
 | Oncologie |  |
DOI: 10.32604/oncologie.2022.022436
CASE REPORT
Surgery Combined with Molecular Targeted Therapy Successfully Treated Giant Esophageal Gastrointestinal Stromal Tumor
1Department of Respiratory Oncology, Renmin Hospital of Qingxian, Cangzhou, 062650, China
2Department of the First Surgery, Wuhan Jin-Yin-Tan Hospital, Wuhan, 430011, China
3Department of Thoracic Cardiovascular Surgery, General Hospital of Central Theater Command of the People’s Liberation Army, Wuhan, 430070, China
*Corresponding Authors: Jian Zhu. Email: zhujian0718@163.com; Ying Liu. Email: xxwkly@163.com
#These authors contributed equally to this work and should be considered as co-first authors
Received: 10 March 2022; Accepted: 06 May 2022
Abstract: Gastrointestinal stromal tumors (GISTs) are rare neoplasms arising from mesenchymal cells of the digestive tract and abdomen. Only a few isolated cases of giant esophageal GISTs (greater than 5 cm in size) have been reported with clinical features and surgical methods. Radical esophagectomy with negative margins, followed by gastric tube reconstruction, is recommended for giant esophageal GISTs. However, patients undergoing this type of surgery experienced a sharp decrease in food intake (due to the removal of most of the stomach) and were prone to eating regurgitation, resulting in poor quality of life. We describe the case of a 65-year-old man with a 16.3-cm giant esophageal GIST. The results of frozen quick pathology during the operation indicated an esophageal stromal tumor. Only resection of the esophageal mass was performed upon no consent for esophageal resection by family members. The patient received oral treatment with 400 mg of imatinib once daily after the operation. After 3 years of follow-up, the patient showed no signs of recurrence or metastasis. The successful management of this case suggests that molecular targeted therapy after surgery would avoid giant esophageal GIST recurrence. Therefore, giant esophageal GISTs probably do not need radical esophagectomy with negative margins, followed by gastric tube reconstruction.
Keywords: Molecular targeted therapy; giant esophageal gastrointestinal stromal tumor; operational styles; imatinib; case report
Gastrointestinal stromal tumors (GISTs) primarily arise in the stomach, with an annual incidence of 7 to 20 per million [1]. Although gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the stomach and small intestine, they can arise anywhere from the entrance of the esophagus to the anus [2]. Only 1%–3% of GISTs occur in the esophagus and are called esophageal GISTs [3], and they are extremely rare [1,4,5]. Due to their rarity, clinical data on esophageal GISTs are extremely limited, with only individual case reports or case series with small numbers [1]. Which surgical procedure should be performed for esophageal GISTs remains subject to debate [1,4,6]. The available literature indicates that enucleation of esophageal GISTs is permitted for smaller tumors (2–5 cm in size), whereas radical esophagectomy with negative margins, followed by gastric tube reconstruction, is recommended for giant esophageal GISTs [1,4,7]. It is time to develop a treatment that minimizes gastrectomy while avoiding tumor recurrence. Targeted drugs, such as imatinib, have shown efficacy in the treatment of GISTs in recent years [8]. Therefore, we report an enlightening case that evaluated the use and efficacy of imatinib in adjuvant treatment for a 16.3 cm esophageal GIST with enucleation.
A 65-year-old man was referred to our hospital for investigation of atypical chest tightness of gradual onset over the previous 6 months. He denied weight loss, dysphagia, upper GI bleeding, reflux, or other symptoms. Blood and urine biochemistry showed almost normal results. However, transthoracic echocardiography revealed a solid echoic mass of approximately 16 cm behind the left atrium and significant compression of the left atrium. Chest computed tomography (CT) with contrast administration was performed. The examination was also completed by volume rendering reconstruction (VR) (Fig. 1) and revealed a solid mass of approximately 163 × 73 mm (the volume was 1308.92 mm3) visible behind the left atrium. The heart was compressed forward, the adjacent left atrium was significantly compressed, and the adjacent bronchi and esophagus were significantly compressed and displaced. A barium swallow study revealed that the lower esophagus was clearly shifted to the left under pressure, partially shifted forward in the left anterior oblique position, and shifted backward in the right anterior oblique position (Fig. 2). The barium meal passed smoothly. Considering that the patient had severe cardiac symptoms, thoracotomy was performed emergently. During the operation, the tumor was found to be approximately 17 cm in diameter and located in the posterior mediastinum, with unclear boundaries in some areas, abundant blood vessels on the surface, and cystic firmness in texture. The tumor was punctured with a thick needle to extract the internal fluid, which was bloody and mixed with sediment. After suction, the cyst wall was sutured and used for traction. The tumor wall was free along the edge of the tumor, with obvious oozing but no obvious blood-supplying artery. Because the source of the tumor was not known before the operation, the operation was very difficult. Further exploration on a subbase revealed that the tumor originated in the esophagus and had a short, wide pedicle on the lateral wall of the esophagus. Although the origin of the tumor has been clarified thus far, it is still difficult to completely remove the tumor. Fortunately, the entire tumor was finally removed with difficulty from meticulous operation. Postoperative pathological examination showed that the tumor was grayish yellow in section, had cystic firmness, was soft in quality and yellowish and sticky and frozen in appearance; part of the gray matter was broken, and part of the envelope was yellowish dark red. The diagnosis was an esophageal gastrointestinal stromal tumor with nuclear division <5/50 HPF (Fig. 3). The tumor basal margins were negative, and lymph node examination showed no cancer metastasis. Immunohistochemical indicators were as follows: SMA (−), S-100 (−), actin (+), desmin (−), PCK (−), EMA (−), VIM (+), CD34 (+), c-kit (+), Dog-1 (+), MDM-2 (+), CDK4 (−), and KI67 (+4%). After a multidisciplinary meeting, the patient received adjuvant therapy. Through multidisciplinary consultation, the patient received oral treatment with 400 mg of imatinib once daily after the operation and was closely followed up with chest CT scans (Fig. 4) or the barium esophagram every 6 months. After 3 years, the patient showed no sign of local recurrence with regular barium esophagram (Fig. 5), and no abnormally enlarged lymph nodes shadows were found on CT scans of the chest and abdomen performed during the follow-up.
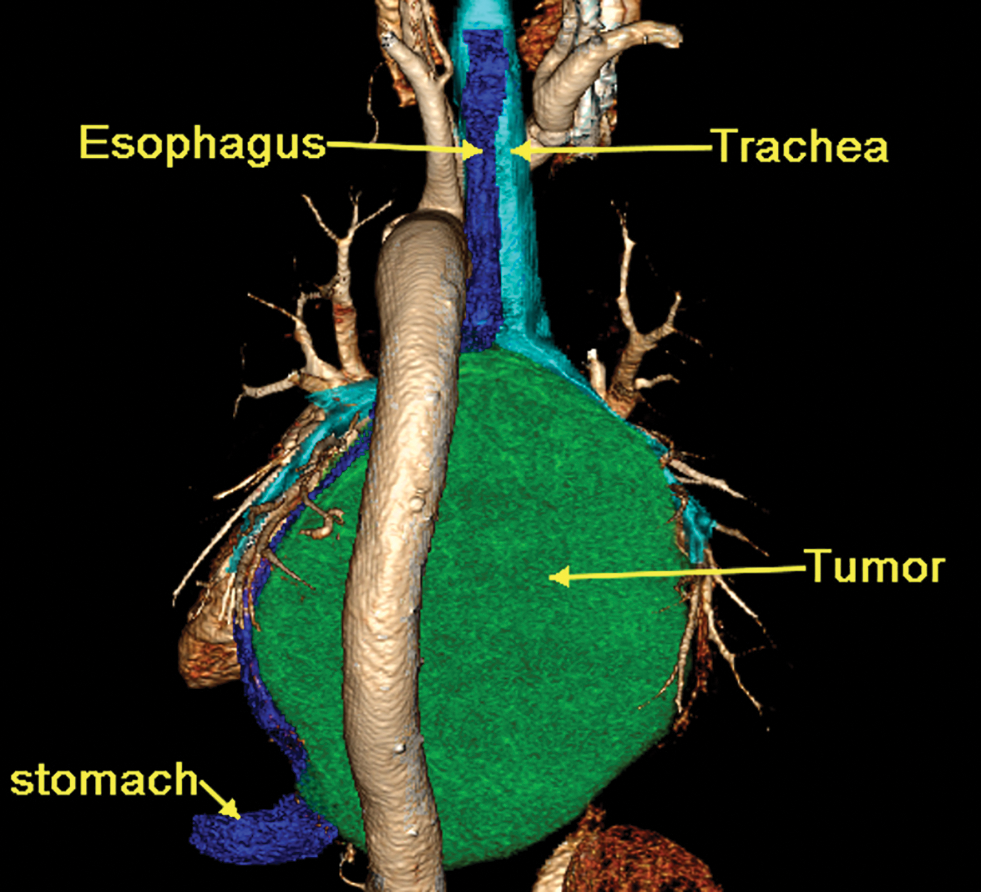
Figure 1: Three-dimensional reconstruction of a 16-slice contrast computed tomographic scan revealed that a solid mass (CT values of 30 HU in the scan phase and 82 HU in the arterial phase) of approximately 163 × 73 mm was visible behind the left atrium, the heart was clearly displaced, and the main bronchus was obviously compressed
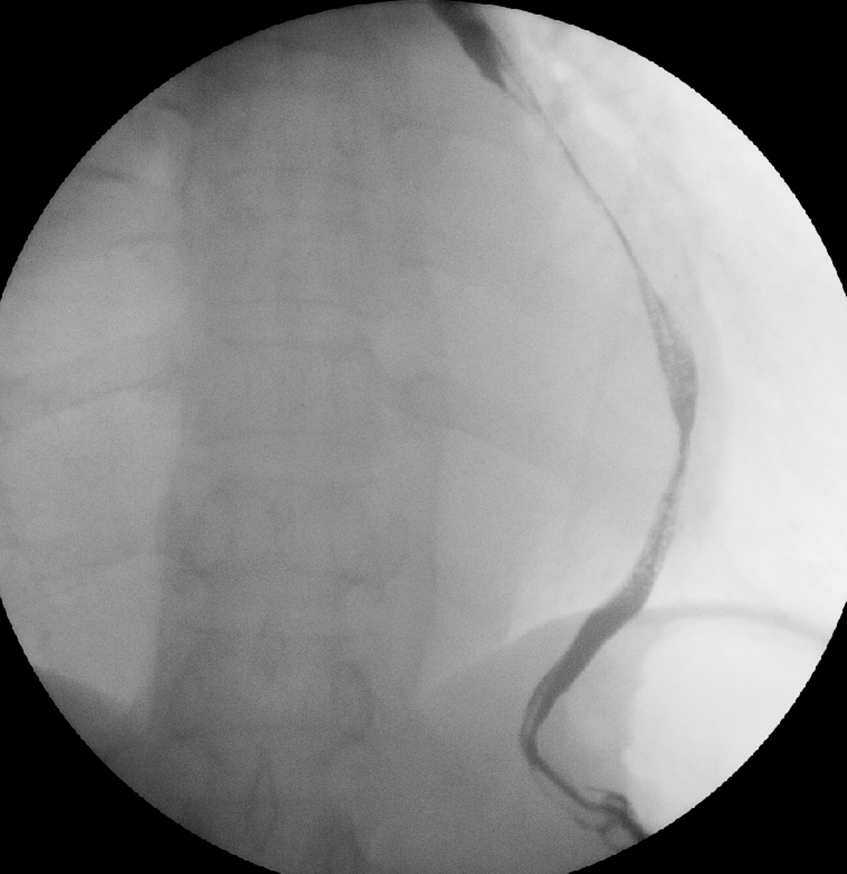
Figure 2: Barium esophagram revealed that the esophagus was clearly shifted to the left and was significantly narrowed
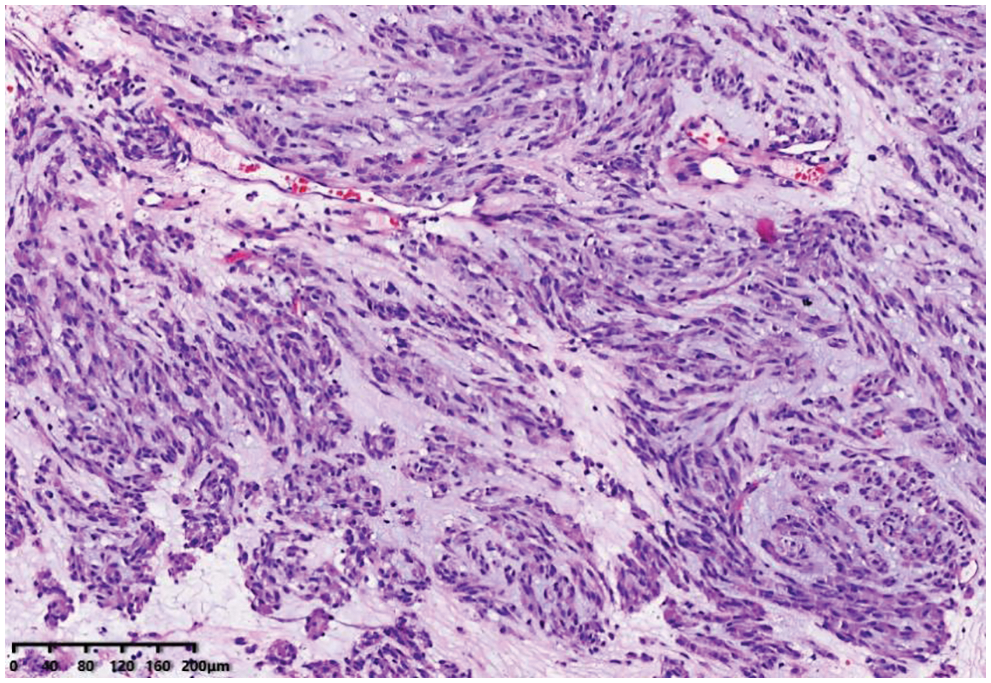
Figure 3: Image of histologic diagnosis using hematoxylin and eosin staining (original × 100)
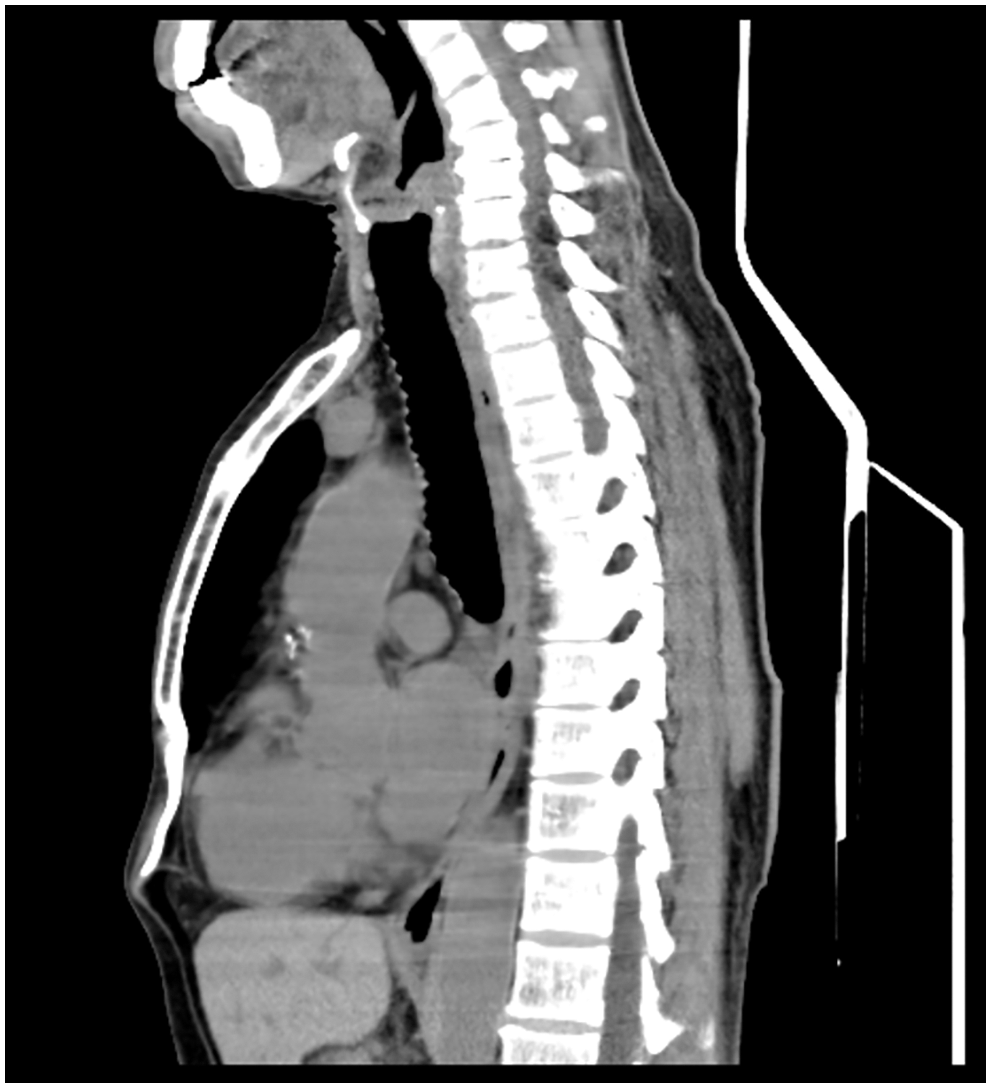
Figure 4: The postoperative 16-slice computed tomographic scan of chest and epigastric examination showed that there was no shadow at the original mass occupying site
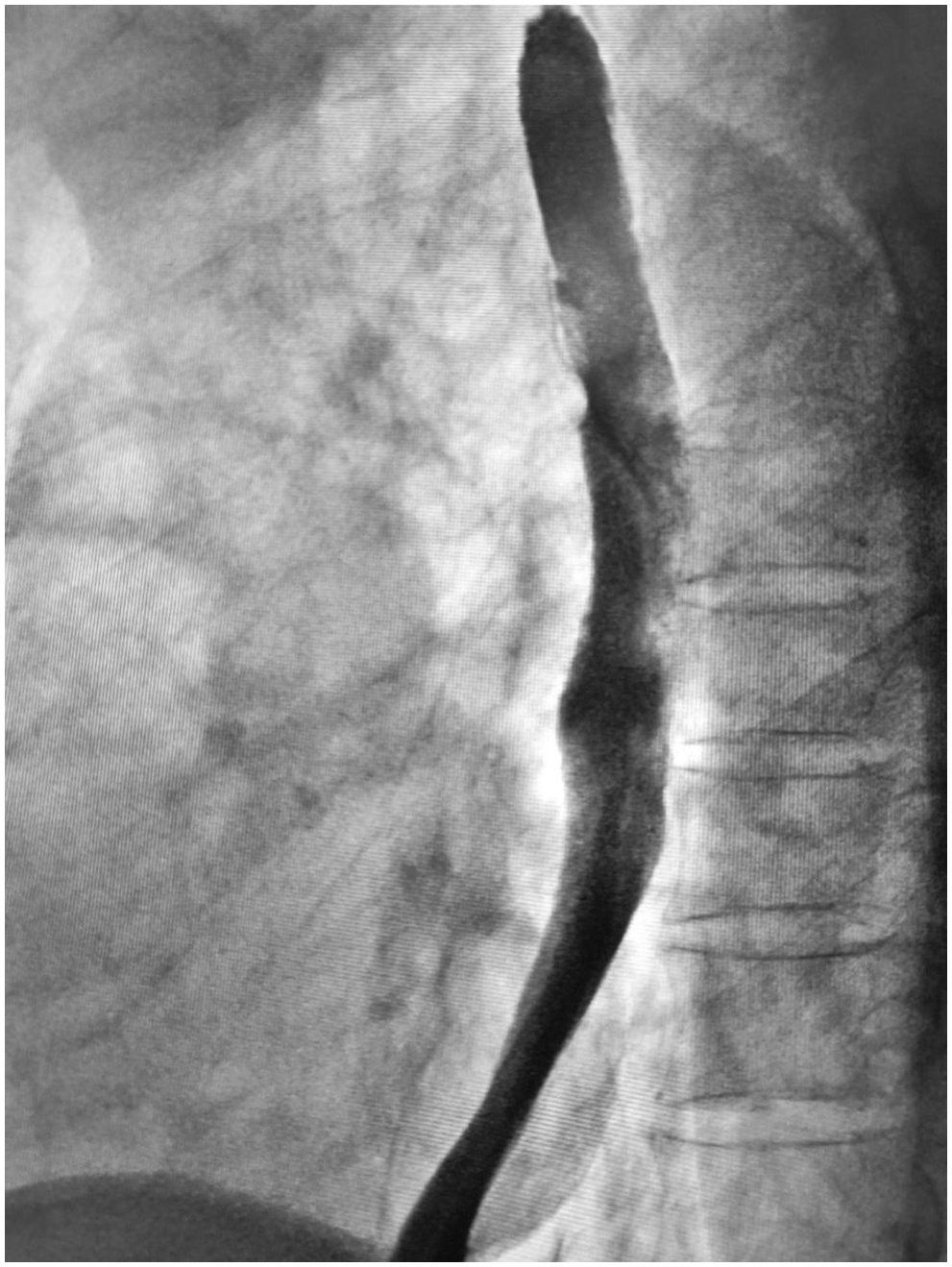
Figure 5: Barium esophagram showed no signs of local recurrence at the three-year follow-up
Gastrointestinal stromal tumors located in the esophagus constitute a very rare subset of GISTs with limited data on the clinicopathologic features and clinical outcomes [9]. Only a few isolated cases of giant esophageal GISTs have been reported with clinical features and surgical methods [1,4,6,7]. The report of this case is aimed at several popular topics in the current discussion of esophageal GISTs.
First, esophageal GISTs are difficult to diagnose preoperatively with no specific findings in clinical features. In its particular location, it can be surprisingly large without causing symptoms. Although dysphagia has been reported to be a common presenting symptom [7], dysphagia is rare even when the mass is larger than 9 cm in the available literature [10]. In our case, the mass was greater than 16 cm, and the only symptom was chest tightness. This fact could be closely related to GISTs being predominantly extraluminal. Of course, esophageal GISTs can present as intraluminal, intramural or exophytic masses [7,9,11]. Regardless of size, esophageal GISTs on the left side of the esophagus are more likely to cause symptoms than those on the right side, possibly due to mass effects or crowding of left-sided mediastinal structures [2]. There are no specific findings to differentiate them from the far more common leiomyomas when their clinical presentation, endoscopy, endoscopic ultrasound, or CT scans are reviewed [12]. Although esophageal GISTs and esophageal leiomyomas have overlapping imaging features, esophageal GISTs tend to be more distal, larger, more heterogeneous, and more enhancing on CT [2].
Second, GISTs are the most common type of gastrointestinal mesenchymal tumors of the stomach and small intestine, but they are rarely found in the thoracic esophagus [7,10]. There is no clear consensus about the optimal treatment of this rare disease. Unlike benign esophageal tumors, esophageal GISTs have unpredictable biological behavior and malignant potential, which must be addressed seriously, including with surgical resection, targeted drug treatment, or a combination. Because of the anatomical peculiarity of the esophagus, the surgical methods for esophageal GISTs are essentially limited to endoscopic resection, enucleation, or radical esophagectomy with negative margins, followed by gastric tube reconstruction [1,4,7,10]. The NCCN guidelines state that enucleation of small (5 cm) esophageal GISTs might be acceptable. When tumors are greater than 5 cm in size or have high-risk features, radical esophagectomy might be an appropriate approach to the resection of esophageal GISTs [13]. However, some studies have reported that, compared with larger tumors after radical esophagectomy, the short-term prognosis of patients was worse than that with enucleation, and the long-term prognosis was not significantly better [13]. When the patient undergoes esophagectomy, complications such as postoperative bleeding and esophagogastric anastomotic leakage are more common and correspondingly extend the postoperative fasting time and the hospital stay [2]. In our case, when the intraoperative rapid frozen pathological examination revealed that the esophageal mass was a stromal tumor, we decided to perform enucleation, considering that the patient was older, had long-term cardiac insufficiency, and the wishes of his family members. The patient recovered smoothly after the operation and quickly underwent targeted therapy for gastrointestinal stromal tumors.
Finally, molecularly targeted therapy has revolutionized the treatment of GISTs and facilitated scientific research on GISTs. Activating mutations of KIT or platelet-derived growth factor receptor alpha (PDGFRA) are found in the majority of GISTs [14]. Imatinib, as a first-line standard therapy for recurrent or metastatic GISTs, has proven clinical benefit in patients, and the standard dosage is 400 mg/day [15]. Unfortunately, it has been reported that more than half of patients develop resistance to imatinib after approximately 2 years, resulting in fatal disease progression [14,15]. Resistance to imatinib can include primary and secondary resistance; hence, mutation testing is recommended when imatinib is being considered for treatment. Other structural inhibitors have been developed, and sunitinib and regorafenib are multitarget inhibitors that inhibit KIT, platelet-derived growth factor receptors, vascular endothelial growth factor receptors and so on [16]. Sunitinib was initially approved for imatinib-resistant disease, and regorafenib was mainly used for imatinib-or sunitinib-resistant GIST with a 3-week-on/1-week-off regimen [17]. In the case presented here, imatinib therapy was used immediately postoperatively. Fortunately, the patient had a pathological response to imatinib treatment (400 mg/day) after resection and has been on the drug ever since. The patient’s postoperative chest CT examination showed that there was no shadow at the original mass-occupying site. On the imaging results, all lesions disappeared, and no signs of metastasis were observed in the esophagus and chest.
In general, we have reported our experience with a rare case of a giant GIST located in the mediastinum, originating from the esophagus. Due to some unusual circumstances, this patient received enucleation of esophageal GISTs combined with molecular targeted therapy (imatinib). This broke common sense but obtained a very good result. This therapy could preserve the normal anatomic structure of the esophagus and stomach to maintain the function of the upper GI tract and thus improve patients’ quality of life. We believe that our case provides new enlightenment for the surgical method and data supplements for giant esophageal GISTs.
Acknowledgement: The authors thank the patient who agreed to be included in this study.
Ethics Approval and Informed Consent Statement: Informed written consent has been obtained from the patient in this case report to publish this paper. The present study involved human participant, and it was conducted considering ethical responsibilities according to the World Medical Association and the Declaration of Helsinki.
Author Contributions: Mengjie Li, Jun Wei, and Guihua Xu contributed equally to this article, and therefore, they are considered co-first authors. They made a substantial contribution to the acquisition, analysis, data interpretation and draft manuscript preparation. Ying Liu and Jian Zhu critically studied conception and designed prepared and revised the manuscript for important intellectual content. Each author gave the final approval of the version to be published and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement: No datasets were generated or analyzed during the current study. All the data are available in the patient’s medical record. The data that support the findings of this case are available from the corresponding author upon reasonable request.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Hihara, J., Mukaida, H., Hirabayashi, N. (2018). Gastrointestinal stromal tumor of the esophagus: Current issues of diagnosis, surgery and drug therapy. Translational Gastroenterology and Hepatology, 3(6). DOI 10.21037/tgh.2018.01.06. [Google Scholar] [CrossRef]
2. Winant, A. J., Gollub, M. J., Shia, J., Antonescu, C., Bains, M. S. et al. (2014). Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. American Journal of Roentgenology, 203(2), 306–314. DOI 10.2214/AJR.13.11841. [Google Scholar] [CrossRef]
3. Tomos, P., Damaskos, C., Dimitroulis, D., Kouraklis, G. (2015). Giant esophageal gastrointestinal stromal tumor mimicking mediastinal tumor treated by thoracic approach. Annals of Gastroenterology, 28(2), 295–296. [Google Scholar]
4. Lee, H. J., Park, S. I., Kim, D. K., Kim, Y. H. (2009). Surgical resection of esophageal gastrointestinal stromal tumors. The Annals of Thoracic Surgery, 87(5), 1569–1571. DOI 10.1016/j.athoracsur.2009.01.051. [Google Scholar] [CrossRef]
5. Ji, F., Wang, Z. W., Wang, L. J., Ning, J. W., Xu, G. Q. (2008). Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography. Journal of Gastroenterology and Hepatology, 23, e318–e324. DOI 10.1111/j.1440-1746.2008.05322.x. [Google Scholar] [CrossRef]
6. Peparini, N., Carbotta, G., Chirletti, P. (2011). Enucleation for gastrointestinal stromal tumors at the esophagogastric junction: Is this an adequate solution? World Journal of Gastroenterology, 17(16), 2159–2160. DOI 10.3748/wjg.v17.i16.2159. [Google Scholar] [CrossRef]
7. Jiang, P., Jiao, Z., Han, B., Zhang, X., Sun, X. et al. (2010). Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. European Journal of Cardiothoracic Surgery, 38(2), 223–227. DOI 10.1016/j.ejcts.2010.01.040. [Google Scholar] [CrossRef]
8. Raut, C. P., Espat, N. J., Maki, R. G., Araujo, D. M., Trent, J. et al. (2018). Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate-or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 clinical trial. JAMA Oncology, 4(12), e184060. DOI 10.1001/jamaoncol.2018.4060. [Google Scholar] [CrossRef]
9. Feng, F., Tian, Y. Z., Liu, Z., Xu, G. H., Liu, S. S. et al. (2016). Clinicopathologic features and clinical outcomes of esophageal gastrointestinal stromal tumor: Evaluation of a pooled case series. Medicine, 95(2), e2446. DOI 10.1097/MD.0000000000002446. [Google Scholar] [CrossRef]
10. Shinagare, A. B., Zukotynski, K. A., Krajewski, K. M., Jagannathan, J. P., Butrynski, J. et al. (2012). Esophageal gastrointestinal stromal tumor: Report of 7 patients. Cancer Imaging, 12(1), 100–108. DOI 10.1102/1470-7330.2012.0017. [Google Scholar] [CrossRef]
11. Miettinen, M., Sarlomo-Rikala, M., Sobin, L. H., Lasota, J. (2000). Esophageal stromal tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. The American Journal of Surgical Pathology, 24(2), 211–222. DOI 10.1097/00000478-200002000-00007. [Google Scholar] [CrossRef]
12. Markakis, C. G., Spartalis, E. D., Liarmakopoulos, E., Kavoura, E. G., Tomos, P. (2013). Esophageal gastrointestinal stromal tumor: Diagnostic complexity and management pitfalls. Case Reports in Surgery, 2013(1), 968394. DOI 10.1155/2013/968394 2013. [Google Scholar] [CrossRef]
13. Zhang, F. B., Shi, H. C., Shu, Y. S., Shi, W. P., Lu, S. C. et al. (2015). Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World Journal of Gastroenterology, 21(18), 5630–5634. DOI 10.3748/wjg.v21.i18.5630. [Google Scholar] [CrossRef]
14. Heinrich, M. C., Corless, C. L., Demetri, G. D., Blanke, C. D., von Mehren, M. et al. (2003). Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. Journal of Clinical Oncology, 21(23), 4342–4349. DOI 10.1200/JCO.2003.04.190. [Google Scholar] [CrossRef]
15. Gold, J. S., Gönen, M., Gutiérrez, A., Broto, J. M., García-del-Muro, X. et al. (2009). Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. The Lancet Oncology, 10(11), 1045–1052. DOI 10.1016/S1470-2045(09)70242-6. [Google Scholar] [CrossRef]
16. Kang, Y. K., Ryu, M. H., Yoo, C., Ryoo, B. Y., Kim, H. J. et al. (2013). Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHTA randomised, placebo-controlled, phase 3 trial. The Lancet Oncology, 14(12), 1175–1182. DOI 10.1016/S1470-2045(13)70453-4. [Google Scholar] [CrossRef]
17. Poveda, A., García, D. M. X., López-Guerrero, J. A., Cubedo, R., Martínez, V. et al. (2017). GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treatment Reviews, 55(6), 107–119. DOI 10.1016/j.ctrv.2016.11.011. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |