
International Journal of
Experimental Botany

 | Phyton- International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2021.016664
REVIEW
Yellow Vein Mosaic Disease in Okra (Abelmoschus esculentus L.): An Overview on Causal Agent, Vector and Management
1Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, 40100, Pakistan
2State Key Laboratory of Agricultural Microbiology and Provincial Key Laboratory of Plant Pathology of Hubei Province, College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, 430070, China
3Department of Agriculture and Agribusiness Management, University of Karachi, Karachi, 75270, Pakistan
4Department of Soil Science, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, 60800, Pakistan
*Corresponding Author: Yasir Iftikhar. Email: yasir.iftikhar@uos.edu.pk
Received: 16 March 2021; Accepted: 30 April 2021
Abstract: Okra (Abelmoschus esculentus L.) belongs to the Malvaceae family and is one of the most essential and popular vegetables globally. It is rich in proteins, carbohydrates, and vitamins. Abiotic and biotic factors threaten okra productivity. Okra yellow vein mosaic disease (OYVMD) is the most destructive disease of okra. The causal agent, [(i.e., Okra yellow vein mosaic virus (OYVMV)] of this disease belongs to the family Geminiviridae and genus Begomovirus. OYVMV is a monopartite with additional ssDNA molecule. This virus has two components DNA-A for protein coding and DNA-B for symptoms induction. Whitefly transmits OYVMV in persistent manner. Characteristic symptoms of OYVMV infected okra plants are chlorosis, dwarfing, and yellowing of veins and fruits. High temperatures with moderate rainfall enhance the development of OYVMV disease and the whitefly population. However, high humidity with low temperature and rainfall has no significant role in developing the OYVMD and whitefly population. Moreover, the virus also affects the secondary metabolites in the infected okra plants. The virus can be managed through various strategies including the application of plant defense activators, the development of resistant varieties and by controlling its vector via pesticides and plant extracts. Various plant defense activators such as monopotassium phosphate (KH2PO), salicylic acid, benzoic acid, and citric acid enhance resistance in okra against OYMVD. In addition, the resistance to OYMVD can also be achieved by successfully incorporating high yielding but resistant cultivars of acceptable quality. In this review, we have discussed history, economic impact, symptomology, disease development under a natural environment, genetics and management of OYVMV.
Keywords: Okra; OYVMV; symptoms; vectors; epidemiological factors; management
1 Historical Background and Economic Impact
Okra (Abelmoschus esculentus L.) belongs to the family Malvaceae and is commonly known as ladyfinger. It is one of the most common vegetables in the world and is edible. The ladyfinger originated in Africa and is being cultivated worldwide, including Asia. Okra can be grown throughout the whole year even in poor soil and arid areas. The okra plants are rich in minerals, carbohydrates, fibers, proteins, fats, phenols, and vitamins A, B, and C [1]. Okra plants cover 1,148,000 hectares with a worldwide annual production of 7,896,300 tons [2]. Okra yield is threatened by different biotic factors including different viruses. Among them Okra yellow vein mosaic virus (OYVMV) is one of the most devastating and widely distributed viruses in the okra fields [3]. In 1924, the virus was initially reported by Kulkarni in Mumbai, India [4]. However, okra plants are severely affected by the OYVMV. The virus is prevalent in all okra growing regions of the world. The virus is transmitted by whitefly (Bemisia tabaci Genn.), which is the most severe pest of many crops in the world. The virus is responsible for 80–90% of yield losses and it affects all the developmental stages of okra plants [3,5]. In India, 100% infection of OYVMV in a field is quite usual with the yield losses ranging between 50% to 94%. In Pakistan, yield losses up to 84% on the susceptible varieties have been reported. In Ghana, Africa, more than 50% disease incidence in okra farms has been reported. In America, the total loss of vegetables due to this virus ranged between 20–30% but in some cases reaches to 80–90%. In Thailand, 50% to 100% okra plants infected with OYVMV were observed in fields. Okra plants infected with OYVMV have also been reported from Bangladesh, Sri Lanka and China.
Although many researchers and scientists have worked on the OYVMV still a lot is needed to be done to characterize the virus. There are reviews available for begomoviruses in general but not for the OYVMV in particular. This review discusses the current status of OYVMV, symptomology, transmission, detection, and recent approaches to tackle the virus and its interaction with the vector and environmental factors.
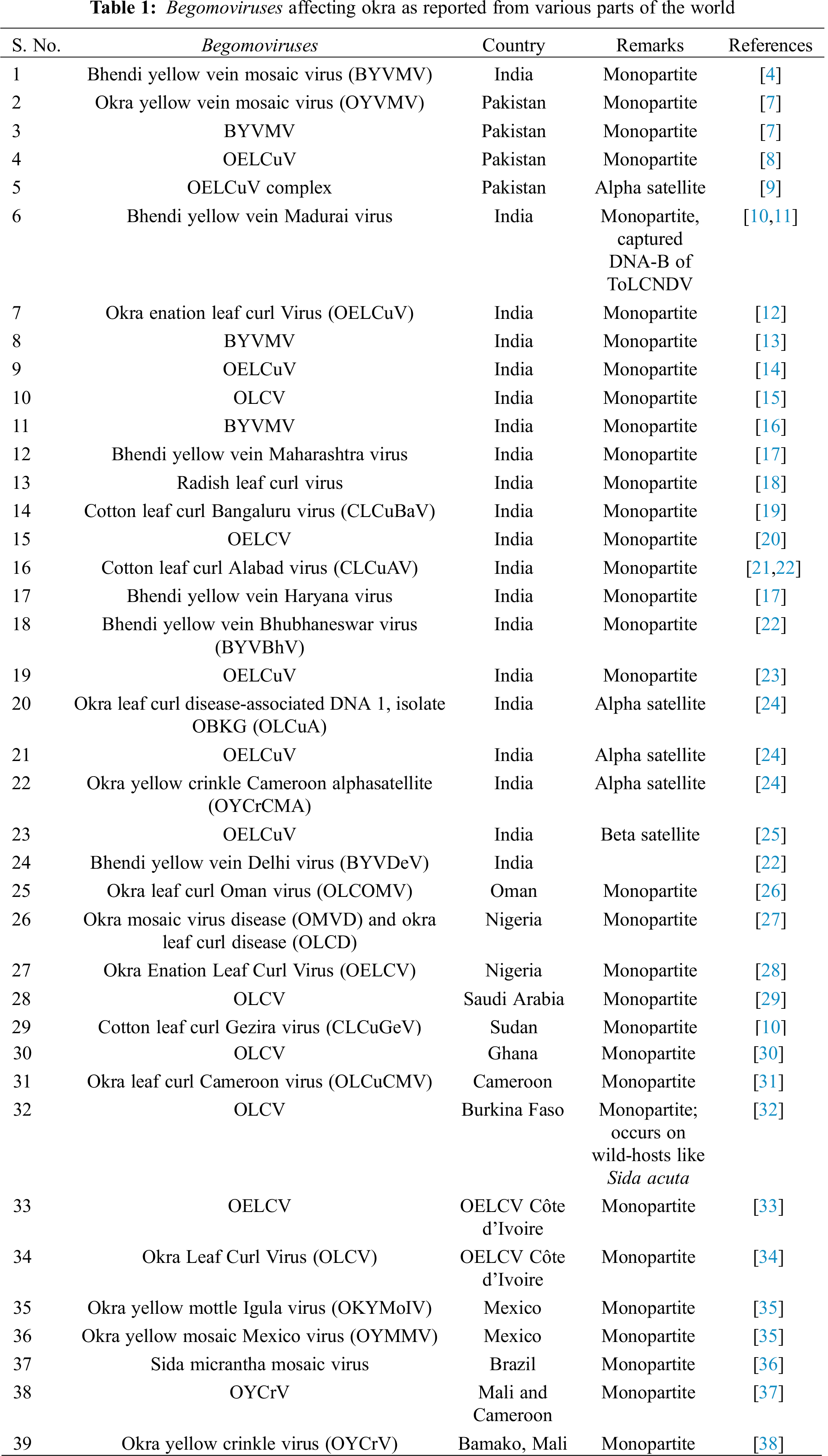
The genomes of begomoviruses usually consist of a single component (DNA-A) which is known as monopartite. Some begomoviruses consist of two components (DNA-A and DNA-B) and are known as bipartite. The DNA-A genome component is responsible for encoding the (1) pre-coat protein, CP, (2) replication-associated protein (Rep), (3) replication enhancer protein (REn) and (4) the transcriptional activator protein (TrAP). The DNA-B genome component is encoding for proteins required for the (1) intracellular movement (BV1) and (2) transport of the viral ssDNA in the host plant (BC1). These begomoviruses belong to the family Geminiviridae. Okra yellow venin mosaic virus is a monopartite begomovirus and also contains additional ssDNA molecules known as beta satellites. These satellites depend on their helper virus for replication, movement, encapsidation, and vector transmission.
The beta-satellite plays an important role in the symptoms development on the affected okra plants. The length of OYVMV particle is about 2741 bp [6]. The begomoviruses which have been reported from various countries are listed in Tab. 1. Most of these begomoviruses are prevalent in India and Pakistan, and these viruses are believed to be originated from India. The majority of them are monopartite begomoviruses. In addition, the viruses have also infected the okra plants of African countries including Sudan, Nigeria, Cameron, and Ghana, and of South and North American countries (Tab. 1).
3 Symptomology and Transmission
Previously the virus was identified based on symptoms (Fig. 1). Firstly, OYVMD symptoms were observed in okra plantations in India [39]. The infected plants were stunted and the infected leaves had green and yellow spots. The fruits were deformed. The characteristic symptoms include the mosaic patterns of leaves, veins clearing, small fruit production, and stunted plant growth in severe cases [40]. Due to high inoculum and very low photosynthetic rates, leaves, veins and fruits became yellow, and consequently, plants showed stunting [41]. It has also been reported that the infected plants produced few leaves and fruits [42]. Furthermore, an intermingling of yellow veins encircling green tissues was also observed. Initially, the veins became yellowish, and at advanced stages, the whole leaves turned yellow [43]. In more severe cases, the entire leaves become creamy and light milk colored. The infected plants at the initial stages exhibited stunting growth. The fruits of infected plants became small but hard, and low in quality, sometimes distorted in shape and became yellowish [44].

Figure 1: Characteristic symptoms of okra yellow vein mosaic virus on the okra plants
The whitefly (Bemisia tabaci) is a natural vector of the OYVMV. It damages the okra plants by sucking the sap from the infected leaves and transmits this lethal viral disease to healthy plants. The whitefly has been reported to be the vector of the virus about 100 years ago, and it is one of the most important vectors in the tropical and subtropical areas and greenhouse farming [45]. The sowing time had significant impacts on the whitefly populations and virus transmission [46]. Betasatellites are required to induce typical disease symptoms in okra. Moreover, when OYVMV was inoculated on indicator plants such as N benthamiana and A esculentus, the plants developed mild symptoms. In addition to symptoms, various biochemical changes have also been observed. Various antioxidative enzymes were found to increase significantly in the virus inoculated into okra plants. Differential responses in various biochemical components (such as photosynthetic pigments, phenol, proline, sugar) in diseased okra plants were also observed. These biochemical changes are very important in understanding the virus pathogenesis and disease development. Numerous whiteflies are responsible for the viral transmission but Bemisia tabaci is the most effective in transmitting the virus efficiently. A minimum of three whiteflies per plant were found to be effective for the virus transmission. Under glasshouse conditions typical symptoms appeared after a minimum incubation period of 9 days. Healthy plants using the back-inoculation assays expressed similar severe symptoms when the inoculation was done after 5–15 days from germination. Moreover, the disease is also transmitted by grafting. For example, in a previous study the disease was transmitted by cleft grafting. After grafting, the yellow vein mosaic symptoms appeared within 7–9 days and in some cases within 10–12 days. However, none of the plants expressed disease symptoms when the virus was inoculated mechanically [47,48]. Under field conditions, the disease incidence was higher during the rainy season [3]. This may be because rainy periods promote lush growth of both weed and crop which are the hosts of whitefly.
4 Factors Affecting the OYVMD Development
Various biotic and abiotic factors are affecting the OYVMD incidence. These factors include crop rotations, nutrients, vectors and various environmental factors (Fig. 2). Among these factors, vectors and environmental factors are contributing a lot in the disease incidence. Furthermore, vectors and environmental factors are highly correlated in the disease incidence. High temperature in the absence of rain was highly favorable for whitefly populations buildup, and consequently there was a high disease incidence [5]. Conversely, low temperatures with high humidity and heavy rainfall are not favorable for the buildup in whitefly populations. As a result, the incidence of the OYVMD will be low. Moreover, the virus is neither sap nor seed transmissible. Besides okra, numerous crops as well as weeds such as the rail weed and goat weeds are also hosting whitefly [49]. In brief, environmental factors are playing a major role in the OYVMD development, which is described in the following section.

Figure 2: Biotic and abiotic factors affecting the OYVMV disease incidence
5 Role of Environmental Factors in Disease Development and Insect Vector
The incidence of the virus, whitefly population, and environmental factors are closely related and interdependent. High temperatures were favorable for OYVMV development without rain, and increase in the whitefly population. Low temperatures with high humidity and heavy rainfall are not appropriate for the buildup in the whitefly population [5]. A study was conducted in India to examine the effects of sowing date on the population of the virus and the associated vector. Early sowings of okra determined a low whitefly population. Moreover, the whitefly, virus incidence, relative humidity, and temperature were positively correlated to each other [49]. The effect of insecticides and sowing time of okra were also observed in the spreading and incidence of the virus [5]. The incidence was not significantly different 45–60 days’ post sowing. Furthermore, the disease incidence was low in okra plants growing in May than on those growing in June and August. The incidence of the disease and vector populations was higher in okra plants growing in April and July, compared to plants growing in October and March. As the crop aged, the virus incidence was reduced. When the infected plant were one week old, the infection level was 100%, and after seven weeks, the level of infection dropped to 31.7%. In conclusion, there was a significant positive correlation between environmental factors, disease severity, and vector populations [50].
Temperature had a significant effect on the virus incidence. High temperatures favored the disease development. Low humidity and high temperatures were also favorable to whiteflies. The whitefly population was reduced with an increase in humidity. Rainfall did not have a significant impact on the disease. Temperature had a substantial effect on the population of the insect vector. Low temperatures were not conducive to crop growth, disease incidence, and whitefly populations. Warm temperatures (March, April, and June) contributed to crop growth, and a greater disease incidence, and vector populations [3]. Because of OYVMV, there was a significant relationship between production loss and crop age. In conclusion, compared to later-stage infections, the initial conditions caused significant losses. The transmission of the virus could be controlled monitoring the vector population [51].
6 Management of OYVMV and Whitefly (Bemisia tabaci Genn.)
The White fly is the main vector which transmit the OYVMV among the different hosts, especially in okra. Hence most managements strategies for controlling the disease turns around the control of this vector [52,53]. In addition, cultural practices and development of genetic resistance also need to minimize the disease incidence. Several and advanced approaches have been used to control the virus [54]. However, most of the studies are focused on vector control, genetic resistance and cultural practices. These strategies are shown in Fig. 3.

Figure 3: Management of OYVMV through various strategies
6.1 Screening of Different Cultivars/Lines/Varieties against OYVMV
Resistant varieties make a significant contribution in the management of OYVMV. Different screening tests have been conducted previously. About twenty-four varieties of okra plants were evaluated against OYVMV under field conditions. Among these varieties, no one was found to be highly resistant. Three varieties were resistant, eight moderately susceptible, three susceptible, and ten highly susceptible [55]. Moreover, a field trial was carried out to check the resistance against OYVMV. About twenty parents and fifty-one hybrids’ cultivars were screened out. The data were collected after 35, 50, and 65-day intervals. Eleven hybrids and one parent cultivar were highly resistant, seven showed moderate resistance, and others were susceptible or highly susceptible to OYVMV [56]. In other studies, twelve okra lines were screened out under natural conditions. The lines, Okra 285 and Ok-292, exhibited resistance against OYVMV. Four lines Ok-317, OK-316, Ok-315, and OK-308, were found tolerant to the disease. Pusa, OK-307, OK-311, Ok-312, OK-310, and Ok-314 were highly susceptible [57]. The resistant varieties were developed against OYVMV through genetic techniques [58].
Okra yellow vein mosaic virus (OYVMV) and whitefly were controlled by changing the sowing dates and applying pesticides, and intercropping okra with the pulses. Previously one spray of foratox and three sprays of methyl demeton 0.025% or phosphamidon 0.02% effectively reduced the whitefly population [59]. Moreover, spray intervals were also very useful in controlling the vector population and the OYVMV. The application of Dimethoate 0.03% after fifteen days of sowing effectively reduced the whitefly population. Three insecticides alone or along with the linseed oil were applied against whitefly and OYVMV. The soil application of Oxydemeton methyl followed by one spray of Carbofuramate effectively reduced the whitefly population, disease incidence and enhanced the yield [60]. The synthetic chemicals were applied for the efficient and effective control of pests; however, these chemicals were health hazards due to their high toxicity [61]. A study was conducted in Bangalore (India) during the Rabi and Kharif seasons to check the efficacy of pesticides, including Thiamethoxan and Imidacloprid, against whiteflies and leafhoppers. Both pesticides effectively controlled the whitefly and were non-phytotoxic to okra plants [62]. However, Imidacloprid gave the best results compared to Thiamethoxan against the vector population and disease incidence [63].
The spray of various plant extracts, i.e., gingers and datura, gave excellent results against OYVMV under field conditions [64]. Furthermore, the disease progression was very low in the treated plants in comparison to the control. The efficacies of two insecticides and six plant extracts were checked against aphid and whitefly populations in the okra field. The seed extract of neem and leaves extract of tobacco (2%) and BudduNarra (5%) considerably reduced the whitefly population in comparison to the pesticides Monocrotophos (0.05%) and Endosulfan (0.06%) [62]. The efficacy of two insecticides (Carbaryl and endosulfan) and three plant extracts (Chinese chaste tree, sacred basil, and Malabar nut) were checked against whitefly, jassid, and fruit borers by spraying them at 10, 25, and 40-day intervals after sowing. These pesticides and the plant extracts suppressed the whitefly population. The sprays of leaf extracts of Bougainvillea spectabilis and Prosopis chilensis on okra plants effectively reduced the OYVMV. These extracts reduced the infection by 81.7–83.3%, respectively [65]. Moreover, the incubation period of the virus was increased to 19 days in those plants treated with leaf extracts of Prosopis chilensis and Bougainvillea spectabilis, compared with ten days in non-treated plants. The virus was controlled by growing the resistant cultivars, applying the plant extracts and effectively rescheduling the insecticides to control the vector population. The spraying of the plant extracts at 15, 30, 45, and 60-day intervals after sowing helped to produce more yield [66]. The efficacy of the plant extracts and insecticides on the disease incidence and whitefly population were checked. Plant extract of Parthenium (10%), pesticide action 100 (0.2%) and soil application of three pesticides [i.e., Roger (0.2%), Metasystox (0.2%) and Carbofuran 3G] were used. Among them, plant extracts followed by Carbofuran 3G effectively controlled the whitefly population [67].
Development of resistance in the okra cultivars against OYVMV is not only economical and ecofriendly but also a durable approach towards OYVMD management (Fig. 4). Moreover, understanding the disease development is very important to formulate the management strategies. Identification of resistant sources in okra cultivars against OYVMV through conventional and molecular breeding leads to Genetic engineering (Fig. 4). The genetic resistance of okra plants was checked against OYVMV in susceptible and resistant parents. The dominant genes were involved in controlling the resistance against the virus [68]. However, the dominant gene effect was less significant than the additive gene effect [69]. Recently it was revealed that tolerance to OYVMV in the okra plants is dependent on duplicating the dominant genes and two complementary dominant genes in the tolerant and susceptible cultivars [70]. Gene silencing is widespread and could occur either through repression of transcription, known as transcriptional gene silencing (TGS), or through mRNA degradation, known as post-transcriptional gene silencing. Thus, RNAi has a solid potential to reduce the infection of OYVMV [71]. As the OYVMV is amenable to transcriptional and post-transcriptional gene silencing (TGS and PTGS) and the genes C2, C4 and βC1 there were different levels of PTGS of OYVMV. The agroinfiltration, polymerase chain reaction (PCR), quantitative PCR (qPCR), and bisulfite next-generation sequencing (NGS) were used to determine the involvement of OYVMV proteins on DNA methylation suppression. The viral genes were involved in TGS suppression, and C2, C4 genes from the virus were selected for further studies. Moreover, the Agroinfiltration experiments with mutant C2 and C4 partial tandem repeat (PTR) constructs of viruses validated to have the role of C2 and C4 in DNA methylation impairment [72].

Figure 4: Keys factors in disease development and management
The protoplast replication assay revealed that C4 genes were not involved in DNA replication. Further agroinfiltration studies confirmed the role of C4 in viral DNA movement [73]. Another vital step that induced resistance to OYVMV has been reported using an mRNA surveillance factor in tomato crops. So far, biotechnological methods are targeted to control helper viruses and not the potentially associated satellites, contributed to assist and help in functions of helper viruses [72]. Recently, a multiplexed clustered regulatory interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (Cas9) system was designed. A cassette of sgRNA has the potential to target viruses and also to incorporate resistance in crops. However, these approaches have been applied against other crops and still need to be explored against okra infected with OYVMV. Thus, the combination of conventional/classical breeding methods with non-conventional/biotechnological methods can be used to identify the resistance genes that can be deployed in okra, which can then impart resistance against OYVMV [74].
Plant defense activators are involved in enhancing the defense against abiotic and biotic factors. Various plant defense activators like KH2PO, citric acid, benzoic acid, and salicylic acid have been used to enhance plant defenses and as a result the disease was minimized and the vector was controlled. Salicylic acid (SA) is associated with the defense genes, and its activation protects the okra plants from the virus. Moreover, the hypersensitive response reduced the virus multiplication at the site of infection and activated the systemic acquired resistance (SAR) [75]. The SAR has a significant role in integrated pest (IPM) and disease management (IDM). The activators aforementioned induced the SAR in okra plants through activation of the plant defense mechanisms against whitefly [76]. In previous studies, the Salicylic acid (0.02%), plant extracts of Calotropis procera (Aak), Azadirachta indica (Neem), Datura stramonium (Datura), Eucalyptus globulus, and Aloe barbadensis (Aloe Vera) were effective against whitefly populations under various field conditions. In addition, salicylic acid and neem extracts were more effective against the whitefly population as compared to the other plant defense activators [77]. By growing resistant varieties, changing sowing dates, weed eradication, proper hoeing, and application of different plant activators and antioxidants can minimize the severity of OYVMV. The plant activators helped activate various types of defense chemicals such as primary and secondary metabolites, alkaloids, and phenolic compounds; thus, plants become resistant to whitefly [78].
6.6 Impact of Biochemical Factors on the Development of OYVMD
Compared to the healthy plants, the amount of nitrogen and sugar in the OYVMV-infected Okra lines were very high. Viral infection increases the sugar and nitrogen levels in okra plants [79]. The minerals and nutrients contribute to the host plant’s growth and production under viral infection [80]. The decreasing potassium concentration on the leaf surface interferes with the photosynthetic rate because it plays a vital role in the opening and closing of Stomata [81]. OYVMV reduced the chemical elements such as phosphorus, potassium, sugar, and chlorophyll; meanwhile, the nitrogen, proteins, total phenol, non-reducing sugar, and total sugar were increased. The phenol, polyphenol oxides, and peroxidase activities played a role in developing resistance in resistant okra cultivars. Quinin, made up of large quantities of phenols and their oxidant products, such as peroxide and polyphenol oxides can reduce viral transmission, eventually leading to the protection of resistant crops from the disease [81]. The application of proper mineral nutrients (Ca, Zn and N, P, K) effectively managed OYVMV [1]. The virus effectively reduced carotenoid and protein levels by 62% and 64%, respectively, compared to healthy leaves. Starch levels were highly accumulated in diseased plants. The excess usage of nitrogen increases plant growth and is directly related to the buildup in whitefly populations. The virus spreads rapidly in the early stages of the crop. Hence, the intensity of the final virus infection is dependent on the initial phase of the disease [82].
7 Conclusions and Future Prospects
In conclusion, Okra yellow vein mosaic disease is one of the most severe diseases of okra. The disease development and population buildup of the vector (Whitefly) is favored by warm and humid conditions for proliferation. It is very hard to change the environmental conditions. However, changing sowing dates and crop rotations can help okra to be free from whiteflies and OYVMD. However, the vector is polyphagous and can survive in several crops. Therefore, it cannot only be managed by insecticides but also different management practices should be used in combination. The development of genetically resistance cultivars which is an ecofriendly and economical approach is one of the important strategies against the OYMVD. However, no resistant cultivars are available against the OYVMD.
More studies should be carried out on the variability of all the accessions of okra in response to OYVMD. Attempts, should also be taken toward the gene pyramiding by incorporating genes from other hosts to the susceptible lines of okra. However, gene pyramiding become useless and time consuming because of the sterility problem. More molecular work is required to gain information about the interaction between the vector and OYVMD. Furthermore, ecofriendly novel chemicals should be developed to minimize the disease incidence. Genome editing has broad applications in the whiteflies’ studies. Therefore, modifications in the genome of whiteflies will be an effort to reduce the transmission of the virus in the environment.
Compliance with Ethical Standards: The authors declare that the review is in compliance with ethical standards of the journal.
Research Involving Human Participants and/or Animals: The authors declare that the manuscript does not contain research involving Human Participants and/or Animals.
Author’s Contribution: All authors have equally contributed in gathering literature and in writing and formatting the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Bilqees, I., Iftikhar, Y., Mubeen, M., Shakeel, Q., Sajid, A. et al. (2020). Use of nutrients and plant extract to manage okra yellow vein mosaic disease (OYVMD) in Sargodha, Punjab, Pakistan. Pakistan Journal of Agricultural Research, 33(4), 754–758. DOI 10.17582/journal.pjar/2020/33.4.754.758. [Google Scholar] [CrossRef]
2. FAO (2019). FAOSTAT database. http://faostat.fao.org. [Google Scholar]
3. Mubeen, M., Iftikhar, Y., Ullah, M. I., Shakeel, Q., Aatif, M. et al. (2017). Incidence of okra yellow vein mosaic disease in relation to insect vector and environmental factors. Environment & Ecology, 35(3C), 2215–2220. [Google Scholar]
4. Kulkarni, G. S. (1924). Mosaic and other related diseases of crops in the Bombay Presidency, pp. 6–12. Poona Agriculture College Magazine, Pune. [Google Scholar]
5. Mohanta, R., Nath, R., Jena, N. K., Mishra, A. (2020). Effect of different dates of sowing and weather parameters on vector whitefly (Bemisia tabaci gennadius) and incidence of yellow vein mosaic disease in okra (Abelmoschus esculentus L. Moench). Journal of Entomology and Zoology Studies, 8(2), 1774–1784. [Google Scholar]
6. Jeyaseelan, T. C., Jeyaseelan, E. C., De Costa, D. M., Shaw, M. W. (2018). Molecular characterization and phylogenetic analysis of betasatellite molecules associated with okra yellow vein mosaic disease in Sri Lanka. Tropical Plant Pathology, 43(5), 468–472. DOI 10.1007/s40858-018-0225-1. [Google Scholar] [CrossRef]
7. Zhou, X., Liu, Y., Robinson, D. J., Harrison, B. D. (1998). Four variants among Pakistani isolates of cotton leaf curl virus and their affinities to DNA-a of geminivirus isolates from okra. Journal of General Virology, 79, 915–923. DOI 10.1099/0022-1317-79-4-915. [Google Scholar] [CrossRef]
8. Hameed, U., Rehman, M. Z., Herrmann, H. W., Haider, M. S., Brown, J. K. (2014). First report of okra enation leaf curl virus and associated cotton leaf curl multan betasatellite and cotton leaf curl multan alphasatellite infecting cotton in Pakistan: A new member of the cotton leaf curl disease complex. Plant Disease, 98, 1447. DOI 10.1094/PDIS-04-14-0345-PDN. [Google Scholar] [CrossRef]
9. Serfraz, S., Amin, I., Akhtar, K. P., Mansoor, S. (2014). Recombination among begomoviruses on malvaceous plants leads to the evolution of okra enation leaf curl virus in Pakistan. Journal of Phytopathology, 163, 764–776. DOI 10.1111/jph.12373. [Google Scholar] [CrossRef]
10. Venkataravanappa, V., Prasanna, H. C., Reddy, C. N. L., Reddy, M. K. (2014). Evidence for two predominant viral lineages, recombination and subpopulation structure in begomoviruses associated with yellow vein mosaic disease of okra in India. Plant Pathology, 64, 508–518. DOI 10.1111/ppa.12292. [Google Scholar] [CrossRef]
11. Venkataravanappa, V., Reddy, C. N. L., Jalali, S., Reddy, M. K. (2015b). Association of tomato leaf curl New Delhi virus dNA-b with bhendi yellow vein mosaic virus in okra showing yellow vein mosaic disease symptoms. Acta Virology, 59, 125–139. DOI 10.4149/av. [Google Scholar] [CrossRef]
12. Singh, S. J. (1996). Assessment of losses in okra due to enation leaf curl virus. Indian Journal Virology, 12, 51–53. [Google Scholar]
13. Jose, J., Usha, R. (2003). Bhendi yellow mosaic disease in India is caused by association of a DNA beta satellite with a begomovirus. Journal of Virology, 30(5), 2310–2317. DOI 10.1006/viro.2002.1768. [Google Scholar] [CrossRef]
14. Venkataravanappa, V., Reddy, C. N. L., Jalali, S., Briddon, R. W., Reddy, M. K. (2015a). Molecular identification and biological characterization of a begomovirus associated with okra enation leaf curl disease in India. European Journal of Plant Pathology, 141, 217–235. DOI 10.1007/s10658-014-0463-0. [Google Scholar] [CrossRef]
15. Sayed, S. S., Rana, D., Krishna, G., Reddy, P. S., Bhattacharya, P. S. (2014). Association of begomovirus with okra (Abelmoschus esculentus L.) leaf curl virus disease in southern India. Scholarena Journal of Biotechnology, 1, 102. DOI 10.18875/23756713.1.102. [Google Scholar] [CrossRef]
16. Venkataravanappa, V., Reddy, M. K. (2013). Begomovirus characterization, and development of phenotypic and dna-based diagnostics for screening of okra genotype resistance against bhendi yellow vein mosaic virus. 3 Biotech, 3, 461–470. DOI 10.1007/s13205-012-0107-z. [Google Scholar] [CrossRef]
17. Brown, J. K., Fauquet, C. M., Briddon, R. W., Zerbini, M., Moriones, E. et al. (2012). Geminiviridae. In: King, A. M. Q., Adams, M. J., Carstens, E. B. and Lefkowitz, E. J. (Eds.), Virus taxonomy-ninth report of the international committee on taxonomy of viruses, pp. 351–373. San Diego, CA: Elsevier Academic Press. [Google Scholar]
18. Kumar, J., Kumar, A., Singh, S. P., Roy, J. K., Lalit, A. (2012). First report of radish leaf curl virus infecting okra in India. New Disease Reports, 7, 13–24. DOI 10.5197/j.2044-0588.2012.025.009. [Google Scholar] [CrossRef]
19. Venkataravanappa, V., Reddy, C. N. L., Devaraju, A., Jalali, S., Reddy, M. K. (2013a). Association of a recombinant cotton leaf curl bangalore virus with yellow vein and leaf curl disease of okra in India. Indian Journal of Virology, 24, 188–198. DOI 10.1007/s13337-013-0141-4. [Google Scholar] [CrossRef]
20. Sanwal, S. K., Singh, M., Singh, B., Naik, P. S. (2014). Resistance to yellow vein mosaic virus and okra enation leaf curl virus: Challenges and future strategies. Current Science, 106, 1470–1471. DOI 10.18520/CS/V106/I11/1470-1471. [Google Scholar] [CrossRef]
21. Venkataravanappa, V., Reddy, C. N. L., Swarnalatha, P., Devaraju, A., Jalali, S. et al. (2012b). Molecular evidence for association of cotton leaf curl alabad virus with yellow vein mosaic disease of okra in north India. Archives of Phytopathology and Plant Protection, 45, 2095–2113. DOI 10.1080/03235408.2012.721682. [Google Scholar] [CrossRef]
22. Venkataravanappa, V., Reddy, C. N. L., Jalali, S., Reddy, M. K. (2013b). Molecular characterization of a new species of begomovirus associated with yellow vein mosaic of bhendi (okra) in bhubhaneswar, India. European Journal of Plant Pathology, 136, 811–822. DOI 10.1007/s10658-013-0209-4. [Google Scholar] [CrossRef]
23. Singh, B., Sanwal, S., Venkataravanappa, V., Halder, J. (2013). Breeding strategies for biotic stresses of okra: Prospects and potential. Abstract Book of National Symposium on Abiotic and Biotic Stress Management in Vegetable Crops, pp. 32–33Varanasi. [Google Scholar]
24. Chandran, S. A., Packialakshmi, R. M., Subhalakshmi, K., Prakash, C., Poovannan, K. et al. (2013). First report of an alphasatellite associated with okra enation leaf curl virus. Virus Genes, 46, 585–587. DOI 10.1007/s11262-013-0898-y. [Google Scholar] [CrossRef]
25. Venkataravanappa, V., Reddy, C. N. L., Swaranalatha, P., Jalali, S., Briddon, R. W. et al. (2011). Diversity and phylogeography of begomovirus-associated beta satellites of okra in India. Virology Journal, 8, 555. DOI 10.1186/1743-422X-8-555. [Google Scholar] [CrossRef]
26. Akhtar, S., Khan, A. J., Singh, A. S., Briddon, R. W. (2014). Identification of a disease complex involving a novel monopartite begomovirus with beta and alphasatellites with okra leaf curl disease in Oman. Archives of Virology, 159, 1199–1205. DOI 10.1007/s00705-013-1926-x. [Google Scholar] [CrossRef]
27. Sergius, U. O., Esther, D. U. (2014). Screening of abelmoschus esculentus and abelmoschus callei cultivars for resistance against okra leaf curl and okra mosaic viral diseases, under field conditions in south eastern Nigeria. African Journal of Biotechnology, 13, 4419–4429. DOI 10.5897/AJB2014.13686. [Google Scholar] [CrossRef]
28. Atiri, G. I. (1984). The occurrence and importance of okra mosaic virus in Nigerian weeds. Annals of Applied Biology, 104, 261–265. DOI 10.1111/j.1744-7348.1995.tb05008. [Google Scholar] [CrossRef]
29. Ghanem, G. A. M. (2003). Okra leaf curl virus: A monopartite begomovirus infecting okra crop in Saudi Arabia. Arab Journal of Biotechnology, 6, 139–152. [Google Scholar]
30. Swanson, M. M., Harrison, B. D. (1993). Serological relationships and epitope profiles of isolates of okra leaf curl geminivirus from Africa and the Middle East. Biochimie, 75, 707–711. DOI 10.1016/0300-9084(93)90101-W. [Google Scholar] [CrossRef]
31. Leke, W. N., Sattar, M. N., Ngane, E. B., Ngeve, J. M., Kvarnheden, A. et al. (2013). Molecular characterisation of begomoviruses and DNA satellites associated with okra leaf curl disease in Cameroon. Virus Research, 174, 116–125. DOI 10.1016/j.virusres.2013.03.010. [Google Scholar] [CrossRef]
32. Konate, G., Barro, N., Fargette, D., Swanson, M. M., Harrison, B. D. (1995). Occurrence of whitefly transmitted geminiviruses in crops in Burkina Faso and their serological detection and differentiation. Annals of Applied Biology, 126, 121–129. DOI 10.1111/j.1744-7348.1995.tb05008. [Google Scholar] [CrossRef]
33. N’guessant, K. P., Fargettet, D., Fauquet, C., Thouvenel, J. C. (1992). Aspects of the epidemiology of okra leaf curl virus in Côte d´Ivoire. Tropical Pest Management, 38, 122–126. DOI 10.1080/09670879209371668. [Google Scholar] [CrossRef]
34. N’guessant, K. P. (1991). Occurrence and spread of okra leaf curl virus (OLCD) disease in Côte d´Ivoire. African Journal of Agronomy, 13, 35–43. DOI 10.4314/aga.v13i1.1625. [Google Scholar] [CrossRef]
35. Hernandez-Zepeda, C., Isakeit, T., Scott, A. J., Brown, J. K. (2010). First report of okra yellow mosaic Mexico virus in okra in the United States. Plant Disease, 94, 924. DOI 10.1094/PDIS-94-7-0924B. [Google Scholar] [CrossRef]
36. Fauquet, C. M., Briddon, R. W., Brown, J. K., Moriones, E., Stanley, J. et al. (2008). Geminivirus strain demarcation and nomenclature. Archives of Virology, 153, 783–821. DOI 10.1007/s00705-008-0037-6. [Google Scholar] [CrossRef]
37. Kon, T., Rojas, M. R., Abdourhamane, I. K., Gilbertson, R. L. (2009). Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. Journal of General Virology, 90, 1001–1013. DOI 10.1099/vir.0.008102-0. [Google Scholar] [CrossRef]
38. Shih, S. L., Green, S. K., Tsai, W. S., Lee, L. M., Levasseur, V. (2007). First report of a distinct begomovirus associated with okra yellow crinkle disease in Mali. Plant Pathology, 56, 718. DOI 10.1111/j.1365-3059.2007.01599.x. [Google Scholar] [CrossRef]
39. Sastry, K. S., Singh, S. J. (1974). Effect of yellow vein mosaic virus infection on growth and yield of okra crop (India). Indian Phytopathology, 27(3), 294–297. [Google Scholar]
40. Naresh, M., Khan, Z. A., Kumar, R., Kale, S. P., Patil, V. M. et al. (2019). Occurrence and variability of begomoviruses associated with bhendi yellow vein mosaic and okra enation leaf curl diseases in south-western India. Virus Disease, 30(4), 511–525. DOI 10.1007/s13337-019-00551-4. [Google Scholar] [CrossRef]
41. Rashid, M. H., Yasmin, L., Kibria, M. G., Malik, A. K., Hossain, S. M. (2002). Screening of okra germplasm for resistance to yellow vein mosaic virus under field conditions. Plant Pathology Journal, 1, 61–62. DOI 10.3923/ppj.2002.61.62. [Google Scholar] [CrossRef]
42. Benchasri, S. (2012). Screening for yellow vein mosaic virus resistance and yield loss of okra under field conditions in southern Thailand. Journal of Animal and Plant Sciences, 3(12), 1676–1686. [Google Scholar]
43. Bag, M. K., Roy, K. K., Dutta, M. (2012). Evaluation of wild okra germplasm against yellow vein mosaic disease for their value-added utilization to sustain livelihood through agriculture. National Bureau of Plant Genetic Resources. [Google Scholar]
44. Jamir, I., Mandal, A. K., Devi, A. P., Bhattacharjee, T., Maurya, P. K. et al. (2020). Screening of genotypes against viral diseases and assessment of yield loss due to yellow vein mosaic virus in okra grown in the eastern part of India. Indian Phytopathology, 73(1), 125–133. DOI 10.1007/s42360-019-00183-0. [Google Scholar] [CrossRef]
45. Sayed, M. K. A., Hossain, M. B., Islam, M. R., Shahriar, S. A., Shathi, M. S. A. (2018). Selection of resistant varieties of okra to yellow vein clearing mosaic virus and its management. Asian Journal of Advances in Agricultural Research, 5(3), 1–9. DOI 10.9734/AJAAR. [Google Scholar] [CrossRef]
46. Liu, T. X. (2000). Population dynamics of bemisia argentifolii (Homoptera: Aleyrodidae) on spring collard and relationship to yield in the lower Rio Grande valley of texas. Journal of Economic Entomology, 93(3), 750–756. DOI 10.1603/0022-0493-93.3.750. [Google Scholar] [CrossRef]
47. Singh, A. K., Yadav, B. K., Krishna, R., Kumar, R. V., Mishra, G. P. et al. (2021). Bhendi yellow vein mosaic virus and bhendi yellow vein mosaic betasatellite cause enation leaf curl disease and alter host phytochemical contents in okra. Plant Disease. DOI 10.1094/PDIS-12-20-2655-RE. [Google Scholar] [CrossRef]
48. Li, K., Wu, G., Li, M., Ma, M., Du, J. et al. (2018). Transcriptome analysis of nicotiana benthamiana infected by tobacco curly shoot virus. Virology Journal, 15(1), 1–15. DOI 10.1186/s12985-018-1044-1. [Google Scholar] [CrossRef]
49. Kumar, D., Sharma, J. K., Meena, S. C., Parewa, H. P., Ratnoo, S. D. (2018). Prevalence of yellow vein mosaic virus of okra [Abelmoschus esculentus (L.) moench] in sheoganj, transitional plain of luni basin (ZoneIIb) of rajasthan. Journal of Entomology and Zoology Studies, 6(4), 1383–1385. [Google Scholar]
50. Mazumder, N., Borthakur, U., Choudhury, D. (1996). Incidence of yellow vein mosaic virus of bhindi (Abelmoschus esculentus (L.) Moench) in relation to cultivar and vector population under different sowing dates. Indian Journal of Virology, 12(2), 137–14 l. [Google Scholar]
51. Ali, S., Khan, M. A., Habib, A., Rasheed, S., Iftikhar, Y. (2005). Correlation of environmental conditions with okra yellow vein mosaic virus and bemisia tabaci population density. International Journal of Agriculture and Biology, 7, 142–144. DOI 1560–8530/2005/07–1–142–144. [Google Scholar]
52. Denholm, I., Cahill, M., Dennehy, T. J., Horowitz, A. R. (1998). Challenges with managing insecticide resistance in agricultural pests, exemplisfied by the whitefly bemisia tabaci. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 353(1376), 1757–1767. DOI 10.1098/rstb.1998.0328. [Google Scholar] [CrossRef]
53. Sani, I., Ismail, S. I., Abdullah, S., Jalinas, J., Jamian, S. et al. (2020). A review of the biology and control of whitefly, bemisia tabaci (Hemiptera: Aleyrodidaewith special reference to biological control using entomopathogenic fungi. Insects, 11(9), 619. DOI 10.3390/insects11090619. [Google Scholar] [CrossRef]
54. Kumar, A., Verma, R. B., Kumar, R., Sinha, S. K., Kumar, R. (2017). Yellow vein mosaic disease of okra: A recent management technique. International Journal of Plant and Soil Science, 19(4), 1–8. DOI 10.9734/IJPSS. [Google Scholar] [CrossRef]
55. Kumari, M., Dubey, A. K., Rao, S. G., Solankey, S. S. (2018). Screening of diverse okra genotypes for yellow vein mosaic virus tolerance under field condition. Journal of Pharmacognosy and Phytochemistry, 7(5), 176–179. [Google Scholar]
56. Dhankhar, S. K., Dhankhar, B. S., Saharan, B. S. (1996). Screening of okra genotypes for resistance to yellow vein mosaic disease. Annals of Biology, 12(1), 90–92. [Google Scholar]
57. Deo, C. J., Singh, K. P., Panda, P. K. (2000). Screening of okra parental lines and their FTs for resistance against yellow vein mosaic virus. Journal of Vegetable Science, 27(1), 78–79. [Google Scholar]
58. Bharathkumar, M. V., Dhankhar, S. K., Dahiya, M. S., Srikanth, M. (2019). Genetic architecture of resistance to yellow vein mosaic virus disease in advance lines of okra (Abelmoschus esculentus). Indian Journal of Agricultural Sciences, 89(4), 640–645. [Google Scholar]
59. Munir, A., Ali, S., Zeshan, M. A., Ghani, M. U., Khan, A. A. (2020). Evaluation of organic amendments and insecticides against okra yellow vein mosaic virus and its vector. Pakistan Journal of Agriculture, Agricultural Engineering and Veterinary Sciences, 36(1), 13–20. DOI 10.47432/pjaaevs.2020.36.1.3. [Google Scholar] [CrossRef]
60. Bhagat, A. P., Yadav, B. P., Prasad, Y. (2001). Rate of dissemination of okra yellow vein mosaic virus in three cultivars of okra. Indian Phytopathology, 54(4), 488–489. [Google Scholar]
61. Varma, J., Dubey, N. K. (2001). Efficacy of essential oils of caesulia axillaris and mentha arvensis against some storage pests causing biodeterioration of food commodities. International Journal of Food Microbiology, 68(3), 207–210. DOI 10.1016/S0168-1605(01)00506-2. [Google Scholar] [CrossRef]
62. Kumar, M., Singh, R. (2002). Potential of pongamia glabra vent as an insecticide of plant origin. Biological Agriculture & Horticulture, 20(1), 29–50. DOI 10.1080/01448765.2002.9754947. [Google Scholar] [CrossRef]
63. Ali, M. I., Khan, M. A., Rashid, A., Haq, M. I., Javed, M. T. et al. (2012). Epidemiology of okra yellow vein mosaic virus (OYVMV) and its management through tracer, mycotal and imidacloprid. American Journal of Plant Sciences, 3, 1741–1745. DOI 10.4236/ajps.2012.312212. [Google Scholar] [CrossRef]
64. Chaudhary, K. A., Biswas, B., Sana, N. K. (1992). Inhibition of bhendi (okra) yellow vein mosaic virus (BYVMV) by different plant extracts. Journal of Mycopathological Research, 30, 97–102. [Google Scholar]
65. Pun, K. B., Doraiswamy, S. (1999). Effect of age of okra plants on susceptibility to okra yellow vein mosaic virus. Indian Journal of Virology, 15, 57–58. [Google Scholar]
66. Debnath, S., Nath, P. S. (2003). Performance of okra varieties in relation to yield and tolerance to YVMV. Annals of Plant Protection Sciences, 11(2), 400–401. [Google Scholar]
67. Ahmed, Z., Patil, M. S. (2004). Incidence of yellow vein mosaic virus on different okra cultivars in karnataka. Karnataka Journal of Agricultural Sciences, 17, 615–616. [Google Scholar]
68. Wasala, S., Senevirathne, S. I., Senanayake, J. B., Navoditha, A. (2019). Genetic analysis of okra yellow vein mosaic virus disease resistance in wild relative of okra Abelmoschus angulosus wall. ex wight & Arn. Plant Genetic Resources, 17(4), 346–351. DOI 10.1017/S1479262119000078. [Google Scholar] [CrossRef]
69. Ali, M., Hossain, M. Z., Sarker, N. C. (2000). Inheritance of yellow vein mosaic virus (YVMV) tolerance in a cultivar of okra (Abelmoschus esculentus). Euphytica, 111, 205–209. DOI 10.1023/A:1003855406250. [Google Scholar]
70. Emmanuel, C. J., Manohara, S., Shaw, M. W. (2020). Molecular characterization of begomovirus–betasatellite–alphasatellite complex associated with okra enation leaf curl disease in Northern Sri Lanka. 3 Biotech, 10(12), 1–13. DOI 10.1007/s13205-020-02502-z. [Google Scholar] [CrossRef]
71. Tharmila, C. J., Emmanuel, C. J., Devika, M. D. C., Michael, W. S. (2020). Detection and absolute quantification of betasatellites associated with okra yellow vein mosaic disease by qPCR. Journal of Virological Methods, 276, 113789. DOI 10.1016/j.jviromet.2019.113789. [Google Scholar] [CrossRef]
72. Venkataravanappa, V., Reddy, C. L., Jalali, S., Reddy, M. K. (2012). Molecular characterization of distinct bipartite begomovirus infecting bhendi (Abelmoschus esculentus L.) in India. Virus Genes, 44(3), 522–535. DOI 10.1007/s11262-012-0732-y. [Google Scholar] [CrossRef]
73. Venkataravanappa, V., Reddy, C. L., Saha, S., Reddy, M. K. (2018). Recombinant tomato leaf curl New Delhi virus is associated with yellow vein mosaic disease of okra in India. Physiological and Molecular Plant Pathology, 104, 108–118. DOI 10.1016/j.pmpp.2018.10.004. [Google Scholar] [CrossRef]
74. Mishra, G. P., Singh, B., Seth, T., Singh, A. K., Halder, J. et al. (2017). Biotechnological advancements and begomovirus management in okra (Abelmoschus esculentus L.): Status and Perspectives. Frontier in Plant Science, 8, 360. DOI 10.3389/fpls.2017.00360. [Google Scholar] [CrossRef]
75. Ryals, J. A., Neucnsch Wander, U. H., Willits, M. G., Molina, A., Steincr, H. Y. et al. (1996). Systemic acquired resistance. Plant Cell, 8, 1809–1819. DOI 10.2307/3870231. [Google Scholar] [CrossRef]
76. Khan, M. A., Afzaal, M., Nasir, M. A. (2003). Evaluation of furnace oil and neem-based products to manage bemisia tabaci and leaf curl virus on cotton. Pakistan Journal of Botany, 35, 983–986. [Google Scholar]
77. Kooner, B. S., Cheema, H. K. (2007). Screening of mungbean germplasm against whitefly (Bemisia tabaci genn) and mungbean yellow mosaic virus. Acta Horticulturae, 752, 307–310. DOI 10.17660/ActaHortic.2007.752.52. [Google Scholar] [CrossRef]
78. Appiah, A. S., Amiteye, S., Boateng, F., Amoatey, H. M. (2020). Evaluation of okra (Abelmoschus esculentus L. Moench) cultivars for resistance to okra mosaic virus and okra yellow vein mosaic virus. Australasian Plant Pathology, 49(5), 541–550. DOI 10.1007/s13313-020-00727-3. [Google Scholar] [CrossRef]
79. Kalaichelvi, K., Prabhaharan, J., Rao, D. S. (2020). Physiology of bhendi yellow vein mosaic disease infected plant. International Journal of Current Microbiology and Applied Sciences, 9(9), 2284–2288. DOI 10.20546/ijcmas. [Google Scholar] [CrossRef]
80. Marschner, H. (1995). Mineral nutrition of higher plants, 2nd edition. London: Academic Press. [Google Scholar]
81. Mahajan, M., Ghai, T. R., Chawla, N. (2004). Phenolic profile and peroxidase activity in relation to yellow vein mosaic virus reaction in okra (Abelmoschus esculentus (L.) Moench). PAU Agricultural Research Journal, 41, 345–351. [Google Scholar]
82. Palanisamy, P., Michael, P. I., Krishna, M. (2009). Physiological response of yellow vein mosaic virus-infected bhendi (Abelmoschus esculentus) leaves. Physiological and Molecular Plant Pathology, 74, 129–133. DOI 10.1016/j.pmpp.2009.10.003. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |