
 | Oncologie |  |
DOI: 10.32604/oncologie.2021.014152
ARTICLE
Value of Mir-1271 and GPC3 in Prognosis Evaluation of Liver Cancer Patients after Liver Transarterial Chemoembolization
1Department of Intervention Section, The First Affiliated Hospital of Suzhou University, Suzhou, 215006, China
2Department of Ultrasound, Lishui Branch, CUHK Affiliated Hospital of Southeast University, Nanjing, 211002, China
*Corresponding Author: Xin Chang. Email: changxin156@sina.com
Received: 03 September 2020; Accepted: 28 December 2020
#These authors contributed equally to this study as co-first author
ABSTRACT:
Objective: This research was designed to observe the value of miR-1271 and GPC3 in evaluating the prognosis of liver cancer (LC) patients after liver transarterial chemoembolization (TACE). Methods: A total of 80 patients diagnosed as LC in our hospital from January 2018 to April 2019 were included in the LC group (LCG), and then assigned into a survival group (SG) and a death group (DG) based on prognosis. Seventy healthy subjects undergoing physical examination simultaneously were included in the normal group (NG). miR-1271 and GPC3 in serum of two groups of subjects were tested via qRT-PCR. ROC curve was drawn. The prognostic factors were assessed via multivariate Cox regression analysis. Results: The expression of miR-1271 in the LCG was notably lower than the NG, while GPC3 expression in the LCG was notably higher (P < 0.001). Patients with low miR-1271 expression and high GPC3 expression had a higher incidence of low differentiation, grade III+IV, lymphatic invasion and distant metastasis (P < 0.05). Both AUC of miR-1271 and GPC3 single diagnosis were higher than 0.8. The relative expression of serum miR-1271 in the SG was markedly higher than the DG, while that of serum GPC3 in the SG was markedly lower (both P < 0.001). ROC was drawn. The AUC of serum miR-1271 and GPC3 in diagnosing prognosis were higher than 0.8, and there was no marked difference in sensitivity and specificity. Cox regression analysis was performed on 5-year survival, and the results manifested that miR-1271 was an independent factor affecting prognosis. Conclusion: miR-1271 and GPC3 may be implicated in LC development and metastasis, and can be employed as potential serum biomarkers for post-TACE evaluation.
Keywords: miR-1271; GPC3; hepatocarcinogenesis; short-term prognosis
Liver Cancer (LC) is a chronic malignant liver tumor. With the influence of people’s unhealthy living habit and poor dietary habit in recent years, the population suffering from LC is becoming younger, and the incidence has been increasing, seriously threatening people’s life and health [1,2]. Early LC patients lack the first symptoms, so they miss timely treatment. Advanced LC patients have a survival rate less than 5% and an extremely high mortality. The annual mortality worldwide is about 30% to 40% [3,4]. With a deeper knowledge of the pathogenesis, a recent study has found fine biomarkers that help diagnose and evaluate the disease [5]. At present, transarterial chemoembolization (TACE) is the first choice for LC treatment. TACE can reduce tumor blood supply and cause tumor necrosis [6,7]. Clinical studies discovered that the recurrence of LC after TACE is particularly important, and the search for more effective biomarkers to predict the prognosis has drawn extensive attention from medical research [8].
microRNAs (miRNAs) are endogenous non-coding single-stranded RNAs [9,10]. Current studies have shown that tumor-related miRNAs in the blood circulation are relevant to tumor development and progression and some pathological processes in the body [11,12]. Some researchers have confirmed that Glypican-3 (GPC3) is a transmembrane protein that is over-expressed in LC cells [13]. Over-expressed GPC3 can significantly promote cell invasion and metastasis [14,15], revealing that it is tied to repressor miR-1271 down-regulation [16]. However, the miR-1271 and GPC3 expression in LC patients and their clinical value are vague. Therefore, we conducted a prospective clinical study, so as to observe serum miR-1271 and GPC3 expression, analyze the clinical value and short term prognosis, thereby supplying a novel theoretical basis for diagnosis and treatment in the aspect of molecular biology.
A total of 80 patients (47 males and 33 females) diagnosed as LC in our hospital from January 2018 to April 2019 were assigned to a LC group (LCG), with a mean age of (55.00 ± 3.19) years. Seventy healthy subjects (40 males and 30 females) undergoing physical examination during the same period were included in the normal group (NG), with an average of (55.12 ± 3.04) years. Inclusion and exclusion criteria were as follows: all LC patients conformed to diagnostic guidelines of LC expert committee [17]; patients with normal hepatic and renal function, without other malignancy. patients received treatment of chemotherapy, immunotherapy or radiotherapy, etc. This study has been approved by the ethical committee. Patients and their families were informed, and they signed the informed consent form.
2.2 Main Reagents, Instruments and Detection Methods
2.2.1 Main Reagents and Instruments
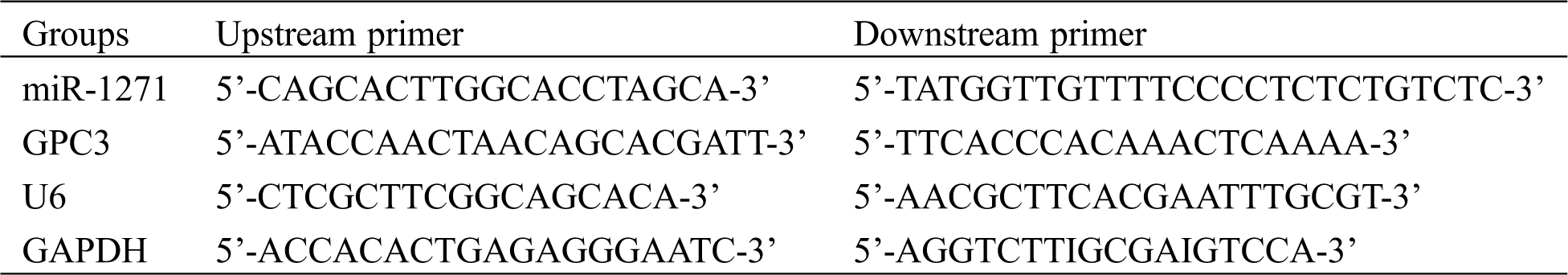
They were: Trizol reagent (Applide Invitrogen, USA), qRT-PCR and minScript reverse transcription kits (TaKaRa, Dalian), HBS-1096A enzyme-labeled analyzer (Nanjing Detie Experimental Equipment Co., Ltd., Nanjing, China), real-time quantitative PCR (BioRad, USA). Primer sequences of miR-1271, GPC3, internal references of U6 and GAPDH, and miRNA negative control were all synthesized and designed by GenePharma Co., Ltd., Shanghai, China (Tab. 1).
Table 1: miR-1271, GPC3 primer sequences and internal references
2.2.2 Detection of miR-1271 and GPC3
Within 24 h after admission, 5 mL elbow venous blood was drawn from subjects and placed in a vacuum blood collection tube without anticoagulant, and centrifuged at 3500 g for 15 min. The serum was retained in EP tube for later use, and then stored in a low-temperature refrigerator at –80°C. The miR-1271 and GPC3 expression in serum were tested by qRT-PCR. Total serum RNA was extracted according to the Trizol reagent instructions and dissolved in 20 μL DEPC water. The total RNA was then reverse-transcribed using a reverse transcription kit: M-MLV 1 μl, Olig (dT) 1 μl, RNA enzyme inhibitor 0.5 μl, d NTPs 1 μl, with the addition of RNAse free water to 15 μl. It was cultivated at 38°C for 60 min, and 1 μl cDNA was collected at 85°C for 5 s. As a template, the synthesized cDNA was employed for qRT-PCR amplification: 10 × PCR buffer 2.5 μl, d NTPs 1 μl, 1 μl upstream primer, 1 μl downstream primers, Taq DNA Polymerase 0.25 μl, with the addition of dd H2O to 25 μl. qRT-PCR reactions were then conducted using 5 µL of 1/20 cDNA dilution, 6.25 µL Power SYBR Green master mix, 0.5 µL of both forward and reverse primers, and 0.25 µL of nuclease-free water. Three duplicate wells were constructed for each sample for three reduplicated experiments. The relative quantification of target gene was counted using 2–ΔCt.
SPSS 10.0 was employed for statistical analysis. The enumeration data was represented by number of cases/percentage [n(%)], and the inter-group comparison was qualified by X2 test. The measurement data was represented by mean (X ± SD), and the inter-group comparison was qualified by t test or F test. The diagnostic value and 5-year survival of patients were drawn by ROC and K-M survival curve, respectively, and they were analyzed through Log-rank test. The independent risk factors affecting prognosis were assessed by multivariate Cox regression analysis; the comparison between multiple groups was analyzed by one-way analysis of variance (ANOVA) and expressed as F. P < 0.05 was statistically significant.
4.1 General Clinical Data of Patients
There was no marked difference in age, gender, drinking, education background and other general clinical data between the LCG and the NG (P > 0.05) (Tab. 2).
Table 2: General clinical data of patients
4.2 miR-1271 and GPC3 Expression in LCG and NG before TACE
miR-1271 expression in the LCG and NG were (0.20 ± 0.10) and (0.41 ± 0.10), respectively, while GPC3 expression in the LCG and NG were (0.8 ± 0.10) and (0.60 ± 0.10), respectively. miR-1271 expression in serum of the LCG was markedly lower than that of the NG (P < 0.001), while GPC3 expression of the LCG was dramatically higher than that of the NG (P < 0.001) (Fig. 1).
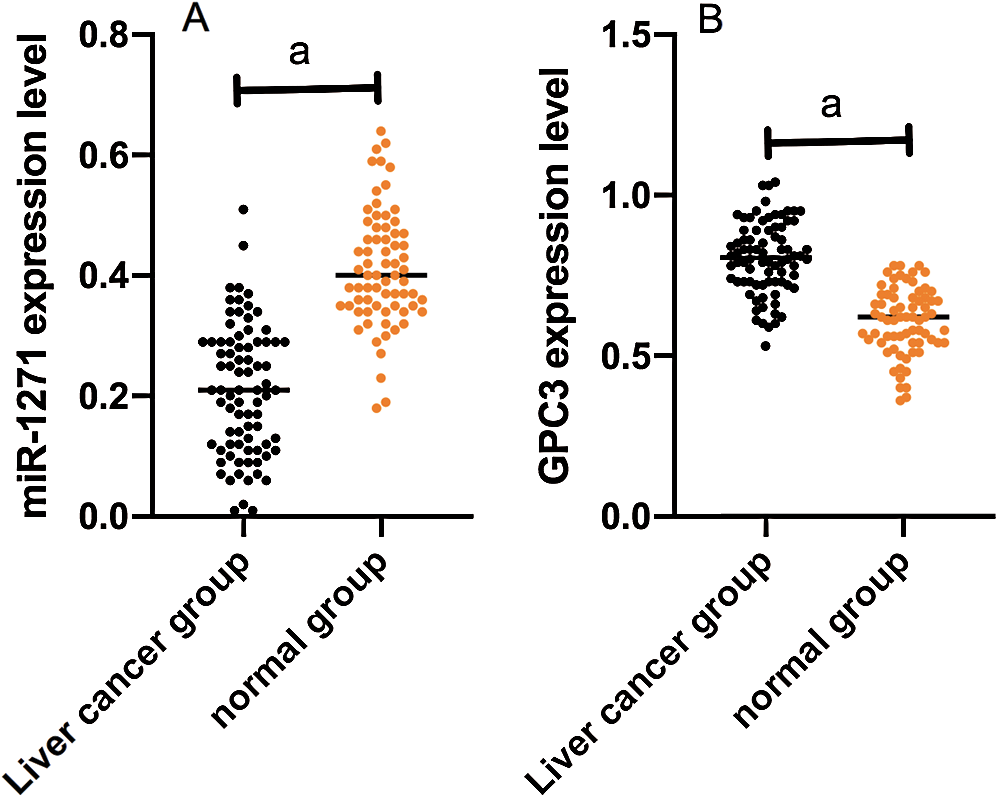
Figure 1: Comparison of miR-1271 and GPC3 relative expression in the LCG and the NG. (A) miR-1271 relative expression in serum of the LCG is markedly higher than that of the NG (P < 0.001). (B) GPC3 relative expression of the LCG is dramatically lower than that of the NG (P < 0.001). Note: A denotes P < 0.001
4.3 miR-1271 and GPC3 Expression and Clinicopathologic Features of LC Patients before TACE
4.3.1 Relationship between miR-1271 and Pathological Data of LC Patients
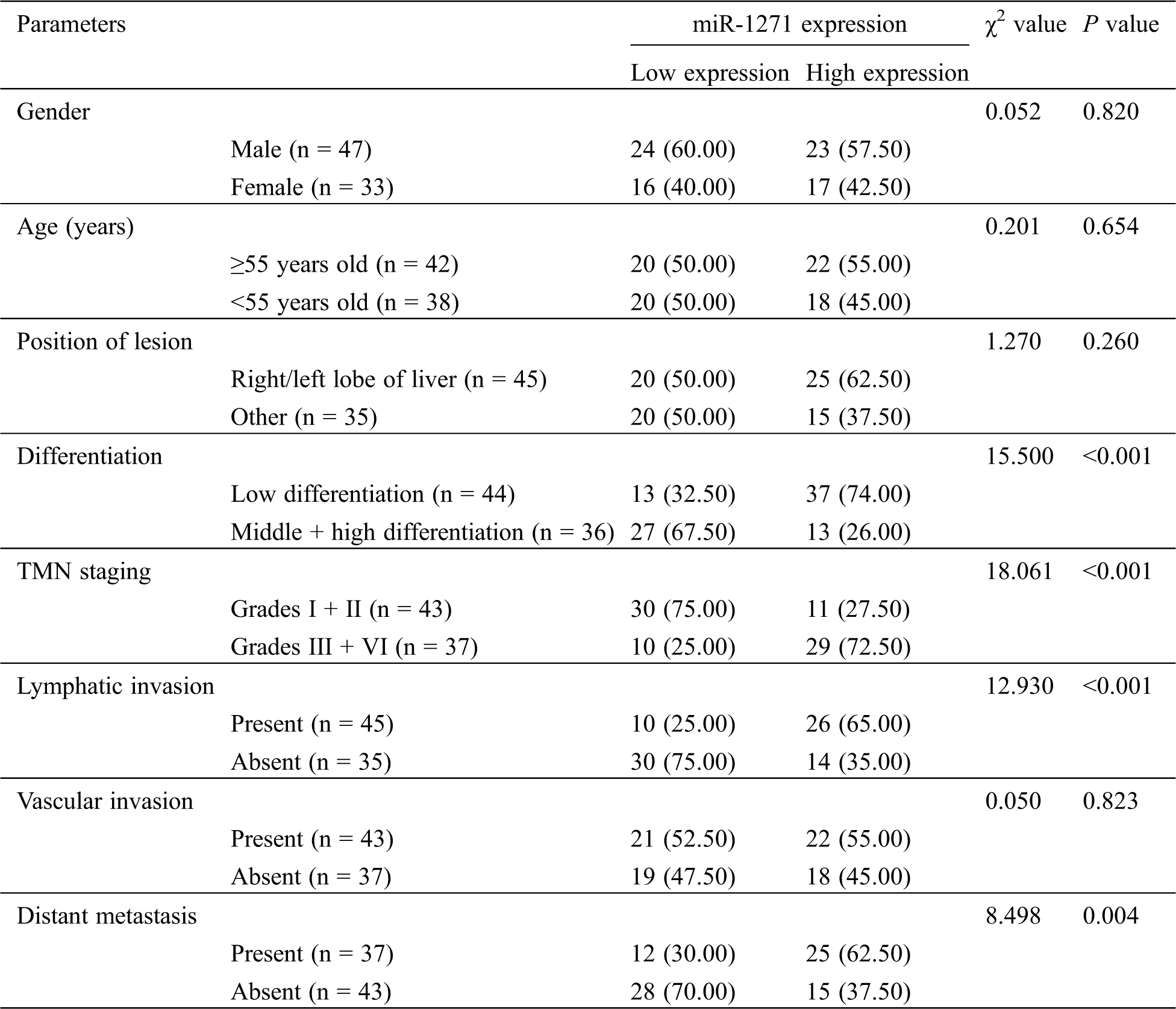
LC patients were divided into a miR-1271 high-expression group and a low-expression group according to the median level of miR-1271. By analyzing the pathological data, we found that patients with low expression were more likely to have higher incidence of low differentiation, Grades III + IV, lymphatic invasion and distant metastasis (P < 0.05) (Tab. 3).
Table 3: Relationship between miR-1271 and pathological data of LC
4.3.2 Analysis of GPC3 Expression and Clinicopathologic Features of LC Patients
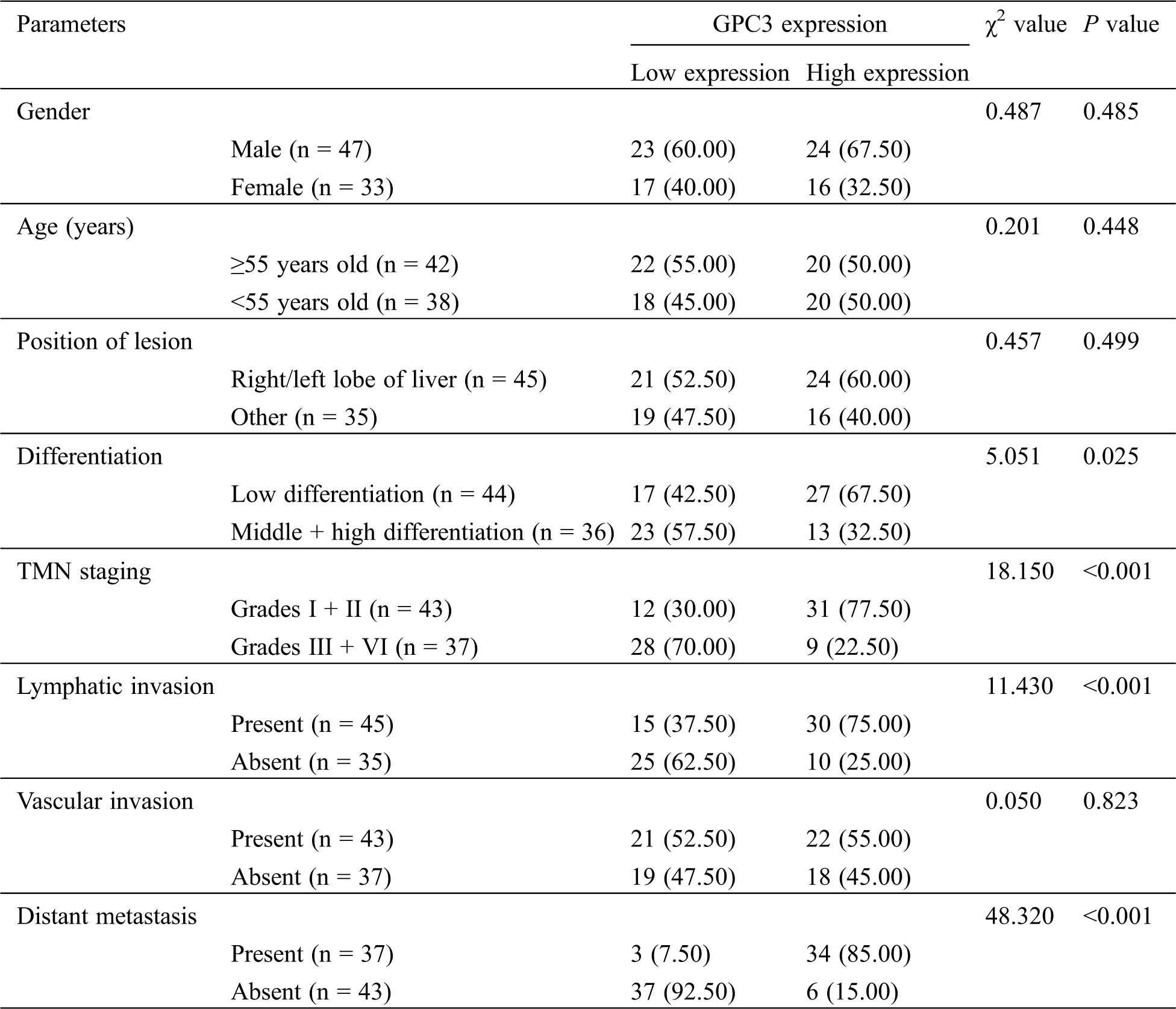
LC patients were divided into a GPC3 high-expression group and a low-expression group in the light of the median level of GPC3. By analyzing the pathological data, we found that patients with high expression were more likely to have a higher incidence of low differentiation, Grades III + IV, lymphatic invasion and distant metastasis (P < 0.05) (Tab. 4).
Table 4: GPC3 expression and clinicopathologic features of LC patients before TACE
4.4 miR-1271 and GPC3’s Value in LC Prognosis
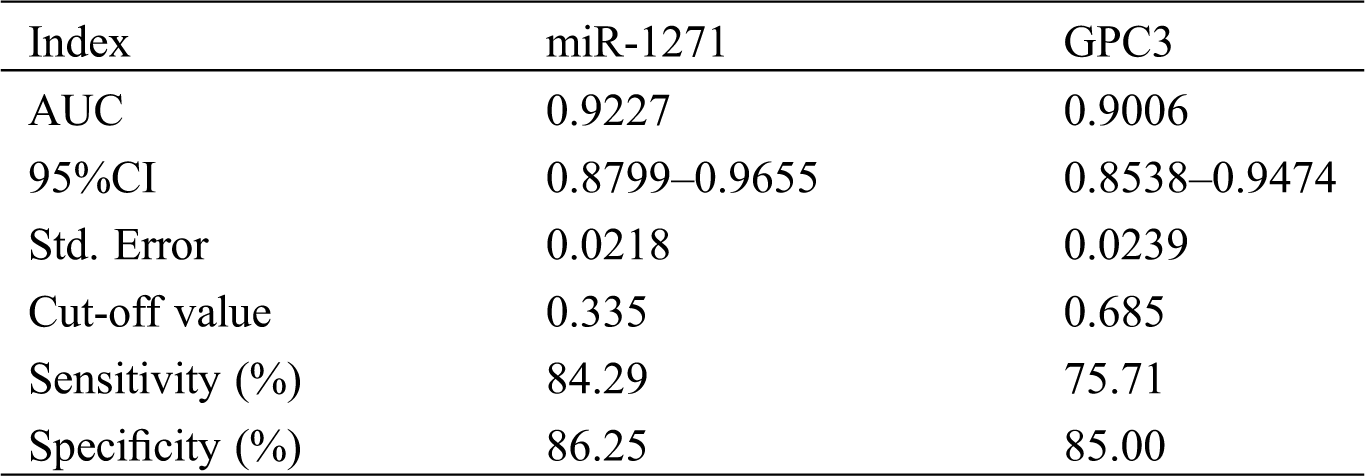
The sensitivity, specificity and AUC of miR-1271 alone in diagnosing LC patients were 84.29%, 86.25% and 0.9227, respectively, while those of GPC3 alone in diagnosis were 75.71%, 85.00%, and 0.9006, respectively (Tab. 5, Fig. 2).
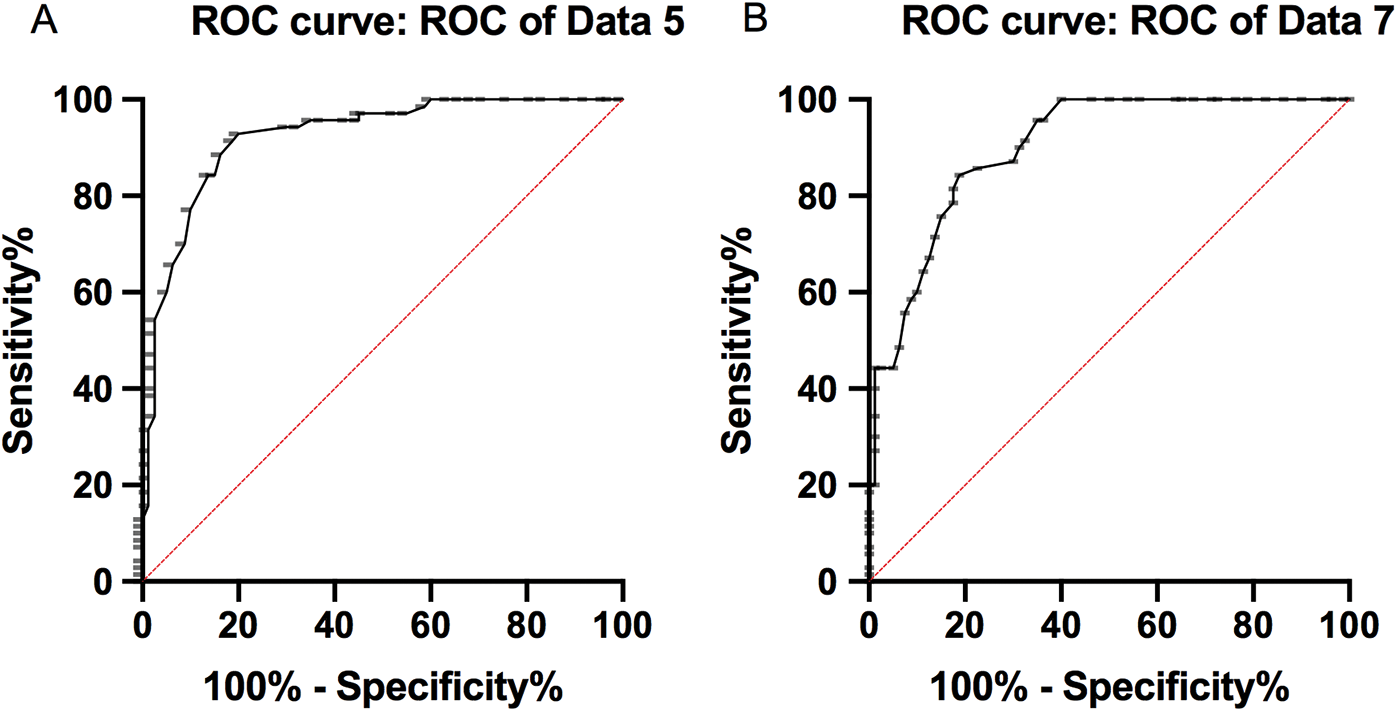
Figure 2: ROC of serum miR-1271 and GPC3 in diagnosing LC. (A) The sensitivity, specificity and AUC of miR-1271 alone in diagnosing LC patients were 84.29%, 86.25%, and 0.9227, respectively. (B) The sensitivity, specificity and AUC of GPC3 alone in diagnosing LC patients were 75.71% , 85.00%, and 0.9006, respectively
Table 5: Serum miR-1271 and GPC3’s diagnostic value on LC before treatment
4.5 Correlation of miR-1271 and GPC3 with Survival of LC Patients
All patients were followed up for 5 years. The 5-year survival rate was 8%. The rate of the miR-1271 high-expression group was dramatically higher than that of the low-expression group. The rate of the GPC3 high-expression group was markedly lower than that of the low-expression group (P < 0.05) (Fig. 3).
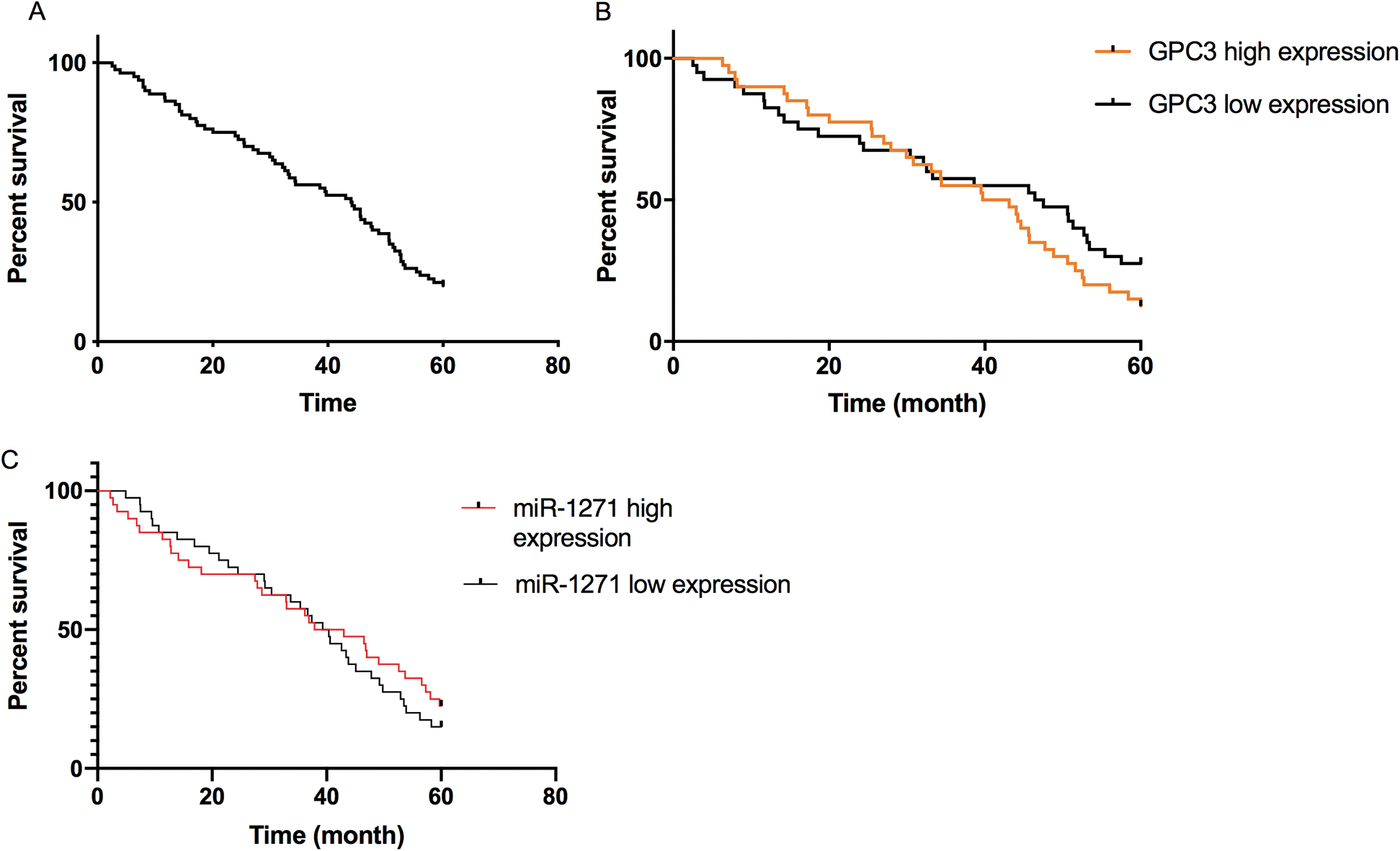
Figure 3: Correlation of miR-1271 and GPC3 with 5-year survival of LC patients. (A) Overall survival rate of LC patients. (B) The 5-year survival rate of the GPC3 high-expression group is lower than the low-expression group (P < 0.05). (C) The 5-year survival rate of the miR-1271 high-expression group is higher than the low-expression group (P < 0.05)
4.6 ROC Analysis of miR-1271 and GPC3 in Predicting Prognosis of LC Patients
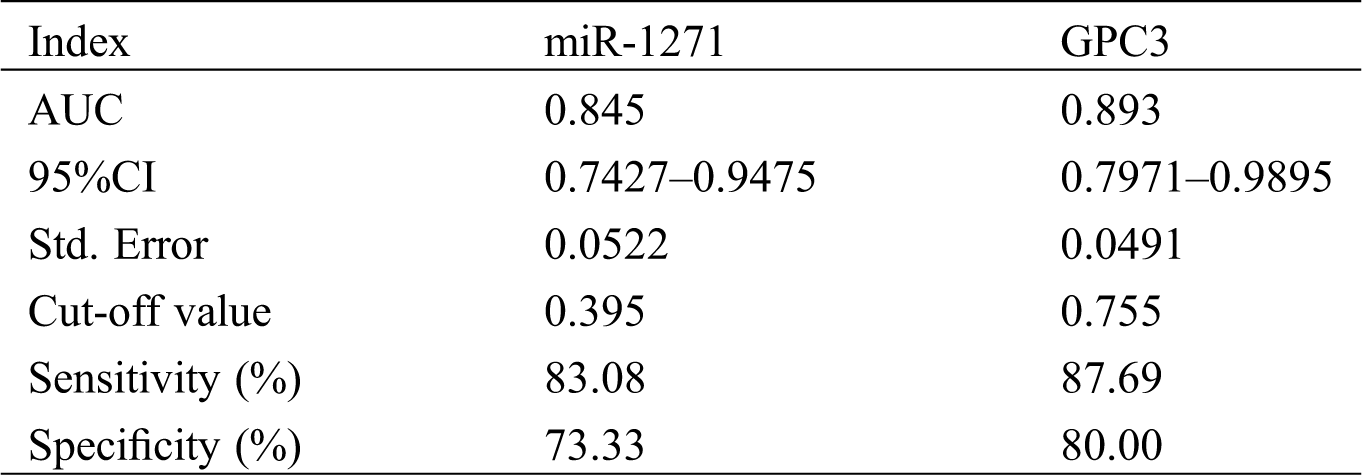
On the basis of 5-year follow-up, LC patients were assigned into a survival group (SG, n = 15) and a death group (DG, n = 65) according to survival. The serum miR-1271 and GPC3 relative expression in the SG were (0.40 ± 0.10) and (0.70 ± 0.10), respectively, and those in the DG were (0.30 ± 0.10) and (0.86 ± 0.10), respectively. The relative expression of serum miR-1271 was markedly higher in the SG than the DG, while that of serum GPC3 was markedly lower in the SG (both P < 0.001). ROC of serum miR-1271 and GPC3 in diagnosing the prognosis of LC patients were drawn. The AUC, sensitivity, specificity, and cut-off value of serum miR-127 were 0.845, 83.08%, 73.33%, and 0.395, respectively, while the four of serum GPC3 were 0.893, 87.69%, 80.00%, and 0.755, respectively (Tab. 6, Fig. 4).
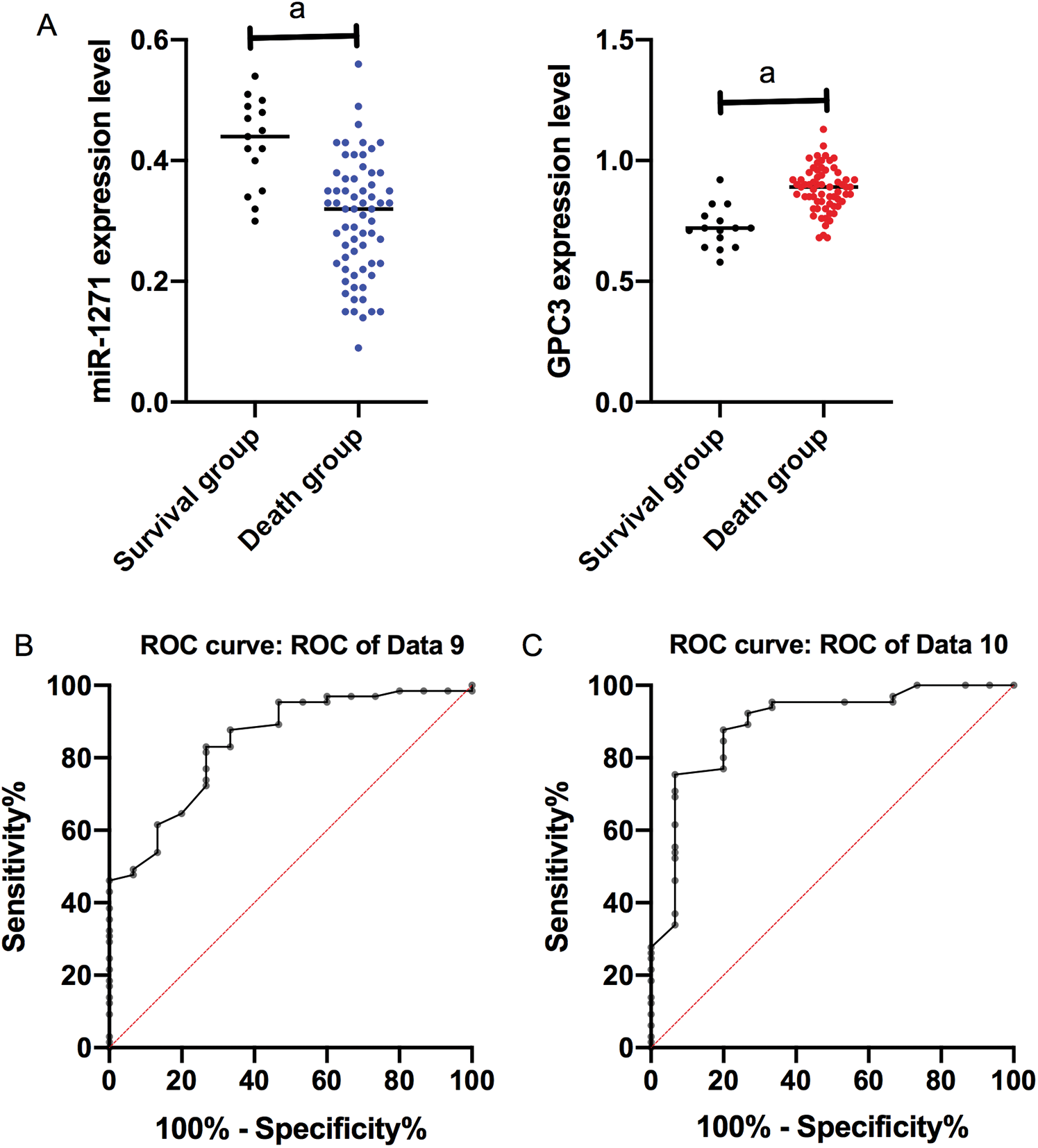
Figure 4: ROC of serum miR-1271 and GPC3 in diagnosing prognosis of LC patients. (A) Relative expression of serum miR-1271 and GPC3 in both groups (a represents P < 0.001). (B) The AUC, sensitivity, specificity and cut-off value of serum miR-1271 in diagnosing prognosis are 0.845, 83.08%, 73.33% and 0.755, respectively. (C) The AUC, sensitivity, specificity, and cut-off value of serum GPC3 in diagnosing prognosis are 0.893, 87.69%, 80.00% and 0.755, respectively
Table 6: Prognostic value of serum miR-1271 and GPC3 in LC patients before treatment
4.7 Cox Regression Analysis of 5-Year Survival of LC Patients
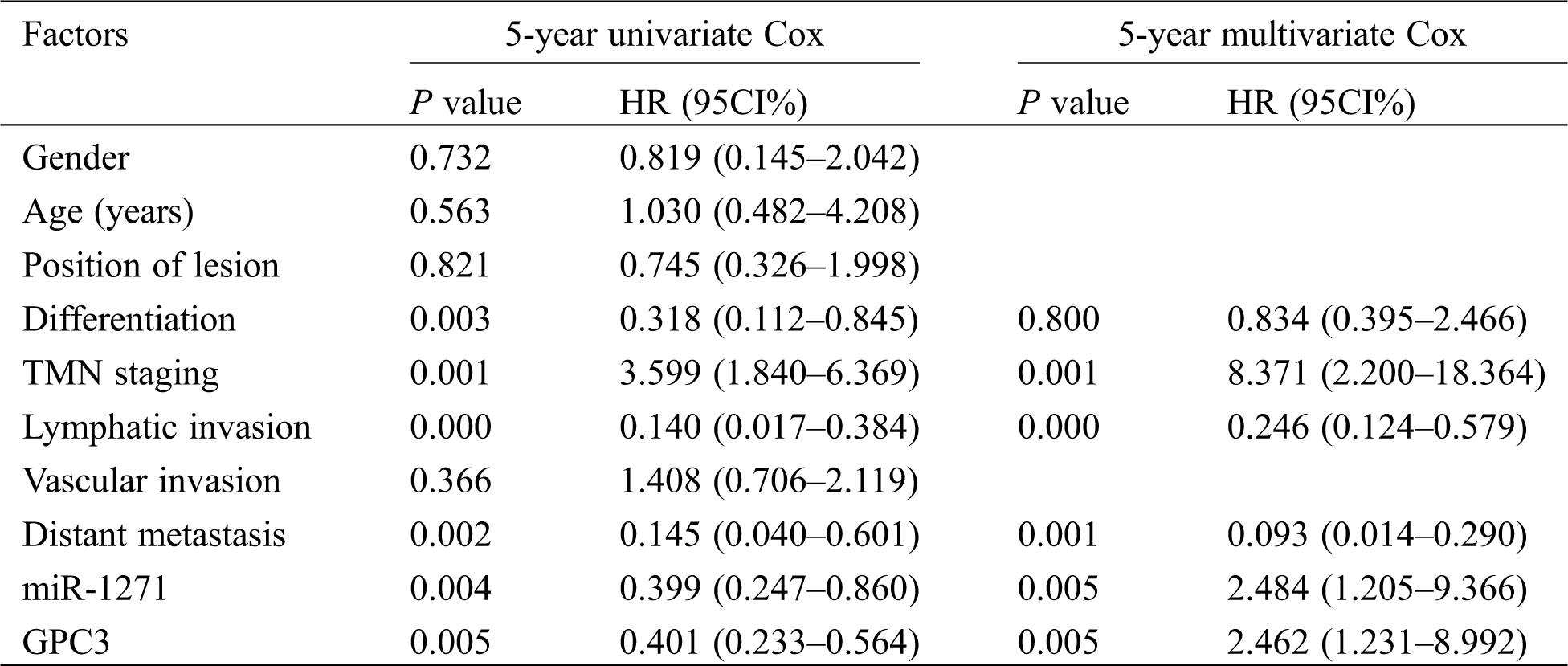
The 5-year survival of LC patients was assessed by Cox regression analysis, so as to observe independent factors affecting prognosis. The results showed that TMN staging, lymphatic invasion, distant metastasis and miR-1271 were independent factors affecting prognosis (Tab. 7).
Table 7: Univariate and multivariate Cox regression analysis of 5-year survival of serum miR-1271 and GPC3 in LC
Currently, we are continuously looking for more sensitive and exact prognosis assessment methods for LC patients after TACE treatment [18,19]. Due to the lack of certain specificity of clinical manifestations of LC patients, conventional diagnostic methods can not provide a comprehensive and complete assessment of the prognosis of LC patients after TACE, resulting in poor prognosis. Therefore, it is critical to explore biomarkers relevant to diagnosis and prognosis [20].
miRNAs are endogenous, non-coding single-stranded RNAs that promote or suppress cancer [21,22]. Relevant studies on LC progression showed that miR-1271, as a new tumor suppressor, was significantly down-regulated in endometrial cancer, LC, prostate cancer and other diseases [23]. Furthermore, miR-1271 inhibits LC cell growth and induces apoptosis by targeting FOXQ1. It is reported that the GPC3 over-expression is related to miR-1271 down-regulation [24]. At present, there are few studies on the significance of miR-1271 and GPC3 expression changes on patient condition change and prognosis after TACE. We analyzed the differences of miR-1271 and GPC3 expression in serum of LC patients and healthy subjects firstly, and found down-regulated miR-1271. In contrast, GPC3 was up-regulated in LC. By analyzing the correlation of miR-1271 and GPC3 expression with clinicopathologic features of patients, we found that the expression was not associated with gender, age and position of lesion, but was related to vascular invasion, differentiation, TMN staging, lymphatic invasion and distant metastasis. Recently, researchers have focused on the biological therapy via regulating miRNAs. Current studies have shown that GPC3 is significantly abnormally expressed in both white blood cells and plasma of hepatocellular carcinoma. With the aggravation of TNM staging, serum GPC3 over-expression is more obvious [25]. However, miR-1271 is hardly expressed in adult normal liver cells and is expressed in LC cells, negatively correlated with the disease condition [26]. Combined with ROC data, we believed that serum miR-1271 and GPC3 could been used in LC patients in evaluating the severity of the disease and the subsequent health monitoring. Then, we analyzed the expression of serum miR-1271 and GPC3 according to different prognosis, and found that the serum miR-1271 in the surviving patients was higher than that in the dead patients, while the relative expression of serum GPC3 was dramatically lower. In the end, we analyzed the prognostic factors affecting the 5-year survival of patients. It was found that TMN staging, lymphatic invasion, distant metastasis, miR-1271 and GPC3 were independent factor affecting 5-year survival prognosis. A previous research has revealed that TNM staging, lymphatic invasion, distant metastasis are independent prognostic factors affecting prognosis [27], but it is the first time that GPC3 and miR-1271 can be employed as independent prognostic factors. So, we preliminarily determined the clinical value of miR-1271 and GPC3, which two were expected to be potential diagnostic and prognostic indicators. Both miR-1271 and GPC3 were critical in clinical diagnosis and prognosis.
We confirmed the expression and prognostic value of miR-1271 and GPC3 in LC patients, but there are still some limitations. For example, there was no specific analysis on the regulatory effect of miR-1271 and GPC3 expression on relevant white blood cells, and no further explanation of biological functions was given. Moreover, miR-1271, GPC3, and conventional clinical inflammatory factors were not analyzed, all of which had certain influence on the refinement of the study design. Hence, we will refer to the latest research in real time at the later stage to add corresponding research solutions, and make up for design flaws, so as to optimize the research.
All in all, miR-1271 was down-regulated in LC, while GPC3 expression was up-regulated. miR-1271 and GPC3 may be implicated in disease development and progression, and can be employed as potential serum biomarkers for TACE evaluation.
Funding Statement: This research is financially supported by Jiangsu Provincial Hospital Association Project (JSYGY-3-2019-504).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, C., Vegna, S., Jin, H., Benedict, B., Lieftink, C. et al. (2019). Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature, 574(7777), 268–272. DOI 10.1038/s41586-019-1607-3. [Google Scholar] [CrossRef]
2. Lleo, A., de Boer, Y. S.,Liberal, R., Colombo, M. (2019). The risk of liver cancer in autoimmune liver diseases. Therapeutic Advances in Medical Oncology, 11, 175883591986191. DOI 10.1177/1758835919861914. [Google Scholar] [CrossRef]
3. Van Haele, M., Moya, I. M., Karaman, R., Rens, G., Snoeck, J. et al. (2019). YAP and TAZ heterogeneity in primary liver cancer: An analysis of its prognostic and diagnostic role. International Journal of Molecular Sciences, 20(3), 638. DOI 10.3390/ijms20030638. [Google Scholar] [CrossRef]
4. Wang, L. T., Wang, S. N., Chiou, S. S., Liu, K. Y., Chai, C. Y. et al. (2019). TIP60-dependent acetylation of the SPZ1-TWIST complex promotes epithelial-mesenchymal transition and metastasis in liver cancer. Oncogene, 38(4), 518–532. DOI 10.1038/s41388-018-0457-z. [Google Scholar] [CrossRef]
5. Basart, H., Paes, E. C., Maas, S. M., van den Boogaard, M. J. H.,van Hagen, J. M. et al. (2015). Etiology and pathogenesis of robin sequence in a large Dutch cohort. American Journal of Medical Genetics, 167(9), 1983–1992. DOI 10.1002/ajmg.a.37154. [Google Scholar] [CrossRef]
6. Sun, Y., Ji, S., Liu, L., Li, C. (2019). Clinical efficacy analysis of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) in primary liver cancer and recurrent liver cancer. Official Journal of the Balkan Union of Oncology, 24(4), 1402–1407. [Google Scholar]
7. Luo, Y., Jiang, Y. (2019). Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer. Journal of the College of Physicians and Surgeons Pakistan, 29(5), 414–417. DOI 10.29271/jcpsp.2019.05.414. [Google Scholar] [CrossRef]
8. Loosen, S. H., Schulze-Hagen, M., Leyh, C., Benz, F., Vucur, M. et al. (2018). IL-6 and IL-8 serum levels predict tumor response and overall survival after TACE for primary and secondary hepatic malignancies. International Journal of Molecular Sciences, 19(6), 1766. DOI 10.3390/ijms19061766. [Google Scholar] [CrossRef]
9. Cong, Y., Yang, X. G., Lv, W., Xue, Y. (2009). Prediction of novel and selective TNF-alpha converting enzyme (TACE) inhibitors and characterization of correlative molecular descriptors by machine learning approaches. Journal of Molecular Graphics and Modelling, 28(3), 236–244. DOI 10.1016/j.jmgm.2009.08.001. [Google Scholar] [CrossRef]
10. Meng, P., Yoshida, H., Matsumiya, T., Imaizumi, T., Tanji, K. et al. (2013). Carnosic acid suppresses the production of amyloid-β 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neuroscience Research, 75(2), 94–102. DOI 10.1016/j.neures.2012.11.007. [Google Scholar] [CrossRef]
11. Mokhlis, H. A., Ozpolat, B. (2019). Nanoparticle delivery of miRNA in cancer. In: MicroRNAs in diseases and disorders. UK: RSC Publishing. [Google Scholar]
12. Sastre, D., Baiochi, J., de Souza Lima, I. M.,de Souza, F. C.,Corveloni, A. C. et al. (2019). Focused screening reveals functional effects of microRNAs differentially expressed in colorectal cancer. BMC Cancer, 19(1), 1239. DOI 10.1186/s12885-019-6468-5. [Google Scholar] [CrossRef]
13. Kolluri, A., Ho, M. (2019). The role of glypican-3 in regulating Wnt, YAP and hedgehog in liver cancer. Frontiers in Oncology, 9, 708. DOI 10.3389/fonc.2019.00708. [Google Scholar] [CrossRef]
14. Wang, C., Gao, W., Feng, M., Pastan, I., Ho, M. (2017). Construction of an immunotoxin, HN3-mPE24, targeting glypican-3 for liver cancer therapy. Oncotarget, 8(20), 32450–32460. DOI 10.18632/oncotarget.10592. [Google Scholar] [CrossRef]
15. Gómez, M. I., Seaghdha, M. O., Prince, A. S. (2007). Staphylococcus aureus protein a activates TACE through EGFR-dependent signaling. EMBO Journal, 26(3), 701–709. DOI 10.1038/sj.emboj.7601554. [Google Scholar] [CrossRef]
16. Xiang, X. J., Deng, J., Liu, Y. W., Wan, L. Y., Feng, M. et al. (2015). MiR-1271 inhibits cell proliferation, invasion and EMT in gastric cancer by targeting FOXQ1. Cellular Physiology and Biochemistry, 36(4), 1382–1394. DOI 10.1159/000430304. [Google Scholar] [CrossRef]
17. Seehawer, M., Heinzmann, F., D’Artista, L., Harbig, J., Roux, P. F. et al. (2018). Necroptosis microenvironment directs lineage commitment in liver cancer. Nature, 562(7725), 69–75. DOI 10.1038/s41586-018-0519-y. [Google Scholar] [CrossRef]
18. Leone Roberti Maggiore, U., Ferrero, S., Candiani, M., Somigliana, E., Viganò, P. et al. (2017). Bladder endometriosis: A systematic review of pathogenesis, diagnosis, treatment, impact on fertility, and risk of malignant transformation. European Urology, 71(5), 790–807. DOI 10.1016/j.eururo.2016.12.015. [Google Scholar] [CrossRef]
19. Ran, R. Z., Chen, J., Cui, L. J., Lin, X. L., Fan, M. M. et al. (2019). miR-194 inhibits liver cancer stem cell expansion by regulating RAC1 pathway. Experimental Cell Research, 378(1), 66–75. DOI 10.1016/j.yexcr.2019.03.007. [Google Scholar] [CrossRef]
20. Si, A., Wang, L., Miao, K., Zhang, R., Ji, H. et al. (2019). miR-219 regulates liver cancer stem cell expansion via E-cadherin pathway. Cell Cycle, 18(24), 3550–3561. DOI 10.1080/15384101.2019.1691762. [Google Scholar] [CrossRef]
21. Burwinkel, B., Cuk, K., Zucknick, M., Madhavan, D. (2013). Circulating miRNAs as markers for breast cancer. U.S. Patent 10,316,367[P]. [Google Scholar]
22. Chan, C., Guo, N., Duan, X., Han, W., Xue, L. et al. (2019). Systemic miRNA delivery by nontoxic nanoscale coordination polymers limits epithelial-to-mesenchymal transition and suppresses liver metastases of colorectal cancer. Biomaterials, 210, 94–104. DOI 10.1016/j.biomaterials.2019.04.028. [Google Scholar] [CrossRef]
23. Xu, H. Y., Shao, J., Yin, B. Z., Zhang, L. M., Fang, J. C. et al. (2020). Bovine bta-microRNA-1271 promotes preadipocyte differentiation by targeting activation transcription factor 3. Biochemistry (Moscow), 85(7), 749–757. DOI 10.1134/S0006297920070032. [Google Scholar] [CrossRef]
24. Zhong, J., Liu, Y., Xu, Q., Yu, J., Zhang, M. (2017). Inhibition of DIXDC1 by microRNA-1271 suppresses the proliferation and invasion of prostate cancer cells. Biochemical and Biophysical Research Communications, 484(4), 794–800. DOI 10.1016/j.bbrc.2017.01.169. [Google Scholar] [CrossRef]
25. Wang, Z., Zhao, K., Zhang, Y., Duan, X., Zhao, Y. (2019). Anti-GPC3 antibody tagged cationic switchable lipid-based nanoparticles for the co-delivery of anti-miRNA27a and sorafenib in liver cancers. Pharmaceutical Research, 36(10), 145. DOI 10.1007/s11095-019-2669-5. [Google Scholar] [CrossRef]
26. Chen, Y., Zhao, Z. X., Huang, F., Yuan, X. W., Deng, L. et al. (2019). MicroRNA-1271 functions as a potential tumor suppressor in hepatitis B virus-associated hepatocellular carcinoma through the AMPK signaling pathway by binding to CCNA1. Journal of Cellular Physiology, 234(4), 3555–3569. DOI 10.1002/jcp.26955. [Google Scholar] [CrossRef]
27. Huang, X. T., Chen, L. H., Huang, C. S., Li, J. H., Cai, J. P. et al. (2019). Establishment of a nomogram by integrating molecular markers and tumor-node-metastasis staging system for predicting the prognosis of hepatocellular carcinoma. Digestive Surgery, 36(5), 426–432. DOI 10.1159/000494219. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |