

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.016434
ARTICLE
Estimation of Growth and Photosynthetic Performance of Two C4 Species (Pennisetum spicatum (L.) Körn. and Zea mays L.) under a Low Temperature Treatment
Department of Biology, Faculty of Science, Taibah University, Almadinah Almunawwarah, 41311, Saudi Arabia
*Corresponding Author: Abdulkhaliq Alshoaibi. Email: Ashoaibi@taibahu.edu.sa
Received: 05 March 2021; Accepted: 12 April 2021
Abstract: Pearl millet (Pennisetum spicatum (L.) Körn.) and maize (Zea mays L.) are C4 grass species grown for feeding humans and animals in Almadinah Almunawwarah, which is in the western part of Saudi Arabia. During the winter, the mean temperature, which drops to 14°C, represents a major problem for the growth of these species in this region. Therefore, the objectives of this research were to investigate the growth response and the photosynthetic performance of P. spicatum and Z. mays under a low temperature stress. The treatments involved daytime and nighttime temperatures of 14/12°C (low temperature) and 24/22°C (optimum temperature). The results indicated that low temperature significantly reduced all growth and physiological parameters, including seed germination, leaf expansion, leaf area, shoot length and root length of the two species compared to those of the control. Additionally, the low temperature significantly decreased the light-saturated assimilation rate (Asat), quantum yield (ϕ), saturated rate of carbon dioxide uptake (Amax) and efficiency of carboxylation on both species compared to those of the control. Moreover, the values of Fv/Fm and the chlorophyll contents of both species were significantly reduced by low temperature compared to those of the control. It can be concluded that both species had little tolerance to low temperatures.
Keywords: Pennisetum spicatum; Zea mays; low temperature; plant growth; CO2 uptake; photochemistry
One of the most critical variables that restricts plant development, distribution and production is low temperature [1–3]. The majority of C4 plants are mainly restricted to regions with relatively warm temperatures because low temperatures adversely influence their physiological processes [2,4]. Sowinski et al. [5] observed that the rates of both leaf cell division and elongation of C4 plants were lower at low than warm temperatures. All stages of plant growth, including germination and early seedling growth, and a wide range of physiological processes are also considered to be influenced by low temperatures [3,6–8]. Several studies have shown that plants have a rapid and sensitive growth response to low temperatures [3,5,8–10]. Z. mays is the world’s most grown C4 species [3]. Exposure of Z. mays to low temperatures impairs leaf growth and photosynthesis and breaks down chlorophyll in developed leaves [11]. The leaf expansion rate was found to be the major factor determining the variations in leaf growth rates of nine varieties of sugar beet at four different temperature treatments [12]. This indicates that low temperature is one of the most important factors influencing C4 plant growth and development.
Under optimum environmental conditions, the efficiency of photosynthesis is 40% greater in C4 than C3 plants [4]. This benefit, however, is not obtained under low temperature conditions in C4 plants. Low temperatures impair thylakoid membranes, reduce chlorophyll contents and decrease the photosynthetic potential of C4 crops such as Z. mays [5,13]. The decrease in photosynthesis ability of leaves of Z. mays under cold temperatures is related to decreases in both the light-saturated assimilation rate (Asat) and the maximum quantum yield (ϕ) [6,8,14]. On the other hand, some C4 crop species are acclimated to and grow in cold climates [15]. The ability of these crop plants to stimulate their physiological processes and photosynthesis or to increase them under cold temperatures allows them to live in these relatively cool environments [5,9,16].
Photoinhibition can occur during photosynthesis when light absorption exceeds that needed by the photosynthetic demands of plants [17]. Several studies have revealed that plants experience low temperature photoinhibition, both under controlled conditions and in the field [1,6,18]. Photoinhibition is described by a decrease in the quantum yield of CO2 absorption (ϕ) and the ratio between the variable and maximum fluorescence of chlorophyll a (Fv/Fm) [1,19]. PSII photoinhibition is demonstrated by a decrease in the Fv/Fm value of dark-adapted leaves. Furthermore, the reduction rate depends on environmental factors sooner than a photo-inhibitory treatment and genotypic variability [5–6,20].
Pearl millet (Pennisetum spicatum) and Maize (Zea mays) are C4 grass species grown for feeding humans and animals in Almadinah Almunawwarah, which is located in the western part of Saudi Arabia. During the winter, the mean temperature, which drops to 14°C, represents a major problem for the growth and photosynthesis of these species in this region (Presidency of Meteorology and Environment, Saudi Arabia; https://ncm.gov.sa/Ar/Weather/Region Weather/Pages/Madinah.aspx). Since there is a lack of information concerning the cold tolerance of P. spicatum, this work was performed to investigate the growth response and photosynthetic performance of the P. spicatum grass at low temperatures. The treatments involved daytime/nighttime temperatures of 14/12°C (low temperature) and 24/22°C (optimum temperature). All growth factors and the photosynthetic performance were compared to those of Z. mays.
Seeds of commercial pearl millet (Pennisetum spicatum L. cv. Madinah) and maize (Zea mays L. cv. Legacy SU; USA) were obtained from Al-Hilali Agricultural Company, Almadinah Almunawwarah, Saudi Arabia. The experiments in this research were carried out in the Biology Department, Science Faculty, University of Taibah, Almadinah Almunawwarah, Saudi Arabia. Seeds of both species were surface sterilized by soaking them for ten minutes in 0.1% mercury chloride. After five washes in sterile distilled water, seeds were placed on two layers of Whatman No. 1 filter paper inside a Petri dish (9 cm × 1.6 cm) and watered with 10 ml of distilled water. Each Petri dish contained 10 seeds, and dishes containing four replicates of each species were placed in an incubator in darkness at two different temperatures (14 and 24°C); there was a total of 16 Petri dishes. The appearance of a radicle from the seeds was considered a sign of germination. The germination process was documented every day for the whole experimental phase, which lasted for one week.
P. spicatum and Z. mays seeds were grown in 12 cm × 20 cm plastic pots containing 2 kg of compost. Pots were located inside an environmentally regulated chamber (JSR 314-240, Inc., JS Research., 40-1 Gumsang-Dong, Gongju Area, Korea) with a light/dark photoperiod of 14/10 h and 60% relative humidity. For lighting, fluorescent and halogen lamps were used, resulting in a total of 400 μmol m−2 s−1 irradiance. The treatments involved daytime/nighttime temperatures of 14/12°C (low temperature) and 24/22°C (optimum temperature). Four replicates for each species were used at each temperature treatment, resulting in a total of 16 pots. Five seeds were planted in each pot, and the seedlings were thinned after emergence, leaving two per pot. The plants were irrigated to field capacity with a full-strength Hoagland solution. The following growth parameters were measured at the end of the experiment (60 days after planting): plant height, leaf number, and root and shoot fresh weight. The area of leaves was measured by a LI-COR leaf area meter (LI-COR Inc., Lincoln, Nebraska, USA). The shoot and root samples were dried in an oven for 48 h at 80°C to estimate dry weight.
Rates of gas exchange of mature fully expanded leaves of P. spicatum and Z. mays were calculated using a LI-COR 6400XT infrared gas analyser (LI-6400, LI-COR Inc., Lincoln, Nebraska, USA). For the measurements of photosynthesis and dark respiration, the fourth youngest fully expanded leaf was used. Light response curves were generated at a 24°C leaf temperature and 410 μmol mol−1 (Ca) atmospheric CO2 concentration. The leaves were steadily illuminated with photon flux densities between 0 and 1500 μmol m−2 s−1.
The Von Caemmerer and Farquar equations were used to calculate the net photosynthesis per unit leaf area and intercellular CO2 concentration ci [21]. Asat was measured at saturating photosynthetic photon flux density (PPFD) of 1500 μmol m−2 s−1 and at ambient CO2 concentration of 410 μmol mol−1. CO2 response curves (A/Ci) within the 50–550 μmol mol−1 range were generated using PPFD of 1500 μmol m−2 s−1 at a 24°C leaf temperature, as defined by Collatz et al. [22].
2.4 Chlorophyll Content Determination
The chlorophyll content was measured and expressed as the chlorophyll content index using a handheld chlorophyll content meter (CCM-200, Opti-Sciences, USA). In the different treatments, the fourth newest completely expanded leaf was used to calculate the chlorophyll content of each species four times, and the average was used for analysis.
2.5 Chlorophyll Fluorescence Measurements
A portable fluorimeter was used to measure the chlorophyll fluorescence of the fourth newest completely expanded leaf of both P. spicatum and Z. mays (PEA, Hansatech, Norfolk, Lynn Kings). The leaves were dark acclimated for 20 min before the fluorescence was measured. As described by Al-shoaibi [23], the ratio of variable fluorescence to maximum fluorescence (Fv/Fm) was measured four times for P. spicatum and Z. mays species.
The data obtained from the different measurements were analysed statistically using one-way and two-way analysis of variance (ANOVA) and with a general linear model for evaluating the primary effects and interactions of the factors investigated (i.e., species and temperature). The significance of the different levels of the variables analysed was tested, and multiple comparisons were carried out using the Tukey test. All analyses were performed using version 15 of Minitab (Brandon Court, Progress Way Unit E1-E2, CV3 2TE, Coventry, Great Britain). Four replicates were used for each temperature treatment, and standard deviations and standard errors were calculated using Microsoft Excel 2016.
The effects of the two temperature treatments on the seed germination percentages of P. spicatum and Z. mays are illustrated in Fig. 1. At 24°C, seed germination of P. spicatum started on the first day and was completed on the third day, while seed germination of Z. mays started on the second day and was completed on the fourth day of the germination trial. On the other hand, at 14°C, seed germination of P. spicatum started on the first day and was completed on the third day, while seed germination of Z. mays started on the second day and was completed on the fifth day of the germination trial. The final germination percentages for both species approached 100% in the two temperature treatments. Thus, temperature had no apparent significant effect on the final germination percentages attained by seeds of both species. However, the germination rate for Z. mays seeds at 14°C significantly decreased compared to that of Z. mays seeds germinated at 24°C (p < 0.01).
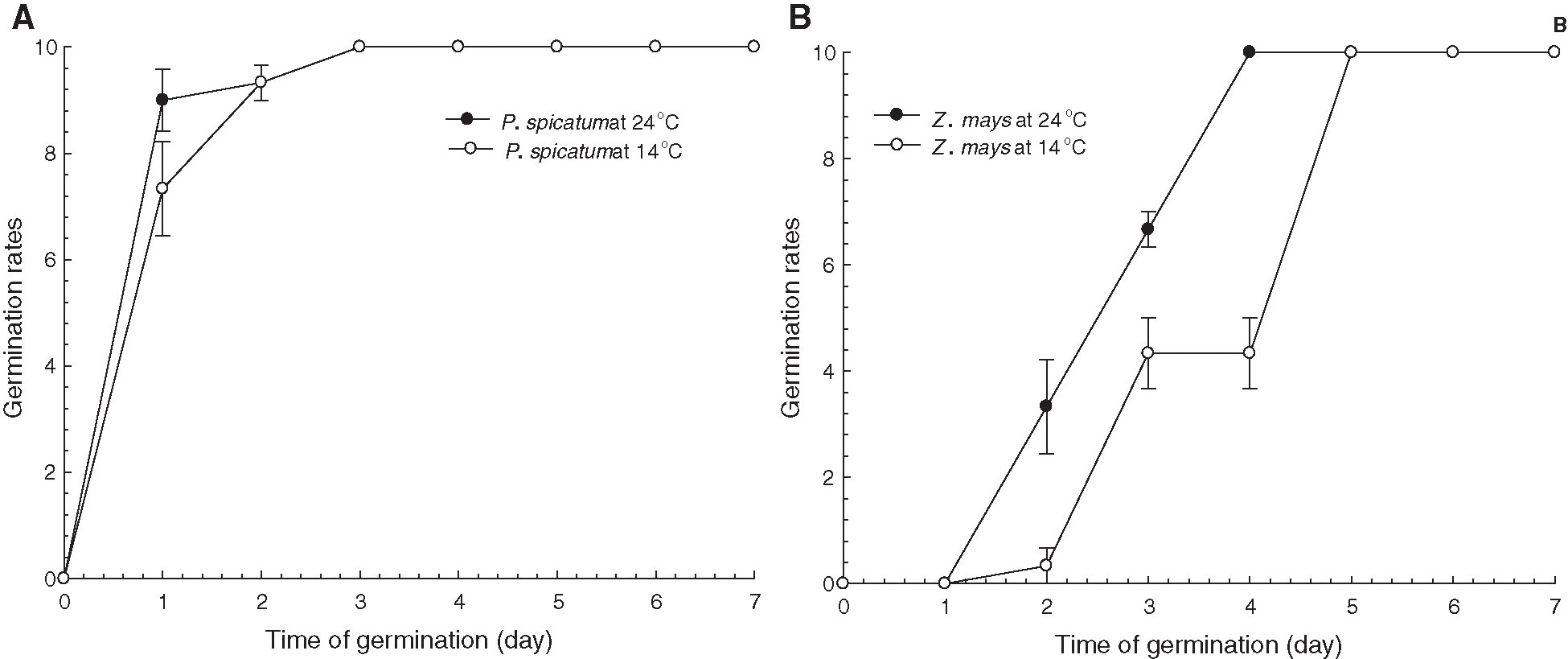
Figure 1: Effects of temperature treatments (14°C and 24°C) on seed germination of the two C4 species; P. spicatum (A), and Z. mays (B); (n = 4, Mean ± S.E.)
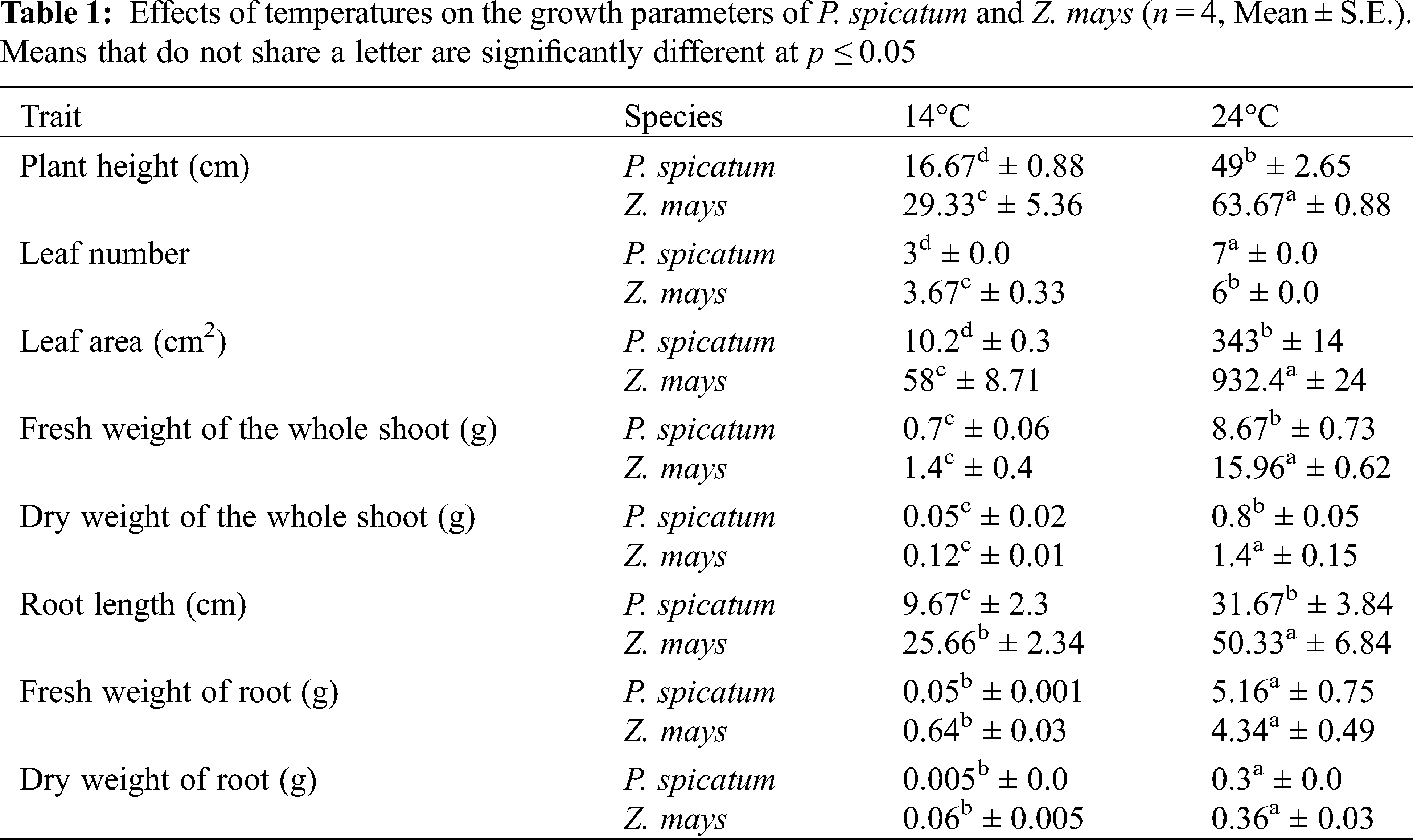
A number of growth parameters of both P. spicatum and Z. mays were measured to determine how they were affected by low-temperature treatment; the results are summarized in Tab. 1. Low temperature significantly affected all growth parameters of the two species (p < 0.001). Moreover, the results showed that all growth parameters of P. spicatum and Z. mays decreased significantly when the plants were grown at 14°C compared to 24°C (p < 0.001; Fig. S1). Between both species, P. spicatum showed the highest percentage of reduction in all growth parameters at 24°C (p < 0.001; Tab. 1). The fresh weight and dry weight of roots of P. spicatum grown at 14°C were 99% and 98.33% lower than those of the same species grown at 24°C; these decreases were the highest observed among these indices. Similarly, the leaf area and dry weight of shoots of Z. mays grown at 14°C were 93.77% and 91.42% lower than those of the same species grown at 24°C (p < 0.001; Tab. 1), representing the largest percent decreases for these indices.
The results presented in Fig. 2 illustrate the photosynthetic CO2 (A) absorption in response to photon flux (Q) for P. spicatum and Z. mays grown in the two temperature treatments. The temperature treatments significantly affected the photosynthetic performance of the two species (p < 0.001). The highest photosynthesis efficiency was observed at 24°C for both species (Fig. 2). The rates of light saturation (Asat) for P. spicatum and Z. mays grown at 14°C were considerably lower than those of the same species grown at 24°C (p < 0.01; Fig. 3A), with percent decreases of 62.8% and 19.7% for P. spicatum and Z. mays, respectively. Additionally, the quantum yields (ϕ) for P. spicatum and Z. mays grown at 14°C were considerably lower than those of the same species grown at 24°C (p < 0.01; Fig. 3B), with percent decreases of 51.9% and 17.9% for P. spicatum and Z. mays, respectively. Additionally, Fig. 3C shows the measurements of the A/ci curve for P. spicatum and Z. mays under the two temperature treatments. Generally, the A/ci curve plateaus (Amax) and the carboxylation efficiency of P. spicatum and Z. mays were considerably lower when plants were grown at 14°C compared with 24°C (p < 0.01; Figs. 3C, 3D). For both species, the highest percent decreases in Amax and carboxylation efficiency were observed for P. spicatum grown at 14°C; these were 62.8% and 67.4% lower than those of the same species grown at 24°C (p < 0.01; Figs. 3C, 3D). Additionally, all the photosynthetic parameters of P. spicatum grown at 14°C were significantly lower than those of Z. mays grown at the same temperature (Fig. 3).
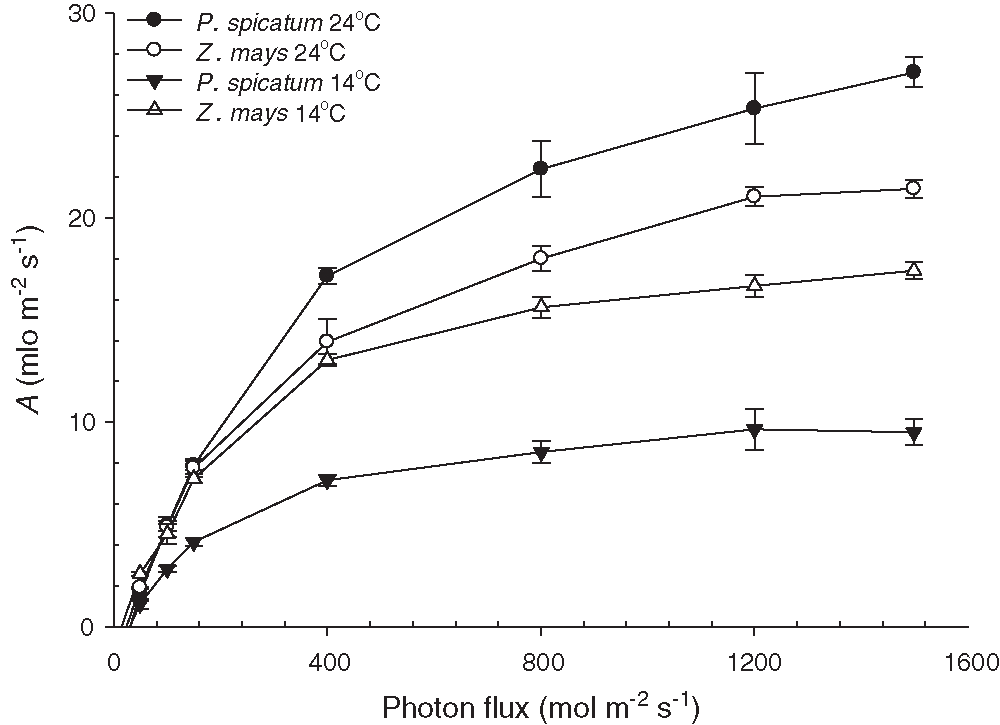
Figure 2: The photosynthetic CO2 absorption response (A) per unit area of the leaf to photon flux (Q) for the two C4 species, P. spicatum and Z. mays (n = 4, Mean ± S.E.). CO2 absorption measurements were all made at 24°C and Ca of 410 μmol mol−1
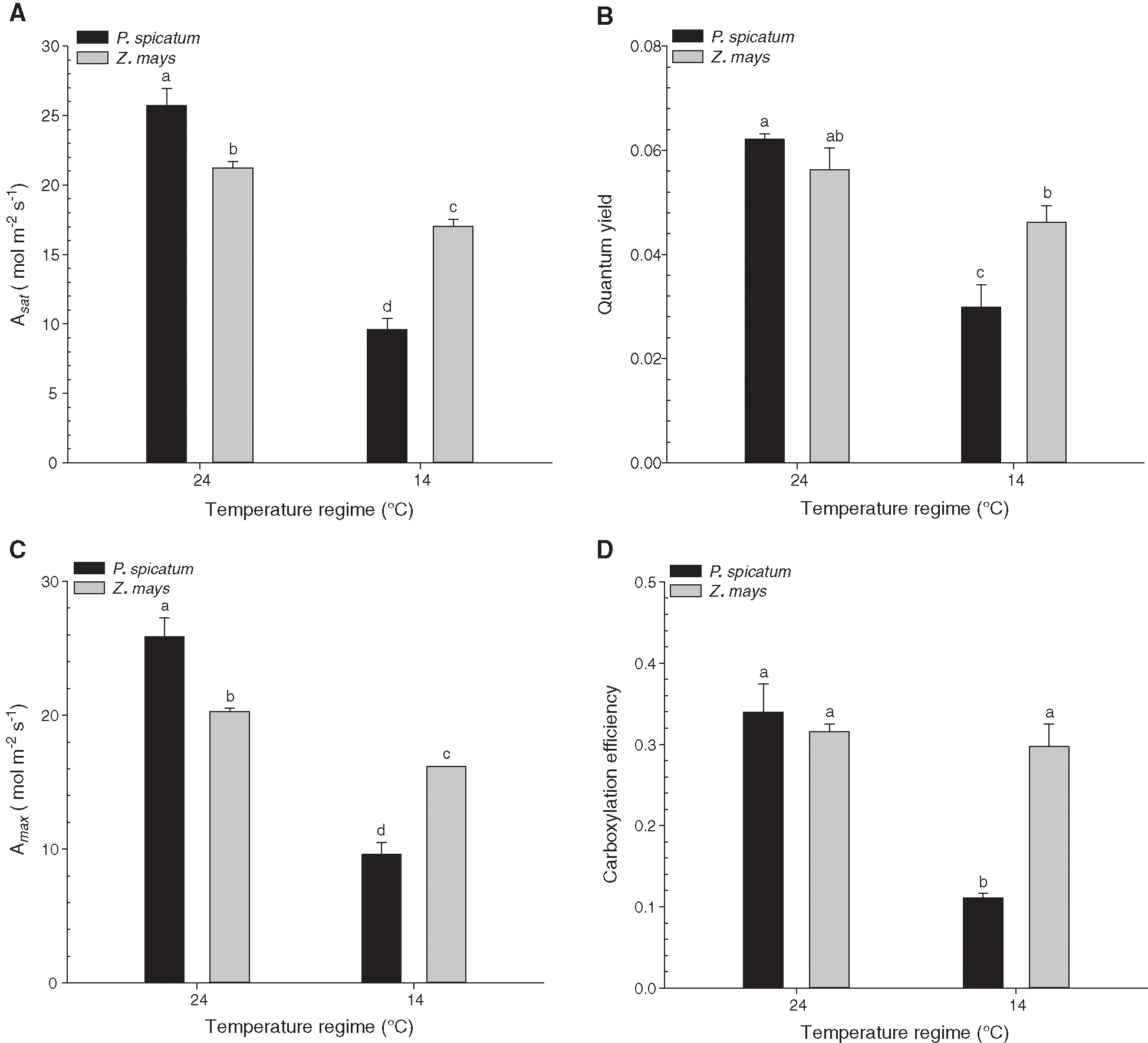
Figure 3: Effects of temperature treatments (14°C, and 24°C) on physiological parameters of the two C4 species; P. spicatum and Z. mays (n = 4, Mean ± S.E.). (A) Asat, (B) Quantum yield, (C) Amax and (D) Carboxylation efficiency. Means that do not share the same letter are significantly different at p ≤ 0.05
3.4 Chlorophyll Content and Chlorophyll Fluorescence
Fig. 4A illustrates the effects of the two temperature treatments on the chlorophyll content of P. spicatum and Z. mays. Compared to the chlorophyll contents of the same species grown at 24°C, the chlorophyll contents of P. spicatum significantly decreased in response to the decreased temperature (14°C) (p < 0.01; Fig. 4A). However, the chlorophyll contents of Z. mays grown at 14°C did not significant decrease compared to those of the same species grown at 24°C (Fig. 4A). The maximum quantum efficiency of PSII (Fv/Fm) photochemistry of P. spicatum and Z. mays grown under the two temperature treatments is shown in Fig. 4B. For both species, the chlorophyll fluorescence values of the species grown at 14°C significantly decreased compared to the values of the two species grown at 24°C (p < 0.01; Fig. 4B). Additionally, the Fv/Fm value of P. spicatum grown at 14°C was significantly greater than the Fv/Fm value of Z. mays grown at the same temperature (p < 0.05; Fig. 4B).
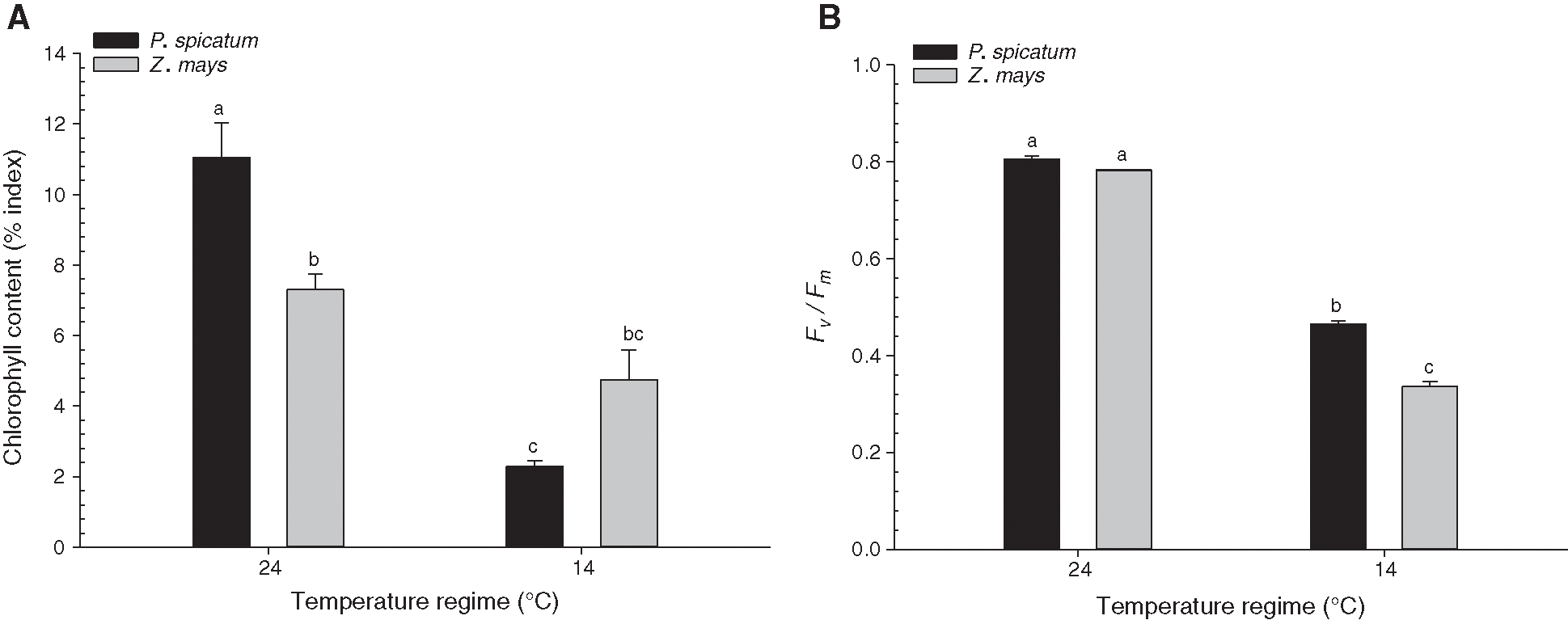
Figure 4: Effects of temperature treatments (14°C, and 24°C) on physiological parameters of the two C4 species, P. spicatum and Z. mays (n = 4, Mean ± S.E.). (A) Chlorophyll content and (B) Fv/Fm. Means that do not share the same letter are significantly different at p ≤ 0.05
One of the most important factors affecting photosynthetic efficiency and plant growth is temperature [24,25]. This research investigated the photosynthesis gas exchange and growth responses of P. spicatum and Z. mays to low temperature. The results showed that all seeds of both P. spicatum and Z. mays were able to complete germination under the two temperature treatments (Fig. 1). The ability of seeds to germinate over a wide range of temperatures is important for plant establishment in many different environments [26]. The results obtained showed that the optimum temperature for quick seed germination for both species was 24°C. Similar findings have been reported for seed germination of two pearl millet cultivars [27]. In addition, compared with Z. mays, P. spicatum presented a faster rate of germination under low temperature.
A substantial environmental variable that governs the development and growth of plants is temperature [24,28]. For both P. spicatum and Z. mays used in this study, all the growth parameters were significantly lower when the plants were grown at 14°C than when they were grown at 24°C (Tab. 1). The optimum temperature for the growth of seedlings of both species was 24°C. It was previously reported that the optimum temperature for Z. mays seedling growth ranged from 25°C to 28°C [2]. Compared with P. spicatum, Z. mays grown at 14°C grew better overall, especially in terms of leaf area and root length (Tab. 1). Muhl et al. [29] found that a considerably greater leaf area of Moringa (Moringa oleifera) increased the whole number of stomata per plant, which resulted in increased vegetative growth and dry matter accumulation. Thus, compared with P. spicatum, Z. mays grown at 14°C presented higher percent increases in fresh and dry weights of both the shoots and roots grown at the same temperature. The differences found can be explained by genetic variations between the two species examined [30].
In addition, the gas exchange parameters of P. spicatum and Z. mays in this study were significantly influenced by low temperature. The photosynthetic measurements showed that the optimal temperature for gas exchange of the two species was 24°C (Fig. 3). Additionally, both P. spicatum and Z. mays exhibited nearly similar quantum yields and carboxylation efficiencies at 24°C. For the leaves of all plants grown at 24°C, the photosynthetic rates of both P. spicatum and Z. mays were comparable and similar to previously documented values for healthy leaves from a variety of NADP-malic enzyme-type C4 grass species [31]. This shows that these two species were unstressed and not experiencing photoinhibition at the optimum temperatures and exhibited relatively high Fv/Fm. However, the 14°C temperature caused a significant decrease in Asat and Amax in Z. mays, and a substantial decrease in Asat, ϕ, Amax and carboxylation efficiency on P. spicatum compared with those of plants grown at 24°C. The photosynthesis inhibition of the two species grown at 14°C may be partly due to a decrease in stomatal conductance or a reduction in photosynthetic pigment content. All of these factors influence the activities of photosystem II (PSII), clearly leading to the deactivation of enzymes that play a key role in photosynthesis [32,33]. In both controlled environments and the field, a reduction in the photosynthetic efficiency of C4 species has previously been observed at low temperatures [2–3,8,33]. The decrease in photosynthesis of these two species could be the result of a reduction in the activity of both phosphoenolpyruvate carboxylase (PEPC) and pyruvate dikinase (PPDK) enzymes [22,34]. Other reasons for the decrease in the photosynthesis might be limitations in the transfer of electrons from the thylakoid membrane or decreases in ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) activity [35,36].
In many crop species that have different responses to low temperatures, a decrease in chlorophyll content has been identified [23,37,38]. Similarly, the findings in Fig. 4A show a decrease in the chlorophyll content of P. spicatum at low temperatures. This decrease may be caused by chlorophyll degeneration at this temperature [38]. Another possibility for this decrease in chlorophyll content may be due to the irregular development of chloroplasts [5,37]. On the other hand, the outcomes of this study provide clear proof that both P. spicatum and Z. mays showed significantly lower resistance to photoinhibition at low temperature. The low Fv/Fm values of both species grown at low temperature may be due to impaired development of the photosynthetic apparatus. Nie et al. [39] demonstrated that some polypeptides of chloroplasts were poorly expressed in Z. mays leaves that developed at 14°C. The abundance of these proteins, including the D1 protein, might account for the low values of Fv/Fm, even during the lack of direct photoinhibition.
This study investigated the growth response of P. spicatum and Z. mays by estimating the photosynthetic performance under low temperature stress. Low temperature significantly reduced all growth and physiological parameters, including seed germination, leaf expansion, leaf area, shoot length and root length, of the two species compared to those of the control plants. Moreover, the photosynthetic performance, the values of Fv/Fm and the chlorophyll contents of both species were significantly reduced by low temperature compared to those of the control. It can be summarize that both species had little tolerance to low temperatures.
Funding Statement: The author received no specific funding for this study.
Conflicts of Interest: The author declares that he has no conflicts of interest to report regarding the present study.
1. Hu, H. W., Zhou, Y. H., Du, Y. S., Xia, X. J., Yu, J. Q. (2006). Differential response of photosynthesis in greenhouse- and field-ecotypes of tomato to long-term chilling under low light. Journal of Plant Physiology, 163, 1238–1246. DOI 10.1016/j.jplph.2005.10.006. [Google Scholar] [CrossRef]
2. Muñoz, B. G., Lekfeldt, J. D. S., Magid, J., Jensen, L. S., De Neergaard, A. (2018). Seed treatment with Penicillium sp. or Mn/Zn can alleviate the negative effects of cold stress in maize grown in soils dependent on soil fertility. Journal of Agronomy and Crop Science, 204, 603–612. DOI 10.1111/jac.12288. [Google Scholar] [CrossRef]
3. Zhang, Q., Liu, Y., Yu, Q., Ma, Y., Gu, W. et al. (2020). Physiological changes associated with enhanced cold resistance during maize (Zea mays) germination and seedling growth in response to exogenous calcium. Crop and Pasture Science, 71, 529–538. DOI 10.1071/CP19510. [Google Scholar] [CrossRef]
4. Long, S. P., East, T. M., Baker, N. R. (1983). Chilling damage to photosynthesis in young Zea mays I. effect of light and temperature variation on photosynthetic CO2 assimilation. Journal of Experimental Botany, 34, 177–188. DOI 10.1093/jxb/34.2.177. [Google Scholar] [CrossRef]
5. Sowinski, P., Rudzinska-Langwald, A., Adamczyk, J., Kubica, I., Fronk, J. (2005). Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. Journal of Plant Physiology, 162, 67–80. DOI 10.1016/j.jplph.2004.03.006. [Google Scholar] [CrossRef]
6. Hund, A., Fracheboud, Y., Soldati, A., Stamp, P. (2008). Cold tolerance of maize seedlings as determined by root morphology and photosynthetic traits. European Journal of Agronomy, 28(3), 178–185. DOI 10.1016/j.eja.2007.07.003. [Google Scholar] [CrossRef]
7. Huang, X. S., Wang, W., Zhang, Q., Liu, J. H. (2013). A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiology, 162, 1178–1194. DOI 10.1104/pp.112.210740. [Google Scholar] [CrossRef]
8. Sun, S., He, Y., Irfan, A. R., Liu, X., Yu, Q. et al. (2020). Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy, 10, 488. DOI 10.3390/agronomy10040488. [Google Scholar] [CrossRef]
9. Naidu, S. L., Moose, S. P., AL-Shoaibi, A. A., Raines, C. A., Long, S. P. (2003). Cold-tolerant C4 photosynthesis in miscanthus x giganteus: Adaptation in amount and sequence of C4 photosynthetic enzymes. Plant Physiology, 132, 1688–1697. DOI 10.1104/pp.103.021790. [Google Scholar] [CrossRef]
10. Chen, Y., Jiang, J., Chang, Q., Gu, C., Song, A. et al. (2014). Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Molecular Biology Reports, 41, 815–822. DOI 10.1007/s11033-013-2921-8. [Google Scholar] [CrossRef]
11. Sage, R. F. (2004). The evolution of C4 photosynthesis. New Phytologist, 161, 341–370. DOI 10.1111/j.1469-8137.2004.00974.x. [Google Scholar] [CrossRef]
12. Milford, G. F., Riley, J. (1980). The effects of temperature on leaf growth of sugar beet varieties. Annals of Applied Biology, 94, 431–443. DOI 10.1111/j.1744-7348.1980.tb03959.x. [Google Scholar] [CrossRef]
13. Tambussi, E. A., Bartoli, C. G., Guiamet, J. J., Beltrano, J., Araus, J. L. (2004). Oxidative stress and photodamage at low temperatures in soybean (Glycine max L. merr.) leaves. Plant Science, 167, 19–26. DOI 10.1016/j.plantsci.2004.02.018. [Google Scholar] [CrossRef]
14. Kościelniak, J., Janowiak, F., Kurczych, Z. (2005). Increase in photosynthesis of maize hybrids (Zea mays L.) at suboptimal temperature (15°C) by selection of parental lines on the basis of chlorophyll a fluorescence measurements. Photosynthetica, 43(1), 125–134. DOI 10.1007/s11099-005-5134-0. [Google Scholar] [CrossRef]
15. Beale, C. V., Long, S. P. (1995). Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates. Plant Cell and Environment, 18, 641–650. DOI 10.1111/j.1365-3040.1995.tb00565.x. [Google Scholar] [CrossRef]
16. Long, S. P. (1999). Environmental responses. in Sage R. F. and Monson R. K. (Eds.C4 plant biology, pp. 215–249. San Diego: Academic Press. [Google Scholar]
17. Savitch, L. V., Massacci, A., Gray, G. R., Huner, N. P. A. (2000). Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: Roles of the calvin cycle and the mehler reaction. Australian Journal of Plant Physiology, 27, 253–264. DOI 10.1071/PP99112. [Google Scholar] [CrossRef]
18. Adams, W. W., Muller, O., Cohu, C. M., Demmig-Adams, B. (2013). May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynthesis Research, 117, 31–44. DOI 10.1007/s11120-013-9849-7. [Google Scholar] [CrossRef]
19. Fryer, M. J., Andrews, J. R., Oxborough, K., Blowers, D. A., Baker, N. R. (1998). Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology, 116, 571–580. DOI 10.1104/pp.116.2.571. [Google Scholar] [CrossRef]
20. Lee, E. A., Staebler, M. A., Tollenaar, M. (2002). Genetic variation and physiological discrimination for cold tolerance in maize (Zea mays L.) during an early autotrophic phase of development. Crop Science, 42, 1919–1929. DOI 10.2135/cropsci2002.1919. [Google Scholar] [CrossRef]
21. Von Caemmerer, S., Farquhar, G. D. (1981). Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta, 53, 376–387. DOI 10.1007/BF00384257. [Google Scholar] [CrossRef]
22. Collatz, G. J., Ribas-Carbo, M., Berry, J. A. (1992). Coupled photosynthesis-stomatal model for leaves of C4 plants. Australian Journal of Plant Physiology, 19, 519–538. DOI 10.1071/PP9920519. [Google Scholar] [CrossRef]
23. Al-Shoaibi, A. A. (2008). Photosynthetic response to the low temperature in elephant grass (Pennisetum purpureum) and Zea mays. International Journal of Botany, 4(3), 309–314. DOI 10.3923/ijb.2008.309.314. [Google Scholar] [CrossRef]
24. Zhao, T. T., Li, Y. X., He, J. D. (2014). Effect of temperature stress on seed germination of Chimonanthus praecox. Journal of Mianyang Normal University, 33, 63–68. [Google Scholar]
25. Wang, Q., Qu, B., Mi, J., Ma, L., Xu, Y. et al. (2020). The effect of temperature on seed germination and seedling growth of two invasive plants in roroppa. Biotechnology Journal International, 24(5), 39–48. DOI 10.9734/bji/2020/v24i530116. [Google Scholar] [CrossRef]
26. Qu, X. X., Huang, Z. Y., Baskin, J. M., Baskin, C. C. (2008). Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Annals of Botany, 101, 293–299. DOI 10.1093/aob/mcm047. [Google Scholar] [CrossRef]
27. Al-Shoaibi, A. A., Al-Sobhi, O. A. (2007). Effect of naCl salinity and incubation temperature on the germination of two cultivars of pearl millet. Bioscience, Biotechnology Research Asia, 4(1), 1–4. [Google Scholar]
28. Jafarzadeh, A. A., Aliasgharzad, N. (2007). Salinity and salt composition effects on seed germination and root length of four sugar beet cultivars. Biología, 2(5), 562–564. DOI 10.2478/s11756-007-0111-7. [Google Scholar] [CrossRef]
29. Muhl, Q. E., Toit, E. S., Robbertse, P. J. (2011). Moringa oleifera (Horseradish tree) leaf adaptation to temperature regimes. International Journal of Agriculture and Biology, 13, 1021–1024. [Google Scholar]
30. Muraya, M. M., Chu, J., Zhao, Y., Junker, A., Klukas, C. et al. (2017). Genetic variation of growth dynamics in maize (Zea mays L.) revealed through automated non-invasive phenotyping. The Plant Journal, 89, 366–380. DOI 10.1111/tpj.13390. [Google Scholar] [CrossRef]
31. Ehleringer, J. R., Pearcy, R. W. (1983). Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology, 73, 555–559. DOI 10.1104/pp.73.3.555. [Google Scholar] [CrossRef]
32. Ashraf, M., Harris, P. J. C. (2013). Photosynthesis under stressful environments: An overview. Photosynthetica, 51, 163–190. DOI 10.1007/s11099-013-0021-6. [Google Scholar] [CrossRef]
33. Sharma, A., Kumar, V., Shahzad, B., Ramakrishnan, M., Sidhu, G. P. S. et al. (2019). Photosynthetic response of plants under different abiotic stresses: A review. Journal of Plant Growth Regulation, 39, 509–531. DOI 10.1007/s00344-019-10018-x. [Google Scholar] [CrossRef]
34. Furbank, R. T., Chitty, J. A., Jenkins, C. L. D., Taylor, W. C., Trevanion, S. J. et al. (1997). Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Australian Journal of Plant Physiology, 24, 477–485. DOI 10.1071/PP97028. [Google Scholar] [CrossRef]
35. Shabala, S., Shabala, L. (2002). Kinetics of net H+, Ca2+, K+, Na+, NH4+, and Cl– fluxes associated with post-chilling recovery of plasma membrane transporters in Zea mays leaf and root tissues. Physiologia Plantarum, 114, 47–56. DOI 10.1046/j.0031-9317.2001.1140108.x. [Google Scholar] [CrossRef]
36. Morales, F., Ancín, M., Fakhet, D., González-Torralba, J., Gámez, A. L. et al. (2020). Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement: A review. Plants, 9(1), 88. DOI 10.3390/plants9010088. [Google Scholar] [CrossRef]
37. Haldimann, P. (1999). How do changes in temperature during growth affect leaf pigment composition and photosynthesis in Zea mays genotypes differing in sensitivity to low temperature? Journal of Experimental Botany, 50, 543–550. DOI 10.1093/jxb/50.333.543. [Google Scholar] [CrossRef]
38. Bhandari, S. R., Kim, Y. H., Lee, J. G. (2018). Detection of temperature stress using chlorophyll fluorescence parameters and stress-related chlorophyll and proline content in paprika (Capsicum annuum L.) Seedlings.Horticultural Science and Technology, 36, 619–629. DOI 10.12972/kjhst.20180062. [Google Scholar] [CrossRef]
39. Nie, G. Y., Robertson, E. J., Fryer, M. J., Leech, R. M., Baker, N. R. (1995). Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant Cell and Environment, 18, 1–12. DOI 10.1111/j.1365-3040.1995.tb00538.x. [Google Scholar] [CrossRef]
Appendix

Figure S1: Pearl millet (Pennisetum spicatum (L.) Körn.) and maize (Zea mays L.) grown at temperature treatments of 24°C and 14°C
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |