zhao, X. P., Su, S. P. (2019). Preparation of lignin maleic acid ester. Fine Chemical Intermediates, 49(2), 36–39. [Google Scholar]
31. Fernandes, D. M., Winkler Hechenleitner, A. A., Job, A. E., Radovanocic, E., Gómez Pineda, E. A. (2006). Thermal and photochemical stability of poly(vinyl alcohol)/modified lignin blends. Polymer Degradation and Stability, 91(5), 1192–1201. DOI 10.1016/j.polymdegradstab.2005.05.024. [Google Scholar] [CrossRef]
32. Uddin, M. J., Alaboina, P. K., Zhang, L. F., Cho, S. J. (2017). A low-cost, environment-friendly lignin-polyvinyl alcohol nanofiber separator using a water-based method for safer and faster lithium-ion batteries. Materials Science and Engineering: B, 223, 84–90. DOI 10.1016/j.mseb.2017.05.004. [Google Scholar] [CrossRef]
33. He, X. Y., Luzi, F., Hao, X. L., Yang, W. J., Torre, L. G. et al. (2019). Thermal, antioxidant and swelling behaviour of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. International Journal of Biological Macromolecules, 127, 665–676. DOI 10.1016/j.ijbiomac.2019.01.202. [Google Scholar] [CrossRef]
34. Wang, X., Ji, S. L., Wang, X. Q., Bian, H. Y., Lin, L. R. et al. (2019). Thermally conductive, super flexible and flame-retardant BN-OH/PVA composite film reinforced by lignin nanoparticles. Journal of Materials Chemistry C, 7(45), 14159–14169. DOI 10.1039/C9TC04961D. [Google Scholar] [CrossRef]
35. Shu, Y., Lin, H. W., Wang, M. L., Huang, R., Hu, Y. J. (2018). Preparation and properties of thermoplastic polyvinyl alcohol. Plastics, 47(4), 32–34+38. [Google Scholar]
36. Li, C. G., Hu, Z. P., Liu, Y. X., Yu, N. S. (2019). Discussion on influencing factors of polymerization rate of ammonium polyphosphate synthesized by phosphoric acid urea method. Phosphate & Compound Fertilizer, 34(1), 7–9. [Google Scholar]
37. Zheng, Q. K., Dong, Q. S. (2011). Structure analysis of lignins from three different sources. Journal of Weifang University, 11(6), 58–61. [Google Scholar]
38. Xing, W. Y., Yuan, H. X., Zhang, P., Yang, H., Song, L. et al. (2013). Functionalized lignin for halogen-free flame retardant rigid polyurethane foam: Preparation, thermal stability, fire performance and mechanical properties. Journal of Polymer Research, 20(9), 1–12. DOI 10.1007/s10965-013-0234-1. [Google Scholar] [CrossRef]
39. Xu, G. H., Ren, S. X., Wang, D., Sun, L. (2013). Fabrication and properties of alkaline lignin/poly (vinyl alcohol) blend membranes. Bioresources, 8(2), 2510–2520. [Google Scholar]
40. Jiang, L., Ye, D. Z., Zhang, M. H., Zhang, X. (2015). Structure and properties of lignosulfonate calcium/polyvinyl alcohol blends. Polymer Materials Science & Engineering, 31(5), 32–38. [Google Scholar]
41. Shu, Y., Xu, Q., Zhao, X. P., Su, S. P. (2019). Preparation and properties of PBS/L-PVAc composites. Fine Chemical Intermediates, 49(1), 34–37+43. [Google Scholar]
42. Wang, X., Yang, Y. J., Li, L. (2013). Structure and properties of poly(vinyl alcohol)/nano-hydroxypatite composites prepared by thermal processing. Acta Polymerica Sinica, 57(10), 1247–1252. [Google Scholar]
43. Wang, M. L., Xiang, B. L., Zhang, H., Zou, H. T., Shu, Y. (2018). Study on plasticization modified of polyvinyl alcohol. Guangzhou Chemical Industry, 46(13), 44–45+49. [Google Scholar]
44. Zhang, Q. R., Lia, Z. W., Lia, X. H., Yu, L. G., Zhang, Z. J. et al. (2019). Zinc ferrite nanoparticle decorated boron nitride nanosheet: Preparation, magnetic field arrangement, and flame retardancy. Chemical Engineering Journal, 356, 680–692. DOI 10.1016/j.cej.2018.09.053. [Google Scholar] [CrossRef]
45. Nabipoura, H., Nieb, S., Wang, X., Song, L., Hua, Y. (2020). Zeolitic imidazolate framework-8/polyvinyl alcohol hybrid aerogels with excellent flame retardancy. Composites Part A: Applied Science and Manufacturing, 129, 105720. DOI 10.1016/j.compositesa.2019.105720. [Google Scholar] [CrossRef]
46. Ong, T. K., Tshai, K. Y., Choo, H. L., Khiew, P. S., Chung, S. L. (2020). Mechanical performance and biodegradability of polyvinyl alcohol nanocomposite films. Materialwissenschaft und Werkstofftechnik, 51(6), 740–749. DOI 10.1002/mawe.202000030. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |