
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.012862
ARTICLE
In Vitro Propagation of Agave guiengola Gentry Using Semisolid Medium and Temporary Immersion Bioreactors
1Departamento de Química, Centro de Ciencias Básicas, Universidad Autónoma de Aguascalientes, Aguascalientes, 20131, México
2Laboratorio de Biología Molecular Vegetal, Centro Universitario de la Ciénega, Universidad de Guadalajara, Ocotlán, 47820, México
*Corresponding Author: Eugenio Pérez-Molphe-Balch. Email: eperezmb@gmail.com
Received: 15 July 2020; Accepted: 09 October 2020
Abstract: Agave guiengola Gentry is an endemic plant from a very small locality in Oaxaca, Mexico. Its conservation status is fragile and can rapidly worsen. Because of its scarcity, this agave has been used solely for ornamental purposes, but it could have other uses if more plants were available. In vitro propagation by enhanced axillary sprouting from stem segments was attained using Murashige and Skoog Basal Medium (MS) as well as basal medium supplemented with cytokinins 6-Benzylaminopurine (BA) or 6-(γ,γ-Dimethylallylamino)purine (2iP). The best treatment for shoot induction in semisolid medium consisted in MS supplemented with 2 mg l–1 BA, obtaining a mean of 3.7 shoots per explant. Other interesting responses were observed, such as nodular callus induction using combinations of BA and 2,4-Dichlorophenoxyacetic acid (2,4-D); root induction without Plant Growth Regulators (PGR); and generation of shoot clusters. These clusters constituted an excellent explant for micropropagation in temporary immersion bioreactors, obtaining a propagation rate of 43 shoots per explant with 1 min immersion and 6 h immersion frequencies. All new plants rooted and survived the transfer to soil. This study developed an in vitro propagation scheme to produce individuals that can be used either for reforestation, economical purposes, or to carry out studies in this species to assess its full potential, avoiding exploitation from wild plants.
Keywords: Agavaceae; temporary immersion systems; callus induction; micropropagation
The genus Agave is native to the American continent. It comprises 200 species, of which 150 of them are endemic to Mexico, which is its center of origin and diversification [1]. These plants are adapted to arid and semiarid environments [1], and therefore to hostile conditions, such as low water availability, intense solar radiation, and extreme temperatures. Agaves are an outstanding part of arid ecosystems, and have important relations with other organisms, especially pollinators such as bats, bees, wasps, ants, among others [1]; conferring them an undisputable ecological importance. On the other hand, agaves were used by man in many ways since ancient times, and they are currently a source of food, alcoholic beverages like tequila and mezcal, fibers and other raw materials, and have great potential in the production of sweeteners and biofuels [1,2].
A. guiengola is micro endemic to the Guiengola limestone formation at the Tehuantepec Isthmus in Oaxaca [3]; this location is not easily accessible, nevertheless, is such a small area that any disturbance could rapidly result in a devastating effect on natural populations. Currently, A. guiengola is used just for ornamental purposes and is considered threatened according to Mexican law [4]. Its potential for economical applications has never been fully assessed, possibly due to its inaccessibility and small population; thence is necessary to carry out studies to have a better knowledge of A. guiengola, since is crucial to avoid overexploitation of wild individuals. The development of efficient systems for its massive propagation is an indispensable requirement for the conservation and rational use of this species.
In vitro plant tissue culture may be part of comprehensive management strategies to preserve threatened and endangered species [5]. Micropropagation allows obtaining individuals that can be used for reforestation; and makes it possible to obtain whole plants, organs, or tissues for economical exploitation, avoiding the need to predate on natural populations. Micropropagation in the Agave genus was studied since the late 1980s within several species. Robert et al. [6] reported A. fourcroydes in vitro regeneration from callus explants using 2,4-Dichlorophenoxyacetic acid (2,4-D) and 6-Benzylaminopurine (BA) [6]. A. tequilana was also regenerated from callus or stem segments using 2,4-D and BA [7,8] or Indole-3-Butyric Acid (IBA) and Kinetin [9]. In vitro regeneration from basal stem segments was reported in several species of the genus, examples of which are A. cantala [10], A. cupreata, A. difformis, A. karwinskii, A. obscura, A. potatorum [11]; and A. tequilana, A. salmiana sub. crassispina, A. durangensis, A. oscura, A. pigmaea, and A. victoriae-raeginae [8]. Other types of explants used were rizome in A. sisalana [12] and complete seedlings in A. salmiana [13]. Also, efficient systems for in vitro mass propagation were reported for A. tequilana [9,14], A. grijalvensis [15], and A. angustifolia [16]. These works used different cytokinins for Agave propagation, but only Domínguez et al. [11] reported the use of 6-(γ,γ-Dimethylallylamino)purine (2iP).
It has been well established that the use of bioreactors presents several advantages over conventional in vitro cultures on semisolid media; such as larger nutrient and Plant Growth Regulators (PGR) availability, increased oxygenation rate, and decreased apical dominance, among others; all of which often results in a higher growth or propagation rate [17]. For differentiated plant organ culture is recommended to use bioreactors with low shear stress and an efficient mass transfer [18]. Both qualities are present in temporary immersion bioreactors (TIBs) [19]. The use of TIBs was reported in A. angustifolia [20]; plantlets cultured in temporary immersion BioMINTTM bioreactors exhibited a better development of stomata and wax deposition compared to plantlets cultured in semi-solid media or permanent immersion.
The aim of this work was to develop efficient in vitro propagation systems for A. guiengola, which is one of the scarcest and most threatened species in the Agave genus. This propagation was achieved both on semisolid culture media and in TIBs, although, the greatest efficiency was obtained by combining both culture systems. As far as we know, there are no previously reported scientific papers about in vitro culture and propagation of this species. Despite the fact that TIBs technology has an increasing relevance in mass propagation of several plant species, there are very few reports using this technology within the Agave genus.
Capsules with viable seeds were collected in April 2016 in Oaxaca, Mexico. These seeds were disinfected and in vitro germinated to obtain axenic seedlings. For this, seeds were washed five times with 1% antiseptic soap with 0.1% benzalkonium chloride (Dermocleen®) in water, then disinfected for 1 min in 70% ethanol, 15 min in 1% sodium hypochlorite in water, and rinsed four times under aseptic conditions with sterile distilled water. Seeds were germinated in culture vessels containing Murashige and Skoog Basal Medium (MS) kept at 25 ± 2°C under white light with a 16/8-h photoperiod (led lamps, 55 μmol m–2 s–1).
Basal medium consisted of MS basal media prepared with analytical grade reagents, supplemented with 30 g l–1 sucrose. When treatments required PGR, these were added at this point; then pH was adjusted to 5.7 ± 0.2 with NaOH or HCl 1 M. For semisolid medium, 9 g l–1 agar (Sigma-Aldrich, St. Louis, MO, USA) were added. Then the media were distributed in glass culture vessels or twin tank bioreactors and autoclaved at 120°C for 20 min.
2.3 Preliminary Multiplication
This step was carried out to obtain enough material for subsequent experiments and to assess the best treatment with cytokinin for shoot multiplication on semisolid media. Explants were taken from the in vitro germinated seedlings by excising leaves and roots, leaving only the stem that contains the apical and lateral meristems. Four treatments were used, consisting of basal MS media supplemented with 1 and 2 mg l–1 BA, and 1 and 2 mg l–1 (2iP), plus a control without PGR. Ten explants were placed in each treatment and maintained for 60–65 days at a constant temperature of 25 ± 2°C under white light with a 16/8-h photoperiod (led lamps, 55 μmol m–2 s–1). The experiment was conducted three independent times, with a completely random experimental design. Data were analyzed with the Minitab® software, v. 19.2020.1; ANOVA analyses were done, and means were compared using a Tukey’s test.
2.4 Effect of Auxin and Citokinin Combinations
Treatments consisted of semisolid basal media plus BA (0, 1, 2 and 3 mg l–1) and 2,4-D (0, 0.1, 0.5 and 1.0 mg l–1) in a 4 × 4 factorial design. We used ten explants per treatment, with three repetitions. The explants were taken from the shoots generated in the preliminary multiplication step, eliminating the leaves, and leaving only the stem. After 60 d of culture, the number of explants that produced shoots, roots, regular callus, and nodular callus were calculated, as well as the number of shoots per explant, and oxidized or necrosed explants. Culture conditions were the same as described above. Data were analyzed with the Minitab® software, v. 19.2020.1; ANOVA analyses were done, and means were compared using a Tukey’s test. Additionally, the data generated in this experiment was used to perform a response surface analysis with a full quadratic model.
2.5 Experiments in Temporary Immersion System
The bioreactors used were of the twin-container design, as described by Monja-Mio et al. [20], which consisted in two 1000 ml flasks for each bioreactor. The volume of culture medium in the reservoir flask was 100 ml. Immersion frequency was 1 min every 12 h. To assess the best type of explant and optimal cytokinin level to be used in the temporary immersion system, one individual shoot or one shoot cluster were placed in each container of the bioreactor, and liquid MS supplemented with 1 mg l–1 BA or 2iP was employed. The multiplication rate was determined after 60 days of culture.
The previous experiment allowed to determine the best PGR. To determine the optimal immersion frequency, one shoot cluster was placed in each container and 1 mg l–1 BA was added to the liquid medium. The compared immersion frequencies were 6, 12 and 24 h. The control treatment consisted of explants cultured on semisolid medium supplemented with 1 mg l–1 BA. The multiplication rate was determined after 90 days; shoot length was measured and hyperhydrated shoots were counted. Data were analyzed with a Kruskal–Wallis test and a Dunn’s multiple comparison test using the GraphPrism® software.
After micropropagation in the temporary immersion system, shoots were excised from the explants and transferred to semisolid medium without PGR for rooting. Plants propagated on semisolid medium were used as control. After two months, the plants developed roots, and once they reached at least 2 cm in length, it was possible to initiate the hardening process.
First, the protocol described by Domínguez et al. [11] for other agave species was used with small modifications; it consisted of gradually opening the lid of the vessel throughout one week to allow the plants to adapt to the exterior environment. Plants were extracted from culture vessels, the remaining medium was gently washed away from the roots, and the plants were placed in 200 cm3 pots, filled with pot soil and sand 1:1 v/v. The survival rate was assessed after two weeks and revised again after 8 months.
A second hardening experiment was conducted. Rooted plants were immediately extracted from the culture vessels; the roots were gently washed with tap water, and the plants were placed in 50 cavities plastic trays and covered with a plastic bag. After two days, plants were transferred to soil; two treatments were tested, one consisted in Sphagnum peat-moss (Premier®) and the second on a mixture of Sphagnum peat-moss (Premier®) and vermiculite 1:1 v/v. Substrate was placed into a double-layer seedling tray; the upper tray was divided into 50 cavities, 90 cm3 each , and provided with a draining hole. The lower tray was not divided and had no draining holes, and it was kept half filled with tap water. The trays were placed in a transparent plastic bag; after one week the bag was lightly perforated, and the bag was completely retired after two months. Then, the survival rate and other measurements were registered.
The germination rate was 84%, this 60 d after disinfection and in vitro inoculation of the seeds. The generated axenic seedlings, 4 to 6 cm high, were used as a source of explants for the first shoot multiplication experiments.
For shoot multiplication, treatments with BA and 2iP presented less-differentiated shoot generation in comparison to the control (Tab. 1). All explants on medium without PGR developed roots (Fig. 1b), as well as 73.7 % of the explants treated with 1 or 2 mg l–1 2iP. Callus formation was observed in some BA-treated explants, and nearly 80% on 2 mg l–1 2iP-treated explants. Interestingly, cytokinins, particularly BA, induced the formation of shoot clusters. These were formed by compact groups of approximately 50–60 poorly differentiated buds (Fig. 1c).
Table 1: Effect of 6-Benzylaminopurine (BA) and 6-(γ,γ-Dimethylallylamino)purine (2iP) on shoot generation in A. guiengola

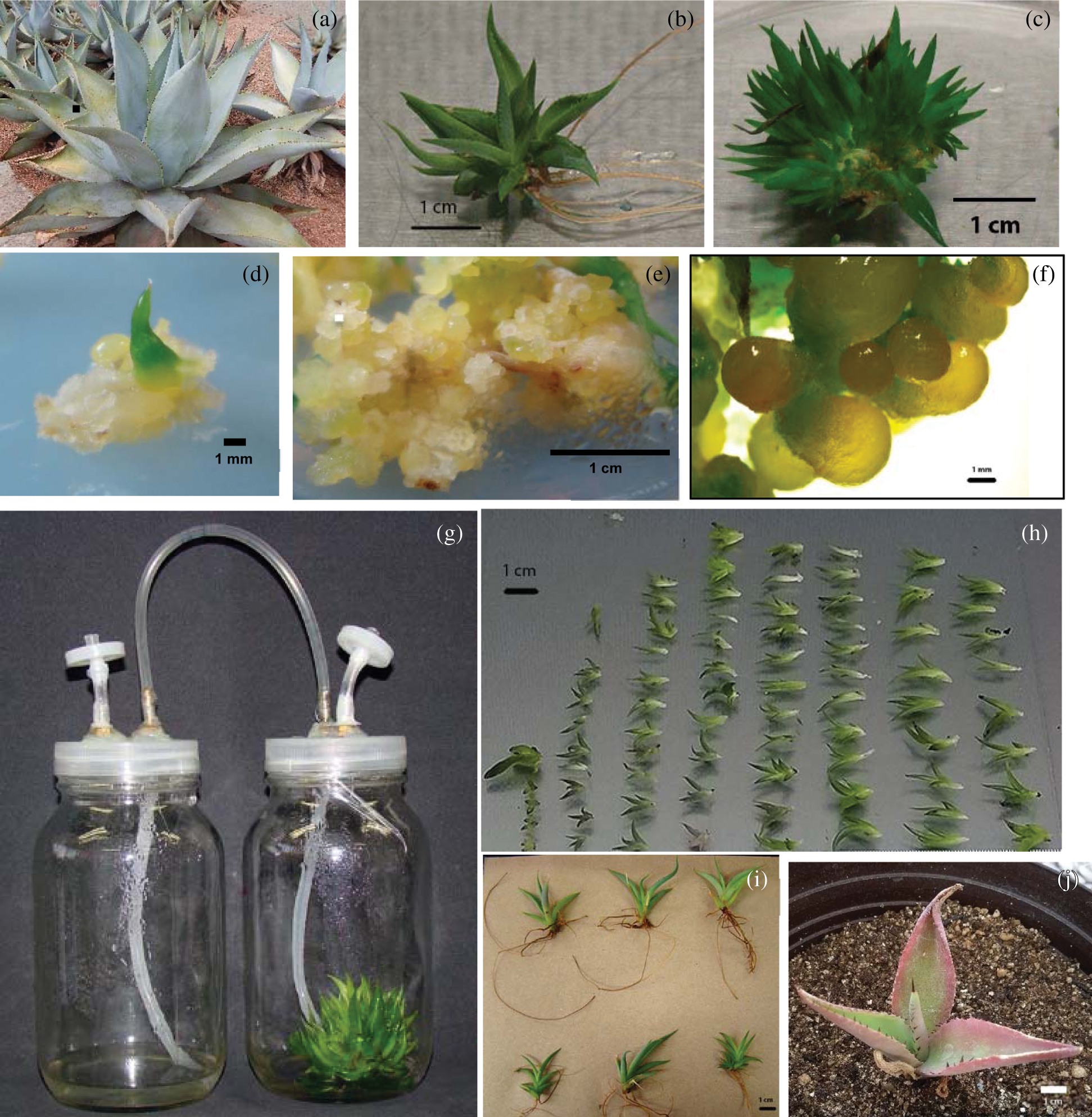
Figure 1: Different characteristics of a A. guiengola-in vitro propagation system. (a) Adult plants; (b) differentiated shoot with well-developed roots from control treatment; (c) shoot conglomerate generated on semisolid medium with 1 mg l–1 BA; (d) compact callus generated on semisolid MS with 1 mg l–1 2,4-D; (e) nodular callus observed on semisolid medium supplemented with 1 mg l–1 BA and 1 mg l–1 2,4-D and (f) globular structures observed on stereo microscope; (g) employed temporary immersion system; (h) shoots generated by a single explant inoculated in a TIBs, with an immersion frequency of 12 h; (i) shoots generated in TIB and rooted on semisolid medium without PGR; (j) shoot generated in TIB, after 8 months of being transferred to soil
Regarding the response to the cytokinin/auxin combination, several authors reported that cytokinins combined with small concentrations of auxins resulted in more vigorous shoots in some Agave species [6,7,8,10,13,21,22]. However, in A. guiengola, combinations of BA/2,4-D and 2,4-D alone always resulted in callus tissue induction (Tab. 2, Figs. 2d and 2e); the combination of 2 mg l–1 BA and 0.1 mg l–1 2,4-D generated the highest amount of callus. Some treatments, particularly 1 mg l–1 BA combined with 0.1, 0.5 and 1.0 mg l–1 2,4-D, produced nodular calluses that exhibit structures with a globose aspect that may be somatic embryos (Figs. 1e and 1f). Embryogenic calluses were reported for such characteristics using 2,4-D and BA in A. tequilana, A. sisalana and A. angustifolia [23]. Synthetic auxins, in particular 2,4-D, induce the development of poorly differentiated tissues but it antagonize development of organized structures. For this reason, they have been used to induce callus and stimulate the early stages of somatic embryogenesis. However, this mechanism of action inhibits shoot regeneration. This has been reported for other Agave species [24] and was confirmed in the experiment carried out with A. guiengola (Figs. 2a, 2c and 2d). Interestingly, the induction of nodular callus required the simultaneous presence of 2,4-D and BA (Fig. 2d). This indicates a synergy between the two types of PGR.
Table 2: Morphogenetic responses in A. guiengola to cytokinin and auxin combinations


Figure 2: Contour response surface graphs of influences of 6-Benzylaminopurine (BA) and 2,4-Dichlorophenoxyacetic acid (2,4-D) on the in vitro morphogenic responses of A. guiengola. (a) Shoots produced per explant; (b) probability of obtaining shoot conglomerates; (c) probability of obtaining regular callus, and; (d) probability of obtaining nodular callus. BA and 2,4-D concentrations in mg l–1
Treatments with BA alone generated shoots in A. guiengola explants (Tab. 2, Fig. 2a); the best treatment was 1 mg l–1 BA, with a mean of 3.7 shoots per explant; this result is similar to those reported for other species, such as in A. salmiana sub. crassispina, in which Ramírez-Malagón et al. [8] reported a mean of 3 shoots per explant, and Angeles-Espino et al. [9] reported 3.67 in A. tequilana. However, Puente-Garza et al. [13] reported 14 shoots per explant in A. salmiana; Ramírez-Malagón et al. [8] reported 12 shoots per explant in A. tequilana and 12.8 in A. oscura. Nevertheless, we observed that some treatments do not produced well-differentiated individual shoots but shoot clusters (Fig. 2b). In these, the shoots were too small and poorly differentiated, so it was not possible to separate them for rooting, but it was necessary to take further steps to attain their growth and full differentiation. Since an average of 3 shoots per explant were fully developed and the rest remained too small, it is possible that the first ones inhibited the development of the rest. As Gahan et al. [25] pointed out, the first meristem formed in vitro may inhibit the development of further meristems.
In this experiment, oxidation and necrosis of some explants exposed to the highest concentrations of PGR were also observed (Tab. 2). This may indicate that the use of higher concentrations would be toxic to tissue.
In many plant species, auxins are necessary for root induction [26], increasing the cost and time during the rooting stage; auspiciously, in A. guiengola explants, roots were observed almost exclusively in the control treatment (Fig. 1b), therefore, in this species it was not necessary to employ exogenous PGR to develop an appropriate in vitro root system.
For the experiments in TIBs, individual shoots and shoot clusters were used as explants, in order to attain A. guiengola propagation trough sprouting of lateral meristems or by direct organogenesis.
In the semisolid system, 2iP induced bigger and more differentiated shoots, but BA produced shoot clusters more often. In TIBs, BA had a better response; apparently, BA added to liquid medium does not generate new shoots but induces enlargement and development of small undifferentiated ones in a shorter period (two to three months). Clearly, shoot clusters are better than individual shoots (Tab. 3) to use as explants in TIBs.
Table 3: Effect of explant type and cytokinin in a temporary immersion system for A. guiengola

For the development of protocols using TIBs, is crucial to assess the optimal immersion times and frequencies. Agaves are adapted to low water availability; therefore, the shortest possible immersion time, 1 min, was employed (Tab. 4). Longer immersion times would favor the intake of nutrients but would also increase the rate of hyperhydration. There were no statistical differences between immersion times of 6, 12 and 24 h and the control on semisolid medium. However, shoots obtained at a 6 h immersion frequency achieved a significant increase in length than shoots from other treatments and were morphologically best differentiated. This probably due to a higher availability of nutrients and cytokinin with respect to the other frequencies tested; only 12.2% shoots were hyperhydrated (Tab. 4). Ríos-Ramírez et al. [16] also used shoot clusters as explants in A. americana. They attained 45.2 shoots per explant; and they achieved their development using BA.
Table 4: Effect of immersion frequency in A. guiengola. shoot conglomerates cultivated on semisolid medium were used as control

After the shoots were rooted, hardened and transferred to soil as described previously [11], the plants obtained from TIB had a survival rate of 81.3% after 14 days, while plants obtained from semisolid cultures had a 77.3% survival rate in the same period; there were no statistical differences. However, after 8 months, the survival rate dropped to 45.8% and 57.1%, respectively.
Due to low survival rates, the second hardening protocol was completed. After 100 d, survival rates of TIB-generated plants were 100% in peat-moss and 80% in peat-moss and vermiculite. Plants from semisolid cultures in both treatments had a survival rate of 80%. The natural habitat of A. guiengola has a warm sub-humid climate, with temperatures between 10°C and 20°C, and an annual precipitation of 600–1000 mm [27], which is more temperate and humid than the habitats of other agaves species, hence the need for a higher environmental humidity for young plants. In vitro culture conditions cause some morphological and physiological alterations, and plantlets exhibit juvenile leaves, impaired stomatic and photosynthetic mechanisms and poor water management, among other deficiencies [28]. Therefore, during the first 30 d after hardening 80% of the plantlets lost some leaves, and the remaining ones had a slight dehydrated appearance; in consequence their size decreased. During the next 30 d there was a slight increase in size, but more important, the emergence of new leaves of healthier appearance was observed, which is clear evidence of the successful hardening of the plantlets.
Eight-month plants obtained from TIB and semisolid medium increased their diameter 276.8% and 206.8%, respectively. Leaves were ovate-lanceolate; terminal spines were dark brown, not decurrent and rounded above and below; the leaf margins presented dark brown teeth, four per cm (Fig. 1g); all of these are consistent with characteristics reported in adult wild plants [3]. Leaves were light green, some of them with reddish margins, and not light gray or white-glaucous as reported for wild individuals [3]. Plants germinated in potted soil from the same seed batch and approximately same age exhibited the same characteristics than in vitro propagated plants, except reddish margins (data not shown). Nevertheless, some species acquire its characteristic coloration until maturity, and color is also highly dependent of light intensity and composition, which could be quite different between the greenhouse and the natural environment. All aspects considered, the morphology of plants micropropagated in temporary immersion bioreactors after hardening was normal.
The use of BA or 2iP on semisolid medium in A. guiengola did not result in a higher generation of well differentiated shoots than the control treatment. However, the use of these cytokinins on semisolid medium, particularly BA, induced the generation of shoot clusters, which are shoot aggregates too small and poorly differentiated, hence it is not possible to separate nor count them. Using BA on these shoot clusters in TIBs instead of semisolid medium, small and indistinguishable shoots were capable of growth and development at higher rates, producing in average 43 shoots per explant. The culture conditions were MS supplemented with 1 mg l–1 BA, 1 min immersion time and 6 h immersion frequency. The shoots obtained in TIBs were rooted on semisolid medium without PGR, and the use of an efficient protocol for hardening resulted in a 100% survival rate of the plantlets transferred to soil; and they exhibited a normal morphology. Hence, we propose that combining several techniques in different stages leads to a more efficient propagation scheme.
Acknowledgement: Thanks to Martha Evelia Pérez Reyes and Adilene Avila Galván for their valuable technical support in plant tissue culture experiments. Thanks to Alberto Isaac Reyes Silva for his suggestions and technical support in greenhouse experiments.
Funding Statement: This work was supported financially by project AGS-2015-02-01-267656, Fondo Mixto CONACYT-Gobierno del Estado de Aguascalientes, and project PIBT-18-2, Universidad Autónoma de Aguascalientes, México.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. García-Mendoza, A. J. (2007). Los agaves de México. Ciencias, 87, 14–23. [Google Scholar]
2. Granados-Sánchez, D. (1993). Los agaves de México. México: Universidad Autónoma de Chapingo. [Google Scholar]
3. Gentry, H. S. (1982). Agaves of Continental North America. Tucson: The University of Arizona Press. [Google Scholar]
4. Norma Oficial Mexicana NOM-059-SEMARNAT-2010 (2010). Protección ambiental-especies nativas de México de flora y fauna silvestres-categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-lista de especies en riesgo. Diario oficial, México. [Google Scholar]
5. Loyola-Vargas, V. M., Ochoa-Alejo, N. (2012). An introduction to plant cell culture: The future ahead. Loyola-Vargas, V. M., Ochoa-Alejo, N., (eds.Plant cell culture protocols, pp. 1–8. New York: Humana Press. [Google Scholar]
6. Robert, M. L., Herrera, J. L., Contreras, F., Scorer, K. N. (1987). In vitro propagation of Agave fourcroydes Lem (Henequen). Plant Cell, Tissue and Organ Culture, 8(1), 37–48. DOI 10.1007/BF00040731. [Google Scholar] [CrossRef]
7. Valenzuela-Sánchez, K. K., Juárez-Hernández, R. E., Cruz-Hernández, A., Olalde-Portugal, V., Valverde, M. E. et al. (2006). Plant regeneration of Agave tequilana by indirect organogenesis. In Vitro Cellular & Developmental Biology–Plant, 42, 336–340. DOI 10.1079/IVP2006788. [Google Scholar] [CrossRef]
8. Ramírez-Malagón, R., Borodanenko, A., Pérez-Moreno, L., Salas-Araiza, M. D., Núñez-Palenius, H. G. et al. (2008). In vitro propagation of three Agave species used for liquor distillation and three for landscape. Plant Cell, Tissue and Organ Culture, 94(2), 201–207. DOI 10.1007/s11240-008-9405-x. [Google Scholar] [CrossRef]
9. Angeles-Espino, A., Valencia-Botin, A. J., Virgen-Calleros, G., Ramírez-Serrano, C., Paredes-Gutiérrez, L. et al. (2012). Micropropagation of agave (Agave tequilana Weber Var. Azul) through axillary buds. Tropical and Subtropical Agroecosystems, 15, 693–698. [Google Scholar]
10. Binh, L. T., Muoi, L. T., Oanh, H. T. K., Thang, T. D., Phong, D. T. (1990). Rapid propagation of agave by in vitro tissue culture. Plant Cell, Tissue and Organ Culture, 23(1), 67–70. DOI 10.1007/BF00116091. [Google Scholar] [CrossRef]
11. Domínguez-Rosales, M. S., Alpuche-Solís, A. G., Vasco-Méndez, N. L., Pérez Molphe-Balch, E. (2008). Efecto de citocininas en la propagación in vitro de agaves mexicanos. Revista Fitotecnia Mexicana, 31, 317–322. [Google Scholar]
12. Das, T. (1992). Micropropagation of Agave sisalana. Plant Cell Tissue Organ Culture, 31, 253–255. DOI 10.1007/BF00036233. [Google Scholar] [CrossRef]
13. Puente-Garza, C. A., Gutiérrez-Mora, A., García-Lara, S. (2015). Micropropagation of Agave salmiana: Means to production of antioxidant and bioactive principles. Frontiers in Plant Science, 6(e42946), 1026. DOI 10.3389/fpls.2015.01026. [Google Scholar] [CrossRef]
14. Barreto, R., Nieto-Sotelo, J., Cassab, G. I. (2010). Influence of plant growth regulators and water stress on ramet induction, rosette engrossment, and fructan accumulationin Agave tequilana Weber var. Azul. Plant Cell, Tissue and Organ Culture, 103(1), 93–101. DOI 10.1007/s11240-010-9758-9. [Google Scholar] [CrossRef]
15. Santíz, J. A., Rincon-Rosales, R., Gutierrez-Miceli, F. A. (2012). In vitro propagation of Agave grijalvensis B. Ullrich, an endemic species from Chiapas under special protection. Gayana Botanica, 69, 23–30. [Google Scholar]
16. Ríos-Ramírez, S. C., Enriquez-del Valle, J. R., Rodríguez-Ortiz, G., Ruiz-Luna, J. (2017). Benzylaminopurine and indol-3-acetic acid concentrations in in vitro proliferation of Agave angustifolia adventitious shoots. Ciencia e Investigación Agraria, 44, 285–294. DOI 10.7764/rcia.v44i3-1810. [Google Scholar] [CrossRef]
17. Takayama, S., Akita, M. (2005). Practical aspects of bioreactor application in mass propagation of plants. Hvoslef-Eide, A. K., Preil, W., (eds.Liquid culture systems for in vitro plant propagation, pp. 6–78. New Dordrecht: Springer. 10.1007/1-4020-3200-5_4. [Google Scholar] [CrossRef]
18. Georgiev, M. I. (2014). Design of bioreactors for plant cell and organ cultures. Paek, K., Murthy, H. N., Zhong, J., (eds.Production of biomass and bioactive compounds using bioreactor technology, pp. 3–15. Dordrecht: Springer. DOI 10.1007/978-94-017-9223-3_1. [Google Scholar] [CrossRef]
19. Escalona, M., Lorenzo, J. C., Gonzalez, B., Daquinta, M., Gonzalez, J. L. et al. (1999). Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Reports, 18(9), 743–748. DOI 10.1007/s002990050653. [Google Scholar] [CrossRef]
20. Monja-Mio, K. M., Barredo-Pool, F., Herrera-Herrera, G., Esqueda-Valle, M., Robert, M. L. (2015). Development of the stomatal complex and leaf surface of Agave angustifolia Haw. ‘Bacanora’ plantlets during the in vitro to ex vitro transition process. Scientia Horticulturae, 189, 32–40. DOI 10.1016/j.scienta.2015.03.032. [Google Scholar] [CrossRef]
21. Chen, Y., Chen, X., Hu, F., Yang, H., Yue, L. et al. (2014). Micropropagation of Agave americana. HortScience, 49(3), 320–327. DOI 10.21273/HORTSCI.49.3.320. [Google Scholar] [CrossRef]
22. Reyes-Zambrano, S. J., Lecona-Guzman, C. A., Barredo-Pool, F. A., Ambrosio, C. J. D., Abud-Archila, M. et al. (2016). Plant growth regulators optimization for maximize shoots number in Agave americana L. by indirect organogenesis. Gayana Botanica, 73(1), 124–131. DOI 10.4067/S0717-66432016000100014. [Google Scholar] [CrossRef]
23. Monja-Mio, K. M., Rober, M. L. (2016). Somatic embryogenesis in Agave: An overview. In: Loyola-Vargas, V. M., Ochoa-Alejo, N., (eds.Somatic embryogenesis: fundamental aspects and applications, pp. 283–296. Cham: Springer. DOI 10.1007/978-3-319-33705-0_1. [Google Scholar] [CrossRef]
24. Tejavathi, D. H., Rajanna, M. D., Sowmya, R., Gayathramma, K. (2007). Induction of somatic embryos from cultures of Agave vera-cruz Mill. In Vitro Cellular & Developmental Biology—Plant. 43, 423–428, 10.1007/s11627-007-9088-8. [Google Scholar] [CrossRef]
25. Gahan, P. B., George, E. F. (2008). Adventitious regeneration. In: George, E. F., Hall, M. A., De Klerk, G., (eds.Plant propagation by tissue culture, pp. 355–401. Third edition, Netherlands: Springer. [Google Scholar]
26. Pérez-Molphe-Balch, E. M., Ramirez, M. R., Nuñez, P. H., Ochoa, A. N. (1999). Introducción al cultivo de tejidos vegetales. First edition. Aguascalientes: Universidad Autónoma de Aguascalientes. [Google Scholar]
27. INEGI (2005). Prontuario de información geográfica municipal de los Estados Unidos Mexicanos. Oaxaca: Santo Domingo Tehuantepec. http://www3.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/20/20515.pdf. [Google Scholar]
28. Nowak, J., Shulaev, V. (2003). Priming for transplant stress resistance in in vitro propagation. In Vitro Cellular & Developmental Biology Plant, 39, 107–124. DOI 10.1079/IVP2002403. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |