
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015996
REVIEW
Carotenoids: New Applications of “Old” Pigments
1Faculty of Agriculture, University of Belgrade, Chair of Agrobotany, Belgrade, 11080, Serbia
2Faculty of Agriculture, University of Belgrade, Chair of Chemistry and Biochemistry, Belgrade, 11080, Serbia
*Corresponding Authors: Aleksandar Ž. Kostić. Email: akostic@agrif.bg.ac.rs; Zora P. Dajić Stevanović. Email: zoradajic@agrif.bg.ac.rs
Received: 29 January 2021; Accepted: 24 March 2021
Abstract: Carotenoids represent a large group of mainly red, orange, and yellow natural metabolites mainly involved in regulation of many metabolic processes. Carotenoids are beneficial for human health. Current study describes the importance, chemical composition and functioning of carotenoids. It is well known that carotenoids support pigments acting in light absorbance mechanisms during photosynthesis, and are known to protect the chlorophyll molecules from oxidative stress and reactive oxygen species (ROS) damage. Carotenoids are involved in signaling processes in plants, responses to environmental stresses, pollination, germination and reproduction, and development regulation. As nutrients of strong antioxidant activity that is primarily linked to their polyene molecular structure, the carotenoids are reported as immune-enhancement and anticancer agents, which are also involved in prevention of eye-, gastric and neurocognitive disorders, and in regulation of obesity and anti-ageing. Concerning the wide prospective applications of carotenoids as pharmaceuticals and nutraceuticals, there are some critical aspects associated with carotenoids’ bioavailability and challenges in their bioengineering. This mostly refers to the needs for identification and cloning of genes responsible for carotenoid biosynthesis and transformation and related development of transgenic carotenoid-rich crops. In the recent years, technologies of micro- and nanoencapsulation have addressed the needs of carotenoid entrapping to enhance their bioavailability, solubility and chemical stability, and to ensure the target delivery and manifestation of their strong antioxidant and other biological activity. Among standard and some advanced analytic tools for carotenoid determination (e.g., High performance liquid chromatography-HPLC, Liquid chromatography–mass spectrometry-LC-MS, Ultra high performance liquid chromatography-UHPLC, High-performance thin-layer chromatography-HPTLC and others), the vibrational spectroscopy techniques, primarily Raman spectroscopy coupled with chemometric modeling, opened a new era in carotenoid research and application.
Keywords: Antioxidant and biological activity; biosynthesis of carotenoids; encapsulation; raman spectroscopy; role in plants
Bioactive molecules present in horticultural crops are widely studied for their beneficial prophylactic and curative effects on the ability to regulate different metabolic processes. Carotenoids comprise a class of natural pigments formed from eight molecules of isoprenoids, the unit blocks of five carbon atoms. Carotenoids are characterized by hydrocarbon polyene chain ending with rings which provide different coloration, i.e., yellow, red and orange [1]. Carotenoids are produced by plant and microbial cells. The biosynthesis and transformation of carotenoids is regulated during the entire life cycle of a plant, which results in significant changes in carotenoids content and composition depending on the developmental needs and responses to environmental stimuli [2]. Enzymatic cleavage of carotenoids results in occurrence of smaller molecules, the apocarotenoids, of some act as important bioactive metabolites in both plants (hormones and signaling molecules) and animals (retinoids) [3]. Carotenoids and their derivatives have an essential importance in photosynthetic organisms in processes of photosynthesis, signaling and stress-related responses, in addition to the role in plant pollination, reproduction and dispersal. There are currently over a thousand of identified carotenoids, about 40 of which are regularly represented in human nutrition [4]. Lately, carotenoids have been widely researched and acknowledged for their health-related benefits, mostly linked to their high antioxidant activity [5] resulting in their extensive utilization in food, cosmetics, pharmaceutical and nutraceutical industries (Fig. 1). Carotenoids exhibit a range of functions in human health; for example β-carotene has a pro-vitamin A function, whereas lutein/zeaxanthin form macular pigment of the eye [6]. There is strong evidence of their role in improvement of cardiovascular, immune and skin disorders, as well as in the anticancer prophylaxis [7].
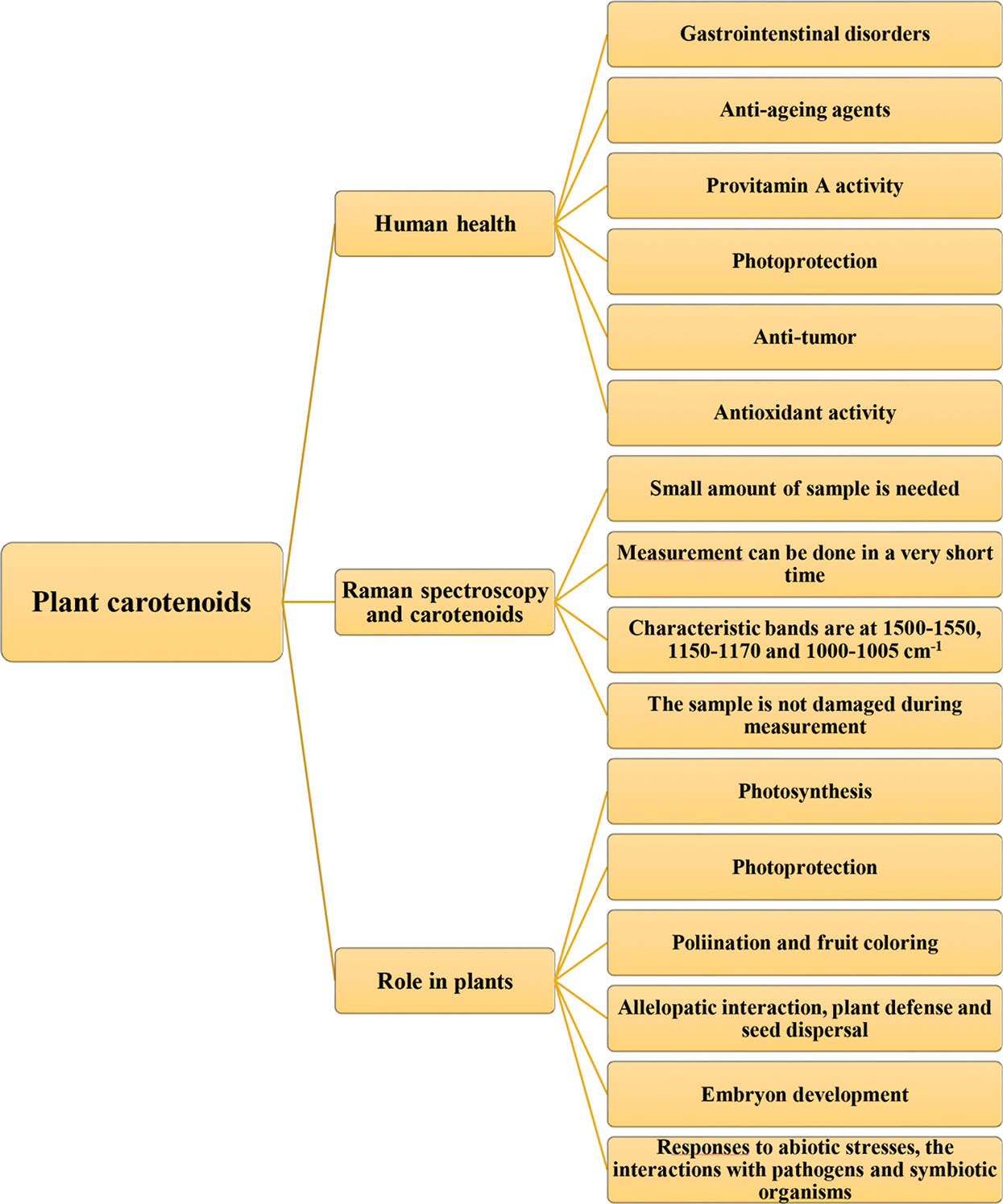
Figure 1: Role of carotenoids in plant and human health with advantages of their determination by Raman spectroscopy
Due to high chemical instability and low solubility and bioavailability, the application of encapsulated carotenoids is recommended for functional foods, fortified food colorants, and various cosmetic products [8]. Encapsulation techniques are needed to protect the carotenoids from oxidation and to maintain their original properties during processing, storage and intake, as well as to enable and enhance their health promoting effects [9]. The global market value for carotenoids was estimated at USD 1,5 billion [10]. Natural carotenoid market is anticipated to achieve significant gain of over 4.5% by 2024, due to growing demand for natural food colorants and additives. Moreover, there is a strong increase in the demand for encapsulated and microcapsulated products estimated at 34 and 12,5 billion US $, respectively, with a share of 400–500 million for carotenoids [11]. However, so far most of the market needs have been met by chemical synthesis and to a minor extent by extraction from natural sources, despite resources in carotenoid-rich plants and the fact that natural carotenoids are more stable and have more expressed biological activity as compared to the synthetic carotenoids [2]. There is a great potential in bioengineering and application of carotenoids in agriculture, food, and pharmaceutical industries.
2 Chemical Structure and Biosynthesis of Carotenoids
Carotenoids are known as an evolutionary old group of molecules exhibiting various roles in plants growth and development. In addition to photosynthetic organisms, such as some photosynthetic bacteria, algae and plants, the carotenoids are also synthesized by archaea, non-photosynthetic bacteria and some fungi [3]. However, the remarkable occurrence of particular carotenoids, i.e., astaxanthin in some marine invertebrates, is linked to the ability of these animals to transform and/or accumulate ingested carotenoids originally synthesized by several microalgae (primarily by Haematococcus pluvialis) or red yeast, the Phaffia rhodozyma [12]. The term “carotene” was derived from the Latin word “carota” (meaning carrot), reflecting the first isolation of one carotenoid in 1831 by Wackenroder, while “xanthophylls” are linked to pigments of the yellowish autumn leaves discovered by Berzelius a couple of years later. The discovery of the chemical structure of carotenoids required exactly one century and was acknowledged by the Nobel prize rewarded to Paul Karrer and Richard Kuhn in 1937 and 1938, respectively [2].
Regarding the chemical structure, all carotenoids are classified into: a) carotenes, the hydrocarbon carotenoids (e.g., lycopene, β-carotene, α-carotene) and b) xanthophylls, the oxygen containing carotenoids, i.e., lutein, astaxanthin and zeaxanthin [13]. Polar groups, i.e., epoxy, keto and hydroxyl and their combinations have capability to modify biological functions of carotenoids [14], while biological behavior related to small chemical modifications is not still well understood. In fact, the high chemical diversity of carotenoids is caused by chemical alterations of the core structure, including the shifting of conjugated double bonds and the addition of different functional groups in the terminal rings of a molecule (Fig. 2) [4]. Apart from the long chain carotenoids (40 and > 40), there is a group of so called apocarotenoids (<C40 ) derived by enzymatic modification reflecting the oxidative cleavage at specific double bonds of the parent carotenoid molecule [15].
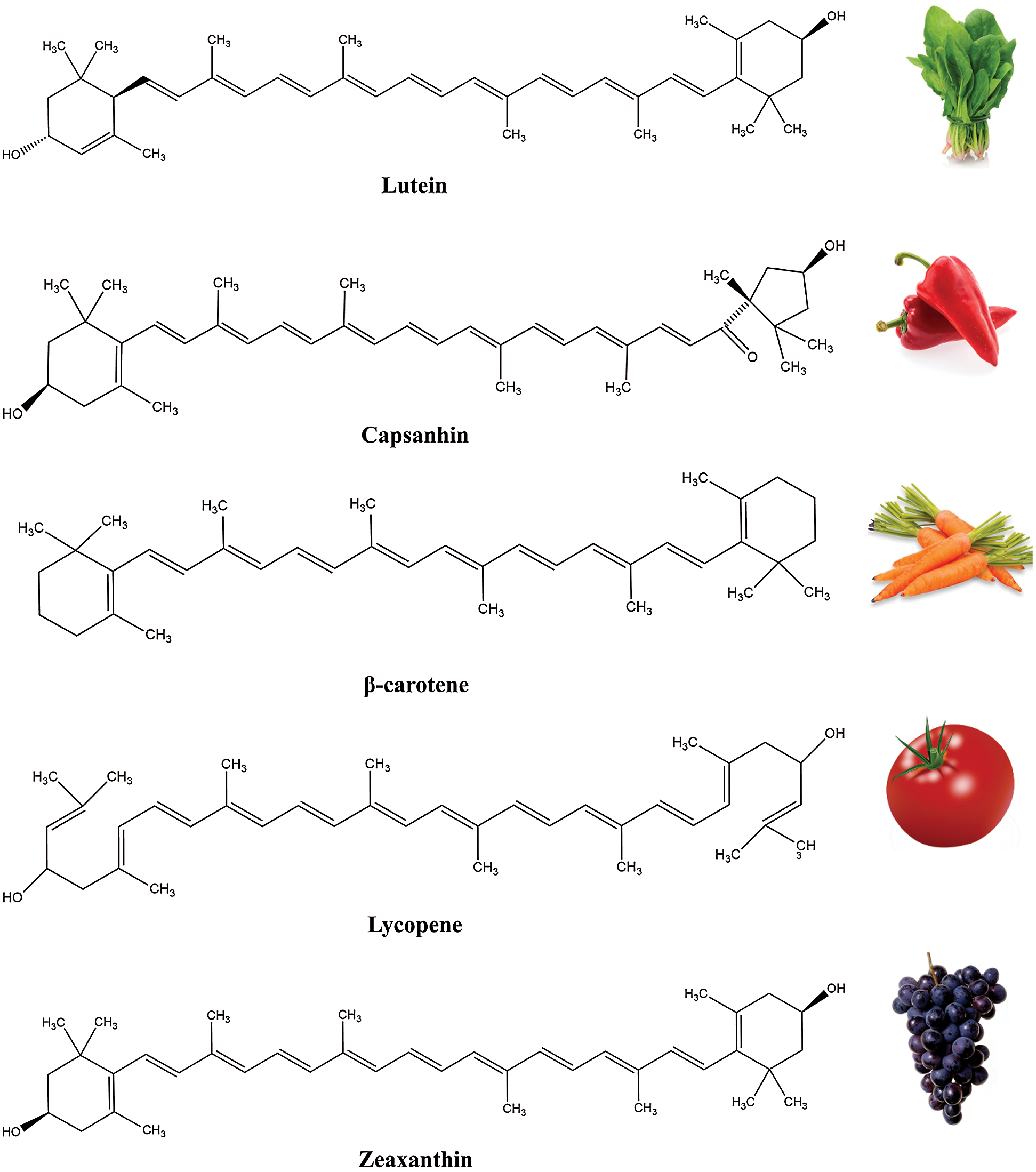
Figure 2: Examples of carotenoids and some of their sources
Carotenoids are mostly synthesized in algal and plant chloroplasts. In contrast to eukaryotes producing C40 or C40-derived carotenoid molecules, the bacterial species produce all C45 and most of the C30 and C50 carotenoids, while the rest of the identified forms are linked to archaea [3]. The biochemical pathway of carotenoid biosynthesis begins with condensation of geranylgeranyl pyrophosphate (GGPP), a precursor of the methylerythritol (MEP) pathway, resulting in production of the C4015-cis-phytoene, further undergoing a series of successive modifications such as desaturation and isomerization to form lycopene, which is later cyclized to carotenes, and carotenes by oxygenation to xanthophylls [16].
The enzymes encoding the carotenoid biosynthesis are predominantly localized in the plant plastids, referring to isopentyl pyrophosphate isomerase, phytoene synthase and geranylgeranyl pyrophosphate synthase found in the plastid stroma, following with the thylakoid membrane enzymes, such as phytoene desaturase, zeta-carotene desaturase, lycopene ε cyclise and some others enzymes [2]. Apocarotenoids are synthesized due to the existence of carotenoid-specific cleavage oxygenases [17], such as CCD (carotenoid cleavage dioxygenases) and NCED (9-cis-epoxycarotenoid dioxygenas, involved in biosynthesis of the phyto-hormone ABA and other apo-carotenoids and their products [18].
The starting steps of the carotenoid biosynthetic pathway lead to appearance of about 40 carbon phytoene intermediate products, undergoing the complex enzymatic processes, such as hydroxylation, epoxidation, desaturation, cyclization, polyene cleavage, etc., resulting in occurrence of a range of structurally different carotenoid products [19].
Carotenoids are characterized by remarkable coloration as a consequence of their chromatophoric molecular structure able for high blue light absorption, although some colorless precursors contribute to their overall chemical diversity [20]. Carotenoids are among the most vital colored phytochemicals, occurring as all-trans and cis-isomers, and accounting for the brilliant colors of a variety of carotenoid-rich sources [21].
The color of carotenoids might be masked by chlorophyll in photosynthetic tissues but during ontogenesis, maturation and ageing of plant body, the amount of carotenoids increases, because of chloroplasts transition into chromoplasts [2]. Among several important features, first of all their conjugated double bond system, the carotenoids are characterized by strong lipophilic nature and their occurrence in a hydrophobic surrounding [22]. Carotenoids’ esterification with fatty acids increases their lipophilic behavior, whereas interaction with proteins or sugars decreases it [3].
3 Role of Carotenoids in Plants
3.1 Photosynthesis and Antioxidant Activity
Photosynthesis is among the most crucial and most miraculous metabolic processes on the Earth, performed by plants and some other photosynthetic organisms, i.e., algae and some bacteria. The final results of photosynthesis are food and oxygen which are used by non-photosynthetic organisms. Chlorophylls play pivotal roles in light absorption of the blue and red spectra, in energy capture, transfer and charge separation during light-depending reactions of the photosynthesis. Apart from chlorophyll, there are other important pigments in plants, such as carotenoids, betalains and anthocyanins. Together with chlorophyll molecules, carotenoids are components of the photosystems I and II, acting as accessory light-harvesting pigments, in addition to photoprotective functions and appearance as precursors for biosynthesis of abscisic acid (ABA) and strigolactone [23,24]. Carotenoids involved in photosynthesis are located in the chloroplast thylakoid membranes in green tissues and are connected with the reaction centers and antenna complex of the photosystems I and II. In order to provide the right positioning for efficient energy transfer, carotenoids are present in the form of pigment protein complexes. Photosystems I and II are rich in β-carotene and lutein, respectively [25].
During the photosynthesis, carotenoids absorb and transfer the light energy towards chlorophylls. Chlorophyll a is able to absorb the light of wavelengths at 430 and 662 nm, while chlorophyll b at 453 and 642 nm. Carotenoids, however, absorb the light at the wavelengths where chlorophyll molecules do not respond, i.e., in the range of 460 and 550 nm, initiating the primary photochemical events of photosynthesis [26]. In this way, carotenoids serve as an “extended arm” of the chlorophyll photosynthetic complex. The energy and electron transfer reactions of the light phase of the photosynthesis are the result of reactions performed by light harvesting antennae molecules (chlorophyll a and b and carotenoids) and reaction centers (photosystem I and photosystem II) [27]. Out of the total of over 1000 naturally occurring carotenoids, only approximately 50 participate in a light-harvesting role in photosynthesis [26].
Beside their function in photosynthesis as a light harvesting molecules, carotenoids have an important photoprotective role, which is reflected in the ability to prevent photo-damage that occurs under conditions of excess light [28]. If chlorophyll absorbs more energy than it could receive, there is a possibility of overheating, disruption of the balance of light-dependent reactions and damage of photosystems, leading to formation of reactive oxygen species (ROS), such as singlet oxygen, superoxide anion radicals, hydroxyl radicals and hydrogen peroxide [29,30]. Proteins, lipids and pigments of the thylakoid membrane may be oxidized by this reactive species, which finally results in the destruction of the photosynthetic apparatus [26]. Because of that, plants evolved several photo-protective strategies. Carotenoids (particularly the β-carotene) are pivotal molecules in these strategies [31]. During sunlight radiation, beta carotene bound in the photosystem II reaction centers is able to transfer an electron to P680+ when it is over-oxidized preventing the harmful oxidation reactions that could damage the PS II [32]. The yield of singlet oxygen is significantly increased if the number of beta carotene molecules bound per isolated complex is reduced from two to one [33]. Therefore, carotenoids are involved in quenching the triplet chlorophylls and scavenging the singlet oxygen and other ROS [34]. The energy level requirements mean that thylakoid carotenoids should have more than eight conjugated double bonds to express the photoprotective ability.
During biosynthesis of carotenoids and chlorophylls and their subsequent binding to pigment-binding proteins, these complexes should be precisely balanced in order to fulfill the photosynthetic demands [35]. Carotenoids regulate the flow of energy within the photosynthetic apparatus and protect the chlorophylls from photo-induced damage caused by excess of light absorption.
Along with beta carotene, the zeaxanthin is also known for its photo-protective role in photosynthesis, according to reports on its role in regulation of photon harvesting and energy dissipation upon irradiance stress [36]. De-epoxidation of zeaxanthin and violaxanthin leads to alterations in structure and properties of these xanthophylls causing significant structural changes in the LHC and light harvesting complex [34].
The ability of carotenoids to scavenge ROS refers not only to the role in photoprotection of chlorophyll and photosystems, but also to the involvement of carotenoids in responses of plants to environmental stresses. ROS, particularly singlet oxygen, which is produced during stress conditions, can oxidize carotenoids resulting in occurrence of different aldehydes, ketones, endoperoxides and lactones. These products (e.g., volatile β-cyclocitral) are very reactive electrophile species which can induce changes in gene expression and lead to responses to stress conditions [37].
3.2 Carotenoids and Plant Stress
Responses of plants to extra-optimal environmental factors imply expression of different tolerance mechanisms, including biosynthesis and accumulation of different antioxidant molecules such as carotenoids, capable to act as quenchers and scavengers of ROS, as well as dissipators of excess harmful energy and the membrane stabilizers [38]. It was shown that particular carotenoid derivatives, such as plant hormones ABA and strigolactones, and apocarotenoid volatiles, mainly the β-cyclocitral and β-ionone play important roles in stress tolerance of plants [39].
Production and alterations in the content of carotenoids and their products under stress conditions is very complex and primarily dependent on the concentration of soluble sugars and H2O2 production as result of photosynthesis and photorespiration rates, respectively [40].
The increase of carotenoids upon stress induction is probably linked to activation of the xanthophyll cycle which can be induced by high light intensity, hypersalinity and drought [41]. The rise of carotenoids and luteolin-7-O-glucoside was noticed in bell pepper cultivars exposed to UV-B radiation and low temperature stress [42]. Salinity stress lead to a significant increase in lycopene content in tomato [43]. Water stress has induced the significant increase in carotenoids content which was observed for several species, including the olive trees for example [44]. It is thought that accumulation of the zeaxanthin may be response to different abiotic stresses [45]. Increase in zeaxanthin and antheraxanthin, and the corresponding decrease in violaxanthin were correlated with high light intensity in microalgae [46]. However, exposure to environmental stresses may also diminish the content of carotenoids. Water deficiency caused a reduction in carotenoids concentration in different crops, as was shown for wheat [47], similarly to effects of salinity stress which was reported for chickpea [48] and beans [49].
Transcription of carotenoid genes is up regulated under abiotic stresses by red-ox balance mediation [40]. The homeostasis and antioxidant activity of carotenoids are linked with the expression of the multifunctional Orange (Or) protein, which was confirmed in studies with transgenic tobacco lines exhibiting increase in carotenoids content under drought, heat, salt, and methyl viologen-mediated oxidative stress [50].
In addition to key roles in photosynthesis, photoprotection and response to environmental stress, carotenoids are involved in interactions with pathogens and symbiotic organisms [23]. Moreover, these pigments play important roles in germination, photomorphogenesis, fruit development and different signaling and regulatory processes [51]. It was shown that apocarotenoids of plants serve as visual or volatile signals to attract pollinators, provide allelopathic interactions, enable plant defense and seed dispersal [52] and act as feeding deterrents in plant-herbivorous insect interaction [53].
Carotenoids are very abundant in flowers and fruits conferring yellow, orange and red colors to attract insects and animals for pollination or seed dispersal [51,54]. Carotenoids as attractants are accumulated in the chromoplasts in a significant amount [55]. The methylerythritol pathway of carotenoid biosynthesis is also activated in case of production of some volatile apocarotenoids, involved in pollinator attraction [56], as reported for the cyclohexenone and mycorradicins derivates [57]. Since chromoplasts are crucial for accumulation of attractant-related roles of carotenoids, the regulation of chromoplast biogenesis is an important aspect in carotenoid production [58]. During enzymatic oxidation of carotenoids some signaling molecules are produced. The ABA hormone is involved in regulation of early embryo development, stomatal regulation, seed germination and stress tolerance responses, and it is derived from the enzymatic oxidation of the xanthophyll neoxanthin [59]. Carotenoid-derived strigolactones are known as terpenoid lactones constituting a class of hormones which were initially reported as signal molecules in establishment of plant-microbial symbioses, arbuscular mycorrhizae and parasitic seed germination [60]. Strigolactones were further evaluated for regulation of shoot development and branching [61], and coordination of shoot and root development [62]. It is known that carotenoids which contain polar groups at the two ends of the molecule (xanthophylls) are localized in the membrane bilayer forming hydrogen bonds with the hydrophilic groups of lipids [63]. These interactions between xanthophyll molecules and membrane lipids result in a decrease in membrane fluidity, an increase in membrane thermo-stability, and a lowered susceptibility to lipid peroxidation [37], which points out some additional functions of carotenoids at subcellular organizational level.
Accumulation of carotenoids in plastids, mainly chromoplasts, is very complex and dependent on transcriptional and post-translational control of biosynthesis pathways, carotenoids’ degradation and transformation, as well as the influence of regulatory networks of different metabolic and physiological processes [64].
3.4 Occurrence of Carotenoids and Carotenoid-Rich Plants
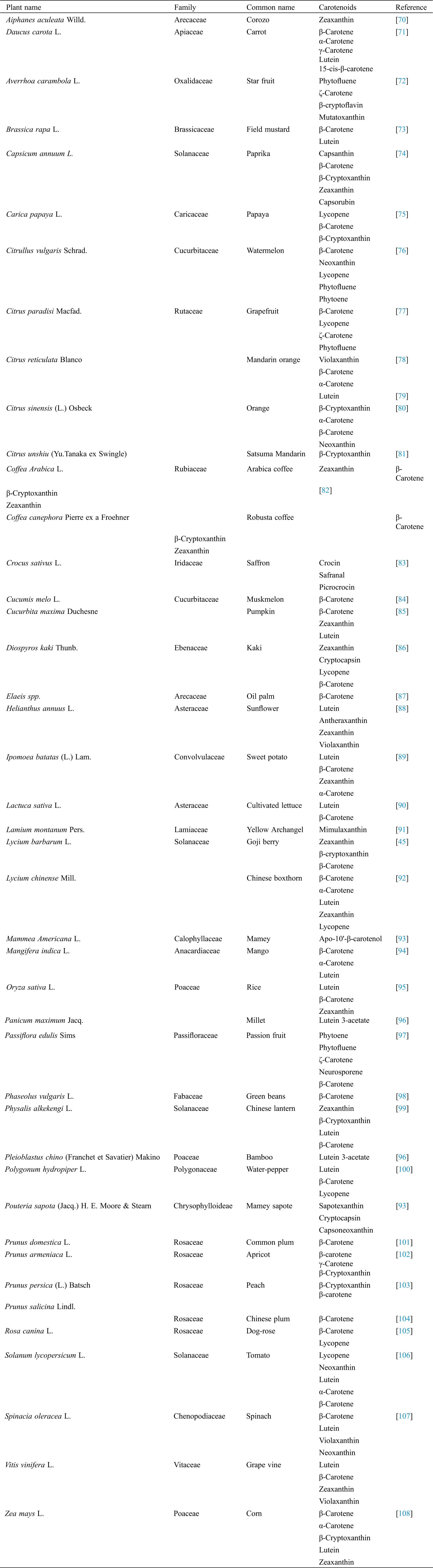
Carotenoids are predominately synthesized by plants, the fruits and vegetables represent their principal sources. Dietary carotenoids are obtained from a number of foods, such as carrots, sweet potato, peach, apricot, tomato, orange, pumpkin and many others. Generally, the type and intensity of a color are in proportion with the composition and concentration of carotenoids, respectively [21]. Yellow-orange vegetables and fruits are generally rich in β-carotene, in addition to α-carotene, and often the xantophylls, while cryptoxanthin and zeinoxanthin can be frequently found in orange fruits [20]. Lutein and zeaxanthin are present in green and dark green leafy vegetables, which also could contain significant portions of β-carotene too [65]. The red and purple vegetables contain a significant amount of non-vitamin A active carotenoids, where tomato and watermelon are major sources of lycopene [66], and red paprika of capsanthin [67]. Different apocarotenoids are typical for saffron plants, for example [68,69]. In general, the content and types of carotenoids are dependent on genotype, maturation phase, cultivation and climatic conditions, as well as postharvest and processing practices [20]. However, the composition and order of the most represented carotenoids in carotenoid-rich sources, is a species-specific feature (Tab. 1).
Tab. 1 shows the list of some edible plants rich in carotenoids (43 species totally).The most dominant carotenoids (minimum 1 and maximum 5) are presented in the table. The most frequent carotenoids were beta-carotene (in 33 species), lutein (in 16 species) and zeaxanthine (in 14 species). Among the less frequent the following could be stated: mutatoxanthin, picrocrocin, sapotexanthin, neurosporene, mimulaxanthin.
Table 1: The most dominant carotenoids in plants
4 Carotenoids and Human Health
Carotenoid-rich sources, mainly fruits and vegetables, have received a lot of attention recently due to health-related benefits expressed after their regular consumption. Carotenoids are associated with enhancement of the immune system functions and lower risk of developing degenerative chronic diseases, such as age-related macular degeneration, type 2 diabetes, obesity, neurological and cognitive disorders, and osteoporosis [5,7]. Deficiency of carotenoids leads to clinical signs of different corneal aberrations, including night, scarring and irreversible blindness, in addition to other eye disorders [109].
Patients with Crohn’s disease and celiac disease have irregular carotenoid levels in blood and certain tissues [110]. Specific dietary recommendations and increased intake of carotenoids is required in case of gastrointestinal disorders [4]. According to the World Cancer Research Fund (WCRF), the intake of carotenoids may reduce cancer risk. The complex anticancer properties of carotenoids are associated with phosphorylation and activation of major signing kinase, modulation of cellular pathways of Nrf2 and NF-κB transcription factors, apoptosis, cell cycle progression, gap junction intercellular communication (GJIC) and angiogenesis [3,10]. It should be stressed that carotenoids are known as anti-ageing agents, the skin photoprotectants and providers of various cosmetic benefits [111]. It is generally assumed that favorable biological effects of carotenoids mainly refer to their provitamin A activity and strong antioxidant properties.
The immune-boosting and prophylactic anticancer effects of carotenoids are most likely linked to their high antioxidant property and ability to reduce the DNA damage induced by reactive oxygen species. Carotenoids are known as antioxidant molecules able to scavenge ROS thanks to polyene backbone with a series of conjugated double-bonds system stabilizing the unpaired electrons after radical quenching [112]. The antioxidant capacity of carotenoid molecule depends on the number of double-bonds in their backbone. Consequently, the lycopene, α-carotene and β-carotene are strong antioxidants and they were the most frequently reported as carotenoids able to lower risks of carcinogenesis of different human organs [113].
5 Bioavailability of Carotenoids
Bioavailability of a nutrient includes two interconnected subsets: the bioaccessibility and the bioactivity. The concept of bioaccessibility is defined as the quantity or fraction that is released from the food matrix in the gastrointestinal tract and able to be absorbed, while bioactivity refers to the processes of a nutrient entering into systemic circulation, its transportation to the target site, resulting in expression of various metabolic and physiological effects [114]. In general, carotenoids are unstable molecules sensitive to light, temperature and extreme pH in the presence of oxygen. Low bioavailability of carotenoids represents a limitation in expression of their potential bioactivity, since bioaccessibility, absorption, and transformation of highly lipophilic compounds are rather low being linked with their weak solubility and sensibility to gastrointestinal fluids [10]. The bioavailability of carotenoids is mainly dependent on carotenoid type, heat treatment, presence of lipids and their interaction [115]. Some processing conditions could induce trans-to-cis carotenoid isomerization. For example, all food trans-β-carotene, which is highly unstable, immediately undergoes isomerization, chemical and thermal oxidation, and photosensitization when exposed to light or high temperature [20,65]. The absorption efficiency of carotenoids is also influenced by food matrix and food formulation [116].
6 Raman Spectroscopy and Carotenoids
Raman is a vibrational spectroscopy technique based on the interaction of high-energy light radiation with molecular vibrations. Raman spectra and images can be used to evaluate physical, chemical, biological [117,118] and sensory properties [76] of food and agricultural products. Current analytic tools for carotenoid determination include HPLC, HPTLC, LC-MS, UHPLC, Orbitrap MS, NMR, etc. [20,35]. All these methods require application of specific and often long extraction procedures, facing high sensitivity of carotenoids and their fast degradation by isomerisation and oxidation. Furthermore, these techniques cannot meet the demand for in situ, fast and multiple analyses as compared to modern optical sensing technologies. Raman spectroscopy allows identifying the species, content and distribution of carotenoids in biological systems, in addition to ability to recognize the isomeric forms [119].
Raman spectroscopy or spectral microscopy has become increasingly common in various fields of research in recent decades due to its many positive characteristics: a) a small amount of sample is needed; b) the sample is not damaged during measurement; c) measurement can be done in a very short time; d) measurement can be repeated several times as needed; e) sample can be used in any physical state, etc.
The application of Raman spectroscopy in solving biochemical problems emerged in the eighties of the 20th century. Carotenoids have shown to be suitable for this type of analysis because vibrational spectra can be obtained even at very low sample concentrations, even if the chromophores are located in a very complex system [120].
Carotenoid molecules are among the most efficient Raman scatterers [121]. Carotenoids are strongly colored as they have an allowed π-π* transition occurring in the visible region. When the laser wavelength coincides with the π-π* (S-S0) electron transition, Raman spectra are obtained [122]. Functional groups of molecules can be identified by their unique pattern of light scattering, called the Raman spectrum, with a signal intensity being proportional to its relative concentration in the sample. Plant tissues contain enzymes and coenzymes that can be absorbed in the visible spectral range, inducing fluorescence that can overlap with Raman carotenoid signals [123]. Raman spectra of carotenoids show strong bands in the range of 1500–1550 cm−1 and in the range of 1150–1170 cm−1 due to C = C (ν1) and C-C vibrations (ν2) in the polyene chain. C-CH3 deformation also occurs, which is denoted by ν3 and occurs in the range of 1000–1005 cm−1 (Fig. 3). Raman spectroscopy was applied for non-destructive and simultaneous determination of carotenoids of very different samples, such as human skin [124,125] and tomato fruit [126] using laser excitations in the visible part of the spectrum (488–514.5 nm). Such experiments enabled rapid resonant signal amplification, but didn’t allow discrimination between lycopene and beta carotene for example. All bands of both carotenoids were observed at the same wave numbers (1525, 1159, 1008 cm−1) [124–126]. However, the use of FT-Raman spectrophotometers with near-infrared excitation (1064 nm) provides discrimination between lycopene (1510 cm−1) and beta-carotene (1520 cm−1) [127]. Therefore, FT-Raman spectroscopy is suitable for simultaneous determination of different carotenoids in carotenoid-rich sources compared to standard Raman spectroscopy tools [128].
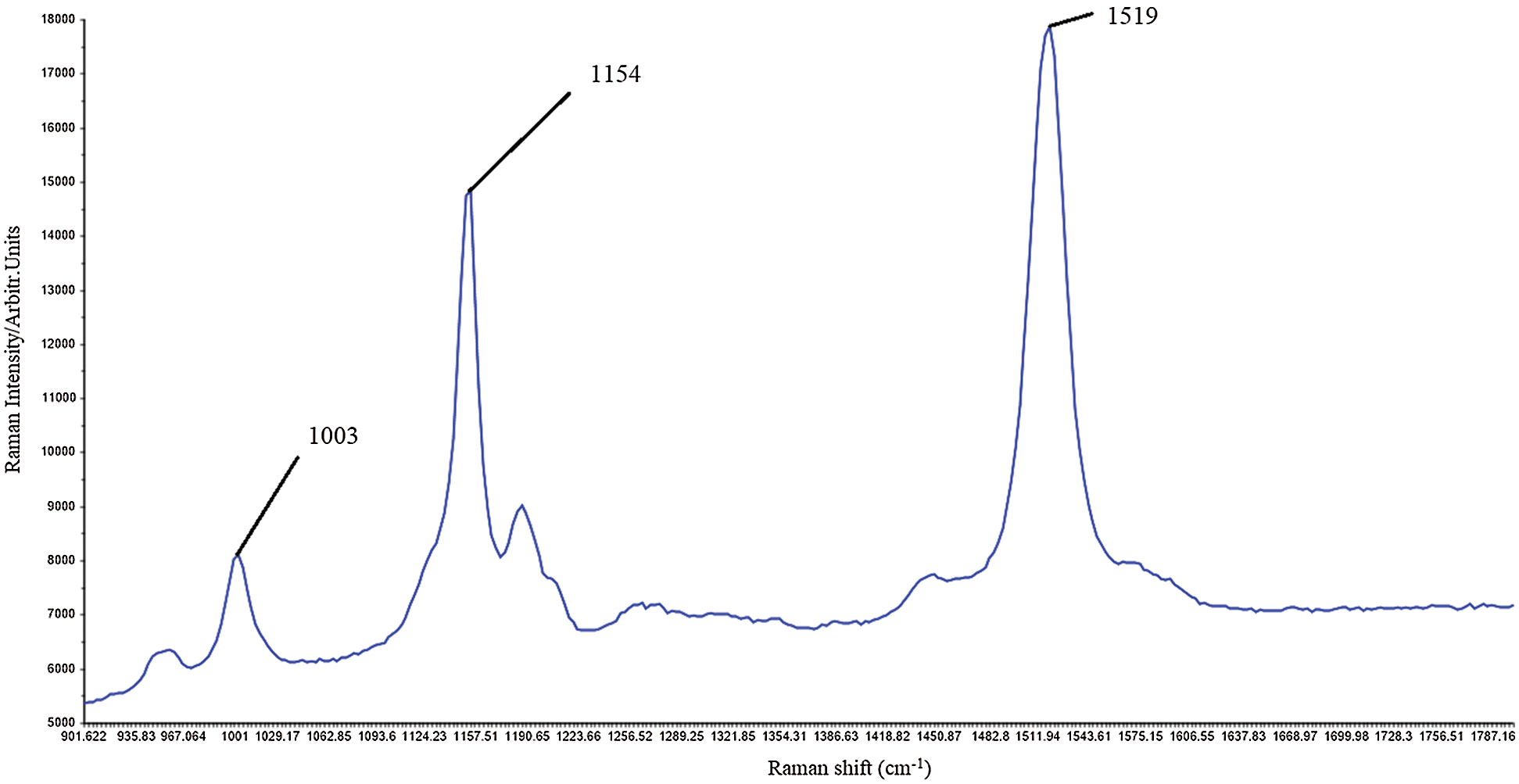
Figure 3: Raman carotenoid spectra of Capsicum anuum fruit (red bell pepper local Balkan variety in full ripening phase (original authors’ unpublished data); strong bands most likely associated with the capsanthin
Schulz et al. [129] used the Raman spectroscopy technique to map carotenoids in carrot tissues. They concluded that β-carotene accumulates in the secondary phloem and gradually increases from the periderm to the cortex, whereas α- carotene and lycopene were stored in younger cells and throughout the secondary phloem, respectively. FT-Raman spectroscopy is also used for in situ analysis of carotenoids in red pepper, nectarine, pumpkin, corn seed and apricot [35].
Application of Raman spectroscopy represents the alternative method in analyses and determination of carotenoids in different samples. It is characterized by speed, simplicity, safety, low operating costs, and non-destructiveness [125,126] and therefore spectra can be obtained directly from the sample without prior carotenoid extraction [130]. In addition to the standard analytics, the frequently used techniques for carotenoids determination refer to FT-Raman (Fourier Transform-Raman Spectroscopy Measurements), ATR-IR (Attenuated Total Reflection Infrared Spectroscopy) and NIR (Near Infrared Spectroscopy) [128]. These methods exhibit a high correlation with the results obtained by the HPLC and other standard analytic methods [35].
Since Raman spectra from biological samples are complex and diverse, the interpretation of such data is difficult. In order to overcome some difficulties and to obtain meaningful information, multivariate chemometric analysis is performed. The first step in multivariate analysis is pretreatment of data which includes spike correction, wavenumber calibration, intensity calibration, smoothing, baseline correction, outlier detection and normalization. After this, data reduction is performed and data are ready for further modeling [131]. Data modeling in Raman-based carotenoid studies includes performance of both unsupervised classification models such as Principal component analysis (PCA) and Hierarchical Cluster Analysis (AHC) and supervised classification models such as Linear discriminant analysis (LDA) [132], Partial least square discrimination analysis (PLS-DA) and k-nearest neighbors (KNN) [133]. In addition to classification, regression models can be performed. The most used regression models are: Multiple least regression (MLR) [134], Principal component regression (PCR) [134]. Artificial neural networks (ANN), Soft independent modeling of class analogy (SIMCA) and Partial least squares regression (PLSR) [135].
7 Biotechnologies Linked with Application of Carotenoids
Modern biotechnologies, colloquially known as “omics”, are widely used in crop modifications aiming to improve their stress tolerance and nutrient quality. There are two main strategies in overcoming the problem of dietary carotenoid deficiency: a) bioengineering for enhancement of the nutritional carotenoid value in staple crops, and b) encapsulation of carotenoids to prevent their degradation and low bioavailability.
Genetic engineering of carotenoid biosynthesis and induction of higher carotenoid accumulation are beneficial for the nutritive improvement of vital crops, where system biology tools, including genome-scale metabolic models with the computational tools, allow the identification of the candidate pathway genes in a genome and characterization of the germplasm diversity [2]. Over the last few decades, range of genes involved in carotenoid biosynthesis, accumulation and transformation have been cloned and used to improve and increase the carotenoid content in different crops [3]. There are already some transgenic crops with enhanced carotenoid content, i.e., rice [136], tomato [137], sweet potato [138], and some others, such as corn, canola, potato, soybean, tobacco, Arabidopsis and wheat [2].
Encapsulation of carotenoids is a relatively new technology relying on entrapment of the bioactive ingredient inside a different polymer carrier(s), i.e., poly(lactic acid) (PLA) and its copolymers, poly(lactide-co-glycolide) (PLGA); and poly(e-caprolactone) (PCL) [139], lipid-based carriers [140], cyclodextrins, glycyrrhizin and arabinogalactan [141], which all are ‘‘generally recognized as safe’’ (GRAS). Encapsulation of carotenoids is a promising approach using different micro- and nano-encapsulation technologies, such as extrusion, homogenization, electrospinning/spraying, (nano) spray drying, and emulsification [9]. Application of encapsulated carotenoids mostly refers to functional foods, fortified food colorants, as well as use in cosmetics.
From nutritional perspective, the encapsulation of carotenoids is advanced technology mainly because of the low bioavailability and low uptake (lower than 1%) of these micronutrients from fresh and processed foods [142]. Encapsulation of carotenoids has two functions: (1) to enhance the oxidative stability, thermo stability, photo stability, shelf-life, and degradation during processing, storage and digestion and (2) to increase their bio-accessibility and ensure the target delivery for performance of desired biological activity [139,143]. Recent advances in encapsulation of carotenoids with bio-polymeric (nano) carriers, solely or in combination (in form of complex carriers), announce the new era of further novel applications and appearance of new formulations and products [9].
8 Future Challenges and Concluding Remarks
Due to the fact that carotenoids have many health beneficial and nutritional effects, the interest for these compounds and their application is gradually increasing. However, the only small portion of a number of carotenoids and their cleavage products that have been already described is being studied in depth. In fact, the mechanisms of carotenoids’ involvement in signaling and developmental processes in plants are not fully understood, as well as the mechanisms related to prophylactic and health beneficial effects in human. There is still many challenges in the field of carotenoid research and further use of new biotechnologies for wider application of carotenoid-rich products. The data on nutritional needs and appropriate supplementation, especially in studies of anticancer, chemoprotection and chemotherapeutical effects of carotenoids are still controversial. Further studies on the safety of nano-encapsulated carotenoids are crucial, as well as the understanding of their complex interactions with the cellular systems and delivery mechanisms to the target sites. Genetic engineering, breeding, and research on transgenic carotenoid-producing crops in addition to development of appropriate carriers for micro/nano-capsulation of carotenoids will be the key approaches to meet the growing global demand for these micronutrients. Recent achievements in encapsulation of carotenoids with biopolymeric (polysaccharides and proteins), and lipid-based carriers (emulsions and liposomes) seem promising for innovative developments in carotenoids application strategies. Finally, wider application of modern analytic tools, first of all the vibrational spectroscopy, mainly referring to Raman (micro)spectroscopy coupled with complex and advanced chemo-metric modeling, will enable more efficient, more comprehensive and more accurate determination of carotenoids in different biological and food systems, which will further facilitate the application of carotenoids as valuable pharmaceuticals and nutraceuticals.
Funding Statement: This work was supported by Ministry of Education, Science and Technological Development of Republic of Serbia, the Contract No. 451-03-68/2020-14/200116 and the EthnoHERBS-H2020-MSCA-RISE-2018 Project.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Namitha, K. K., Negi, P. S. (2010). Chemistry and biotechnology of carotenoids. Critical Reviews in Food Science and Nutrition, 50(8), 728–760. DOI 10.1080/10408398.2010.499811. [Google Scholar] [CrossRef]
2. Sankari, M., Rao, P. R., Hemachandran, H., Pullela, P. K., Doss, C. G. P. et al. (2018). Prospects and progress in the production of valuable carotenoids: Insights from metabolic engineering, synthetic biology, and computational approaches. Journal of Biotechnology, 266, 89–101. DOI 10.1016/j.jbiotec.2017.12.010. [Google Scholar] [CrossRef]
3. Rodriguez-Concepcion, M., Avalos, J., Bonet, M. L., Boronat, A., Gomez-Gomez, L. et al. (2018). A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research, 70, 62–93. DOI 10.1016/j.plipres.2018.04.004 [Google Scholar] [CrossRef]
4. Von Lintig, J., Moon, J., Lee, J., Ramkumar, S. (2020). Carotenoid metabolism at the intestinal barrier. BBA-Molecular and Cell Biology of Lipids, 1865, 158580. DOI 10.1016/j.bbalip.2019.158580. [Google Scholar] [CrossRef]
5. Madrid, R., Carballo-Uicab, R., Cárdenas-Conejo, V. M., Aguilar-Espinosa, Y., Siva, M., R. (2020). Overview of carotenoids and beneficial effects on human health. Carotenoids: Properties, Processing and Applications. Amsterdam: Elsevier. [Google Scholar]
6. Eggersdorfer, M., Wyss, A. (2018). Carotenoids in human nutrition and health. Archives of Biochemistry and Biophysics, 652, 18–26. DOI 10.1016/j.abb.2018.06.001. [Google Scholar] [CrossRef]
7. Khalid, M.Saeed-ur-Rahman, Bilal, M., Iqbal, H. M. N., Huang, D. F. (2019). Biosynthesis and biomedical perspectives of carotenoids with special reference to human health-related applications. Biocatalysis and Agricultural Biotechnology, 17, 399–407. DOI 10.1016/j.bcab.2018.11.027. [Google Scholar] [CrossRef]
8. Neri-Numa, I. A., Arruda, H. S., Geraldi, M. V., Maróstica Júnior, M. R., Pastore, G. M. (2020). Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Current Opinion in Food Science, 33, 98–107. DOI 10.1016/j.cofs.2020.03.004. [Google Scholar] [CrossRef]
9. Rehman, A., Tong, Q., Jafari, S. M., Assadpour, E., Shehzad, Q. et al. (2020). Carotenoid-loaded nanocarriers: A comprehensive review. Advances in Colloid and Interface Science, 275, 102048. DOI 10.1016/j.cis.2019.102048. [Google Scholar] [CrossRef]
10. Saini, R. K., Keum, Y.-S., Daglia, M., Rengasamy, K. R. (2020). Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacological Research, 157, 104830. DOI 10.1016/j.phrs.2020.104830. [Google Scholar] [CrossRef]
11. Ahuja, K., Rawat, A. (2019). Diverse applicability of encapsulated ingredients along with increasing encapsulation capacity will foster the product demand. https://www.gminsights.com/industry-analysis/food-encapsulation-market. [Google Scholar]
12. Yang, Y., Kim, B., Lee, J. (2013). Astaxanthin structure, metabolism, and health benefits. Journal of Human Nutrition & Food Science, 1(1), 1003. https://www.jscimedcentral.com/Nutrition/Articles/nutrition-1-1003.php#tab2. [Google Scholar]
13. Cheng, S.-H., Khoo, H. E., Kong, K. W., Prasad, K. N., Galanakis, C. M. (2020). Carotenoids: Properties, processing and applications, Amsterdam: Elsevier. [Google Scholar]
14. Britton, G. (2020). Carotenoid research: History and new perspectives for chemistry in biological systems. BBA-Molecular and Cell Biology of Lipids, 1865(11), 158699. DOI 10.1016/j.bbalip.2020.158699. [Google Scholar] [CrossRef]
15. Harrison, P. J., Bugg, T. D. H. (2014). Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Archives of Biochemistry and Biophysics, 544, 105–111. DOI 10.1016/j.abb.2013.10.005. [Google Scholar] [CrossRef]
16. Cuttriss, A. J., Cazzonelli, C. I., Wurtzel, E. T., Pogson, B. J. (2011). Carotenoids, advances in botanical research. Amsterdam: Elsevier. [Google Scholar]
17. Tran, P. T., Sharifi, M. N., Poddar, S., Dent, R. M., Niyogi, K. K. (2012). Intragenic enhancers and suppressors of phytoene desaturase mutations in chlamydomonas reinhardtii. PLoS One, 7, e42196. DOI 10.1371/journal.pone.0042196. [Google Scholar] [CrossRef]
18. Walter, M. H., Floss, D. S., Strack, D. (2010). Apocarotenoids: Hormones, mycorrhizal metabolites and aroma volatiles. Planta, 232(1), 1–17. DOI 10.1007/s00425-010-1156-3. [Google Scholar] [CrossRef]
19. Ruiz-Sola, M. Á., Rodríguez-Concepción, M. (2012). Carotenoid biosynthesis in arabidopsis: A colorful pathway. The Arabidopsis Book, 10, e0158. DOI 10.1199/tab.0158. [Google Scholar] [CrossRef]
20. Saini, R. K., Nile, S. H., Park, S. W. (2015). Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, 76(Pt3), 735–750. DOI 10.1016/j.foodres.2015.07.047. [Google Scholar] [CrossRef]
21. Prakash, D., Sharma, G. (2014). Phytochemicals of nutraceutical importance. UK: CABI. [Google Scholar]
22. Britton, G. (1995). Structure and properties of carotenoids in relation to function. Official Publication of the Federation of American Societies for Experimental Biology, 9(15), 1551–1558. DOI 10.1096/fasebj.9.15.8529834. [Google Scholar] [CrossRef]
23. Hirschberg, J. (2001). Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology, 4(3), 210–218. 10.1016/S1369-5266(00)00163-1. [Google Scholar] [CrossRef]
24. Cazzonelli, C. I. (2001). Carotenoids in nature: Insights from plants and beyond. Functional Plant Biology, 38(11), 833–847. DOI 10.1071/FP11192. [Google Scholar] [CrossRef]
25. Demmig-Adams, B., Adams, W. W. (1996). The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends in Plant Science, 1(1), 22–26. DOI 10.1016/S1360-1385(96)80019-7. [Google Scholar] [CrossRef]
26. Polívka, T., Frank, H. A. (2010). Molecular factors controlling photosynthetic light harvesting by carotenoids. Accounts of Chemical Research, 43(8), 1125–1134. DOI 10.1021/ar100030m. [Google Scholar] [CrossRef]
27. Fiedor, L., Zbyradowski, M., Pilch, M. (2019). Tetrapyrrole pigments of photosynthetic antennae and reaction centers of higher plants: structures, biophysics, functions, biochemistry, mechanisms of regulation, applications. advances in botanical research. Amsterdam: Elsevier. [Google Scholar]
28. Green, B. R., Parson, W. W. (2019). Light-harvesting antennas in photosynthesis, Switzerland. Springer. [Google Scholar]
29. Niyogi, K. K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology, 50, 333–359. DOI 10.1146/annurev.arplant.50.1.333. [Google Scholar] [CrossRef]
30. Shen, Y., Li, J., Gu, R., Yue, L., Wang, H. et al. (2018). Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere, 197, 513–525. DOI 10.1016/j.chemosphere.2018.01.036. [Google Scholar] [CrossRef]
31. Braslavsky, S. E., Holzwarth, A. R. (2012). Role of carotenoids in photosystem II (PSII) reaction centers. International Journal of Thermophysics, 33, 2021–2025. DOI 10.1007/s10765-012-1274-1. [Google Scholar] [CrossRef]
32. Hashimoto, H., Uragami, C., Cogdell, R. J. (2016). Carotenoids and photosynthesis. Carotenoids in Nature. Switzerland. Springer. [Google Scholar]
33. Telfer, A., Dhami, S., Bishop, S. M., Phillips, D., Barber, J. (1994). Β-carotene quenches singlet oxygen formed by isolated photosystem II reaction centers. Biochemistry, 33(48), 14469–14474. DOI 10.1021/bi00252a013. [Google Scholar] [CrossRef]
34. Choudhury, N. K., Behera, R. K. (2001). Photoinhibition of photosynthesis: Role of carotenoids in photoprotection of chloroplast constituents. Photosynthetica, 39, 481–488. DOI 10.1023/A:1015647708360. [Google Scholar] [CrossRef]
35. Rodriguez-Amaya, D. B. (2016). Food carotenoids: Chemistry, biology and technology. USA. Wiley-Blackwell. [Google Scholar]
36. Kress, E., Jahns, P. (2017). The dynamics of energy dissipation and xanthophyll conversion in arabidopsis indicate an indirect photoprotective role of zeaxanthin in slowly inducible and relaxing components of non-photochemical quenching of excitation energy. Frontiers in Plant Science, 8, 2094. DOI 10.3389/fpls.2017.02094. [Google Scholar] [CrossRef]
37. Havaux, M. (2014). Carotenoid oxidation products as stress signals in plants. The Plant Journal for Cell and Molecular Biology, 79(4), 597–606. DOI 10.1111/tpj.12386. [Google Scholar] [CrossRef]
38. Uarrota, V. G., Stefen, D. L. V., Leolato, L. S., Gindri, D. M., Nerling, D. (2018). Antioxidants Antioxid. Enzymes. High. Plants, Berlin, Springer, Cham. [Google Scholar]
39. Felemban, A., Braguy, J., Zurbriggen, M. D., Al-Babili, S. (2019). Apocarotenoids involved in plant development and stress response. Frontiers in Plant Science, 10, 1168–1184. DOI 10.3389/fpls.2019.01168. [Google Scholar] [CrossRef]
40. Fanciullino, A. L., Bidel, L. P. R., Urban, L. (2014). Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant, Cell & Environment, 37, 273–289. DOI 10.1111/pce.12153. [Google Scholar] [CrossRef]
41. Eismann, A. I., Perpetuo Reis, R., Ferreira da Silva, A., Negrão Cavalcanti, D. (2020). Ulva spp. carotenoids: Responses to environmental conditions. Algal Research, 48, 101916. DOI 10.1016/j.algal.2020.101916. [Google Scholar] [CrossRef]
42. León-Chan, R. G., López-Meyer, M., Osuna-Enciso, T., Sañudo-Barajas, J. A., Heredia, J. B. et al. (2017). Low temperature and ultraviolet-b radiation affect chlorophyll content and induce the accumulation of UV-b-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environmental and Experimental Botany, 139, 143–151. DOI 10.1016/j.envexpbot.2017.05.006. [Google Scholar] [CrossRef]
43. Borghesi, E., González-Miret, M. L., Escudero-Gilete, M. L., Malorgio, F., Heredia, F. J. et al. (2011). Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. Journal of Agricultural and Food Chemistry, 59, 11676–11682. DOI 10.1021/jf2021623. [Google Scholar] [CrossRef]
44. Doupis, G., Bertaki, M., Psarras, G., Kasapakis, I., Chartzoulakis, K. (2013). Water relations, physiological behavior and antioxidant defence mechanism of olive plants subjected to different irrigation regimes. Scientia Horticulturae (Amsterdam153, 150–156. DOI 10.1016/j.scienta.2013.02.010. [Google Scholar] [CrossRef]
45. Inbaraj, B. S., Lu, H., Hung, C. F., Wu, W. B., Lin, C. L. et al. (2008). Determination of carotenoids and their esters in fruits of Lycium barbarum linnaeus by HPLC-dAD-aPCI-mS. Journal of Pharmaceutical and Biomedical Analysis, 47(4–5), 812–818. DOI 10.1016/j.jpba.2008.04.001. [Google Scholar] [CrossRef]
46. Couso, I., Vila, M., Vigara, J., Cordero, B. F., Vargas, M. Á. et al. (2012). Synthesis of carotenoids and regulation of the carotenoid biosynthesis pathway in response to high light stress in the unicellular microalga Chlamydomonas reinhardtii. European Journal of Phycology, 47, 223–232. DOI 10.1080/09670262.2012.692816. [Google Scholar] [CrossRef]
47. Hammad, S. A. R., Ali, O. A. M. (2014). Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Annals of Agricultural Sciences, 59, 133–145. DOI 10.1016/j.aoas.2014.06.018. [Google Scholar] [CrossRef]
48. Shankar, V., Kumar, D., Agrawal, V. (2016). Assessment of antioxidant enzyme activity and mineral nutrients in response to NaCl stress and its amelioration through glutathione in chickpea. Biotechnology and Applied Biochemistry, 178, 267–284. DOI 10.1007/s12010-015-1870-1. [Google Scholar] [CrossRef]
49. Taïbi, K., Taïbi, F., Ait Abderrahim, L., Ennajah, A., Belkhodja, M. et al. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany, 105, 306–312. DOI 10.1016/j.sajb.2016.03.011. [Google Scholar] [CrossRef]
50. Wang, Z., Xu, W., Kang, J., Li, M., Huang, J. et al. (2018). Overexpression of alfalfa orange gene in tobacco enhances carotenoid accumulation and tolerance to multiple abiotic stresses. Plant Physiology and Biochemistry, 130, 613–622. DOI 10.1016/j.plaphy.2018.08.017. [Google Scholar] [CrossRef]
51. Cazzonelli, C. I., Pogson, B. J. (2010). Source to sink: Regulation of carotenoid biosynthesis in plants. Trends in Plant Science, 15(5), 266–274. DOI 10.1016/j.tplants.2010.02.003. [Google Scholar] [CrossRef]
52. Bouvier, F., Isner, J. C., Dogbo, O., Camara, B. (2005). Oxidative tailoring of carotenoids: A prospect towards novel functions in plants. Trends in Plant Science, 10(4), 187–194. DOI 10.1016/j.tplants.2005.02.007. [Google Scholar] [CrossRef]
53. Gruber, M. Y., Xu, N., Grenkow, L., Li, X., Onyilagha, J. et al. (2009). Responses of the crucifer flea beetle to Brassica volatiles in an olfactometer. Environmental Entomology, 38, 1467–1479. DOI 10.1603/022.038.0515. [Google Scholar] [CrossRef]
54. Yuan, H., Zhang, J., Nageswaran, D., Li, L. (2015). Carotenoid metabolism and regulation in horticultural crops. Horticulture Research, 2, 15036. DOI 10.1038/hortres.2015.36. [Google Scholar] [CrossRef]
55. Nisar, N., Li, L., Lu, S., Khin, N. C., Pogson, B. J. (2015). Carotenoid metabolism in plants. Molecular Plant, 8(1), 68–82. DOI 10.1016/j.molp.2014.12.007. [Google Scholar] [CrossRef]
56. McQuate, G. T., Peck, S. L. (2001). Enhancement of attraction of alpha-ionol to male Bactrocera latifrons (Diptera: Tephritidae) by addition of a synergist, cade oil. Journal of Economic Entomology, 94(1), 39–46. DOI 10.1603/0022-0493-94.1.39. [Google Scholar] [CrossRef]
57. Esteban, R., Moran, J. F., Becerril, J. M., García-Plazaola, J. I. (2015). Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environmental and Experimental Botany, 119, 63–75. DOI 10.1016/j.envexpbot.2015.04.009. [Google Scholar] [CrossRef]
58. Sun, T., Yuan, H., Cao, H., Yazdani, M., Tadmor, Y. et al. (2018). Carotenoid metabolism in plants: The role of plastids. Molecular Plant, 11(1), 58–74. DOI 10.1016/j.molp.2017.09.010. [Google Scholar] [CrossRef]
59. Nambara, E., Marion-Poll, A. (2005). Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology, 56, 165–185. DOI 10.1146/annurev.arplant.56.032604.144046. [Google Scholar] [CrossRef]
60. Mostofa, M. G., Li, W., Nguyen, K. H., Fujita, M., Tran, L. S. P. (2018). Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant, Cell & Environment, 41, 2227–2243. DOI 10.1111/pce.13364. [Google Scholar] [CrossRef]
61. Gomez-Roldan, V., Fermas, S., Brewer, P. B., Puech-Pagès, V., Dun, E. A. et al. (2008). Strigolactone inhibition of shoot branching. Nature, 455(7210), 189–194. DOI 10.1038/nature07271. [Google Scholar] [CrossRef]
62. Koltai, H. (2011). Strigolactones are regulators of root development. New Phytologist, 190, 545–549. DOI 10.1111/j.1469-8137.2011.03678.x. [Google Scholar] [CrossRef]
63. Gruszecki, W. I., Strzałka, K. (2005). Carotenoids as modulators of lipid membrane physical properties. Biochimica et Biophysica Acta, 1740(2), 108–115. DOI 10.1016/j.bbadis.2004.11.015. [Google Scholar] [CrossRef]
64. Sun, T., Li, L. (2020). Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Science: An International Journal of Experimental Plant Biology, 290, 110331. DOI 10.1016/j.plantsci.2019.110331. [Google Scholar] [CrossRef]
65. Mezzomo, N., Ferreira, S. R. S., (2016). Carotenoids functionality, sources, and processing by supercritical technology: A review. Journal of Chemistry, 2016, 1–16. DOI 10.1155/2016/3164312. [Google Scholar] [CrossRef]
66. Del Campo, J. A., García-González, M., Guerrero, M. G. (2007). Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Applied Microbiology and Biotechnology, 74(6), 1163–1174. DOI 10.1007/s00253-007-0844-9. [Google Scholar] [CrossRef]
67. Kim, J. S., An, C. G., Park, J. S., Lim, Y. P., Kim, S. (2016). Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chemistry, 201, 64–71. DOI 10.1016/j.foodchem.2016.01.041. [Google Scholar] [CrossRef]
68. Popović-Djordjević, J.B., Kostić, A.Ž., Kiralan, M. (2020). Antioxidant activities of bioactive compounds and various extracts obtained from Saffron. In: Saffron, Galanakis, C. M. Chapter, 2, 41–97. Elsevier Inc./ Academic Press, London, UK. DOI 10.1016/B978-0-12-821219-6.00002-6. [Google Scholar] [CrossRef]
69. Rosati, C., Diretto, G., Giuliano, G. (2009). Biosynthesis and engineering of carotenoids and apocarotenoids in plants: State of the art and future prospects. Biotechnology & Genetic Engineering Reviews, 26, 139–162. DOI 10.5661/bger-26-139. [Google Scholar] [CrossRef]
70. Murillo, E., Meléndez-Martínez, A. J., Portugal, F. (2010). Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chemistry, 122, 167–172. 10.1016/j.foodchem.2010.02.034. [Google Scholar] [CrossRef]
71. Heinonen, M. I. (1990). Carotenoids and provitamin a activity of carrot (Daucus carota L.) cultivars. Journal of Agricultural and Food Chemistry, 38(3), 609–612. DOI 10.1021/jf00093a005. [Google Scholar] [CrossRef]
72. Gross, J., Ikan, R., Eckhardt, G. (1983). Carotenoids of the fruit of Averrhoa carambola. Phytochemistry, 22(6), 1479–1481. DOI 10.1016/S0031-9422(00)84040-6. [Google Scholar] [CrossRef]
73. Jung, H. J., Manoharan, R. K., Park, J. I., Chung, M. Y., Lee, J. et al. (2014). Identification of yellow pigmentation genes in Brassica rapa ssp. pekinensis using br300 microarray. International Journal of Genomics, 2014, 204969. DOI 10.1155/2014/204969. [Google Scholar] [CrossRef]
74. Hassan, M., Yusof, N., Yahaya, N. A., Mohd Rozali, A. F., Othman, N. N. (2019). Carotenoids of Capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants, 8(10), 469. DOI 10.3390/antiox8100469. [Google Scholar] [CrossRef]
75. Schweiggert, R. M., Steingass, C. B., Heller, A., Esquivel, P., Carle, R. (2011). Characterization of chromoplasts and carotenoids of red- and yellow-fleshed papaya (Carica papaya L.). Planta, 234(5), 1031–1044. DOI 10.1007/s00425-011-1457-1. [Google Scholar] [CrossRef]
76. Zhao, W., Lv, P., Gu, H. (2013). Studies on carotenoids in watermelon flesh. Agricultural Sciences, 4, 13–20. DOI 10.4236/as.2013.47A003. [Google Scholar] [CrossRef]
77. Khan, M. U. D., Mackinney, G. (1953). Carotenoids in grapefruit, Citrus paradisi. Plant Physiology, 28(3), 550–552. DOI 10.1104/pp.28.3.550. [Google Scholar] [CrossRef]
78. Xue, F., Li, C., Pan, S. (2012). Subacute toxicity assessment of carotenoids extracted from citrus peel (Nanfengmiju. Citrus Reticulata Blanco) in Rats. Regulatory Toxicology and Pharmacology: RTP, 62(1), 16–22. DOI 10.1016/j.yrtph.2011.12.003. [Google Scholar] [CrossRef]
79. MacLachlan, D. J., Mueller, U. (2012). A refined approach to estimate exposure for use in calculating the maximum residue limit of veterinary drugs. Regulatory Toxicology and Pharmacology: RTP, 62(1), 99–106. DOI 10.1016/j.yrtph.2011.12.006. [Google Scholar] [CrossRef]
80. Lux, P. E., Carle, R., Zacarías, L., Rodrigo, M. J., Schweiggert, R. M. et al. (2019). Genuine carotenoid profiles in sweet orange [Citrus sinensis (L.) Osbeck cv. Navel] Peel and Pulp at Different Maturity Stages. Journal of Agricultural and Food Chemistry, 67(47), 13164–13175. DOI 10.1021/acs.jafc.9b06098. [Google Scholar] [CrossRef]
81. Ma, G., Zhang, L., Yungyuen, W., Tsukamoto, I., Iijima, N. et al. (2016). Expression and functional analysis of citrus carotene hydroxylases: Unravelling the xanthophyll biosynthesis in citrus fruits. BMC Plant Bology, 16(1), 148. DOI 10.1186/s12870-016-0840-2. [Google Scholar] [CrossRef]
82. Simkin, A. J., Moreau, H., Kuntz, M., Pagny, G., Lin, C. et al. (2008). An investigation of carotenoid biosynthesis in Coffea canephora and Coffea arabica. Journal of Plant Physiology, 165(10), 1087–1106. DOI 10.1016/j.jplph.2007.06.016. [Google Scholar] [CrossRef]
83. Tarantilis, P. A., Beljebbar, A., Manfait, M., Polissiou, M. (1998). FT-Ir, FT-Raman spectroscopic study of carotenoids from saffron (Crocus sativus L.) and some derivatives. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 54(4), 651–657. DOI 10.1016/S1386-1425(98)00024-9. [Google Scholar] [CrossRef]
84. Medeiros, I., de Oliveira, G., de Queiroz, J., de Carvalho Gomes, C., de Carvalho, F. et al. (2020). Safety and bioactive potential of nanoparticles containing cantaloupe melon (Cucumis melo L.) Carotenoids in an Experimental Model of Chronic Inflammation. Biotechnology Reports, 28, e00567. DOI 10.1016/j.btre.2020.e00567. [Google Scholar] [CrossRef]
85. Kulczyński, B., Gramza-Michałowska, A. (2019). The profile of carotenoids and other bioactive molecules in various pumpkin fruits (Cucurbita maxima duchesne) cultivars. Molecules, 24(18), 3212. DOI 10.3390/molecules24183212. [Google Scholar] [CrossRef]
86. Brossard, J., Mackinney, G. (1963). Fruit pigments, carotenoids of diospyros kaki (Japaneses persimmons). Journal of Agricultural and Food Chemistry, 11(6), 501–503. DOI 10.1021/jf60130a019. [Google Scholar] [CrossRef]
87. Ribeiro, B. D., Barreto, D. W., Coelho, M. A. Z. (2011). Technological aspects of β-carotene production. Food and Bioprocess Technology, 4(5), 693–701. DOI 10.1007/s11947-011-0545-3. [Google Scholar] [CrossRef]
88. Ohmiya, A. (2011). Diversity of carotenoid composition in flower petals. Japan Agricultural Research Quarterly: JARQ, 45(2), 163–171. DOI 10.6090/jarq.45.163. [Google Scholar] [CrossRef]
89. Kammona, S., Othman, I., Jaswir, I., Jamal, P. (2015). Characterisation of carotenoid content in diverse local sweet potato (Ipomoea batatas) flesh tubers. International Journal of Pharmacy and Pharmaceutical Sciences, 2, 347–351. https://innovareacademics.in/journals/index.php/ijpps/article/view/3643. [Google Scholar]
90. Cruz, R., Baptista, P., Cunha, S., Pereira, J. A., Casal, S. (2012). Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules, 17(2), 1535–1547. DOI 10.3390/molecules17021535. [Google Scholar] [CrossRef]
91. Buchecker, R., Eugster, C. H. (1980). Mimulaxanthin, hauptcarotinoid von lamium montanum, und seine absolute konfiguration; konfigurative verknüpfung von deepoxyneoxanthin mit neoxanthin. Helvetica Chimica Acta, 63, 2531–2537. DOI 10.1002/hlca.19800630848. [Google Scholar] [CrossRef]
92. Bunghez, I. R., Avramescu, S. M., Marian, N., Georgeta, R., Rodica-Mariana, I. (2012). Obtaining of carotenoid extract from Lycium chinense and characterization using spectometrical analysis. Digest Journal of Nanomaterials and Biostructures, 7(2), 523–528. http://web.a.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=1&sid=e35981f4-7f61-4889-91b3-6a23b27fd457%40sdc-v-sessmgr03. [Google Scholar]
93. Chacón-Ordóñez, T., Schweiggert, R. M., Bosy-Westphal, A., Jiménez, V. M., Carle, R. et al. (2017). Carotenoids and carotenoid esters of orange- and yellow-fleshed mamey sapote (Pouteria sapota (Jacq.) H.E. Moore & Stearn) Fruit and Their Post-Prandial Absorption in Humans. Food Chemistry, 221, 673–682. DOI 10.1016/j.foodchem.2016.11.120. [Google Scholar] [CrossRef]
94. Haque, S., Begum, P., Khatun, M., Islam, N. S. (2015). Total carotenoid content in some mango (Mangifera indica) varieties of Bangladesh. International Journal of Pharmaceutical Sciences and Research, 6, 4875. DOI 10.13040/IJPSR.0975-8232.6(11). 4875-78. [Google Scholar] [CrossRef]
95. Kim, J. K., Lee, S. Y., Chu, S. M., Lim, S. H., Suh, S. C. et al. (2010). Variation and correlation analysis of flavonoids and carotenoids in Korean pigmented rice (Oryza sativa L.) cultivars. Journal of Agricultural and Food Chemistry, 58(24), 12804–12809. DOI 10.1021/jf103277g. [Google Scholar] [CrossRef]
96. Kusaba, M., Maoka, T., Morita, R., Takaichi, S. (2009). A novel carotenoid derivative, lutein 3-acetate, accumulates in senescent leaves of rice. Plant & Cell Physiology, 50(8), 1573–1577. DOI 10.1093/pcp/pcp096. [Google Scholar] [CrossRef]
97. Marcadante, A. Z., Rodriguez-Amaya, G. B. D. (1998). Carotenoids from yellow passion fruit (Passiflora edulis). Journal of Agricultural and Food Chemistry, 46(10), 4102–4106. DOI 10.1021/jf9801724. [Google Scholar] [CrossRef]
98. Lopez-Hernandez, J., Vazquez-Oderiz, L., Vazquez-Blanco, E., Romero-Rodriguez, A., Simal-Lozano, J. (1993). HPLC determination of major pigments in the bean Phaseolus vulgaris. Journal of Agricultural and Food Chemistry, 41(10), 1613–1615. DOI 10.1021/jf00034a017. [Google Scholar] [CrossRef]
99. Wen, X., Hempel, J., Schweiggert, R. M., Ni, Y., Carle, R. (2017). Carotenoids and carotenoid esters of red and yellow physalis (Physalis alkekengi L. and P. pubescens L.) fruits and calyces. Journal of Agricultural and Food Chemistry, 65(30), 6140–6151. DOI 10.1021/acs.jafc.7b02514. [Google Scholar] [CrossRef]
100. Mihaylova, D., Vrancheva, R., Petkova, N., Ognyanov, M., Desseva, I. et al. (2018). Carotenoids, tocopherols, organic acids, charbohydrate and mineral content in different medicinal plant extracts. Zeitschrift fur Naturforschung C—Journal of Biosciences, 73(11–12), 439–448. DOI 10.1515/znc-2018-0057. [Google Scholar] [CrossRef]
101. Vio-Michaelis, S., Feucht, W., Gómez, M., Hadersdorfer, J., Treutter, D. et al. (2020). Histochemical analysis of anthocyanins, carotenoids, and flavan−3-ols/proanthocyanidins in Prunus domestica L. fruits during ripening. Journal of Agricultural and Food Chemistry, 68(10), 2880–2890. DOI 10.1021/acs.jafc.9b01954. [Google Scholar] [CrossRef]
102. Ruiz, D., Egea, J., Tomás-Barberán, F. A., Gil, M. I. (2005). Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. Journal of Agricultural and Food Chemistry, 53(16), 6368–6374. DOI 10.1021/jf0480703. [Google Scholar] [CrossRef]
103. Dabbou, S., Maatallah, S., Castagna, A., Guizani, M., Sghaeir, W. et al. (2017). Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Foods for Human Nutrition, 72(1), 103–110. DOI 10.1007/s11130-016-0585-y. [Google Scholar] [CrossRef]
104. Cuevas, F. J., Pradas, I., Ruiz-Moreno, M. J., Arroyo, F. T., Perez-Romero, L. F. et al. (2015). Effect of organic and conventional management on bio-functional quality of thirteen plum cultivars (Prunus salicina lindl.). PLoS One, 10(8), e0136596. DOI 10.1371/journal.pone.0136596. [Google Scholar] [CrossRef]
105. Ghazghazi, H., Miguel, M. G., Hasnaoui, B., Sebei, H., Ksontini, M. et al. (2010). Phenols, essential oils and carotenoids of Rosa canina from Tunisia and their antioxidant activities. African Journal of Biotechnology, 9(18), 2709–2716. DOI 10.5897/AJB2010.000-3092. [Google Scholar] [CrossRef]
106. Perveen, R., Suleria, H. A., Anjum, F. M., Butt, M. S., Pasha, I. et al. (2015). Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims-a comprehensive review. Critical Reviews in Food Science and Nutrition, 55(7), 919–929. DOI 10.1080/10408398.2012.657809. [Google Scholar] [CrossRef]
107. Bunea, A., Andjelkovic, M., Socaciu, C., Bobis, O., Neacsu, M. et al. (2008). Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chemistry, 108(2), 649–656. DOI 10.1016/j.foodchem.2007.11.056. [Google Scholar] [CrossRef]
108. Kurilich, A. C., Juvik, J. A. (1999). Quantification of carotenoid and tocopherol antioxidants in Zea mays. Journal of Agricultural and Food Chemistry, 47(5), 1948–1955. DOI 10.1021/jf981029d. [Google Scholar] [CrossRef]
109. Sommer, A. (2008). Vitamin a deficiency and clinical disease: An historical overview. The Journal of Nutrition, 138(10), 1835–1839. DOI 10.1093/jn/138.10.1835. [Google Scholar] [CrossRef]
110. Drai, J., Borel, P., Faure, H., Galabert, C., Le Moël, G. et al. (2009). Fasting plasma carotenoids concentrations in crohn’s and pancreatic cancer patients compared to control subjects. International Journal for Vitamin and Nutrition Research, 79(2), 87–94. DOI 10.1024/0300-9831.79.2.87. [Google Scholar] [CrossRef]
111. Meléndez-Martínez, A. J., Stinco, C. M., Mapelli-Brahm, P. (2019). Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the uv radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients, 11(5), 1093. DOI 10.3390/nu11051093. [Google Scholar] [CrossRef]
112. Kaulmann, A., Bohn, T. (2014). Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutrition Research, 34(11), 907–929. DOI 10.1016/j.nutres.2014.07.010. [Google Scholar] [CrossRef]
113. Rowles, J. L., Erdman, J. W. J. (2020). Carotenoids and their role in cancer prevention.Biochimica et Biophysica Acta: Molecular and Cell Biology of Lipids, 1865(11), 158613. DOI 10.1016/j.bbalip.2020.158613 [Google Scholar] [CrossRef]
114. Stevanovic, D., Sieniawska, Z., Glowniak, E., Obradovic, K., Pajic-Lijakovic, N. I. (2020). Natural macromolecules as carriers for essential oils: From extraction to biomedical application. Frontiers in Bioengineering and Biotechnology, 8, 563. DOI 10.3389/fbioe.2020.00563. [Google Scholar] [CrossRef]
115. Gehse, S., Knorr, F., Patzelt, A., Zastrow, L., Meinke, M. C. et al. (2018). Determination of the effect of boiling on the bioavailability of carotenoids in vegetables using resonance Raman spectroscopy. Laser Physics, 28(10), 105602. DOI 10.1088/1555-6611/aad1b4. [Google Scholar] [CrossRef]
116. Bohn, T., Desmarchelier, C., Dragsted, L. O., Nielsen, C. S., Stahl, W. et al. (2017). Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Molecular Nutrition & Food Research, 61(6DOI 10.1002/mnfr.201600685. [Google Scholar] [CrossRef]
117. Yang, D., Ying, Y. (2011). Applications of Raman spectroscopy in agricultural products and food analysis: A review. Applied Spectroscopy Reviews, 46(7), 539–560. DOI 10.1080/05704928.2011.593216. [Google Scholar] [CrossRef]
118. Qin, J., Kim, M. S., Chao, K., Dhakal, S., Cho, B. K. et al. (2019). Advances in Raman spectroscopy and imaging techniques for quality and safety inspection of horticultural products. Postharvest Biology and Technology, 149, 101–117. DOI 10.1016/j.postharvbio.2018.11.004. [Google Scholar] [CrossRef]
119. Subramanian, B., Tchoukanova, N., Djaoued, Y., Pelletier, C., Ferron, M. et al. (2014). Investigations on the geometrical isomers of astaxanthin: Raman spectroscopy of conjugated polyene chain with electronic and mechanical confinement. Journal of Raman Spectroscopy, 45, 299–304. DOI 10.1002/jrs.4459. [Google Scholar] [CrossRef]
120. Merlin, J. C. (1985). Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure and Applied Chemistry, 57(5), 785–792. DOI 10.1351/pac198557050785. [Google Scholar] [CrossRef]
121. Feltl, L., Pacakova, V., Stulik, K., Volka, K. (2005). Reliability of carotenoid analyses: A review. Current Analytical Chemistry, 1(1), 93–102. DOI 10.2174/1573411052948424. [Google Scholar] [CrossRef]
122. Withnall, R., Chowdhry, B. Z., Silver, J., Edwards, H. G., de Oliveira, L. F. (2003). Raman spectra of carotenoids in natural products. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 59(10), 2207–2212. DOI 10.1016/S1386-1425(03)00064-7. [Google Scholar] [CrossRef]
123. Baranska, M., Roman, M., Cz, D., Schulz, J., Baranski, H. R. (2012). Recent advances in Raman analysis of plants: Alkaloids, carotenoids, and polyacetylenes. Current Analytical Chemistry, 9(1), 108–127. DOI 10.2174/1573411011309010108. [Google Scholar] [CrossRef]
124. Hata, T. R., Scholz, T. A., Pershing, L. K., Ermakov, I. V., McClane, R. W. et al. (2000). Non-invasive Raman spectroscopic detection of carotenoids in human skin. Journal of Investigative Dermatology, 115(3), 441–448. DOI 10.1046/j.1523-1747.2000.00060.x. [Google Scholar] [CrossRef]
125. Darvin, M. E., Gersonde, I., Meinke, M., Sterry, W., Lademann, J. (2005). Non-invasive in vivo determination of the carotenoids β-carotene and lycopene concentrations in the human skin using the Raman spectroscopic method. Journal of Physics D: Applied Physics, 38(15), 2696–2700. DOI 10.1088/0022-3727/38/15/023. [Google Scholar] [CrossRef]
126. Bhosale, P., Ermakov, I. V., Ermakova, M. R., Gellermann, W., Bernstein, P. S. (2004). Resonance Raman quantification of nutritionally important carotenoids in fruits, vegetables, and their juices in comparison to high-pressure liquid chromatography analysis. Journal of Agricultural and Food Chemistry, 52(11), 3281–3285. DOI 10.1021/jf035345q. [Google Scholar] [CrossRef]
127. Baranski, R., Baranska, M., Schulz, H. (2005). Changes in carotenoid content and distribution in living plant tissue can be observed and mapped in situ using NIR-fT-Raman spectroscopy. Planta, 222(3), 448–457. DOI 10.1007/s00425-005-1566-9. [Google Scholar] [CrossRef]
128. Baranska, M., Schütze, W., Schulz, H. (2006). Determination of lycopene and β-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-iR, and NIR spectroscopy. Analytical Chemistry, 78(24), 8456–8461. DOI 10.1021/ac061220j. [Google Scholar] [CrossRef]
129. Schulz, H., Baranska, M., Baranski, R. (2005). Potential of NIR-fT-Raman spectroscopy in natural carotenoid analysis. Biopolymers, 77(4), 212–221. DOI 10.1002/(ISSN)1097-0282. [Google Scholar] [CrossRef]
130. Schrader, B., Klump, H. H., Schenzel, K., Schulz, H. (1999). Non-destructive NIR FT Raman analysis of plants. Journal of Molecular Structure, 509(1–3), 201–212. DOI 10.1016/S0022-2860(99)00221-5. [Google Scholar] [CrossRef]
131. Ryabchykov, O., Guo, S., Bocklitz, T. (2019). Analyzing Raman spectroscopic data. Physical Sciences Reviews, 4(2). DOI 10.1515/psr-2017-0043. [Google Scholar] [CrossRef]
132. Cetó, X., Serrano, N., Aragó, M., Gámez, A., Esteban, M. et al. (2018). Determination of HPLC-uV fingerprints of spanish paprika (Capsicum annuum L.) for its classification by linear discriminant analysis. Sensors, 18(12), 4479. DOI 10.3390/s18124479. [Google Scholar] [CrossRef]
133. Ivleva, N. P., Niessner, R., Panne, U. (2005). Characterization and discrimination of pollen by Raman microscopy. Analytical and Bioanalytical Chemistry, 381(1), 261–267. DOI 10.1007/s00216-004-2942-1. [Google Scholar] [CrossRef]
134. Nikbakht, A. M., Tavakkoli, H. T., Malekfar, R., Gobadian, B. (2011). Nondestructive determination of tomato fruit quality parameters using Raman spectroscopy. Journal of Agricultural Science and Technology, 13(4), 517–526. http://jast.modares.ac.ir/article-23-4685-en.html. [Google Scholar]
135. Akpolat, H., Barineau, M., Jackson, K. A., Akpolat, M. Z., Francis, D. M. et al. (2020). High-throughput phenotyping approach for screening major carotenoids of tomato by handheld Raman spectroscopy using chemometric methods. Sensors, 20(13), 3723. DOI 10.3390/s20133723. [Google Scholar] [CrossRef]
136. Zhai, S., Xia, X., He, Z. (2016). Carotenoids in staple cereals: Metabolism, regulation, and genetic manipulation. Frontiers in Plant Science, 7, 1197. DOI 10.3389/fpls.2016.01197. [Google Scholar] [CrossRef]
137. Fraser, P. D., Romer, S., Shipton, C. A., Mills, P. B., Kiano, J. W. et al. (2002). Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proceedings of the National Academy of Sciences of the United States of America, 99(2), 1092–1097. DOI 10.1073/pnas.241374598. [Google Scholar] [CrossRef]
138. Goo, Y. M., Han, E. H., Jeong, J. C., Kwak, S. S., Yu, J. et al. (2015). Overexpression of the sweet potato ibOr gene results in the increased accumulation of carotenoid and confers tolerance to environmental stresses in transgenic potato. Comptes Rendus Biologies, 338(1), 12–20. DOI 10.1016/j.crvi.2014.10.006. [Google Scholar] [CrossRef]
139. dos Santos, P. P., de Aguiar Andrade, L., Flôres, S. H., de Oliveira Rios, A. (2018). Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. Journal of Food Science and Technology, 55(10), 3851–3860. DOI 10.1007/s13197-018-3316-6. [Google Scholar] [CrossRef]
140. Rostamabadi, H., Falsafi, S. R., Jafari, S. M. (2019). Nanoencapsulation of carotenoids within lipid-based nanocarriers. Journal of Controlled Release: Official Jurnal of the Controlled Release Society, 298, 38–67. DOI 10.1016/j.jconrel.2019.02.005. [Google Scholar] [CrossRef]
141. Polyakov, N. E., Kispert, L. D. (2015). Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydrate Polymers, 128, 207–219. DOI 10.1016/j.carbpol.2015.04.016. [Google Scholar] [CrossRef]
142. Courraud, J., Berger, J., Cristol, J. P., Avallone, S. (2013). Stability and bioaccessibility of different forms of carotenoids and vitamin a during in vitro digestion. Food Chemistry, 136(2), 871–877. DOI 10.1016/j.foodchem.2012.08.076. [Google Scholar] [CrossRef]
143. Gutiérrez, F. J., Albillos, S. M., Casas-Sanz, E., Cruz, Z., García-Estrada, C. et al. (2013). Methods for the nanoencapsulation of β-carotene in the food sector. Trends in Food Science & Technology, 32(2), 73–83. DOI 10.1016/j.tifs.2013.05.007. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |