
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015024
ARTICLE
Colonization Characteristics and Diversity of Arbuscular Mycorrhizal Fungi in the Rhizosphere of Iris lactea in Songnen Saline-alkaline Grassland
College of Landscape Architecture, Northeast Forestry University, Harbin, 150040, China
*Corresponding Author: Chunxue Yang. Email: senxiu99@163.com
Received: 16 November 2020; Accepted: 08 January 2021
Abstract: To understand arbuscular mycorrhizal (AM) fungi resources and develop AM fungal species in ornamental plants with saline-alkaline tolerances, Iris lactea, which grows in the Songnen saline-alkaline grassland with a high ornamental value, was selected as the experimental material, and the colonization characteristics of its roots and the AM fungal diversity in its rhizosphere were explored. The results of the observations and calculations of mycorrhizae from ten different samples showed that AM fungi colonized the roots of I. lactea and formed Arum-type mycorrhizal structures. There was a significant correlation between soil spore density and pH value, while the colonization rate showed a fluctuating trend with increasing pH values. The observed colonization intensities were of Levels II (1%–10%) or III (11%–50%), and the vesicle abundances were of grades A2 or A3 among different sites. AM fungi produced a large number of mycelia and vesicles in the roots of I. lactea after colonization. Thirty-seven species belonging to 15 genera of AM fungi were isolated from the rhizosphere of I. lactea and identified by morphological identification. Funneliformis and Glomus were the dominant genera, accounting for 21.79% and 20.85% of the total number, respectively. F. mosseae and Rhizophagus intraradices were isolated in all samples with importance values of 58.62 and 51.19, respectively. These results are expected to provide a theoretical basis for the analysis of the salt tolerance mechanism of I. lactea and for the discovery, exploration and further screening of AM fungal resources with salinity tolerances in saline-alkaline soils.
Keywords: Iris lactea; colonization characteristics; morphology of AM fungal spores; saline-alkaline soils
Salinization is considered to be the most important stress factor affecting crop yield. In the 20th century, the global salinization area was approximately 955 million hm2, accounting for 7% of the Earth’s surface. Since the beginning of the 21st century, 1/3 of the world’s farmland has been affected by salinization, and it is expected that 30% of all cultivated land will be lost as a result of salinization within 25 years [1,2]. The Songnen Plain is one of the largest plains in China and one of the three soda salinized soil distribution areas in the world [3]. The low-lying noncontributing area in the western Songnen Plain experienced salinization most severely. Soda salt (Na2CO3 and NaHCO3, etc.) are the main saline components, and small amounts of chloride and sulfate are also contained in the soils. The physicochemical properties of the soil are poor, and it is difficult to improve the soil quality [4]. With the changing natural environment and the influence of human activities, the area and degree of salinization in the Songnen Plain grassland is gradually increasing. There are some particularities in saline-alkaline environments that cause plant growth difficulties, which severely affect the development of agricultural and environmental quality in this region [5,6].
As the most active part of soil, microorganisms can promote the circulation of nutrients such as carbon, nitrogen, phosphorus and sulfur, repair environmental pollution, and maintain the stability of terrestrial ecosystems [7]. As the most widely distributed microorganism in soil, arbuscular mycorrhizal (AM) fungi can form symbionts with most higher plants to help them obtain nutrients, promote plant growth and development, enhance the resistance of plants to abiotic stress (e.g., salinity) and reduce the damage that saline environments cause to plants by altering the soil structure of saline-alkaline land, subsequently mutually benefiting host plants and maintaining the stability of the rhizosphere environments of host plants under adverse circumstances. There are approximately 300 AM fungal species at present. Many AM fungal species have been discovered in the deserts of Inner Mongolia, and species in mining areas seriously polluted by heavy metals, plateaus, coastal regions and typical karst areas have been reported in previous explorations. Many scholars have found that alkaline soils are rich in AM fungal resources; for example, 7 genera of AM fungi were found in the rhizospheres of 4 halophytes in saline-alkali land located in the Yellow River Delta [8], 28 species of 4 genera were isolated from saline-alkaline soils in Gansu, Ningxia and Inner Mongolia [9], and 21 species belonging to 6 genera were observed in the saline-alkaline grassland of Songnen Plain [10]. AM fungal community structures are often unique in saline-alkaline soils and are likely to contain AM fungal species with special functions, especially species with strong tolerances to saline-alkaline stress.
Iris lactea, which can form symbionts with AM fungi, is a plant that is indicative of degradation in saline grasslands on the Songnen Plain [11], and it has a strong salt tolerance and high ornamental value [12]. The salinity tolerance of I. lactea has been widely confirmed. Previous studies showed that overexpression of the Na+/H+ antiporter gene in the membrane of I. lactea improved its salinity tolerance [13], 129 upregulated genes and 1609 downregulated genes were detected during salt treatment [14], and the activity of antioxidant enzymes improved under saline-alkaline stress [15]. However, little research has been performed to investigate the relationship between the salinity tolerance of I. lactea and AM fungal resources in the rhizosphere [16]. Therefore, I. lactea, which grows in Songnen saline-alkaline grassland, was observed, aiming to provide a theoretical basis for the analysis of the salt tolerance mechanism of I. lactea and for the exploration and discovery of AM fungal resources in saline-alkaline soils as well as for the further screening of AM fungal resources with salt tolerances.
The materials for this study were collected from Zhaodong city (46°2’5’’-46°2’51’’N, 125°54’6’’-125°42’31’’E) in Heilongjiang Province, China, which is located in the middle of the Songnen Plain and has a temperate continental monsoon climate. The landscape is flat, rainfall is mainly distributed in summer, and winter is dry and cold. The soil can be roughly sorted into saline soil, alkaline soil and meadow soil.
Five I. lactea samples were selected from each site according to the “multipoint parallel sampling method” and the “five sampling method”, and the roots and rhizosphere soils of samples from 10 sites were collected on July 6, 2016. The rhizosphere soil samples (approximately 1.5 Kg each) were collected from a soil depth of 0 cm–30 cm after the superficial rubbish was removed, and then the soil was packed in sealed bags marked with sample numbers, latitude and longitude. The samples were placed in a ventilated and dark place and air-dried until the roots could be separated from the soil completely. The roots were cut into 0.5-1-cm-long segments and soaked in FAA fixative solution (formaldehyde-glacial acetic acid) after they were washed with distilled water several times, and the water on their surface was sucked dry using filter paper. The air-dried soils were sieved through a 20-mesh sieve, numbered and then stored: the soils and root segments were stored at 4°C.
2.3 Determination of Sample Soil pH
Fifty grams of soil was weighed from each single sample and mixed into suspension liquid (water:soil = 2:1). After the solution precipitated for 0.5 h, its pH value was measured using a pH meter (METTLER TOLEDO FE20). The average of three measurements, determined after the pH meter reading stabilized, was taken as the value of each sample.
2.4 Measurement of AM Fungal Colonization Rate in I. lactea Roots
The root segments were rinsed with clean water after they were removed from the FAA fixed solution and then stained with Alkaline Trypan Blue [17]. The cleaned root segments were placed in 10% KOH solution and bathed for 1 h to 1.5 h until they became soft and transparent. In addition, the root segments were dipped into alkaline hydrogen peroxide to soften, after which the roots were neutralized with 2% hydrochloric acid for 5 min and then washed before they were stained using a 0.05% Trypan Blue reagent and incubated for 0.5 h. The structural characteristics of the roots, including the characteristics of vesicles, hyphae, and arbuscules, were observed under a microscope (OLYMPUS-DSX500). The number of each structure was counted and recorded according to the method of Trouvelot et al. [18]. The AM fungal colonization rate in I. lactea roots and other indexes were analyzed using MYCOCALC, and the measurement results of the colonization intensities of the root segments were divided into five levels: (1) 0%–1% as Level I, (2) 1%–10% as Level II, (3) 11%–50% as Level III, (4) 51%–90% as Level IV, and (5) 91%–100% as Level V. The colonization assessment of each site was described using the colonization intensity, which was calculated based on the mycorrhizal colonization grade. Similarly, the arbuscular abundance was calculated based on the level of vesicular abundance: A1 indicated few arbuscles, at <5%, A2 indicated frequent arbuscles, at 5%–50%, and A3 indicated abundant arbuscles, at >50%).
The colonization rate (F%) was calculated by dividing the number of colonized segments by the total number of root segments and multiplying the result by 100%.
The colonization intensity (M%) was calculated as follows: M% = (95 × NV + 70 × NIV + 30 × NIII + 5 × NII + NI)/(total number of root segments × 100) × 100%, where N is the number of roots at the same colonization level.
The arbuscular abundance (A%) was calculated as follows: A% = (100 × m × NA3 + 50 × m× NA2 + 10 × m × NA1)/100, where m% = M × total number of root segments/amounts of colonized root segments and N is the number of roots at the same grade of vesicular abundance.
2.5 Isolation and Identification of AM Fungal Spores
The quantity of spores per sampling site was counted according to the amount of spores in 50 g soil. In this experiment, we separated AM fungal spores in the rhizosphere soil of I. lactea using wet screening and sucrose density-gradient centrifugation. The isolated AM fungal spores were placed in a petri dish before the spores were enumerated under a double-tube solid-state dissecting microscope. Single spores were picked out with sterile tips; subsequently, the diameter, color, superficial decoration and thickness of each spore were observed under a microscope after a PVLG (a mixture of polyvinyl alcohol, lactic acid and glycerin) floating carrier had been added to the glass slide. The spores were identified and classified on the basis of the specific response of the spores to Melzer’s reagent. The morphological descriptions, pictures and newly published literature that were used to identify spore species in this experiment were provided by the INVAM International website (http://invam.wvu.edu/the-fungi/species-descriptions).
2.6 Index Determination of Fungal Spore Diversity
Diversity indexes were calculated according to the method of Yang et al. [19], including indexes of dominance grade classification, spore density, relative abundance, importance value and separation frequency. The equations used to calculate these indexes are as follows:
Spore Density (SD) = Spore number of all AM fungal species/soil sample number
Separation Frequency (F) = Occurrence frequency of certain pecies/total sample number × 100%
Relative Abundance (RA) = Spore number of certain species/total quantity of AM fungal spores × 100%
Importance Value (IV) = (F + RA)/2 × 100%
3.1 Symbiotic Structural Characteristics of I. lactea Colonized Roots
All symbiotic structures observed in the roots of I. lactea in this study were Arum-type (Fig. 1). Mycelia were abundant in roots, including septa hyphae (Figs. 1b and 1g) and nonsepta hyphae (Fig. 1a). The hyphae colonized the roots from outside (Fig. 1c) and formed large numbers of intercellular hyphae in the cortical cells. The lateral dichotomous branches directly penetrated the cell walls of the cortexes into the cells (Fig. 1d) and formed dendritic arbuscular structures (Fig. 1e); hyphae circles could be observed in the cells (Fig. 1f). The intercellular hyphae expanded and shaped vesicles that mostly presented as circles (Figs. 1e and 1i), ovals (Figs. 1g, 1h and 1l) or other irregular shapes (Fig. 1i). colonial structures formed by large numbers of vesicles bound together tightly were also seen (Fig. 1j), and internal spores were visible occasionally (Fig. 1k).
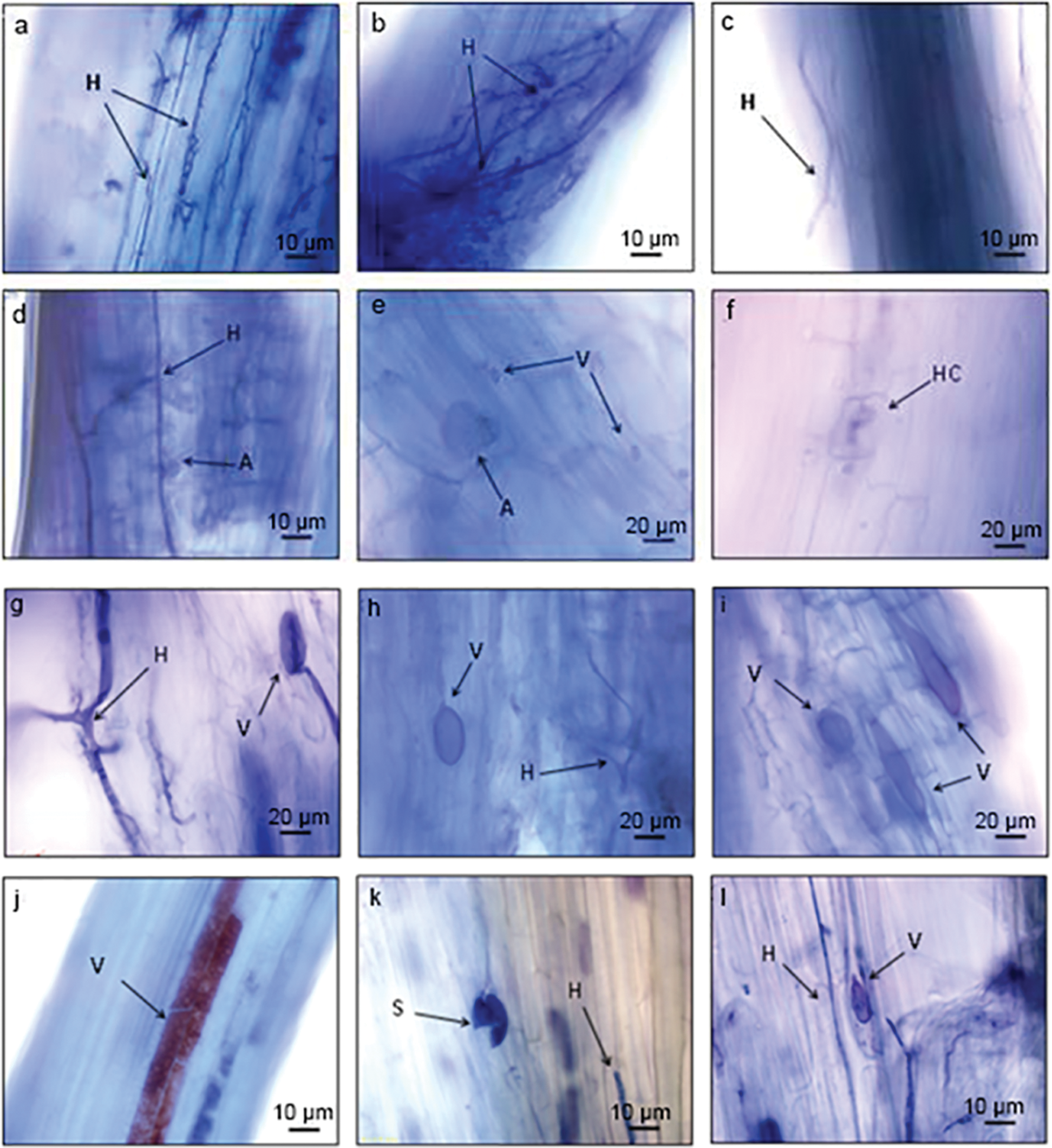
Figure 1: Mycorrhizal characteristics of I. lactea colonized roots. Note: H-Hypha, V-Vesicule, S-Spore, A-Arbuscule, HC-Hyphal Coil
3.2 AM Fungal Colonization Characteristics in Roots of I. lactea
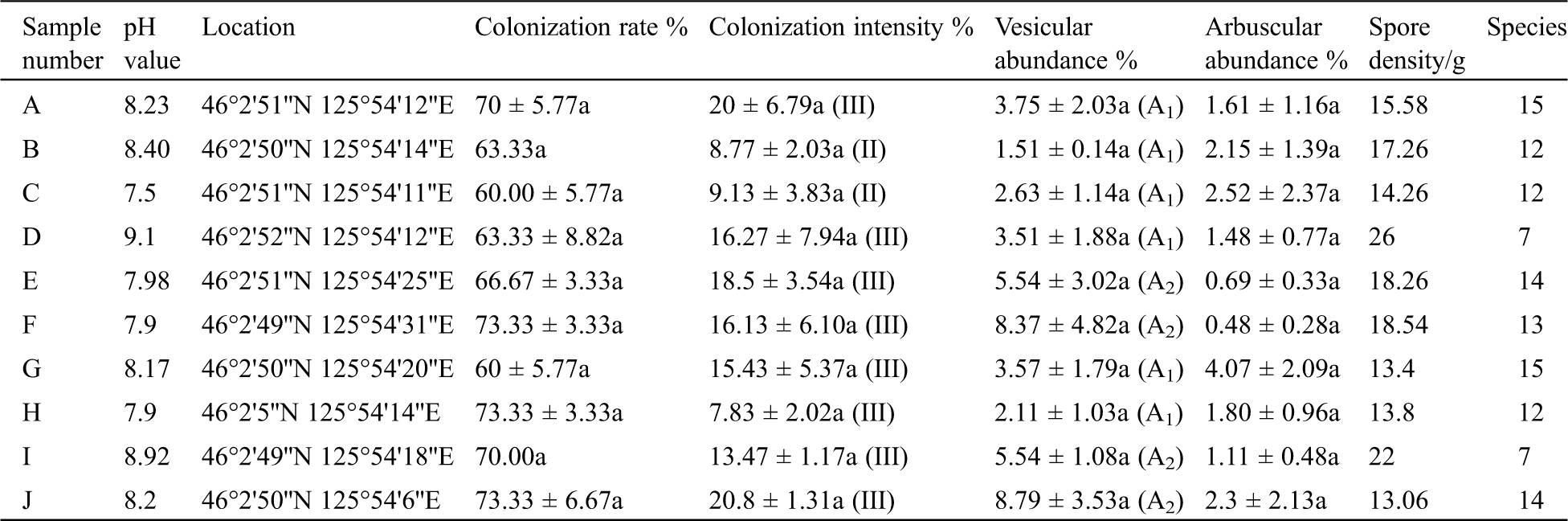
The Arum-type mycorrhizal structures formed after I. lactea was colonized by AM fungus could be seen through observations of mycorrhizae from 10 different sampling sites. As shown in Tab. 1, both the colonization rate and the colonization intensity reached maximum values at pH 8.2, and these maximum values were 73.33% and 20.8%, respectively. The maximal vesicular abundance (8.79%) and arbuscular abundance (4.07%) were observed when the soil pH reached 8.20 and 8.17, respectively. Overall, the colonization rate showed a fluctuating trend with increasing pH values, the colonization intensities were of level II or III, and the vesicle abundances were of grade A2 or A3 at the ten sampling sites. The colonization characteristics were dissimilar when the symbionts were under different pH conditions. Spearman analysis results showed that there was a significant and strong correlation between arbuscular abundance and spore density (P = 0.013, R = 0.748) as well as between vesicular abundance and colonization intensity (P = 0.008, R = 0.782). The spore density of AM fungi in the rhizosphere was 13–19/g at lower pH values, while it was 22/g at a pH value of 8.92 and up to 26/g at a pH value of 9.1. A significant strong correlation existed between pH and spore density (P = 0.010, R = 0.767) according to Pearson correlation analysis. However, there was no significant correlation observed between spore density and mycorrhizal colonization rate in this experiment, although both may be affected by pH.
Table 1: The colonization of AM fungi in I. lactea roots
3.3 Morphological Identification of AM Fungi in the Rhizosphere of I. lactea
AM fungi of 39 species in 15 genera were isolated from the samples taken from the Songnen saline-alkaline grassland (Fig. 2), among which 2 species were not identified by morphological identification. The 37 species of AM fungi that were identified included 10 species of Glomus, 5 species of Acaulospora, 4 species of Funneliformis, 3 species of Rhizophagus, 2 species each of Sclerocystis, Pacispora, Gigaspora and Claroideoglomus, and 1 species of Entropnospora, Scutellospora, Ambispora, Diversispora, Dominikia, Septoglomus and Dentiscutata.
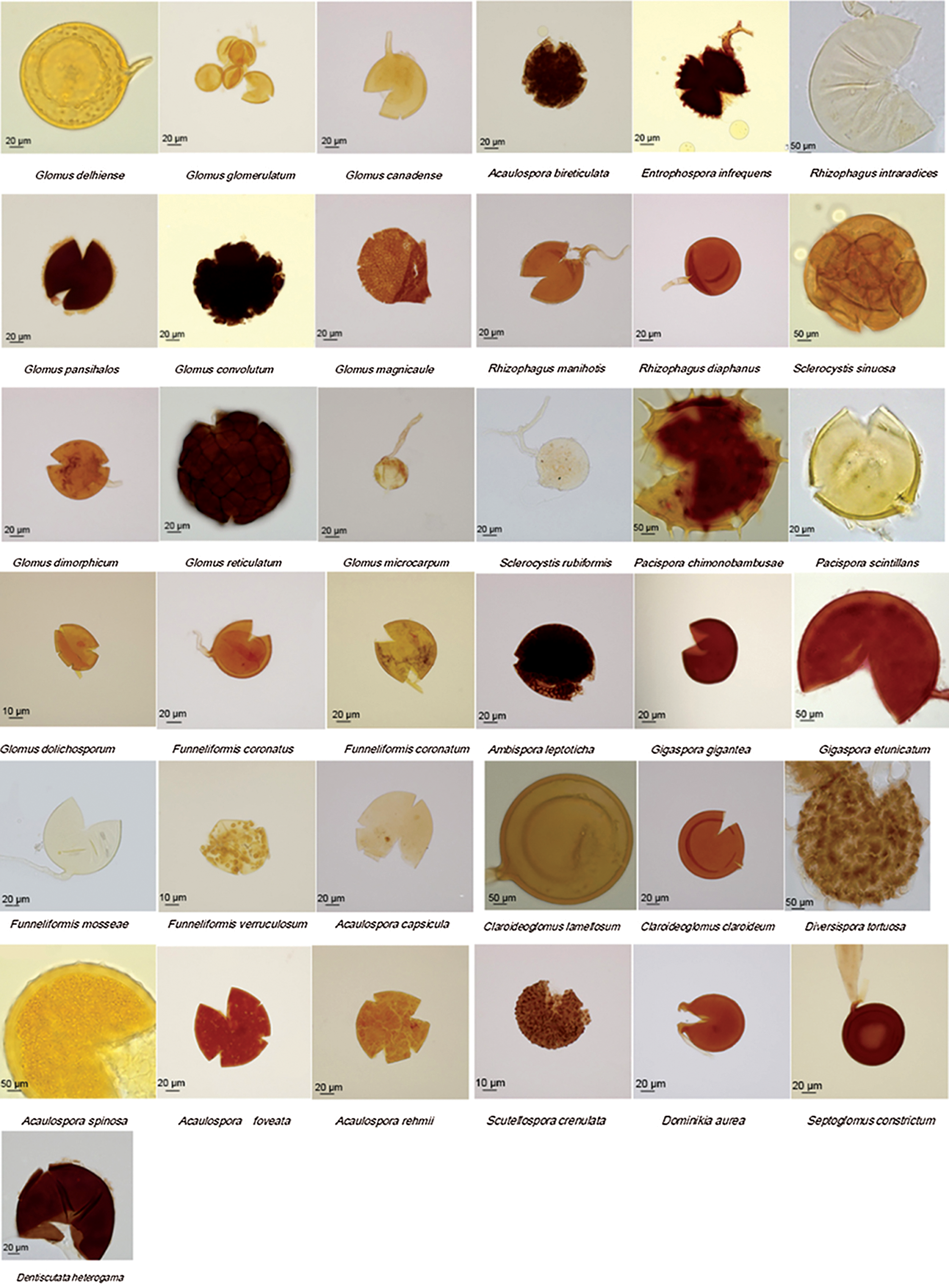
Figure 2: The spore morphology of AM fungi identified in the rhizosphere of I. lactea belonged to 15 genera and 37 species
3.4 AM Fungal Distribution and Diversity in the Rhizosphere of I. lactea
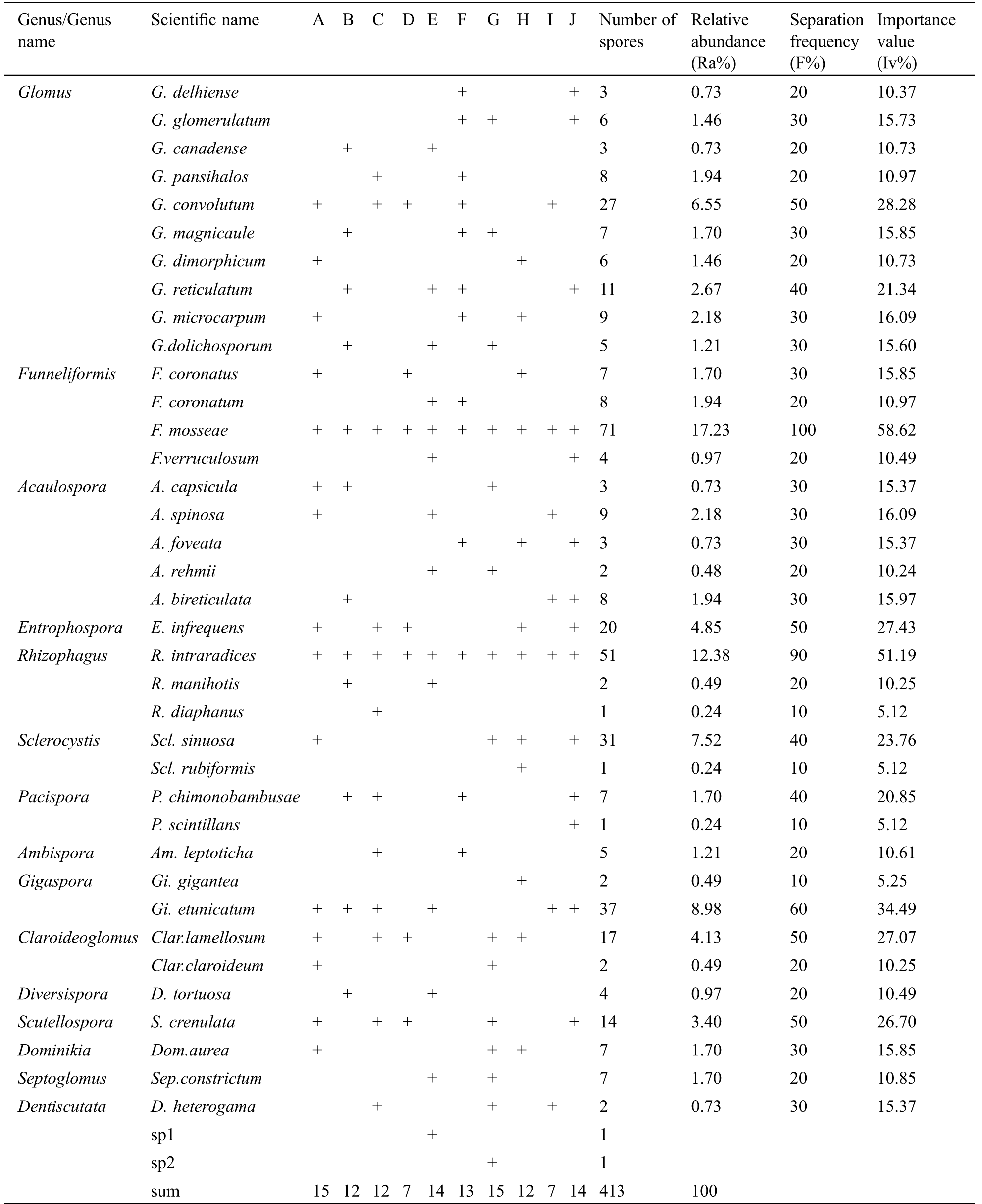
Thirty-seven species of 15 AM fungal genera were isolated and identified from the soil samples (Tab. 2). Among these species, the dominant genera were Funneliformis and Glomus, which accounted for 21.97% and 20.58% of the total number of identified genera, respectively. Claroideoglomus and Rhizophagus were isolated from the rhizosphere with proportions of 13.56% and 13.08%, respectively, ranking second in dominance. Funneliformis mosseae and Rhizophagus intraradices were observed in all soil samples with importance values of 58.62 and 51.19, respectively, and these were the dominant species observed in the samples. Gigaspora etunicatum, Entrophospora infrequens, Glomus convolutum, Claroideoglomus lamellosum, and Sclerocystis sinuosa were common species, with spore proportions of 8.98%, 4.85%, 6.55%, 4.13% and 7.52%, respectively. Pacispora scintillans, Gigaspora gigantean, Sclerocystis rubiformis, and Rhizophagus diaphanus were occasional AM fungal species detected in the rhizospheres of I. lactea sampled in the Songnen saline-alkaline grassland.
Table 2: The distribution and diversity indexes of AM fungi in the rhizosphere of I. lactea
4.1 Differences in AM Fungal Colonization Characteristics in the Roots of I. lactea
AM fungi colonize host plants by recognizing signal substances from roots [20]. According to the colonization rate statistics and the identification of AM fungal species observed in this study, it was found that the species showed complex variation with changing soil pH, spore density was significantly and positively correlated with pH, and the colonization rate had no correlation with pH value. The colonization rate reached as low as 60% at the lowest soil pH (7.5) because the growth of I. lactea might not be inhibited and the secretion of signal substances would decrease under low pH circumstances, which would weaken the colonization of AM fungi and thus reduced the species number [21]. The experimental results showed that there was no significant correlation between spore density and mycorrhizal colonization rate, which was similar to the research results of Yang et al. [22]. However, many scholars have pointed out that there is a significant positive correlation between these two factors [23]. This inconsistency may be because the irregular spatial distributions of pores and the complex structures of roots are the most important factors affecting spore density [24], and these factors also lead to variations in the colonization rate among different conditions [25]. Inconsistent research conclusions appeared when symbionts were studied under different experimental conditions. In addition, it might be possible that there is no obvious correlation between the spore-producing ability of AM fungi and their colonization ability in host plants, and a strong spore- producing ability does not correlate to a strong colonization ability [22]. Colonization and spore production are two stages of the life cycle of AM fungi, the conditions required for these two stages are different, and there is no inevitable connection between them. Finally, the tolerance of different AM fungal spores to the same pH value varies, the AM fungal effectiveness could be affected by pH [26], and the mycelial growth and colonization capacity of AM fungi may be influenced under high pH conditions [27]. All these explanations are possible reasons for the inconsistencies between our results and the results of previous studies.
4.2 Diversity of AM Fungal Spores in the Rhizosphere of I. lactea
According to the statistics, the dominant observed species were F. mosseae and R. intraradices, and these species were isolated in all sampling sites, while the distributions of other AM fungi and spore densities showed some variance among different sampling sites, these variations not only reflected the diversity of AM fungi in Songnen saline-alkaline grassland but also showed the uneven distributions and varying colonization intensities of AM fungi. Some selectivity and adaptability were shown in the process of AM fungal recognition and colonization, and the mycorrhizal characteristics were determined jointly by the present AM fungi, host plants and environmental conditions [28]. The main ecological problem faced by Songnen saline-alkaline grassland is salinization, which directly manifests as increased pH values, and the total salinity of soil fluctuates with seasonal changes [29]. Subsequently, the content and availability of inorganic ions and heavy metal ions in soil are affected. The N application experiment showed that high N levels can increase the value of the N:P ratio and decrease the value of the C:N ratio, subsequently reducing the relative abundance of mycorrhizae. In addition, the colonization rate is enhanced at lower P levels [30]. The colonization indexes of AM fungi showed a trend of increased-decreased with the worsened heavy metal stress, such as stress due to the presence of cadmium or zinc [31]. These secondary effects of salinization may be factors affecting AM fungal diversity and spore density among different sites. However, different mycorrhizal characteristics have direct impacts on the physiological and ecological effects of AM fungi-host plant symbionts [32]. Therefore, it is of great significance for the application of AM fungal symbionts to select efficient AM fungi suitable for host plants.
4.3 Relationship between AM Fungal Colonization and the Saline-Alkaline Tolerance of I. lactea
It has been confirmed that many AM fungal species, such as F. mosseae [33], S. constrictum [34] and G. etunicatum [35], have strong salt-alkali tolerance in previous studies. Furthermore, these species could significantly improve the vegetal salt-alkali tolerances by enhancing the antioxidant enzymatic activities, increasing the proline, soluble sugar, soluble protein, and chlorophyll contents, reducing the malondialdehyde (MDA) contents, and regulating the palladone, dionine, quercetin 3’ –methyl ether and apigenin 7-O-neohesperidin concentrations in leaves [36]. In addition, these fungal species can also influence the expression of proteins related to active oxygen metabolism, phenylpropane biosynthesis, carbohydrate and energy metabolism, translation, sulfur metabolism, photosynthesis, nitrogen metabolism, and amino acid metabolism in plants [37] to improve the salt-alkali tolerances of host plants. The abovementioned AM fungal species were isolated in this research, and F. mosseae was distributed in all sampling sites, which indicates that AM fungal colonization improves the saline-alkaline tolerance of I. lactea growing in the Songnen saline grassland to a certain extent. Further verification of the roles of the other AM fungal species identified in this research in ecological restoration is needed.
Availability of Data and Material: All data generated or analysed during this study are included in this manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (31601986), the Fundamental Research Funds for the Central Universities (2572018BK02), and Heilongjiang Postdoctoral Scientific Research Developmental Fund (LBH-Q16005).
Conflicts of Interest: The authors declare that we have no conflicts of interest to report regarding the present study.
1. Neera, G., Amrit, B. (2018). Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza, 28(8), 727–746. DOI 10.1007/s00572-018-0856-6. [Google Scholar] [CrossRef]
2. Yang, H. X., Guo, S. X., Liu, R. J. (2015). Characteristics of arbuscular mycorrhizal fungal diversity and functions in saline-alkali land. Chinese Journal of Applied Ecology, 26(1), 311–320. [Google Scholar]
3. Li, Q. S., Li, X. J., Wang, Z. C., Song, C. C., Zhang, G. X. (2003). Sodium bicarbonate soil management and utilization in Songnen Plain. Resouces Science, 25(1), 15–19. [Google Scholar]
4. Yan, N. N., Cui, G. W., Zhang, X., Li, J. X. (2015). The Influence of straw covering and reseeding on soil ion content in saline and alkaline grassland in Songnen Plain. Chinese Journal of Grassland, 37(2), 112–116. [Google Scholar]
5. Su, X., Lu, M., Feng, C. C., Guo, Y. L., Yue, Z. H. (2020). Responses of seasonal dynamic of soil enzyme activity to soil salinity in Songnen saline-alkali grassland. Chinese Journal of Grassland, 42(1), 127–134. [Google Scholar]
6. Sun, Y. F., Song, F. Q., Chang, W., Fan, X. X. (2016). Effect of arbuscular mycorrhizal fungi on growth and physiology of Elaeagnus angustifolia seedlings subjected to salinity stress. Scienctia Silvae Sinicae, 52(6), 18–27. [Google Scholar]
7. Yang, L., Zou, Y. N., Tian, Z. H., Wu, Q. S., Kuča, K. (2021). Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Scientia Horticulturae, 277, 109815. DOI 10.1016/j.scienta.2020.109815. [Google Scholar] [CrossRef]
8. Wang, Y. Y., Guo, D. F. (2016). AM fungal diversity in saline alkali soil of yellow river delta. Molecular Plant Breeding, 14(8), 2232–2238. [Google Scholar]
9. Sheng, M., Tang, M., Zhang, F. F., Huang, Y. H. (2011). Effect of soil factors on Arbuscular Mycorrhizal fungi in saline alkaline soils of Gansu, Inner Mongolia and Ningxia. Biodiversity Science, 19(1), 85–92. [Google Scholar]
10. Gao, X. M. (2019). Research on diversity and function of Arbuscular Mycorrhizal fungi from Taraxacun mongolicum rhizosphere in Songnen Saline-alkali grassland (Ph.D. Thesis). Northeast forestry university, Harbin. [Google Scholar]
11. Wang, Y. (2009). Response of indicator species (Iris lactea Pall. Var.chinensis Koidz) of grassland degradation in Songnen Plain to saline-alkali environment (Ph.D. Thesis). Northeast Normal University, Changchun. [Google Scholar]
12. Yi, C. S., Yu, J. P., Ren, Q. J., Ni, X. J. (2015). The characteristics and application of saline-alkali-tolerant Iris chinensis. Modern Agricultural Science and Technology, 2015(14), 169–170. [Google Scholar]
13. Guo, Q., Tian, X. X., Mao, P. C., Meng, L. (2020). Overexpression of Iris lactea tonoplast Na+/H+ antiporter gene IlNHX confers improved salt tolerance in tobacco. Biologia Plantarum, 64, 50–57. DOI 10.32615/bp.2019.126. [Google Scholar] [CrossRef]
14. Gu, C. S., Xu, S., Wang, Z. Q., Liu, L. Q., Zhang, Y. X. et al. (2018). De novo sequencing, assembly, and analysis of Iris lactea var. chinensis roots’ transcriptome in response to salt stress. Plant Physiology and Biochemistry, 125, 1–12. DOI 10.1016/j.plaphy.2018.01.019. [Google Scholar] [CrossRef]
15. Yuan, Z. B., Mou, C. H., Wang, B., Sun, X. L. (2020). Effects of NaCl and NaHCO3 mixed stress on physiological characteristics of Iris lacteal seedling. Journal of Zhejiang Agricultural Sciences, 61(1), 91–95. [Google Scholar]
16. Yang, C. X., Chen, F., Yue, Y. N., Yan, X. F. (2015). Diversity characteristics of arbuscular mycorrhizal fungi in the rhizosphere of twenty six species of plants in Songnen saline-alkaline grassland. Pratacultural Science, 32(12), 2008–2020, 2008. [Google Scholar]
17. Phillips, J. M., Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vasicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55(1), 158–161. [Google Scholar]
18. Trouvelot, A., Kough, J. L., Gianinazzi-Pearson, V. G. (1986). Mesure du taux de mycorhization VA d’un systeme radiculaire.Recherche de methodes d’estimation ayant une significantion fonctionnelle. Paris: INRA Press. [Google Scholar]
19. Yang, J., He, X. L., Zhao, L. L. (2011). Species diversity of arbuscular mycorrhizal fungi in the rhizosphere of Salix psammophila in Inner Mongolia desert. Biodiversity Science, 19(3), 377–385. [Google Scholar]
20. Sun, X. G. (2014). Mechanisms of AM symbiosis: Recognition between AM fungi and plant roots and characterization of AM functional related genes (Ph.D. Thesis). Northwest A&F University, Yangling. [Google Scholar]
21. Yang, C. X., Chen, F., Li, L. L., Li, C., Yu, D. F. et al. (2016). Isolation and identification of Glomus intraradices in saline-alkaline glassland and its effects on salt tolerance of Trifolium repens L. Fresenius Environmental Bulletin, 10(25), 4439–4446. [Google Scholar]
22. Yang, C. X., Huang, S. C., Chen, F., Li, L. L. (2017). Infection characteristics and diversity of arbuscular mycorrhizal fungi in the rhizosphere of Inula japonica in Songnen saline-alkaline grassland. Pratacultural Science, 34(2), 231–239. [Google Scholar]
23. Birhane, E., Gebremedihin, K. M., Tadesse, T., Hailemariam, M., Solomon, N. (2017). Exclosures restored the densityand root colonization of arbuscular mycorrhizal fungi in Tigray, Northern Ethiopia. Ecological Processes, 6(1), 121. DOI 10.1186/s13717-017-0101-9. [Google Scholar] [CrossRef]
24. Muthukumar, T., Sha, L., Yang, X., Cao, M., Tang, J. et al. (2003). Distribution of roots and arbuscular mycorrhizal associations in tropical forest types of Xishuangbanna, Southwest China. Applied Soil Ecology, 22(3), 241–253. DOI 10.1016/S0929-1393(02)00156-7. [Google Scholar] [CrossRef]
25. Zhao, Z., Xia, Y., Qin, X., Li, X., Cheng, L. et al. (2001). Arbuscular mycorrhizal status of plants and the spore density of arbuscular mycorrhizal fungi in the tropical rain forest of Xishuangbanna, Southwest China. Mycorrhiza, 11(3), 159–162. DOI 10.1007/s005720100117. [Google Scholar] [CrossRef]
26. Dumbrell, A. J., Nelson, M., Helgason, T., Dytham, C., Fitter, A. H. (2010). Relative roles of niche and neutral processes in structuring a soil microbial community. ISME Journal, 4(3), 337–345. [Google Scholar]
27. Zhang, C. L., Li, W. J., Yao, H. Y., Luo, C., Xu, C. C. et al. (2017). Correlation study on the diversity of the AM fungi and soil nutrients in the rhizosphere of different kiwifruit cultivars. Journal of Fruit Science, 34(3), 344–353. [Google Scholar]
28. Dickson, S. (2004). The Arum-Paris continuum of mycorrhizal symbioses. New Phytologist, 163(1), 187–200. DOI 10.1111/j.1469-8137.2004.01095.x. [Google Scholar] [CrossRef]
29. Su, X., Lu, M., Feng, C. C., Guo, F. L., Yue, Z. H. (2020). Responses of seasonal dynamic of soil enzyme activity to soil salinity in Songnen Saline-Alkali Grassland. Chinese Journal of Grassland, 42(1), 127–134. [Google Scholar]
30. Sarmiento-Lopez, L. G., Lopez-Meyer, M., Sepulveda-Jimenez, G., Cardenas, L., Rodriguez-Monroy, M. (2020). Photosynthetic performance and stevioside concentration are improved by the arbuscular mycorrhizal symbiosis in Stevia rebaudiana under different phosphate concentrations. PeerJ, 8(3), e10173. DOI 10.7717/peerj.10173. [Google Scholar] [CrossRef]
31. Sarkar, A., Asaeda, T., Wang, Q. Y., Kaneko, Y., Rashid, M. H. (2017). Response of Miscanthus sacchariflorus to zinc stress mediated by arbuscular mycorrhizal fungi. Flora, 234, 60–68. DOI 10.1016/j.flora.2017.05.011. [Google Scholar] [CrossRef]
32. Tian, M., Chen, Y. L., Li, M., Liu, R. J. (2013). Structure and function of arbuscular mycorrhiza: A review. Chinese Journal of Applied Ecology, 24(8), 2369–2376. [Google Scholar]
33. Wang, Y. N., Tao, S., Hua, X. Y., Yu, X. Y., Yan, X. F. et al. (2018). Effects of arbuscular mycorrhizal fungi on the growth and physiological metabolism of Leymus chinensis under salt-alkali stress. Acta Ecologica Sinica, 38(6), 2187–2194. DOI 10.1016/j.chnaes.2017.08.004. [Google Scholar] [CrossRef]
34. Hu, Z. H. (2020). Effects of AM Fungi to saline-alkali tolerance of four plants under saline-alkali stress and phosphorus addition (Ph.D. Thesis). Northeast Normal University, Changchun. [Google Scholar]
35. Wang, Y. D. (2020). Research on diversity and function of AM Fungi from the rhizosphere of potentilla anserine in songnen salt-alkali grassland (Ph.D. Thesis). Northeast Forestry University, Harbin. [Google Scholar]
36. Yang, C. X., Zhao, W. N., Wang, Y. N., Zhang, L., Huang, S. C. et al. (2020). Metabolomics analysis reveals the alkali tolerance mechanism in Puccinellia tenuiflora plants inoculated with arbuscular mycorrhizal fungi. Microorganisms, 8(3), 327. DOI 10.3390/microorganisms8030327. [Google Scholar] [CrossRef]
37. Wang, Y. N., Lin, J. X., Huang, S. C., Zhang, L., Zhao, W. N. et al. (2019). Isobaric tags for relative and absolute quantification-based proteomic analysis of Puccinellia tenuiflora inoculated with arbuscular mycorrhizal fungi reveal stress response mechanisms in alkali-degraded soil. Land Degradation & Development, 30(13), 1584–1598. DOI 10.1002/ldr.3346. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |