

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.016227
ARTICLE
Azospirillum brasilense and Saccharomyces cerevisiae as Alternative for Decrease the Effect of Salinity Stress in Tomato (Lycopersicon esculentum) Growth
1Botany Department, Faculty of Agriculture, Menoufia University, Shebin El-Kom, Po 32511, Egypt
2Instituto de Ciencias Agrícolas, Universidad Autónoma de Baja California, Carretera a Delta s/n Ejido Nuevo León, Baja California, CP 21705, México
3Department of Soil Science, Faculty of Agriculture, Menoufia University, Shebin El-Kom, Po 32511, Egypt
*Corresponding Author: Daniel Gonzalez-Mendoza. Email: danielg@uabc.edu.mx
Received: 18 February 2021; Accepted: 17 May 2021
Abstract: The salinity stress is one of the most relevant abiotic stresses that affects the agricultural production. The present study was performed to study the improvement of the salt tolerance of tomato plants which is known for their susceptibility to salt stress. The present study aimed to assess to what extent strain Azospirillum brasilense (N040) and Saccharomyces cerevisiae improve the salt tolerance to tomato plants treated with different salt concentration. The inoculant strain A. brasilense (N040) was previously adapted to survive up to 7% NaCl in the basal media. A greenhouse experiment was conducted to evaluate the effect of this inoculation on growth parameter such as: plant height, root length, fresh and dry weight, fruits fresh weight, chlorophyll content, proline and total soluble sugar in tomato plants under salt stress condition. The results revealed that co-inoculation of Azospirillum brasilense (N040) and Saccharomyces cerevisiae significantly increased the level of proline (8.63 mg/g FW) and total soluble sugar (120 mg/g FW) of leaves under salinity condition comparing to non-inoculated plants (2.3 mg/g FW and 70 mg/g FW, respectively). Plants co-inoculated with adapted strain of A. brasilense and S. cerevisiae showed the highest significant (p < 0.01) increase in fruit yield (1166.6 g/plant), plant high (115 cm) and roots length (52.6) compared whit un-inoculated control plants (42 g/pant, 43.3 cm and 29.6 cm, respectively). In contrast, Na+ ion content was significantly decreased in the leaves of salt stressed plants treated with the A. brasilense (N040) and S. cerevisiae. Finally, the results showed that dual benefits provided by both A. brasilense (N040) and S. cerevisiae can provide a major way to improve tomato yields in saline soils.
Keywords: Salinity stress; tomato; proline; exopolysaccharides; microorganism
Salinity represents the major challenge
The application of Azospirillum is an alternative approach, eco-friendly that contribute to the plant response regulation of salt stress increasing agriculture productivity [10]. The growth stimulation of tomato, rapeseed, pepper and corn plants under salt stress by inoculation with PGPR has been reported [9,11]. Tomato (Solanum lycopersicum) is one of the best crops to study salt tolerance in the dicotyledonous crops due to its well-known genetics [12]. High salinity adversely affects seed germination and inhibit growth and fruit development in tomato. Therefore, the alleviation of adverse effects of salinity on tomato plants will significantly impact the enhancement of vegetable productivity, particularly in arid and semi-arid regions.
This study extends our understanding of microbially mediated systemic tolerance in plants and motivates us to use microbial inoculants for the reclamation of salt lands. Therefore, this work was conducted to adapt A. brasilense to high salt concentrations and investigate PGPR properties of adapted strain under different salinity levels. It also evaluated the ability of wild type and adapted strain of A. brasilense in single inoculants and combined with Saccharomyces cerevisiae to alleviate harmful effect of salinity, and their role in enhancing tomato growth and yield under salt stress in a greenhouse experiment. We propose a positive outcome of tomato plants interaction with A. brasilense and S. cerevisiae during salt stress and represent a promising way to improve tolerance of this plant’s productivity in saline soils.
2.1 Microorganism Strains Used in the Experiment
Azospirillum brasilense NO40 was obtained from Microbiology Dep., Soil, Water and Environmental Research Institute, Agriculture Research Center. Saccharomyces cerevisiae Y-389 were kindly received from the National center for Agriculture Utilization Research, USDA, Illinois, USA.
2.2 Sequential Adaptation Approach
For the preparation of bacterial inoculum, the culture was grown up to a mid-exponential growth phase in nutrient broth and then, the grown bacterial culture was centrifuged, the cell pellets were washed and resuspended in water and adjusted to a cell density of 108 CFU mL−1. The adaptation of A. brasilense NO40 for tolerance to high salt concentration was conducted through the sequential transfers approach with gradually increasing salt concentration in the culture media [13]. Overnight bacterial culture was inoculated in liquid media supplemented with 2% NaCl and incubated at 30 ± 2°C and 130 rpm for three days. After incubation, the grown culture was inoculated with a cell density of 107 CFU mL−1 into fresh liquid media containing 2% NaCl under the same growth conditions, followed by three such transfers. Part of the earlier bacterial culture was inoculated in fresh media increasing NaCl concentration to 3%, followed by three such sequential transfers in fresh media under similar growth conditions. The sequential transfer strategy was used for bacterial adaptation to 4%, 5%, 6% and 7% NaCl by the same approach. The bacterial growth rate for each NaCl concentration was quantified in triplicates by measuring the optical density (OD) at 600 nm. Finally, the adapted culture was re-isolated on agar plates, and then a single colony was picked up and cultivated in liquid media to store in 30% glycerol at −20°C. To confirm adapted bacteria’s stability to high salt concentration, an aliquot of frozen glycerol stock was taken after 30, 45, 60 and 75 days of preservation, and grown in broth media with zero additional salt. The bacterial culture was transferred once into the same media, and then transferred into a growth medium containing NaCl (7%).
2.3 Acetylene Reduction Assay (ARA) under Salinity Condition
The quantitative estimation of nitrogenase enzyme activity was conducted by gas chromatography (GC) [9]. ARA was determined by Hewlett Packard chromatograph (Hp 6890 GC) fitted with a dual flame detector and a 150 cm × 0.4 cm diameter stainless steel column fitted with a Propa XR 100–120 mesh. The adaptive bacteria inoculated the tubes (7 mL capacity) containing 3 mL nitrogen-free broth media amended with different concentrations of NaCl. The tubes were stoppered with cotton plugs, and then exchanged with rubber stoppers after observing visible bacterial growth.
The air (gas phase) in the headspace was replaced with acetylene 10% (v/v), and after incubation in the dark at 28°C, the ethylene production was measured, and ARA was expressed as μmoL of C2H4/mg protein/h.
2.4 Quantification of IAA Production under Salinity Condition
Quantitative estimation of IAA production by the adaptive tolerance bacteria was determined in the presence of 0%, 1%, 3%, 5% and 7% NaCl concentration in the growth media. 50 mL broth media supplemented with L-Tryptophan (100 mg/L) was inoculated with 1 mL overnight bacterial culture and incubated at 30 ± 2°C for 3 days 150 rpm under dark condition. After incubation time, the cultures were centrifuged at 12000 rpm for 10 min at 4°C. IAA was measured through mixing1 mL of culture supernatant with 2 mL of Salkowski reagent (2% 0.5 M FeCl3 in 35% perchloric acid); and incubated the mixture in the dark for 30 min at room temperature for pink color development as an indicator of IAA production. The optical density of color was spectrophotometrically measured at 530 nm, and a standard curve of authentic IAA was used for calibration of the quantity of produced IAA [14].
2.5 Production of Exopolysaccharides (EPS) by Adapted Bacteria under Salt Condition
The adapted bacteria were inoculated in 250 mL Erlenmeyer flasks containing 100 mL broth media supplemented with 0%, 1%, 3%, 5% and 7% NaCl at 28°C and 150 rpm for 3 days of incubation. After incubation, the supernatant was collected by centrifugation at 7000 rpm and 4°C for 15 min to remove the bacterial cells. Crude EPS was prepared by mixing the supernatant with two volumes of absolute ethanol at 4°C for 2 days, and then the mixture was centrifuged at 8000 rpm and 4°C for 15 min. EPS solution was prepared by re-suspending crude EPS with distilled water. The sugar content was assessed via phenol sulfuric acid scheme by mixing 200 μL of EPS solution with 200 μL of phenol solution (5%), then addition 1 mL-sulfuric acid solution. After 15 min at room temperature, the optical density of the mixture was measured at 490 nm. The results were compared to glucose standard curve to quantify the EPS concentrations [15].
Green house experiment was carried out in the faculty of agriculture, Menoufia University, Egypt; to evaluate wild-type and adapted A. brasilense inoculation on growth and yield of tomato plants under saline condition. The complete experiment had a randomized block design, where three replications for each treatment were present under controlled conditions. Inoculum preparation was conducted by cultivating of wild-type strain of A. brasilense, adapted A. brasilense and Saccharomyces cerevisiae individual in LB broth at 28 ± 2°C and 150 rpm for 48 h. Bacterial cells were harvested by centrifugation at 10000 rpm for 5 min and the supernatant was discarded. The pellet was washed twice with sterile distilled water and re-suspended in sterile distilled water to get approximately 108 CFU/mL. Co-inoculants were prepared by mixing previously prepared single inoculants at 1:1 rate. Plant inoculation was carried out by dipping seedling roots three times in broth inoculants before transplanting them in the pots. For non-inoculated control, seedling roots were dipped three times in sterile distilled water [16]. Seedlings of tomato cv. Alisa were transplanted into 25 cm diameter black polyethylene bags filled with 7 kg of non-sterile sandy soil. Pots experiments plan was based on six treatments as follows:
1-1-Control without any inoculation (200 mM NaCl)
2-2-Inoculation with wild type strain of A. brasilense (W)
3-3-Co-inoculation with wild type strain of A. brasilense and Saccharomyces cerevisiae (W+S)
4-4-Inoculation with Saccharomyces cerevisiae (S)
5-5-Inoculation with adapted strain of A. brasilense (A)
6-6-Co-inoculation with adapted strain of A. brasilense and S. cerevisiae (A+S)
A saline soil is generally defined as one in which the electrical conductivity (EC) of the saturation extract (ECe) in the root zone exceeds 4 dS m−1 (approximately 40 mM NaCl) at 25°C and has an exchangeable sodium of 15% [17]. In this sense to induce salinity-resulted osmotic stress, all pots were watered by 200 mM NaCl dissolved in Hoagland’s nutrient solution [18]. Plants were grown under greenhouse condition for 90 days to evaluate the effect of applied inoculants on biometric parameters (shoot and root length, fresh and dry weight of plants (in a forced-air oven at 70°C for 72 h to constant weight) and fresh weight of fruits.
2.7 Determination of Photosynthetic Pigments and Ion Content in Tomato Leaves
For estimation of photosynthetic pigments content (chlorophyll a, b and carotenoids), about 1 g of small leaves of each plant (after 90 days of sowing) were homogenized in 8 mL acetone (80%), and then centrifuged at 10,000 rpm for 10 min at 4°C. The absorbance of the supernatant was measured using a UV/VIS spectrophotometer in the range of 450–750 nm against ethanol as a blank test [19]. To ion content dried leaves were ground and acid digested for the analysis of cations and phosphorus. Leaf digestions was analyzed using atomic absorption spectrometry [19]. Leaf N was measured using a micro-Kjeldahl procedure.
2.8 Determination of Free Proline Content and Total Soluble Sugar
0.2 g of fresh leaves were frozen and homogenized in 4 mL sulfosalicylic acid (3%), and the homogenates were centrifuged at 11,000 ×g for 15 min. Equal volumes from the supernatant, glacial acetic acid and acidic ninhydrin were mixed and incubated at 90°C for 1 h in a water bath. The mixture has been transferred to ice to stop the reaction, and then 1 ml of toluene was added. After vortexing, the solution was left at room temperature for separating the two phases. The absorbance of the upper phase (toluene) was measured at 520 nm. Proline content (mg/g FW) was calculated using the standard curve [10]. Total soluble sugars were determined according to phenol sulfuric acid method [19].
After 90 days of cultivation, 0.5 g of fresh leaves of each plant was homogenized in deionized water; and then filtered. 1 mL of the resulted extract was mixed with 1 mL 5% phenol and 5 mL sulfuric acid 98%. After one hour, the mixture’s absorbance of mixture was recorded at 490 nm by spectrophotometer, and soluble sugar content was calculated against the glucose standard curve.
Statistical analysis was performed using SPSS software ver. 21.0 (SPSS, Inc., USA) for windows. The data were processed using analysis of variance (ANOVA) and Turkey’s multiple range tests (p < 0.05) according to Steel et al. [20]. The results were expressed as mean of triplicates ± standard division (SD).
3.1 Effect of Salinity on the Growth and Biochemical Parameters of A. brasilense
Our results showed that A. brasilense exhibited an increase in the growth rate combined with the increase of salt concentration up to 5% NaCl, and then the growth rate was decreased after that with increasing NaCl concentrations above 5% (Fig. 1). The highest significant (p < 0.05) growth rate was recorded at 5% NaCl, while the lowest (p < 0.05) significant growth was observed at 0% NaCl. No significant difference was observed between the growth rates at 1%, 3% and 7% NaCl (p ≤ 0.05).
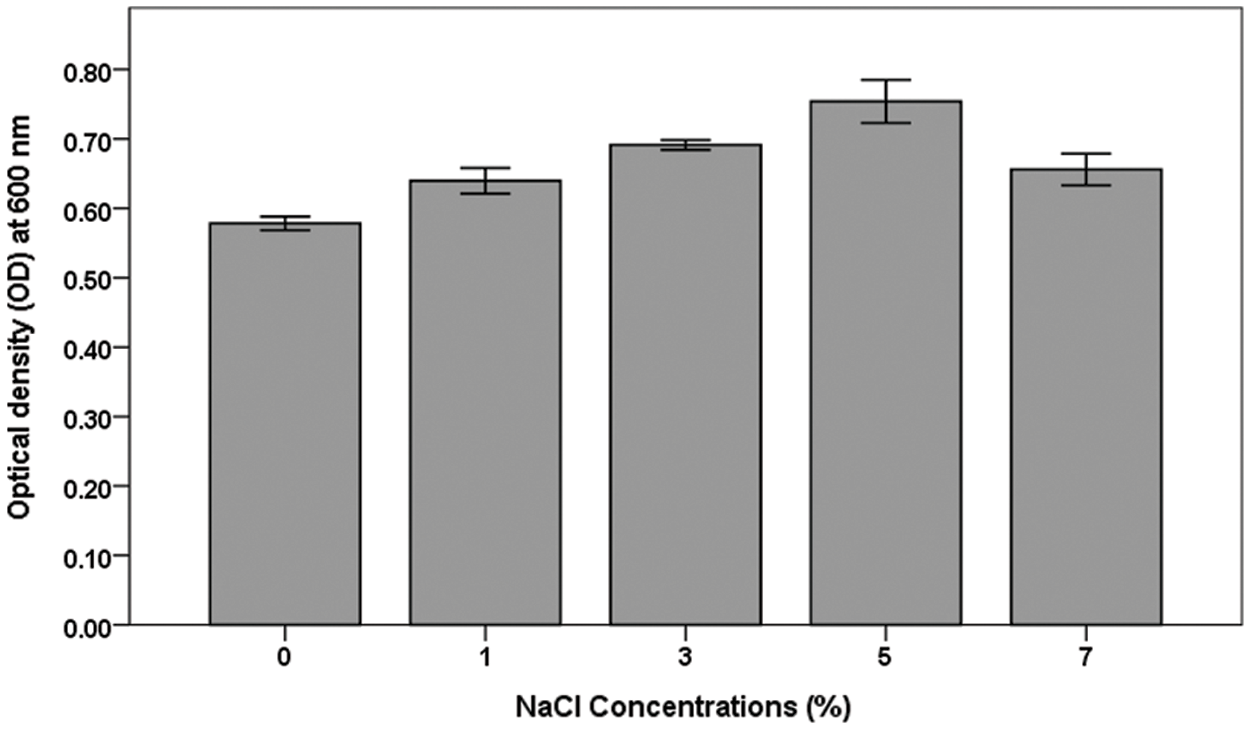
Figure 1: Growth of adapted A. brasilense at different NaCl concentrations
The results of the effects of different NaCl concentrations on ARA activity (nitrogenase activity), IAA and the production of exopolysaccharides (EPS) are shown in Tab. 1. ARA activity was increased with an increase of NaCl concentration up to 3%. Generally, 3% and 5% NaCl have been significantly (p < 0.05) among different salt concentrations, which enhanced the ARA activity compared to 0% NaCl. No significant difference (p < 0.05) between both 1% and 7% NaCl with 0% NaCl was observed. On the other hand, the highest production of IAA (43.2 ± 3.83 μg/mL) was recorded at 3% NaCl, while the lowest IAA production (29.1 ± 2.85 μg/mL) was observed at 7% NaCl concentrations (Tab. 1). On the other hand, the results showed that increasing NaCl concentration increased EPS production by adapted A. brasilense. The highest significant (p < 0.01) of EPS (646.73 ± 8.72 μg/mL) was recorded at 5% NaCl, after that it was slightly decreased. The lowest significant (p < 0.01) production of EPS (380.26 ± 10.75 μg/mL) was observed in the absence of salt (0% NaCl).

3.2 Effect of Adapted A. brasilense on Vegetative Parameters and Yield of Tomato
The effect of adapted A. brasilense on the tomato plant’s growth promotion was evaluated under salt stress condition, and the result has presented in Tab. 2 and Fig. 2. All vegetative parameters (plant height, root length, fresh and dry weight, and fruit yield) of tomato plants showed significant differences between the treatments. Among all treatments, plants co-inoculated with adapted strain of A. brasilense and S. cerevisiae (A+S) showed the highest significant (p < 0.01) increase in all growth parameters and yield as compared with un-inoculated control plants. Plants inoculated with adapted strain of A. brasilense (A) alone, also demonstrated a significant increase in all growth parameters and yield of tomato, except plant high. In contrast, the plants inoculated with wild type A. brasilense alone (W) did not show any significant differences with control in all measured parameters.


Figure 2: Effect of A. brasilense inoculants on the growth of tomato plants under salinity stress
However, co-inoculation with wild type and S. cerevisiae (W+S) significantly (p < 0.05) enhanced growth parameters and fruit yield of tomato when compared to control. Also, the plants treated with S. cerevisiae (S) alone showed significant increase in fresh and dry weight, while did not show significant plant high, root length and fruit yield of tomato compared with control. These results revealed that the growth characteristics (plant high, root length, fresh and dry weight and fruit yield) of tomato plants co-inoculated with (A+S) had been increased to 1.65, 0.77, 2.78, 2.06 and 26.7 folds respectively as compared to control, under salt stress condition.
3.3 Photosynthetic Pigments (Chlorophyll a, b and Carotenoids)
The results of the effect of PGPR inoculants on photosynthetic pigments (Chlorophyll a, b and carotenoid) in tomato leaves pointed to the existence of significant differences among treatments (Fig. 3). All treatments, except inoculation with wild type strain of A. brasilense (W), significantly stimulated photosynthetic pigments’ content under 4% NaCl concentration. Plants co-inoculated with an adapted strain of A. brasilense and S. cerevisiae (A+S) showed the highest significant (p < 0.05) values of chlorophyll a, b and carotenoid which were 1.35 ± 0.08, 0.45 ± 0.02 and 0.41 ± 0.02 mg/g fresh weight, respectively, as compared to un-inoculated plants, followed by plants inoculated with an adapted strain of A. brasilense alone (A). The lowest significant (p < 0.05) chlorophyll content was recorded with the wild type of strain A. brasilense (W), which was non-significant with un-inoculated control. No significant difference between plants treated with S. cerevisiae (S) alone and plants co-inoculated with both S. cerevisiae and wild type of A. brasilense (W+S) in chlorophyll a, b and carotenoid contents was observed.
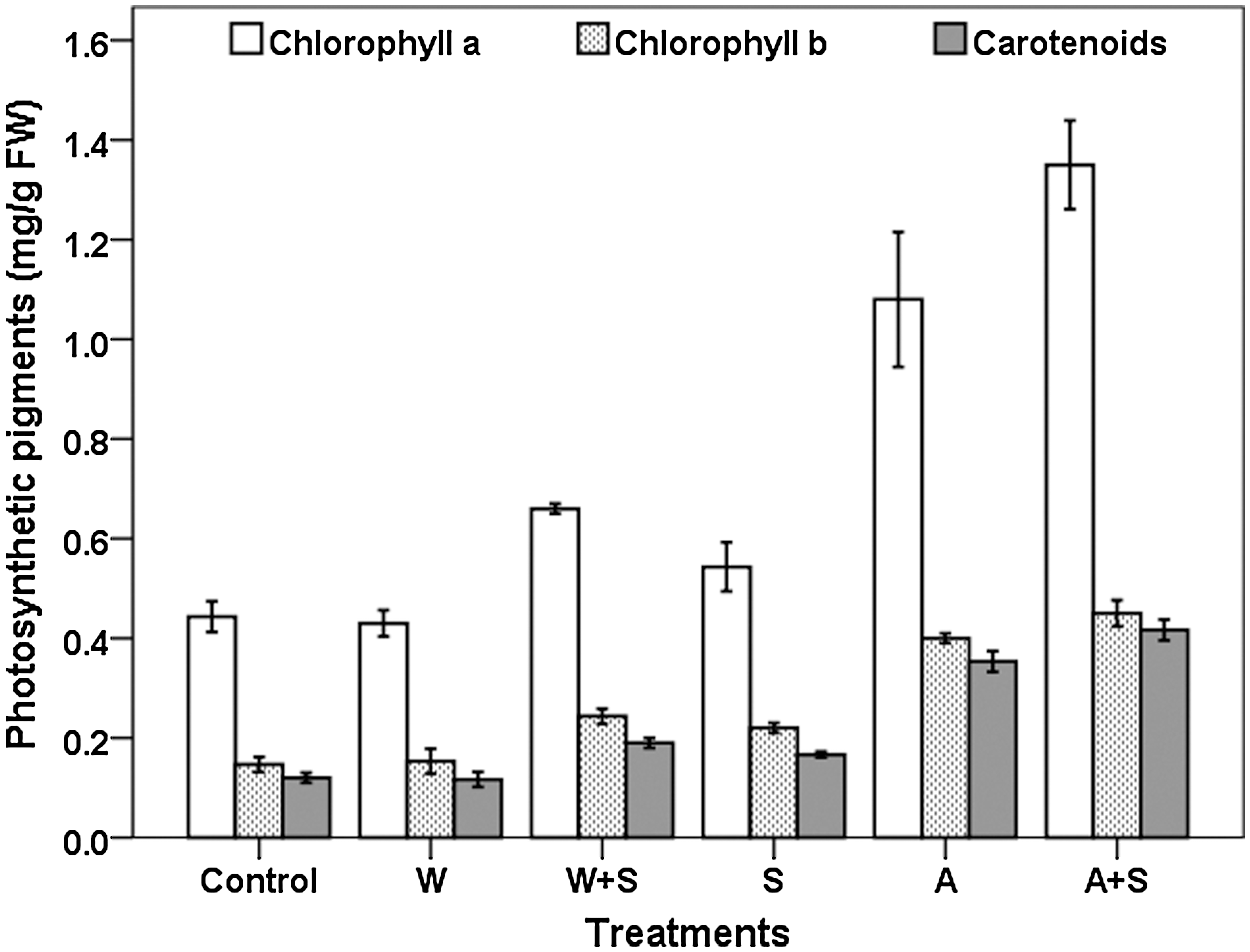
Figure 3: Effect of bacterial inoculants on photosynthetic pigments of tomato plants under salt stress condition
3.4 Proline and Total Soluble Sugar (TSS) Content
Application of bacterial inoculants had significant impacts on proline and total soluble sugar content in tomato plant leaves under salinity condition (Figs. 4a and 4b). Proline content was significantly (p < 0.01) increased in leaves of plant treated with an adapted strain of A. brasilense, whether in the case of single inoculant (A) or co-inoculant with an adapted strain of A. brasilense and S. cerevisiae (A+S), in comparison to un-inoculated control. No significant difference in proline content between plants treated with the wild type strain of A. brasilense and S. cerevisiae in both single inoculation and co-inoculation and un-treated plants was observed. On the other hand, all treatments, except inoculation with the wild type of strain A. brasilense (W), were significantly (p < 0.05) stimulated TSS content in tomato leaves in comparison to control. Plants treated with both adapted strain of A. brasilense and S. cerevisiae (A+S) exhibited the highest significant (p < 0.01) leaf contents of both proline and TSS, followed by plant treated with an adapted strain of A. brasilense alone (A) when compared with un-treated plants.
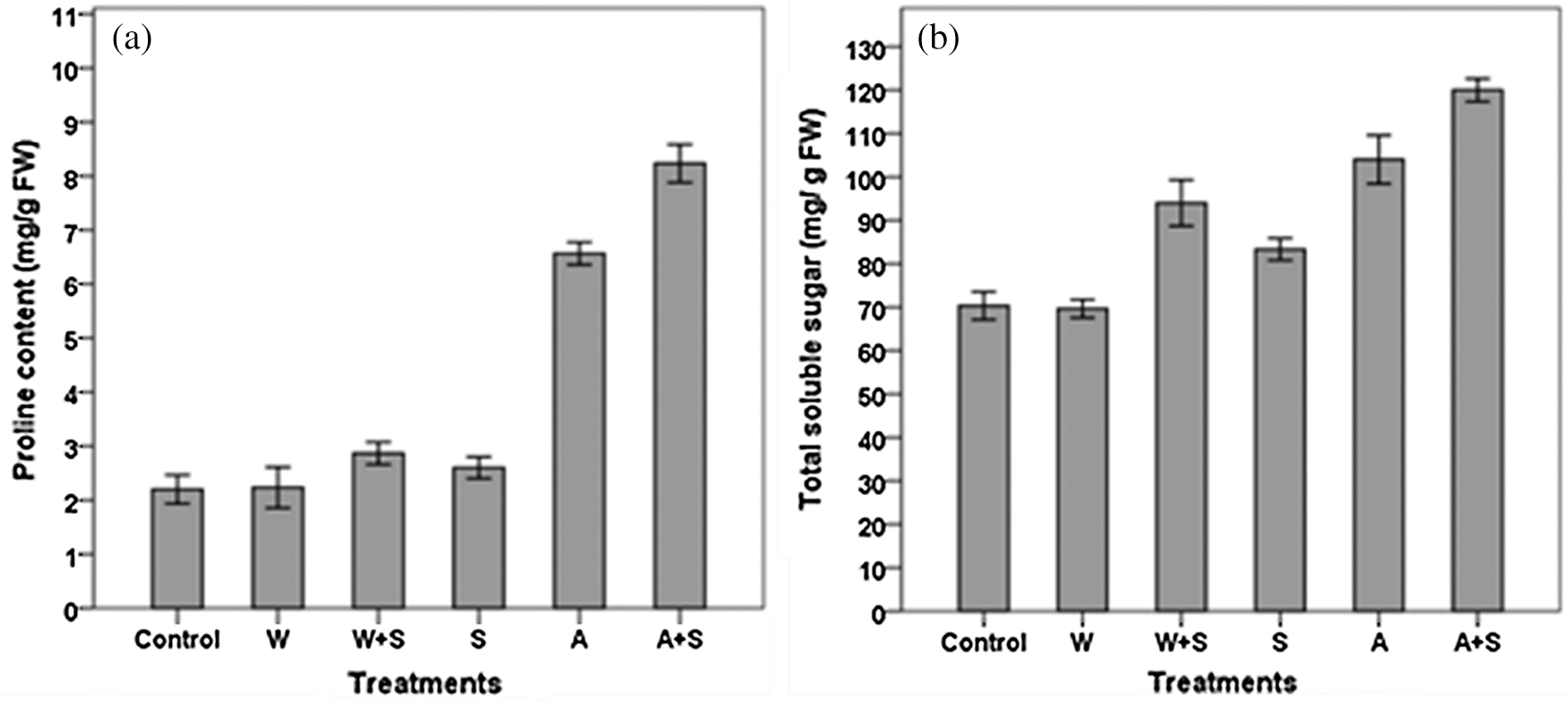
Figure 4: Effect of bacterial inoculants on proline content (a), and total soluble sugar content (b) in leaves of tomato plants under salt stress condition
Data illustrated in Fig. 5 exhibit that leaf ion contents (Na, Ca, Mg, K, P and N) were significantly affected by microbial treatments. Leaf Na+ content was significantly (p < 0.01) decreased in tomato plants treated with an adapted strain of A. brasilense whether alone (A) or combined with S. cerevisiae (A+S) in comparison to control. On the other hand, all other ions have been significantly (p < 0.01) increased in plant treated with A and A+S. A+S treated plants showed the lowest significant (p < 0.01) Na+ content and the highest significant (p < 0.01) content of all other ions as compared to un-treated plants. There was no significant difference between the wild strain of A. brasilense treated plants (W) and un-treated plants for all ions content. Ca, Mg, P and N leaf contents were significantly increased in plant co-inoculated with a wild strain of A. brasilense and S. cerevisiae (W+S) and plant treated with S. cerevisiae alone (S) as compared to control, without significant difference between the two treatments S and W+S. Co-inoculation with W+S led to significant increase of K content and a significant decrease of Na content, while inoculation with S alone did not show any significant difference in K and Na content compared to un-inoculated control.

Figure 5: Effect of bacterial inoculants on leaf mineral contents under salt stress conditions
A.brasilensis based biofertilizers, alone or in combination with other microbial-based products, show promise as alternatives to chemical fertilization, especially in salinity affected areas [1,18]. Our results showed that co-inoculation with (A. brasilense strain N040 and S. cerevisiae) clearly benefited the tomato plant’s by enhancing growth, productivity, biochemical parameters (proline and chlorophyll) and leaf Na+ reduction. Extreme accumulation of sodium ion (Na+) in plants could affect different mineral nutrients by limitation of their uptake, transport and distribution, and cause to nutrient imbalance, a decrease of K+ and Ca2+ uptake and induce Na+ toxicity in plant [1,10]. Our results indicated that tomato plants’ inoculation with adapted strain of A. brasilense and S.cereviseae induced significant decrease in Na+ and increase in K, Ca, Mg, P and N content in tomato leaves, under salinity stress. Similarly, A. brasilense significantly relieved the salinity stress in white clover plants, where shoot fresh and shoot dry weight and root dry weight of inoculated plants were significantly increased compared to un-inoculated plants under different NaCl concentrations [18]. In this concern, tomato plants treated with adapted Pseudomonas species to 6% NaCl concentration showed an increase of tomato growth parameters such as root length, shoot length, fresh weight, and dry weight in the presence of 2% NaCl concentration [21]. Inoculation of tomato roots with Azotobacter chroococcum 76A led to promoting of salt stress tolerance, fruit number/plant, fruit weight, shoot dry weight and nutrient uptake efficiency under severe salinity [2]. Catharanthus roseus plants treated with mutant strains mixture of P. stutzeri, P. mendocina, B. flexus and B. subtilis, showed a significant increase in length, fresh and dry weight of root and shoot as compared to un-inoculated plants, and plants treated with such mixture of wild strains, under 4% NaCl concentration [16]. On the other hand, the increase of chlorophyll and carotenoid content in PGPR-inoculated plant leaves under salt stress condition has been reported by numerous researchers [6,22–24]. In this sense, in our study, increase of chlorophyll in the tomato plants inoculated with A. brasilense strain N040 and S. cerevisiae could be results of inhibition Na transport, thus improving Mg2+ ions absorption leading to intensifying chlorophyll synthesis under salt stress conditions [25]. In this sense, the PGPR could enhance chlorophyll pigments in plants via increase of proline levels in plant tissues, which decreased chlorophyll degradation [26]. Another option is that the increased chlorophyll content in tomato plants-co-inoculated exposed to salts stress may be due to the increased leaf area compared to un-inoculated plants where photosynthetic leaf area reduced due to stress (Fig. 2) [21]. The accumulation of proline in plant tissues is an adaptive and common response of plants to salinity stress, which assists in osmotic adjustment and scavenging of hydroxyl radicals, thus protecting intracellular macromolecule’s structure and cell membranes stabilization [23,27]. Under salinity stress, proline play a vital role in reducing the oxidative stress caused by salinity through several strategies such as regulation of cytosolic acidity, maintenance of proteins, ROS scavenging and reduced lipid peroxidation [5]. In the present study, both proline and TSS contents were increased in tomato plants treated with an adapted strain of A. brasilense under salt stress conditions, suggesting that this strain may stimulate plant growth under salinity condition inducing metabolic defense mechanisms in plants. Similarly, halotolerant Bacillus aryabhattai H19-1 and B. mesonae H20-5 increased proline and total soluble sugar contents, as well as induction of salinity tolerance in tomato plants [10,28]. Our results suggested that the adapted strain of A. brasilense and S.cereivisae could play a vital role in alleviating salinity stress and enhancing nutrients uptake by plants, under salinity stress. In this regard, the tomato plant’s coinoculation with Bacillus aryabhattai H19-1 and B. mesonae H20-5 induced the salinity tolerance in plants through Ca2+ accumulation, where Ca2+ accumulation contributed to osmoregulation by enhancing water uptake efficiency and increasing proline [10]. Finally, we suggest, coinoculation of A. brasilense and S.cereivisae induced the activation of important biochemical and physiological mechanism in tomato plants under salt stress.
The used PGPR strain in the present study was adapted to 7% NaCl concentration, which can enhance tomato plant growth and yield in the presence of 200 mM NaCl, as well as alleviate harmful impact caused by salt stress. From the study, we can conclude that co-inoculation A. brasilense with S. cerevisiae exhibited grateful results in improving tomato plant growth and yield under salinity stress. Our results showed that co-inoculation with (A. brasilense strain N040 and S. cerevisiae) clearly benefited the tomato plant’s by enhancing growth, productivity, biochemical parameters (proline and chlorophyll) and leaf Na+ reduction. Though much more in-depth study is needed to find out the importance of these bacteria’s in plant-microorganisms interaction under salinity stress.
Authors’ Contributions: Ali Abdelmoteleb and Ahmed Mohamed Elbaalawy conceived and designed the research. Daniel Gonzalez-Mendoza analyzed the experimental data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Funding Statement: This work was funded by the Faculty of Agriculture, Menoufia University (Grant No. 000212).
Conflicts of Interest: The authors declare that they have no interest in reporting regarding the present study.
1. Kumar, A., Patel, J. S., Meena, V. S., Ramteke, P. W. (2019). Plant growth-promoting rhizobacteria: Strategies to improve abiotic stresses under sustainable agriculture. Journal of Plant Nutrition, 42(11–12), 1402–1415. DOI 10.1080/01904167.2019.1616757. [Google Scholar] [CrossRef]
2. van Oosten, M. J., Di Stasio, E., Cirillo, V., Silletti, S., Ventorino, V. et al. (2018). Root inoculation with Azotobacter chroococcum 76A enhances tomato plants adaptation to salt stress under low N conditions. BMC Plant Biology, 18(1), 205. DOI 10.1186/s12870-018-1411-5. [Google Scholar] [CrossRef]
3. Suhail, F. M., Mahdi, I. A. (2011). Response of Azospirillum brasilense bacteria to the types and concentrations of different salts. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca-Agriculture, 68(1), 356–362. DOI 10.15835/buasvmcn-agr:6466. [Google Scholar] [CrossRef]
4. Shrivastava, P., Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal Bioloical Sciences, 222(2), 123–31. DOI 10.1016/j.sjbs.2014.12.001. [Google Scholar] [CrossRef]
5. Abbas, R., Rasul, S., Aslam, K., Baber, M., Shahid, M. et al. (2019). Halotolerant PGPR: A hope for cultivation of saline soils. Journal of King Saud University–Science, 31(4), 1195–1201. DOI 10.1016/j.jksus.2019.02.019. [Google Scholar] [CrossRef]
6. Abdel Motaleb, N. A., Abd Elhady, S. A., Ghoname, A. A. (2020). AMF and Bacillus megaterium neutralize the harmful effects of salt stress on bean plants. Gesunde Pflanzen, 72(1), 29–39. DOI 10.1007/s10343-019-00480-8. [Google Scholar] [CrossRef]
7. Viscardi, S., Ventorino, V., Duran, P., Maggio, A., De Pascale, S. et al. (2016). Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. Journal of Soil Science and Plant Nutrition, 16(3), 848–863. DOI 10.4067/S0718-95162016005000060. [Google Scholar] [CrossRef]
8. Acosta-Motos, J. R., Penella, C., Hernández, J. A., Díaz-Vivancos, P., Sánchez-Blanco et al. (2020). Towards a sustainable agriculture: Strategies involving phytoprotectants against salt stress. Agronomy, 10(2), 1–32. DOI 10.3390/agronomy10020194. [Google Scholar] [CrossRef]
9. Misra, S., Chauhan, P. S. (2020). ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech, 10(3), 1–14. DOI 10.1007/s13205-020-2104-y. [Google Scholar] [CrossRef]
10. Yoo, S. J., Weon, H. Y., Song, J., Sang, M. K. (2019). Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19–1 and B. mesonae H20–5 in tomato plants. Journal of Microbiology and Biotechnology, 29(7), 1124–1136. DOI 10.4014/jmb.1904.04026. [Google Scholar] [CrossRef]
11. Farhangi-Abriz, S., Tavasolee, A., Ghassemi-Golezani, K., Torabian, S., Monirifar, H. et al. (2020). Growth-promoting bacteria and natural regulators mitigate salt toxicity and improve rapeseed plant performance. Protoplasma, 257(4), 1035–1047. DOI 10.1007/s00709-020-01493-1. [Google Scholar] [CrossRef]
12. Ahmed, N. U., Mahmud, N. U., Zaman, M. A. U., Ferdous, Z., Halder, S. C. (2017). Effect of different salinity level on tomato (Lycopersicon esculentum) production under climate change condition in Bangladesh. Annual Research and Review in Biology, 13(3), 1–9. DOI 10.9734/ARRB/2017/33613. [Google Scholar] [CrossRef]
13. Puranik, S., Shaligram, S., Paliwal, V., Raje, D. V., Kapley, A. et al. (2012). Demonstration of sequential adaptation strategy for developing salt tolerance in bacteria for wastewater treatment: A study using Escherichia coli as model. Bioresource Technology, 121, 282–289. DOI 10.1016/j.biortech.2012.06.037. [Google Scholar] [CrossRef]
14. Khamna, S., Yokota, A., Peberdy, J. F., Lumyong, S. (2010). Indole-3-acetic acid production by streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian Journal of Biosciences, 32, 23–32. DOI 10.5053/ejobios.2010.4.0.4. [Google Scholar] [CrossRef]
15. Saad, M. M. G., Kandil, M., Mohammed, Y. M. M. (2020). Isolation and identification of plant growth-promoting bacteria highly effective in suppressing root rot in fava beans. Current Microbiology, 77, 2155–2165. DOI 10.1007/s00284-020-02015-1. [Google Scholar] [CrossRef]
16. Hingole, S. S., Pathak, A. P. (2016). Saline soil microbiome: A rich source of halotolerant PGPR. Journal of Crop Science and Biotechnology, 19(3), 231–239. DOI 10.1007/s12892-016-0035-2. [Google Scholar] [CrossRef]
17. Shrivastava, P., Kumar, R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences, 22(2), 123–131. DOI 10.1016/j.sjbs.2014.12.001. [Google Scholar] [CrossRef]
18. Khalid, M., Bilal, M., Hassani, D., Iqbal, H. M. N., Wang, H. et al. (2017). Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Botanical Studies, 58(1), 5. DOI 10.1186/s40529-016-0160-8. [Google Scholar] [CrossRef]
19. Dimkpa, C., Weinand, T., Asch, F. (2009). Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell & Environment, 32(12), 1682–1694. DOI 10.1111/j.1365-3040.2009.02028.x. [Google Scholar] [CrossRef]
20. Steel, R. G., Torrie, J. H., Dickey, D. A. (1997). Principles and procedures of statistics. A biometrical spproach, 3rd edition. New York, USA: McGraw Hill Book Co., Inc. [Google Scholar]
21. Tank, N., Saraf, M. (2010). Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. Journal of Plant Interactions, 5(1), 51–58. DOI 10.1080/17429140903125848. [Google Scholar] [CrossRef]
22. Velázquez-Márquez, S., Conde-Martínez, V., Trejo, C., Delgado-Alvarado, A., Carballo, A. et al. (2015). Effects of water deficit on radicle apex elongation and solute accumulation in Zea mays L. Plant Physiology and Biochemistry, 96, 29–37. DOI 10.1016/j.plaphy.2015.07.006. [Google Scholar] [CrossRef]
23. Hahm, M. S., Son, J. S., Hwang, Y. J., Kwon, D. K., Ghim, S. Y. (2017). Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. Journal of Microbiology and Biotechnology, 27(10), 1790–1797. DOI 10.4014/jmb.1609.09042. [Google Scholar] [CrossRef]
24. Akram, W., Aslam, H., Ahmad, S. R., Anjum, T., Yasin, N. A. et al. (2019). Bacillus megaterium strain A12 ameliorates salinity stress in tomato plants through multiple mechanisms. Journal of Plant Interactions, 14(1), 506–518. DOI 10.1080/17429145.2019.1662497. [Google Scholar] [CrossRef]
25. Chowdhury, S. P., Nagarajan, T., Tripathi, R., Mishra, M. N., Le Rudulier, D. et al. (2007). Strain-specific salt tolerance and osmoregulatory mechanisms in Azospirillum brasilense. FEMS Microbiology Letters, 267(1), 72–79. DOI 10.1111/j.1574-6968.2006.00540.x. [Google Scholar] [CrossRef]
26. Grover, M., Ali, S. Z., Sandhya, V., Rasul, A., Venkateswarlu, B. (2011). Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World Journal of Microbiology and Biotechnology, 27(5), 1231–1240. DOI 10.1007/s11274-010-0572-7. [Google Scholar] [CrossRef]
27. Rabie, G. H., Almadini, A. M. (2005). Role of bioinoculants in development of salt-tolerance of vicia faba plants under salinity stress. African Journal of Biotechnology, 4(3), 210–222. DOI 10.5897/AJB2005.000-3041. [Google Scholar] [CrossRef]
28. Kaushal, M., Kaushal, R. (2015). Acetylene reductase activity and molecular characterization of plant growth promoting rhizobacteria to know efficacy in integrated nutrient management system. Indian Journal of Biotechnology, 14, 221–227. http://nopr.niscair.res.in/handle/123456789/31813. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |