
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.014232
ARTICLE
Soil Fungal Diversity and Community Composition in Response to Continuous Sweet Potato Cropping Practices
1Key Laboratory of Degraded and Unused Land Consolidation Engineering, Ministry of Natural Resources, Xi’an, 710075, China
2College of Resources and Environment, Qingdao Agricultural University, Qingdao, 266109, China
3College of Resources and Environment, Baoshan University, Baoshan, 678000, China
*Corresponding Author: Liang Xu. Email: xuliang@qau.edu.cn
Received: 11 September 2020; Accepted: 07 December 2020
Abstract: Soil fungi are extremely important for maintaining soil health and plant production in agricultural systems. Currently, the effect of continuous cropping of sweet potato on soil fungal communities and physiochemical parameters has not been well documented. In the present study, four sweet potato fields consecutively monocultured for 1, 2, 3, and 4 years were selected to investigate the effect of monoculture on soil fungal communities through Illumina MiSeq sequencing. Continuous cropping of sweet potatoes dramatically altered the fungal community composition, whereas fungal diversity was almost unchanged. Ascomycota and Basidiomycota were the most abundant phyla in all soil samples, accounting for 32.59% and 21.14% of the average relative abundance, respectively. The abundance of some potential pathogens, such as Ascobolus spp, specifically Ascobolus stercorarius, and some unknown fungi increased significantly as the sweet potato monoculture period increased, and their presence were highly positively correlated with disease incidence. In contrast, Basidiomycota, Bullera, Fusarium and Trichocladium most likely play roles as antagonists of sweet potato disease development, as their relative abundance decreased significantly over time and were negatively correlated with disease incidence. Redundancy and correlation analyses revealed that soil pH and organic carbon content were the most important factors driving these changes. Our findings provided a dynamic overview of the fungal community and presented a clear scope for screening beneficial fungi and pathogens of sweet potato.
Keywords: Soil fungal community; internal transcribed spacers; continuous cropping; Illumina Miseq sequencing; soil pH
Sweet potatoes are an important root crop used as food and feed and as a renewable energy source. China is the largest producer of sweet potatoes in the world and the area used for their cultivation ranks fourth among China’s agricultural crops [1]. However, the limited amount of arable land and expanding population centers have resulted in widespread use of continuous cropping for sweet potato cultivation. This has resulted in increased disease that has seriously restricted the development of the sweet potato industry [2,3]. Our previous field studies determined that there is a substantial reduction in sweet potato production and plant health after 3 years of continuous cropping [4].
Continuous cropping is the cultivation of the same crop in the same field year after year without crop rotation [5,6]. The result is a decrease in soil quality that lowers yields while increasing soil-borne diseases. This process has been defined as the “continuous cropping obstacle” [7,8]. The factors causing this obstacle include nutrient deficiency, accumulation of toxic substances and an imbalance in soil microfloral populations [9,10]. However, there is an increasing body of evidence that alterations in the microflora are a primary cause for this effect [11–13]. Soil ecosystem balance and function are largely governed by rhizosphere microbial dynamics as well as microbial composition and diversity. All these affect geochemical cycles, humus dynamics and soil structure [14]. In turn, microbial diversity and dynamics in soil is largely determined by soil health and this is related to both the outbreak or suppression of soil-borne plant diseases [15].
The fungi play an important role in soil ecosystems that are responsible for plant diseases and plant disease suppression. Fungi are major causes of soil borne diseases [16], while many fungal populations have been identified as antagonists of soil borne plant pathogens and play important roles in promoting plant growth [17,18]. In addition, fungal diversity both has beneficial effect [19,20] or harmful effect [13] on plant pathogen suppression and plant productivity. Since soil fungal communities and diversity play crucial roles in soil health, understanding how continuous cropping influences soil fungal communities may provide potentially useful guidance for regulating and maintaining soil health. Until now, although there are more and more reports on soil microbes and plants, there are relatively few reports on soil fungal communities and physiochemical parameters in sweet potato [21–25].
Establishing the link between fungal communities and the physicochemical properties of soil is essential to uncover the mechanism of the continuous cropping obstacle for sweet potatoes. This will also assist in the development of sustainable crop cultivation practices [5,25]. Numerous studies have concluded that continuous cropping alters soil fungal community structures but only a handful of these have focused on the links between fungal communities and soil properties. For instance, soil pH had the greatest impact on fungal community composition in rhizosphere soil under continuous cropping of C. chinensis while soil organic matter composition was a primary driver of diversity in banana monocultures [13,25]. On the other hand, it has not detected obvious relationship between soil properties and soil fungal community in Panax notoginseng continuous cropping soils [20]. Thus, the mechanisms for fungal community shifts are most likely complex interactions that are plant species, microorganism and soil property dependent [26–28].
We studied the succession of soil fungal communities under continuous sweet potato cultivation. Four fields were used for consecutive sweet potato monocultures for 1, 2, 3 and 4 years to investigate the effects of this practice on the composition and diversity of soil fungal communities using Illumina MiSeq sequencing. The objectives of our study were to (1) examine the changes in abundance and composition of the soil fungal community over monoculture time (2) determine which soil properties contribute to the soil fungal community composition and (3) identify potential pathogenic fungi as well as pathogen antagonists under sweet potato continuous cropping. This study will assist in improving sweet potato farming using continuous cultivation.
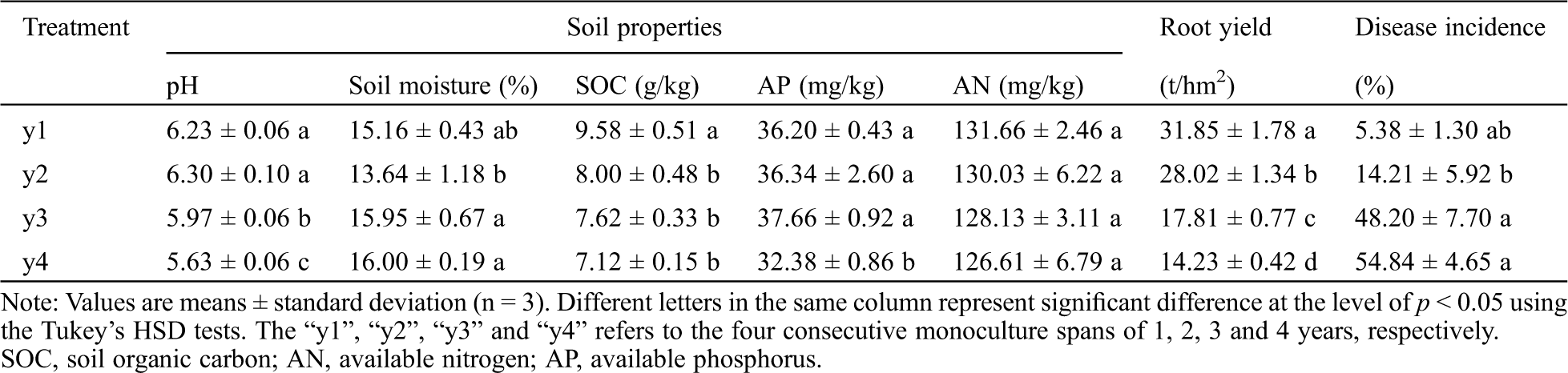
The study was conducted at the modern agricultural science and technology demonstration garden of Qingdao Agricultural University, Jiaozhou, Shandong Province, China (150°25’E, 36°13’N). This area is characterized by a warm temperate monsoon climate with an average temperature of 11–14°C and mean annual precipitation of 695 mm. The experimental soil samples were collected on September 25, 2016 from 4 sweet potato fields containing the same sweet potato cultivar (Yan25) that had been continuously cultivated for 1, 2, 3 and 4 years, referred below as y1, y2, y3, and y4, respectively. For each field, the area was 3600 m2 (120 m × 30 m) and three representative subplots within each field, with an area of 100 m2 (10 m × 10 m) were selected for soil collection and estimation of disease incidence and crop yield. The distance between subplots was 20 meters and the distance between subplots and plot edges were at least 10 meters. For each subplot, 15 sweet potato plants were randomly selected for monitoring and soil core samples (20 cm × 2.5 cm) were collected from each plant after carefully removing the sweet potatoes and surface coverings. Fifteen (15) cores were mixed as a single soil sample. All 12 soil samples (4 fields × 3 replicates) were put into sterile plastic bags, placed into ice box, and transported to the laboratory. After passing through a 2 mm sieve, each sample was divided into two subsamples: one portion was air-dried for soil characterization and the remainder was stored at –80°C for DNA analysis. The methods for determination of soil physicochemical parameters, sweet potato yield and disease incidence have previously been described [4], and these data were shown in Tab. S1.
2.2 DNA Extraction, ITS rRNA Gene Amplification, and Miseq Sequencing
For each soil sample, DNA was isolated from 500 mg of soil using the FastDNA Spin kit for soil (MP Biomedicals, Vista, CA, USA) according to the manufacturer’s recommendations. The primer sets of ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′) were used to amplify the internal transcribed spacers 1 (ITS1) region [29]. Transposase sequences (5’-TCGTCGGCAGCGTCAGATGTGTA TAAGAGACAG-3’) and (5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3’) were added to the 5’ end of ITS primers, respectively. The PCR reactions contained 12.5 μL 2x KAPA HiFi HotStart Ready Mix (Kapa Biosystems, Woburn, MA, USA), 0.5 μL of each primer (10 μM), and 2.5 μL DNA template (5 ng/μL) with the following cycling conditions: 95°C for 3 min for initial melting; 25 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s; final extension at 72°C for 10 min. PCR products were purified using the Agencourt Ampure XP beads (Beckman Coulter, Danvers, MA, USA) and used for Miseq sequencing. Paired-end sequencing (2 ×300 bp) was carried out on the Illumina MiSeq platform with MiSeq Reagent Kit v3 (Illumina, San Diego, CA, USA) at the Chinese National Human Genome Center in Shanghai.
The initial quality filtering and assembling of paired-end reads were performed by the Chinese National Human Genome Center. Briefly, low quality sequences with average quality scores <30, ambiguous nucleotides, nucleotide mismatches with the barcode or primer and those <200 bp were removed [4]. The modified sequences were OTUs (Operational Taxonomic Units) clustered with a cutoff of 97% similarity, and phylogenetically assigned according to their best matches to the sequences in the ITS reference database, UNITE+INSD database (https://unite.ut.ee/repository.php) [30]. Representative sequences have been deposited in GenBank (accession numbers MT924327–MT925512).
We calculated various indexes (Coverage, Sobs, Chao1, Shannon indices) after resampling the read number for each sample to 18920 reads in Mothur (Version 1.39.5) (http://www.mothur.org) (for rarefaction curves of OTU and Shannon diversity see Fig. S1). One-way analysis of variance (ANOVA) was conducted using Turkey’s HSD test for multiple comparisons. Pearson correlation coefficients between fungal taxa and soil properties and yield and disease incidence were calculated using SPSS v20.0 (IBM, Armonk, NY, USA). All fungal community-related analyses were based on relative abundances of OTUs per sample. The Bray-Curtis index was used as a distance measure unless otherwise stated. Principal Coordinate Analysis (PCoA), redundancy analysis (RDA) and Monte Carlo test of fungal communities were conducted using Canoco 5.0 software (Microcomputer Power, Ithaca, NY, USA).
3.1 Overall Miseq Sequencing Information
Coverage for our samples was all >99% and the rarefaction curve of each library approached a saturation plateau indicating the sequencing depth was reasonable and sufficient for the needs of our experiments (Tab. 1 and Fig. S1). We obtained 239,878 ITS sequences from 12 samples and the sequence number per sample ranged from 18,920 to 22,061. All of the obtained sequences were divided into 1186 OTUs using a 3% dissimilarity threshold. The fungal communities were taxonomically classified into 6 phyla, 26 classes, 62 orders, 119 families and 199 genera. Ascomycota and Basidiomycota were the dominant phyla in the soil of sweet potato consecutive monoculture.
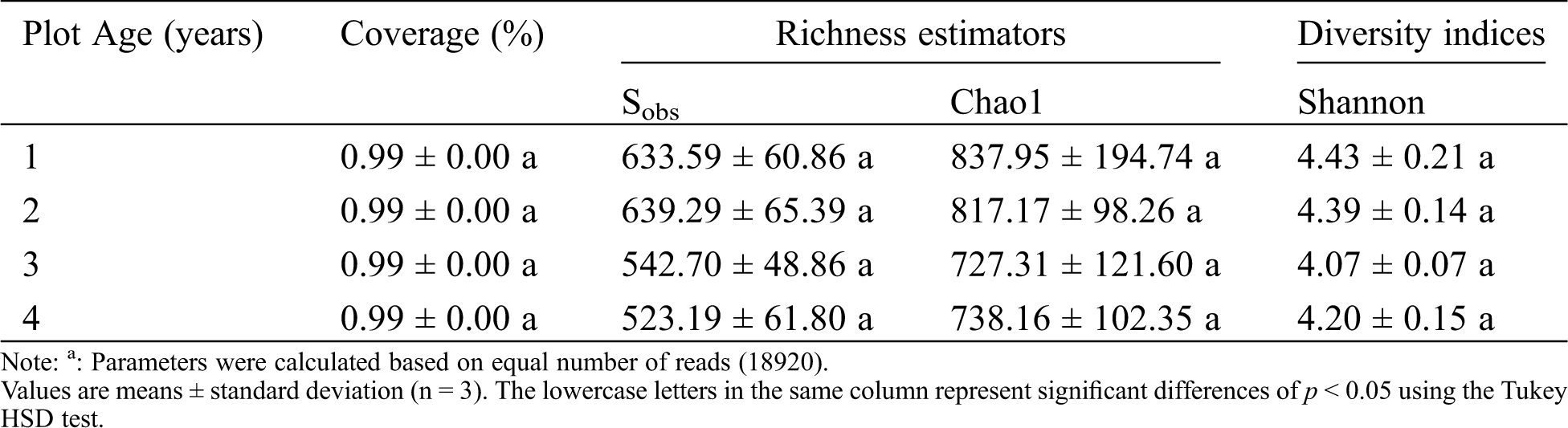
Table 1: Alpha-diversitya of treatment groups for sweet potato monocultures
3.2 Fungal α-Diversity and Community Composition
We analyzed the richness and diversity of these samples using the coverage, Chao1, sobs and Shannon indexes. None of these indices showed any significant differences between the soil samples from the four sweet potato fields (Tab. 1). However, UniFrac-weighted Principal Coordinate Analysis (PCoA) based on the OTU composition revealed distinct differences in fungal community composition between plot ages and similar patterns for triplicate subplot samples (Fig. 1). The first two principal components explain 64.52% of the total variation for the fungal data. The first principal coordination axis (accounting for 43.76% of the variation) clearly separated the “y3” and “y4” fields from the “y1” and “y2” fields, suggesting that the third year might be considered as the critical period for the occurrence of fungal disease in sweet potato monoculture soils.
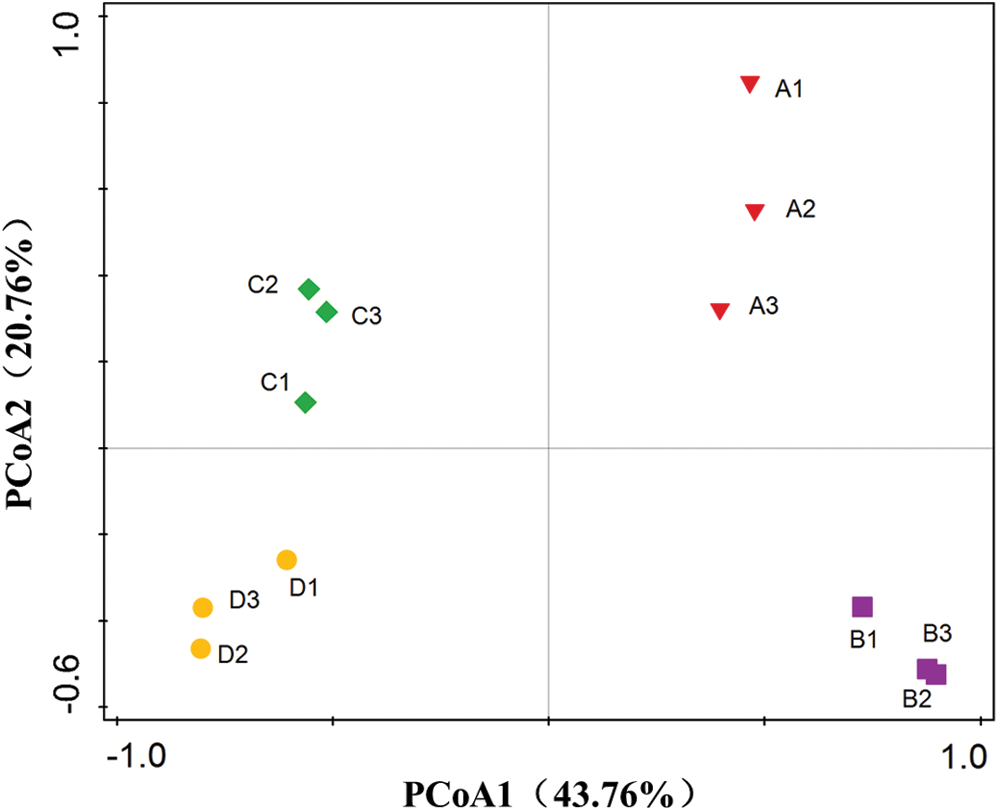
Figure 1: UniFrac-weighted PCoA plots for fungal communities over time in four sweet potato fields. “A”, “B”, “C” and “D” refers to the four consecutive monoculture spans of 1, 2, 3 and 4 years, respectively. Numbers represent replicates for each year
3.3 Shifts in Soil Fungal Community Composition Under Consecutive Monoculture
Five genera were changed significantly along with continuous cropping years (Fig. 2a). The relative abundance of Bullera (belongs to Basidiomycota), Fusarium (Ascomycota) and Trichocladium (Ascomycota) decreased significantly with the increasing continuous cropping years; whereas the abundance of Ascobolus (Ascomycota) and Macrorhabdus (Ascomycota) increased significantly over time (Fig. 2a).
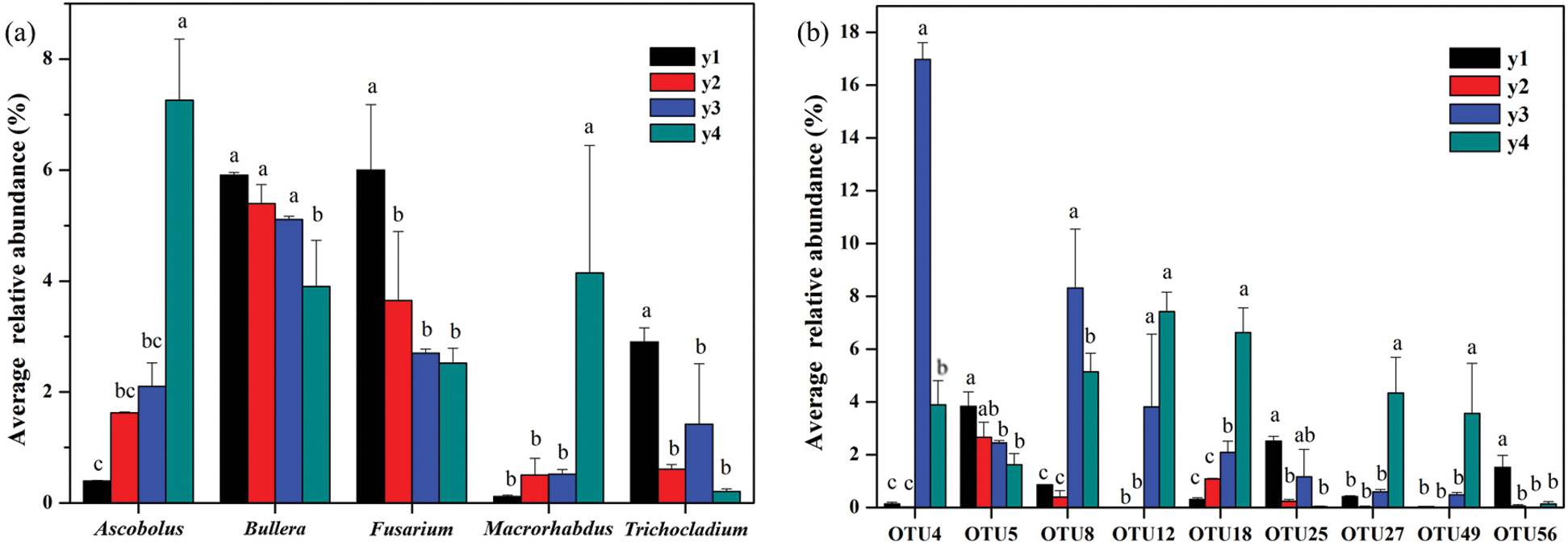
Figure 2: Dominant genera (a, with the relative abundance value in at least one sample above 1%) and Dominant OTUs (b, with relative abundance >1% in at least one sample) with significant differences among treatments (Tukeys HSD, p < 0.05). “y1”, “y2”, “y3” and “y4” refers to the four consecutive monoculture spans of 1, 2, 3 and 4 years, respectively
With respect to OTU level, ten OTUs were identified to be significantly changed with continuous cropping years (Fig. 2b). Among them, OTU18 (Ascobolus stercorarius), OTU25 (uncultured Trichocladium), OTU56 (uncultured Fusarium) and OTU 5 (uncultured Bullera), had the same trend in their relative abundance trend as their respective genera. The relative abundance of OTU18 increased significantly with increasing cropping years whereas OTU25, OTU56 and OTU 5 had the reverse trend. Moreover, four unclassified fungi (OTU4, OTU8, OTU12, OTU27 and OTU49) increased significantly over time (Fig. 2b).
3.4 Relationship between Soil Fungal Community Composition and Soil Properties, Root Yield and Disease Incidence of Sweet Potato
Monte Carlo tests based on soil properties and the soil fungal community revealed that soil pH and organic carbon (SOC) were significantly correlated with variations in the whole fungal community (p > 0.05). On the other hand, available nitrogen, phosphorus and soil moisture were not significant factors affecting the fungal community composition in response to continuous cropping practices. Redundancy analysis (RDA) showed that the first and second RDA components explained 40.7% and 15.1% of the total variation (Fig. 3). Moreover, Pearson’s correlation analysis revealed that the soil pH and SOC correlated significantly with many fungal phyla (Tab. 2). In addition, this correlation analysis between disease incidence and soil properties also revealed that only soil pH and SOC were significantly correlated with sweet potato disease incidence with correlation coefficients of −0.879 (p < 0.001) and −0.827 (p < 0.005), respectively (data not shown). These results indicated that soil pH and SOC were strong predictors of fungal community composition and disease incidence in these monocropped sweet potato fields.
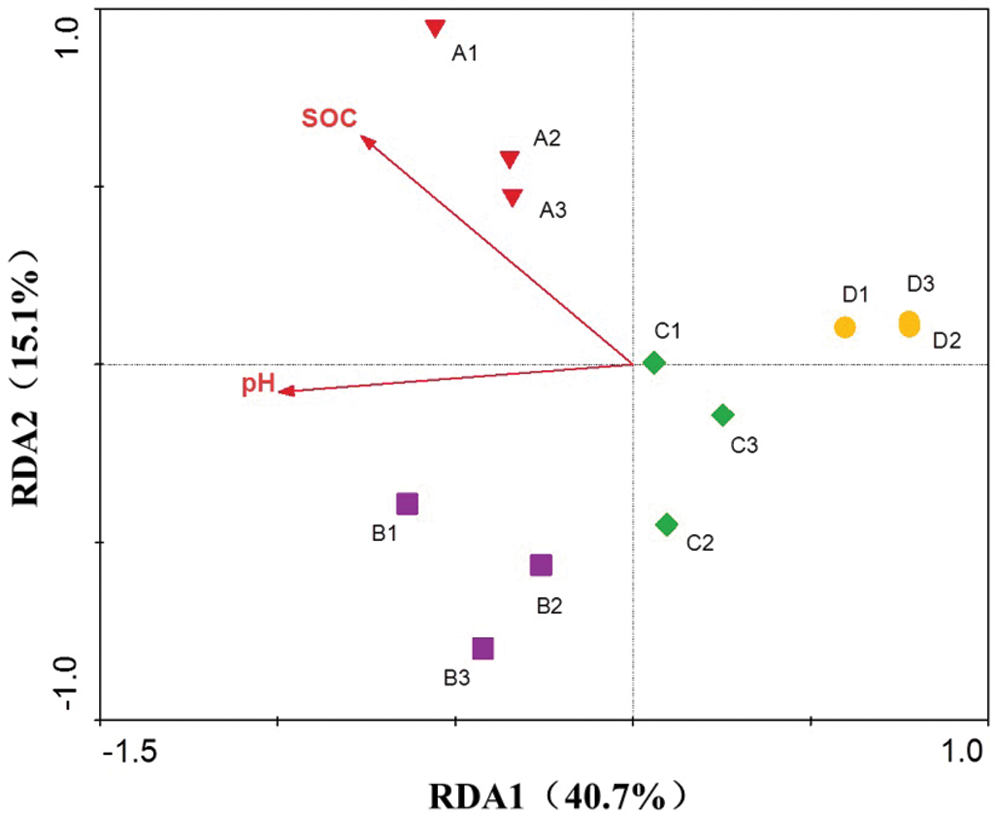
Figure 3: Redundancy analysis (RDA) of fungal community and soil properties over time in four sweet potato fields. The vectors represent statistically significant variables that explain observed patterns (p < 0.05). “A”, “B”, “C” and “D” refers to the four consecutive monoculture spans of 1, 2, 3 and 4 years, respectively. Numbers represent replicates for each year. SOC, soil organic carbon
Table 2: Soil properties and Pearson’s correlations between significantly different fungal taxa
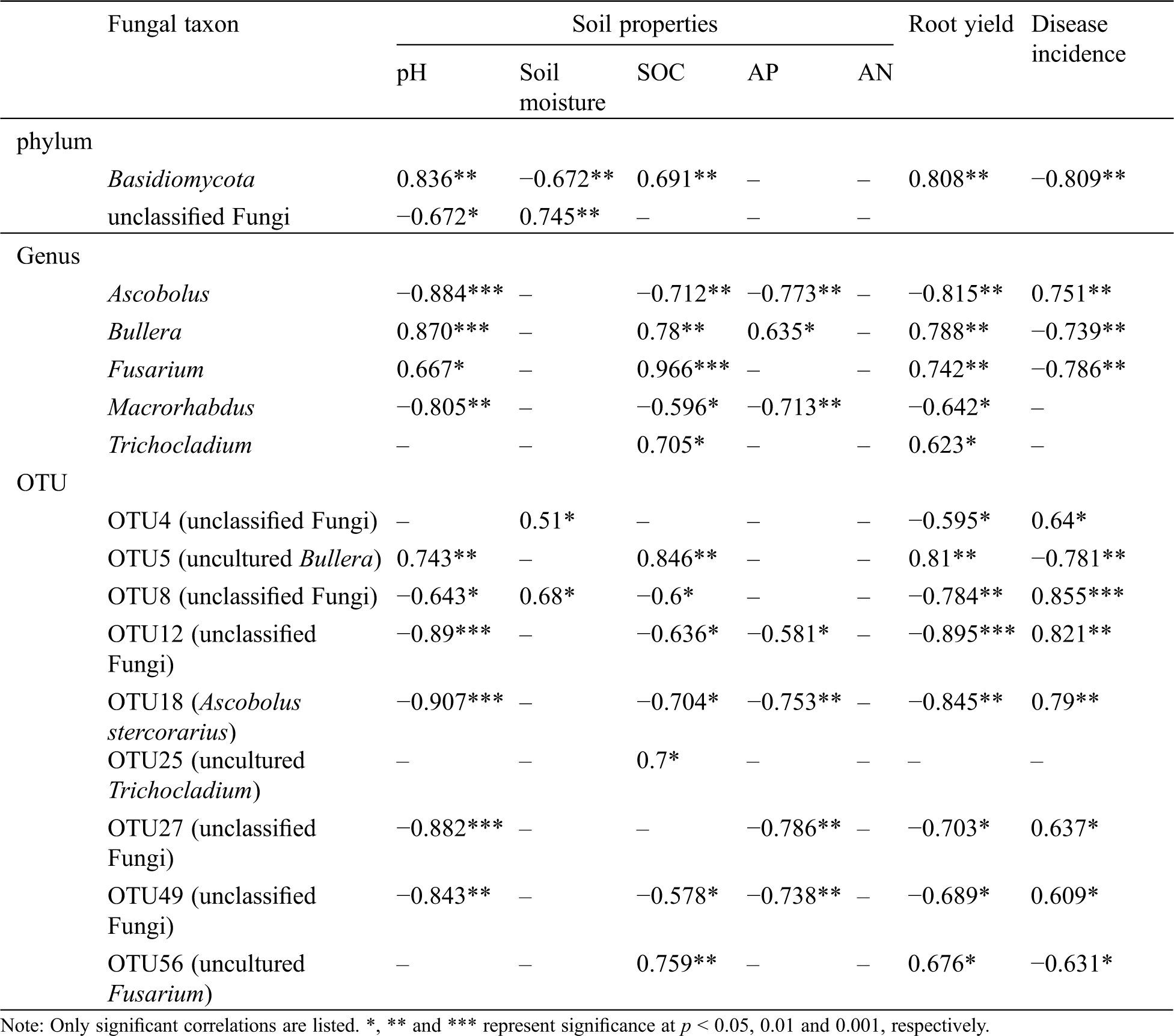
Interestingly, we also identified relationships between fungal taxa and disease incidence. The abundance of the Basidiomycota as well as Bullera, Fusarium, OTU5 (uncultured Bullera) and OTU56 (uncultured Fusarium) were negatively correlated with disease incidence. In contrast, the abundance of Ascobolus and the unclassified fungi represented by OTU4, OTU8, OTU12, OTU27, OTU49 and OTU18 (A. stercorarius) were positive correlated with disease incidence (Tab. 2).
Illumina MiSeq sequencing targeting the ITS1 region was employed to detect the soil fungal community composition and diversity. Long-term consecutive sweet potato monocropping significantly altered fungal community composition, whereas fungal diversity remained stable. Our results were consistent with previous findings in other crops, which showed no significant differences in the fungal diversity indices among different continuous cropping spans, even though the fungal community structures were quite different [31–33]. Our findings suggest that the fungal community composition responded more sensitive to consecutive monoculture than did the fungal diversity.
Analysis at the phylum level revealed that Ascomycota and Basidiomycota were the most abundant phyla, accounting for 32.59% and 21.14% of the average relative abundance, respectively. This finding agreed with previous results on peanut [21,34], soybean [23], and Salvia miltiorrhiza [24] monocultures. In addition, Ascomycota and Basidiomycota were reported as important fungal groups in most soils [35]. These studies suggested the ubiquity of these species and their important role in agroecosystems.
Ascomycota subgroups, including Ascobolus, Macrorhabdus, and OTU18 (A. stercorarius), accumulated with time and were significantly and positively correlated with disease incidence, thereby suggesting that these taxa may be potential pathogens that contribute to the formation of obstacles in sweet potato continuous cropping. In contrast, we found that four subgroups of Ascomycota decreased significantly over time and were negatively correlated with sweet potato disease incidence. These subgroups were Fusarium, Trichocladium (Ascomycota), OTU56 (uncultured Fusarium), and OTU25 (uncultured Trichocladium). Interestingly, members of the genus Fusarium have been commonly identified as plant pathogens [20,36,37]. The relative abundance of these species has increased in the continuous cropping of cucumbers [32], peanuts [34], vanilla [30], and Panax notoginseng [12]. In our study, Fusarium and its subgroup OTU56 were negatively associated with disease in sweet potatoes and their relative abundance decreased over time. This finding agreed with a previous report that Fusarium abundance was lower in a five-year continuously cropped field than in a one-year field of Coptis chinensis [25]. These discrepancies could partly be attributed to the broad range of fungi in this genus, because Fusarium is a functionally important component of agricultural soil ecosystems [38], and both soil pathogenic and nonpathogenic species exist [32]. It could also be attributed partly to different surrounding environment. A fungal species has different functions at different ecotypes because of the interaction between soil fungi and local soil’s physical, chemical, and biological characteristics [39,40]. Therefore, the same Fusarium species could possibly be either pathogenic or nonpathogenic species according to the different crop species and soil environments. In our study, the species of Fusarium could be beneficial to the rhizosphere of sweet potato and may play an important role in its cultivation. However, the exact mechanism for this observation has not been detected in our experiments and requires further study.
The abundance of Basidiomycota and its subgroups, including Bullera and OTU5 (uncultured Bullera), decreased significantly as the sweet potato monoculture period increased. Moreover, their relative abundance was highly negatively correlated with disease incidence. This result was consistent with previous reports, thereby indicating that Basidiomycota was depleted as time progressed and showed a negative correlation with the incidence for vanilla stem rot [30,34]. These results indicated that these species may be a potentially beneficial fungi for sweet potato. In addition, Basidiomycota dominated all our soil samples, thereby suggesting its vital role in maintaining the microbial ecological balance of sweet potato cultivation.
Besides these known species, the ratios of unclassified fungi and their subgroups, including OTU4, OTU8, OTU12, OTU27, and OTU49, increased markedly along with the monuculture years, which indicated that these fungal phyla may be the potential pathogens of sweet potato. Despite their unknown taxonomy and function, they must be associated with continuous cropping stress and should not be dismissed. With the development of next-generation DNA sequencing and the improvement of fungal gene database, their function may be revealed, as well as their role in regulating continuous cropping obstacles.
Soil properties, especially pH and soil organic matter, greatly affect the composition of soil microbial communities [41,42]. In our study, the RDA and correlation analysis revealed that soil pH and SOC had the most significant effect on fungal community composition in monocropped sweet potato fields. This result agreed with previous observations in soils of the monocultures of P. notoginseng [12] and C. chinensis [25]. Moreover, our results indicated that pH and SOC were negatively correlated with disease incidence, which was consistent with results of several previous studies [5,8,13,43]. In addition, these two factors were positively correlated with the relative abundance of some potential beneficial taxa, such as Basidiomycota, Bullera, Fusarium, and OTU5, but were negatively correlated with some harmful taxa, such as Ascobolus, Macrorhabdus, and OTU18. These findings suggested that the decrease of soil pH and SOC can lead to an imbalance of soil fungal communities and thus limit sweet potato growth. Soil organic matter plays a key role in maintaining soil quality and disease suppression by promoting soil physical, chemical, and biological properties and long-term productivity [13,44]. However, in China, organic fertilizers have been ignored for a long time, because growers are more inclined to use large amounts of chemical fertilizers to increase yield [5,8]. The reduced organic material input and excessive application of chemical fertilizers not only result in unbalanced soil fertility but also decrease the soil pH significantly [8,45]. The decrease in soil pH may impose physiological constraints on fungal survival and growth, which directly alter fungal community composition [46,47]. Therefore, exploring sustainable agricultural measures to improve soil pH and SOC is extremely important for sweet potato production.
In conclusion, this study demonstrated that the continuous cropping of sweet potatoes affected fungal community composition, whereas fungal diversity remained unchanged. Alterations of the fungal community in the sweet potato monoculture soils were mainly driven by soil pH and soil organic carbon. Some potential pathogens, such as Ascobolus, Macrorhabdus, OTU18 (A. stercorarius), and some unknown fungi, accumulated with increasing time and were positively correlated with sweet potato disease incidence. By contrast, the potentially beneficial taxa, including Basidiomycota, Bullera, Fusarium, and Trichocladium, decreased considerably along with monoculture time and were negatively correlated with disease incidence. Our findings helped elucidate the mechanism of fungal community alteration under the sweet potato monoculture system and presented an outline for screening beneficial fungi and pathogens. Further exploration of these potential microbe and pertinent fertilization measures might be useful for biological control in sweet potato continuous cropping.
Funding Statement: This work was supported by Key laboratory of Degraded and Unused Land Consolidation Engineering, the Ministry of Land and Resources (SXDJ2018-06), National Natural Science Foundation of China (Nos. 41501271 and 41601339), China Agriculture Research System (No. CARS-10-B10) and Support Plan on Youth Innovation Science and Technology for Higher Education of Shandong Province (2019KJD014).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ma, D. F., Li, Q., Cao, Q. H., Niu, F. X. (2012). Development and prospect of sweetpotato industry and its technologies in China. Jiangsu Journal of Agricultural Sciences, 28(5), 969–973 (in Chinese). [Google Scholar]
2. Jia, Z. D., Xie, Y. Z., Yin, Q. H., Guo, X. D. (2010). Study progress and perspective of black rot resistant germplasm in sweetpotato. Journal of Plant Genetic Research, 11, 424–427 (in Chinese). [Google Scholar]
3. Qiao, Y. J., Wang, X. J., Wu, B. Y., Wang, Z. H. (2014). Effect of different planting patterns on yield of sweet potato and the number of nematodes in rhizosphere. Research of Agricultural Modernization, 35, 800–803 (in Chinese). [Google Scholar]
4. Huan, L., Wang, J. Q., Liu, Q., Zhou, Z. F. (2019). Effects of consecutive monoculture of sweet potato on soil bacterial community as determined by pyrosequencing. Journal of Basic Microbiology, 59(2), 181–191. DOI 10.1002/jobm.201800304. [Google Scholar] [CrossRef]
5. Xiong, W., Li, Z., Liu, H., Xue, C. (2015). The effect of long-term continuous cropping of black pepper on soil bacterial communities as determined by 454 pyrosequencing. PLoS One, 10(8), e0136946. DOI 10.1371/journal.pone.0136946. [Google Scholar] [CrossRef]
6. Li, Y. C., Li, Z. W., Arafat, Y., Lin, W. X. (2020). Studies on fungal communities and functional guilds shift in tea continuous cropping soils by high-throughput sequencing. Annals of Microbiology, 70(1), 7. DOI 10.1186/s13213-020-01583-8. [Google Scholar] [CrossRef]
7. Chen, P., Wang, Y. Z., liu, Q. Z., Zhang, Y. T., li, X. Y. et al. (2020). Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Applied Soil Ecology, 155, 103626. DOI 10.1016/j.apsoil.2020.103626. [Google Scholar] [CrossRef]
8. Li, Z. G., Zu, C., Wang, C., Yang, J. F. (2016). Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Reproductive Sciences, 6(1), 52. DOI 10.1038/srep35825. [Google Scholar] [CrossRef]
9. Huang, H. C., Chou, C. H., Erickson, R. S. (2006). Soil sickness and its control. Allelopathy Journal, 18(1), 1–21. [Google Scholar]
10. Ling, N., Deng, K. Y., Song, Y., Wu, Y. C. (2014). Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Research in Microbiology, 169(7–8), 570–578. DOI 10.1016/j.micres.2013.10.004. [Google Scholar] [CrossRef]
11. Li, X. G., Panke-Buisse, K., Yao, X. D., Coleman-Derr, D., Ding, C. F. et al. (2020). Peanut plant growth was altered by monocropping-associated microbial enrichment of rhizosphere microbiome. Plant and Soil, 446(1–2), 655–669. DOI 10.1007/s11104-019-04379-1. [Google Scholar] [CrossRef]
12. Tan, Y., Cui, Y. S., Li, H. Y., Kuang, A. X. (2017). Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiological Research, 194, 10–19. DOI 10.1016/j.micres.2016.09.009. [Google Scholar] [CrossRef]
13. Shen, Z. Z., Penton, C. R., Lv, N., Xue, C. (2018). Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microbial Ecology, 75(3), 739–750. DOI 10.1007/s00248-017-1052-5. [Google Scholar] [CrossRef]
14. Huang, L. F., Song, L. X., Xia, X. J., Mao, W. H. (2013). Plant-soil feedbacks and soil sickness: From mechanisms to application in agriculture. Journal of Chemical Ecology, 39(2), 232–242. DOI 10.1007/s10886-013-0244-9. [Google Scholar] [CrossRef]
15. Garbeva, P., Van Veen, J. A., Van Elsas, J. D. (2004). Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology, 242(1), 243–270. DOI 10.1146/annurev.phyto.42.012604.135455. [Google Scholar] [CrossRef]
16. Schardl, C. L., Craven, K. D. (2003). Interspecific hybridization in plant-associated fungi and oomycetes: A review. Molecular Ecology, 12(11), 2861–2873. DOI 10.1046/j.1365-294X.2003.01965.x. [Google Scholar] [CrossRef]
17. Ownley, B. H., Gwinn, K. D., Vega, F. E. (2010). Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. Biocontrol, 55(1), 113–128. DOI 10.1007/s10526-009-9241-x. [Google Scholar] [CrossRef]
18. Blakney, A. J., Patten, C. L. (2011). A plant growth-promoting pseudomonad is closely related to the Pseudomonas syringae complex of plant pathogens. FEMS Microbiology Ecology, 77(3), 546–557. DOI 10.1111/j.1574-6941.2011.01136.x. [Google Scholar] [CrossRef]
19. Wehner, J., Antunes, P. M., Powell, J. R., Mazukatow, J. (2010). Plant pathogen protection by arbuscular mycorrhizas: A role for fungal diversity? Pedobiologia, 53(3), 197–201. DOI 10.1016/j.pedobi.2009.10.002. [Google Scholar] [CrossRef]
20. Dong, L. L., Xu, J., Feng, G. Q., Li, X. W. (2016). Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system. Scientific Reports, 6(1), 31802. DOI 10.1038/srep31802. [Google Scholar] [CrossRef]
21. Chen, M. N., Li, X., Yang, Q. L., Chi, X. Y. (2012). Soil eukaryotic microorganism succession as affected by continuous cropping of reanut-pathogenic and beneficial fungi were selected. PLoS One, 7(7), e40659. DOI 10.1371/journal.pone.0040659. [Google Scholar] [CrossRef]
22. Gao, Z. Y., Han, M. K., Hu, Y. Y., Li, Z. Q., Liu, C. F. et al. (2020). Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Frontiers in Microbiology, 10, 49. DOI 10.3389/fmicb.2019.02269. [Google Scholar] [CrossRef]
23. Bai, L., Cui, J. Q., Jie, W. G., Cai, B. Y. (2015). Analysis of the community compositions of rhizosphere fungi in soybeans continuous cropping fields. Microbiological Research, 180, 49–56. DOI 10.1016/j.micres.2015.07.007. [Google Scholar] [CrossRef]
24. Tang, J., Xue, Z. Q., Daroch, M., Ma, J. (2015). Impact of continuous Salvia miltiorrhiza cropping on rhizosphere actinomycetes and fungi communities. Annals of Microbiology, 65(3), 1267–1275. DOI 10.1007/s13213-014-0964-2. [Google Scholar] [CrossRef]
25. Song, X. H., Pan, Y., Li, L. Y., Wu, X. L. (2018). Composition and diversity of rhizosphere fungal community in Coptis chinensis Franch. continuous cropping fields. PLoS One, 13(3), e0193811. DOI 10.1371/journal.pone.0193811. [Google Scholar] [CrossRef]
26. Liliensiek, A. K., Thakuria, D., Clipson, N. (2012). Influences of plant species composition, fertilisation and lolium perenne ingression on soil microbial community structure in three Irish grasslands. Microbial Ecology, 63(3), 509–521. DOI 10.1007/s00248-011-9985-6. [Google Scholar] [CrossRef]
27. Perotto, S., Angelini, P., Bianciotto, V., Bonfante, P. (2013). Interactions of fungi with other organisms. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology, 147(1), 208–218. DOI 10.1080/11263504.2012.753136. [Google Scholar] [CrossRef]
28. Wang, M., Shi, S., Lin, F., Jiang, P. (2014). Response of the soil fungal community to multi-factor environmental changes in a temperate forest. Applied Soil Ecology, 81, 45–56. DOI 10.1016/j.apsoil.2014.04.008. [Google Scholar] [CrossRef]
29. Bucbe, M., Reich, M., Murat, C., Morin, E. (2009). 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytologist, 184(2), 449–456. DOI 10.1111/j.1469-8137.2009.03003.x. [Google Scholar] [CrossRef]
30. Xiong, W., Zhao, Q. Y., Zhao, J., Xun, W. B. (2015). Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microbial Ecology, 70(1), 209–218. DOI 10.1007/s00248-014-0516-0. [Google Scholar] [CrossRef]
31. Li, C., Li, X., Kong, W., Wu, Y. (2010). Effect of monoculture soybean on soil microbial community in the Northeast China. Plant and Soil, 330(1–2), 423–433. DOI 10.1007/s11104-009-0216-6. [Google Scholar] [CrossRef]
32. Zhou, X. G., Wu, F. Z. (2012). Dynamics of the diversity of fungal and Fusarium communities during continuous cropping of cucumber in the greenhouse. FEMS Microbiology Ecology, 80(2), 469–478. DOI 10.1111/j.1574-6941.2012.01312.x. [Google Scholar] [CrossRef]
33. Zhu, S. Y., Wang, Y. Z., Xu, X. M., Liu, T. M. (2018). Potential use of high-throughput sequencing of soil microbial communities for estimating the adverse effects of continuous cropping on ramie (Boehmeria nivea L. Gaud). PLoS One, 13(5), e0197095. DOI 10.1371/journal.pone.0197095. [Google Scholar] [CrossRef]
34. Li, X. G., Ding, C. F., Zhang, T. L., Wang, XX. (2014). Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biology and Biochemistry, 72, 11–18. DOI 10.1016/j.soilbio.2014.01.019. [Google Scholar] [CrossRef]
35. Hussain, Q., Liu, Y., Zhang, A., Pan, G. (2011). Variation of bacterial and fungal community structures in the rhizosphere of hybrid and standard rice cultivars and linkage to CO2 flux. FEMS Microbiology Ecology, 78(1), 116–128. DOI 10.1111/j.1574-6941.2011.01128.x. [Google Scholar] [CrossRef]
36. Yu, J. Q., Shou, S. Y., Qian, Y. R., Zhu, Z. Z. (2000). Autotoxic potential of cucurbit crops. Plant and Soil, 223(1/2), 147–151. DOI 10.1023/A:1004829512147. [Google Scholar] [CrossRef]
37. Pinaria, A. G., Liew, E. C. Y., Burgess, L. W. (2010). Fusarium species associated with vanilla stem rot in Indonesia. Australasian Plant Pathology, 39(2), 176–183. DOI 10.1071/AP09079. [Google Scholar] [CrossRef]
38. Wakelin, S. A., Warren, R. A., Kong, L., Harvey, P. R. (2008). Management factors affecting size and structure of soil Fusarium communities under irrigated maize in Australia. Applied Soil Ecology, 39(2), 201–209. DOI 10.1016/j.apsoil.2007.12.009. [Google Scholar] [CrossRef]
39. Sylvia, D. M., Wilson, D. O., Graham, J. H., Maddox, J. J. (1993). Evaluation of vesicular-arbuscular mycorrhizal fungi in diverse plants and soils. Soil Biology and Biochemistry, 25(6), 705–713. DOI 10.1016/0038-0717(93)90111-N. [Google Scholar] [CrossRef]
40. Klironomos, J. N. (2003). Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology, 84(9), 2292–2301. DOI 10.1890/02-0413. [Google Scholar] [CrossRef]
41. Drenovsky, R. E., Vo, D., Graham, K. J., Scow, K. M. (2004). Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microbial Ecology, 48(3), 424–430. DOI 10.1007/s00248-003-1063-2. [Google Scholar] [CrossRef]
42. Sari, S., Anu, E., Minna, K. M. (2012). Regulation of microbial community composition and activity by soil nutrient availability, soil pH, and herbivory in the Tundra. Ecosystems, 15(1), 18–33. DOI 10.1007/s10021-011-9491-1. [Google Scholar] [CrossRef]
43. Liu, X., Zhang, J., Gu, T., Zhang, W. Shen, Q. et al. (2014). Microbial community diversities and taxa abundances in soils along a seven-year gradient of potato monoculture using high throughput pyrosequencing approach. PLoS One, 9(1), e86610. DOI 10.1371/journal.pone.0086610. [Google Scholar] [CrossRef]
44. Bausenwein, U., Gattinger, A., Langer, U., Embacher, A. (2008). Exploring soil microbial communities and soil organic matter: Variability and interactions in arable soils underminimum tillage practice. Applied Soil Ecology, 40(1), 67–77. DOI 10.1016/j.apsoil.2008.03.006. [Google Scholar] [CrossRef]
45. Schroder, J. L., Zhang, H., Girma, K., Raun, W. R. Penn, C. J. et al. (2011). Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Science Society of America Journal, 75(3), 957–964. DOI 10.2136/sssaj2010.0187. [Google Scholar] [CrossRef]
46. Zhang, T., Wang, N. F., Liu, H. Y., Zhang, Y. Q. (2016). Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund Region, Svalbard (High Arctic). Frontiers Microbiology, 7, 227. [Google Scholar]
47. Glassman, S. I., Wang, I. J., Bruns, T. D. (2017). Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Molecular Ecology, 26(24), 6960–6973. DOI 10.1111/mec.14414. [Google Scholar] [CrossRef]
1.1 Sweet Potato Yield and Disease Incidence Determinations
For each subplot (10 m × 10 m), sweet potato disease incidence was assessed and calculated as the percentage of infected fruits among the total number of fruits after harvest by carefully observing the potato block. All mature and edible sweet potato in each subplot were weighed and used to estimate the total yield at harvest.
1.2 Soil Physical and Chemical Properties
Soil pH, soil organic carbon (SOC), available phosphorus (AP) and available nitrogen (AN) were determined in the same manner as reported in our previous paper [1]. Briefly, Soil pH was determined with a soil to water ratio of 1:2.5 (w:v). SOC was determined by the K2Cr2O7 oxidation method. AP was extracted with 0.5 M NaHCO3 and measured with Mo-Sb Anti-spectrophotometry Method. AN was assayed through the alkaline hydrolysis method.
References
1. Xiang, D., Verbruggen, E., Hu, Y. J., Veresoglou, S. D. Rillig, M. C. et al. (2014). Land use influences arbuscular mycorrhizal fungal communities in the farming-pastoral ecotone of northern China. New Phytologist, 204(4), 968–978. DOI 10.1111/nph.12961.
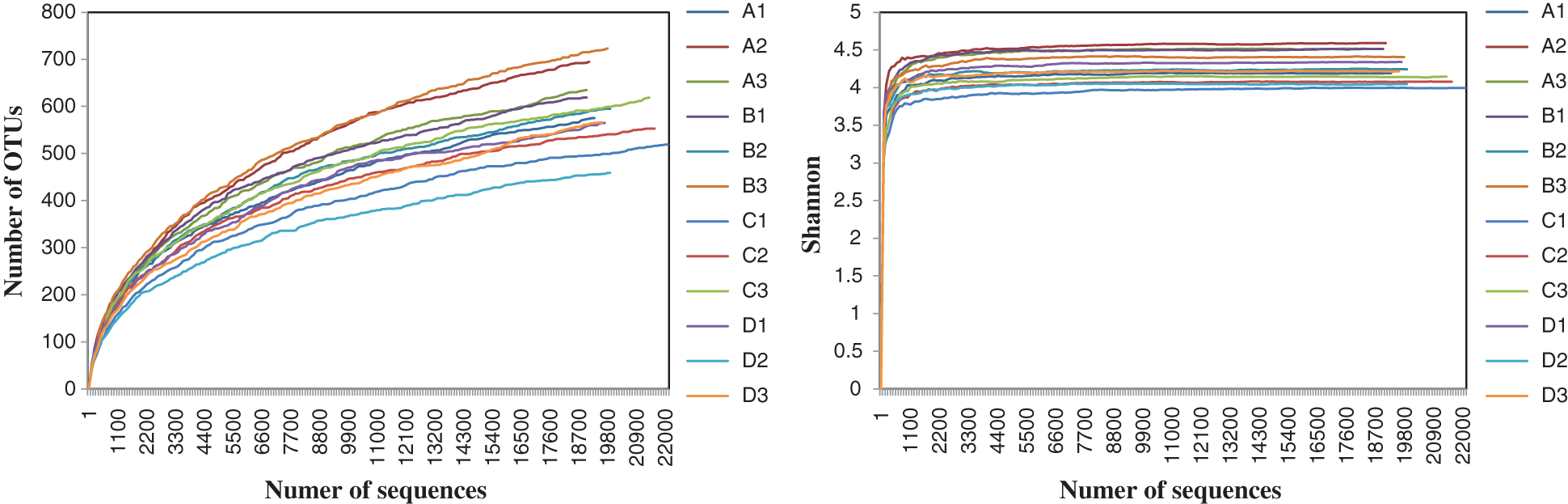
Figure S1: Rarefaction curves of Number of OTUs and Shannon-Weiner index for all samples
Table S1: Selected soil properties, storage root yield and disease incidence (%) for sweet potatoes under consecutive monoculture
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |