

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.021273
ARTICLE
In vitro Germination and Micropropagation of Aconitum vilmorinianum: An Important Medicinal Plant in China
1School of Ecology and Environmental Science, Yunnan University, Kunming, 650504, China
2Biocontrol Engineering Research Center of Plant Disease and Pest, Yunnan University, Kunming, 650504, China
3Biocontrol Engineering Research Center of Crop Disease and Pest, Yunnan University, Kunming, 650504, China
4Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, 650500, China
*Corresponding Author: Dake Zhao. Email: zhaodk2012@ynu.edu.cn
Received: 05 January 2022; Accepted: 21 February 2022
Abstract: Aconitum vilmorinianum, a well-known traditional Chinese herb, is recently being threatened by overexploitation and environment disturbance. This study was conducted to provide propagation methods through in vitro germination and explant cultivation. Germination was stimulated up to 66.00% on Murashige and Skoog (MS) medium containing 2.0 mg L−1 6-benzylaminopurine (BAP), 0.1 mg L−1 1-napthaleneacetic acid (NAA), and 30 g L−1 sucrose. Three bacteria (Pantoea agglomerans, Erwinia persicina, and Pseudomonas tolaasii) would be responsible for consistent contamination during germination. The latter two were effectively eradicated after disinfected. The influence of explant types and hormone combinations on direct and indirect organogenesis was evaluated in the present work. The frequency of shoot induction from axillary bud explants was 100% on the MS fortified with 2.0 mg L−1 BAP and 0.3 mg L−1 NAA. Shoots multiplication was optimized on MS medium supplemented with 0.1 mg L−1 thidiazuron (TDZ) and 0.1 mg L−1 NAA. High callus induction percentage (96.67%) was obtained from stem segments on MS medium with 2.0 mg L−1 2,4-D, then successfully regenerated into shoots on MS medium in the presence of 0.1 mg L−1 TDZ and 0.2 mg L−1 NAA. The present work could be useful for the utilization and conservation of this valuable species.
Keywords: Aconitum vilmorinianum; seed surface bacteria; in vitro multiplication; organogenesis
Aconitum vilmorinianum Kom. (Ranunculaceae) is a perennial herb mainly distributed in southwestern China at 1800–2500 m elevation. The tuberous roots of A. vilmorinianum are an ideal medicine for treating rheumatoid arthritis and various types of pains [1–3]. Since antiquity, the species has been broadly used in many traditional Chinese medicines, and one of the most representatives is Yunnan Baiyao (famous for treating wounds). Pharmacological studies demonstrated that the major components of tuberous roots of A. vilmorinianum are alkaloids, most of which showed significant analgesic and anti-inflammatory effects [4,5]. However, some of these alkaloids, such as yunaconitine, are highly toxic [3,6,7]. The BAHD (an acronym for the first four biochemically characterized enzymes of acyltransferase family, namely benzylalcohol O-acetyltransferase, anthocyanin O-hydroxycinnamoyltransferase, N-hydroxycinnamoyl/benzoyltransferase, and deacetylvindoline 4-O-acetyltransferase) acyltransferases which were closely associated with the biosynthesis of acute toxicity of A. carmichaelii were identified, and future research into the molecular basis for toxicant biosynthesis can be achieved by genetic transformation [8].
Due to the great biological activities of A. vilmorinianum, this species is jeopardized by heavy exploitation from local residents and pharmaceutical companies in natural habitats. Moreover, along with environmental disturbance, the wild resources of A. vilmorinianum have been dramatically reduced. Regeneration from seeds is a common way of reproduction in many plant species. For A. vilmorinianum, seeds are difficult to germinate under common conditions, and seedling survival is quite poor under natural conditions. The conventional approach of A. vilmorinianum propagation based on the cultivation of tuberous roots frequently results in various problems such as virus infection and degeneration of genetics, which can lead to a decrease in medicinal parts yield and quality. For the reasons mentioned above, establishing a high-efficiency regeneration system is indispensable for species conservation, pharmaceutical industry, reduction of toxicity by genetic modification, and investigations on bioactive component production of A. vilmorinianum.
Propagation protocols have been developed for many economically important plants, and several studies based-on in vitro regeneration of the genus Aconitum have been reported [9–15]. However, there are limited published works on a regeneration for A. vilmorinianum [16]. The present study was aimed to establish the regeneration system of A. vilmorinianum through in vitro germination and explant cultivation. To be specific, the effect of plant hormones on seed germination, direct shoot organogenesis, and indirect shoot organogenesis were evaluated, and the interaction of explant sources and plant hormones was also studied. This represents the first comprehensive report of an in vitro study of this valuable medicinal plant.
Healthy growing seeds and bulbils of A. vilmorinianum were collected in November, Kunming, Yunnan Province, China.
2.2 Culture Medium and Experimental Conditions
Murashige and Skoog (MS) medium supplemented with 30 g L−1 sucrose and 6.5 g L−1 agar were used as a basic culture medium in this study. The pH of the medium was adjusted to 5.8 prior to autoclaving at 121°C and 15 psi pressure for 15 min. The cultures were incubated at 20 ± 2°C under a 12 h photoperiod with a light intensity of 2000 lux. The humidity was 70%–80%.
Seeds were air-dried at room temperature and stored in a refrigerator at 4°C for two months. The experiments of seed’s water uptake and effects of GA3 on dormancy break tests were conducted with 100 seeds per replication, and five replicates per treatment. The effects of sterilization agents, plant growth regulators, and sucrose on germination were evaluated using six replicates of 10 seeds.
For the seed’s water uptake experiment, seeds were immersed in distilled water at 25°C after weighting, and then taken out at 3 h-intervals up to 36 h. Wet weight of each replicate was recorded at every time point after drained on a filter paper.
Effect of gibberellic acid (GA3) on seed dormancy break
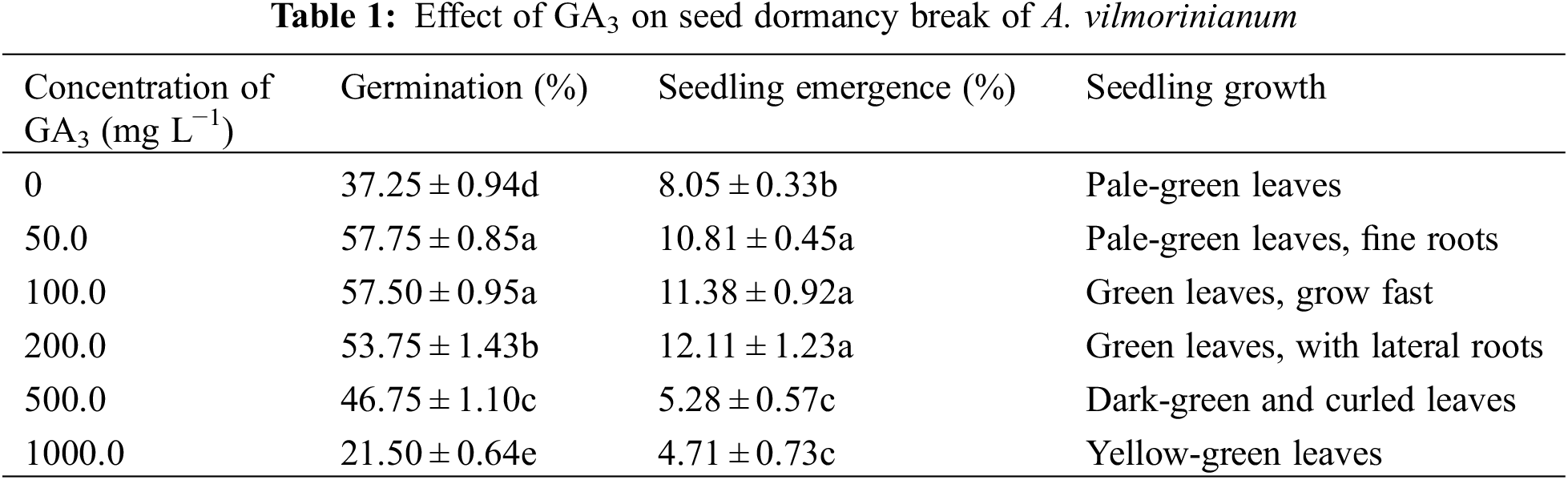
The ability of GA3 to overcome dormancy was examined. Seeds were soaked in 0, 50.0, 100.0, 200.0, 500.0, and 1000.0 mg L−1 GA3 solutions for 21 h, then placed on two layers of filter paper in 9.0 cm diameter Petri-dishes and moistened with distilled water. Seeds were tested for germination and seedling growth after 30 days.
Seed sterilization
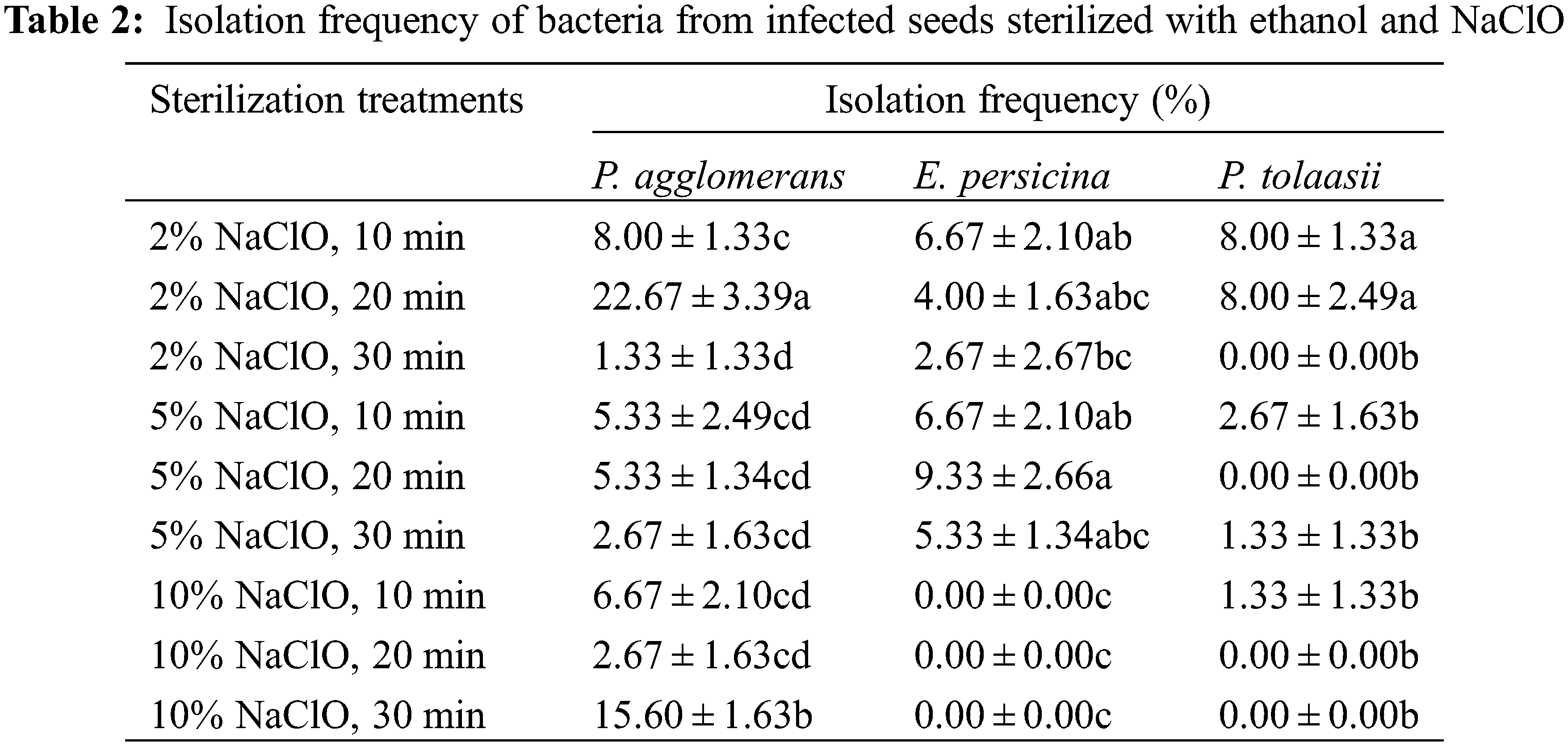
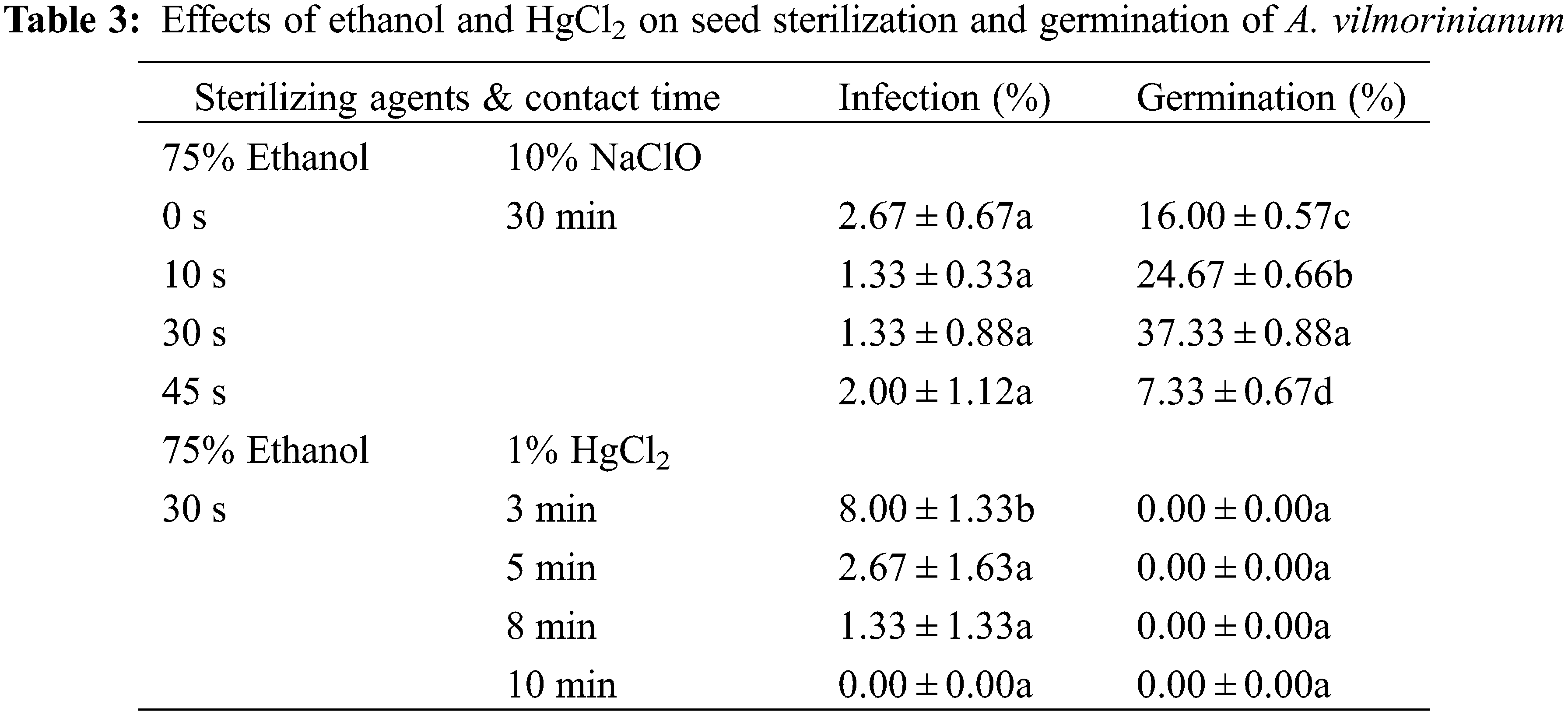
In view of the severe bacterial contamination in pretest studies of in vitro germination, investigations on seed sterilization had been focused on three aspects to determine the optimal sterilization protocol. All tested seeds had been soaked in 100.0 mg L−1 GA3 solution for 21 h before being treated. Firstly, morphology characteristics of seeds were observed. Seeds were cleaned with 75% (W/V) alcohol solution and kept for drying. The general morphology of the seed coat was characterized by a scanning electron microscope (KYKY 1000B, Apparatus Factory, KYKY Technology Co., Ltd., Beijing, China) after vacuum coating with a thin layer of gold-palladium by a sputtering apparatus (SBC-1, Apparatus Factory, KYKY Technology Co., Ltd., Beijing, China). In the second test, the sterilization effect of sodium hypochlorite (NaClO) was evaluated. Seeds were surface sterilized in a 75% ethanol solution for 30 s, then in 2%, 5%, or 10% (W/V) NaClO for 10, 20, or 30 min, respectively. After the process, seeds were rinsed with sterile distilled water for several times, then cultured on MS basal medium for 30 days. Infected seeds were counted after incubation. To further address the contamination during the germination process, strains from the infected culture media were incubated in Luria-Bertani medium [17] for one week and then genomic DNA was extracted by the CTAB method [18]. PCR was taken using the universal primers 1492R and 27F. After that, sequencing of the16S rRNA gene was performed. All of the sequences were deposited in the GenBank of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/). The isolation frequency of a single strain was calculated as the ratio of the number of media from which the strain was isolated to the total number of tested media. In the third test, the sterilization protocols were optimized by varying the contact time of 75% ethanol from 0 to 45 s and replacing NaClO with 0.1% (W/V) mercuric chloride (HgCl2) from 3 to 10 min. Germinated seeds and infected seeds were counted after 30 days of incubation.
Effects of plant growth regulators on seed germination
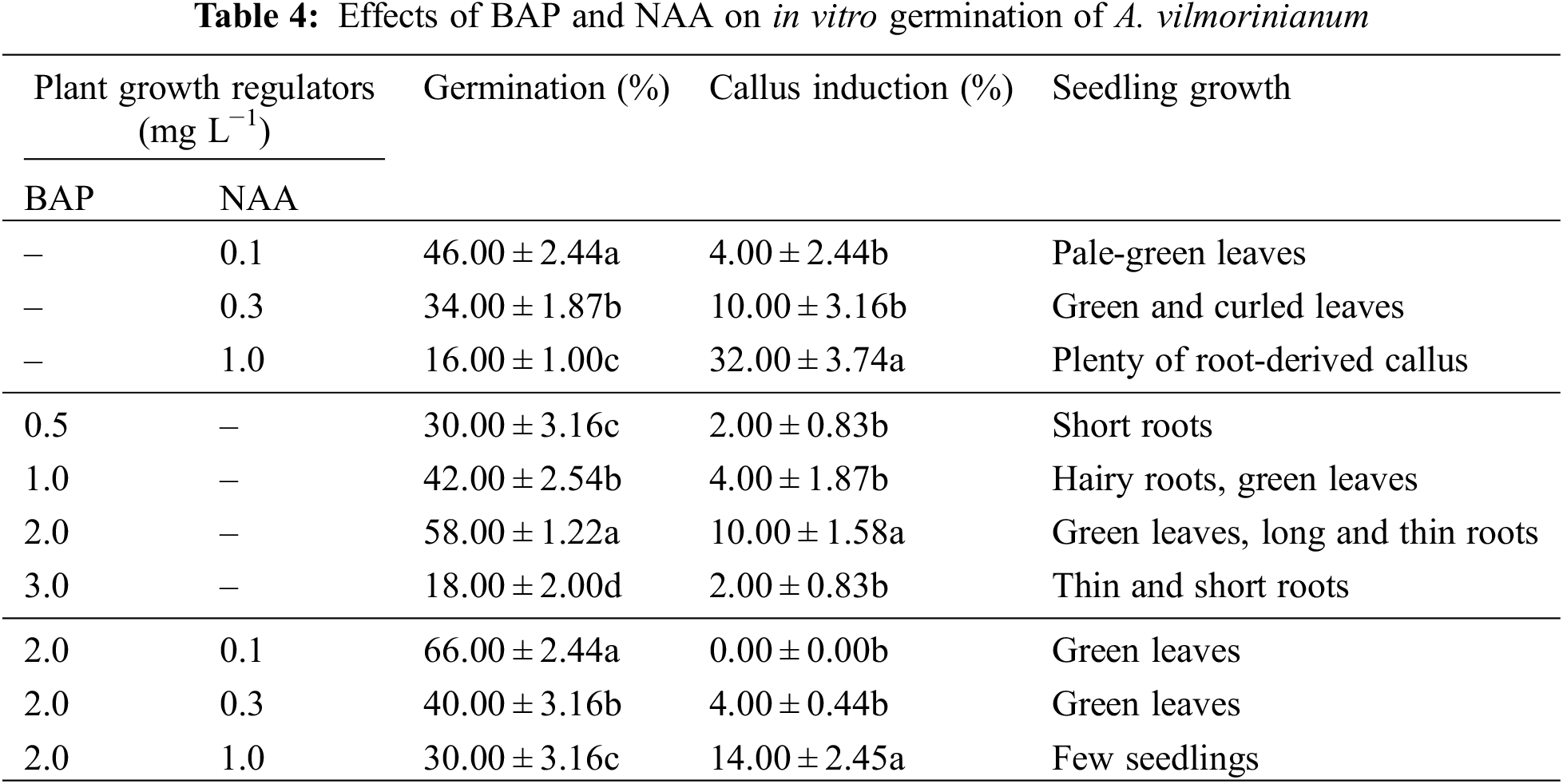
Seeds were disinfected with 75% ethanol for 30 s and then with 10% NaClO for 30 min, followed by repeated rinses with sterilized distilled water. Seeds were placed on MS basal medium supplemented with different concentrations of 6-benzylaminopurine (BAP) (0.5, 1.0, 2.0, 3.0 mg L−1) or 1-napthaleneacetic acid (NAA) (0.1, 0.3, 1.0 mg L−1). The combined effects of BAP (2.0 mg L−1) and NAA (0.1, 0.3, 1.0 mg L−1) on seed germination were setup to find out the optimal plant growth regulator combinations for seed germination. Four weeks after culture initiation, germination percentage, callus induction frequency, and seedling performance were recorded.
Effects of sucrose concentration on seed germination
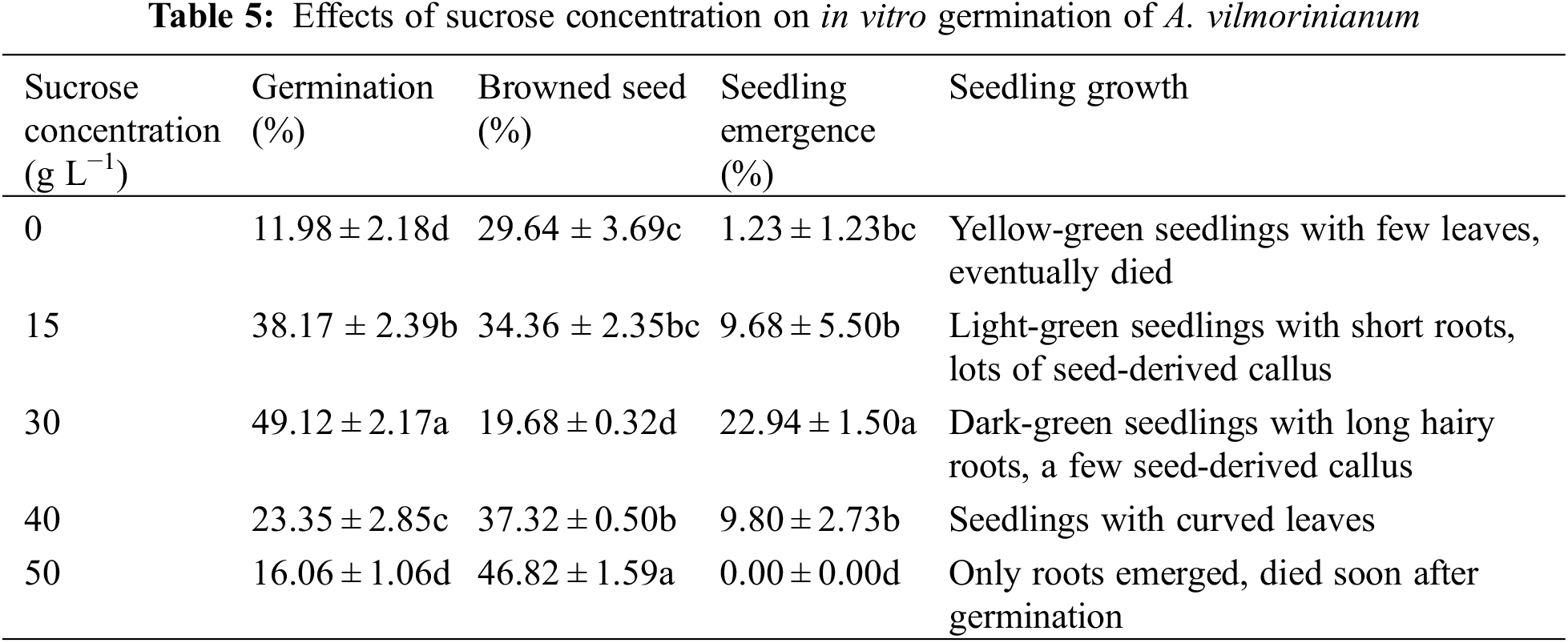
Seeds were pretreated with 100 mg L−1 GA3 solution for 21 h. Subsequently, seeds were surface sterilized with 75% ethanol for 30 s and 10% NaClO for 30 min, and then washed several times by sterilized distilled water. Seeds were placed on MS basal medium (without sucrose) supplemented with different concentrations of sucrose (0, 15.0, 30.0, 40.0, or 50.0 g L−1) in combination with 2.0 mg L−1 BAP and 0.1 mg L−1 NAA. After 30 days of incubation, the percentage of germination, browned seed, and seedling emergence were calculated.
Bulbils were planted in pots filled with sterile humus, vermiculite and perlite (1:1:1, V/V), and kept inside a greenhouse. Two weeks after planting, bulbil-producing plants were used as a source of explants for shoot multiplication. Three explants were cultured per flask and 5 replications were prepared for each treatment.
Explant sterilization
Nodal explants with axillary buds were washed thoroughly by running water and dried on filter paper. After that, explants were surface sterilized in 0.1% (W/V) HgCl2 for 3, 6, 10, and 15 min, followed by 12 rinses with sterile distilled water. The explants were cut into 1 cm segments with one axillary bud. They were placed vertically on MS basal medium. One week later, the number of infected or browned explants, the percentage of shoot induction, and the growth status of new shoots were recorded.
Effects of explant sources on shoots induction
Shoot tips, the uppermost axillary buds (with a mature leaf), and the second axillary buds (with a mature leaf) were used as different explant sources to test for the induction of shoots. Explants were surface sterilized in 0.1% (W/V) HgCl2 for 6 min, rinsed 12 times with sterile distilled water and used for culturing on MS basal medium supplemented with 2.0 mg L−1 BAP and 0.3 mg L−1 NAA for 15 days. The number of browned explants, the percentage of shoot induction, and the growth status of new shoots were recorded.
Effects of plant growth regulators on shoots induction and multiplication
The uppermost axillary buds (with a mature leaf) were used for shoot regeneration on MS basal medium with different concentrations and combinations of plant growth regulators. Explants were surface sterilized in 0.1% (W/V) HgCl2 for 6 min, rinsed 12 times with sterile distilled water and used for culturing on MS basal medium supplemented with various combinations of BAP (1.0, 2.0, 3.0 mg L−1) and NAA (0.1, 0.3, 0.5 mg L−1). After 15 days cultivation, the percentage of shoot induction, the number of shoots per explant, and shoot growth were recorded. Where after, single shoots, separated from the newly formed shoot clumps, were transferred to a multiplication medium supplemented with thidiazuron (TDZ) (0.1, 1.0, 2.0 mg L−1) and NAA (0.1, 0.3 mg L−1). The number of multiplied shoots of each culture was counted after 15 days.
2.2.3 Callus Induction and Re-Differentiation
The stem, leaf, and petiole explants from bulbil-producing plantlets were tested for their capability of callus formation by using a serious of auxins and cytokines including BAP, kinetin, NAA, and 2, 4-dichlorophenoxyacetic acid (2,4-D), either singly or in combination. All trials were performed using five replicates of 5 explants. Explants were surface sterilized in 0.1% (W/V) HgCl2 for 6 min, rinsed 12 times with sterile distilled water and cut into 1 cm segments, then cultured on MS basal media supplemented with 2,4-D and kinetin singly or in combination, at the concentration of 0.1, 0.5, 1.0, 2.0, and 3.0 mg L−1. In addition, NAA (0.1, 0.5 mg L−1) and 2,4-D (1.0, 2.0 mg L−1) were used to make combinations with BAP (1.0, 2.0 mg L−1). The combinations were listed in Tables 10 and 11. After 30 days of culture initiation, the frequency of callus induction was recorded. Induced callus was further used for shoot induction and transferred to a regeneration medium consisting of TDZ (0.5, 1.0, 2.0 mg L−1) and NAA (0.1, 0.2, 0.5 mg L−1). The re-differentiation of shoots from callus was observed after 30 days.
Statistical analysis
The effects of different treatments were quantified as mean ± SE and the data were subjected to statistical analysis using ANOVA and Duncan test at 5% level significance by SPSS 16.0 software. Figures were plotted using SigmaPlot 14.0.
The uptake of water by A. vilmorinianum seed was triphasic (Fig. 1). The initial uptake during 3 to 9 h was rapid, followed by a slowly rising phase at 9 to 18 h, and came to plateau phase after 18 h. The peak of water uptake rate was at 21 h.
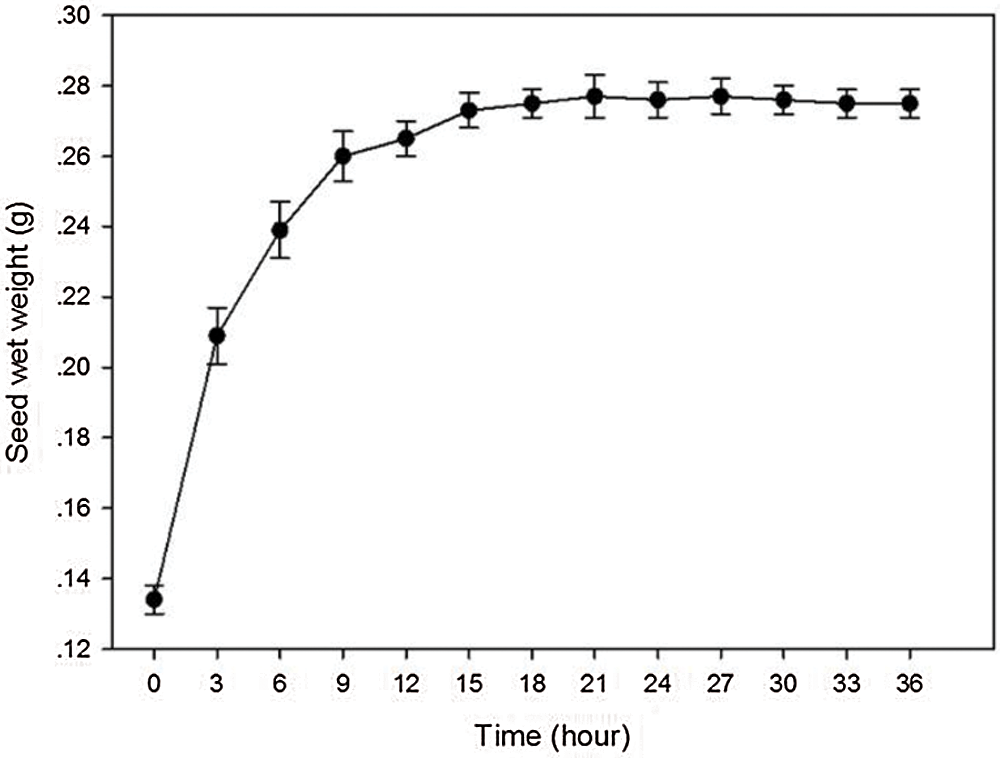
Figure 1: Seed’s water uptake rate of A. vilmorinianum
3.1.1 Effect of GA3 on Seed Dormancy Break
Most of the GA3 treated seeds germinated after 7 days whereas untreated seeds required 20 days (data not shown). When treated with 50.0 and 100.0 mg L−1 GA3, germination was mostly enhanced up to 57.75% and 57.50%, respectively, compared with untreated seeds of 37.25% (Table 1). The 100.0 mg L−1 GA3 treated seeds performed better than 50.0 mg L−1 GA3 on seedling formation, and seedlings grew fast and robustly. Medium containing 200.0, 500.0, and 1000.0 mg L−1 GA3 showed gradually decreased effects on germination. The highest seedling emergence rate of 57.75% was found when seeds were treated with 50.0 mg L−1 GA3, although this value was not significantly different to that of seeds treated with 100.00 mg L−1 GA3. Higher concentrations of GA3 (500.0 and 1000.0 mg L−1) determined significant inhibiting effects on germination, seedling emergence, and seedling growth when compared to untreated and lower GA3 concentration solution treated seeds.
Morphology characteristics of seeds have a significant effect on sterilization efficiency. The observations on seed coat structure of A. vilmorinianum revealed that the seeds had a flat kidney shape and wrinkled surface. Furrows on seed surface were observed at 1000 times of magnification, which was much clearer at 5000 times of magnification (Fig. 2). These dense furrows were most likely to provide shelters for microorganisms in the case of using different sterilizing agents.
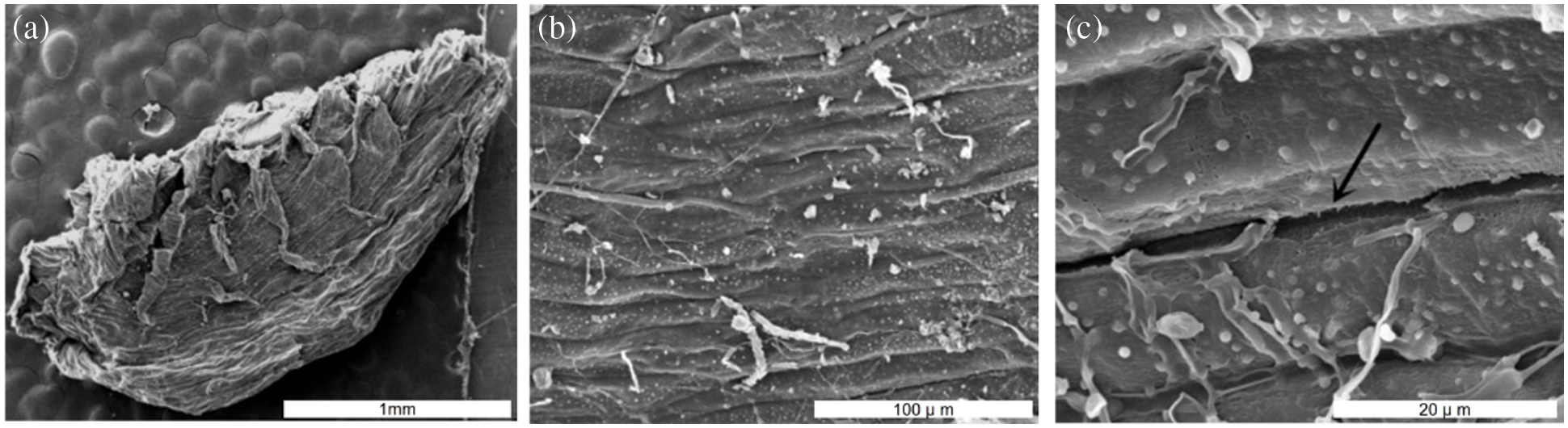
Figure 2: Scanning electron microscopy photographs of A. vilmorinianum seeds. a entire seed at 20 times magnification; b seed surface 1000 times magnification; c seed surface at 5000 times magnification
Ethanol and NaClO were used in tandem to determine the efficiency of sterilization against infection during germination. Treatment with 75% ethanol for 30 s and then with 2% NaClO for 10 to 30 min resulted in a high infection rate of more than 50% (Fig. 3). Increasing concentration and contact time of NaClO showed a remarkable reduction in infection. Using 10% NaClO for 30 min reduced the infection to the minimum (1.33%).
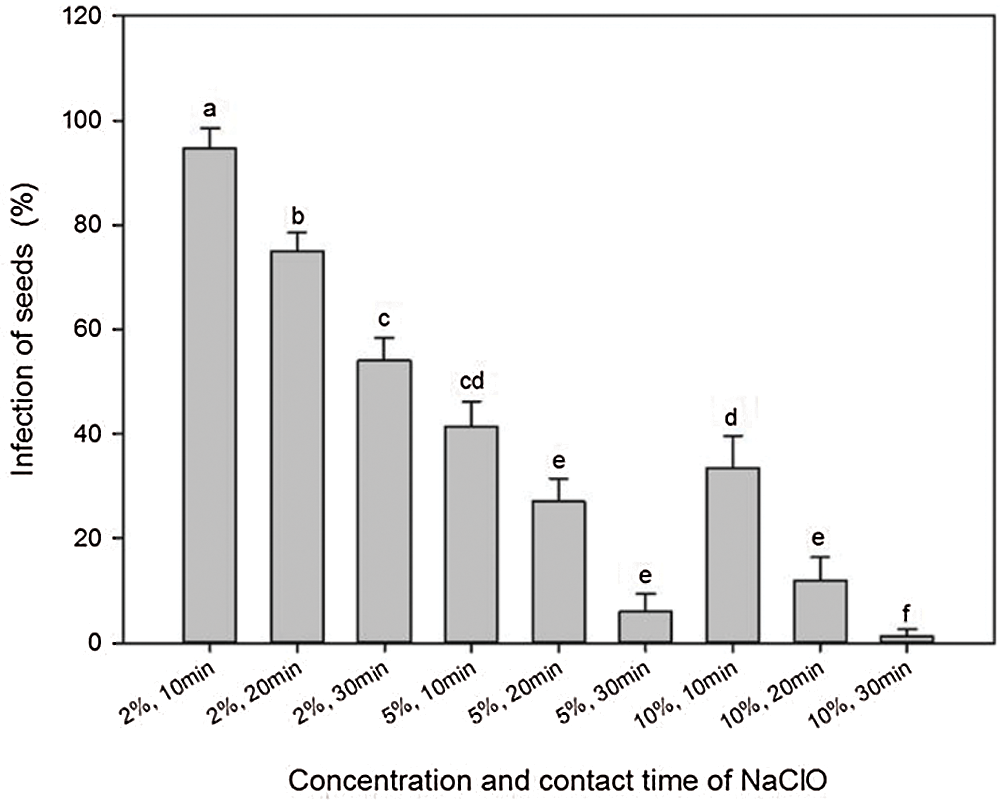
Figure 3: Effects of concentration and contact time of NaClO on seed sterilization of A. vilmorinianum
To further address the infection problem, strains were isolated from infected seeds sterilized with ethanol and NaClO. Analysis of 16S rRNA gene sequences for three strains showed high levels of similarity to the species of Pantoea agglomerans (99.58%), Erwinia persicina (99.93%), and Pseudomonas tolaasii (99.78%). The sequences reported here have been deposited in the GenBank database, under the accession numbers MT561437, MT561438, and MT561439, respectively. All three bacteria are gram-negative. Table 2 showes that E. persicina and P. tolaasii were eradicated with increasing contact time and concentration of NaClO, and the optimal concentration was 10.00%. P. agglomerans could not be eliminated by NaClO soak in this study.
Optimizations of the sterilization protocol were conducted by varying the contact time of 75% ethanol from 0 to 45 s and replacing NaClO with 0.1% HgCl2 from 3 to 10 min. The results showed no significant difference among the ethanol treatments with respect to the percentage of infection (Table 3). Nevertheless, germination percentage increased with sterilization time when seeds were treated with ethanol from 0 up to 30 s, and then reduced in the treatment of 45 s. The highest germination percentage (37.33%) was in the 30 s treatment with 75% ethanol. On the contrary, no germination occurred when seeds were disinfected with 0.1% HgCl2 while minor seeds still got infected.
In general, seeds sterilized with 75% ethanol for 30 s and then with 10% NaClO for 30 min performed best on germination, with an acceptable infection rate and the highest germination percentage.
3.1.3 Effects of Plant Growth Regulators on in vitro Germination
BAP and NAA were tested for their effects on germination. Seed-derived callus was obtained in most of the treatments. When NAA was used singly or in combination with BAP, germinated percentage decreased with the NAA increasing concentrations whereas callus induction frequency increased with increasing concentrations of NAA (Table 4). The maximum callus induction (32.00%) was obtained at 1.0 mg L−1 NAA alone. BAP had positive effect on seed development. When BAP was used alone, the highest germination percentage and callus induction frequency, i.e., 58.00% and 10.00%, were observed in the 2.0 mg L−1 treatment, but in higher concentration of BAP (3.0 mg L−1) the percentage of germination and callus induction were reduced to 18.00% and 2.00%, respectively. The synergistic effect of NAA with BAP on seed germination was observed. Germination was effectively enhanced up to a maximum of 66.00%, without any callus induction, by adding 0.1 mg L−1 NAA and 2.0 mg L−1 BAP to the medium.
3.1.4 Effects of Sucrose Concentration on in vitro Germination
Few seeds germinated and most of them failed to grow into healthy seedlings without sucrose (Table 5). The addition of sucrose to the medium improved seed germination and seedling growth. Seeds performed best with regard to germination (49.12%), browned seed (19.68%), and seedling emergence (22.94%) on MS medium supplemented with 30 g L−1 sucrose. With further increasing concentration of sucrose, the percentage of germination and seedling emergence decreased significantly. For instance, increasing sucrose concentration up to 50 g L−1, only 16.06% of the seeds germinated but failed to establish as seedlings after 30 days of incubation.
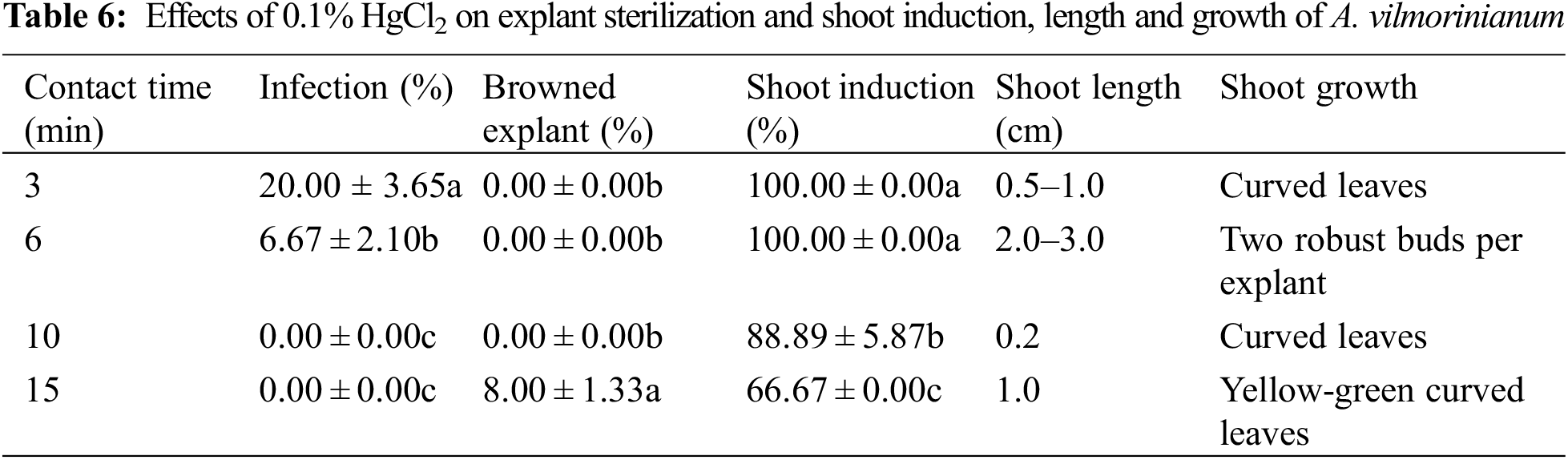
It was found that increasing contact time of 0.1% HgCl2 from 3 to 15 min significantly reduced infection but showed adverse effects on shoot formation (Table 6). Infection was completely eliminated by extending contact time over 10 min. However, increasing contact time to 15 min caused explant browning, reduction of shoot induction capability, and poorly grown shoots. Disinfected with 0.1% HgCl2 for 6 min was proved to be the most efficient sterilization method due to acceptable percentage of infection (6.67%), perfect adventitious shoot induction (100.00%), and well-grown new shoots.
3.2.2 Effects of Explant Sources on Shoot Induction
Different sources of explants were cultured on MS basal medium supplemented with 2.0 mg L−1 BAP and 0.3 mg L−1 NAA. Data in Table 7 show that 100% of the uppermost axillary bud explants and 91.67% of the second axillary bud explants produced new shoots. The newly formed shoots from the uppermost axillary buds were obviously stronger than those from the second axillary buds in terms of robust stems and green leaves (Fig. 4a). A small percentage of explants turned brown in both explants. Meanwhile, none of the shoot tips turned brown but they developed much less shoots (8.33%) than the other two explants with axillary buds. Abundant calluses were formed at the basal end of the shoot tip explants, which were not conducive to shoot induction. The results suggested that the uppermost axillary buds seemed to be the most suitable explants for shoot induction among the three explants.
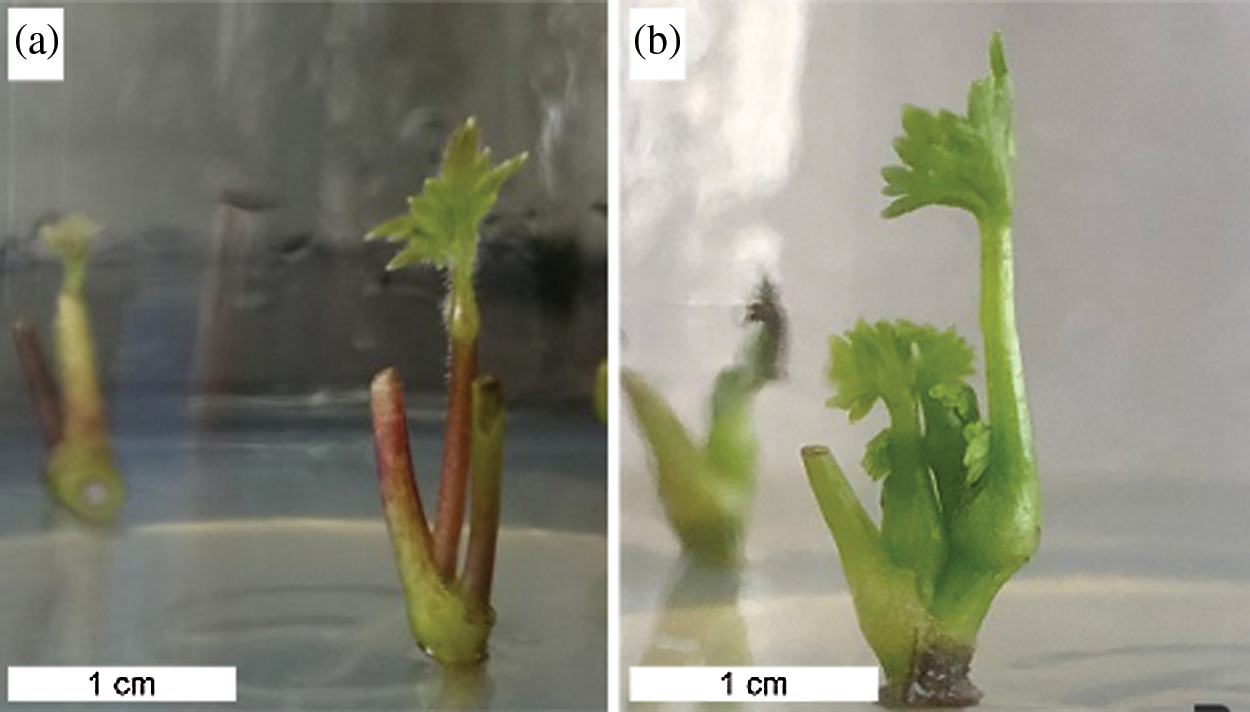
Figure 4: Shoots regeneration of A. vilmorinianum. a shoots induction from the uppermost axillary buds; b shoots multiplication
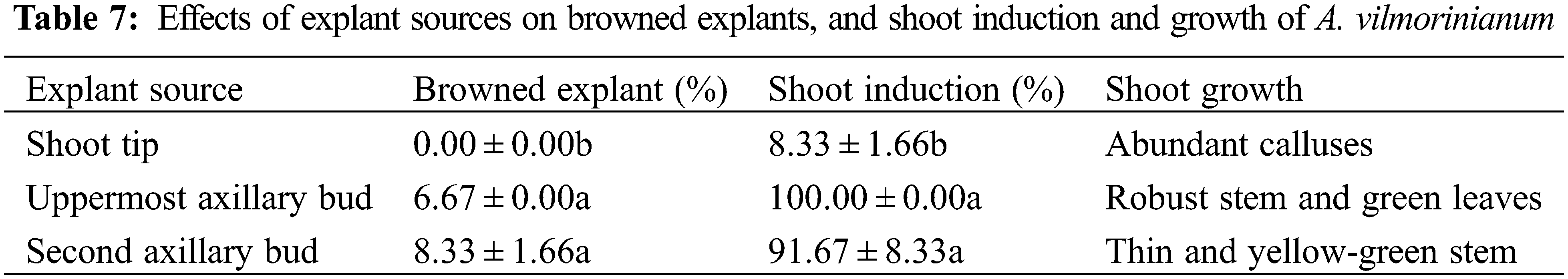
3.2.3 Effects of Plant Growth Regulators on Shoot Induction and Multiplication
All of the uppermost axillary bud explants produced shoots on MS medium supplemented with 1.0 mg L−1 BAP and 0.5 mg L−1 NAA, 2.0 mg L−1 BAP and 0.1 mg L−1 NAA, and 2.0 mg L−1 BAP and 0.3 mg L−1 NAA (Table 8). Among the three treatments aforementioned, 2.0 mg L−1 BAP in combination with 0.3 mg L−1 NAA performed best on shoot induction because of the highest number of shoots per explant (2.39) and well-grown healthy shoots.
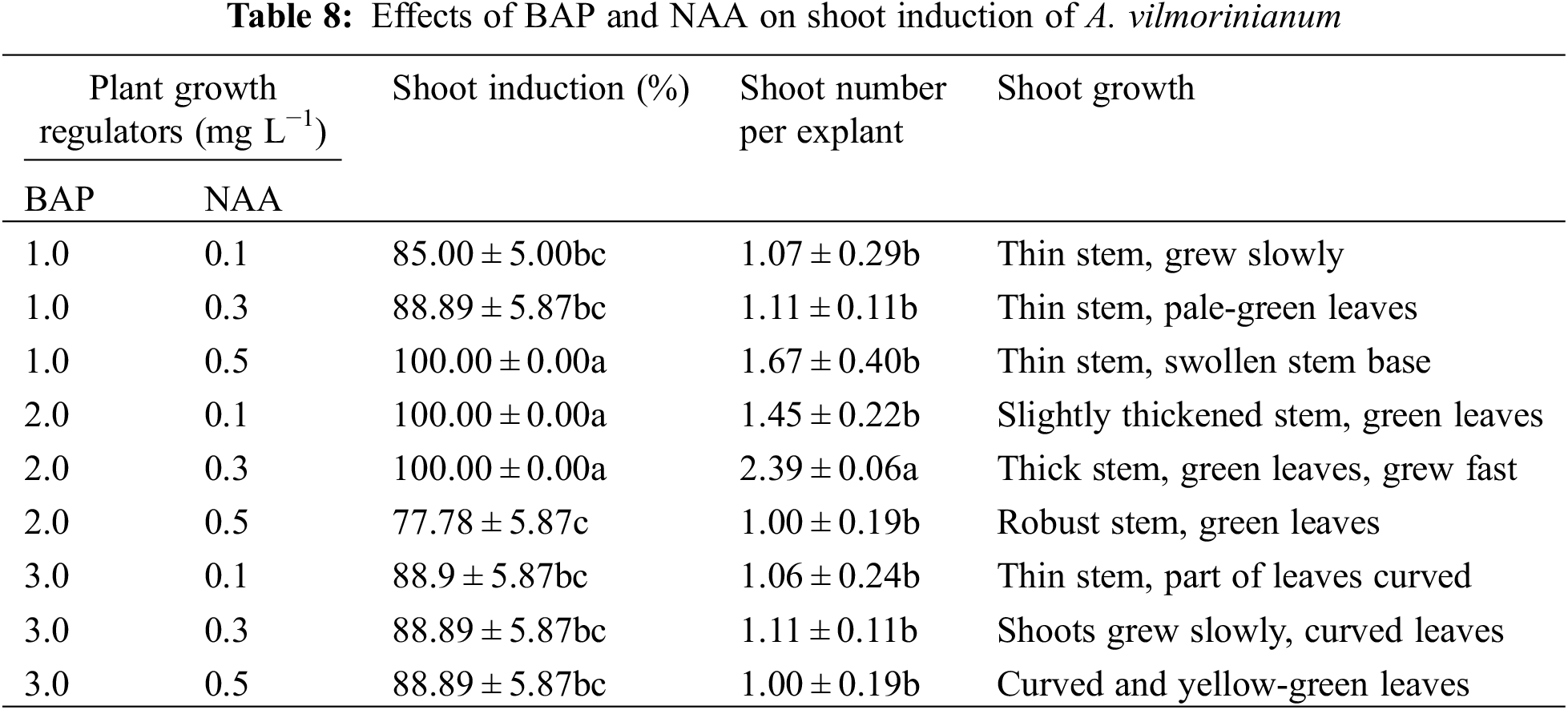
Newly formed shoots were transferred to a multiplication medium supplemented with various concentrations of TDZ and NAA for multiple shoot regeneration. Shoot multiplication was induced in four of the nine treatments (Table 9). The number of multiplied shoots was highest (1.78) on MS containing 0.1 mg L−1 TDZ and 0.1 mg L−1 NAA, which gradually decreased with increasing concentrations of TDZ and NAA (Fig. 4b).
3.3 Callus Induction and Re-Differentiation
Different concentrations and combinations of auxins and cytokines were used to observe callus induction from the stem, leaf, and petiole explants. The texture of the callus formed from all the three explants was soft, incompact, and light green.
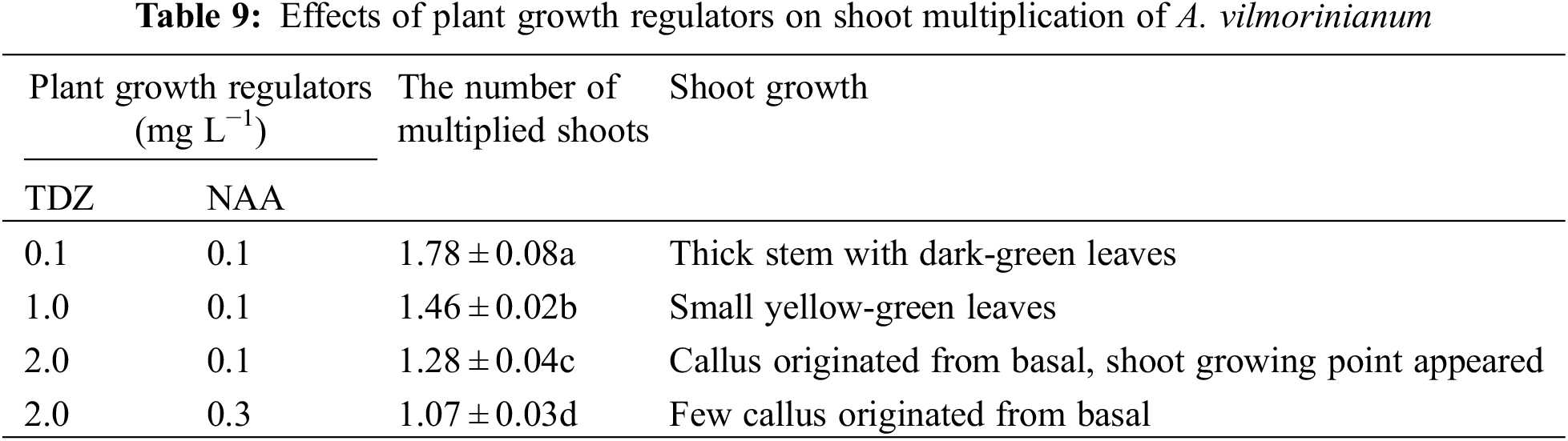
Callus formation capability varied when explants were treated with 2,4-D and kinetin singly or in combination (Table 10). Not significant but a variable trend was found in terms of callus induction frequency in stem explants among treatments of 0.5, 1.0, 2.0, and 3.0 mg L−1 2,4-D, with a relatively high value of 96.67% in the 2.0 mg L−1 treatment (Fig. 5a). The addition of kinetin to the medium supplemented with 2.0 mg L−1 2,4-D reduced callus formation. The percentage of callus induction decreased with increasing kinetin concentrations from 0.1 to 2.0 mg L−1 in the treatments of 2,4-D + kinetin. The callus induction frequency of leaf explants presented an inverted-U relation with the concentration of 2,4-D, and the peak of 83.33% occurred in 1.0 mg L−1 (Fig. 5b). No callus was induced from leaf explants on MS medium containing kinetin or in combination of 2,4-D with kinetin. For petiole explants, the highest callus induction frequency of 73.33% was in 2.0 mg L−1 2,4-D treatment while a higher concentration of 2,4-D (3.0 mg L−1) significantly reduced callus induction (17.73%) (Fig. 5c). Kinetin at 1.0 mg L−1 induced a small amount of callus at the ends of petiole segments while the other kinetin concentrations failed in inducing callus induction. The frequency of callus induction was 24.00–34.00% when 2,4-D was combined with kinetin. These results indicated that using 2,4-D singly was suitable for callus induction from the stem, leaf, and petiole explants, and the optimal concentrations were 2.0, 1.0, and 2.0 mg L−1, respectively.
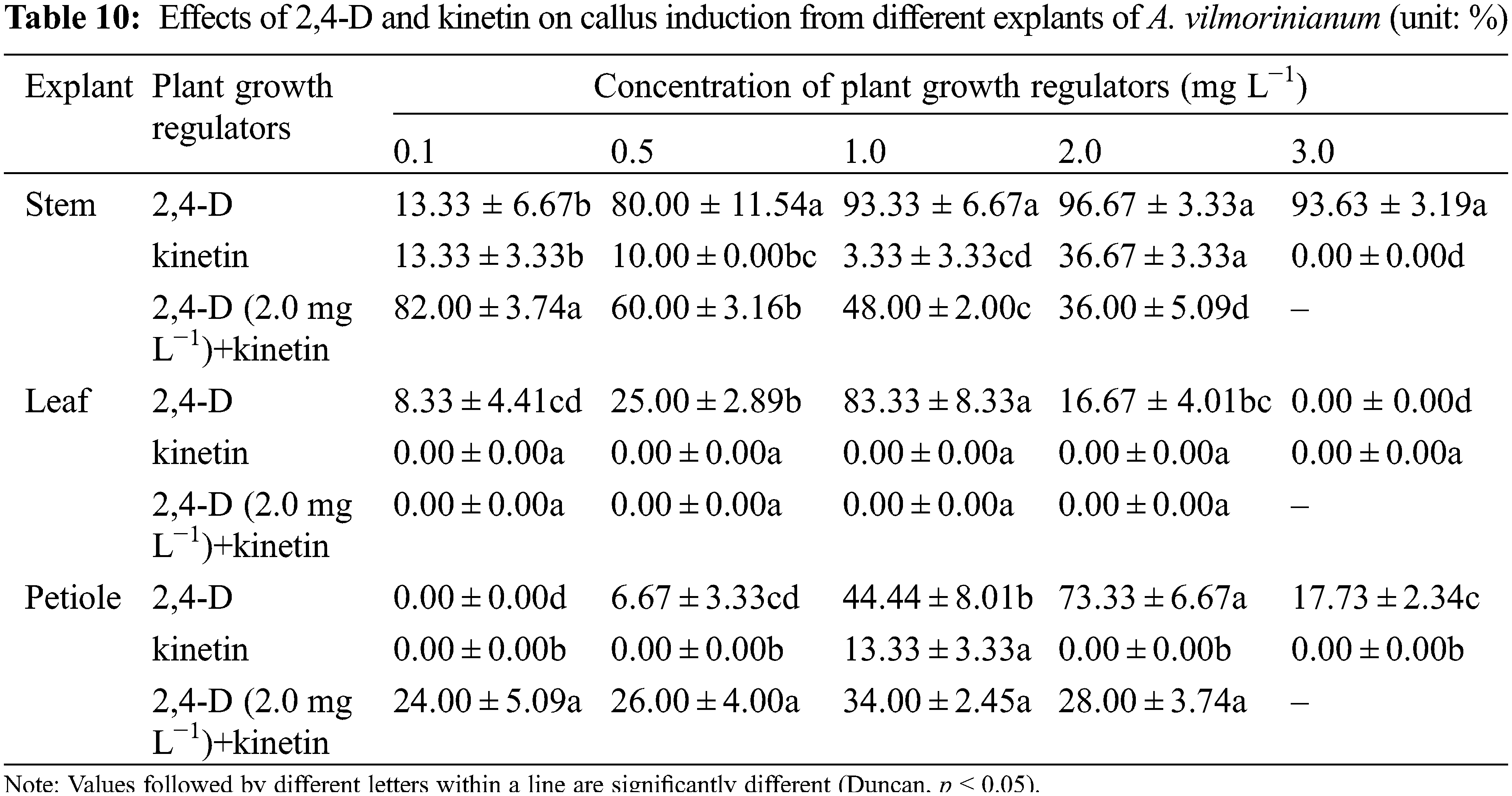
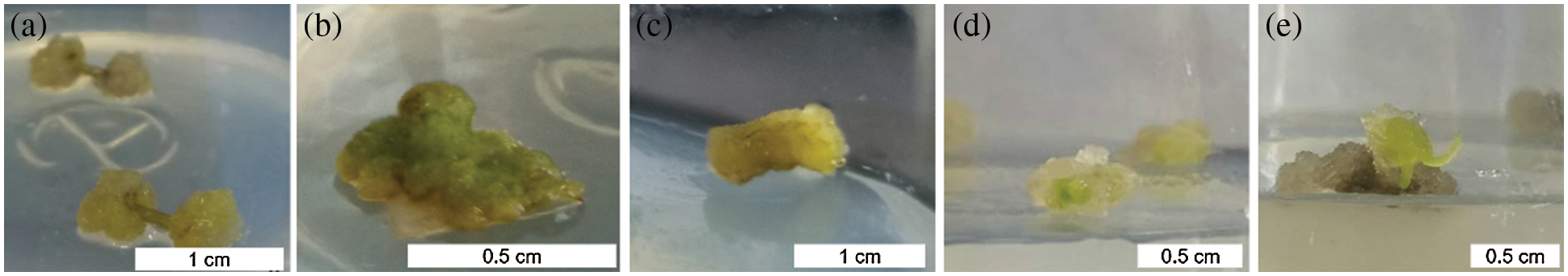
Figure 5: Callus induction and re-differentiation of A. vilmorinianum. a stem-derived callus; b leaf-derived callus; c petiole-derived callus; d initiation stage of callus re-differentiation; e callus re-differentiation
Effects of BAP in combination with NAA or 2,4-D were also examined on callus induction by using the three explants types (Table 11). Callus was induced from all tested stem explants. At a constant BAP concentration (0.5, 1.0, or 2.0 mg L−1), the increase of auxin concentrations, NAA from 0.1 to 0.5 mg L−1 or 2,4-D from 1.0 to 2.0 mg L−1, alleviated in general explants browning and enhanced callus production. The maximum callus induction frequency of 80.00% and lowest browned explant percentage of 20% were obtained by the use of 2.0 mg L−1 BAP with 2.0 mg L−1 2,4-D, which was found to be the optimum combination of plant growth regulators for callus induction from stem segments.
No callus formed from leaf explants on MS medium containing BAP (1.0–2.0 mg L−1) in combination with NAA (0.1–0.5 mg L−1). When replacing NAA with 2,4-D, explants produced a small percentage of callus (0–36.00%) and the induction frequency was higher in 2.0 mg L−1 2,4-D than in 1.0 mg L−1 2,4-D regardless of the concentration of BAP. In the presence of 2.0 mg L−1 2,4-D, increasing the concentration of BAP had prompt effect on callus formation, but aggravated browning of explants. The maximum callus induction frequency was recorded as 36.00% in MS medium with 2.0 mg L−1 2,4-D and 2.0 mg L−1 BAP, and it was far less than that of stem and petiole explants, i.e., 80.00% and 84.00%, respectively.
The tested combinations of BAP and NAA were not suitable for callus induction from petiole explants due to a small amount of callus formation. The combination of BAP and 2,4-D was better than that of BAP and NAA. Increasing concentrations of 2,4-D alleviated the browning of petiole explants and enhanced callus production. The highest amount of callus (84.00%) and the lowest explants browning rate (16.00%) were obtained on MS medium supplemented with 1.0 mg L−1 BAP combined with 2.0 mg L−1 2,4-D.
It seemed like that the leaf explants were not suitable for plant regeneration because of low callus-formation capability. The optimal plant growth regulator combinations for callus induction from the stem and petiole explants in every treatment were listed in Table 12. Put all together, the stem was considered as the most suitable explant source for callus induction, and the presence of 2,4-D at 2.0 mg L−1 was found to be the most effective.
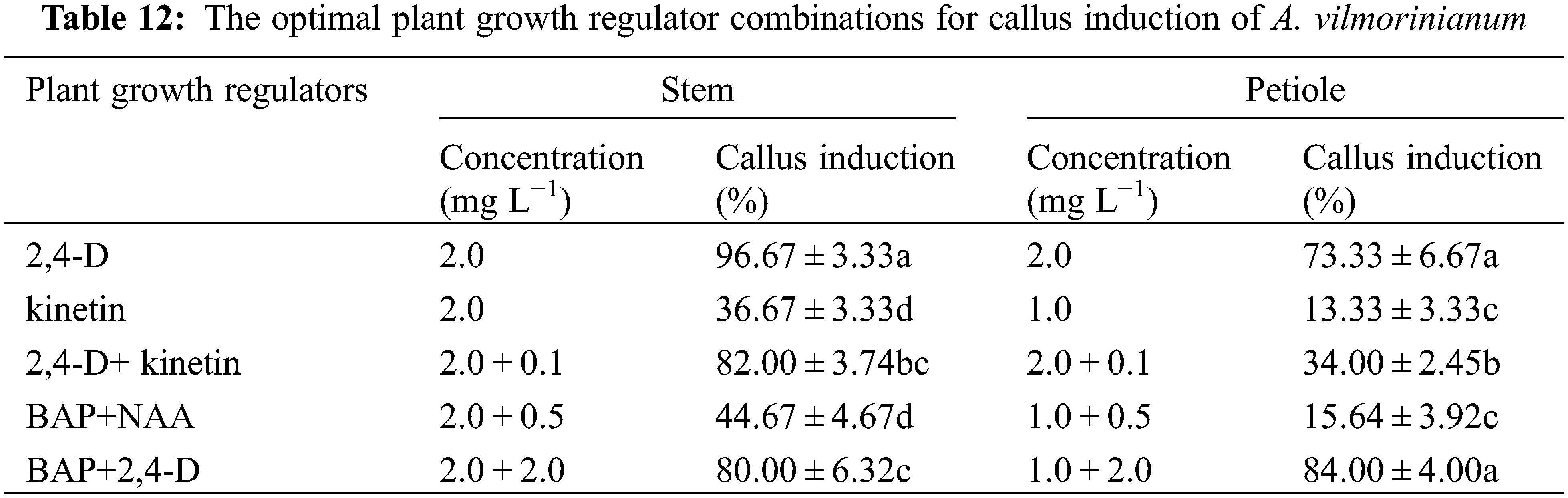
3.3.2 Callus Re-Differentiation
After 30 days of culture on regeneration medium, 8% of pale-green callus eventually showed further differentiation on medium supplemented with 0.1 mg L−1 TDZ and 0.2 mg L−1 NAA (data not shown, Figs. 5d, 5e).
As the sources of A. vilmorinianum are getting depleted for its medicinal value, there is an urgent need for its rapid regeneration and conservation. Plant regeneration through in vitro germination and micropropagation has evolved as valuable method for a constant supply of plantlets.
Seed dormancy caused by underdeveloped embryo has limited the application of some Aconitum species, e.g., A. napellus, A. lycoctonum, and A. heterophyllum [19–22]. It has been shown that some Aconitum species require chilling for dormancy break, such as A. heterophyllum and A. sinomontanum [23,24]. Similar findings that 35 days of storage at 4°C facilitated germination have been reported in A. vilmorinianum, and the results indicated that it may present one or more type of dormancy during seed maturation [16]. Generally, GA3 is commonly used to break seed dormancy, and the effect varied among different species. Studies have indicated that the application of GA3 had positive effects on seed germination of A. balfourii, A. lycoctonum, and A. napellu, but was inhibitory in A. heterohyllum [19,22,25]. In the present study, 100 mg L−1 GA3 for 21 h stimulated germination of A. vilmorinianum and facilitated seedling establishment, while GA3 concentration of more than 200.0 mg L−1 was inhibitory. Moreover, the treatment of GA3 facilitated germination, for instance, the GA3 treated seeds germinated after 7 days whereas untreated seeds required 20 days.
Aseptic seedlings raised from sterilized seeds were used as ideal explants for plant regeneration because their levels of genetic differentiation were lower than mature plants. Surface sterilization of seeds is obligatory before culture initiation. Severe visible bacterial contamination was observed in a pretest study of in vitro germination of A. vilmorinianum. Therefore, searching for an efficient seed sterilization method is critical. Morphology characteristics of the seed coat and the concentration and contact time of sterilizing agents have significant effects on seed sterilization, and these factors were evaluated in this study. The observations on seed coat structure suggested that the dense furrows on the seed surface were most likely to provide shelters for microorganisms in the case of using different sterilizing agents. The seed coat micro-morphology of A. vilmorinianum is described for the first time. Three types of bacteria were isolated from infected seeds. E. persicina and P. tolaasii were effectively eradicated with 75% ethanol for 30 s and then with 10% NaClO for 30 min while the procedure took less effect on elimination of P. agglomeran. NaClO was a better sterilization agent than HgCl2 which showed a completely inhibitory effect on germination. For further investigation, it is possible to achieve high sterilization efficiency by using specific disinfectants, like antibiotics represented by rifampicin which were extremely effective in controlling contamination of Aconitum cultures [9,12,26]. Besides, the stimulation of germination was observed from the contact time of ethanol ranging from 0 up to 30 s. Likewise, ethanol exhibited a dormancy breaking effect on seeds of A. heterophyllum due to protein changes during germination [27,28]. It can be concluded that surface sterilization of A. vilmorinianum seeds with 75% ethanol for 30 s and then with 10% NaClO for 30 min performed best on germination.
The effects of plant growth regulators either singly or in combination for enhancing germination of A. vilmorinianum were examined. Up to 66.00% of the seeds germinated in MS medium containing BAP at 2.0 mg L−1 and NAA at 0.1 mg L−1. The promoting effect of BAP on in vitro germination has been demonstrated in A. heterophyllum [22]. Presumably, BAP has significant roles on seed germination of the Aconitum genus, giving clues for enhancing the in vitro germination for other Aconitum spp.
Given the above, the optimal germination protocol is summarized below for A. vilmorinianum. Seeds should be pre-chilled at 4°C for two months, followed by soaking in 100.00 mg L−1 GA3 for 21 h, sterilized with 75% ethanol for 30 s and then with 10% NaClO for 30 min. Next, the seeds should be transferred to MS medium supplemented with 2.0 mg L−1 BAP, 0.1 mg L−1 NAA and 30 g L−1 sucrose. Further research still needs to be done to improve seed propagation protocols. It is worthwhile to examine the effects of specific disinfectants, cold stratification, temperature, and other plant growth regulators on in vitro germination of A. vilmorinianum.
4.3 Direct Shoot Organogenesis
Multiplied shoots were generated through direct shoot organogenesis in the present study. A distinct difference in the shoot induction response among the three explants (the shoot tip, the uppermost axillary buds, and the second axillary buds) was observed. Since the uppermost axillary buds are rich in meristematic tissues, the shoot induction potential was higher in the uppermost axillary buds (100%) compared with apical buds and the second axillary buds [29]. Since this potential tends to decrease as the structure matures [30], the second axillary buds were less efficient than the uppermost axillary buds in shoot induction. The theory of apical dominance suggest that auxin produced by apical buds inhibits axillary bud outgrowth [31]. This could be the reason for the extremely low shoot induction capability (8.33%) of apical buds.
The combination of several types of auxin and cytokine is usually considered to induce multiple shoots on plant regeneration, and the ratio of auxin to cytokine plays a crucial role in the morphogenetic response of cultured tissues. NAA at lower concentrations along with higher BAP concentrations always resulted in optimal shoot induction through direct organogenesis [14,32,33]. The synergistic effect of NAA at 0.1 μM and BAP at 0.5 μM led to the highest shoot proliferation of A. violaceum [14]. In the present study, the use of BAP at 2.0 mg L−1 in combination with NAA at 0.3 mg L−1 resulted in 100% of shoot induction as well as the maximum number of shoots per explant (2.39) in stem segments. TDZ is the most frequently used cytokine for shoot regeneration and promotion of callus growth, which can induce greater axillary bud proliferation and has fewer side effects than other cytokines, such as BAP [34,35]. The effectiveness of TDZ in promoting in vitro organogenesis depends on the genotype and other factors [36]. It is noteworthy that the high efficiency of TDZ on callus induction has been reported in many highly recalcitrant species [34–35,37]. In the case of A. vilmorinianum, optimal shoot multiplication (1.78 per explant) was achieved on medium containing 0.1 mg L−1 TDZ and 0.1 mg L−1 NAA. However, either the number of shoots induced or the number of shoots multiplied in A. vilmorinianum was much less than that in A. carmichaeli, A. heterophyllum, A. napellus, A. balfourii, and A. violaceum, in which BAP was used alone or in combination with NAA during the shoot proliferation process [10–12,38,39]. Further investigations in plant growth regulator composition may provide a method to increase shoot induction rate and improve shoot number and quality for micropropagated A. vilmorinianum.
4.4 Indirect Shoot Organogenesis
The indirect organogenesis via callus is an effective method for generating whole plant regeneration of explants and establishing genetic transformation systems. In this study, the stem was proven as the most suitable explant source for callus induction of A. vilmorinianum. Meanwhile, leaf and petiole showed lower ability to generate callus. However, leaf and petiole have been used as explants to develop efficient micropropagation protocols through indirect adventitious shoot formation and somatic embryogenesis induction via callus for A. heterophyllum, A. balforii, and A. violaceum [11,13,15,39].
The type, concentration, and ratio of plant growth regulators play significant roles during the process of callus induction in the in vitro conditions. The percentage of callusing reduced when kinetin was used alone or mixed with 2,4-D as compared to 2,4-D alone. Using only 2,4-D turned out to be the most effective way for inducing callus from stem, leaf, and petiole segments of A. vilmorinianum, and the optimal concentrations were 2.0, 1.0, and 2.0 mg L−1, respectively. The higher concentration of 2,4-D (3.0 mg L−1) showed inhibitory effects on callus induction compared to 2,4-D at 2.0 mg L−1. This finding agrees with the reports of Pandey et al. [13] and Rawat et al. [39] that increasing concentrations of auxin (NAA or 2,4-D) alone resulted in a decrease in the frequency of callus induction and caused blackening of cultures in A. balfourii and A. violaceum [13,39].
In most published works of Aconitum, the combination of BAP and NAA always gave a good response on in vitro shoot regeneration through indirect organogenesis [11,13,38,40]. Some of these works also showed adverse effects of 2,4-D on callus maintenance due to excessive leaching [11,40]. In contrast with these reports, the combination of BAP and 2,4-D was better on callus induction than that of BAP and NAA in the present study. About 8% of pale-green callus eventually showed further differentiation on medium supplemented with 0.1 mg L−1 TDZ and 0.2 mg L−1 NAA. The results conform to the previous study of A. violaceum [39]. Moreover, in the report of A. balfourii regeneration, MS medium containing 0.5 mg L−1 TDZ alone stimulated the maximum shoot induction and formation via callus-mediated organogenesis [15].
Somaclonal variation might be a potential problem in the in vitro micropropagation of A. vilmorinianum. Generally speaking, the direct formation of plant structures from cultured plant tissue, without any intermediate callus phase, minimizes the frequency of somaclonal variation, while indirect organogenesis usually induces more somaclonal variants than direct organogenesis [41]. Regarding the regeneration of A. vilmorinianum, direct organogenesis from seeds and the uppermost axillary buds could be used for large-scale propagation, while indirect organogenesis through callus induction and re-differentiation is an alternative method for the genetic transformation to define gene function. In addition, the investigations on micropropagation of Aconitum spp. give some clue to the degree of somaclonal variation in A. vilmorinianum. No somaclonal variation was detected in micropropagated clones of A. heterophyllum by the genetic approach of ISSR markers [42]. Moreover, in vitro raised A. balforii plants showed no difference from seed raised plants in terms of the number of chromosomes, protein profile, and amounts of diterpenoid alkaloids [13]. For the clonally propagated plants of A. noveboracense [9], A. carmechaeli [10], A. heterophyllum [11,40], A. napellus [12], A. violaceum [14,43], A. balforii [15,38,44,45], and A. chasmanthum [46], no phenotype/behavior or genetic variation were reported because of the absence of a specific test for somaclonal variation in these studies. Clearly, after the propagation of A. vilmorinianum seedlings based on the protocol presented here, these in vitro raised plants should be further screened according to the purpose of application.
The methods developed here present an effective way to multiply A. vilmorinianum through in vitro germination and micropropagation. Moreover, the procedures provide tools for further improvement of propagation techniques and other investigations. To modify and standardize the propagation protocols that could be used for large-scale multiplication, more work (e.g., in vitro shoots rooting and ex vitro acclimatization) needs to be done.
Funding Statement: This research was funded by Yunnan Fundamental Research Projects (Nos. 202101AS070021; 202101AT070265), the National Natural Science Foundation of China (Grant Nos. 31960082; 32160249), and the Scientific Research Fund Project of Yunnan Education Department (No. 2021J0002).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Wang, J., Shen, X., Chen, Q., Qi, G., Wang, W. et al. (2009). Structure-analgesic activity relationship studies on the C18- and C19- diterpenoid alkaloids. Chemical & Pharmaceutical Bulletin, 57(8), 801–807. DOI 10.1248/cpb.57.801. [Google Scholar] [CrossRef]
2. Xiao, P. G., Wang, F. P., Gao, F., Yan, L. P., Chen, D. L. et al. (2006). A pharmacophylogenetic study of Aconitum L. (Ranunculaceae) from China. Acta Phytotaxonomica Sinica, 44(1), 1–46. DOI 10.1360/aps050046. [Google Scholar] [CrossRef]
3. Nyirimigabo, E., Xu, Y., Li, Y., Wang, Y., Agyemang, K. et al. (2015). A review on phytochemistry, pharmacology and toxicology studies of Aconitum. Journal of Pharmacy and Pharmacology, 67(1), 1–19. DOI 10.1111/jphp.12310. [Google Scholar] [CrossRef]
4. Li, M., He, J., Jiang, L., Ng, E. S., Wang, H. et al. (2013). The anti-arthritic effects of Aconitum vilmorinianum, a folk herbal medicine in southwestern China. Journal of Ethnopharmacology, 147(1), 122–127. DOI 10.1016/j.jep.2013.02.018. [Google Scholar] [CrossRef]
5. Oyama, T., Isono, T., Suzuki, Y., Hayakawa, Y. (1994). Antinociceptive effects of Aconite tuber and its alkaloids. American Journal of Chinese Medicine, 22(2), 175–182. DOI 10.1142/S0192415X94000218. [Google Scholar] [CrossRef]
6. Fu, M., Wu, M., Qiao, Y., Wang, Z. (2006). Toxicological mechanisms of Aconitum alkaloids. Pharmazie, 61(9), 735–741. [Google Scholar]
7. Shen, Y., Liang, W., Shi, Y., Kennelly, E. J., Zhao, D. (2020). Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Natural Product Reports, 37(6), 763–796. DOI 10.1039/D0NP00002G. [Google Scholar] [CrossRef]
8. Zhao, D., Shen, Y., Shi, Y., Shi, X., Qiao, Q. et al. (2018). Probing the transcriptome of Aconitum carmichaelii reveals the candidate genes associated with the biosynthesis of the toxic aconitine-type C19-diterpenoid alkaloids. Phytochemistry, 152, 113–124. DOI 10.1016/j.phytochem.2018.04.022. [Google Scholar] [CrossRef]
9. Cervelli, R. (1987). In vitro propagation of Aconitum noveboracense and Aconitum napellus. Hortscience, 22(2), 304–305. [Google Scholar]
10. Hatano, K., Kamura, K., Shoyama, Y., Nishioka, I. (1988). Clonal multiplication of Aconitum carmichaeli by tip tissue culture and alkaloid contents of clonally propagated plants. Planta Medica, 2, 152–155. [Google Scholar]
11. Giri, A., Ahuja, P. S., Kumar, P. V. A. (1993). Somatic embryogenesis and plant regeneration from callus culture of Aconitum heterophyllum wall. Plant Cell Tissue and Organ Culture, 32(2), 213–218. DOI 10.1007/BF00029845. [Google Scholar] [CrossRef]
12. Watad, A. A., Kochba, M., Nissim, A., Gaba, V. (1995). Improvement of Aconitum napellus micropropagation by liquid culture on floating membrane rafts. Plant Cell Reports, 14(6), 345–348. DOI 10.1007/BF00238594. [Google Scholar] [CrossRef]
13. Pandey, H., Nandi, S. K., Kumar, A., Palni, U. T., Chandra, B. et al. (2004). In vitro propagation of Aconitum balfourii stapf.: An important aconite of the himalayan alpines. Journal of Horticultural Science & Biotechnology, 79(1), 34–41. DOI 10.1080/14620316.2004.11511733. [Google Scholar] [CrossRef]
14. Rawat, J. M., Rawat, B., Agnihotri, R. K., Chandra, A., Nautiyal, S. (2013). In vitro propagation, genetic and secondary metabolite analysis of Aconitum violaceum Jacq.: A threatened medicinal herb. Acta Physiologiae Plantarum, 35(8), 2589–2599. DOI 10.1007/s11738-013-1294-x. [Google Scholar] [CrossRef]
15. Gondval, M., Chaturvedi, P., Gaur, A. K. (2016). Thidiazuron-induced high frequency establishment of callus cultures and plantlet regeneration in Aconitum balfourii Stapf.: An endangered medicinal herb of north-west Himalayas. Indian Journal of Biotechnology, 15(2), 251–255. [Google Scholar]
16. Ai, H., He, H., Yang, M., Sha, B., Yang, S. et al. (2015). Characteristic of seed germination of Aconitum vilmorinianum komarov. Seed, 34(12), 80–82. [Google Scholar]
17. Sambrock, J., Russel, D. W. (2001). Molecular cloning: A laboratory manual (3ed. edition). New York: Cold Spring Harbor Laboratory. [Google Scholar]
18. William, S., Feil, H., Copeland, A. (2012). Bacterial genomic DNA isolation using CTAB. USA: Sigma. [Google Scholar]
19. Herranz, J. M., Copete, M. A., Ferrandis, P., Copete, E. (2010). Intermediate complex morphophysiological dormancy in the endemic iberian Aconitum napellus subsp castellanum (Ranunculaceae). Seed Science Research, 20(2), 109–121. DOI 10.1017/S0960258510000048. [Google Scholar] [CrossRef]
20. Vandelook, F., Lenaerts, J., Jozef, A. V. (2009). The role of temperature in post-dispersal embryo growth and dormancy break in seeds of Aconitum lycoctonum L. Flora, 204(7), 536–542. DOI 10.1016/j.flora.2008.11.003. [Google Scholar] [CrossRef]
21. Engell, K. (1995). Embryo morphology of the ranunculaceae. Plant Systematics and Evolution, 9, 207–216. [Google Scholar]
22. Pandey, K., Nandi, S. K., Nadeem, M., Palni, L. M. S. (2000). Chemical stimulation of seed germination in Aconitum heterophyllum wall. and A. balfourii Stapf.: Important himalayan species of medicinal value. Seed Science and Technology, 28(1), 39–48. [Google Scholar]
23. Beigh, S. Y. (2005). Cultivation and conservation of Aconitum heterophyllum: A critically endangered medicinal herb of the northwest Himalayas. Journal of Herbs, Spices & Medicinal Plants, 11(4), 47–56. DOI 10.1300/J044v11n04_06. [Google Scholar] [CrossRef]
24. Dosmann, M. S. (2002). Stratification improves and is likely required for germination of Aconitum sinomontanum. Horttechnology, 12(3), 423–425. DOI 10.21273/HORTTECH.12.3.423. [Google Scholar] [CrossRef]
25. Sarasan, V., Cripps, R., Ramsay, M. M., Atherton, C., McMichen, M. et al. (2006). Conservation in vitro of threatened plants-progress in the past decade. In Vitro Cellular & Developmental Biology–Plant, 42(3), 206–214. DOI 10.1079/IVP2006769. [Google Scholar] [CrossRef]
26. Scortichini, M., Chiariotti, A. (1988). In vitro culture of Prunus persica var. laevis gray (nectarineDetection of bacterial contaminants and possibility of decontaminants by means of antibiotics. Acta Horticulturae, 225, 109–118. DOI 10.17660/ActaHortic.1988.225.12. [Google Scholar] [CrossRef]
27. Sreenivasulu, Y., Chanda, S. K., Ahuja, P. S. (2008). Ethanol induced seed germination in Aconitum heterophyllum Wall.: An endangered medicinal herb of the North-West Himalayas. Indian Journal of Plant Physiology, 13(2), 159–165. [Google Scholar]
28. Rana, B., Sreenivasulu, Y. (2013). Protein changes during ethanol induced seed germination in Aconitum heterophyllum. Plant Science, 198, 27–38. DOI 10.1016/j.plantsci.2012.09.013. [Google Scholar] [CrossRef]
29. Taiz, L., Zeiger, E. (2002). Plant physiology. Massachusetts: Sinauer Associates. [Google Scholar]
30. Fehér, A. (2005). Why somatic plant cells start to form embryos. In: Mujib, A. (Ed.Omatic embryogenesis, pp. 85–101. Springer: Berlin. [Google Scholar]
31. Bennett, T., Leyser, O. (2006). Something on the side: Axillary meristems and plant development. Plant Molecular Biology, 60(6), 843–854. DOI 10.1007/s11103-005-2763-4. [Google Scholar] [CrossRef]
32. Agnihotri, R. K., Mishra, J., Nandi, S. K. (2009). Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. Ex Munro: Production of genetically uniform plants and field evaluation. Acta Physiologiae Plantarum, 31, 961–967. DOI 10.1007/s11738-009-0311-6. [Google Scholar] [CrossRef]
33. Giri, L., Jugran, A., Rawat, S., Dhyani, P., Andola, H. et al. (2012). In vitro propagation, genetic and phytochemical assessment of Habenaria edgeworthii: An important astavarga plant. Acta Physiologiae Plantarum, 34(3), 869–875. DOI 10.1007/s11738-011-0884-8. [Google Scholar] [CrossRef]
34. Kahia, J., Kirika, M., Lubabali, H., Mantel, S. (2016). High-frequency direct somatic embryogenesis and plantlet regeneration from leaves derived from in vitro-germinated seedlings of a Coffea arabica hybrid cultivar. Hortscience, 51(9), 1148–1152. DOI 10.21273/HORTSCI10771-16. [Google Scholar] [CrossRef]
35. Verma, S. K., Sahin, G., Yucesan, B., Eker, I., Sahbaz, N. et al. (2012). Direct somatic embryogenesis from hypocotyl segments of Digitalis trojana ivan and subsequent plant regeneration. Industrial Crops and Products, 40, 76–80. DOI 10.1016/j.indcrop.2012.02.034. [Google Scholar] [CrossRef]
36. Magyar-Tabori, K., Dobranszki, J., da Silva, J. A. T., Bulley, S. M., Hudak, I. (2010). The role of cytokinins in shoot organogenesis in apple. Plant Cell Tissue and Organ Culture, 101(3), 251–267. DOI 10.1007/s11240-010-9696-6. [Google Scholar] [CrossRef]
37. Gill, R., Saxena, P. K. (1993). Somatic embryogenesis in Nicotiana tabacum L. induction by thidiazuron of direct embryo differentiation from cultured leaf discs. Plant Cell Reports, 12(3), 154–159. DOI 10.1007/BF00239097. [Google Scholar] [CrossRef]
38. Pandey, H., Chandra, B., Mohammad, N., Kumar, A., Nandi, S. et al. (2002). In vitro propagation of some alpine medicinal herbs of Himalaya. In: Nandi, S. K. (Ed.Role of plant tissue culture in biodiversit, pp. 297–323. Gyanodaya Prakashan: Nainital. [Google Scholar]
39. Rawat, J. M., Rawat, B., Nautiyal, S. (2013). Influence of growth regulators on indirect shoot organogenesis and secondary metabolite production of Aconitum violaceum Jacq. African Journal of Biotechnology, 12, 6287–6293. DOI 10.5897/AJB2013.13390. [Google Scholar] [CrossRef]
40. Jabeen, N., Shawl, A. S., Dar, G. H. (2006). Callus induction and organogenesis from explants of Aconitum heterophyllum-medicinal plant. Biotechnology, 5, 287–291. DOI 10.3923/biotech.2006.287.291. [Google Scholar] [CrossRef]
41. Bairu, M. W., Aremu, A. O., van Staden, J. (2011). Somaclonal variation in plants: Causes and detection methods. Plant Growth Regulation, 63(2), 147–173. DOI 10.1007/s10725-010-9554-x. [Google Scholar] [CrossRef]
42. Belwal, N. S., Kamal, B., Sharma, V., Gupta, S., Dobriyal, A. K. et al. (2016). Production of genetically uniform plants from shoot tips of Aconitum heterophyllum wall–A critically endangered medicinal herb. Journal of Horticultural Science & Biotechnology, 91(5), 529–535. DOI 10.1080/14620316.2016.1184434. [Google Scholar] [CrossRef]
43. Rawat, J. M., Rawat, B., Mehrotra, S. (2013). Plant regeneration, genetic fidelity, and active ingredient content of encapsulated hairy roots of Picrorhiza kurrooa royle ex benth. Biotechnology Letters, 35(6), 961–968. DOI 10.1007/s10529-013-1152-3. [Google Scholar] [CrossRef]
44. Bist, R., Pathak, K., Hatwal, D., Punetha, H., Gaur, A. K. (2011). In vitro propagation of Aconitum balfourii Stapf: A rare medicinal herb of the alpine Himalayas. Indian Journal of Horticulture, 68(3), 394–398. [Google Scholar]
45. Sharma, E., Gaur, A. K. (2012). In vitro regeneration of Aconitum balfourii Stapf: A rare medicinal herb from Himalayan alpine through root explants. Research Journal of Medicinal Plant, 6(4), 318–325. DOI 10.3923/rjmp.2012.318.325. [Google Scholar] [CrossRef]
46. Rafiq, S., Wagay, N. A., Bhat, I. A., Kaloo, Z. A., Rashid, S. et al. (2021). In vitro propagation of Aconitum chasmanthum Stapf ex holmes: An endemic and critically endangered plant species of the western himalaya. Horticulturae, 7(12), 586. DOI 10.3390/horticulturae7120586. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |