
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014218
ARTICLE
Rapid Propagation of Rhynchostylis retusa in Vitro
1Yunnan Breeding and Cultivation Research and Development Center of Endangered and Daodi Chinese Medicinal Materials, Yunnan University of Chinese Medicine, Kunming, 650000, China
2Chemistry Department, Guizhou University of Traditional Chinese Medicine, Guiyang, 550000, China
3Yunnan Academy of Agricultural Science, Kunming, 650000, China
4Qiucheng Breeding Company Ltd., Lijiang, 674100, China
*Corresponding Author: Hengyu Huang. Email: hhyhhy96@163.com
Received: 10 September 2020; Accepted: 09 December 2020
Abstract: An efficient regeneration system of Rhynchostylis retusa was established to provide technical reference for the application of tissue culture tube seedlings in production. The mixtures of callus and protocorm from aseptic germination were used as explants. The optimal media of each stage was selected for callus proliferation, protocorm occurrence and growth, rejuvenation and rooting via a single, complete combination and orthogonal experiment. The results showed that the optimal medium for callus proliferation, protocorms occurrence and growth was 1/2 Murashige and Skoog (MS) medium adding 50 g·L−1 banana puree, 0.1 mg·L−1 α-naphthaleneacetic acid (NAA), 1.5 mg·L−1 6-benzylaminopurine (6-BA) and 1.0 mg·L−1 kinetin (KT) with 17.33 proliferation coefficient of callus and 19.63 occurrence coefficient of buds after 90 days. Then the buds occurred from protocorm were cultured on 1/2 MS medium including 100 g·L−1 banana puree, 1.0 mg·L−1 NAA, 2.0 mg·L−1 6-BA and 0.05 mg·L−1 KT, in which the proliferation coefficient of callus was 10.32 and occurrence coefficient of buds reached 17.87. In the further subculture, the same medium was simultaneously used for callus proliferation, protocorm occurrence and bud growth. The plantlets developed roots in 1/2 MS medium containing 70 mL·L−1 coconut water and 1.5 mg·L−1 NAA with 100% rooting rates after 90 days. The survival rate was more than 90% after domestication and transplantation. This regeneration protocol will provide technique foundation for protecting wild resource and developing artificial cultivation.
Keywords: Rhynchostylis retusa; callus; protocorm; proliferation coefficient; rapid propagation
Rhynchostylis is a perennial epiphytic herb of Orchidaceae with about 6 species, mainly distributed in Tropical Asia. There are two species in China, namely R. gigantea and R. retusa [1]. The floral characteristics of this genus contains light color with perfume, plump inflorescence and pendulous or upright, which looks like a furry fox tail. Therefore, its commodity is commonly known as foxtail orchid with high ornamental value. Furthermore, the color of R. retusa is polymorphism, including red, pink, white, blue, orange and other colors [2]. In China, owing to the unique flower shape, long flowering period and the opening around the Spring Festival can be used as a spring festival gift, deeply loved by people.
R. retusa is found in the south of China, it is also distributed in Sri Lanka, India, Bangladesh, Laos, Vietnam, Cambodia, Malaysia, Indonesia and Philippines Fig. 1, commonly grows in open forest or forest margin trunks ascending up to 310–1400 elevation [1]. The traditional breeding methods of R. retusa are division propagation and seed propagation. Although the division propagation can maintain the parent traits, it has low efficiency, only 1–3 times of proliferation and a long cycle, which is not suitable for factory production [3]. Besides, the seeds of most species of Orchidaceae are small and lack cotyledon and endosperm to cause incomplete embryo development. Usually, under field conditions, it is necessary to be with related symbiotic bacteria in order to germination, which results the long germination time, low germination rate, low seedling rate, and generally 4–5 years or even longer from seed germination to flowering plants that can conduct trait identification. Since 1960, Morel [4] from France used the plant tissue culture technology to induce protocorm from the stem tip meristem of orchids and finally differentiate into the intact plants. The artificial propagation mode of modern orchids is mostly tissue culture, which can rapidly propagate excellent materials, tens to hundreds of thousands of times faster than traditional methods. Meanwhile, this method can overcome problems of distant incompatibility and stunted development of hybrid embryos, and not restricted by season, region and climate [5].
At present, some reports established tissue culture through leaves, stem tips, stem-segments and roots etc. as explants in the study of R. retusa [6–8]. However, some studies have pointed out that the effective path was the rapid propagation of seedlings for the regeneration, rational development and utilization of endangered orchid plant resources, and the core of the technology was the aseptic germination of seeds [5,9,10]. Aseptic culture of seeds is a tedious process, and its development process is crudely as follows: the seed expands with water, and the chalazal end of the embryo distends the seed coat longitudinally to form the protocorm, which then forms a protocorm with terminal or lateral meristem. The rhizoid first appears from the tip of the protocorm and then overgrows the entire surface. Next, a cotyledon forms on one side of the apical meristem. Afterward, true leaves begin to appear, protocorm elongate into rhizomes, and eventually grow into seedlings.
This study explored the regeneration pathway suitable for rapid reproduction via the mixture of callus and protocorm from aseptic germination of seeds as the material coupled with the complete combination and orthogonal test which suited the hormone combination, natural additive type and concentration. The technique system of callus proliferation, protocorm induction and plant regeneration of R. retusa was established to lay a technical foundation for the protection of wild resources and the development of artificial cultivation. Meanwhile, this protocol can also provide a reference for the study of in vitro rapid propagation of other orchidaceae plants.

Figure 1: The distribution map for Rhynchostylis retusa in the world
The mixtures of callus and protocorm from aseptic germination of seeds (Rhynchostylis retusa (L.) Bl.) were provided by Professor Chang-Chun Ding (Yunnan Wenshan University, Kunming, China).
The basic medium for all cultures of R. retusa was 1/2 Murashige and Skoog (MS) containing 1.0 g·L−1 activated carbon (AC) and 0.47% agar. The pH of the media was adjusted to 5.4–5.8 and then autoclaved at 122°C for 20 min.
2.3 Screening of Natural Additives and Initial Culture
Refer to the research experience of Yunnan Breeding and Cultivation Research and Development Center of Endangered and Daodi Chinese Medicinal Materials for other orchid, the mixtures of callus and protocorm from aseptic germination were used as explants [11]. The callus proliferation and protocorm occurrence experiment of R. retusa were conducted in the medium adding 1.5 mg·L−1 6-benzylaminopurine (6-BA), apple puree (30, 50 and 70 g·L−1) and banana puree (30, 50 and 70 g·L−1) of different mass concentrations and coconut water (50, 100 and 150 mL·L−1) of different volume concentrations, respectively [12,13]. Apple, banana and coconut were purchased from fruit store (Yunnan University of Chinese Medicine, Kunming, China). Thirty, fifty and seventy grams of peeled apple and banana pulp were weighed, respectively. Then, they were homogenized with a blender and added into the medium. Similarly, green coconut water (50, 100 and 150 mL) were added into the medium. The mixtures of callus and protocorm were cut into 1 × 1 cm in size. The natural additions suitable for the growth of R. retusa were selected based on the growth conditions of each group after 90 days.
2.4 Synchronous Medium for Callus Proliferation and Protocorm Occurrence
Orthogonal experiment is the most successful multifactorial and multilevel experiment, and it is a statistical experiment rather than a visual experiment. A few representative experiments were used to achieve the purpose of comprehensive experiment, and then the statistical method was used to get the best experimental results. Therefore, according to the results of initial culture, banana puree (30, 50, 70 and 100 g·L−1), α-naphthaleneacetic acid (NAA: 0.1, 0.5, 1.0 and 1.5 mg·L−1), 6-benzylaminopurine (6-BA: 0.5, 1.0, 1.5 and 2.0 mg·L−1) and kinetin (KT: 0.05, 0.1, 0.5 and 1.0 mg·L−1) were used as factors. L16 (45) orthogonal test was established to study the influence of various factors on callus proliferation and protocorm occurrence, among which the protocorm occurrence efficiency was expressed by bud occurrence coefficient [14]. The mixture of callus and protocorm was about 1 × 1 cm in size. The callus proliferation coefficient and bud occurrence coefficient were calculated after 90 days. The statistical criteria were as follows: a) The callus with green and compact in structure was effective callus; b) The effective bud was 2–3 lobules with 0.5–1.5 cm plant in height.
2.5 Culture Medium of Growth and Proliferation of Buds
The optimal medium for bud occurrence was obtained based on the above L16 (45) orthogonal test. Two to three buds with 2–3 lobules and 0.5–1.5 cm height were selected as the material for bud proliferation culture. The proliferation coefficient of bud was calculated 90 days later.
2.6 The Medium of Rejuvenation and Rooting
In the case of few factors and levels, the complete combination can get the most accurate result. Thus, the complete combination test was conducted in the basic medium adding NAA of different mass concentration and coconut water of different volume concentration, which included NAA (1.0, 1.5 and 2.0 mg·L−1) and coconut water (50, 70 and 100 mL·L−1) [14]. The well-grown seedlings with 3 cm high and 3–4 leaves were selected as rooting materials and then the roots were cut to 2/3 for cultivation. After 90 days, the rooting rate, average number of roots and average root diameter were recorded. The root diameter was measured at 1 cm of the base of the root by vernier caliper.
2.7 Acclimatization and Transplanting
When the regenerated plants in the culture bottle grow to a 5–6 cm in height and the root reached to 4–5 cm with 4–6 leaves, they were exposed to natural light and acclimated for 3 days. Then, the seedlings were removed from the culture bottle, carefully washed the agar of root with water, and placed them in the room to dry the water. After the roots become white, transplanted them into crushed pine bark sterilized with 0.1% chlorothalonil. The culture was carried out in a greenhouse with a temperature of 20~25°C and a humidity of 75~95%. After 90 days, the survival rate and the growth of seedlings were recorded.
The cultivation temperature was maintained between 21°C and 23°C. The photoperiod was 12 h with 1500~2000 lx irradiance provided by cool-white fluorescence tubes. Every group contained 7 culture bottles with 5 materials and all experiments were repeated 3 times.
Dates were subjected to analysis of variance (ANOVA) using SPSS software (IBM Corp, Armonk, USA). The minor difference among every treatment method was determined using Least Significant Differences Test at 5% probability (p ≤ 0.05), the mean values were further separated using analysis of range. The calculation method of the data in each stage was as follows:
Callus proliferation coefficient = the number of effective callus after 90 days of growth/the total number of effective callus of initial inoculations;
Bud occurrence coefficient = the number of available buds produced/the number of callus of initial inoculations;
Rooting rate (%) = (the number of seedlings with new roots/the total number of seedlings inoculations) × 100%;
The survival rate (%) = (the number of survival seedlings /the total number of transplantion) × 100%
In the blank group with 6-BA mg·L−1 alone, after 30 days of culture, although the material had a certain growth and bud occurrence, the growth was slow and the material gradually turned yellow and aged. After 60 days of culture, growth condition was not seen obviously improved in this medium (Fig. 2a). Additionally, in the apple puree group, although the callus proliferation effect was significant, the vitrification phenomenon was obvious with abnormal seedlings (Fig. 2b). In the coconut water group, callus had a considerable amount of proliferation with the signs of aging. More buds appeared and the roots grew rapidly (Fig. 2c). In the banana puree group, the growth condition was the best, with rapid callus proliferation and significant growth, and a lot of protocorms occurred, especially the concentration of banana puree was 70 g·L−1 (Fig. 2d) (Fig. 3).

Figure 2: Initial culture of R. retusa. (a) Growth condition after 60 days in the blank group with 6-BA mg·L−1. (b) Growth condition after 90 days with 70 g·L−1 apple puree. (c) Growth condition after 90 days with 100 mL·L−1 coconut water. (d) Growth condition after 90 days with 70 g·L−1 banana puree group

Figure 3: Screening of natural additives
3.2 Callus Proliferation and Protocorm Occurrence
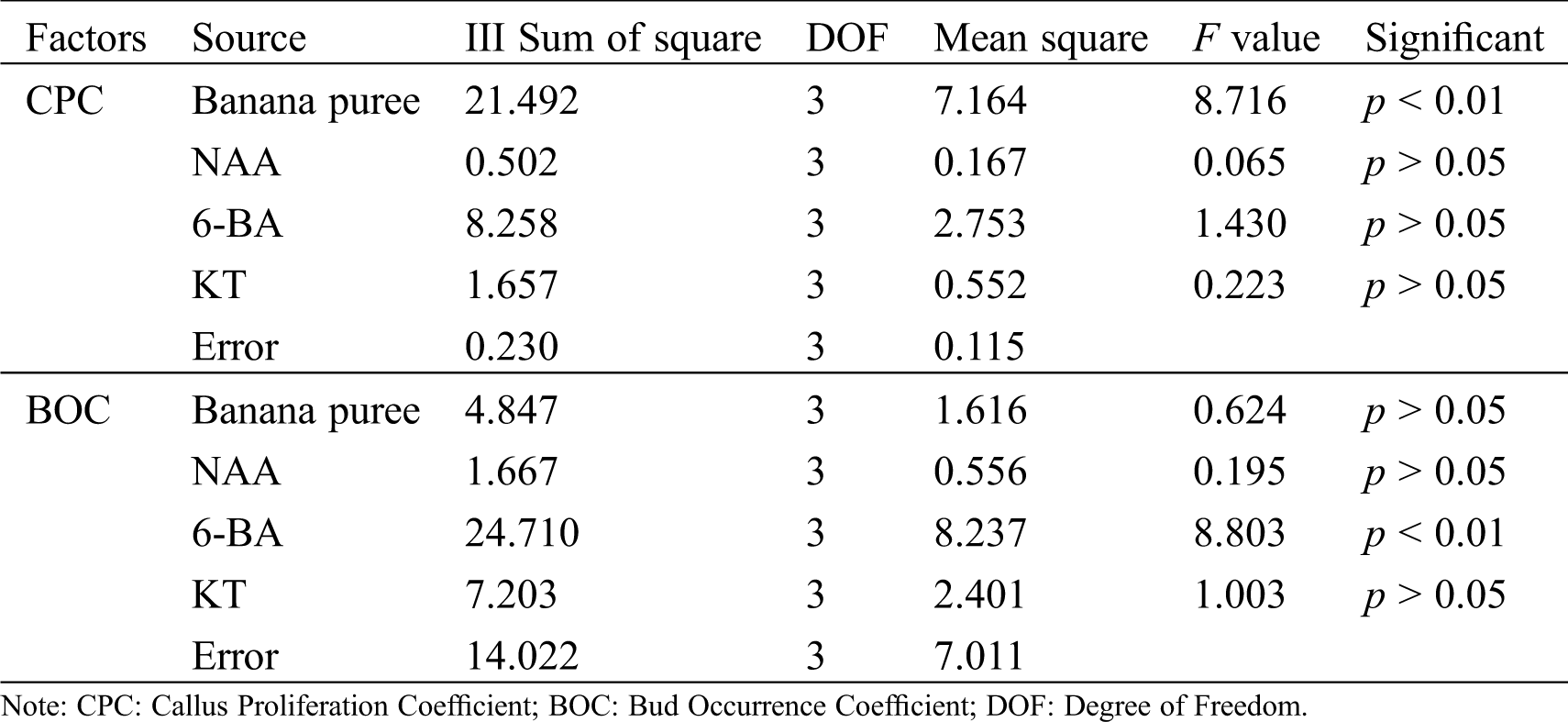
Orthogonal experiment (Tab. 1) and its analysis results (Tab. 2) showed that the main factors for callus proliferation were banana puree, followed by 6-BA, KT and NAA. The range of four factors was more than that of the blank column (0.549), indicated that the influence of the four factors on callus proliferation were reliable. According to the analysis of variance (Tab. 3), an extremely significant influence was observed in banana puree for the proliferation coefficient of callus (p < 0.01), while NAA, 6-BA and KT had no significant influence (p > 0.05). According to the mean value analysis, the best combination of callus proliferation of R. retusa was A2B1C3D4, namely 50 g·L−1 banana puree + 0.1 mg·L−1 NAA + 1.5 mg·L−1 6-BA + 1.0 mg·L−1 KT.
As could be seen from Tabs. 1 and 2, 6-BA was the most important influence factor for bud occurrence coefficient, followed by KT, banana puree and NAA. The range of these four factors was more than the blank column (0.422), indicated that 6-BA, KT, banana puree and NAA had reliable effects on bud occurrence. Analysis of variance (Tab. 3) showed that an extremely significant influence was observed in 6-BA for the bud occurrence coefficient (p < 0.01), while KT, banana puree and NAA had no significant influence (p > 0.05). By the mean value analysis, the optimal combination of bud occurrence of R. retusa was A4B3C4D1 (100 g·L−1 banana puree + 1.0 mg·L−1 NAA + 2.0 mg·L−1 6-BA + 0.05 mg·L−1 KT).
Table 1: L16 (45) results of callus proliferation and protocorm occurrence in R. retusa
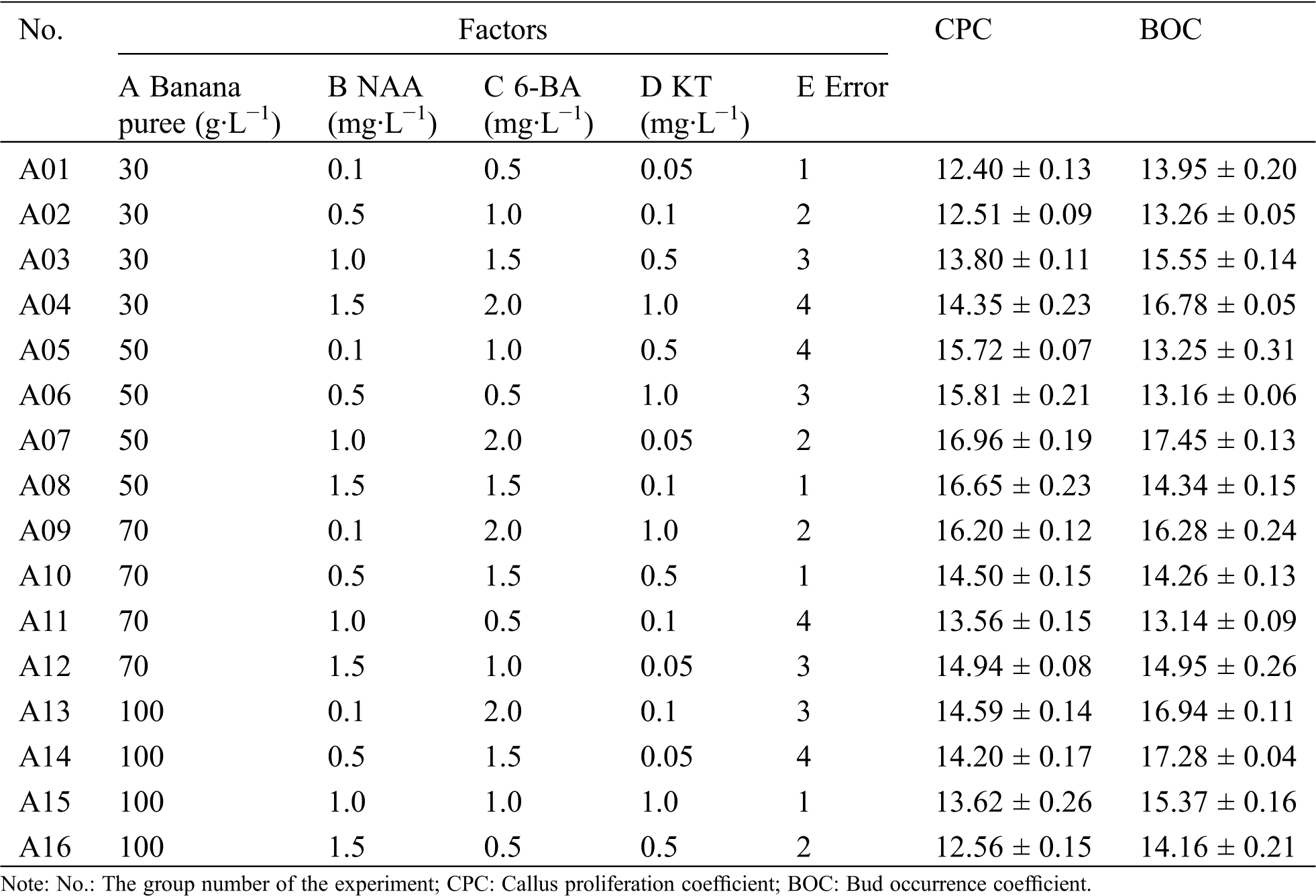
Table 2: Results of mean and range in callus proliferation coefficient and bud occurrence coefficient of R. retusa
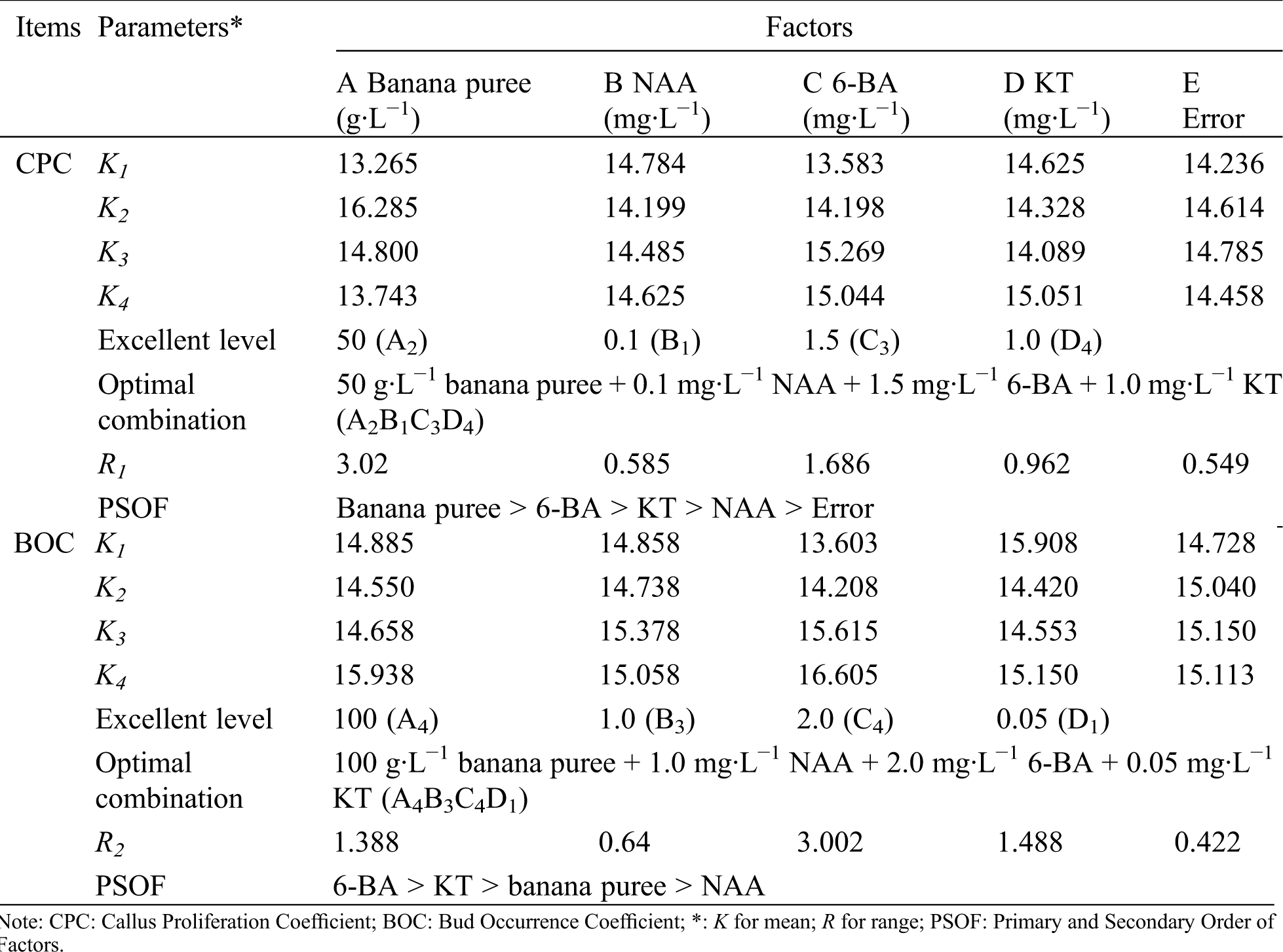
Table 3: Variance analysis of callus proliferation coefficient and bud occurrence coefficient in R. retusa
Based on the two parameters of callus proliferation coefficient and bud occurrence coefficient in the orthogonal experiment, and combined with the fact that the callus proliferation culture was the main culture in this culture stage, so the culture medium for callus proliferation and bud occurrence was A2B1C3D4 (1/2 MS + 50 g·L−1 banana puree + 0.1 mg·L−1 NAA + 1.5 mg·L−1 6-BA + 1.0 mg·L−1 KT + 1.0 g·L−1 AC). In this medium, callus grew significantly after material transfer for 20 days, and embryogenic callus appeared (Fig. 4a). The protocorm developed into bud and callus further proliferated after 30 days (Fig. 4b). With the occuerrence of protocorm, cluster buds were generated on each callus block and obvious rhizoids were seen on the surface of callus after 50 days (Fig. 4c). Then, lots of cluster buds produced from the protocorm increased, callus and protocorm proliferated continuously after 70 days (Fig. 4d). After 90 days, with the callus proliferation, the produced cluster buds from the protocorm grew rapidly. Simultaneously, the callus proliferation coefficient and the bud occurrence coefficient were 17.33 and 19.63, respectively (Fig. 4e). In the process of callus proliferation and bud induction, callus of R. retusa had a strong embryogenesis and a lot of protocorms occurred on each callus, accompanied by the production of rhizoids (Fig. 4f). The protocorm grown into seedlings by first inducted buds and then rooting (Fig. 4g). Meanwhile, callus also continuously proliferated, produced more protocorms and appeared similar to the phenomenon of adventitious cluster buds (Fig. 4h).

Figure 4: Callus proliferation and bud occurrence of R. retusa in the 1/2 MS medium with 50 g·L−1 banana puree, 0.1 mg·L−1 NAA, 1.5 mg·L−1 6-BA, 1.0 mg·L−1 KT and 1.0 g·L−1 AC (a) Callus began to proliferate after 20 days. (b) The protocorm developed into bud and callus further proliferated after 30 days. (c) Embryonic callus and protocorm with rhizoids after 50 days. (d) Lots of cluster buds produced from the protocorm increased after 70 days. (e) The cluster buds from the protocorm grew rapidly with the proliferation of callus after 90 days. (f) Protocorm derived from embryogenic callus. (g) The protocorm developed as a seedling by first growing buds and then rooting. (h) Adventitious cluster buds inducted from lots of protocorms
3.3 Buds Growth and Proliferation
When the material was transferred into the 1/2 MS medium with 100 g·L−1 banana puree, 1.0 mg·L−1 NAA, 2.0 mg·L−1 6-BA, 0.05 mg·L−1 KT and 1.0 g·L−1 AC around 20 days, the callus at the base began to proliferate, the roots of the buds grew rapidly and the leaves began to extend (Fig. 5a). After that, the leaves of the buds were further extended, and the callus at the base was also significantly increased about 40 days (Figs. 5b and 5c). After 50 days, the buds had 4–6 new leaves, and callus at the base turned green due to the large number of protocorms proliferated (Fig. 5d). Then a lot of protocorms occurred on callus after 70 days (Figs. 5e and 5f). After 90 days, the young buds grew into small plants and the basal was covered by new buds arising from the protocorm (Figs. 5g and 5h). At this time, the bud occurrence coefficient was 17.87, and the callus proliferation coefficient was 10.32.
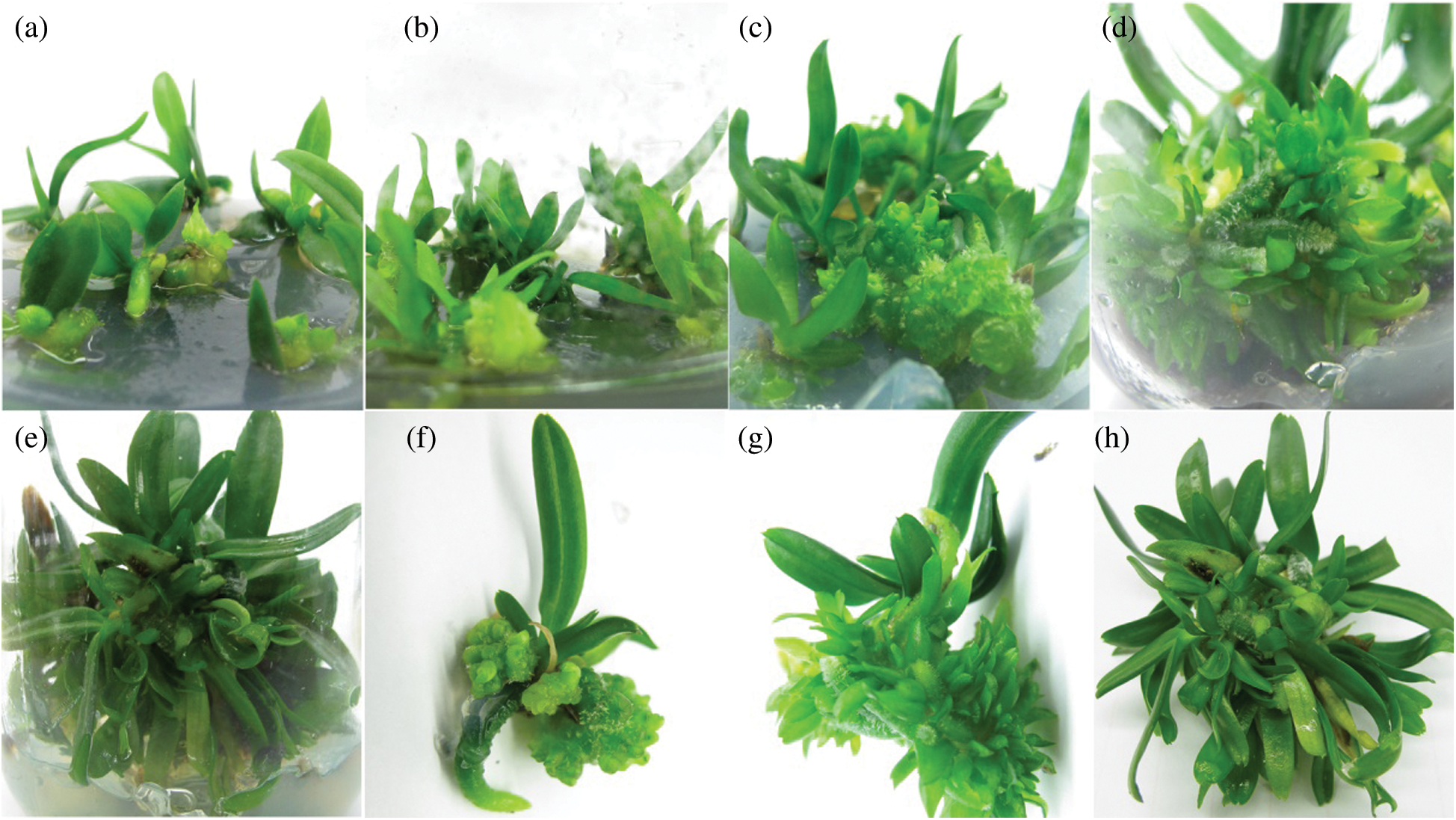
Figure 5: Bud growth and proliferation of R. retusa in the 1/2 MS medium with 100 g·L−1 banana puree, 1.0 mg·L−1 NAA, 2.0 mg·L−1 6-BA, 0.05 mg·L−1 KT and 1.0 g·L−1 AC (a) Growth condition after 20 days transplantation culture. (b) and (c) Leaves of the buds began to extend and callus at the base proliferated rapidly about 40 days. (d) Callus at the base turned green due to the large number of protocorms proliferated. (e) and (f) A lot of protocorms occurred on callus after 70 days. (g) and (h) Growth condition after around 90 days
In view of the actual situation of R. retusa, in order to reduce the steps and shorten the time at this stage of culture, callus proliferation and bud occurrence could be eliminated in the future culture. Therefore, callus proliferation, bud occurrence, growth and proliferation could be completed in the 1/2 MS medium adding 100 g·L−1 banana puree, 1.0 mg·L−1 NAA, 2.0 mg·L−1 6-BA, 0.05 mg·L−1 KT and 1.0 g·L−1 AC. The culture time of 180 days or even longer could be shortened to about 90 days.
3.4 Rejuvenation, Rooting, Domestication and Transplanting
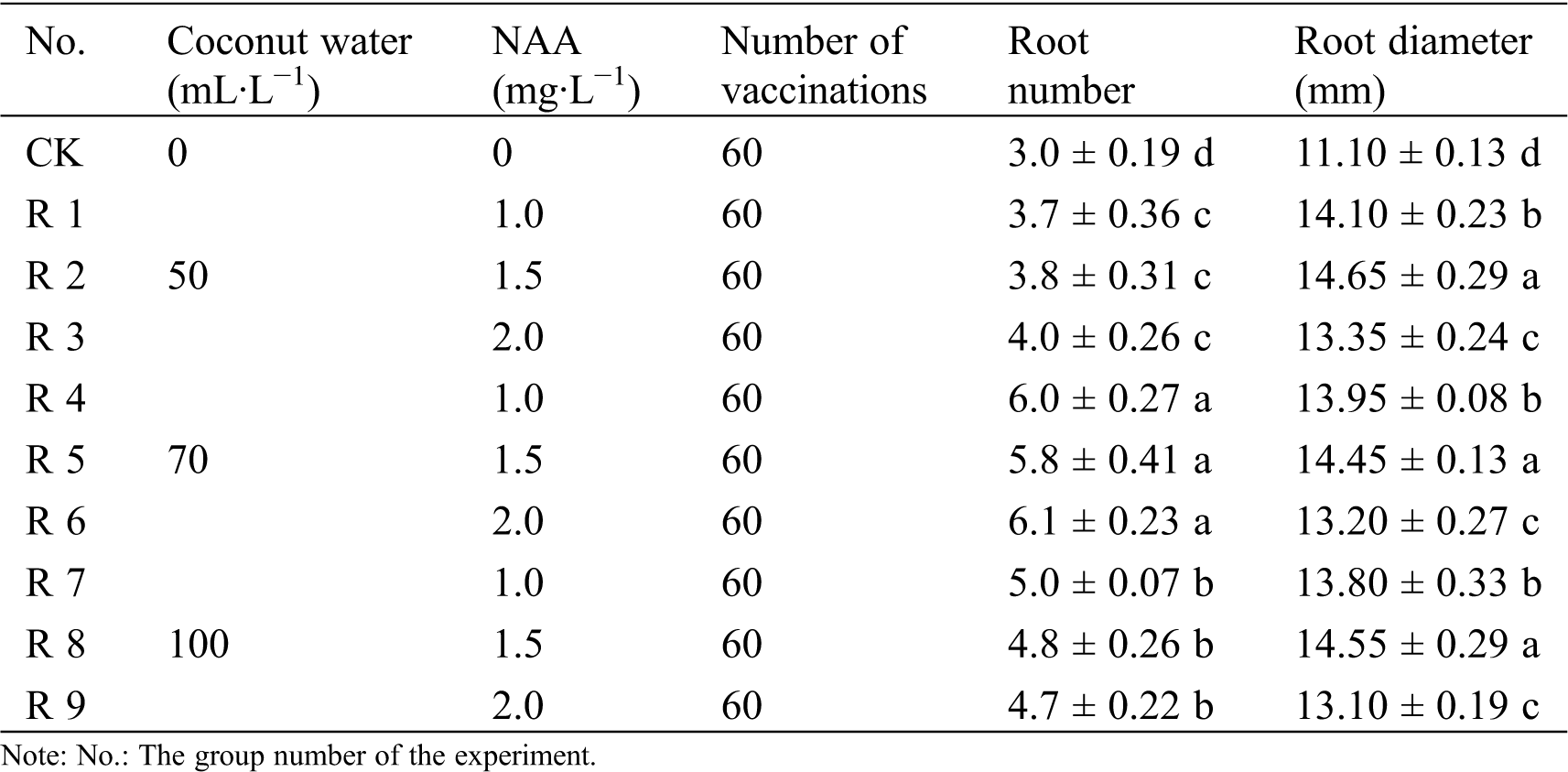
Single seedling of R. retusa rooted easily, the rooting rate of all the test groups could reach 100%, but the rooting number and root diameter had obvious differences (Tab. 4).
The experiment showed that although the rooting rate of the blank group was up to 100%, the single seedlings not only had fewer and thinner roots, but also had slower growth. Additionally, culture must exceed 120 days before transplanting. Coconut water had significant influence for the number of roots per seedling, but NAA had no significant influence. When the volume concentration of coconut water was the same, NAA treatments did not significant influence (p > 0.05), while the coconut water treatments with different volume concentrations had significant influence (p < 0.05). In terms of root diameter of R. retusa, coconut water treatments did not significant influence, but the NAA treatments had significant influence (p < 0.05). After the single seedling was transferred into the 1/2 MS medium containing 70 mL·L−1 coconut water, 1.5 mg·L−1 NAA and 1.0 g·L−1 AC for 20 days, new leaves appeared and new roots at the base occurred (Figs. 6a and 6b). After 50 days, growth-rapid tube seedlings with 4–5 new roots could be observed (Figs. 6c and 6d). Next, the leaves of the test-tube seedlings were completely unfolded with 5–6 new roots, and the roots were about 14 mm in diameter after 90 days (Figs. 6e and 6f). In the whole culture of rejuvenation and rooting, there were no callus, protocorm and cluster bud, which showed that the culture medium was very suitable for this stage of R. retusa cultivation. After 90 days of domestication and transplanting, the survival rate was more than 90% (Figs. 6g and 6h).

Figure 6: Rejuvenation, rooting domestication and transplantation of R. retusa in the 1/2 MS medium containing 70 mL·L−1 coconut water, 1.5 mg·L−1 NAA and 1.0 g·L−1 AC (a) and (b) Rooting seedlings after rejuvenation in 20 days. (c) and d Growth-rapid tube seedlings with 4–5 new roots after 50 days. (e) and (f) Growth condition after 90 days. (g) and (h) Tube seedlings after 90 days domestication
Table 4: Effects of different volume concentrations of coconut water and NAA on rooting of seeding of R. retusa
4.1 Effect of Natural Additives on Rapid Propagation in Vitro of R. retusa
Some natural organic substances, such as coconut water, banana puree, apple puree, potato puree, corn endosperm, malt extract, peptone, tomato juice, yeast extract etc., they mostly contain amino acids, enzymes, vitamins, plant hormones and other substances, can promote the orchid plant seed germination and explants proliferation, differentiation. As is known, these natural additives have complex components, which make it difficult to determine the real effective factors, but they are effective in tissue culture and have high production value [15–20]. In this study, apple puree, banana puree and coconut water were used as additives for initial culture, and three additives promoted the proliferation of callus of R. retusa. But, apple puree could cause serious vitrification. As reported by Sen et al. [21], the content of phenolics and superoxide dismutas in highly hydrogenated plants was significantly higher than that in normal plants. It was speculated that apple puree might contain a large amount of such substances, which promoted callus proliferation of R. retusa and accumulated in large quantities at the same time, leading to excessive hydrogenation and then showing vitrified state. Coconut water is considered as an important organic substance in orchidaceae culture. It not only promotes the protocorm occurrence obviously, but also promotes root development. It was reported that diphenyl urea, present in coconut water could promote growth and development by inducing cell division. In addition, IAA, which is abundant in coconut water, has been shown to be beneficial for root development [13]. Similarly, banana pulp is also commonly used in orchids, where it can be used as an antacid to neutralize acidic conditions and maintain PH. Besides, banana puree with the higher level of inorganic ions, vitamins and peptone can promote callus proliferation, protocorm differentiation and bud growth [22], which was consistent with this study. In contrast, in the blank control group, few callus proliferated and little protocorm occurred. These results indicated that the natural organic substances were indispensable in tissue culture of R. retusa.
4.2 Protocorm and Rhizoids of R. retusa
Generally, the rhizoids formation in the protocorm is a unique characteristic of orchidaceae. As reported by Stewart et al. [9], the emergence of the rhizoids during the protocorm growth was related to photoperiod. According Johnson et al. [23], the emergence of the rhizoids might be influenced by sugar. The study observed that the occurrence of protocorm shortly after seed germination, with the emergence of non-obvious rhizoids. The rhizoids are usually a single cell structure produced by epiphytes in order to absorb nutrients. Nevertheless, in the present tissue culture of R. retusa, there were no reports of rhizoids [5,6,24–26]. It was speculated that the medium composition affected the emergence of the rhizoids. However, the number of the rhizoids of R. retusa was small and the existence time was short. This phenomenon might be to better absorb nutrients from the medium to meet the rapid development of protocorm in earlier stage, but, in the late, the rhizoids were no longer required due to the emergence of roots.
4.3 Breeding Methods of R. retusa
In generally, special protocorm, protocorm-like bodies or succulent rhizomes should are rapid in vitro propagation of orchidaceae, callus is rarely produced [27]. The proliferation of protocorm, protocorm-like bodies and rhizome had been widely reported [28,29]. In Dendrobium officinale, callus could differentiate into leaf primordium and apical meristem, and finally formed adventitious cluster bud [30]. In Bletilla striata, after the callus occurrence, the early proliferation was completely dependent on the mode of callus-adventitious buds [11]. Unlike these orchids, there was no indirect pathway in R. retusa. After the occurrence of callus, one part of callus continued to proliferate, and the other part transformed into embryonic cell masses, which directly differentiated into protocorm and developed into seedlings in appropriate medium, thus produced a phenomenon similar to cluster buds. A large number of protocorms could be obtained via the callus proliferation culture in a short time. Hence, the mixture of callus and protocorm of R. retusa was the optimal material for rapid propagation in vitro and preservation of germplasm resources.
4.4 Effects of Plant Hormones on Various Stages of R. retusa
In the process of rapid propagation in vitro, the plant hormone types and concentrations influenced callus proliferation, protocorm occurrence, rejuvenation and rooting of R. retusa. According to report, 6-BA showed better results in the regeneration of most orchids [31], and most researchers combined it with the growth elements in a certain proportion to achieve better results in the protocorm regeneration of Dendrobium firmbriatum [32] and Dendrobium Candidum [33]. In this study, when 6-BA was used alone, the callus proliferated rapidly, but the differentiation rate of protocorm was low. After NAA and KT were added, both the callus proliferation and the protocorm coefficient were greatly improved, which indicated that synergy between hormone was greater than the single action. It had been reported that the protocorm occurrence required high concentration of 6-BA (4.0–6.0 mg·L−1) [34]. However, in the protocorm induction of R. retusa, lower concentration of 6-BA (1.5 mg·L−1) induced a strong impact, which was estimated to be caused by different sensitivity of different species to 6-BA. In addition, this study found that after the protocorm occurrence, the mixture of callus and protocorm could synchronously carry out callus proliferation, protocorm differentiation and bud proliferation in the medium adding 100 g·L−1 banana puree, 1.0 mg·L−1 NAA, 2.0 mg·L−1 6-BA and 0.05 mg·L−1 KT. This synchronous culture method could reduce the steps and shorten the time of seedling formation.
In rejuvenation and rooting culture, the tube seedlings were easy to root due to they were developed from the protocorm, but the diameter of the root was closely associated with the NAA concentration. In term of the results of the Tab. 4, the effect of NAA on the root was mainly reflected in the root diameter, which was not significantly related to root number and rooting rate. The results indicated that NAA could not only promote cell dedifferentiation and rooting, but also promote the rapid succulence of roots in orchidaceae, which was similar to the study of Rodrigues et al. [35].
4.5 Effect of Activated Carbon on Rapid Propagation in Vitro of R. retusa
AC played a crucial role in rapid propagation of R. retusa. In the present orchidaceae tissue culture, AC can promote the growth and development of plants [5,35,36]. In generally, adding AC to tissue culture can create a dark environment suitable for seed germination and plant root growth [16]. Another research showed that AC had a strong adsorption effect, which could adsorb phenol, quinone and other harmful substances in the medium, promoted the development and improved the survival rate [37,38]. However, some researchers believed that AC could also adsorb plant growth regulators or some substances produced in the process of plant growth, or released some substances contained in AC and previously adsorbed substances, thereby promoting the growth of orchidaceae [39].
In the present study, an efficient regeneration system of R. retusa was established. The results indicated that the optimal medium for callus proliferation, protocorms occurrence and growth was 1/2 MS medium adding 50 g·L−1 banana puree, 0.1 mg·L−1 NAA, 1.5 mg·L−1 6-BA and 1.0 mg·L−1 KT. However, the best combination of bud occurrence was A4B3C4D1 (100 g·L−1 banana puree + 1.0 mg·L−1 NAA + 2.0 mg·L−1 6-BA + 0.05 mg·L−1 KT). In the further subculture, the same medium was simultaneously used for callus proliferation, protocorm occurrence and bud growth. The plantlets developed roots in 1/2 MS medium containing 70 mL·L−1 coconut water and 1.5 mg·L−1 NAA with 100% rooting rate. The survival rate was more than 90% after domestication and transplantation. This study can provide a technical foundation for the protection of wild resources and the development of artificial cultivation of R. retusa. Meanwhile, this protocol can also provide a reference for the study of in vitro rapid propagation of other orchidaceae plants.
Funding Statement: The research was supported by Yunnan Breeding and Cultivation Research and Development Center of Endangered and Daodi Chinese Medicinal Materials (No. 2016DH011).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Flora of China Editorial Committee (1999). Flora Reipublicae Popularis Sinicae. Beijing, China. [Google Scholar]
2. Arditti, J. (1967). Factors affecting the germination of orchid seeds. Botanical Review, 33(1), 1–97. DOI 10.1007/BF02858656. [Google Scholar] [CrossRef]
3. Li, C. H., Li, T. C., Li, K. C. (2015). The factory cultivation of fox tail orchid. Flowers & Plantlet, 3, 36–38. [Google Scholar]
4. Morel, G. (1960). Producing virus-free Cymbidium. American Orchid Society Bulletin, 29(7), 495–497. [Google Scholar]
5. Thomas, T. D., Michael, A. (2007). High-frequency plantlet regeneration and multiple shoot induction from cultured immature seeds of Rhynchostylis retusa Blume., an exquisite orchid. Plant Biotechnology Reports, 1(4), 243–249. DOI 10.1007/s11816-007-0038-z. [Google Scholar] [CrossRef]
6. Naing, A. H., Park, I. S., Hwang, Y. J., Chung, J. D., Lim, K. B. (2010). In vitro micropropagation and conservation of Rhynchostylis retusa BL. Horticulture Environment and Biotechnology, 51, 440–444. [Google Scholar]
7. Sinha, P., Jahan, M. A. A. (2012). Clonal propagation of Rhynchostylis retusa (Lin.) Blume through in vitro culture and their establishment in the nursery. Plant Tissue Culture and Biotechnology, 22(1), 1–11. DOI 10.3329/ptcb.v22i1.11242. [Google Scholar] [CrossRef]
8. Islam, S. S., Bhattacharjee, B. (2015). Plant regeneration through somatic embryogenesis from leaf and root explants of Rhynchostylis retusa (L.) Blume. Applied Biological Research, 17(2), 158–165. DOI 10.5958/0974-4517.2015.00025.7. [Google Scholar] [CrossRef]
9. Stewart, S. L., Kane, M. E. (2006). Asymbiotic seed germination and in vitro seedling development of Habenaria macroceratitis (Orchidaceaea rare Florida terrestrial orchid. Plant Cell, Tissue and Organ Culture, 86(2), 147–158. DOI 10.1007/s11240-006-9098-y. [Google Scholar] [CrossRef]
10. Johnson, T. R., Stewart, S. L., Dutra, D., Kane, M. E., Richardson, L. (2007). Asymbiotic and symbiotic seed germination of Eulophia alta (Orchidaceae)—Preliminary evidence for the symbiotic culture advantage. Plant Cell, Tissue and Organ Culture, 90(3), 313–323. DOI 10.1007/s11240-007-9270-z. [Google Scholar] [CrossRef]
11. Zhang, A. L., Wang, Y. Z., Huang, H. Y. (2018). The study of effective proliferative protocol in artificial propagation of Bletilla striata test-tube. Journal of Chinese Medicinal Materials, 41, 275–280. [Google Scholar]
12. Seon, K. M., Kim, D. H., Kang, K. W., Sivanesan, I. (2018). Highly competent in vitro propagation of Thrixspermum japonicum (Miq.) Rchb. f., a rare epiphytic orchid. In Vitro Cellular & Developmental Biology-Plant, 54(3), 302–308. DOI 10.1007/s11627-018-9890-5. [Google Scholar] [CrossRef]
13. Huh, Y. S., Lee, J. K., Nam, S. Y., Paek, K. Y., Suh, G. U. (2016). Improvement of asymbiotic seed germination and seedling development of Cypripedium macranthos Sw. with organic additives. Journal of Plant Biotechnology, 43(1), 138–145. DOI 10.5010/JPB.2016.43.1.138. [Google Scholar] [CrossRef]
14. Xi, Y. K., Wang, Y., Zeng, B., Huang, H. Y., Yang, W. D. (2020). Callus induction and adventitious bud differentiation of Cyclocodon lancifolius (Roxb.) Kurz. Botanical Sciences, 98(4), 534–544. DOI 10.17129/botsci.2609. [Google Scholar] [CrossRef]
15. Ge, L., Yong, J. W. H., Goh, N. K., Chia, L. S., Tan, S. N. et al. (2005). Dentification of kinetin and kinetin riboside in coconut (Cocos nucifera L.) water using a combined approach of liquid chromatography-tandem mass spectrometry, high performance liquid chromatography and capillary electrophoresis. Journal of Chromatography B, 829(1–2), 26–34. DOI 10.1016/j.jchromb.2005.09.026. [Google Scholar] [CrossRef]
16. Teixeira-da-Silva, J. A., Chan, M. T., Sanjaya Chai, M. L., Tanaka, M. (2006). Priming abiotic factors for optimal hybrid Cymbidium (Orchidaceae) PLB and callus induction, plantlet formation, and their subsequent cytogenetic stability analysis. Scientia Horticulturae, 109(4), 368–378. DOI 10.1016/j.scienta.2006.05.016. [Google Scholar] [CrossRef]
17. Ge, L., Yong, J. W. H., Tan, S. N., Hua, L., Ong, E. S. (2008). Analyses of gibberellins in coconut (Cocos nucifera L.) water by partial filling-micellar electrokinetic chromatography-mass spectrometry with reversal of electroosmotic flow. Electrophoresis, 29(10), 2126–2134. DOI 10.1002/elps.200700717. [Google Scholar] [CrossRef]
18. Ge, L., Peh, C. Y. C., Yong, J. W. H., Tan, S. N., Hua, L. et al. (2007). Analyses of gibberellins by capillary electrophoresis–mass spectrometry combined with solid-phase extraction. Journal of Chromatography A, 1159(1–2), 242–249. DOI 10.1016/j.chroma.2007.05.041. [Google Scholar] [CrossRef]
19. Vyas, S., Guha, S., Bhattacharya, M., Rao, I. U. (2009). Rapid regeneration of plants of Dendrobium lituiflorum Lindl. (Orchidaceae) by using banana extract. Scientia Horticulturae, 121(1), 32–37. DOI 10.1016/j.scienta.2009.01.012. [Google Scholar] [CrossRef]
20. Hossain, M. M., Sharma, M., Pathak, P. (2012). In vitro propagation of Dendrobium aphyllum (Orchidaceae)—seed germination to flowering. Journal of Plant Biochemistry and Biotechnology, 22(2), 157–167. DOI 10.1007/s13562-012-0124-3. [Google Scholar] [CrossRef]
21. Sen, A., Alikamanoglu, S. (2013). Antioxidant enzyme activities, malondialdehyde, and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture. In Vitro Cellular & Developmental Biology-Plant, 49, 396–404. [Google Scholar]
22. Gnasekaran, P., Rathinam, X., Sinniah, U. R., Subramaniam, S. (2010). A study on the use of organic additives on the protocorm-like bodies (PLBs) growth of Phalaenopsis violacea orchid. Journal of Phytology, 2, 29–33. [Google Scholar]
23. Johnson, T. R., Kane, M. E., Pérez, H. E. (2010). Examining the interaction of light, nutrients and carbohydrates on seed germination and early seedling development of Bletia purpurea (Orchidaceae). Plant Growth Regulation, 63(1), 89–99. DOI 10.1007/s10725-010-9516-3. [Google Scholar] [CrossRef]
24. Kumar, A., Palni, L. M. S. (2003). The effect of light source and gelling agent on micropropagation of Rosa damascena Mill. and Rhynchostylis retusa (L.) Bl. Journal of Horticultural Science and Biotechnology, 78(6), 786–792. DOI 10.1080/14620316.2003.11511700. [Google Scholar] [CrossRef]
25. Attri, L. K., Nayyar, H., Bhanwra, R. K., Vij, S. P. (2007). Post-pollination biochemical changes in the floral organs of Rhynchostylis retusa (L.) Bl. and Aerides multiflora Roxb. (Orchidaceae). Journal of Plant Biology, 50, 548–556. [Google Scholar]
26. Parab, G. V., Krishnan, S. (2012). Rapid in vitro mass multiplication of orchids Aerides maculosa Lindl. and Rhynchostylis retusa (L.) Bl. from immature seeds. Indian Journal of Biotechnology, 11, 288–294. [Google Scholar]
27. Ding, L., Zhang, L., Guo, L., Sang, J., Qin, L. X. et al. (2014). Asymbiotic seed germination and rapid seedling regeneration of endangered. Plant Physiology Journal, 50, 77–82. [Google Scholar]
28. Chen, T. Y., Chen, J. T., Chang, W. C. (2004). Plant regeneration through direct shoot bud formation from leaf cultures of Paphiopedilum orchids. Plant Cell, Tissue and Organ Culture, 76(1), 11–15. DOI 10.1023/A:1025858211320. [Google Scholar] [CrossRef]
29. Chugh, S., Guha, S., Rao, I. U. (2009). Micropropagation of orchids: A review on the potential of different explants. Scientia Horticulturae, 122(4), 507–520. DOI 10.1016/j.scienta.2009.07.016. [Google Scholar] [CrossRef]
30. Pan, C. M., Tong, J. Y., Liu, D. X., Hu, A. Q., Yang, S. Q. (2008). Histocytological observation of somatic embryogenesis in vitro cultured Dendrobium candidum wall. Ex Lindl. Journal of Guangzhou University of Traditional Chinese Medicine, 25, 74–76. [Google Scholar]
31. Nayak, N., Sahoo, R., Patnaik, S., Rath, S. P. (2002). Establish-ment of thin cross section (TCS) culture method for rapid micropropagation of Cymbidium aloifolium (L.) Sw. and Dendrobium nobile Lindl. (Orchidaceae). Scientia Horticulturae, 94(1–2), 107–116. DOI 10.1016/S0304-4238(01)00372-7. [Google Scholar] [CrossRef]
32. Roy, J., Banerjee, N. (2003). Induction of callus and plant regeneration from shoot-tip explants of Dendrobium fimbriatum Lindlvar. oculatum Hk. f. Scientia Horticulturae, 97(3–4), 333–340. DOI 10.1016/S0304-4238(02)00156-5. [Google Scholar] [CrossRef]
33. Zhao, P., Wang, W., Feng, F. S., Wu, F., Yang, Z. Q. et al. (2007). High-frequency shoot regeneration through transverse thin cell layer culture in Dendrobium Candidum Wall Ex Lindl. Plant Cell, Tissue and Organ Culture, 90(2), 131–139. DOI 10.1007/s11240-006-9181-4. [Google Scholar] [CrossRef]
34. Feng, Y., Lai, Z. X. (2009). Effect of hormone and sugar on the establishment of transgenic system. Journal of Fujian Agriculture and Forestry University (Natural Science Edition), 5, 495–499. [Google Scholar]
35. Rodrigues, L. A., de Paiva Neto, V. B., Boaretto, A. G., de Oliveira, J. F. de Aguiar Torrezan, M. et al. (2015). In vitro propagation of Cyrtopodium saintlegerianum rchb. f. (orchidaceaea native orchid of the Brazilian savannah. Crop Breeding and Applied Biotechnology, 15, 10–17. [Google Scholar]
36. Ket, N. V., Hahn, E. J., Park, S. Y., Chakrabarty, D., Paek, K. Y. (2004). Micropropagation of an endangered orchid Anoectochilus formosanus. Biologia Plantarum, 48(3), 339–344. DOI 10.1023/B:BIOP.0000041084.77832.11. [Google Scholar] [CrossRef]
37. Thomas, T. D. (2008). The role of activated charcoal in plant tissue culture. Biotechnology Advances, 26(6), 618–631. DOI 10.1016/j.biotechadv.2008.08.003. [Google Scholar] [CrossRef]
38. Sáenz, L., Herrera-Herrera, G., Uicab-Ballote, F., Chan, J. L., Oropeza, C. (2010). Influence of form of activated charcoal on embryogenic callus formation in coconut (Cocos nucifera). Plant Cell, Tissue and Organ Culture, 100(3), 301–308. DOI 10.1007/s11240-009-9651-6. [Google Scholar] [CrossRef]
39. Van-Winkle, S. C., Pullman, G. S. (2005). Achieving desired plant growth regulator levels in liquid plant tissue culture media that include activated carbon. Plant Cell Reports, 24(4), 201–208. DOI 10.1007/s00299-005-0931-2. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |