
 | Oncologie |  |
DOI: 10.32604/oncologie.2021.019738
ARTICLE
In vivo Effects of Aqueous Extract of Gongronema latifolium Benth on the Tumor Necrosis Factor-α, Transforming Growth Factor-β, and Hepatic Enzymes
1Department of Medical Biotechnology, National Biotechnology Development Agency, Abuja, Nigeria
2Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Agulu, Anambra, Nigeria
3Department of Pharmaceutical Microbiology and Biotechnology, National Institute for Pharmaceutical Research and Development, Abuja, Nigeria
4Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
5Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
6Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Agulu, Anambra, Nigeria
*Corresponding Author: Morteza Saki. Email: mortezasaki1981@gmail.com
Received: 12 October 2021; Accepted: 16 December 2021
Abstract: Nonsteroidal anti-inflammatory drugs have a number of side effects. However, some medicinal herbs have anti-inflammatory properties with few side effects. This study aimed to determine the effect of the aqueous extract of Gongronema latifolium (AEGL) leaves on the level of tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) in rabbits. Enzyme-linked immunosorbent assay (ELISA) was performed to determine the effect of the AEGL on the activities of pro-inflammatory cytokine (TNFα), and regulatory cytokine (TGF-β) in rabbits. Also, a colorimetric method was performed to evaluate the levels of AST and ALT. Overall, the weight of the animals increased until the 21st day and then slightly decreased in the last week of treatment. AEGL showed significant (p < 0.05) inhibitory activity against pro-inflammatory and regulatory cytokines. The greatest inhibitory activities against TNF-α (21 days) and TGF-β (14 days) were obtained at a dose of 400 mg/kg of AEGL. No hepatotoxicity was observed within a 28-day period. This study showed that AEGL can be used as a safe herbal anti-inflammatory and immunomodulatory treatment. Further clinical trials are needed to approve this potential.
Keywords: Inflammation; Gongronema latifolium Benth; TNF-α; TGF-β
Inflammation causes the immune system to respond to invading factors such as toxins, pathogens, and damaged cells. While the initial inflammatory response serves to heal, repair tissues, and maintain homeostasis, persistent inflammation can be problematic [1]. These invading factors trigger acute or chronic inflammatory responses in the body’s organs, leading to cellular damage and disease [1].
Two cytokines that play an important role in the inflammation process are tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) [2–4]. TNF-α or cachectin is a pro-inflammatory cytokine involved in inflammation-associated carcinogenesis. Depending on the cellular signaling pathway, TNF-α can be a tumor promoter or a cancer destroyer [5,6]. TNF-α plays contradictory roles in cancer immunology due to its multifunctionality [5,6]. Its pleiotropic nature could be due to the fact that TNF-α is produced by various cellular sources such as macrophages, monocytes, T lymphocytes, and natural killer (NK) cells [5–7].
TGF-β is a regulatory immunosuppressive cytokine that blocks interleukin-15 induced activation of the serine and threonine kinase, mTOR (mammalian target of rapamycin) and elicits an anti-inflammatory activity [8]. TGF-β suppresses the cytotoxic activity of NK cells as it downregulates the production of interferon-γ (IFN-γ), TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [8].
Plants, drugs, and potential therapeutic agents that combat inflammation alter cytokine secretion by modulating various protein complexes and pathways [3,9]. Nonsteroidal anti-inflammatory drugs have a number of side effects. However, some medicinal herbs have anti-inflammatory properties with few side effects [10,11]. So far, the anti-inflammatory effects of various plants including Curcuma longa, Zingiber officinale, Salvia officinalis, Ribes nigrum, and Rosmarinus officinalis have been reported [11]. A wide variety of diseases including inflammation have been treated by Africans for centuries using medicinal plants. A variety of medicinal plants on the African continent are known to have anti-inflammatory properties, few of which have been studied [11]. Plants have several constituents with anti-inflammatory properties including terpenes, alkaloids, and phenolic compounds such as lignans, coumarins, tannins, saponins, and especially flavonoids [12]. These compounds can affect several enzymes involved in inflammatory pathways such as ATPase, metallopeptidases, and prostaglandin synthesis by inhibiting the arachidonic acid pathway [12].
Gongronema latifolium Benth commonly called utazi in southeastern Nigeria belongs to the family of non-woody plants called Asclepiadaceae. It grows as an herb in tropical and subtropical Africa, South America, and some parts of Asia [13]. In traditional medicine, it is used as a spice and vegetable and in the treatment of chronic diseases like diabetes mellitus, hypertension, mental disorder, and infectious diseases like malaria [13,14]. Phytochemical compounds found in G. latifolium include saponins, glycosides, alkaloids, and tannins (flavonoids) [15]. The effects of this plant on immunological factors including TNF-α and TGF-β have rarely been studied in vivo. Hence, this study aimed to investigate the in vivo effects of this plant on TNF-α and TGF-β. The results obtained from this research may initiate the process of possible development of nutraceutical products that can support the immune system of cancer patients.
Twenty mixed breed male weaner rabbits (Oryctolagus cuniculus) each weighing 1.0–1.5 kg were used in the study. The rabbits were obtained from the Animal Bioresources Division of the Bioresources Development Center, Isanlu (Kogi state, Nigeria), handled by an experienced veterinarian. The rabbits were in good health and were conditioned for two weeks in the cages before the commencement of the experiment. No antibiotics were administered during this period. The room temperature was 28 ± 2°C, relative humidity was in the range of 36%–40%, and there was a 12 h light/dark cycle. The rabbits were fed with standard commercial pelleted feed (Vital feed® Nigeria). Roughage and water were also provided ad libitum. The handling of the rabbits and experimental design were approved by the proposal/animal Ethics Committee of the Faculty of Pharmaceutical sciences, Nnamdi Azikiwe University, Agulu, Anambra state, Nigeria and conformed to international standard.
2.2 Preparation of Aqueous Extract of Gongronema latifolium (AEGL)
The leaves of G. latifolium were obtained from a farm in Ndi Ibe village, Ohafia, Abia State, Nigeria and identified by the taxonomist in the herbarium of the National Institute for Pharmaceutical Research and Development (NIPRD) and assigned a voucher number NIPRD/H/6968. The leaves were dried at room temperature for 4 weeks, de-stemmed and pulverized. A total of 1800 g powdered leaves of G. latifolium were soaked in 24 L water for 24 h. The solution was filtered and concentrated using a rotary evaporator. The sticky extract was dried on a water bath and then stored in a refrigerator at 4°C. Different concentrations were later prepared using distilled water [16].
2.3 Experimental Design and Sample Collection
The rabbits were divided into 5 groups of 4 rabbits each and treated as follows: Group 1: untreated control (no extract; no multivitamins), Group 2: treated control (0.33 ml of multivitamins per kg body weight), Group 3: 200 mg/kg of extract, Group 4: 400 mg/kg of extract, and Group 5: 600 mg/kg of extract. Treatments were administered daily by intubation for a period of 28 days. Animals were weighed just before treatment and then weekly to adjust the volume of the AEGL administered for as long as treatment continued. The 100 ml of EMVITE multivitamin syrup (Emzor, Nigeria) contained: vitamin A 100 IU, vitamin B1 150 µg, vitamin B2 1500 µg, vitamin B12 2500 µg, nicotinamide 10 mg, vitamin C 50 mg, and vitamin D 200 IU. Blood samples (1 ml) were obtained from the ear vein or lateral saphenous veins of the rabbits by an experienced veterinarian on Days 0, 7, 14, 21 and 28 of treatment. The blood was centrifuged at 2000 rpm for 10 min and the serum obtained. The animals were not sacrificed and none died after the experiment. They were therefore used for other studies after a waning period.
2.4 Assay of TGF-β and TNF-α by Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
The TGF-β and TNF-α were assayed using the sandwich ELISA kit (Elabscience, China) according to the manufacturer instructions. Briefly, 100 μl of each standard or serum samples were added to the wells of the microplates and incubated at 37°C for 90 min. The microplates were washed 3 times. Then, 100 μl of the conjugate working solution was added and incubated at 37°C for 30 min. Then, 90 μl of substrate reagent was added and incubated at 37°C for 15 min. The reaction was terminated with the addition of 50 μl of the stop solution. The optical density of the ELISA reaction was read at 450 nm (GF-N3000 Microplate reader, England) and the results were calculated.
2.5 Assay of Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT)
A colorimetric method was performed for the evaluation of the aminotransferases level using AST and ALT Fortress Diagnostics, UK kit according to the manufacturer’s instructions. Briefly, 100 μl of each sample was added to 0.8 of buffer/enzyme reagent and 200 μl of coenzyme reagent and mixed thoroughly. After 1 min, the absorbance was measured at a wavelength of 340 nm.
The results were expressed as mean ± standard error in the mean (SEM). Experiments were laid out in a completely randomized design and subjected to analysis of variance (ANOVA) using GraphPad prism 5 software. Significant means were separated using the Duncan multiple range test at the 95% confidence interval (p < 0.05).
The schematic findings of the current study are shown in Fig. 1. The yield of the plant extract was 185.1 g (10.32%). Until the 21st day, the weights of the animals increased overall, (Fig. 2), then slightly decreased in all groups in the last week of the experiment. A 2-Way ANOVA of Fig. 2 showed that the interaction between treatments and time of evaluation accounted for approximately 3.23% of the total variance in the animals’ weight (p-value = 0.9996). The interaction was not considered significant. However, the treatments (controls and extracts) accounted for about 19.89% of the total variance in the animals’ weight with a p-value of 0.0016. The effect was considered highly significant. Therefore, it can be concluded that the treatments significantly increased the animals’ weights. However, the effects of the extracts were not significantly better (p-value > 0.05) than those of the controls (positive or negative controls). Also, the time (days) of evaluation of the animals’ weight accounted for about 13.38% of the total variance in the animals’ weight with a p-value of 0.0156. The effect was considered significant. The time of assessment had a significant effect on the weight of the animals.
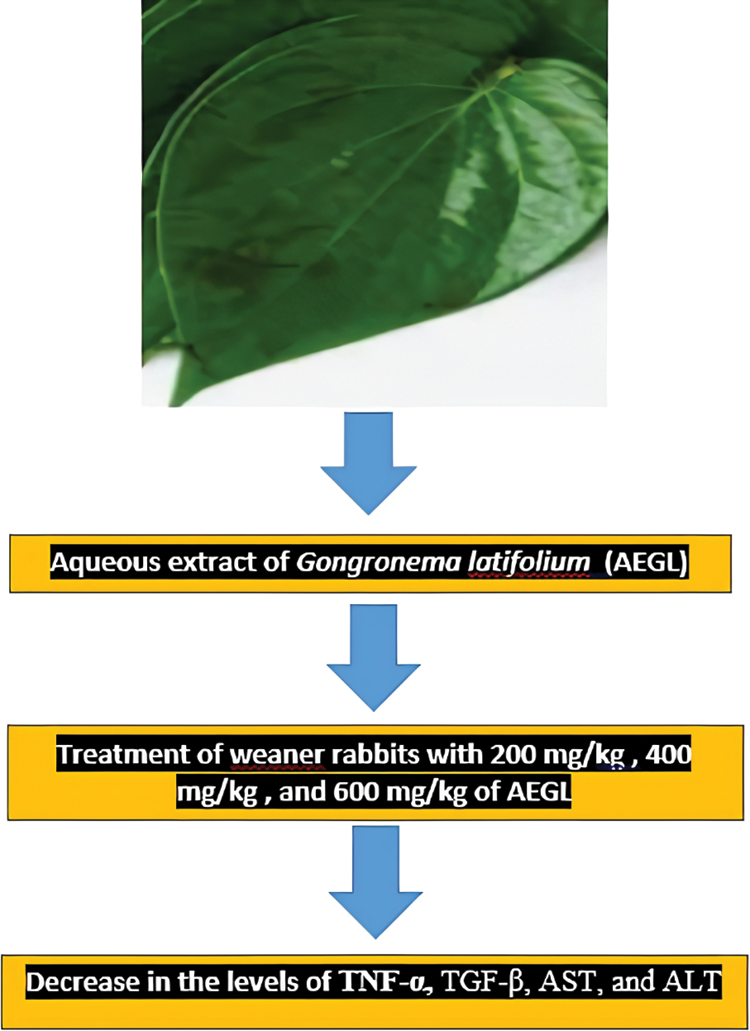
Figure 1: Schematic findings of treatment of weaner rabbits with 200, 400, and 600 mg/kg AEGL on the levels of TNF-α, TGF-β, AST, and ALT
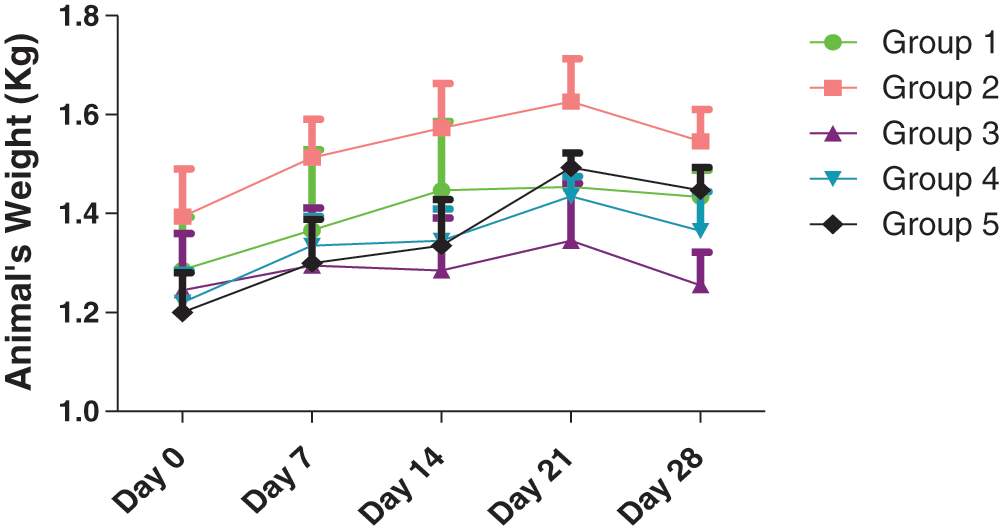
Figure 2: Effects of treatments on the animals’ weight. Group 1: Untreated control (no extract; no multivitamins), Group 2: Treated control (0.33 ml of multivitamins per kg body weight)*, Group 3: 200 mg/kg of extract, Group 4: 400 mg/kg of extract, and Group 5: 600 mg/kg of extract
The data in Fig. 3 indicated that there was a significant (p < 0.05) difference in TNF-α production on Days 0, 7, and 21 in all groups. There was no significant (p > 0.05) difference in TNF-α levels on Days 14 and 28. There was no significant (p > 0.05) effect of time on TNF-α production in Group 2 and Group 5 over the period of 28 days. On the contrary, there was a significant (p < 0.05) effect of time on TNF-α production in Group 1. Fig. 3 also revealed a marginally significant effect in Group 3 and Group 4 over the period of 28 days. The trend revealed that Group 4 maintains an almost steady decline from baseline levels until Day 21. However, undulating trends are seen as cytokine levels decrease from Day 0 to Day 7 (Groups 1, 2, and 3), increase from Day 7 to Day 14 (Groups 1, 2, and 3), and decrease from Days 14 to 21 (Groups 1, 2, and 3). High baseline values were also observed in Group 3 (838.75 pg/ml) and Group 4 (1,385 pg/ml).
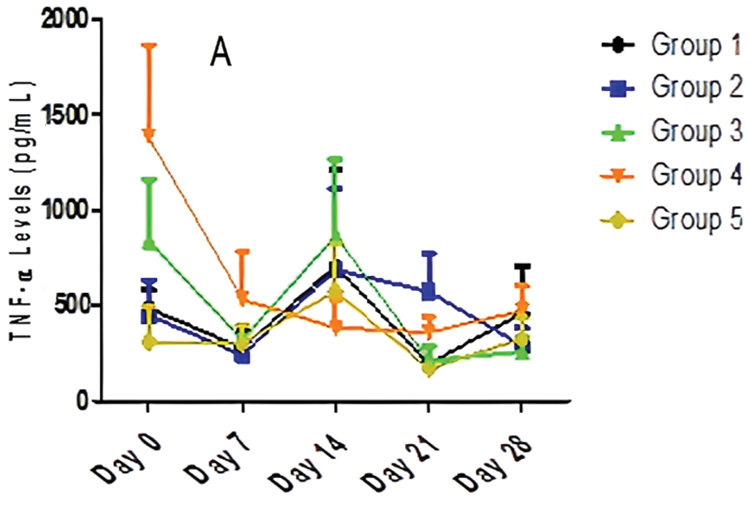
Figure 3: The effect of different doses of AEGL and time on TNF-α levels
Fig. 4 revealed that TGF-β levels were significantly (p < 0.05) different in all the groups, in Days 0 and 7, while they were not significantly (p > 0.05) different on Days 14, 21 and 28. The data from Fig. 4 also showed that there was no significant (p > 0.05) effect of time on TGF-β production in Group 2 and Group 5 over the period of 28 days. On the contrary, there was a significant (p < 0.05) effect of time on TGF-β production in Group 1, Group 3, and Group 4 over the 28-day period. The trend as seen in Fig. 4 revealed that Group 4 maintained a steady decline from baseline levels up to Day 14. However, undulating trends were seen as cytokine levels decreased from Day 0 to Day 7 (Groups 1, 2, 3, and 5) and increased from Day 7 to Day 14 (Groups 1, 2, 3, and 5). Fig. 4 also revealed high baseline values for Groups 3 and 4. There was a significant decrease in TGF-β levels in Groups 3 and 4, suggesting that AEGL is a natural TGF-β inhibitor. Over the period of 28 days, the greatest TGF-β inhibitory activity occurred in Group 4 (400 mg/kg AEGL). TGF-β levels decreased from 1455 to 386.5 pg/ml in a period of 21 days. This suggests a maximum effective dose of 400 mg/kg of AEGL administered at 21 days. The highest TGF-β level was seen on Day 0 in Group 4; while lowest TGF-β level was seen on Day 21 in Group 5.
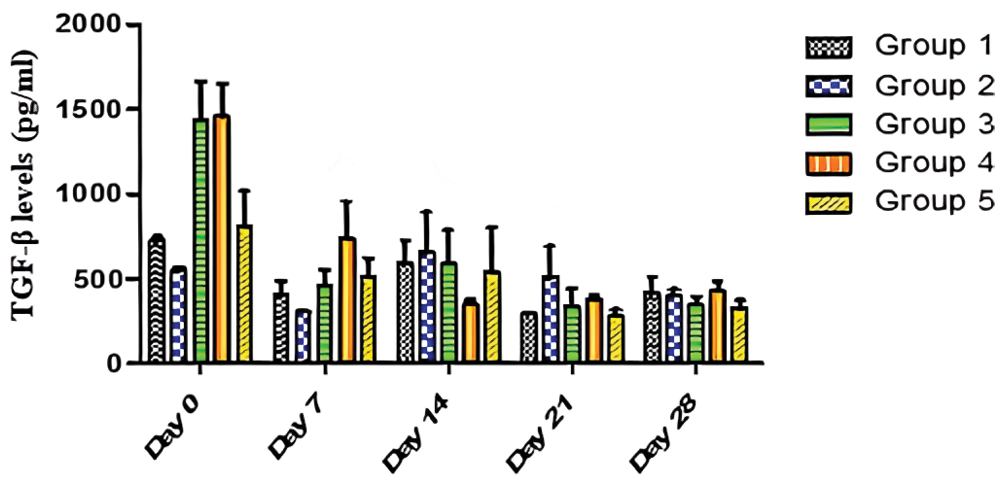
Figure 4: Effects of AEGL and time on TGF-β levels
A two-way analysis of variance (ANOVA) of Fig. 5 showed that the interaction between AEGL treatments with different doses and treatment duration (days) accounted for about 16.15% of the total variance in the AST levels with a p-value of 0.1685. The interaction was not considered significant. Also, the treatments accounted for about 3.40% of the total variance with a p-value of 0.3255, so the effect of treatments with different doses was not considered significant. However, the duration of treatment (days) accounted for approximately 29.18% of the total variance with a p-value < 0.0001. The effect of duration of treatment on AST level was considered highly significant. Therefore, it can be concluded that the decrease in the AST level is due to the duration of treatment. The effect of time (duration) was considered highly significant. The Fig. 5 showed the effect of different doses of AEGL and time on AST activity (U/L). The AST values on Days 0 and 7 were significantly (p < 0.05) different in all groups, while they were insignificant (p > 0.05) on Day 14. The AST values on Days 21 and 28 were moderately significant in all groups. Also, the AST values in Groups 1, 3, 4, and 5 were significantly different (p < 0.05) over the period of 28 days, whereas the values in Group 2 were not significant (p > 0.05). The Fig. 5 revealed a downward trend in all groups in the first 7 days of the experiment. The value of Group 4 continues to decrease, indicating that the greatest antioxidant activity is attained at the dose of 400 mg/kg AEGL.
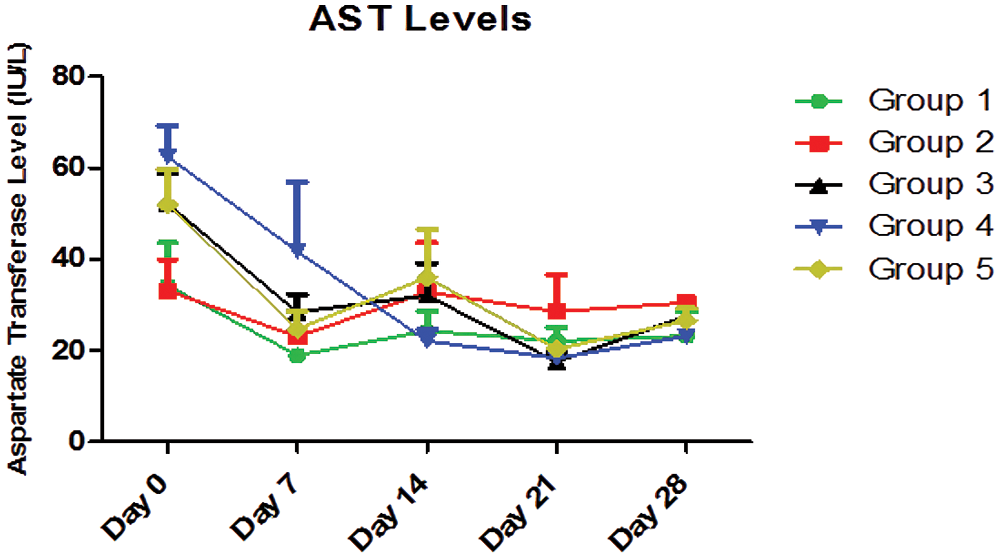
Figure 5: AST levels (IU/L) for a period of 28 days
Two-Way ANOVA of Fig. 6 showed that the interaction between the treatment with different doses of AEGL and time accounted for approximately 57.49% of the total variance in the ALT levels with a p-value < 0.0001. The interaction was considered highly significant. This means that the combined effects of the different doses of AEGL and time significantly affected the ALT levels.
The AEGL doses accounted for approximately 17.63% of the total variance in the ALT levels with a p-value < 0.0001. The effect of the AEGL on the ALT activity was considered highly significant. Also, time (duration) accounted for approximately 13.38% of the total variance with a p-value < 0.0001.
In Fig. 6, ALT values were significantly different (p < 0.05) on Days 0, 7, 14, 21, and 28. Also the ALT values in Groups 1, 2, 3, and 5 were significantly different (p < 0.05) over the period of 28 days, while the values in Group 4 were not significantly different (p > 0.05).
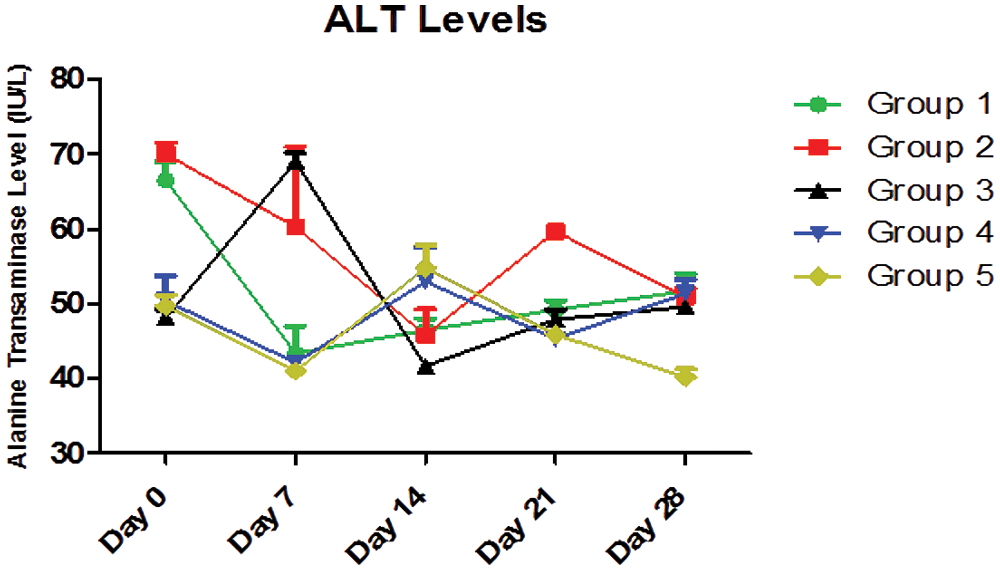
Figure 6: ALT levels (IU/L) for a 28-day period
A majority of plant species have not yet been chemically or biologically studied, especially when it comes to anti-inflammatory action, making them an important source of molecules for the development of new drugs, which increases the interest of various industries in looking for compounds derived from these sources [12]. In this study, the G. latifolium effects were investigated on the levels of TNF-α and TGF-β. The overall weight gain of the animals up to the Day 21 showed that the extracts had no negative effects on the animals, while the slight decrease in mean weights in all groups could be attributed to physical stress.
Also, the results indicated that TNF-α levels decreased significantly (p < 0.05) from Day 0 to Day 7 in all groups. This down-regulation of TNF-α seen in the first week may be due to the anti-inflammatory properties of AEGL [17]. Antagonists of Th1-derived TNF-α can be obtained from many natural resources including Stryphnodendron adstringens, Campomanesia lineatifolia, Terminalia glabrescens, and Illicium verum [18].
According to the anti-inflammatory effect (reduction of Th1-derived TNF-α production), the undulating pattern in Groups 2, 3, and 5 may be due to a dynamic interplay between Th1-derived TNF-α and NK cells derived TNF-α production between Day 7 and Day 21. Simultaneously (although it takes longer) the ligands that trigger the activating receptors of NKC initiate TNF-α production which peaks at Day 14. An upward trend observed in any group over the period of 28 days may indicate NK cell-induced TNF-α production. In this NKC-associated process, TNF-α interacts with TNF receptor 2 (TNFR-2) to trigger the c-Jun N-terminal kinase (JNK) signaling pathway, which promotes apoptosis [19,20]. Over the period of 28 days, the greatest TNF-α inhibitory activity occurred in Group 4 (400 mg/kg of AEGL). TNF-α levels decreased from 1385 to 358.5 pg/ml in a period of 21 days. This indicated a maximum effective dose of 400 mg/kg AEGL administered after 21 days. The initial high TNF-α levels in Groups 3 and 4 may be due to an ongoing inflammatory process in the rabbits produced by Th1 cells. In this process, TNF-α interacts with TNF receptor 1 (TNFR-1), which triggers the nuclear factor κB (NF-κB) pathway which promotes cell survival and inflammation [20–23]. Inflammation is usually characterized by an increased TNF-α and a deregulation of TNFR signaling [24–26]. The highest TNF-α level was observed on Day 0 in Group 4; while the lowest TNF-α level was seen on Day 21 in Group 5. In line with our results, a previous study by Okesola et al. [27], reported the anti-inflammatory effects of AEGL on reduction of TNF-α and C-reactive protein (CRP) in diabetic Wistar rat model. Also, Ojo et al. [28], showed that G. latifolium leaf extract can exhibit anti-inflammatory effects by lowering the levels of interleukin-6 (IL-6) and IL-2 in alloxan-induced diabetic Wistar rats. The anti-inflammatory activity of AEGL may be explained by the presence of multiple anti-inflammatory compounds such as flavonoids and saponins that act synergistically [12,15].
There was a significant reduction in TGF-β levels in Groups 3, and 4 suggesting that AEGL is a natural TGF-β inhibitor. Several herbal products are known to inhibit TGF-β production in target cells. Like corilagin extracted from Phyllanthus niruri L., AEGL could also block TGF-β/AKT/ERK/Smad signaling pathways which culminates in the inhibition of the growth of the ovarian cancer cell lines SKOv3ip and Hey [29]. TGF-β is a regulatory cytokine that promotes metastasis and its inhibition is therefore a therapeutic strategy in the treatment of cancer [23,30]. So far, the effect of various plants including Scutellaria baicalensis and Fritillaria cirrhosa extracts has been reported on reducing the levels of TGF-β [31]. But by searching scientific databases, no study was found that showed the effect of the AEGL on TGF-β.
In this study, the production of two examined cytokines was affected in a dose and time dependent manner. Rabbits receiving 400 mg/kg, showed significant activity for the two cytokines, indicating the maximum effective dose for AEGL (21 days for TNF-α; 14 days for TGF-β). Although known for its remarkable antioxidant and NK cell stimulatory properties, the administration of 0.33 ml of multivitamins per kg body weight did not show sufficient efficacy compared to 400 mg/kg AEGL. High baseline values in Groups 3 and 4 could indicate an inflammatory process before the start of the experiment. However, it is important to note that the results and interpretations may have been negatively affected by several factors, including the complex interactions of the cytokine network, the nature of cytokines, genetic differences, experimental methodology, and sample related issues [32]. The greatest inhibitory activities against TNF-α (21 days), and TGF-β (14 days) were achieved at a dose of 400 mg/kg of AEGL.
All studied groups had physiological ranges for AST (10–98 IU/L) and ALT (19–73 IU/L). However, AEGL significantly decreased the activity of AST and ALT in a dose and time-dependent manner. The decreasing levels of aminotransferases indicated a reduction in hepatocyte damage and the presence of hepatoprotective compounds in the AEGL. The phytochemical constituents of G. latifolium have been reported to have effects on cellular proteins possessing some enzymatic activities [15,33]. Also, a previous study by Meng et al. [34] from China, showed the hepatoprotective effect of 10 medicinal plants including Amomum kravanh and Pueraria lobata by lowering the levels of AST and ALT. Likewise, the extracts of plants such as Hibiscus sabdariffa and Zingiber officinale are known to significantly reduce the levels of AST and ALT due to their antioxidant effects in combating lipid peroxidation in the liver [35–37]. All aminotransferase results in all groups were within the physiological range, indicating that there was no hepatotoxicity within the 28-day period. This indicated a remarkable nutraceutical potential of AEGL. The results of the current study confirmed the previous findings by Sulaiman et al. [38] that showed a drop in the levels of AST and ALT in G. latifolium-treated Wistar rats. Their study found that ethanolic extract of G. latifolium leaves and stems had no hepatotoxic effects.
While these results may indicate activation of T cells and NK cells responsible for TNF-α production, further work is needed to validate the direct efficacy of the bioactive compounds on these groups of immune cells. It is also possible that the activity of unwanted bioactive compounds could overshadow the effect of the activity of the other compounds. It is therefore necessary to identify and isolate the important bioactive compounds for in vitro assays.
The current study had some limitations due to financial constraints and traffic restrictions caused by the COVID-19 virus pandemic. In this study, other inflammatory markers including CRP and IL-6 were not assayed. Also, other hepatotoxicity markers such as alkaline phosphatase, glutamate dehydrogenase, and Micro RNA 122 were not evaluated. The third limitation was that the constituents of the plant were not investigated by gas chromatography mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC). The fourth limitation was the lack of an inflammatory animal model.
The AEGL showed significant anti-inflammatory and immunomodulatory activities with no hepatotoxicity. To further investigate NK cell mediated cytokine production of TNF-α, the use of cultured NK cells and cancer cell lines would be required. This would be more specific as only the activities of NK cell derived cytokines would be revealed. This study has shown that AEGL can be used as a safe herbal anti-inflammatory and immunomodulatory treatment. Further clinical trials are needed to approve this potential.
Availability of Data and Materials: All analyzed data within this study can be obtained from the corresponding author on request.
Author Contributions: ABR, MON and ANO designed the experiments. TA, ICU, and AA performed the experiments. MS, SA, COE analyzed the data. ANO, SA and MS wrote the manuscript. COE, ANO, SA and MO revised the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Ethics Approval and Informed Consent Statement: The handling of the rabbits and experimental design were approved by the proposal/animal Ethics Committee of the Faculty of Pharmaceutical sciences, Nnamdi Azikiwe University, Agulu, Anambra state, Nigeria (Code: 21.4.2020). No human was examined in the current study to obtain the written informed consent.
Funding Statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z. et al. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. DOI 10.18632/oncotarget.23208. [Google Scholar] [CrossRef]
2. Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S. et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25(12), 1822–1832. DOI 10.1038/s41591-019-0675-0. [Google Scholar] [CrossRef]
3. Yahfoufi, N., Alsadi, N., Jambi, M., Matar, C. (2018). The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients, 10(11), 1618. DOI 10.3390/nu10111618. [Google Scholar] [CrossRef]
4. Parisi, L., Bassani, B., Tremolati, M., Gini, E., Farronato, G. et al. (2017). Natural killer cells in the orchestration of chronic inflammatory diseases. Journal of Immunology Research, 2017(2), 1–13. DOI 10.1155/2017/4218254. [Google Scholar] [CrossRef]
5. Silva, L. B., dos Santos Neto, A. P.,Maia, S. M., dos Santos Guimarães, C., Quidute, I. L. et al. (2019). The role of TNF-α as a proinflammatory cytokine in pathological processes. The Open Dentistry Journal, 13(1), 332–338. DOI 10.2174/1874210601913010332. [Google Scholar] [CrossRef]
6. Martínez-Reza, I., Díaz, L., García-Becerra, R. (2017). Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. Journal of Biomedical Science, 24(1), 1–8. DOI 10.1186/s12929-017-0398-9. [Google Scholar] [CrossRef]
7. Mercogliano, M. F., Bruni, S., Elizalde, P. V., Schillaci, R. (2020). Tumor necrosis factor α blockade: An opportunity to tackle breast cancer. Frontiers in Oncology, 10, 584. DOI 10.3389/fonc.2020.00584. [Google Scholar] [CrossRef]
8. Regis, S., Dondero, A., Caliendo, F., Bottino, C., Castriconi, R. (2020). NK cell function regulation by TGF-β-induced epigenetic mechanisms. Frontiers in Immunology, 11, 311. DOI 10.3389/fimmu.2020.00311. [Google Scholar] [CrossRef]
9. Heimfarth, L., Serafini, M. R., Martins-Filho, P. R. S., Quintans, J. S. S., Júnior, L. J. Q. (2020). Drug repurposing and cytokine management in response to COVID-19: A review. International Immunopharmacology, 88, 106947. DOI 10.1016/j.intimp.2020.106947. [Google Scholar] [CrossRef]
10. Oguntibeju, O. O. (2018). Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. Journal of Inflammation Research, 11, 307–317. DOI 10.2147/JIR. [Google Scholar] [CrossRef]
11. Ghasemian, M., Owlia, S., Owlia, M. B. (2016). Review of anti-inflammatory herbal medicines. Advances in Pharmacological Sciences, 2016, 9130979. DOI 10.1155/2016/9130979. [Google Scholar] [CrossRef]
12. Nunes, C. D. R., Barreto Arantes, M., Menezes de Faria Pereira, S., Leandro da Cruz, L.,de Souza Passos, M. et al. (2020). Plants as sources of anti-inflammatory agents. Molecules, 25(16), 3726. DOI 10.3390/molecules25163726. [Google Scholar] [CrossRef]
13. Al-Hindi, B., Yusoff, N. A., Ahmad, M., Atangwho, I. J., Asmawi, M. Z. et al. (2019). Safety assessment of the ethanolic extract of Gongronema latifolium Benth. leaves: A 90-day oral toxicity study in Sprague Dawley rats. BMC Complementary and Alternative Medicine, 19, 152. DOI 10.1186/s12906-019-2573-x. [Google Scholar] [CrossRef]
14. Morebise, O. (2015). A review on Gongronema latifolium, an extremely useful plant with great prospects. European Journal of Medicinal Plants, 10(1), 1–9. DOI 10.9734/EJMP/2015/19713. [Google Scholar] [CrossRef]
15. Eze, S. O., Nwanguma, B. C. (2013). Effects of tannin extract from Gongronema latifolium leaves on lipoxygenase Cucumeropsis manii seeds. Journal of Chemistry, 2013, 864095. DOI 10.1155/2013/864095. [Google Scholar] [CrossRef]
16. Oli, A. N., Obaji, M., Enweani, I. B. (2019). Combinations of Alchornea cordifolia, Cassytha filiformis and Pterocarpus santalinoides in diarrhoegenic bacterial infections. BMC Research Notes, 12(1), 649. DOI 10.1186/s13104-019-4687-0. [Google Scholar] [CrossRef]
17. Deree, J., Martins, J. O., Melbostad, H., Loomis, W. H., Coimbra, R. (2008). Insights into the regulation of TNF-α production in human mononuclear cells: The effects of non-specific phosphodiesterase inhibition. Clinics, 63(3), 321–328. DOI 10.1590/s1807-59322008000300006. [Google Scholar] [CrossRef]
18. Kumar Verma, P., Bala, M., Kumar, N., Singh, B. (2012). Therapeutic potential of natural products from terrestrial plants as TNF-α antagonist. Current Topics in Medicinal Chemistry, 12(13), 1422–1435. DOI 10.2174/156802612801784425. [Google Scholar] [CrossRef]
19. Hayden, M. S., Ghosh, S. (2014). Regulation of NF-κB by TNF family cytokines. Seminars in Immunology, 26, 253–266. DOI 10.1016/j.smim.2014.05.004. [Google Scholar] [CrossRef]
20. Henriques, B. O., Corrêa, O., Azevedo, E. P. C., Pádua, R. M., Oliveira, V. L. S. D. et al. (2016). In vitro TNF-inhibitory activity of Brazilian plants and anti-inflammatory effect of Stryphnodendron adstringens in an acute arthritis model. Evidence-Based Complementary and Alternative Medicine, 2016, 9872598. DOI 10.1155/2016/9872598. [Google Scholar] [CrossRef]
21. Holbrook, J., Lara-Reyna, S., Jarosz-Griffiths, H., McDermott, M. F. (2019). Tumour necrosis factor signalling in health and disease. F1000Research, 8. DOI 10.12688/f1000research.17023.1. [Google Scholar] [CrossRef]
22. van Horssen, R., Ten Hagen, T. L., Eggermont, A. M. (2006). TNF-α in cancer treatment: Molecular insights, antitumor effects, and clinical utility. The Oncologist, 11(4), 397–408. DOI 10.1634/theoncologist.11-4-397. [Google Scholar] [CrossRef]
23. Yang, S., Wang, J., Brand, D. D., Zheng, S. G. (2018). Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Frontiers in Immunology, 9, 784. DOI 10.3389/fimmu.2018.00784. [Google Scholar] [CrossRef]
24. Yang, L. (2010). TGFβ and cancer metastasis: An inflammation link. Cancer and Metastasis Reviews, 29(2), 263–271. DOI 10.1007/s10555-010-9226-3. [Google Scholar] [CrossRef]
25. Brenner, D., Blaser, H., Mak, T. W. (2015). Regulation of tumour necrosis factor signalling: Live or let die. Nature Reviews Immunology, 15(6), 362–374. DOI 10.1038/nri3834. [Google Scholar] [CrossRef]
26. Ruder, B., Atreya, R., Becker, C. (2019). Tumour necrosis factor alpha in intestinal homeostasis and gut related diseases. International Journal of Molecular Sciences, 20(8), 1887. DOI 10.3390/ijms20081887. [Google Scholar] [CrossRef]
27. Okesola, M. A., Ojo, O. A., Onikanni, S. A., Ajiboye, B. O., Oyinloye, B. E. et al. (2020). Ameliorative effect of Gongronema latifolium leaf extract on alloxan-induced diabetic cardiomyopathy in Wistar rats model. Comparative Clinical Pathology, 29(4), 865–872. DOI 10.1007/s00580-020-03134-8. [Google Scholar] [CrossRef]
28. Ojo, O. A., Osukoya, O. A., Ekakitie, L. I., Ajiboye, B. O., Oyinloye, B. E. et al. (2020). Gongronema latifolium leaf extract modulates hyperglycaemia, inhibits redox imbalance and inflammation in alloxan-induced diabetic nephropathy. Journal of Diabetes and Metabolic Disorders, 19(1), 469–481. DOI 10.1007/s40200-020-00533-0. [Google Scholar] [CrossRef]
29. Ortega, F. J., Fernández-Real, J. M. (2017). Inflammation in the spotlight—Clinical relevance of genetic variants affecting nuclear factor κB and tumor necrosis factor receptor 1. Annals of Translational Medicine, 5(10), 219. DOI 10.21037/atm.2017.02.13. [Google Scholar] [CrossRef]
30. Berlin Grace, V. M., Viswanathan, S., David Wilson, D., Jagadish Kumar, S., Sahana, K. et al. (2020). Significant action of Tridax procumbens L. leaf extract on reducing the TNF-α and COX-2 gene expressions in induced inflammation site in Swiss albino mice. Inflammopharmacology, 28(4), 929–938. DOI 10.1007/s10787-019-00634-0. [Google Scholar] [CrossRef]
31. Bokhari, A. A., Syed, V. (2015). Inhibition of transforming growth factor-β (TGF-β) signaling by Scutellaria baicalensis and Fritillaria cirrhosa extracts in endometrial cancer. Journal of Cellular Biochemistry, 116(8), 1797–1805. DOI 10.1002/jcb.25138. [Google Scholar] [CrossRef]
32. Wojdasiewicz, P., Poniatowski, Ł.A., Szukiewicz, D. (2014). The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation, 2014, 561459. DOI 10.1155/2014/561459. [Google Scholar] [CrossRef]
33. Eleyinmi, A. F. (2007). Chemical composition and antibacterial activity of Gongronema latifolium. Journal of Zhejiang University Science B, 8(5), 352–358. DOI 10.1631/jzus.2007.B0352. [Google Scholar] [CrossRef]
34. Meng, X., Tang, G. Y., Liu, P. H., Zhao, C. J., Liu, Q. et al. (2020). Antioxidant activity and hepatoprotective effect of 10 medicinal herbs on CCl4-induced liver injury in mice. World Journal of Gastroenterology, 26(37), 5629. DOI 10.3748/wjg.v26.i37.5629. [Google Scholar] [CrossRef]
35. Abou Seif, H. S. (2016). Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-suef University Journal of Basic and Applied Sciences, 5(2), 134–146. DOI 10.1016/j.bjbas.2016.03.004. [Google Scholar] [CrossRef]
36. Elijah, J. P., Young, U. C., Chuma, E., Chinelo, N. C., Onyiagoziri, O. J. et al. (2017). Biochemical effects of the aqueous extract of Hibiscus sabdariffa on liver marker enzymes and lipid profiles in acetaminophen-challenged Rats. Research Journal of Medicinal Plants, 11, 48–54. DOI 10.3923/rjmp.2017.48.54. [Google Scholar] [CrossRef]
37. Motawi, T. K., Hamed, M. A., Shabana, M. H., Hashem, R. M., Naser, A. F. A. (2011). Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutrition & Metabolism, 8, 40. DOI 10.1186/1743-7075-8-40. [Google Scholar] [CrossRef]
38. Sulaiman, F. A., Yusuf, B. O., Omar, S. A., Muritala, H. F., Adisa, J. M. et al. (2022). Ethanolic extracts of the Gongronema latifolium stem and leaves caused mild renal injury and modulated serum triglycerides in rats. Biointerface Research in Applied Chemistry, 12(4), 5045–5053. DOI 10.33263/BRIAC124.50455053. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |