
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.015828
ARTICLE
Formononetin Inhibits Non-Small Cell Lung Cancer Proliferation via Regulation of mir-27a-3p through p53 Pathway
Institute of Chinese Medicine Chemistry, Zhejiang University of Traditional Chinese Medicine, Hangzhou, 310053, China
*Corresponding Author: Yu He. Email: heyu0923@sina.com
Received: 17 January 2021; Accepted: 25 March 2021
Abstract: Objective: The aim of the present study was to investigate the anti-tumor effects of formononetin on human non-small cell lung cancer (NSCLC) and its potential molecular mechanism. Methods: A549 cells were treated with different concentrations of formononetin, then detected the cell proliferation, apoptosis and the expression of HIPK2 respectively by MTT assay, flow cytometry analysis and RT-qPCR. Then the interaction between miR-27a-3p and its target gene HIPK2 was verified through luciferase reporter assay. The expression of miR-27a-3p, HIPK2, and p53 was detected after being treated with different formononetin concentrations by RT-qPCR and western blot. Results: The results showed that formononetin significantly inhibited the proliferation and induced the apoptosis of A549 cells in a time- and dose-dependent manner. miR-27a-3p targeted HIPK2 3’UTR and inhibited the expression of HIPK2. Also, formononetin-treated A549 cells were remarked with a significant decline in the expression of miR-27a-3p, accompanied with growth of HIPK2, and subsequently a reduction of p53. Conclusions: The study indicates that miR-27a-3p might pose regulated effects on the HIPK2/p53 pathway, resulting in formononetin’s anti-carcinogenic effects on NSCLC in vitro.
Keywords: Non-small cell lung cancer (NSCLC); formononetin; miRNA; anti-cancer; p53 pathway
Lung cancer, a leading cause of global cancer deaths and a leading public health issue in China, accounts for more than 28% death toll caused by all types of malignant tumors [1]. Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) which are recognized as two prime lung cancer types, accounting for roughly 15% and 85% of all lung cancer instances, respectively [2]. NSCLC usually develops resistance to radiation and chemotherapy, and the rate of patients who could survive for more than five years is only 15% [3]. Out of the high fatality rate of NSCLC, it is crucial and urgent to discover innovative and functional remedial procedures.
MicroRNA (miRNA) is a type of endogenous small non-coding RNAs, which has various critical regulatory effects in cells of both animals and plants [4,5]. By combining with genes responsible for protein-coding at mRNAs, miRNAs can result in translational capability decline or post-transcriptional levels modulation of the target mRNAs [6]. miRNAs play as regulators in various bioprocesses [7], but different target genes could trigger contrasting results concerning the genesis of tumors and the process of cancerous growth. From evidential studies, miRNAs can either suppress the tumor or act as oncogenes [8,9]. miR-27a-3p was known as an oncogene involved in many cancers, including gastric cancer, breast cancer, thyroid cancer, etc. [10,12]. However, the role of miR-27a-3p in NSCLC is remaining unclear.
With the promotion of healthy lifestyles, the public’s focus has increasingly shifted into the field of plant food-derived phytochemicals to act as potential anti-cancer drugs. Astragalus membranaceus (Huangqi), which is a broadly used Chinese traditional herb and has been believed to have the ability to enhance the immune system by one of its major components—flavonoids [13]. Among various flavonoids, a recently found type, formononetin, extracted from A. membranaceus has arisen scholarly attention for its special effects on preventing tumors and neuron damages [14,15]. Many recent studies showed the substantial potentiality of formononetin in inhibiting the speed of cell proliferation by inducing programmed death of tumor cells through multiple signaling pathways [16,17]. Formononetin also manifests abilities of cancer-preventing in various body parts, like the prostate [18,19], breast [20,21], colon [22], to name just a few. However, the potency of formononetin in human NSCLC is still unsure, as well as its role as a mediator in the interfering process of miRNAs during the transformation of tumor cells.
Therefore, the present study aimed to investigate the influence of formononetin on proliferation and apoptosis in NSCLC cells, and further elucidate the underlying mechanisms involved in the anticancer effects of formononetin.
2.1 Cell Culture and Formononetin Treatment
A549 cells were obtained from American Type Culture Collection (ATCC, USA). The cell culture was set in DMEM (HyClone, USA), added with 100 U/ml penicillin/streptomycin and 15% fetal bovine serum (FBS; Gibco, USA), under a moisturized atmosphere with a 5% CO2 level. The test group processed cells (60%–70% confluent) in dimethyl sulfoxide (DMSO) with formononetin (Sigma, USA), while the control group set cells with vehicle (DMSO). Before the experiments, all cells had been starved for a day in a low-serum medium (containing 0.5% FBS).
A549 cells were seeded in 6-well plates with a 3 × 106 cells per well density in the environment of DMEM. Followed the manufacturer’s protocol of Lipofectamine 2000 Transfection Kit (Thermo Fisher Scientific, Waltham, MA, USA), transfection was conducted with final concentrations of miRNA mimic, negative control (NC), inhibitors, and inhibitors negative control (I-NC) were 100 nM. After that, the cells were set for 48 h in order for future analysis.
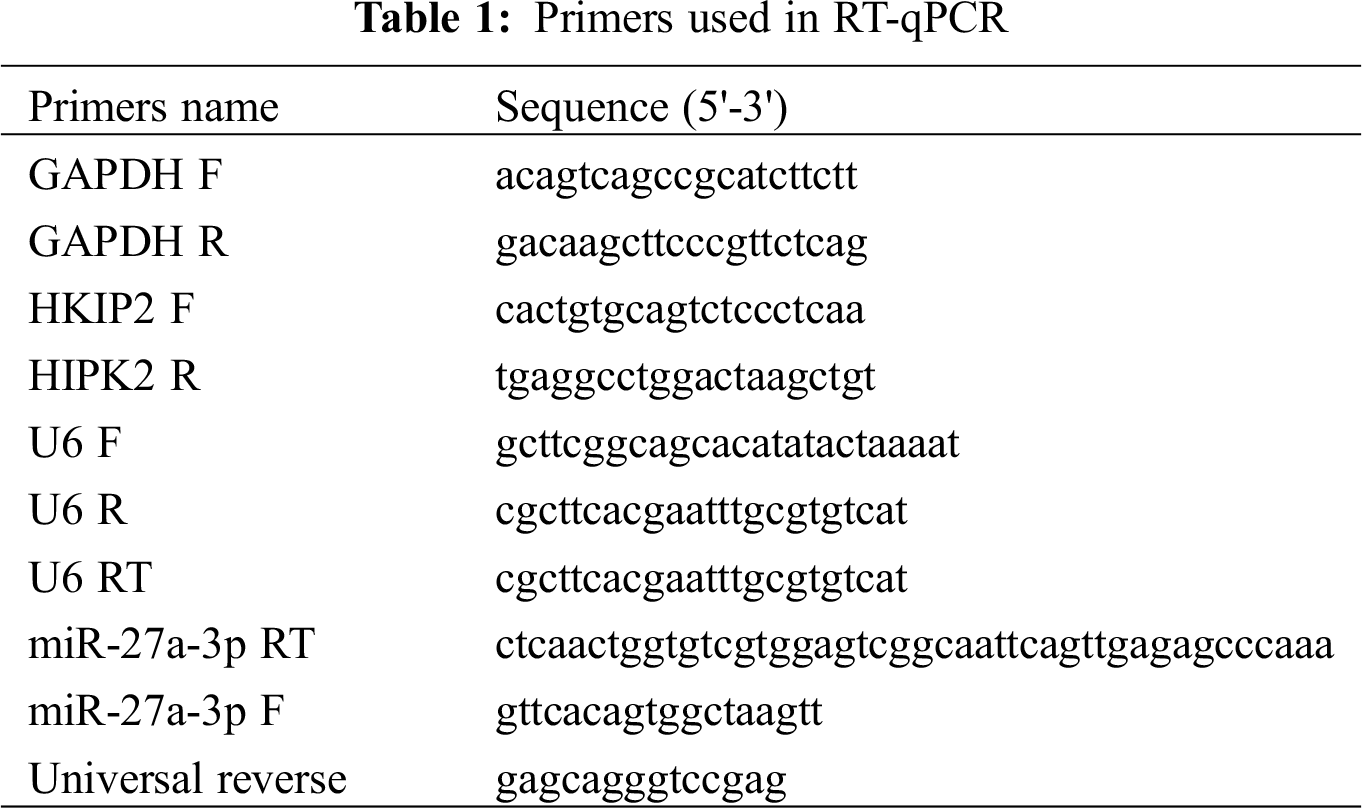
TRIzol (Invitrogen) was used as a separating reagent for total RNA to get the first-strand cDNA using a FastKing RT Kit (With gDNase) (Tiangen) following the official instructions. The qRT-PCR analysis was carried out by SYBR Green (Tiangen), and the primers are demonstrated in Tab. 1. GAPDH and U6 were taken as endogenous controllers for mRNAs and miRNAs, respectively.
2.4 MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide) Assay
A549 cells were seeded in a 96-well plate at a 1 × 105/mL dose and incubated for 22 h. Subsequently, cells were starved for another 2 h. Culture medium was replaced with 20 μL of methyl thiazolyl tetrazolium (MTT) solution (5 g/L) (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C for 4 h. The supernatant was discarded, and 100 μL of DMSO (dimethyl sulfoxide) (Sigma-Aldrich, St. Louis, MO, USA) was put on to each well. The absorbance value was tracked at the wavelength of 570 nm with a microplate reader.
A549 cells were undergone lysis for protein extraction. Each protein sample concentration was determined by a BCA (bicinchoninic acid) kit (Beyotime, Shanghai, China). The protein sample was divided by gel electrophoresis and moved into PVDF (polyvinylidene difluoride) membranes (Roche, Basel, Switzerland). After the primary and secondary antibody incubating process, immunoreactive bands were exposed by intensified chemiluminescence (ECL) method.
2.6 Flow Cytometry Analysis for Cell Apoptosis
The programmed death of A549 cells was identified by an Annexin V-FITC/PI Apoptosis Kit (Multi Sciences, Hangzhou, China). The specific process was as follows: first, obtained cell pellets from digested cells which had been undergoing transfection for 48 h; secondly, resuspended cell pellets in 200 µl of 1 × working solution; thirdly, added annexin V-FITC (5 μl) and propidium iodide (PI; 5 μl) into two single dye tubes; then 5 μl of annexin V-FITC and 5 μl of PI were mixed into sample tubes for 10 min reaction. The flow cytometry analysis was conducted subsequently for 1 h.
To detect the target miRNA, we co-transfected 293T cells with miR-27a-3p mimics under the condition of negative controls, pmiR-HIPK2-3’UTR-WT, and pmiR-RB-REPORTTM. Moreover, for miR-27a-3p, 293T cells were co-transfected under the condition of the mimics, negative controls, pmiR-HIPK2-3’UTR-WT, pmiR-HIPK2-3’UTR-MUT, and pmiR-RB-REPORTTM. The 293T cells were seeded in 96-well plates, which fill with 100 µl of DMEM, by a 1.5 × 104 cells/well density. The transfection was set in Lipofectamine 2000 reagent (Invitrogen, USA) for 48 h; then as the luciferase activity was shown out, a fluorescence intensity meter was used for measuring (Veritas 9100-002). The above process was repeated at least three times. To exclude the experimental uncertainties, Renilla luciferase was applied as an internal reference. Reporter plasmid construction and luciferase reporter assay were performed by Guangzhou Ribobio Biotech Co., Ltd., Guangzhou, China.
In this study, we used SPSS 17.0 as the experimental data analysis tool, and single-tailed p < 0.05 was regarded as significant statistically.
3.1 Formononetin Suppresses the Proliferation and Induces Apoptosis in NSCLC
To discover the anti-tumor mechanism of formononetin in NSCLC, we detected the proliferation of A549 cells by MTT assay and apoptosis by flow cytometry analysis. Compared with the negative control, formononetin exhibited with significantly inhibitory effect on the proliferation of A549 cells in both time- and dose-dependent manner (Fig. 1A), while formononetin showed the opposite effect on the apoptosis process, namely, it can significantly induced A549 cells programmed death as compared with the negative control (Fig. 1B).
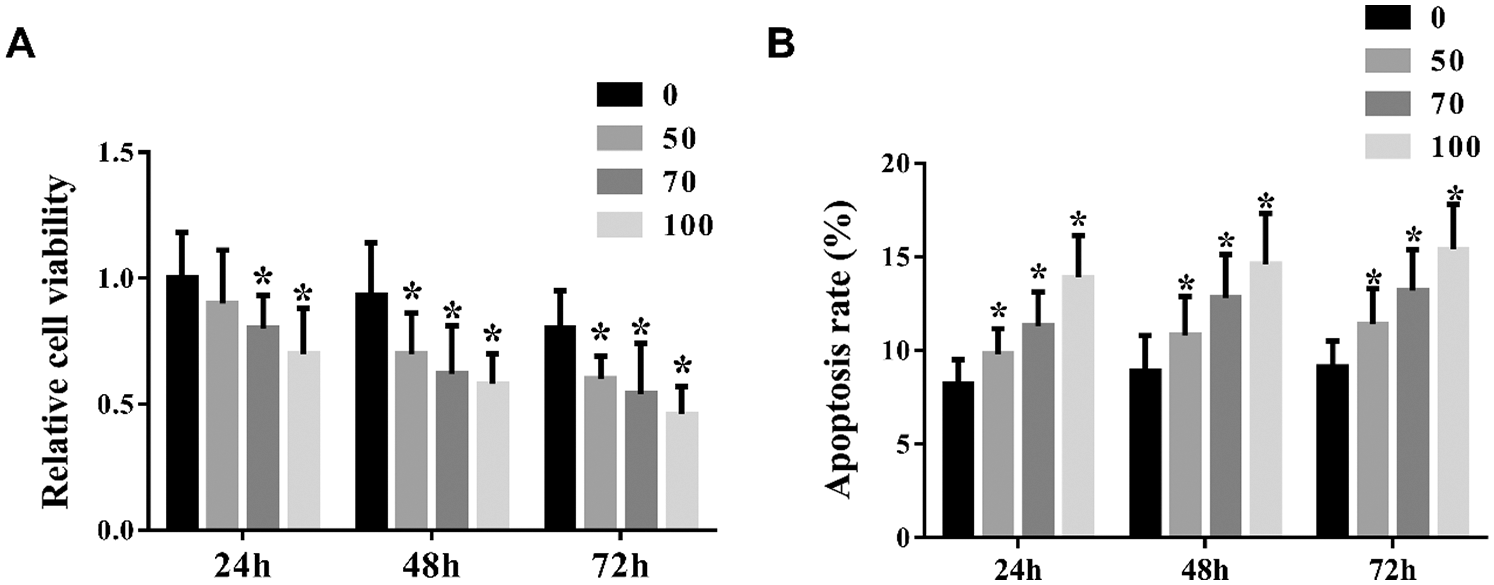
Figure 1: Formononetin suppresses the proliferation and induces apoptosis in NSCLC. (A) Formononetin-treated A549 cells were analyzed using MTT assay for determining cell viability at a time of 24 h, 48 h, and 72 h. (B) Formononetin-treated A549 cells were analyzed using flow cytometry analysis for determining cell apoptosis at a time of 24 h, 48 h, and 72 h. All experiments were repeated at least three times, and the data are shown in the form of M (mean) ± SD (standard deviation) and processed by one-way ANOVA analysis (*p < 0.05)
3.2 HIPK2 is the Key Gene Changed in NSCLC after Treated with Formononetin
Based our previous study, we constructed an mRNA profiles in NSCLC cell lines (including A549, PC-3, T24, PKO cell lines) after treated with formononetin. Furthermore, 84 genes related to cell apoptosis were expressed differentially. Formononetin also changed the protein level in VEGF and p53 pathways. Because HIPK2 was involved in p53 pathways and differentially expressed, so we chose this gene for the next experiment. We detected the expression of HIPK2 after formononetin treatment in both mRNA and protein levels. The result showed that formononetin increased HIPK2 expression in a dose-dependent manner (Figs. 2A and 2B) .
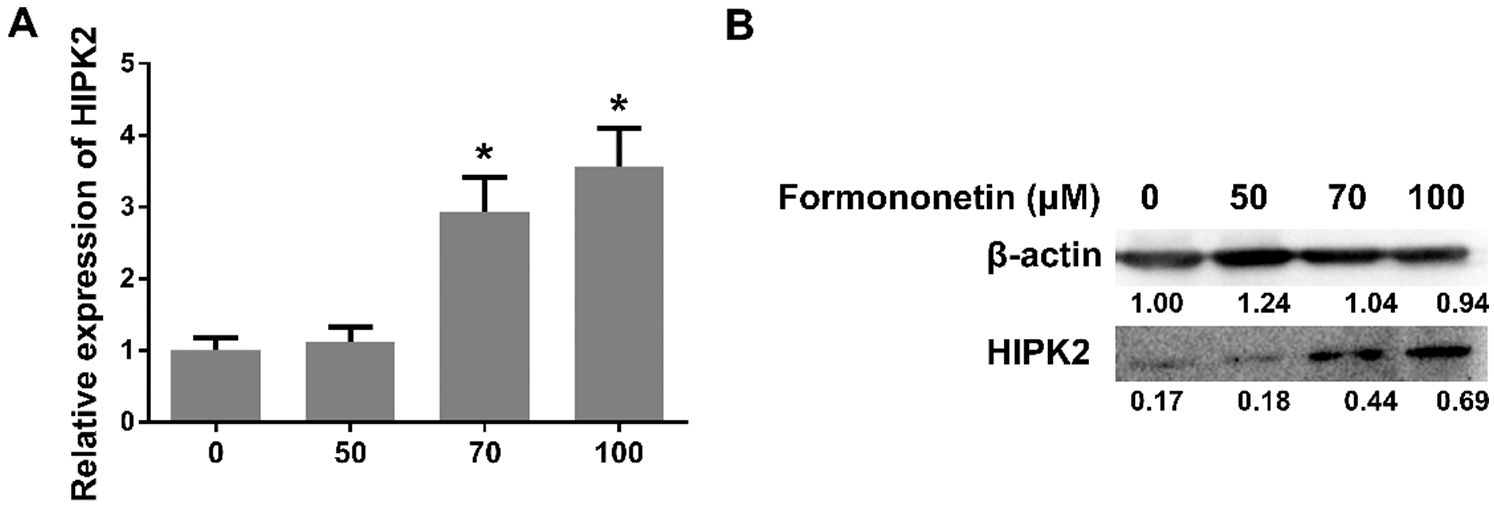
Figure 2: HIPK2 is the key gene changed in NSCLC after treated with formononetin. (A) The expression of HIPK2 after treating with formononetin at different concentrations (0, 50, 70, and 100 μM) for 24 h by RT-qPCR. (B) The protein expression of HIPK2 was analyzed by western blot. β-Actin was used as an internal control. All experiments were repeated at least three times, and the data are shown in the form of M (mean) ± SD (standard deviation) and processed by one-way ANOVA analysis (*p < 0.05)
3.3 miR-27a-3p Targets HIPK2 3’UTR
Carcinogenesis is considered to connect with miR-27a-3p. To determine the relationship between miR-27a-3p and lung cancer, we used the Targetscan program as the detection method. The target site of miR-27a-3p in the HIPK2 3’ UTR was shown as Fig. 3A. We used a luciferase reporter assay to detect the interaction between miR-27a-3p and HIPK2. As a result, the luciferase activity of 293T cells showed a decline of more than 40% under the condition of miR-27a-3p mimics and pmiR-HIPK2-3’ UTR-WT co-transfection; additionally, compared with the control group, an increase showed in luciferase activity when replaced the miR-27a-3p binding site, HIPK2 3’UTR originally, with a mutant sequence pmiR-HIPK2-3’UTR-MUT (Fig. 3B). Therefore, a direct interaction can be seen between miR-27a-3p and HIPK2, which suggests the regulatory ability of miR-27a-3p for HIPK2 expression.
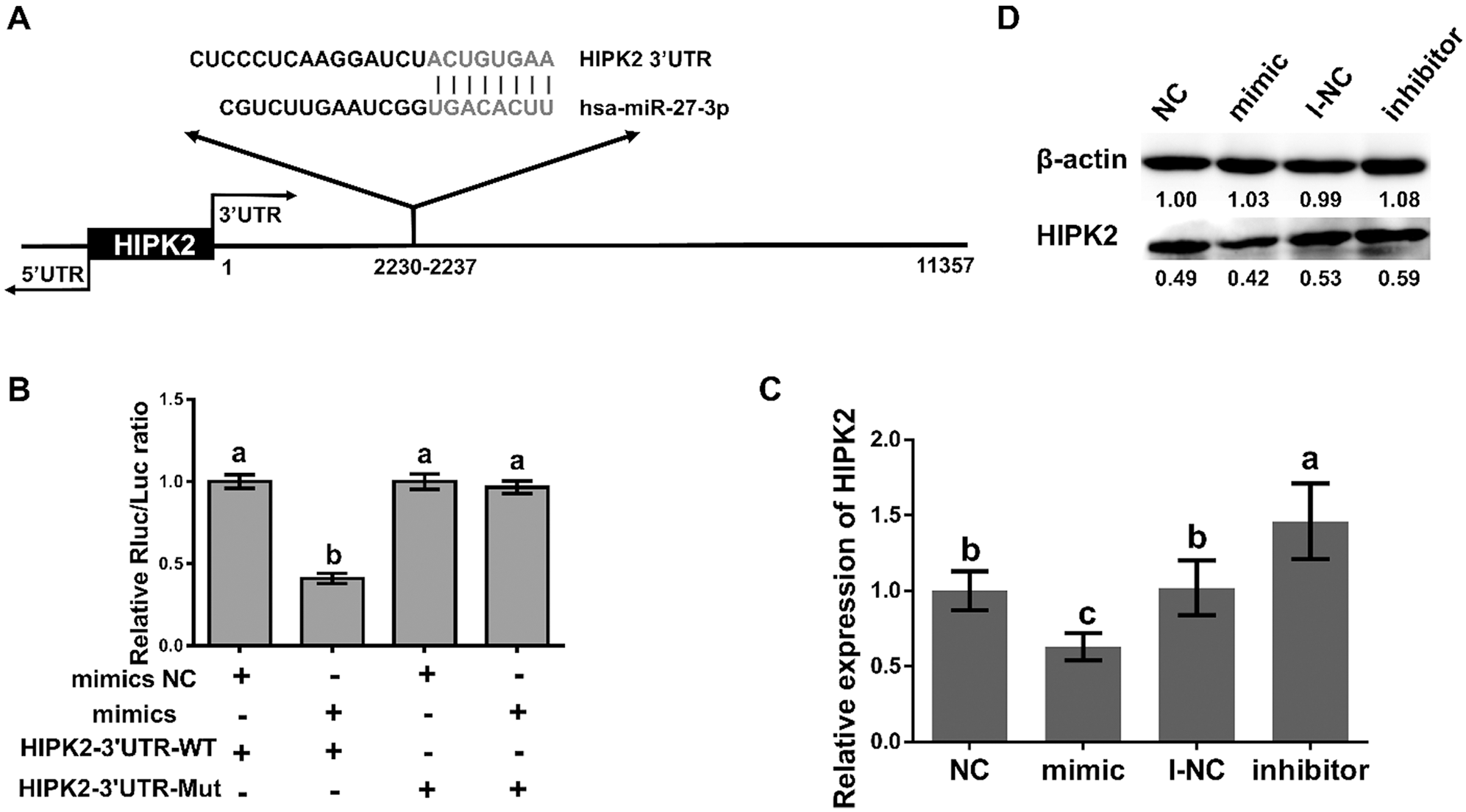
Figure 3: miR-27a-3p inhibits the expression of HIPK2. (A) The sequences marked in red are regions of matching seed between the HIPK2 3’UTR and miR-27a-3p from TargetScan’s prediction. (B) The relative luciferase activity of miR-27a-3p with negative control and mimic under 48 h pmiR-HIPK2-3’UTR-WT and pmiR-HIPK2-3’UTR-MUT co-transfection. (C) The relative expression of HIPK2 in miR-27a-3p mimic negative control (NC), mimic, inhibitor negative control (I-NC), and inhibitor groups. (D) The protein expression of HIPK2, analyzed by western blot under transfection conditions of 24 h with miR-27a-3p NC, mimic, I-NC, and inhibitor. All experiments were repeated at least three times, and the data are shown in the form of M (mean) ± SD (standard deviation) and processed by one-way ANOVA analysis, the single-tailed significance is identified as p < 0.05, and dissimilarities are distinct with differential symbols
3.4 miR-27a-3p Inhibits HIPK2 Expression
The interaction between miR-27a-3p and HIPK2 in A549 cells has been detected accordingly. Specifically, A549 cells were treated in two ways: 100 nM negative control (NC) with miR-27a-3p mimic; the other was inhibitor negative control (I-NC) with miR-27a-3p inhibitor; then, we examined the level of HIPK2 after 24 h by the method of quantitative RT-PCR. Compared with the NC group, the decline levels of HIPK2 mRNA showed statistical significance (0.63-fold, p < 0.05; Fig. 3C) after the overexpression of miR-27a-3p. On the contrary, the HIPK2 expression levels of miR-27a-3p increased significantly in the inhibitor group compared with the I-NC group (1.46-fold, p < 0.05; Fig. 3C). Due to the overexpression and inhibition of miR-27a-3p can trigger opposite fluctuation in HIPK2 levels, we western blotted the 24 h transfected cells to observe the trend of HIPK2. It showed the same tendency in both protein and mRNA levels (Fig. 3D).
3.5 Formononetin Regulates the Expression of miR-27a-3p and HIPK2 through the p53 Pathway in NSCLC
To clarify the anti-tumor mechanism of formononetin on A549 cells through the mediator miR-27a-3p, different concentrations of formononetin was used to detect the differential effects on miR-27a-3p, HIPK2 expression, and p53 phosphorylation in A549 cells, respectively. As the results showed, compared with the untreated cells, the expression level of miR-27a-3p decreased in A549 cells as the concentrations of formononetin increased (p < 0.05; Fig. 4A). Meanwhile, the changing of HIPK2 protein level in A549 cells was positively correlated with formononetin concentrations (Fig. 2B). Simultaneously, the phosphorylation of p53 went down after incubation with formononetin, especially in the 70 μM and 100 μM formononetin-treated group (p < 0.05) (Fig. 4B). These data showed that formononetin concentration can significantly alter the reaction of miR-27a-3p expression by down-regulating, HIPK2 by up-regulating, and p53 phosphorylation by inhibition.
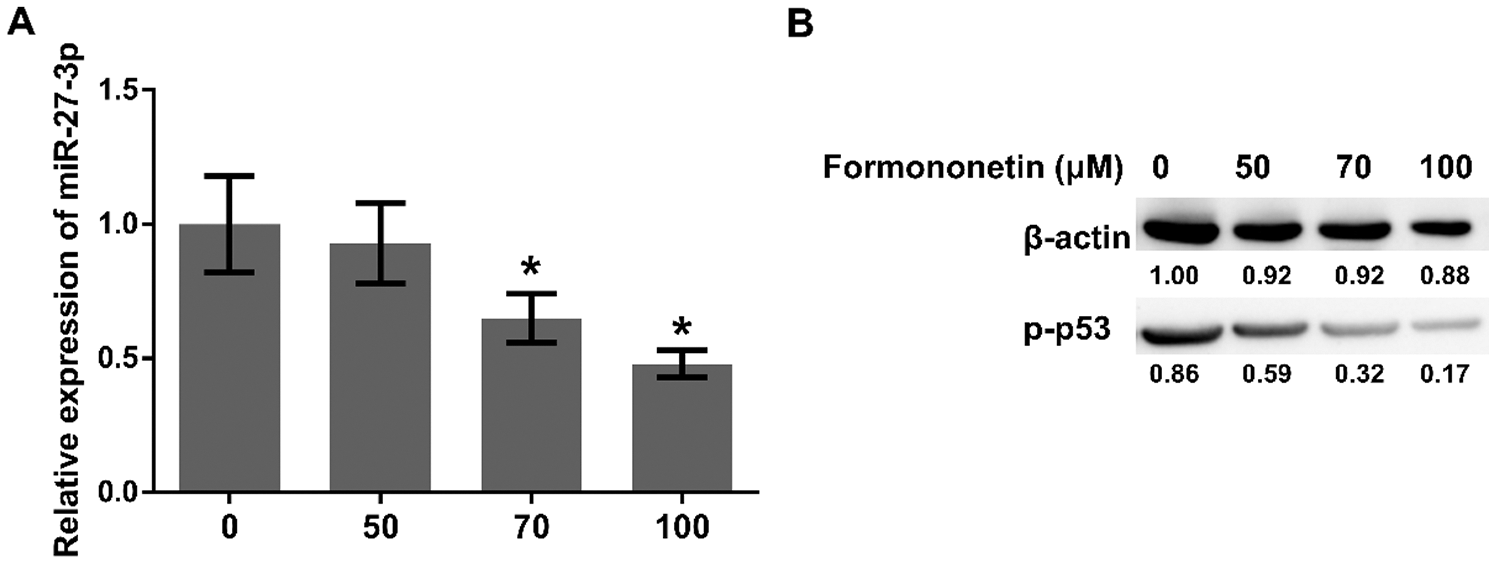
Figure 4: Formononetin regulates the expression of miR-27a-3p and HIPK2 through p53 pathway in NSCLC. (A) The expression of miR-27a-3p for 24 h by RT-qPCR and (B) the protein expression of p-p53 by western blot after treating with formononetin at different concentrations (0, 50, 70, and 100 μM). All experiments were repeated at least three times, and the data are shown in the form of M (mean) ± SD (standard deviation) and processed by one-way ANOVA analysis (*p < 0.05)
Currently, the efficacy of chemoradiotherapy in the clinical treatment of cancer is limited due to drug toxicity. The good news is that there is growing evidence that traditional Chinese medicine has alleviating effects on malignancy with low toxicity and side effects [23,24]. The isoflavone formononetin, an active component of Astragalus membranaceus, has attracted much attention due to its potential anti-cancer potency. The anti-tumor function of formononetin in vitro and in vivo is performed by causing programmed death of cancer cells (by inherent pathway including Bax, Bcl-2, and caspase-3 proteins) or halting their cell cycle(by mediating cyclins like cyclin A, B1, and D1) by interfering with certain signaling pathways, to slow down the speed of cell multiplication and prevent cell invasion [25]. In 2018, Zhang et al. [26] reported that the expression levels of cleaved caspase-3 and caspase-9 in ovarian cancer cells went up after exposure to formononetin. Formononetin inhibits the DNA repairing ability by resulting in the cell division of poly (ADPribose) polymerase (PARP), which consequentially induces apoptosis in human multiple myeloma and nasopharyngeal carcinoma cells [16,27]. In our study, we found that formononetin could induce cell apoptosis. This result is consistent with previous reports [28]. Moreover, formononetin could increase cell proliferation in time- and dose-dependent manner. These results suggested that formononetin might function as an oncogene in NSCLC cells.
Homologous domain interaction protein kinases (HIPKs) are Ser/Thr kinases that interrelate homologous box proteins and other transcription factors and act as transcription co-activators or co-suppressors, HIPK2 is known as the “caretaker” gene because of its activating properties [29,30]. HIPK responds to various extracellular stimuli and regulates a variety of biological processes, such as signal transduction, apoptosis, embryonic development, DNA damage response, and cell proliferation [31]. HIPK2 activation could induce apoptotic function of p53 onco-suppressor, which could further trigger cancer cell death and misfolding in p53 [32,33]. In 2019, Hu et al. [34] reported that exosomal miR-1229 targeting HIPK2 promoted colorectal cancer cell angiogenesis. One study reported that the crystal structure of HIPK’s kinase domain bound to CX-4945, a casein kinase 2α (CK2α) inhibitor currently in clinical trials against several cancers [35]. Another study indicated HIPK2 suppressed pancreatic cancer cell proliferation and aerobic glycolysis via ERK/cMyc axis [36]. Our study found the expression of HIPK2 increased after formononetin treatment, and HIPK2 inhibited p53 phosphorylation in a dose-dependent manner in NSCLC.
miRNAs can regulate post-transcription activity in a wide range of bioprocess, participating in stem cell differentiation, physical growth, disease development, and cancer formation [37–39]. Moreover, miRNAs function as discrete and preserved controllers of tissue identity, the process of which is achieved by its specialized expression based on various tissue and time [40]. Furthermore, miRNAs play dual roles, as oncogenes or tumor suppressors, in the development of cancerous growth [41]. In gastric cancer, miR-27a-3p played an essential role as an oncogene by targeting BTG2 [10], it can also promote GC cell metastasis by causing the transition from epithelial to mesenchymal [42]. Zhang et al. [43] reported that miRNA polymorphisms miR-27 rs895819 were associated with relatively lower evidence of malignancies in Caucasians. Tanaka et al. [44] indicated that miR-27a/b could cause mutation of normal fibroblast into cancer-associated fibroblasts to resist chemotherapy in esophageal cancer. Pei Yue’s data suggested that miR-27-3p could suppress the expression of ING5 by promoting the G1-S phase transition, which proliferates cells [45]. Furthermore, high expression of miR-27a is associated with the remarkable malignancy of breast cancer which causes the overall survival rate to go down with high miR-27a expression [11]. The increased level of miR-27 in thyroid cancer cells imposes its impact on the metastasis, invasion, and angiogenesis activity of cancer cells by targeting downstream genetic coding regions. By this phenomenon, miR27a is likely to be an indictive biocomponent of thyroid cancer [12]. However, the related information of miR-27a-3p in NSCLC is still limited. The results indicated that miR-27a-3p decreased in a dose-dependent manner after formononetin treatment in NSCLC, and we also verified that miR-27a-3p could target HIPK2 3’UTR and further inhibited the expression of HIPK2. These outcomes collectively pointed out that miR-27a-3p might play an important role in NSCLC.
Collectively, our findings demonstrate that formononetin exerts an anti-carcinogenic effect on NSCLC in vitro, in which the underlying mechanisms may be associated with reducing miR-27a-3p, and subsequent upregulation of HIPK2 inhibited p53 pathway (Fig. 5). Thus, these findings improve our understanding of the anti-tumor activity of formononetin, and support that formononetin may have curative and prophylactic applications in NSCLC.
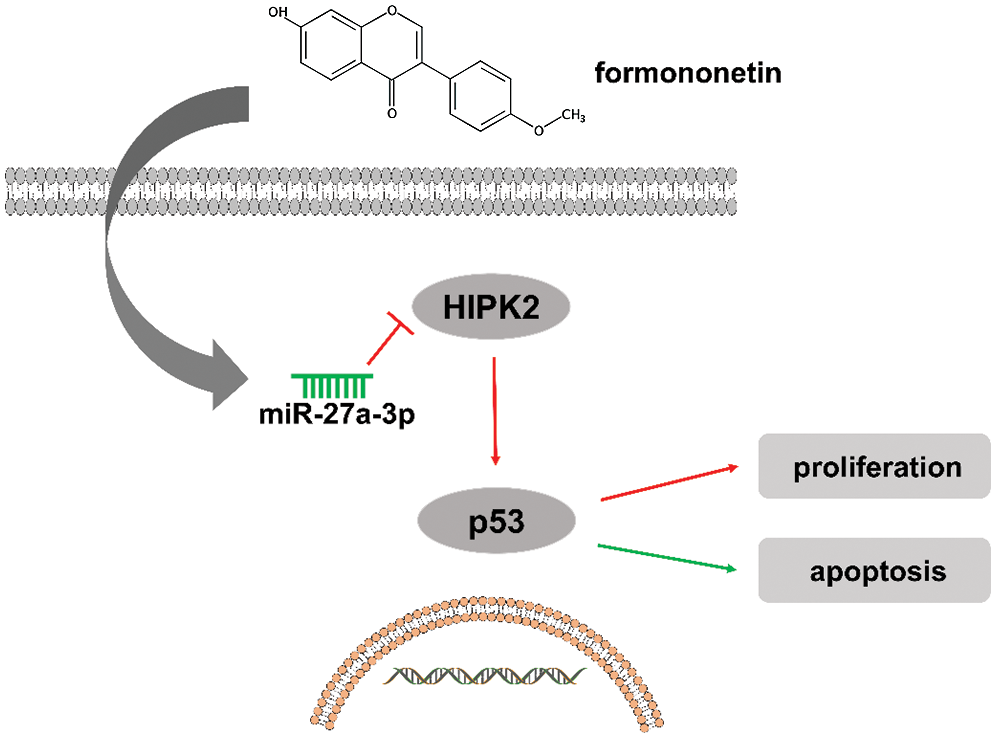
Figure 5: Schematic of the proposed mechanism of formononetin in NSCLC
Formononetin exerts an anti-carcinogenic effect on NSCLC in vitro, in which the underlying mechanisms may be associated with reducing miR-27a-3p, and subsequent upregulation of HIPK2 inhibited p53 pathway.
Funding Statement: This study was supported by the Key Project of Zhejiang Provincial Natural Science Foundation (No. LZ18H270001).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Jonna, S., Subramaniam, D. S. (2019). Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLCAn update. Discovery Medicine, 27(148), 167–170. [Google Scholar]
2. Lee, S. S., Cheah, Y. K. (2019). The interplay between microRNAs and cellular components of tumour microenvironment (TME) on non-small-cell lung cancer (NSCLC) progression. Journal of Immunology Research, 2019(4), 1–12. DOI 10.1155/2019/3046379. [Google Scholar] [CrossRef]
3. Herbst, R. S., Morgensztern, D., Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature, 553(7689), 446–454. DOI 10.1038/nature25183. [Google Scholar] [CrossRef]
4. He, L., Hannon, G. J. (2004). MicroRNAs: Small RNAs with a big role in gene regulation. Nature Reviews Genetics, 5(7), 522–531. DOI 10.1038/nrg1379. [Google Scholar] [CrossRef]
5. Zhang, B., Wang, Q., Pan, X. (2007). MicroRNAs and their regulatory roles in animals and plants. Journal of Cellular Physiology, 210(2), 279–289. DOI 10.1002/jcp.20869. [Google Scholar] [CrossRef]
6. Guo, H., Ingolia, N. T., Weissman, J. S., Bartel, D. P. (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature, 466(7308), 835–840. DOI 10.1038/nature09267. [Google Scholar] [CrossRef]
7. Ambros, V. (2004). The functions of animal microRNAs. Nature, 431(7006), 350–355. DOI 10.1038/nature02871. [Google Scholar] [CrossRef]
8. Qadir, M. I., Faheem, A. (2017). miRNA: A diagnostic and therapeutic tool for pancreatic cancer. Critical Reviews in Eukaryotic Gene Expression, 27(3), 197–204. DOI 10.1615/CritRevEukaryotGeneExpr.2017019494. [Google Scholar] [CrossRef]
9. Lee, Y. S., Dutta, A. (2009). MicroRNAs in cancer. Annual Review of Pathology-Mechanisms of Disease, 4(1), 199–227. DOI 10.1146/annurev.pathol.4.110807.092222. [Google Scholar] [CrossRef]
10. Zhou, L., Liang, X., Zhang, L., Yang, L., Nagao, N. et al. (2016). MiR-27a-3p functions as an oncogene in gastric cancer by targeting BTG2. Oncotarget, 7(32), 51943–51954. DOI 10.18632/oncotarget.10460. [Google Scholar] [CrossRef]
11. Tang, W., Zhu, J., Su, S., Wu, W., Liu, Q. et al. (2012). MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One, 7(12), e51702. DOI 10.1371/journal.pone.0051702. [Google Scholar] [CrossRef]
12. Wang, Y. L., Gong, W. G., Yuan, Q. L. (2016). Effects of miR-27a upregulation on thyroid cancer cells migration, invasion, and angiogenesis. Genetics and Molecular Research, 15(4), 15. [Google Scholar]
13. Liu, P., Zhao, H., Luo, Y. (2017). Anti-aging implications of Astragalus membranaceus (HuangqiA well-known Chinese Tonic. Aging And Disease, 8(6), 868–886. DOI 10.14336/AD.2017.0816. [Google Scholar] [CrossRef]
14. Li, Z., Zeng, G., Zheng, X., Wang, W., Ling, Y. et al. (2018). Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomedicine & Pharmacotherapy, 106(2), 349–354. DOI 10.1016/j.biopha.2018.06.041. [Google Scholar] [CrossRef]
15. Chen, J., Zhao, X., Ye, Y., Wang, Y., Tian, J. (2013). Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cellular Physiology and Biochemistry, 32(6), 1790–1797. DOI 10.1159/000356612. [Google Scholar] [CrossRef]
16. Kim, C., Lee, S. G., Yang, W. M., Arfuso, F., Um, J. Y. et al. (2018). Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Letters, 431, 123–141. [Google Scholar]
17. Park, S., Bazer, F. W., Lim, W., Song, G. (2018). The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation. Journal of Cellular Biochemistry, 119(9), 7377–7387. DOI 10.1002/jcb.27041. [Google Scholar] [CrossRef]
18. Li, T., Zhao, X., Mo, Z., Huang, W., Yan, H. et al. (2014). Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cellular Physiology and Biochemistry, 34(4), 1351–1358. DOI 10.1159/000366342. [Google Scholar] [CrossRef]
19. Liu, X. J., Li, Y. Q., Chen, Q. Y., Xiao, S. J., Zeng, S. E. (2014). Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by formononetin in vitro. Asian Pacific Journal of Cancer Prevention, 15(6), 2835–2839. DOI 10.7314/APJCP.2014.15.6.2835. [Google Scholar] [CrossRef]
20. Zhou, Q., Zhang, W., Li, T., Tang, R., Li, C. et al. (2019). Formononetin enhances the tumoricidal effect of everolimus in breast cancer MDA-MB-468 cells by suppressing the mTOR pathway. Evidence-based Complementary and Alternative Medicine, 2019(3), 1–8. DOI 10.1155/2019/9610629. [Google Scholar] [CrossRef]
21. Xin, M., Wang, Y., Ren, Q., Guo, Y. (2019). Formononetin and metformin act synergistically to inhibit growth of MCF-7 breast cancer cells in vitro. Biomedicine & Pharmacotherapy, 109(5), 2084–2089. DOI 10.1016/j.biopha.2018.09.033. [Google Scholar] [CrossRef]
22. Auyeung, K. K., Law, P. C., Ko, J. K. (2012). Novel anti-angiogenic effects of formononetin in human colon cancer cells and tumor xenograft. Oncology Reports, 28(6), 2188–2194. DOI 10.3892/or.2012.2056. [Google Scholar] [CrossRef]
23. Luo, H., Vong, C. T., Chen, H., Gao, Y., Lyu, P. et al. (2019). Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chinese Medicine, 14(48), 14–48. [Google Scholar]
24. So, T. H., Chan, S. K., Lee, V. H., Chen, B. Z., Kong, F. M. et al. (2019). Chinese medicine in cancer treatment—How is it practised in the east and the west? Clinical Oncology (Royal College of Radiologists), 31(8), 578–588. DOI 10.1016/j.clon.2019.05.016. [Google Scholar] [CrossRef]
25. Tay, K. C., Tan, L. T., Chan, C. K., Hong, S. L., Chan, K. G. et al. (2019). Formononetin: A review of its anticancer potentials and mechanisms. Frontiers in Pharmacology, 10, 820. DOI 10.3389/fphar.2019.00820. [Google Scholar] [CrossRef]
26. Zhang, J., Liu, L., Wang, J., Ren, B., Zhang, L. et al. (2018). Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. Journal of Ethnopharmacology, 221, 91–99. DOI 10.1016/j.jep.2018.04.014. [Google Scholar] [CrossRef]
27. Sun, T., Cao, L., Ping, N. N., Wu, Y., Liu, D. Z. et al. (2016). Formononetin upregulates nitric oxide synthase in arterial endothelium through estrogen receptors and MAPK pathways. Journal of Pharmacy and Pharmacology, 68(3), 342–351. DOI 10.1111/jphp.12519. [Google Scholar] [CrossRef]
28. Yang, Y., Zhao, Y., Ai, X., Cheng, B., Lu, S. (2014). Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. International Journal of Experimental Pathology, 7(12), 8453–8461. [Google Scholar]
29. Garufi, A., Traversi, G., Cirone, M., D’Orazi, G. (2019). HIPK2 role in the tumor-host interaction: Impact on fibroblasts transdifferentiation CAF-like. IUBMB Life, 71(12), 2055–2061. DOI 10.1002/iub.2144. [Google Scholar] [CrossRef]
30. Feng, Y., Zhou, L., Sun, X., Li, Q. (2017). Homeodomain-interacting protein kinase 2 (HIPK2A promising target for anti-cancer therapies. Oncotarget, 8(12), 20452–20461. DOI 10.18632/oncotarget.14723. [Google Scholar] [CrossRef]
31. Conte, A., Pierantoni, G. M. (2018). Update on the regulation of HIPK1, HIPK2 and HIPK3 protein kinases by microRNAs. Microrna, 7(3), 178–186. DOI 10.2174/2211536607666180525102330. [Google Scholar] [CrossRef]
32. Puca, R., Nardinocchi, L., Gal, H., Rechavi, G., Amariglio, N. et al. (2008). Reversible dysfunction of wild-type p53 following homeodomain-interacting protein kinase-2 knockdown. Cancer Research, 68(10), 3707–3714. DOI 10.1158/0008-5472.CAN-07-6776. [Google Scholar] [CrossRef]
33. Stanga, S., Lanni, C., Govoni, S., Uberti, D., D’Orazi, G. et al. (2010). Unfolded p53 in the pathogenesis of Alzheimer’s disease: Is HIPK2 the link? Aging, 2(9), 545–554. DOI 10.18632/aging.100205. [Google Scholar] [CrossRef]
34. Hu, H. Y., Yu, C. H., Zhang, H. H., Zhang, S. Z., Yu, W. Y. et al. (2019). Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. International Journal of Biological Macromolecules, 132, 470–477. DOI 10.1016/j.ijbiomac.2019.03.221. [Google Scholar] [CrossRef]
35. Agnew, C., Liu, L., Liu, S., Xu, W., You, L. et al. (2019). The crystal structure of the protein kinase HIPK2 reveals a unique architecture of its CMGC-insert region. Journal of Biological Chemistry, 294(37), 13545–13559. DOI 10.1074/jbc.RA119.009725. [Google Scholar] [CrossRef]
36. Qin, Y., Hu, Q., Ji, S., Xu, J., Dai, W. et al. (2019). Homeodomain-interacting protein kinase 2 suppresses proliferation and aerobic glycolysis via ERK/cMyc axis in pancreatic cancer. Cell Proliferation, 52(3), e12603. DOI 10.1111/cpr.12603. [Google Scholar] [CrossRef]
37. Bhaskaran, M., Mohan, M. (2014). MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Veterinary Pathology, 51(4), 759–774. DOI 10.1177/0300985813502820. [Google Scholar] [CrossRef]
38. Wienholds, E., Plasterk, R. H. (2005). MicroRNA function in animal development. FEBS Letters, 579(26), 5911–5922. DOI 10.1016/j.febslet.2005.07.070. [Google Scholar] [CrossRef]
39. Houbaviy, H. B., Murray, M. F., Sharp, P. A. (2003). Embryonic stem cell-specific MicroRNAs. Developmental Cell, 5(2), 351–358. DOI 10.1016/S1534-5807(03)00227-2. [Google Scholar] [CrossRef]
40. Wienholds, E., Kloosterman, W. P., Miska, E., Alvarez-Saavedra, E., Berezikov, E. et al. (2005). MicroRNA expression in zebrafish embryonic development. Science, 309(5732), 310–331. DOI 10.1126/science.1114519. [Google Scholar] [CrossRef]
41. Acunzo, M., Romano, G., Wernicke, D., Croce, C. M. (2015). MicroRNA and cancer—A brief overview. Advanced Biological Regulation, 57, 1–9. DOI 10.1016/j.jbior.2014.09.013. [Google Scholar] [CrossRef]
42. Zhang, Z., Liu, S., Shi, R., Zhao, G. (2011). miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genetics, 204(9), 486–491. DOI 10.1016/j.cancergen.2011.07.004. [Google Scholar] [CrossRef]
43. Zhang, H., Zhang, Y., Zhao, X., Ma, X., Yan, W. et al. (2017). Association of two microRNA polymorphisms miR-27 rs895819 and miR-423 rs6505162 with the risk of cancer. Oncotarget, 8(29), 46969–46980. DOI 10.18632/oncotarget.16443. [Google Scholar] [CrossRef]
44. Tanaka, K., Miyata, H., Sugimura, K., Fukuda, S., Kanemura, T. et al. (2015). miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis, 36(8), 894–903. DOI 10.1093/carcin/bgv067. [Google Scholar] [CrossRef]
45. Ye, P., Ke, X., Zang, X., Sun, H., Dong, Z. et al. (2018). Up-regulated MiR-27-3p promotes the G1-S phase transition by targeting inhibitor of growth family member 5 in osteosarcoma. Biomedicine & Pharmacotherapy, 101(9), 219–227. DOI 10.1016/j.biopha.2018.02.066. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |