

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.018118
REVIEW
Role of Reactive Oxygen Species in the Initiation of Plant Retrograde Signaling
1Centro de Investigación en Alimentación y Desarrollo, A.C. Tecnología de Alimentos de Origen Vegetal, Laboratorio de Fisiología y Biología Molecular de Plantas, Hermosillo, 83304, México
2Department of Food Science and Plant Research and Innovation Center, Laval University, Quebec, QC, G1V 0A6, Canada
*Corresponding Author: Martín Ernesto Tiznado-Hernández. Email: tiznado@ciad.mx
Received: 30 June 2021; Accepted: 22 November 2021
Abstract: The interaction between the nucleus and the different organelles is important in the physiology of the plant. Reactive oxygen species (ROS) are a by-product of the oxidation of organic molecules to obtain energy by the need to carry out the electron transfer between the different enzymatic complexes. However, they also have a role in the generation of what is known as retrograde signaling. This signal comes from the different organelles in which the oxidation of molecules or the electron transference is taking place such as mitochondria and chloroplasts. Furthermore, ROS can also induce the release of signals from the apoplast. It seems that these signals plays a role communicating to the nucleus the current status of the different parts of the plant cell to induce a changes in gene expression. In this review, the molecular mechanism of ROS retrograde signaling is described.
Keywords: Reactive oxygen species; retrograde signaling; mitochondria; chloroplast
Reactive oxygen species (ROS) is a group of unstable molecules which shows either different levels of oxygen oxidation states or unpaired electrons which is characteristic of free radicals. Most common ROS include: singlet oxygen (1O2), ozone (O3), hydrogen peroxide (H2O2), superoxide (O2.-), hydroxyl free radical (HO.) and perhydroxyl radical (HOO.), which is the conjugate acid of the superoxide [1].
These highly chemically reactive molecules are generated by the metabolism of mitochondria [2], peroxisomes [3], chloroplasts [4,5], endoplasmic reticulum, glyoxysomes, and apoplast which can be related to cell wall and plasma membrane [6]. However there are enzymes that convert ROS into stable molecules such as oxygen or water to prevent catastrophic events [1]. Reactive oxygen species are also employed to carry out several physiological functions [7]. In this regard, ROS plays an important role in signal transduction, including the control of several metabolic pathways [8,9], responses to pathogen attack, as well as to biotic and abiotic stresses, and retrograde signaling [10,11].
The present review will describe the outcomes on retrograde signaling from 2005 to date. There are also excellent reviews of the topic done before this time period for interested readers [12–14].
2 Role of Reactive Oxygen Species in Plant Cell Signal Transduction
There are several signaling phenomena in which ROS plays a role in retrograde signaling [15]. Among the most important are: ROS release by the plant vascular system [16,17], signaling by reactive electrophile species created by lipid oxidation [18], cell wall changes [19] and signaling based on H2O2 [4]. Fig. 1 shows a schematic description of the different findings included in the following sections of this review.
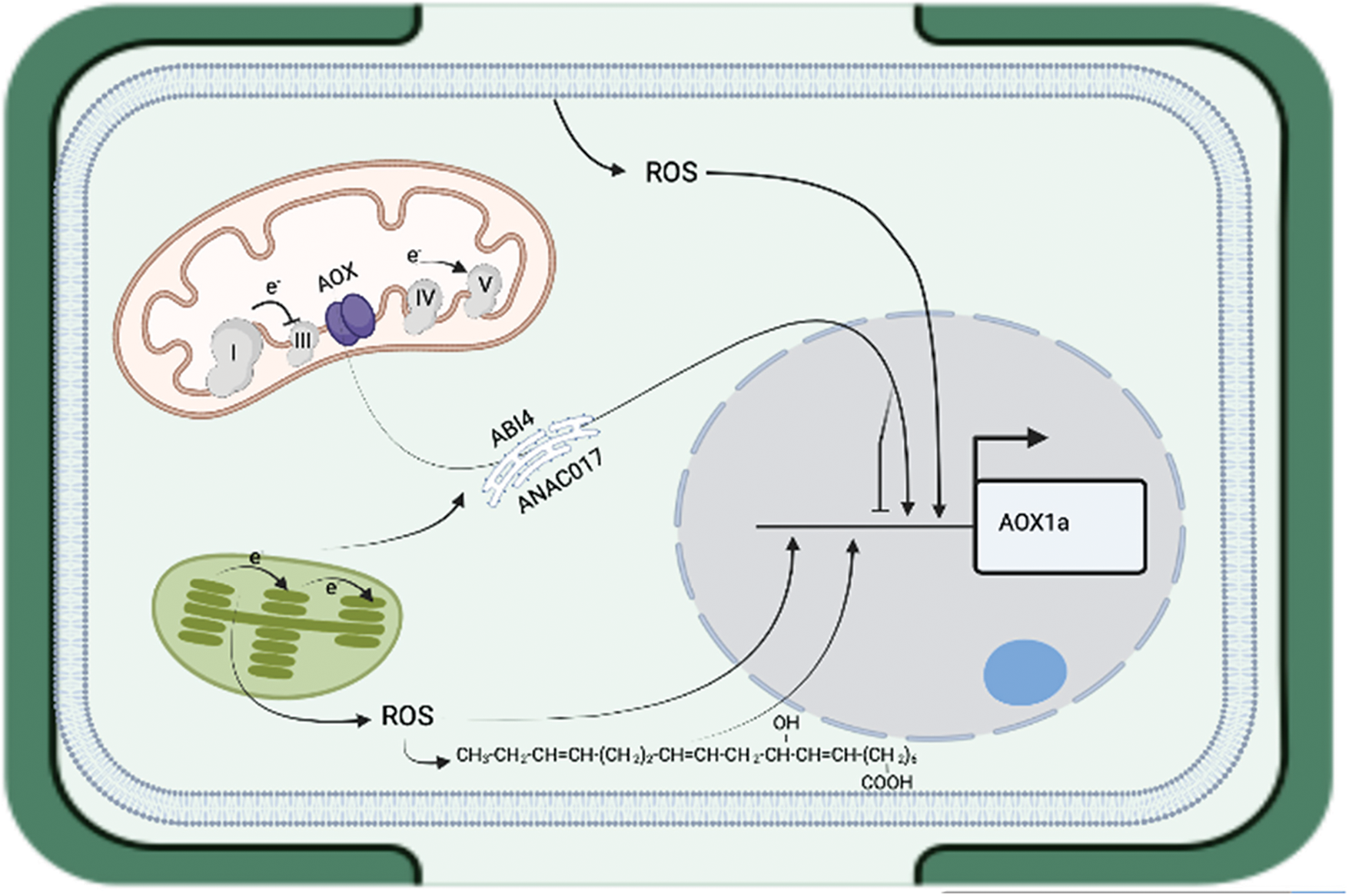
Figure 1: It is shown the different pathways and sources of the intracellular reactive oxygen species which can move from plasma membrane, mitochondria, chloroplast to the nucleus and alter the expression of nuclear genes. In the figure, it is included the gene AOX1a which had been utilized during several studies. Lines with arrows means induction and with a horizontal line means repression of activity
2.1.1 Retrograde Signal from Mitochondria
The retrograde signal phenomenon consists in the generation of ROS in different organelles, which is used as a relay signal to the nucleus to induce a response, although the receptors for these molecules remain unidentified [19]. It is assumed, that organelles can send signals to the nucleus using ROS, however, in some instances, such as peroxisomes, the molecular mechanism is unknown [3,20]. Also, in plant mitochondria, the nature of the retrograde signals, and the transduction to nucleus is largely unknown, but efforts have been made following gene regulation, as well as studying post-translational modifications [21]. In this context, ten components involved in retrograde signaling have been identified by analyzing the expression of the ALTERNATE OXIDASE 1a gene. The ABSCISIC ACID INSENSITIVE4 (ABI4) transcription factor represses the transcriptional activation of the gene ALTERNATIVE OXIDASE 1a by binding to the B element located in the promoter of the gene [22], whereas the transcription factor WRKY40, which binds to the W-box of the promoter, maintains the level of gene activity after being induced [23]. On the other hand, CYCLIN-DEPENDENT KINASE E appears to control the signals between the transcription factors bound to the ALTERNATIVE OXIDASE 1a promoter and the RNA polymerase II enzyme. This can be used as a checkpoint to maintain plant growth, or to activate stress response mechanisms [24]. Furthermore, the NAC (NO APICAL MERISTEM/ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR/CUPSHAPED COTYLEDON) family transcription factor: ANACO17 was found to be a positive regulator of the ALTERNATIVE OXIDASE 1a gene. ANACO17 interacts with the endoplasmic reticulum via a transmembrane domain located in the C-terminus of the protein, and during mitochondria alteration, a protease cleaves the N-terminus domain of the protein that targets the nucleus and interacts with NAC promoter binding site of the gene [25]. It was revealed that other transcription factor of the same family, ANACO13, can trigger a positive regulation of mitochondrial retrograde signaling genes by binding to the mitochondria disfunction motif (MDM) cis-regulatory responsive elements of the gene promoters. Besides ANACO13, it was identified that the transcription factors ANAC017, ANAC053, and ANAC078 also binds to the MDM responsive element [26]. In addition, the presence of the MDM response element was located in the promoter of the ALTERNATIVE OXIDASE 1a gene. The transcription factor MYB29 (MYB domain protein 29) is a negative regulator of the ALTERNATIVE OXIDASE 1a gene. However, there are not responsive elements located in the promoter of this gene, and it seems that MYB29 action is carried out by using a network of 18 transcription factors including several WRKY transcription factors with WRKY40 being one of them [27]. Based of the above, it is clear that there is a complex regulation of the mitochondria anterograde signal.
2.1.2 Retrograde Signal from Chloroplasts
In chloroplasts, it appears that H2O2 as well as 1O2 play a role in the retrograde signaling to the nucleus [28]. Hydrogen peroxide is the longest half life ROS with about 1 microsecond, thus, it can be transferred directly to the nucleus in which some genes or maybe transcription factors can be modulated. Evidences supporting this statement came from studies with Nicotiana benthamiana, where the transference of H2O2 to the nucleus was concomitant with the activation of the ascorbate peroxidase gene NbAPXa [29]. Alternative, the singlet oxygen most likely is unable to leave the chloroplast to carry out the signal because it can be quickly quenched by water. Likewise, it can react with pigments, proteins and lipids which makes the long distance diffusion of this molecule pretty much unlikely [30]. Therefore, two different pathways had been observed: 1) by the oxidation of molecules such as the carotenoids inducing the production of β-cyclocitral, α-ionene, α-ionone and dihydroactinidiolide. Among these compounds, it appears that β-cyclocitral is the most important by upregulating 706 genes and downregulating 439 genes. Most of the positively regulated genes were found within the categories of environmental interaction, cell rescue, defense and virulence [31]; 2) phosphoadenosine 5’-phosphate is degraded by phosphoadenosine 5’-phosphate phosphatase, which could be be deactivated by changes in the redox state of the chloroplast by oxidation of two cysteine residues leading to accumulation of substrate. The prior can migrate to the nucleus to induce genes known as nuclear genes related with the plastid redox [32]. Moreover, it seems that the ABSCISIC ACID INSENSITIVE4 (ABI4) transcription factor also plays a role in the activation of genes in response to ROS activated retrograde signal from chloroplast, and in general it had been found to be involved in other signal pathways to control plant development such as: ABA signaling, sugar signaling, pathogen response, lipid mobilization, among others [33].
Most likely the retrograde signals carry out the control of ABI4 expression at both the transcription and post-translation levels, although this phenomenon is fairly unknown. However, it was found by using treatments to induce retrograde signals, that the transcriptional regulator known as PTM is released from the chloroplast envelop and transported to the nucleus. Moreover, in the nucleus, this regulator induce ABI4 gene expression [34]. The final outcome depends upon the different signals perceived by the nucleus such as ABA presence, redox status and retrograde signals from mitochondria [33].
The GUN acronym means genomes uncoupled and this phenomena was studied for first time in mutants of C. reinhardtii [35]. After that, gun mutants in Arabidopsis were found designated as GUN 3, GUN4 and GUN5 and it was shown that it is a signal from chloroplast to nucleus controlling the activity of Lhcb1 gene. This message was important for controlling the biosynthesis of chlorophyl. GUN5 gene was cloned and shown to be a chloroplast subunit of the Mg-Chelatase enzyme. Furthermore, it appears that the precursors of the chlorophyll biosynthesis pathway control the gene expression rather than the enzyme subunit [36]. It had been mentioned that this retrograde signal is still not well understood [37] although it is related with the biotic stress during cucumber mosaic virus infection [38].
The molecule methylerythritol cyclodiphosphate (MEcPP) is an isoprenoid precursor which is involved in the response to the Pseudomonas syringae, a biotrophic pathogen, and to abiotic stresses such as wounding and high light stress [39]. The ceh1 mutant which does not express the enzyme 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase that carries out the enzymatic conversion of MEcPP to hydroxymethylbutenyl diphosphate shows large levels of MEcPP. This mutant also shows high levels of HDL which is a nuclear gene encoding the plastidial located enzyme hydroxyperoxide lyase, which is important in the biosynthesis of metabolites playing a role as stress responsive signal. Studies with this mutant concluded that MecPP is responsible for the induction of the HDL nuclear gene and the large levels of salicylic acid which explains the high resistance of this mutant to biotrophic pathogens such as Pseudomonas syringae [40]. Protemic and transcriptomic analysis of the ceh1 mutant showed that MEcPP induces the accumulation of some unfolded protein response in the endoplasmic reticulum which is important for the cell to face the endoplasmic reticulum stress which is related with the presence of stress conditions [41].
Executer 1 and Executer 2 play a role in the retrograde signal initiated by singlet oxygen generated by chloroplast. The Executers 1 and 2 are required for suppression of the singlet oxygen induced gene expression. This particular ROS is highly unstable and that is why it is unlikely to move from the chloroplast to the nucleus. Because of this, EXECUTER protein plays a role as a second messenger initiated by the singlet oxygen. However, it seems that the primary function of singlet oxygen is not the control of chloroplast performance but the activation of an stress related pathway in response to pathogen attack, wounding and drought stress [28,42–44] and programmed cell death [45]. In this regard, it had been mentioned that this response initiated by the singlet oxygen is similar to retrograde signalling [46].
2.2 Auto-Induced ROS Release by the Plant Vascular System
This phenomenon was first described in the communication between organelles within cells [47]. However, in plants it turns out that this phenomenon mediates the communication from cell to cell, leading to a systemic spread of a signal in presence of different stress factors [16]. It appears that this response is triggered by ROS generated by the respiratory oxidase homologue (RBOH), which plays the same role as the NOX protein in mammalian cells [48]. This enzyme is located at the cell apoplast, and it is activated by the increase of calcium concentration of (Ca2+)cys in the cytoplasm. It appears that the intracellular calcium can bind to the EF hand motifs of the RBOH present in the N terminal side [48]. Moreover, RBOH keeps active by inducing the opening of the calcium channels [49]. The increment in ROS in the apoplast of a cell induces the increase of ROS by the same mechanism on the neighbor cell. In this way, the system signal is spread throughout the plant tissues. Furthermore, it has been shown that this mechanism of signaling plays a role in seed germination, lignification, abiotic stress responses, plant defense, root hair formation, closing stomata, pollen tube growth, and responses to wounding [48].
2.3 Signaling by Reactive Electrophile Species Created by Lipid Oxidation Induced by ROS
It has been shown that 1O2 can oxidize the polyunsaturated fatty acids, which leads to the production of 10-hydroxy-8,12,15(E,Z,Z) octadecatrienoic acid and 15-hydroxy-8,12,15(E,Z,Z) octadecatrienoic. Besides, the production of 9-hydroxy-8,12,15(E,Z,Z) octadecatrienoic, 13-hydroxy-8,12,15(E,Z,Z) octadecatrienoic, 12-hydroxy-8,12,15(E,Z,Z) octadecatrienoic, and 16-hydroxy-8,12,15(E,Z,Z) octadecatrienoic, was found to be the result of the oxidation of the polyunsaturated fatty acids by either 1O2 or free radicals [50]. Based on this fact, it was suggested that lipid oxidation can be another way to carry out a retrograde signaling from chloroplasts [51]. Experimental evidences supporting this hypothesis were generated studying the fluorescent (flu) mutant of Arabidopsis. Moreover, it was found that 1O2 can specifically induce an increase of 2.5 fold, or increase the expression of 70 genes. Within these genes, it was recorded proteins playing a role in plant cell wall, calcium responses, membrane transport, initiation of signal transduction, response to disease, among others [52]. In general, the molecules created by lipid oxidation are known as oxylipin. The best know oxylipin retrograde signal from chloroplast is carried out by the phytohormone jasmonate which is derived from lipid degradation. It had been shown that this oxylipin can interact with the nuclear protein CORONATINE INSENSITIVE1 inducing in this way the degradation of the jasmonate ZIM domain by the 26S proteosome. This degradation induces the expression of MYC transcription factors that activate the jasmonate activated genes. However, still the mechanism by which oxylipin carries out the retrograde signal from plastids is largely unknown [53].
2.4 ROS Induce a Signal from the Cell Wall
The apoplast is the region composed of the cell walls between the cells, and it is delimited by the plasma membrane. In the apoplast, the production of H2O2 and 1O2, is carried out by cell wall peroxidases, and NADPH oxidases, also known as respiratory burst oxidase homologs [54]. The apoplast can start a retrograde signaling by the launch of H2O2 apoplastic oxidative burst outside of the cytoplasm which can be recognized by the HPCA1, which is a leucine-rich-repeat receptor kinase. This receptor includes two cysteine residues in the extracellular domain which can undergo an oxidation which in turn induces the autophosphorylation of the receptor and the activation of calcium channels [55]. In mammalian cells, the need for the presence of anion channels in the membrane to transport 1O2 to the cytoplasm has been observed [56]. However, within plants, the presence of anion channels remains to be demonstrated. On the other hand, the transport of H2O2 into the cytoplasm can be carried out by aquaporins and indeed, specific aquaporins have been identified in Arabidopsis [57]. Furthermore, it appears that the difference in the oxidative status between apoplast and symplast can play a role in signal transduction. The protein GRIM REAPER which includes a signal peptide for exportation to the extracellular space, can be an example of the aforementioned. The proteolysis of this protein carried out by metacaspase 9 requires production of ROS by the respiratory burst oxidase homolog. In addition, the small peptide released is a ligand for the pollen specific receptor like kinase 5, which initiates the signal transduction to induce cell death [58].
It is clear that ROS are not only by-products of molecule oxidation, but rather play an important role in sending messages to the nucleus. This is called retrograde signal, and it plays a role by alerting the nucleus about the physiological status of a particular organelle. The study of these molecules will help in the elucidation of the role that plays the mechanism of the interaction between nucleus and organelles on plant physiology.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Apel, K., Hirt, H. (2004). REACTIVE OXYGEN SPECIES: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55(1), 373–399. DOI 10.1146/annurev.arplant.55.031903.141701. [Google Scholar] [CrossRef]
2. Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochemical Journal, 417(1), 1–13. DOI 10.1042/BJ20081386. [Google Scholar] [CrossRef]
3. Su, T., Li, W., Wang, P., Ma, C. (2019). Dynamics of peroxisome homeostasis and its role in stress response and signaling in plants. Frontiers in Plant Science, 10, 705. DOI 10.3389/fpls.2019.00705. [Google Scholar] [CrossRef]
4. Smirnoff, N., Arnaud, D. (2019). Hydrogen peroxide metabolism and functions in plants. New Phytologist, 221(3), 1197–1214. DOI 10.1111/nph.15488. [Google Scholar] [CrossRef]
5. Wang, L., Apel, K. (2019). Dose-dependent effects of 1O2 in chloroplasts are determined by its timing and localization of production. Journal of Experimental Botany, 70(1), 29–40. DOI 10.1093/jxb/ery343. [Google Scholar] [CrossRef]
6. Janků, M., Luhová, L., Petřivalský, M. (2019). On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants, 8(4), 105. DOI 10.3390/antiox8040105. [Google Scholar] [CrossRef]
7. Shapiguzov, A., Vainonen, J. P., Wrzaczek, M., Kangasjärvi, J. (2012). ROS-talk—How the apoplast, the chloroplast, and the nucleus get the message through. Frontiers in Plant Science, 3, 292. DOI 10.3389/fpls.2012.00292. [Google Scholar] [CrossRef]
8. Couturier, J., Chibani, K., Jacquot, J. P., Rouhier, N. (2013). Cysteine-based redox regulation and signaling in plants. Frontiers in Plant Science, 4, 105. DOI 10.3389/fpls.2013.00105. [Google Scholar] [CrossRef]
9. Skryhan, K., Gurrieri, L., Sparla, F., Trost, P., Blennow, A. (2018). Redox regulation of starch metabolism. Frontiers in Plant Science, 9, 1344. DOI 10.3389/fpls.2018.01344. [Google Scholar] [CrossRef]
10. Møller, I. M., Sweetlove, L. J. (2010). ROS signalling—Specificity is required. Trends in Plant Science, 15(7), 370–374. DOI 10.1016/j.tplants.2010.04.008. [Google Scholar] [CrossRef]
11. Suzuki, N., Koussevitzky, S., Mittler, R., Miller, G. (2012). ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell & Environment, 35(2), 259–270. DOI 10.1111/j.1365-3040.2011.02336.x. [Google Scholar] [CrossRef]
12. Bolwell, G. P., Wojtaszek, P. (1997). Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiological and Molecular Plant Pathology, 51(6), 347–366. DOI 10.1006/pmpp.1997.0129. [Google Scholar] [CrossRef]
13. Bolwell, G. P. (1999). Role of active oxygen species and NO in plant defence responses. Current Opinion in Plant Biology, 2(4), 287–294. DOI 10.1016/S1369-5266(99)80051-X. [Google Scholar] [CrossRef]
14. Hippeli, S., Heiser, I., Elstner, E. F. (1999). Activated oxygen and free oxygen radicals in pathology: New insights and analogies between animals and plants. Plant Physiology and Biochemistry, 37(3), 167–178. DOI 10.1016/S0981-9428(99)80031-X. [Google Scholar] [CrossRef]
15. Czarnocka, W., Karpiński, S. (2018). Friend or foe? reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radical Biology and Medicine, 122, 4–20. DOI 10.1016/j.freeradbiomed.2018.01.011. [Google Scholar] [CrossRef]
16. Zandalinas, S. I., Mittler, R. (2018). ROS-induced ROS release in plant and animal cells. Free Radical Biology and Medicine, 122, 21–27. DOI 10.1016/j.freeradbiomed.2017.11.028. [Google Scholar] [CrossRef]
17. Kollist, H., Zandalinas, S. I., Sengupta, S., Nuhkat, M., Kangasjärvi, J. et al. (2019). Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends in Plant Science, 24(1), 25–37. DOI 10.1016/j.tplants.2018.10.003. [Google Scholar] [CrossRef]
18. Farmer, E. E., Mueller, M. J. (2013). ROS-mediated lipid peroxidation and RES-activated signaling. Annual Review of Plant Biology, 64(1), 429–450. DOI 10.1146/annurev-arplant-050312-120132. [Google Scholar] [CrossRef]
19. Novaković, L., Guo, T., Bacic, A., Sampathkumar, A., Johnson, K. (2018). Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants, 7(4), 89. DOI 10.3390/plants7040089. [Google Scholar] [CrossRef]
20. Sandalio, L. M., Romero-Puertas, M. C. (2015). Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Annals of Botany, 116(4), 475–485. DOI 10.1093/aob/mcv074. [Google Scholar] [CrossRef]
21. Hartl, M., Finkemeier, I. (2012). Plant mitochondrial retrograde signaling: Post-translational modifications enter the stage. Frontiers in Plant Science, 3, 253. DOI 10.3389/fpls.2012.00253. [Google Scholar] [CrossRef]
22. Giraud, E., van Aken, O., Ho, L. H. M., Whelan, J. (2009). The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiology, 150(3), 1286–1296. DOI 10.1104/pp.109.139782. [Google Scholar] [CrossRef]
23. Van Aken, O., Zhang, B., Law, S., Narsai, R., Whelan, J. (2013). AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiology, 162(1), 254–271. DOI 10.1104/pp.113.215996. [Google Scholar] [CrossRef]
24. Ng, S., Giraud, E., Duncan, O., Law, S. R., Wang, Y. et al. (2013). Cyclin-dependent kinase E1 (CDKE1) provides a cellular switch in plants between growth and stress responses. Journal of Biological Chemistry, 288(5), 3449–3459. DOI 10.1074/jbc.M112.416727. [Google Scholar] [CrossRef]
25. Ng, S., Ivanova, A., Duncan, O., Law, S. R., van Aken, O. et al. (2013). A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. The Plant Cell, 25(9), 3450–3471. DOI 10.1105/tpc.113.113985. [Google Scholar] [CrossRef]
26. De Clercq, I., Vermeirssen, V., van Aken, O., Vandepoele, K., Murcha, M. W. et al. (2013). The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. The Plant Cell, 25(9), 3472–3490. DOI 10.1105/tpc.113.117168. [Google Scholar] [CrossRef]
27. Zhang, X., Ivanova, A., Vandepoele, K., Radomiljac, J., van de Velde, J. et al. (2017). The transcription factor MYB29 is a regulator of ALTERNATIVE OXIDASE1a. Plant Physiology, 173(3), 1824–1843. DOI 10.1104/pp.16.01494. [Google Scholar] [CrossRef]
28. Dogra, V., Kim, C. (2020). Singlet oxygen metabolism: From genesis to signaling. Frontiers in Plant Science, 10, 1640. DOI 10.3389/fpls.2019.01640. [Google Scholar] [CrossRef]
29. Exposito-Rodriguez, M., Laissue, P. P., Yvon-Durocher, G., Smirnoff, N., Mullineaux, P. M. (2017). Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Communications, 8(1), 49. DOI 10.1038/s41467-017-00074-w. [Google Scholar] [CrossRef]
30. Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141(2), 391–396. DOI 10.1104/pp.106.082040. [Google Scholar] [CrossRef]
31. Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylidès, C. et al. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences of the United States of America, 109(14), 5535–5540. DOI 10.1073/pnas.1115982109. [Google Scholar] [CrossRef]
32. Chan, K. X., Mabbitt, P. D., Phua, S. Y., Mueller, J. W., Nisar, N. et al. (2016). Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proceedings of the National Academy of Sciences of the United States of America, 113(31), E4567–E4576. DOI 10.1073/pnas.1604936113. [Google Scholar] [CrossRef]
33. León, P., Gregorio, J., Cordoba, E. (2013). ABI4 and its role in chloroplast retrograde communication. Frontiers in Plant Science, 3, 304. DOI 10.3389/fpls.2012.00304. [Google Scholar] [CrossRef]
34. Sun, X., Feng, P., Xu, X., Guo, H., Ma, J. et al. (2011). A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nature Communications, 2(1), 477. DOI 10.1038/ncomms1486. [Google Scholar] [CrossRef]
35. Johanningmeier, U., Howell, S. H. (1984). Regulation of light-harvesting chlorophyll-binding protein mRNA accumulation in Chlamydomonas reinhardi. Possible involvement of chlorophyll synthesis precursors. Journal of Biological Chemistry, 259(21), 13541–13549. DOI 10.1016/S0021-9258(18)90727-1. [Google Scholar] [CrossRef]
36. Mochizuki, N., Brusslan, J. A., Larkin, R., Nagatani, A., Chory, J. (2001). Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proceedings of the National Academy of Sciences of the United States of America, 98(4), 2053–2058. DOI 10.1073/pnas.98.4.2053. [Google Scholar] [CrossRef]
37. Nott, A., Jung, H. S., Koussevitzky, S., Chory, J. (2006). Plastid-to-nucleus retrograde signaling. Annual Review Plant Biology, 57(1), 739–759. DOI 10.1146/annurev.arplant.57.032905.105310. [Google Scholar] [CrossRef]
38. Fu, F. Q., Zhang, D. W., Deng, X. G., Li, J. Y., Peng, X. J. et al. (2015). Role of plastid signals in modulating Arabidopsis responses to cucumber mosaic virus. Plant Growth Regulation, 75(3), 761–769. DOI 10.1007/s10725-014-9979-8. [Google Scholar] [CrossRef]
39. Estavillo, G. M., Chan, K. X., Phua, S. Y., Pogson, B. J. (2013). Reconsidering the nature and mode of action of metabolite retrograde signals from the chloroplast. Frontiers in Plant Science, 3, 300. DOI 10.3389/fpls.2012.00300. [Google Scholar] [CrossRef]
40. Xiao, Y., Savchenko, T., Baidoo, E. E., Chehab, W. E., Hayden, D. M. et al. (2012). Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell, 149(7), 1525–1535. DOI 10.1016/j.cell.2012.04.038. [Google Scholar] [CrossRef]
41. Walley, J., Xiao, Y., Wang, J. Z., Baidoo, E. E., Keasling, J. D. et al. (2015). Plastid-produced interorgannellar stress signal MEcPP potentiates induction of the unfolded protein response in endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America, 112(19), 6212–6217. DOI 10.1073/pnas. [Google Scholar] [CrossRef]
42. Danon A., Miersch O., Felix G., Camp R. G. L., Apel K. (2005). Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. The Plant Journal, 41(1), 68–80. DOI 10.1111/j.1365-313X.2004.02276.x. [Google Scholar] [CrossRef]
43. Danon, A., Coll, N. S., Apel, K. (2006). Cryptochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America, 103(45), 17036–17041. DOI 10.1073/pnas. [Google Scholar] [CrossRef]
44. Ochsenbein, C., Przybyla, D., Danon, A., Landgraf, F., Göbel, C. et al. (2006). The role of EDS1 (enhanced disease susceptibility) during singlet oxygen-mediated stress responses of Arabidopsis. The Plant Journal, 47(3), 445–456. DOI 10.1111/j.1365-313X.2006.02793.x. [Google Scholar] [CrossRef]
45. Matilla, A. J. (2021). Cellular oxidative stress in programmed cell death: Focusing on chloroplastic 1O2 and mitochondrial cytochrome-c release. Journal of Plant Research, 134(2), 179–194. DOI 10.1007/s10265-021-01259-7. [Google Scholar] [CrossRef]
46. Lee, K. P., Kim, C., Landgraf, F., Apel, K. (2007). EXECUTER1-and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America, 104(24), 10270–10275. DOI 10.1073/pnas.0702061104. [Google Scholar] [CrossRef]
47. Zorov, D. B., Juhaszova, M., Sollott, S. J. (2006). Mitochondrial ROS-induced ROS release: An update and review. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1757(5–6), 509–517. DOI 10.1016/j.bbabio.2006.04.029. [Google Scholar] [CrossRef]
48. Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M. A. et al. (2011). Respiratory burst oxidases: The engines of ROS signaling. Current Opinion in Plant Biology, 14(6), 691–699. DOI 10.1016/j.pbi.2011.07.014. [Google Scholar] [CrossRef]
49. Choi, W.-G., Miller, G., Wallace, I., Harper, J., Mittler, R. et al. (2017). Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. The Plant Journal, 90(4), 698–707. DOI 10.1111/tpj.13492. [Google Scholar] [CrossRef]
50. Triantaphylides, C., Krischke, M., Hoeberichts, F. A., Ksas, B., Gresser, G. et al. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiology, 148(2), 960–968. DOI 10.1104/pp.108.125690. [Google Scholar] [CrossRef]
51. Mullineaux, P. M., Exposito-Rodriguez, M., Laissue, P. P., Smirnoff, N. (2018). ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radical Biology and Medicine, 122(33), 52–64. DOI 10.1016/j.freeradbiomed.2018.01.033. [Google Scholar] [CrossRef]
52. op den Camp, R. G. L., Przybyla, D., Ochsenbein, C., Laloi, C., Kim, C. et al. (2003). Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. The Plant Cell, 15(10), 2320–2332. DOI 10.1105/tpc.014662. [Google Scholar] [CrossRef]
53. Muñoz, P., Munné-Bosch, S. (2020). Oxylipins in plastidial retrograde signaling. Redox Biology, 37, 1–5. DOI 10.1016/j.redox.2020.101717. [Google Scholar] [CrossRef]
54. Daudi, A., Cheng, Z., O’Brien, J. A., Mammarella, N., Khan, S. et al. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. The Plant Cell, 24(1), 275–287. DOI 10.1105/tpc.111.093039. [Google Scholar] [CrossRef]
55. Wu, F., Chi, Y., Jiang, Z., Xu, Y., Xie, L. et al. (2020). Hydrogen peroxide sensor HPCA1 is an LRR Receptor Kinase in Arabidopsis. Nature, 578(7796), 577–581. DOI 10.1105/tpc.014662. [Google Scholar] [CrossRef]
56. Browning, E. A., Chatterjee, S., Fisher, A. B. (2012). Stop the flow: A paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annual Review of Physiology, 74(1), 403–424. DOI 10.1146/annurev-physiol-020911-153324. [Google Scholar] [CrossRef]
57. Hooijmaijers, C., Rhee, J. Y., Kwak, K. J., Chung, G. C., Horie, T. et al. (2012). Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. Journal of Plant Research, 125(1), 147–153. DOI 10.1007/s10265-011-0413-2. [Google Scholar] [CrossRef]
58. Wrzaczek, M., Brosche, M., Kollist, H., Kangasjarvi, J. (2009). Arabidopsis GRI is involved in the regulation of cell death induced by extracellular ROS. Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5412–5417. DOI 10.1073/pnas.0808980106. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |