

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.011724
ARTICLE
Polysaccharide Elicitor from the Endophyte Bionectria sp. Fat6 Improves Growth of Tartary Buckwheat under Drought Stress
1Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, Chengdu University, Chengdu, 610106, China
2College of Medicine (School of Nursing), Chengdu University, Chengdu, 610106, China
*Corresponding Authors: Gang Zhao. Email: zhaogang@cdu.edu.cn; Jianglin Zhao. Email: jlzhao@cdu.edu.cn
Received: 26 May 2020; Accepted: 28 July 2020
Abstract: Drought can limit the growth and reduce the yield of crops, but the safe and effective bio-approach to improve the drought resistance of crops is very little. We conducted an experiment in which we monitored the effects of polysaccharide from the endophyte Bionectria sp. Fat6 on the growth of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) seedlings under control and drought-stressed conditions by determining gas exchange, photosynthesis parameters, photosynthetic pigment contents, and metabolite accumulation. Results indicated that the polysaccharide from endophyte stimulated plant growth and increased the aboveground biomass, root mass, and root/shoot ratio of Tartary buckwheat. Application of the polysaccharide to drought-stressed plants resulted in a significant increase in the net photosynthetic rate, stomatal conductance, and transpiration rate of Tartary buckwheat and decreased the intercellular CO2 concentration. The contents of chlorophyll a, chlorophyll b, chlorophyll a + b, and carotenoids in leaves were higher in polysaccharide-treated seedlings than that in control. Polysaccharide notably increased the soluble protein and proline content and decreased the malondialdehyde content in Tartary buckwheat leaves. The endophytic polysaccharide may protect Tartary buckwheat against drought by improving leaf gas exchange and photosynthetic capacity, and altering concentrations of protective metabolites. Together, these changes may compensate for the negative impacts of drought stress on the growth of Tartary buckwheat. Thus, the polysaccharide from the endophyte Bionectria sp. Fat6 may be an effective biotic elicitor and a promising bio-approach to improve Tartary buckwheat production worldwide.
Keywords: Endophyte; physiological response; plant growth; polysaccharide; Tartary buckwheat
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) is a dicotyledonous plant in the family Polygonaceae (Fig. 1). It is widely grown in many countries, including India, Russia, Japan, France, Canada, Poland, Slovenia, and Nepal, for its medicinal and nutritive value. It could be more fully utilized as a resource [1,2]. It is rich in minerals, vitamins, protein, fiber, amino acids, trace elements, and various bioactive phytochemicals [3,4]. The major functional components of Tartary buckwheat are flavonoids, polyphenols, phytosterols, D-chiro-inositol, and D-fagomine. There is potential to extract and refine these components to develop high-value products. Recent studies have revealed that some of these bioactive compounds are beneficial for human health, showing notable antioxidant, hypocholesterolemic, antidiabetic, antimicrobial, and antitumor activities [5,6].

Figure 1: Endophyte Bionectria sp. Fat6 and F. tataricum
Currently, there are many buckwheat-based products available on the market, such as buckwheat pillows, protein, chewable tablets, tea, and sprouts [7,8]. Because of its potential health benefits, Tartary buckwheat consumption is increasing on a global scale [9]. However, the most high-quality arable land is used to grow staple food crops (e.g., rice and wheat), resulting in limited cultivation area and relatively low yield of Tartary buckwheat [2,10]. About 60% of the world’s Tartary buckwheat crop is grown under high-cold mountainous conditions with infrequent rain, which belongs to arid and semi-arid zones [11,12]. A previous study found that Tartary buckwheat could not tolerate drought stress during its initial growth stages [12]. Therefore, it is crucial to increase the drought resistance at the early stage to improve plant growth and yield.
Endophytic fungi is a special group of microorganisms that resides within plant tissues intercellularly or intracellularly without causing any apparent symptoms of disease. Endophytes may produce functional constituents such as antimicrobial, insecticidal, cytotoxic, and growth-regulating compounds. They can protect the host from pathogen infection and promote the growth and adaptation of the host to remain healthy [13]. It has become a hot research topic due to the great potential in agriculture for improving plant resistance. However, few studies have focused on how endophytic fungi function as elicitors of growth and stress resistance in their host plants.
Polysaccharides are macromolecular compounds with complex structures. As a treatment agent, they have promising prospects for use in agricultural production because of their strong efficacy and low toxicity. Previous studies have reported that the polysaccharides can be used to improve the stress tolerance and yield of crops. Bai et al. [14] found that a polysaccharide treatment could increase leaf chlorophyll content, delay leaf senescence, improve leaf photosynthesis, and increase the grain yield of soybean. A microbial polysaccharide enhanced the drought resistance of bluegrass to drought stress [15]. In our previous studies, we acquired an endophytic fungus from Tartary buckwheat and found that polysaccharides, produced by the endophyte, could increase the germination of Tartary buckwheat and promote flavonoid production in buckwheat seedlings [16–18]. The present study aimed to determine the effect of the polysaccharide on the growth of drought-stressed Tartary buckwheat and explore the mechanism by which the polysaccharide improves plant growth under drought stress.
The specific objectives of this study were as follows: (i) To analyze the effect of an endophytic polysaccharide on the biomass, root/shoot ratio and leaf photosynthetic characteristics of Tartary buckwheat under drought stress; (ii) To evaluate the effect of the endophytic polysaccharide on leaf photosynthetic pigment contents of Tartary buckwheat under drought stress; and (iii) To investigate the effect of drought stress on the content of soluble protein, proline and malondialdehyde (MDA) in Tartary buckwheat seedling leaves. The results of these analyses provided information about the physiological mechanism by which the endophytic polysaccharide promoted the growth of Tartary buckwheat, and highlighted the potential of this polysaccharide to improve Tartary buckwheat growth and yields in arid or semi-arid regions.
The Tartary buckwheat cultivar XiQiao-1, the most widely planted cultivar in southwest China, was obtained from the National Research and Development Center for Coarse Cereal Processing at Chengdu University. The sandy soil in texture and alkaline (pH 7.84) was used in this experiment, which consisted of 49.3, 21.5, and 30.4 mg kg–1 available N, P, and K, respectively; 0.75, 0.42, and 13.4 g kg–1 total N, P, and K, respectively; and 9.8 g kg–1 organic matter. The collected soil was air-dried and sieved (2 mm) to remove large stones, plant roots, and other litter. Tartary buckwheat seeds were surface-sterilized for 5 min in 10% (v/v) hydrogen peroxide and rinsed four times with deionized-H2O, and then collected and stored for further use.
2.2 Cultivation of Endophytic Fungus and Preparation of Polysaccharide
The endophytic fungus Bionectria pityrodes Fat6 (GenBank accession number KC218450) (Fig. 1) was isolated from a healthy Tartary buckwheat plant as reported previously [16]. The living culture has been maintained on potato dextrose agar (PDA) slants at 4°C, and in 40% (v/v) glycerol at −70°C at the Coarse Cereal Processing Center of Chengdu University. The endophytic fungus B. pityrodes Fat6 was cultured as described in Zhao et al. [19].
The collected mycelia of B. pityrodes was washed twice with deionized water and then lyophilized. The lyophilized mycelia were powdered and subjected to heat circumfluence extraction at 50°C in 95% ethanol-petroleum ether at 1:1 (v/v) to remove the lipids, monosaccharides, and disaccharides. The ratio of mycelia powder (g) to refluxing solvent (ml) was 1:6 (w/v). Defatted mycelia powder was obtained by centrifugation and drying at 40°C–45°C to constant weight. The pretreated mycelia powder was immersed in distilled water and extracted at 90°C for 120 min (1 g material to 30 mL water). The mixture was centrifuged at 8000 rpm for 15 min to collect the supernatant. The supernatant was concentrated to a certain volume, mixed with three volumes of 95% ethanol, and then kept at 4°C for 48 h. After that, the solution was centrifuged at 8000 rpm for 15 min to collect the precipitate as the crude mycelia polysaccharide.
This experiment included control and drought stress treatments. The seeds (200 grains per treatment) used for each of these main treatments were immersed in polysaccharide solution (0, 75, 150, and 225 mg L–1) for 12 h. Then, three replications, which consisted of 104 pots (5 seeds/pot) for each replication, were built for each treatment. The seedlings were thinned to three plants per pot. For the drought treatment, water was withheld for seven days as described in Bauddh et al. [20] before harvesting. The control plants were watered routinely every 2–3 days. The trial was carried out in a phytotron (RXZ-300B, Dongnan Co., Ltd., Ningbo, China) with 23°C days (07:00–20:00) and 17°C nights (20:00–07:00), 50%–60% relative humidity, and light intensity of 400 μmol−2 s−1. The pots were placed randomly and moved to a different place every week.
2.4 Biomass and Root/Shoot Ratio
Fifteen representative plants per treatment were collected and measured at the seedling stage. Three biological replicates were used for each measurement. The root, stem, and leaves were dried to a constant weight at 65°C after initial drying at 105°C for 0.5 h, and then the dry weight was measured. The root/shoot ratio was calculated as dry weight of roots/dry weight of shoots.
The leaf photosynthesis measurements were conducted on the 7th day of the drought treatment measuring three fully opened leaves of each plant using a portable GFS-3000 photosynthesis system (WALZ Inc., Effeltrich, Germany). Photosynthetically active radiation was provided by a red-blue light source at 1200 μmol m−2 s−1, the CO2 concentration at 350 μmol mol−1, and the leaf temperature at 25°C. All measurements were performed between 09:30 and 12:00 h. The net photosynthetic rate (Pn), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were automatically recorded by the photosynthesis system.
2.6 Content of Photosynthetic Pigments
After the leaf photosynthesis measurements, the leaf pigments were quantified according to the description in Lichtenthaler [21]. Briefly, three fully expanded leaves from each plant were collected in each treatment and wrapped immediately in aluminum foil to avoid pigment degradation. Leaf samples were powdered in liquid nitrogen then incubated in 80% acetone and absolute alcohol at a ratio 1:1 at −20°C for 12 h in darkness. Extracts were centrifuged at 12 000 g for 5 min at 4°C. The absorbance of the solution from leaf extract was recorded at 645, 663, and 470 nm using a UV-3200S spectrophotometer (MAPADA Instruments, Shanghai, China) for calculating the content of chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid. Three biological replicates were used for each measurement. Leaf pigment contents were estimated according to the following equations [21] and expressed as mg g−1 fresh weight (FW−1).
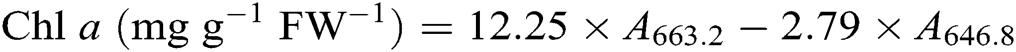
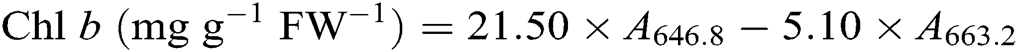

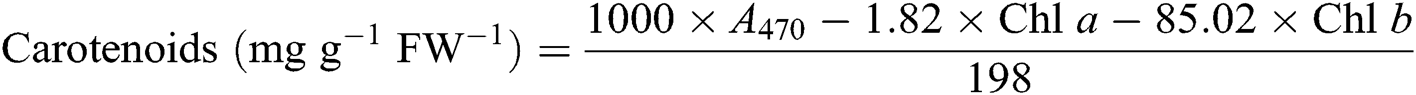
2.7 Contents of Leaf Soluble Protein, Proline, and Malondialdehyde
The leaf soluble protein content was measured using the Coomassie brilliant blue G-250 staining method described by Bradford [22]. Fresh leaf samples (0.5 g) were homogenized with 4 ml Na-phosphate buffer (pH 7.2) and then centrifuged at 4°C. The absorbance of the supernatant containing the dye was determined at 595 nm using a UV-3200S spectrophotometer (MAPADA Instruments). Proline content in the leaf was determined using the method mentioned from Bates et al. [23], and the MDA content was measured as described by Heath et al. [24].
Microsoft Excel 2010 was used to process data and draw figures. All data were statistically analyzed by analysis of variance using the software SPSS 17.0 (SPSS, Chicago, IL, USA). The significance test was performed using a t-test at the p < 0.05 level.
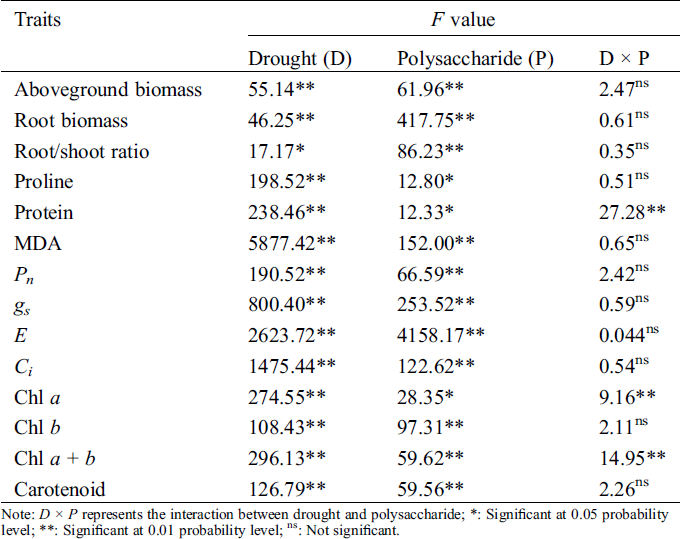
The polysaccharide enhanced the plant growth of Tartary buckwheat under both control and drought treatments (Fig. 2). The aboveground biomass of Tartary buckwheat seedlings was 1.52 g in control and 1.33 g in the drought treatment with 150 mg/L of endophytic polysaccharide, about 1.43-fold and 1.46-fold higher than their respective counterparts in the 0 mg/L polysaccharide treatment. The root biomass and root/shoot ratio were significantly higher in the treatments with 150 mg/L endophytic polysaccharide than in other treatments. We did not detect significant interactive effects of drought × polysaccharide (p > 0.05, Tab. 1) on aboveground biomass, root biomass, or root/shoot ratio.
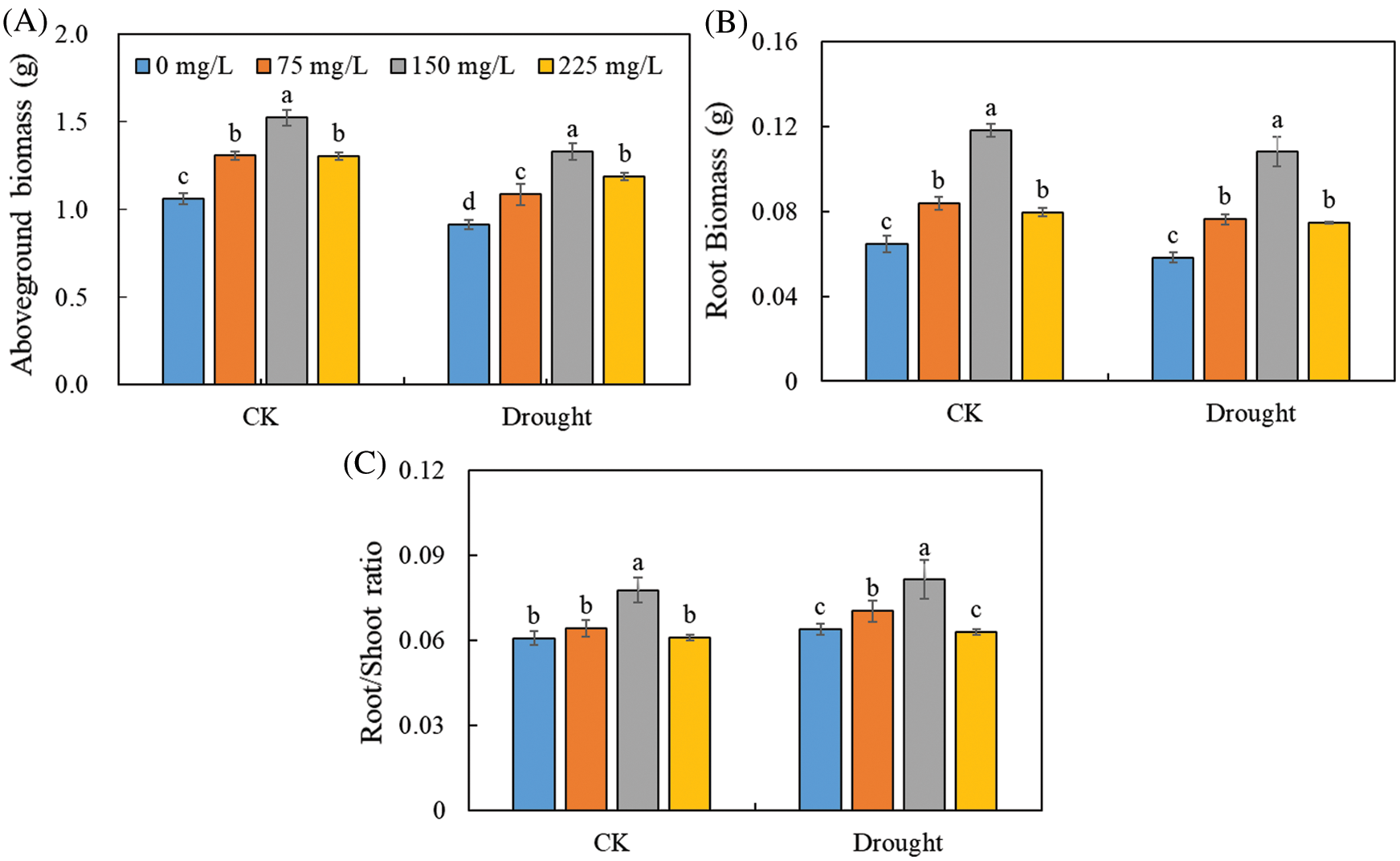
Figure 2: Effects of polysaccharide elicitor on aboveground biomass (A), root biomass (B), and root/shoot ratio (C) of Tartary buckwheat under control (CK) and drought conditions. Vertical bars indicate the standard deviation of the replicates (n = 3). Different lowercase letters indicate significant differences among different polysaccharide elicitor treatments under control and drought conditions at the 0.05 significance level
Table 1: Analysis of variance (ANOVA) on effects of drought, polysaccharide, and their interactions on studied traits in Tartary buckwheat
3.2 Leaf Photosynthetic Pigments
A significant decrease was observed in the levels of Chl a (Fig. 3A), Chl b (Fig. 3B), Chl a + b (Fig. 3C), and carotenoid (Fig. 3D) in the leaves of Tartary buckwheat under drought stress. However, the leaf pigment contents were increased after applying endophytic polysaccharide in control and drought treatment (Fig. 3, Tab. 1). The contents of Chl a, Chl b, Chl a + b, and carotenoids tended first to increase and then decrease with an increasing amount of polysaccharide (Fig. 3). The highest contents of Chl a, Chl b, Chl a + b, and carotenoids were always found in the leaves of plants treated with 150 mg/L endophytic polysaccharide. Under drought and control conditions, the application of 150 mg/L polysaccharide significantly increased Chl a, Chl b, Chl a + b, and carotenoid contents by 56.3%, 76.5%, 63.4%, and 98.5%, respectively, compared with the 0 mg/L treatment. We did not detect significant interactive effects of drought × polysaccharide (p > 0.05) on the content of Chl b and carotenoids. However, the significant (p < 0.05) interactive effects of drought × polysaccharide were observed on Chl a and Chl a + b contents (Tab. 1).
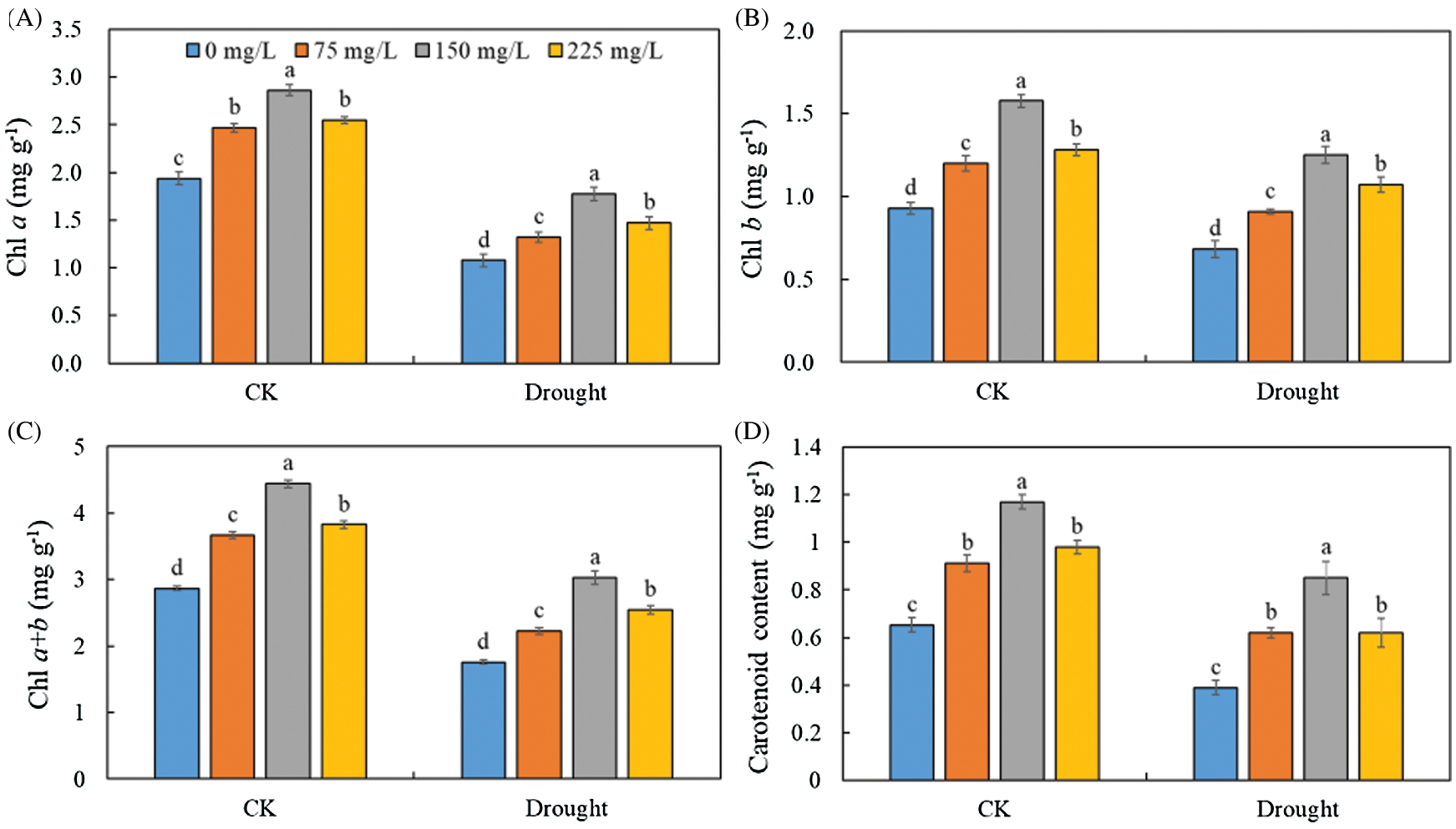
Figure 3: Effects of polysaccharide elicitor on chlorophyll a (A), chlorophyll b (B), chlorophyll a + b (C), and carotenoid content (D) of Tartary buckwheat under control (CK) and drought conditions. Vertical bars indicate the standard deviation of the replicates (n = 3). Different lowercase letters indicate significant differences among different polysaccharide elicitor treatments under control and drought conditions at the 0.05 significance level
3.3 Leaf Photosynthesis and Gas Exchange Parameters
Drought significantly decreased Pn (Fig. 4A), gs (Fig. 4B), and E (Fig. 4C), but increased the Ci (Fig. 4D) of Tartary buckwheat seedlings. The application of endophytic polysaccharide increased the photosynthetic performance of Tartary buckwheat seedlings in both control and drought conditions (Fig. 3, Tab. 1). The Pn, gs, and E tended first to increase and then decrease with an increasing amount of polysaccharide (Fig. 4). However, the Ci showed the opposite trend with them. Application of 150 mg/L polysaccharide in control and drought treatment significantly increased the mean values of Pn, gs, and E by 41.1%, 71.5%, and 71.8%, respectively, and significantly decreased Ci by 24.4%, compared to the 0 mg/L of polysaccharide. We did not find significant interactive effects of drought × polysaccharide (p > 0.05, Tab. 1) on leaf photosynthesis parameters.
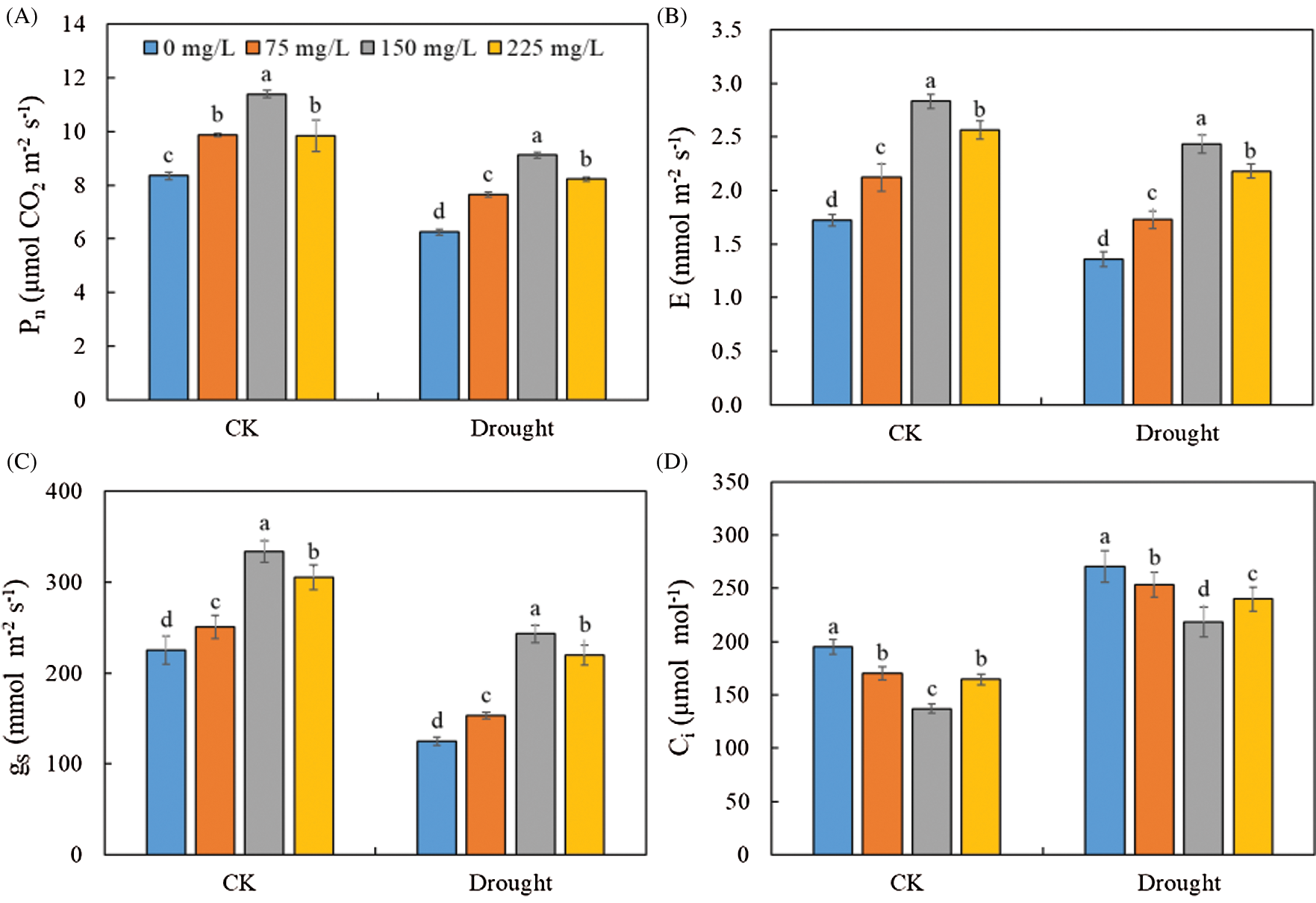
Figure 4: Effects of polysaccharide elicitor on net photosynthesis (Pn) (A), transpiration rate (E) (B), stomatal conductance (gs) (C), and intercellular CO2 concentration (Ci) (D) of Tartary buckwheat under control (CK) and drought conditions. Vertical bars indicate the standard deviation of the replicates (n = 3). Different lowercase letters indicate significant differences among different polysaccharide treatments under control and drought conditions at the 0.05 significance level
3.4 Leaf Soluble Protein, Proline, and MDA Contents
Drought stress decreased soluble protein content (Fig. 5A), but increased proline (Fig. 5B) and MDA contents (Fig. 5C) without the application of endophytic polysaccharide. Application of the endophytic polysaccharide significantly increased soluble protein and proline contents of Tartary buckwheat seedlings under drought conditions (Fig. 4, Tab. 1). Compared with untreated plants, those treated with the polysaccharide showed a significant increase in protein and proline contents by 27.0% and 46.6%, and significantly (p < 0.05) decreased MDA content by 21.6% under drought condition. In control, the polysaccharide treatment significantly increased proline content by 67.2% and decreased the MDA content by 40.6%. However, no significant effect was found on the soluble protein content. We detected significant interactive effects of drought × polysaccharide (p < 0.05) on soluble protein content, but no significant interactive effects of drought × polysaccharide were found on proline and MDA contents (Tab. 1).
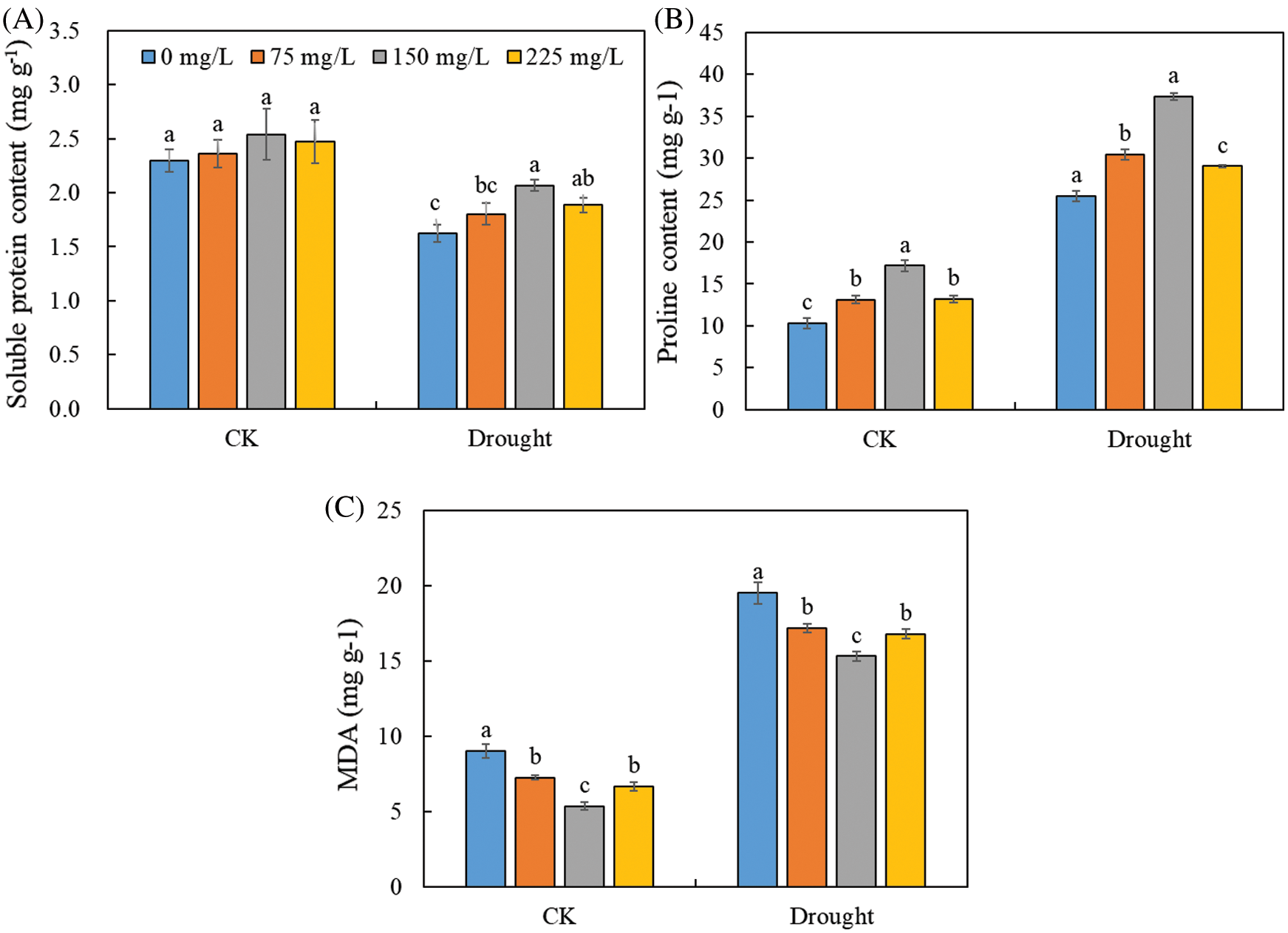
Figure 5: Effects of polysaccharide elicitors on soluble protein content (A), proline content (B), and malondialdehyde (MDA) content (C) in leaves of Tartary buckwheat under control (CK) and drought stress conditions. Vertical bars indicate the standard deviation of the replicates (n = 3). Different lowercase letters indicate significant differences among different polysaccharide elicitor treatments under control and drought conditions at the 0.05 significance level
Photosynthesis is very important for plant growth, especially in extreme environments. Many studies have demonstrated that drought can reduce the levels of Pn, gs, and E [25–27]. Our results indicated that drought decreased the leaf Pn, gs, and E of Tartary buckwheat (Fig. 4), consistent with the results reported in the study of potato seedlings [28]. These results suggested that drought can suppress the growth of Tartary buckwheat via the reduction of leaf gas exchange at the early seedling stage.
We found that the polysaccharide from endophytic Bionectria sp. Fat6 increased the root/shoot ratio, root and aboveground biomass, and plant growth of drought-stressed Tartary buckwheat (Fig. 2). It showed that the polysaccharide plays a strong part in the development process of Tartary buckwheat, which may improve the growth performance by increasing the photosynthetic capacity [10]. These results also indicated that the polysaccharide improved leaf gas exchange in Tartary buckwheat by increasing the gs and E (Fig. 4). These results might be explained by the increase in nutrient and water uptake caused by application of the polysaccharide [14]. Our results indicated that the Pn of leaf was significantly lower under drought conditions than that in control (Fig. 4), which might be the response to the part closure of stomata, or to the decrease of photosynthetic activity of mesophyll cells that increased Ci [29,30]. The drought stress could result in part closure of stomata, which is an important way for plants to protect themselves from stress [31]. We found that the Ci was significantly higher under drought conditions than that in control (Fig. 4). So, the main reason for the decrease of Pn of Tartary buckwheat was due to the decrease of photosynthetic activity of mesophyll. Interestingly, we also found that the difference of Ci between control and drought conditions was significantly decreased by the polysaccharide application (Tab. 1). Thus, our results suggest that the endophytic polysaccharide might alleviate the reduction in photosynthesis caused by drought, thereby enhancing the growth of Tartary buckwheat. Polysaccharide application increased the Pn, gs, and E, but decreased the Ci of Tartary buckwheat seedlings (Fig. 4). Together, these results suggested that the polysaccharide decreased the non-stomatal limitation of Tartary buckwheat photosynthesis and increased the photosynthetic activity of mesophyll cells. Polysaccharide, as elicitors, could improve the enzymatic activity, related to the process of photophosphorylation, and enhance the photophosphorylation activity, and thereby improving the synthetic efficiency of ATP [32]. This may prompt the operation of photosynthetic carbon cycle and increase of photosynthetic activity [33], and thus improve the Pn of Tartary buckwheat seedling.
Chlorophyll plays a key role in determining the intensity of photosynthesis, which is strongly influenced by adverse conditions [34]. Mafakheri et al. [35] found that drought stress significantly decreased chlorophyll content, consistent with our results (Fig. 3). In this study, polysaccharide increased the chlorophyll content in Tartary buckwheat leaves under control and drought conditions (Fig. 3, Tab. 1). The increase in chlorophyll content might be related to increased uptake of magnesium, higher activity of chlorophyll synthetase, and less destruction of chlorophyll [36,37]. The polysaccharide of endophytic Bionectria sp. Fat6, as elicitor, may interact with specific receptors on the cells of Tartary buckwheat leaf. As a result, the signaling transduction could be triggered and enhanced downstream genes expression to improve the content of intermediates-related synthetase, thus increasing the final chlorophyll content [38–40]. Simultaneously, the polysaccharide elicitor also might be as growth regulator to activate certain enzymes which could alleviate the destruction of chlorophyll structure [41,42]. Consequently, the promotion of the gene expression of the enzymes related to the chlorophyll synthesis by the polysaccharide elicitor might be an important reason for increasing the chlorophyll content of Tartary buckwheat leaf. However, further research is required to clarify which of these processes is most strongly affected by the polysaccharide.
The high chlorophyll content indicated that the polysaccharide increased the rate of chlorophyll synthesis or decreased the rate of chlorophyll degradation to increase leaf photosynthesis under drought condition. The increases in chlorophyll content and gas exchange indicate that the polysaccharide increased carbon fixation by photosynthesis. In addition, the polysaccharide may induce resistance proteins in Tartary buckwheat to enhance protective enzyme activity and increase the levels of osmotic adjustment substances and secondary metabolites. Together, these changes could mitigate the adverse effects of stress [43].
A previous study showed that drought-stressed plants produce excess H2O2, which could cause oxidative damage through the formation of reactive oxygen species that damages proteins [44,20]. In the current study, the polysaccharide-treated seedlings showed significant increase in soluble protein content under drought stress (Fig. 5), suggesting that polysaccharide application stimulated protein synthesis and/or reduced protein degradation under drought. Proline is an important metabolite, and its accumulation can help to protect plant growth under drought condition [45]. Proline helps to maintain optimal turgor and protein conformation, and accumulates in plants subjected to different types of stress [46]. In our study, polysaccharide application significantly increased the proline content in leaves, especially under drought stress (Fig. 5, Tab. 1), suggesting that the polysaccharide may play an important role in the protection of Tartary buckwheat seedlings against drought. Thus, the polysaccharide may achieve its protective function by increasing proline content, altering the osmo-regulation and osmo-tolerance of Tartary buckwheat. We also monitored the content of MDA, which is a product of lipid peroxidation and an indicator of oxidative damage. We detected marked decrease in MDA content in the polysaccharide-treated seedlings under drought stress. This was consistent with the results of Li et al. [15], who found that a microbial polysaccharide reduced lipid peroxidation under drought stress. The lower MDA content in leaves of polysaccharide-treated than untreated Tartary buckwheat under drought stress suggested that these treated plants were better protected against oxidative damage than untreated plants. Thus, the polysaccharide might reduce plasma membrane lipid peroxidation in Tartary buckwheat seedlings under drought stress, which would improve plant growth.
In our study, we found that polysaccharide application improved the growth of Tartary buckwheat, showing significantly higher root and aboveground biomass and the root/shoot ratio of polysaccharide-treated plants than that of untreated plants (Fig. 2, Tab. 1). Our findings showed that plant drought tolerance can be improved by applying the polysaccharide from Bionectria sp. Fat6. The polysaccharide may be applied for promoting the growth of Tartary buckwheat in drought-affected regions. The optimum polysaccharide concentration was 150 mg L−1 amount for Tartary buckwheat in this study. The fungal polysaccharide obtained from endophyte Bionectria sp. Fat6 is a complicated mixture, and its chemical structure, composition, physiochemical properties, and regulatory mechanisms are still needed to be clarified. Therefore, further studies are required to determine the composition and the mechanism of action of the polysaccharide from the endophyte Bionectria sp. Fat6.
Application of polysaccharide from the endophyte Bionectria sp. Fat6 increased the drought resistance of Tartary buckwheat seedlings by improving leaf gas exchange properties (Pn, gs, E), increasing the chlorophyll, protein, and proline contents, and reducing the MDA contents. The results showed that the polysaccharide can promote the growth of Tartary buckwheat seedlings. Thus, it has potential applications in increasing the yield of Tartary buckwheat crops in drought-affected regions. These findings also provide valuable information about how a fungal polysaccharide elicitor stimulates responses to drought stress in Tartary buckwheat.
Funding Statement: This work was funded by National Key R&D Program of China (Grant No. 2019YFD1001302/2019YFD1001300) and Supported by National Natural Science Foundation of China (31771716; 31601260; 31701358), the earmarked fund for China Agriculture Research System (CARS-07-02A).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Peng, L. X., Huang, Y. F., Liu, Y., Zhang, Z. F., Lu, L. Y. et al. (2014). Evaluation of essential and toxic element concentrations in buckwheat by experimental and chemometric approaches. Journal of Integrative Agriculture, 13(8), 1691–1698. DOI 10.1016/S2095-3119(13)60724-8. [Google Scholar] [CrossRef]
2. Xiang, D. B., Zhao, G., Wan, Y., Tan, M. L., Song, C. et al. (2016). Effect of planting density on lodging-related morphology, lodging rate, and yield of Tartary buckwheat (Fagopyrum tataricum). Plant Production Science, 19(4), 479–488. DOI 10.1080/1343943X.2016.1188320. [Google Scholar] [CrossRef]
3. Zhao, G., Zhao, J. L., Peng, L. X., Zou, L., Wang, J. B. et al. (2012). Effects of yeast polysaccharide on growth and flavonoid accumulation in Fagopyrum tataricum sprout cultures. Molecules, 17(10), 11335–11345. DOI 10.3390/molecules171011335. [Google Scholar] [CrossRef]
4. Song, C., Xiang, D. B., Yan, L., Song, Y., Zhao, G. et al. (2016). Changes in seed growth, levels and distribution of flavonoids during Tartary buckwheat seed development. Plant Production Science, 19(4), 518–527. DOI 10.1080/1343943X.2016.1207485. [Google Scholar] [CrossRef]
5. Siatka, T., Kašparová, M. (2010). Seasonal variation in total phenolic and flavonoid contents and DPPH scavenging activity of Bellis perennis L. flowers. Molecules, 15(12), 9450–9461. DOI 10.3390/molecules15129450. [Google Scholar] [CrossRef]
6. Zielińska, D., Turemko, M., Kwiatkowski, J., Zieliński, H. (2012). Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and Tartary buckwheat plants. Molecules, 17(8), 9668–9682. DOI 10.3390/molecules17089668. [Google Scholar] [CrossRef]
7. Ikeda, K. (2002). Buckwheat: Composition, chemistry, and processing. Advances in Food and Nutrition Research, 44, 395–434. [Google Scholar]
8. Qin, P. Y., Ma, T. J., Wu, L., Shan, F., Ren, G. X. (2011). Identification of Tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. Journal of Food Science, 76(6), 401–407. DOI 10.1111/j.1750-3841.2011.02223.x. [Google Scholar] [CrossRef]
9. Li, S. Q., Zhang, Q. H. (2001). Advances in the development of functional foods from buckwheat. Critical Reviews in Food Science and Nutrition, 41(6), 451–464. DOI 10.1080/20014091091887. [Google Scholar] [CrossRef]
10. Xiang, D. B., Song, Y., Wu, Q., Ma, C. R., Zhao, J. L. et al. (2019). Relationship between stem characteristics and lodging resistance of Tartary buckwheat (Fagopyrum tataricum). Plant Production Science, 22(2), 201–210. DOI 10.1080/1343943X.2019.1577143. [Google Scholar] [CrossRef]
11. Ohmi, O., Mitsuyuki, T. (2005). Distribution of cultivated and wild buckwheat species in the Nu river valley of southwestern China. Fagopyrum, 22, 1–5. [Google Scholar]
12. Zhao, G., Shang, F. (2009). Tartary buckwheat of China, pp. 50–75. Beijing: Science Press. [Google Scholar]
13. Rodriguez, R. J., White, J. F., Amold, A. E., Redman, R. S. (2009). Fungal endophytes: Diversity and functional roles. New Phytologist, 182(2), 314–340. DOI 10.1111/j.1469-8137.2009.02773.x. [Google Scholar] [CrossRef]
14. Bai, W. B., Song, J. Q., Guo, J. Y., Liu, X. H., Li, J. H. (2012). Effects of plant polysaccharide compound agents on the photosynthetic characteristics and dry matter of soybean. Chinese Journal of Applied Ecology, 23, 1861–1868. [Google Scholar]
15. Li, Y., Jin, Q., Yang, D., Cui, J. (2018). Molybdenum sulfide induce growth enhancement effect of rice (Oryza sativa) through regulating the synthesis of chlorophyll and the expression of aquaporin gene. Journal of Agricultural and Food Chemistry, 66(16), 4013–4021. DOI 10.1021/acs.jafc.7b05940. [Google Scholar] [CrossRef]
16. Zhao, J. L., Zhong, L. Y., Zou, L., Zhang, C., Peng, L. et al. (2014). Efficient promotion of the sprout growth and rutin production of Tartary buckwheat by associated fungal endophytes. Cereal Research Communication, 42(3), 401–412. DOI 10.1556/CRC.2013.0068. [Google Scholar] [CrossRef]
17. Zhong, L. Y., Niu, B., Tang, L., Chen, F., Zhao, G. et al. (2016). Effects of polysaccharide elicitors from endophytic Fusarium oxysporum Fat9 on the growth, flavonoid accumulation and antioxidant property of Fagopyrum tataricum. Molecules, 21(12), 1590. DOI 10.3390/molecules21121590. [Google Scholar] [CrossRef]
18. Zhong, L. Y., Zou, L., Tang, X. H., Li, W. F., Li, X. et al. (2017). Community of endophytic fungi from the medicinal and edible plant Fagopyrum tataricum and their antimicrobial activity. Tropical Journal of Pharmaceutical Research, 16(2), 387–396. DOI 10.4314/tjpr.v16i2.18. [Google Scholar] [CrossRef]
19. Zhao, J. L., Zou, L., Zhong, L. Y., Peng, L. X., Ying, P. L. et al. (2015). Effects of polysaccharide elicitors from endophytic Bionectria pityrodes Fat6 on the growth and flavonoid production in Tartary buckwheat sprout cultures. Cereal Research Communication, 43(4), 661–671. DOI 10.1556/0806.43.2015.013. [Google Scholar] [CrossRef]
20. Bauddh, K., Singh, R. P. (2012). Growth: Tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicology and Environmental Safety, 85, 13–22. DOI 10.1016/j.ecoenv.2012.08.019. [Google Scholar] [CrossRef]
21. Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: The pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382. [Google Scholar]
22. Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. DOI 10.1016/0003-2697(76)90527-3. [Google Scholar] [CrossRef]
23. Bates, L. S., Waldern, R. P., Tear, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39(1), 205–207. DOI 10.1007/BF00018060. [Google Scholar] [CrossRef]
24. Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I: Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198. DOI 10.1016/0003-9861(68)90654-1. [Google Scholar] [CrossRef]
25. Darvishzadeh, R., Pirzad, A., Hatami, M. H. (2010). Evaluation of the reaction of sunflower inbred lines and their F1 hybrids to drought conditions using various stress tolerance indices. Spanish Journal of Agricultural Research, 8(4), 1037–1046. DOI 10.5424/sjar/2010084-1398. [Google Scholar] [CrossRef]
26. Hura, T., Hura, K., Grzesiak, M., Rzepka, A. (2007). Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiologiae Plantarum, 29(2), 103–113. DOI 10.1007/s11738-006-0013-2. [Google Scholar] [CrossRef]
27. Yan, W., Zhong, Y., Shangguan, Z. (2016). A meta-analysis of leaf gas exchange and water status responses to drought. Scientific Reports, 6(1), 20917. DOI 10.1038/srep20917. [Google Scholar] [CrossRef]
28. Zhang, S. H., Xu, X. F., Sun, Y. M., Zhang, J. L., Li, C. Z. (2018). Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. Journal of Integrative Agriculture, 17(2), 336–347. DOI 10.1016/S2095-3119(17)61758-1. [Google Scholar] [CrossRef]
29. He, W., Adachi, S., Sage, R. F., Ookawa, T., Hirasawa, T. (2017). Leaf photosynthetic rate and mesophyll cell anatomy changes during ontogenesis in backcrossed indica × japonica rice inbred lines. Photosynthesis Research, 134(1), 27–38. DOI 10.1007/s11120-017-0403-x. [Google Scholar] [CrossRef]
30. Xiang, D. B., Ma, C. R., Song, Y., Wu, Q., Wu, X. Y. et al. (2019). Post-anthesis photosynthetic properties provide insights into yield potential of Tartary buckwheat cultivars. Agronomy, 9(3), 149. DOI 10.3390/agronomy9030149. [Google Scholar] [CrossRef]
31. Koffler, B. E., Luschin-Ebengreuth, N., Stabentheiner, E., Müller, M., Zechmann, B. (2014). Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Science, 227, 133–144. DOI 10.1016/j.plantsci.2014.08.002. [Google Scholar] [CrossRef]
32. Han, T., Zhan, W., Gan, M. X., Liu, F. R., Yu, B. T. et al. (2018). Phosphorylation of glutaminase by PKCε is essential for its enzymatic activity and critically contributes to tumorigenesis. Cell Research, 28(6), 655–669. DOI 10.1038/s41422-018-0021-y. [Google Scholar] [CrossRef]
33. Bauerle, W. L., Oren, R., Way, D. A., Qian, S. S., Stoy, P. C. et al. (2012). Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proceedings of the National Academy of Sciences, 109(22), 8612–8617. DOI 10.1073/pnas.1119131109. [Google Scholar] [CrossRef]
34. Ghosh, P. K., Ajay, K. K., Bandyopadhyay, M. C., Manna, K. G., Mandal, A. K. et al. (2004). Comparative effectiveness of cattle manure, poultry manure, phosphocompost and fertilizer-NPK on three cropping system in vertisols of semi-arid tropics П. Dry matter yield, nodulation, chlorophyll content and enzyme activity. Bioresource Technology, 95(1), 85–93. DOI 10.1016/j.biortech.2004.02.012. [Google Scholar] [CrossRef]
35. Mafakheri, A., Siosemardeh, A., Bahramnejad, B., Struik, P., Sohrabi, Y. (2010). Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian Journal of Crop Science, 4, 580–585. [Google Scholar]
36. Tanaka, Y., Tanaka, A., Tsuji, H. (1993). Effects of 5-aminolevulinic acid on the accumulation of chlorophyll b and apoproteins of the light-harvesting chlorophyll a/b-protein complex of photosystem II. Plant and Cell Physiology, 34, 465–472. [Google Scholar]
37. Xiang, D. B., Wei, W., Ouyang, J. Y., Le, L. Q., Zhao, G. et al. (2020). Nitrogen alleviates seedling stage drought stress response on growth and yield of Tartary buckwheat. International Journal of Agriculture and Biology, 24(5), 1167–1177. DOI 10.17957/IJAB/15.1546. [Google Scholar] [CrossRef]
38. Liu, D., Kong, D. D., Fu, X. K., Ali, B., Xu, L. et al. (2016). Influence of exogenous 5-aminolevulinic acid on chlorophyll synthesis and related gene expression in oilseed rape de-etiolated cotyledons under water-deficit stress. Photosynthetica, 54(3), 468–474. DOI 10.1007/s11099-016-0197-7. [Google Scholar] [CrossRef]
39. Shmakov, N. A., Vasiliev, G. V., Shatskaya, N. V., Doroshkov, A. V., Khlestkina, E. K. (2016). Identification of nuclear genes controlling chlorophyll synthesis in barley by RNA-seq. BMC Plant Biology, 16(S3), 119–138. DOI 10.1186/s12870-016-0926-x. [Google Scholar] [CrossRef]
40. Li, Y., Jin, Q., Yang, D., Cui, J. (2018). Molybdenum sulfide induce growth enhancement effect of rice (Oryza sativa) through regulating the synthesis of chlorophyll and the expression of aquaporin gene. Journal of Agricultural and Food Chemistry, 66(16), 4013–4021. DOI 10.1021/acs.jafc.7b05940. [Google Scholar] [CrossRef]
41. Zhang, C., He, P., Li, Y., Li, Y., Li, S. (2015). Exogenous diethyl aminoethyl hexanoate, a plant growth regulator, highly improved the salinity tolerance of important medicinal plant Cassia obtusifolia L. Journal of Plant Growth Regulation, 35(2), 330–344. DOI 10.1007/s00344-015-9536-3. [Google Scholar] [CrossRef]
42. Tsuchiya, T., Ohta, H., Okawa, K., Iwamatsu, A., Shimada, H. et al. (1999). Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proceedings of the National Academy of Sciences, 96(26), 15362–15367. DOI 10.1073/pnas.96.26.15362. [Google Scholar] [CrossRef]
43. Zhang, Z. G., Shang, Q. M. (2010). Regulation of salicylic acid and chitosan on photosynthetic parameters of cucumber leaves under salt stress. Acta Agriculturae Boreali-occidentalis Sinica, 19, 174–178. [Google Scholar]
44. Hamid, M., Mansour, G., Marian, B. (2018). Exogenous putrescine changes redox regulations and essential oil constituents in field-grown, Thymus vulgaris L. under well-watered and drought stress conditions. Industrial Crops and Products, 122, 119–132. DOI 10.1016/j.indcrop.2018.05.064. [Google Scholar] [CrossRef]
45. Kaur, G., Asthir, B. (2015). Proline: A key player in plant abiotic stress tolerance. Biologia Plantarum, 59(4), 609–619. DOI 10.1007/s10535-015-0549-3. [Google Scholar] [CrossRef]
46. Misra, N., Gupta, A. K. (2005). Effect of salt stress on proline metabolism in two high yielding genotypes of green gram. Plant Science, 169(2), 331–339. DOI 10.1016/j.plantsci.2005.02.013. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |