
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.013775
ARTICLE
Antifeedant Activity of Caesalpinia coriaria Essential Oil Against Incisitermes marginipennis (Latreille)
1Instituto de Investigaciones Químico Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, 58030, Mexico
2Departamento de Botánica y Zoología, CUCBA Universidad de Guadalajara, Nextipac, 45200, Mexico
*Corresponding Author: Mauro M. Martínez Pacheco. Email: mpacheco@umich.mx
Received: 20 August 2020; Accepted: 06 November 2020
Abstract: This study scrutinized the possibility of finding toxicant or deterrent plant metabolites against the dry wood termite Incisitermes marginipennis (Latreille). Plant deterrent agents act as repellents or antifeedants to prevent wood decay and increase its useful life. The potential of the tree Caesalpinia coriaria (Fabaceae) as a biological source of molecules with deterrent effects against the dry wood termite was assessed by a phytochemical fractionation guided by repellence and antifeedant activities. The gas chromatography-mass spectrometry analysis of the leaf essential oil showed geraniol to be one of the major components and its repellent and antifeedant effects were determined. Geraniol had only an antifeedant effect without affecting the body weight or survival of the dry wood termite. Unlike the leaf essential oil, geraniol did not exhibit a repellency effect. An in-silico approach of the activity of acetylcholinesterase in interaction with geraniol resulted in an affinity energy of −7.5 Kcal/mol. Geraniol interacted with the amino acid tyrosine 324 located in the enzyme’s active site while citronellol (negative control) interacted with tryptophan 83 located adjacent to the active site. These deterring terpenes have not been implemented for the preservation and restoration of wood products exposed to the attack of the dry wood termite. However, they are an important natural control alternative.
Keywords: Termite; geraniol; repellent; antifeedant; deterrent
Traditionally, plants have been recognized to have insecticidal and repellent activities that have been used to annihilate or repel competitors or aggressors, among them, termites. The species in the genus Caesalpinia–in the family Fabaceae, one of the better represented flowering plant families in the tropical and temperate regions of the world–possess such characteristics, because of which they are used in traditional medicine. Several species of Caesalpinia are used as multipurpose trees and shrubs as ornamentals and in agroforestry practices including shading, living fence, or fodder. Used in living fences, they provide a physical and chemical barrier against the fauna, in particular, the insects. Of the eleven species of Caesalpinia that grow and reproduce in the region [1], C. coriaria, C. eriostachys, C. platyloba, C. pulcherrima and C. sclerocarpa [2] are the most abundant. C. coraria (locally known as cascalote) is used in local medicine for its antiseptic and antibiotic properties. Although the metabolome of the species in the genus has been little studied, their pods are known to contain 20%–30% tannins, stigmasterol, ethyl gallate, and gallic acid [3–5]. Cassane diterpenes are other natural products characteristic of the genus Caesalpinia having antimalarial [6], cytotoxic [7], anti-inflammatory [8], antibacterial [9] and antiviral [10] bioactivities.
The concept of natural deterring agents safe and adequate for safeguarding the integrity of sensitive genotypes or in ecologically important organisms. It is by its early developmental phase has gained relevance in recent times. Therefore, insect deterrents have been searched from multipurpose trees. That is the cases of the compounds extracted from the heartwood of Thuja plicata, Chamaecyparis nootkatensis, and Enterolobium cyclocarpum, which when applied to softwood of the same tree species or pinewood, avoided degradation by fungi and deterioration by termites [11,12]. Of specific interest in this search are deterrent molecules of cryptic lifestyle insects like the dry wood termites.
The dry wood termite Incisitermes marginipennis (Latrillé)–a xylophagous insect that bores into pinewood– is considered a pest causing major damage to wooden products. Their presence in the wood can be known only when considerable damage has occurred as evident from feeding frass and excreta [13]. This termite exhibits a slow population growth in colonies with few individuals that live inside the wood, a caste system, with minimal mobility; only winged individuals exit from the wood to form new colonies [14]. Due to its cryptic lifestyle, the dry wood termite is seen as a competitor of anthropogenic activities and an extremely destructive and difficult to control pest that damages cellulosic and wooden assets of people by feeding on them [15]. For that reason, there is great value in the use of substances less toxic to mammalians and ecofriendly for the protection of the domestic, cultural, and historical heritage made of wood or cellulose, requiring frequent maintenance, preservation, and attention.
The living fences of Caesalpinia coriaria have traditionally been used as a physical and chemical barrier against predatory and crop pest animals [1] which motivated the study of the effect of metabolites extracted from Caesalpinia spp. in the control of the dry wood termite I. marginipennis. In this study, it is especially interesting to find in the metabolome of Caesalpinia coriaria possible toxicant deterrent metabolites–including repellent substances that prevent the approach of pest organisms to the substrate due to irritability and antifeedant substances that inhibit normal feeding behavior –effective against the dry wood termite having potential application to prevent the decay of hemicellulosic materials and increase their useful life. We assumed that the sensory modalities of insects, like smell and taste, play a role in the toxic and repellent effect of compounds used for pest control.
Caesalpinia coriaria leaves were collected on October 18, 2016 at 204.5 Km of the Morelia-Buena Vista-Apatzingan highway (N 19°42.851; W 101°36.729) in the state of Michoacán (Mich.), México, at an elevation of 302 metres above sea level (m a.s.l.). The species was determined taxonomically in the Biology Faculty of the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH) by Xavier Madrigal botanist and a voucher specimen was deposited in the Instituto de Investigaciones Químico Biológicas of UMSNH.
2.2 Dry Wood Termite Incisitermes marginipennis (Latreille)
The authors collected colonies of dry wood termites from infested pinewood from the city of Morelia, Mich., at 1921 m a.s.l. The insect species was determined at the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) by Amalia Ojeda Aguilera, according to the taxonomic morphological keys for soldier termite [16,17]. Dry wood termites identified as I. marginipennis were cultivated in a 40 cm × 50 cm × 150 cm container and maintained in the laboratory in darkness at 25°C for a month for their acclimatization prior to the repellency test. Insect and plant specimens were manipulated according to the official Mexican standards related to natural resource management [18].
2.3 Leaf Essential Oil Extraction
Leaf essential oils from C. coriaria (CcLEO) were obtained by steam distillation, carried out by passing steam into a 5-litre round-bottomed flask (195 g–230 g) for 180 min and collecting the condensate (water and oil) in a round-bottomed flask. The condensate was extracted three times with dichloromethane completely to extract the leaf essential oil. Sodium sulfate was added to the dichloromethane to remove moisture. Dichloromethane then was removed by rotary evaporation, stored in sealed vials at 4°C in the dark until its analysis, and tested. The leaf essential oil yield was 1.3%. Geraniol and citronellol were purchased from a commercial supplier (Sigma-Aldrich, Inc., German), see the Fig. 1.
Gas chromatography-mass spectrometry was done using a Hewlett Packard brand model 5890 Series II Plus gas chromatograph equipped with a Hewlett Packard model 5989B (70 eV direct inlet) mass detector and a nonpolar capillary HP-5 MS [(5-phenyl) methylpolysiloxane] (30 m × 25 mm × 0.25 µm) column. The column temperature was initially programmed at 50°C for five minutes, then increased by 20°C every minute until reaching a temperature of 200°C, which was maintained for five minutes. Every minute thereafter, the temperature was increased by 20°C up to 250°C, which was sustained for 10 min.
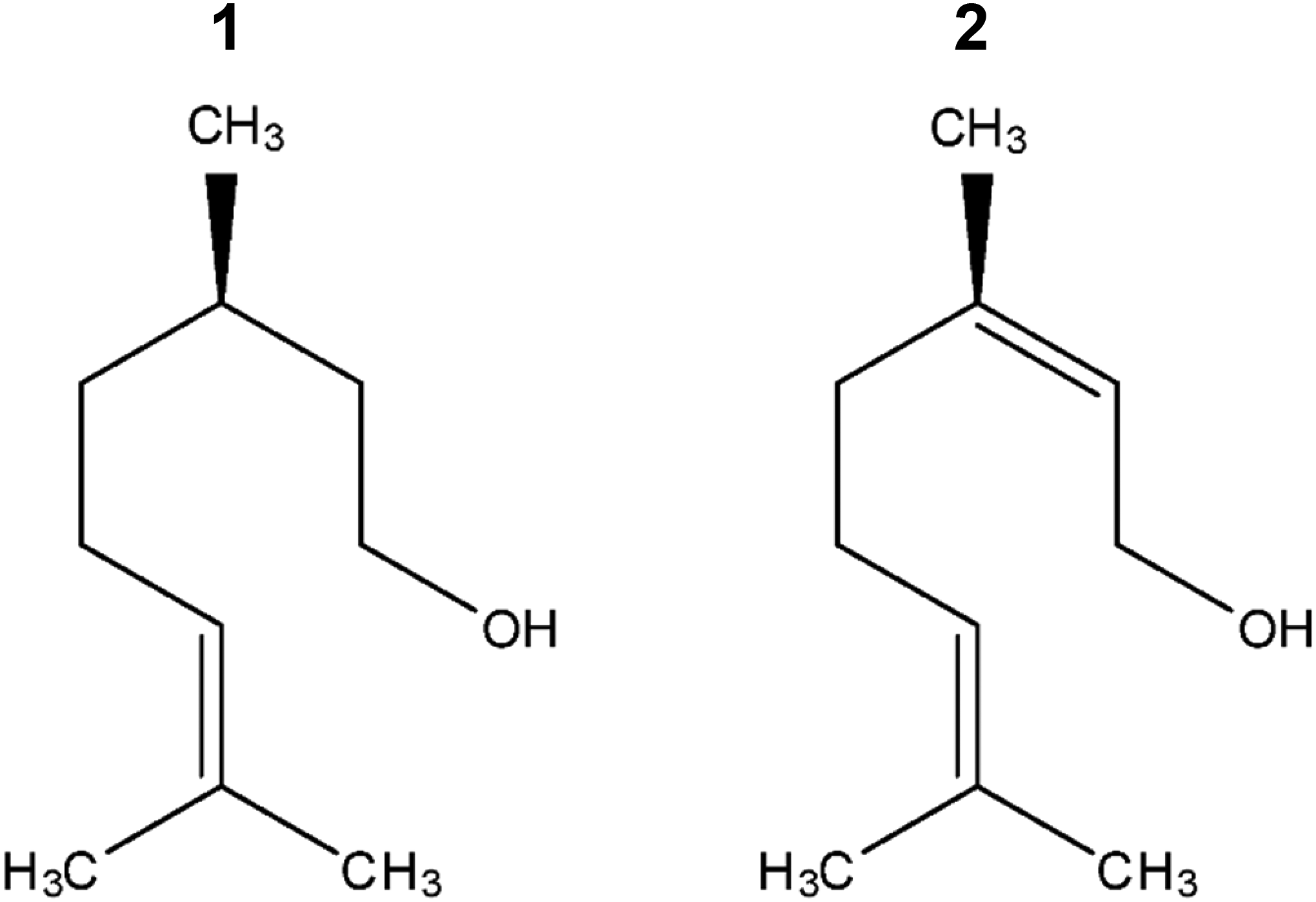
Figure 1: Chemical structures of citronellol (1) and geraniol (2)
The total run time was 30 min. In accordance with the method reported by Adams [19], the individual compounds were identified based on comparison of their retention indexes (RI)–relative to that of n-alkanes (C6-C32) as a standard– and retention time. The identity of compounds was confirmed by comparison of their mass spectra with the NIST02 library and literature data [20]. Relative percentage amounts were calculated from the total ion chromatogram (TIC) using a computer.
The fumigant or repellent activity tests were done according to Martínez-Muñoz et al. [20]. The CcLEO, citronellol and geraniol were dissolved in ethanol. The outer edge of No. 3 filter paper disks was impregnated with 50 µL of serial concentrations of plant essential oils, geraniol or citronellol and air dried to remove the solvent. The impregnated filter paper disks were placed in the bottom of Petri dishes and ten workers and three soldier dry wood termites were released inside each one. The Petri dishes were placed in an isolated chamber in darkness. The dry wood termites distributed randomly in the petri dish, but with a tendency to be in contact with the vertical wall of the petri dish, perhaps, this behavior occurs because termites in a colony inside wood need the security of the tunnel and contact with the tunnel walls. Essential oil and terpenes in the outer edge of the disks act as an irritant obstacle between the termites and the vertical walls of the Petri dish. The repellent activity of plant essential oils was determined by recording the number of termites in the center of the paper disc each 10 min.
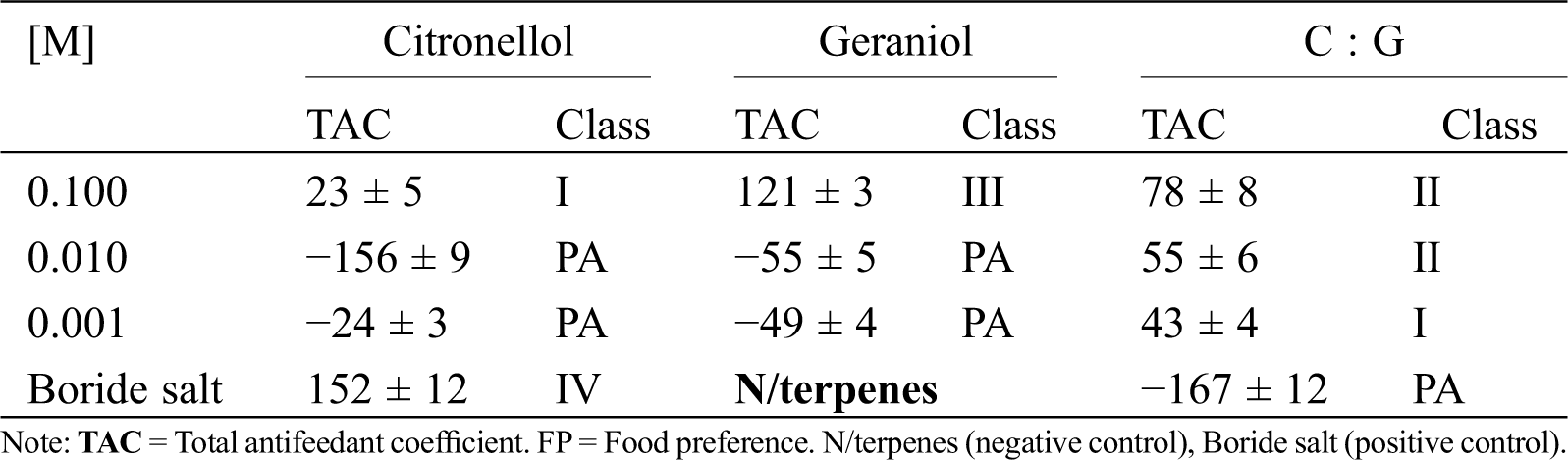
To know the antifeedant effect of CcLEO, forced feeding and food selection assays were made. Food probes were prepared with 10 mm × 10 mm × 2 mm blocks of unpreserved pine wood veneer as substrate, each impregnated by immersion in CcLEO, citronellol, or geraniol at a concentration of 1 mg/mL and in 1:10 dilutions until 1 µg/mL. The positive control probes were impregnated in a mixture of boride salts and negative control probes were unpreserved blocks. Five nymphs and two soldier termites were used by treatment, which were kept at room temperature in darkness for 45 to 50 days. Probes impregnated with each plant compound and exposed to the voracity of termites were weighted every two or three days. The quantity of food substrate consumed by the dry wood termite in each treatment was determined by the differences in probe weight. The total antifeedant coefficient (T) was determined using the data from the forced feeding (A) and food selection (R) assays according to Daniewski et al. [21]. Briefly, the percent antifeedant coefficient in forced feeding conditions was calculated by A% = [(KK – EE)•(KK+ EE)−1]100, where A = antifeedant coefficient in forced feeding assays, KK = weight loss in control probes, and EE = weight loss in treated probes. The percent food selection antifeedant coefficient was estimated by Rn% = [(K−E)•(K + E)−1]100, where R = antifeedant coefficient in food selection assays, K = weight loss in control probes, and E = weight loss in treated probes. The total antifeedant coefficient was calculated by T = A + R. Food preference was classified as Class I (0 > T < 50), Class II (50 > T < 100), Class III (100 < T < 150), and Class IV (150 > T < 200), 200 being the highest value of T for an effective antifeedant. In both assays, the appetite, viability and weight loss of dry wood termites were determined.
2.5 Determination of the Effect on the Viability, Appetite, and Weight Loss of the Dry Wood Termite I. marginipennis
Counting of death and alive individuals was done each sampling day for each treatment (forced feeding and food selection assays) during the 6 days of the experiment: Molar concentrations tested were 0.1, 0.01, and 0.001. The surviving individuals in each sampling were weighed every three days in an XPR Mettler Toledo precision ultra-microbalance to determine the weight gain or loss of the insects in the treatments. The consumption of substrate of the dry wood termite in presence of terpenes was measured every other day by weighing the filter paper disks impregnated with each compound from the beginning to the end of the experiment. The quantity in milligrams of the paper consumed by the termites was determined by weight difference.
2.6 Acetylcholinesterase Assay
Acetylcholinesterase (AChE) assay was estimated by colorimetric method described by Ellman et al. [22]. Homogenates of ten dry wood termites were prepared in phosphate buffer as described and 40 µl of the supernatant from everyone was loaded in a microplate well, with two replicates of each. The volume in each well was brought to 100 µl using pH 7.4 phosphate buffer. Then 200 µl of 1 mM of ATChI/DTNB dissolution was added. The microtitre plate was quickly transferred to Epoch BioTek microplate reader and activity of AChE monitored at 405 nM and 25°C for 10 min. Protein was determined according to the method of Bradford, using bovine serum albumin (fraction 5) as a standard.
2.7 Molecular Docking Analysis of Acetylcholinesterase and Geraniol
The molecular structure of acetylcholinesterase (AChE, EC 3.1.1.7) was obtained from the Protein Data Bank with code 1QO9, corresponding to the enzyme in Drosophila melanogaster found forming complexes with the inhibitors 1,2,3,4-tetrahydro-N-(phenylmethyl)-9-acridinamine and 1,2,3,4-tetrahydro-N-(3-iodophenyl-methyl)-9-acridinamine. Both inhibitors and water molecules were removed from the enzyme’s structure in the AutoDockTools program. The coordinates for the search map were, first, the location of the enzyme’s active site according to Harel et al. [23] and 12 more areas with different shapes, covering the whole enzyme. The structures of citronellol and geraniol were obtained from the Chemspider database and adjusted to their minimal energy configuration with the Maestro software. The molecular docking was made in the AutoDockVina program and the obtained results were viewed in Discovery Studio Visualizer [24].
Each treatment was made by triplicate and the obtained results were analyzed by pairwise comparison of means in a Tukey test with α = 0.01 using the Statistica 6.0 software for Windows (Statsoft Inc., USA)
3.1 Deterrent Effect on Incisitermes marginipennis of C. coriaria Leaf Essential Oil
The CcLEO extract had a higher antifeedant effect and a lower repelling effect on dry wood termites (Figs. 2b and 2f) compared to the corresponding controls (Figs. 2a and 2e).
3.2 Volatile Components in C. coriaria Leaf Essential Oil
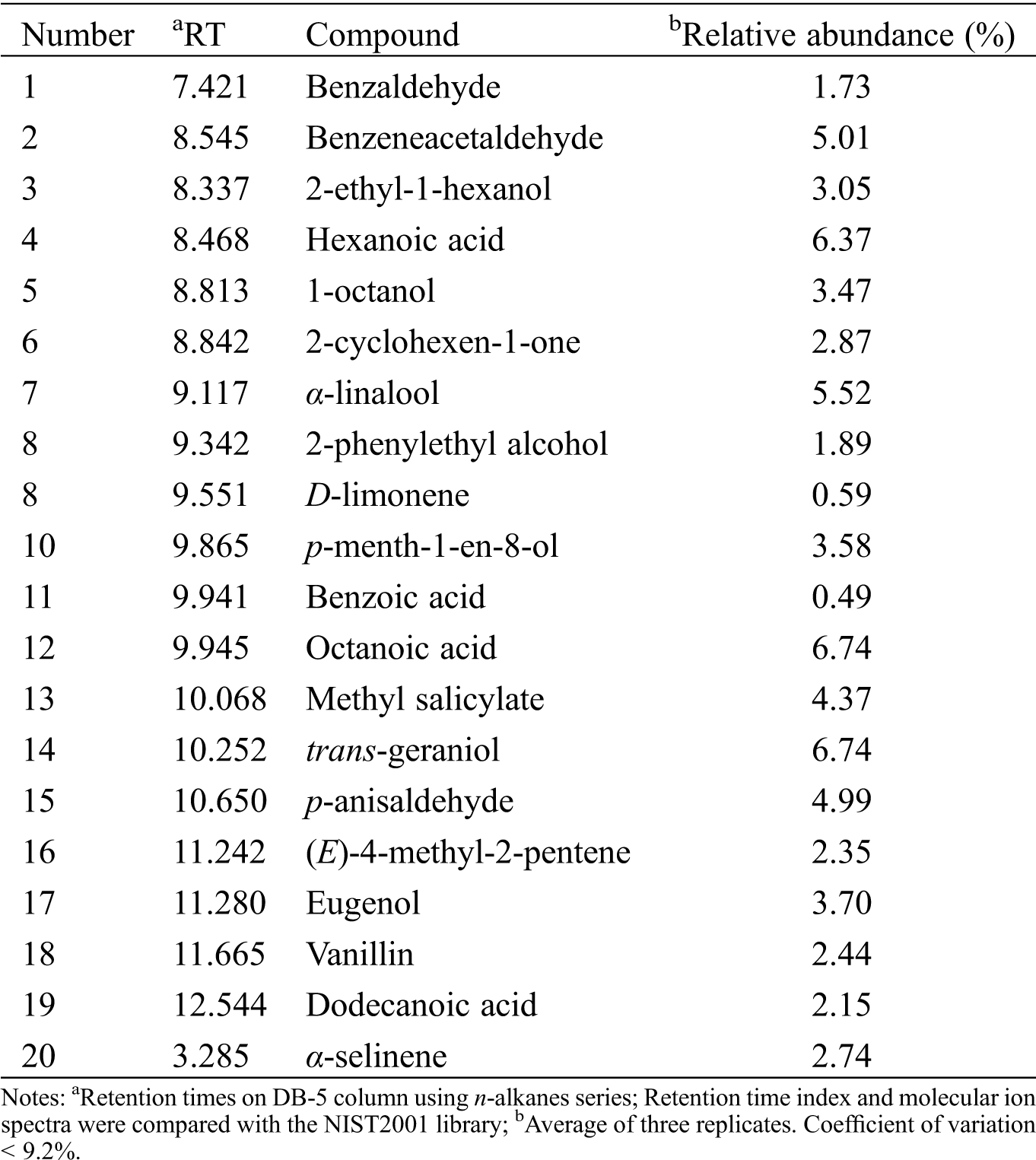
Twenty components were determined in the chromatographic analysis of CcLEO, geraniol being one of the most abundant components with a relative abundance of 6.74% followed by octanoic and hexanoic acids, linalool, and benzeneacetaldehyde (Tab. 1).
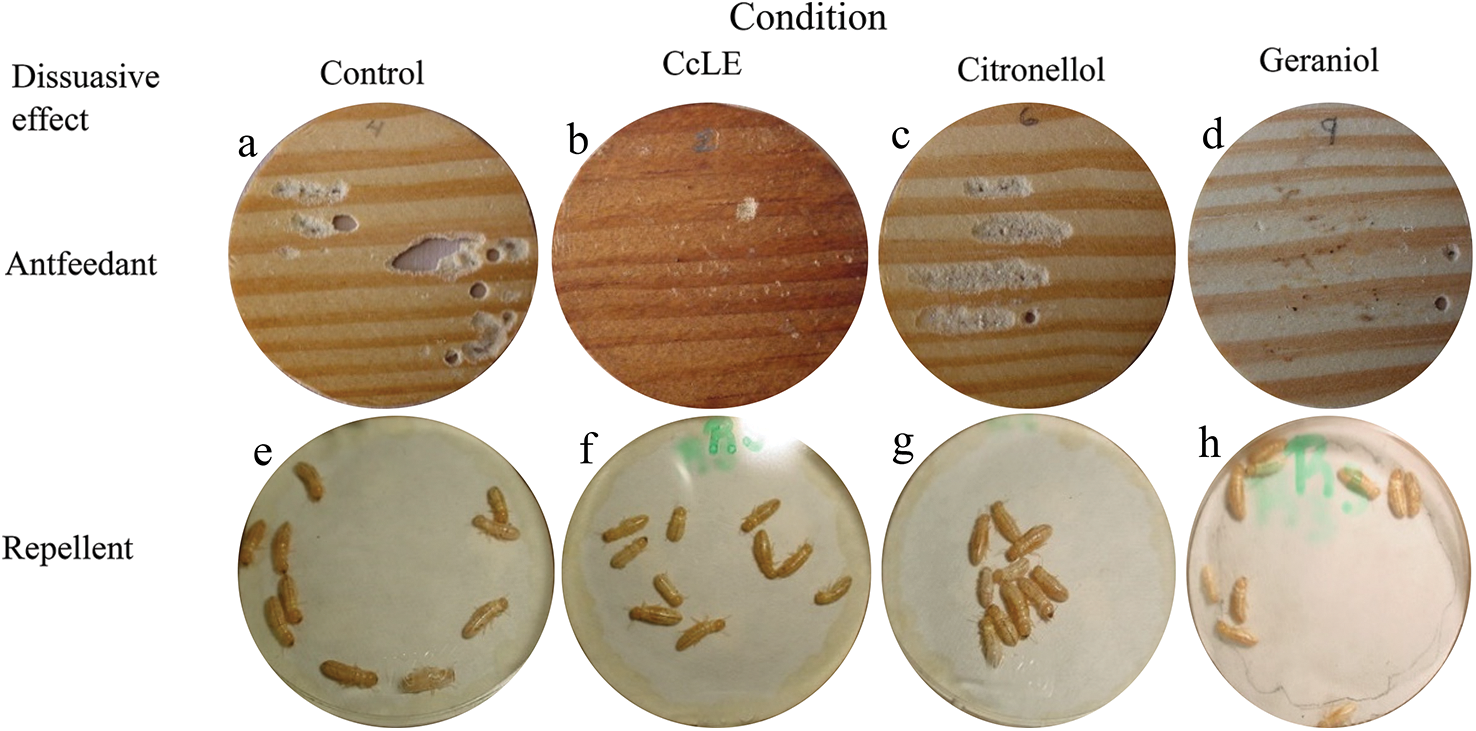
Figure 2: Repellent effect of the leaf essential oil from C. coriaria (CcLEO)
Table 1: Major components from the essential oil of C. coriaria
3.3 Deterrent Effect of Geraniol on the Dry Wood Termite I. marginipennis
Repellent effect. Tab. 2 and Fig. 2h considers the assessment of the repellent effect of geraniol compared with citronellol (Fig. 1).
Table 2: Repellent effect of geraniol on dry wood termite I. marginipennis
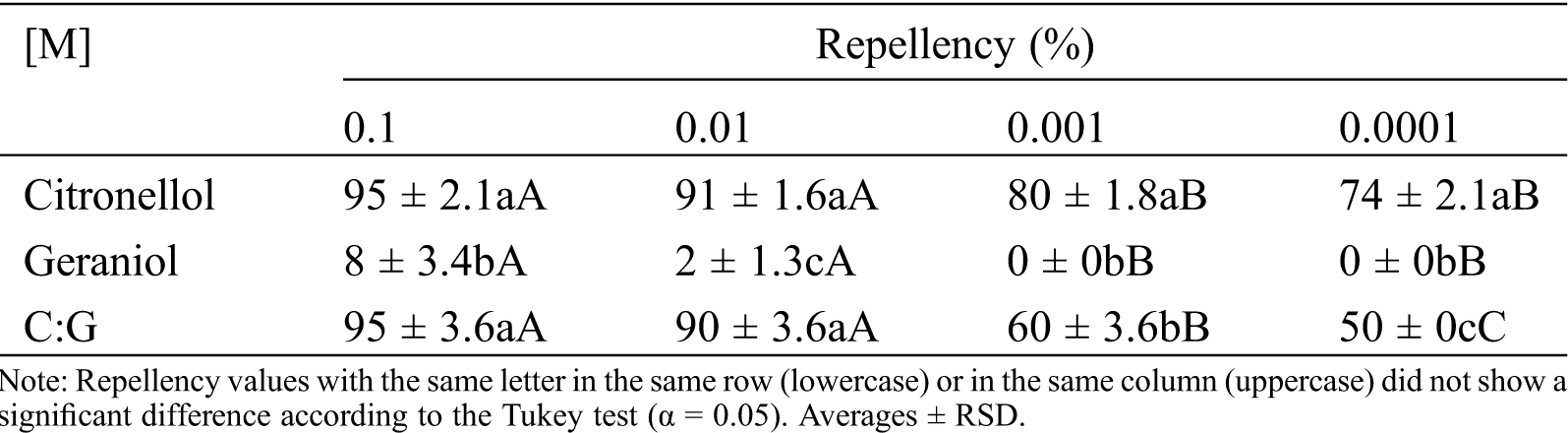
Geraniol had not repellent effect on the dry wood termite at the evaluated concentrations relative to citronellol, which had a repellent effect of 95% to 74%. Although there are structural similarities between geraniol and citronellol, differing in the presence of a double bond, a repellent biological activity was only found with citronellol (control). In the equimolar combination, the repellent effect of citronellol was observable. Geraniol was not considered to be a compound having an irritant repellent effect on the insect.
Antifeedant effect. Tab. 3 shows the effect of geraniol on the voracity of the dry wood termite. The effect on the feeding behavior of the termite was made according to Daniewsky by measuring the voracity in relation to substrate availability (forced feeding and food selection). Under that criterion, geraniol had an antifeedant effect on the termite and, at the highest concentration, it acted as a Class III antifeedant. However, at the maximum assayed concentration, citronellol had a minimal antifeedant effect on the termite classifying in Class I. In the combined compounds, the effect of geraniol is observable as Class II at the 1−1 and 1−2 molar concentrations. This result exhibits geraniol as an inhibitor of feeding in the dry wood termite.
Table 3: Termite antifeedant class of the citronellol
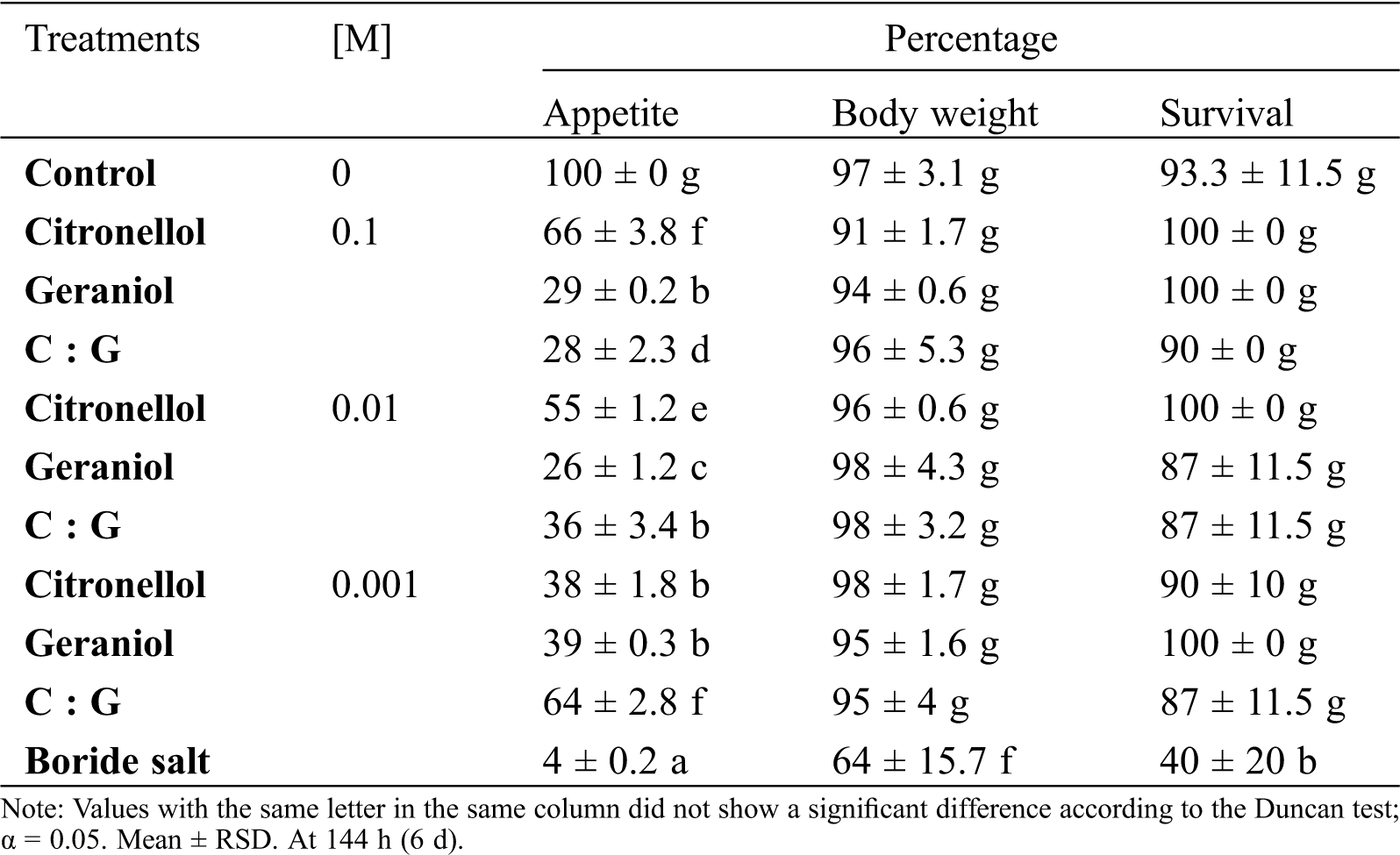
3.4 Intake Behavior of the Dry Wood Termite I. marginipennis in Presence of Geraniol
Deterrent agents should affect the intake behavior of the insects to a considerable extent. The deterrent activity on the dry wood termite was determined for geraniol and citronellol in force feeding conditions during 144 h by measuring the survival, voracity, and body weight of the insect (Tab. 4). Geraniol, citronellol, and their equimolar mixture had no effect on the survival and body weight of the termites. The presence of geraniol decreased the voracity of the insects by 70%–the termites consumed only 29% of the substrate. In force feeding conditions, citronellol had a moderate inhibitory effect in substrate intake of up to 66%. These data suggest that geraniol has an inhibitory effect on the voracity of the dry wood termite.
Table 4: Intake behavior of dry wood termite I. marginipennis in the presence of geraniol and citronellol
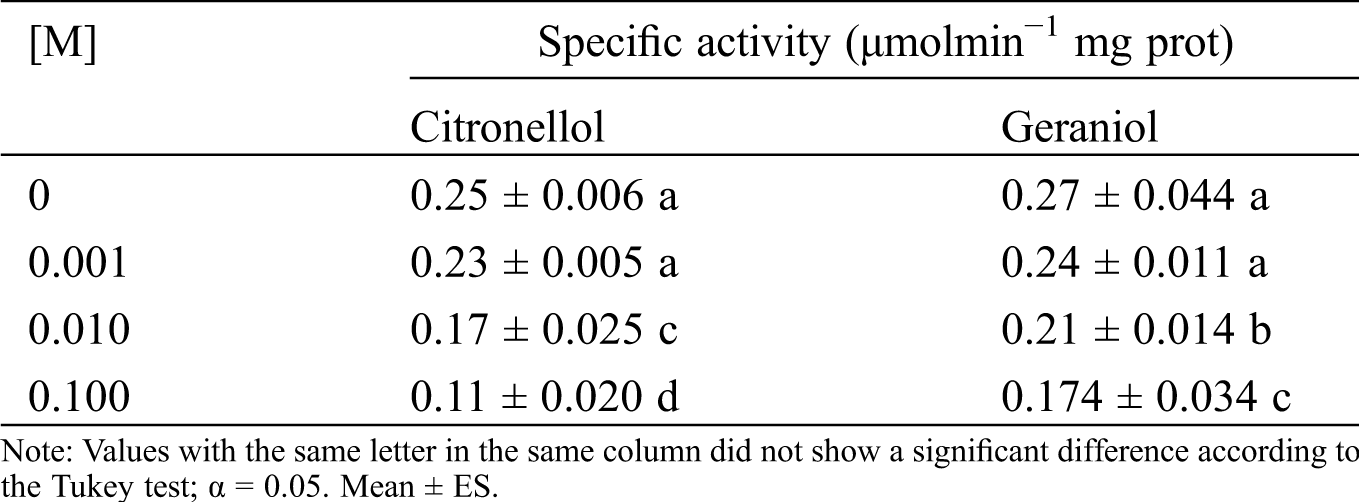
3.5 Effect of Geraniol and Citronellol on I. marginipennis Acetylcholinesterase
It was hypothesized that acetylcholinesterase has a central role in the sensory modalities of the dry wood termite, such as smell and taste. So, the toxic and repellent effect of the plant essential oil was due to inhibition of acetylcholinesterase by geraniol and citronellol. To prove this assumption, the I. marginipennis acetylcholinesterase was assayed with geraniol or citronellol, see Tab. 5. The acetylcholinesterase was inhibited 36% and 57% by 5 mM geraniol and 5 mM citronellol, respectively. In addition to this observation, these terpenes have other pharmacological targets.
3.6 Molecular Docking Analysis of Acetylcholinesterase and Geraniol
Molecular docking with a rigid Acethylcholinesterase was performed to obtain the zone where citronellol and geraniol interact with less affinity energy. With these data, flexible molecular docking was performed.
Table 5: Effect of the citronellol and geraniol on I. marginipennis acetylcholinesterase
The search map for flexible molecular docking with citronellol and geraniol was with dimensions of 30.172. Å x 38.906 Å x 27.79 Å and coordinates of 31.761, 63.577 and 8.821. Molecular docking between the D. melanogaster Acetylcholinesterase and the citronellol and geraniol ligands had an interaction energy of −6.7 Kcal/mol and −7.5 Kcal/mol, respectively. The interaction was carried out near to same site, see Fig. 3. This site known as the active site [23].
Botanical insecticides were used in Mesoamerica by the Aztecs and Mayans since Pre-Hispanic times and, in Europe, since the 17th century essential oils from plants were obtained by hydrodistillation, to which medicinal properties were later attributed. In the recent past, these compounds were displaced by synthetic and semisynthetic insecticides that have been extremely effective for pest control, but also for causing adverse effects in ecosystems, animal health–including that of humans, and generating resistance in insects; circumstances that lead back to the study of botanical insecticides.
Because of that, the native plants of the traditional pharmacopoeias, their extracts, and their essential oils have gained new interest [25–27]. Some successful botanical insecticides based on plant essential oils or its components are those extracted from Tanacetum cinerariifolium–containing esters of chrysanthemic acid (pyrethrin I, cinerin I, and jasmolin I) and pyrethric acids (pyrethrin II, cinerin II, and jasmolin II), nicotine extracted from tobacco, and rotenone extracted from Derris elliptica [28], secondary metabolites which became models for the obtention of potent semisynthetic insecticides which lose their natural characteristics.
In the case of the dry wood termite, the insect is controlled with insecticides formulated based on metallic salts, boron, and semisynthetic and synthetic compounds. The use of deterrent agents for termite pest control, including confusing agents, oviposition inhibitors, antifeedants, and repellents, became aids for solving the dilemma of having to control a beneficial insect turned into a plague [29]; the biological function of termites is extremely important for the maintenance of the delicate natural balance in the different ecosystems that they inhabit, playing key roles in the carbon, hydrogen, nitrogen, oxygen, and phosphorous geochemical cycles during soil formation, because of which their populations must not be drastically affected [30].
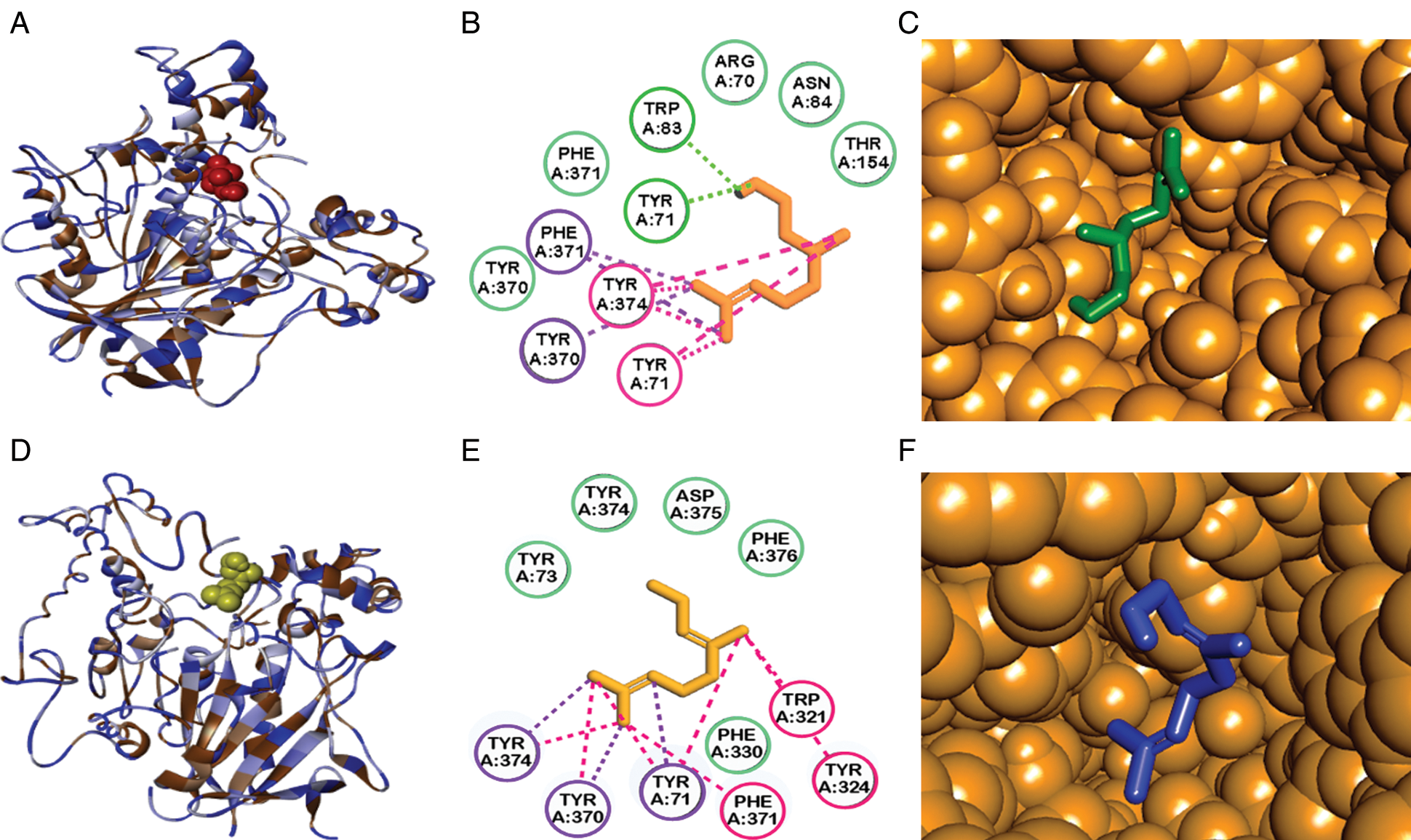
Figure 3: Molecular docking studies showing the best binding conformation of acetylcholinesterase with terpenes tested. Enzyme interactions with citronellol (A, B, C) and geraniol (D, E, F). A and D are 3D ligand interaction diagrams. B and E are 1D ligand interaction diagrams. The dotted lines in color are an interaction kind: in green (hydrogen bridges), in pink (pi-alkyl interactions) and in purple (pi-sigma interactions). Amino acids in green have van der Waals forces. C are F are enzyme pocket binding sites and their interactions with citronellol and geraniol
The traditional use of C. coraria in Michoacán, Mexico as a physical and chemical barrier against predators and harmful animals led us to study metabolites obtained from species in the genus Caesalpinia for the control of the dry wood termite. For that, a bioguided phytochemical fractionation was made to find possible repellent and antifeedant effects. Our results found no obtained fraction had repellent effects on the dry wood termite; however, a fraction–shown by GC/MS to be mostly composed of geraniol–showed an antifeedant deterring activity on the insects. The geraniol is one of the major components of the essential oils of aromatic plants and has bioactive properties [31,32]. The detection of geraniol in C. coraria led to assume that this most abundant component in the plant’s extract was the responsible for its deterrent effect, and to establish the possible effects of geraniol as a repellent and antifeedant agent against the dry wood termite.
To determine the repelling activity, we used citronellol as a positive control, which is the oldest and better-known natural insect repellent [31]. The connection between geraniol and its derived terpenes like citronellol raises interest regarding their structural differentiation and, therefore, the differentiated functionality of each compound. As geraniol, citronellol is a commercial monoterpene used in the food and perfume industries because of its rose scent [33]. In this study, we compared the repelling efficacy of geraniol and citronellol. Geraniol is the biosynthetic precursor of citronellol, nerol, and their esters. The oxidation products of geraniol, known as citral, are characteristic of citric scent. In nature, citronellol (3,7-dimethyl-6-octen-1-ol or hydro geraniol) exists in the R and S enantiomeric forms.
Our results showed geraniol had no repellent effect on the dry wood termite. However, we did observe that geraniol inhibited the appetite of the dry wood termite relative to boride salts–used as a mineral preservative to protect pine wood from the biodeterioration caused by boring xylophagous insects.
The mixed terpenes had the same antifeedant and repellent effect than those exhibited by geraniol and citronellol individually. The differential biological activity of compounds like citronellol and geraniol had insecticidal activity on Anisakis simplex causing alterations in their digestive tract [34]. Therefore, the mixture of these compounds can be applied to pine wood in service. Recent studies demonstrated that citronellol (hydrogeraniol) and geraniol had insecticidal activity [35]. Plant terpenes are currently a potential tool for insect control and wood preservation. In that regard, some terpenes like citronellol are widely used and considered to be botanical repellents and insecticides in the control of insects that inhabit open spaces like the mosquito Aedes aegypti [34].
Our results of the evaluation of the analyzed terpenes at the three concentrations we used showed that citronellol had no effect on the viability and substrate consumption–being of 76% at 0.1 M concentration, a similar effect to that in the untreated negative control, therefore not affecting body weight loss of the insect.
However, geraniol at 0.1 M concentration inhibited substrate consumption in the same degree as the boride salts positive control, both at less than 10%, because of which the body weight loss was higher than in the other assayed treatments. The geraniol-citronellol mixture at 0.01 M concentrations and 0.1 M geraniol caused similar body weight losses in the dry wood termites due to diminished substrate consumption (average values of 20% and 19%, respectively), but the viability observed in both treatments was 40% compared to 27% in treatment with boride salts.
Several explanations are possible to understand the effects of geraniol and citronellol in two sensory modalities of dry wood termite such as taste and smell. Possible explanations are the roles of the insect metabolism of geraniol and citronellol, acetylcholinesterase, and transient receptor potential melastatin (TRPM) channels, another possible explanation is the movement of intracellular Ca2+. The reduction of geraniol to citronellol –formation of a simple bond in carbon 2– is the first step in the synthesis of natural phytol in the production of tocopherols, and genes related to the enzyme in charge of that reduction have been detected [36].
In insects, acetylcholinesterase is present only in the synapses of the central nervous system, where it hydrolyses the neurotransmitter acetylcholine. Both citronellol and geraniol inhibit 60% of the acetylcholinesterase activity in larvae of Anisakis simplex, Sitophilus oryzae, Rhyzopertha dominica, and Cryptolestes pusillus causing their death [34,37]. Our results agree with the previously observed antagonism between acetylcholinesterase and geraniol, and it is possible that other geraniol targets are involved. The relationship between inhibition of AChE and the deterrent effects of terpenes exhibited by I. marginipennis behavior is not clear.
One way to study the interaction of acetylcholinesterase with geraniol is to apply bioinformatic tools, for which we used the acetylcholinesterase of Drosophila melanogaster (accession number 1QO9) because of its similarities in the biochemical behavior of other insect acetylcholinesterases.
Our results of molecular docking between acetylcholinesterase and geraniol found the best bonding energy between acetylcholinesterase and geraniol of −7.5 Kcal/mol, one in the active site search map without formation of hydrogen bonds, and another one adjacent to the active site of the enzyme, therefore involving the enzyme’s activity. The affinity energy of −6.7 Kcal/mol that we found for citronellol was similar to those found for geraniol, but the formation of hydrogen bonds differed widely. The hydrogen bond being formed with tyrosine 71 and tryptophan 83, both aminoacids located adjacent to the active site. The primary modes of action of these hydrogen bonds remain unclear, and further research is needed to understand the mechanism of toxicity.
In our evaluation of variables causing physiological alterations on the dry wood termite we considered that the development and survival of the insects would not be affected to a large extent, but that the insects should avoid consuming the substrate, therefore protecting wood deterioration; an effect that was similar in geraniol and boride salts (positive control) treatments.
The sensory modalities of insects–sight, touch, hearing, smell, taste, and others–are comparable to those of mammals [38]. It has been shown that some of these sensory modalities are dependent on transient receptor potential (TRP) channels in fruit flies [39]. The toxic and repellent properties of plant monoterpenoids used as alternatives to synthetic insecticides against stored products pests have been studied [40].
Recently, a comparative genomics study of insect TRP channels using Drosophila melanogaster, Bombyx mori, Tribolium castaneum, Apis mellifera, Nasonia vitripennis, and Pediculus humanus showed that all the examined insects contained two TRPV (vanilloid), one TRPN, one TRPM, three TRPC (canonical), and one TRPML (mucolipin) channels [41]. Interestingly, a single TRPM was detected in those insects. We assumed that the dry wood termite contains a putative TRPM channel like other insects. The functions of the ubiquitous TRPM channels in insects have not been identified. Some mammalian TRPM channels are associated with taste [42] and cold perception [43,44].
The TRPM subfamily has been shown to have vastly differing modes of activation, cation selectivity, and tissue distributions [45]. The TRPM subfamily is presented in four groups based on structural similarity: Group I, TRPM1 and TRPM3; Group II, TRPM4 and TRPM5; Group III, TRPM2, TRPM6, and TRPM7, and Group IV, TRPM8. In the mammalian taste buds, TRPM5 has been shown to be a transducer of bitter, sweet, and umami taste sensation [46]. This was confirmed using mice models lacking functional TRPM5. Also, mammalian TRPM8 is modulated by terpenes such as menthol [47,48]. Our data are in correlation with the function of insect TRPM versus mammalian TRPM.
Another possibility is that geraniol could mobilize [Ca2+]cyt in insect taste bulbs in a TRPM nonselective cation channel, as in HEK-293 cells and PC-3 cells [49]. Changes in the cytosolic free Ca2+ concentration ([Ca2+]i), play a central role in many fundamental cellular processes.
Our work demonstrated the efficacy of leaf extracts from C. coriaria as a deterrent insecticide against the dry wood termite I. marginipennis. The essential oil of C. coriaria has antifeedant effect due to its terpene content and geraniol was found to be the major component. Geraniol decreased the appetite of the dry wood termite, because of which it is proposed to be a deterrent agent, while citronellol had a repellent effect on the dry wood termite. This is the first report of the evaluation of the effectiveness of geraniol and citronellol as control agents of the dry wood termite, the compounds showing deterrent effect as repellent and antifeedant, respectively.
Acknowledgement: C. B. Ramírez López, R. Beltrán Sánchez, A. Hernández Izquierdo and J. L. Salvador Hernández are grateful with CONACyT for the scholarship.
Authorship and Contribution: C. B. Ramírez López extracted and analyzed the volatile compounds. J. L. Salvador Hernández assessed repellent effects. R. Beltrán Sánchez carried out the in-silico analysis by molecular docking. A. Hernández Izquierdo carried out the acetylcholinesterase assay. M. M. Martínez Pacheco and E. Salcedo Pérez designed this project, coordinated all the activities, and contributed with the preparation of the manuscript. R. E. del Río supervised the chemical studies.
Funding Statement: This research was funded by the institutional research programs of UMSNH, University of Guadalajara and the philanthropic program of the M3P Foundation.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Gutiérrez Vázquez, E., Villalba Sánchez, C. A., Juárez Caratachea, A. (2018). Propagación sexual y asexual de especies arbóreas forrajeras de la selva baja caducifolia de la región de Tierra Caliente, Michoacán, Méx. Avances en Investigación Agropecuaria, 22, 1–6 (Abstract in English). [Google Scholar]
2. Gómez-Hurtado, M. A., Álvarez-Esquivel, F. E., Rodríguez-García, G., Martínez-Pacheco, M. M., Espinoza-Madrigal, R. M. et al. (2013). Cassane diterpenes from Caesalpinia platyloba. Phytochemistry, 96, 397–403. DOI 10.1016/j.phytochem.2013.09.028. [Google Scholar] [CrossRef]
3. Lokeswari, N., Sujatha, P. (2011). Isolation of tannins from Caesalpinia coriaria and effect of physical parameters. International Research Journal of Pharmacy, 2(2), 146–152. [Google Scholar]
4. Sánchez-Carranza, J. N., Alvarez, L., Marquina-Bahena, S., Salas-Vidal, E., Cuevas, V. et al. (2017). Phenolic compounds isolated from Caesalpinia coriaria induce S and G2/M phase cell cycle arrest differentially and trigger cell death by interfering with microtubule dynamics in cancer cell lines. Molecules, 22(4), 666–679. DOI 10.3390/molecules22040666. [Google Scholar] [CrossRef]
5. Gilani, S. M. U., Ahmed, S., Baig, S. G., Hasan, M. M. (2019). Ethnopharmacognosy, phytochemistry and pharmacology of genus Caesalpinia: A review. Journal of Pharmacognocy and Phytochemistry, 8(4), 2222–2229. [Google Scholar]
6. Ma, G., Wu, H., Chen, D., Zhu, N., Zhu, Y. et al. (2015). Antimalarial and antiproliferative cassane diterpenes of Caesalpinia sappan. Journal of Natural Products, 78(10), 2364–2371. DOI 10.1021/acs.jnatprod.5b00317. [Google Scholar] [CrossRef]
7. Wang, D. S., Nie, W., Jiang, T. T., Ding, L. F., Song, L. D. et al. (2020). Caesalpanins A-C, three dimeric cassane diterpenoids from the seeds of Caesalpinia sappan L. Chemistry and Biodiversity, 17(5), e2000103. [Google Scholar]
8. Kumbhare, M. R., Sivakumar, T. (2019). Caesalpinia pulcherrima extract on blood glucose in normal and alloxan monohydrate-induced diabetic rats. Journal of Biological Sciences, 19(1), 34–39. DOI 10.3923/jbs.2019.34.39. [Google Scholar] [CrossRef]
9. Olayé, T., Tchobo, F. P., Chabi, N. W. (2018). Phytochemical potential, antiradical and antimicrobial activity of crude extracts of Caesalpinia benthamiana roots used for oral hygiene in Benin republic. Journal of Pharmacognosy and Phytochemistry, 7(5), 1939–1944. [Google Scholar]
10. Oh, M., Park, S., Song, J. H., Ho, H. J., Kim, S. H. (2020). Chemical components from the twigs of Caesalpinia latisiliqua and their antiviral activity. Journal of Natural Medicines, 74(1), 26–33. DOI 10.1007/s11418-019-01335-2. [Google Scholar] [CrossRef]
11. Taylor, A. M., Gartner, B. L., Morrell, J. J., Tsunoda, K. (2006). Effects of heartwood extractive fractions of Thuja plicata and Chamaecyparis nootkatensis on wood degradation by termites or fungi. Journal of Wood Science, 52(2), 147–155. DOI 10.1007/s10086-005-0743-6. [Google Scholar] [CrossRef]
12. Raya-González, D., Martínez-Muñoz, R. E., Ron-Echeverría, O. A., Flores-García, A., Macías-Rosdríguez, L. I. et al. (2013). Dissuasive effect of an extract aqueous from Enterolobium cyclocarpum (Jacq) Griseb on the dry wood termite Incisitermes marginipennis (Isoptera: Kalotermitidae) (Latreille). Emirates Journal of Food and Agriculture, 25(7), 524–530. DOI 10.9755/ejfa.v25i7.15987. [Google Scholar] [CrossRef]
13. Scheffrahn, R. H. (2019). Expanded new world distributions of genera in the termite Family Kalotermitidae. Sociobiology, 66(1), 136–153. DOI 10.13102/sociobiology.v66i1.3492. [Google Scholar] [CrossRef]
14. Cibri, D., Mez Montiel, J. T., Campos Bolaos, R., Yates, O. H., Lara Flores, J. (1995). Insectos Forestales de México/Forest Insects of México. Universidad Autnoma Chapingo. Subsecretaa Forestal y de Fauna Silvestre, SARH, Mexico. Forest Service, USDA, USA. Natural Resources Canada, FAO. Pub. Esp. 6. pp. 453. [Google Scholar]
15. Mahapatro, G. K., Chatterjee, D. (2018). Termites as structural pest: Status in Indian scenario. Proceeding of the National Academy of Science, India, Section B: Biological Science, 88, 977–994. [Google Scholar]
16. Krishna, K., Weesner, F. M. (1970). Biology of termites. vol. II. New York and London: Academic Press. [Google Scholar]
17. Nickle, D. A., Collins, M. (1988). The termite fauna (Isoptera) in the vicinity of Chamela, State of Jalisco, Mexico. Folia Entomologica Mexicana, 77, 85–122. [Google Scholar]
18. Mexican Standards. NOM-059-ECOL-1994. Secretaría de Desarrollo Social. NOM-005-RECNAT-1997, NOM-007-RECNAT-1997 and NOM-009 RECNAT-1997. Secretaría de Recursos Naturales. México. http://www.siicex.gob.mx/PortalSiicex/SICITECA/Acuerdos/NOMS/nomsx.htm. [Google Scholar]
19. Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry. 4th Edition. Allured Pub Corp. Carol Stream. [Google Scholar]
20. Martínez Muñoz, R. E., Raya González, D., Cajero Juárez, M., Calderón Raya, M., del Río, R. E. et al. (2009). Innocuous use of aqueous extract obtained from the heartwood of Enterolobium cyclocarpum (Jacq.) Griseb. Pharmacologyonline, 2, 1091–1096. [Google Scholar]
21. Daniewski, W. M., Gumulka, M., Przesmycka, D., Ptaszyńska, K., Błoszyk, E. et al. (1995). Sesquiterpenes of Lactarius origin, antifeedant structure-activity relationships. Phytochemistry, 38(5), 1161–1168. DOI 10.1016/0031-9422(94)00781-N. [Google Scholar] [CrossRef]
22. Ellman, G. L., Courtney, K. D., Valentino, A. J., Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88–95. DOI 10.1016/0006-2952(61)90145-9. [Google Scholar] [CrossRef]
23. Harel, M., Kryger, G., Rosenberry, T., Mallender, W. D., Lewis, T. et al. (2000). Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Science, 9(6), 1063–1072. DOI 10.1110/ps.9.6.1063. [Google Scholar] [CrossRef]
24. Trott, O., Olson, A. J. (2010). Software news and update AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computer Chemistry, 31(2), 455–461. [Google Scholar]
25. Smith, R. L., Cohen, S. M., Doull, J., Feron, V. J., Goodman, J. I. et al. (2005). A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food and Chemical Toxicology, 43(3), 345–363. DOI 10.1016/j.fct.2004.11.007. [Google Scholar] [CrossRef]
26. Saxena, M., Saxena, J., Nema, R., Dharmendra, S., Abhishek, G. (2013). Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry, 1(6), 168–182. [Google Scholar]
27. Ramírez-López, C. B., García-Sánchez, E., Martínez-Muñoz, R. E., del Río, R. E., Martínez-Pacheco, M. M. (2016). Chemical composition of the essential oil from Ageratina jocotepecana and its repellent effect on dry wood termite Incisitermes marginpennis. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 15(1), 53–60. [Google Scholar]
28. Oguh, C. E., Okpaka, C. O., Ubani, C. S., Okekeaji, U., Joseph, P. S. et al. (2019). Natural pesticides (biopesticides) and uses in pest management. A critical review. Asian Journal of Biotechnology and Genetic Engineering, 2(3), 1–18. [Google Scholar]
29. Konradsen, F., van der Hoek, W.,Gunnell, D., Eddleston, M. (2005). Missing deaths from pesticide self-poisoning in the IFCS forum IV. Bulletin of World Health Organization, 83(2), 157–158. [Google Scholar]
30. Maynard, D. S., Crowther, T. W., King, J. R., Warren, R. J., Bradford, M. A. (2015). Temperate forest termites: Ecology, biogeography, and ecosystem impacts. Ecological Entomology, 40(3), 199–210. DOI 10.1111/een.12185. [Google Scholar] [CrossRef]
31. Bakkali, F., Averbeck, S., Averbeck, D., Idaomar, M. (2008). Biological effects of essential oils–A review. Food and Chemical Toxicology, 46(2), 446–475. DOI 10.1016/j.fct.2007.09.106. [Google Scholar] [CrossRef]
32. Nana, W. L., Eke, P., Fokom, R., Bakanrga-Via, I., Begoude, D. et al. (2015). Antimicrobial activity of Syzygium aromaticum and Zanthoxylum xanthoxyloides essential oils against Phytophthora megakarya. Journal of Phytopathology, 163(7–8), 632–641. DOI 10.1111/jph.12363. [Google Scholar] [CrossRef]
33. Guenther, E. (1950). The essential oils: History-origin in plants production, analysis. D. Van Nostrand Company. New York, USA. [Google Scholar]
34. Hierro, I., Valero, A., Pérez, P., González, P., Cabo, M. M. et al. (2004). Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine, 11(1), 77–82. DOI 10.1078/0944-7113-00375. [Google Scholar] [CrossRef]
35. Anderson, J. A., Coats, J. R. (2012). Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pesticide Biochemistry and Physiology, 102(2), 124–128. DOI 10.1016/j.pestbp.2011.12.002. [Google Scholar] [CrossRef]
36. Yuan, T. T., Chen, Q. Q., Zhao, P. J., Zeng, Y., Liu, X. Z. et al. (2011). Identification of enzymes responsible for the reduction of geraniol to citronellol. Natural Products and Bioprospecting, 1(3), 108–111. DOI 10.1007/s13659-011-0032-6. [Google Scholar] [CrossRef]
37. López, M. D., Pascual Villalobos, M. J. (2010). Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Industrial Crops and Products, 31(2), 284–288. DOI 10.1016/j.indcrop.2009.11.005. [Google Scholar] [CrossRef]
38. Cosens, D. J., Manning, A. (1969). Abnormal electroretinogram from a Drosophila mutant. Nature, 224(5216), 285–287. DOI 10.1038/224285a0. [Google Scholar] [CrossRef]
39. Lee, Y., Lee, Y., Lee, J., Bang, S., Hyun, S. et al. (2005). Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nature Genetics, 37(3), 305–310. DOI 10.1038/ng1513. [Google Scholar] [CrossRef]
40. García, M., Donadel, O. J., Ardanaz, C. E., Tonn, C. E., Sosa, M. E. (2005). Toxic and repellent effects of Baccharis salicifolia essential oil on Tribulium castaneum. Pest Management Science, 61(6), 612–618. DOI 10.1002/ps.1028. [Google Scholar] [CrossRef]
41. Matsuura, H., Sokabe, T., Kohno, K., Tominaga, M., Kadowake, T. (2009). Evolutionary conservation and changes in insect TRP channels. BMC Evolutionary Biology, 9(1), 228–239. DOI 10.1186/1471-2148-9-228. [Google Scholar] [CrossRef]
42. Zhang, Y., Hoon, M. A., Chandrashekar, J., Mueller, K. L., Cook, B. et al. (2003). Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell, 112(3), 293–301. DOI 10.1016/S0092-8674(03)00071-0. [Google Scholar] [CrossRef]
43. Fujiwara, Y., Minor, D. L.Jr (2008). X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. Journal of Molecular Biology, 383(4), 854–870. DOI 10.1016/j.jmb.2008.08.059. [Google Scholar] [CrossRef]
44. Hashimoto, A., Kambe, T. (2015). Mg, Zn and Cu transport proteins: A brief overview from physiological and molecular perspectives. Journal of Nutritional Science and Vitaminology, 61(Supplement), S116–S118. DOI 10.3177/jnsv.61.S116. [Google Scholar] [CrossRef]
45. Hofmann, T., Chubanov, V., Gudermann, T., Montell, C. (2003). TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Current Biology, 13(13), 1153–1158. DOI 10.1016/S0960-9822(03)00431-7. [Google Scholar] [CrossRef]
46. Liman, E. R. (2007). TRPM5 and taste transduction. In: Flockerzi, V., Nilius, B. (eds.Transient Receptor Potential (TRP) channels. Handbook of experimental pharmacology, vol. 179. Berlin, Heidelberg: Springer, 287–298. [Google Scholar]
47. Bautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I. et al. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature, 448(7150), 204–208. DOI 10.1038/nature05910. [Google Scholar] [CrossRef]
48. Yin, Y., Wu, M., Zubcevic, L., Borschel, W. F., Lander, G. C. et al. (2018). Structure of the cold- and menthol-sensing ion channel TRPM8. Science, 359(6372), 237–241. DOI 10.1126/science.aan4325. [Google Scholar] [CrossRef]
49. Kim, S. H., Bae, H. C., Park, E. J., Lee, C. R., Kim, B. J. et al. (2011). Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochemical and Biophysical Research Communications, 407(1), 129–134. DOI 10.1016/j.bbrc.2011.02.124. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |