
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.015719
ARTICLE
Nanosilver-Promoted Lateral Root Development in Rice is Mediated through Hydrogen Peroxide
Department of Plant Industry, National Pingtung University of Science and Technology, Pingtung, 912301, Taiwan
*Corresponding Author: Yun-Yang Chao. Email: chaoyy@mail.npust.edu.tw
Received: 17 January 2021; Accepted: 29 March 2021
Abstract: Nanosilver (10−9 m) refers to particles comprising 20–15,000 silver atoms, exhibiting high stability and specific surface area. At present, nanosilver has been used in agricultural cultivation and production. This study examined the effects of nanosilver on growth and development of rice root systems. Study results showed that fresh weight of rice belowground organs and root length both increased significantly by 5% and 25%, respectively, after rice radicles were treated with 2 ppm of nanosilver for three days. However, the H2O2 level reached its peak at 2 days from treatment, but the activities of the antioxidant enzymes CAT, APX, and GR were inhibited by 2 ppm of nanosilver treatment. The results showed that nanosilver treatment inhibited the antioxidant enzyme activity of rice roots. The treatment of rice radicles with 5 μM H2O2 promoted root development and the same was observed when nanosilver was used for treatment. Moreover, ascorbic acid (AsA) is a H2O2 scavenger and therefore rice root development was inhibited when AsA was added to rice radicles together with either treatment of nanosilver or H2O2. In summary, nanosilver treatment of rice radicles promoted root growth and development via the regulation of H2O2 and not the O2•− pathway.
Keywords: Rice; nanosilver; antioxidant enzyme; root development; H2O2
Rice (Oryza sativa), a grass from the Gramineae family, is the primary staple food in Asia, and one of the world’s five major grains. In recent years, global climate changes, climate abnormalities, and plant diseases have reduced rice output by 10%–16%. Therefore, improving the stress resistance of rice has become an important topic [1].
Nanosilver (10−9 m) refers to particles comprising 20–15,000 silver atoms, exhibiting high stability and specific surface area. Furthermore, nanosilver shows strong bactericidal activity and is used in medicine, such as in wound dressing or catheter, to decrease the risk of bacterial infection. Moreover, nanosilver is often used for various industrial and domestic purposes, including water filtration and disinfection, dye catalysis and degradation and on domestic applications such as cosmetics manufacturing, textiles, detergents and deodorants to inhibit bacterial growth and kill bacteria [2,3].
Nanosilver can improve crop biomass; publications in recent years have described the use of nanosilver in agricultural cultivation and production. Burman et al. [4] sprayed nanosilver on the leaves of chickpeas and spraying 1.5 ppm of nanosilver on chickpeas significantly increased the biomass of aboveground and belowground organs as well as the total biomass. Shams et al. [5] sprayed nanosilver on different parts of cucumbers and nanosilver improved the morphological characteristics and fruit traits of cucumbers. Baskar et al. [6] treated napa cabbages with 100, 250, or 500 ppm of nanosilver and treatment with 100 ppm of nanosilver promoted plant growth, but treatment with 250 or 500 ppm of nanosilver inhibited root and stem growth and decreased biomass.
Application of nanosilver during crop growth not only promoted crop growth but increased the lifespan of cut flowers. Jung et al. [7] treated Welsh onion with three different types of nanosilver solution (WA-CV-WA13B, WA-AT-WB13R, and WA-PR-WB13R) to assess the antifungal ability of nanosilver: the bacteriostatic activity was 90% after treatment with 7 ppm of nanosilver and the biomass and dry weight of treated Welsh onion were significantly increased. Sah et al. [8] treated borage with nanosilver during its growth phase to assess the effects of nanosilver on borage growth. Borage seed yield increased when nanosilver concentration was increased from 20 to 60 ppm. Hassan et al. [9] treated roses with 25, 50, or 100 ppm of nanosilver after harvesting to assess the effects of nanosilver on postharvest quality: treatment with 50 ppm of nanosilver significantly inhibited microbial growth in the flowerpot solution, maintained fresh weight and relative water concentration, and prolonged the vase life of cut roses.
Moreover, many studies indicate that spraying crops with nanosilver can increase the antioxidant capacity. Leaf mustard seedlings were treated with different concentrations of nanosilver one week after growth and the fresh weight, root length, shoot length, and seed activity were increased in leaf mustard seedlings. Furthermore, the antioxidant activity such as of the guaiacol peroxidase, catalase and ascorbate peroxidase were significantly increased [10]. Juárez-Maldonado et al. [11] compared the effects of adding nutrient solution containing silver nitrate nanoparticles vs. foliar spraying of silver nitrate nanoparticles. They found that treatment with the nutrient solution containing silver nitrate nanoparticles increased the total antioxidant capacity. Aghajani et al. [12] treated thymes with different concentrations (0, 20, 40, 60, 80, and 100 ppm) of nanosilver to investigate the effects of nanosilver on the survival rate, plant height, canopy area, flowering duration, yield, essential oil yield and composition of thymes. These authors showed that survival rate, canopy diameter, flowering duration, yield, and essential oil yield were the highest when the nanosilver concentration was 20, 40, or 60 ppm. However, an additional increase in the nanosilver concentration to 100 ppm posed significant inhibitory effects. At 60 ppm, the α-terpinyl acetate concentration was significantly increased. The above results show that spraying low concentrations of nanosilver during crop growth not only increased crop yield but also the antioxidant capacity of plants.
At present, there are a limited number of papers on the treatment of rice with nanosilver. In particular, the effects of nanosilver on the growth and development of rice belowground organs remain unknown. Belowground organs are important organs that are used by plants to absorb water and nutrients. The growth and development of roots and root hairs are associated with the initial plant growth and later yield. Therefore, this study aimed to examine the effects of nanosilver on the growth, development, and antioxidant activity of rice belowground organs.
2.1 Preparation of Experimental Materials and Nanosilver Treatment
Rice seeds (TCN-1, indica rice) were disinfected with 3% sodium chlorate for 30 min and completely washed with distilled water. After washing, rice seeds were placed in culture dishes (20 cm) containing distilled water and incubated at 37°C in the dark for 24 h. Germinated seeds were selected and placed in 20 cm culture dishes containing two pieces of filter paper. After two days, plants with a root length of approximately 3 cm were placed in culture dishes (9 cm) containing distilled water and 1.0, 1.5 or 2 ppm of nanosilver (TS&T International Corporation, Taoyuan City, Taiwan). Nanosilver concentration: Sliver (15–20 nm), polyvinylpyrrolidone (medical grade): 1.02 ± 0.05%, water: 98.68 ± 0.5% (Fig. 1). Every culture dish contained five rice roots and four duplicates were set up for every treatment. Rice fresh weight was recorded after seedlings were grown in the dark for three days.

Figure 1: Nanosilver particle size in transmission electron microscope. (Information provided by TS&T International Corporation)
2.2 Measurement of H2O2 Concentration
The method of Jana et al. [13] was referenced for measurement. Briefly, 0.05 g of rice roots was ground in sodium phosphate buffer (50 mM, pH 6.8; containing 1 mM hydroxylamine). Following that, the homogenate was centrifuged at 4°C and 6,000 g for 20 min. Then, 1 mL of TiCl4 was added to 2 mL of the supernatant and mixed evenly. Then, the solution was centrifuged at 5,000 rpm for 15 min. The supernatant was collected and A410 was measured.
Calculation formula:
2.3 Measurement of Superoxide Radical, (O2•−) Concentration
The method of Panda [14] was referenced for measurement. Five rice roots were homogenized in sodium phosphate (65 mM pH 7.8) and centrifuged at 4°C and 12,000 g for 20 min. Following that, 0.45 mL of sodium phosphate buffer (65 mM, pH 7.8) and 0.05 mL of hydroxylamine (10 mM) were added to 0.5 mL of the supernatant and mixed evenly. The solution was allowed to react at room temperature for 20 min. Following that, 0.45 mL of sulfanilic acid (17 mM) and 0.5 mL of α-naphthylamine (7 mM) were added to 0.5 mL of the reaction solution and mixed evenly. The solution was allowed to react at room temperature for 20 min. Following that, 0.7 mL of the reaction solution was added to 0.7 mL of ether and centrifuged at 4°C and 1,500 g for 5 min. The bottom layer was collected and A530 was measured.
2.4 Measurement of Superoxide Dismutase (SOD) Activity
The assay method of Paoletti et al. [15] was used as reference. Five rice roots were homogenized in sodium phosphate buffer (50 mM, pH 7.4) and centrifuged at 4°C and 1,5000 g for 30 min. Then, 1.6 mL of Tea-Dea buffer (triethanolamine-diethanolamine, 100 mM, pH 7.4), 0.08 mL of NADH (7.5 mM), 0.05 mL of EDTA/MnCl2 (100 mM/50 mM, pH 7.0), and 1 mL of 2-mercaptoethanol (10 mM) were successively added to 0.2 mL of the supernatant to start the reaction. After evenly mixing, a spectrophotometer was used for measurement at 340 nm.
Calculation formula:
2.5 Measurement of Catalase (CAT) Activity
The method of Kato et al. [16] was referenced for measurement. Five rice roots were homogenized in sodium phosphate buffer (50 mM, pH 6.8) and centrifuged at 4°C and 12,000 g for 20 min. Following that, 2.7 mL of sodium phosphate buffer (100 mM, pH 7.0) and 0.1 mL of H2O2 (1 M) were added to 0.2 mL of the supernatant. After mixing evenly, A240 measurement was carried out.
Calculation formula:
2.6 Measurement of Ascorbate Peroxidase (APX) Activity
The method of Nakano et al. [17] was referenced for measurement. Five rice roots were homogenized in sodium phosphate buffer (50 mM, pH 6.8) and centrifuged at 4°C and 12,000 g for 20 min. Then, 1 mL of potassium phosphate (150 mM, pH 7.0), 1 mL of ascorbate (1.5 mM), 0.4 mL of EDTA (0.75 mM), and 0.5 of H2O2 (6 mM) were successively added to 0.1 mL of the supernatant. After mixing evenly, a spectrophotometer was used for measurement at 290 nm.
Calculation formula:
2.7 Measurement of Glutathione Reductase (GR) Activity
The method of Foster et al. [18] was referenced for measurement. Five rice roots were homogenized in 4 mL of sodium phosphate buffer (50 mM, pH 6.8) and centrifuged at 4°C and 12,000 g for 20 min. Then, 1 mL of Tris-HCl buffer (150 mM, pH 7.5), 0.3 mL of MgCl2 (30 mM), 0.5 mL of GSSG (3 mM, freshly prepared), and 1 mL of NADPH (0.45 mM) were successively added to 0.2 mL of the supernatant. After mixing evenly, a spectrophotometer was used for measurement at 340 nm.
Calculation formula:
Rice germination was performed based on the aforementioned sections. After two days, rice roots of length ∼3 cm were placed in culture dishes (9 cm) containing distilled water and 2.5, 5, or 7.5 μM of H2O2. Every culture dish contained 5 rice roots and 3 duplicates were set up for every treatment. Rice fresh weight was recorded after seedlings were grown in the dark for three days.
2.9 Ascorbic Acid (AsA) Pre-Treatment
Rice germination was performed based on the aforementioned sections. After two days, rice roots of length ∼3 cm were placed in culture dishes (9 cm) containing distilled water and 2.0 mM of AsA. Every culture dish contained 5 rice roots and 3 duplicates were set up for every treatment. After pre-treatment in the dark for 3 h, nanosilver or H2O2 was used for treatment. Following that, the change in rice fresh weight was observed.
All the assays were performed on the basis of the completely randomized design. SAS 9.4 (SAS Institute Inc., USA.) was employed to calculate the least significant difference to determine the differences between various treatments (p ≤ 0.05).
This study aims to examine the effects of nanosilver on the growth and development of rice roots and to further discuss the possible effector mechanism of nanosilver on rice roots.
3.1 Effects of Nanosilver on the Growth of the Rice Root System
After rice germination, seedlings that were 3 cm in root length were placed in nanosilver solutions of 0, 1, 1.5, or 2 ppm for 3 days. Rice root length and fresh weight were higher than those in the control group (Figs. 2A, 2B). After rice roots were treated with 1.5, or 2 ppm nanosilver, the number of crown roots and that of lateral roots significantly, increased and the treatment did not compromise root appearance (Fig. 2C).
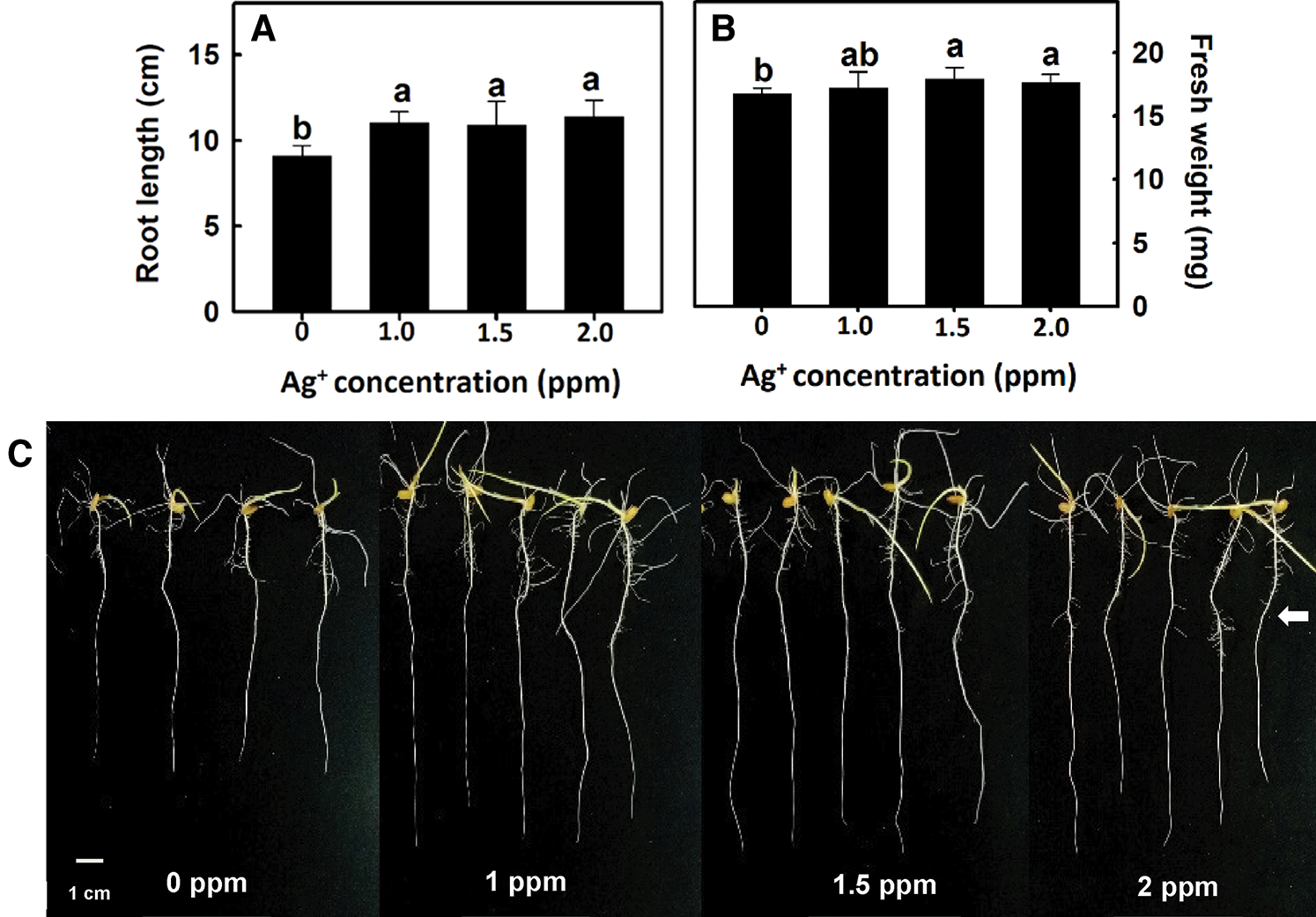
Figure 2: Effect of nanosilver on root length and fresh weight in rice. Selected plant material with 3 cm root length after the germination of rice, placed in 0, 1, 1.5, 2 ppm concentration of nanosilver solution for 3 days. (A) root length and (B) root fresh weight of rice were measured and recorded, (C) rice root appearance. The arrow points to the lateral roots. Bars show means ± SE (n = 5), repeated four times. Values with the same letter are not significantly different at p < 0.05
The root imaging analysis software DigiRoot V2.5 [19] was used to further analyze total root length, root area, and root volume to elucidate the effect of nanosilver on rice root growth. Root length, root area, and root volume were significantly increased after treatment with 1.5 or 2 ppm of nanosilver (Fig. 3). Therefore, 2 ppm nanosilver was used as the treatment concentration in subsequent experiments.
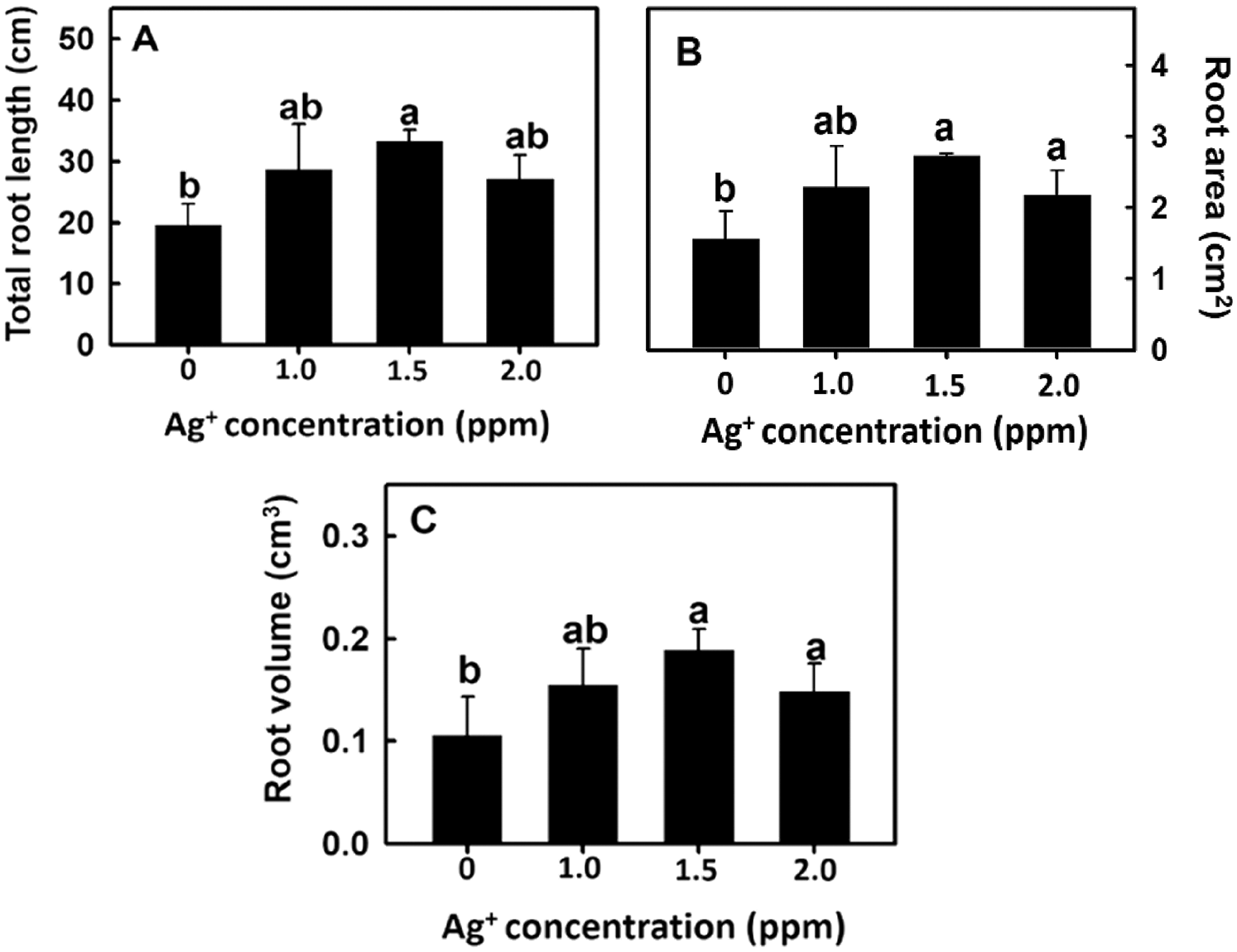
Figure 3: Effect of nanosilver on (A) the total root length, (B) root area and (C) root volume in rice. Selected plant material with 3 cm root length after the germination of rice, placed in 0, 1, 1.5, 2.0 ppm concentration of nanosilver solution for 3 days. Bars show means ± SE (n = 5), repeated four times. Values with the same letter are not significantly different at p < 0.05
According to the results in Fig. 2C, the number of lateral roots in the 2 ppm nanosilver treatment was greater than that in the control treatment. Therefore, rice roots were treated with 2 ppm nanosilver for 1, 2, 3, or 4 days, and the root length was significantly increased on Day 3 of treatment (Figs. 4C, 4E). These results show that treatment with nanosilver at a concentration of 2 ppm for 3 days was the optimal treatment condition.
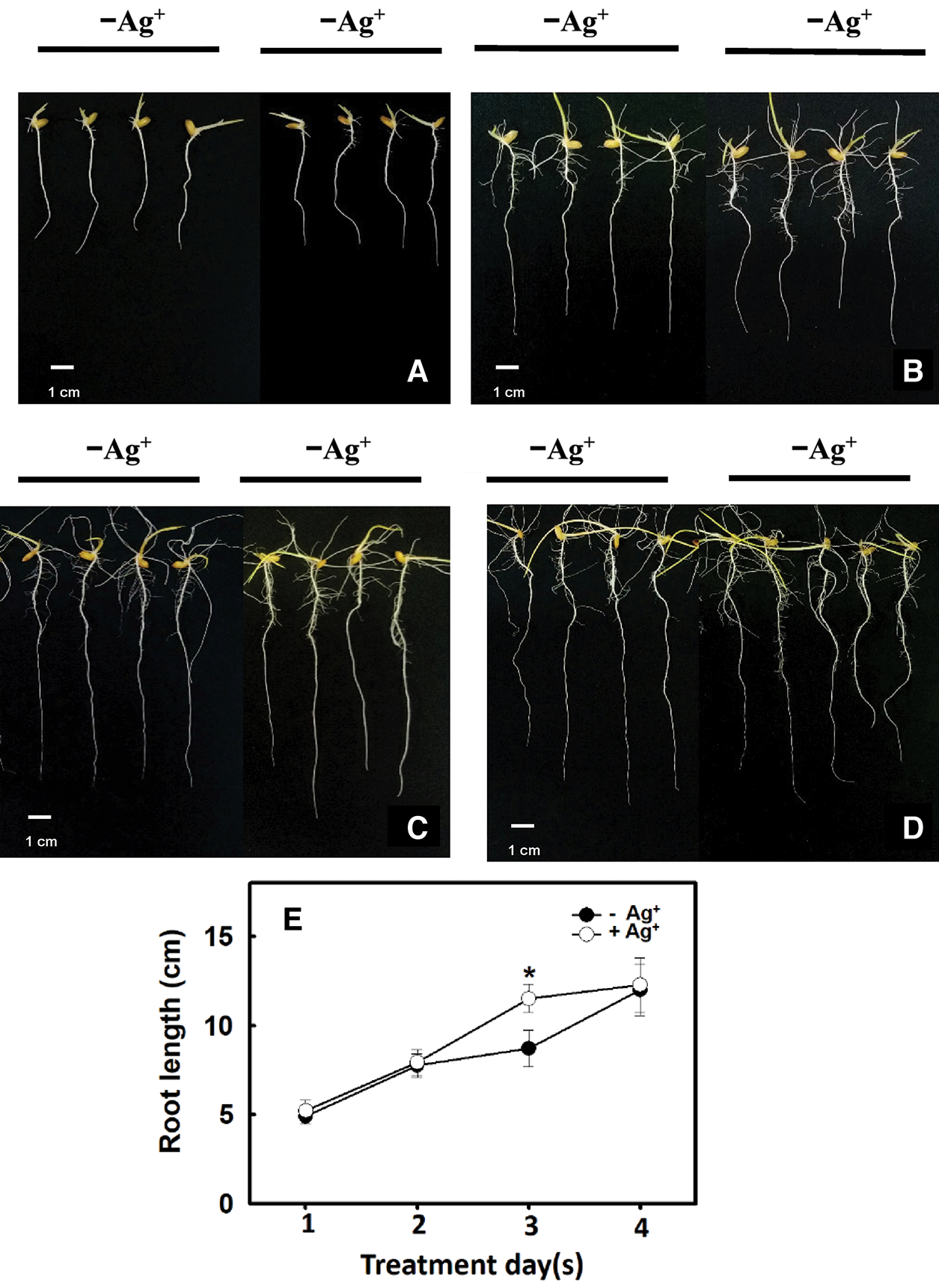
Figure 4: Effect of different times of treatments with/without nanosilver on the root length in rice. Selected plant material with 3 cm root length after the germination of rice was placed in 2 ppm concentration of nanosilver solution for 3 days. The growth at (A) 1 day, (B) 2 days, (C) 3 days and (D) 4 days of root appearance were recorded, and the change of root length (E) was measured. Symbols show means ± SE (n = 5), repeated four times. Asterisks represent values that were significantly different between –Ag+ and +Ag+ at p < 0.05
3.2 Effects of Nanosilver on Rice Antioxidant Enzyme Activity
Hydrogen peroxide can regulate plant growth and signal transduction during plant growth and development or when plants experience stress. Therefore, the influence of nanosilver treatment on the H2O2 concentration was studied. Experimental results show that H2O2 concentration significantly increased on Day 2 after rice roots were treated with nanosilver. On Day 3 of treatment, H2O2 concentration decreased (Fig. 5A). Changes in superoxide concentration followed a similar trend as that of H2O2 concentration, i.e., reaching a peak on Day 2 and significantly decreasing on Day 3 (Fig. 5B). However, no significant differences were observed in superoxide concentration between treated and untreated groups (Fig. 5B). The above results demonstrated that nanosilver treatment of rice can increase H2O2 concentration but did not affect superoxide concentration.
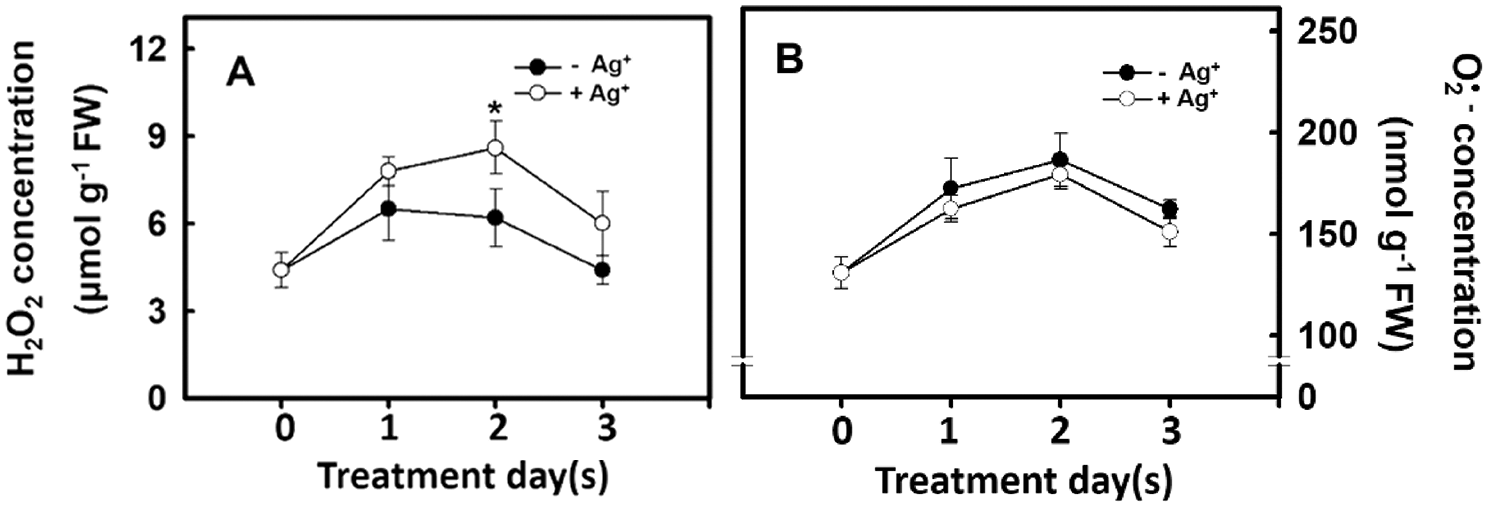
Figure 5: Effect of nanosilver on reactive oxygen species concentration in rice. Selected plant material with 3 cm root length after the germination of rice, placed in 2 ppm concentration of nanosilver solution for 3 days. The (A) hydrogen peroxide concentration and (B) superoxide concentration of rice were measured. Symbols show means ± SE (n = 5), repeated four times. Asterisk on Fig. 5A represent values that are significantly different between –Ag+ and +Ag+ at p < 0.05
The H2O2 concentration in the rice root differed depending on the antioxidant enzyme activity. Therefore, the effect of nanosilver treatment on antioxidant enzyme activity in rice roots was studied. Experimental results showed that the SOD activities in the rice roots of the untreated group and the nanosilver treated group did not differ (Fig. 6A). However, CAT, APX, and GR activities in the nanosilver treated group were significantly lower on Days 2 and 3 of treatment compared with the untreated group (Figs. 6B, 6C, 6D), showing that nanosilver treatment reduced CAT, APX, and GR activities.
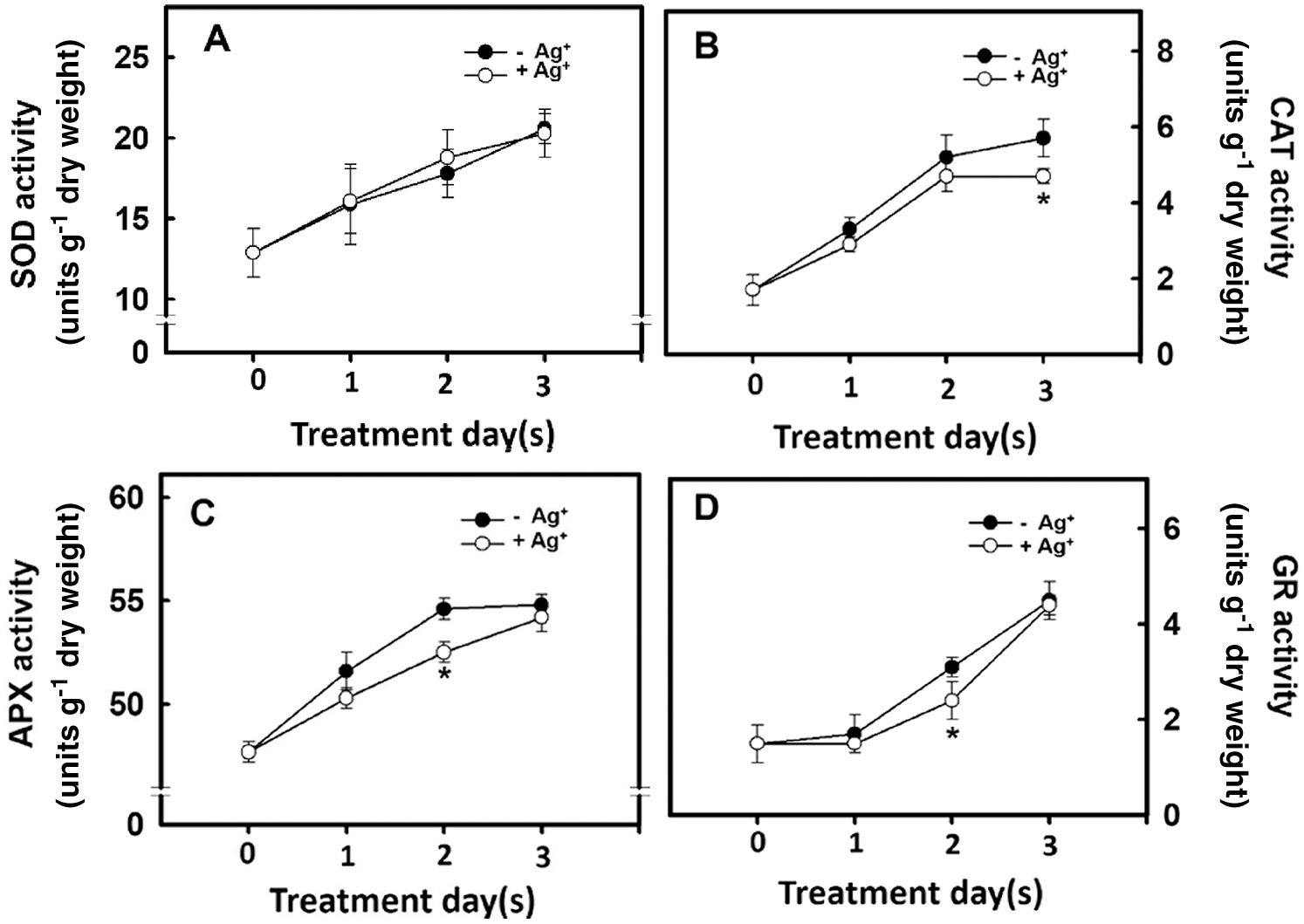
Figure 6: Effect of nanosilver on antioxidant enzyme activities in rice. Selected plant material with 3 cm root length after the germination of rice was placed in 2 ppm concentration of nanosilver solution for 3 days. The (A) SOD (B) CAT (C) APX and (D) GR activities of rice were measured. Symbols show means ± SE (n = 5), repeated four times. Asterisks represent values that are significantly different between –Ag+ and +Ag+ at p < 0.05
3.3 Effects of H2O2 on the Growth of Rice Root Systems
After rice roots were treated with 0, 2.5, 5.0, or 7.5 μm of H2O2, rice root fresh weight and change in H2O2 concentration were measured. Experimental results showed that rice root fresh weight and H2O2 concentration increased as the H2O2 treatment concentration also increased (Fig. 7). The above results show that H2O2 treatment of rice roots can promote root growth.
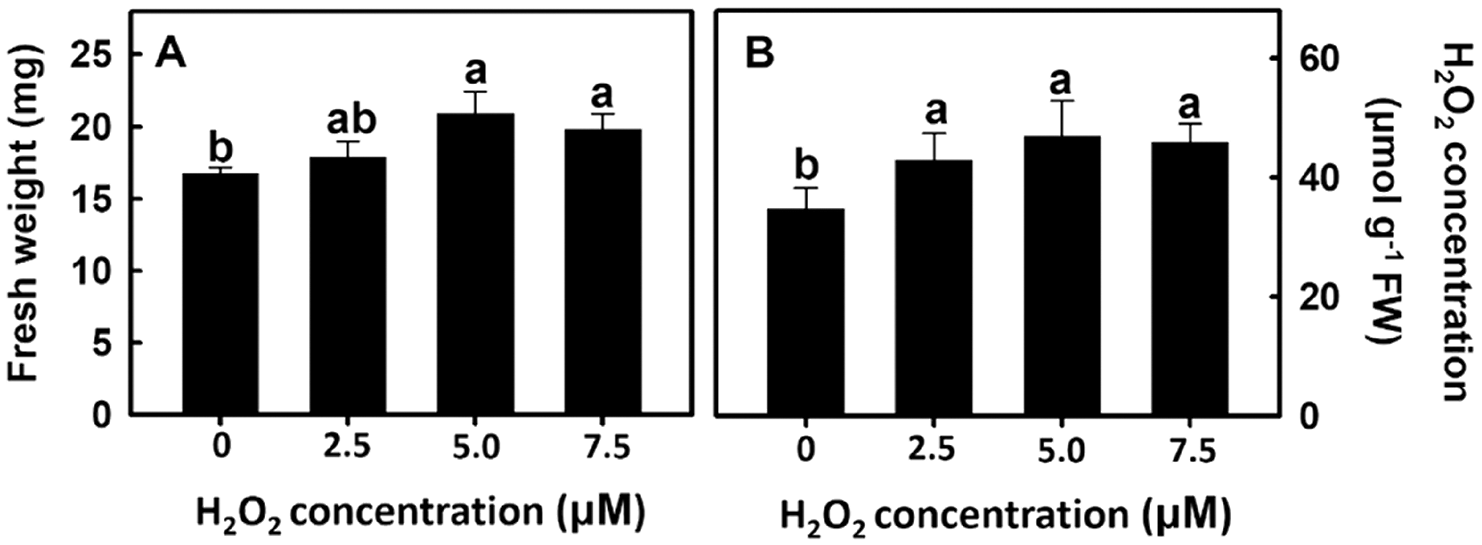
Figure 7: Effect of hydrogen peroxide on root fresh weight and H2O2 concentration in rice. Selected plant material with 3 cm root length after the germination of rice was placed in 0, 2.5, 5.0, 7.5 ppm concentration of peroxide solution for 3 days. The (A) root fresh weight and (B) peroxide concentration of rice were measured. Bars show means ± SE (n = 5), repeated four times. Values with the same letter are not significantly different at p < 0.05
AsA is a H2O2 scavenger and the addition of AsA in the experiment can reduce H2O2 concentration in rice roots. Furthermore, H2O2 can play a dual role in cells. When the concentration is high, it becomes a free radical that damages the cell membrane; when the concentration is low, it becomes a message-transmitting molecule. In this experiment, the H2O2 concentration is 5 μM as the intermediate value (2.5 μM∼7.5 μM), which is the subsequent treatment concentration.
Experimental results showed that root fresh weight was increased after H2O2 and nanosilver treatments (Fig. 8). This demonstrates that the effect of nanosilver on rice roots is the same as the H2O2 treatment. When 2 mM of AsA was added, root fresh weight decreased regardless of whether roots were treated with H2O2, nanosilver, or both (Fig. 8). This shows that AsA decreased H2O2 concentration in rice roots, thereby abolishing its growth promoting effects on rice roots.
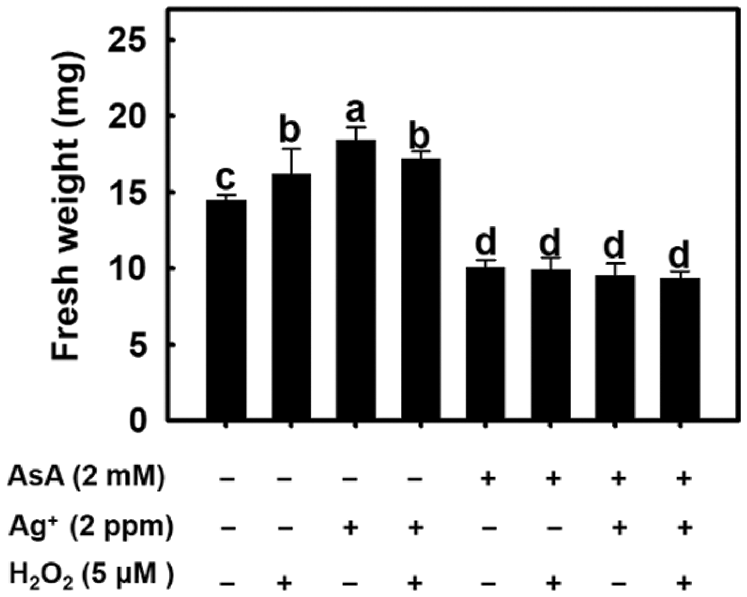
Figure 8: Effect of ascorbate, nanosilver and hydrogen peroxide on root fresh weight in rice. Selected plant material with 3 cm root length after the germination of rice with or without 2 mM ascorbate, 2 ppm nanosilver or 5 μM hydrogen peroxide solution for 3 days. Bars show means ± SE (n = 5), repeated four times. Values with the same letter are not significantly different at p < 0.05.
Nanosilver, which features unique physical and chemical properties, high stability, large specific surface areas, and favorable disinfection capability, is widely utilized in industrial and medical fields [20,21]. In recent years, nanosilver has also been used in fields of agricultural cultivation and production [4–6]. However, to induce crop growth, the appropriate nanosilver concentrations must be used. Lin et al. [22]. investigated the effects of nanoparticles on plant germination rates and root growth by using six crops and five different types of nanoparticles, respectively. The results revealed that a high nanoparticle concentration (i.e., 2,000 mg/L) inhibited root growth among all crops; those of nanozinc and nanozinc oxide concentrations of 50% inhibited radish at 50 mg/L; and those of nanozinc and nanozinc oxide inhibited edible rape and perennial ryegrass at 20 mg/L. The results of subjecting Thymus plants to nanosilver of varying concentrations (i.e., 0, 20, 40, 60, 80, and 100 ppm) revealed that at concentrations of 20, 40, and 60 ppm, nanosilver considerably affected survival rates, canopy area diameters, flowering days, yields, and essential oil volumes [12]. In vitro barley experiments revealed that adding 6 and 8 mg dm–3 nanosilver to the Murashige and Skoog medium limited the number of infected barley embryos, and that the adding of 4 and 8 mg dm–3 nanosilver yielded the most seedlings with tall plant height and long root lengths [23]. Additionally, the selection of nanosilver particle sizes is critical. Mustafa et al. [24] treated soybeans using nanosilver particles (i.e., 2, 15, and 50–80 nm) of varying concentrations (i.e., 0.2, 2, and 20 ppm) for six days, and discovered that 15-nm nanoparticles with a concentration of 2 ppm yielded the highest soybean seed fresh weight; and that 20-nm nanosilver particles with concentrations between 1 and 10 ppm produced the most favorable rice root lengths, dryness, and fresh weights [25]. In this study, 15−20 nm nanosilver particles with concentrations of 0, 1, 5, 10 ppm were used to treat rice, where those with a concentration of 5 ppm resulted in brown roots, signaling plant poisoning (data not shown). Accordingly, this study selected only nanosilver particles with concentrations of 0, 1, 1.5, and 2 ppm. The results indicated that treating rice with nanosilver with concentrations of 1.5 and 2 ppm nanosilver substantially increased rice root lengths, fresh weights (Figs. 2, 4), root areas, and root volumes (Fig. 3). This implies that treating rice with nanosilver can induce early-stage root growth and development, supporting the findings of Thuesombat et al. [25].
Nanosilver treatment promotes crop growth, enhances crop quality [7–9], and elevates crop antioxidant capacity, increasing crop tolerance to adverse environments. Hazel tree cells treated using nanosilver with concentrations of 2.5 or 5 ppm exhibit higher enzyme (e.g., Ascorbate peroxidase (APX), Catalase (CAT), Peroxidase (POD)) activities [26]. Onions treated using nanosilver with a concentration of 20 ppm demonstrate considerably higher total antioxidant capacity [11]. Pelargonium treated using nanosilver display elevated enzyme (e.g., APX and POD) activities, effectively preventing its flower petals from falling off when stored in the dark [27,28]. Soybeans treated using nanosilver exhibit improved tolerance to flooding [24]. Gupta et al. [29] discovered that by treating rice seedlings in culture mediums using nanosilver with concentrations of 0, 10, 20, and 40 ppm, the rice demonstrated improved dry and fresh weights, elevated CAT and APX gene expressions and enzyme activities, and decreased, but not significantl, H2O2 concentration. Therefore, they maintained that adding of nanosilver enhanced rice antioxidant enzyme activities and facilitated (but not significant) H2O2 because the rice was not in adverse environments. This study treated rice roots using nanosilver, where the APX and Glutathione Reductase (GR) showed decreased activities by Day 2; and decreased CAT activities by Day 3, increasing H2O2 concentration and inducing root growth. The results of this study supported those of Gupta et al. [29] in that the nanosilver treatment facilitated rice growth; and did not support those of Gupta et al. [29] in that antioxidant enzyme activities dropped in this study. Possible reasons for such a difference may be because of the different treatment materials and nanosilver concentrations employed.
Reactive Oxygen Species (ROS) play an important role in plant growth and development. The growth and development of belowground organs are regulated by ROS. When the ROS level reaches that of micromolar, root growth and elongation are promoted [30]. Xu et al. [31] used salicylic acid to inhibit the expression of ROS scavenging-related genes and increase ROS level to promote root meristem activity. He et al. [32] treated wheat under drought stress with H2O2 and found that wheat germination rate, leaf area, dry weight, moisture utilization efficiency, net photosynthetic rate, and antioxidant enzyme activity were higher than those recorded in the control group. This proves that H2O2 can act as a signaling molecule under stress to induce stress resistance of the plant antioxidant system. In addition, O2•− can regulate plant growth and development. Kim et al. [33] transformed potatoes with antisense-sequences of the lily chloroplast-localized Cu, Zn SOD and found that the O2•− level in potatoes was increased: this regulated gibberellin (GA) synthesis to promote potato growth and tuber development. The present study examined the effects of nanosilver treatment on the antioxidant enzyme activity in rice. Results showed that nanosilver treatment of rice resulted in no significant change in SOD activity (Fig. 6A). This result showed that there was no significant difference in the O2•− level (Fig. 5B). However, APX and GR activities were inhibited on Day 2 after nanosilver treatment (Figs. 6C, 6D), and CAT activity was inhibited on Day 3 of treatment (Fig. 6B). Results showed that there were significant differences between H2O2 levels on Day 2 of treatment (Fig. 5A). These results suggested that nanosilver treatment promoted rice root growth and elongation through H2O2 and not O2•−. Therefore, we further treated rice with 0, 2.5, 5, or 7.5 μM of H2O2. Results show that the treatment with H2O2 increased fresh root weight (Fig. 7A). This result is the same as that the treatment of nanosilver promoted the root length and fresh weight of rice (Figs. 2A, 2B). These findings show that nanosilver promotes the growth of rice roots, and this was related to H2O2.
AsA is a H2O2 scavenger [34]. Beltagi [35] treated chickpeas with AsA under salinity stress and the resulting dry weight and fresh weight of plants were significantly higher than those recorded in the control group. This shows that AsA can scavenge excess of free radicals under oxidative stress conditions to protect plants from oxidative damage. In this study, AsA pre-treatment significantly inhibited the development of rice roots, and inhibited the promoting effect of nanosilver on root elongation. When nanosilver and 5 μM of H2O2 were simultaneously added after AsA pre-treatment, the resulting root length was similar as that recorded in the AsA pre-treatment group (Fig. 8). Therefore, results suggest that AsA pre-treatment allows simultaneous scavenging of nanosilver and additional H2O2, thereby abolishing their promoting effects on root growth and elongation.
In summary, the above results show that the nanosilver promotion of rice root growth in the early stage of development is regulated by H2O2 and not O2•−. Moreover, 2 ppm nanosilver treatment for 3 days increased rice root H2O2 levels and inhibited the activities of antioxidant enzymes (CAT, GR, and APX) to increase fresh weight and promote root growth (Fig. 9). Furthermore, the development of rice root systems were similar after treatment with 5 μM of H2O2 or nanosilver treatment. As AsA is a H2O2 scavenger, AsA pre-treatment significantly inhibited the development of rice roots and at the same time abolished the promoting effects of nanosilver on root elongation. The mechanism by which nanosilver affects rice root growth is still unclear. From the results obtained in this study, it can be deduced that nanosilver increases the H2O2 level in rice roots to affect root development (Fig. 9).
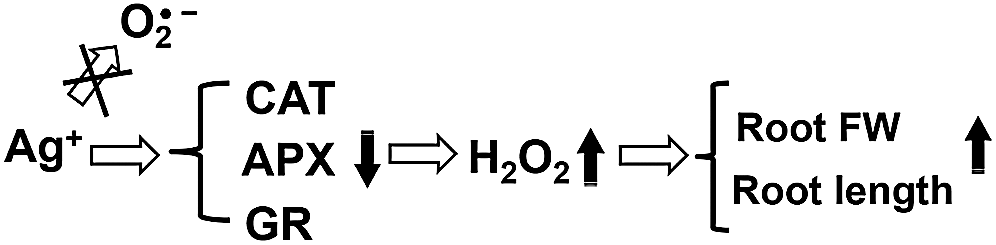
Figure 9: Mechanism diagram of nanosilver to promote root growth of rice
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Trebicki, P. (2020). Climate change and plant virus epidemiology. Virus Research, 286, 198059. DOI 10.1016/j.virusres.2020.198059. [Google Scholar] [CrossRef]
2. Ravi, S. S., Christena, L. R., SaiSubramanian, N., Anthony, S. P. (2013). Green synthesized silver nanoparticles for selective colorimetric sensing of Hg2+ in aqueous solution at wide pH range. Analyst, 138(15), 4370. DOI 10.1039/c3an00320e. [Google Scholar] [CrossRef]
3. Wijnhoven, S. W., Peijnenburg, W. J., Herberts, C. A., Hagens, W. I., Oomen, A. G. et al. (2009). Nano-silver–A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology, 3(2), 109–138. DOI 10.1080/17435390902725914. [Google Scholar] [CrossRef]
4. Burman, U., Saini, M., Kumar, P. (2013). Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicological and Environmental Chemistry, 95(4), 605–612. DOI 10.1080/02772248.2013.803796. [Google Scholar] [CrossRef]
5. Shams, G., Ranjbar, M., Amiri, A. (2013). Effect of silver nanoparticles on concentration of silver heavy element and growth indexes in cucumber (Cucumis sativus L. negeen). Journal of Nanoparticle Research, 15(5), 1630. DOI 10.1007/s11051-013-1630-5. [Google Scholar] [CrossRef]
6. Baskar, V., Venkatesh, J., Park, S. W. (2015). Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in brassica rapa ssp. pekinensis. Environmental Science and Pollution Research, 22(22), 17672–17682. DOI 10.1007/s11356-015-4864-1. [Google Scholar] [CrossRef]
7. Jung, J. H., Kim, S. W., Min, J. S., Kim, Y. J., Lamsal, K. et al. (2010). The effect of nano-silver liquid against the white rot of the green onion caused by sclerotium cepivorum. Mycobiology, 38(1), 39–45. DOI 10.4489/MYCO.2010.38.1.039. [Google Scholar] [CrossRef]
8. Sah, S., Sorooshzadeh, A., Rezazadehs, H., Naghdibadi, H. A. (2011). Effect of nano silver and silver nitrate on seed yield of borage. Medicinal Plants Research, 5(2), 171–175. DOI 10.1186/s13588-014-0011-0. [Google Scholar] [CrossRef]
9. Hassan, F. S. A., Ali, E. F., El-Deeb, B. (2014). Improvement of postharvest quality of cut rose cv.‘First red’ by biologically synthesized silver nanoparticles. Scientia Horticulturae, 179, 340–348. DOI 10.1016/j.scienta.2014.09.053. [Google Scholar] [CrossRef]
10. Sharma, P., Bhatt, D., Zaidi, M. G. H., Saradhi, P. P., Khanna, P. K. et al. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of brassica juncea. Applied Biochemistry and Biotechnology, 167(8), 2225–2233. DOI 10.1007/s12010-012-9759-8. [Google Scholar] [CrossRef]
11. Juárez-Maldonado, A., Rosales-Velázquez, J. L., Ortega-Ortiz, H., Cabrera De-la-Fuente, M., Ramírez, H. et al. (2013). Accumulation of silver nanoparticles and its effect on the antioxidant capacity in allium cepa L. Phyton-International Journal of Experimental Botany, 82, 91–97. DOI 10.32604/phyton.2013.82.091. [Google Scholar] [CrossRef]
12. Aghajani, Z., Ekhtiyari, R. (2013). Effect of nano-silver on stages of plant growth and yield and composition of essential oil of thymus kotschyanus boiss. Hohen. African Journal of Agricultural Research, 8(8), 707–710. DOI 10.5897/AJAR12.1762. [Google Scholar] [CrossRef]
13. Jana, S., Choudhuri, M. A. (1981). Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquatic Botany, 11, 67–77. DOI 10.1016/0304-3770(81)90047-4. [Google Scholar] [CrossRef]
14. Panda, S. K. (2007). Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. Journal of Plant Physiology, 164(11), 1419–1428. DOI 10.1016/j.jplph.2007.01.012. [Google Scholar] [CrossRef]
15. Paoletti, F., Aldinucci, D., Mocali, A., Caparrini, A. (1981). A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Analytical Biochemistry, 154(2), 536–541. DOI 10.1016/0003-2697(86)90026-6. [Google Scholar] [CrossRef]
16. Kato, M., Shimizu, S. (1985). Chlorophyll metabolism in higher plants. VII. Chlorophyll Degradation in Senescing Tobacco Leaves; Phenolic-Dependent Peroxidative Degradation. Canadian Journal of Botany, 65(4), 729–735. DOI 10.1139/b87-097. [Google Scholar] [CrossRef]
17. Nakano, Y., Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880. DOI 10.1093/oxfordjournals.pcp.a076232. [Google Scholar] [CrossRef]
18. Foster, J. G., Hess, J. L. (1980). Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiology, 66(3), 482–487. DOI 10.1104/pp.66.3.482. [Google Scholar] [CrossRef]
19. Stefanelli, D., Fridman, Y., Perry, R. L. (2009). Digiroot™: New software for root studies. European Journal of Horticultural Science, 74(4), 169–174. [Google Scholar]
20. Haider, A., Kang, I. K. (2015). Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Advances in Materials Science and Engineering, 2015(3), 1–16. DOI 10.1155/2015/165257. [Google Scholar] [CrossRef]
21. Murphy, M., Ting, K., Zhang, X., Soo, C., Zheng, Z. (2015). Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. Journal of Nanomaterials, 2015, 1–12. DOI 10.1155/2015/696918. [Google Scholar] [CrossRef]
22. Lin, D., Xing, B. (2007). Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environmental Pollution, 150(2), 243–250. DOI 10.1016/j.envpol.2007.01.016. [Google Scholar] [CrossRef]
23. Krupa-Małkiewicz, M., Oszmiański, J., Lachowicz, S., Szczepanek, M., Jaśkiewicz, B. et al. (2019). Effect of nanosilver (nAg) on disinfection, growth and chemical composition of young barley leaves under in vitro. Journal of Integrative Agriculture, 18(8), 1871–1881. DOI 10.1016/S2095-3119(18)62146-X. [Google Scholar] [CrossRef]
24. Mustafa, G., Sakata, K., Hossain, Z., Komatsu, S. (2015). Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. Journal of Proteomics, 122(2015), 100–118. DOI 10.1016/j.jprot.2015.03.030. [Google Scholar] [CrossRef]
25. Thuesombat, P., Hannongbua, S., Akasit, S., Chadchawan, S. (2014). Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicology and Environmental Safety, 104(2014), 302–309. DOI 10.1016/j.ecoenv.2014.03.022. [Google Scholar] [CrossRef]
26. Jamshidi, M., Ghanati, F., Rezaei, A., Bemani, E. (2014). Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology, 68(3), 525–530. DOI 10.1007/s10616-014-9808-y. [Google Scholar] [CrossRef]
27. Hatami, M., Ghorbanpour, M. (2013). Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. Journal of Horticultural Research, 21(1), 15–20. DOI 10.2478/johr-2013-0003. [Google Scholar] [CrossRef]
28. Hatami, M., Ghorbanpour, M. (2014). Defense enzyme activities and biochemical variations of pelargonium zonale in response to nanosilver application and dark storage. Turkish Journal of Biology, 38(1), 130–139. DOI 10.3906/biy-1304-64. [Google Scholar] [CrossRef]
29. Gupta, S. D., Agarwal, A., Pradhan, S. (2018). Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicology and Environmental Safety, 161, 624–633. DOI 10.1016/j.ecoenv.2018.06.023. [Google Scholar] [CrossRef]
30. Wang, L., Sun, J., Lin, L., Fu, Y., Alenius, H. et al. (2020). Silver nanoparticles regulate arabidopsis root growth by concentration-dependent modification of reactive oxygen species accumulation and cell division. Ecotoxicology and Environmental Safety, 190, 110072. DOI 10.1016/j.ecoenv.2019.110072. [Google Scholar] [CrossRef]
31. Xu, L., Zhao, H., Ruan, W., Deng, M., Wang, F. et al. (2017). Abnormal inflorescence meristem 1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. The Plant Cell, 29(3), 560–574. DOI 10.1105/tpc.16.00665. [Google Scholar] [CrossRef]
32. He, L., Gao, Z. (2009). Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. African Journal of Biotechnology, 8(22), 6151–6157. DOI 10.5897/AJB09.490. [Google Scholar] [CrossRef]
33. Kim, M. S., Kim, H. S., Kim, Y. S., Baek, K. H., Oh, H. W. et al. (2007). Superoxide anion regulates plant growth and tuber development of potato. Plant Cell Reports, 26(10), 1717–1725. DOI 10.1007/s00299-007-0380-1. [Google Scholar] [CrossRef]
34. Pérez, F. (2002). Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry, 60(6), 573–580. DOI 10.1016/S0031-9422(02)00146-2. [Google Scholar] [CrossRef]
35. Beltagi, M. S. (2008). Exogenous ascorbic acid (vitamin C) induced anabolic changes for salt tolerance in chick pea (Cicer arietinum L.) plants. African Journal of Plant Science, 2(10), 118–123. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |