

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019774
ARTICLE
The Effects of Fertilizers on Rabbiteye Blueberry (Vaccinium ashei Reade.) Root Distribution and Fruit Yield
1College of Forestry, Guizhou University, Guiyang, 550025, China
2Provincial Key Laboratory for Agricultural Pest Management of Mountainous Regions, 9 Institute of Entomology, Guizhou University, Guiyang, 550025, China
*Corresponding Author: Delu Wang. Email: deluwang23@aliyun.com
Received: 14 October 2021; Accepted: 08 December 2021
Abstract: The root system plays an important role in the growth and development of blueberry. The aim of this study was to assess the impacts of different fertilizers on the root growth and root–yield relationship of blueberry to provide insight into the regulation of root growth and fruit yield by fertilizing from the perspective of the root system. Rabbiteye blueberry variety ‘Britewell’ as the test material, and six fertilizers, including BF, OR, CF, SF, HF, and RT were used in single-factor fertilization experiments to analyze the effects of different fertilizer treatments on the root morphology, root distribution, and fruit yield of blueberry. Fertilization overall increased the root length density and root surface area in most soil layers, and the RT treatment significantly increased the total root length density and total root surface area 98.6% and 98.5%, respectively, compared with a control lacking fertilizer. In addition, the effect of fertilization on the blueberry root system was mainly observed in the 0–20 cm layer. Fruit yield was positively correlated with total root length density and total root surface area, and negatively correlated with average root diameter. In summary, the SF and RT treatments increased the morphological indexes of the root system, particularly in the shallow soil layers, leading to an increase in blueberry fruit yield.
Keywords: Fertilization; membership function; root morphology; rabbiteye blueberry
The root system is the primary organ for nutrient absorption in plants that synthesizes and transports physiological activators [1] and also regulates plant growth and development [2,3]. Roots are therefore of great importance for crop growth, and their growth affects shoot growth and crop yield [4,5] as they act as “receptors” for the perception of environmental changes in crop plants. Root growth is sensitive to changes in the soil nutrient environment. For instance, ammonium nutrition inhibits root growth and nitrogen (N) absorption on citrus seedlings [6], while Arabidopsis thaliana modulates root N acquisition efficiency in response to the N demands of shoots [5]. One study suggests that fertigation with 50% of the recommended fertilizer could be most effective for enhancing the growth and N use efficiency (NUE) of rabbiteye blueberry [7]. The appropriate amount of P fertilizer application is known as key nutrients for tomato production, and maintaining its adequate levels leads to maximizing tomato yield [8]. By increasing the amount of P fertilizer, the tomato plant growth was also improved [9]. In addition, N deficiency is more likely to promote water absorption and N accumulation at the same root surface area level, which has been shown to lead to a higher dry mass in maize seedlings [10]. Therefore, fertilization plays an important role in the communication between plant roots, plant growth, and fruit yield. In recent years, most of the studies have focused on the factors affecting root growth and distribution [11,12]. The root–shoot system is affected by irrigation, fertilization, and straw management [13–15], among which fertilization has been regarded as the key factor in root system development.
Rabbiteye blueberries prefer ammonium nitrogen (NH4+-N) over nitrate nitrogen (NO3−-N) [16]. N is a key factor in blueberry production, and while high blueberry yields can be acquired via the application of optimal fertilization rates [17], the type of fertilizer that achieves the greatest blueberry fruit yield remains unknown. Insufficient N fertilizer application causes premature senescence, while excessive application results in late ripening and increases environmental pollution. The improper application of phosphorous (P) and potassium (K) fertilizers affect the normal growth of plants [18]. Therefore, selecting the correct fertilizer is of great importance for promoting growth and increasing fruit yield of blueberry plants. Recent studies have measured the impacts of compound fertilizer on blueberry [19–21]. Marty et al. [22] showed that applying chipped ramial wood (CRW) compost to low bush blueberry crops may not only be a mean of increasing growth and fruit production, but also alleviate the need for chemical weed control. In berry crops, N status strongly affects orchard longevity, berry quality and productivity, and root and shoot growth rate [23]. At present, compound fertilizer (N, P, and K) is applied widely. Recent studies suggested that N, P, and K can affect the distribution of roots in the soil in cotton [24] and tomato [25]. The optimal fertilizer application rate might increase root distribution in the layer in which the fertilizer is applied, thus promoting nutrient absorption and increasing the photosynthetic capacity of plants. Phosphate fertilizer was found to induce deeper root growth into the soil within the 0–24 cm layer in maize resulting in a significant increase in the N absorption and yield of the plants. Root growth regulates above ground plant growth, and the impact of fertilization on the vertical distribution of the roots is an important factor influencing the photosynthetic ability of the leaves [13].
The above observations indicated that shoot growth and yield formation are significantly influenced by root growth. Rabbiteye blueberry is an economically important acid-loving plant with a shallow root system [26] and is thus sensitive to fertilizer selection. Plants are primarily dependent on the spatial and temporal distribution of roots for fertilizer absorption [27]. Insufficient or excessive fertilization can affect both blueberry yield and quality [28]. Therefore, it is an urgent need to identify suitable fertilizer regimes for blueberry varieties in Guizhou. As of 2017, Guizhou’s blueberry cultivation area was 13,000 ha, and its yield reached 30,000 t, which ranked first in China. At present, most studies were focused on root growth and structure of blueberry [29,30]. However, no report has assessed the spatial distribution of the root system of blueberry. Therefore, the present study was performed to evaluate the effects of different fertilizers on the root distribution characteristics and yield of seven-year-old rabbiteye blueberry ‘Britewell’ plants in the field. Our findings shall provide insight into a suitable fertilization regime for cultivating a productive root–shoot system in blueberry.
The research site was located in Wuyang Maritt Blueberry Base, Xuanwei Town, Majiang County, Guizhou Province, China (26° 37′–26° 49′ N and 107° 66′–107° 74′ E). The site was located in a subtropical monsoon humid zone with a warm and humid climate throughout the year. The mean annual temperature, rainfall, sunshine hours, and frost-free period were 15°C, 1250 mm, 1200 h, and 310 days, respectively. The thickness of the soil layer exceeds 100 cm. The base soil was acid yellow soil, and the soil pH was 4.5–5.5, which is suitable for the cultivation of rabbiteye blueberry in Guizhou [31]. We were promised the right to use the plant collection as the cooperation base of the Forestry College of Guizhou University.
In this experiment, 7-year-old rabbiteye ‘Britewell’ well-grown blueberries were selected as the research objects. The plant row spacing, ground diameter, and tree height were about 1.5 m × 2 m, 18.4–44 mm, and 1.3–1.6 m, respectively. Another field management measures were daily blueberry management. Six types of fertilizers named as blueberry special fertilizer (SF), Haohuahong organic fertilizer (OR), fulvic acid chelated compound fertilizer (HF), soybean meal biological bacterial fertilizer (BF), potassium sulfate compound fertilizer (CF), and Runtian cattle microbial compound fertilizer (RT) were used. The various fertilizer components and related information are shown in Table 1; they were purchased from the market.
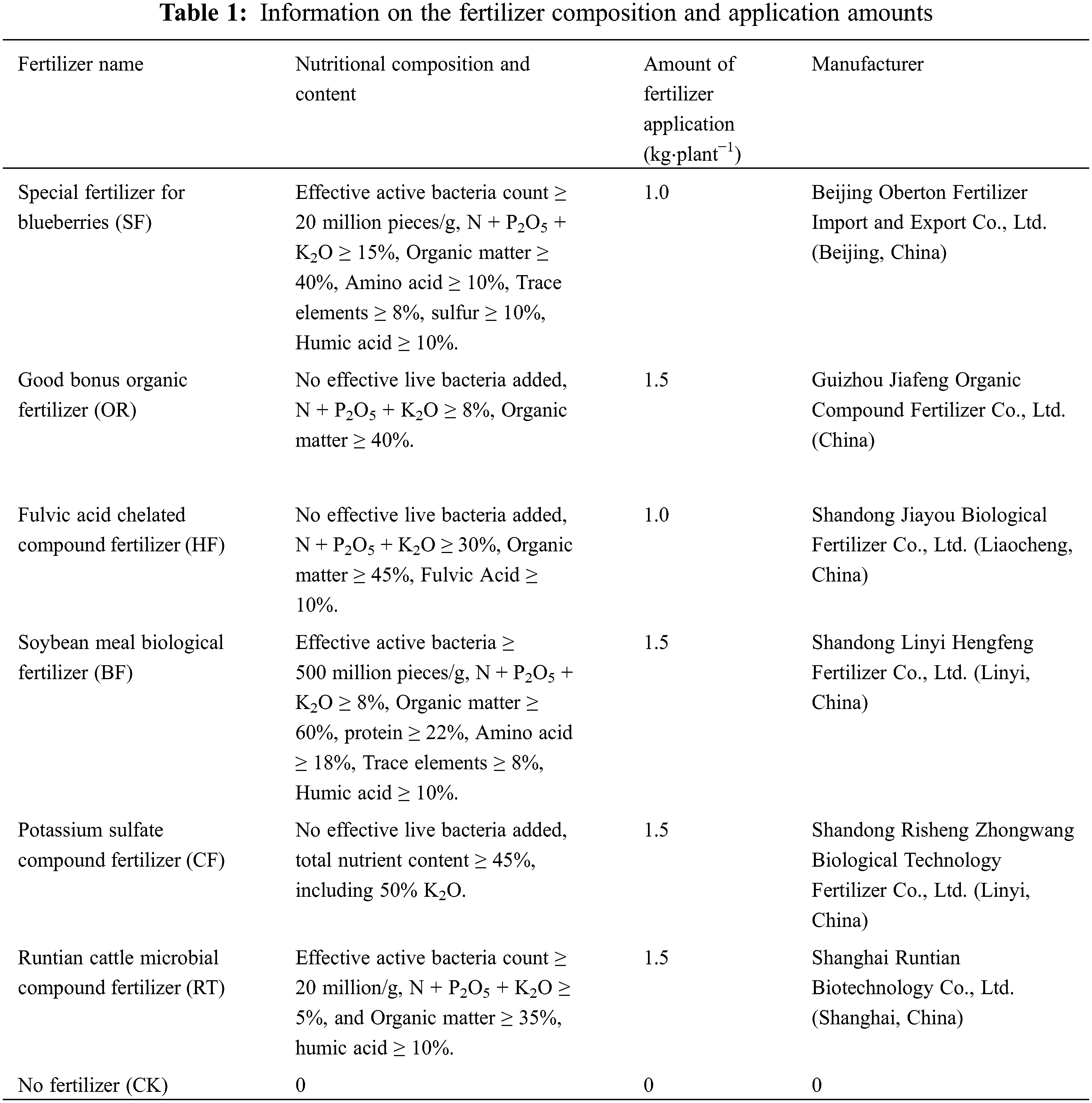
This experiment included six single-factor treatments, with no fertilizer as the control (CK). A random block design was used. There were 3 replicates for each fertilization treatment, 3 plants for each repetition, and a total of 63 plants, including 9 plants without fertilization (CK). The fertilizer application was consistent with the daily cultivation management practices of the region. The dry ditching fertilization method was used, whereby a ditch of 20 cm width and 20 cm depth was dug around the periphery of the canopy projection; it was covered with soil following fertilization. The fertilizer was applied once every December from 2015 to 2017, with the amount of fertilizer application following the optimal fertilizer quantity recommended by the fertilizer manufacturer. All samples were collected in 2018.
Three bushes demonstrating typical growth were selected from each treatment to sample the root distribution. Sampling was initiated vertically at four diagonal vertices at 20, 40, and 60 cm away from the main stem of the root in a horizontal direction (Fig. 1a). A soil auger of 7 cm core diameter was used. There was no interval between each soil layer. Each point was sampled in layers of 10 cm depth until a depth of 40 cm was reached. For each plant, 12 tubes were sampled from each layer, and 48 tubes were sampled in total from the four layers (Fig. 1b). The roots and soil were placed into plastic bags and transported back to the laboratory for refrigeration after labeling. After sifting most of the soil with a 10-mesh screen in the laboratory, the remaining soil adhering to the root system was rinsed off with running water. The blueberry roots were distinguished from other roots based on characteristics such as shape, color, and elasticity. With the aid of tweezers, the live roots were rinsed in a small plastic basin filled with distilled water and then air-dried. Please clarify how did you separate live from dead roots.
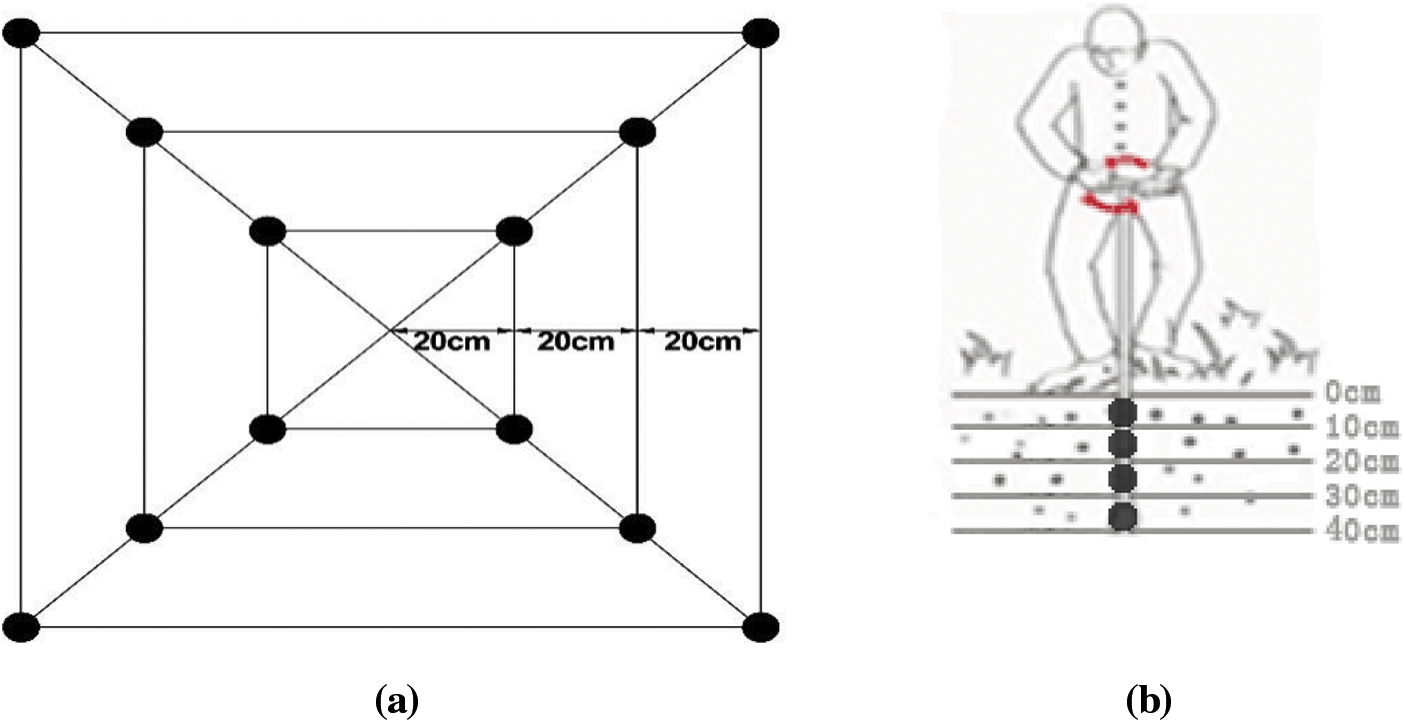
Figure 1: (a) Horizontal sampling legend. Note:  Represents the root sampling point. (b) Vertical sampling legend.
Represents the root sampling point. (b) Vertical sampling legend.  Represents the sampling point of the soil auger. Note: Each layer of soil was 10 cm in depth, with a total of 40 cm depth
Represents the sampling point of the soil auger. Note: Each layer of soil was 10 cm in depth, with a total of 40 cm depth
For the determination of the root morphology index, the washed blueberry roots were placed into a clean scan tray and spread out as much as possible. Roots were then scanned with a Microtek ScanMaker i800 Plus equipment (MICROTEK), using a ScanWizard EZ software. Thereafter, the Wanshen LA Root Analysis System was used to analyze root length, root surface area, root volume, average root diameter, and branch number. Root biomass (mg) was obtained using the drying method, whereby roots were dried at 65°C until constant weight (about 48 h). Afterwards, their dry weight was determined. The root parameter calculation formulae were as follows:
A one-way analysis of variance (ANOVA) was conducted using SPSS version 19.0 (IBM Corp., Armonk, USA). Post-hoc tests were conducted using Duncan’s test, and differences between treatments were considered significant at P < 0.05. Pearson’s correlation coefficients were calculated using the correlation procedure in SPSS.
The membership function evaluation method used here was based on the principle of fuzzy mathematics. The membership function is a fuzzy control system that converts clear quantities into fuzzy quantities, and performs fuzzy logic operations. Membership function analysis comprehensively evaluates plant characteristics based on multiple indexes. The membership function calculation formulae were as follows:
In the formulae, X is the measured value, Xmax is the maximum value, and Xmin is the minimum value. The membership function values are cumulative, and average values were obtained. Treatment efficacy increases with membership value [32].
3.1 Impact of Fertilizer on Root Length Density in the Different Soil Layers
The blueberry root system was distributed primarily in the 0–20 cm soil layer. The six fertilizer applications significantly promoted root length density at all soil depths (0–40 cm) compared with CK (Table 2). The application of RT fertilizer treatment resulted in the highest root length density in most soil layers (0–10, 10–20, and 30–40 cm). At soil depths of 0–10, 10–20, and 30–40 cm, the RT fertilizer treatment increased the root length density by 1843%, 1508%, and 848.00%, respectively, compared with controls. At a depth of 20–30 cm, the OR fertilizer treatment increased the root length density by 448.76% compared to CK.
3.2 Impact of Fertilizer on the Root Surface Area in the Different Soil Layers
The six fertilizer applications significantly promoted the root surface area at most soil depths (0–30 cm) compared to the controls (Table 2). The application of RT fertilizer treatment resulted in the greatest root surface area in the 0–10 cm soil layer. At a soil depth of 0–10 cm, the RT fertilizer treatment increased the root surface area by 774.83% compared with no fertilizer application. At depths of 10–20, 20–30, and 30–40 cm, the SF fertilizer treatment increased the root surface area by 531.36%, 1145.50%, and 120.44%, respectively, compared with CK.
3.3 Impact of Fertilizer on the Root Volume in the Different Soil Layers
The six fertilizer applications significantly decreased the root volume at 0–10 cm soil depth compared with the control (Table 2), with the greatest root volume detected under no fertilizer application. Conversely, at soil depths of 10–20, 20–30, and 30–40 cm, the SF fertilizer treatment increased the root volume by 131.40%, 722.78%, and 181.19%, respectively, compared with CK.
3.4 Impact of Different Fertilizers on the Root Biomass Density in the Different Soil Layers
At a depth of 0–10 cm, OR increased the root biomass density the most, being 10.31% higher than CK and 221.19% higher than BF; the latter obtained the lowest root biomass. At 10–20 cm depth, SF was the most important contributor to root biomass density, and CF showed the lowest biomass at this depth, with SF being 133.56% higher than CK and 308.37% higher than CF. At 20–30 cm depth, SF had the greatest contribution to root biomass density, while BF had the lowest contribution, with SF being 783.85% higher than CK and 890.75% higher than BF. At 30–40 cm depth, the most important contributor to root biomass density was SF, while OR had the lowest contribution. SF was 58.49% higher than CK and 2073.95% higher than OR (Table 2).
3.5 Impact of Fertilizer on the Average Root Diameter in Different Soil Layers
The six fertilizer applications significantly decreased the average diameter of the roots at 0–10 and 30–40 cm soil depths compared to CK (Table 2). At 10–20 and 20–30 cm soil depth, SF had the greatest effect on root diameter, being 47.01% and 180.99% higher than CK, respectively.
3.6 Impact of Fertilizer on Root Branching in the Different Soil Layers
At 0–10 cm depth, RT promoted root branching number the most, being 60.14% higher than in CK and 171.89% higher than in HF. At the depths of 10–20 and 30–40 cm, OR resulted in the greatest increase in root branching number. At 0–40 cm soil depth, HF had the least effect on the number of root branching.
3.7 Impact of Fertilizer on Average Values of Root Distribution Parameters
The total root length density and total root surface area were significantly increased under the six fertilization treatments (Fig. 2). The values from the RT fertilization treatment were the highest among the different treatments and increased the total root length density and total root surface area by 98.6% and 98.5%, respectively, compared with CK. The maximum total root volume and total root biomass values were achieved under SF, being 74.5% and 87.8% higher, respectively, than in CF, which achieved the lowest values. Root biomass was similar between BF and CF. The maximum total root bifurcation number was achieved under OR and BF, while HF showed the lowest. Total root bifurcation number was not significant difference in SF and CF.
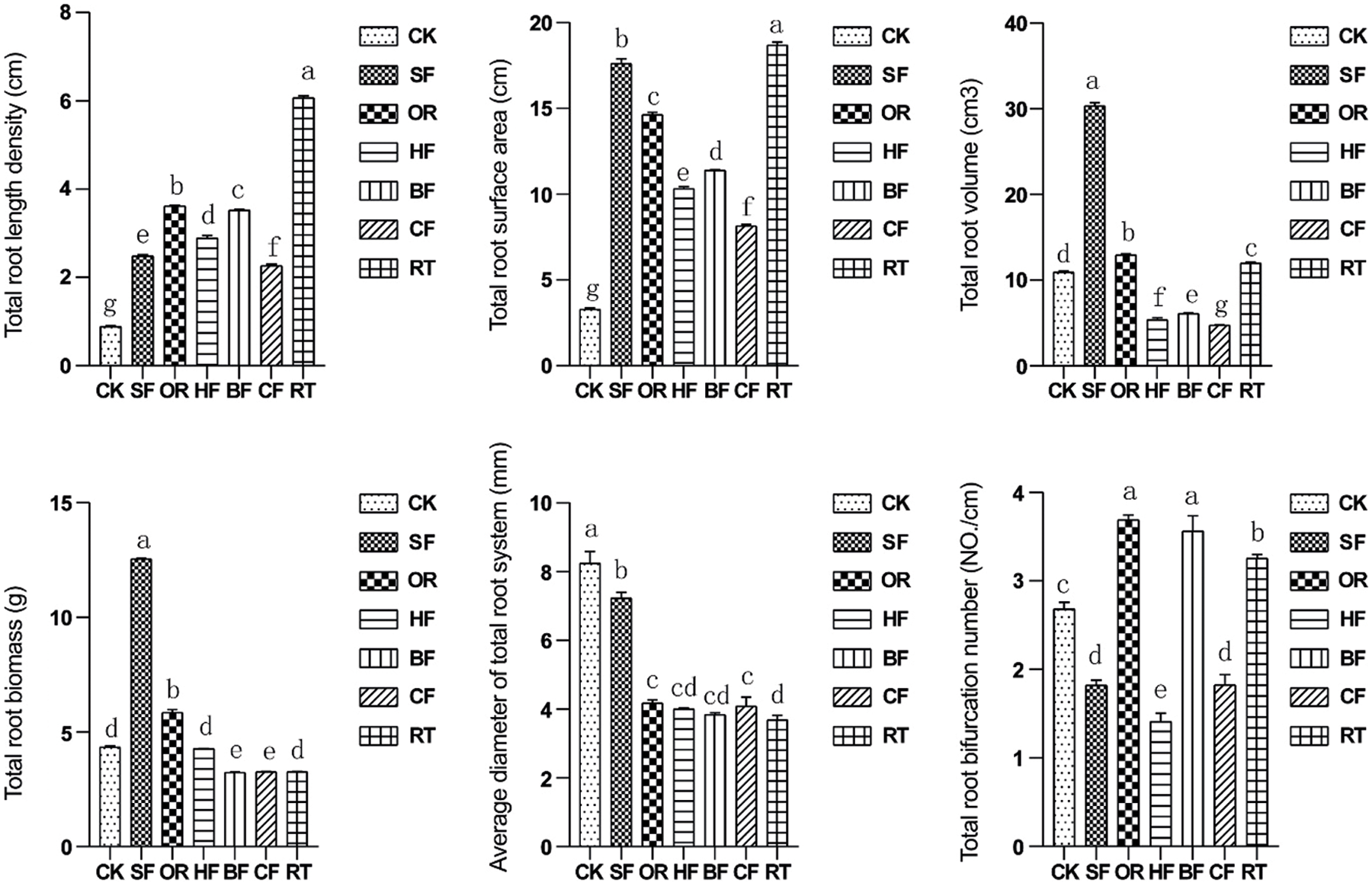
Figure 2: Bar graphs showing the impact of different fertilizers on total root length density, total root surface area, total root volume, total root biomass, average root diameter, and total number of root branches within the 0–40 cm soil depth. Different letters among treatments indicate significant differences at P < 0.05. Error bars represent the standard deviation. Total: It is the sum of individual values for each depth. Abbreviations for treatments are as described in the material and methods section
3.8 Impact of Fertilizer on Fruit Yield
Fertilization significantly increased fruit yield. The RT treatment had the highest fruit yield. It did not differ significantly from SF, HF, and BF, with an increase of 99.6% compared with CK (Fig. 3).
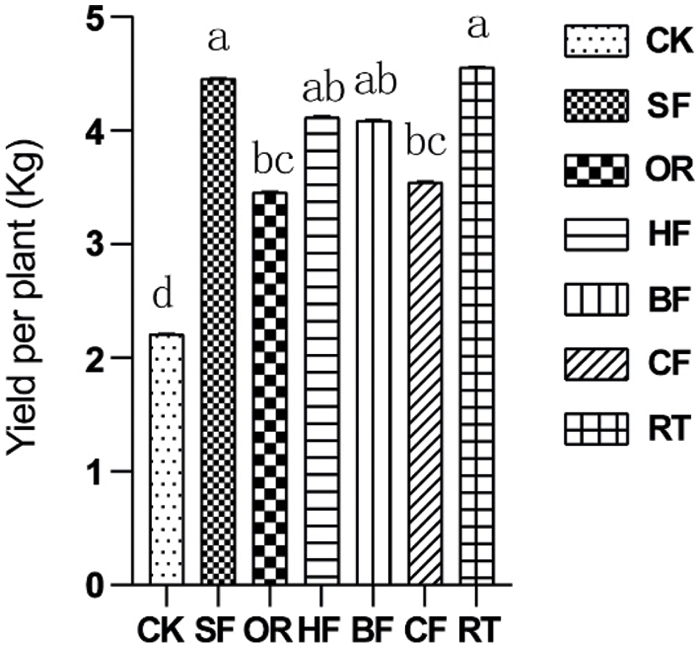
Figure 3: Bar chart showing the effect of different fertilizers on fruit yield. Different letters among treatments indicate significant differences at P < 0.05. Error bars represent the standard deviation. Abbreviations for treatments are as described in the material and methods section
3.9 Membership Function Value and Ranking
With the exception of CF and HF, the average values of all treatments were higher than in CK, which indicated that fertilization overall promoted root growth and fruit yield (Fig. 3). Among the treatments, SF had the best effect on promoting root growth and fruit yield, followed by RT. The root systems were found to be largest under the SF treatment (Table 3).
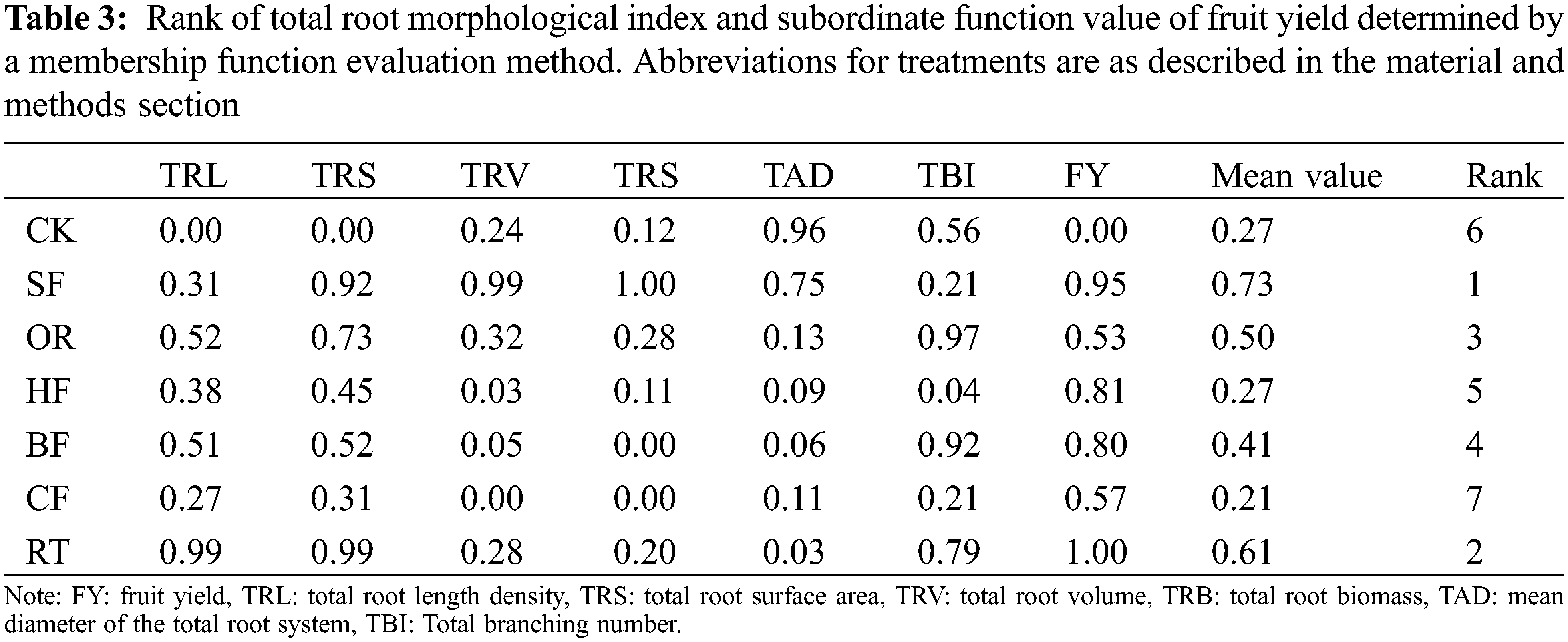
3.10 Correlation between Total Root Morphology Index and Fruit Yield
Fruit yield was positively correlated with total root length density and total root surface area, but negatively correlated with average root diameter. Total root length density was positively correlated with total root surface area and total root bifurcation number, and negatively correlated with the average root diameter. Total root surface area was significantly positively correlated with total root biomass density and total root volume. Total root volume was positively correlated with total root biomass density and average root diameter. And total root biomass was significantly positively correlated with mean diameter of the total root system. These results indicate that the root morphological index (total root length density, total root surface area and mean diameter of the total root system) influenced each other and significantly affected fruit yield (Table 4).
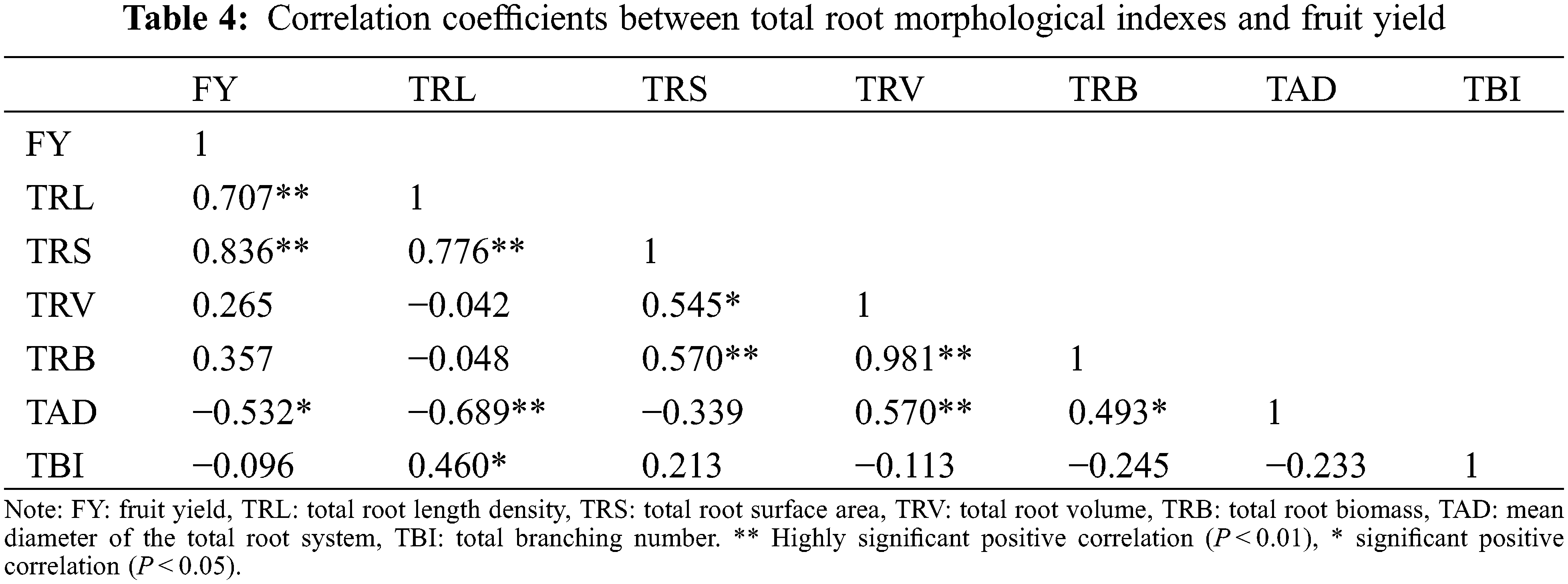
Some researchers believe that the content of fertilizers affects root morphology index. For example, Chen et al. found that the richness of the bacterial community was reduced under both low- and high-nitrogen fertilizers compared to the control treatment, and increased in high-N fertilizers plus P or K treatments [33]. Under laboratory conditions, both stimulating and reducing effects of P and K deficiencies on root traits have been reported [34–36]. Therefore, the components of compound fertilizers play a key role in plant growth. This study revealed how different fertilizers affected blueberry root morphology and distribution, as well as how the root system impacted blueberry fruit yield. The root morphology indexes (surface area, volume, biomass density), and average root diameter in most soil layers under the SF treatment were significantly higher than in the control treatment. The root length density in most soil layers was the highest under the RT treatment, and it was also significantly higher than in CK. Fruit yield was positively correlated with total root length density and total root surface area, but negatively correlated with average root diameter. This is consistent with the results of Wang et al. on the morphological development and yield of the peanut root system [37].
Root morphology is the foundation of nutrient and water uptake by plants [38,39]. Root length density and surface area are appropriate indexes for describing a root system [40]. In this study, we found that root length density and surface area generally increased with fertilization and were the highest in the 10–20 cm layer, which is consistent with the findings of Bryla et al. [41] in northern highbush blueberry. The application of fertilizers in this study significantly increased root elongation and resulted in the greatest root length density, surface area, and volume in most soil layers. This root length density finding corroborates the results of Liu et al. [42], who used the Minirhizotron technique to identify root morphological traits in rice under different fertilizer treatments throughout an entire growth period. Similarly, Zhang et al. [24] showed that fertilization increased the root length density of cotton under a drip irrigation system; we observed that the highest root length density, root surface, and fruit yield were obtained in the fertilization treatments. Fertilization deficiency has been shown to lead to low root activity and water consumption [24] as well as a reduction in the production of reactive oxygen species (ROS) in the roots [15], resulting in low root biomass accumulation.
We found that the root system was primarily distributed in the shallow soil layer, with the highest values for root length density, surface area, and biomass density observed in the 0–20 cm layer, accounting for approximately 50% of the corresponding totals. This is in agreement with the results of Min et al. [43]. In this study, relative to the control, the root length density in the OR treatment was lower in the surface and deeper studied soils, but significantly higher in the 10–30 cm soil layer. These results indicate that the fertilization treatment increased the root length distribution in the middle-deep soil layers, which can be expected to promote photosynthesis and water potential in the leaves [44]. In contrast, root biomass density was increased in the surface soil layers but decreased in the deeper ones.
Reports on these areas showed that the growth medium had an effect on plant height, crown diameter, and surface area and biomass densities [45,46]. Total surface area, volume, and biomass density were affected by fertilization, with the greatest values observed in the SF treatment. Blueberry fruit yield was strongly associated with total root length and surface area, illustrating that root morphology significantly affected blueberry fruit yield. Our research results are similar to previous ones. The root morphology indexes were overall highest in the SF and RT treatments, and lowest in the OR and CF treatments. As the biological fertilizer contains microbes, the HF, OR, and CF fertilizers, which are not bio-fertilizers, did not add effective live bacteria. Because of this, they might have had little effects on blueberry root morphology; this is consistent with Yuan et al.’s conclusion that bio-organic fertilizers can promote plant growth [47]. The analysis of the fertilizer components in Table 1 shows that the contents of N, P, K, and organic matter in RT were the lowest among all fertilizers, and the effect of organic fertilizers (RT, OR, BF, SF, HF) was better than that of the inorganic fertilizer (CF) (Table 2). This is not surprising given that blueberry is an oligotrophic plant. In comparison with other species, rabbiteye blueberry is particularly sensitive to chemical fertilizers [48]. The contents of N, P, calcium, manganese, and K in the leaves of Vaccinium uliginosum were previously found to be lower than those of other small berry tree species [49]. In summary, our research results are in line with previous thoughts that fertilizers increase plant growth, biomass, root morphology index and yield [50].
The different fertilizers studied significantly affected the root morphology and root distribution of blueberries in the field. The RT treatment improved root growth in each soil layer, and increased the total root length, surface area, volume, fruid yield and biomass. Root morphology and biomass were the most strongly affected by the application of SF and RT in the upper soil layers. These findings indicate that SF and RT can promote root growth, especially for shallower roots (0–20 cm), thereby increasing root biomass and promoting fruit yield.
Conceptualization: Xiaolan Guo: Writing-original draft preparation and Methodology, Chenyan Liu: Formal analysis and investigation, Muhammad Shakeel, Writing-review and editing. Delu Wang: Supervision.
Permission for Plant Material Collection: We have obtained permission from Wuyang Maritt Blueberry Base institute for samples collection. The study was carried out with relevant institutional, national, and international guidelines and legislation.
Funding Statement: This research was funded by the National Natural Science Foundation of China, Grant No. “31760205”.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Mohd-Radzman, N. A., Djordjevic, M. A., Imin, N. (2013). Nitrogen modulation of legume root architecture signaling pathways involves phytohormones and small regulatory molecules. Frontiers in Plant Science, 4, 385. DOI 10.3389/fpls.2013.00385. [Google Scholar] [CrossRef]
2. King, J., Gay, A., Sylvester-Bradley, R. O. G. E. R., Bingham, I. A. N., Foulkes, J. et al. (2003). Modelling cereal root systems for water and nitrogen capture: Towards an economic optimum. Annals of Botany, 91(3), 383–390. DOI 10.1093/aob/mcg033. [Google Scholar] [CrossRef]
3. Wang, F. H., Wang, X. Q., Ken, S. (2004). Comparison of conventional, flood irrigated, flat planting with furrow irrigated, raised bed planting for winter wheat in China. Field Crops Research, 87(1), 35–42. DOI 10.1016/j.fcr.2003.09.003. [Google Scholar] [CrossRef]
4. Wang, C., Liu, W., Li, Q., Ma, D., Lu, H. et al. (2014). Effects of different irrigation and nitrogen regimes on root growth and its correlation with above-ground plant parts in high-yielding wheat under field conditions. Field Crops Research, 165, 138–149. DOI 10.1016/j.fcr.2014.04.011. [Google Scholar] [CrossRef]
5. Ota, R., Ohkubo, Y., Yamashita, Y., Ogawa-Ohnishi, M., Matsubayashi, Y. (2020). Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nature Communications, 11(1), 1–9. DOI 10.1038/s41467-020-14440-8. [Google Scholar] [CrossRef]
6. Chen, H., Jia, Y., Xu, H., Wang, Y., Zhou, Y. et al. (2020). Ammonium nutrition inhibits plant growth and nitrogen uptake in citrus seedlings. Scientia Horticulturae, 272, 109526. DOI 10.1016/j.scienta.2020.109526. [Google Scholar] [CrossRef]
7. Kwack, Y. B., Kim, H. L., Choi, Y. H., Lee, J. H., Kim, J. G. et al. (2012). Fruit quality and fruit locule air hole of kiwifruit (Actinidia deliciosa cv. Hayward) affected by early defoliation. Korean Journal of Environmental Agriculture, 31(3), 229–234. DOI 10.5338/KJEA.2012.31.3.229. [Google Scholar] [CrossRef]
8. Zhu, Q., Ozores-Hampton, M., Li, Y., Morgan, K., Liu, G. et al. (2017). Effect of phosphorus rates on growth, yield, and postharvest quality of tomato in a calcareous soil. HortScience, 52(10), 1406–1412. DOI 10.21273/HORTSCI12192-17. [Google Scholar] [CrossRef]
9. Higo, M., Azuma, M., Kamiyoshihara, Y., Kanda, A., Tatewaki, Y. et al. (2020). Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms, 8(2), 178. DOI 10.3390/microorganisms8020178. [Google Scholar] [CrossRef]
10. Niu, X. L., Zhou, H. M., Wang, X. K., Hu, T. T., Feng, P. Y. et al. (2020). Changes in root hydraulic conductance in relation to the overall growth response of maize seedlings to partial root-zone nitrogen application. Agricultural Water Management, 229, 105839. DOI 10.1016/j.agwat.2019.105839. [Google Scholar] [CrossRef]
11. Ren, B., Li, X., Dong, S., Liu, P., Zhao, B. et al. (2018). Soil physical properties and maize root growth under different tillage systems in the North China Plain. The Crop Journal, 6(6), 669–676. DOI 10.1016/j.cj.2018.05.009. [Google Scholar] [CrossRef]
12. Nacry, P., Bouguyon, E., Gojon, A. (2013). Nitrogen acquisition by roots: Physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant and Soil, 370(1), 1–29. DOI 10.1007/s11104-013-1645-9. [Google Scholar] [CrossRef]
13. Luo, H., Zhang, H., Han, H., Hu, Y., Zhang, Y. et al. (2014). Effects of water storage in deeper soil layers on growth, yield, and water productivity of cotton (Gossypium hirsutum L.) in arid areas of Northwestern China. Irrigation and Drainage, 63(1), 59–70. DOI 10.1002/ird.1793. [Google Scholar] [CrossRef]
14. Pu, X. Z., Zhang, G. J., Zhang, P. P., Liu, Y. J., Zhang, W. F. (2016). Effects of straw management, inorganic fertiliser, and manure amendment on soil microbial properties, nutrient availability, and root growth in a drip-irrigated cotton field. Crop and Pasture Science, 67(12), 1297–1308. DOI 10.1071/CP16230. [Google Scholar] [CrossRef]
15. Chen, Z., Tao, X., Khan, A., Tan, D. K., Luo, H. (2018). Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Frontiers in Plant Science, 9, 173. DOI 10.3389/fpls.2018.00173. [Google Scholar] [CrossRef]
16. Poonnachit, U., Darnell, R. (2004). Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Annals of Botany, 93(4), 399–405. DOI 10.1093/aob/mch053. [Google Scholar] [CrossRef]
17. Bryla, D. R., Strik, B. C. (2015). Nutrient requirements, leaf tissue standards, and new options for fertigation of northern highbush blueberry. HortTechnology, 25(4), 464–470. DOI 10.21273/HORTTECH.25.4.464. [Google Scholar] [CrossRef]
18. Chen, J., Liu, L., Wang, Z., Zhang, Y., Sun, H. et al. (2020). Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Frontiers in Plant Science, 11, 880. DOI 10.3389/fpls.2020.00880. [Google Scholar] [CrossRef]
19. Williamson, J. G., Miller, E. P. (2009). Effects of fertilizer rate and form on vegetative growth and yield of southern highbush blueberry in pine bark culture. HortTechnology, 19(1), 152–157. DOI 10.21273/HORTSCI.19.1.152. [Google Scholar] [CrossRef]
20. Halligan, A. C., Becotte, A. M., Crane, A. M., Dougherty, E. T. (2018). A mathematical model for the effects of fertilization on nitrogen concentrations in unsaturated soil on blueberry farms in Southern New Jersey. Environmental Modeling & Assessment, 23(5), 583–595. DOI 10.1007/s10666-018-9589-3. [Google Scholar] [CrossRef]
21. Clark, M. J., Zheng, Y. (2020). Fertilization methods for organic and conventional potted blueberry plants. HortScience, 55(3), 304–309. DOI 10.21273/HORTSCI14416-19. [Google Scholar] [CrossRef]
22. Marty, C., Lévesque, J. A., Bradley, R. L., Lafond, J., Paré, M. C. (2019). Lowbush blueberry fruit yield and growth response to inorganic and organic N-fertilization when competing with two common weed species. PLoS One, 14(12), e0226619. DOI 10.1371/journal.pone.0226619. [Google Scholar] [CrossRef]
23. Osorio, R., Cáceres, C., Covarrubias, J. I. (2020). Vegetative and physiological responses of “Emerald” blueberry to Ammoniacal sources with a nitrification inhibitor. Journal of Soil Science and Plant Nutrition, 20(2), 507–515. DOI 10.1007/s42729-019-00135-7. [Google Scholar] [CrossRef]
24. Zhang, H., Khan, A., Tan, D. K., Luo, H. (2017). Rational water and nitrogen management improves root growth, increases yield and maintains water use efficiency of cotton under mulch drip irrigation. Frontiers in Plant Science, 8, 912. DOI 10.3389/fpls.2017.00912. [Google Scholar] [CrossRef]
25. Zhang, J., Jiao, X., Du, Q., Song, X., Ding, J. et al. (2021). Effects of vapor pressure deficit and potassium supply on root morphology, potassium uptake, and biomass allocation of tomato seedlings. Journal of Plant Growth Regulation, 40(2), 509–518. DOI 10.1007/s00344-020-10115-2. [Google Scholar] [CrossRef]
26. Perez, A., Ferreira, G., Minor, T. (2017). Fruit and tree nuts outlook (No. 1494-2017-5276). [Google Scholar]
27. Shen, Y., Li, S., Shao, M. (2013). Effects of spatial coupling of water and fertilizer applications on root growth characteristics and water use of winter wheat. Journal of Plant Nutrition, 36(4), 515–528. DOI 10.1080/01904167.2012.717160. [Google Scholar] [CrossRef]
28. Michel, L., Peña, Á., Pastenes, C., Berríos, P., Rombolà, A. D. et al. (2019). Sustainable strategies to prevent iron deficiency, improve yield and berry composition in blueberry (Vaccinium spp.). Frontiers in Plant Science, 10, 255. DOI 10.3389/fpls.2019.00255. [Google Scholar] [CrossRef]
29. Bryla, D. R., Valenzuela-Estrada, L. R., Vargas, O. L. (2016). Root production, distribution, and turnover in conventional and organic northern highbush blueberry systems. Acta Horticulturae, 1180, 169–176. DOI 10.17660/ActaHortic.2017.1180.23. [Google Scholar] [CrossRef]
30. Nunez, G. H., Rodríguez-Armenta, H. P., Darnell, R. L., Olmstead, J. W. (2016). Toward marker-assisted breeding for root architecture traits in southern highbush blueberry. Journal of the American Society for Horticultural Science, 141(5), 414–424. DOI 10.21273/JASHS03798-16. [Google Scholar] [CrossRef]
31. Milivojević, J., Radivojević, D., Maksimović, J. D., Urošević, S., Koron, D. et al. (2019). Field performance of ‘Bluecrop’ highbush blueberry in a soilless growing system by using different fertilizers. Acta Horticulturae, 26(1265), 187–193. DOI 10.17660/ActaHortic.2019.1265.26. [Google Scholar] [CrossRef]
32. Roy, R., Mostofa, M. G., Wang, J., Fornara, D., Sarker, T. et al. (2021). Revegetation intervention of drought-prone coal-mined spoils using Caragana korshinskii under variable water and nitrogen-phosphorus resources. Agricultural Water Management, 246, 106712. DOI 10.1016/j.agwat.2020.106712. [Google Scholar] [CrossRef]
33. Chen, Y. L., Liu, J. T., Liu, S. T. (2018). Effect of long-term mineral fertilizer application on soil enzyme activities and bacterial community composition. Plant Soil Environ, 64, 571–577. DOI 10.17221/PSE. [Google Scholar] [CrossRef]
34. McLachlan, J. W., Haling, R. E., Flavel, R. J., Guppy, C. N., Simpson, R. J. et al. (2020). Root proliferation in response to P stress and space: Implications for the study of root acclimation to low P supply and P acquisition efficiency. Plant Soil, 452, 1–16. DOI 10.1007/s11104-020-04535-y. [Google Scholar] [CrossRef]
35. Nguyen, V. L., Stangoulis, J. (2019). Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiologiae Plantarum, 41, 109. DOI 10.1007/s11738-019-2891-0. [Google Scholar] [CrossRef]
36. Roshani, G., Narayanasamy, G. (2010). Effects of potassium on temporal growth of root and shoot of wheat and its uptake in different soils. International Journal of Plant Production, 4, 25–32. DOI 10.1016/j.indcrop.2009.09.012. [Google Scholar] [CrossRef]
37. Wang, J. G., Zhang, Y., Li, L., Liu, D. W., Yi, J. (2017). Effects of different calcium fertilizer gradients and film mulching on root morphological development and yield of peanuts in Low calcium Red soil. Chinese Journal of Oil Crop Sciences, 39(6), 820–826. DOI 10.7505/j.issn.1007-9084.2017.06.013. [Google Scholar] [CrossRef]
38. Cheng, L., Chen, W., Adams, T. S., Wei, X., Li, L. et al. (2016). Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology, 97(10), 2815–2823. DOI 10.1002/ecy.1514. [Google Scholar] [CrossRef]
39. Chen, W., Koide, R. T., Eissenstat, D. M. (2018). Root morphology and mycorrhizal type strongly influence root production in nutrient hot spots of mixed forests. Journal of Ecology, 106(1), 148–156. DOI 10.1111/1365-2745.12800. [Google Scholar] [CrossRef]
40. Amato, M., Ritchie, J. T. (2002). Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure. Crop Science, 42, 773–780. DOI 10.2135/cropsci2002.7730. [Google Scholar] [CrossRef]
41. Bryla, D. R., Strik, B. C. (2015). Nutrient requirements, leaf tissue standards, and new options for fertigation of northern highbush blueberry. HortTechnology, 25(4), 464–470. DOI 10.21273/HORTTECH.25.4.464. [Google Scholar] [CrossRef]
42. Liu, K., He, A., Ye, C., Liu, S., Lu, J. et al. (2018). Root morphological traits and spatial distribution under different nitrogen treatments and their relationship with grain yield in super hybrid rice. Scientific Reports, 8(1), 1–9. DOI 10.1038/s41598-017-18576-4. [Google Scholar] [CrossRef]
43. Min, W., Guo, H., Zhou, G., Zhang, W., Ma, L. et al. (2014). Root distribution and growth of cotton as affected by drip irrigation with saline water. Field Crops Research, 169, 1–10. DOI 10.1016/j.fcr.2014.09.002. [Google Scholar] [CrossRef]
44. Qi, D. L., Wu, X., Hu, T. T. (2014). Effects of nitrogen supply methods on root growth, yield and nitrogen use of maize. Scientia Agricultura Sinica, 47(14), 2804–2813. [Google Scholar]
45. Tariq, R., Qureshi, K. M., Hassan, I., Rasheed, M., Qureshi, U. S. (2013). Effect of planting density and growing media on growth and yield of strawberry. Pakistan Journal of Agriculture Research, 26, 113–123. [Google Scholar]
46. Adak, N., Tozlu, I., Gubbuk, H. (2018). Influence of different soilless substrates to morpho-physiological characteristics and yield relations in strawberries. Erwerbs-Obstbau, 60, 341–348. DOI 10.1007/s10341-018-0382-x. [Google Scholar] [CrossRef]
47. Yuan, J., Ruan, Y., Wang, B. B., Shen, Q. (2013). Plant growth-promoting rhizobacteria strain bacillus amyloliquefaciens NJN-6-Enriched Bio-organic fertilizer suppressed fusarium wilt and promoted the growth of banana plants. Journal of Agricultural and Food Chemistry, 61, 3774–3780. DOI 10.1021/jf400038z. [Google Scholar] [CrossRef]
48. Medvecký, M., Daniel, J., Vollmannová, A., Zupka, S., Kopernická, M. (2021). The content of polyphenols in fruit of highbush blueberry (Vaccinium corymbosum L.) relating to different fertilizer application. Journal of Microbiology, Biotechnology and Food Sciences, 2021, 109–113. DOI 10.15414/jmbfs.2015.4.special3.109-113. [Google Scholar] [CrossRef]
49. Chen, L., Bai, Y., Hou, Z., Li, B., Gong, Z. et al. (2017). Characteristics of mineral nutrition in basal branches of vaccinium uliginosum in Daxing’an Mountains. Acta Botanica Boreali-Occidentalia Sinica, 37(10), 2042–2051. DOI 10.7606/j.issn.1000-4025.2017.10.2042. [Google Scholar] [CrossRef]
50. Chan, K. Y., van Zwieten, L., Meszaros, I., Downie, A., Joseph, S. (2008). Agronomic values of green waste biochar as a soil amendment. Soil Research, 45, 629–634. DOI 10.1071/SR07109. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |