
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.015234
ARTICLE
Analysis of Antioxidant Potential of Trigonella foenum-graecum (L.) Extract Against Tumorigenesis
1Department of Biotechnology, IIS (Deemed to be University), SFS, Gurukul Marg, Mansarovar, Jaipur, Rajasthan, 302020, India
*Corresponding Author: Nidhi Gupta. Email: nidhi.gupta@iisuniv.ac.in
Received: 02 December 2020; Accepted: 18 January 2021
#Contributed equally to the research work and manuscript
ABSTRACT:
Background: Trigonella foenum-graecum Linn. has been extensively used for medicinal purposes. The current study deals with in vitro and in vivo correlation of free radical quenching activity and anticancer potential of seed extracts of Trigonella. Materials and methods: Antioxidant activity was evaluated against DPPH, NO and ABTS via in vitro radical scavenging assay. Cytotoxicity effect of Trigonella seed extract was studied in human embryonic kidney HEK 293 cell line by alamar blue assay. In vivo antioxidant activity in Swiss albino mice model was assessed by studying endogenous antioxidant enzymes such as GSH, GPx, SOD and LPO. Antitumor effect was observed by studying parameters like total number of tumors, tumor size in mice. Further, expression of tumor suppressor gene, p53 in treated mice was investigated. Results: In vitro antioxidant assays had shown methanolic extract to possess higher radical scavenging activity than aqueous and to be minimal cytotoxic. In vivo study has shown a significant reduction in total number of tumors and tumor size for the mice group treated with extract in comparison to DMBA-TPA treated group. An increase in the levels of GSH, SOD and GPx was observed with a significant reduction in LPO levels. Expression of p53 was found to be upregulated in Swiss albino mice treated with extract emphasizing possible antitumor effect of Trigonella. Conclusion: The present study helped in understanding the reducing potential and antitumorigenic activity of Trigonella seed extract and its probable therapeutic effect in skin papillomagenesis.
Keywords: Trigonella; anti-tumorigenic; antioxidants; free radicals; p53; skin papillomagenesis
Skin cancer is one of the most prevalent cancers with 5 million incidences occurring every year [1]. Annually 2–3 million cases of non-melanoma skin cancer have been registered globally. Cases of non-melanoma skin cancer, which comprise of basal cell carcinoma and squamous cell carcinoma, vary according to the geographic locations and population. The primary risk factors for all types of skin cancer include environmental factors, occupational factors, diet, viral agents, genetic makeup etc. that might generate reactive oxygen species (ROS) which in turn disturbs the homeostatic balance, ultimately led to tumor formation [2]. Among pollutants that exist in the environment are polycyclic aromatic hydrocarbons (PAHs), responsible factor for carcinogenesis, mutagenesis and also suppression of immune system [3]. One of the PAHs that has been used in the current study is 7,12 dimethylbenz (a) anthracene (DMBA), which is known to be an organ specific carcinogen and it has been studied by researchers as one of the potent tumor initiators [4]. These tumor initiators work in coordination with promoters such as tetradecanoyl phorbol acetate (TPA) to complete the process of tumor formation. The mechanism for tumorigenesis involves formation of ROS that could change structure of DNA by forming adducts or cross linking with proteins. Henceforth, damage to DNA can either activate oncogenes or inactivate tumor suppressor genes resulting in incidences of cancer [5].
Obliteration of free radicals by the antioxidant systems can be an effective approach at the grass-root level and can be exploited as a preventive measure against diseases like cancer, cardiovascular disease, neurodegenerative disorders, cataracts and inflammation [6]. Antioxidants are molecules that have both monohydroxy or polyhydroxy phenols and work by donating the electrons to the free radicals making them a stable entity [7,8]. Antioxidants can be divided into two forms namely; endogenous antioxidant systems and the exogenous antioxidant systems. Superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) are some of the endogenous antioxidant enzymes that aid in achieving a reduced state of cell, essential for the cell survival. When there is a higher level of oxidative stress, the body’s defense mechanism, i.e., the endogenous antioxidants are impaired and hence caters the need for the exogenous antioxidant systems [9]. Researchers worldwide have successfully exploited medicinal plants for their antioxidant constituents [10]. Various plants like Phyllanthus niruri [11], Aloe barbadensis [12,13], Clerodendrum serratum [14] contain flavonoids, phenols, terpenoids and saponins exhibited the property of scavenging ROS. Methanolic extract of Pandanus odoratissimus lim leaf showed an increase in the level of endogenous antioxidants in Wistar albino rats [15]. Bioactive compounds of Pennisetum glaucum exhibited reducing potential against radicals such as DPPH, ABTS, FRAP, etc. Twelve cultivars of Pennisetum glaucum (pearl millet) also showed protective effect against damaged DNA [16]. Trigonella foenum graecum is rich in saponins, alkaloids and flavonoids. This plant is widely used for treatment of diabetes, inflammation, reducing cholesterol level, cancer and acts as a dietary herb [17]. Major alkaloid trigonellin from the seeds are responsible for its antidiabetic role [18]. This plant is yet to be fully explored in order to exploit its ROS scavenging potential and anticancer activity.
The current study employed analysis of antioxidant and anticancer potential of Trigonella foenum-graecum seed extract (TFGS). Biochemical estimations, such as reduced glutathione (GSH), lipid peroxidation (LPO), SOD and GPx, were carried out with a view to assess antioxidant activity. Extracts for their probable toxicity were tested on human embryonic kidney cell line, HEK 293 by alamar blue assay. Expression of tumor suppressor gene, p53, was studied in order to understand the probable mechanism underlying anticancer activity of the extract. Also, a comparative study was conducted to evaluate between oral intake of extract and topical application in terms of better mode of administration for more efficient antitumorigenic activity.
2.1 Collection of Trigonella Seeds
The seeds of Trigonella were procured from National Research Centre for Seeds and Spices (NRCSS) Tabiji farm, Ajmer, Rajasthan, India. The voucher specimen collection number RUBL 20658 was deposited in the herbarium of Department of Botany, University of Rajasthan, Jaipur, Rajasthan, India.
2.2 Preparation of Trigonella Seed Extract
Methanolic and aqueous extracts of Trigonella seeds were prepared for which, seeds were shade dried and grinded to powder. For methanolic extract preparation, soxhlet extraction procedure was followed in which 250 ml methanol was added to 50 g of TFG powder and heated for 5–6 h at 70°C. Extracts were filtered using Whatman no. 1 filter paper and the resulting slurry was dried, weighed and stored at 4°C [19]. For aqueous extract, 60 g of powder was dissolved in 1 L of distilled water and kept on mechanical shaker for 48 h. The slurry obtained was filtered using Whatmann filter paper no. 1. It was dried, weighed and was kept at 4°C till further investigations were carried out [20]. The calculated yield was 30% and 27% for aqueous and methanolic extracts, respectively.
2.3 Determination of in Vitro Antioxidant Activity
In vitro antioxidant activity of aqueous and methanolic extract was determined by ROS scavenging assays namely 2,2-diphenyl-1-picrylhydrazyl (DPPH), Nitric oxide (NO) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay. Ascorbic acid was taken as standard ROS scavenger for all the three independent assays.
2.3.1 DPPH Radical Scavenging Activity
The antioxidant activity was evaluated against commercially available DPPH free radicals. For this equal volume of extract and 0.135 mM DPPH in methanol was mixed and incubated at room temperature in dark for 30 min. The absorbance was then observed at 517 nm [21]. DPPH in methanol was taken as control.
2.3.2 NO Radical Scavenging Activity
The extracts were also tested for their ability to quench nitrogen radicals. Griess Illosvoy reagent was employed for this study which was prepared by adding naphthyl ethylene diamine dihydrochloride (0.1% w/v) and sulfanilic acid (0.33% in 20% of glacial acetic acid) in equal volumes [22]. Phosphate buffer saline (0.5 ml) was added to 0.5 ml of extract and volume was made up to 3 ml by adding 10 mM of sodium nitroprusside solution. The solution was then incubated at 25°C for 150 min. After incubation, 1 ml of sulfanilic acid reagent was added to 0.5 ml of reaction mixture and further incubated for 5 min. 1 ml of naphthyl ethylene diamine dihydrochloride was then added and mixture was incubated at room temperature for 30 min. Post incubation pink color was developed and absorbance recorded at 540 nm. For the control, all the reagents taken in mentioned concentrations without seed extract and volume was make up with water.
2.3.3 ABTS Radical Scavenging Assay
ABTS is a colorless molecule that oxidizes to form an ABTS cation (ABTS+). ABTS solution was prepared by adding 7 mM of ABTS in 2.4 mM of potassium persulphate and incubated in dark at room temperature for 15–16 h. Equal volume of seed extract and ABTS solution was then mixed and incubated at 30°C for 7 min and absorbance was measured at 734 nm [23]. As the control, ABTS reagent in potassium persulphate was set.
Percentage radical scavenging activity for all the three different assays was calculated by:
[(Absorbance of control – absorbance of sample)/absorbance of control] × 100
Mammalian cell line namely HEK was procured from National Centre for Cell Science (NCCS), Pune, India. Cells were routinely maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin and incubated at 37°C in 5% CO2 incubator.
To assess the toxic nature of TFGS, alamar blue assay was conducted on HEK 293 cells. Alamar blue consists of resazurin, a blue colour cell permeable compound, and virtually non-fluorescent. Reduction of resazurin to resorufin, a red color compound and highly fluorescent, by the cells indicates the viability of the cells. The cells were cultured at the rate of 10,000 cells/well. After 16 h of incubation, the culture medium was changed with fresh medium, followed by addition of seed extract at varying concentrations (2 μg/μl–40 μg/μl). After 24 h 10% of pre warmed (37°C) alamar blue reagent was added and incubated in CO2 incubator for 3–4 h and absorbance was recorded at 495 nm. Cells without alamar blue were set as blank and cells untreated with extract were taken as control with 100% viability. The relative cell viability (%) compared to control cells was calculated by [O.D. sample/[O.D. control] × 100.
2.5 Mouse Skin Papilloma Model
Male Swiss albino mice of age 6–7 weeks, weighing 25–30 g were procured from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) approved animal house facility of IIS (deemed to be University), Jaipur, Rajasthan. Animals were fed with standard pellet mice diet (Aashirwad Industries, Mohali, Punjab, India) and fresh drinking water ad libitum. These mice were maintained in animal house under hygienic conditions (25 ± 5°C with 12 h day: Night cycle) in polypropylene cages and sacrificed by euthanization. All the experiments conducted in mice were strictly adhered with the guidelines of the Animal Welfare Committee of the University in compliance with CPCSEA norms which was duly approved by institutional animal ethical committee with reference number CPCSEA 1689/PO/a/13.
All the mice were divided into ten different groups with 5 mice in each group and the experiment was carried out for a total duration of 16 weeks. Removal of hair from the dorsal area of the mice was done before starting the treatment regime, in order to facilitate better distribution of the chemicals that were applied topically.
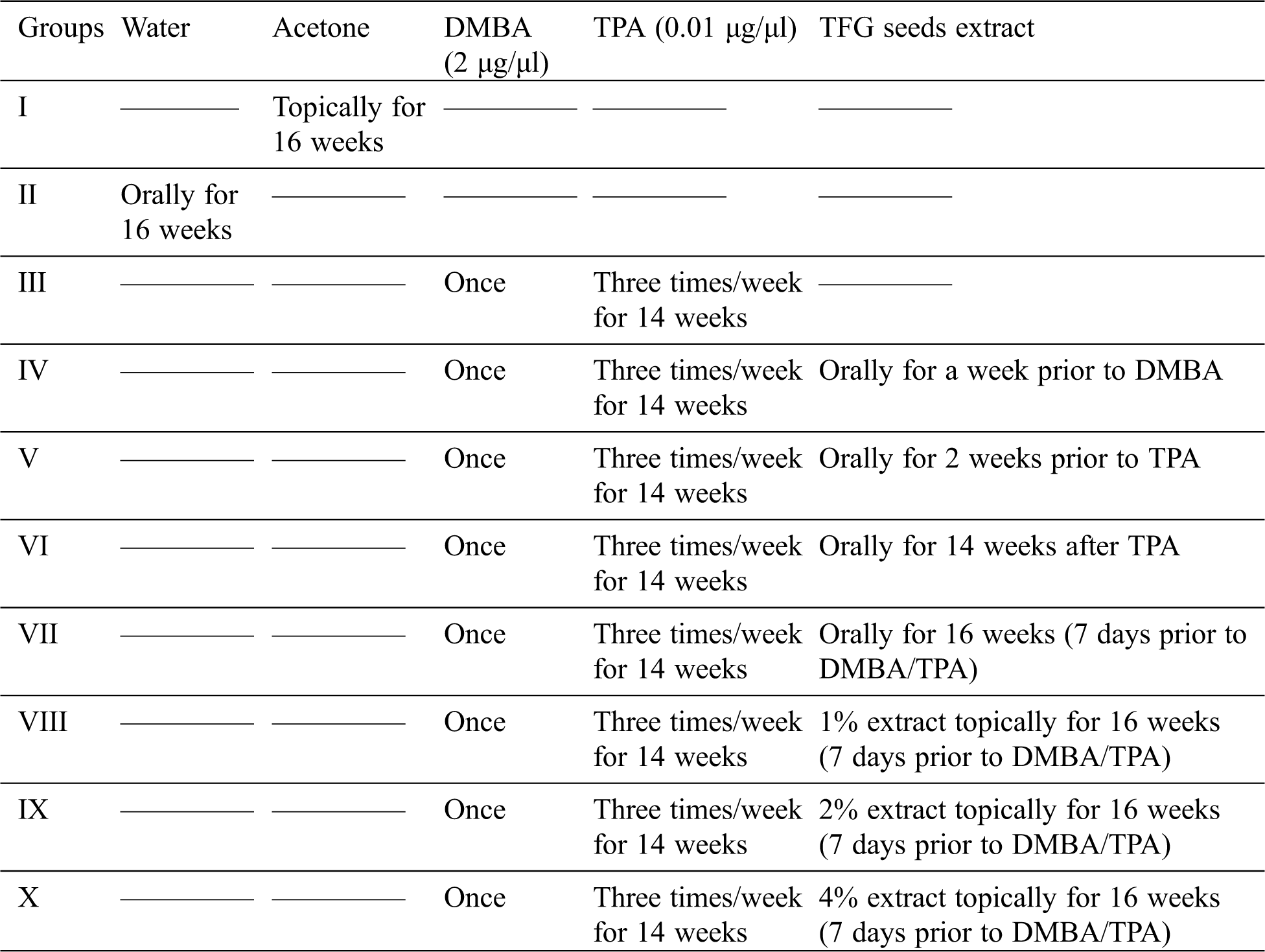
Development of two stage skin carcinogenesis model was followed according to the method described by Qiblawi and co-workers. Initially, DMBA (initiator) solution prepared in acetone was applied topically at a concentration of 2 μg/μl and after a gap of two weeks; TPA (promoter) solution in acetone was applied thrice a week for 14 weeks at a concentration of 0.01 μg/μl [24]. As shown in Tab. 1 the experimental group of mice was given TFGS extract at a dose level of 400 mg/kg body weight/day at different stages of study set up [25].
Table 1: Categorization of mice into various groups as per the requirement of the study
2.5.1 Parameters Studied for Skin Tumor Model System
To study the anti-carcinogenic activity of TFGS, different parameters related to skin tumor model system were studied. All the mice of various groups were sacrificed at the end of the experiment by euthanization in compliance with CPCSEA guidelines. After recording general parameters such as number and size of tumor, time of occurrence of tumor; biochemical parameters like endogenous antioxidant status and molecular parameters such as expression of p53 were studied. Continuous monitoring of papillomagenesis in mice to record weekly onset of tumor, number of tumors formed and their size in terms of diameter was performed throughout the treatment regimen.
2.5.2 Determination of in Vivo Antioxidant Activity
In order to investigate in vivo antioxidant potential of TFGS extract, Swiss albino mice were divided into ten groups and treated as summarized in Tab. 1.
For biochemical estimation, liver was collected, perfused with chilled 0.9% NaCl (pH 7.4), air dried and weighed. A 10% liver homogenate was made in 0.15M Tris KCl buffer pH 7.4. Homogenate was centrifuged at 2500 rpm, 10 min, 4°C and supernatant was estimated for endogenous antioxidant potential by studying levels of GSH, SOD, LPO and GPx.
To 1 ml of liver tissue homogenate, 100 µl of 25% trichloroacetic acid (TCA) was added. The mixture was centrifuged at 15000 g for 15 min at room temperature and supernatant collected. To 0.1 ml of supernatant collected, 0.9 ml of 0.2M sodium phosphate buffer (pH 8.0) and 2 ml of 0.6M Ellmann’s reagent were added. The absorbance was immediately measured at 412 nm and estimated GSH expressed as µM/g tissue [26].
To 10 μl of 10% liver homogenate, 1 ml of 50 mM sodium carbonate pH 10.0, 0.5 ml of 96 μM nitrobluetetrazolium (NBT) and 0.1 ml of 0.6% triton X-100 in water was added and mixture was incubated for 10 min at 37°C. 20 mM hydroxylamine hydrochloride was added to the incubated mixture and absorbance measured at 560 nm. SOD level was calculated and expressed as U/g tissue [27].
Thiobarbituric acid reactive substances (TBARS) method was used to estimate LPO level [28]. 0.2 ml of 10% tissue homogenate was used to which the following chemicals were added: thiobarbituric acid (TBA) (0.6%), sodium dodecyl sulphate (SDS) (0.1%), and trichloroacetic acid (TCA) (20%). This mixture was incubated at 80°C in water bath for 1 hr, followed by immediate cooling; after which N-butanol-pyridine in the ratio of 15:1 was added. A pink color solution along with precipitation was formed. Precipitate was removed by centrifugation (4000 rpm, 10 min, and room temperature) and absorbance of pink color solution was measured at 532 nm. LPO levels were expressed as nmole/mg of tissue.
0.2 ml of tissue homogenate in 0.4M phosphate buffer (pH 7.0) was mixed with 0.1 ml of 10 mM sodium azide, 0.1 ml of 0.2 mM hydrogen peroxide and incubated at 37°C for 10 min. To cease the reaction, 10% TCA (0.4 ml) was added to the mixture and centrifuged at 3200 g for 20 min. 0.6M Ellman’s reagent was used to assay glutathione in the supernatant collected. The activity was expressed as µg of GSH consumed/min/g tissue [29].
To assess the antitumor effect of TFGS extract on p53 expression, whole liver tissue homogenates were prepared and analyzed by western blot. Liver homogenate was resolved on 10% SDS-PAGE and transferred to nitrocellulose membrane at 15–20 mA, 4°C for overnight. The membrane was blocked with 2% bovine serum albumin (BSA) in TBST for 1 h at room temperature. Blots were incubated overnight at 4°C on a rocker with primary antibodies, namely, anti-p53 and anti-actin at 1:1000 dilutions in TBST. The blots were then washed with TBST and incubated at room temperature for 1 h in anti-rabbit HRP conjugated secondary antibody (1:10,000 dilution). The blot was then washed with TBST and developed using luminol and p-coumaric acid as chemiluminescent agents as per manufactures’ instructions.
3.1 In vitro Antioxidant Activity
Methanolic and aqueous extracts were evaluated for their radical quenching efficacy against commercially available radicals such as DPPH, NO and ABTS. The experiment was set thrice, independently for all the in vitro antioxidant assays. Results were compiled and standard deviation was accordingly calculated.
Methanolic and aqueous extracts of Trigonella along with standard Ascorbic acid exhibited their hydrogen donating capability by reducing stable DPPH radical in a concentration dependent manner. Pairing of the spare unpaired electron of DPPH with hydrogen ion of the extracts resulted in decolourisation of its deep violet color and the reaction was monitored at 517 nm. At 1 mg/ml concentration, methanolic extract showed about 90% inhibition whereas aqueous extract showed ~60% (Fig. 1a). Henceforth, it could be concluded that methanolic extract of Trigonella seeds had more potential to scavenge free radicals.
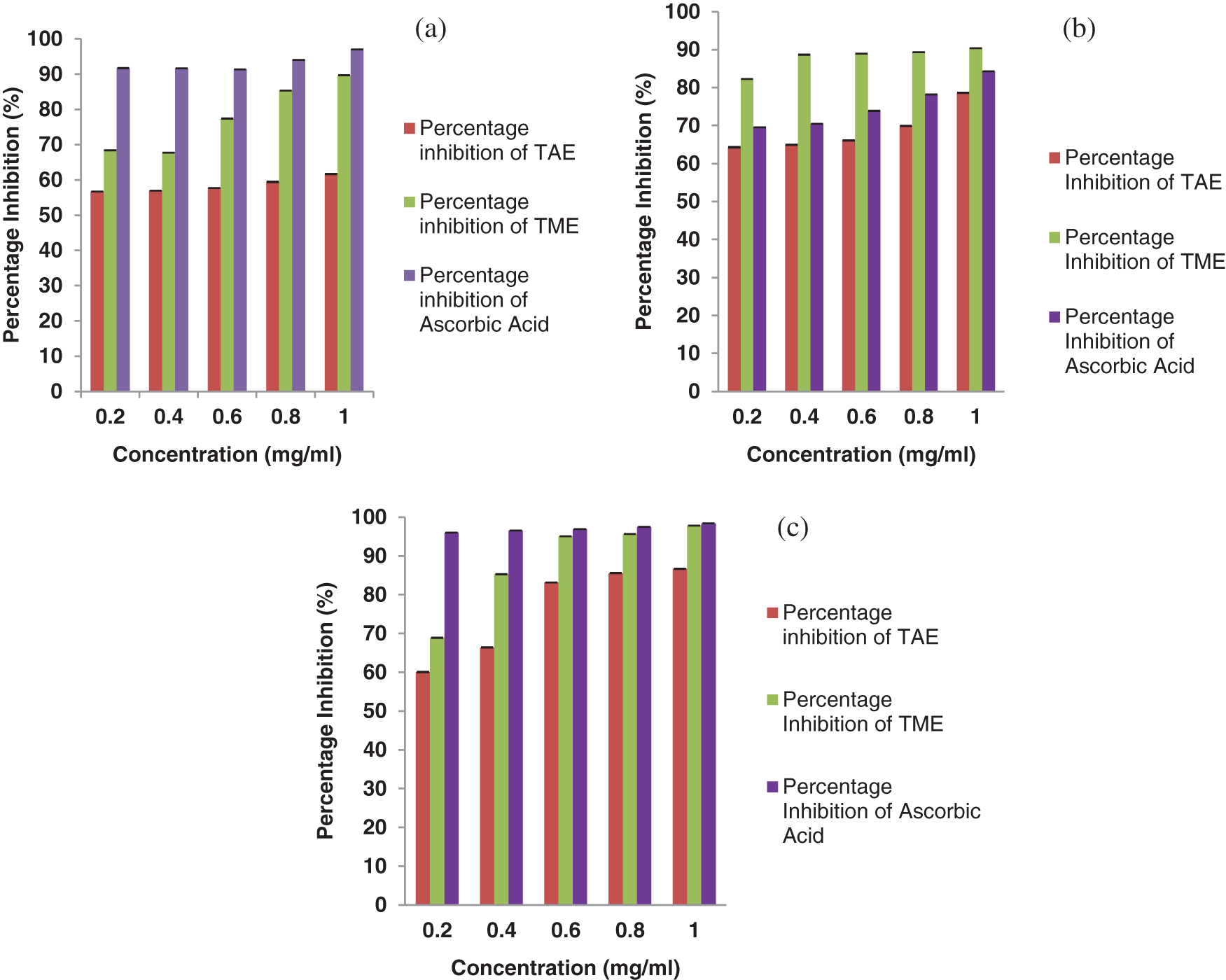
Figure 1: DPPH radical scavenging activity (a), NO radical scavenging activity (b) and ABTS radical scavenging activity (c) of TFGS extracts (TAE: Trigonella aqueous extract, TME: Trigonella methanolic extract) compared with ascorbic acid as standard antioxidant in terms of free radical inhibition percentage. All assays were done in triplicates and results were expressed as mean ± SD
Further, antioxidant potential of Trigonella seed extract was confirmed by nitric oxide scavenging assay. Trigonella extract resulted in a dose dependent inhibition of formation of nitrite by competing with oxygen to react with nitric oxide, generated from sodium nitroprusside. It was found that methanolic extract exhibited about 90% antioxidant activity as illustrated in Fig. 1b. Several studies have had shown correlation of nitric oxide with diseases like cancer, inflammation, multiple sclerosis, etc. [30]. Nitric oxide when reacts with super oxide radical forms peroxynitrite anion (ONOO-) which renders harmful effects to the tissues and cells by causing oxidative damage [31]. In our study Trigonella seed extract was able to remarkably scavenge nitric oxide radical, showing more than 90% inhibition at 1 mg/ml concentration.
As illustrated in Fig. 1c, both the extracts of Trigonella seeds were able to scavenge ABTS radicals. It was observed that at concentration of 1 mg/ml, inhibition by methanolic extract and standard was 97.8% and 98.4%; respectively. At the same concentration inhibition by the aqueous extract was 86.7%. Even at the lower concentrations of 0.2 mg/ml, methanolic extract showed more than 50% radical scavenging activity. The quenching of ABTS radical by the extracts is in contrast with the studies done by few scientists who found out that extracts had the inhibitory effect towards either DPPH or ABTS but never both.
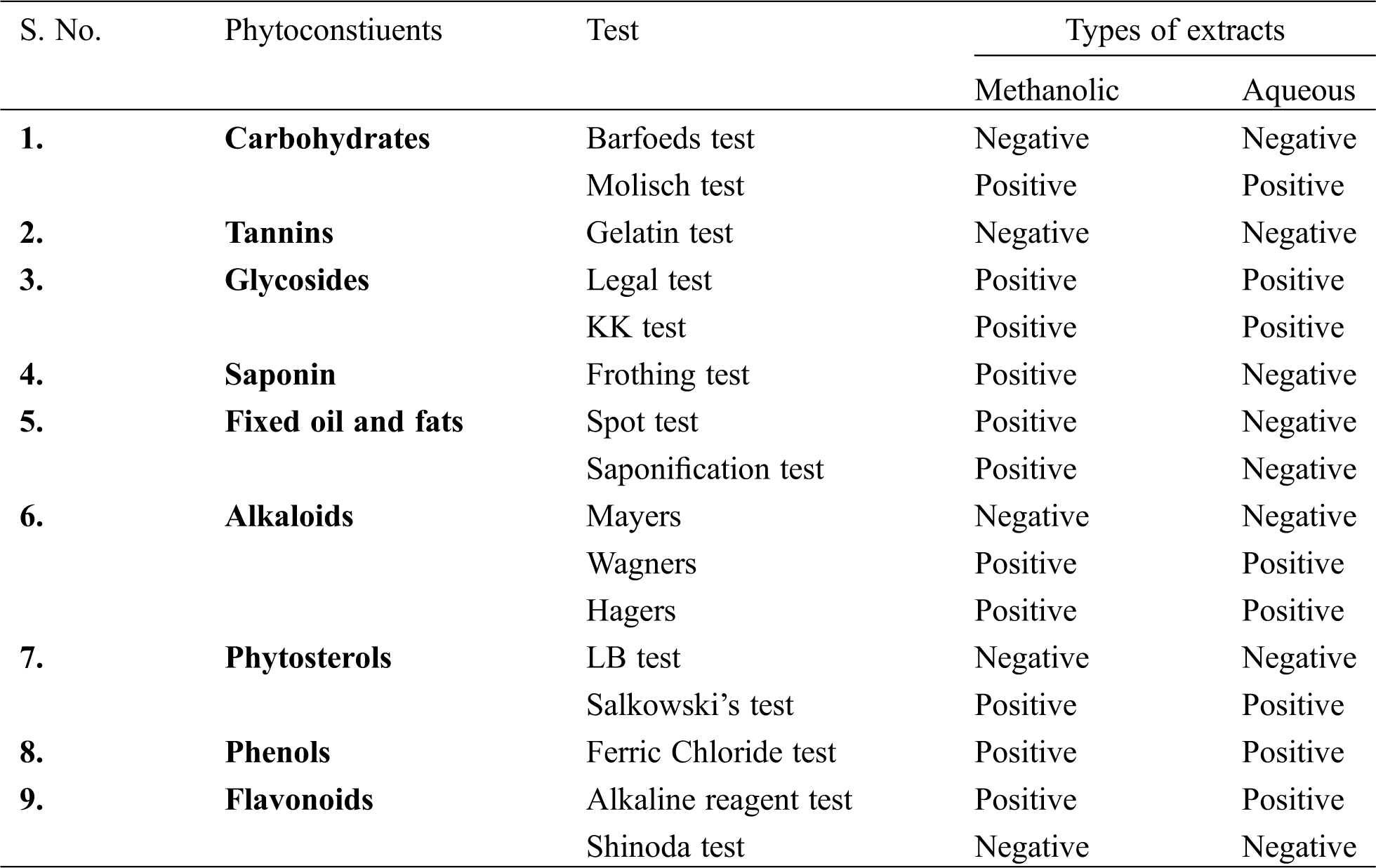
Methanolic extract showed better antioxidant potential than the aqueous extract in all the antioxidant assays performed. This could be due to presence of higher percentage of phytochemicals in the methanolic extract (Supplementary Table). Thus methanolic extract was chosen to further conduct in vitro and in vivo experiments.
Supplementary Table: Qualitative analysis of the phytoconstituents present in methanolic and aqueous TFGS extracts
To assess the toxicity of TFGS, alamar blue cytotoxicity assay was conducted on HEK 293 cells. Cells treated with seed extract were incubated in CO2 incubator at 37°C for 24 h. These were then incubated with alamar blue and change in color was monitored. Absorbance was noted at 495 nm and toxicity calculated as mentioned in methodology. It was observed that Trigonella seed extract at various concentrations (2–24 μg/μl) was not toxic to the cells though slight toxicity at a higher concentration of 40 μg/μl was noticed. Thus, it can be inferred that extract could be used over a variable range of concentrations (Fig. 2).
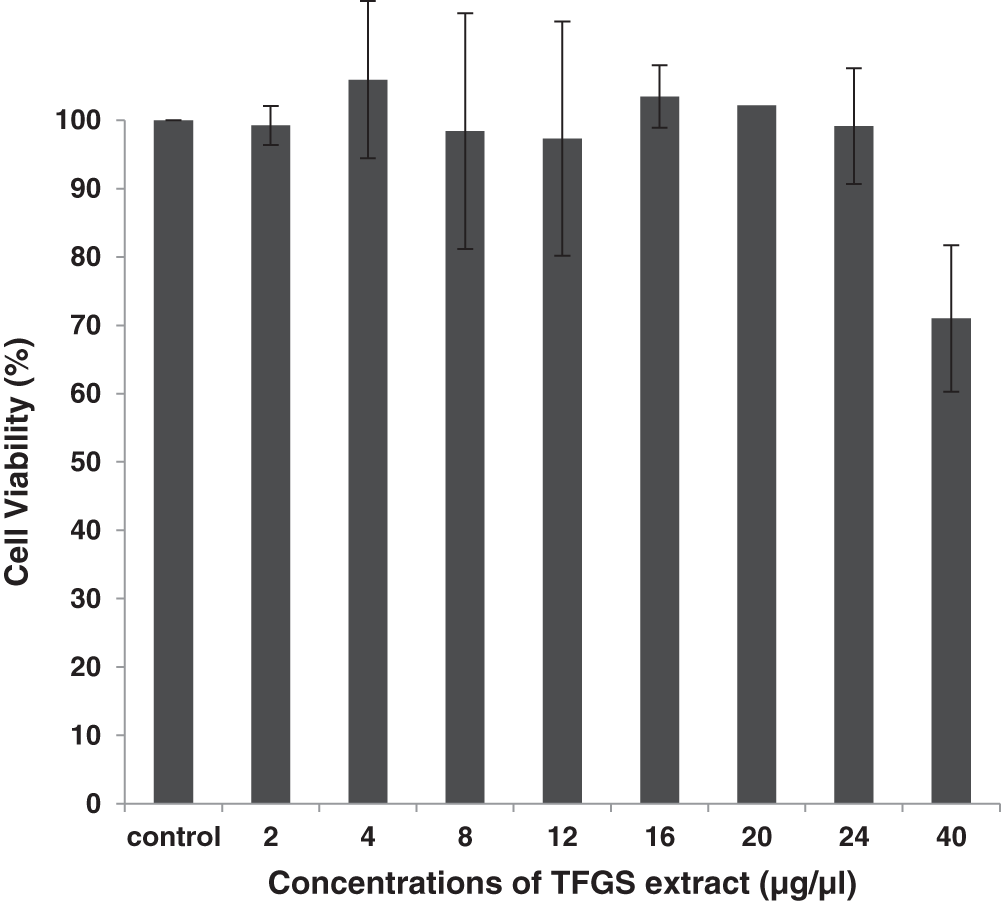
Figure 2: Percentage cell viability in HEK 293 cells against varying concentrations of TFG seeds extract (concentration range: 2–40 μg/μl). The experiment was done thrice and SD obtained
3.3 Mouse Skin Papilloma Model
As described in materials and methods, DMBA and TPA were successfully used to develop two-stage skin papilloma in Swiss albino mice. The occurrence of skin tumors were observed in experimental mice, Groups III-X. As predicted, no tumors were observed in control mice (Groups I & II) which were fed with normal diet and treated with acetone or water, respectively. As shown in Tab. 2, appearance of tumors on the skin was in the form of outward projections originated from the epithelial tissues. Parameters like total number of tumors and tumor size were studied in all the groups.
Table 2: In vivo study of tumor incidences, tumor burden, and average latent period recorded for various experimental groups of mice
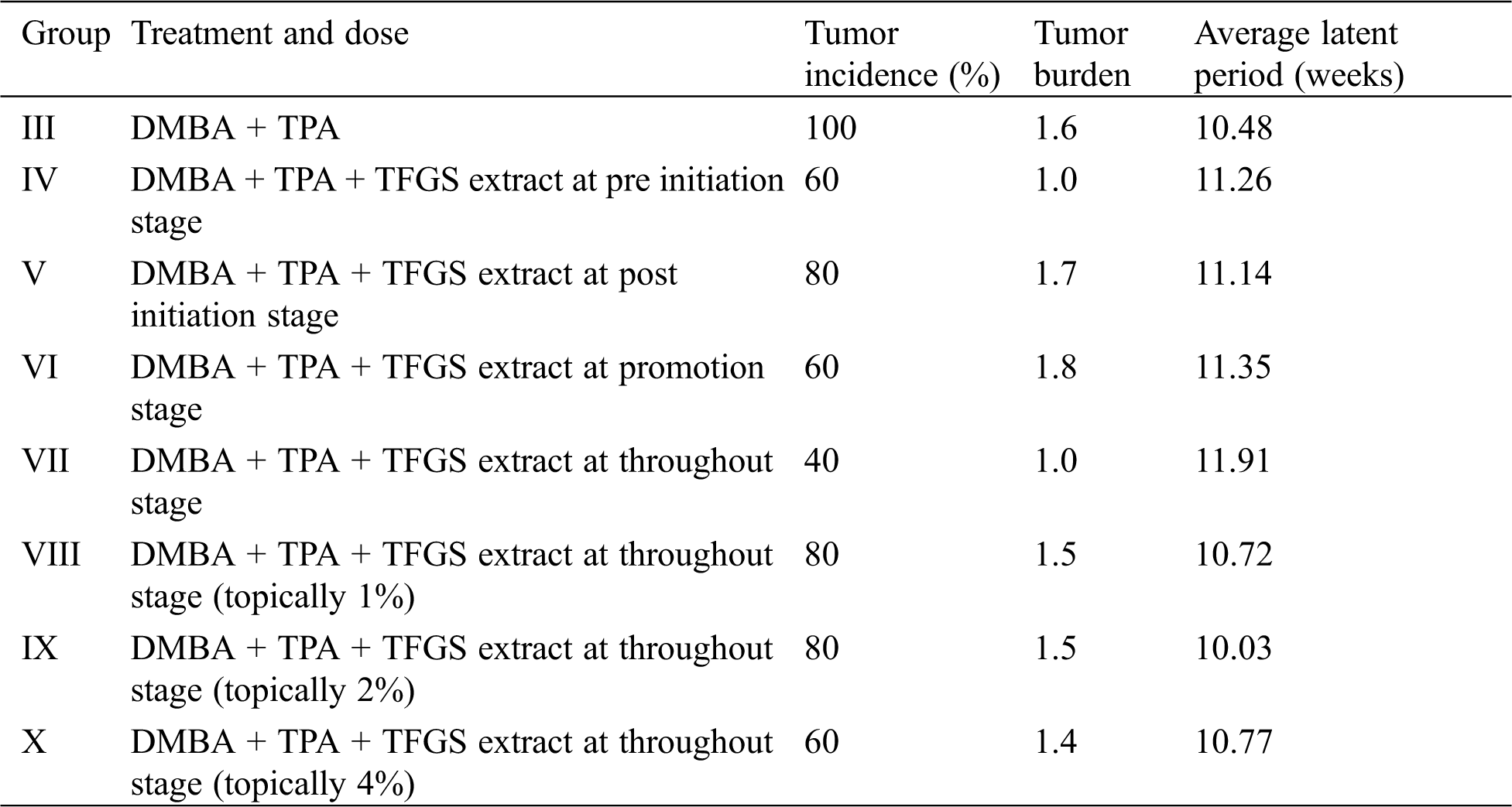
As shown in Tab. 2, a significant reduction in total number of tumors for Group VII was observed as compared to Group III. Also tumors appeared in the 8th week in Group VII mice in contrast to other groups in which tumors started appearing from 4th week onward.
As a measure of antioxidant status, levels of GSH were measured in liver homogenates of different experimental mice groups. Levels of GSH for acetone and water treated groups (Groups I and II) were 37.83 and 36.89 µM/g tissue, respectively. However, for Group III which was given DMBA and TPA and no seed extract, there was a sharp decrease in GSH value to 16.67 µM/g tissue. Group VII, that was orally administered with the extract for 16 weeks showed a significant elevation in the levels of GSH (34.33 µM/g tissue). All the other groups that were orally administered showed insignificant increase in the level of GSH as compared to the control groups. An increase in GSH level was also observed for (28.4 µM/g tissue) Group IV mice that were initially treated with extract for a week and then discontinued. This observation led us to infer that Trigonella possessed antioxidant potential and its radical quenching activity was maintained to an extent, eventually responsible for elevation in GSH levels (Fig. 3a). Groups that were topically applied with the extract (Groups VIII, IX and X) also exhibited an insignificant increase in the levels of GSH (Fig. 3b).
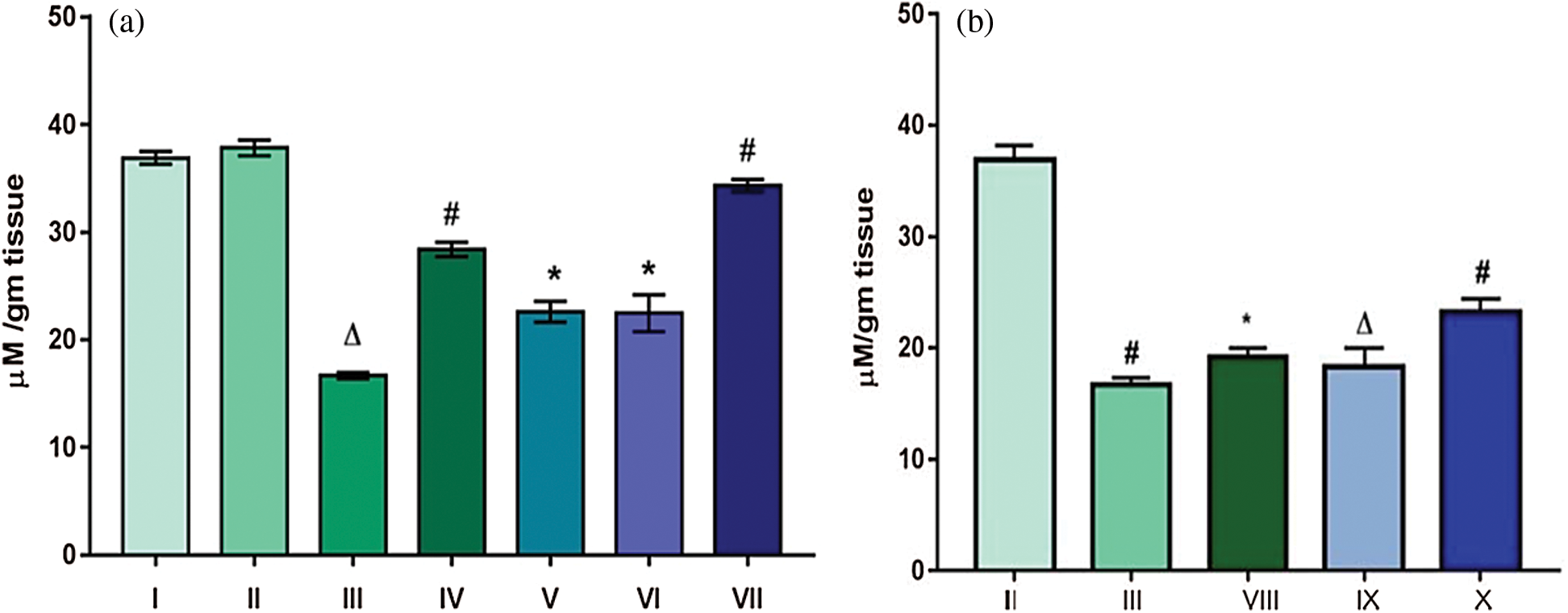
Figure 3: Effect of TFG seeds extract on expression of GSH in liver tissues of mice (a) Groups given the dose orally; (b) Groups given tropical application. Significant difference with p ∆ and # < 0.0001, moderately significant difference: p* falls between 0.01–0.001
Therefore it can be inferred that TFGS extract exhibited its reducing potential as indicated by an increase in GSH. Increased GSH level is an indicator of reduced state of cell. Furthermore it also conjugates with the epoxides (intermediate produced during DMBA metabolism) and renders them inactive. Thus the antioxidant effect by the extract, i.e., restoration of the enzymatic level can account for possibilities such as inhibition of metabolic activation of the carcinogen to its active form.
Activity of SOD was found to be decreased by three folds in Group III (12.37 U/g tissue) that was treated with DMBA and TPA as compared to the control Groups, i.e., Group I (36.51 U/g tissue) and Group II (36.15 U/g tissue). SOD activity was restored in Group VII (33.93 U/g tissue) that were fed with extracts throughout the experimental regime. In DMBA-TPA treated Group III, generation of surplus free radicals might be responsible for decreased concentration of SOD as observed. For Group IV, though the extract was effective in inducing an increase in the activity of SOD measured to be 25.91 U/g tissue, yet was less than compared to Group VII (Fig. 4a). For all the other groups no significant increase in the level of SOD was observed. Similar results were observed with the groups which were subjected to topical application of the extract, i.e., no significant increase in the level of SOD was observed (Fig. 4b). Superoxide dismutase is one of the key antioxidant enzymes present in aerobic cells, and has been extensively studied by the researchers in detoxifying process. SOD was found to reduce oxygen radicals such as superoxide radical to hydrogen peroxide which is further acted upon by catalases [27]. Thus the elevated level of the enzyme in Group VII indicated that the extract possesses antioxidant potential.
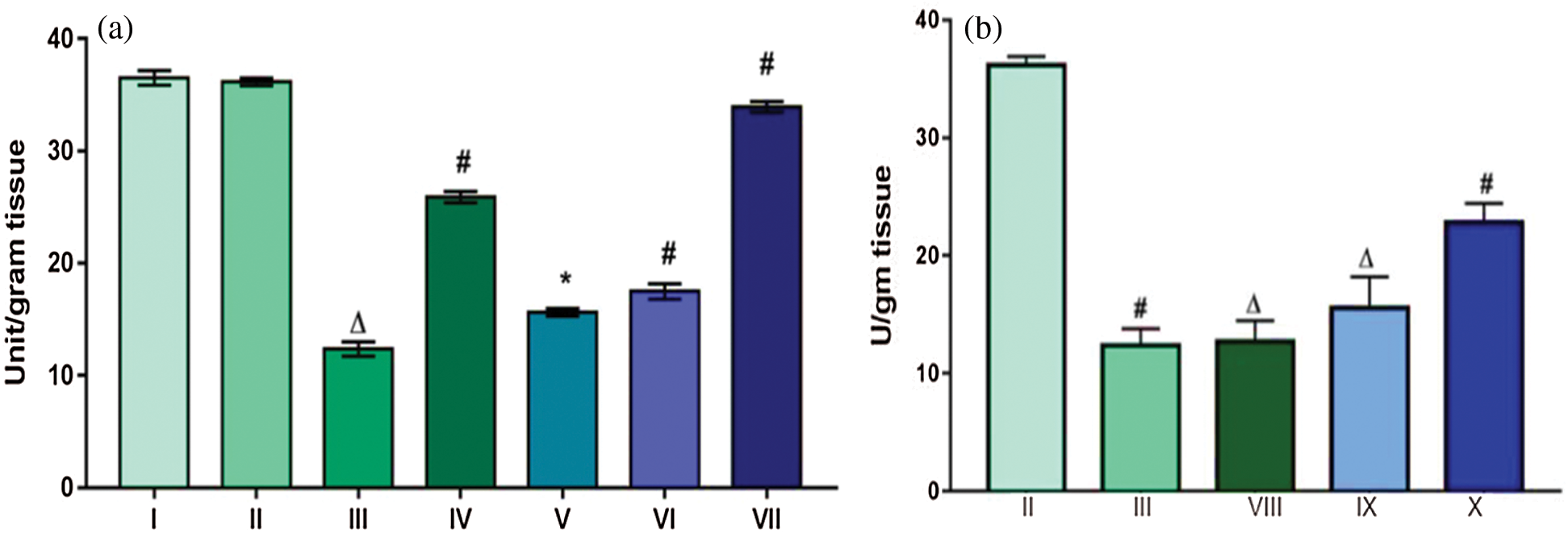
Figure 4: Effect of TFG seeds extract on SOD levels in liver tissues of mice (a) Groups given the dose orally; (b) Groups given tropical application. Significant difference with p ∆ and # < 0.0001, moderately significant difference: p* falls between 0.01–0.001
An indicator of lipid peroxidation, malondialdehyde (MDA) was found to be increased more than three folds in Group III (3.66 nmol/mg tissue) in comparison to control groups. MDA levels for control Groups I, II and Group VII, the one treated with extract throughout, were found to be almost equal 1.06, 1.08 and 1.36 nmol/mg tissue; respectively (Fig. 5a). MDA levels, an indicator of lipid oxidation, were found to be elevated in topically applied groups (Groups VIII, IX, X) as compared to control group (Fig. 5b) rendering topical application as ineffective. Thus it can be understood that the topical application of the extract was unable to alter the level of peroxidation in the cell. The increase in the value of MDA in Group III indicated oxidation of lipids of cellular membrane. When lipids are oxidized, the two by products namely malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) generated that are the indicators of lipid peroxidation [32]. Oxidation of lipids could be understood in a view of actions carried out by reactive oxygen species which in turn were produced by fat soluble compound DMBA. The free radicals thus generated have an ability to attack cell membrane and oxidize polyunsaturated fatty acids. As a result several free radicals are produced which further decompose to form secondary products like MDA and HAE [33]. The extract however, exhibited its reducing potential by decreasing LPO levels in mice treated with DMBA-TPA along with continuous dosing of extract.
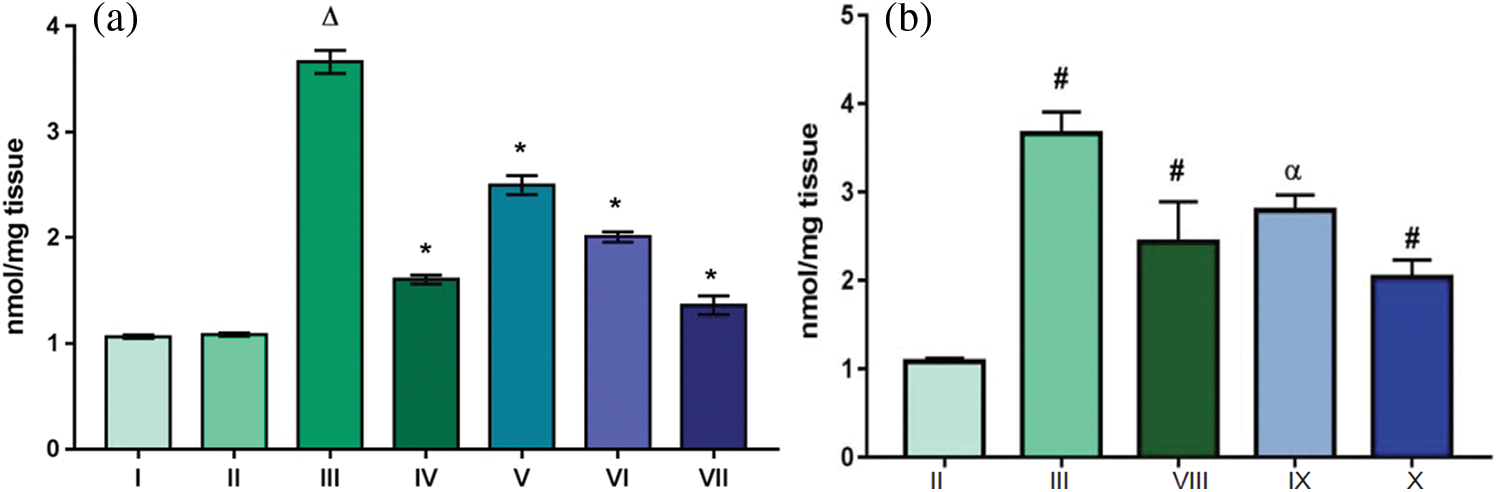
Figure 5: Effect of TFG seeds extract on LPO concentrations in liver tissues of mice (a) Groups given the dose orally; (b) Groups given tropical application. Significant difference among groups is with p ∆ and # < 0.0001, moderately significant difference: p* and α falls between 0.01–0.001
Levels of GPx in tissue homogenate for Groups I and II was found to be 33.74 and 33.57 µg GSH consumed/min/g tissue, respectively. It was observed that there was three fold decreases in the GPx level for Group III (12.61 µg GSH consumed/min/g tissue). However, extract administration in Group VII, i.e., throughout treatment helped in restoring the level of GPx to a nearly normal level (31.36 µg GSH consumed/min/g tissue (Fig. 6a). Topical application of extract for 16 weeks in the Groups VIII, IX and X, exhibited a decrease in the level of GPx upto ~19.2 ± 0.61. Level of GPx VIII, IX & X groups were found to be very less as compared to Group II. Rather the values were nearly equal to that observed in Group III (Fig. 6b). Glutathione peroxidase, one of the important antioxidant enzymes, is involved with detoxification of free radicals generated from the oxidation of lipids. Henceforth, it could be claimed that low levels of GPx in the cells, would make cells more prone to debilitated state as was the case with Groups III, VIII, IX and X. However treatment by TFGS throughout the experiment (Group VII) has significantly recuperated the antioxidant mechanism in terms of GPx concentrations.
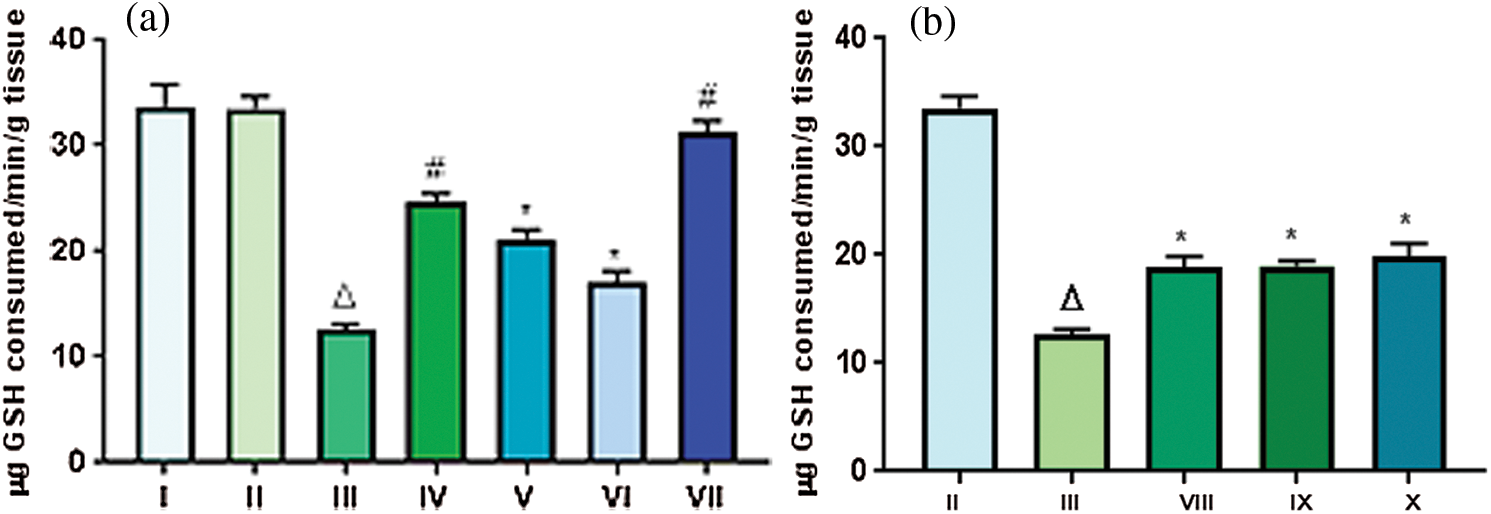
Figure 6: Effect of TFG seeds extract on expression of GPx in liver tissues of mice: (a) Mice groups that were administered extract doses orally; (b) Mice groups in which extract doses were applied tropically. Significant difference with p ∆ and # < 0.0001, moderately significant difference: p* falls between 0.01–0.001
With an aim to evaluate the possible mode of action of TFGS, expression of tumor suppressor gene, p53, was analyzed in liver tissue homogenates of Swiss albino mice treated with DMBA and TPA with further or no treatment of TFGS.
For in vivo analysis of expression of pro apoptotic gene such as p53, liver homogenates were prepared and subjected to western blot analysis. It was observed that the expression of p53 was downregulated in case of Group III that was treated with DMBA-TPA as compared to control group. p53 expression has to be downregulated or controlled in order to allow tumorigenesis to occur. However, a significant increase in the expression was observed in case of Group VII which was given extract throughout the experiment duration as compared to Group III. The expression of p53 in Group VII was also found to be higher than that of control. Significant increase in p53 expression can be correlated with activation of apoptosis of cancer cells and thus prevention of tumorigenesis. In case of Groups IV, V and VI no significant change in expression of p53 was observed as compared to control group. This study is therefore a confirmation of beneficial aspect of TFGS if taken throughout lifetime. The upregulated expression of p53 in Group VII mice in comparison with Group I (control group) and Group III (DMBA-TPA) clearly indicated the inactivation of tumor generation process that was induced by DMBA-TPA as shown in Fig. 7.
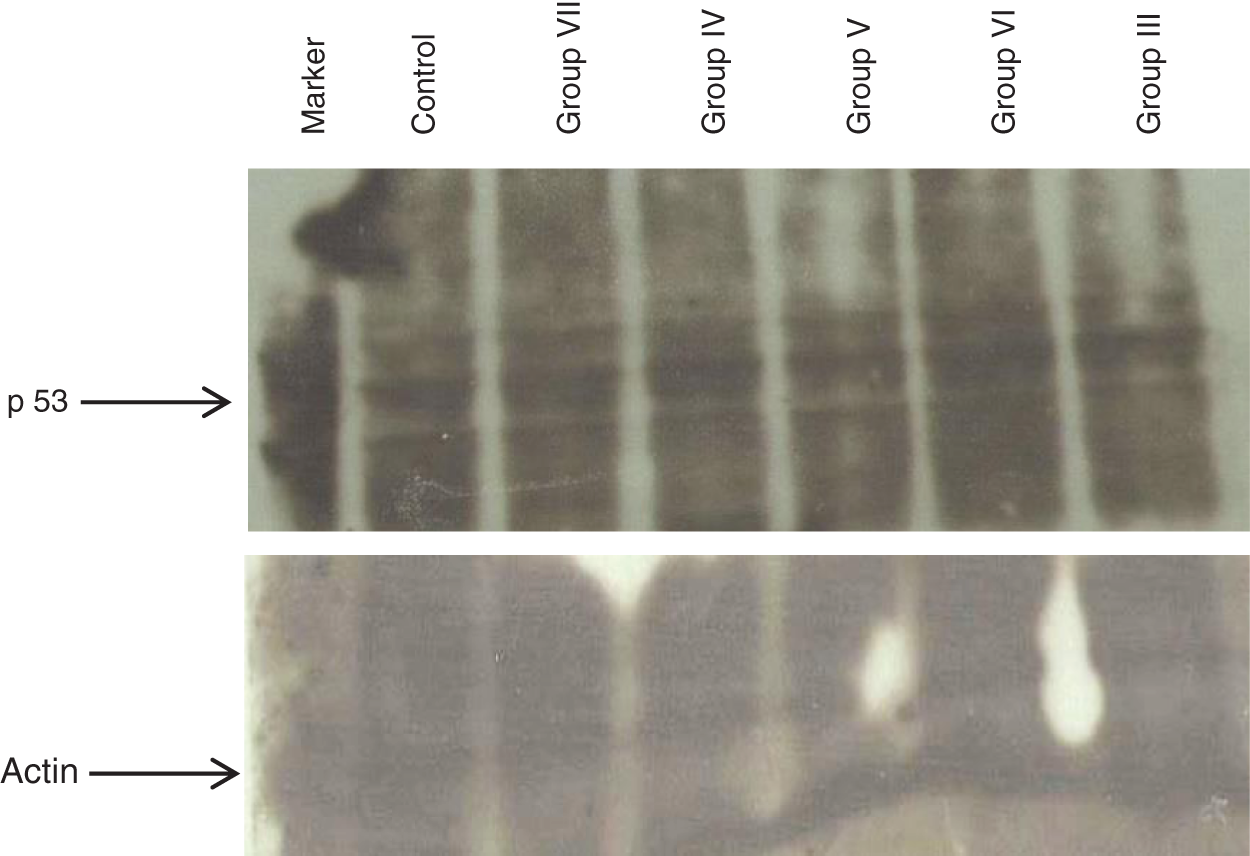
Figure 7: Western blot analysis of expression of P53 in liver tissue homogenates of various relevant groups of mice to observe the potential of TFG seed extracts as anti-tumorigenic agent
Incidences of skin carcinogenesis, has been increasing in recent years all over the world. Skin is the most common site of malignancy and represents 33% of all human cancers with tremendous impact on health and morbidity. DMBA induced mouse skin carcinogenesis is commonly used to test chemopreventive efficacy of medicinal plants and their constituents. In recent years, profound interest has been focused in the identification of non-toxic natural products that are capable of reducing the tumorigenicity of the environmental carcinogen. The present study, evaluated the antioxidant and antitumor potential of Trigonella by monitoring the change in tumor numbers and tumor size as well as by analysing the status of endogenous antioxidant enzymes such as GSH, LPO, SOD and GPx.
In vitro analysis of ROS quenching assays have shown that methanolic extract possessed more antioxidant potential compared to the aqueous extract. Presence of higher concentrations of phytochemicals in the methanolic extract of TFGS might be responsible for the better efficacy. In vitro cytotoxicity by alamar blue assay has evaluated the two extracts of Trigonella seeds to be quite safe over a varying range of concentration.
General parameters that were taken into account showed maximum number of tumors in the Group III which was applied only DMBA-TPA. While there was a 60% reduction in the Group VII which was administered extract throughout experimental period. Moreover a delay in the onset of tumors was also observed with Group VII with onset of tumors started from 7th week, as compared to Group III for which it was 4th week. However groups subjected to topical application i.e., Groups VIII, IX and X did not show any significant reduction in tumor numbers.
The endogenous antioxidant status for the groups studied, revealed reducing potential of Trigonella extract in terms of an increase in the levels of GSH, SOD and GPx with a simultaneous decrease in MDA level. This was more significantly observed with animals, administered Trigonella extract throughout the experimental duration (Group VII). In contrast, animals subjected to the carcinogens DMBA and TPA dose, showed elevation of malondialdehyde formation, a measure of lipid peroxidation. A significant decrease in the levels of GSH, SOD and GPx was observed in comparison to control groups. An important finding was also observed for the Group IV which was given extract for 7 days before DMBA application. This finding suggested antioxidant potential of TFGS extract even when given for short duration.
Topical application of the extract on the contrary resulted in insignificant increase in the level of antioxidant enzymes. Moreover, the levels of MDA formed were found to be nearly equal to that of Group III rendering topical application of extract, an inefficient method to curb generation of ROS. In the present study the inefficacy of topical extract to reduce the number of tumors could be due to the poor absorption of the drug in the skin epidermis.
The oral administration on the contrary ensures the drug to enter the bloodstream and various other organs and thus help in rendering systemic effect. Thus in the current work oral administration was found to give better results than the topical mode of administration.
The various groups studied in terms of parameters like total number of tumors, tumor size, antioxidant potential in terms of GSH, SOD, GPx and LPO levels, and expression of tumor suppressor gene; suggested Trigonella consumption as one of the possible chemopreventive strategy against ROS induced adverse effects and diseases like cancer.
The current study demonstrated antioxidant and antitumor potential of Trigonella extract against DMBA-TPA mediated skin papillomagenesis in Swiss albino mice. It was observed that methanolic extract possessed higher radical scavenging activity than the aqueous extract. This may be due to the presence of higher percentage of phytochemicals in the methanolic extract. Oral consumption of the extract had shown better efficacy than the topical application in delaying the onset of tumors and decreasing the incidences of tumor. Moreover, a detailed investigation would be required to understand the mechanism of action of chemopreventive nature of Trigonella. A descriptive study would prevail way to utilize potential of natural resources such as spices and herbs as possible therapeutic agent against a plethora of human diseases.
Acknowledgement: Authors acknowledge kind contribution of Dr. Akhil C. Banerjea, National Institute of Immunology, New Delhi, India for providing us antibodies for conduction of western blot.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. American Cancer Society (2019). Cancer facts & figures. Atlanta: American Cancer Society. [Google Scholar]
2. Fechete, O., Ungureanu, L., Şenilă, S., Vornicescu, D., Dănescu, S. et al. (2019). Risk factors for melanoma and skin health behaviour: An analysis on Romanian melanoma patients. Oncology Letters, 17(5), 4139–4144. [Google Scholar]
3. Rajendran, P., Jayakumar, T., Nishigaki, I., Ekambaram, G., Nishigaki, Y. et al. (2013). Immunomodulatory effect of Mangiferin in experimental animals with benzo (a) pyrene-induced lung carcinogenesis. International Journal of Biomedical Science, 9(2), 68–74. [Google Scholar]
4. Manoharan, S., Muneeswaran, M., Baskaran, N. (2010). Chemopreventive efficacy of berberine in 7, 12-dimethylbenz[a]anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. International Journal of Research in Pharmaceutical Sciences, 1(4), 521–529. [Google Scholar]
5. Kumari, S., Badana, A. K., Murali Mohan, G., Shailender, G., Malla, R. R. (2018). Reactive oxygen species: A key constituent in cancer survival. Biomarker Insights, 13, 1–9. DOI 10.1177/1177271918755391. [Google Scholar] [CrossRef]
6. Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., Kalayci, O. (2012). Oxidative stress and antioxidant defense. World Allergy Organization Journal, 5(1), 9–19. DOI 10.1097/WOX.0b013e3182439613. [Google Scholar] [CrossRef]
7. Shafaq, N. (2012). An overview of oxidative stress and antioxidant defensive system. Open Access Scientific Reports, 1(8), 2–9. [Google Scholar]
8. German, J. B. (1999). Food processing and lipid oxidation. Advances in experimental medicine and biology. Boston MA: Springer. [Google Scholar]
9. Moussa, Z., Judeh, Z. M. A., Ahmed, S. A. (2019). Nonenzymatic exogenous and endogenous antioxidants. Free radical medicine and biology. Rijeka: IntechOpen. [Google Scholar]
10. Reed, J. C., Pellecchia, M. (2005). Apoptosis-based therapies for hematologic malignancies. Blood, 106(2), 408–418. DOI 10.1182/blood-2004-07-2761. [Google Scholar] [CrossRef]
11. Sharma, P., Parmar, J., Verma, P., Sharma, P., Goyal, P. K. (2010). Chemopreventive effect of Phyllanthus niruri on DMBA induced skin papillomagenesis in Swiss albino mice. International Journal of Biological and Medical Research, 1(4), 158–164. [Google Scholar]
12. Chaudhary, G. (2011). Inhibition of dimethylebenz (a) anthracene (DMBA)/croton oil induced skin tumorigenesis in Swiss albino mice by Aloe vera treatment. International Journal of Biological and Medical Research, 2(3), 671–678. [Google Scholar]
13. Hęś, M., Dziedzic, K., Górecka, D., Jędrusek-Golińska, A., Gujska, E. (2019). Aloe vera (L.) Webb.: Natural sources of antioxidants–A review. Plant Foods for Human Nutrition, 74(3), 255–265. DOI 10.1007/s11130-019-00747-5. [Google Scholar] [CrossRef]
14. Chinchali, J. F., Sanakal, R. D., Kaliwal, B. B. (2011). Evaluation of anticarcinogenic activity of Clerodendrum serratum leaf extract on liver and kidney of 7, 12-dimethylbenz[a]anthracene (DMBA) induced skin carcinogenesis in mice. European Journal of Experimental Biology, 1(4), 130–141. [Google Scholar]
15. Londonkar, R. L., Kamble, A. (2011). Hepatotoxic and in vivo antioxidant potential of Pandanus odoratissimus against carbon tetrachloride induced liver injury in rats. Oriental Pharmacy and Experimental Medicine, 11(4), 229–234. DOI 10.1007/s13596-011-0033-3. [Google Scholar] [CrossRef]
16. Salar, R. K., Purewal, S. S. (2017). Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. Journal of Food Measurement and Characterization, 11(1), 126–133. DOI 10.1007/s11694-016-9379-z. [Google Scholar] [CrossRef]
17. Goyal, S., Gupta, N., Chatterjee, S. (2016). Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. Journal of Toxicology, 2016, 10. [Google Scholar]
18. Zhou, J., Chan, L., Zhou, S. (2012). Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Current Medicinal Chemistry, 19(21), 3523–3531. DOI 10.2174/092986712801323171. [Google Scholar] [CrossRef]
19. Javed, S., Shahid, A., Muhammad, S., Umeera, A., Ahmad, R. et al. (2012). Nutritional, phytochemical potential and pharmacological evaluation of Nigella sativa (Kalonji) and Trachyspermum ammi (Ajwain). Journal of Medicinal Plants Research, 6(5), 768–775. [Google Scholar]
20. Oyedemi, S. O., Bradley, G., Afolayan, A. J. (2010). In vitro and in vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. African Journal of Pharmacy and Pharmacology, 4(2), 70–78. [Google Scholar]
21. Liyana-Pathirana, C. M., Shahidi, F. (2005). Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. Journal of Agricultural and Food Chemistry, 53(7), 2433–2440. DOI 10.1021/jf049320i. [Google Scholar] [CrossRef]
22. Garratt, D. C. (1964). The quantitative analysis of drugs. USA: Springer. [Google Scholar]
23. Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M. et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237. DOI 10.1016/S0891-5849(98)00315-3. [Google Scholar] [CrossRef]
24. Qiblawi, S., Kumar, A. (1999). Chemopreventive action by an extract from Brassica compestris (var Sarason) on 7, 12-dimethylbenz(a)anthracene induced skin papillomagenesis in mice. Phytotherapy Research, 13(3), 261–263. [Google Scholar]
25. Chatterjee, S., Kumar, M., Kumar, A. (2012). Chemomodulatory effect of Trigonella foenum graecum (L.) seed extract on two stage mouse skin carcinogenesis. Toxicology International, 19(3), 287–294. DOI 10.4103/0971-6580.103670. [Google Scholar] [CrossRef]
26. Moron, M. S., Depierre, J. W., Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta (BBA)-General Subjects, 582(1), 67–78. DOI 10.1016/0304-4165(79)90289-7. [Google Scholar] [CrossRef]
27. Kono, Y. (1978). Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Archives of Biochemistry and Biophysics, 186(1), 189–195. DOI 10.1016/0003-9861(78)90479-4. [Google Scholar] [CrossRef]
28. Ohkawa, H., Ohishi, N., Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 351–358. DOI 10.1016/0003-2697(79)90738-3. [Google Scholar] [CrossRef]
29. Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G. et al. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179(4073), 588–590. DOI 10.1126/science.179.4073.588. [Google Scholar] [CrossRef]
30. Taylor, B. S., Kim, Y. M., Wang, Q., Shapiro, R. A., Billiar, T. R. et al. (1997). Nitric oxide down-regulates hepatocyte–inducible nitric oxide synthase gene expression. Archives of Surgery, 132(11), 1177–1183. DOI 10.1001/archsurg.1997.01430350027005. [Google Scholar] [CrossRef]
31. Ischiropoulos, H., al-Mehdi, A. B., Fisher, A. B. (1995). Reactive species in ischemic rat lung injury: Contribution of peroxynitrite. American Journal of Physiology, 269(2 Pt 1), L158–L164. [Google Scholar]
32. Esterbauer, H., Schaur, R. J., Zollner, H. (1991). Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine, 11(1), 81–128. DOI 10.1016/0891-5849(91)90192-6. [Google Scholar] [CrossRef]
33. Barrera, G. (2012). Oxidative stress and lipid peroxidation products in cancer progression and therapy. Oncology, 2012(10), 137289. DOI 10.5402/2012/137289. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |