
Materials

 | Journal of Renewable Materials |  |
DOI: 10.32604/jrm.2021.015231
ARTICLE
Carbonation Reaction of Lithium Hydroxide during Low Temperature Thermal Energy Storage Process
1Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou, 510640, China
2Guangdong Key Laboratory of New and Renewable Energy Research and Development, Guangzhou, 510640, China
3Southern Marine Science and Engineering Guangdong Laboratory, Guangzhou, 511458, China
4Department of Chemical Engineering, Nagoya University, Nagoya, 464-8603, Japan
*Corresponding Authors: Tao Zeng. Email: zengtao@ms.giec.ac.cn; Hongyu Huang. Email: huanghy@ms.giec.ac.cn
Received: 02 December 2020; Accepted: 20 February 2021
Abstract: In order to apply lithium hydroxide (LiOH) as a low temperature chemical heat storage material, the carbonation reaction of LiOH and the prevention method are focused in this research. The carbonation of raw LiOH at storage and hydration condition is experimentally investigated. The results show that the carbonation reaction of LiOH with carbon dioxide (CO2) is confirmed during the hydration reaction. The carbonation of LiOH can be easily carried out with CO2 at room temperature and humidity. LiOH can be carbonated at a humidity range of 10% to 20%, a normal humidity region that air can easily be reached. Furthermore, the carbonation reaction rate has not nearly affected by the increase of reaction temperature. An improved storage method by storing LiOH at a low humidity less than 1.0% can be effectively prevented the carbonation of LiOH. The hydration reaction ratio of LiOH at the improved storage method shows a better result compared to the ordinary storage method. Therefore, the humidity should be carefully controlled during the storage of LiOH before hydration and dehydration reaction when apply LiOH as a low heat chemical storage material.
Keywords: LiOH; heat storage; low temperature; carbonation reaction; hydration reaction
Enormous amounts of exhaust heat are discharged from various industries process with the consumption of fuels. The effective utilization of exhaust heat can improve the overall energy efficiency of the whole society [1]. Sensible heat storage [2,3], latent heat storage [4,5], and chemical heat storage [6,7], are the main thermal energy storage technologies. Among them, chemical heat storage systems can be applied to store exhaust heat at various temperature levels with a high heat storage density and long-term heat storage ability. Different reversible chemical reactions can be applied to storage exhaust heat at a wide range of storage temperature. For exhaust heat below 100°C, MgSO4 [8,9], MgCl2 [9–11], SrBr2 [12,13], and CaCl2 [14–17] are available. For exhaust heat between 100°C to 300°C, CaO/Ca(OH)2 [18,19], CaSO4 [20,21], and CaC2O4/CaC2O4•H2O [22] show promising results. These potential salts have been investigated in detail, varying from lab-scale to field demonstrations. LiOH is a typic chemical heat storage material for low temperature heat with a relative high heat storage density, where the reversible hydration reaction is as follows:
The LiOH/LiOH•H2O reaction pair is promising low temperature heat storage. However, it has a list of technical problems, especially this reaction pair has a low hydration reaction rate, a low final reaction ratio, and sub reaction with CO2. In detail, the hydration ratio, which is the ratio of the amount of hydrated water with the stoichiometric one was only 0.142 at 480 s [23]. Kubota et al. [24] focused on a composite of LiOH and porous carbon (MPC). They found that LiOH supported on mesoporous carbon was very effective in improving the hydration rate of LiOH. Li et al. [25] modified LiOH by the hydrophilic substance of polyethylene glycol, lithium chloride, 13X-zeolite and NaY-zeolite. Among those hygroscopic materials, LiOH•H2O/13X-zeolite had a lowest apparent activation energy of 21.5 kJ/mol and highest heat storage density of 1949 kJ/kg. Carbon nanospheres (CNSs) and multi-walled carbon nanotubes (MWCNTs) have been applied to modify LiOH by Yang et al. [26]. The heat density can be reached to 2020 kJ/kg, 1804 kJ/kg, and 1236 kJ/kg for LiOH·H2O/CNSs, LiOH·H2O/MWCNTs, and LiOH·H2O/AC (activated carbon), respectively. Li et al. [27] also modified LiOH by 3D-nickel-carbon nanotubes (Ni-CNTs). They found that the selection of 3D carbon nano-additives was a very efficient way to enhance comprehensive performance of heat storage activity of LiOH. The hydration reaction ratio and heat density can be improved by the mentioned methods. However, we confirmed that the finial hydration reaction cannot be completely reached in our previous studies [28]. The relationship between the carbonation of LiOH and the humidity of the air was investigated by Williams et al. [29,30]. They found that LiOH can be reacted with CO2. Therefore, the limit of the final hydration reaction ratio of LiOH was depended by the carbonation reaction of LiOH, because the sub reaction of LiOH can be active with CO2 in air, leading to the conversion of LiOH to Li2CO3, but the reverse reaction was hard to carry out at the dehydration process. The carbonization reaction process of LiOH, such as the carbonization reaction conditions, and the prevention of carbonization are still unclear. Therefore, it is necessary to enhance the hydration rate and prevent the carbonization process of LiOH when apply LiOH as a low temperature heat storage material.
In this study, the carbonation reaction process of LiOH is focused. The carbonation reaction of LiOH at room temperature and humidity conditions has been confirmed. In order to improve hydration performance of LiOH during chemical heat storage process, an improved storage method of LiOH before hydration and dehydration reaction is proposed. The prevention of carbonation reaction of LiOH before hydration and dehydration reaction has been achieved. Furthermore, the hydration reaction performance of LiOH by the improved storage method is also evaluated.
2 Experimental Apparatus and Method
The experimental diagram for carbonation and hydration reaction of LiOH is shown in Fig. 1. A thermogravimetric system (TG, Shimadzu Corporation TGA-50) is applied to measure the weight and temperature change of material. LiOH (Kishida Chemical, purity 98%) was used as the heat storage material in this research. The thermophysical properties are shown in Tab. 1. In order to confirm the carbonation reaction of LiOH, the experiments under various conditions as shown in Tab. 2 have been carried out by TG measurement. The SEM-EDX (SEM: JEOL Ltd., JSM-7800F; EDX: Oxford Instruments plc, AZtec Energy X-Max20) is applied to measure the SEM image and the element of O and C, while Li and H cannot be measured with this device.
Table 1: The properties of LiOH
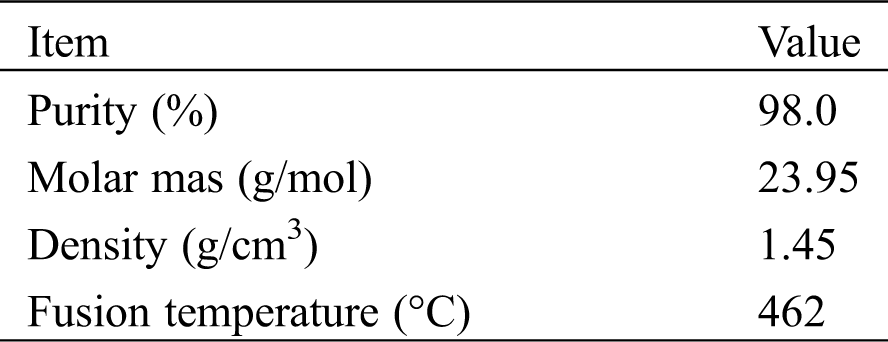
Table 2: Experimental conditions for evaluation of carbonation rate
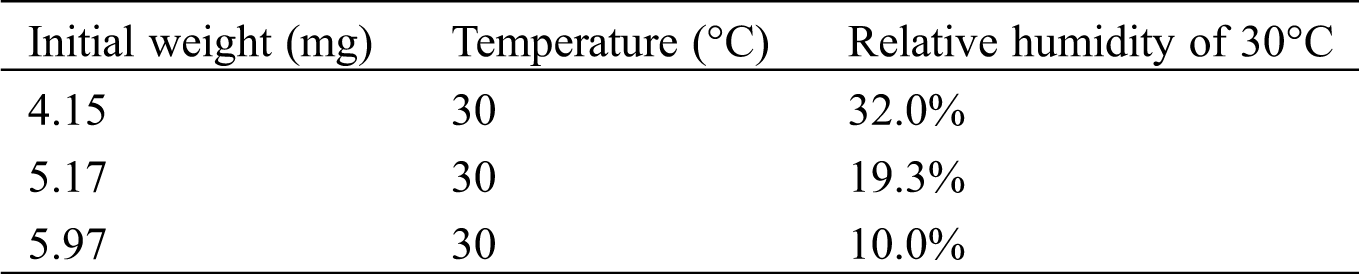
The experimental procedures of the measurements are shown as the following four steps. 1) Nitrogen was supplied into TG to prevent carbonation before the measurement. The sample of LiOH was heated to 80°C and stayed for about an hour to dehydrate the LiOH•H2O. 2) TG internal temperature was reduced to 30°C, then the supplying of nitrogen was stopped. 3) The TG device was opened to expose LiOH in the air. The temperature and humidity meter were used to measure the temperature and humidity of air at that time. The weight and temperature changes have been recorded during the reaction process. 4) After 3 h of the measurement, nitrogen gas was injected into TG to replace the air. At the same time, the temperature of TG was raised to 80°C, the weight and temperature changes were also measured during the high temperature treatment process.
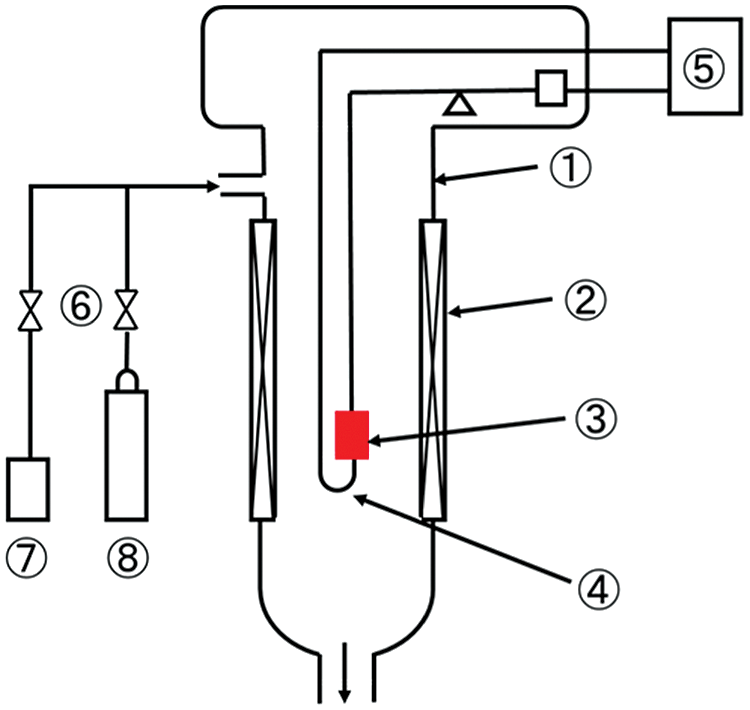
Figure 1: Experimental setup for thermogravimetric measurement method
3.1 Validation of Carbonation of LiOH
In order to confirm whether the carbonation has occurred in the sample bottle or not, raw material of LiOH before hydration reaction was directly analyzed by SEM-EDX. The raw material of LiOH produced by Kishida Chemical with a purity of 98% is analyzed. The SEM and EDX results of the raw material in the sample bottle are shown in Fig. 2 and Tab. 3. As can be seen from Tab. 2, at the measurement areas (a, b, and c) as shown in Fig. 2, the oxygen contents are 79.3 At%, 96.2 At%, and 94.7 At% (proportion of atom number), and the carbon contents are 20.7 At%, 3.8 At%, and 5.3 At%, respectively. It indicated that carbonation process of LiOH had occurred before the hydration reaction, which made the hydration reaction incomplete [27,28]. Once the purchased sample bottle of LiOH was opened, it can be carbonated with CO2 in air. Therefore, the carbonation reaction of LiOH can be easily carried out at room temperature and humidity. The XRD analysis as shown in Fig. 3 can also indicate this results. The raw material of LiOH should be properly stored in air when apply LiOH as a low temperature heat storage material.
Table 3: Summarization of EDX results at different area of the SEM as shown in Fig. 2
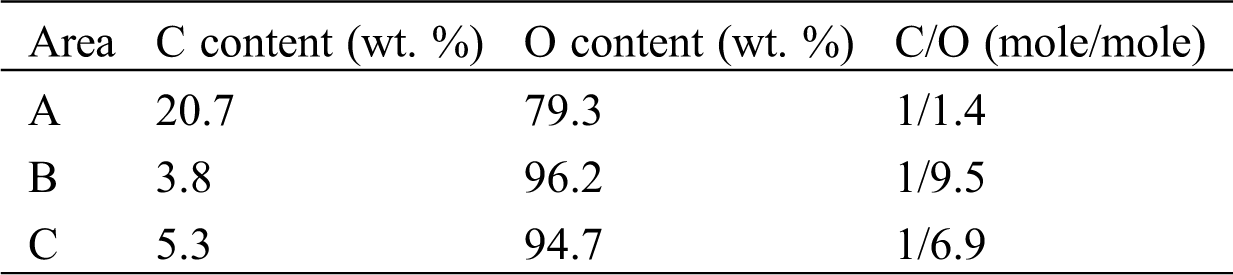
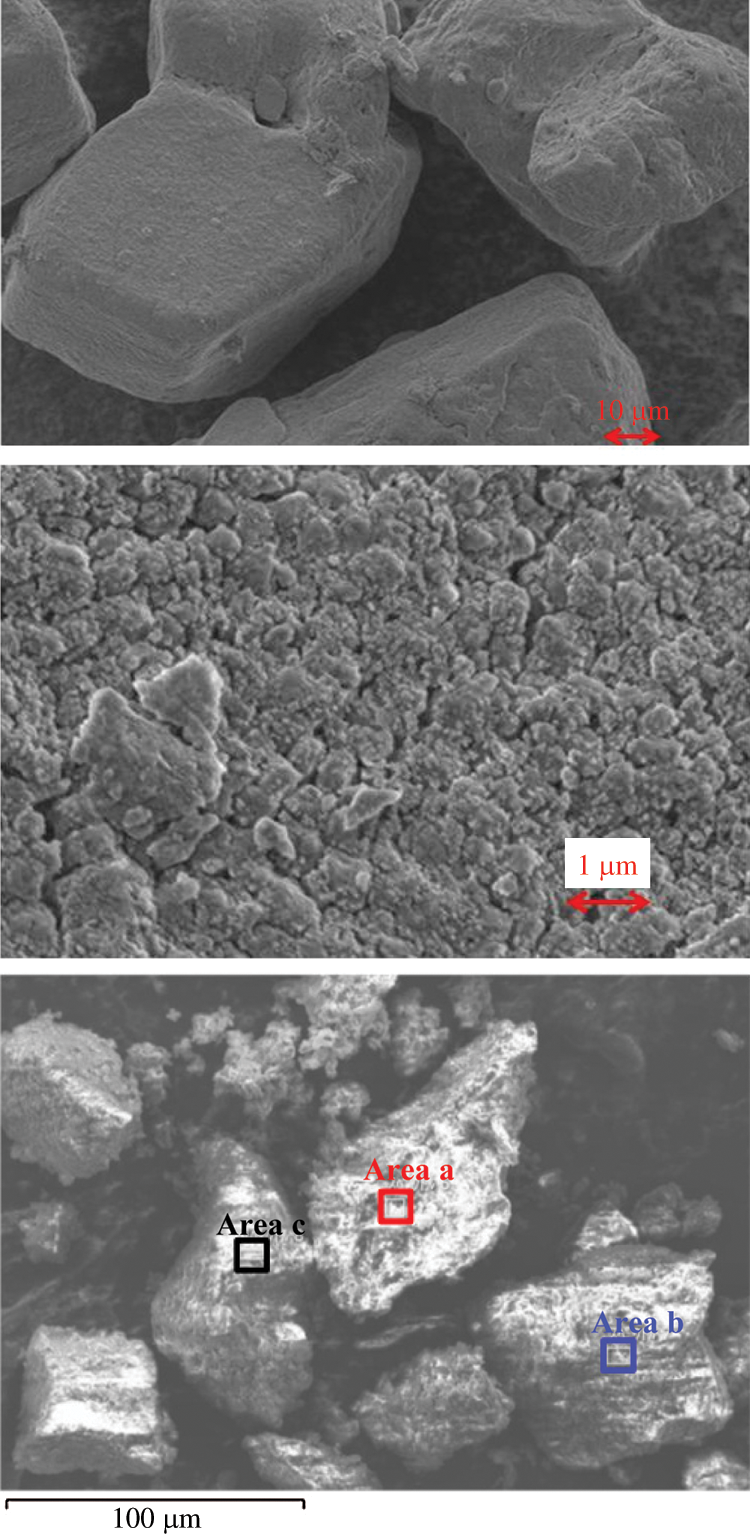
Figure 2: SEM-EDX photos of LiOH from the sample bottle and the element selected analysis area
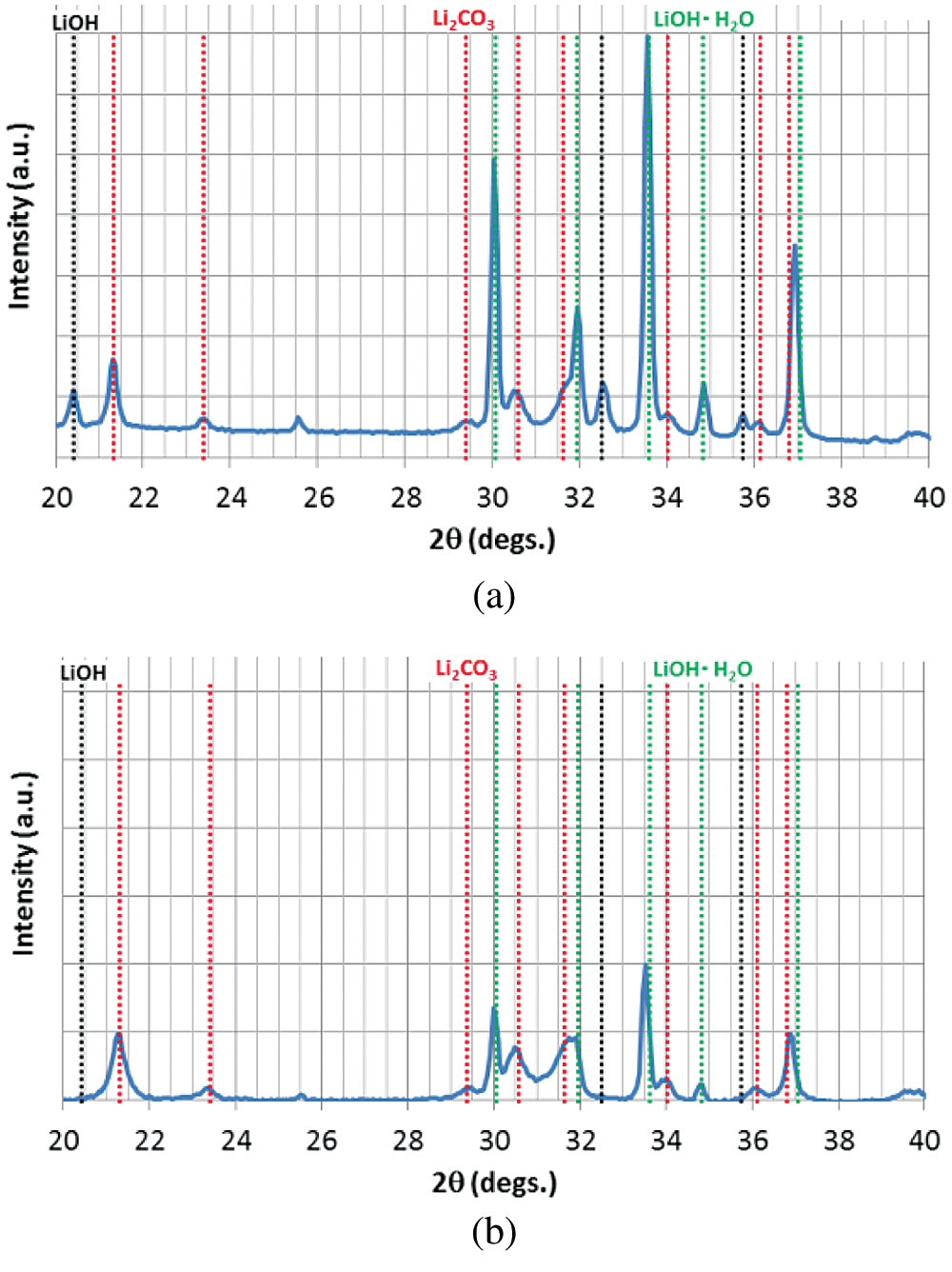
Figure 3: The XRD analysis of samples: (a) Raw material before hydration reaction, (b) Material after 15 times of hydration/dehydration reactions
3.2 LiOH Carbonation Rate in Air
In order to confirm the carbonation ratio of LiOH, the measurements of LiOH at the conditions as shown in Tab. 1 were carried out by TG. The measurement time for these measurements was around 2 to 3 h, while CO2 in air was considered as the reactant. The relative humidity of air is varied as 32.0%, 19.3%, and 10.2%. The results are shown in Fig. 4 to Fig. 6. The weight change of the reaction sample can be from hydration reaction of LiOH with vapor in air, and carbonation reaction with CO2 in air.
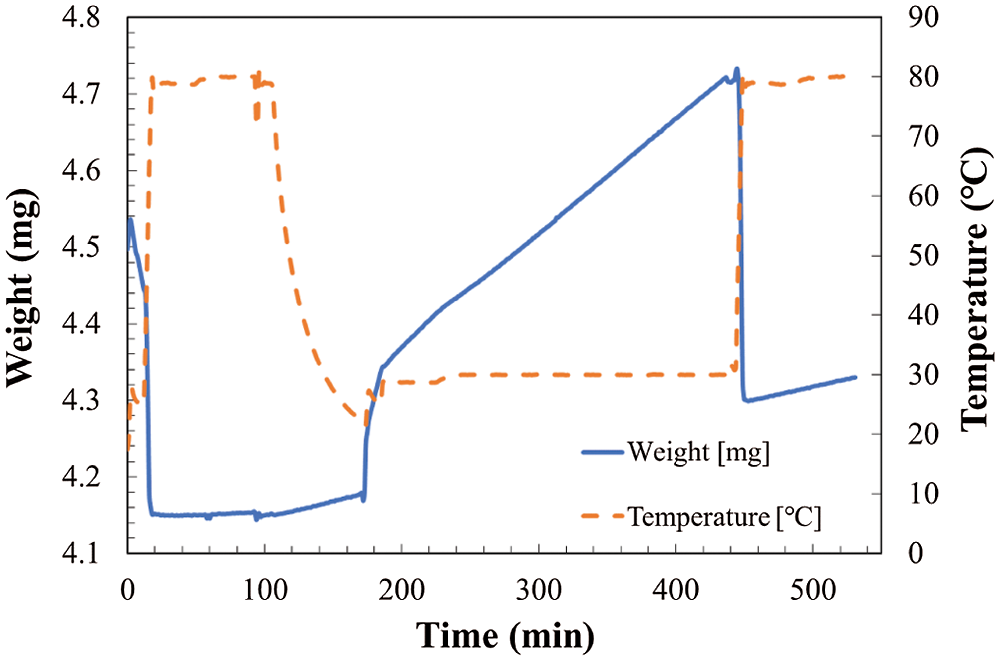
Figure 4: Temperature and weight change in TG at reaction temperature of 30°C and relative humidity of 32.0%
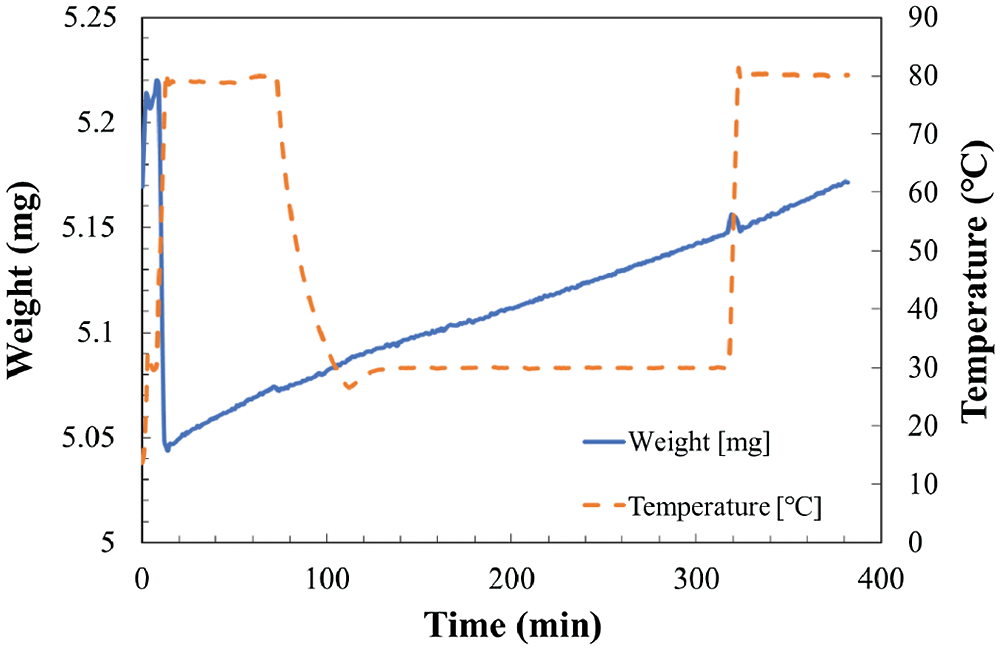
Figure 5: Temperature and weight change in TG at reaction temperature 30°C and relative humidity of 19.3%
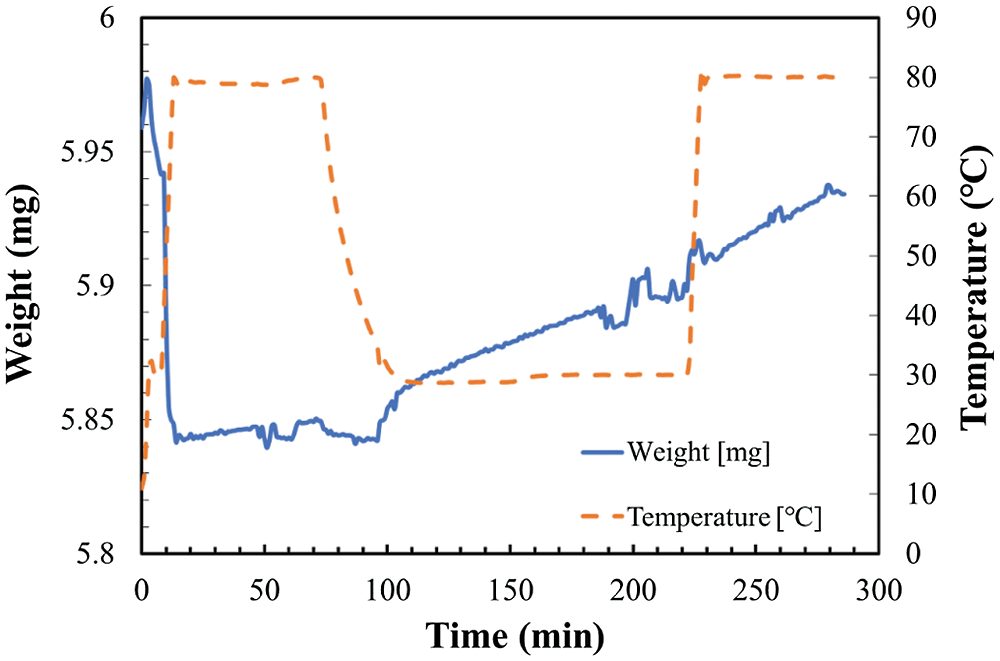
Figure 6: Temperature and weight change in TG at reaction temperature 30°C and relative humidity of 10.2%
As shown in Fig. 4, the total weight change of the sample can be divided to two stages. The increase of weight from hydration and/or carbonation reaction stage as temperature kept at 30°C, and the decrease of weight from dehydration stage as temperature increased to 80°C. However, as shown in Figs. 5 and 6, the total weight of LiOH has only one stage even at high temperature of 80°C. As shown in Fig. 4, the total weight of LiOH has increased 0.15 mg for 5 h during the reaction process. At the first step, there were two main reasons for the weight increase of material. The first one is the absorption of CO2 by the carbonation reaction of LiOH with CO2 in air. The second one is the absorption of H2O by the hydration reaction of LiOH with vapor in air. In the high temperature treatment process (dehydration condition for LiOH•H2O), the weight of material was not decreased in Figs. 5 and 6. It can be indicated that the hydration reaction of LiOH was not carried out without enough water vapor to produce LiOH•H2O, because no LiOH•H2O is formed at low temperature treatment process (According to Pressure-Temperature equilibrium diagram of LiOH [26], the water vapor pressure at hydration reaction temperature of 30°C should be over 2 kPa, which is relative humidity of 20%, the relative humidity condition in Figs. 5 and 6 is lower than 2 kPa, which is away from the hydration conditions of LiOH). Therefore, the weight increase was only related to carbonation as shown in Figs. 5 and 6. The air condition in Fig. 4 is suitable for hydration reaction as relative humidity is as high as 32.0%. However, the relative humidity of air in Figs. 5 and 6 are 19.3% and 10.2%, which is lower than the necessary relative humidity condition for hydration reaction. Therefore, we can conclude that the total weight change of LiOH in Figs. 5 and 6 is only from the carbonation reaction of LiOH. From the above discussion, it can be predicted that LiOH could be carbonated at the humidity range of 10% to 20%, which is the most normally humidity region of air. Furthermore, the curve of the change of weight can be considered as the reaction speed of carbonation. The slope is almost the same as the slop of weight change at 30°C and 80°C during measurement process as shown in Figs. 5 and 6. Therefore, the carbonation reaction rate has not nearly affected by the increase of reaction temperature.
3.3 Prevention of LiOH Carbonation Reaction
As discussed in the previous section, the carbonation reaction of LiOH is related to CO2 and humidity of air. In order to prevent the carbonation reaction of LiOH, the humidity condition of air should be well considered. For example, LiOH could directly insulate the air, such as using a vacuum desiccator or injecting CO2-free shielding gas. In this research, we propose a storage method for LiOH as shown in Fig. 7.

Figure 7: Experimental pretreatment method of carbonation prevention
As for the storage method in Fig. 7, the same sample of LiOH anhydrate material was used. Generally, the material would be placed in a sealed glass bottle and stored for about 2 weeks. For comparison, an improved storage method to store LiOH in a glass bottle with dried air by using silica gel is applied. In the improved storage method, the relative humidity is controlled to be less than 1.0%. All samples have stored for 2 weeks. After this storage process, the comparison hydration reactions of LiOH between the materials with original pretreatment and those with improved pretreatment are carried out at the conditions of reaction temperature of 47°C, particle size of 32–40 μm, and initial pressure of 9 kPa. The result is shown in Fig. 8. As can be seen from Fig. 8, the hydration reaction ratio has increased by using the improved method when compared with the ordinary method, which was possible due to the prevention of carbonation, leading to an increased final reaction ratio. In the improved storage method, the raw materials can be kept dry, which is effective to prevent the sample from absorbing the water vapor in the air. Therefore, the structure and particle size can stay at a condition without the influence of vapor. The improved storage method of keeping LiOH in silica gel under a low relative humidity of 1.0% can be considered as an effective method to prevent the carbonation of LiOH before hydration reaction.
The carbonation reaction of LiOH can be considered as a two-stage reaction as follows [29,30]. LiOH is firstly hydrated to the monohydrate (LiOH•H2O), which is proved to be a necessary intermediary product to the carbonation reaction as shown in Eq. (3).
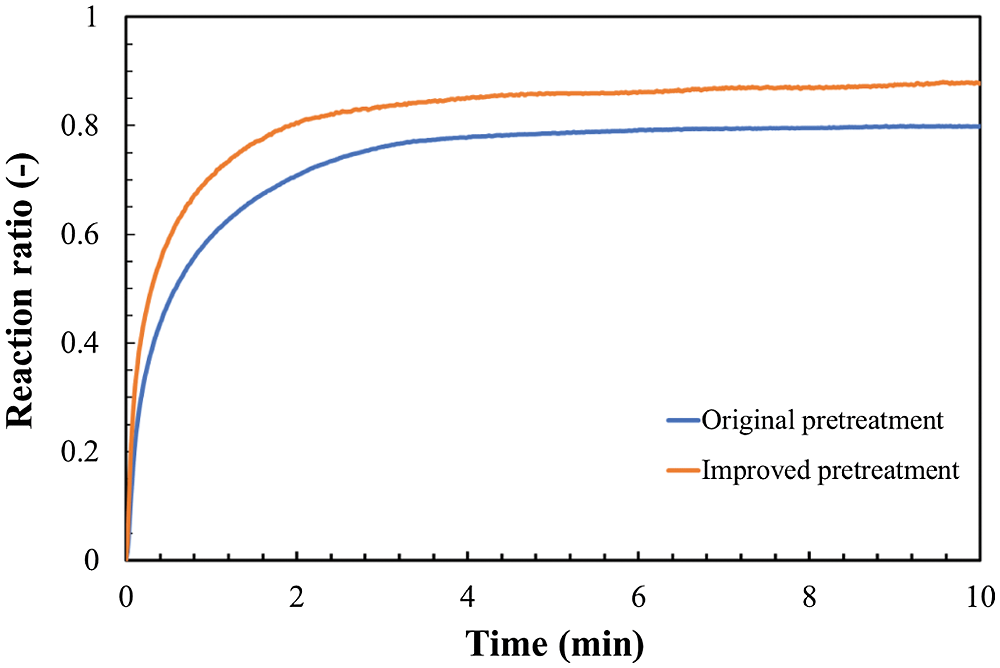
Figure 8: Effect of reducing carbonation (Temperature 47°C, steam pressure 9 kPa, particle size 32–40 μm)
In the second stage, The LiOH•H2O can be reacted with CO2 to form Li2CO3 as shown in Eq. (3). As can be seen from the carbonation reaction of LiOH, the relative humidity plays a key role during this process. In our improved storage method, the carbonation of LiOH can be prevented by cutting down the first stage of carbonation reactions. Therefore, the hydration reaction ratio of LiOH at the improved storage method shows a better result compared to the ordinary method. Considering with the relation between the reaction and relative humidity, the carbonation reaction of LiOH can be reduced by storing raw LiOH at a low humidity condition.
To apply LiOH as a low temperature chemical heat storage material, the carbonation reaction of LiOH is focused. The carbonations of raw LiOH during storage and hydration reaction process are experimentally investigated. An improved method to prevent the carbonation of LiOH during the storage process of LiOH before hydration and dehydration reaction is proposed. The main results are summarized as follows.
(1) The carbonation reaction of LiOH is confirmed during the hydration reaction. The carbonation reaction of LiOH can be easily carried out at room temperature and humidity;
(2) LiOH can be carbonated at a humidity range of 10% to 20%, a normal humidity region that air can easily be reached. Furthermore, the carbonation reaction rate has not nearly affected by the increase of reaction temperature;
(3) An improved storage method by storing LiOH at a low humidity less than 1.0% can be effectively prevented the carbonation of LiOH. The hydration reaction ratio of LiOH at the improved storage method shows a better result compared to the ordinary method.
Funding Statement: This work was supported by “Knowledge Hub Aichi,” Priority Research Project from Aichi Prefectural Government, Japan, Leading Key Projects of Chinese Academy of Sciences (No. QYZDY-SSW-JSC038), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory, Guangzhou (GML2019ZD0108), and Science and Technology Planning Project of Guangdong Province, China (No. 2017A050501046).
Conflicts of Interest: The authors declare that they have no conflicts of interest to regarding the present study.
1. Donkers, P. A. J., Sögütoglu, L., Huinink, H., Fischer, H., Adan, O. (2017). A review of salt hydrates for seasonal heat storage in domestic applications. Applied Energy, 199(3), 45–68. DOI 10.1016/j.apenergy.2017.04.080. [Google Scholar] [CrossRef]
2. Deshmukh, H., Thombre, S. (2017). Solar distillation with single basin solar still using sensible heat storage materials. Desalination, 410(11), 91–98. DOI 10.1016/j.desal.2017.01.030. [Google Scholar] [CrossRef]
3. Li, G. (2016). Sensible heat thermal storage energy and exergy performance evaluations. Renewable and Sustainable Energy Reviews, 53(8), 897–923. DOI 10.1016/j.rser.2015.09.006. [Google Scholar] [CrossRef]
4. Amin, M., Putra, N. K., Kosashih, E., Prawiro, E., Luanto, E. et al. (2017). Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Applied Thermal Engineering, 112, 273–280. DOI 10.1016/j.applthermaleng.2016.10.085. [Google Scholar] [CrossRef]
5. Jiang, Z., Jiang, F., Li, C., Leng, G., Zhao, X. et al. (2019). A form stable composite phase change material for thermal energy storage applications over 700°C. Applied Sciences, 9(5), 814. DOI 10.3390/app9050814. [Google Scholar] [CrossRef]
6. Yan, T., Wang, R., Li, T., Wang, W., Fred, I. (2015). A review of promising candidate reactions for chemical heat storage. Renewable and Sustainable Energy Reviews, 43, 13–31. DOI 10.1016/j.rser.2014.11.015. [Google Scholar] [CrossRef]
7. Kuznik, F., Johannes, K. (2020). Thermodynamic efficiency of water vapor/solid chemical sorption heat storage for buildings: Theoretical limits and integration considerations. Applied Sciences, 10(2), 489. DOI 10.3390/app10020489. [Google Scholar] [CrossRef]
8. Donkers, P., Beckert, S., Pel, L., Stallmach, F., Steiger, M. et al. (2015). Water transport in MgSO4•7H2O during dehydration in view of thermal storage. Journal of Physical Chemistry C, 119(52), 28711–28720. DOI 10.1021/acs.jpcc.5b08730. [Google Scholar] [CrossRef]
9. Posern, K., Kaps, C. (2010). Calorimetric studies of thermochemical heat storage materials based on mixtures of MgSO4 and MgCl2. Thermochimica Acta, 502(1-2), 73–76. DOI 10.1016/j.tca.2010.02.009. [Google Scholar] [CrossRef]
10. Rammelberg, H., Schmidt, T., Ruck, W. (2012). Hydration and dehydration of salt hydrates and hydroxides for thermal energy storage—kinetics and energy release. Energy Procedia, 30, 362–369. DOI 10.1016/j.egypro.2012.11.043. [Google Scholar] [CrossRef]
11. Pathak, A., Tranca, I., Nedea, S., Zondag, H., Rindt, C. et al. (2017). First-principles study of chemical mixtures of CaCl2 and MgCl2 hydrates for optimized seasonal heat storage. Journal of Physical Chemistry C, 121(38), 20576–20590. DOI 10.1021/acs.jpcc.7b05245. [Google Scholar] [CrossRef]
12. Michel, B., Mazet, N., Mauran, S., Stitou, D. Xu, J. et al. (2012). Thermochemical process for seasonal storage of solar energy: characterization and modeling of a high density reactive bed. Energy, 47(1), 553–563. DOI 10.1016/j.energy.2012.09.029. [Google Scholar] [CrossRef]
13. Michel, B., Neveu, P., Mazet, N. (2014). Comparison of closed and open thermochemical process, for long-term thermal energy storage application. Energy, 72, 702–716. DOI 10.1016/j.energy.2014.05.097. [Google Scholar] [CrossRef]
14. Esaki, T., Yasuda, M., Kobayashi, N. (2017). Experimental evaluation of the heat output/input and coefficient of performance characteristics of a chemical heat pump in the heat upgrading cycle of CaCl2 hydration. Energy Conversion and Management, 150(2), 365–374. DOI 10.1016/j.enconman.2017.08.013. [Google Scholar] [CrossRef]
15. Fujioka, K., Suzuki, H. (2013). Thermophysical properties and reaction rate of composite reactant of calcium chloride and expanded graphite. Applied Thermal Engineering, 50(2), 1627–1632. DOI 10.1016/j.applthermaleng.2011.08.024. [Google Scholar] [CrossRef]
16. Barreneche, C., Fernández, A., Cabeza, L., Cuypers, R. (2015). Thermophysical characterization and thermal cycling stability of two TCM: CaCl2 and zeolite. Applied Energy, 137(2), 726–730. DOI 10.1016/j.apenergy.2014.09.025. [Google Scholar] [CrossRef]
17. Kito, T., Kobayashi, N. (2012). Evaluation of output power characteristics of chemical heat pump by using CaO-LiCl compound reactant. Journal of Chemical Engineering of Japan, 38, 172–175. [Google Scholar]
18. Criado, Y., Alonso, M., Abanades, J. (2014). Kinetics of the CaO/Ca(OH)2 hydration/dehydration reaction for thermochemical energy storage applications. Industrial & Engineering Chemistry Research, 53(32), 12594–12601. DOI 10.1021/ie404246p. [Google Scholar] [CrossRef]
19. Schmidt, M., Gutierrez, A., Linder, M. (2017). Thermochemical energy storage with CaO/Ca(OH)2–experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor. Applied Energy, 188, 672–681. DOI 10.1016/j.apenergy.2016.11.023. [Google Scholar] [CrossRef]
20. Lee, J., Ogura, H., Sato, S. (2014). Reaction control of CaSO4 during hydration/dehydration repetition for chemical heat pump system. Applied Thermal Engineering, 63(1), 192–199. DOI 10.1016/j.applthermaleng.2013.10.043. [Google Scholar] [CrossRef]
21. Huang, H., Li, J., Huhetaoli, O., Osaka, Y., Wang, C. et al. (2016). Porous-resin-supported calcium sulfate materials for thermal energy storage. Energy Technology, 4(11), 1401–1408. DOI 10.1002/ente.201600174. [Google Scholar] [CrossRef]
22. Knoll, C., Müller, D., Artner, W., Welch, J., Werner, A. et al. (2017). Probing cycle stability and reversibility in thermochemical energy storage–CaC2O4•H2O as perfect match? Applied Energy, 187, 1–9. DOI 10.1016/j.apenergy.2016.11.053. [Google Scholar] [CrossRef]
23. Pablo, J., Aadersson, J., Azoulay, M. (1987). Kinetic investigation of the sorption of water by lithium hydroxide. Thermochimica Acta, 113(12), 87–94. DOI 10.1016/0040-6031(87)88311-9. [Google Scholar] [CrossRef]
24. Kubota, M., Massumoto, S., Matsuda, H. (2019). Enhancement of hydration rate of LiOH by combining with mesoporous carbon for low-temperature chemical heat storage. Applied Thermal Engineering, 150(3), 858–863. DOI 10.1016/j.applthermaleng.2019.01.049. [Google Scholar] [CrossRef]
25. Li, S., Huang, H., Yang, X., Bai, Y., Li, J. et al. (2018). Hydrophilic substance assisted low temperature LiOH•H2O based composite thermochemical materials for thermal energy storage. Applied Thermal Engineering, 128, 706–711. DOI 10.1016/j.applthermaleng.2017.09.050. [Google Scholar] [CrossRef]
26. Yang, X., Li, S., Huang, H., Li, J., Kobayashi, N. et al. (2017). Effect of carbon nanoadditives on lithium hydroxide monohydrate-based composite materials for low temperature chemical heat storage. Energies, 10(5), 644. DOI 10.3390/en10050644. [Google Scholar] [CrossRef]
27. Li, S., Huang, H., Li, J., Kobayashi, N., Osaka, Y. et al. (2018). The effect of 3D carbon nanoadditives on lithium hydroxide monohydrate based composite materials for highly efficient low temperature thermochemical heat storage. RSC Advances, 8(15), 8199–8208. DOI 10.1039/C8RA00269J. [Google Scholar] [CrossRef]
28. Li, J., Zeng, T., Kobayashi, N., Xu, H., Bai, Y. et al. (2019). Lithium hydroxide reaction for low temperature chemical heat storage: Hydration and dehydration reaction. Energies, 12(19), 3741. DOI 10.3390/en12193741. [Google Scholar] [CrossRef]
29. Williams, D., Miller, R. (1969). The effect water vapor on the LiOH-CO2 reaction. Part 1. Dynamic isothermal system. Washington DC, USA: Naval Research Laboratory. [Google Scholar]
30. Williams, D., Miller, R. (1970). Effect of water vapor on the LiOH-CO2 reaction. Dynamic isothermal system. Industrial & Engineering Chemistry Fundamentals, 9(3), 454–457. DOI 10.1021/i160035a024. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |