

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020642
REVIEW
Sorghum: Nutritional Factors, Bioactive Compounds, Pharmaceutical and Application in Food Systems: A Review
1Biological and Geological Sciences Department, Faculty of Education, Ain Shams University, Cairo, 11575, Egypt
2Department of Horticulture, Faculty of Crop Production Sciences, University of Agriculture, Peshawar, 25120, Pakistan
3Department of Agricultural Mechanization and Renewable Energy Technologies, Faculty of Crop Production Sciences, University of Agriculture, Peshawar, 25120, Pakistan
4Department of Botany, Central University of Kashmir, Ganderbal, 474011, India
5Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, 11884, Egypt
*Corresponding Author: Heba I. Mohamed. Email: hebaibrahim79@gmail.com; hebaebrahem@edu.asu.edu.eg
Received: 04 December 2021; Accepted: 18 January 2022
Abstract: After wheat, rice, maize, and barley, sorghum is the fifth most widely grown cereal on the planet. Due to its high production, drought resistance, and heat tolerance, this crop is replacing maize in some areas. Sorghum is available in a variety of colors, including cream, lemon-yellow, red, and even black. The principal grain anatomical components are pericarp, germ or embryo and endosperm. This review provides an overview of key sorghum grain components, including starches, fiber, proteins, lipids, and vitamins. Also, we summarized phenolic compounds, flavonoids, tannins, carotenoids, vitamin E, amines, Policosanols and Phytosterols in sorghum grains. Sorghum is used to manufacture bread and porridge, and it provides a significant source of energy and nutrition for humans; sorghum is extensively farmed for animal feed. However, because the natural components in sorghum are useful in the development of healthy and functional foods, sorghum farming for both biofuel production and human consumption is gaining popularity. Pigmented sorghum grain is high in antioxidants such as polyphenols, primarily tannins, which have a variety of health benefits, including antiproliferative properties linked to the prevention of certain cancers, antioxidant activities linked to the prevention of diseases linked to oxidative stress, and anti-inflammatory effects, as well as improving glucose metabolism. Because these chemicals cannot be assimilated, their application in the food business has been limited, as sorghum is regarded as a low-nutritional grain due to the presence of anti-nutritional components such as strong tannins, which form complexes with proteins and iron, limiting their digestibility. This review aims to show the utilization of sorghum as a source of bioactive chemicals and the value they bestow on human health due to the general biological potential it possesses.
Keywords: Antioxidant; anticancer; food; anti-inflammatory; phenolic compounds; sorghum
Cereal whole grains are crucial for human health because they provide considerable amounts of energy, minerals, and dietary fibre [1,2]. Numerous prospective studies show that eating whole grains regularly reduces the risk of heart disease and diabetes by 20%–30% [3], improves blood glucose regulation [4], improves weight management over time [5], and lowers the risk of some forms of cancer [5]. Multiple national dietary guidelines reflect a critical assessment of the body of data, advising people to eat more “grain items, particularly whole grain cereals”, and to consume less processed grains. Bioactive substances in cereals have been shown to have a variety of biological activities in various studies, including antioxidant, anti-inflammatory, and antibacterial characteristics, all of which aid in the prevention of human diseases [6]. One of the tropical species in the Poaceae family and one of Africa, Asia and Latin America’s leading species [7] is Sorghum [Sorghum bicolor (L.)]. Over 35% of sorghum is cultivated directly for consumption by people. The rest is mainly used for animal feed, and alcohol and industrial products [8]. The current annual worldwide production is increasing approximately by 60 million tons, due to improved species and conditions of breeding. Sorghum breeders produce several enhanced sorghum varieties each year adapted to semi-arid and tropical environments. Selection of species from this great biodiversity that fulfil specific local food and industrial requirements is important for food safety. Demand for sorghum is growing in developing countries generally, and especially in West Africa. This is not only because the population is increasing, but also because of the countries’ policies to improve their processing and industrial use [9]. There have been over 7,000 types of sorghum [10] identified and, consequently, a further molecular qualification with regard to food quality is needed. In order to produce acceptable sorghum food products, the procurement of good quality grain is essential. Sorghum is a major source of income for households while playing an important role in food security in Africa [11].
Sorghum is well-known for its high agronomic productivity, or ability to grow in a variety of environments. It has the ability to grow at high elevations from the soil surface, salinity and barren soils, as well as in environments that contain rare water and high temperatures. This is owed to the sorghum’s well-developed root system, which has a high root to leaf ratio, and its wax-coated leaves, which can roll in response to external threat/stimulus [12,13]. Sorghum grain is a good choice since it is gluten-free, high in resistant starch, and a good source of minerals, as well as a variety of bioactive phenolic compounds [12]. Sorghum contains more abundant and diverse phenolic compounds than other major cereal crops; it comprises a lot of types of phenolic compounds, with simple phenolic acids, flavonoids, and tannins being the most prominent [14]. Bioactive chemicals in sorghum grains enhance the gut microbiota and exhibit a wide range of biological activities, including anti-inflammatory, antioxidant, antithrombotic, and antidiabetic qualities [15–17]. This review provides a summary of the bioactive chemicals found in sorghums, as well as the numerous health benefits they provide due to their biological potential and food applications.
The principal anatomical grain components are pericarp, germ or embryo and endosperm (Fig. 1).
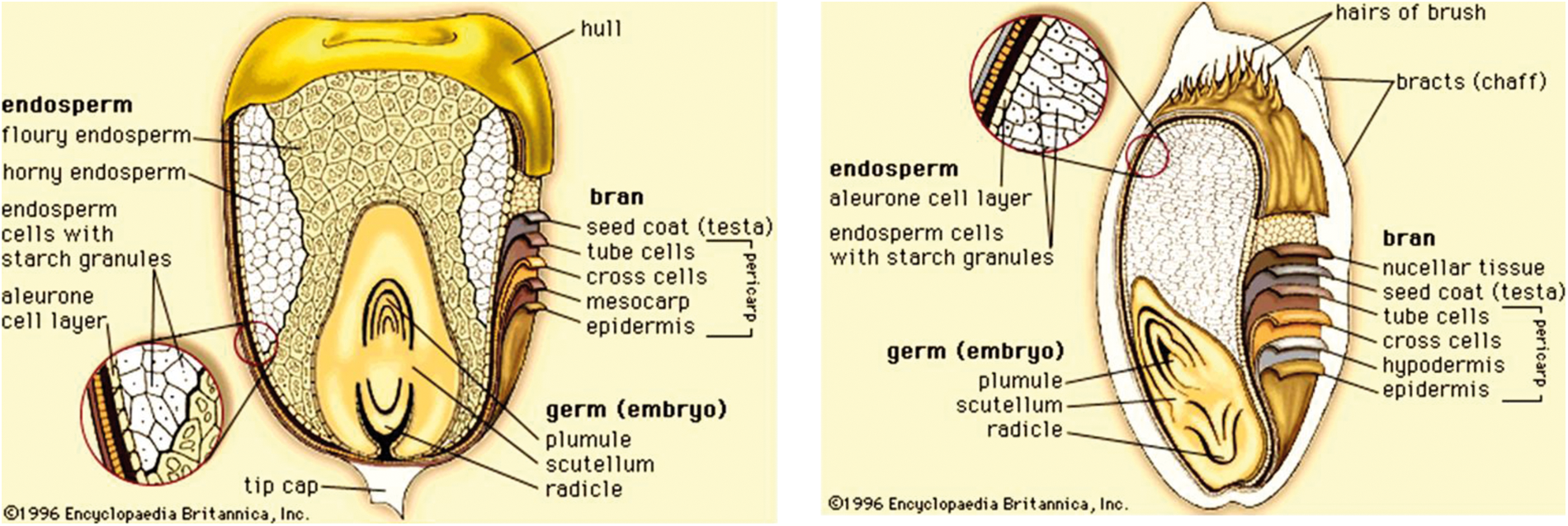
Figure 1: Structure of a sorghum grain [18]
The pericarp is the most external caryopsis structural component, consisting of three layers: epicarp, mesocarp and endocarp. The epidermis and hypodermis make up the epicarp. The epidermis in the sorghum caryopsis is made up of dense, elongated, rectangular cells that have an outer surface coating of cutin. The epidermis usually contains a pigment. Various pericarp pigments are reflective of the certain phytochemical levels which may include anthocyanins, carotenoids, and condensed tannins. The hypodermis consists of relatively smaller cells than the epidermis and has a thickness of one to three cells. The mesocarp is the thickest layer in the pericarp of sorghum, but its thickness varies significantly among genotypes. The endocarp, the innermost sub-layer of the pericarp, comprises cross-cells and a tube layer that transports moisture into the kernel. The cross- and tube-cell layers are broken during the dry milling of sorghum [18].
The test layer or seed coat is just below the endocarp. The test is intensely pigmented in some genotypes of sorghum. A genetics character is the presence of the pigment and the color. The test layer is not consistent in thickness. It is thick close to the kernel crown area and thin close to the part of the embryo. There is a partial Testa in some genotypes while this part does not appear or is lacking in other genotypes [18].
The endosperm, a main storage tissue, is the greatest grain cereal component. It has an aleurone layer and a peripheral corneous and floury zone. The aleurone cells contain several hydrolyzing enzymes and they are rich in minerals, vitamins of the B complex, and oil [18]. The peripheral endosperm is characterized by long rectangular cells, which are densely embedded and contain starch granules and protein bodies in the protein material. There is therefore no easy access to starch in these cells for digestion of enzymes without also reducing their associated protein [19]. The matrix protein is generally alkaline-soluble, and the protein bodies are prolamins that are alcohol-soluble and make up the biggest proportion of the total protein in the kernel. In sorghum, the number of protein bodies reduces as the content of starch boosts from the peripheral zone to the central core of the flour endosperm.
The two main parts of the germ are the embryonic axis and the scutellum. The scutellum is a lipid, protein, enzyme, and mineral storage tissue. The fatty acids rich in polyunsaturated oil are similar to those in the maize oil [20].
3 Nutritional Value of Sorghum Grain
Starch, like in other cereals, is the primary storage form of carbohydrates in sorghum, with a starch concentration of 69.5% on average. Arabinoxylans (pentosans) in grains have been shown to alter the water balance and structural features of dough, as well as starch retrogradation [21–23]. They are polysaccharides that have a xylan backbone and arabinose residues branching off of them. The carbohydrate composition and structural characteristics of arabinoxylans from sorghum with good roti-making quality have been studied [24]. When compared to wheat flour, sorghum has similar levels of starch but much less activity of –amylase (40%–50%) and amylolytic (10%) enzymes. Due to significant quantities of resistant and gradually biodegradable starch, as well as significant associations among starch granules, endosperm proteins, and condensed tannins, sorghum has the least potential starch digestibility among the cereal crops [25,26].
Sorghum has a high fiber content. It is mostly made up of insoluble (75%–90%) and soluble fibers (10%–25%), which are present on cell walls in the pericarp and endosperm and have an amount of about 6–15 g per 100 g of grain [27]. Non-starch sorghum carbohydrates consist mainly of β-glucan and arabinoxylans. Arabinoxylans are mainly glucuronoarabinoxylans and contain important sorghum phenolic acids, and bound p-coumaric and ferulic acids [28].
Prolamin (such as kafirins) and non-prolamin proteins (such as globulins, glutelins, and albumins) are two types of sorghum proteins. Kafirin accounts for 70% of the sorghum grain protein storage, and 30% of it are albumins, glutelins, and globulins [29]. There are four types of molecular-weight kafirins, (i.e., α-, β-, γ -, and δ-kafirin) which are hydrophobic proteins, they are stored in the endosperm in strongly wrapped protein bodies [29]. Sorghum grains are rich in glutamic acid, proline and leucine but they may be deficient in lysine, similar to other cereal grains; however, sorghum breeding or food fortification could help to improve this problem [30]. Sorghum kafirins have high polymetry levels and comprehensive disulfide bridges which makes them resistant to enzymatic digestion by the digestive tract, and also hamper protein digestion because of their strong interaction with tannins and starch.
The lipids consist mainly of unsaturated fatty acids in sorghum grains, the most readily available being polyunsaturated fatty acids. Oleic, linoleic, palmitic, linolenic, and stearic acids are the principal fatty acids in sorghum; the lipid profile of maize is similar but is more unsaturated [22].
Sorghum, like other cereals, is a good source of B-complex vitamins (including thiamin, riboflavin, vitamin B6, biotin, and niacin), which are lost through grain refining methods like decortication. Sorghum has a mineral content that is similar to millet, greater than maize but less than wheat, and contains primarily K and S. Sorghum-based diets are high in Fe and Zn, while phytoconstituents like phytates can reduce bioavailability, a problem that is not specific to sorghum but is widespread to all grains and plant foods [22,27].
4 Bioactive Compounds of Sorghum Grains
Except for white sorghums, most sorghum varieties contain high levels of phytochemicals, especially phenolic compounds, which have a high antioxidant capacity and are related to health benefits [31]. In fact, according to reports, the bran of some sorghum grain varieties has the potent antioxidant activity of all cereal crop fractions [14]. The distinctive phenolic composition has high medicinal effects, like oxidative managing stress and antitumor [32]. Fig. 2 illustrates the structure and profile of the principal sorghum phenolic compounds.
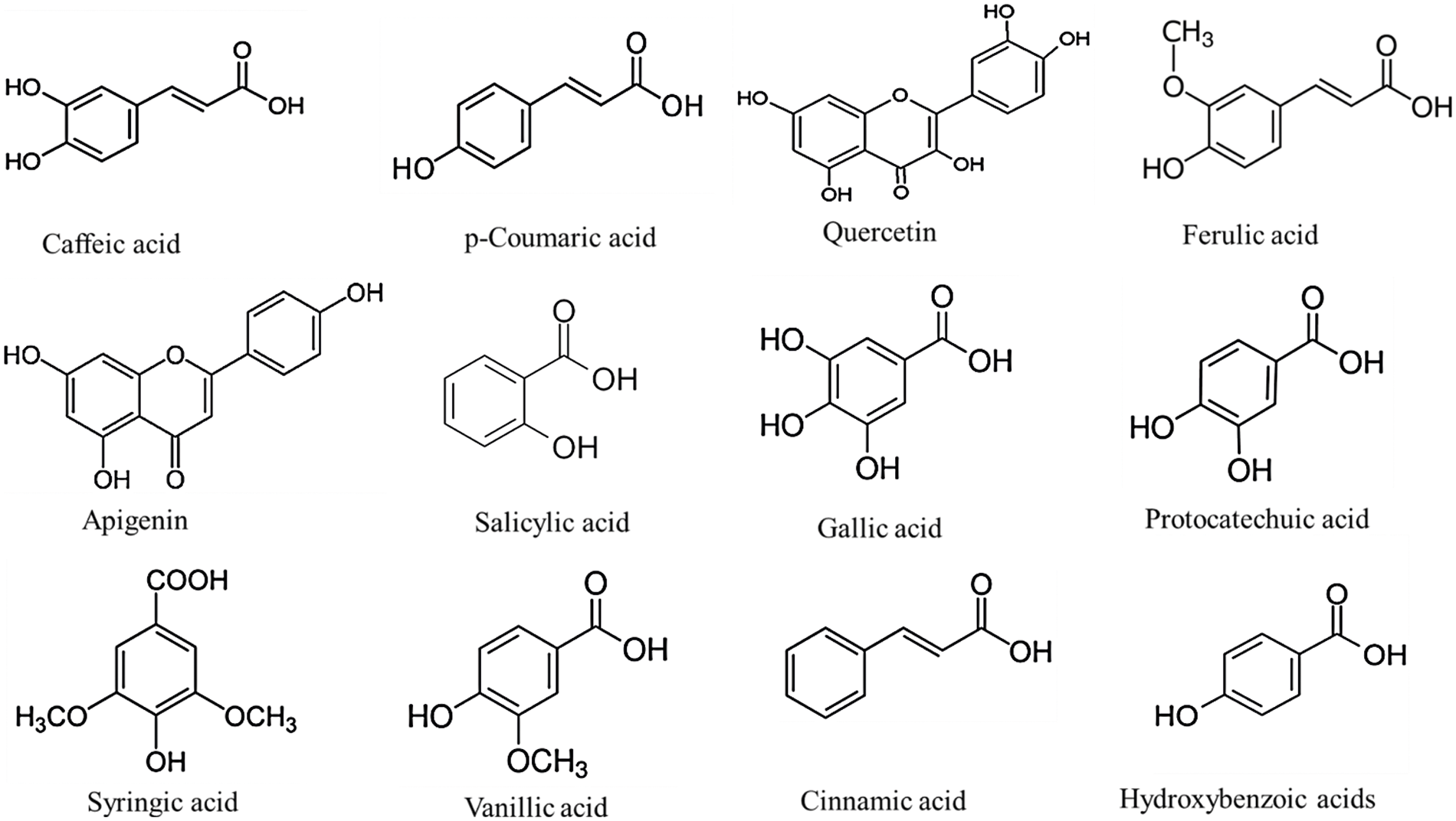
Figure 2: Structure of major sorghum phenolic compounds
Sorghum grain has the simplest, but abundant phenolic compounds at a total concentration of 445 to 2,850 μg g−1 [33]. Phenolic acids may be subdivided into 2 categories, depending on their structure: benzoic and cinnamic acid. Gallic, vanillic, protocatechuic, cinnamic, p-coumaric, p-hydroxybenzoic, syringic, ferulic, caffeic, and sinapic acids constitute the principal phenolic acids in the sorghum grain [34,35]. The phenolic acids occur in both free and bound forms and are present in the endosperm, pericarp and testa. Freely extractable phenolic acids, organically solvent-extractable, are not bound to the cell wall and can be found mainly in pericarp and testa. They are frequently combined with monomeric carbohydrates and glycerol and are present freely as esters or aldehydes (unconjugated). The ester conjugates are the main phenolic acids extracted from sorghum [36]. The bonded phenolic acids are however linked by covalent bonds to the cell wall (lignin) and they belong to the cell wall and require acidic or alkaline conditions, as well as high temperatures to break covalent bonds [37]. The majority of phenolic acids in sorghum (about 70%−95%) are in bound form. Ferulic acid is one of the most commonly available (100–500 μg g−1 in the grain) and can account for up to 90% of the total phenolic acids [36]. In addition, the content of bound phenolic acids is also strongly tied to grain hardness, since the bound phenolic acids form part of the cell wall components [38]. Here, we summarize the phenolic compounds (Table 1) in sorghum grains.
Researchers are particularly interested in the anthocyanin flavonoids present in colored sorghums but not in white sorghums because some are specific to sorghum grain and have powerful antioxidant effects [33]. The primary flavonoid class is 3-deoxyanthocyanins (3-DAs and derivatives), which are found in the pericarp [39]. 3-DAs, such as apigenidin and luteolinidin, lack the hydroxyl group in the 3-position of the C-ring and are responsible for the color of certain sorghum grain kinds [36,45], especially red and black sorghums [45]. An in vitro study of red sorghum flour extracts revealed high free radical scavenging activity as measured by an oxygen radical absorbance capacity (ORAC) assay, as well as defense against LDL oxidation, adding to the factual basis for red sorghum’s potential as a valuable health-promoting food grain [46–48].
Flavanones are abundant in the naringenin food crops and their derivatives, which are the most common. They are the primary intermediary in the production of flavonoids; however, they are uncommon in cereal grains [46]. Sorghum flavanone is found in concentrations ranging from 0 to 2000 μg g−1. The minimum quantity was found in white sorghum, whereas the maximum amount was found in sorghum with a yellow pericarp [47]. Sorghum’s primary flavanons are naringenin and eriodictyol glycosides, with a modest number of aglycones and derivatives including O-methyl [36,47]. The phenolic composition and antioxidant capacity in three sorghum varieties grown in the Mediterranean, consisting of different pericarp colors: white, red, and black were compared. The results showed that the investigated sorghum varieties contained polyamines, such as spermidines, and polyphenolic compounds belonging to different classes: hydroxycinnamic acids, flavanols, flavones, and flavanones. Pyrano-flavanone-flavanols were detected only in the black sorghum [49,50].
The anthocyanin content is the distinctive characteristic of sorghum flavonoids and source of natural water-soluble pigments and antioxidants [50]. Most natural anthocyanins in plants are C-3-hydroxylated anthocyanins, while C-3-deoxylated analogues, i.e., 3-deoxyanthocyanidins, which are an uncommon subclass of anthocyanins are found actually largely in sorghum [31]. The 3-deoxyanthocyanidie and anthocyanins are obtained from the biosynthetic pathway of flavanone but is different from the intermediate flavanone naringenin [51]. 3-deoxyanthocyanidins are among the most abundant sorghum flavonoids, accounting for up to 80% of total grain flavonoids, and ranging in total concentration from 200 to 4,500 μg g−1 in some sorghums [33,52]. Black sorghum bran has the greatest amount of 3-deoxyanthocyanidins (1.790 to 6.120 μg g−1), at least double the amount of red and brown sorghum bran among the red pericarp sorghum genotypes [53]. In addition, in other plant parts, such as sheath and leaves, 3-deoxyanthocyanidins are also found at amounts of up to 90,000 μg g−1 [54,55]. 3-deoxyanthocyanidins are powerful, antimicrobial active antioxidants and have several other advantages [31].
Tannins are one of the sorghum polyphenols most widely distributed. However, sorghum tannins are condensed in the form of high-molecular weight, high-grade polymerization and are not common between most cereals [56]. Sorghum tannins vary considerably between genotypes. In general, sorghums have high amounts of condensed tannin in the pigmented testa [33,53]. High amounts of tannins found in sorghums have agronomic advantages to protect the plant from diseases and birds, they have been frequently cultivated in underdeveloped regions with a food security problem [56].
Carotenoids are C40 isoprenoids that provide a variety of health benefits [57]. The most studied carotenoids in sorghum grains are lutein, zeaxanthin, and β-caroteneis (Table 2), and the predominant sorghum carotenoids are xanthophylls (lutein and zeaxanthin) [57]. Carotenoids have been found in a variety of investigations [58]. This could be because of differences in genotypes, extraction procedures, detection methods, and sorghum grain fractions. The expression status of nine genes involved in carotenoid production or degradation is responsible for the considerable diversity in carotenoid concentration in sorghum grains [58]. Furthermore, carotenoids are extremely susceptible to heat, air, light, acids, and other environmental factors [57]. The negative effects of sorghum grain processing on carotenoid compounds should be avoided or minimized.
The most investigated tocochromanols in sorghum are α-Tocopherol, β-tocopherol, γ-tocopherol, and δ-tocopherol (Table 2). According to Cardoso et al. [58], the primary tocochromanols in sorghum are γ- tocopherol, followed by α-tocopherol, and the vitamin E concentration of sorghum varies greatly [58]. Chung et al. [59] discovered that β-tocopherol is the most abundant tocopherol in sorghum, and that the vitamin E content of sorghum grains varies with genotype. As a result, sorghum’s total vitamin E concentration and profile differ greatly. Furthermore, the vitamin E profile and levels in sorghum grains are influenced by the farming environment or region [59].
Amines are nitrogenous bases with a low molecular mass that are split into biogenic amines and polyamines. The composition and concentration of bioactive amines in distinct sorghum lines were initially described by Paiva et al. [60]. According to their study, the polyamines accounted for 60%–100% of the total amines [60]. Spermine and spermidine were the most common amines, followed by putrescine and cadaverine. As a result, sorghum is a significant source of polyamines.
4.9 Policosanols and Phytosterols
Plant-derived steroids are known as phytosterols. β-sitosterol, campesterol, and stigmasterol have all been identified, with β-sitosterol being the most abundant phytosterol in sorghum grains (Table 2). The concentration of phytosterols can be affected by sorghum genotype, growing site, and extraction procedure [61]. Policosanols are a type of high-molecular-weight aliphatic alcohol with a variety of bioactivities [59]. C26 policosanol, C28 policosanol, C30 policosanol, and C32 policosanol were extracted and identified, with C28 policosanol being the most abundant in sorghum (Table 2). The extraction and detection methods of phytosterols need to be researched in the future since the method of determination can impact the content of phytosterols [61].
Sorghum was frequently grown in tropical and subtropical climates. Sorghum is used to make bread and porridge and it is a substantial source of energy and nourishment for people, particularly in impoverished and semi-arid regions [66,67]. Sorghum is commonly grown for animal feed in western countries. However, because the natural components in sorghum are useful in the development of healthful and functional foods, there is a growing interest in farming sorghum for both biofuel production and human consumption [68]. Also, Sorghum has a mild flavor and can be ground into flour for baking gluten-free goods [69].
5.1 Sweet Sorghum as Biofuel Feedstock
As a promising target for both sugar and lignocellulosic biofuel production, sorghum bicolor was developed. The input requirements for it are relatively low and can expand on marginal lands. There are various phenotypical and morphological characteristics of cultivated sorghum varieties. These have been divided into four groups, namely grain, forage, energy and sweet sorghum based on their production characteristics and use. Sorghum grain varieties with large ear heads are between three and six feet high and mainly serve as food for people or feed for cattle. The rapidly growing varieties of coarse sorghum forage are used for food, silage and grazing [70]. Energy sorghum is generated especially for the high lignocellulosic biomass which can be converted to biofuels, whereas sorghum, also known as sweet stalk sorghum, specifically refers to genotypes which are used to accumulate soluble sugars [71].
Sweet sorghum may grow up to twenty feet tall and produce significantly higher biomass yields compared to grain sorghum. Sorghum stems are thicker and fleshlier than grains, although their seed production is relatively low [72]. Sweet sorghum is one of the leading feedstock crops for new-age biofuels, given its high sugar content and its easy extractability. Sweet stalk sugars mainly contain sucrose (∼75%), some fructose and glucose (∼2.6%) [73]. Soluble sugars in form of glucose, fructose and sucrose in sweet sorghum are easily and frequently fermentable as compared to lignocellulosic biomass plants as switchgrass and Miscanthus [74]. Special advantages for the use as a biofuel feedstock include other agronomic features like a short 4- months life cycle, the capability to grow under adverse environmental conditions, less input requirements and low cultivation costs.
Furthermore, C4 photosynthesis is particularly important as it contributes to higher nitrogen and water use efficiency as well as overall robustness of sweet sorghum making it better equipped to survive in the dry regions with higher light intensity/temperatures [72]. Also, sweet sorghum varieties are taller, have larger leaf canopy surface area, and are equipped with a better light interception and high radiation use efficiency compared to grain and energy sorghums [72]. The energy ratio invested in the extraction of biofuels from sweet sorghum has been estimated at 1:8, according to the US Department of Agriculture, Billings [75] which can be further improved by technological and molecular breeding technologies. Because of low sulphur content, low biological and chemical oxygen demand and high-octane level [76], ethanol produced from sweet sorghum is safer for the environment. Although the annual output of sweet sorghum ethanol depends on a range of factors such as genetic history, year time, soil quality and other environmental factors, sweet sorghum cultivation is estimated to yield 8000 liters/ha/year of ethanol [77].
Besides the stem sugars which are a major commodity for the cultivation of sweet sorghum, coproducts in grains, bagasse, vinasse, steam, foam, and froth are also used for various purposes as raw materials. Juice is fermented into ethanol for biofuel purposes that can be used as a substitute for conventional fuels. Bagasse can also be applied to lignocellulosic biomass in the production of ethanol and biodegradable plastic. Bagasse silage is rich in micronutrients and minerals, therefore a nutritious source of animal feed, particularly for dairy livestock. In fact, following extraction of ethanol from sweet sorghum, known as vinasse or stillage, the liquid distillate left is used as a fertilizer for agricultural purposes that reduces the waste disposal problem [78]. Anaerobic digestion is used for the production of methane gas for combustion in order to produce heat energy. Sweet sorghum grains can be used to replace gluten-free wheat or maize flour. Although the reserves of starch can be used for the production of ethanol and vinegar, poor quality grain is used primarily in animal feed [78].
5.2 Potential Health Benefits of Sorghum Grains
The major cause of different chronic illnesses is oxidative stress, which is an imbalance between free radicals and antioxidants [79]. In health promotion and disease prevention, sorghum-related phenolic compounds appear to be of major importance for their antioxidant activity. Sorghum grain phenolic compounds represent the highest antioxidant activity when compared with that in other cereal grains (e.g., wheat, rice, and maize) and compared to common fruits and vegetables (Fig. 3). The activity of antioxidants is strongly linked to the overall phenolic contents, especially condensed sorghum tannins [8,80–85]. Condensed tannin sorghum has consistently demonstrated high antioxidant activity in vitro, particularly in the bran with concentrated phenolics [86]. The main properties of tannin are the ability to chelate with metals, which reduces their bioavailability with proteins and lipids. Because of this property, they are mainly excluded in feeding stuffs but can be used as energy reducers in human foodstuffs. Tannins have many positive effects on human health, they prevent chronic conditions, for instance nitric oxide (NO), synthetase and xanthin oxidases, or anti-inflammatory effects of cardiovascular diseases. They also have a prooxidative enzymes inhibition effect [86]. In addition, Wu et al. [87] have studied cell antioxidant activity in Chinese steamed bread and have demonstrated a substantial increase in cellular antioxidant activity by incorporating sorghum into the bread. Also, brown and black sorghums showed a strong activity as antioxidants with the highest antioxidant activity for brown-bran-free and black-bran-free phenolic extracts. Free black-bran-free phenolics also had high cell-based antioxidant quality and high cell membrane and intracellular antioxidant effects with the brown-bran-free phenolics [88]. Besides, two novel black-sowed sorghum phenolic contents (SC84 and PI570481) were investigated, and their antioxidant and anti-inflammatory activity assessed using different extraction systems (water, ethanol, and their acidified counterpart). Results showed that phenolic compounds and sorghum samples had different effect of solvent types as well as HCL on extraction efficiency. In the studied extracts, tannins were the most dominant polyphenols (11, 11,136, 11 mg of the epicatechin equivalent/g of sorghum). Sorghum extracts have been more powerful than NO radicals in DPPH scavenging activity [89]. In the meantime, the capacity to inhibit DPPH production seemed much greater as all sorghum extracts compared to a further study with an average of 90% of antioxidant activity against DPPH for three separate genotypes. DPPH has been reported to be averaged by 90% DPPH [90].
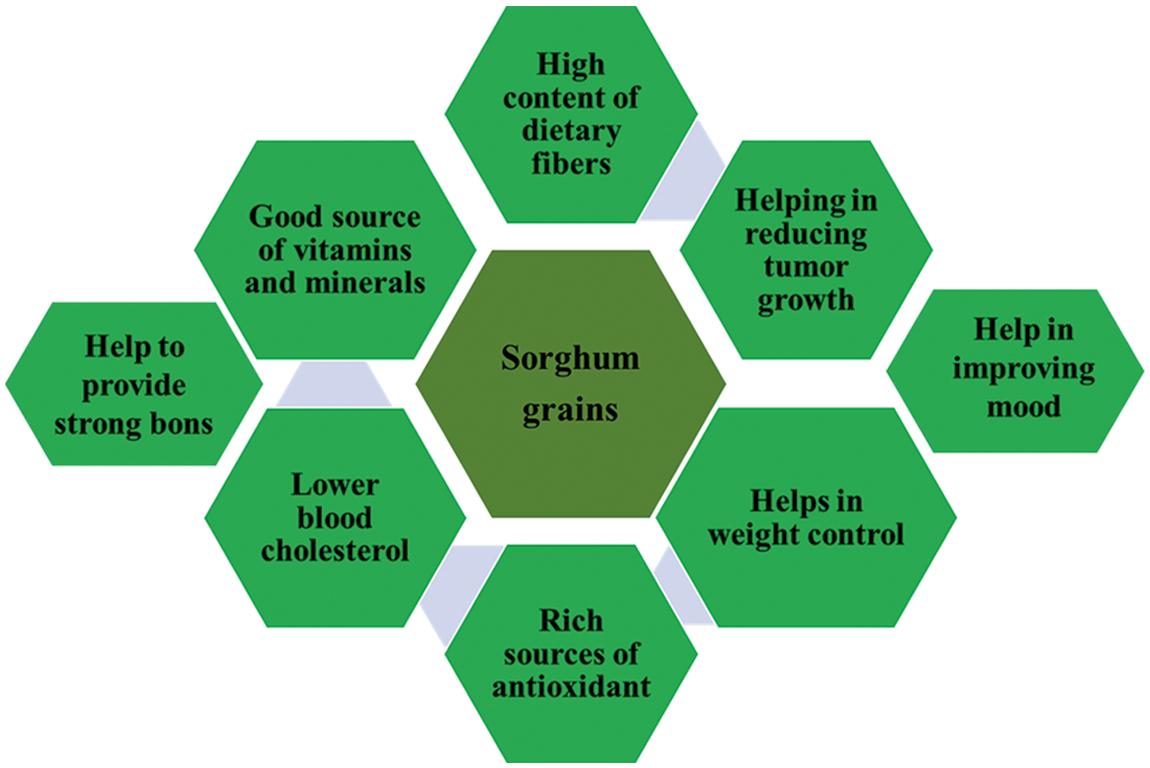
Figure 3: Different health benefits of sorghum grains
5.2.2 Anti-Inflammatory Activity
Long-term oxidative stress can cause chronic inflammation, which can lead to a variety of chronic diseases. During inflammation, inflammatory substances such interleukin (IL), cyclooxygenase (COX)-2, tumor necrosis factor (TNF)-, and prostaglandin E2 (PG-E2) are generated. Several phenolic chemicals found in sorghum grain can inhibit the synthesis of these pro-inflammatory molecules [91,92]. For example, phenolic acids such as gallic acid and ferulic acid have been shown to reduce the COX-2 enzyme, while ferulic acids have been shown to decrease the synthesis of (TNF)-α, and prostaglandin E2 (PG-E2). The flavones apigenin and luteolin suppress COX-2 production as well as the transcription factor (nuclear factor kappa B) that controls the production of pro-inflammatory chemicals [93]. The formation of COX-2 and PG-E2 was suppressed with 3-Deoxyanthocyanidins [92]. Furthermore, the combination of flavone apigenin and flavonol quercetin, as well as the apigenin-rich extract from sorghum and cowpea rich in quercetin have strong synergistic anti-inflammatory effects by improving its bioavailability by reducing the metabolism of Phase II and binding ATP cassette membrane transporter function in cellular models [93,94]. Apigenin and quercetin can play a key role in improving the anti-inflammatory effect by the configuration of the conjugation C2 = C3 [94]. The crude phenolic extract from the sorghum bran, particularly black, has also shown a strong inhibitory effect on the pro-inflammatory activity of COX-2, IL-1β and TNF-α and is similar to the anti-inflammatory medicine indomethacin [91]. Recent research has demonstrated the significant decrease in IL 1β, IL-6, IL-8 and TNF α pro-inflammatory compounds over a period of 12 weeks by introducing full-grain sorghum biscuits in the human diet (overweight adults) [95]. In addition, phenolic extraction from sorghum bran inhibited the hyaluronidase enzyme, an enzyme involved in chronical joint inflammation. All sorghum varieties showed significant inhibition against hyaluronidase activity, regardless of the pericarp colors and condensed tannin content. Aside from phenols, triacylglycerols, tocopherols, carotenoids, and unsaturated fatty acids from sorghum have also shown inhibitory effects on the inflammatory response, through reducing expression of specific genes in lipopolysaccharides (LPS) [96].
Sorghum phenolic chemicals can have anti-cancer properties, and whole-grain sorghum consumption may reduce the incidence of some malignant diseases [97]. Sorghum’s anticancer potential may be attributed to its powerful antioxidant activities and Phase II enzyme inductions of its phenolic component [98]. Colon, hepatoma, esophageal, intestinal epithelial, leukaemia, breast, and stomach cancer cells can be resistant to the formation of 3-deoxyanthocyanidin and 3-deoxyanthocyanidin-rich extracts. These chemicals fight against cancer directly by causing cell death and suppressing cancer cell proliferation [99]. This characteristic is more effective in 3-deoxyanthocyanidins than in their anthocyanidin counterparts. Apigeninidin and luteolinidin, for example, were found to be more cytotoxic than their anthocyanin analogues pelargonidine and cyanidin in human epithelial (HEP-G2) and leukaemia (HL-60) cancer cells [100]. Cancer protection may also be aided by sorghum tannin. Aromatase (a breast cancer enzyme) can be blocked, preventing the creation of an unwelcome cancer development stimulus [101]. The tannin-rich extract from brown sorghum bran was shown to be substantially more effective than the 3-deoxyanthocyanidin-rich [101] in reducing aromatase activity at low levels. Tannins derived from sorghum are also more efficient than grape seed tannins at inhibiting colon cancer cell proliferation [102]. Other phenolics found in sorghum, such as flavones and flavanones, have also anti-cancer properties, particularly flavone apigenin, which can stimulate estrogenic activity and cause apoptosis in colon cancer cells [36,47]. Recently, Lee et al. [9] found that a new high level of phenolic sorghum (PI570481 accession) originating in Sudan showed pro-apoptotic activeness with increased cleavage of caspase-3 and PARP in human colorectal cancer cells (HCT15, SW480, HCT116, and HT-29). This increased apoptosis is linked to the activation of damage to the DNA pathway and the following proapoptotic protein expression, ATF3. The positive effect of the sorghum component, on the other hand, could be linked to a p53-independent mechanism [9]. The higher amounts of phenolic compounds of sorghum (accession PI570481) also stopped human colorectal cancer cells from proliferating by arresting the S phase [9].
5.2.4 Antidiabetic and Obesity Prevention
Obesity and diabetes patients will benefit from a diet rich in whole grain sorghum. Sorghum is a grain with a low digestion. The resistant and poorly digested starch content of sorghum endosperm is high [25]. A cross-linking occurs between sorghum protein (kafirin) and starch, and it is primarily composed of strong disulfide bonds that resist digestion during the hydrothermal feeding process [103]. Sorghum tannins may react with carbohydrates and proteins in the gastrointestinal tract to form bulk complexes that are even less digestible and nondigestible [25,104]. Sorghum tannins interact with starch in a much more complex way than phenolics, and the higher the tannin molecular weight, the stronger the connection [25]. The combination induces satiety, reduces calorie intake, and produces a low glycemic response, all of which are beneficial to obese and diabetic individuals [96]. Healthy people who ate all-grain sorghum biscuits had higher hunger and satiety ratings than those who ate wheat biscuits [105]. Sorghum also has antidiabetic potential. The inhibitory activity of the phenol extract from sorghum grain has been shown to be anti-digestion enzymes like Bacillus stearothermophilus α-glucosidase, porcine pancreatic α-amylase, and human salivary α-amylase, which reduce the level of glycemia. Some sorghums showed stronger inhibitory activities of α-glucosidase than the common antidiabetic medicinal acarbose [106]. A significant decrease in plasma glucose concentration has been found in the use of phenolic extract in streptozotocin-induced diabetic rats, and it has been as effective as the antidiabetics drug glibenclamide [107]. Sorghum phenolics can act as an adjuvant in diabetic treatment and play a role in insulin regulation [107]. In addition, sorghum muffins have been used to influence blood glucose and insulin levels and enhance glycemic responses in healthy people [108]. Sorghum can partly be attributed to the antidiabetic activity of condensed tannins. Links et al. [109] demonstrated the strong inhibitory activity of the raw extract from tannin sorghum type III against yeast α-glucosidase, which is approximately 20,000 times greater than acarbose, but the inhibition of pancreatic alpha-amylase is better with acarboses. Sorghum phenolics, particularly sorghum with high tannin levels, effectively inhibit these enzyme activities.
Antidiabetic mechanisms may be initially inhibited by digestive Enzymes to prevent glucose digestion [109]. The inclusion of sorghum in mainstream diets could contribute to obesity and diabetes prevention and human health improvements. In a further study, the effect of extruded sorghum was assessed for anthropometry, body composition and clinical measures in overweight men, which found a lower weight loss and decreasing waist and body fat percentages of the extracted sorghum consumed [110]. Findings from these studies show the potential for sorghum as a checking ingredient to be used in foods, however, in-vitro and in-vivo studies still need to clarify in depth the mechanisms under which sorghum control the obesity-related parameters in the human body. Sorghum extract reversed a rise in blood sugar and triacylglycerol associated with the initiation of AMPK and a decline of macrophages infiltration to the liver according to a recent study using STZ-induced diabetic rats [111].
5.2.5 Dyslipidemia and Cardiovascular Disease Prevention
Sorghum grain has a variety of bioactive phenolic chemicals that can help to prevent dyslipidemia and cardiovascular disease. Sorghum lipids of phytosterols and polycosanols were shown to regulate cholesterol absorption, discharge, and synthesis, demonstrating cardiovascular health. For example, adding sorghum lipids to a hamster’s diet boosted cholesterol elimination and metabolites, lowering plasma and hepatic cholesterol levels [112]. The phenolic chemicals in sorghum may also help with cholesterol metabolism. Sorghum phenolic chemicals can also be used in cholesterol metabolism. When hyperlipidemic or diabetic rats were administered sorghum phenolic extract, their cholesterol and triacylglycerol levels were significantly reduced [107].
5.3 Sorghum in Livestock Nutrition
51% of sorghum is used for livestock feeding, while 49% is used for humans and other purposes [113]. Further, 48% of sorghum production is produced in animals that are often compared to maize, which is a close replacement for according to Dowling et al. [114]. While sorghum in whole grains has more protein than maize, vitamin A is lower. The intake of grain, although sometimes it limits productivity, ranges from 90% to almost the same as maize. For drilling or silage, sorghum is often grown. The dried leaves are a useful roughage for bovine animals and horses. The mature plant can be used for the purposes of green forage or silage. However, since they contain dhurrin, a cyanogenic glycoside which hydrolysis produces cyanide hydrogen (HCN), it is unsafe to feed young green plants [115]. The sorghum kernel is slightly like maize, but smaller. Sheep, pigs and even poultry may be given entire sorghum grains, but they are often ground for cattle [116].
6 Conclusion and Future Perspectives
Sorghum is a crop that has become highly significant around the world, and innovative approaches of boosting its utilization as food for humans had already arisen. For example, it can be used as bread, pasta, cookies, or cereal substitutes for celiac disease persons. These persons suffer from health problems related to an immunological response to gluten proteins. Sorghum’s grains lack these proteins, which are observed in other cereals such as wheat, oats, barley, and rye. However, due to the presence of phenolic compounds such as tannins, which are considered anti-nutritional factors, sorghum’s use in food production is still limited. An excessive intake of these compounds can have a negative effect on iron absorption and low protein digestibility, primarily due to complex formation. It is worth noting that phenolic chemicals have exceptionally low toxicity when consumed in moderate amounts, owing to their limited absorption and fast metabolism. Sorghum, on the other hand, has a lot of potential as a nutraceutical and functional component for food processing because of the tannins and other phenolics it contains. Because sorghum is high in polyphenolic substances such as procyanidins (condensed tannins), 3-deoxyantocyanidins, and phenolic acids, it has a high antioxidant activity to mitigate diseases associated with oxidative stress, antiproliferative properties related to the prevention of certain cancers, antimicrobial properties, and improves glucose metabolism, which is an activity linked to diabetes. As a results, sorghum could be considered a valuable source of bioactive ingredients. Because of the rapid population growth around the world, there is a need for nutritious foods that can improve the quality of life of low-income people who suffer from nutritional deficiencies and health problems. Incorporating sorghum and its co-products into human diets would be a good alternative. This is the result of the frequent viral epidemics that have been highly contagious and easily transmissible in the previous two decades, such as the latest COVID-19 outbreak, which has killed tens of thousands of people around the world. As a result, safe and effective interventions to prevent, decrease susceptibility and minimize all types of viruses are urgently needed. Nutraceuticals, functional foods, and herbal plants may aid in the prevention and treatment of viral infections. Furthermore, the presence of numerous bioactive substances (or natural products) in sorghum grains could be linked to preventative actions of viral diseases. Several studies are needed to study the effects of bioactive substances in sorghum grains as antiviral.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. Ghonaim, M. M., Mohamed, H. I., Omran, A. A. (2021). Evaluation of wheat (Triticum aestivum L.) salt stress tolerance using physiological parameters and retrotransposon-based markers. Genetic Resources and Crop Evolution, 68(1), 227–242. DOI 10.1007/s10722-020-00981-w. [Google Scholar] [CrossRef]
2. Mohamed, H. I., Ashry, N. A., Ghonaim, M. M. (2019). Physiological and biochemical effects of heat shock stress and determination of molecular markers related to heat tolerance in maize hybrids. Gesunde Pflanzen, 71(3), 213–222. DOI 10.1007/s10343-019-00467-5. [Google Scholar] [CrossRef]
3. Puoane, T. R., Swaminathan, S., Dehghan, M. (2021). Associations of cereal grains intake with cardiovascular disease and mortality across 21 countries in prospective urban and rural epidemiology study: Prospective cohort study. BMJ, 372, m4948. DOI 10.1136/bmj.m4948. [Google Scholar] [CrossRef]
4. Li, S., Cheng, C. S., Zhang, C., Tang, G. Y., Tan, H. Y. et al. (2021). Edible and herbal plants for the prevention and management of COVID-19. Frontiers in Pharmacology, 12, 656103. DOI 10.3389/fphar.2021.656103. [Google Scholar] [CrossRef]
5. Lee, S. H., Lee, H. S., Lee, J., Amarakoon, D., Lou, Z. et al. (2021). Polyphenol containing sorghum brans exhibit an anti-cancer effect in Apc Min/+ mice treated with dextran sodium sulfate. International Journal of Molecular Sciences, 22(15), 8286. DOI 10.3390/ijms22158286. [Google Scholar] [CrossRef]
6. Verma, D. K., Srivastav, P. P. (2020). Bioactive compounds of rice (Oryza sativa L.Review on paradigm and its potential benefit in human health. Trends of Food Science and Technology, 97, 355–365. DOI 10.1016/j.tifs.2020.01.007. [Google Scholar] [CrossRef]
7. Anglani, C. (1998). Sorghum for human food—A review. Plant Foods for Human Nutrition, 52(1), 85–95. DOI 10.1023/A:1008065519820. [Google Scholar] [CrossRef]
8. Awika, J. M., Rooney, L. W. (2004). Sorghum phytochemicals and their potential impact on human health. Phytochemistry, 65(9), 1199–1221. DOI 10.1016/j.phytochem.2004.04.001. [Google Scholar] [CrossRef]
9. Lee, S. H., Lee, J., Herald, T., Cox, S., Noronha, L. et al. (2020). Anticancer activity of a novel high phenolic sorghum bran in human colon cancer cells. Oxidative Medicine and Cellular Longevity, 2020, 2890536. DOI 10.1155/2020/2890536. [Google Scholar] [CrossRef]
10. Zhang, Y., Li, M., Gao, H., Wang, B., Tongcheng, X. et al. (2019). Triacylglycerol, fatty acid, and phytochemical profiles in a new red sorghum variety (Ji Liang No. 1) and its antioxidant and anti-inflammatory properties. Food Science and Nutrition, 7, 949–958. DOI 10.1002/fsn3.886. [Google Scholar] [CrossRef]
11. Espitia-Hernandez, P., Chavez Gonzalez, M. L., Ascacio-Valdes, J. A., Davila-Medina, D., Flores-Naveda, A. et al. (2020). Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Critical Reviews in Food Science and Nutrition, 2020, 1–12. DOI 10.1080/10408398.2020.1852389. [Google Scholar] [CrossRef]
12. Punia, H., Tokas, J., Malik, A., Sangwan, S. (2021). Characterization of phenolic compounds and antioxidant activity in sorghum [Sorghum bicolor (L.) moench] grains. Cereal Research Communications, 49, 343–353. DOI 10.1007/s42976-020-00118-w. [Google Scholar] [CrossRef]
13. Miafo, A. P. T., Koubala, B. B., Kansci, G., Muralikrishna, G. (2020). Antioxidant properties of free and bound phenolic acids from bran, spent grain, and sorghum seeds. Cereal Chemistry, 97, 1236–1243. DOI 10.1002/cche.10348. [Google Scholar] [CrossRef]
14. Shen, S., Huang, R., Li, C., Wu, W., Chen, H. et al. (2018). Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules, 23(5), E1203. DOI 10.3390/molecules23051203. [Google Scholar] [CrossRef]
15. Romeilah, R. M., El-Beltagi, H. S., Shalaby, E. A., Younes, K. M., Moll, E. H. et al. (2021). Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla L. essential oils. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 49(1), 12233–12233. DOI 10.15835/nbha49112233. [Google Scholar] [CrossRef]
16. Nguyen, P. H., Dung, V. V., Zhao, B. T., Kim, Y. H., Min, B. S. et al. (2014). Antithrombotic and antidiabetic flavonoid glycosides from the grains of Sorghum bicolor (L.) Moench var. hwanggeumchal. Archives of Pharmacal Research, 37, 1394–1402. DOI 10.1007/s12272-014-0422-5. [Google Scholar] [CrossRef]
17. Dia, V. P., Pangloli, P., Jones, L., McClure, A., Patel, A. (2016). Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Function, 7, 3410–3420. DOI 10.1039/C6FO00757K. [Google Scholar] [CrossRef]
18. Earp, C. F., Donough, C. M. M., Awika, J., Rooney, L. W. (2004). Testa development in the caryopsis of Sorghum bicolor (L.) moench. Journal of Cereal Science, 39(2), 303–311 DOI 10.1016/j.jcs.2003.11.005. [Google Scholar] [CrossRef]
19. Chandrashekar, A., Kirleis, A. W. (1988). Influence of protein on starch gelatinization in sorghum. Cereal Chemistry, 65, 457–462. [Google Scholar]
20. Rooney, L. W. (1978). Sorghum and pearl millet lipids. Cereal Chemistry, 55, 584–59. [Google Scholar]
21. Hill, H., Lee, L. S., Henry, R. J. (2012). Variation in sorghum starch synthesis genes associated with differences in starch phenotype. Food Chemistry, 131(1), 175–183. DOI 10.1016/j.foodchem.2011.08.057. [Google Scholar] [CrossRef]
22. USDA (2019). National nutrient database for standard reference legacy release: Full report (all nutrients) 20067, sorghum grain. [Google Scholar]
23. Li, Z., Zhao, X., Zhang, X., Liu, H. (2021). Bioactive compounds and biological activities of sorghum grains. Foods, 10, 2868. DOI 10.3390/foods10112868. [Google Scholar] [CrossRef]
24. Dicko, M. H., Gruppen, H., Zouzouho, O. C., Traoré, A. S., van Berkel, W. J. H. et al. (2006). Effects of germination on the activities of amylases and phenolic enzymes in sorghum varieties grouped according to food end-use properties. Journal of the Science of Food and Agriculture, 86(6), 953–963. DOI 10.1002/(ISSN)1097-0010. [Google Scholar] [CrossRef]
25. Barros, F., Awika, J. M., Rooney, L. W. (2012). Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. Journal of Agricultural and Food Chemistry, 60(46), 11609–11617. DOI 10.1021/jf3034539. [Google Scholar] [CrossRef]
26. Barros, F., Awika, J., Rooney, L. W. (2014). Effect of molecular weight profile of sorghum proanthocyanidins on resistant starch formation. Journal of the Science of Food and Agriculture, 94(6), 1212–1217. DOI 10.1002/jsfa.6400. [Google Scholar] [CrossRef]
27. MartinoI, H. S. D., Tomaz, P. A., Moraes, É. A., Conceição, L. L. D., Oliveira, D. D. S. et al. (2012). Chemical characterization and size distribution of sorghum genotypes for human consumption. Revista do Instituto Adolfo Lutz, 71(2), 337–344. [Google Scholar]
28. Verbruggen, M. A., Spronk, B. A., Schols, H. A., Beldman, G., Voragen, A. G. J. et al. (1998). Structures of enzymically derived oligosaccharides from sorghum glucuronoarabinoxylan. Carbohydrate Research, 306(1–2), 265–274. DOI 10.1016/S0008-6215(97)10064-7. [Google Scholar] [CrossRef]
29. Belton, P. S., Delgadillo, I., Halford, N. G., Shewry, P. R. (2006). Kafirin structure and functionality. Journal of Cereal Science, 44(3), 272–286. DOI 10.1016/j.jcs.2006.05.004. [Google Scholar] [CrossRef]
30. Galili, G., Amir, R. (2013). Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnology Journal, 11(2), 211–222. DOI 10.1111/pbi.12025. [Google Scholar] [CrossRef]
31. Xiong, Y., Zhang, P., Luo, J., Johnson, S., Fang, Z. (2019). Effect of processing on the phenolic contents, antioxidant activity and volatile compounds of sorghum grain tea. Journal of Cereal Science, 85, 6–14. DOI 10.1016/j.jcs.2018.10.012. [Google Scholar] [CrossRef]
32. González-Montilla, F. M., Chávez-Santoscoy, R. A., Gutiérrez-Uribe, J. A., Serna-Saldivar, S. O. (2012). Isolation and identification of phase II enzyme inductors obtained from black shawaya sorghum [Sorghum bicolor (L.) moench] bran. Journal of Cereal Science, 55(2), 126–131. DOI 10.1016/j.jcs.2011.10.009. [Google Scholar] [CrossRef]
33. Girard, A. L., Awika, J. M. (2018). Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. Journal Cereal Science, 84, 112–124. DOI 10.1016/j.jcs.2018.10.009. [Google Scholar] [CrossRef]
34. Althwab, S., Carr, T. P., Weller, C. L., Dweikat, I. M., Schlegel, V. (2015). Advances in grain sorghum and its co– products as a human health promoting dietary system. Food Research International, 77, 349–359. DOI 10.1016/j.foodres.2015.08.011. [Google Scholar] [CrossRef]
35. Vanamala, J. K. P., Massey, A. R., Pinnamaneni, S. R., Reddivari, L., Reardon, K. F. (2018). Grain and sweet sorghum (Sorghum bicolor L. moench) serves as a novel source of bioactive compounds for human health. Critical Reviews in Food Science and Nutrition, 58(17), 2867–2881. DOI 10.1080/10408398.2017.1344186. [Google Scholar] [CrossRef]
36. Yang, L., Allred, K. F., Geera, B., Allred, C. D., Awika, J. M. (2012). Sorghum phenolics demonstrate estrogenic action and induce apoptosis in nonmalignant colonocytes. Nutrition and Cancer, 64(3), 419–427. DOI 10.1080/01635581.2012.657333. [Google Scholar] [CrossRef]
37. Wu, G., Johnson, S. K., Bornman, J. F., Bennett, S. J., Fang, Z. (2017). Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chemistry, 214, 199–207. DOI 10.1016/j.foodchem.2016.07.089. [Google Scholar] [CrossRef]
38. Chiremba, C., Taylor, J. R. N., Rooney, L. W., Beta, T. (2012). Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chemistry, 134(1), 81–88. DOI 10.1016/j.foodchem.2012.02.067. [Google Scholar] [CrossRef]
39. Li, M., Xu, T., Zheng, W., Gao, B., Zhu, H. et al. (2021). Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chemistry, 340, 128123. DOI 10.1016/j.foodchem.2020.128123. [Google Scholar] [CrossRef]
40. Lohani, U. C., Muthukumarappan, K. (2020). Influence of fermentation followed by ultrasonication on functional properties of sorghum extrudates. Journal of Food Process Engineering, 2020, 43. DOI 10.1111/jfpe.13390. [Google Scholar] [CrossRef]
41. Hithamani, G., Srinivasan, K. (2014). Bioaccessibility of polyphenols from wheat (Triticum aestivumsorghum (Sorghum bicolorgreen gram (Vigna radiataand chickpea (Cicer arietinum) as influenced by domestic food processing. Journal of Agriculture and Food Chemistry, 62, 11170–11179. DOI 10.1021/jf503450u. [Google Scholar] [CrossRef]
42. Przybylska-Balcerek, A., Frankowski, J., Stuper-Szablewska, K. (2018). Bioactive compounds in sorghum. European Food Research and Technology, 245, 1075–1080. DOI 10.1007/s00217-018-3207-0. [Google Scholar] [CrossRef]
43. N’Dri, D., Mazzeo, T., Zaupa, M., Ferracane, R., Fogliano, V. et al. (2013). Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal african cereals. Journal of the Science of Food and Agriculture, 93, 29–36. DOI 10.1002/jsfa.5837. [Google Scholar] [CrossRef]
44. Luo, X., Cui, J., Zhang, H., Duan, Y. (2018). Subcritical water extraction of polyphenolic compounds from sorghum (Sorghum bicolor L.) bran and their biological activities. Food Chemistry, 262, 14–20. DOI 10.1016/j.foodchem.2018.04.073. [Google Scholar] [CrossRef]
45. Buer, C. S., Imin, N., Djordjevic, M. A. (2010). Flavonoids: New roles for old molecules. Journal of Integrative Plant Biology, 52(1), 98–111. DOI 10.1111/j.1744-7909.2010.00905.x. [Google Scholar] [CrossRef]
46. Awika, J. M. (2017). Sorghum: Its unique nutritional and health-promoting attributes. In: Taylor, J. R. N., Awika, J. M. (Eds.Gluten-free ancient grains, pp. 21–54. Elsevier, Amsterdam, The Netherlands. [Google Scholar]
47. Yang, L., Allred, K. F., Dykes, L., Allred, C. D., Awika, J. M. (2015). Enhanced action of apigenin and naringenin combination on estrogen receptor activation in non-malignant colonocytes: Implications on sorghum-derived phytoestrogens. Food and Function, 6(3), 749–755. DOI 10.1039/C4FO00300D. [Google Scholar] [CrossRef]
48. Dykes, L., Peterson, G. C., Rooney, W. L., Rooney, L. W. (2011). Flavonoid composition of lemon-yellow sorghum genotypes. Food Chemistry, 128(1), 173–179. DOI 10.1016/j.foodchem.2011.03.020. [Google Scholar] [CrossRef]
49. Bhagwat, S., Haytowitz, D. B., Holden, J. M. (2014). USDA database for the flavonoid content of selected foods, release 3.1. U.S. Department of Agriculture, Agricultural Research Service. Nutrient Data Laboratory. BARC-West Beltsville, Maryland. http://www.ars.usda.gov/nutrientdata/flav. [Google Scholar]
50. Pontieri, P., Pepe, G., Campiglia, P., Merciai, F., Basilicata, M. G. et al. (2021). Comparison of content in phenolic compounds and antioxidant capacity in grains of white, red, and black sorghum varieties grown in the Mediterranean area. ACS Food Science and Technology, 1(6), 1109–1119. DOI 10.1021/acsfoodscitech.1c00115. [Google Scholar] [CrossRef]
51. Kawahigashi, H., Kasuga, S., Sawada, Y., Yonemaru, J. I., Ando, T. et al. (2016). The sorghum gene for leaf color changes upon wounding (P) encodes a flavanone 4-reductase in the 3-deoxyanthocyanidin biosynthesis pathway. G3: Genes, Genomes, Genetics, 6(5), 1439–1447. DOI 10.1534/g3.115.026104. [Google Scholar] [CrossRef]
52. de Morais Cardoso, L., Pinheiro, S. S., Martino, H. S., Pinheiro-Sant’Ana, H. M. (2017). Sorghum (Sorghum bicolor L.Nutrients, bioactive compounds, and potential impact on human health. Critical Reviews in Food Science and Nutrition, 57(2), 372–390 DOI 10.1080/10408398.2014.887057. [Google Scholar] [CrossRef]
53. Dykes, L., Rooney, W. L., Rooney, L. W. (2013). Evaluation of phenolics and antioxidant activity of black sorghum hybrids. Journal of Cereal Science, 58(2), 278–283. DOI 10.1016/j.jcs.2013.06.006. [Google Scholar] [CrossRef]
54. Geera, B., Ojwang, L. O., Awika, J. M. (2012). New highly stable dimeric 3-deoxyanthocyanidin pigments from sorghum bicolor leaf sheath. Journal of Food Science, 77(5), C566–C572. DOI 10.1111/j.1750-3841.2012.02668.x. [Google Scholar] [CrossRef]
55. Petti, C., Kushwaha, R., Tateno, M., Harman-Ware, A. E., Crocker, M. et al. (2014). Mutagenesis breeding for increased 3-deoxyanthocyanidin accumulation in leaves of Sorghum bicolor (L.) moench: A source of natural food pigment. Journal of Agricultural and Food Chemistry, 62(6), 1227–1232. DOI 10.1021/jf405324j. [Google Scholar] [CrossRef]
56. Wu, Y., Li, X., Xiang, W., Zhu, C., Lin, Z. et al. (2012). Presence of tannins in sorghum grains is conditioned by different natural alleles of tannin. Proceedings of the National Academy of Sciences of the United States of America, 109, 10281–10286 DOI 10.1073/pnas.1201700109. [Google Scholar] [CrossRef]
57. Trono, D. (2019). Carotenoids in cereal food crops: Composition and retention throughout grain storage and food processing. Plants, 2019(8), 551. DOI 10.3390/plants8120551. [Google Scholar] [CrossRef]
58. Cardoso, L., Pinheiro, M., da Silva, S. S., de Menezes, L. L., de Carvalho, C. et al. (2015). Tocochromanols and carotenoids in sorghum (Sorghum bicolor L.Diversity and stability to the heat treatment. Food Chemistry, 172, 900–908. DOI 10.1016/j.foodchem.2014.09.117. [Google Scholar] [CrossRef]
59. Chung, I. M., Yong, S. J., Lee, J., Kim, S. H. (2013). Effect of genotype and cultivation location on β-sitosterol and α, β-, γ-, and δ-tocopherols in sorghum. Food Research International, 51, 971–976. DOI 10.1016/j.foodres.2013.02.027. [Google Scholar] [CrossRef]
60. Paiva, C. L., Evangelista, W. P., Queiroz, V. A., Gloria, M. B. (2015). Bioactive amines in sorghum: Method optimisation and influence of line, tannin and hydric stress. Food Chemistry, 173, 224–230. DOI 10.1016/j.foodchem.2014.10.039. [Google Scholar] [CrossRef]
61. Leguizamón, C., Weller, C. L., Schlegel, V. L., Carr, T. P. (2009). Plant sterol and policosanol characterization of hexane extracts from grain sorghum, corn and their DDGS. Journal of the American Oil Chemists’ Society, 86, 707–716. DOI 10.1007/s11746-009-1398-z. [Google Scholar] [CrossRef]
62. Kean, E. G., Ejeta, G., Hamaker, B. R., Ferruzzi, M. G. (2007). Characterization of carotenoid pigments in mature and developing kernelsof selected yellow-endosperm sorghum varieties. Journal of Agricultural and Food Chemistry, 55, 2619–2626. DOI 10.1021/jf062939v. [Google Scholar] [CrossRef]
63. Afify, A. E. M. M., El-Beltagi, H. S., Abd El-Salam, S. M., Omran, A. A. (2012). Biochemical changes in phenols, flavonoids, tannins, vitamin E, β–carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pacific Journal of Tropical Biomedicine, 2(3), 203–209. DOI 10.1016/S2221-1691(12)60042-2. [Google Scholar] [CrossRef]
64. Pinheiro-Sant’Ana, H. M., Guinazi, M., Oliveira, D. D. S., Della Lucia, C. M., Reis, B. D. L. et al. (2011). Method for simultaneous analysis of eight vitamin E isomers in various foods by high performance liquid chromatography and fluorescence detection. Journal of Chromatography A, 1218, 8496–8502. DOI 10.1016/j.chroma.2011.09.067. [Google Scholar] [CrossRef]
65. Liu, H., Li, Z., Zhang, X., Liu, Y., Hu, J. et al. (2021). The effects of ultrasound on the growth, nutritional quality and microbiological quality of sprouts. Trends in Food Science and Technology, 111, 292–300. DOI 10.1016/j.tifs.2021.02.065. [Google Scholar] [CrossRef]
66. Espitia-Hernández, P., Chávez González, M. L., Ascacio-Valdés, J. A., Dávila-Medina, D., Flores-Naveda, A. et al. (2020). Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Critical Reviews in Food Science and Nutrition, 1–12. DOI 10.1080/10408398.2020.1852389. [Google Scholar] [CrossRef]
67. Rooney, L. W., Waniska, R. D. (2000). Sorghum food and industrial utilization. In: Smith, C. W., Frederiksen, R. A., Hoboken, N. J., John Wiley Sons (Eds.Sorghum: Origin, history, technology, and production, vol. 2, pp. 689–729. [Google Scholar]
68. Rooney, W. L., Portillo, O., Hayes, C. (2013). Registration of ATx3363 and BTx3363 black sorghum germplasms. Journal of Plant Registrations, 7(3), 342–346. DOI 10.3198/jpr2013.01.0006crg. [Google Scholar] [CrossRef]
69. Pontieri, P., Mamone, G., de Caro, S., Tuinstra, M. R., Roemer, E. et al. (2013). Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analyses. Journal of Agricultural and Food Chemistry, 61(10), 2565–2571. DOI 10.1021/jf304882k. [Google Scholar] [CrossRef]
70. Mathur, S., Umakanth, A. V., Tonapi, V. A., Sharma, R., Sharma, M. K. (2017). Sweet sorghum as biofuel feedstock: recent advances and available resources. Biotechnology for Biofuels, 10(1), 1–19. DOI 10.1186/s13068-017-0834-9. [Google Scholar] [CrossRef]
71. Codesido, V., Vacas, R., Macarulla, B., Gracia, M. P., Igartua, E. (2013). Agronomic and digital phenotyping evaluation of sweet sorghum public varieties and F1 hybrids with potential for ethanol production in Spain. Maydica, 58, 42–53. [Google Scholar]
72. Whitfield, M. B., Chinn, M. S., Veal, M. W. (2013). Processing of materials derived from sweet sorghum for biobased products. Industrial Crops and Products, 37(1), 362–75. DOI 10.1016/j.indcrop.2011.12.011. [Google Scholar] [CrossRef]
73. Kawahigashi, H., Kasuga, S., Okuizumi, H., Hiradate, S., Yonemaru, J. I. (2013). Evaluation of brix and sugar content in stem juice from sorghum varieties. Grassland Science, 59(1), 11–9. DOI 10.1111/grs.12006. [Google Scholar] [CrossRef]
74. Regassa, T. H., Wortmann, C. S. (2014). Sweet sorghum as a bioenergy crop: Literature review. Biomass and Bioenergy, 64, 348–355. DOI 10.1016/j.biombioe.2014.03.052. [Google Scholar] [CrossRef]
75. Billings, M. (2015). Biomass sorghum and sweet sorghum data gathering report. WA Crop Insurance. USDA-RMA, CTOR: Jaime Padget, Missouri Watts and Associates Inc. [Google Scholar]
76. Reddy, B. V. S., Kumar, A. A., Ramesh, S. (2007). Sweet sorghum: A water saving bio-energy crop. International Conference on Linkages between Energy and Water Management for Agriculture in Developing Countries, pp. 29–30. Patancheru, India. [Google Scholar]
77. Rutto, L. K., Xu, Y., Brandt, M., Ren, S., Kering, M. K. (2013). Juice, ethanol, and grain yield potential of five sweet sorghum (Sorghum bicolor [L.] moench) cultivars. Journal of Sustainable Bioenergy Systems, 3(2), 113–118. DOI 10.4236/jsbs.2013.32016. [Google Scholar] [CrossRef]
78. Rao, P. S., Kumar, C. G., Reddy, B. V. S. (2012). Sweet sorghum: From theory to practice. In: Rao, P. S., Kumar, C.G. (Eds.Characterization of improved sweet sorghum cultivars, pp. 1–15. Springer, Berlin. DOI 10.1007/978-81-322-0783-2_1. [Google Scholar] [CrossRef]
79. Lee, S., Park, Y., Zuidema, M. Y., Hannink, M., Zhang, C. (2011). Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World Journal of Cardiology, 3(1), 18–24. DOI 10.4330/wjc.v3.i1.18. [Google Scholar] [CrossRef]
80. El-Beltagi, H. S., Mohamed, H. I., Safwat, G., Megahed, B. M. H., Gamal, M. (2018). Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of Beta vulgaris L. root. Fresenius Environmental Bulletin, 27(9), 6369–637881. [Google Scholar]
81. El-Beltagi, H. S., Mohamed, H. I., Abdelazeem, A. S., Youssef, R., Safwat, G. (2019a). GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 47(2), 493–505. DOI 10.15835/nbha47211405. [Google Scholar] [CrossRef]
82. El-Beltagi, H. S., Mohamed, H. I., Elmelegy, A. A., Eldesoky, S. E., Safwat, G. (2019b). Phytochemical screening, antimicrobial, antioxidant, anticancer activities and nutritional values of cactus (Opuntia Ficus Indicia) pulp and peel. Fresenius Environmental Bulletin, 28(2A), 1534–1551. [Google Scholar]
83. El-Beltagi, H. S., Mohamed, H. I., Safwat, G., Gamal, M., Megahed, B. M. H. (2019c). Chemical composition and biological activity of Physalis peruviana L. Gesunde Pflanzen, 71, 113–122. DOI 10.1007/s10343-019-00456-8. [Google Scholar] [CrossRef]
84. Hamed, M. M., Abd El-Mobdy, M. A., Kamel, M. T., Mohamed, H. I., Bayoumi, A. E. (2019). Phytochemical and biological activities of two asteraceae plants Senecio vulgaris and Pluchea dioscoridis L. Pharmacology Online, 2, 101–121. [Google Scholar]
85. Moustafa-Farag, M., Mohamed, H. I., Mahmoud, A., Elkelish, A., Misra, A. N. et al. (2020). Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants, 9, 724, DOI 10.3390/plants9060724. [Google Scholar] [CrossRef]
86. Nagy, R., Szőllősi, E., Molnár, P. B., Murányi, E., Czimbalmos, R. et al. (2021). Condensed tannin content and antioxidant activity of Hungarian sorghum varieties grown at research institute in Karcag. Acta Agraria Debreceniensis, (1), 155–160. DOI 10.34101/actaagrar/1/8467. [Google Scholar] [CrossRef]
87. Wu, G., Shen, Y., Qi, Y., Zhang, H., Wang, L. et al. (2018). Improvement of in vitro and cellular antioxidant properties of Chinese steamed bread through sorghum addition. LWT—Food Science and Technology, 91, 77–83. DOI 10.1016/j.lwt.2017.12.074. [Google Scholar] [CrossRef]
88. Xiong, Y., Teixeira, T. V. D., Zhang, P., Warner, R. D., Shen, S. et al. (2021). Cellular antioxidant activities of phenolic extracts from five sorghum grain genotypes. Food Bioscience, 41, 101068. DOI 10.1016/j.fbio.2021.101068. [Google Scholar] [CrossRef]
89. Hong, S., Pangloli, P., Perumal, R., Cox, S., Noronha, L. E. et al. (2020). A comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolysaccharide-induced RAW 264.7 macrophages. Antioxidants, 9(12), 1297. DOI 10.3390/antiox9121297. [Google Scholar] [CrossRef]
90. Moraes, É. A., Natal, D. I. G., Queiroz, V. A. V., Schaffert, R. E., Cecon, P. R. et al. (2012). Sorghum genotype may reduce low-grade inflammatory response and oxidative stress and maintains jejunum morphology of rats fed a hyperlipidic diet. Food Research International, 49, 553–559. DOI 10.1016/j.foodres.2012.07.029. [Google Scholar] [CrossRef]
91. Shim, T. J., Kim, T. M., Jang, K. C., Ko, J. Y., Kim, D. J. (2013). Toxicological evaluation and anti-inflammatory activity of a golden gelatinous sorghum bran extract. Bioscience, Biotechnology, and Biochemistry, 77(4), 697–705. DOI 10.1271/bbb.120731. [Google Scholar] [CrossRef]
92. Makanjuola, S. B. L., Ogundaini, A. O., Ajonuma, L. C., Dosunmu, A. (2018). Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-e2 blockade. International Journal of Rheumatic Diseases, 21(8), 1487–1495. DOI 10.1111/1756-185X.13355. [Google Scholar] [CrossRef]
93. Agah, S., Kim, H., Mertens-Talcott, S. U., Awika, J. M. (2017). Complementary cereals and legumes for health: Synergistic interaction of sorghum flavones and cowpea flavonols against LPS-induced inflammation in colonic myofibroblasts. Molecular Nutrition Food Research, 61(7). DOI 10.1002/mnfr.201600625. [Google Scholar] [CrossRef]
94. Ravisankar, S., Agah, S., Kim, H., Talcott, S., Wu, C. et al. (2019). Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chemistry, 279, 88–97. DOI 10.1016/j.foodchem.2018.12.006. [Google Scholar] [CrossRef]
95. Stefoska-Needham, A., Beck, E. J., Johnson, S. K., Batterham, M. J., Grant, R. et al. (2017). A diet enriched with red sorghum flaked biscuits, compared to a diet containing white wheat flaked biscuits, does not enhance the effectiveness of an energy-restricted meal plan in overweight and mildly obese adults. Journal of the American College of Nutrition, 36(3), 184–192. DOI 10.1080/07315724.2016.1237314. [Google Scholar] [CrossRef]
96. Mohamed, H. I., Elsherbiny, E. A., Abdelhamid, M. T. (2016). Physiological and biochemical responses of Vicia faba plants to foliar application with zinc and iron. Gesunde Pflanzen, 68, 201–212. DOI 10.1007/s10343-016-0378-0. [Google Scholar] [CrossRef]
97. Isaacson, C. (2005). The change of the staple diet of black South Africans from sorghum to maize (CORN) is the cause of the epidemic of squamous carcinoma of the oesophagus. Medical Hypotheses, 64(3), 658–660. DOI 10.1016/j.mehy.2004.09.019. [Google Scholar] [CrossRef]
98. Awika, J. M., Yang, L., Browning, J. D., Faraj, A. (2009). Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT—Food Science and Technology, 42(6), 1041–1046. DOI 10.1016/j.lwt.2009.02.003. [Google Scholar] [CrossRef]
99. Massey, A. R., Reddivari, L., Vanamala, J. (2014). The dermal layer of sweet sorghum (Sorghum bicolor) stalk, a byproduct of biofuel production and source of unique 3-deoxyanthocyanidins, has more antiproliferative and proapoptotic activity than the pith in p53 variants of HCT116 and colon cancer stem cells. Journal of Agricultural and Food Chemistry, 62(14), 3150–3159. DOI 10.1021/jf405415u. [Google Scholar] [CrossRef]
100. Shih, C. H., Siu, S. O., Ng, R., Wong, E., Chiu, L. C. et al. (2007). Quantitative analysis of anticancer 3-deoxyanthocyanidins in infected sorghum seedlings. Journal of Agricultural and Food Chemistry, 55(2), 254–259. DOI 10.1021/jf062516t. [Google Scholar] [CrossRef]
101. Hargrove, J. L., Greenspan, P., Hartle, D. K., Dowd, C. (2011). Inhibition of aromatase and α-amylase by flavonoids and proanthocyanidins from Sorghum bicolor bran extracts. Journal of Medicinal Food, 14(7–8), 799–807. DOI 10.1089/jmf.2010.0143. [Google Scholar] [CrossRef]
102. Awika, J. M. (2011). Sorghum flavonoids: Unusual compounds with promising implications for health. In: Awika, J. M., Piironen, V., Bean, S. (Eds.Advances in cereal science: Implications to food processing and health promotion, pp. 171–200. Washington DC, American Chemical Society. [Google Scholar]
103. Ezeogu, L. I., Duodu, K. G., Emmambux, M. N., Taylor, J. R. N. (2008). Influence of cooking conditions on the protein matrix of sorghum and maize endosperm flours. Cereal Chemistry, 85(3), 397–402. DOI 10.1094/CCHEM-85-3-0397. [Google Scholar] [CrossRef]
104. Amoako, D. B., Awika, J. M. (2019). Resistant starch formation through intrahelical V-complexes between polymeric proanthocyanidins and amylose. Food Chemistry, 285, 326–333. DOI 10.1016/j.foodchem.2019.01.173. [Google Scholar] [CrossRef]
105. Stefoska-Needham, A., Beck, E. J., Johnson, S. K., Chu, J., Tapsell, L. C. (2016). Flaked sorghum biscuits increase postprandial GLP-1 and GIP levels and extend subjective satiety in healthy subjects. Molecular Nutrition Food Research, 60(5), 1118–1128. DOI 10.1002/mnfr.201500672. [Google Scholar] [CrossRef]
106. Kim, J. S., Hyun, T. K., Kim, M. J. (2011). The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chemistry, 124(4), 1647–1651. DOI 10.1016/j.foodchem.2010.08.020. [Google Scholar] [CrossRef]
107. Kim, J., Park, Y. (2012). Anti-diabetic effect of sorghum extract on hepatic gluconeogenesis of streptozotocin-induced diabetic rats. Nutrition Metabolism, 9(1), 106. DOI 10.1186/1743-7075-9-106. [Google Scholar] [CrossRef]
108. Poquette, N. M., Gu, X., Lee, S. O. (2014). Grain sorghum muffin reduces glucose and insulin responses in men. Food Function, 5(5), 894–899. DOI 10.1039/C3FO60432B. [Google Scholar] [CrossRef]
109. Links, M. R., Taylor, J., Kruger, M. C., Taylor, J. R. N. (2015). Sorghum condensed tannins encapsulated in kafirin microparticles as a nutraceutical for inhibition of amylases during digestion to attenuate hyperglycaemia. Journal of Functional Foods, 12, 55–63. DOI 10.1016/j.jff.2014.11.003. [Google Scholar] [CrossRef]
110. Anunciação, P. C., de Morais Cardoso, L., Alfenas, R. D. C. G., Queiroz, V. A. V., Carvalho, C. W. P. et al. (2019). Extruded sorghum consumption associated with a caloric restricted diet reduces body fat in overweight men: A randomized controlled trial. Food Research International, 119, 693–700. DOI 10.1016/j.foodres.2018.10.048. [Google Scholar] [CrossRef]
111. Mukai, Y., Kataoka, S., Sato, S. (2020). Sorghum (Sorghum bicolor) extract affects plasma lipid metabolism and hepatic macrophage infiltration in diabetic rats. Current Nutrition Food Science, 16(5), 824–832. DOI 10.2174/1573401315666190114153933. [Google Scholar] [CrossRef]
112. Hoi, J. T., Weller, C. L., Schlegel, V. L., Cuppett, S. L., Lee, J. Y. et al. (2009). Sorghum distillers dried grain lipid extract increases cholesterol excretion and decreases plasma and liver cholesterol concentration in hamsters. Journal of Functional Foods, 1(4), 381–386. DOI 10.1016/j.jff.2009.09.005. [Google Scholar] [CrossRef]
113. Maunder, B., (2002). Sorghum–The global grain of the future. http://www.sorghumgrowers.com/maunder.htm. [Google Scholar]
114. Dowling, L. F., Arndt, C., Hamaker, B. R. (2002). Economic viability of high digestibility sorghum as feed for market broilers. Agronomy Journal, 94, 1050–1058. DOI 10.2134/agronj2002.1050. [Google Scholar] [CrossRef]
115. Adamu, A. M., Alhassan, W. S. (1993). Improving the nutritive value of sorghum stover by treatment with organic ash, urea and poultry waste. Proceedings 18th Annual Conference Nigerian Society Animal Production, pp. 92. Federal University of TechnologyOwerri. [Google Scholar]
116. Sofy, A. R., Sofy, M. R., Hmed, A. A., Dawoud, R. A., Refaey, E. E. et al. (2021). Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and the control of its deleterious effects with melatonin and salicylic acid. Plants, 28, 10(3), 459. DOI 10.3390/plants10030459. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |