

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013588
ARTICLE
Foliar Application of Phosphorus Enhances Photosynthesis and Biochemical Characteristics of Maize under Drought Stress
1Department of Field Crops, Faculty of Agriculture, Çukurova University, Adana, 01330, Turkey
2Department of Botany, University of Central Punjab (UCP), Punjab Group of Colleges, Bahawalpur, 63100, Pakistan
3Department of Agronomy, University of Agriculture, Faisalabad, 38000, Pakistan
4Institute of Soil and Environmental Science, University of Agriculture, Faisalabad, 38000, Pakistan
5Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, 21577, Saudi Arabia
6College of Agronomy, Sichuan Agricultural University, Chengdu, 611130, China.
7Department of Field Crops, Faculty of Agriculture, Siirt University, Siirt, 56100, Turkey
8Department of Agronomy, Faculty of Agriculture, University of Kafrelsheikh, Kafrelsheikh, 33516, Egypt
*Corresponding Authors: Zahoor Ahmad. Email: zahoorahmadbwp@gmail.com; Ayman El Sabagh. Email: aymanelsabagh@gmail.com
Received: 11 August 2020; Accepted: 07 November 2020
Abstract: Water is essential for the growth period of crops; however, water unavailability badly affects the growth and physiological attributes of crops, which considerably reduced the yield and yield components in crops. Therefore, a pot experiment was conducted to investigate the effect of foliar phosphorus (P) on morphological, gas exchange, biochemical traits, and phosphorus use efficiency (PUE) of maize (Zea mays L.) hybrids grown under normal as well as water deficit situations at the Department of Agronomy, University of Agriculture Faisalabad, Pakistan in 2014. Two different treatments (control and P @ 8 kg ha−1) and four hybrids (Hycorn, 31P41, 65625, and 32B33) of maize were tested by using a randomized complete block design (RCBD) with three replications. Results showed that the water stress caused a remarkable decline in total soluble protein (9.7%), photosynthetic rate (9.4%) and transpiration rate (13.4%), stomatal conductance (10.2%), and internal CO2 rate (20.4%) comparative to well-watered control. An increase of 37.1%, 36.8%, and 24.5% were recorded for proline, total soluble sugar, and total free amino acid, respectively. However, foliar P application minimized the negative impact of drought by improving plant growth, physio-biochemical attributes, and PUE in maize plants under water stress conditions. Among the hybrids tested, the hybrid 6525 performed better both under stress and non-stress conditions. These outcomes confirmed that the exogenous application of P improved drought stress tolerance by modulating growth, physio-biochemical attributes, and PUE of maize hybrids.
Keywords: Photosynthetic attributes; biochemical characters; water stress; foliar P; Maize
Abbreviations
| TSP = | Total Soluble Protein, |
| A = | Photosynthetic rate, |
| E = | Transpiration rate, |
| gs = | Stomatal conductance, |
| Ci = | Internal CO2 rate, |
| TSS = | Total soluble sugars, |
| TFA = | Total free amino acid, |
| PUE = | Phosphorus use efficiency. |
Water is an essential constituent of life, has endless uses and functions in the plant body, and its adequate amount is mandatory for the normal functioning of plant biochemical and physiological functions [1]. Water is necessary for plant survival. That’s why its shortage can lead to devastating negative impacts on plant growth, development, and functioning depending upon the severity and duration of water stress [2]. Maize is an important crop worldwide based upon its production and diversity in utilization; it stands 3rd among cereal crops [3]. Maize is used as a staple food, as animal feed and processed food like corn flop, gruels, and corn flour bread [3]. Maize is a water-sensitive crop, but both male (tassel) and female (cob) parts of the crop respond to water stress differently once the crop is shifting to the reproductive stage. Upon the onset of water stress, the female part of maize is more negatively influenced, leading to a noticeable delay in silking initiation. The male part remains unaffected; thus, pollen shed time remains unaffected [4]. Water stress also significantly reduces carbon assimilation during photosynthesis in plant and plant tissue water potential due to decreased relative water content of plant tissue [5,6]. Reviewing the importance of maize crop and the impact of water stress on crop its need of the hour to improve maize growth under water deficit conditions. Improvement of plant water stress tolerance is coupled with the loss of turgor pressure in the water, limiting conditions enabling the plant to withstand stress temporarily without significantly affecting chloroplast functioning [7]. Variation in leaf turgor pressure is an adaptive strategy to tolerate water stress in maize plants. Drought stress causes a remarkable reduction in the physiological characters and the biochemical attributes of maize. Still, the duration and severity of drought stress are important for creating a negative impact on maize physiology [8].
Phosphorous (P) is a second most important macronutrient of plants involved in energy transfer thus can play a leading role in maintaining photosynthesis under water deficit conditions, and this is a well-documented fact that it does so [3] P deficiency in maize crop often compromise plant root and shoot biomass by causing a decrease in net photosynthesis activity [9,10]. P deficiency similarly causes stunted growth of leaf associated with lower leaf area, which ultimately reduces the absorption of photosynthetic absorption rate resulting in compromised photosynthesis and maize growth [11,12]. Yaseen et al. [13] have reported that the potential of exogenous applied P in mitigating water stress in plants by increasing plant growth and yield. At the same time, foliar application of P is more effective in increasing phosphorus use efficiency (PUE), which ultimately decreases the usage of P via fertigation and increase growth significantly [14,15]. Based on all the above features, the present study was planned to evaluate the impact of foliarly applied P on maize physio-biochemical traits, growth, and PUE under water stress conditions to mitigate water stress boosting photosynthetic and biochemical characteristics in maize plants.
2 Materials under Circumstances
The current research study was performed at the wirehouse of Department of Agronomy, University of Agriculture Faisalabad, Pakistan, in the growing season of spring 2014. Collected sand was medium for current research work, which was dried under the sun, grinded, and sieved to remove any contamination before filling pots. The electrical balance was used to weigh 4 kg of sand for each pot, and recommended doses of N, P, and K (150-100-100 kg ha−1) were added in pots. Four seeds of each hybrid maize cultivars (Hycorn, 31P41, 32B33, and 6525) were sown in each pot, and distilled water was used for irrigation to help seeds imbibe and germinate. Once germinated, the plant population per pot was maintained at 3 using thinning. At the 8th leaf stage of plants, the pots were separated into two distinct groups, one was applied water stress, and the other was non-stressed. Each pot was weighed at 9 am daily to calculate water loss in evapotranspiration (weight pots daily and add water that maintained the daily water loss through evaporation transpiration); an equivalent amount was added predetermined pot weight. Foliar application of P (8 kg ha−1) was plant canopy with surfactant (Tween) started at the 10th day of application of water stress. Water stress was maintained at 40% field capacity throughout maize growth. Physio-chemical and PUE parameters of crop plants were recorded after 40 days of maize sowing using the following data recording methods.
The measuring tape was used to measure the root shoot length of plants after harvesting, and electrical balance was used to estimate plant fresh and dry weights.
Analytical Development Company, Hoddesdon, England Company developed LCA04 ADC portable infrared gas analyzer (IRGA) was used for the determination of photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs) and internal CO2 rate (Ci) of youngest and fully expanded leaf of crop plant at 8th leaf stage. For using IRGA, morning time (9.00 to 11.00 a.m.) was selected to keep uniformity in measurement as it may get affected by sunlight after 11 am. IRGA was set at 99.9 kPa atmospheric pressure, 403.3 mmol m−2s−1 of molar air flow rate, and 6.0 to 8.9 m bar of water vapor pressure in the chamber of IRGA.
2.3 Biochemical Parameters Determination
Proline contents in leaf tissue were measured using methodology reported by Falcioni et al. [16].
Other biochemical parameters were total soluble sugar (TSS) measured using the method of Sinay et al. [17], total free amino acids (TFA) measured following Sood et al. [18], and total soluble proteins (TSP) were measured using the methodology reported by Mohd Rosni et al. [19].
Plant tissue phosphorus contents were measured in plant shoot and root samples using the spectrophotometric technique described by Waraich et al. [20].
2.5 Samples Analysis by Using Stat Software
A randomized complete block design (RCBD) was applied to determine the statistical significance of applied treatments using MSTAT-C software. Analysis of variance (ANOVA) under RCBD factorial design was performed. The least significant difference (LSD) test was used to check significant differences among different treatment results at a 5% probability level.
Maize plants exhibited significantly lower (p ≤ 0.001) growth characters such as root and shoot length and their fresh-dry weights in maize subjected to normal as well as drought stress (Tab. 1). The highest root-shoot length and their fresh and dry weight (RL = 59.20 cm, SL = 84.45 cm, SFW = 132.41 g, RFW = 70.66 g, SDW = 29.91 g, and RDW = 13.34 g) were noted in full availability of water for the growth of plant such as normal conditions. In contrast, the lowest length of root-shoot length and their fresh and dry weight (RL = 49.83 cm, SL = 76.04 cm, SFW = 106.46 g, RFW = 44.00 g, SDW = 17.93 g, and RDW = 12.30 g) were observed in plants where limited water was given to the plant, such as water stress (Tab. 1). Recorded data of root-shoot length and their fresh and dry weights performed better. They showed significant enhanced tolerance among all hybrids of maize in limited availability of water to the plants. The maize genotype 6525 and 32B33 maintained significantly higher root and shoot length and fresh and dry weights than other genotypes (Tab. 1).
Table 1: Growth characters of maize responses by foliar P application under well-watered and water-deficit situations

Note: aShoot length (cm), Root length (cm), Shoot fresh weight (g), Root fresh weight (g), Shoot dry weight (g) and Root dry weight (g), bmean values across four hybrids; cWW = Well-watered, WS = Water stress; dmean values across two levels; F0 = No foliar applied P (Control), F1 = Foliar applied P; NS = Non Significant; *’**’***significant at p ≤ at 0.05, p ≤ at 0.01, p ≤ at 0.001, respectively. eH = Hybrid, T = Treatment and W = Water level
Exogenous P application significantly (p ≤ 0.001) improved length of root-shoot and their fresh and dry weight of hybrids maize compared to control. The maximum value of root-shoot length and their fresh and dry weight (RL = 61.33 cm, SL = 89.27 cm, SFW = 136.40 g, RFW = 70.92 g, SDW = 32.06 g, and RDW = 17.53 g) was observed in P treatment while the lowest length of root-shoot and their fresh and dry weight (RL = 47.70 cm, SL = 71.12 cm, SFW = 102.47 g, RFW = 43.74 g, SDW = 15.78 g, and RDW = 8.11 g) was noted in that treatment where P was not applied. Non-significant interactions were shown in the growth characters (Tab. 1).
3.2 Gaseous Exchange Parameters
Water stressed conditions had shown significant reduction (p < 0.01) in IRGA characters such as A, E, gs, and Ci of all maize hybrid parameters (Tab. 2). A drop of 9.4, 13.4, 10.2, and 20.4% in different attributes of A, E, gs, and Ci were observed on an average basis in all hybrids of maize subjected to limited availability of water such as water stress conditions to normal availability of water such as normal conditions shown in Fig. 1. Maize hybrids 6525 and 32B33, which were being exposed to supplemental P applied via a foliar application, have shown better A (4.19 & 3.71 µmol CO2 m−2 s−1) and E (2.76 & 2.36 mmol H2O m−2 s−1) under non-stress and water stress conditions. While the lowest values of A (3.74 & 3.26 µmol CO2 m−2 s−1) and E (2.38 & 2.01 mmol H2O m−2 s−1) were observed by Hycorn hybrid in Fig. 2. Among all hybrid tested, 6525 had proven to be most responsive to exogenous P application when the plant was grown in well-watered and water-deficit situations. More gs (4.50 & 3.78 mmol H2O m−2 s−1) and Ci (292 & 214.67 µ mol H2O m−2 s−1) were recorded in water enough and stress conditions through foliar P application presented in Fig. 1.
Table 2: Analysis of variance for different maize parameters and foliar P in both water stress and non-stress situations

Note: A = Leaf Photosynthetic rate; E = Leaf Transpiration rate; gs = Stomatal conductance; Ci = Substomatal CO2 rate; TPC = Total proline contents; TFA = Total free amino acid; TSS = Total soluble sugar; TSP = Total soluble protein; Leaf P = Phosphorus concentration in leaf; Stem P = Phosphorus concentration in stem; Root P = Phosphorus concentration in root; grain P = Phosphorus concentration in grain; PUE = Phosphorus use efficiency; N.S = Non-significant; *’**’***significant at p ≤ at 0.05, p ≤ at 0.01, p ≤ at 0.001, respectively.
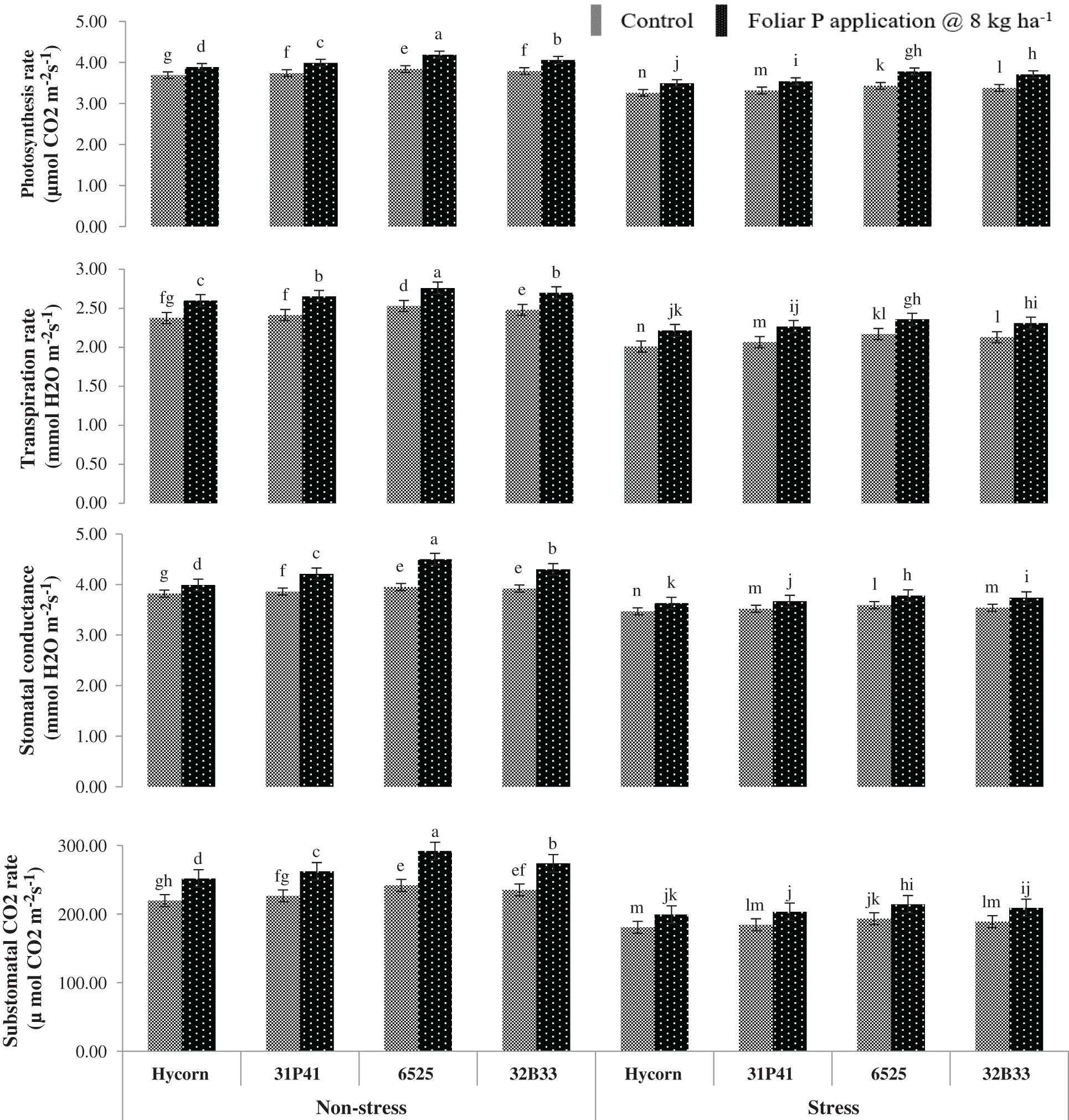
Figure 1: Impact of foliar-applied P on physiological characters of maize under well-watered and water deficit (mean values ± S.E)
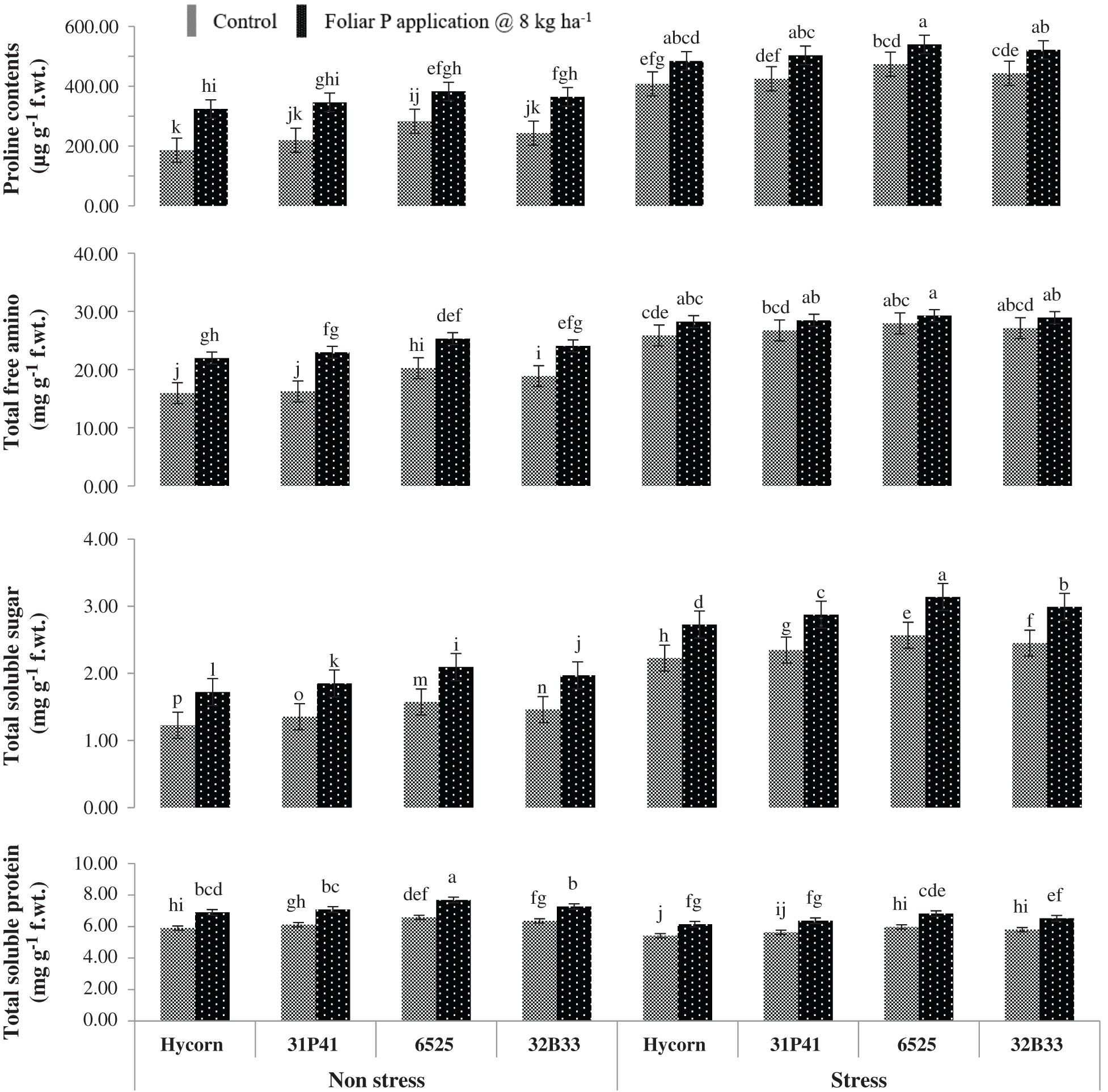
Figure 2: Impact of foliar-applied P on biochemical attributes of maize under well-watered and water deficit (mean values ± S.E)
Among biochemical parameters, plant tissue proline contents have shown to be significantly increased p < 0.001, but non-significant interaction was found between hybrid and foliar-applied P (Tab. 2). The cultivars experiencing water stress have shown a 37.1% increase in proline contents compared to well-watered (non-stressed) conditions. Plant proline content was found to be maximum in hybrid 6525 was 539.68 µg g−1 FW under low water conditions and maintained comparatively better (382.56 µg g−1 FW) in well water conditions when P was applied via foliar way. Foliar used P was non-effective in improving proline consents in Hycorn and showed the lowest value (407.62 µg g−1 FW) under water stress situations (Fig. 2).
Besides proline, TSS and TFA were also increased significantly in all hybrids, which were unsuccessful in showing any higher TSP value under water stress conditions (Tab. 2). Overall water stress has increased TSS and TFA (36.8% and 24.5%) in different maize hybrids, while minimum TSP was 9.7% compared to well-watered control (Fig. 3). The best-performing hybrid among all tested genotypes was hybrid 6525. The highest level of TSS and TFA were recorded in hybrid 6525 under water stress (3.13 & 29.27 mg g−1 FW) and normal conditions (2.09 & 25.30 mg g−1 FW) with exogenous application of P. While in similar conditions, Hycorn was poor performing with minimum TSS (2.72 & 1.72 mg g−1 FW) and TFA (28.24 & 21.97 mg g−1 FW under water stress and control conditions (Fig. 2).
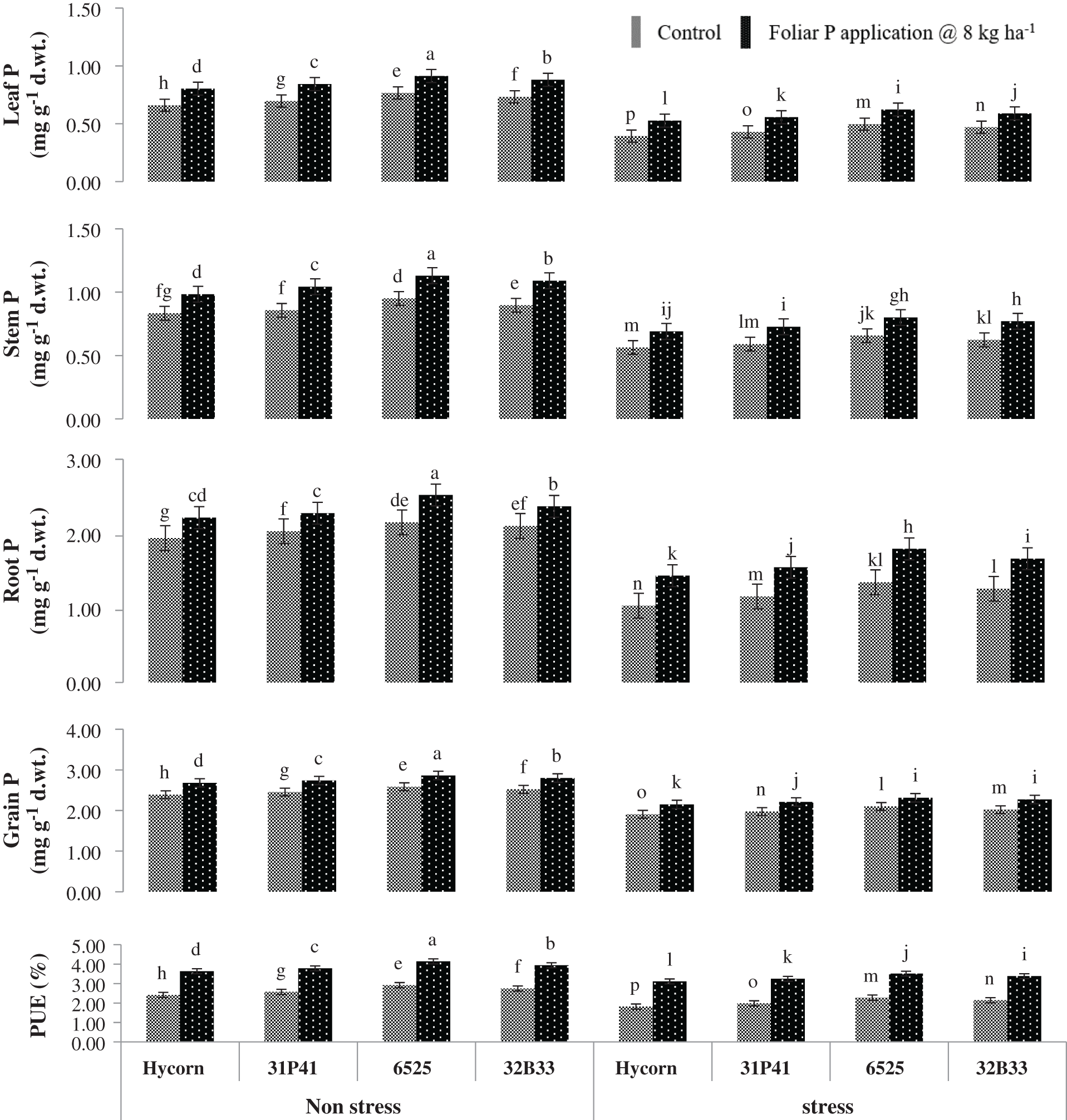
Figure 3: Impact of foliar-applied P on P absorption in different maize plant parts under well-watered and water deficit (mean values ± S.E). Data represented in figures are the mean of three replicates for each treatment. The vertical bar indicates the standard error. The different letter on top of each bar denotes a statistically significant difference at p < 0.05 following the LSD test
There was a non-significant interaction between TSP, P treatment, and water stress (Tab. 2), but a clear reduction in TSP was monitored in water-stressed conditions compared to well water conditions. TSP in maize hybrids was improved with P application under non-stress and water stress conditions. Recorded data revealed that hybrid 6525, when supplemented with foliar applied P, showed maximum TSP value (7.68 & 6.82 mg g−1 FW) under optimum and stressed water conditions, respectively. While hybrid Hycorn has shown the lowest values (5.90 mg g−1 FW) and (6.41 mg g−1 FW) under enough and stressed water conditions, respectively (Fig. 2).
Water stress has significantly (p < 0.01) decreased plant leaf, stem, root, and grain P concentration, and PUE was severely affected by all hybrids (Tab. 2). Plants grown up to 8th leaf in water deficit environment significantly decreased the absorption of P in plant leaves (24.5%), stem (29.8%), root (35%), grain (18.4%), and PUE (33.4%) as compared to those plants which were grown under well-watered situations (Fig. 3). Among all tested genotypes, hybrid 6525 has shown maximum tissue P concentration under well-watered and water stress conditions. Hybrid 6525 contained leaf P as 0.91 & 0.62 mg g−1 DW, stem P as 1.13 & 0.80 mg g−1 DW, root P as 2.10 & 1.49 mg g−1 DW and grain P as 2.86 & 2.31 mg g−1 DW in control as well as in water stress conditions. Maize hybrid Hycron showed the poorest performance in PUE under water sufficient conditions. Similarly, under water-stress and optimal water conditions, hybrid 6525 has shown the highest percent increase in PUE (4.14% & 3.51%) when applied with supplemented foliar P. In contrast, Hycorn hybrid of maize showed the lowest performance subjected to drought stress as well as the foliar P application and noted minimum values of PUE (2.41% & 1.82%) presented in Fig. 3.
The present study showed that a remarkable reduction in all growth parameters such as root-shoot length and their fresh and dry weight were noted where water stress present in maize plant. The decrease in the growth of maize plants due to death of the plant cells or minimize the growth of cells in water stress. While in the present study, the death of the cells was not observed, and the reduction in the plant growth is due to suppressed plant growth under water stress conditions. Similar results were observed in wheat under water stress conditions. The growth of wheat plants minimizes under water stress [20]. Foliar application of P play a significant role in enhancing the growth of maize plants under normal and water stress conditions observed in the current study. The current study was supported by the finding of Girma et al. [14]. He stated that the exogenous application of P helpful for the better growth of corn when it was applied at the proper time. The increase in the growth of maize plants is due to P as P is the major constituent of the cell membrane and also essential for nucleic acids and proteins [3]. Similarly, the P is one of the major nutrients used for energy and helpful in activating many enzymes that play an important role in the growth and development of plants [21]. P is useful for the better growth of crops because P is one of the macronutrients that is constituted of nucleic acid and nucleic acid and main components of RNA and DNA molecules, and it also plays a major role in transportation and translation of various information of genetics to the plants which promote the growth of the plant.
Water stress significantly reduced the physiological attributes such as A, gs, E, and Ci in maize plants in the current study. The reduction of physiological characters in maize plants is due to the closure of stomata under water stress conditions, which leads to reduced intake of CO2 and outgoing of O2 from the leaf and reduces photosynthesis of plants. A similar observation was noted by Pieters et al. [22] in rice and Ahmed et al. [23] in olive; they reported that water stress hampered the metabolic processes in plants, which results in closure of stomata and reduce the process of photosynthesis in plants which ultimately decreased the production of crops. The different researchers reported the similar results in other crops, which were observed in the present research work on maize plants. They stated that the water stress is an important factor that is responsible for the reduction of A, gs, E, and Ci in the case of maize [24], canola [25], and in Vigna radiata [26]. While the foliar P application significantly improved the physiological characters (A, gs, E, and Ci) of maize plants in well-watered and water-deficit situations, which were recorded in the current research experiment. The improvement in the physiological attributes of maize plants under both normal and water stress conditions is due to the foliage application of P, because of the foliage P application helpful in ATP production as well as RNA and DNA molecules, which are the main constituents of nucleic acids. The foliar P enhanced the leaf growth, which improved the assimilation rate of plants that ultimately improved maize plants’ physiological characters under normal and water stress conditions, which was observed by the Girma et al. [14] in the Triticum aestivum plant. Among all hybrid maize, the 6525 performed the better by improving all physiological processes under both well-watered and water stress conditions compared to other hybrids of maize plants.
Water stress caused a negative impact on the growth and development of the crops while proline was produced under drought stress conditions and minimize the adverse effect of drought reported by Bajji et al. [27]. Related outcomes were observed in the current research work in which proline content increased under water stress conditions. An increase in the proline contents under drought was also reported by many other scientists in different crops [28–30]. Our study showed that TSP’s content was minimum in water stress situations as associated with non-stress cases. The decrease in TSP contents is due to more accumulation of proline contents under water stress conditions. The present study was supported by Cechin et al. [29], who stated that the more accumulation of proline contents under drought, decrease the TSP in water-stressed leaf. However, the contents of proline and TSP were improved in plants’ leaf under normal, and water deficit situations by exogenous P at the rate of 8 kg ha−1 applied when maize plants grown up to the 8th leaf observed in the current study. Present outcomes are similar to the conclusion of Ahmad et al. [3]. They stated that the foliar P application helped improve proline and TSP contents under a water-stressed environment.
The data regarding TFA and TSS showed that the water stress conditions favorable for increasing TFA and TSS contents in this study. Similar outcomes concluded by Hsu et al. [28] and Ashraf et al. [31], reported that the TFA contents enhanced under different environmental stresses like drought stress. TFA contents increased under water stress are due to the conversion of proteins into amino acids that were essential for osmotic adjustment in plants [28]. Moreover, the present study indicated that the foliar P application @ 8 K ha−1 significantly improved the TFA and TSS under a normal and water-stressed environment in maize when maize plants grew up to 8th leaf. In water deficit situations TSS and TFA with other related compatible solutes were enhanced significantly in plants reported by Nawaz et al. [32]. The protein biosynthesis is low compared to the breakdown of protein in water deficit situations [33]. Our results revealed a similar conclusion under water stress with foliar P application in maize.
4.4 Phosphorus Concentration and PUE
Outcomes of current research work showed that the water deficit significantly affected the concentration of P in maize plants and reduced the P concentration in all parts such as leaf, stem, and root. Similarly, the PUE was also suppressed under water stress conditions in all hybrids of maize. At the same time, the foliar application exhibited the best performance in enhancing the P concentration in leaf, stem, root. Also, PUE increased under both normal under water stress conditions. Enhanced concentration of P in maize plants is due to the positive impact of foliar P application, which is absorbed through the leaf to provide energy and increase the P concentration in plants. P concentration in maize enhanced by the application of foliar P under water deficit environment showed in a current research experiment. The same result concluded that the foliar P application increases the P concentration in maize [34], which supports the finding of the current research study. PUE decreases under water-limited conditions, while the foliar P application plays an important role in improving the PUE in plants. Foliar P application responsible for enhancing the PUE up to 16% in wheat reported by Mosali et al. [35]. Girma et al. [14] experimented on corn and stated that the foliar P application increased the P concentration in corn when applied at the proper leaf stage of corn.
The present study was concluded that the exogenous applied P @ 8 kg ha−1 improve all measured characters of growth (root-shoot length and their fresh and dry weights), physiological (A, gs, E and Ci), biochemical attributes (proline contents, TSS, TSP, and TFA) and PUE significantly under both normal as well as water stress conditions. The improvement in these characters in maize plants is due to P application through the foliage, helpful in the plant for osmotic adjustment under the water stresses conditions. The 6525 maize hybrid showed the best performance among the genotypes compared to all other tested hybrids using foliar P application under normal and water stress conditions. Finally, we concluded that, the exogenous application of P improved water stress tolerance in maize plants by boosting photosynthetic and biochemical attributes in maize. However, further studies using different doses of P will give us important clues about the functional role of exogenous P in modulating drought stress tolerance in maize.
Funding Statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, X., Cao, J., Dai, X., Xiao, J., Wu, Y. et al. (2017). Total flavonoid concentrations of bryophytes from Tianmu Mountain, Zhejiang Province (ChinaPhylogeny and ecological factors. PLoS One, 12(6), e0179837. DOI 10.1371/journal.pone.0179837. [Google Scholar] [CrossRef]
2. Ahmad, Z., Anjum, S., Warrich, E., Ayub, M., Ahmad, T. et al. (2018). Growth, physiology, and biochemical activities of plant responses with foliar potassium under drought stress-A review. Journal of Plant Nutrition, 41(13), 1734–1743. DOI 10.1080/01904167.2018.1459688. [Google Scholar] [CrossRef]
3. Ahmad, Z., Waraich, E. A., Ahmad, R., Shahbaz, M. (2017). Modulation in water relations, chlorophyll contents and antioxidants activity of maize by foliar phosphorus application under drought stress. Pakistan Journal of Botany, 49(1), 11–19. [Google Scholar]
4. Ahmed, Z., Waraich, E. A., Ahmad, R., Shahbaz, M. (2017). Morpho-physiological and biochemical responses of camelina (Camelina Sativa Crantz) genotypes under drought stress. International Journal of Agriculture and Biology, 19(1), 1–7. DOI 10.17957/IJAB/15.0141. [Google Scholar] [CrossRef]
5. Lawlor, D. W., Cornic, G. (2002). Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell & Environment, 25(2), 275–294. DOI 10.1046/j.0016-8025.2001.00814.x. [Google Scholar] [CrossRef]
6. Hao, Z., Singh, V. P. (2015). Drought characterization from a multivariate perspective: A review. Journal of Hydrology, 527, 668–678. DOI 10.1016/j.jhydrol.2015.05.031. [Google Scholar] [CrossRef]
7. Waraich, E. A., Ahmad, R., Saifullah, Ahmad, A. (2011). Water stress and nitrogen management effects on gas exchange, water relations, and water use efficiency in wheat. Journal of Plant Nutrition, 34(12), 1867–1882. DOI 10.1080/01904167.2011.600413. [Google Scholar] [CrossRef]
8. Sabagh, A. El, Hossain, A., Barutçular, C., Khaled, A. A., Fahad, S. et al. (2018). Sustainable maize (Zea mays L.) production under drought stress by understanding its adverse effect, survival mechanism and drought tolerance indices. Journal of Experimental Biology and Agricultural Science, 6(2), 282–295. DOI 10.18006/2018.6(2). 282.295. [Google Scholar] [CrossRef]
9. Wissuwa, M., Gamat, G., Ismail, A. M. (2005). Is root growth under phosphorus deficiency affected by source or sink limitations? Journal of Experimental Botany, 56(417), 1943–1950. DOI 10.1093/jxb/eri189. [Google Scholar] [CrossRef]
10. Kavanova, M. (2006). Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Physiology, 141(2), 766–775. DOI 10.1104/pp.106.079699. [Google Scholar] [CrossRef]
11. Pellerin, S., Mollier, A., Plénet, D. (2000). Phosphorus deficiency affects the rate of emergence and number of maize adventitious nodal roots. Agronomy Journal, 92(4), 690–697. DOI 10.2134/agronj2000.924690x. [Google Scholar] [CrossRef]
12. Shi, Y., Zhang, Y., Han, W., Feng, R., Hu, Y. et al. (2016). Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum Lycopersicum L. Frontiers in Plant Science, 7(376), 1–15. DOI 10.3389/fpls.2016.00196. [Google Scholar] [CrossRef]
13. Yaseen, M., Malhi, S. S. (2009). Variation in yield, phosphorus uptake, and physiological efficiency of wheat genotypes at adequate and stress phosphorus levels in soil. Communications in Soil Science and Plant Analysis, 40(19–20), 3104–3120. DOI 10.1080/00103620903261643. [Google Scholar] [CrossRef]
14. Girma, K., Martin, K. L., Freeman, K. W., Mosali, J., Teal, R. K. et al. (2007). Determination of optimum rate and growth stage for foliar-applied phosphorus in corn. Communications in Soil Science and Plant Analysis, 38(9–10), 1137–1154. DOI 10.1080/00103620701328016. [Google Scholar] [CrossRef]
15. Ali, M. S., Sutradhar, A., Edano, M. L., Edwards, J. T., Girma, K. (2014). Response of winter wheat grain yield and phosphorus uptake to foliar phosphite fertilization. International Journal of Agronomy, 2014(5), 1–8. DOI 10.1155/2014/801626. [Google Scholar] [CrossRef]
16. Falcioni, F., Blank, L. M., Frick, O., Karau, A., Bühler, B. et al. (2013). Proline availability regulates proline-4-hydroxylase synthesis and substrate uptake in proline-hydroxylating recombinant Escherichia coli. Applied and Environmental Microbiology, 79(9), 3091–3100. DOI 10.1128/AEM.03640-12. [Google Scholar] [CrossRef]
17. Sinay, H., Karuwal, R. L. (2014). Proline and total soluble sugar content at the vegetative phase of six corn cultivars from Kisar Island Maluku, grown under drought stress conditions. International Journal of Agronomy and Agricultural Research, 2, 77–82. [Google Scholar]
18. Sood, V., Rawat, D., Khanna, R., Sharma, S., Gupta, P. K. et al. (2017). Study of carnitine/acylcarnitine and amino acid profile in children and adults with acute liver failure. Journal of Pediatric Gastroenterology and Nutrition, 64(6), 869–875. DOI 10.1097/MPG.0000000000001510. [Google Scholar] [CrossRef]
19. Mohd Rosni, S., Fisal, A., Azwan, A., Chye, F. Y., Matanjun, P. (2015). Crude proteins, total soluble proteins, total phenolic contents and SDS-PAGE profile of fifteen varieties of seaweed from Semporna. International Food Research Journal, 22(4), 1483–1493. [Google Scholar]
20. Waraich, E. A., Ahmad, Z., Ahmad, R., Saifullah, Ashraf, M. Y. (2015). Foliar applied phosphorous enhanced growth, chlorophyll contents, gas exchange attributes and PUE in Wheat (Triticum aestivum L.). Journal of Plant Nutrition, 38(12), 1929–1943. DOI 10.1080/01904167.2015.1043377. [Google Scholar] [CrossRef]
21. Zhang, Y., Huang, L., Zhang, Z., Wei, L., Sun, C. et al. (2016). Phosphorus fractions and phosphorus adsorption characteristics of soils from the water-level fluctuating zone of Nansi Lake, China. Polish Journal of Environmental Studies, 25(2), 865–872. DOI 10.15244/pjoes/61007. [Google Scholar] [CrossRef]
22. Pieters, A. J., El Souki, S. (2005). Effects of drought during grain filling on PS II activity in rice. Journal of Plant Physiology, 162(8), 903–911. DOI 10.1016/j.jplph.2004.11.001. [Google Scholar] [CrossRef]
23. Ahmed, C. B., Rouina, B. B., Sensoy, S., Boukhris, M., Abdallah, F. B. (2009). Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environmental and Experimental Botany, 67(2), 345–352. DOI 10.1016/j.envexpbot.2009.07.006. [Google Scholar] [CrossRef]
24. Ashraf, M., Nawazish, S., Athar, H. U. R. (2007). Are chlorophyll fluorescence and photosynthetic capacity potential physiological determinants of drought tolerance in maize (Zea mays L.). Pakistan Journal of Botany, 39(4), 1123–1131. [Google Scholar]
25. Kauser, R., Athar, H. U. R., Ashraf, M. (2006). Chlorophyll fluorescence: A potential indicator for rapid assessment of water stress tolerance in Canola (Brassica Napus L.). Pakistan Journal of Botany, 38, 1501–1509. [Google Scholar]
26. Ali, A. A., Areej, A. A., Fahad, A. A. Y., Shahira, S. R., Ahmed, A. D. et al. (2017). Effect of combined biotic and abiotic stress on some physiological aspects and antioxidant enzymatic activity in mungbean (Vigna radiata L.). African Journal of Agricultural Research, 12(9), 700–705. DOI 10.5897/AJAR2016.12084. [Google Scholar] [CrossRef]
27. Bajji, M., Lutts, S., Kinet, J. M. (2001). Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Science, 160(4), 669–681. DOI 10.1016/S0168-9452(00)00443-X. [Google Scholar] [CrossRef]
28. Hsu, Y. T., Kao, C. H. (2003). Changes in protein and amino acid contents in two cultivars of rice seedlings with different apparent tolerance to cadmium. Plant Growth Regulation, 40(2), 147–155. DOI 10.1023/A:1024248021314. [Google Scholar] [CrossRef]
29. Cechin, I., Corniani, N., De Fátima Fumis, T., Cataneo, A. C. (2008). Ultraviolet-B and water stress effects on growth, gas exchange and oxidative stress in sunflower plants. Radiation and Environmental and Biophysics, 47(3), 405–413. DOI 10.1007/s00411-008-0167-y. [Google Scholar] [CrossRef]
30. Ahmadizadeh, M. (2013). Physiological and agro-morphological response to drought stress. Middle-East Journal of Science and Research, 13(8), 998–1009. DOI 10.5829/idosi.mejsr.2013.13.8.3531. [Google Scholar] [CrossRef]
31. Ashraf, M., Iram, A. (2005). Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora-Morphology, Distribution, Functional Ecology of Plants, 200(6), 535–546. DOI 10.1016/j.flora.2005.06.005. [Google Scholar] [CrossRef]
32. Nawaz, F., Ahmad, R., Ashraf, M. Y., Waraich, E. A., Khan, S. Z. (2015). Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicology and Environmental Safety, 113, 191–200. DOI 10.1016/j.ecoenv.2014.12.003. [Google Scholar] [CrossRef]
33. Guardado-Félix, D., Serna-Saldivar, S. O., Cuevas-Rodríguez, E. O., Jacobo-Velázquez, D. A., Gutiérrez-Uribe, J. A. (2017). Effect of sodium selenite on isoflavonoid contents and antioxidant capacity of chickpea (Cicer arietinum L.) sprouts. Food Chemistry, 226, 69–74. DOI 10.1016/j.foodchem.2017.01.046. [Google Scholar] [CrossRef]
34. Harder, H. J., Carlson, R. E., Shaw, R. H. (1982). Corn grain-yield and nutrient response to foliar fertilizer applied during grain fill. Agronomy Journal, 74(1), 106–110. DOI 10.2134/agronj1982.00021962007400010027x. [Google Scholar] [CrossRef]
35. Mosali, J., Desta, K., Teal, R. K., Freeman, K. W., Martin, K. L. et al. (2006). Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. Journal of Plant Nutrition, 29(12), 2147–2163. DOI 10.1080/01904160600972811. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |