
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.016480
ARTICLE
A Nomogram for Predicting Lateral Lymph Node Metastasis in Cases of Papillary Thyroid Micro-Carcinoma with Suspected Lymph Node Metastasis
1Department of Thyroid and Breast Surgery, Shenzhen Second People’s Hospital, Shenzhen, 518000, China
2Department of Ultrasound, Shenzhen Second People’s Hospital, Shenzhen, 518000, China
3School of Biomedical Engineering, Health Science Center, Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, Shenzhen University, Shenzhen, 518060, China
*Corresponding Author: Weixiang Liu. Email: wxliu@szu.edu.cn
Received: 11 March 2021; Accepted: 04 May 2021
Abstract: The elevation for lateral lymph node metastasis (LLNM) plays an important role in therapeutic decision-making for thyroid carcinoma. A reliable forecasting model for LLNM in patients with papillary thyroid micro-carcinoma (PTMC) is needed, using clinicopathological characteristics. A total of 576 PTMC patients with suspicious lateral cervical lymph node (II, III, IV or V region) metastasis and known clinicopathological variables were randomly collected at Shenzhen Second People’s Hospital. Cervical lymph node status of every patient was assessed by ultrasonography (US). The patients in this cohort study underwent thyroidectomy and lateral neck lymph node dissection. Univariate analysis and logistic regression analysis were performed to screen out the predictive variables associated with LLNM, and a nomogram was constructed by integrating clinicopathological features collected in our study. The overall LLNM rate was 23.0% (133/576). After statistical analysis, central lymph node metastasis (CLNM), prelaryngeal lymph node metastasis (PLNM), bilateral lesions, tumor location in thyroid (upper or lower), and gross extrathyroidal extension (ETE) were found to be independent predictive factors for LLNM (P < 0.01). The nomogram built to predict LLNM in PTMC patients passed the calibration step and the area under the receiver operating characteristic curve was 0.967, which showed that the nomogram we used had a good predictive effect. The nomogram constructed in this study has a good predictive value for LLNM, which will help thyroid surgeons to make a more accurate surgical plan for patients with PTMC. A strict preoperative evaluation and total thyroidectomy and lateral neck dissection may be indicated when patients with PTMC have a high score.
Keywords: Papillary thyroid micro-carcinoma; lateral lymph node metastasis; clinical predictive model; nomogram
The detection of thyroid carcinoma has increased with the development of ultrasound (US) scanning, and among thyroid carcinoma cases, papillary thyroid micro-carcinoma (PTMC) accounts for a large proportion. PTMC is defined as papillary thyroid carcinoma (PTC) with a size equal to or less than 1 cm [1]. It is reported that PTMC can make up as much as 50% of newly-diagnosed thyroid carcinomas [2]. Surgery is the main treatment for PTMC, but the necessary extent of neck lymph node dissection remains controversial, especially for patients with lateral lymph node metastasis (LLNM). Large studies have reported that the rate of LLNM can be as high as 40% [3]. Furthermore, a large retrospective analysis confirmed that LLNM is an adverse prognostic factor [4].
Deep cervical lymph nodes have been divided into seven regions in an internationally-recognized system, and are identified by Roman numerals [5]. Among them, areas VI and VII constitute the central region, while the lateral region consists of areas II, III, IV and V. Lymphatic metastasis in area I is not in the scope of this research due to its low metastasis rate (<3%) [6,7].
There is a general consensus that therapeutic lateral neck dissection (LND) is a routine treatment in PTC patients with LLNM, so as to improve the regional control rate [8]. Whether total thyroidectomy (TT) and lateral neck dissection (LND) should be performed in PTMC still remains controversial [9].
The preoperative detection rate of lateral neck lymph nodes is unreliable, making it difficult to determine the scope of the operation. The rates of recurrence and distant metastasis are significantly higher in patients with LLNM than in patients without LLNM [10]. Consequently, the thoroughness of the initial operation is important. Meanwhile, with the expanded scope of surgery, a series of surgical complications might arise. As the scope of the operation expands, scar formation is a problem that cannot be ignored. In addition, it may lead to laryngeal nerve injury (temporary or permanent), hypoparathyroidism (temporary or permanent), or lifelong thyroid hormone replacement therapy as a result of surgery. Furthermore, a report pointed out that PTMC patients are concerned about neck lymph node metastasis and permanent thyroid hormone replacement therapy [11].
Many studies have shown that greater tumor size, central lymph node metastasis, increased numbers of both central lymph node metastasis and extrathyroidal extension (ETE) were predictors for LLNM of papillary thyroid cancer [12]. In this study, we analyzed the details of clinical information of PTMC patients, and screened out the most valuable risk factors related to LLNM. Then we performed further analysis to build a clinical predictive model to calculate the risk of LLNM in PTMC patients, which might help to estimate the necessity of total thyroidectomy and lateral neck LN dissection.
2.1 Patient Population and Inclusion Criteria
A total of 576 PTMC patients with suspected lymph node metastasis, assessed by US, in the lateral cervical region who underwent thyroidectomy and lateral neck lymph node dissection in Shenzhen Second People’s Hospital between January 2013 and January 2020 were enrolled in this study. Patients who had received previous thyroidectomy treatment were excluded. The data retrieved from a database in our center included patients’ general characteristics, preoperative US findings, and histopathology reports of thyroidectomy and lateral neck dissection specimens.
US examinations were performed by a sonographer with at least 5 years’ experience in thyroid US. The blood flow signal classification was defined according to the following criteria: grade 0: there is no blood flow signal in the mass, Grade 1: there is a small amount of blood flow signal in the mass, or there are 1–2 point or rod blood flow signals, Grade 2: there are 3–4 point blood flow signals in the mass, or there are clearly visible blood vessels passing through, Grade 3: there are more than 5 points of blood flow signals in the mass, or there are more than 2 clear blood vessels in the mass. In addition, if microcalcification is found on US, it will be defined as the presence of microcalcification.
If the patient has positive results of thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb) accompanied by typical US manifestations of Hashimoto’s thyroiditis (HT), it will be defined as positive for Hashimoto’s thyroiditis. In our study, a tumor located in the upper part of the entire thyroid gland was defined as upper by US. In contrast, a tumor in the lower part of the entire thyroid gland was defined as lower by US. If a macroscopic invasion of the extracellular membrane was found during surgery, it was defined as gross ETE.
2.2 Inclusion Criteria and Exclusion Criteria
Inclusion criteria: (1) primary PTMC patients confirmed by pathology (with size measured less than 1 cm); (2) adenoidectomy and isthmectomy or total thyroidectomy plus central area and lateral area lymph node dissection (dissection of II–IV or elective dissection of the affected side) were performed; (3) complete preoperative US image data available.
Exclusion criteria: (1) patients underwent a second operation; (2) the postoperative pathology was not PTC or was PTC with a diameter of more than 1 cm; (3) age less than 18 years old; (4) patients with incomplete preoperative US data.
Statistical analysis was completed using R for Windows (version 3.6.3, https://cran.r-project.org/). The χ2-test was used to determine the significant risk factor differences between LLNM and non-LLNM cases. Multiple logistic regression analysis was modelled to compute the predictive value of variables. The performance of the nomogram was calculated based on discrimination and calibration. The area under the receiver operating characteristic (ROC) curve (AUC) was measured by the discrimination (predictive accuracy). The best cutoff point for the risk of LLNM was checked using the ROC curve. Diagnostic values of ROC curve analysis were presented with accuracy, sensitivity, specificity, positive predictive value, and negative predictive value. Calibration was judged by a calibration curve which shows how closely the nomogram predicted risk correlated with the actual risk. Statistical significance of univariate analysis and multiple logistic regression was set at P < 0.05 and P < 0.01, respectively.
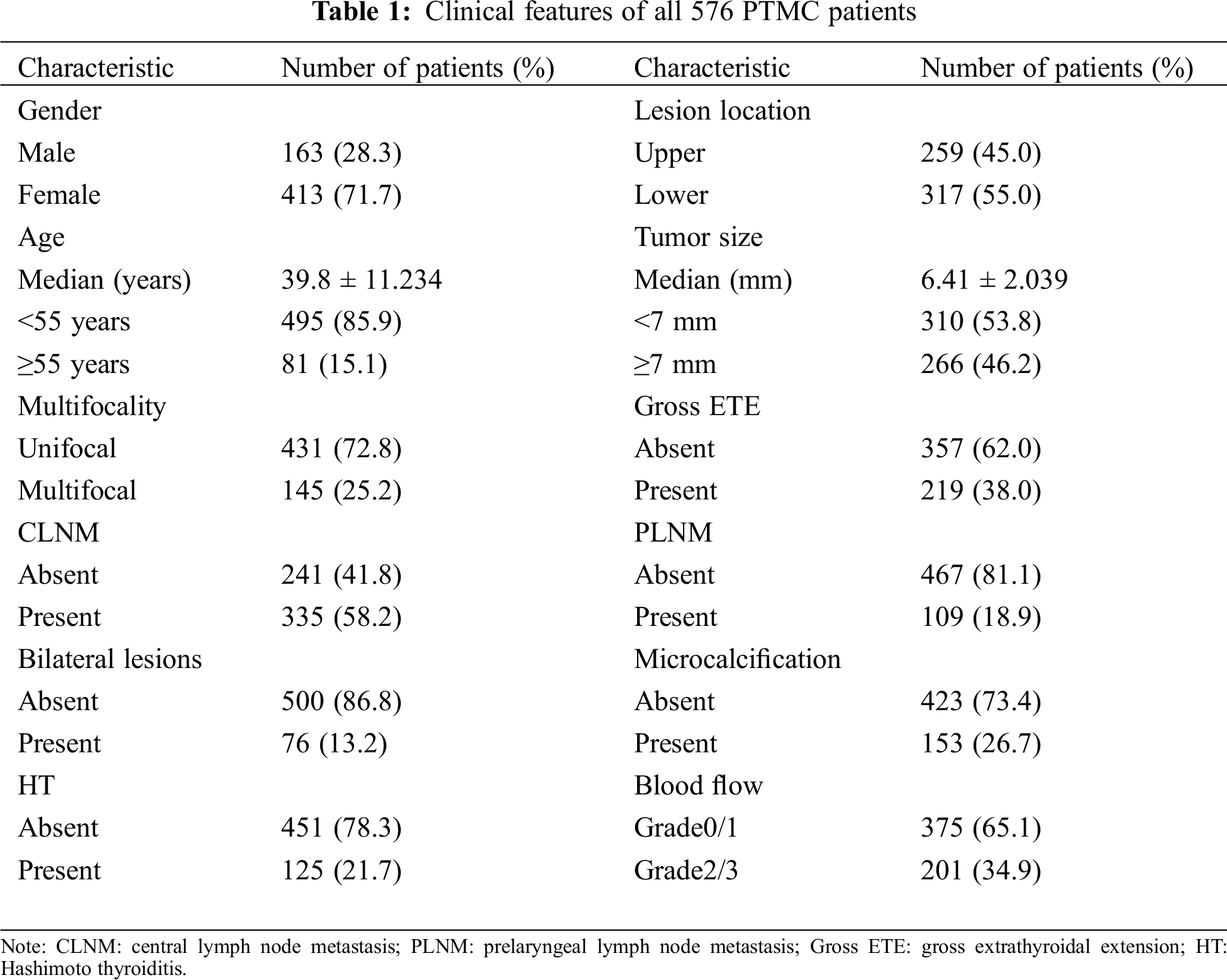
Among the 576 PTC patients who underwent unilateral LND, 163 were male and 413 were female. The mean size of the primary lesion was 6.41 ± 2.039 mm, while the mean age of our cohort was 39.8 ± 11.234 years. Multifocality and gross ETE were observed in 25.1% (145/576) and 38.0% (219/576) of the patients, respectively. Ultrasonic features of microcalcification and grade 2/3 blood flow were observed in 26.7% (153/576) and 34.9% (201/576) of the patients. Hashimoto’s thyroiditis (HT) was found in 125 patients (21.7%). Bilateral lesions were found in 73 patients (13.2%), while 259 patients had a tumor located in the upper side of the thyroid (45.0%). CLNM had occurred in 335 patients (335/576; 58.1%), while PLNM occurred in 109 patients (109/576; 18.9%). The total rate of LLNM was 23.0% (133/576) (Tab. 1).
3.2 Univariate Analysis and Multivariate Analysis
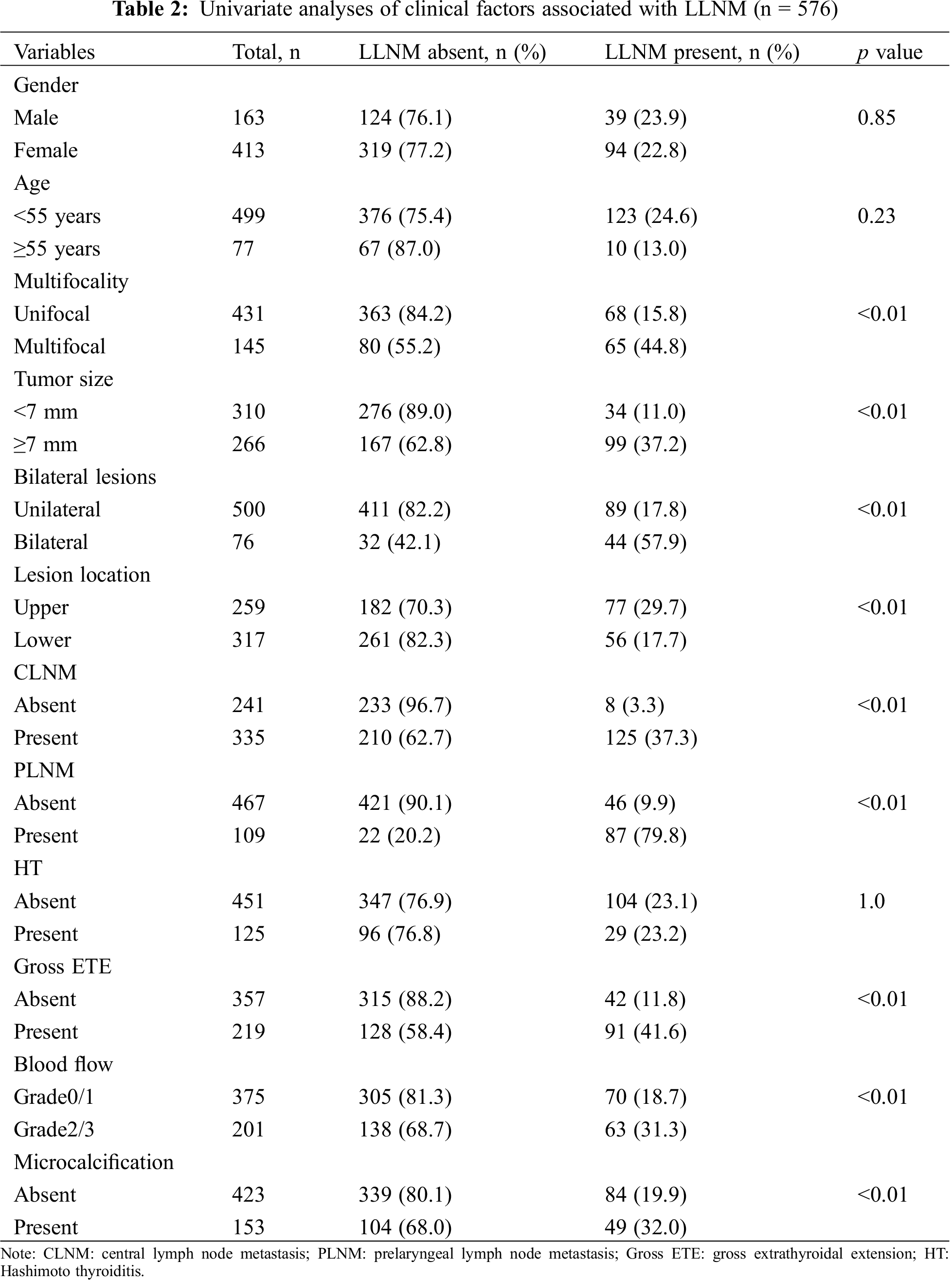
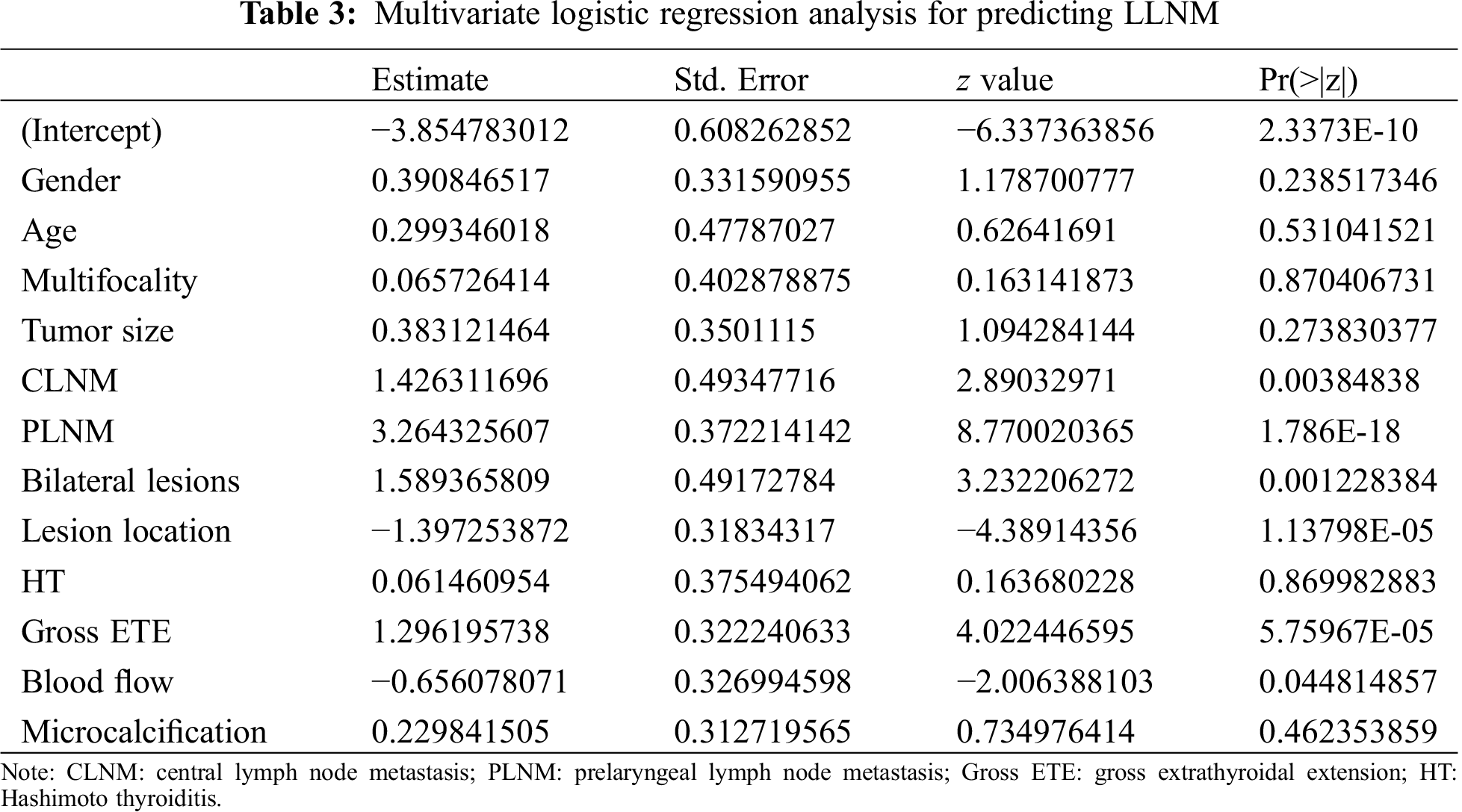
Univariate analysis showed that age, multifocality, tumor size, bilateral lesions, lesion location, CLNM, PLNM, gross ETE, blood flow and microcalcification are risk factors for LLNM (P < 0.05, Tab. 2), while multiple logistic regression analysis indicated that CLNM, PLNM, bilateral lesions, location in the thyroid and gross ETE are independent predictive factors for LLNM (P < 0.01, Tab. 3). Further, for nomogram modelling we included CLNM, PLNM, bilateral lesions, lesion location, and gross ETE (Tab. 4).
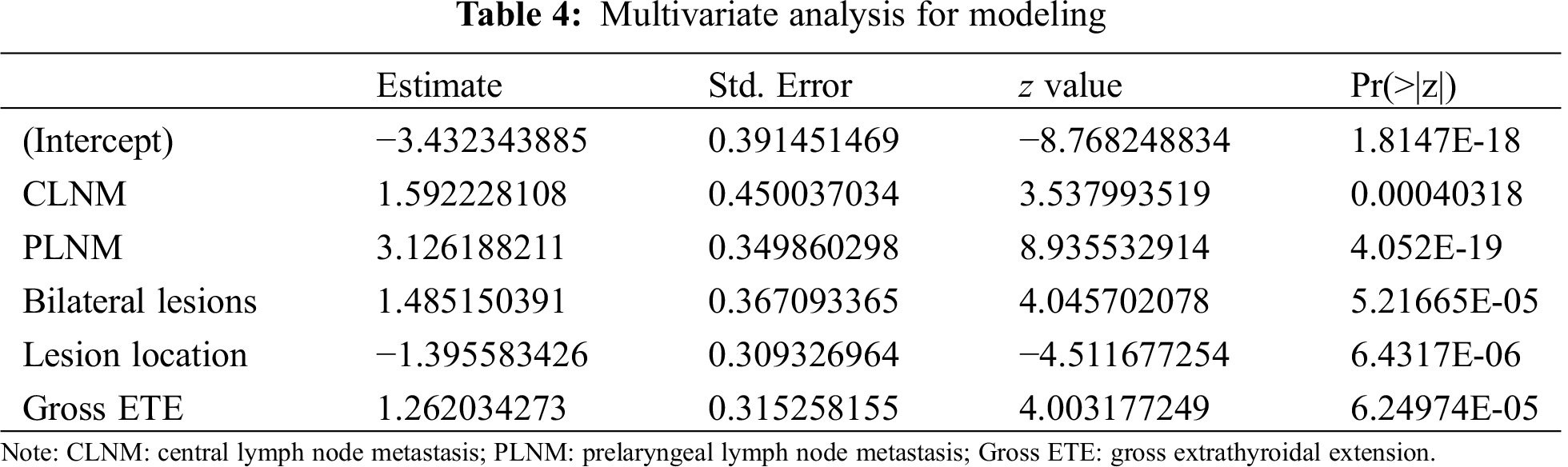
3.3 Construction of the Predictive Model
A nomogram was developed using logistic regression. During the build process, we calculated regression coefficients for each variable and created a scale ranging from 0 to 100 for each predictive variable to be assigned to it in the nomogram (Fig. 1). The risk of LLNM was calculated for each patient according to the predictive model in the form of a nomogram. For example, the risk of LLNM in a patient with CLNM (+50 points) and PLNM (+100 points) and has a tumor located on the left (+0 points) upper side (+45 points) of the thyroid with gross ETE (+40 points, total points = 235) is over 90%. The optimal cutoff point was determined to be 0.036 using the ROC curve. At this cutoff point, the sensitivity and the specificity were 0.888 and 1.000, respectively. The AUC turned out to be 0.967 (Fig. 2).
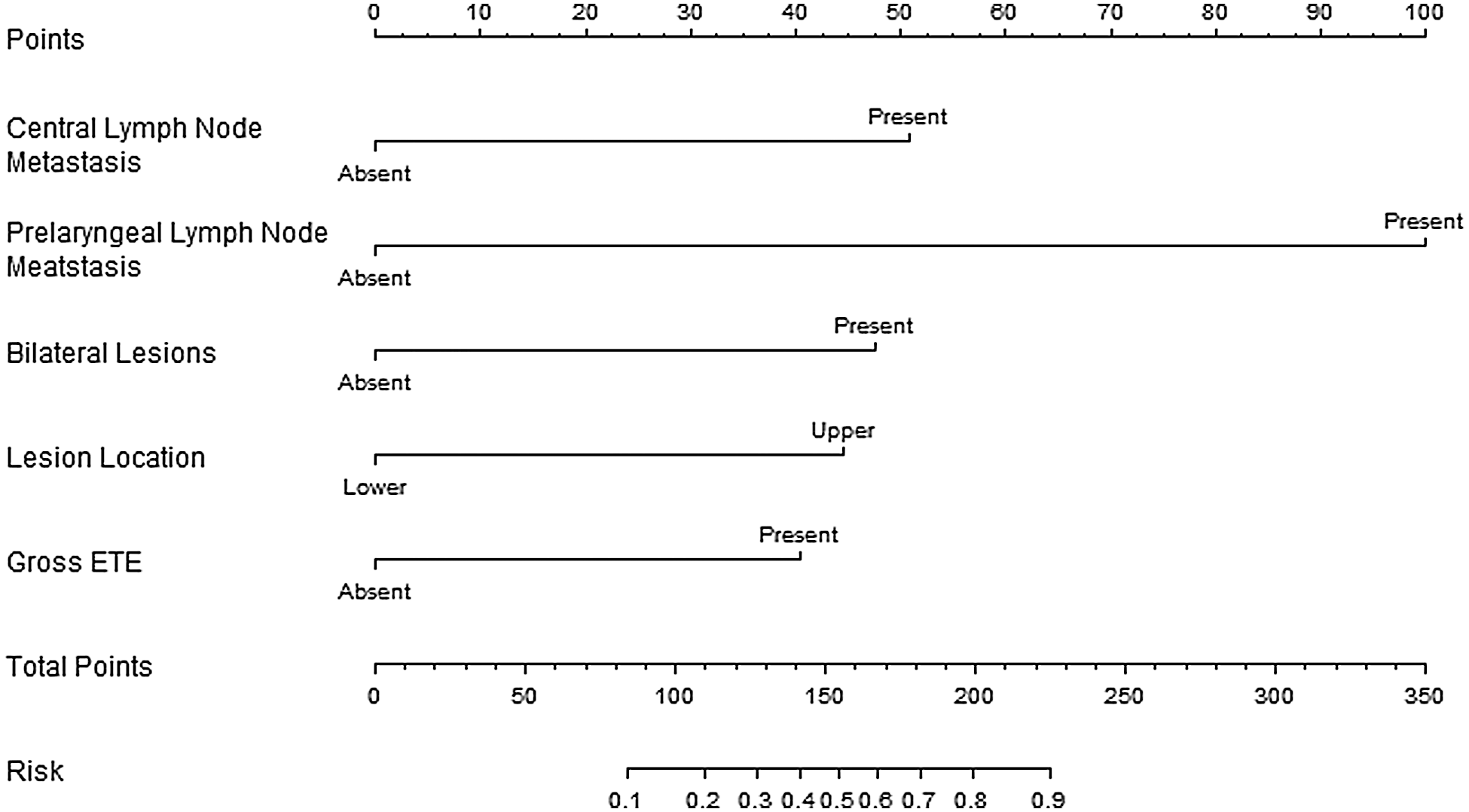
Figure 1: The nomogram scale
A nomogram for predicting the probability of LLNM. To use the nomogram, the patient variable is located on each axis. Then, a vertical line is drawn to read the point on the axis and obtain the arithmetic sum of the points for all variables. This cumulative score is then located on the total point line to assess the individual probability of LLNM.
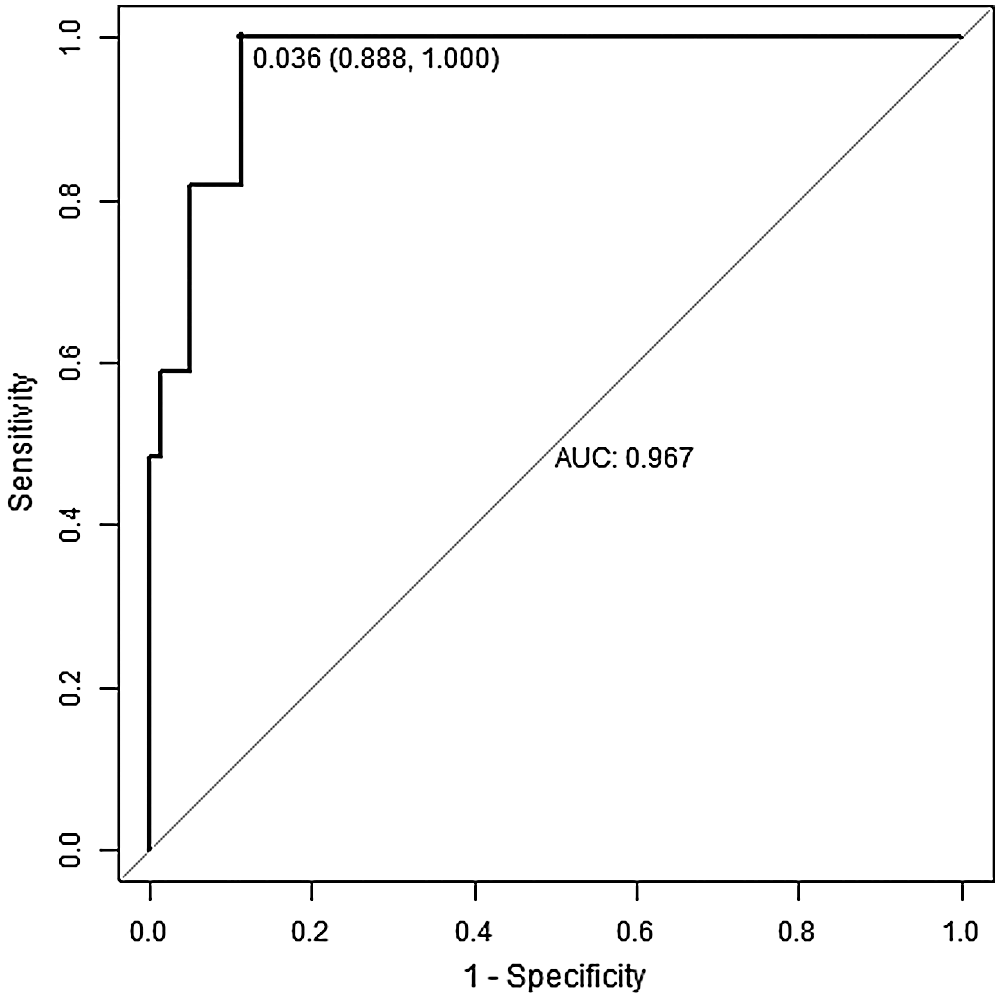
Figure 2: The ACU curve analysis
The optimal cutoff point was determined as 0.036. At this cutoff point, the sensitivity and the specificity were calculated as 0.888, and 1.000, respectively. The AUC was found to be 0.967.
In the verification, we found that the curve was close to the 45° line, and the calibration plot for predicting LLNM risk was in good agreement with the predicted and observed probabilities of LLNM (Fig. 3).
Figure 3: The calibration plot analysis
Calibration plot (curve): closer distance to the 45° straight line indicates a higher accuracy of the nomogram. B = 1000 repetitions, boot; Mean absolute error = 0.02, n = 576.
The incidence of PTMC is increasing. Some scholars have applied the nomogram to the prediction of central lymph node metastasis and level V lymph node metastasis in PTC, and research by Wang et al. [13–15] successfully predicted the skip metastasis in papillary thyroid cancer using a nomogram, which had obtained reliable prediction data and verified the feasibility of this method. Therefore, we applied this method to the prediction of LLNM in PTMC, and achieved a relatively good predictive effect.
Studies have shown that PTMC share the same metastatic lateral Lymph node spread pattern with PTC [16]. A small size does not guarantee a lower metastasis rate. Our analysis showed that CLNM, PLNM, bilateral lesions, location in the thyroid (upper or lower), and gross ETE were independent predictive factors for LLNM, which were consistent with previous studies [17–19]. The advantage of this study is that we were able to build a risk predictive model for LLNM in PTMC patients using clinical indicators, and to form a quantifiable nomogram rating scale. In our verification, the prediction sensitivity was 0.888, and the prediction specificity was 1.000, which showed a reliable prediction effect and a great value for application and research.
Generally speaking, excision of the thyroid gland and the isthmus gland on the affected side plus central area lymph node dissection are widely accepted. However, whether this excision range is sufficient for patients with LLNM is controversial. The predictive model we created in our study provides a better prediction of the risk of LLNM in PTMC, which is helpful for enabling clinicians to make better surgical decisions.
More critically, better evaluation of the risk of LLNM will help surgeons decide on the suitable scope of PTMC surgery. If the risk of LLNM is high according to the model evaluation, imaging examination may be needed to further evaluate the situation, and total thyroidectomy and the dissection of lymph nodes in the lateral neck area should be considered; in contrast, for those who are at low risk of LLNM, exemption from total thyroidectomy and lateral lymph node dissection ought to be considered, which has favorable clinical practicability.
However, our research still leaves much to be explored. Our results may be limited by the sample size, so it is necessary to further expand the sample size for validation. Besides, we defined LLNM as lymph node metastasis which occurred in one or more areas of the lateral neck (II, III, IV or V), which may lead to bias of the predictive result.
Studies done by Tongtong Liu and his colleagues had explored a radiomics model to assessing the risk of papillary thyroid carcinoma metastasis using US and CT images, making artificial intelligence technology possible in evaluating metastasis of PTMC [20–21]. Noninvasive imaging intelligent assessment may greatly improve diagnostic efficiency and accuracy. In future research, it will be necessary to divide the LLNM into different subgroups and adding more features such as data of CT images into account, so as to improve the accuracy of our predictive model.
The nomogram presented herein helps surgeons to establish more precise surgical procedures for thyroidectomy and neck lymph node dissection for PTMC patients. In our study cohort, this predictive model has a good discrimination (AUC of 0.967) and accurate calibration, which helps patients with PTMC get the most out of treatment with the least trauma.
This predictive model will help us to identify PTMC patients who need total thyroidectomy and lateral neck lymph node dissection.
Statement of Ethics:The subjects (or their parents or guardians) provided written informed consent to participate in this study, and the study protocol was approved by the institute’s committee on human research.
Funding Statement: This study was sponsored by fund from the National Key R&D Program of China(2019YFA0906000).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Pasqualino, M., Gabriella, P., Marco, A., Antonia, V. M., Carla, G. et al. (2013). Papillary thyroid microcarcinomas: A comparative study of the characteristics and risk factors at presentation in two cancer registries. Journal of Clinical Endocrinology & Metabolism, 98(4), 1427–1434. DOI 10.1210/jc.2012-3728. [Google Scholar] [CrossRef]
2. Vigneri, R., Malandrino, P., Vigneri, P. (2015). The changing epidemiology of thyroid cancer: Why is incidence increasing? Current Opinion in Oncololgy, 27, 1–7. DOI 10.1097/CCO.0000000000000148. [Google Scholar] [CrossRef]
3. Hay, I. D., Thompson, G. B., Grant, C. S., Bergstralh, E. J., Dvorak, C. E. et al. (2002). Papillary thyroid carcinoma managed at the mayo clinic during six decades (1940–1999Temporal trends in initial therapy and long-term out come in 2444 consecutively treated patients. World Journal of Surgery, 26, 879–885. DOI 10.1007/s00268-002-6612-1. [Google Scholar] [CrossRef]
4. Xue, S., Han, Z., Lu, Q. Y., Wang, P. S., Chen, G. (2020). Clinical and ultrasonic risk factors for lateral lymph node metastasis in papillary thyroid microcarcinoma: A systematic review and meta-analysis. Frontiers in Oncology, 10, 436. DOI 10.3389/fonc.2020.00436. [Google Scholar] [CrossRef]
5. American Thyroid Association Surgery Working Group, American Association of Endocrine Surgeons, American Academy of Otolaryngology-Head and Neck Surgery (2009). Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid, 19, 1153–1158. DOI 10.1089/thy.2009.0159. [Google Scholar] [CrossRef]
6. Kupferman, M. E., Patterson, M., Mandel, S. J., LiVolsi, V., Weber, R. S. (2004). Patterns of lateral neck metastasis in papillary thyroid carcinoma. Archives of Otolaryngology—Head and Neck Surgery, 130, 857–860. DOI 10.1001/archotol.130.7.857. [Google Scholar] [CrossRef]
7. Wada, N., Duh, Q. Y., Sugino, K., Iwasaki, H., Kameyama, K. et al. (2003). Lymph node metastasis from 259 papillary thyroid microcarcinomas: Frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Annals of Surgery, 237, 399–407. DOI 10.1097/01.SLA.0000055273.58908.19. [Google Scholar] [CrossRef]
8. Haugen, B. R., Alexander, E. K., Bible, K. C., Doherty, G. M., Mandel, S. J. et al. (2016). 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid, 26, 1–133. DOI 10.1089/thy.2015.0020. [Google Scholar] [CrossRef]
9. Zhang, X. J., Liu, D., Xu, D. B., Mu, Y. Q., Chen, W. K. (2013). Should level V be included in lateral neck dissection in treating papillary thyroid carcinoma. World Journal of Surgical Oncology, 25, 304. DOI 10.1186/1477-7819-11-304. [Google Scholar] [CrossRef]
10. Sapuppo, G., Palermo, F., Russo, M., Tavarelli, M., Masucci, R. et al. (2017). Latero-cervical lymph node metastases (N1b) represent an additional risk factor for papillary thyroid cancer outcome. Journal of Endocrinological Investigation, 40, 1355–1363. DOI 10.1007/s40618-017-0714-y. [Google Scholar] [CrossRef]
11. Nickel, B., Brito, J. P., Moynihan, R., Barratt, A., Jordan, S. et al. (2018). Patients’ experiences of diagnosis and management of papillary thyroid microcarcinoma: A qualitative study. BMC Cancer, 18, 242. DOI 10.1186/s12885-018-4152-9. [Google Scholar] [CrossRef]
12. Zhao, H. Q., Huang, T., Li, H. H. (2019). Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery, 166, 55–60. DOI 10.1016/j.surg.2019.01.025. [Google Scholar] [CrossRef]
13. Wang, Y. J., Guan, Q., Xiang, J. (2018). Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study of 8668 patients. International Journal of Surgery, 55, 98–102. DOI 10.1016/j.ijsu.2018.05.023. [Google Scholar] [CrossRef]
14. Wang, W. L., Yang, Z., Ouyang, Q. H. (2020). A nomogram to predict skip metastasis in papillary thyroid cancer. World Journal of Surgical Oncology, 18, 167. DOI 10.1186/s12957-020-01948-y. [Google Scholar] [CrossRef]
15. Wang, Y. J., Guan, Q., Xiang, J. (2019). Nomogram for predicting level V lymph node metastases in papillary thyroid carcinoma with clinically lateral lymph node metastases: A large retrospective cohort study of 1037 patients from FDUSCC. Journal of Cancer, 10, 772–778. DOI 10.7150/jca.28527. [Google Scholar] [CrossRef]
16. Lee, J. H., Chun, Y. S., Chung, Y. S. (2019). Extent of lateral neck dissection for papillary thyroid microcarcinomas. Head & Neck, 41(5), 1367–1371. DOI 10.1002/hed.25570. [Google Scholar] [CrossRef]
17. Zhao, W. J., Chen, S. B., Hou, X. M., Liao, Q., Chen, G. et al. (2019). Predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Pathology Oncology Research, 25(3), 1245–1251. DOI 10.1007/s12253-018-0511-8. [Google Scholar] [CrossRef]
18. Zhang, X., Zhang, L., Xue, S., Wang, P. S., Chen, G. (2019). Predictive factors of lateral lymph node metastasis in solitary papillary thyroid microcarcinoma without gross extrathyroidal extension. Asian Journal of Surgery, 42(4), 563–570. DOI 10.1016/j.asjsur.2018.07.003. [Google Scholar] [CrossRef]
19. Back, K., Kim, J. S., Kim, J. H., Choe, J. H. (2019). Superior located papillary thyroid microcarcinoma is a risk factor for lateral lymph node metastasis. Annals of Surgical Oncology, 26(12), 3992–4001. DOI 10.1245/s10434-019-07587-2. [Google Scholar] [CrossRef]
20. Liu, T. T., Zhou, S. C., Yu, J. H., Guo, Y., Wang, Y. Y. et al. (2019). Prediction of lymph node metastasis in patients with papillary thyroid carcinoma: A radiomics method based on preoperative ultrasound images. Technology in Cancer Research & Treatment, 18, 1533033819831713. DOI 10.1177/1533033819831713. [Google Scholar] [CrossRef]
21. Tong, Y. Y., Li, J., Huang, Y. X., Zhou, J., Liu, T. T. et al. (2020). Ultrasound-based radiomic nomogram for predicting lateral cervical lymph node metastasis in papillary thyroid carcinoma. Academic Radiology. DOI 10.1016/j.acra.2020.07.017. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |