
 | Oncologie |  |
DOI: 10.32604/Oncologie.2021.018296
ARTICLE
An Overview on the Anticancer Potential of Punarnavine: Prediction of Drug-Like Properties
1Department of Pharmacy, Abasyn University, Peshawar, 25000, Pakistan
2Department of Pharmacy, Abdul Wali Khan University, Mardan, 23200, Pakistan
3Department of Botany, Islamia College University, Peshawar, 25120, Pakistan
4Chitkara College of Pharmacy, Chitkara University, Punjab, 140401, India
*Corresponding Author: Haroon Khan. Email: hkdr2006@gmail.com; haroonkhan@awkum.edu.pk
Received: 14 July 2021; Accepted: 09 September 2021
Abstract: Punarnavine (PN) has been isolated from the roots of Boerhaavia diffusa L. (Nyctaginaceae). It is an important medicinal plant with a vast historical ethnopharmacological background. Its identification resulted from the interrogation of ‘Punarnava’, a tribal medicinal product. The molecule exhibited its position as incipient anticancer therapeutic agent. The inhibition of NFκB, ATF-2, c-Fos, and CREB-1 are one of the underlying mechanisms of anticancer action along with modification of the immune system. These signalling molecules are upregulated in the cancer microenvironment. Punarnavine also modified the release of interleukins, i.e., upregulated IL-2, IL-7, IL-10, IL-12, IFN-γ, while downregulated IL-1β, IL-6, TNF-α, VEGF, and GM-CSF. Punarnavine has exhibited its anticancer potential in murine melanoma cell lines, breast cancer and its metastasis to lungs, liver, and lymph nodes by modifying apoptotic pathways and even suppressing metastatic genes like TIMP-1, TIMP-2, MMP-2, MMP-9 and VEGF. The pharmacokinetic and basic drug discovery properties are also discussed concerning in silico databases. This approach exhibited PN as a successful molecule for drug development. The drug-likeness, solubility, GIT absorption, drug metabolism, and bioavailability of PN are promising of its drug candidature and therefore need further detailed studies to ascertain its clinical potential. This article is a preview of the preclinical anticancer profile of Punarnavine, including the computational approach of its drug-like properties.
Keywords: Punarnavine; plant alkaloid; therapeutic potential; drug likeness
Boerhaavia diffusa L. (BD; family: Nyctaginaceae) is an important ethno-herbal medicine used in Ayurvedic and Unani system of therapeutics in many parts of the world, including India. It is widely distributed in the tropics and subtropics [1].
Medicinal properties of Boerhaavia diffusa have been utilized since long in the indigenous system of medicine in India. It is a perennial, creeping herb found throughout wasteland and grasslands of India; also grown in Western Africa, Angola, Ghana, Nigeria, Sudan and Australia [2]. The roots of Boerhaavia diffusa possess diuretic, laxative, antistress, antioxidant, fibrinolytic, nephroprotective, anti-inflammatory and anticonvulsant activities [2–8]. The roots of BD is commonly known as ‘Punarnava’, which is used by numerous tribes in India for the treatment of hepatic disorders, i.e., liver enlargement, jaundice, ascites, anasarca [9,10]. Different parts of the plant are used as an appetizer, alexiteric, eye tonic, for flushing out the renal system, and to treat blood pressure [11–13].
Punarnavine (PN) is a quinolizidine alkaloid found in B. diffusa. It is isolated from the whole plant-in the alkaloidal fraction [10,14,15]. Syringaresinol mono-β-D-glucoside along with PN was also isolated from roots of BD [16]. The chemical formula of PN is C18H15NO4 and its molecular weight is 309.32 Da (Fig. 1) [17].
The purpose of this article is to summarize the preclinical anticancer potential of PN as a newly emerged drug candidate from natural source. The source of this plant is already used in traditional medicine. Therefore, this natural product will be a new approach of anticancer drug discovery.
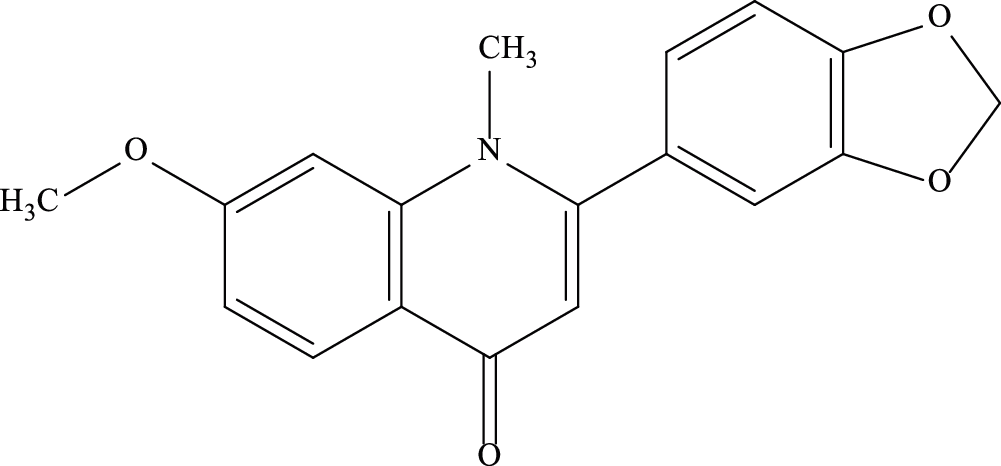
Figure 1: Structure of Punarnavine
In a recent study, analytical method has been developed for PN found in plant extract [18]. A quantitative method based on high performance thin layer chromatography has been developed for the estimation of PN (Rf = 0.73). The gradient mobile phase was used containing toluene, ethyl acetate and formic acid in the ration of 7.0:2.5:0.7 at 262 nm. This validated method was linear in a wide range [(r2 = 0.9971) (100–1000 ng/spot)], precise, and accurate with robustness. The robustness was proved with the Box–Behnken design (BBD). The limit of detection and limit of quantification was 30.3 ng spot−1 and 100.0 ng spot−1, respectively.
3 Preclinical Anticancer Profile of Punarnavine
Manu et al. [18] explored the mechanism of apoptosis in B16F-10 melanoma cells. At nontoxic dose dependent concentrations PN resulted in the formation of apoptotic bodies and DNA fragmentation. This observation was confirmed by cell cycle analysis and TUNEL assays. The apoptotic genes p53 and caspase-3 were upregulated, while the antiapoptotic gene Bcl-2 was downregulated in PN treated cells. The mechanism is shown in Fig. 2. The nuclear translocation of NFκB-c-Rel, NFκBp50, NFκBp65, ATF-2, c-Fos, and CREB-1 was inhibited in PN treated B16 F-10 cells which suggest suppression of NFκB signalling. This is a demonstration of apoptosis induction via p53-induced caspase-3 mediated pro-apoptotic signalling along with suppression of NFκB induced Bcl-2 mediated survival signalling [19].
The NFκB is involved in pathological events, especially cancer. The stimulation of NFκB signalling pathway track towards progression and development of cancer. NFκB upregulation in cancer cells is also involved in resistance to chemotherapy [20–22]. Since it is known that NFκB stimulates expression of oncogenic miR-21 (microRNA) to inhibit PTEN (phosphatase and tensin homolog) expression, creating CP resistance in lung cancer cells. PTEN is a cancer related protein which is encoded by PTEN gene. This PTEN significantly suppress PI3K/Akt and ends up the proliferation and growth of lung cancer cells [23]. The suppression of NFκB signalling is desirable for anticancer mechanism as well as to halt the inflammatory process.
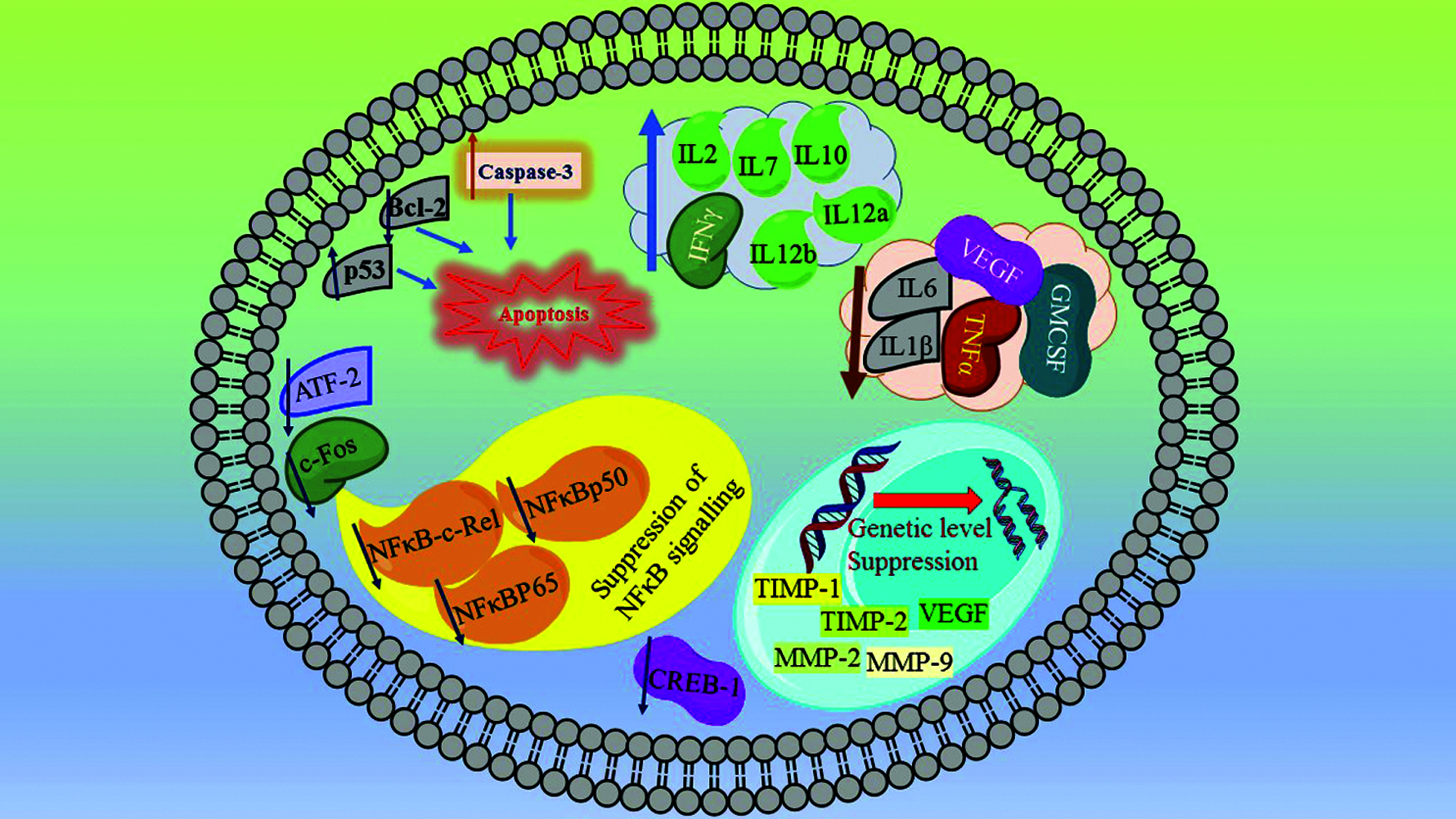
Figure 2: Punarnavine inhibit NFκB signalling-suppress nuclear translocation of NFκB-c-Rel, NFκBp50, NFκBp65, ATF-2, c-Fos, and CREB-1 which ultimately ends up in the inhibition of cancerous proliferation. The activation of p53 and caspase-3, along with suppression of Bcl-2 leads to apoptosis pathway resulting in the death of cancerous cells. From genetic level the suppression of TIMP-1, TIMP-2, MMP-2, and MMP-9 and VEGF arrest the metastatic progression and proliferation of cancer cells. Further the reduction in the cytokines like VEGF, TNF-α, IL-1β, IL-6, and GM-CSF reduce tumour growth and metastasis. Increasing the level of antitumorigenic and antiangiogenic cytokines like IL-2, IL-7, IL-10, IL-12a and IL-12b further synergise the overall anticancer mechanistic spectrum of Punarnavine
Breast cancer become fatal when cancerous cells spread from site of tumour origin and colonise in distant organs. This is followed by systemic plantation wherever it is circulated via blood. PN has already proven its antimetastatic effect on spontaneous B16F10 pulmonary metastasis. To further extend anticancer spectrum, Kallivalappil et al. [23] evaluated its effect on one of a drastic organ-specific breast cancer. A syngenic mouse model (4T1 breast tumour model) was used for the study which mimics the staged-four human breast cancer. PN (40 mg/kg) administration to BALB/c mice reduced metastatic progression of 4T1 to the lymph nodes, liver, and lungs. This was evidenced from the histopathology of these organs. However, metastatic cell density of cultured 6-thioguanine-resistant 4T1 cells followed a declining pattern in the PN-treated group as compared to the control group. Similarly, a significant inhibition (p < 0.0001) of the primary breast tumour growth also confirmed in the orthotopic site of induction. A concomitant increase (p < 0.0001) in the life span of treated animals also confirmed it. Various biochemical parameters, i.e., hydroxyproline, hexosamine, uronic acid, sialic acid, and γ-glutamyl transferase were found reduced in the PN-treated group. The analysis of cytokines like VEGF, TNF-α, IL-1β, and GM-CSF exhibited a similar reduction pattern in PN treated group (p < 0.0001) as compared to the control group.
Following genomics, it was also revealed that PN can suppress metastatic genes. The inhibitory effect was observed for tissue inhibitor matrix metalloproteinase-1 (TIMP-1), TIMP-2, matrix metalloproteinase-2 (MMP-2), MMP-9 and VEGF, that are involved in the metastatic process [24]. TIMPs and MMPs are involved in breast cancer prognosis [25,26]. All these findings are a summation of arresting the metastatic behaviour of breast cancer as well as its progression. The studies are carried out in a murine model, so giving strong evidence for Punarnavine as a drug candidate against breast cancer. TIMP1 is also an important promoter of human colon cancer tumorigenesis and metastasis and a potential prognostic indicator for colon cancer [27].
Immunomodulators modify the activities of immune system. Mostly, synthetic chemotherapeutic agents which are immunosuppressants produce adverse effects. The Immunomodulators derived from plant source are used generally as adaptogens and immunity enhancers with low risk of undesired effects [28,29]. In this extension, PN was also tested following this approach.
PN provoke a non-specific immune response, and increase the phagocytic activity of polymorphonuclear cells, exhibited by the engulfment of candida cells. There was enhancement in the humoral immune response in Plaque Forming Assay. PN increase the synthesis of antibodies, macrophages, and B lymphocyte subsets; bone marrow cellularity and α-esterase positive cells indicating proliferation of stem cells. PN increase the mRNA gene expression of various cytokines, i.e., IL-7; IL-10; IL-12a, and IL-12b. Hence all these effects project PN among potent immunomodulatory agents. Along with these effects PN did not exhibit any toxic effect (1000 mg/kg body weight, oral dose) in mice which classify it as safe ‘drug’ [30].
The immune cells are capable to kill malignant cells which are abnormal and being stressed. Since they can differentiate normal cells from the cancerous cells especially from the activated and inhibited receptor expressions. Upon binding of the activated receptors, the NK cells (immunoregulatory) release pro-inflammatory cytokines, i.e., interferon (IFN)-γ, while the cytotoxic type of NK cells directly attack the target cell [31]. Therefore, anticancer agents with precise immune molecules as targets that support oncogenesis or tumour progression are of importance. This approach is followed by various scientists as well who design anticancer immunomodulatory agents [32,33].
Manu et al. [10] studied the effect of PN on the cell-mediated immune response in metastatic condition using C57BL/6 mice model. Administration of PN increased the activity of natural killer cells, antibody-dependent cellular cytotoxicity, and antibody-dependent complement mediated cytotoxicity. Production of cytokines (IL-2, IFN-γ) is enhanced after PN administration as compared to the metastatic tumour-bearing control group. However, as compared to metastatic control group, the pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α are lowered. This enhanced immune response provoked against B16F-10 melanoma cells’ metastatic progression in mice [10]. In a similar study by Manu et al. [33] in Balb/c mice, PN (40 mg/kg i.p.) enhanced total WBC count. After PN treatment along with sheep red blood cells (antigen), the circulating antibody titre and number of plaques forming cells in the spleen were increased. The proliferation of thymocytes, splenocytes, and bone marrow cells also increased in both the presence and absence of specific mitogens. This shows the immunomodulatory activity of PN [34]. The pathways and molecular mechanisms targeted by Punarnavine in a cancerous cell are exhibited in Fig. 2.
4 Other Cancer Related Activities
The in vivo genotoxic potential of PN was evaluated as per OECD guideline 474, 1997. The activity was performed using the comet assay on liver, brain, lymphocytes, spleen, and bone marrow together with micronucleus test in bone marrow cells including in vitro chromosomal aberration test. There were no genotoxic effects observed at all doses exhibited in the comet assay, or any clastogenic effect in the micronucleus test [35]. The three tested doses of PN inhibited DNA damage induced by cyclophosphamide for all cells evaluated. Hence, no genotoxic or clastogenic effects were identified in these experiments. However, this significant decrease in cyclophosphamide induced DNA damage is beneficial protective effect. The antigenotoxic properties of PN may be of great value in cancer prevention and pharmacological intervention [35].
4.2 Antimetastatic Potential of Punarnavine
The anti-metastatic activity of PN was studied in B16F-10 melanoma cells in C57BL/6 mice. PN administration at 40 mg/kg body weight could inhibit the metastatic colony formation of melanoma in lungs; prophylactically the inhibition was 95.25%, with simultaneous administration it was 93.9% and 10 days after tumour inoculation 80.1% inhibition was observed. Survival rate of the animals bearing metastatic tumour was increased significantly in all modalities as compared to the metastatic tumour bearing untreated control group. The biochemical parameters and histopathological studies also were in line with pharmacological actions, i.e., lung collagen hydroxyl proline, hexosamine, uronic acid, serum γ-glutamyl transpeptidase serum sialic acid, and serum vascular endothelial growth factor (VEGF) levels. PN administration down regulated the expression of matrix metalloproteinases, i.e., MMP-2 and MMP-9. The expression of extracellular signal regulated kinases, i.e., ERK-1, ERK-2, and VEGF were also down regulated in the lung tissue of metastatic tumour bearing animals. This inhibition of MMP-2 and MMP-9 protein expression was also exhibited in gelatin zymographic analysis of B16F-10 cells [15].
Saraswati et al. [36] determined the in vivo antiangiogenic activity using sponge implant angiogenesis assay and antitumor activity against Ehrlich ascites carcinoma tumour [36]. PN inhibited the migration and invasion of endothelial cell and capillary structure formation in Human umbilical vein endothelial cells (HUVECs). PN significantly inhibited the expression of MMP-2 and MMP-9 at 50 µM in HUVECs in an in vitro experiment. In sponge implant assay PN also inhibited neovascularization. PN treatment exhibited a dose-dependent decrease in the ascitic fluid volume (60.94% reduction) and tumour volume (86.40% reduction) in Ehrlich ascites model at 15 mg/kg/day. There was a reduction in peritoneal angiogenesis as well. And this effect can be extended to develop a therapeutic tool for treatment of cancer [36].
All these anticancer activities and the underlying mechanisms are summarized in Tab. 1.

5 Prediction of Drug-Like Properties
‘‘Drug-like’’ property depends upon mode of administration. The original rule of five (RO5) deal with orally active compounds and define four simple physicochemical parameter ranges, i.e., MWT ≤ 500, log P ≤ 5 H-bond donors ≤ 5, and H-bond acceptors ≤ 10. These properties are associated with 90% of the orally active drugs that have achieved phase II clinical status and have acceptable aqueous solubility with intestinal permeability which are the first steps in oral bioavailability [37].
The physicochemical parameters and drug like properties (Tab. 2) were calculated with SwissADME, a free web tool used in medicinal chemistry [38]. The structure of Punarnavine is composed of carbon, hydrogens, nitrogen, and oxygen. The molecular weight is 309.32 g/mol. Drugs having molecular weight < 500 are more easily absorbed through git and can be formulated in oral dosage form. Rotatable bond count is a widely used ‘drug filter’ which guides that > 10 rotatable bonds correlates with decreased rat oral bioavailability [39]. While PN have only 2 rotatable bonds. The mechanistic basis for the ‘rotatable bond filter’ is unclear since its count does not correlate with in vivo clearance rate in the rat. But still the filter is reasonable from a viewpoint of in vitro screening because ligand affinity decreases 0.5 kcal (average) for each two rotatable bonds [40]. The H-bond acceptor and donor are 4 and zero, respectively. Both are within accurately strict limit. Oral drugs have fewer H-bond acceptors, donors and rotatable bonds [37].
The molar refractivity was found to be 87.46, total polar surface area (TPSA) 49.69 Å2, Log Po/w (average of iLOGP, XLOGP3, WLOGP, MLOGP, SILICOS-IT) 2.87, and the molecule is moderately water soluble [41,42].
The molecule satisfies all the Lipinski’s drug likeness parameters and there is no violation observed. Lead likeness shows the capability to serve as ‘lead’ molecule in the drug discovery process. PN has not been synthesized in the lab yet. However, the swiss database predicted its synthetic accessibility of 2.78, which suggest very easy step reactions of synthesis. The difficult molecules to synthesize are those having a score of 10.
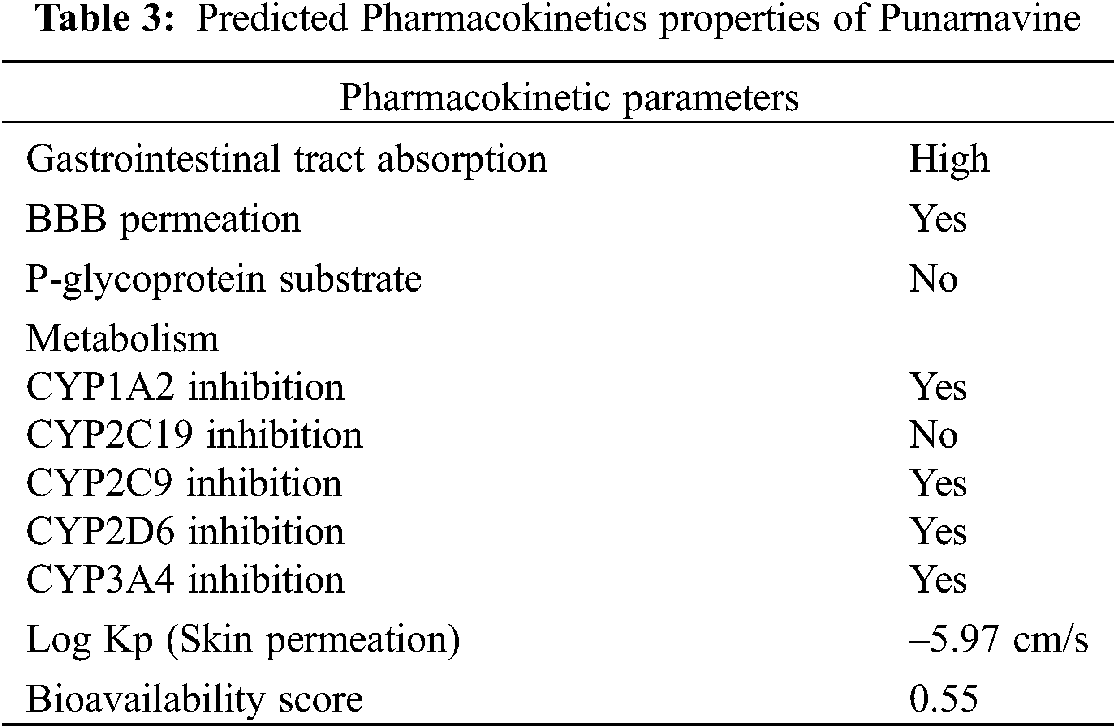
Pharmacokinetics (PK) plays an important role in achieving the pharmacological goals of drug administration. Every parameter has its own limiting criteria. Absorption is the first phase to gain access to body tissues, followed by distribution throughout body, getting biotransformation and move out via excretion routes. However, the PK parameters of PN are not studied. Therefore, an initial in silico screening is desirable (Tab. 3). The predicted pharmacokinetics with SwissADME database, revealed high GI absorption [38]. The forecast of bioavailability and permeability is very important before proceeding towards purchasing, synthesising or any advanced testing. Since a probability-based score is assigned to a drug candidate that it will have, F > 10% in rat. The Bioavailability score obtained for PN is 0.55 [43]. It was identified as permeable through blood brain barrier and not a substrate for P-glycoprotein. This implies the lower potential of developing pharmacokinetic drug interactions [44]. The molecule can inhibit some of the major Cytochrome P450 enzymes, i.e., CYP1A2, CYP2C9, CYP2D6, CYP3A4. However, it is not an inhibitor of CYP2C19. The molecule has skin permeation as well, exhibited as Log Kp −5.97 cm/s [45].
6 Outlook: Challenges and Approaches
In the wet lab PN has demonstrated a range of anticancer mechanisms. PN modify NFκB which is a cellular signal transduction pathway involved in physiologic and pathologic pathways. This is a family of homodimeric and/or heterodimeric transcription factors (TFs) formed by the combination of RelA, RelB, cRel, p50 and p52. The combinatorial association of RelA/p50 upregulates a broad spectrum of innate immunity genes, which encode for antimicrobial peptides (like β-defensin), cytokines (e.g., TNF) and their receptors, pro-inflammatory chemokines and cell adhesion molecules [46,47]. The inhibition of NFκBp65 nuclear translocation is also been involved in reducing apoptosis of the hippocampal neurons, alleviating their inflammation and oxidative stress [48]. Similarly, cyclic AMP-response element-binding protein 1 (CREB-1) is a stimulus-induced 43 kDa transcription factor which is overexpressed in hematopoietic and solid tumours (CREB-associated cancers; CACs). Some of the CACs are acute myeloid leukaemia, acute lymphoblastic leukaemia, chronic lymphatic leukaemia, Hodgkin’s lymphoma, melanoma; oesophageal, gastric, hepatocellular, pancreatic, lung, breast renal cell, ovarian and prostate carcinoma, including brain tumours [49–52].
Activating Transcription Factor-2 (ATF-2) is a sequence-specific DNA binding protein. It is stress induced and upon stimulation it activates gene targets like cyclin A, cyclin D, and c-jun, which cause oncogenesis in various tissues. ATF-2 expression has been correlated with maintenance of a cancer cell phenotype [53]. Therefore, inhibition of NFκB, ATF-2, c-Fos, and CREB-1 by PN could be a new strategy of drug development for the treatment of human cancers and inflammatory diseases. IL-1β, a potent inflammatory cytokine promote tumour growth and metastasis both in animals and human breast cancer model [54]. This IL-1β is also one of the targets of PN, which diminish its level, promoting anticancer interplay.
PN modify the release of various cytokines which are discussed in the subsequent paragraphs and summarized in Fig. 3. PN increase the level of interleukin-2, a key cytokine approved for the treatment of cancer immunotherapy like metastatic renal cell carcinoma, and metastatic melanoma. The IL-2 might be used as monotherapy or in combination with other anticancer immunotherapies [55].
Interleukin-6 is overexpressed in the tumour microenvironment and is known to be deregulated in cancer. The high level of IL-6 promotes tumorigenesis in the tumour microenvironment by modifying multiple signalling pathways, i.e., apoptosis, proliferation, invasiveness, angiogenesis, metastasis, metabolism and the survival [56]. Further, the upregulation of IL-6 promotes multidrug resistance (MDR) via activation of Janus kinases/signal transducer and activator of transcription 3 (JAK/STAT3), phosphatidylinositol-3 kinase/protein kinase B (PI3K/Akt), and mitogen-activated protein kinase pathways (Ras-MAPK) [57]. Therefore, blocking of IL-6 by PN is important from therapeutic perspective as well as to encounter chemo-resistance.
Upregulated gene IL-7 is previously correlated with human oral cancer-associated Tertiary lymphoid structure. The tertiary lymphoid structure offers a local microenvironment for cellular and humoral immunity. The TLS support effective antigen presentation and lymphocyte activation [58,59]. Li et al. [58] favours the upregulation of IL-7 by PN, enhancing its anticancer mechanistic spectrum.
PN increase the level of Interleukin-10 (IL-10), an immune regulatory cytokine with both immunosuppressive and antiangiogenic functions. IL-10 is released by immune cells, i.e., T lymphocytes, natural killer cells, macrophages. However, it is also noted that IL-10 promotes tumour cell proliferation and metastasis via immunosuppression. IL-10 inhibits NFκB translocation which is in favour of its anti-inflammatory and anticancer potential [60]. These findings make IL-10 as a possible therapeutic target in the prognosis of cancer.
IL-12 is an immune-activating cytokine, which increased the infiltration by NK cells and CD8α+ T cells, while decreased CD4+Foxp3+ regulatory T cells in mice tumours [61]. IL-12 also inhibits tumour angiogenesis. IL-12 expression locally in the tumours or utilizing various dosage forms/vectors can devoid systemic toxicity. This is capable of efficient anti-tumour immunity which can be synergized with anti-angiogenic or immunomodulator drugs with safety [62].
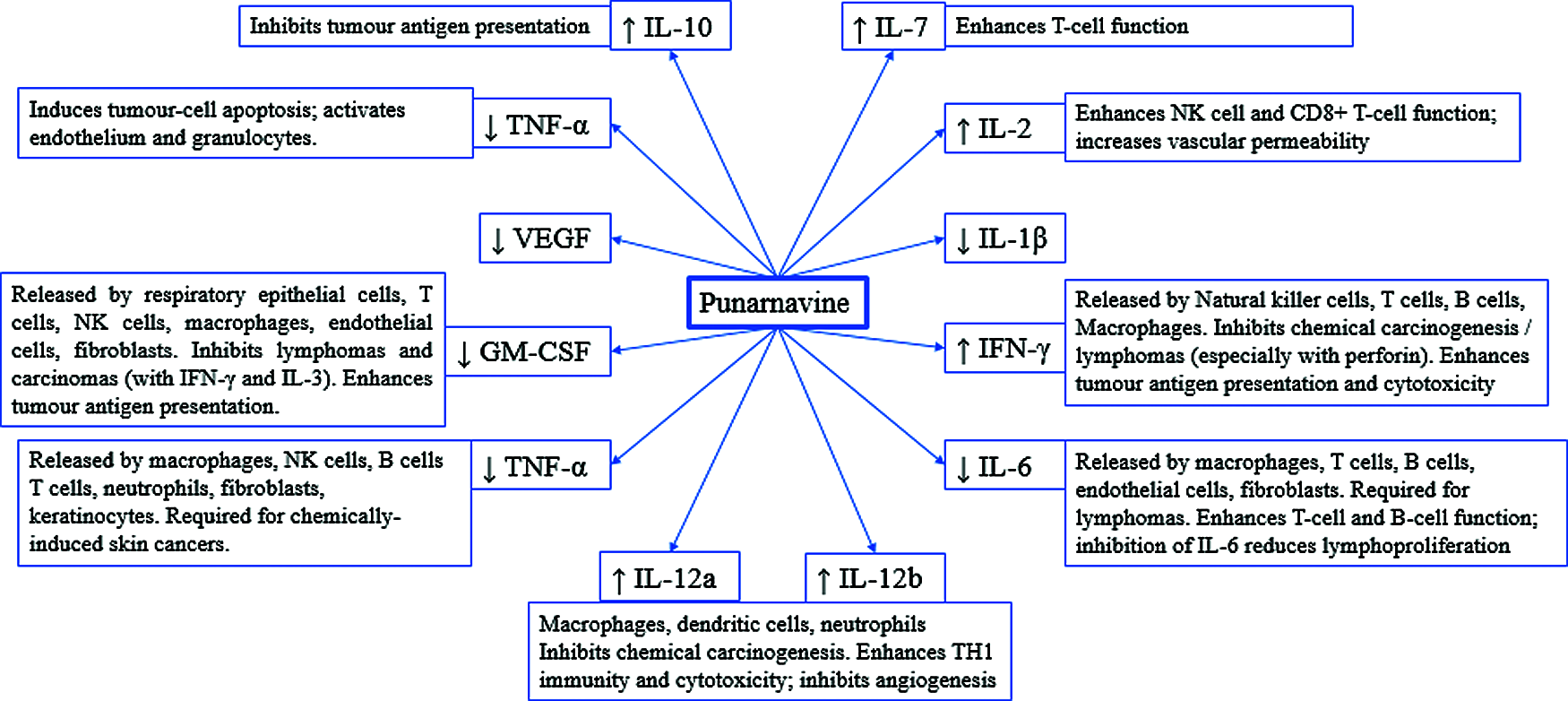
Figure 3: Effect of Punarnavine on cytokines, ultimately modifying the fate of cancer
Summarizing this writing by presenting Punarnavine to the drug discovery arena which has just caught in the net of a pharmaceutical scientist. The molecule has proved itself preclinically and pharmacologically, especially its anticancer drug-ability by modifying molecular mechanisms. From modifying cytokines to oncogenic pathways and oncogenes, Punarnavine can be explored further both mechanistically and pharmacologically. Its anticancer spectrum also needs to explore in several other cancers like prostate cancer, adenocarcinoma, cervical cancer etc. The pharmacokinetic profile of PN is lacking which may halt or slow down its drug development process. However, the computational approach has given a green signal for its drug candidature. Due to its small size, it can be a successful drug candidate during pharmacokinetic profiling. And if any difficulty arises, novel drug delivery approaches can be employed to solve the bioavailability problem. Chemical modification is another way of improving the existing molecule safety, efficacy, and pharmacokinetics. These are some of the challenges that need attention. Punarnavine is used as traditional medicine since long time by the name of ‘punarnava’. This article tends to rationalise its use as anticancer, based on studies related to cancer. The studies revealed about the pharmacologic effects of Punarnavine in pure form. The article is also helpful in drug development process for a medicinal chemist as well to synthesize derivatives and further rationalise the structure-activity relationship.
Funding Statement: The authors did not get any funding.
Conflicts of Interest: The authors of this article have no conflicts of interest.
1. Murti, K., Panchal, M. A., Lambole, V. (2010). Pharmacological properties of Boerhaavia diffusa–A review. International Journal of Pharmaceutical Sciences Review and Research, 5(2), 107–110. [Google Scholar]
2. Adesina, S. K. (2008). Anticonvulsant properties of the roots of Boerhaavia diffusa Linnaeus. Quarterly Journal of Crude Drug Research, 17(2), 84–86. DOI 10.3109/13880207909067455. [Google Scholar] [CrossRef]
3. Kirtikar, K., Basu, B. (1933). Indian medicinal plants, vol. 3, pp. 1823–1824. Deheradun: International Book Distributors. [Google Scholar]
4. Rawat, A., Mehrotra, S., Tripathi, S., Shome, U. (1997). Hepatoprotective activity of Boerhaavia diffusa L. roots—A popular Indian ethnomedicine. Journal of Ethnopharmacology, 56(1), 61–66. DOI 10.1016/S0378-8741(96)01507-3. [Google Scholar] [CrossRef]
5. Bhalla, T. (1971). Anti-inflammatory activity of Boerhaavia diffusa L. Indian Journal of Medical Research, 6, 11–15. [Google Scholar]
6. Sumanth, M., Mustafa, S. (2007). Antistress, adoptogenic and immunopotentiating activity roots of Boerhaavia diffusa in mice. International Journal of Pharmacology, 3(5), 416–420. DOI 10.3923/ijp.2007.416.420. [Google Scholar] [CrossRef]
7. Jain, G., Khanna, N. (1989). Punarnavoside: A new antifibrinolytic agent from Boerhaavia diffusa Linn. ChemInform, 20(34), 163. DOI 10.1002/chin.198934353. [Google Scholar] [CrossRef]
8. Olaleye, M. T., Akinmoladun, A. C., Ogunboye, A. A., Akindahunsi, A. A. (2010). Antioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in rats. Food and Chemical Toxicology, 48(8–9), 2200–2205. DOI 10.1016/j.fct.2010.05.047. [Google Scholar] [CrossRef]
9. Singh, V., Pandey, R. (1980). Medicinal plant lore of the tribals of Eastern Rajasthan. Journal of Economic and Taxonomic Botany, 1, 137–147. [Google Scholar]
10. Manu, K., Kuttan, G. (2008). Effect of punarnavine, an alkaloid from Boerhaavia diffusa, on cell-mediated immune responses and TIMP-1 in B16F-10 metastatic melanoma-bearing mice. Immunopharmacology and Immunotoxicology, 29(3–4), 569–586. DOI 10.1080/08923970701692676. [Google Scholar] [CrossRef]
11. Hiruma-Lima, C., Gracioso, J., Bighetti, E., Robineou, L. G., Brito, A. S. (2000). The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. Journal of Ethnopharmacology, 71(1–2), 267–274. DOI 10.1016/S0378-8741(00)00178-1. [Google Scholar] [CrossRef]
12. Riaz, H., Raza, S. A., Hussain, S., Mahmood, S., Malik, F. (2014). An overview of ethnopharmacological properties of Boerhaavia diffusa. African Journal of Pharmacy and Pharmacology, 8(2), 49–58. DOI 10.5897/AJPP2013.3718. [Google Scholar] [CrossRef]
13. Aruna, R., Ethirajulu, S., Pandian, J. S. J. (2014). Pharmacognostical studies on siddha medicinal plant Boerhaavia diffusa L. Research Journal of Pharmacognosy and Phytochemistry, 6(4), 156. [Google Scholar]
14. Shrivastava, N., Padhya, M. (1995). ‘Punarnavine’ profile in the regenerated roots of Boerhaavia diffusa L. from leaf segments. Current Science, 68(6), 653–656. [Google Scholar]
15. Manu, K., Kuttan, G. (2009). Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology, 214(4), 245–255. DOI 10.1016/j.imbio.2008.10.002. [Google Scholar] [CrossRef]
16. Mishra, S., Aeri, V., Gaur, P. K., Jachak, S. M. (2014). Phytochemical, therapeutic, and ethnopharmacological overview for a traditionally important herb: Boerhavia diffusa Linn. BioMed Research International, 2014(5), 1–19. DOI 10.1155/2014/808302. [Google Scholar] [CrossRef]
17. Udo, U. E., Echeme, J. O., Igwe, O. U., Sunday, T. P. (2020). β-Stigmasterol is present in the stembark of Lonchocarpus sericeus Poir. (Papilionaceae). Journal of Pharmacognosy and Phytochemistry, 9(1), 1750–1755. [Google Scholar]
18. Ahmad, W., Husain, I., Ahmad, N., Amir, M., Sarafroz, M. et al. (2020). Box-Behnken supported development and validation of robust HPTLC method: An application in estimation of punarnavine in leaf, stem, and their callus of Boerhavia diffusa Linn. 3 Biotech, 10(4), 1–10. DOI 10.1007/s13205-020-2154-1. [Google Scholar] [CrossRef]
19. Manu, K. A., Kuttan, G. (2009). Punarnavine induces apoptosis in B16F-10 melanoma cells by inhibiting NF-kB signaling. Asian Pacific Journal of Cancer Prevention, 10(6), 1031–1038. [Google Scholar]
20. Su, R. L., Qiao, Y., Guo, R. F., Lv, Y. Y. (2019). Cyr61 overexpression induced by interleukin 8 via NF-kB signaling pathway and its role in tumorigenesis of gastric carcinoma in vitro. International Journal of Clinical and Experimental Pathology, 12(9), 3197. [Google Scholar]
21. Khongthong, P., Roseweir, A. K., Edwards, J. (2019). The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocrine-Related Cancer, 26(6), R369–R380. DOI 10.1530/ERC-19-0087. [Google Scholar] [CrossRef]
22. Chang, K. S., Tsui, K. H., Lin, Y. H., Hou, C. P., Feng, T. H. et al. (2019). Migration and invasion enhancer 1 is an NF-ĸB-inducing gene enhancing the cell proliferation and invasion ability of human prostate carcinoma cells in vitro and in vivo. Cancers, 11(10), 1486. DOI 10.3390/cancers11101486. [Google Scholar] [CrossRef]
23. Matloob, A. M., Abd El-Hafiz, D. R., Saad, L., Mikhail, S., Guirguis, D. (2019). Metal organic framework-graphene nano-composites for high adsorption removal of DBT as hazard material in liquid fuel. Journal of Hazardous Materials, 373(9), 447–458. DOI 10.1016/j.jhazmat.2019.03.098. [Google Scholar] [CrossRef]
24. Kallivalappil, G. G., Kuttan, G. (2019). Efficacy of punarnavine in restraining organ-specific tumour progression in 4T1-induced murine breast tumour model. Inflammopharmacology, 27(4), 701–712. DOI 10.1007/s10787-018-0490-0. [Google Scholar] [CrossRef]
25. Jinga, D., Blidaru, A., Condrea, I., Ardeleanu, C., Dragomir, C. et al. (2006). MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in breast cancer: Correlations with prognostic factors. Journal of Cellular and Molecular Medicine, 10(2), 499–510. DOI 10.1111/j.1582-4934.2006.tb00415.x. [Google Scholar] [CrossRef]
26. Dofara, S. G., Chang, S. L., Diorio, C. (2020). Gene polymorphisms and circulating levels of MMP-2 and MMP-9: A review of their role in breast cancer risk. Anticancer Research, 40(7), 3619–3631. DOI 10.21873/anticanres.14351. [Google Scholar] [CrossRef]
27. Song, G., Xu, S., Zhang, H., Wang, Y., Xiao, C. et al. (2016). TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. Journal of Experimental & Clinical Cancer Research, 35(1), 7. DOI 10.1186/s13046-016-0427-7. [Google Scholar] [CrossRef]
28. Baroroh, H. N., Nugroho, A. E., Lukitaningsih, E., Nurrochmad, A. (2020). Water-soluble fiber from Bengkoang (Pachyrhizus erosus (L.) urban) tuber modulates immune system activity in male mice. Scientia Pharmaceutica, 88(3), 34. DOI 10.3390/scipharm88030034. [Google Scholar] [CrossRef]
29. Sobhani, M., Farzaei, M. H., Kiani, S., Khodarahmi, R. (2021). Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Reviews International, 37(8), 759–811. DOI 10.1080/87559129.2020.1717523. [Google Scholar] [CrossRef]
30. Aher, V. D., Chattopadhyay, P., Patra, A. (2020). Immunomodulatory activity of Punarnavine Alkaloid from Boerhaavia diffusa. Current Bioactive Compounds, 16(4), 460–468. DOI 10.2174/1573407214666181119122711. [Google Scholar] [CrossRef]
31. Vogler, M., Shanmugalingam, S., Särchen, V., Reindl, L. M., Grèze, V. et al. (2021). Unleashing the power of NK cells in anticancer immunotherapy. Journal of Molecular Medicine, 1–13. [Google Scholar]
32. El-Zahabi, M. A., Sakr, H., El-Adl, K., Zayed, M., Abdelraheem, A. S. et al. (2020). Design, synthesis, and biological evaluation of new challenging thalidomide analogs as potential anticancer immunomodulatory agents. Bioorganic Chemistry, 104(12), 104218. DOI 10.1016/j.bioorg.2020.104218. [Google Scholar] [CrossRef]
33. Petroni, G., Formenti, S. C., Chen-Kiang, S., Galluzzi, L. (2020). Immunomodulation by anticancer cell cycle inhibitors. Nature Reviews Immunology, 20(11), 669–679. DOI 10.1038/s41577-020-0300-y. [Google Scholar] [CrossRef]
34. Manu, K. A., Kuttan, G. (2009). Immunomodulatory activities of Punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacology and Immunotoxicology, 31(3), 377–387. DOI 10.1080/08923970802702036. [Google Scholar] [CrossRef]
35. Aher, V., Chattopadhyay, P., Goyary, D., Veer, V. (2013). Evaluation of the genotoxic and antigenotoxic potential of the alkaloid punarnavine from Boerhaavia diffusa. Planta Medica, 79(11), 939–945. DOI 10.1055/s-00000058. [Google Scholar] [CrossRef]
36. Saraswati, S., Alhaider, A. A., Agrawal, S. (2013). Punarnavine, an alkaloid from Boerhaavia diffusa exhibits anti-angiogenic activity via downregulation of VEGF in vitro and in vivo. Chemico-Biological Interactions, 206(2), 204–213. DOI 10.1016/j.cbi.2013.09.007. [Google Scholar] [CrossRef]
37. Lipinski, C. A. (2004). Lead-and drug-like compounds: The rule-of-five revolution. Drug Discovery Today: Technologies, 1(4), 337–341. DOI 10.1016/j.ddtec.2004.11.007. [Google Scholar] [CrossRef]
38. Daina, A., Michielin, O., Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7(1), 40. DOI 10.1038/srep42717. [Google Scholar] [CrossRef]
39. Veber, D. F., Johnson, S. R., Cheng, H. Y., Smith, B. R., Ward, K. W. et al. (2002). Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, 45(12), 2615–2623. DOI 10.1021/jm020017n. [Google Scholar] [CrossRef]
40. Andrews, P., Craik, D., Martin, J. (1984). Functional group contributions to drug-receptor interactions. Journal of Medicinal Chemistry, 27(12), 1648–1657. DOI 10.1021/jm00378a021. [Google Scholar] [CrossRef]
41. Daina, A., Michielin, O., Zoete, V. (2014). iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. Journal of Chemical Information and Modeling, 54(12), 3284–3301. DOI 10.1021/ci500467k. [Google Scholar] [CrossRef]
42. Moriguchi, I., Hirono, S., Liu, Q., Nakagome, I., Matsushita, Y. (1992). Simple method of calculating octanol/water partition coefficient. Chemical and Pharmaceutical Bulletin, 40(1), 127–130. DOI 10.1248/cpb.40.127. [Google Scholar] [CrossRef]
43. Martin, Y. C. (2005). A bioavailability score. Journal of Medicinal Chemistry, 48(9), 3164–3170. DOI 10.1021/jm0492002. [Google Scholar] [CrossRef]
44. Zhang, H., Xu, H., Ashby, C. R.Jr, Assaraf, Y. G., Chen, Z. S. et al. (2021). Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Medicinal Research Reviews, 41(1), 525–555. DOI 10.1002/med.21739. [Google Scholar] [CrossRef]
45. Potts, R. O., Guy, R. H. (1992). Predicting skin permeability. Pharmaceutical Research, 9(5), 663–669. DOI 10.1023/A:1015810312465. [Google Scholar] [CrossRef]
46. Dorrington, M. G., Fraser, I. D. (2019). NF-κB signaling in macrophages: Dynamics, crosstalk, and signal integration. Frontiers in Immunology, 10, 921. DOI 10.3389/fimmu.2019.00705. [Google Scholar] [CrossRef]
47. Chawla, M., Roy, P., Basak, S. (2021). Role of the NF-κB system in context-specific tuning of the inflammatory gene response. Current Opinion in Immunology, 68(2), 21–27. DOI 10.1016/j.coi.2020.08.005. [Google Scholar] [CrossRef]
48. Zhang, L., Zhou, Q., Zhou, C. L. (2021). RTA-408 protects against propofol-induced cognitive impairment in neonatal mice via the activation of Nrf2 and the inhibition of NF-κB p65 nuclear translocation. Brain and Behavior, 11(1), e01918. [Google Scholar]
49. Chen, P., Li, M., Hao, Q., Zhao, X., Hu, T. (2018). Targeting the overexpressed CREB inhibits esophageal squamous cell carcinoma cell growth. Oncology Reports, 39(3), 1369–1377. [Google Scholar]
50. Siu, Y. T., Jin, D. Y. (2007). CREB−A real culprit in oncogenesis. FEBS Journal, 274(13), 3224–3232. DOI 10.1111/j.1742-4658.2007.05884.x. [Google Scholar] [CrossRef]
51. Xia, Y., Zhan, C., Feng, M., Leblanc, M., Ke, E. et al. (2018). Targeting CREB pathway suppresses small cell lung cancer. Molecular Cancer Research, 16(5), 825–832. DOI 10.1158/1541-7786.MCR-17-0576. [Google Scholar] [CrossRef]
52. Steven, A., Friedrich, M., Jank, P., Heimer, N., Budczies, J. et al. (2020). What turns CREB on? And off? And why does it matter? Cellular and Molecular Life Sciences, 77, 4049–4067. [Google Scholar]
53. Vlahopoulos, S. A., Logotheti, S., Mikas, D., Giarika, A., Gorgoulis, V. et al. (2008). The role of ATF-2 in oncogenesis. BioEssays, 30(4), 314–327. DOI 10.1002/(ISSN)1521-1878. [Google Scholar] [CrossRef]
54. Guo, B., Fu, S., Zhang, J., Liu, B., Li, Z. (2016). Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Scientific Reports, 6(1), 646. DOI 10.1038/srep36107. [Google Scholar] [CrossRef]
55. Jiang, T., Zhou, C., Ren, S. (2016). Role of IL-2 in cancer immunotherapy. OncoImmunology, 5(6), e1163462. DOI 10.1080/2162402X.2016.1163462. [Google Scholar] [CrossRef]
56. Kumari, N., Dwarakanath, B., Das, A., Bhatt, A. N. (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biology, 37(9), 11553–11572. DOI 10.1007/s13277-016-5098-7. [Google Scholar] [CrossRef]
57. Ghandadi, M., Sahebkar, A. (2016). Interleukin-6: A critical cytokine in cancer multidrug resistance. Current Pharmaceutical Design, 22(5), 518–526. DOI 10.2174/1381612822666151124234417. [Google Scholar] [CrossRef]
58. Li, K., Guo, Q., Zhang, X., Dong, X., Liu, W. et al. (2020). Oral cancer-associated tertiary lymphoid structures: Gene expression profile and prognostic value. Clinical & Experimental Immunology, 199(2), 172–181. DOI 10.1111/cei.13389. [Google Scholar] [CrossRef]
59. Gao, J., Zhao, L., Liu, L., Yang, Y., Guo, B. et al. (2017). Disrupted fibroblastic reticular cells and interleukin‐7 expression in tumor draining lymph nodes. Oncology Letters, 14(3), 2954–2960. DOI 10.3892/ol.2017.6537. [Google Scholar] [CrossRef]
60. Sheikhpour, E., Noorbakhsh, P., Foroughi, E., Farahnak, S., Nasiri, R. et al. (2018). A survey on the role of interleukin-10 in breast cancer: A narrative. Reports of Biochemistry & Molecular Biology, 7(1), 30. [Google Scholar]
61. Hwang, M. P., Fecek, R. J., Qin, T., Storkus, W. J., Wang, Y. (2020). Single injection of IL-12 coacervate as an effective therapy against B16-F10 melanoma in mice. Journal of Controlled Release, 318, 270–278. DOI 10.1016/j.jconrel.2019.12.035. [Google Scholar] [CrossRef]
62. Nguyen, H. M., Guz-Montgomery, K., Saha, D. (2020). Oncolytic virus encoding a master pro-inflammatory cytokine interleukin 12 in cancer immunotherapy. Cells, 9(2), 400. DOI 10.3390/cells9020400. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |