

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019129
ARTICLE
Proline and Oxidative Metabolism in Young Pecan Trees Associated with Sulphate Accumulation
1Facultad de Ciencias Agrotecnológicas, Universidad Autónoma de Chihuahua, Chihuahua, 31350, México
2Centro de Investigación en Alimentación y Desarrollo, A.C., Delicias, 33088, México
3InstitutoTecnologico Nacional de México-Instituto Tecnológico de Torreón, Coahuila, 27170, México
*Corresponding Author: Dámaris Leopoldina Ojeda-Barrios. Email: dojeda@uach.mx
Received: 04 September 2021; Accepted: 17 November 2021
Abstract: Pecan [Carya illinoinensis (Wangenh.) K. Koch.] is a deciduous tree whose fruits (nuts) are of high economic value and offer excellent nutritional benefits. However, soils high in sulphates can limit its growth and development. Working with 5-year-old trees of ‘Western Schley’ pecan grown in soils high in sulphates, the levels of proline and oxidative metabolism were recorded in the leaflets. Results showed that different levels of visible leaflet damage (‘sufficiency’, ‘low’, ‘moderate’ or ‘severe’) were associated with different levels of leaflet sulphates (mg kg−1): ‘sufficiency’ (≤40), ‘low’ (41–60), ‘moderate’ (61–80) and ‘severe’ (80–100). ‘Severe’ sulphate damage was associated with significant reductions in chlorophyll (TChl) (17.04 μg g−1), relative water content (RWC) (50%) and leaf area (LA), and with increases in the concentrations of total carotenoids (TC) and proline (Prl). Increases were also observed in the activities of the oxidative metabolism enzymes: superoxide dismutase (SOD) (1.82 units min−1 g−1), catalase (CAT) (2.86 μmol H2O2 min−1 g−1) and antioxidant capacity (AC) (87% DPPH inhibition). However, guaiacol peroxidase (GP) showed a reduction (2.97 nmol GSH min−1 g−1). An inverse relationship was found between the sulphate concentration in the leaflets with respect to the evaluated parameters of TChl, TC, RWC, LA, AC, and GP. Proline synthesis and antioxidant enzymatic activity indicate salt stress in pecan leaflets in orchards irrigated with deep-well water high in sulphates.
Keywords: Carya illinoinensis; catalase; guaiacol peroxidase; salt stress; photosynthetic pigments; superoxide dismutase
In many arid and semi-arid regions of the world, salinity stress limits agricultural and horticultural production. With pecans, [Carya illinoinensis (Wangenh.) K. Koch], this occurs especially where poor-quality irrigation water contributes to the accumulations of various salts in the upper soil layers [1,2]. Excessive accumulations of Na+, Mg2+, Ca2+, Cl−, SO42− or HCO3−/CO32− are all associated with salt stress. In many areas of commercial pecan production, SO42− is the dominant salt at issue [3]. Among tree fruit species, the pecan is one of the most sensitive to salt stress [1,2]. The preferred ecological niche for this tree is on the banks of rivers with alluvial soils. Therefore, its cultivation has led to the establishment of many orchards on soils with high salt content, low moisture retention and poor drainage [4].
The main areas for pecan production are in northern Mexico and the southeastern USA, where the irrigation tends to be either gravity fed or pumped [5]. The irrigation water is often high in salts, and these tend to be deposited as a crust on the soil surface because in these areas evaporation greatly exceeds precipitation [6,7]. Other factors that contribute to the accumulation of salts in soils are excessive applications of chemical fertilizers and inappropriate tillage, especially in clay soils with poor drainage [4]. Pecan trees growing in soils with excessive salts show signs of distress indicated by stomatal closure, reduced vegetative growth, necrosis of leaf margins and early leaf senescence [8,9]. Consequently, the accumulation of biomass and the yield and quality of the nuts are all reduced [1].
Salt stress affects tree water relations by reducing the osmotic potential in the rhizosphere, which consequently reduces water uptake and significantly reduces the uptake of nutrients (Na+, Cl− and SO42−) [10]. A more negative soil osmotic potential also reduces photosynthesis and thus the availability of energy (carbohydrates) for essential metabolic functions and interfering with the trees’ normal growth and development [11,12]. Among the range of defense mechanisms that reduce damage caused by accumulations of salts in plant tissues are the synthesis and accumulation of proline, an amino acid that serves as an osmotic and protective agent that reduces tissue dehydration and the consequent damage to cell organelles [2,13].
Another defense mechanism is the modification of the activity and the physiological behavior of the tree’s antioxidant enzyme systems, mainly superoxide dismutase, catalase and guaiacol peroxidase [14]. These enzymes are among the most efficient in reducing or eliminating oxidative stress due to the presence of reactive oxygen species (ROS) produced under conditions of high salt concentrations [15]. There is little information related to salt stress and its effects on the physiological behavior of pecan. A reason for this is the difficulty of working on immature trees in their different phenological stages under greenhouse conditions, and the associated short exposure times to salinity [1,5]. The objective of this study was to evaluate the effects of salt stress on young, productive pecan trees induced by sulphates and their impact on photosynthetic pigments, proline accumulation and oxidative metabolism.
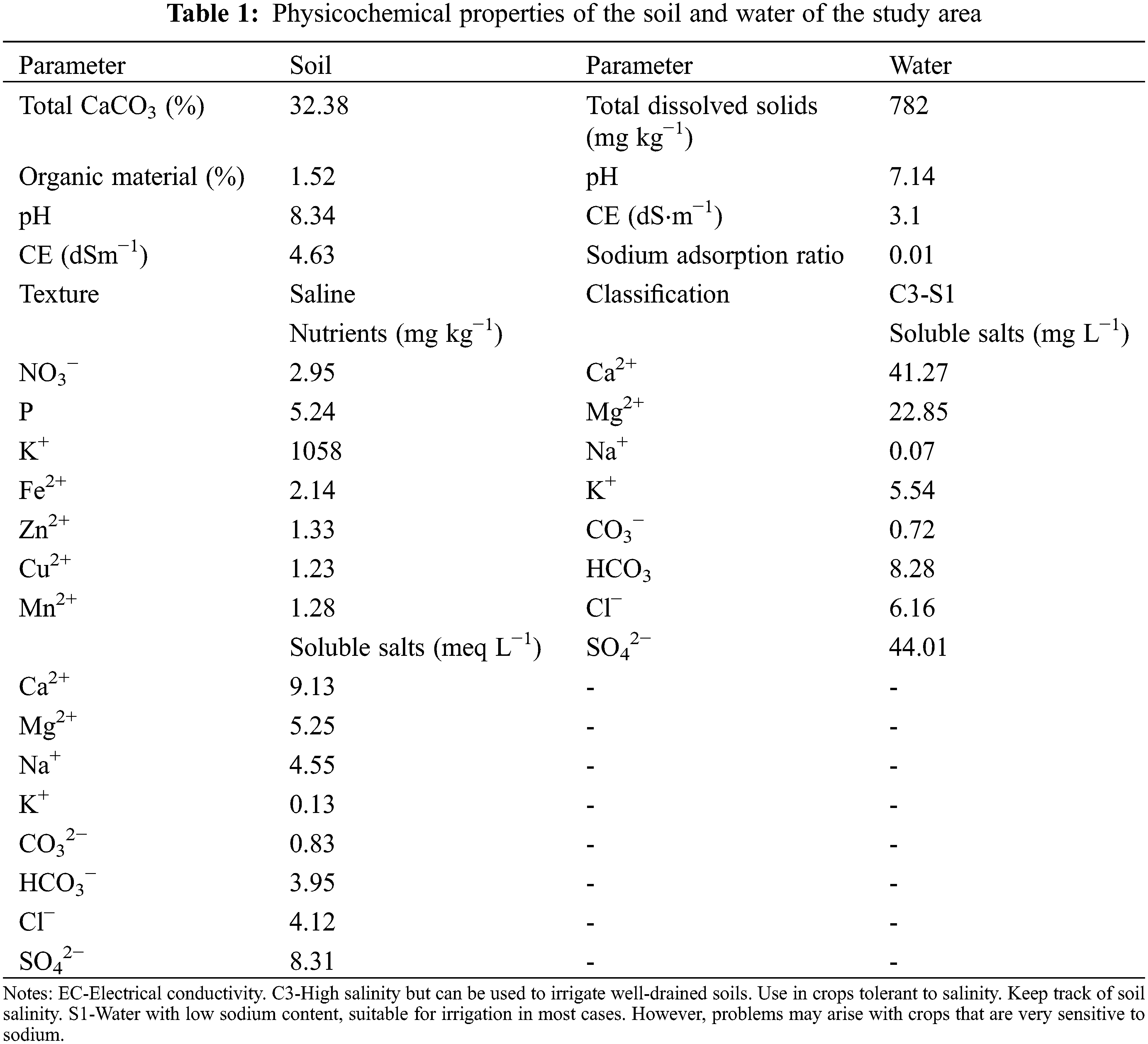
Study area, plant material and orchard management. The experiment was carried out during the 2019 and 2020 production cycles in Jiménez, Chihuahua, Mexico. The geographical location of the experimental site is 27°16′26.65″N, 104°49′44.52″W. The orchard was at an altitude of 1262 m.a.s.l., a mean annual temperature of 27°C and a mean annual rainfall of 350 mm. The plant material consisted of pecan trees [Carya illinoinensis (Wangenh.) K. Koch ‘Western Schley’]. Trees were established in 2014 on native rootstocks at a 12 × 12 m spacing (70 trees ha−1). Nutrient supply was by a surface application of dry fertilizer (120 N: 100 P2O5: 96 K2O). Irrigation during the production phase (April-October) was by sprinkler with average applications of 130 mm at 20-day intervals. Analysis of the soil physicochemical properties and irrigation water are presented in Table 1. During flowering in 2019, 24 young, productive trees were selected with mean trunk diameters of 11.96 ± 2 cm, mean heights at 5 ± 0.5 m and different severities of leaflet margin necrosis–presumably caused by accumulations of sulphates. From the four cardinal points of the canopy, at mid-height (1.5 m), 30 pairs of leaflets were collected from vegetative and fruiting shoots of the previous season’s growth. Leaflets were analyzed for sulphate using the method described by Novozamsky et al. [16]. Subsequently, the measured sulphate concentrations were correlated with the severity of marginal damage in the leaflets [17]. Four levels of sulphate visible damage were identified: ‘sufficiency’ (Sf), ‘low’ (L), ‘moderate’ (M) or ‘severe’ (S). These four visual damage levels (Sf, L, M and S) were found to correspond to four ranges of sulphate concentration (mgkg−1): Sf ≤ 40, L = 41–60, M = 61–80 and S = 80–100.
At the hardening stage of the shell (July 25 of the study years), 50 pairs of leaflets were collected from each experimental unit, using the method of Rodríguez-Jiménez et al. [18]. Briefly, the leaflets were divided into two groups: the first group was placed in transparent plastic bags and frozen at −80°C for later enzymatic analysis. The other group was immediately taken to the laboratory for determination of total chlorophyll, carotenoids, relative water content, leaf area and proline.
A completely randomized experimental design was used with trees homogenized by trunk diameter and height. Six replications were used in which the measured sulphate levels were considered as levels.
The foliar pigment content (total chlorophyll and total carotenoids) was determined by the method described by the AOAC [19]. Acetone (80%) was used for the extraction and the absorbance was measured at 470, 666 and 653 nm using a spectrophotometer. Data are reported in μg g−1. For each replicate, leaflet length and width were measured and the product length × width × 0.70 was used to estimate leaflet area [20]. The relative leaf water content (RWC) was determined by the method proposed by Ings et al. [21] with slight modifications. For this, 10 leaflets were weighed per replicate and recorded as fresh weight (FW). They were subsequently immersed in distilled water and left to rest for 24 h. After this period, the saturation weight (S) was recorded. They were then dehydrated in a drying oven) at 70°C for 24 h. The weight after dehydration was recorded as the dry weight (DW). The calculation of relative water content used the expression:
The extraction and quantification of proline used the method proposed by Ings et al. [21] with slight modifications. Briefly, 0.5 g of plant material was homogenized with 5 mL of 96% ethanol (v:v) and then with 5 mL of 70% ethanol (v/v). The mixture was centrifuged at 3740 rpm for 10 min. Next, 2.5 mL of ninhydrin reagent, 2.5 mL of 99% glacial acetic acid and 4 mL of deionized water were added to the supernatant and vigorously stirred for 1 min. The mixture was placed in a water bath for 45 min. Last, 5 mL of 99% benzene was added and it was allowed to stand for 15 min. Once this period had elapsed the absorbance was measured at 515 nm using a spectrophotometer. Proline concentration was expressed as μg g−1 of fresh weight based on a standard proline curve [22].
The activity of superoxide dismutase (SOD EC 1.15.1.1) was determined by the control method involving the photochemical reduction inhibition of nitroblue tetrazolium (NBT) described by Yu et al. [23] with modifications. Samples (20 g) of leaflets were homogenized in a mortar for 2 min with 1.5 g of quartz sand and 10 mL of a solution composed of 50 mmol HEPES buffer at pH 7.6 and 0.1 mmol Na2EDTA. The mixture was first centrifuged at 11,000 rpm for 10 min at 4°C and then filtered with qualitative filter paper MN 617. The extract obtained was used for the analysis of SOD and protein concentration, the latter by the Bradford method (1976), in which bovine serum albumin (BSA) was used as standard. Total SOD activity was determined in a 5 mL reaction mixture containing 50 mmol HEPES at pH 7.6, 0.1 mmol EDTA, 50 mmol Na2CO3 (pH 10), 13 mmol methionine, 0.25% Triton X-100 (w/v), 63 μM NBT, 1.3 μM riboflavin and an appropriate aliquot of enzyme extract. The reaction mixtures were illuminated for 15 min at a photosynthetic photon flux density of 380 μmol m−2 s−1. Identical reaction mixtures that were not illuminated were used to correct for background absorbance. The enzymatic activity was reported as unit min−1 g−1, where one unit of SOD activity corresponds to the amount of enzyme required to cause 50% inhibition of the reduction of NBT evaluated at 560 nm.
The extraction and analysis of total H2O2 were carried out with the method modified by Brennan et al. [24]. In this method, the hydroperoxides form a specific complex with titanium (Ti4+), which can be measured by colorimetry at 415 nm. The peroxide concentration in the extracts was determined by comparing the absorbance against a standard curve representing a 0.1 to 1 mmol titanium-H2O2 complex. The results were expressed in μmol H2O2 min−1 g−1.
The extraction and determination of catalase activity (CAT EC 1.11.1.6) were carried out using the method described by Sanchez et al. [25] with some modifications. The fresh plant material was macerated in a 50 mmol Tris-acetate buffer solution at pH 7.5 and with 5 mmol 2-mercaptoethanol, 2 mmol 1,4-dithio-DL-threitol (DTT), 2 mmol ethylenediaminetetraacetic acid (EDTA), 0.5 mmol phenylmethylsulfonyl fluoride (PMSF) and 1% (p:v) polyvinylpyrrolidone (PVP). The mixture was placed on a two-layer filter and a sample was centrifuged at 11500 rpm for 20 min at 4°C. The supernatant was used for CAT analysis, for which bovine serum albumin was used as a standard. CAT enzyme activity was determined following guaiacol oxidation and H2O2 consumption by changing A485 and A240, respectively. Control extracts were boiled and subsequently analyzed to confirm that the reactions were due to enzyme activity.
The extraction and determination of the guaiacol peroxidase (GP) activity were carried out as described by Sanchez et al. [25]. A sample (0.5 g) of foliar material was macerated with liquid nitrogen and homogenized with 5 mL of 25 mmol HEPPES-HCl buffer at pH 7.8 and 0.2 mmol EDTA-Na was added with 70% PVPP. The homogenate was filtered, and the supernatant was recovered by centrifugation at 11,500 rpm for 10 min at 4°C. The GP activity was determined using a 3 mL reaction mixture containing 500 μL of 25 mmol sodium phosphate buffer at pH 7.0, 500 μL of 0.4 M EDTA-Na, 750 μL of 0.05% guaiacol (v/v), 750 μL of 10 mmol H2O2 and finally 500 μL of the enzyme extract. The enzyme extract was then added, and the absorbance change readings (485 nm) were taken at 3 min, both for the plant tissue samples and the blanks. The same reaction mixture was used as a blank, in which the enzyme extract was replaced with 25 mmol of sodium phosphate buffer. The results were expressed as nmol GSH min−1g−1.
The antioxidant capacity was evaluated according to the method described by Brand-Williams et al. [26] with modifications. To 0.3 mL of extract was added 5.7 mL of DPPH compound (2,2-diphenyl-1-picrylhydrazyl at a concentration of 0.0375 g L−1). The mixture was kept in the dark for 30 min. The decrease in the DPPH radical was measured at 515 nm with a UV-visible spectrophotometer. The results were expressed as % inhibition of DPPH. The following equation was used for their calculation:
With the information obtained, a test of homogeneity of variances was carried out with the Kolmogorov-Smirnov test. When the variances were homogeneous, the analysis of variance was performed for a completely randomized design with repeated measures (years). Tukey’s multiple comparison test of means was used (P ≤ 0.05) to detect treatment effects. For heterogeneous variances, the Kruskal-Wallis test was used and after a treatment effect, the Mann-Whitney test was used to assess significant differences (P ≤ 0.05) between treatments. Subsequently, a Pearson correlation analysis was performed to determine the degree of association between the evaluated variables. The SAS (Statistical Analysis System) version 9.3 was used in all cases.
No significant effect was found for years (P ≤ 0.05), which enables the analysis to be performed by the treatment. Sustained strong growth in the commercial demand for nuts of all species has encouraged an increase in the area planted in pecans. However, productivity is low for orchards established on soils suffering salinity problems [1]. In this study, the lowest TChl values corresponded to leaflets from trees showing ‘moderate’ (23.66 μg g−1) to severe (17.04 μg g−1) salinity damage (Table 2). This behavior is often associated with stomatal closure and a reduction in intracellular CO2 concentration [13]. Likewise, previous studies have reported the negative effects of high concentrations of NaCl and Na2SO4 on the activity of RuBisCO (ribulose−1,5-bisphosphate carboxylase/oxygenase), PEP (phosphoenolpyruvate carboxylase) and AC (carbonic anhydrase) all of which impact the efficiency of photosynthesis in leaflets of pecan tree [27]. Our results are like those reported for pistachio (Pistacia vera L.) [28] and pecan [(Carya illinoinensis (Wangenh.) K. Koch)] [2]. On the other hand, this behavior may also be associated with the hydrolysis of the ester bond of the chlorophyll molecule, because of increases in the enzymatic activity of chlorophyllase [27,28]. Similarly, a significant increase in the concentration of carotenoids was also observed, with values fluctuating between 9.95 and 14.25 μg g−1 (Table 2). In this sense, a response to various stress conditions is associated with the synthesis and accumulation of secondary metabolites, including carotenoids [29]. These compounds actively participate in inhibiting, reducing, and eliminating superoxide, hydroxyl and singlet oxygen ions generated by the plant under conditions of salinity stress [8,30]. In addition, they are a precursor of the synthesis of abscisic acid which is involved in the opening and closing of stomata [31].
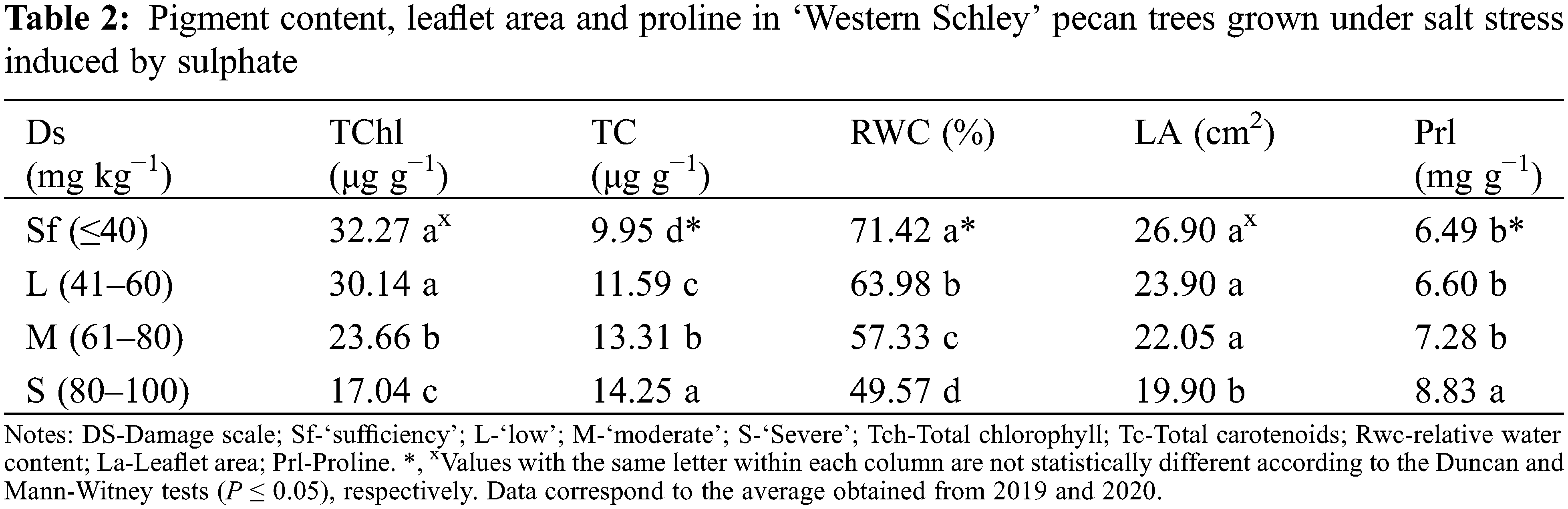
The RWC is an appropriate estimate of the water status of the plant in terms of cellular hydration (water potential and osmotic adjustment) [32]. The results here show an inverse relationship between the observed damage and the RWC with mean values of 50%, 57%, 64% and 71% corresponding to severe, medium, low and sufficiency foliar damage, respectively (see Table 2). Various studies report that a high concentration of sulphates hinders the movement of water from the soil to the plants by reducing the conduction of water from the roots and affecting the relative water content of the shoot at the cellular level [10]. This is attributed to a reduction in the partial pressure of intracellular CO2 in the leaves, induced by the accumulations of Na+, Cl−, SO42− and their negative effects on leaf expansion and stomatal opening [15,31]. Except for the sufficiency damage, the pecan leaflets with sulphate damage showed severe problems of wilting and marginal necrosis, which coincides with the report of Moreno-Izaguirre et al. [2].
A high concentration of salts is correlated with reductions in biomass, leaflet area and height by modifying the ionic balance, mineral nutrition, stomatal behavior, and photosynthetic efficiency [15]. In this study, severely damaged leaflets showed a significant reduction in the leaflet area (26.90 cm2) (Table 2). Hoang et al. [33] reported a 43% reduction in a foliar area with respect to the controls when evaluating the application of calcium sulphate (Ca, 2.5 mmol) to the soil under salinity conditions (100 mmol NaCl) in red amaranth (Amaranthus tricolor L.). On the other hand, Stoeva et al. [34] reported significant reductions in the foliar area of 64% and 67% when applying NaCl (50 mM) and Na2SO4 (100 mmol), respectively, in beans (Phaseolus vulgaris L.) ‘Lody’. Previous studies in corn (Zea mays L.) and soybean (Glycine max (L.) Merr.) report inhibition of N uptake under salinity conditions [34]. Nitrogen is a macronutrient linked to the structure of the chlorophyll molecule, amino acids and nucleic acids [35].
Proline level can be an indicator of salt stress in plants [29]. Here, a direct relationship was found between the concentration of sulphates and Prl (Table 2). These results agree with previous research that affirms that proline increases in concentration as a response to stress due to drought, salinity, extreme temperatures, or high light intensity [2,22]. A previous study by Moreno-Izaguirre et al. [2] with edaphic applications of Na2SO4 (1000, 2000, 3000 or 4000 mg L−1) in pecan nut seedlings, reports a direct relationship between the increasing concentrations of this salt and proline. However, these authors reported that it was from 2000 mg L−1 of Na2SO4 that a significant variation was found in the accumulation of biomass and in concentrations of chlorophyll and sulphates. The synthesis and accumulation of proline as an osmotic agent are among the protection mechanisms against the loss of turgor due to the presence of salts [8]. Furthermore, Prl can also be an initial source of N for recovery from salinity-induced stress [11,36].
The results related to the enzymatic activity and AC are shown in Table 3. Pecan trees planted in areas with excess soil sulphates present different adaptation mechanisms. Among these is their antioxidant enzymatic activity (superoxide dismutase, catalase, guaiacol peroxidase, among others) [6]. These enzymes play important roles in the detoxification of ROS (i.e., O2−, •OH, ROO•, H2O2) generated by this stress factor, and these ROS are harmful to proteins, lipids, and nucleic acids [27,37]. Our results showed that the enzymatic activity of SOD was higher in those leaflets showing ‘medium’ and ‘severe’ damage with values of 1.89- and 1.82-units min−1 g−1, respectively (Table 3). This agrees with the results of Wang et al. [7] in Juglans regia L. seedlings, irrigated with 50 mmol NaCl. The NaCl treatments (0, 300, 450 and 600 mmol) evaluated by Mohammadi et al. [8] in Leptochloa fusca, indicate that the highest SOD enzymatic activity (4.0 U mg of protein) was observed in the 450 mmol treatment, which is associated with the ability of this species to buffer the negative effects of ROS. There is no evidence to indicate the behavior of SOD with respect to the presence of sulphates in pecan. However, it has been reported that pecan is a species sensitive to the presence of salts in the soil [4]. In this sense, pecan has a vigorous root system, and feeder roots develop near the plant neck and are naturally associated with ectomycorrhiza [38], which could be linked to adaptations to water stress and salinity.
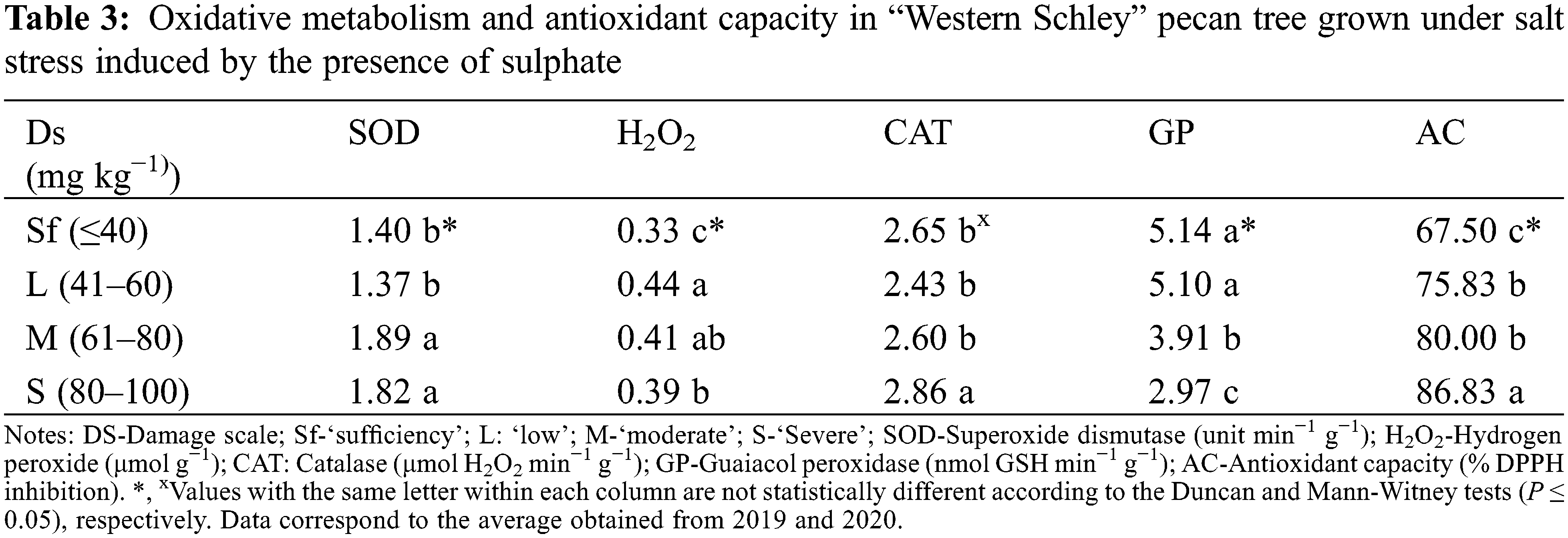
The presence of sulphates in the soil significantly impacted the production of H2O2 in pecan nut leaflets (Table 3). One of the physiological responses to stomatal closure and low CO2 availability is associated with the production of H2O2 for the oxygenation of RuBisCO and the increase in photorespiration [9]. In this sense, the behavior in the production of H2O2 is associated with the activity of SOD through the Haber Weiss reaction [13,39].
The damage induced by the oxidative metabolism and the generation of H2O2 is regulated by CAT (E.C.1.11.1.6), which catalyzes the dismutation of H2O2 to H2O and O2 [35]. The ‘severe’ damage condition significantly increased CAT activity (2.86 μmol H2O2 min−1 g−1). Similar values (2.06 μmol H2O2 min−1 g−1 and 3.54 μmol H2O2 min−1 g−1) were reported by Balandrán-Valladares et al. [40] in ‘Western Scheley’ pecan tree. This also fits with findings of Karimi et al. [41] who reported a five-fold increase in CAT activity with respect to the controls when evaluating treatments of 100 mM Na2SO4, CaCl2 and KNO3, 50 mmol Na2SO4 + CaCl2, 50 mmol Na2SO4 + KNO3 and 50 mmol CaCl2 + KNO3 in Juglans regia ‘Chandler’.
It has been observed that oxidative stress activates the antioxidant enzymatic systems (SOD, CAT, GP, and others) which help regulate the levels of ROS [21]. However, the efficiency of these systems is affected by the severity of the stress, the types of ROS present and the physiological condition of the plant [37]. Previous studies with halophytes (Atriplex lentiformis, Tamarix aphylla, Sporobolus marginatus, Suaeda nudiflora, Urochondra setulosa, Arundo donax, Aeluropus lagopoides, Heliotropium ramossimum, Atriplex nummularia, and Lecaptachloa under saline conditions (300 mmol NaCl) report increases in the activity of the antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase and peroxidase). This work highlights the genetic capacity of these species to minimize the negative effects of ROS [14]. In an experiment with corn seedlings (Zea mays L.) subjected to treatment with NaCl (75 and 150 mmol), Karimi et al. [41] report significant increases in oxidative stress in roots and mature leaves but not in young leaves. This result may be because of the synthesis and joint activities of polyphenols (non-enzymatic antioxidants) and the enzymatic activity of CAT.
In our study, leaflets with sufficiency levels of sulphate damage showed a maximum reduction in H2O2 level through the activity of the enzyme guaiacol peroxidase (GP) (5.14 nmol GSH min−1 g−1). However, this value was not significantly different from the low range (Table 3). According to previous physicochemical analyses of soil and water, sulphate concentrations were 8.31 meq L−l (soil) and 44.10 meq L−l (water). These results indicate that the trees in our pecan orchard may be subjected to oxidative stress induced by the presence of sulphates. This could explain the low activity of GP under conditions of ‘moderate’ and even ‘severe’ damage. In this regard, Alayafi [37] point out an inverse relationship between oxidative stress and the efficiency of the antioxidant enzyme system. This is, ‘severe’ damage under stress conditions can minimize the activities of SOD, CAT and GP. In contrast, when the concentration of H2O2 is increased in Panax ginseng seedlings, greater tolerance to salinity has been demonstrated by the activities of CAT and GP [42]. This behavior may be linked to the homeostasis generated by the specific production of H2O2 and the activities of the antioxidant systems [9]. This contrasts with our observations for GP in leaflets suffering ‘moderate’ and ‘severe’ damage.
Our leaflets showing ‘severe’ damage presented the highest AC value (87% DPPH inhibition) (Table 3). These results do not agree with those reported in various genotypes of ‘beef’ tomatoes (Solanum lycopersicum L.) cultivated under three levels of electric conductivity (2, 2.5 and 3 dS m−1) [43]. On the other hand, an increase in antioxidant capacity is associated with an increased enzyme activity of SOD, CAT and GP [39]. A direct relationship with proline concentration in leaflet tissues has been observed previously [12]. This behavior affects the tolerance of pecan trees to the high concentration of sulphates. Some pecan orchards in northern Mexico and southeast USA are exposed to salinity conditions due to Na2SO4 or CaSO4 (among others) [4]. Pecan is known to be sensitive to Na+ salinity with a tolerance threshold between 2 and 3 dS m−1. However, it has been reported that values of 3 dS m−1 can cause significant depressions of tree growth [11]. Moreno-Izaguirre et al. [2] when evaluating applications of Na2SO4 (1000, 2000, 3000 or 4000 mg L−1) to native pecan genotypes indicate reductions in biomass accumulation, shoot length and tree height.
According to Pearson’s correlation analysis, an inverse relationship was found between the sulphate concentration in the leaflets with respect to the evaluated parameters of TChl (−0.907), TC (−0.911), RWC (−0.948), LA (−0.828), AC (−0.845) and GP (−0.845). These results suggest that the presence of sulphates is an abiotic factor of saline stress that increases the oxidative metabolism and limits the production of photoassimilates by pecan, also reducing stomatal conductance and growth of branches and leaflets and reducing the uptake of soil water [40]. Significant reductions in chlorophyll concentration in four-year-old ‘Wichita’ pecan have been reported by Ben Ali et al. [6]; however, these authors did not observe effects on the growth (height or diameter) or development of trees irrigated with saline water (4 dS m−1) or reverse osmosis water (8 dS m−1). On the other hand, accumulations of osmolytes (proline, carotenes, and soluble sugars) of low molecular weight in mandarin ‘Kinnow’ Citrus reticulata (White) increases the tolerance to reductions of water in the tissues due to the presence of salts [31]. Here, we found that sulphate-damaged ‘Western Schley’ pecan leaflets showed significant increases in the synthesis of proline and antioxidant enzyme activities along with changes in growth and development.
Pecan orchards planted in northern Mexico and the southeastern United States have soil problems with high concentrations of salts, including sulphates. In this study, the lowest total chlorophyll values corresponded to leaflets from trees showing ‘moderate’ (23.66 μg g−1) to severe (17.04 μg g−1) salinity damage. Similarly, there was an inverse relationship between the observed damage and the RWC with mean values of 50%, 57%, 64% and 71% corresponding to severe, medium, low and sufficiency foliar damage, respectively. Severely damaged leaflets showed a significant reduction in the leaflet area (26.90 cm2). A direct, positive relationship was found between the increasing concentrations of sulphates and proline. Our results showed that the enzymatic activity of SOD was higher in those leaflets showing ‘medium’ (1.89 units min−1 g−1) and ‘severe’ (1.82 units min−1 g−1) salinity levels. The ‘severe’ damage condition significantly increased CAT activity (2.86 μmol H2O2 min−1 g−1). Leaflets with sufficiency levels of sulphate damage showed a maximum reduction in H2O2 levels through the activity of the enzyme guaiacol peroxidase (5.14 nmol GSH min−1 g−1); however, this later value was not significantly different from the low salinity range. Our leaflets showing ‘severe’ damage presented the highest AC values (87% DPPH inhibition). Proline synthesis and the antioxidant enzymatic activity indicate salt stress in pecan leaflets in orchards irrigated with deep-well water high in sulphates.
Author Contributions: D.J.E.A and D.L.O.B. designed and supervised the experiment. D.J.E.A. performed the experiment, harvested plant materials and collected field data. O.C.A and J.L.J.C. oversaw experiment, analyzed data and drafted manuscript with input from O.A.H.R., E.S.C and P.P.R. performed biochemical analysis and analyzed data. D.L.O.B and O.C.A. wrote, revised and finalized the manuscript.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Deb, S. K., Sharma, P., Shukla, M. K., Sammis, T., Ashigh, J. (2013). Drip-irrigated pecan seedlings response to irrigation water salinity. HortScience, 48(12), 1548–1555. DOI 10.21273/hortsci.48.12.1548. [Google Scholar] [CrossRef]
2. Moreno-Izaguirre, E., Ojeda-Barrios, D., Ávila-Quezada, G., Guerrero-Prieto, V., Parra-Quezada, R. et al. (2015). Sodium sulfate exposure slows growth of native pecan seedlings. Phyton-International Journal of Experimental Botany, 84(1), 80–85. DOI 10.32604/phyton.2015.84.080. [Google Scholar] [CrossRef]
3. Huang, X., Soolanayakanahally, R. Y., Guy, R. D., Shunmugam, A. S. K., Mansfield, S. D. (2020). Differences in growth and physiological and metabolic responses among Canadian native and hybrid willows (Salix spp.) under salinity stress. Tree Physiology, 40(5), 652–666. DOI 10.1093/TREEPHYS/TPAA017. [Google Scholar] [CrossRef]
4. Miyamoto, S., Nesbitt, M. (2011). Effectiveness of soil salinity management practices in basin-irrigated pecan orchards. HortTechnology, 21(5), 569–576. DOI 10.21273/horttech.21.5.569. [Google Scholar] [CrossRef]
5. Valles-Aragón, M. C., Ojeda-Barrios, D. L., Guerrero-Prieto, V. M., Prieto-Amparan, J. A., Sánchez-Chávez, E. (2017). Calidad del agua para riego en una zona nogalera del estado de chihuahua. Revista Internacional de Contaminación Ambiental, 33(1), 85–97. DOI 10.20937/RICA.2017.33.01.08. [Google Scholar] [CrossRef]
6. Ben Ali, A. R., Shukla, M. K., Schutteb, B. J., Gard, C. C. (2020). Irrigation with RO concentrate and brackish groundwater impacts pecan tree growth and physiology. Agricultural Water Management, 240, 106328. DOI 10.1016/j.agwat.2020.106328. [Google Scholar] [CrossRef]
7. Wang, B., Zhang, J., Pei, D., Yu, L. (2020). Combined effects of water stress and salinity on growth, physiological, and biochemical traits in two walnut genotypes. Physiologia Plantarum, 172(12), 1–12. DOI 10.1111/ppl.13316. [Google Scholar] [CrossRef]
8. Mohammadi, F., Kavousi, H. R., Mansouri, M. (2019). Effects of salt stress on physio-biochemical characters and gene expressions in halophyte grass Leptochloa fusca (L.) kunth. Acta Physiologiae Plantarum, 41(8), 1–10. DOI 10.1007/s11738-019-2935-5. [Google Scholar] [CrossRef]
9. Leng, X., Xue, L., Wang, J., Li, S., Yang, Z. et al. (2020). Physiological responses of Handeliodendron bodinieri (Levl.) Rehd. to exogenous calcium supply under drought stress. Forests, 11(1), 1–14. DOI 10.3390/f11010069. [Google Scholar] [CrossRef]
10. Saha, J., Brauer, E. K., Sengupta, A., Popescu, S. C., Gupta, K. et al. (2015). Polyamines as redox homeostasis regulators during salt stress in plants. Frontiers in Environmental Science, 3(21), 1–13. DOI 10.3389/fenvs.2015.00021. [Google Scholar] [CrossRef]
11. Campos-Villarreal, A., Arreola-Ávila, J., Chávez-Simental, J., Trejo-Calzada, R., Borja de la Rosa, A. et al. (2017). Physiological response, ion accumulation and dry weight of pecan (Carya illinoinensis (Wangenh) K. koch) rootstocks grown under saline stress conditions. Interciencia, 42(11), 744–749. [Google Scholar]
12. Aguilar-Carpio, C., Juárez-López, P., Campos-Aguilar, I. H., Alia-Tejacal, I., Sandoval-Villa, M. et al. (2018). Analysis of growth and yield of cape gooseberry (Physalis peruviana L.) grown hydroponically under greenhouse conditions. Revista Chapingo Serie Horticultura, 24(3), 191–202. DOI 10.5154/r.rchsh.2017.07.024. [Google Scholar] [CrossRef]
13. Naeem, M., Naeem, M. S., Ahmad, R., Ihsan, M. Z., Ashraf, M. Y. et al. (2018). Foliar calcium spray confers drought stress tolerance in maize via modulation of plant growth, water relations, proline content and hydrogen peroxide activity. Archives of Agronomy and Soil Science, 64(1), 116–131. DOI 10.1080/03650340.2017.1327713. [Google Scholar] [CrossRef]
14. Kumar, A., Mann, A., Kumar, A., Kumar, N., Meena, B. L. (2021). Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. International Journal of Phytoremediation, 2(3), 1–11. DOI 10.1080/15226514.2021.1874289. [Google Scholar] [CrossRef]
15. Hand, M. J., Taffouo, V. D., Nouck, A. E., Nyemene, K. P. J., Tonfack, B. (2017). Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 45(2), 481–490. DOI 10.15835/nbha45210928. [Google Scholar] [CrossRef]
16. Novozamsky, I., van Eck, R. (1977). Total sulphur determination in plant material. Fresenius’ Zeitschrift Für Analytische Chemie, 286(5), 367–368. DOI 10.1007/BF00431199. [Google Scholar] [CrossRef]
17. Vélez-Carvajal, N. A., Melo-Martínez, S. E., Flórez-Roncancio, V. J. (2014). Behavior of Ca, Mg and S in a soilless culture system for carnation. Revista Chapingo Serie Horticultura, 20(2), 171–185. DOI 10.5154/r.rchsh.2013.10.038. [Google Scholar] [CrossRef]
18. Rodríguez-Jiménez, T., Ojeda-Barrios, D. J., Blanco-Macías, D. L., Valdez-Cepeda, F., Parra-Quezada, R. D. (2016). Urease and nickel in plant physiology. Revista Chapingo Serie Horticultura, 22(2), 69–82. DOI 10.5154/r.rchsh.2014.11.051. [Google Scholar] [CrossRef]
19. AOAC. Association of Official Analytical Chemists (2005). Official methods of analysis. Association of Official Analytical Chemists, Arlington. [Google Scholar]
20. Ojeda-Barrios, D. L., Hernández-Rodríguez, O. A., Martínez-Téllez, J., Núñez-Barrios, A., Perea-Portillo, E. (2009). Foliar application of zinc chelates on pecan. Revista Chapingo Serie Horticultura, 15(2), 205–210. DOI 10.5154/R.RCHSH.2009.15.028. [Google Scholar] [CrossRef]
21. Ings, J., Mur, L. A. J., Robson, P. R. H., Bosch, M. (2013). Physiological and growth responses to water deficit in the bioenergy crop Miscanthus x giganteus. Frontiers in Plant Science, 4(11), 1–12. DOI 10.3389/fpls.2013.00468. [Google Scholar] [CrossRef]
22. Almansa, E. M., Chica, R. M., Plaza, B. M., Lao, M. T. (2017). Proline test to evaluate light stress in tomato seedlings under artificial light. Acta Horticulturae, 1170, 1019–1026. DOI 10.1016/j.scienta.2020.109473. [Google Scholar] [CrossRef]
23. Yu, Q., Osborne, L., Rengel, Z. (1998). Micronutrient deficiency changes activities of superoxide dismutase and ascorbate peroxidase in tobacco plants. Journal of Plant Nutrition, 21(7), 1427–1437. DOI 10.1080/01904169809365493. [Google Scholar] [CrossRef]
24. Brennan, T., Frenkel, C. (1977). Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology, 59(3), 411–416. DOI 10.1104/pp.59.3.411. [Google Scholar] [CrossRef]
25. Sanchez, E., Soto, J. M., García, P. C., Lopez-Lefebre, L. R., Rivero, R. M. et al. (2000). Phenolic compounds and oxidative metabolism in green bean plants under nitrogen toxicity. Australian Journal of Plant Physiology, 27(10), 973–978. DOI 10.1071/pp00008. [Google Scholar] [CrossRef]
26. Brand-Williams, W., Cuvelier, M. E., Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. DOI 10.1016/S0023-6438(95)80008-5. [Google Scholar] [CrossRef]
27. Shahid, M. A., Sarkhosh, A., Khan, N., Balal, R. M., Ali, S. et al. (2020). Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy, 10(7), 1–34. DOI 10.3390/agronomy10070938. [Google Scholar] [CrossRef]
28. Karimi, S., Rahemi, M., Maftoun, M., Eshghi, S., Tavallali, V. (2009). Effects of long-term salinity on growth and performance of two pistachio (Pistacia vera L.) rootstocks. Australian Journal of Basic and Applied Sciences, 3(3), 1630–1639. [Google Scholar]
29. Mendoza-Tafolla, R. O., Juarez-Lopez, P., Ontiveros-Capurata, R. E., Sandoval-Villa, M., Alia-Tejacal, I. et al. (2019). Estimating nitrogen and chlorophyll status of romaine lettuce using SPAD and at LEAF readings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 47(3), 751–756. DOI 10.15835/nbha47311589. [Google Scholar] [CrossRef]
30. Shereen, A., Asma-Shirazi, M. U., Arif, M., Mahboob, W., Khan, M. A. (2020). Salinity induced variability in morpho-physiological traits and their relationship with grain yield in rice (Oryza sativa L.). Pakistan Journal of Botany, 52(5), 1615–1623. DOI 10.30848/PJB2020-5(44). [Google Scholar] [CrossRef]
31. Sabzmeydani, E., Sedaghathoor, S., Hashemabadi, D. (2020). Salinity response of kentucky bluegrass (Poa pratensis L.) as influenced by salicylic acid and progesterone. Revista Chapingo Serie Horticultura, 26(1), 49–63. DOI 10.5154/r.rchsh.2019.08.012. [Google Scholar] [CrossRef]
32. Dong, Y., Hu, G., Yu, J., Thu, S. W., Grover, C. E. et al. (2020). Salt-tolerance diversity in diploid and polyploid cotton (Gossypium) species. Plant Journal, 101(5), 1135–1151. DOI 10.1111/tpj.14580. [Google Scholar] [CrossRef]
33. Hoang, H. L., Guzman, C. C., Cadiz, N. M., Hoang, T. T. H., Tran, D. H. et al. (2020). Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). Journal of Soil Science and Plant Nutrition, 20(4), 1759–1769. DOI 10.1007/s42729-020-00248-4. [Google Scholar] [CrossRef]
34. Stoeva, N., Kaymakanova, M. (2008). Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). Journal of Central European Agriculture, 9(3), 385–391. DOI 10.5513/jcea.v9i3.682. [Google Scholar] [CrossRef]
35. Thapa, R., Wick, A., Chatterjee, A. (2017). Response of spring wheat to sulfate-based salinity stress under greenhouse and field conditions. Agronomy Journal, 109(2), 442–454. DOI 10.2134/agronj2016.07.0384. [Google Scholar] [CrossRef]
36. Das, K., Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science, 2(53), 1–13. DOI 10.3389/fenvs.2014.00053. [Google Scholar] [CrossRef]
37. Alayafi, A. A. M. (2020). Exogenous ascorbic acid induces systemic heat stress tolerance in tomato seedlings: Transcriptional regulation mechanism. Environmental Science and Pollution Research, 27(16), 19186–19199. DOI 10.1016/j.scienta.2020.109473. [Google Scholar] [CrossRef]
38. Blasco, B., Graham, N. S., Broadley, M. R. (2015). Antioxidant response and carboxylate metabolism in Brassica rapa exposed to different external Zn, Ca, and Mg supply. Journal of Plant Physiology, 176(15), 16–24. DOI 10.1016/j.jplph.2014.07.029. [Google Scholar] [CrossRef]
39. Bonito, G., Brennema, T., Vilgalys, R. (2011). Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; juglandaceae). Mycorrhiza, 21(7), 601–612. DOI 10.1007/s00572-011-0368-0. [Google Scholar] [CrossRef]
40. Balandrán-Valladares, M. I., Cruz-Alvarez, O., Jacobo-Cuellar, J. L., Hernández-Rodríguez, O. A., Flores-Córdova, M. A. et al. (2021). Changes in nutrient concentration and oxidative metabolism in pecan leaflets at different doses of zinc. Plant, Soil and Environment, 67(1), 33–39. DOI 10.17221/525/2020-PSE. [Google Scholar] [CrossRef]
41. Karimi, S., Karami, H., Vahdati, K., Mokhtassi-Bidgoli, A. (2020). Antioxidative responses to short-term salinity stress induce drought tolerance in walnut. Scientia Horticulturae, 267(12), 109322. DOI 10.1016/j.scienta.2020.109322. [Google Scholar] [CrossRef]
42. Sathiyaraj, G., Srinivasan, S., Kim, Y. J., Lee, O. R., Parvin, S. et al. (2014). Acclimation of hydrogen peroxide enhances salt tolerance by activating defense-related proteins in Panax ginseng. Molecular Biology Reports, 41(6), 3761–3771. DOI 10.1007/s11033-014-3241-3. [Google Scholar] [CrossRef]
43. Martínez-Damián, M. T., Cruz-Alvarez, O., Rodríguez-Pérez, J. E., Colinas-León, M. T., Góngora-Canto, M. A. (2017). Yield, physicochemical quality, and antioxidant capacity of “beef” and wild tomato fruits (Solanum lycopersicum L.) as a function of the electrical conductivity of the nutrient solution. Agronomía Colombiana, 35(3), 330–339. DOI 10.15446/agron.colomb.v35n3.64905. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |