
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.016277
ARTICLE
Prolonged Survival in Patients with Human Epidermal Growth Factor Receptor-2-Overexpressed Metastatic Breast Cancer after Targeted Therapy is Dominantly Contributed by Luminal-Human Epidermal Growth Factor Receptor-2 Population
1Department of Thoracic, Breast and Endocrine Surgery, Kagawa University Faculty of Medicine, 1750-1 Miki-cho, Kita-gun, 761-0793, Japan
2Department of Surgery, Takamatsu Red Cross Hospital, 4-1-3 Ban-cho, Takamatsu, 760-0017, Japan
3Kagawa Health Service Association, Health Care Center, 148 Fuseishi, Takamatsu, 761-8071, Japan
4Department of Radiology, Osaka Neurosurgery Hospital, 378-1 Sanmyo-cho, Takamatsu, 761-8083, Japan
5Department of Surgery, Date Hospital, 588-8 Kanko-cho, Takamatsu, 760-0076, Japan
6Department of Surgery, Shiga University of Medical Science, 1-1 Seta, Otsu, 520-2191, Japan
*Corresponding Author: Keiichi Kontani. Email: konbat@med.kagawa-u.ac.jp
Received: 22 February 2021; Accepted: 14 May 2021
Abstract: The prognosis of patients with human epidermal growth factor receptor-2 (HER2)-overexpressed metastatic breast cancer (MBC) has improved drastically following the development of anti-HER2 therapies. We question what factors are involved in the improved outcome by the treatment. One hundred and two MBC patients who received chemotherapy were classified into groups according to breast cancer subtype: luminal/HER2-negative (n = 50), HER2 (n = 26), and triple-negative subtypes (n = 26). Clinicopathologic features and clinical outcomes of the groups were compared. Disease-free intervals in the triple-negative group were significantly shorter than those in the other two groups. Age, tumor grade, the number of disease sites, and prior chemotherapeutic regimens did not differ among the groups. As a result, median overall survival was significantly longer in the HER2 and luminal/HER2-negative groups than in the triple-negative group (114, 68, and 18 months, respectively, p < 0.001). To determine key factors underlying favorable outcomes of the HER2 group, the HER2 group was further divided into two subgroups, luminal-HER2 and non-luminal-HER2 groups, according to their hormone receptor status, and clinical outcomes were compared. Median overall survival from the time of diagnosis of MBC in the luminal-HER2 group was significantly longer than that in the non-luminal-HER2 group (not reached and 39 months, respectively, p = 0.048), and was as favorable as shown in the luminal/HER2-negative group. In conclusion, the prognosis and survival of patients with HER2-overexpression receiving anti-HER2 therapy improved considerably in the luminal-HER2, but not in the non-luminal-HER2 group.
Keywords: Metastatic breast cancer; anti-HER2 therapy; prognosis; luminal-HER2 type
The clinical and biological heterogeneity of breast cancer has necessitated classification into three subtypes based on biomarker expression of hormone receptor and human epidermal growth factor receptor-2 (HER2): luminal/HER2-negative, HER2, and triple-negative breast cancer. Compared with the luminal/HER2-negative subtype, the HER2 and triple-negative subtypes exhibit aggressive behaviors and poor prognoses [1,2]. However, in a recent paradigmatic shift, differences in prognosis for the different subtypes have become more distinct because of novel, improved treatments [3]. In particular, developments of anti-HER2 therapies have drastically improved survival in patients with HER2-overexpressed breast cancer in the metastatic and adjuvant settings [4–7].
Combined administration of trastuzumab and anti-cancer cytotoxic agents has shown good therapeutic efficacy in HER2-overexpressed primary breast cancer and metastatic breast cancer (MBC) [8]. As previously reported, the mortality rate in patients with HER2-overexpression after operation has decreased to 35%–50% in adjuvant settings, and survival of patients with HER2-overexpressed MBC has doubled following the introduction of trastuzumab in combination with cytotoxic agents [5]. Since it became available, trastuzumab has become part of the standard therapeutic regimen for HER2-overexpressed breast cancer. Subsequently, the tyrosine kinase inhibitor lapatinib has become available more recently: it has demonstrated good efficacy to improve survival in patients with HER2-overexpressed MBC [9]. More recently, the humanized monoclonal antibody pertuzumab directed against the HER2 extracellular domain [10,11], and the antibody-drug conjugate trastuzumab emtansine (TDM-1), which incorporates trastuzumab with the microtubule-inhibitory agent emtansine [12], have demonstrated favorable therapeutic efficacies. In the Phase 3 trials, both therapeutics prolonged the survival of patients with HER2-overexpressed MBC relative to conventional anti-HER2 therapeutics. Thus, physicians have a variety of treatment options in anti-HER2 therapeutics at their disposal for specific patient subpopulations.
Here, we compared clinicopathological features and therapeutic outcomes of patients with HER2-overexpressed MBC, with those of patients with other (i.e., luminal type and triple-negative) breast cancer subtypes.
Data from 102 patients with recurrent or MBC who were treated with various chemotherapeutic regimens at Kagawa University Hospital between February 2005 and October 2017, were retrospectively analyzed. Patients with MBC who had not received chemotherapy were not included in this study. The patients were allocated to one of three groups according to their hormone receptor and HER2 status: luminal/HER2-negative (n = 50), HER2 (n = 26), and triple-negative subtypes (n = 26). Clinicopathological features and treatment outcomes were compared between subtypes. All patients with HER2-overexpressed MBC received anti-HER2-targeted agents such as trastuzumab, lapatinib, pertuzumab, or TDM-1 in the metastatic settings.
The research protocol for this study complied with the guidelines of the Ethics Committee at Kagawa University Hospital and was approved by the review board (HEISEI23-085), and conformed to the provisions in the Declaration of Helsinki in 1995. We received written informed consent from all of the study participants.
2.2 Evaluation of Therapeutic Efficacy and Safety
During treatment, tumor responses were assessed every 2 to 3 months by physical examination and computed tomography, magnetic resonance imaging, or bone scan according to the Response Evaluation Criteria in Solid Tumors. Complete response (CR) was defined as the absence of evidence of disease; partial response (PR) was defined as a reduction of ≥50% in the product of the two largest perpendicular diameters of the target lesions; progressive disease (PD) was defined as an increase in tumor size by ≥25% or presence of a new lesion. Clinical responses that did not meet any of the abovementioned definitions were classified as stable disease (SD). CR and PR are classified as objective responses (OR); CR, PR, and SD are defined as disease control (DC); and CR, PR, and SD observed for a duration of ≥6 months are defined as clinical benefit (CB). Responses were evaluated to the most effective therapeutic regimen if patients received two or more regimens for MBC. Several clinical outcome parameters examined: time to treatment failure (TTF) was defined as the duration from initiation to treatment discontinuation; time to progression (TTP) was defined as the duration between initiation of treatment and disease progression or death by any cause; overall survival (OS) was defined as the duration between initiation of treatment and death by any cause. For patients who had received two or more cytotoxic regimens for MBC, time to events was calculated from the start of the most effective regimen. Additionally, OS was calculated from the time of diagnosis of MBC to death by any cause, designated as OS from MBC. Toxicity was assessed according to version 3.0 of the National Cancer Institute Common Toxicity Criteria.
We used Mann-Whitney U test or standard chi-square procedures for comparisons of two groups. The effects of baseline characteristics, clinical responses, and prognostic parameters on the risk of progression or death were assessed using Kaplan-Meier survival analysis and log-rank tests of significance. A 95% confidence interval for the median of each variable was calculated using the method of Brookmeyer et al. [13]. Differences with P < 0.05 were considered as to be significant; all performed statistical tests were two-sided. SPSS statistical software (SPSS Inc., Tokyo, Japan) was used for all analyses.
3.1 Baseline Characteristics of Patients
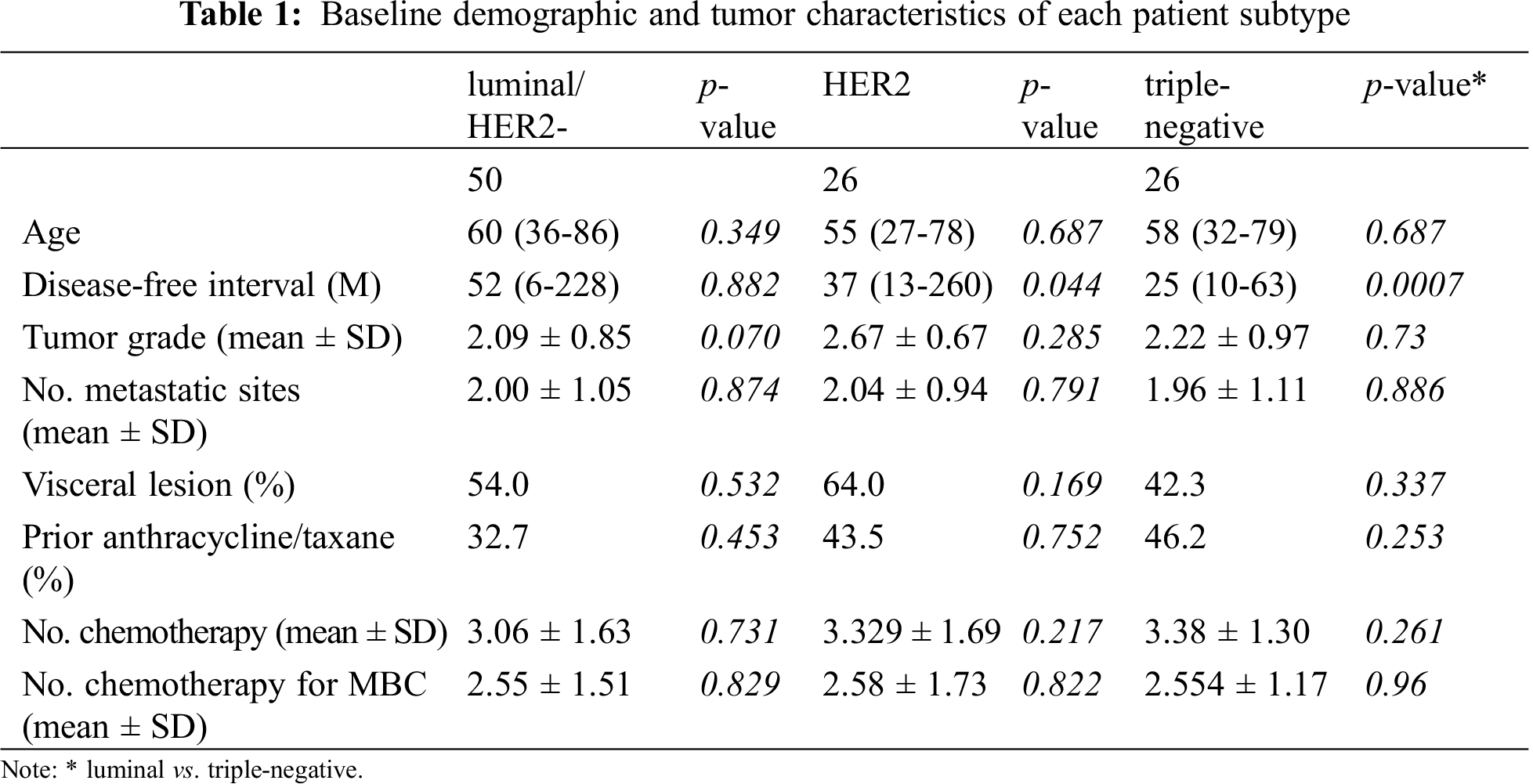
Patients were allocated to three subtype groups according to the hormone receptor and HER2 status of their tumors: luminal/HER2-negative, HER2, and triple-negative subtype groups. Baseline characteristics of patients of each group were established (Tab. 1). Disease-free intervals of patients were significantly shorter in patients of the triple-negative subtype group (25 months) than those of patients in the luminal/HER2-negative (52 months, p = 0.0007) or HER2 groups (37 months, p = 0.044). Other factors such as tumor grade, numbers of metastatic sites or administered chemotherapeutic regimens, and the proportion of patients with visceral diseases were not different among the groups.
3.2 Comparison of the Efficacy of Treatment and Clinical Outcomes among Cancer Subtypes
Response rates to treatment in the HER2 group were significantly higher than those in the triple negative group (76% and 26.9%, respectively, p = 0.0017; Tab. 2) but not significantly different from those in the luminal/HER2-negative group (76% and 50.0%, respectively, p = 0.077). As evident from the response rates, clinical benefit and disease control rates were significantly lower in the triple negative group than in the other two groups. The median OS from the time of the most effective administered regimen as well as TTF or TTP were significantly shorter in the triple-negative group than in the HER2 and luminal/HER2-negative groups (OS: 18, 114, and 68 months, respectively, p < 0.001; TTF: 5, 12, and 15 months, respectively, p < 0.001; TTP: 6, 25, and 35 months, respectively, p < 0.001; Tab. 2 and Fig. 1). Moreover, the median OS from MBC was significantly shorter in the triple-negative group than in the HER2 and luminal/HER2-negative groups (20, 114, and 102 months, respectively, p < 0.001; Tab. 2 and Fig. 1). The prognostic factors described above were not significantly different in the HER2 and luminal/HER2-negative groups. These results indicate that patients with HER2-overexpressed MBC, specifically those with luminal-HER2 MBC showed improved prognosis due to advances in anti-HER2 therapy. The frequency of grade 3 or 4 adverse events that occurred during treatment for MBC did not differ between subtypes.
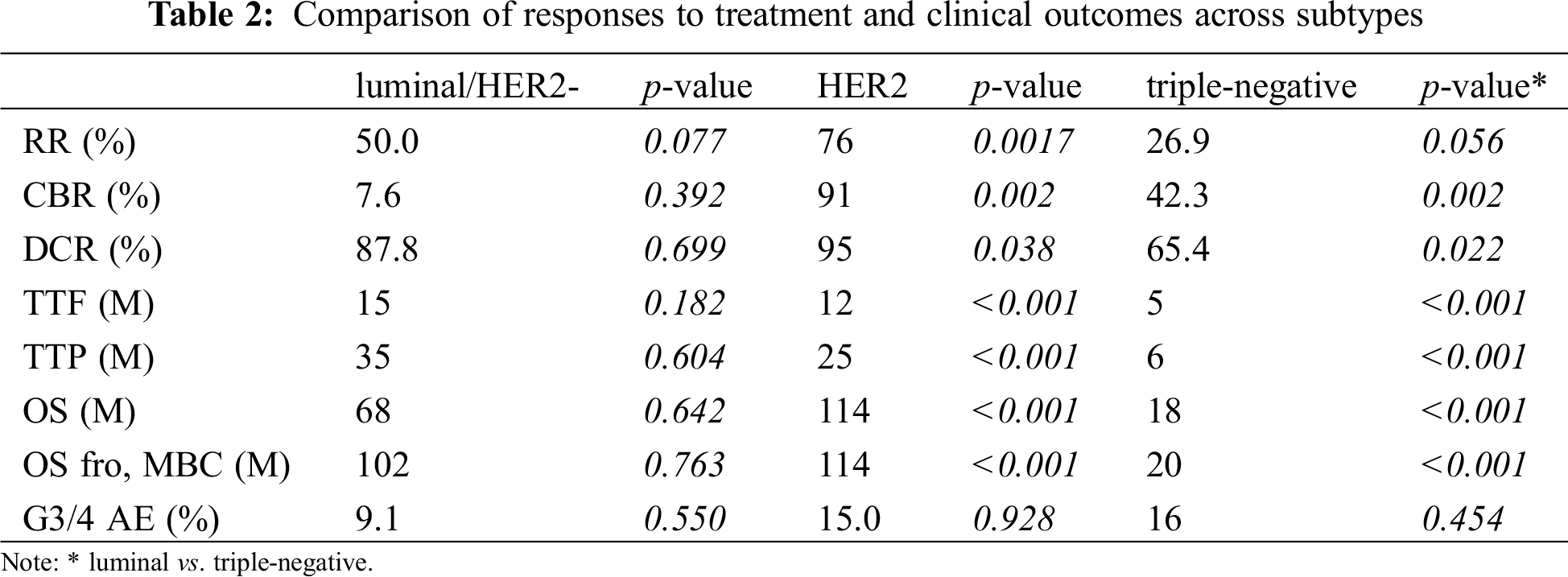
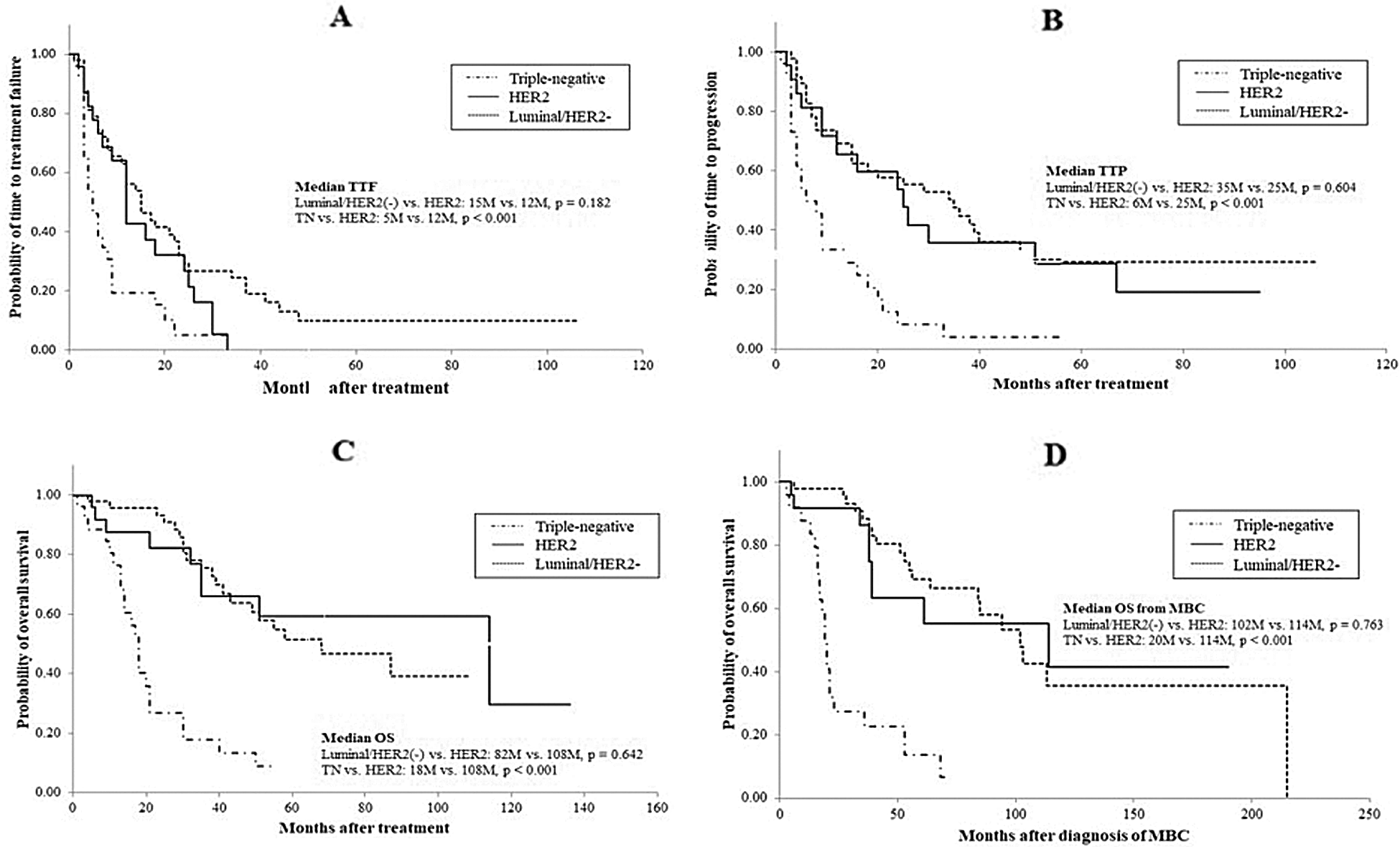
Figure 1: Comparison of clinical outcomes of patients across subtypes: A, time to treatment failure; B, time to progression; C, overall survival; D, overall survival from the time of diagnosis of MBC
3.3 Identification of Key Factors Responsible for Favorable Outcomes in the HER2 Group by Anti-HER2 Therapy
To determine if a particular subpopulation of the HER2 group was prone to favorable outcomes, the HER2 group was further stratified into two subgroups: luminal-HER2 and non-luminal-HER2 according to their hormone receptor status, and clinical outcomes were compared. Demographic and tumor characteristics were balanced across the two subgroups (Tab. 3). Although there was a trend that patients with luminal-HER2 MBC were younger than those with non-luminal-HER2 MBC, there were no significant subgroup differences. The proportion of patients who were pretreated with anthracycline and taxane or the number of chemotherapeutic interventions for breast cancer or MBC also did not differ between the two subgroups.
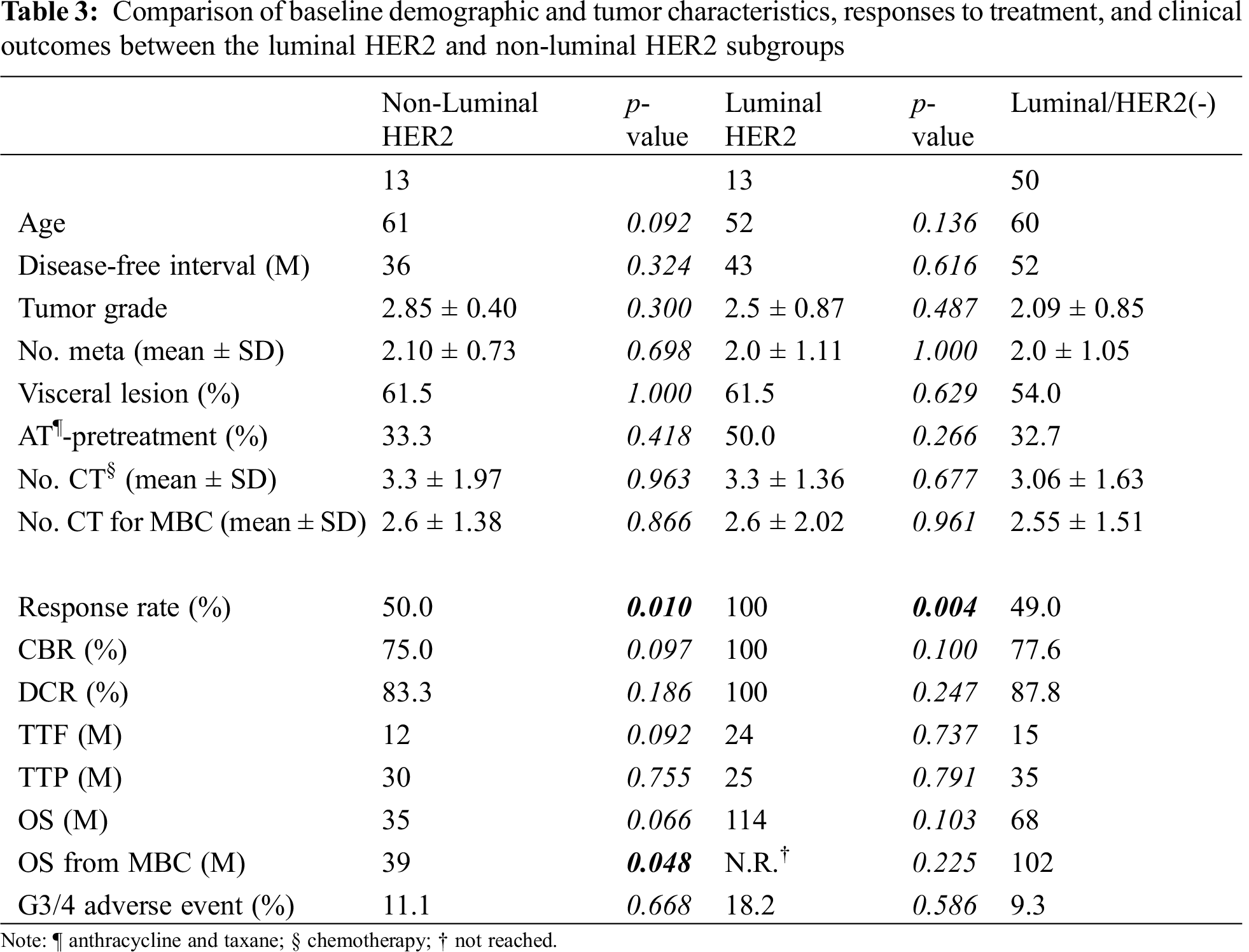
As to the efficacy of treatment in patients with HER2-overexpressed MBC, response rates in patients with luminal-HER2 MBC were significantly higher than those with non-luminal HER2 or luminal/HER2-negative MBC (luminal-HER2 vs. non-luminal-HER2, 100% vs. 50.0%, respectively, p = 0.01; luminal-HER2 vs. luminal/HER2-negative, 100% vs. 49.0%, respectively, p = 0.004, Tab. 3). There were no significant differences in response rates between the non-luminal-HER2 and luminal/HER2-negative groups. Although median TTF, TTP, or OS from the date of the most effective administered regimens did not differ between the luminal-HER2 and non-luminal-HER2 groups, median OS from the date of diagnosis of MBC was significantly longer in the luminal-HER2 group than in the non-luminal-HER2 group (not reached vs. 39 months, respectively, p = 0.048, Tab. 3 and Fig. 2). No difference was found in median OS from the date of diagnosis of MBC between the luminal-HER2 and luminal/HER2-negative group (not reached vs. 102 months, respectively, p = 0.225).
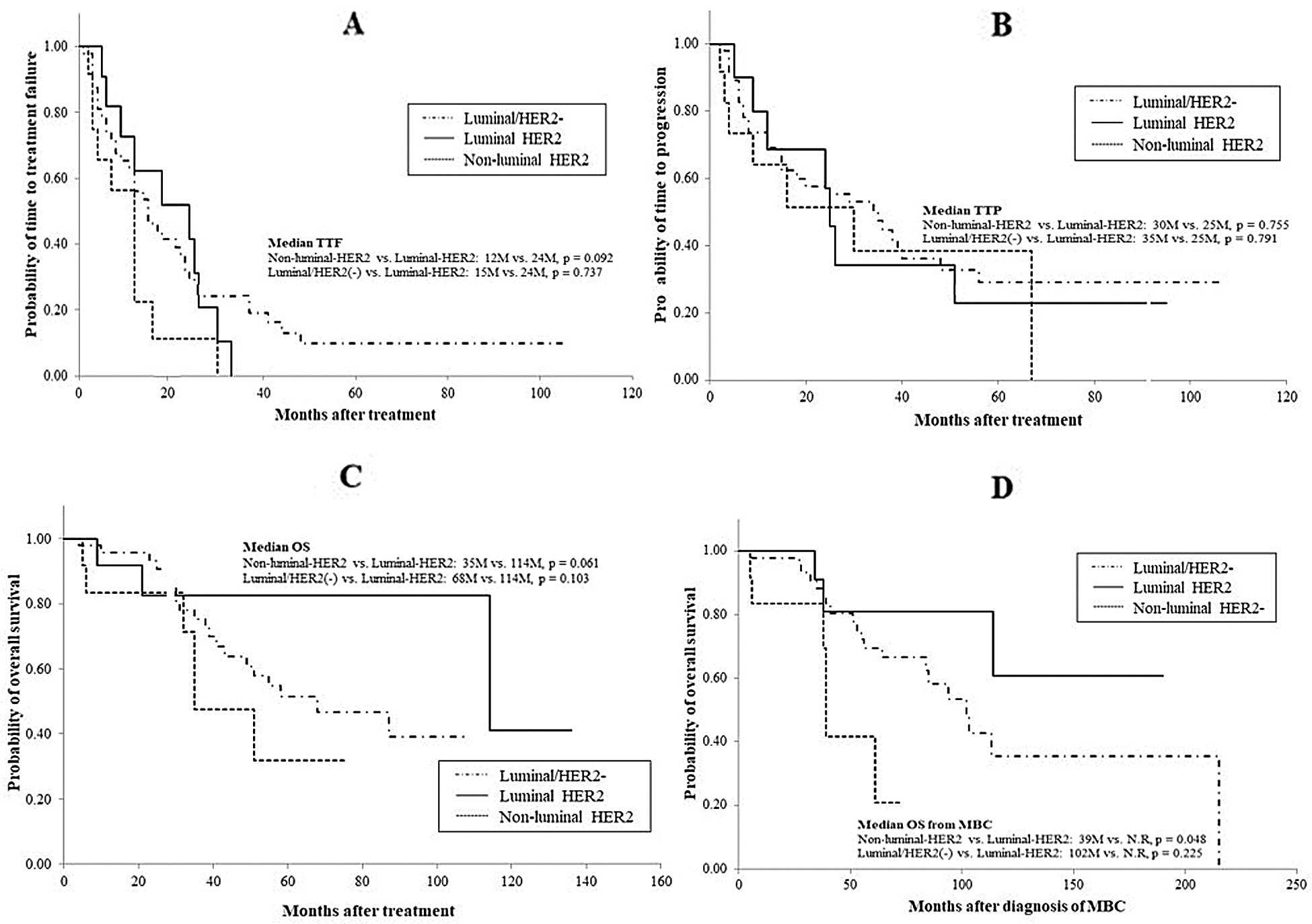
Figure 2: Comparison of clinical outcomes between patients with luminal HER2 and patients with nonluminal HER2 MBC: A, time to treatment failure; B, time to progression; C, overall survival; D, overall survival from the time of diagnosis of MBC
Of 102 patients studied, eighty-nine patients (87.3%) showed at least one grade 3 or 4 adverse events (data not shown). There was no patient who died as a result of adverse events. The most frequent adverse events were neutropenia (74 out of 89 patients, 85.4%). The frequency of grade 3 or 4 adverse events during treatment for MBC did not differ among the subtypes (Tab. 2). Also, the frequency of grade 3 or 4 adverse events did not differ between the luminal-HER2 and luminal/HER2-negative group (p = 0.668, Tab. 3).
Amplification of HER2 genes or overexpression of HER2 proteins occur in 15% to 30% of primary breast tumors and are associated with short survival or poor prognosis for patients [14–16]. However, once anti-HER2 targeted therapy such as trastuzumab, lapatinib, or pertuzumab became clinically available, prognosis of patients has considerably improved [8–12]. More recently, an antibody-drug conjugate of trastuzumab and cytotoxic agents such as trastuzumab emtansine and trastuzumab deruxtecan demonstrated substantial improvements in survival of patients with resistance to anti-HER2 therapy [17,18]. In this study, clinical outcomes of three patient groups with MBC, based on the breast cancer subtypes, were compared: luminal/HER2-negative, HER2, and triple-negative MBC. The prognosis of patients in the HER2 and in the luminal/HER2-negative group were equally favorable (Fig. 1 and Tab. 2). Several studies have documented that clinical outcomes of patients with HER2-overexpressed MBC receiving trastuzumab (i.e., anti-HER2 treatment) combined with chemotherapy greatly improved compared with those in patients treated with standard chemotherapy (i.e., without anti-HER2 treatment) [1,4,5,8]. These findings suggest that the biological aggressiveness of the HER2-overexpressed breast cancer controlled predominantly by HER2 gene products may be attenuated by the inhibition of HER2 signaling and that aggressive tumors may be turned into slower-growing ones, like the ones seen in patients with luminal/HER2-negative cancer. In the present study, the proportion of patients responding to treatment in the HER2 group (76%) was much higher than that in the luminal/HER2-negative (50.0%) and triple negative groups (26.9%) (Tab. 2). In response to anti-HER2 agents combined with cytotoxic agents, three quarters of the patients showed apparent shrinkage of tumors, regardless of tumor grades, number of disease sites, prior treatment with anthracycline/taxane, or the number of previous chemotherapeutic interventions (Tab. 1). These results support previous findings that combined regimens of standard chemotherapy and trastuzumab improved multiple clinical outcome parameters in patients with HER2-overexpressed MBC, including response rates, TTP, and OS compared with standard chemotherapy alone [19]. Reflecting the favorable responses to treatment in patients with HER2-overexpressed MBC, all therapeutic outcome parameters, i.e., TTF, TTP, and OS, of these patients were significantly prolonged compared with those of patients with triple-negative cancer and similar to those of patients with luminal/HER2-negative cancer (Fig. 1 and Tab. 2).
To identify which factors mediate favorable outcomes in the HER2 group following anti-HER2 targeted therapy, we compared clinical outcomes of the luminal-HER2 and non-luminal HER2 groups. The response rates of luminal-HER2 patients receiving anti-HER2 therapy were significantly higher than those of non-luminal HER2 patients and luminal/HER2-negative patients (Tab. 3). This indicates that all patients with luminal HER2 breast cancer experienced favorable responses at least once in metastatic settings. As a result, survival from the time of diagnosis with MBC was significantly longer in the luminal-HER2 group than in the non-luminal HER2 group, but did not differ from survival in the luminal/HER2-negative group. The data suggest that the improved prognosis of patients with HER2-overexpressed MBC, as shown in this study, mainly depends on patients with luminal-HER2 MBC showing improved survival, but not on patients with non-luminal HER2 MBC who did not show any benefit from the anti-HER2 therapy. To the best of our knowledge, only a few studies have investigated the factors responsible for the improved prognosis of HER2-overexpressed MBC by anti-HER2 targeted therapy. In these studies, patients with primary breast cancer were examined for their prognosis after adjuvant therapy comprising chemotherapeutic agents and trastuzumab [1,6,20]. Two studies demonstrated that patients with luminal-HER2 breast cancer benefited from trastuzumab-containing regimens in contrast to patients with non-luminal HER2 cancer. However, the second analysis of the Hera trial demonstrated that patients with high estrogen receptor expression/low HER2 expression did not benefit from trastuzumab treatment [20]. In metastatic settings, Bonotto et al. [3] reported that among HER2-overexpressed patients, those with hormone receptor-positive MBC showed significantly longer PFSs and OSs than those with hormone receptor-negative MBC. This lends support to our hypothesis that cancer cells expressing hormone receptors tend to behave less aggressively than cancer cells without hormone receptor expression if HER2-associated signaling cascades are inhibited by anti-HER2 therapy. Further analyses should clarify the exact molecular mechanisms underlying these therapeutic effects. This is the first report to demonstrate in detail (a) that patients with luminal-HER2 MBC receiving anti-HER2 therapy showed improvements in survival to levels seen in patients with luminal/HER2-negative MBC and (b) that patients with non-luminal HER2 MBC did not benefit from anti-HER2 therapy.
In conclusion, prospects for patients with HER2-overexpressed MBC, particularly of the luminal-HER2 subtype, have greatly improved since anti-HER2 targeted therapy became available. However, because tumors of the non-luminal HER2 cancer subtype are unresponsive to this treatment, novel approaches are required for these patients.
Acknowledgement: We thank Hiromi Kita and Miho Takigawa for editorial assistance for an earlier version of the manuscript.
Funding Statement: This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan (Nos. 10671249, 13671380, 14571262 and 15591340).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hugh, J., Hanson, J., Cheang, M. C., Nielsen, T. O., Perou, C. M. et al. (2009). Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. Journal of Clinical Oncology, 27(8), 1168–1176. [Google Scholar]
2. Liedtke, C., Mazouni, C., Hess, K. R., Andre, F., Tordai, A. et al. (2008). Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. Journal of Clinical Oncology, 26, 1275–1281. [Google Scholar]
3. Bonotto, M., Gerratana, L., Poletto, E., Driol, P., Giangreco, M. et al. (2014). Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist, 19(6), 608–615. [Google Scholar]
4. Dawood, S., Broglio, K., Buzdar, A. U., Hortobagyi, G. N., Giordano, S. H. (2010). Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. Journal of Clinical Oncology, 28(1), 92–98. [Google Scholar]
5. Sundquist, M., Brudin, L., Tejler, G. (2017). Improved survival in metastatic breast cancer 1985–2016. Breast, 31, 46–50. [Google Scholar]
6. Smith, I., Procter, M., Gelber, R. D., Guillaume, S., Feyereislova, A. et al. (2007). HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet, 369(9555), 29–36. [Google Scholar]
7. Romond, E. H., Perez, E. A., Bryant, J., Suman, V. J., Geyer, C. E., Jr. et al. (2005). Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine, 353(16), 1673–1684. [Google Scholar]
8. Slamon, D., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V. et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine, 344, 783–792. [Google Scholar]
9. Cameron, D., Casey, M., Press, M., Lindquist, D., Pienkowski, T. et al. (2008). A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Research and Treatment, 112(3), 533–543. [Google Scholar]
10. Baselga, J., Gelmon, K. A., Verma, S., Wardley, A., Conte, P. et al. (2010). Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. Journal of Clinical Oncology, 28(7), 1138–1144. [Google Scholar]
11. Swain, S. M., Kim, S. B., Cortés, J., Ro, J., Semiglazov, V. et al. (2013). Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA studyOverall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncology, 14(6), 461–471. [Google Scholar]
12. Verma, S., Miles, D., Gianni, L., Krop, I. E., Welslau, M. et al. (2012). Trastuzumab emtansine for HER2-positive advanced breast cancer. New England Journal of Medicine, 367(19), 1783–1791. [Google Scholar]
13. Brookmeyer, R., Crowley, J. (1982). A confidence interval for the median survival time. Biometrics, 38, 29–41. [Google Scholar]
14. Slamon, D. J., Clark, G. M., Wong, S. G., Levin, W. J., Ullrich, A. et al. (1987). Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science, 235, 177–182. [Google Scholar]
15. Sjgren, S., Ingans, M., Lindgren, A., Holmberg, L., Bergh, J. (1998). Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. Journal of Clinical Oncology, 16, 462–469. [Google Scholar]
16. Gabos, Z., Sinha, R., Hanson, J., Chauhan, N., Hugh, J. et al. (2006). Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. Journal of Clinical Oncology, 24, 5658–5663. [Google Scholar]
17. Von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P. et al. (2019). Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New England Journal of Medicine, 380(7), 617–628. [Google Scholar]
18. Modi, S., Saura, C., Yamashita, T., Park, Y. H., Kim, S. B. et al. (2020). Trasutuzumab deruxtecan in previously treated HER2-positive breast cancer. New England Journal of Medicine, 382(7), 610–621. [Google Scholar]
19. Roche, P. C., Ingle, J. N. (1999). Increased HER2 with U.S. Food and Drug Administration-approved antibody. Journal of Clinical Oncology, 17(1), 434. [Google Scholar]
20. Loi, S., Dafni, U., Karlis, D., Polydoropoulou, V., Young, B. M. et al. (2016). Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of Trastuzumab: A secondary analysis of the HERA trial. Journal of Americal Medical Association Oncology, 2(8), 1040–1047. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |