
 | Oncologie |  |
DOI: 10.32604/oncologie.2021.018532
ARTICLE
Synthesis, Pharmacological Evaluation, and In-Silico Studies of Thiophene Derivatives
1Institute of Pharmaceutical Research, GLA University, Mathura, 281406, India
2School of Pharmaceutical Sciences, IFTM University, Moradabad, 244102, India
3Saraswathi College of Pharmacy, Anwarpur, 245304, India
*Corresponding Author: Raghav Mishra. Email: raghav.mishra@gla.ac.in
Received: 31 July 2021; Accepted: 20 October 2021
Abstract: The relevance of Retinoic acid receptor-related orphan receptors in cancer progression has sparked interest in developing multifunctional therapeutics. In the search for potentially active novel compounds with anticancer characteristics, the Gewald reaction was employed to develop different thiophene derivatives (8a–8i). Physicochemical and spectroanalytical investigations verified the molecular structures of the synthesized derivatives. Using an in vitro primary anticancer assay, NCI chose all of the synthesized molecules as prototypes and assessed their anticancer efficacy against a panel of various cancer cell lines representing nine distinct neoplasms. The compounds were found to have a wide range of anticancer activity. Following significant anticancer efficacy against all cell lines in the initial screening, compound 8e was chosen for a five-dose test. Compound 8e inhibited growth at concentrations ranging from 0.411 to 2.8 μM. The antioxidant activity of the compounds was further evaluated using the radical scavenging action of the stable DPPH free radical. In comparison to Ascorbic Acid, compounds 8e and 8i showed outstanding antioxidant activity, while the remaining compounds in the series demonstrated acceptable antioxidant activity. In a molecular docking investigation, 8e demonstrated excellent docking scores inside the binding pocket of the specified pdb-id (6q7a), complementing the results of anticancer screening. Based on our results, novel ethyl 5-acetyl-2-amino-4-methylthiophene-3-carboxylate derivatives could be useful in the development of potential anticancer treatments.
Keywords: Thiophene; anticancer; antioxidant; RORγt inhibitors; SAR; in-silico studies
| Abbreviations | |
| DPPH: | 2,2-diphenyl-2-picrylhydrazyl |
| GI50: | Growth inhibition of 50% cell |
| LC50: | Lethal concentration that gives 50% cell kill |
| MG_MID: | Full panel mean-graph midpoint |
| NCI: | National Cancer Institute |
| ppm: | Parts per million |
| RORs: | Retinoic acid receptor-related orphan receptors |
| ROS: | Reactive oxygen species |
| SAR: | Structure activity relationship |
| SRB: | Sulforhodamine B protein assay |
| TGI: | Total Growth Inhibition |
| TMS: | Tetramethylsilane |
In the modern medical field, cancer is the most prevalent, particularly complex, and deadly illness [1]. It has been one of the world’s main sources of mortality over the last decade. According to the World Health Organization, an estimated 9.6 million people died of cancer in 2018 [2,3]. To provide better and more successful cancer therapy and cure, the medical scientific community confronts a significant challenge [4–6]. This task involves the development of new medications, therapies, and care for cancer patients. These neoplastic tumor cells are varied and diversified, with the ability to proliferate rapidly. These malignant neoplasms may infiltrate or spread to other parts of the body through the blood circulation and lymphatic networks [7–9].
Chemotherapeutic drugs are one treatment option for different types of cancer. However, these medicines are associated with many drawbacks, including drug tolerance, systemic cytotoxicity, and a narrow therapeutic efficacy [10–13]. Novel chemotherapeutic drugs with well-established mechanisms must be developed to overcome these limitations. The identification of cytotoxic molecules, or agents that may kill cancer cells, has led to advances in anticancer treatment. These medicines improve cancer patients’ survival and well-being [14,15]. Numerous compounds having heterocyclic rings have already shown significant antiproliferative activity, especially those containing thiophene rings. Thiophene derivatives stand out among biomolecules employed in research to evaluate biological activity due to their diverse properties [16].
To improve specificity and safety profiles, several synthetic routes are being used to develop various novel thiophene derivatives [17]. 2-amino-thiophenes have earned a significant interest among the thiophene compounds. A great deal of attention has been paid to 2-amino-thiophenes recently, owing to improvements in their synthetic methods, stability, availability, and structural simplicity, which makes them an important moiety in pharmaceuticals [18,19]. Furthermore, antifungal [20], antibacterial [21,22], antileishmanial [23], anxiolytic [24], anti-inflammatory [25], antiplatelet [26], antioxidant [27], antiandrogenic [28], and anti-diabetes [29] actions have been revealed for thiophene and its analogues.
A key element in the growth of cancer and as potential therapeutic targets for different malignancies has been detected with nuclear receptors. Retinoic acid receptor-related orphan receptors (RORs) are a subfamily of the thyroid hormone receptor and belong to the orphan nuclear receptor family, which is a nuclear receptor subfamily. RORα, RORβ, and RORγ are members of the ROR subfamily. These receptors are involved in the development of autoimmune diseases, inflammatory diseases, circadian rhythm, secondary lymphoid tissue, and homeostasis-metabolism by triggering transcription through ligand-dependent interactions with co-regulators [30].
According to recent studies, RORγ and its isoforms are expressed in the thymus and lymphoid organs, but also tend to be involved in some cancers, including lymphoma, melanoma, lung, and ovarian cancer [31].
RORγt is an immune cell-specific isoform of ROR with a ligand-binding domain receptor. Cellular differentiation and activation of TH-17 cells result in the production of pro-inflammatory mediators such as interleukin-17 (IL-17), which itself is linked to malignancies such as lung, colon, and ovarian cancer [32–35]. As a result of this, researchers are always looking for new molecules that have the potential to block RORγt and use them to treat cancer-related conditions. Ursolic acid, digoxin, azole-type fungicides, and SR211 are all known RORγt receptor inhibitors.
The approach for designing the compound was based on heterocyclic structures previously reported as RORγt inhibitors (Fig. 1) [36].
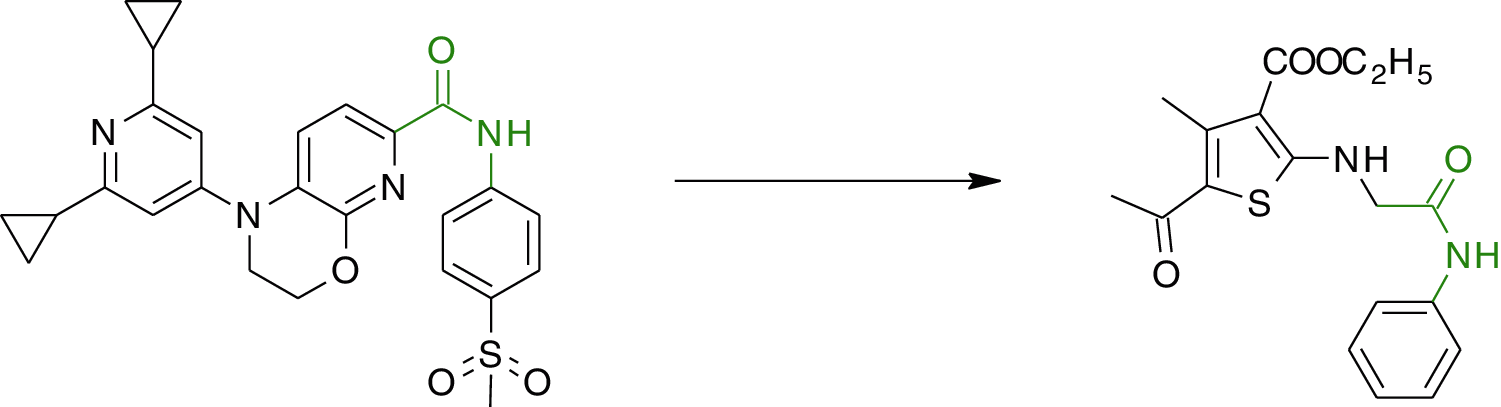
Figure 1: Design of target compounds
Inflammatory disorders, cancer, rheumatoid arthritis and aging are among the pathological situations where reactive oxygen species (ROS) are involved. Oxidative stress is linked with an increase in free radical consumption or a decrease in antioxidant concentration, which affects cell membranes and other components such as DNA, lipids, proteins, and lipoproteins [37]. Excess hydroxyl radicals and peroxynitrite, for example, may induce lipid peroxidation by causing damage to cell membranes and lipoproteins. Malondialdehyde and conjugated diene compounds are produced during this process, both of which are cytotoxic and mutagenic.
Experimental investigations on animals, as well as in vitro studies, have indicated that ROS is an important factor in carcinogenesis and that there is a critical balance between free radical production and antioxidant defense as a preventative force against cancer. However, the exact mechanism through which antioxidants exert their anti-carcinogenic actions is still unclear. Based on the findings so far, it seems that the most probable mechanism for antioxidants anticancer action is by [38–40]:
1. possibility of reducing the production of “activated” carcinogenic species through Phase I biotransformation enzymes,
2. scavenging free radicals, ROS, and electrophiles; and
3. enhancement of electrophile detoxification via induction of Phase II detoxification enzymes such as glutathione S-transferases and NAD(P)H: quinone reductase.
ROS is known to induce a variety of human malignancies, and given the severity of the disease, the anticancer profile of thiophene-based compounds has mostly been thoroughly studied [41–46]. The present research was carried out as an outcome of this finding to design strong RORγt inhibitors via the introduction of various substituted aromatic moieties at the thiophene terminal (Scheme 1) and assess their therapeutic potential to continue our search for novel anticancer agents.
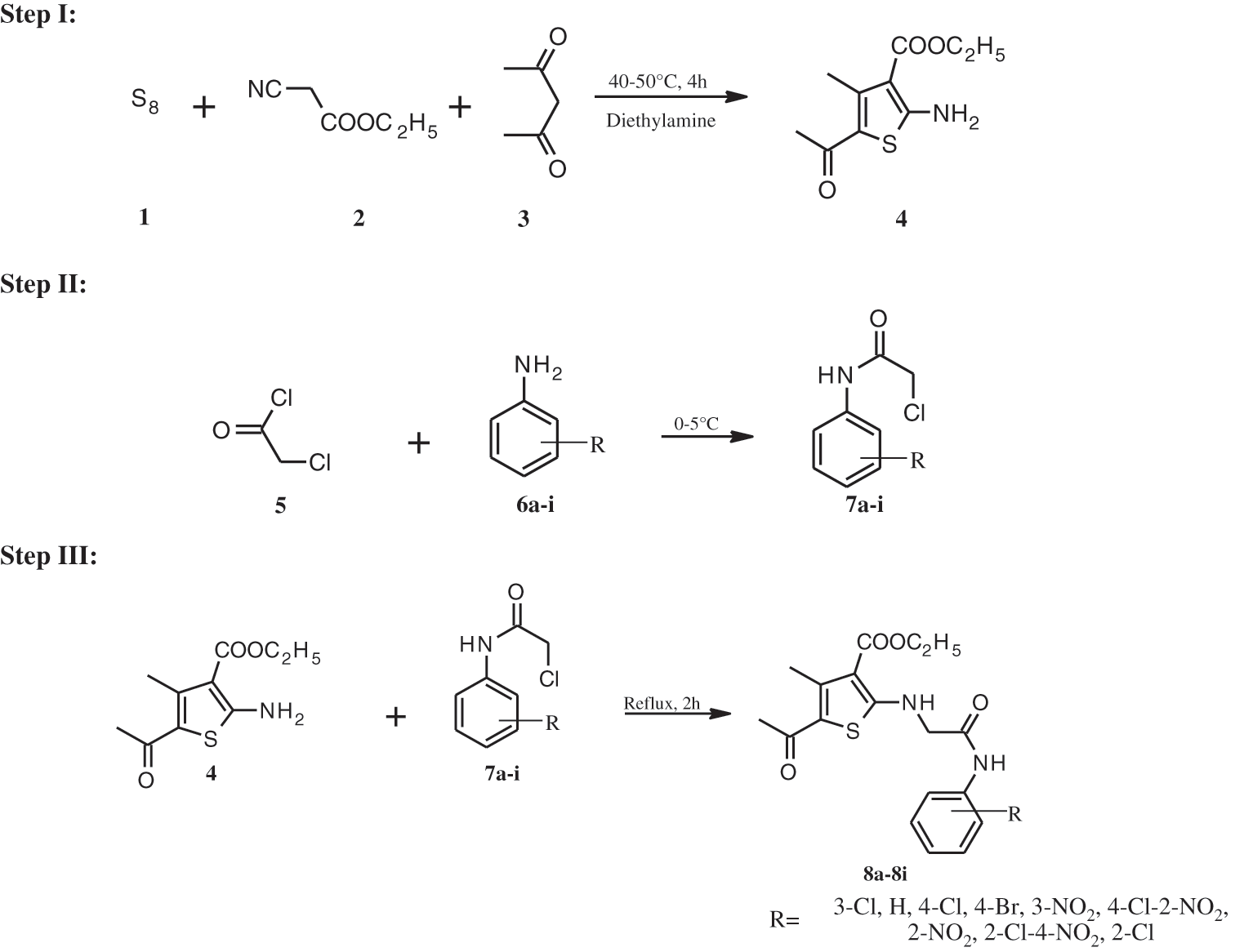
Scheme 1: Synthetic pathway for compounds 8a-i
2.1.1 Chemicals and Instrumentations
Preliminary material required for experimental work was procured from the authorized suppliers and used without further purification. For tracking the reaction progress, TLC was carried out using glass plates coated with silica gel G using mobile phase {ethyl acetate/n-hexane (1:2) and ethyl acetate/benzene (1:1)}. The plates were visualized in an iodine chamber. Open capillary melting point apparatus was used to determine melting points and reported as uncorrected. IR spectra (in KBr) were acquired using the DRS 8000A accessory technique on a Shimadzu IR Affinity-1 FTIR spectrophotometer. Both 1H and 13C NMR spectral analysis of synthesized compounds in CDCl3 with tetramethylsilane (TMS) as an internal standard were recorded on Bruker Avance-II 400 NMR Spectrometer operating at 500 MHz. Chemical shift (δ) values were reported in parts per million (ppm). Using Waters Q-TOF (ESI–MS) micromass, mass spectra were recorded. SAIF, Panjab University, Chandigarh conducted spectral analysis, including mass spectroscopy and NMR studies.
2.1.2 Step 1: Synthesis of ethyl 5-acetyl-2-amino-4-methylthiophene-3-carboxylate (4)
At room temperature, sulphur (0.06 mol) was added with stirring to an equimolar (0.05 mol) mixture of ethyl cyanoacetate and acetylacetone. Diethylamine (0.05 mol) was added dropwise to this heterogeneous mixture. The reaction mixture was agitated for 4 h at a temperature of 40–50°C. TLC was used to monitor the reaction’s progress, and the mixture was left at room temperature overnight. After filtering, washing with water, drying, and recrystallization from ethanol, the brown precipitate was obtained.
Compound 4: Yield: 34%; M.P.: 150–152°C; Rf = 0.66; IR (KBr, cm–1): 785 (C-S-C str.), 1257 (C-O-C str.), 1583 (C=C str.), 1666 (C=O str.), 2968 (C-H str.), 3408 (N-H str.)
2.1.3 Step 2: Synthesis of N-substituted α-chloro Acetanilides (7a-i)
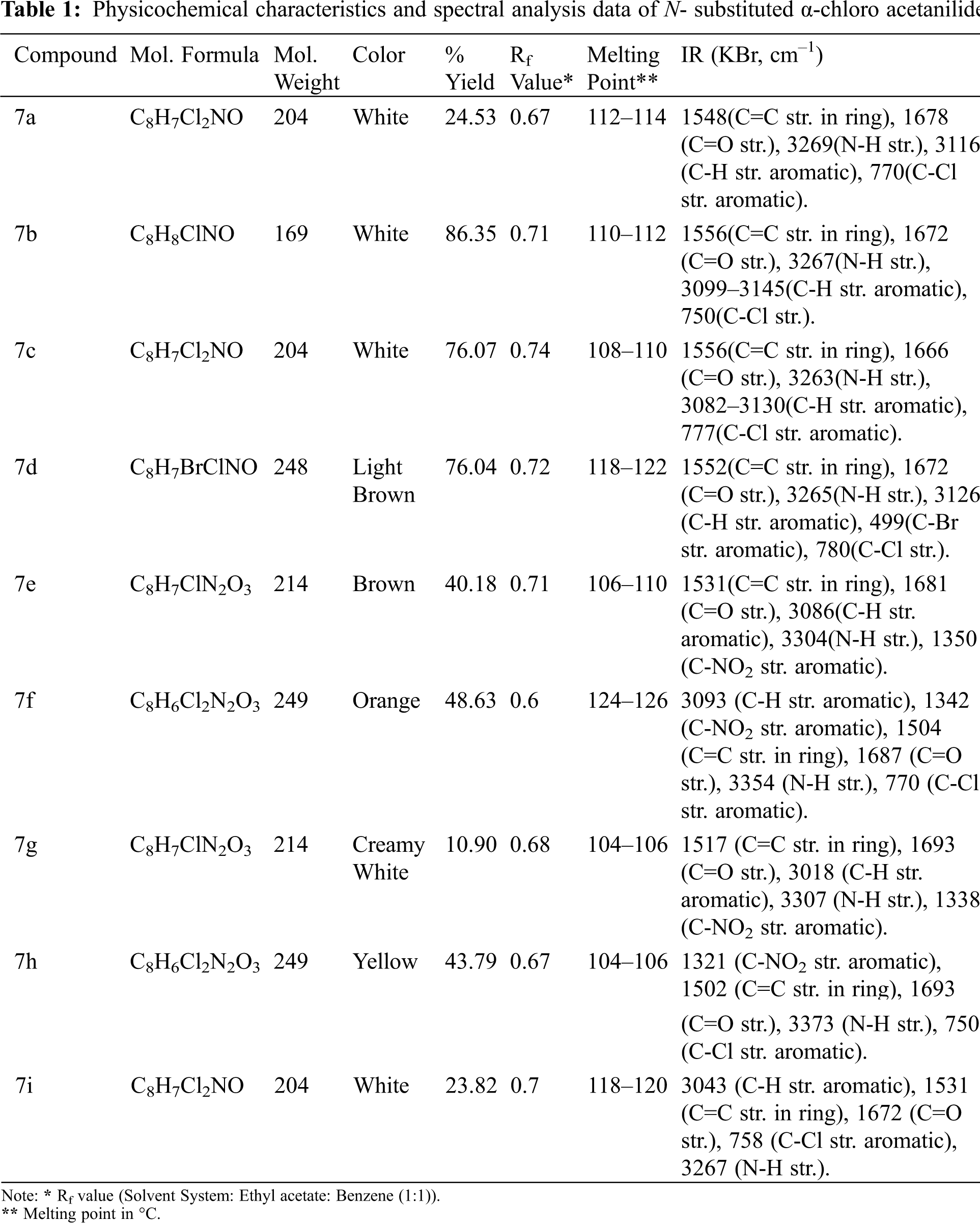
In a saturated sodium acetate solution (25 mL), a suitable substituted aromatic amine (0.05 mol) was dissolved. If the substance is not completely dissolved, the mixture is warmed up until it is dissolved. It was subsequently cooled in an ice bath with stirring. To this reaction mixture, chloroacetyl chloride (0.07 mol) was added dropwise to avert a vigorous reaction. For 5–6 h, the mixture was kept at room temperature. The product obtained was then filtered, washed with distilled cold water, dried, and recrystallized from aqueous ethanol. Physicochemical characteristics and spectral analysis data of N- substituted α-chloro acetanilides are included in Table 1.
2.1.4 Step 3: Synthesis of Ethyl 5-acetyl-2-({2- [(substitutedphenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3- carboxylate (8a-8i)
In equimolar proportions (0.05 mol), various N-substituted -chloro acetanilides (7A1–A9) and compound 4 were mixed in 1,4-dioxane (15 mL). Following the addition of triethylamine solution (0.005 mol), the reaction mixture was refluxed for 2 h. The reaction mixture was poured over crushed ice after cooling. The resulting product was filtered and dried after being washed with potassium bicarbonate (1%). The reaction was monitored and Rf values for 8a-8i were determined using the ethyl acetate/n-hexane solvent system (1:2).
Compound 8a: Ethyl 5-acetyl-2-({2-[(3-chlorophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Brown Solid (77.7%); M.P.: 108–110°C; Rf = 0.7; IR (KBr, cm–1): 3296 (N-H str.), 3408 (N-H str. coupled), 1603 (C=C str.), 3087 (Ar-H str.), 877 (C-C str.), 1664 (C=O str.), 1477 (C-N str.), 819 (C-Cl str.), 785 (C-S str.); 1H NMR (500 MHz, CDCl3) δ 4.49 (s, 2H, CH2), 8.19 (s, 1H, -CONH), 7.13-7.47 (m, 4H, Ar-H), 4.38 (q, 2H, OCH2CH3), 4.20 (s, 1H, NH), 1.42 (t, 3H, OCH2CH3), 2.55 (s, 3H, -COCH3), 2.19 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 182.8, 167.7, 166.3, 164.0, 149.4, 139.5, 134.3, 129.9, 124.8, 120.4, 120.3, 109.1, 109.1, 61.5, 47.8, 27.2, 15.1, 14.6; ESI-MS (m/z): 396.37 (M+1).
Compound 8b: Ethyl 5-acetyl-4-methyl-2-{[2-oxo-2-(phenylamino)ethyl] amino}thiophene-3-carboxylate: Creamy solid (73.33%); M.P.: 130–132°C; Rf = 0.66; IR (KBr, cm–1): 786 (C-S str.), 3297 (N-H str.), 3405 (N-H str. coupled), 1663 (C=O str.), 3097 (Ar-H str.), 1499 (C-N str.), 1618 (C=C str.), 859 (C-C str.), 1284 (C-O str.), 691 (Monosubsti. Ring); 1H NMR (500 MHz, CDCl3) δ 4.47 (s, 2H, CH2), 7.50–7.14 (m, 5H, Ar-H), 4.30 (q, 2H, OCH2CH3), 4.40 (s, 1H, NH), 2.57 (s, 3H, -COCH3), 1.40 (t, 3H, OCH2CH3), 2.43 (s, 3H, -CH3), 8.46 (s, 1H, -CONH); 13C NMR (125 MHz, CDCl3) δ 194.2, 167.7, 166.3, 164.0, 149.4, 137.7, 129.3, 126.4, 123.7, 121.4, 109.1, 86.3, 61.5, 47.8, 27.2, 15.1, 16.87, 14.6; ESI-MS (m/z): 361.61 (M+1).
Compound 8c: Ethyl 5-acetyl-2-({2-[(4-chlorophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Buff colored solid (92%); M.P.: 122–124°C; Rf = 0.67; IR (KBr, cm–1): 778 (C-S str.), 826 (C-Cl str.), 863 (C-C str.), 1275 (C-O str.), 1474 (C-N str.), 1606 (C=C str.), 1665 (C=O str.), 3408 (N-H str. coupled), 3085 (Ar-H str.), 3297 (N-H str.); 1H NMR (500 MHz, CDCl3) δ 7.28–7.48 (m, 4H, Ar-H), 8.43 (s, 1H, -CONH), 4.48 (s, 2H, CH2), 4.33 (q, 2H, OCH2CH3), 4.24 (s, 1H, NH), 1.39 (t, 3H, OCH2CH3), 2.55 (s, 3H, -COCH3), 2.43 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 194.2, 181.7, 167.7, 166.3, 164.1, 137.79, 149.4, 136.5, 129.2, 128.6, 126.4, 122.2, 109.1, 61.5, 47.8, 27.2, 15.1, 14.6; ESI-MS (m/z): 396.16 (M+1).
Compound 8d: Ethyl 5-acetyl-2-({2-[(4-bromophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Light Yellow (93%); M.P.: 134–136°C; Rf = 0.6; IR (KBr, cm–1): 3300 (N-H str.), 3410 (N-H str. coupled), 1606 (C=C str.), 862 (C-C str.), 3081 (Ar-H str.), 1663 (C=O str.), , 657 (C-S str.), 1488 (C-N str.), 1250 (C-O str.), 773 (C-Br str. coupled); 1H NMR (500 MHz, CDCl3) δ 9.24 (s, 1H, -CONH), 4.36 (s, 2H, CH2), 4.34 (q, 2H, OCH2CH3), 7.26-7.64 (m, 4H, Ar-H), 4.42 (s, 1H, NH), 2.15 (s, 3H, -COCH3), 2.56 (s, 3H, -CH3), 1.39 (t, 3H, OCH2CH3); 13C NMR (125 MHz, CDCl3) δ 194.2, 167.7, 166.3, 164.0, 149.4, 136.2, 131.7, 136.2, 131.7, 126.4, 122.1, 118.2, 109.1, 61.5, 47.8, 27.2, 15.1, 14.6; ESI-MS (m/z): 440.61 (M+1), 441.41 (M+2).
Compound 8e: Ethyl 5-acetyl-2-({2-[(3-nitrophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Light Brown solid (67%); M.P.: 122–124°C; Rf = 0.66; IR (KBr, cm–1): 2986 (Ar-H str.), 1310 (C-NO2 str. aromatic), 3415 (N-H str. coupled), 3295 (N-H str.), 786 (C-S str.), 1684 (C=O str.), 1476 (C-N str.), 1526 (N–O asymmetric stretch), 1273 (C-O str.), 835 (C-C str.); 1H NMR (500 MHz, CDCl3) δ 7.2704 (s, 1H, -CONH), 7.56–7.89 (m, 4H, Ar-H), 4.54 (s, 2H, CH2), 4.34 (q, 2H, OCH2CH3), 1.39 (t, 3H, OCH2CH3), 3.96 (s, 1H, NH), 2.55 (s, 3H, -COCH3), 2.44 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 183.5, 167.7, 166.2, 164.1, 149.4, 148.7, 139.6, 129.2, 127.0, 126.4, 120.2, 115.8, 109.2, 61.5, 47.8, 27.2, 15.1, 14.3; ESI-MS (m/z): 406.24 (M+1).
Compound 8f: Ethyl 5-acetyl-2-({2-[(4-chloro-2-nitrophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Yellowish Orange solid (94.1%); M.P.: 130–132°C; Rf = 0.66; IR (KBr, cm–1): 3410 (N-H str. coupled), 3296 (N-H str.), 2873 (C-H str.), 1661 (C=O str.), 1455 (N–O asymmetric stretch), 1589 (C=C str.), 914 (C-C str.), 1254 (C-O str.), 2993 (Ar-H str.), 891 (C-Cl str.), 786 (C-S str.); 1H NMR (500 MHz, CDCl3) δ 7.46–7.85 (m, 3H, Ar-H), 8.84 (s, 1H, -CONH), 1.39 (t, 3H, OCH2CH3), 4.49 (s, 2H, CH2), 4.35 (q, 2H, OCH2CH3), 4.36 (s, 1H, NH), 2.47 (s, 3H, -COCH3), 2.59 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 194.2, 167.7, 166.3, 164.0, 149.4, 148.7, 139.6, 129.2, 127.0, 126.4, 120.2, 115.8, 109.8, 84.6, 47.8, 27.2, 15.1, 14.7; ESI-MS (m/z): 440.51 (M+1).
Compound 8g: Ethyl 5-acetyl-2-({2-[(2-nitrophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Light Yellow solid (91.7%); M.P.: 110–112°C; Rf = 0.67; IR (KBr, cm–1): 1588 (C=C str.), 2986 (Ar-H str.), 3410 (N-H str. coupled), 3295 (N-H str.), 2854 (C-H str.), 1665 (C=O str.), 1513 (N-O asymmetric stretch), 1458 (C-N str.), 1311 (C-NO2 str. aromatic), 1271 (C-O str.), 1257 (C-N str.); 847 (C-C str.), 738 (C-S str.); 1H NMR (500 MHz, CDCl3) δ 7.43–8.31 (m, 4H, Ar-H), 8.32 (s, 1H, -CONH), , 1.39 (t, 3H, OCH2CH3), 4.41 (s, 2H, CH2), 4.34 (q, 2H, OCH2CH3), 4.39 (s, 1H, NH), 2.09 (s, 3H, -COCH3), 2.54 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 194.2, 183.5, 167.7, 164.9, 149.4, 148.7, 139.6, 129.2, 127.0, 126.4, 120.0, 115.8, 109.8, 84.6, 47.3, 27.4, 15.0, 14.9; ESI-MS (m/z): 406.1 (M+1).
Compound 8h: Ethyl 5-acetyl-2-({2-[(2-chloro-4-nitrophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Yellow solid (88.6%); M.P.: 118–120°C; Rf = 0.60; IR (KBr, cm–1): 947 (C-C str.), 3376 (N-H str.), 1510 (N-O asymmetric stretch), 893 (C-Cl str.), 3496 (N-H str. coupled), 1262(C-O str.), 3117 (Ar-H str.), 1665 (C=O str.), 1588 (C=C str.), 1310 (C-NO2 str. aromatic), 786 (C-S str.); 1H NMR (500 MHz, CDCl3) δ 7.52–8.32 (m, 3H, Ar-H), 6.48 (s, 1H, -CONH), 4.52 (s, 2H, CH2) , 1.39 (t, 3H, OCH2CH3), 4.35 (q, 2H, OCH2CH3), 5.99 (s, 1H, NH), 2.13 (s, 3H, -COCH3), 2.57 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 194.2, 183.0, 166.3, 164.0, 149.4, 148.7, 139.6, 129.2, 127.0, 126.4, 120.2, 115.8, 109.1, 61.5, 47.8, 27.2, 15.1, 14.9; ESI-MS (m/z): 440.73 (M+1).
Compound 8i: Ethyl 5-acetyl-2-({2-[(2-chlorophenyl)amino]-2-oxoethyl}amino)-4-methylthiophene-3-carboxylate: Light Brown solid (75.44%); M.P.: 120–122°C; Rf = 0.72; IR (KBr, cm–1): 1504 (C-N str.), 3296 (N-H str.), 3410 (N-H str. coupled), 2986 (C-H str.), 3070 (Ar-H str.), 1665 (C=O str.), , 758 (C-S str.), 1604 (C=C str.), 1256 (C-O str.), 810 (C-Cl str.), 836 (C-C str.); 1H NMR (500 MHz, CDCl3) δ 7.07–7.39 (m, 4H, Ar-H), 5.18 (s, 1H, -CONH), 4.48 (s, 2H, CH2), 1.40 (t, 3H, OCH2CH3), 4.34 (q, 2H, OCH2CH3), 4.40 (s, 1H, NH), 2.13 (s, 3H, -COCH3), 2.55 (s, 3H, -CH3); 13C NMR (125 MHz, CDCl3) δ 194.7, 183.5, 167.7, 166.3, 164.0, 149.4, 148.7, 139.6, 129.2, 127.0, 126.4, 120.2, 109.2, 61.5, 47.8, 27.4, 15.0, 14.6; ESI-MS (m/z): 396.42 (M+1).
2.2 Pharmacological Evaluation of the Synthesized Derivatives
2.2.1 Assessment of Anticancer Activity
Full NCI 60 Cell Panel Assay in Single Doses
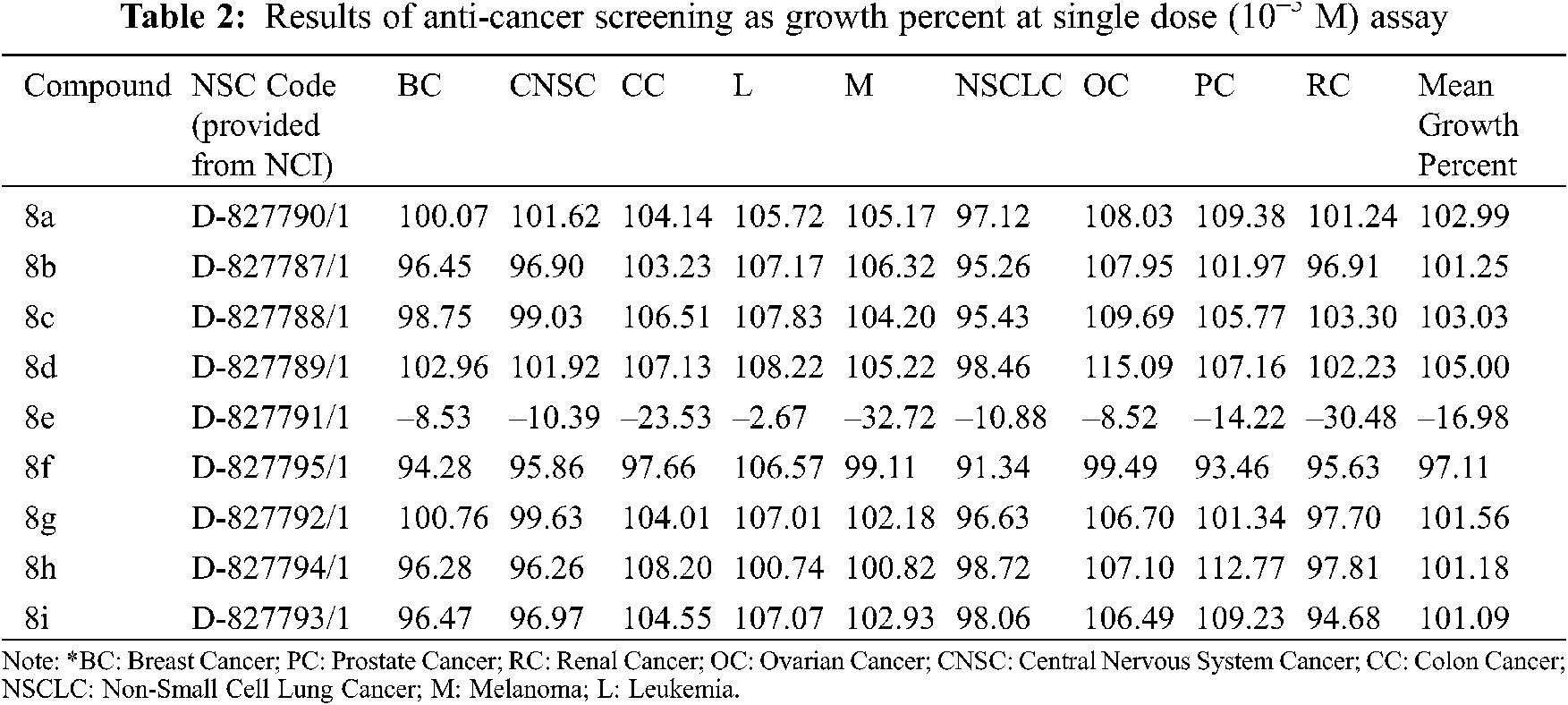
National Cancer Institute (NCI) identified nine compounds with anticancer efficacy in the full NCI 60 human tumor cell screen program. The compounds were first evaluated on 60 cancer cell lines, including breast, CNS, colon, leukemia, melanoma, lung, ovarian, prostate, and renal cancer cell lines, at a concentration of 10–5 M. A mean graph of the percentage growth of treated cells showed the behavior of the chosen compounds. The percentage growth was determined using spectrophotometry in contrast to controls that were not administered the test entities. The continuous drug exposure procedure was evaluated for 48 h using a Sulforhodamine B (SRB) protein assay and cell viability was determined [47–49].
Full NCI 60 Cell Panel Assay in Five Doses
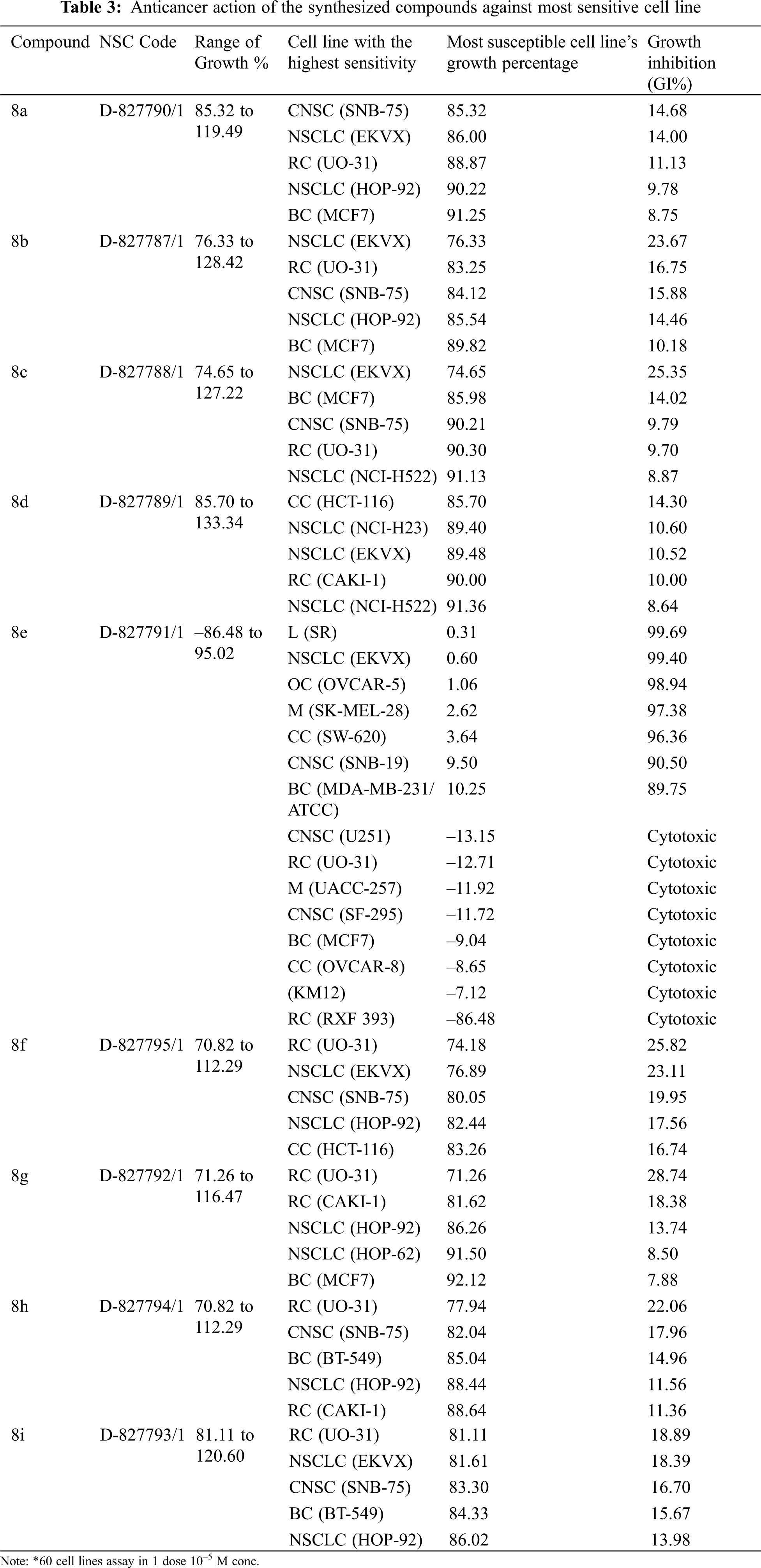
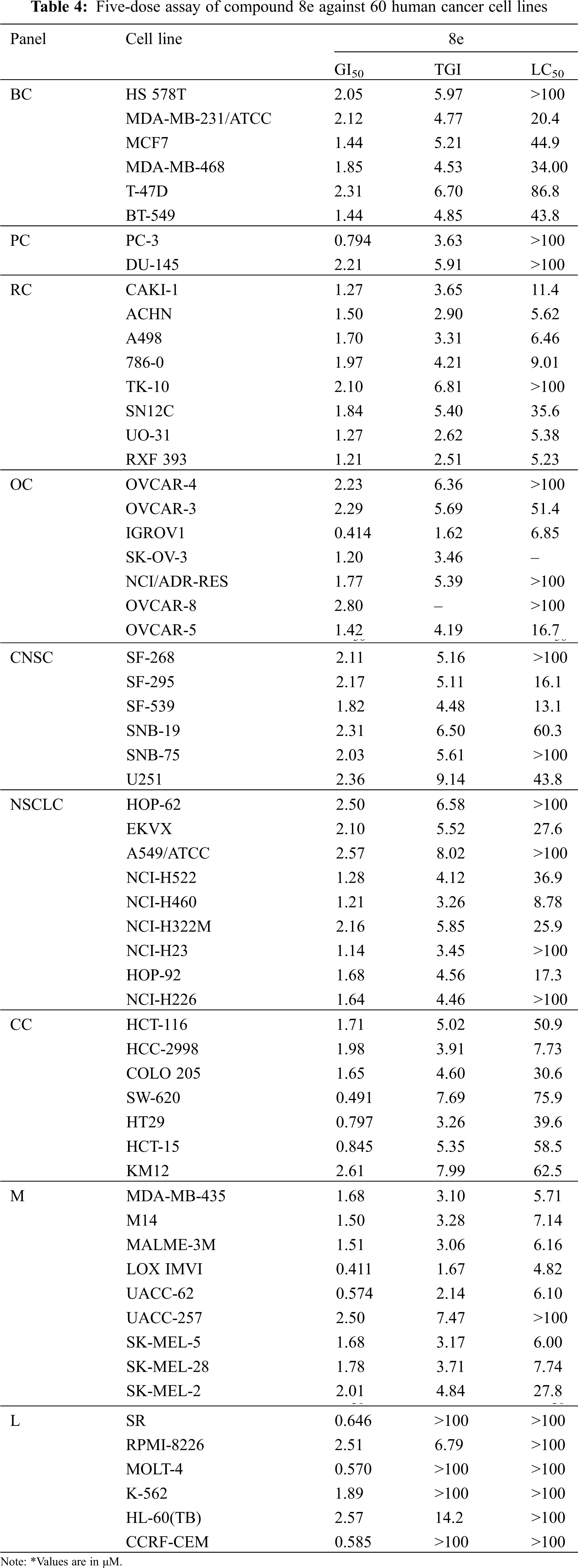
Following the significant suppression of growth by compound 8e, further study was performed at 10-fold dilutions of five doses (10–4 to 10–8 M). The dose-response parameters GI50, TGI, and LC50 were used to evaluate the drug. GI50 value (Growth Inhibition of 50% of Cells) indicates a compound concentration that prevents net cell growth by 50%. TGI (Total Growth Inhibition) is the concentration of a substance that inhibits total growth and therefore indicates its cytostatic effect. LC50 value (Lethal concentration that kills 50% of cells) shows cytotoxicity and refers to the concentration of a compound that results in a net loss of 50% of the initial cells following a 48-hour incubation period.
The percentage growth curve is calculated as follows:
where,
T is the cell count at day 3 at the test concentration,
To is the cell count at day 0, and
C is the vehicle control (without drug) cell count
The GI50 and TGI values are calculated utilizing drug concentrations that resulted in 50% and 0% growth after 48 h of drug intake, respectively.
when T < To.
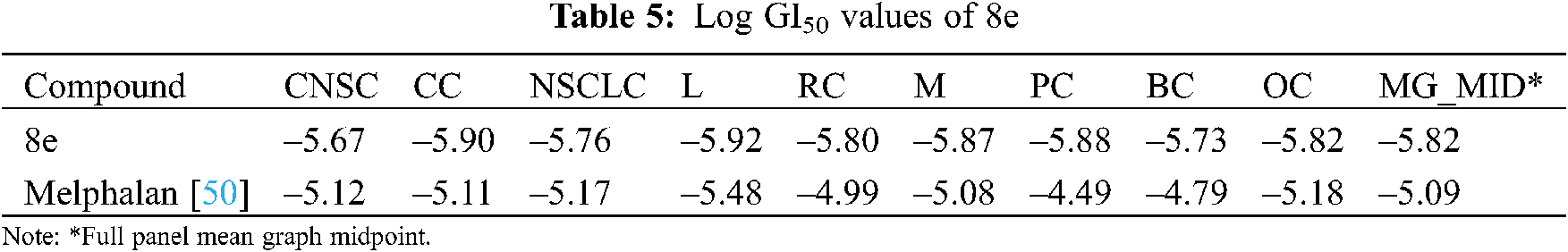
The results were derived by using methods specified by the NCI/NIH Development Therapeutic Program. Following the determination of log GI50 values, mean-graph midpoint (MG_MID) values for the whole panel were computed. Because these numbers are presented in terms of concentration, they are more relevant for evaluating the activity. The obtained results are logarithmic concentration values showing 50% inhibition based on the test protocol. If the value is higher than –4, the compound is inactive [50].
2.2.2 Assessment of Antioxidant Activity
The Shimada method, which is based on the theory of scavenging the 2,2-diphenyl-2-picrylhydrazyl (DPPH) radical, was used to evaluate the free radical scavenging activities of synthesized compounds in reference to ascorbic acid [51]. Different concentrations of synthesized derivatives and ascorbic acid (10–100 μg/ml) were prepared in methanol, and 1 mL of each concentration of test compound and ascorbic acid was added to 1 mL of 0.1 mM DPPH solution. The absorbance was assessed using UV at 517 nm once the mixture was held in a dark place at room temperature for 30 minutes after intense shaking [52].
The following formula was used to calculate the % scavenging of the free radical DPPH.
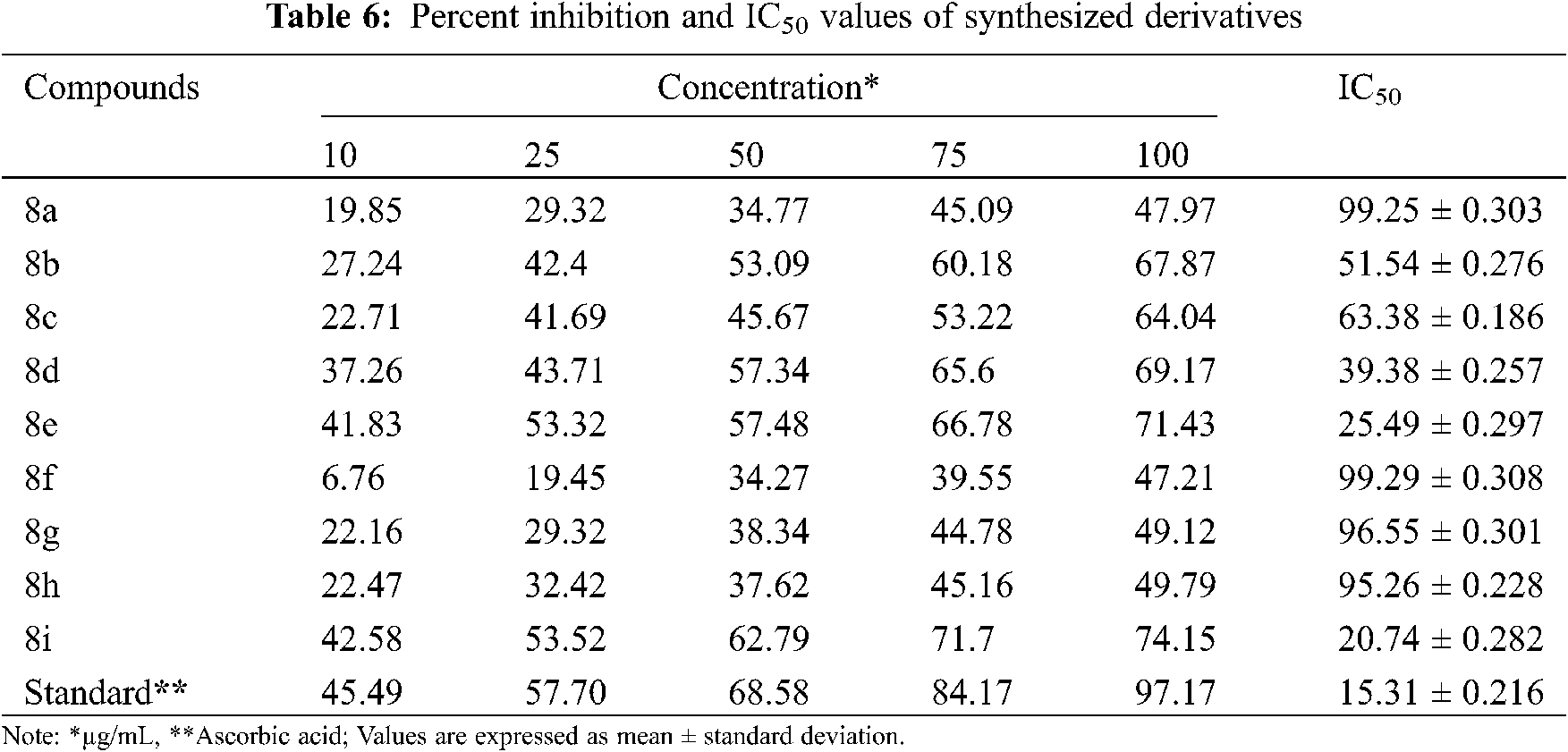
A graph depicting percent inhibition against various concentrations of synthesized derivatives was used to calculate the IC50 value for each sample. The IC50 value is the test sample concentration that causes a 50% reduction in the initial DPPH concentration. A higher free radical scavenging activity is indicated by a lower mean concentration value of the inhibitor.
For molecular docking studies, the preferred target protein (PDB id-6q7a) was retrieved from the RCSB Protein data bank [53]. Protein Preparation Wizard was used to prepare the chosen PDB file for the molecular docking study. By incorporating polar hydrogen bonds, removing redundant water molecules, and then adding and distributing charge, the receptor molecule was optimized. With the help of the AutoDock software, the processed receptor molecule was saved in *.pdbqt format. The amino acids Phe388 and His479 aided in the active binding of ligand to the RORγt receptor. A grid around the co-crystallized ligand is created, allowing it to be eliminated and new compounds to be bound to the same active site to analyze their receptor interactions. The overlay method and chemical resemblance were used to verify the molecular docking process when docking a specific ligand with a relevant macromolecule. The docking score obtained using molecular docking software was used to examine the results. The docking score was designated by a negative value. Lower the docking score, the greater the ligand’s affinity for the receptor [54].
In this study, nine new thiophene derivatives (8a–8i) were synthesized using Gewald synthesis. Intermediate (4) was synthesized by reacting ethyl cyanoacetate, acetylacetone, and sulfur with diethylamine as a base. Under cold conditions, aromatic amines and chloroacetyl chloride were reacted to give N-substituted alpha-chloroacetanilides (7a-i). Target compounds were produced in 75–86% yields in the presence of triethylamine by reacting equimolar quantities of intermediate and various N-substituted chloroacetanilides. The synthesized derivatives were characterized using a variety of spectroscopy approaches, all of which were consistent with the assigned chemical structures.
3.2 Pharmacological Evaluation of the Synthesized Derivatives
The NCI, Bethesda, USA, investigated the anticancer activity of the selected nine compounds (8a–8i) on 60 human cancer cell lines. Table 2 includes the behavior of the identified compounds as a mean graph of the percent growth of treated cells. The highest mean activity was observed to be –16.98% for 8e when the percentage of growth inhibition of substance-treated tumor cells (10–5 M concentration of compounds) was compared to non-treated tumor cells. Some cell lines were, in particular, more sensitive to the compounds examined and major individual results were obtained. When contrasting the percentage of growth inhibition of substance-treated tumor cells to those that were not treated, as seen in Table 3, it was established that lung cancer, renal cancer, leukemia, CNS, and breast cancer were the most vulnerable cancer cell lines. Compound 8e was investigated further with a 5-log dose molar range after inhibiting cell growth at 10–5 M concentration in a variety of cell lines. The findings are described in Table 4 in terms of GI50, TGI, and LC50 values. 8e reported GI50 values ranging from 0.411 to 2.8 μM. TGI values >100 μM were seen in all leukemia cancer cell lines for the specified compound. The MG_MID values in Table 5 were estimated after calculating their log GI50 values in reference to Melphalan. These values are more relevant for assessing the activity as they are revealed in terms of concentration. The compound is inactive if the value exceeds –4. The substantial activity was seen in compound 8e with a MG_MID value of –5.82. These concentration values were comparable to the percentage growth values stated earlier. It was also selective against all cancer cell lines, with logGI50 values varying from –5.67 to –5.92. Those obtained concentration values corresponded to the previously indicated percentage growth values.
In vitro antioxidant activity of the synthesized derivatives was investigated in terms of percentage (%) inhibition by DPPH assay using ascorbic acid as a reference (Fig. 2). By plotting concentrations against percent inhibition of the test compound, the IC50 value of synthesized compounds was calculated. Only a few synthesized compounds had significant antioxidant activity, while others had moderate to strong antioxidant activity, according to the results. The antioxidant properties of the compounds 8e and 8g were excellent, with IC50 values and inhibition percentages equivalent to ascorbic acid (Fig. 3). The findings of the antioxidant screening are presented in Table 6.
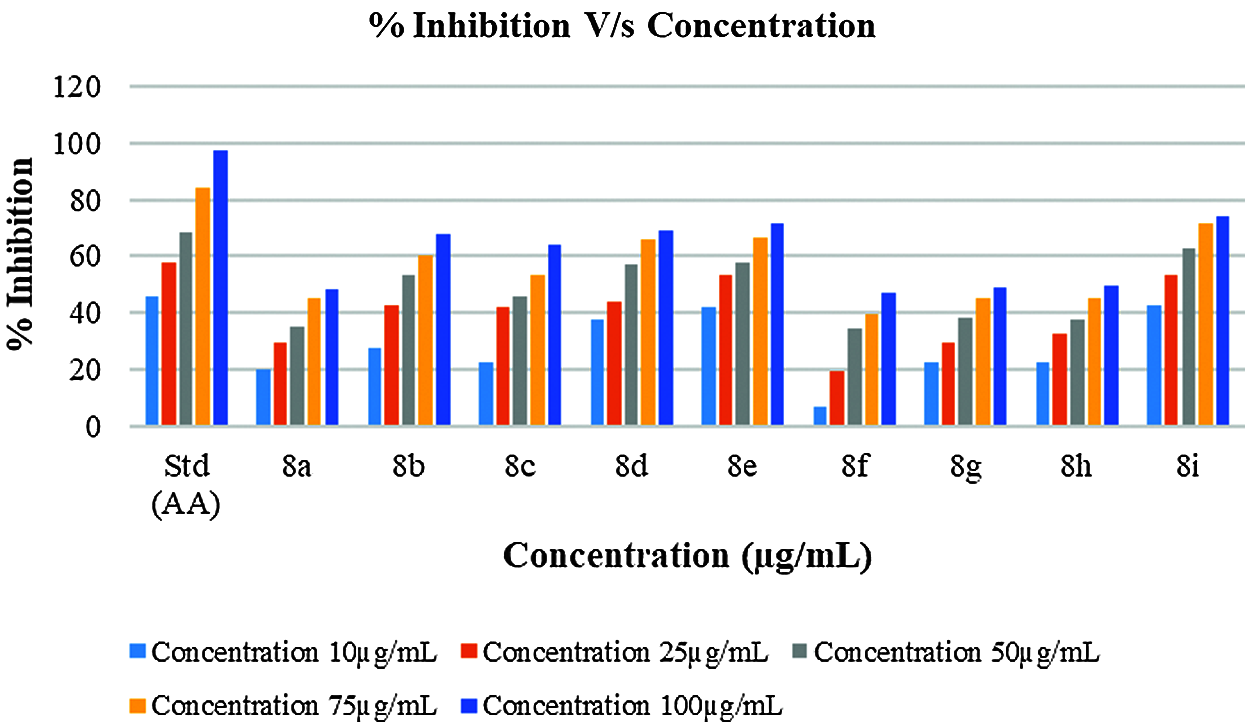
Figure 2: Antioxidant behavior of synthesized derivatives and Ascorbic acid
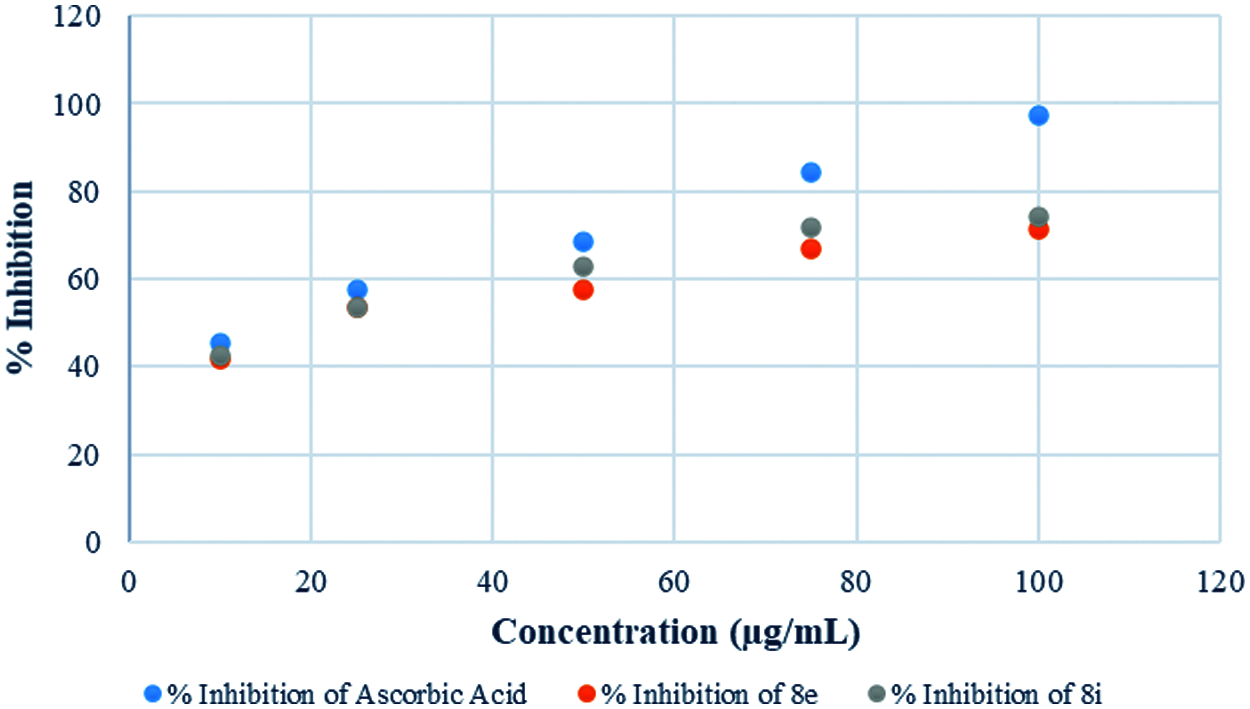
Figure 3: Antioxidant behavior of potent derivatives and Ascorbic acid
To explore prospective RORγt receptor inhibitor molecules, a molecular docking simulation-based in silico virtual screening was carried out. A ligand library of nine thiophene derivatives was virtually screened against the validated RORγt receptor. The binding affinity of all ligand molecules was determined by calculating the binding energy for each ligand’s top-ranked pose, and the interactions of docked compounds were visualized. Against the RORγt receptor, the outcomes of AutoDock-based molecular docking simulations of the nine ligand molecules are summarized in Table 7. Based on the lowest binding energy, the best ligand molecule was chosen. As per empirical information, the free binding energy should be between –5 to –15 kcal/mol. Figs. 4 and 5 show a ligand interaction diagram (2D) and a pictorial representation (3D) of 8e.
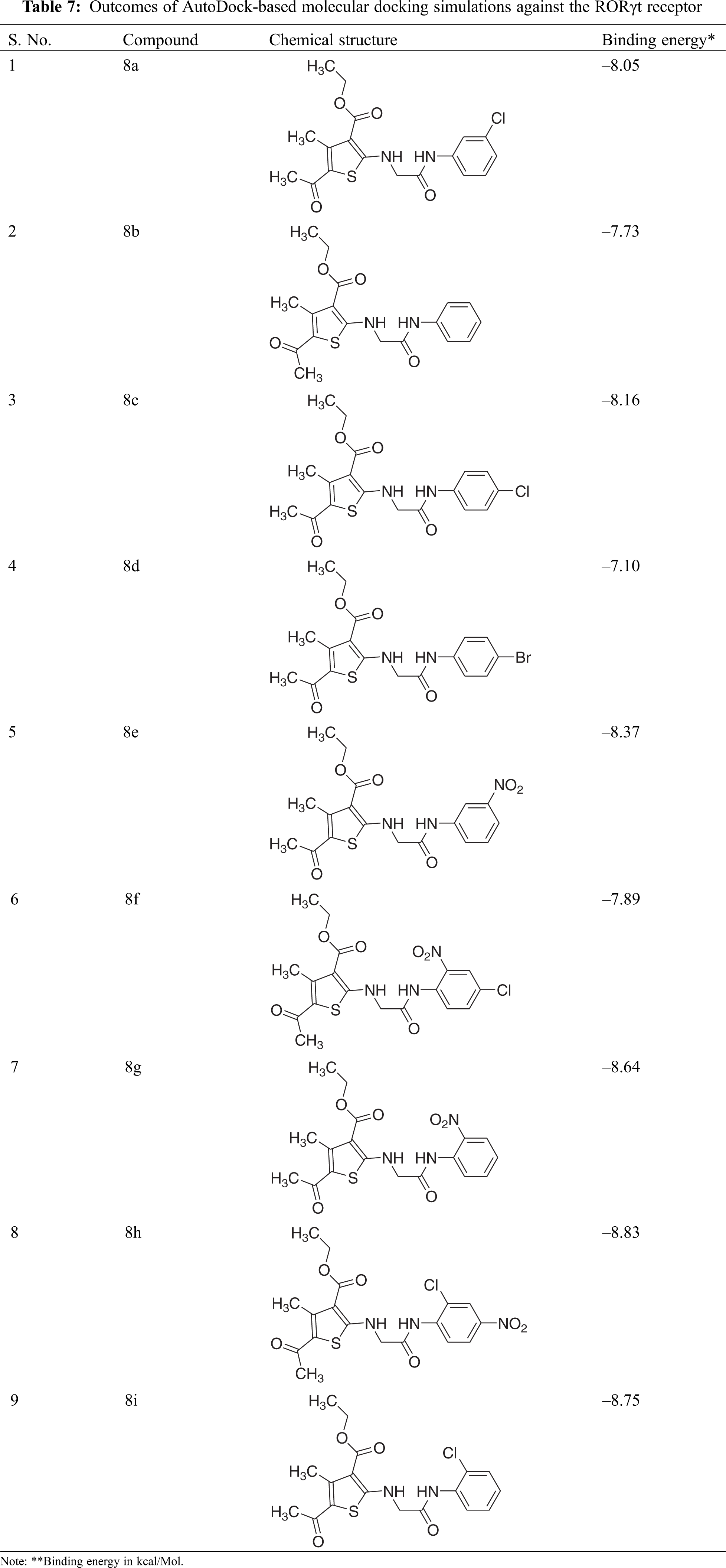
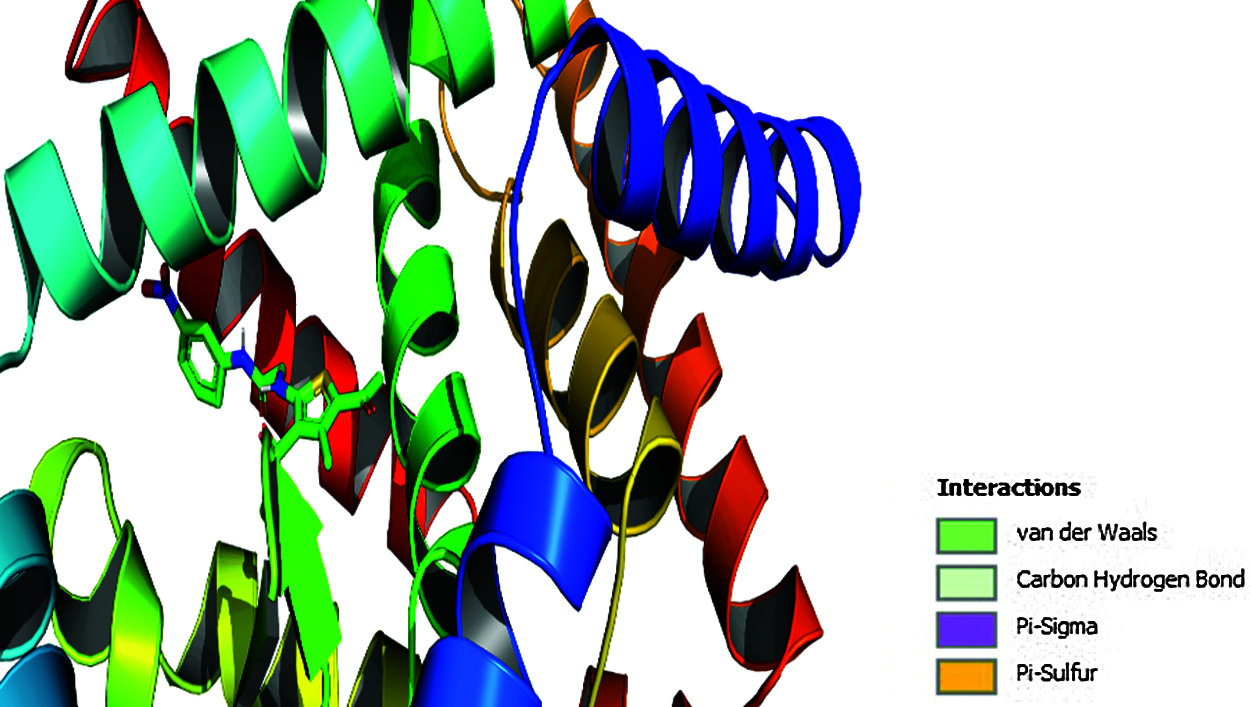
Figure 4: Ligand interaction diagram (2D) of 8e
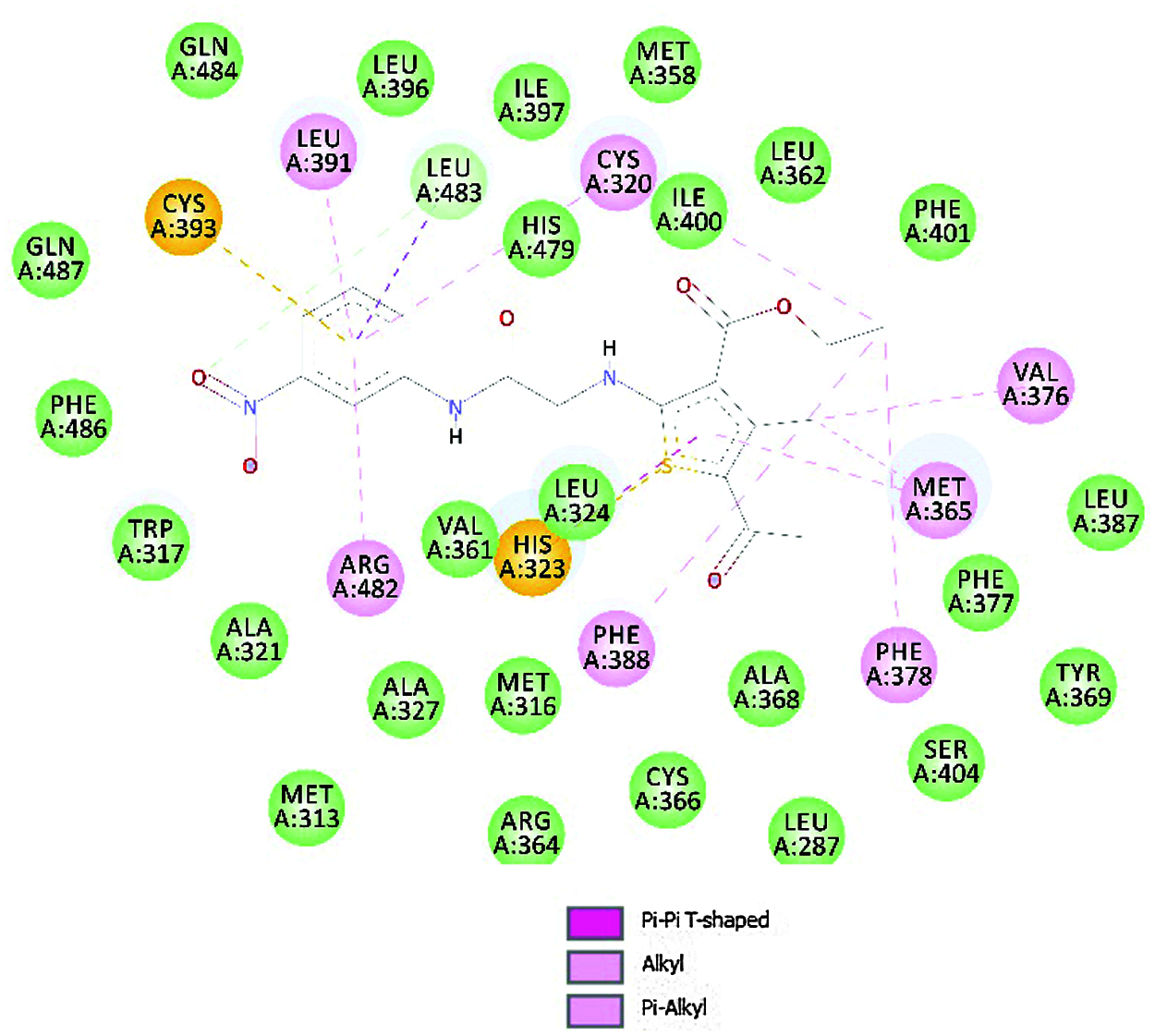
Figure 5: Pictorial presentation (3D) of 8e
3.4 Structure-Activity Relationship (SAR) Studies
Based on antioxidant and anti-cancer data, the SAR of synthesized thiophene derivatives may be interpreted as follows:
• The results of the antiproliferative research indicated that electron-withdrawing groups at the meta-position are required for activity, which complemented the in-silico docking analysis precisely.
• In compounds containing an electron-withdrawing group in the ortho- and meta-positions of the phenyl group, significant antioxidant activity was observed.
• The antioxidant function of the synthesized compounds may work synergistically to combat the enhanced oxidative stress in cancerous conditions.
• As a result of the resonating effect, the derivative having a nitro group at meta-position in the aromatic ring exhibited substantial anticancer and antioxidant activities.
To summarize, this paper discusses the synthesis, pharmacological prospects, and in silico studies of novel thiophene derivatives. In vitro antiproliferative and antioxidant functions of synthesized compounds were assessed. Among the synthesized derivatives, compound 8e revealed notable anticancer activity, while compounds 8d, 8e, and 8i evinced promising antioxidant activity. In addition, inside the binding pocket, all the compounds revealed a good docking score in the molecular docking study. The findings of the in-silico study correspond to the cytotoxicity tests. In compound 8e, the presence of an electron-withdrawing (m-NO2) group at benzylidene ring conferred upon it the highest anticancer and antioxidant activity. It is suggested that antioxidant activity may play a role in cancer progression control, while cytotoxic ability may be utilized over cancer cells, leading them to apoptosis and cell death. These remarkable biological screening results of synthesized derivatives will help provide a strong basis in this field and pave the way for the establishment of effective therapeutics.
Ethics Approval and Informed Consent Statement: Not Applicable.
Availability of Data and Materials: The data supporting the article is available within the article.
Authors’ Contribution: All authors contributed equally to this work. Raghav Mishra, Nitin Kumar and Neetu Sachan prepared the manuscript.
Acknowledgement: Thanks to GLA University for providing the facility to conduct research as well as for furnishing literature survey facility to accomplish the study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Irfan, A., Batool, F., Zahra Naqvi, S. A., Islam, A., Osman, S. M. et al. (2020). Benzothiazole derivatives as anticancer agents. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), 265–279. [Google Scholar]
2. Abdel-Rahman, S. A., El-Damasy, A. K., Hassan, G. S., Wafa, E. I., Geary, S. M. et al. (2020). Cyclohepta[b]thiophenes as potential antiproliferative agents: Design, synthesis, in vitro, and in vivo anticancer evaluation. ACS Pharmacology & Translational Science, 3(5), 965–977. [Google Scholar]
3. World Health Organization (2021). Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. [Google Scholar]
4. Anand, P., Kunnumakkara, A. B., Sundaram, C., Harikumar, K. B., Tharakan, S. T. et al. (2008). Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research, 25(9), 2097–2116. [Google Scholar]
5. Syed, T., Asiri, Y. I., Shaheen, S., Gangarapu, K. (2021). Design, synthesis and anticancer evaluation of structurally modified substituted aryl-quinazoline derivatives as anticancer agents. Synthetic Communications, 51(18), 2782–2795. [Google Scholar]
6. Chaudhary, A., Sharma, P. K., Kashyap, S., Gupta, J. K., Dudhe, R. et al. (2011). Insignificant anticancer activity of novel substituted pyrimidine derivatives. Journal of Pharmaceutical Negative Results, 2(2), 62–68. DOI 10.4103/0976-9234.90213. [Google Scholar] [CrossRef]
7. Li, Q., Chen, L., Jian, X. E., Lv, D. X., You, W. W. et al. (2021). Design, synthesis and antiproliferative activity of novel 2,4-Diamino-5-Methyleneaminopyrimidine derivatives as potential anticancer agents. Bioorganic & Medicinal Chemistry Letters, 47(128213), 128213. DOI 10.1016/j.bmcl.2021.128213. [Google Scholar] [CrossRef]
8. Kumar, N., Bhatnagar, A., Dudhe, R. (2017). Synthesis of 3-(4, 5-Dihydro-1-Phenyl-5-Substituted Phenyl-1H-Pyrazol-3-Yl)-2H-Chromen-2-One derivatives and evaluation of their anticancer activity. Arabian Journal of Chemistry, 10(3), S2443–S2452. DOI 10.1016/j.arabjc.2013.09.008. [Google Scholar] [CrossRef]
9. Dudhe, R., Dudhe, A. C., Dudhe, R., Sakarkar, S. N., Porwal, O. (2021). AI–New avenue for drug discovery and optimization. Clinical Oncology and Research, 2021, 1–9. DOI 10.31487/J.COR.2021.01.02. [Google Scholar] [CrossRef]
10. Sui, X., Chen, R., Wang, Z., Huang, Z., Kong, N. et al. (2013). Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death & Disease, 4(10), e838. DOI 10.1038/cddis.2013.350. [Google Scholar] [CrossRef]
11. Marin, J. J. G., Romero, M. R., Blazquez, A. G., Herraez, E., Keck, E. et al. (2009). Importance and limitations of chemotherapy among the available treatments for gastrointestinal tumours. Anti-Cancer Agents in Medicinal Chemistry, 9(2), 162–184. DOI 10.2174/187152009787313828. [Google Scholar] [CrossRef]
12. Vanneman, M., Dranoff, G. (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nature Reviews Cancer, 12(4), 237–251. DOI 10.1038/nrc3237. [Google Scholar] [CrossRef]
13. Zimmermann, S., Dziadziuszko, R., Peters, S. (2014). Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treatment Reviews, 40(6), 716–722. DOI 10.1016/j.ctrv.2014.03.005. [Google Scholar] [CrossRef]
14. Ismael, G. F. V., Rosa, D. D., Mano, M. S., Awada, A. (2008). Novel cytotoxic drugs: Old challenges, new solutions. Cancer Treatment Reviews, 34(1), 81–91. DOI 10.1016/j.ctrv.2007.08.001. [Google Scholar] [CrossRef]
15. Lu, Y., Mahato, R. I. (2016). Pharmaceutical perspectives of cancer therapeutics. New York, NY: Springer. [Google Scholar]
16. Mishra, R., Jha, K. K., Kumar, S., Tomer, I. (2011). Synthesis, properties and biological activity of thiophene: A review. Der Pharma Chemica, 3(4), 38–54. [Google Scholar]
17. Mohammad, A. I. C., Satyendra, D., Apurba, T., Patel, M., Monika, K. et al. (2012). Synthesis and antimicrobial screening of some novel substituted thiophenes. Hygeia: Journal for Drugs and Medicines, 4(1), 112–118. [Google Scholar]
18. Véras of Aguiar, A.C., Of Moura, R. O., Junior, J. F. B. M., de Oliveira Rocha, H. A., Câmara, R. B. G. et al. (2016). Evaluation of the antiproliferative activity of 2-amino thiophene derivatives against human cancer cells lines. Biomedicine & Pharmacotherapy, 84, 403–414. [Google Scholar]
19. Liang, C., Tang, Z., Qian, W., Shi, C., Song, H. (2014). Ultrasound-promoted synthesis of 2-aminothiophenes accelerated by DABCO utilizing PEG-200 as solvent. Journal of Chemical and Pharmaceutical Research, 6(4), 798–802. [Google Scholar]
20. Abo-Salem, H., El-Sawy, E., Fathy, A., Mandour, A. (2014). Synthesis, antifungal activity, and molecular docking study of some novel highly substituted 3-indolylthiophene derivatives. Egyptian Pharmaceutical Journal, 13(2), 71–86. [Google Scholar]
21. Rao, S. D., Rasheed, S., Basha, T. S. K., Raju, N. C., Naresh, K. (2013). SiO2/ZnCl2 catalyzed a-aminophosphonates and phosphonated N-(substitued phenyl) sulfonamides of 2-aminothiophene synthesis and biological evaluation. Der Pharma Chemica, 5(1), 61–74. [Google Scholar]
22. Arora, M., Saravanan, J., Mohan, S., Bhattacharjee, S. (2013). Synthesis, characterization and antimicrobial activity of some schiff bases of 2-amino-n-(p-acetamidophenyl carboxamido)-4,5,6,7-tetramethylene thiophenes. International Journal of Pharmacy and Pharmaceutical Sciences, 5(1), 315–319. [Google Scholar]
23. Rodrigues, K. A. D. F., Dias, C. N. D. S., Neris, P. L. D. N., Rocha, J. D. C., Scotti, M. T. et al. (2015). derivatives present antileishmanial activity mediated by apoptosis and immunomodulation in vitro. European Journal of Medicinal Chemistry, 106, 1–14. [Google Scholar]
24. Fortes, A. C., Almeida, A. A. C., Mendonça-Júnior, F. J. B., Freitas, R. M., Soares-Sobrinho, J. L. et al. (2013). Anxiolytic properties of new chemical entity, 5TIO1. Neurochemical Research, 38(4), 726–731. [Google Scholar]
25. Khan, K. M., Ullah, Z., Lodhi, M. A., Jalil, S., Choudhary, M. I. et al. (2006). Synthesis and anti-inflammatory activity of some selected aminothiophene analogs. Journal of Enzyme Inhibition and Medicinal Chemistry, 21(2), 139–143. [Google Scholar]
26. Jagadish, E. R., Mohan, S., Saravanan, J., Satyendra, D., Sree, S. P. et al. (2013). Synthesis and in-vitro anti-platelet aggregation activity of some new substituted thiophenes. Hygeia: Journal for Drugs and Medicines, 5(2), 87–96. [Google Scholar]
27. Gouda, M. A. (2013). Synthesis and antioxidant activity of novel series of naphthoquinone derivatives attached to benzothiophene moiety. Medicinal Chemistry, 3(2), 2228–2232. [Google Scholar]
28. Hana, H. Y., Khalil, W. K. B., Elmakawy, A. I., Elmegeed, G. A. (2008). Androgenic profile and genotoxicity evaluation of testosterone propionate and novel synthesized heterocyclic steroids. The Journal of Steroid Biochemistry and Molecular Biology, 110(3–5), 284–294. DOI 10.1016/j.jsbmb.2007.11.006. [Google Scholar] [CrossRef]
29. Duffy, J. L., Kirk, B. A., Konteatis, Z., Campbell, E. L., Liang, R. et al. (2005). Discovery and investigation of a novel class of thiophene-derived antagonists of the human glucagon receptor. Bioorganic & Medicinal Chemistry Letters, 15(5), 1401–1405. DOI 10.1016/j.bmcl.2005.01.003. [Google Scholar] [CrossRef]
30. Ortiz, M. A., Piedrafita, F. J., Pfahl, M., Maki, R. (1995). TOR: A new orphan receptor expressed in the thymus that can modulate retinoid and thyroid hormone signals. Molecular Endocrinology, 9(12), 1679–1691. [Google Scholar]
31. Fan, J., Lv, Z., Yang, G., Liao, T. T., Xu, J. et al. (2018). Retinoic acid receptor-related orphan receptors: Critical roles in tumorigenesis. Frontiers in Immunology, 9, 1187. DOI 10.3389/fimmu.2018.01187. [Google Scholar] [CrossRef]
32. Wu, F., Xu, J., Huang, Q., Han, J., Duan, L. et al. (2016). The role of Interleukin-17 in lung cancer. Mediators of Inflammation, 2016(1), 1–6. DOI 10.1155/2016/8494079. [Google Scholar] [CrossRef]
33. Saddawi-Konefka, R., Seelige, R., Gross, E. T., Levy, E., Searles, S. C. et al. (2016). Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Reports, 16(9), 2348–2358. DOI 10.1016/j.celrep.2016.07.075. [Google Scholar] [CrossRef]
34. Lee, E. J., Park, H. J., Lee, I. J., Kim, W. W., Ha, S. J. et al. (2014). Inhibition of IL-17A suppresses enhanced-tumor growth in low dose pre-irradiated tumor beds. PLoS One, 9(9), e106423. DOI 10.1371/journal.pone.0106423. [Google Scholar] [CrossRef]
35. Huh, J. R., Littman, D. R. (2012). Small molecule inhibitors of ROR γt: Targeting T h17 cells and other applications. European Journal of Immunology, 42(9), 2232–2237. DOI 10.1002/eji.201242740. [Google Scholar] [CrossRef]
36. Kargbo, R. B. (2018). ROR(GMMA)T modulating activity for the treatment of cancers. ACS Medicinal Chemistry Letters, 9(7), 590–591. DOI 10.1021/acsmedchemlett.8b00216. [Google Scholar] [CrossRef]
37. Halliwell, B. (2007). Biochemistry of oxidative stress. Biochemical Society Transactions, 35(5), 1147–1150. DOI 10.1042/BST0351147. [Google Scholar] [CrossRef]
38. Pham-Huy, L. A., He, H., Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. International Journal of Biomedical Science, 4(2), 89–96. [Google Scholar]
39. Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., Kalayci, O. (2012). Oxidative stress and antioxidant defense. The World Allergy Organization Journal, 5(1), 9–19. DOI 10.1097/WOX.0b013e3182439613. [Google Scholar] [CrossRef]
40. Rahman, K. (2007). Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging, 2(2), 219. [Google Scholar]
41. Singh, P. K. (2019). Histone methyl transferases: A class of epigenetic opportunities to counter uncontrolled cell proliferation. European Journal of Medicinal Chemistry, 166, 351–368. DOI 10.1016/j.ejmech.2019.01.069. [Google Scholar] [CrossRef]
42. Archna, Pathania, S., Chawla, P. A. (2020). Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorganic Chemistry, 101, 104026. DOI 10.1016/j.bioorg.2020.104026. [Google Scholar] [CrossRef]
43. Kolli, S. K., Nakhi, A., Medishetti, R., Yellanki, S., Kulkarni, P. et al. (2014). NaSH in the construction of thiophene ring fused with N-heterocycles: A rapid and inexpensive synthesis of novel small molecules as potential inducers of apoptosis. Bioorganic & Medicinal Chemistry Letters, 24(18), 4460–4465. DOI 10.1016/j.bmcl.2014.07.096. [Google Scholar] [CrossRef]
44. Cai, G., Wang, S., Zhao, L., Sun, Y., Yang, D. et al. (2019). Thiophene derivatives as anticancer agents and their delivery to tumor cells using albumin nanoparticles. Molecules, 24(1), 192. DOI 10.3390/molecules24010192. [Google Scholar] [CrossRef]
45. Zhao, M., Cui, Y., Zhao, L., Zhu, T., Lee, R. J. et al. (2019). Thiophene derivatives as new anticancer agents and their therapeutic delivery using folate receptor-targeting nanocarriers. ACS Omega, 4(5), 8874–8880. DOI 10.1021/acsomega.9b00554. [Google Scholar] [CrossRef]
46. Zhang, B., Li, Y. H., Liu, Y., Chen, Y. R., Pan, E. S. et al. (2015). Design, synthesis and biological evaluation of novel 1,2,4-triazolo [3,4-b][1,3,4] thiadiazines bearing furan and thiophene nucleus. European Journal of Medicinal Chemistry, 103, 335–342. DOI 10.1016/j.ejmech.2015.08.053. [Google Scholar] [CrossRef]
47. Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paull, K. et al. (1991). Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute, 83(11), 757–766. DOI 10.1093/jnci/83.11.757. [Google Scholar] [CrossRef]
48. Boyd, M. R., Paull, K. D. (1995). Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Development Research, 34(2), 91–109. [Google Scholar]
49. Devita, V., Hellman, S., Rosenberg, S. (1989). Cancer: Principles and practice of oncology: Two-volume set. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins. [Google Scholar]
50. Gundogdu-Karaburun, N., Karaburun, A., Demirayak, S., Kayagil, I., Yurttas, L. (2014). Synthesis and anticancer activity of some 2-[3/4-(2-Substituted Phenyl-2- oxoethoxy)benzylidene]-6-substituted-2,3-dihydro-1H-inden-1-one derivatives. Letters in Drug Design & Discovery, 11(5), 578–585. [Google Scholar]
51. Shimada, K., Fujikawa, K., Yahara, K., Nakamura, T. (1992). Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry, 40(6), 945–948. [Google Scholar]
52. Shah, R., Verma, P. K. (2019). Synthesis of thiophene derivatives and their anti-microbial, antioxidant, anticorrosion and anticancer activity. BMC Chemistry, 13(1), 54. [Google Scholar]
53. Protein Data Bank (2021). A structural view of biology. http://www.rcsb.org/pdb/home/home.do. [Google Scholar]
54. Sharma, D., Kumar, S., Narasimhan, B., Ramasamy, K., Lim, S. M. et al. (2019). Synthesis, molecular modelling and biological significance of N-(4-(4-bromophenyl) thiazol-2-yl)-2-chloroacetamide derivatives as prospective antimicrobial and antiproliferative agents. BMC Chemistry, 13(1), 46. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |