

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.019728
ARTICLE
Effects of Chemical Products on Fire Blight Suppression, and Fruit Production and Quality in Apple (Malus domestica Borkh.) cv. Golden Glory
1Facultad de Ciencias Agrotecnológicas, Universidad Autónoma de Chihuahua, Pascual Orozco, Chihuahua, 31350, México
2Centro de Investigación y Desarrollo A.C., Delicias, Chihuahua, 33089, México
*Corresponding Author: Loreto Robles-Hernández. Email: lrobles@uach.mx
Received: 11 October 2021; Accepted: 20 December 2021
Abstract: Antibiotics are widely used in fire blight management programs, yet there are no studies that demonstrate the evaluation of their efficacy in Mexico. Therefore, the present study was conducted to investigate the effects of the active ingredients in five commercial products (Kasumin® 2L, Agrygent Plus®, Agricultural Terramycin®, Agrimicin® 100, and Actigard®) on fire blight suppression, and fruit yield and quality of apple (Malus domestica Borkh.) cv. Golden Glory. The experiment was conducted in a commercial orchard using a completely randomized block design, with six treatments: (1) Oxytetracycline [Ox], 110 mg L−1; (2) Kasugamycin [Kas], 4.7 mL L−1; (3) Oxytetracycline + Gentamicin [Ox + Gen], 48 mg L−1 + 12 mg L−1; (4) Streptomycin + Oxytetracycline [Str + Ox], 90 mg L−1 + 9 mg L−1; (5) Acibenzolar-S-methyl [ASM], 70 mg L−1; and (6) Control, only water, with four replications, and three 11-year-old trees as an experimental unit. Variables of infection including flowers, shoots and fruits, yield and fruit quality were evaluated. All treatments suppressed infection in flowers, shoots, and fruits. ASM provided the highest levels of reduction of flower and shoot infection, while Kas had the least effect on the reduction of infection in these variables. The Ox + Gen treatment had the greatest suppression of fruit infection, and the best results on fruit yield and quality, followed by Ox and ASM. This is the first study conducted to evaluate the efficacy of the active ingredients of five commercial products used for the management of fire blight in apple trees in Mexico.
Keywords: Erwinia amylovora; oxytetracycline; gentamicin; kasugamycin; streptomycin; Acibenzolar-S-methyl
Apple production has been increasing over recent years. Worldwide, more than 89 million tons are produced. China is the largest apple producer in the world with a production volume over 44 million tons [1]. In Mexico, apple production during 2019 was 714,203 tons, with a production value greater than 6,500 million pesos. The state of Chihuahua is one of the primary apple producers with 594,710 tons and a production value above 5,500 million pesos, equivalent to 84.6% of national production value. The municipality of Cuauhtémoc stands out among the primary producing municipalities, where this research was performed [2]. However, the productivity of this crop is affected by various diseases, including fire blight, caused by Erwinia amylovora (Burril), one of the most important diseases.
Erwinia amylovora is a facultative anaerobic gram-negative bacterium, with a doubling time of 20 min, and has peritrichous flagella. The growth and development of the bacterium is favored by the presence of high relative humidity, temperatures above 15.5°C, heavy soils with acidic pH, susceptibility of varieties and rootstocks, nutritional imbalances, and the presence of insect disease vectors. The symptomatology of fire blight in flower clusters shows a rapid brown wilting and, in the shoots and twigs, this wilting occurs in a descending direction, forming a “shepherd’s crook”-like structure. Blighted fruits turn brown to black and become covered with droplets of whitish tan-colored abundant bacterial ooze in the presence of high relative humidity (70%) and temperatures above 26°C [3,4].
Fire blight causes high economic losses, especially in apple orchards in many fruit-growing areas worldwide. In 1998, Greece reported the destruction of 50% of infected trees over a period of 8 years; in 1991, the USA recorded losses of USD $3.8 million; in 1997, Italy lost 500,000 trees; in 2000, Switzerland lost € 4.11 million [5]. During 2004, in Chihuahua, Mexico, losses of up to 30% of apple production occurred; an investment of 22% of production costs was estimated for fire blight management [6]. Fire blight is also present in most Central and Northern European countries, in the Mediterranean region [7] and in the Middle East [8].
In countries where fire blight occurs, several methods for its management have been reported [9], with antibiotics being the most used [10]. Effective control of fire blight can be achieved by bactericide sprays prior to infection, or upon arrival of the pathogen to the flowers. However, these products are inefficient when E. amylovora enters its endophytic phase [11]. The inappropriate use of antibiotics has caused resistance to appear in E. amylovora, limiting control of fire blight [12]. Moreover, in Mexico there are no studies focused on the evaluation of the effectiveness of commercial products based on antibiotics and non-antibiotics on the suppression of fire blight, including Kasumin® 2L (I.A., Kasugamycin) and the non-antibiotic Actigard® (A.I., Acibenzolar-S-methyl), which were recently included in the management of this disease. Therefore, the present study was carried out to investigate the effects of the active ingredients of five commercial products (Kasumin® 2L, Agrygent Plus®, Agricultural Terramycin®, Agrimicin® 100 and Actigard®) on the suppression of fire blight on tree fruit yield and quality of apple (Malus domestica Borkh.) cv. Golden Glory.
The experiment site was located in the municipality of Cuauhtémoc, Chihuahua, Mexico (28°26’17” N and 106°55’15.20” W; 2,063 m.a.s.l.) [13]. The research was carried out during the 2019 growing season on 11-year-old apple trees (Malus domestica Borkh.) cv. Golden Glory, grafted on a rootstock franco, with a planting density of 833 trees per hectare in the orchard “FM Hermanos” (40 ha). This orchard has a history record of fire blight for at least five consecutive years prior to this study. Thus, infections of blossoms, fruits and young shoots under natural conditions relied on the endogenous pathogen spread within the orchard.
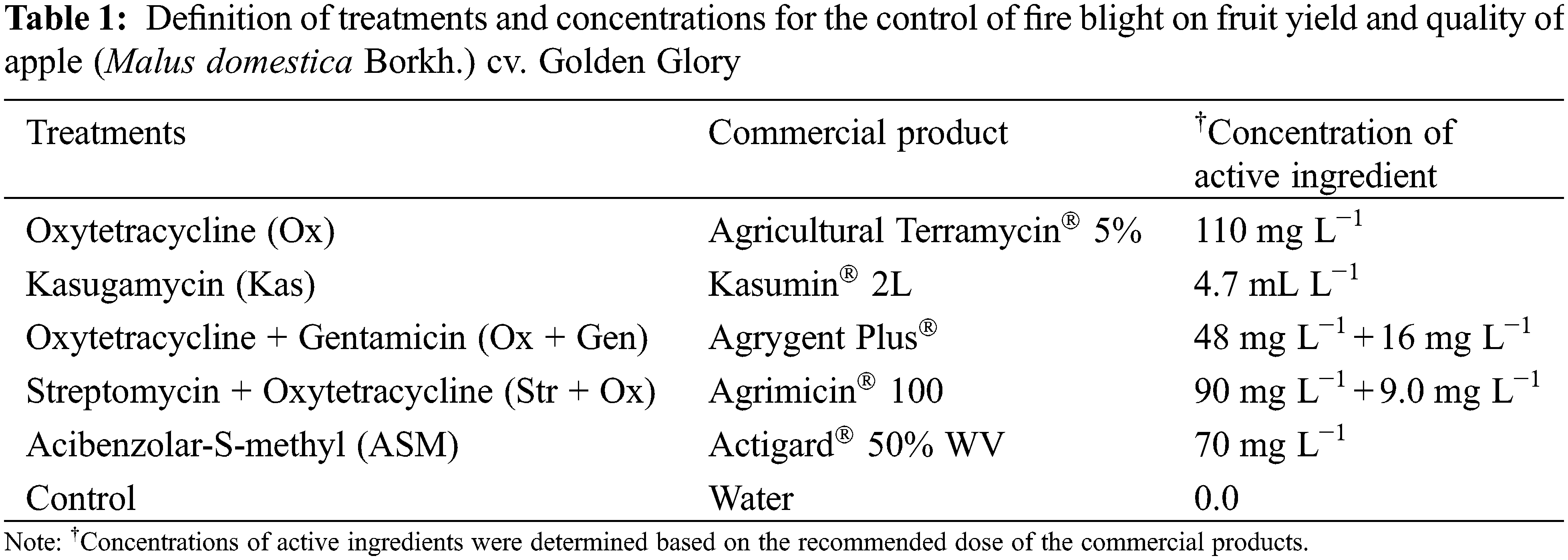
A completely randomized block design was used for field trials, with six treatments, four replications, and three 11-year-old trees per experimental unit. Each tree was marked, by designating two branches for measurement from the East and West directions. All blocks were distributed over almost half of the orchard surface to cover more microclimate conditions. Table 1 presents the definition of treatments and concentrations. All concentrations of active ingredients were calculated according to the recommended dose of the commercial products.
The spraying of treatments was performed according to the results of floral infection and the risk levels provided by the Maryblight and Cougarblight prediction models. Floral infection was determined daily throughout the flowering period according to the stigma printing method proposed by Thomson et al. [14]. Prior to stigma printing, a four-hectare lot was marked within the commercial orchard, and floral phenology was monitored (Fig. 1) to determine the optimal time to start sampling. Using this information, floral infection measurements began on April 03 and ended on April 30. Inside the orchard, an agroclimatic station programmed with the Maryblight and Cougarblight risk models was installed, which have been widely used to predict the risk of fire blight appearance in apple and pear trees [15]. The applications were made when the floral infection levels were equal to or greater than 5% and the Maryblight and Cougarblight models reported high levels of disease risk; these conditions matched on April 08 and 14, 2019.
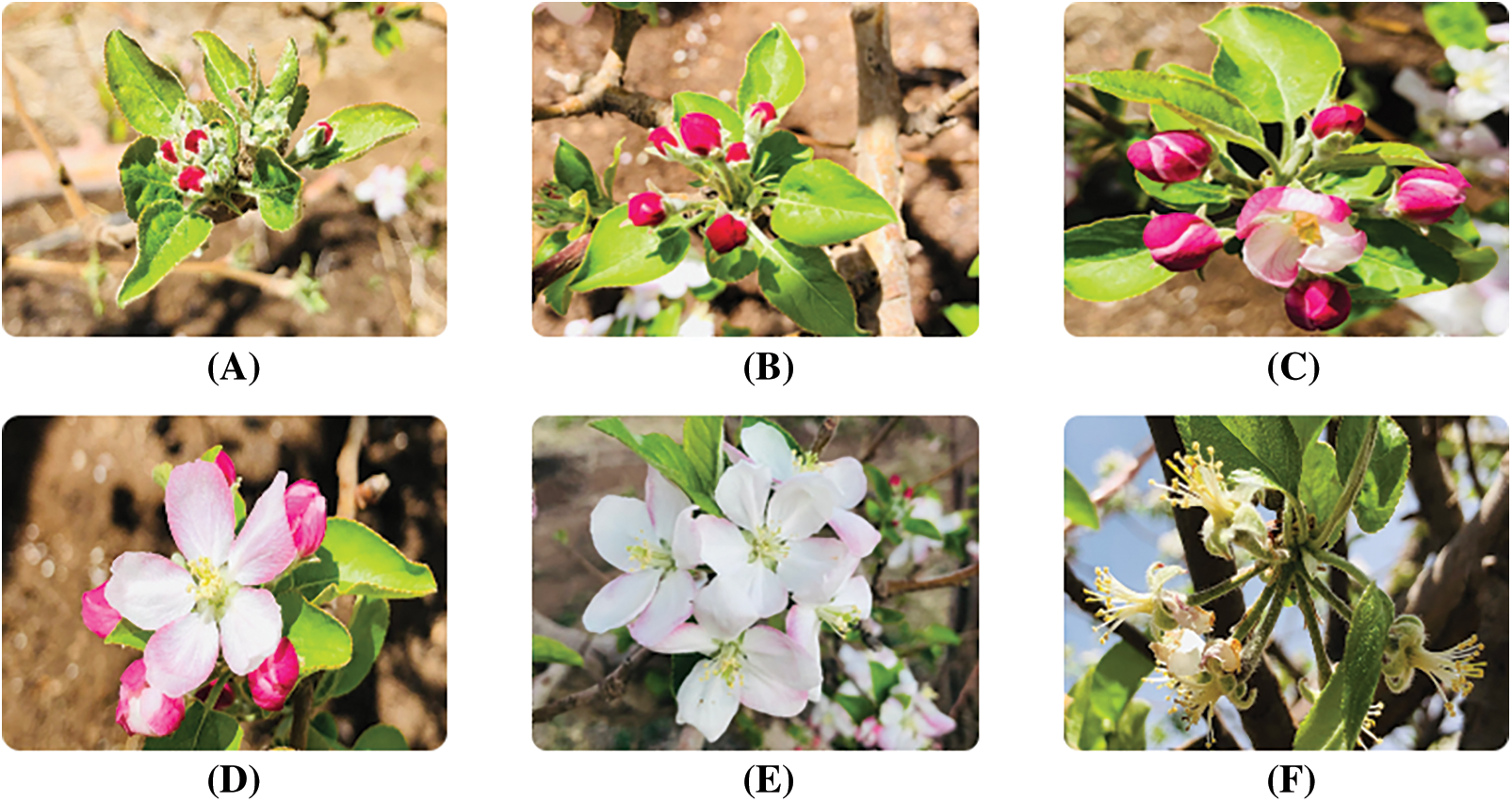
Figure 1: Apple tree flowering phenology. Closed bunch (A), pink tip (B), pink button (C), open king flower (D), full bloom (E) and petal drop (F). Elaboration with own images
2.4 Measurement of Infection in Flowers, Shoots, and Fruits
The percentage of infected flowers was determined using the stigma printing method [14], with a Cycloheximide-Crystal Violet Tergitol (CCT) medium [16,17], which has a good level of selectivity. The medium contains sucrose and sorbitol as carbon sources, as well as anionic tergitol, thallium nitrate, cycloheximide and crystal violet as inhibitors of other bacteria and fungi. The monitoring of flower infection was carried out five days after each treatment application (April 08 and 14). A total of 108 flowers were sampled per treatment (9 flowers per CCT plate), which were emasculated to perform the stigma printing. The CCT plates were transferred to the laboratory at 4°C and incubated for 24 h at 28 ± 1°C. After incubation, the plates were analyzed using a stereoscope (Nikon brand, model SMZ745T). Morphological identification of E. amylovora was performed in the stigma printing spaces for each flower. The print spaces for the presence of E. amylovora were counted and the infection percentage was determined based on the total number of flowers sampled by treatment and repetition according to Manulis et al. [18]. Fruit and shoot infection were determined 35 days after the last treatment application. The evaluation was performed on each treatment repetition. The number of infected shoots and fruits was counted, and the infection percentage was determined based on the total number of shoots and fruits in each treatment replication.
According to Melvin [19], yield per hectare estimates the efficiency of both bearing surface per tree and land surface. In our study, yield from each treatment replication was calculated as follows: three 11-year-old trees were used as experimental units in each treatment replication (four replications). Each tree was marked, by designating two branches for measurement in the East and West directions. The average number of fruits, and the average fruit weight were obtained from each branch. The average of branches in the three trees was also calculated. The yield per tree was calculated as follows:
where,
TY = tree yield (kg)
ANFB = average number of fruits per branch
AFWB = average fruit weight per branch
ANBT = average number of branches per tree
Yield in tons ha−1 was calculated as follows:
where,
Y = yield in tons ha−1
TD = tree density ha−1
At the end of the growing cycle, fruit samples were collected from each treatment repetition. Six representative fruits were collected. A total of 864 fruits were evaluated for the determination of the fruit quality parameters following Musacchi et al. [20], with modifications. Color was determined using the color scale: 1) green; 2) slightly green; 3) transition between green to yellow; 4) yellow. This variable was expressed in percentage units. Diameter was measured with a vernier (Truper brand, Stainless Steel) in the equatorial zone of the fruit and was expressed in cm. Firmness was measured with a penetrometer (Effe-Gi brand, model 327) from 0 to 28 lb in2, using a punch diameter of 11.3 mm, taking two readings on opposite sides of the circumference. The total soluble solids content was read with a refractometer (Atago brand) from 0 to 32 °Brix. Malic acid content (%) was determined by titration, where ten mL of juice were collected, 3 drops of phenolphthalein indicator were added, and then titrated with a 0.1 N NaOH solution. The malic acid content was calculated as follows [20]:
where,
N = Normality of NaOH
V = Volume (mL) of NaOH used
Meg = Milliequivalent gram (0.067) of malic acid
v = Sample volume (10 mL)
The sugar-acidity ratio parameter was obtained by dividing the total soluble solids content by the malic acid percentage.
Statistical data analysis was conducted using the InfoStat Software package [21], performing analyses of variance (ANOVAs) and comparisons of means using Fisher’s Least Significant Difference (LSD) test with a significance level of α = 0.05, n = 4.
Significant differences were observed among treatments (P < 0.05). The ASM treatment significantly (P< 0.05) prevented floral infection. The treatments Ox, Ox + Gen and Str + Ox reduced floral infection by 60%. In contrast, Kas only inhibited floral infection by 5% (Fig. 2). DuPont [22] mentions that prior to antibiotic application, copper applications should be made during the pre-flowering stage to reduce bacterial inoculum concentration. The author also mentions that the best results for reducing the disease are obtained when antibiotics are administered 24 h before flowering. If infections occur during flowering, antibiotics should be applied as soon as possible within 24 h after the infections, making it necessary to repeat antibiotic applications for prolonged periods with short application intervals. However, in our research only two applications were made (April 08 and 14), according to the prediction models and the results of floral infection, dates on which a higher percentage of floral infection was recorded, as well as higher risk levels according to the Maryblight and Cougarblight models. Smith et al. [23] and Pusey et al. [24] reported that these models project risk for the next three days, based on temperature, relative humidity, and the predicted bacterial population. Most antibiotics only protect open flowers to defend new flowers as they open in warm weather. New applications must be made within a few days of the risk period recorded by the models or shortly before rain or heavy dew occurs. Adaskaveg et al. [25] also reported that Kasugamycin and Streptomycin can be applied up to 12 h after a rain or presence of strong mists in the orchard, but their efficacy is reduced, since both antibiotics only have activity against bacteria that are outside the floral cup. ASM is a product that mimics pathogen-host interaction capable of inducing Systemic Acquired Resistance (SAR) in plants, allowing control of various plant diseases by inducing the synthesis of PR proteins, which give the plant greater resistance to microbial infections [26]. Maxson et al. [27] reported a significant reduction of fire blight in apple trees with Acibenzolar-S-methyl at concentrations of 150 and 300 mg L−1, while in this study ASM prevented floral infection with a concentration of 75 mg L−1.
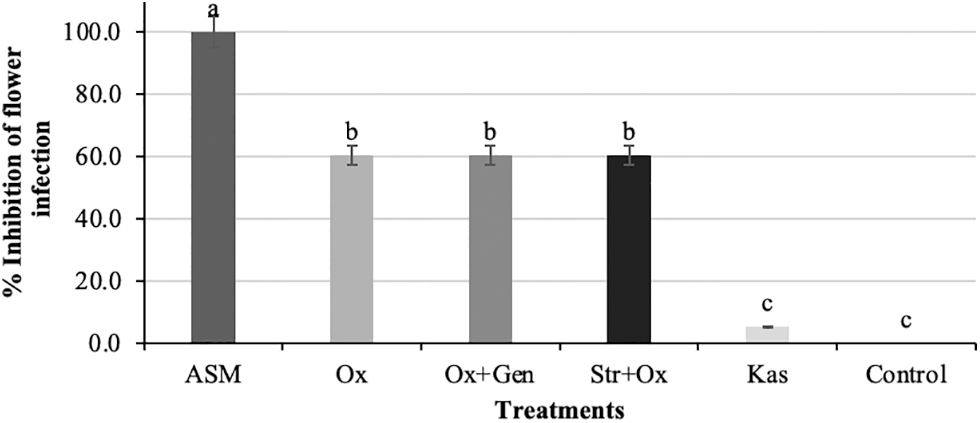
Figure 2: Percentage inhibition of flower infection in apple trees (Malus domestica Borkh.) cv. Golden Glory 6 days after each application. Means with the same letter on the shaded bars are statistically similar (P > 0.05) according to the LSD fisher test. ASM = Acibenzolar-S-methyl, Ox = Oxytetracycline, Ox + Gen = Oxytetracycline + Gentamicin, Str + Ox = Streptomycin + Oxytetracycline, Kas = Kasugamycin
The ASM treatment showed the highest inhibition of shoot infection. The treatments Ox + Gen and Str + Ox resulted in a reduction of shoot infection by 78.4% and 74.4%, respectively. The treatments Ox and Kas only inhibited the infection by 50% and 45%, respectively (Fig. 3). Results from ASM were similar to those reported by Maxson et al. [27], who achieved a lower incidence of fire blight in Jonathan and Fuji apple cultivars treated with 75 and 150 mg L−1 of ASM. The action of ASM is related to its mechanism of action [28]. Kunz et al. [29] mention that ASM induces systemic acquired resistance in the plant and provides better control of the disease. Bastas [30] remarks that ASM is a broad spectrum and long-lasting product, providing greater plant protection against pathogen attack, but it must be applied a few weeks before infections occur to allow the plant to activate its defense mechanisms in advance. The results of shoot infection inhibition shown by the combination of Ox + Gen and Str + Ox were similar to those reported by Palacio et al. [31], who mention that when antibiotics are applied in mixtures, they have a synergistic effect on the suppression of E. amylovora, inhibiting its multiplication by up to 50% in pear trees, apple trees and ornamental plants. The results obtained by Kas were different from those described by Adaskaveg et al. [25], who, in a similar study, reported that Kasugamycin reduced the disease by up to 95%.
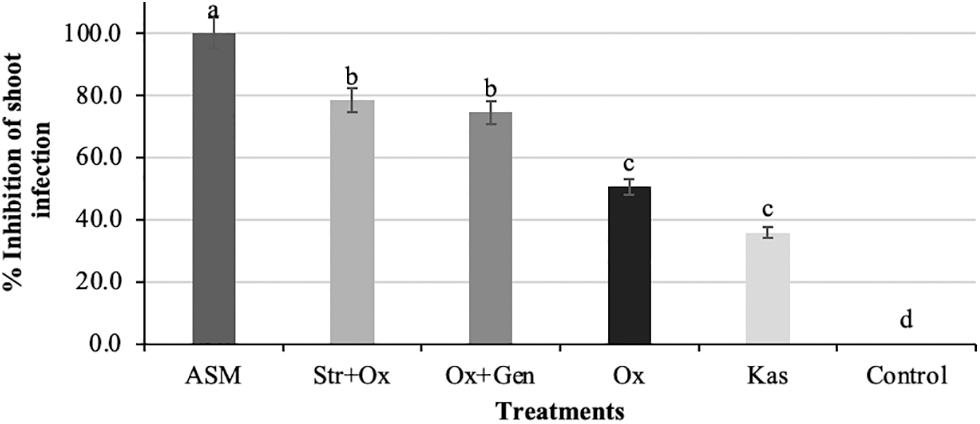
Figure 3: Percentage inhibition of shoot infection in apple trees (Malus domestica Borkh.) cv. Golden Glory 35 days after the last application. Means with the same letter on the shaded bars are statistically similar (P > 0.05) according to the LSD Fisher test. ASM = Acibenzolar-S-methyl, Ox = Oxytetracycline, Ox + Gen = Oxytetracycline + Gentamicin, Str + Ox = Streptomycin + Oxytetracycline, Kas = Kasugamycin
The Ox + Gen treatment had the highest (P < 0.05) inhibition of fruit infection with a suppression of 82.4%, followed by Ox and Kas with levels of fruit infection suppression over 50%, while ASM and Str + Ox presented the lowest levels of suppression of fruit infection (Fig. 4). The results from Ox + Gen agree with those reported by McManus et al. [32], who mention that in field tests of the Agrygent Plus product, whose active ingredients are Oxytetracycline and Gentamicin, results showed a suppression of fire blight above 95%. This product is one of the most used in Mexico for fire blight management programs. The efficiency of the Kas treatment was different from that reported by Adaskaveg et al. [25], who in a comparative study on the management of fire blight in pear (Pyrus communis L.) cv. Bartlett, found that Kasugamycin reduced the disease by up to 95%, when doses of 3.5 to 18.3 mL L−1 were used. The variation in the efficiency of Kasugamycin in the suppression of E. amylovora is because this antibiotic does not penetrate the nectaries, the site of infection by the bacteria. Therefore, good suppression of the bacteria is not achieved once they penetrate these flower organs [33].
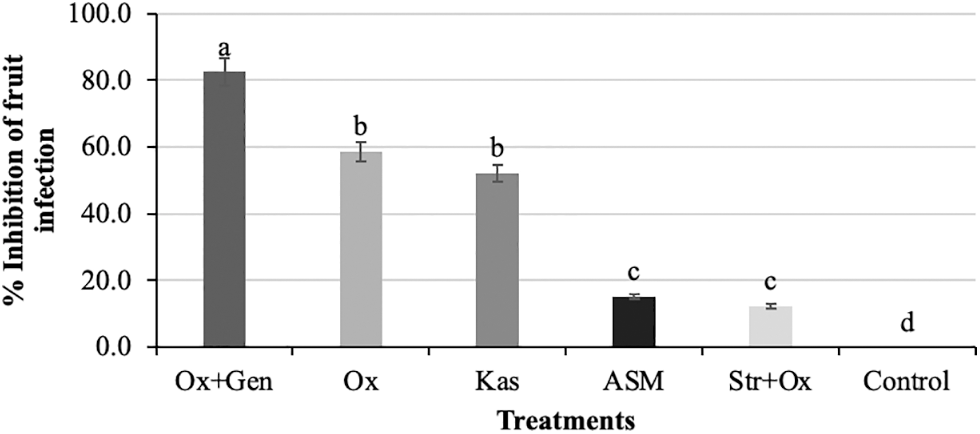
Figure 4: Percentage of inhibition of fruit infection in apple trees (Malus domestica Borkh.) cv. Golden Glory 35 days after the last application. Means with the same letter on the shaded bars are statistically similar (P > 0.05) according to the LSD fisher test. ASM = Acibenzolar-S-methyl, Ox = Oxytetracycline, Ox + Gen = Oxytetracycline + Gentamicin, Str + Ox = Streptomycin + Oxytetracycline, Kas = Kasugamycin
All treatments were significantly (P < 0.05) superior to the control, with a range in yield from 25.81 to 46.78 tons ha−1, averages higher than that reported in Mexico (12.59 tons ha−1), and that for the state of Chihuahua (18.71 tons ha−1) [2]. The Ox + Gen treatment presented the highest production values with an average of 46.78 tons ha−1, followed by the treatments Ox, ASM, Str + Ox, and Kas, which were statistically similar with values of 37.11, 34.37, 32.62, and 25.81 tons ha−1, respectively (Fig. 5). The results of Ox + Gen agree with those reported by Aldwinckle et al. [34] and Forster et al. [35], who observed a direct effect from the use of antibiotics on increases in crop yield. In our study, all treatments were superior to the control, which also had an important effect in the reduction of the disease, resulting in greater fruit set, fruit development, and higher production, as mentioned by Blazek et al. [36], who indicate that crop yield depends on disease management, since diseases significantly reduce crop production.
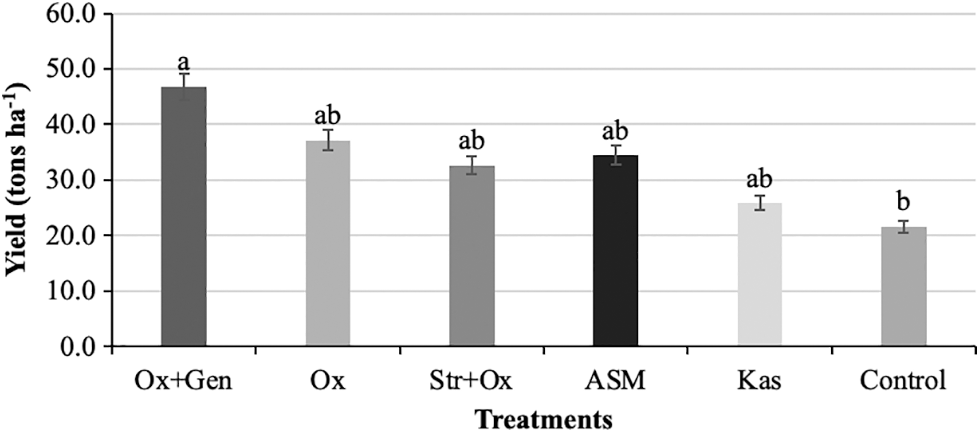
Figure 5: Effect of chemical treatments on yield (tons ha−1) in apple (Malus domestica Borkh.) cv. Golden Glory. Means with the same letter on the shaded bars are statistically similar (P > 0.05) according to the LSD fisher test. ASM = Acibenzolar-S-methyl, Ox = Oxytetracycline, Ox + Gen = Oxytetracycline + Gentamicin, Str + Ox = Streptomycin + Oxytetracycline, Kas = Kasugamycin
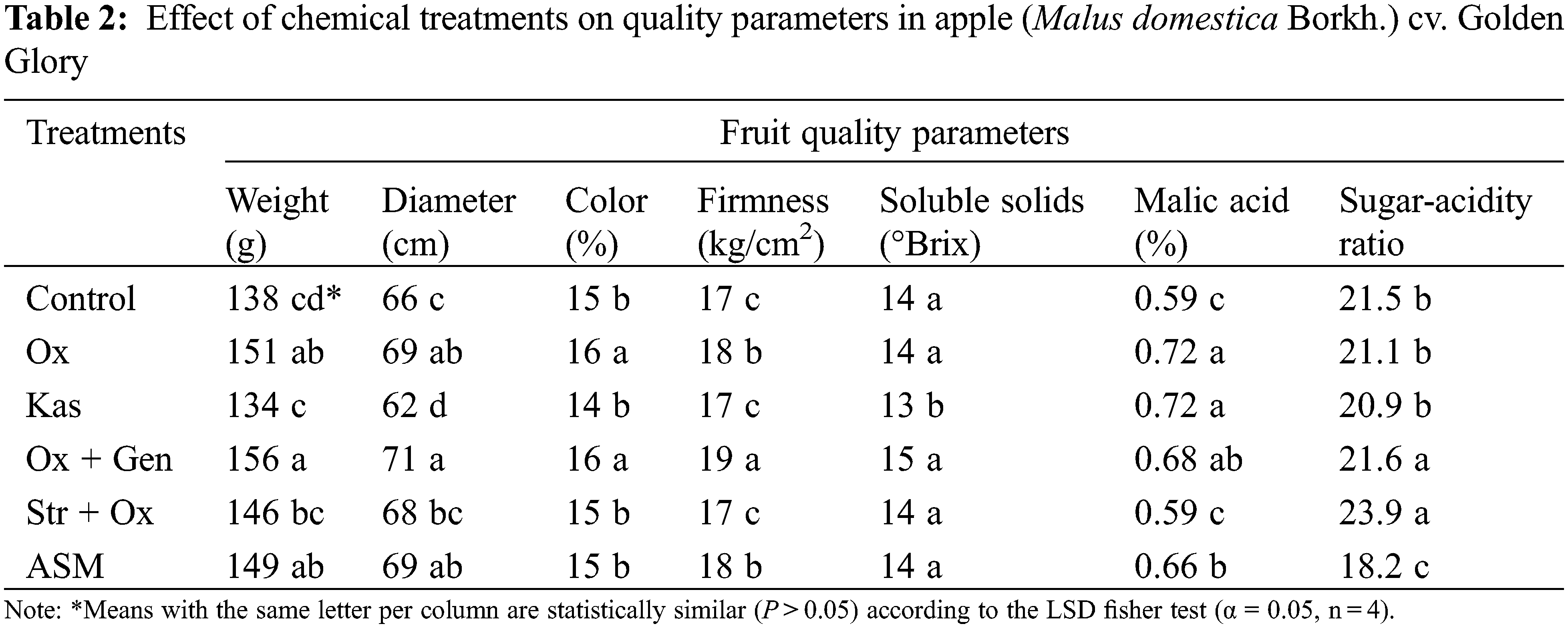
Significant differences (P < 0.05) among treatments were shown in all quality parameters. The Ox + Gen treatment was superior to all remaining treatments regarding diameter, weight, firmness, soluble solids, and sugar acidity ratio, followed by Ox and ASM. The treatment Ox produced higher color scores and higher malic acid content (Table 2). Values obtained with Ox + Gen, Ox and ASM coincide with those reported by González et al. [37]. Diameter, weight, firmness, soluble solids content and sugar acidity ratio are the main parameters determining apple fruit quality [38,39]. Values of soluble solids obtained in this study agreed with those reported by Mratinić et al. [40] who found values for this variable between 12.55 °Brix and 19.24 °Brix. It is worth mentioning that other studies that focused on biochemical characteristics had lower values for sugar content [41,42] than those reported in this study. Our results agree with those reported by Nischwitz et al. [43], who mention that the effect of Agrygent Plus (Ox + Gen) on fruit quality is due to its active ingredients Gentamicin Sulfate and Oxytetracycline. Thus, it is the current marketed product with the highest efficacy on fire blight suppression during flowering, favoring a better set, development, and quality of apple fruit. Both firmness and soluble solids content are very important quality parameters for the acceptance of the varieties by consumers [44,45].
All treatments suppressed fire blight infection in flowers, shoots, and fruits. ASM presented the best results in the suppression of fire blight in flowers and shoots, while Kas had the lowest values for all variables. Treatments based on Ox, Str and Gen alone or in combination, showed positive effects on inhibition of fruit infection, yield and fruit quality. The best results in the suppression of fruit infection, as well as in the production and quality of fruit were obtained using the Ox + Gen treatment. This is the first study that shows effects from the active ingredients of five of the commercial products with the greatest use in the management of fire blight on apple trees in Mexico. ASM could be an alternative to reducing the use of antibiotics, not only because of the suppression of fire blight on apple trees, but also because it does not induce resistance in the pathogen. However, more studies are needed to estimate the optimal application timing to allow the plant to activate its defense mechanisms before the disease begins. In addition, it would be better to conduct extensive field trials over several years to define the effectiveness of treatments under varying environmental conditions in the apple growing regions in Chihuahua, Mexico.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. FAO (2020). Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data. [Google Scholar]
2. SAGARPA (2020). Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Servicio de Información Agroalimentaria y Pesquera. Sistema de Información Agroalimentaria y Pesquera. https://nube.siap.gob.mx/cierreagricola/. [Google Scholar]
3. Eastgate, J. A. (2000). Erwinia amylovora: The molecular basis of fire blight disease. Molecular Plant Pathology, 1, 325–329. DOI 10.1046/j.1364-3703.2000.00044.x. [Google Scholar] [CrossRef]
4. Beer, S. V. (1997). Fire blight. In: Compendium of apple and pear diseases, pp. 61–63. St. Paul, Minnesota, USA: American Phytopathological Society Press. [Google Scholar]
5. EPPO (2013). List of pests recommended for regulation as quarantine pests. EPPO (version 2014-09). http://www.eppo.org/QUARANTINE/listA2.html. [Google Scholar]
6. Ramírez, M. R., Jacobo, J. L., Ávila, M. R., Parra, R. A. (2004). Efficiency of pesticide use in apple orchards [Malus sylvestris (L.) Mill. Var. domestica (Borkh.) Mansf. in Chihuahua, Mexico. Revista Mexicana de Fitopatología, 22(3), 403–413. [Google Scholar]
7. van der Zwet, T. (2000). Distribution and economic importance of fire blight. In: Vanneste, J. L. (Ed.Fire blight: The disease and its causative agents, pp. 37–54. Wallingford, UK: CABI Publishing. [Google Scholar]
8. van der Zwet, T. (2002). Present worldwide distribution of fire blight. Acta Horticulturae, 590, 33–34. DOI 10.17660/ActaHortic.2002.590.1. [Google Scholar] [CrossRef]
9. Steiner, P. W. (2000). Integrated orchard and nursery management for the control of fire blight. In: Vanneste, J. L. (Ed.Fire blight: The disease and its causative agents, pp. 339–354. Wallingford, UK: CABI Publishing. [Google Scholar]
10. Ordax, M., Marco, E., López, M. M., Biosca, E. G. (2006). Survival strategy of Erwinia amylovora against copper: Induction of the viable-but-nonculturable state. Applied and Environmental Microbiology, 72(5), 3482–3488. DOI 10.1128/AEM.72.5.3482-3488.2006. [Google Scholar] [CrossRef]
11. Aćimović, S. G., Zeng, Q., McGhee, G. C., Sundin, G. W., Wise, J. C. (2015). Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Frontiers in Plant Science, 6(16), 1–10. DOI 10.3389/fpls.2015.00016. [Google Scholar] [CrossRef]
12. McGhee, G. C., Sundin, G. W. (2011). Evaluation of kasugamycin for fire blight management, effect on nontarget bacteria, and assessment of kasugamycin resistance potential in Erwinia amylovora. Phytopathology, 101, 192–204. DOI 10.1094/PHYTO-04-10-0128. [Google Scholar] [CrossRef]
13. INEGI (2020). México-Directorio Estadístico Nacional de Unidades Económicas. https://www.inegi.org.mx/app/mapa/denue/default.aspx. [Google Scholar]
14. Thomson, S. V., Gouk, S. C. (2003). Influence of age of apple flowers on growth of Erwinia amylovora and biological control agents. Plant Disease, 87, 502–509. DOI 10.1094/PDIS.2003.87.5.502. [Google Scholar] [CrossRef]
15. Dewdney, M. M., Biggs, A. R., Turechek, W. W. (2007). A statistical comparison of the blossom blight forecasts of maryblight and cougarblight with receiver operating characteristic curve analysis. Phytopathology, 97(9), 1164–1176. DOI 10.1094/PHYTO-97-9-1164. [Google Scholar] [CrossRef]
16. Schaad, N. W., Jones, J. B., Chun, W. (2001). Laboratory guide for identification of plant pathogenic bacteria (3rd edition). St. Paul, Minnesota, USA: APS Press. [Google Scholar]
17. Ishimaru, C., Klos, E. J. (1984). New medium for detection of Erwinia amylovora and its use in epidemiological studies. Phytopathology, 74, 1342–1345. DOI 10.1094/Phyto-74-1342. [Google Scholar] [CrossRef]
18. Manulis, S., Kleitman, F., Dror, O., David, I., Zutra, D. (1998). Characterization of the Erwinia amylovora population in Israel. Phytoparasitica, 26, 39–46. [Google Scholar]
19. Melvin, N. W. (2009). Temperate-zone pomology: Physiology and culture. Portland, USA: Timber Press. [Google Scholar]
20. Musacchi, S., Serra, S. (2018). Apple fruit quality: Overview on pre-harvest factors. Scientia Horticulturae, 234, 409–430. DOI 10.1016/j.scienta.2017.12.057. [Google Scholar] [CrossRef]
21. di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M. et al. (2008). InfoStat, versión 2008, Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. [Google Scholar]
22. DuPont, T. (2019). Fire blight of apple and pear. http://s3-us-west-2.amazonaws.com/treefruit.wsu.edu/wp-content/uploads/2016/03/31090741/WSU-Fire-Blight-Factsheet-2019.04.01.pdf. [Google Scholar]
23. Smith, T. J., Pusey, P. L. (2011). CougarBlight 2010, a significant update of the CougarBlight fire blight infection risk mode. Acta Horticulturae, 896, 331–336. DOI 10.17660/ActaHortic.2011.896.45. [Google Scholar] [CrossRef]
24. Pusey, P. L., Curry, E. A. (2004). Temperature and pomaceous flower age related to colonization by Erwinia amylovora and antagonists. Phytopathology, 94(8), 901–911. DOI 10.1094/PHYTO.2004.94.8.901. [Google Scholar] [CrossRef]
25. Adaskaveg, J. E., Forster, H., Wade, M. (2011). Effectiveness of kasugamycin against Erwinia amylovora and its potential use for managing fire blight of pear. Plant Disease, 95(4), 448–454. DOI 10.1094/pdis-09-10-0679. [Google Scholar] [CrossRef]
26. Johnson, K. B., Temple, T. N. (2016). Comparison of methods of Acibenzolar-S-methyl application for post-infection fire blight suppression in pear and apple. Plant Disease, 100(6), 1125–1131. DOI 10.1094/PDIS-09-15-1062-RE. [Google Scholar] [CrossRef]
27. Maxson, S. K., He, S. Y., Hammerschmidt, R., Jones, A. L. (2002). Effect of treating apple trees with Acibenzolar-S-methyl on fire blight and expression of pathogenesis-related protein genes. Plant Disease, 86 (7), 785–790. DOI 10.1094/PDIS.2002.86.7.785. [Google Scholar] [CrossRef]
28. Brisset, M. N., Cesbron, S., Thomson, S. V., Paulin, J. P. (2000). Acibenzolar-S-methyl induces the accumulation of defense-related enzymes in apple and protects from fire blight. European Journal of Plant Pathology, 106, 529–536. DOI 10.1023/A:1008728119087. [Google Scholar] [CrossRef]
29. Kunz, W., Schurter, R., Maetzke, T. (1997). The chemistry of benzothiadiazole plant activators. Pesticide Sciences, 50, 275–282. DOI 10.1002/(SICI)1096-9063(199708)50:4<275::AID-PS593>3.0.CO;2-7. [Google Scholar] [CrossRef]
30. Bastas, K. K. (2011). An integrated management program for fire blight disease on apples. HortScience, 46(9), S129. [Google Scholar]
31. Palacio, A., Cambra, M., Milagros, M., Ordax, M. M., Peñalver, T. (2009). The fire blight of rosaceae (Erwinia amylovora). Madrid España: Ministerio de Medio Ambiente y Medio Rural y Marino. [Google Scholar]
32. McManus, P., Stockwell, V. (2000). Antibiotics for plant diseases control: Silver bullets or rusty sabers. USA: APSnet Features. DOI 10.1094/APSnetFeature-2000-0600. [Google Scholar] [CrossRef]
33. Nottingham, L., Kangiser, J., Amiri, A., Daniels, C. H., Steffen, G. R. et al. (2019). Crop protection guide for tree fruits in Washington. Pullman, Washington, USA: Washington State University Extension. EB0419. [Google Scholar]
34. Aldwinckle, H. S., Norelli, J. L. (1990). Evaluation of kasumin for control of fire blight. Acta Horticulturae, 273, 391–391. DOI 10.17660/ActaHortic.1990.273.58. [Google Scholar] [CrossRef]
35. Forster, H., McGhee, G. C., Sundin, G. W., Adaskaveg, J. E. (2015). Characterization of streptomycin resistance in isolates of Erwinia amylovora in California. Phytopathology, 105 (10), 1302–1310. DOI 10.1094/phyto-03-15-0078-r. [Google Scholar] [CrossRef]
36. Blazek, J., Paprštein, F. (1988). Genetical aspects of yields and their quality within tree fruit crops. Acta Horticulturae, 224, 437–440. DOI 10.17660/ActaHortic.1988.224.58. [Google Scholar] [CrossRef]
37. González, J., Yuri, J. A., Del Pozo, A. (2013). Relations among pigments, color and phenolic concentrations in the peel of two gala apple strains according to canopy position and light environment. Scientia Horticulturae, 151, 83–89. DOI 10.1016/j.scienta.2012.12.007. [Google Scholar] [CrossRef]
38. Seipel, M., Pirovani, M. E., Güemes, D. R., Gariglio, N. F., Piagentini, A. M. (2009). Physicochemical characteristics of the fruits of three varieties of apples grown in the central-eastern region of the Province of Santa Fe. Revista FAVE-Ciencias Agrarias, 8, 27–36. DOI 10.14409/fa.v8i1.1340. [Google Scholar] [CrossRef]
39. Feng, F., Li, M., Ma, F., Cheng, L. (2014). The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Scientia Horticulturae, 165, 123–131. DOI 10.1016/j.scienta.2013.11.008. [Google Scholar] [CrossRef]
40. Mratinić, E., Akšić, M. F. (2012). Phenotypic diversity of apple (Malus sp.) germplasm in south serbia. Brazilian Archives of Biology and Technology, 55, 349–358. DOI 10.1590/S1516-89132012000300004. [Google Scholar] [CrossRef]
41. Campeanu, G., Neata, G., Darjanschi, G. (2009). Chemical composition of the fruits of several apple cultivars growth as biological crop. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 37, 161–164. DOI 10.15835/nbha3723465. [Google Scholar] [CrossRef]
42. Minnocci, A., Lacopini, P., Martinelli, F., Sebastiani, L. (2010). Micromorphological, biochemical, and genetic characterization of two ancient, late-bearing apple varieties. European Journal of Horticultural Science, 75, 1–7. [Google Scholar]
43. Nischwitz, C., Dhiman, C. (2013). Streptomycin resistance of Erwinia amylovora isolated from apple (Malus domesticus) in Utah. Plant Health Progress, 14(1), 1. DOI 10.1094/PHP-2013-1025-01-RS. [Google Scholar] [CrossRef]
44. Feliciano, R. P., Antunes, C., Ramos, A., Serra, A. A. T., Figueira, M. E. et al. (2010). Characterization of traditional and exotic apple varieties from Portugal. Part 1–Nutritional, phytochemical and sensory evaluation. Journal of Functional Foods, 2, 35–45. DOI 10.1016/j.jff.2009.12.004. [Google Scholar] [CrossRef]
45. Jakopic, J., Slatnar, A., Stampar, F., Veberic, R., Simoncic, A. (2012). Analysis of selected primary metabolites and phenolic profile of ‘Golden Delicious’ apples from four production systems. Fruits, 67, 377–386. DOI 10.1051/fruits/2012032. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |