

 | Phyton-International Journal of Experimental Botany |  |
DOI: 10.32604/phyton.2022.020935
ARTICLE
Triterpenoids from Uncaria rhynchophylla and Their PTP1B Inhibitory Activity
1Guizhou University of Traditional Chinese Medicine, Guiyang, 550025, China
2State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming, 650201, China
*Corresponding Authors: Juan Zou. Email: zoujuan466@gzy.edu.cn; Chang-An Geng. Email: gengchangan@mail.kib.ac.cn
Received: 20 December 2021; Accepted: 18 January 2022
Abstract: Uncaria rhynchophylla (Gouteng) is a famous traditional Chinese medicine used for psychiatric and hypotensive purposes in China. In this study, the ethyl acetate (EtOAc) part of U. rhynchophylla was revealed with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. Subsequent investigation on the EtOAc part yielded one new triterpenoid, 3β-formyloxy-6β,19α-dihydroxyurs-12-en-28-oic acid (1) and four known ones, 3β,6β,19α-trihydroxyurs-12-en-28-oic acid (2), 2-oxopomolic acid (3), 3β,19α-dihydroxy-6-oxo-olean-12-en-28-oic acid (4) and sumaresinolic acid (5). The structure of compound 1 was determined by extensive HRESIMS, IR, 1D and 2D NMR spectroscopic analyses. Two ursane-type triterpenoids (2 and 3) showed selective inhibition on PTP1B with IC50 values of 48.2 and 178.7 μM. The enzyme kinetic study suggested that compounds 2 and 3 were mix-type inhibitors on PTP1B with Ki values of 15.6 and 132.5 μM. This investigation manifests the antidiabetic potency of U. rhynchophylla with triterpenoids as the active constituents.
Keywords: Uncaria rhynchophylla; triterpenoids; antidiabetic effects; PTP1B inhibitors
Diabetes mellitus as one of the most serious chronic diseases in the world is characterized by high levels of blood glucose (hyperglycemia) [1]. Of the 425 million people suffering from diabetes mellitus, more than 90% cases are type 2 diabetes and lead to 1.6 million deaths every year. According to the World Health Organization (WHO), diabetes mellitus will reach to the seventh cause of death by 2030 [2]. Although many antidiabetic drugs with diverse mechanism of action are available on the market, the inevitable side effects including hypoglycemia, allergic reactions, weight gain, gastrointestinal reactions, liver damage and nutritional disorders hinder their application. Thus, different therapeutic targets are being gradually discovered along with the in-depth study of diabetes mellitus. Protein tyrosine phosphatase 1B (PTP1B), a negative regulator of insulin and leptin signal transduction, has been regarded as a hot target in treating diabetes mellitus. Recently, many kinds of PTP1B inhibitors including alkaloids, terpenoids, phenolics, fatty acids, and steroids have been reported, which provide valuable clues for the discovery of novel antidiabetic drugs [3,4]. However, it is a great challenge to discover inhibitors specific to each PTPs due to their high homology. Especially, T-cell protein tyrosine phosphatase (TCPTP) sharing about 74% identity with PTP1B in catalytic domain is a major obstacle in the search of PTP1B selective inhibitors.
Traditional Chinese medicines are important sources for drug discovery [5]. Uncaria rhynchophylla (Gouteng) is a famous traditional Chinese medicine used for psychiatric and hypotensive purposes in China [6,7]. Alkaloids, triterpenoids, flavonoids and organic acids are the main constituents in U. rhynchophylla [8]. Our previous study disclosed that different parts of U. rhynchophylla could be well differentiated by their chemical profiles [9], from which two pairs of dimeric isoechinulin-type alkaloids [10], two flavanols [11], and one indole alkaloid triglycoside [12] with antidepressant potency were isolated under the guidance of bioassay. As a comparison, the antidiabetic constituents of U. rhynchophylla have been little investigated. A recent study reported that hirsutine, the characteristic constituents in Uncaria plants, showed antidiabetic effects by regulating glucose homeostasis and ameliorating hepatic and cardiac insulin resistance in diabetic mice [13]. As a continuous search for PTP1B selective inhibitors from natural sources [14], this investigation yielded one new triterpenoid, 3β-formyloxy-6β,19α-dihydroxyurs-12-en-28-oic acid (1), along with four known ones, 3β,6β,19α-trihydroxyurs-12-en-28-oic acid (2), 2-oxopomolic acid (3), 3β,19α-dihydroxy-6-oxo-olean-12-en-28-oic acid (4) and sumaresinolic acid (5) from U. rhynchophylla (Fig. 1). Herein, we describe their isolation, structural characterization, and inhibitory activities on PTP1B and TCPTP.
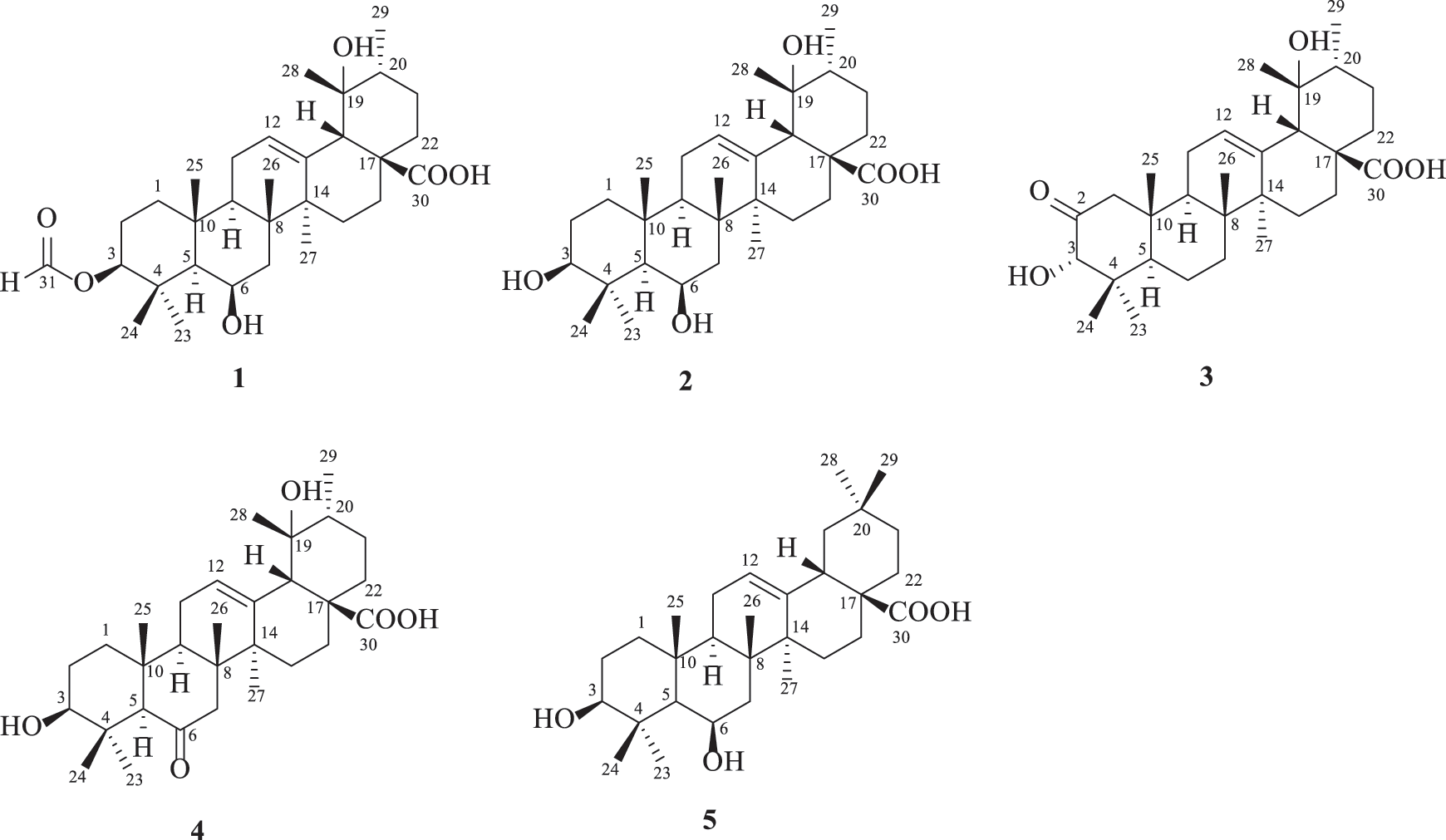
Figure 1: Structures of compounds 1–5
2.1 General Experimental Procedure
1D and 2D NMR spectra were performed on an Advance III-600 spectrometer (Bruker, Bremerhaven, Germany). HRESIMS data were recorded on a LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan). Thin-layer chromatography (TLC) analyses were performed on silica gel GF254 plates (Yantai Jiangyou Silicon Development Company, Yantai, China), and spots were detected by heating after spraying with 10% H2SO4 in EtOH. Chromatographic silica gel (200∼300 mesh, Qingdao Makall Chemical Company, Qingdao, China) and Sephadex LH-20 gel (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used for chromatography.
The aboveground parts of Uncaria rhynchophylla (Miq.) Miq. ex Havil. were collected from Pingxiang City, Jiangxi Province of China, which were authenticated by Dr. Li-Gong Lei (Kunming Institute of Botany, Chinese Academy of Sciences). A voucher specimen (2015080102) was deposited in the Laboratory of Antivirus and Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences.
The air-dried sample (1.5 kg) was powdered and extracted with 70% aqueous EtOH (10 L) under reflux for two times (each 2 h). The combined EtOH extract was evaporated under reduced pressure and partitioned between H2O and EtOAc. The EtOAc part (48 g) was fractionated by silica gel column chromatography (Si CC) using a petroleum ether-acetone gradient (90:10, 80:20, 70:30, 90:40, 0:100, v/v) to give eight fractions, Frs. 1~8. Fr. 3 (14.7 g) was separated by Si CC and eluted with CHCl3-acetone to provide five subfractions, Frs. 3-1~3-5. Fr. 3-3 (3.8 g) was further chromatographed on a silica gel column using petroleum ether-EtOAc (70:30) to afford compounds 1 (0.7 g) and 2 (1.1 g). Fr. 3-4 (3.6 g) was purified by repeated Si CC using petroleum ether-EtOAc and CHCl3-acetone elution, and Sephadex LH-20 CC (CHCl3-MeOH) to afford compounds 3 (15.3 mg), 4 (21.5 mg), and 5 (20.0 mg).
Compound 1: white powder; IR (KBr) νmax: 3632, 3550-3400 (broad peak), 1719, 1691, 1450, 1369, 1209, 1183, 1149, 1037, 968, 934, 903, 768, 656 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z 515.3382 ([M‒H]‒, C31H47O6, +0.4 mDa).
Inhibitory activity against PTP1B and TCPTP was performed in accordance with our previous report [15]. In brief, a mixture containing tested samples, enzymes, and working buffer was transferred into 96-well plates and preincubated at 37°C. The reaction was initiated by adding p-nitrophenyl phosphate, and further incubated for 30 min. The reaction was stopped by adding 100 μL of Na2CO3 solution (0.1 M). The cell plates were monitored at 405 nm using a BIO-RAD Model 680 microplate reader (Bio-Rad Laboratories, CA, USA). Sodium orthovanadate was used as the positive control.
Compound 1 was deduced with the molecular formula of C31H48O6 by the deprotonated ion at m/z 515.3382 ([M‒H]‒, +0.4 mDa) in the high-resolution electrospray ionization mass spectrometry (HRESIMS), suggesting eight indices of hydrogen deficiency. Its IR spectrum indicated the presence of hydroxyl (3632 cm–1), carbonyl (1719 cm−1) and formyl (1691 cm−1) groups. In accordance with the molecular formula, all the 31 carbons were well recognized in the 13C NMR (DEPT) spectrum, including seven methyls, eight methylenes, eight methines, and eight non-protonated carbons. In the 1H NMR spectrum, seven methyls at δH 1.71 (3H, s, H-27), 1.43 (3H, s, H-28), 1.11 (3H, J = 6.0 Hz, H-29), 1.09 (3H, s, H-26), 1.05 (3H, s, H-23), 1.02 (3H, s, H-25), and 0.99 (3H, s, H-24), one olefinic proton at δH 5.39 (t, J = 3.4 Hz), and two oxygenated methines at δH 4.58 (1H, dd, J = 11.8, 4.4 Hz) and 4.57 (1H, m) were obviously recognized. Taking the characteristic carbons of seven methyls at δC 27.7, 26.1, 23.9, 18.1, 17.6, 16.8, and 16.1, one tri-substituted double bond at δC 129.1 and 137.1, one carboxyl at δC 181.4 into consideration, an ursane-type triterpenoid was deduced. The 1H and 13C NMR data (Table 1) of compound 1 were similar with those of 3β,6β,19α-trihydroxyurs-12-en-28-oic acid (2) [16], except for an extra formyl group (δC 161.6 and δH 8.14) in 1, and the de-shielded shift of C-3 from δC 79.4 to 81.5. Thus, compound 1 was reasonably proposed to be the 3-O-formyl derivative of 2. This deduction was fully confirmed by the HMBC correlations from H-31 to C-3 and from H-3 to C-31. The α-orientation of H-3 was verified by the dd splitting (J = 11.8, 4.4 Hz) and the ROESY correlation between H-3 and H-5 (Fig. 2). Through the above analysis, the structure of 1 was determined to be 3β-formyloxy-6β,19α-dihydroxyurs-12-en-28-oic acid.
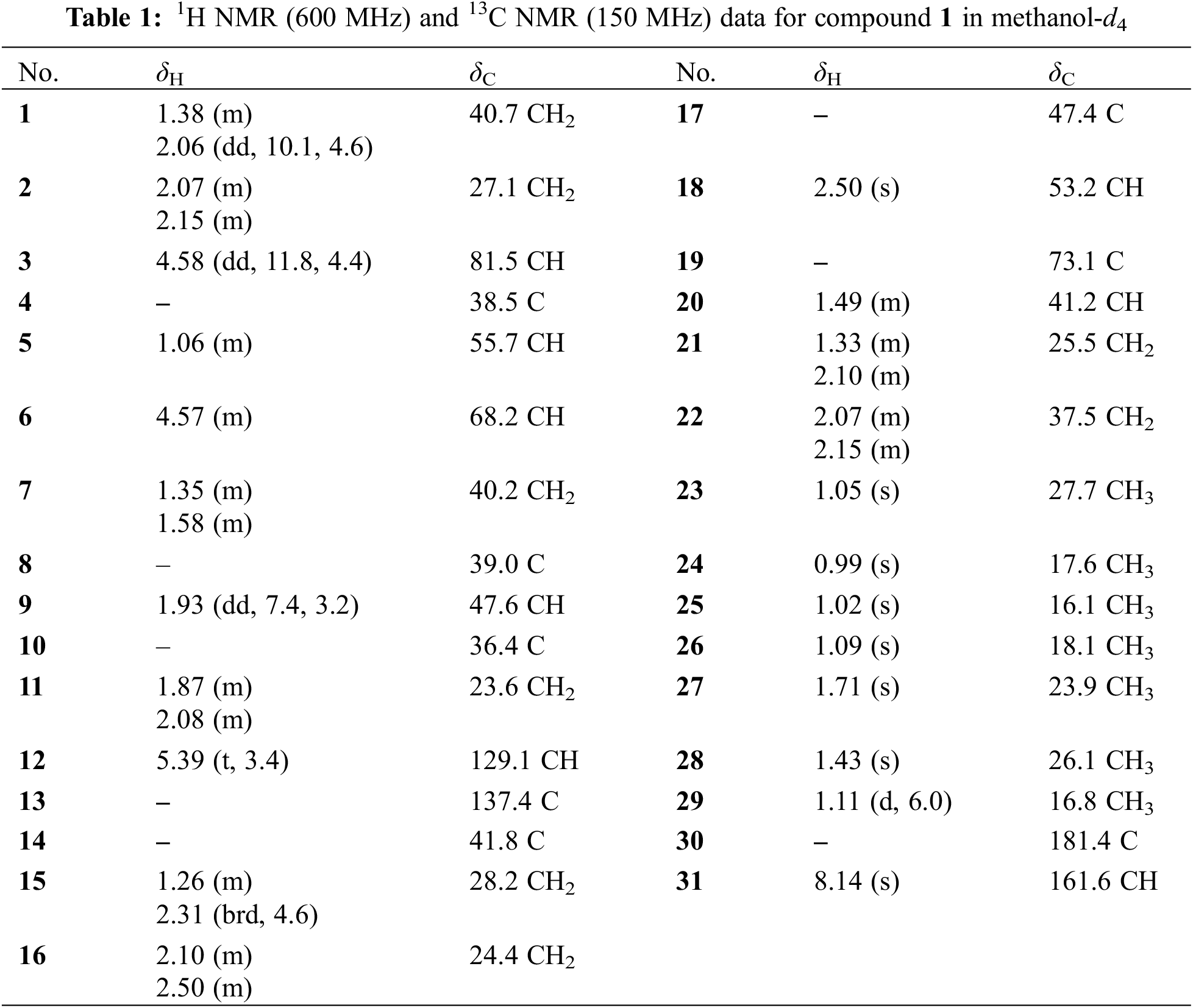
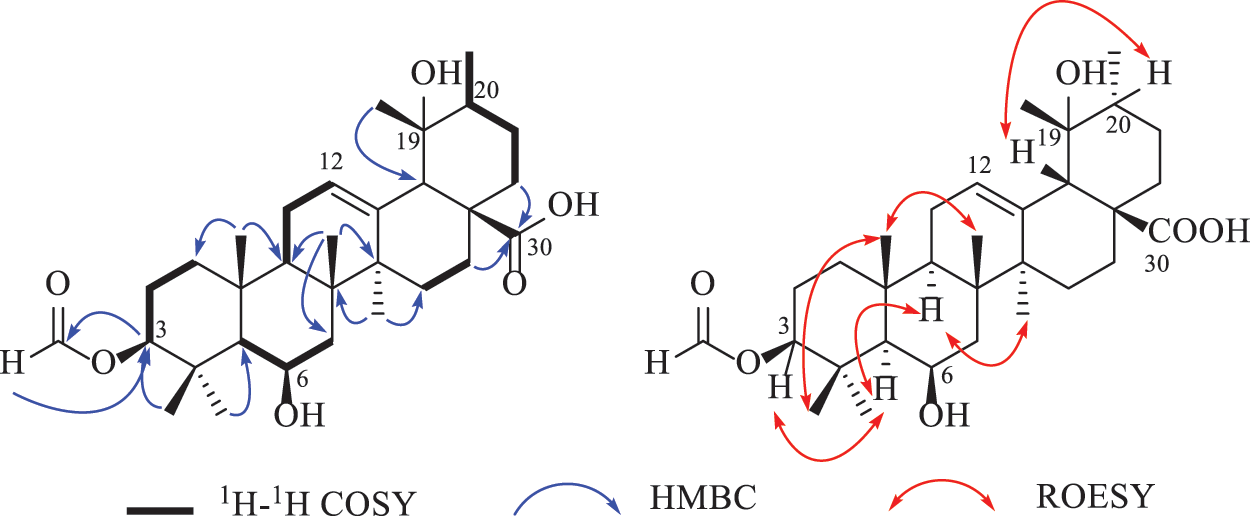
Figure 2: Key 2D NMR correlations of compound 1
The known compounds were determined as 3β,6β,19α-trihydroxyurs-12-en-28-oic acid (2) [16], 2-oxopomolic acid (3) [17], 3β,19α-dihydroxy-6-oxo-olean-12-en-28-oic acid (4) [18] and sumaresinolic acid (5) [19], by comparing with the spectroscopic data reported in literatures.
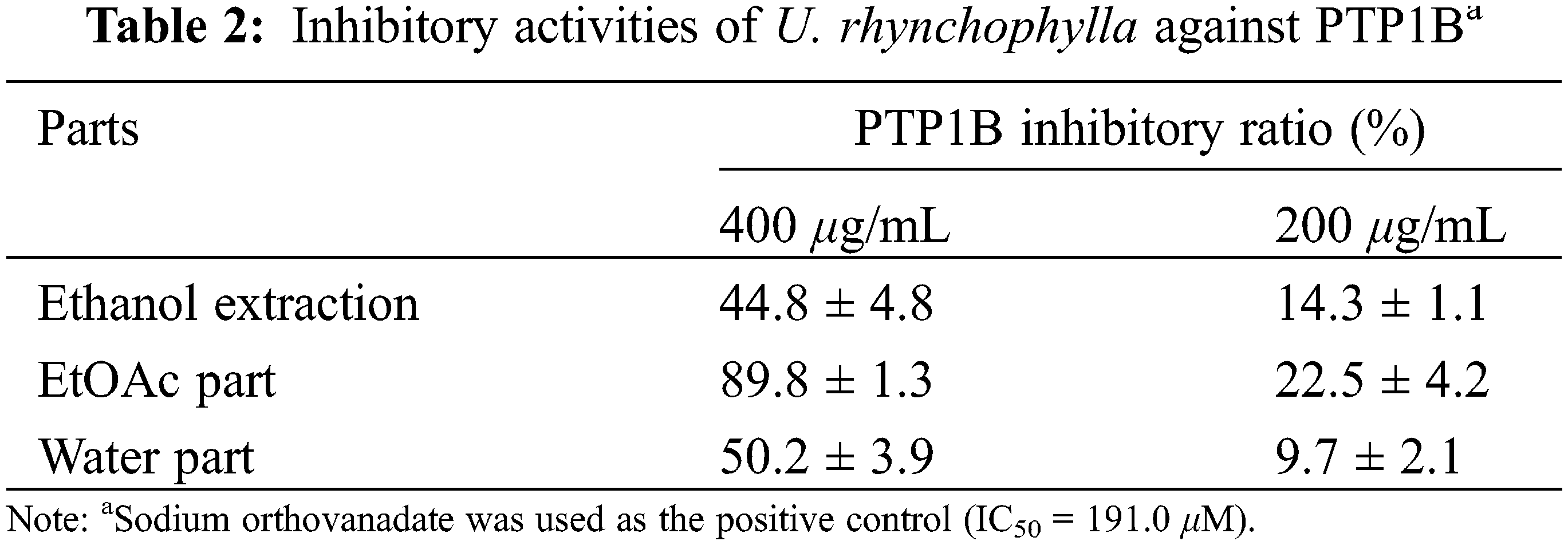
In this study, the ethanol extraction of U. rhynchophylla was revealed with PTP1B inhibition with inhibitory ratios of 44.8% and 14.3% at concentrations of 400 and 200 μg/mL, respectively. After extracted with EtOAc, the EtOAc part showed increased activity with inhibitory ratios of 89.8% and 22.5%, whereas the water part maintained the similar activity with the ethanol extraction (Table 2).
In order to evaluate the antidiabetic effects of the isolates, compounds 1–5 were assayed for their inhibition on PTP1B and TCPTP. As shown in Table 3, compound 2 showed significant inhibition on PTP1B with inhibitory ratios of 96.9% and 87.6% at concentrations of 200 and 100 μM, respectively. Compounds 3 and 4 exhibited moderate activity against PTP1B with inhibitory ratios of 56.2% and 29.8% at 200 μM, and 36.3% and 21.7% at 100 μM, whereas compounds 1 and 5 were inactive at the tested concentrations. All the isolates showed moderate to weak inhibition on TCPTP, indicating a PTP1B selectivity.
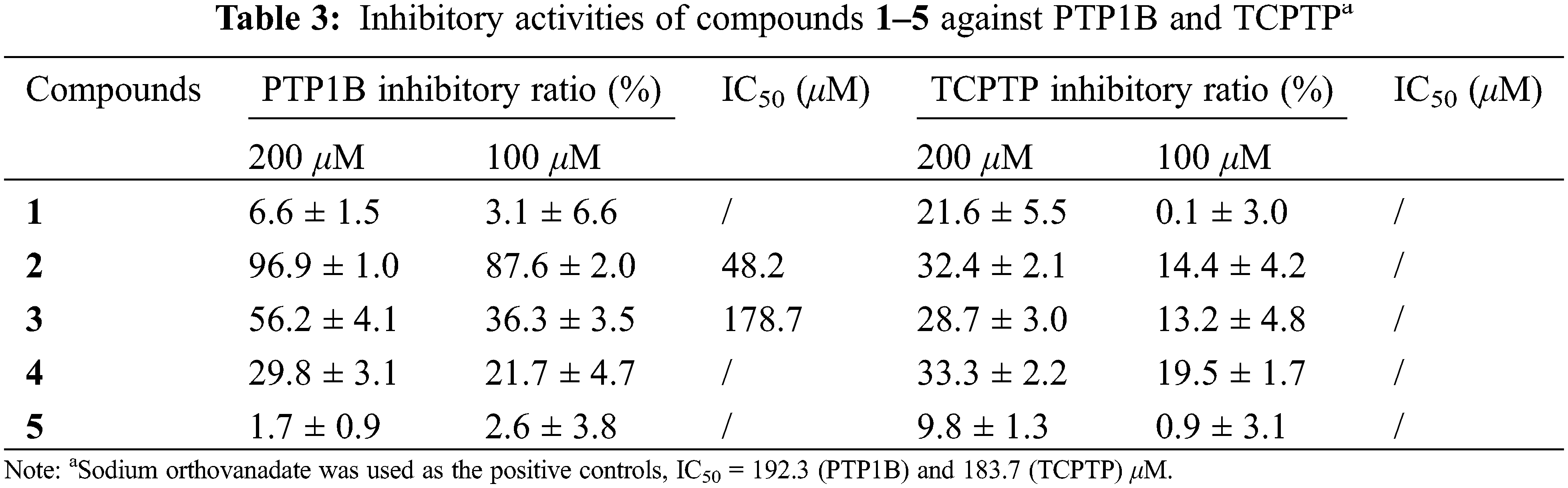
The dose-effect relationships of compounds 2 and 3 on PTP1B were further evaluated at five concentrations to provide their respective IC50 value of 48.2 and 178.7 μM, more potent than the positive control, sodium orthovanadate (IC50, 192.3 μM).
The inhibition mode of compounds 2 and 3 on PTP1B was studied using Lineweaver-Burk double reciprocal and Dixon single reciprocal plots (Fig. 3). In the Lineweaver-Burk plots, the lines of compounds 2 and 3 intersected on the second quadrant, indicating the mixed-type inhibition. The Ki values of compounds 2 and 3 were calculated as 15.6 and 132.5 μM by the Dixon plots.
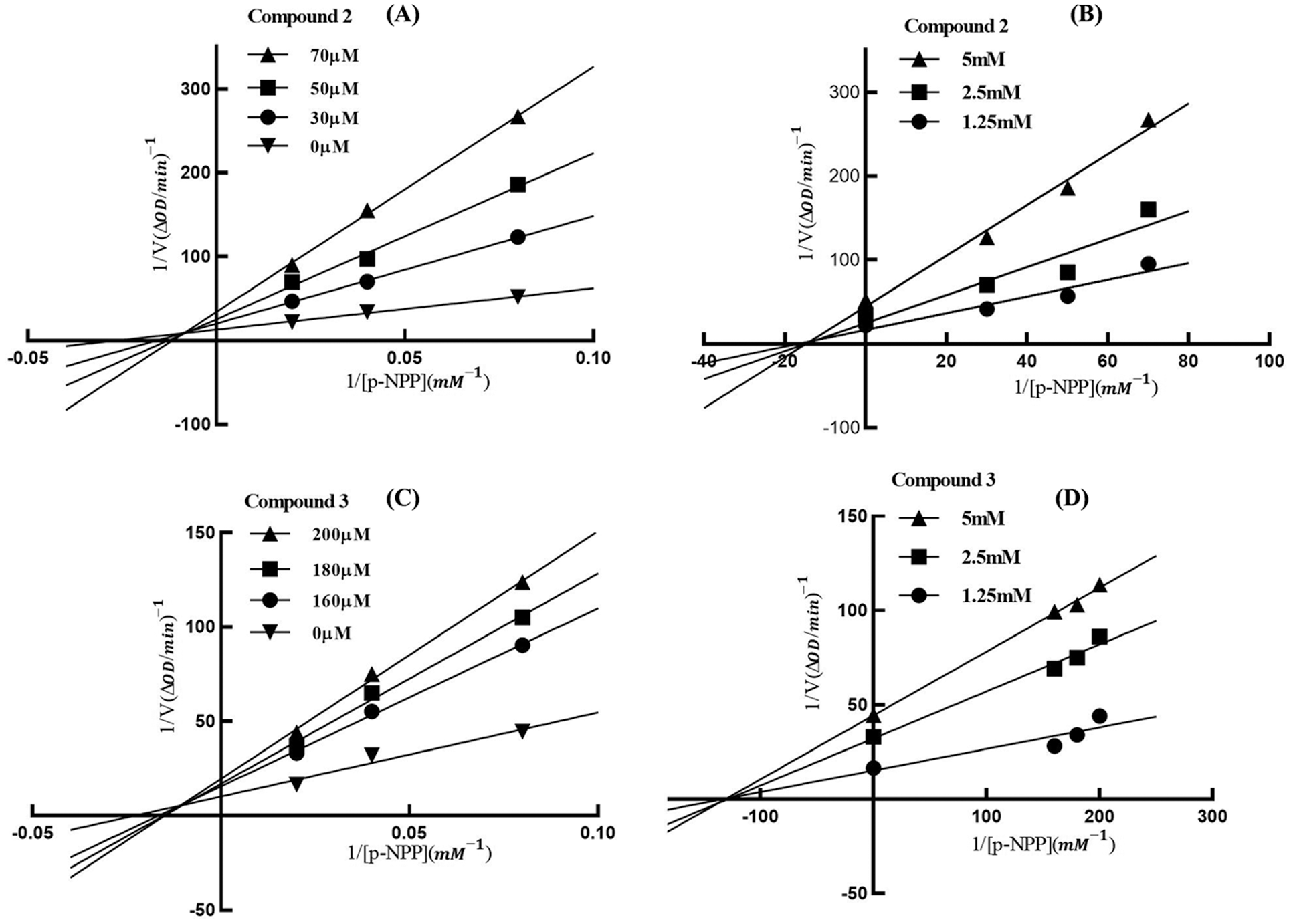
Figure 3: Lineweaver-Burk (A/C) and Dixon (B/D) plots of compounds 2 and 3 against PTP1B. In the Lineweaver-Burk plots (A/C), the lines of compounds 2 and 3 intersected on the second quadrant meaning that the Km was increased but Vmax was decreased with the increased concentration of inhibitors, which indicated 2 and 3 as mixed-type inhibitors
In this investigation, the ethanol extraction of U. rhynchophylla was revealed with PTP1B inhibitory effects. Under the guidance of bioassay, one new and four known triterpenoids were isolated from the EtOAc part. Two ursane-type triterpenoids 2 and 3 showed selective inhibition on PTP1B with IC50 values of 48.2 and 178.7 μM, respectively. The enzyme kinetic study manifested that compounds 2 and 3 were mix-type inhibitors on PTP1B with Ki values of 15.6 and 132.5 μM. This investigation provides valuable clues for understanding the antidiabetic effects of U. rhynchophylla and searching new PTP1B selective inhibitors from natural sources.
Funding Statement: This work was supported by the Yunnan Wanren Project (YNWR-QNBJ-2018-061), the Yunnan Science Fund for Excellent Young Scholars (2019FI017), the Reserve Talents of Young and Middle-aged Academic and Technical Leaders in Yunnan Province (2018HB067), and the National Natural Science Foundation of China (81573322).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
1. He, X. F., Zhang, X. K., Geng, C. A., Hu, J., Zhang, X. M. et al. (2020). Tsaokopyranols A-M, 2,6-epoxydiarylheptanoids from Amomum tsao-ko and their α-glucosidase inhibitory activity. Bioorganic Chemistry, 96, 103638. DOI 10.1016/j.bioorg.2020.103638. [Google Scholar] [CrossRef]
2. Roden, M., Shulman, G. I. (2019). The integrative biology of type 2 diabetes. Nature, 576, 51–60. DOI 10.1038/s41586-019-1797-8. [Google Scholar] [CrossRef]
3. Kyriakou, E., Schmidt, S., Dodd, G. T., Pfuhlmann, K., Simonds, S. et al. (2018). Celastrol promotes weight loss in diet-induced obesity by inhibiting the protein tyrosine phosphatases PTP1B and TCPTP in the hypothalamus. Journal of Medicinal Chemistry, 61, 11144–11157. DOI 10.1021/acs.jmedchem.8b01224. [Google Scholar] [CrossRef]
4. Xie, F., Yang, F., Liang, Y., Li, L., Xia, Y. et al. (2019). Investigation of stereoisomeric bisarylethenesulfonic acid esters for discovering potent and selective PTP1B inhibitors. European Journal of Medicinal Chemistry, 164, 408–422. DOI 10.1016/j.ejmech.2018.12.032. [Google Scholar] [CrossRef]
5. Geng, C. A., Wang, L. J., Guo, R. H., Chen, J. J. (2013). Small-molecule inhibitors for the treatment of hepatitis B virus documented in patents. Mini-Reviews in Medicinal Chemistry, 13, 749–776. DOI 10.2174/1389557511313050012. [Google Scholar] [CrossRef]
6. Chinese Pharmacopoeia Commission (2015). Pharmacopoeia of the People’s Republic of China I, pp. 257. Beijing: China Medical Science Press. [Google Scholar]
7. Heitzman, M. E., Neto, C. C., Winiarz, E. (2005). Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry, 66, 5–29. DOI 10.1016/j.phytochem.2004.10.022. [Google Scholar] [CrossRef]
8. Ndagijimana, A., Wang, X., Pan, G. (2013). A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia, 86, 35–43. DOI 10.1016/j.fitote.2013.01.018. [Google Scholar] [CrossRef]
9. Zhang, J. G., Geng, C. A., Huang, X. Y. (2017). Chemical and biological comparison of different sections of Uncaria rhynchophylla (Gou-Teng). European Journal of Mass Spectrometry, 23, 11–21. DOI 10.1177/1469066717694044. [Google Scholar] [CrossRef]
10. Geng, C. A., Huang, X. Y., Ma, Y. B., Hou, B., Li, T. Z. et al. (2017). (±)-Uncarilins A and B, dimeric isoechinulin-type alkaloids from Uncaria rhynchophylla. Journal of Natural Products, 80, 959–964. DOI 10.1021/acs.jnatprod.6b00938. [Google Scholar] [CrossRef]
11. Geng, C. A., Yang, T. H., Huang, X. Y., Ma, Y. B., Zhang, X. M. et al. (2019). Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. Journal of Ethnopharmacology, 232, 39–46. DOI 10.1016/j.jep.2018.12.013. [Google Scholar] [CrossRef]
12. Zhang, J. G., Huang, X. Y., Ma, Y. B., Zhang, X. M., Chen, J. J. et al. (2018). Dereplication-guided isolation of a new indole alkaloid triglycoside from the hooks of Uncaria rhynchophylla by LC with ion trap time-of-flight MS. Journal of Separation Science, 41, 1532–1538. DOI 10.1002/jssc.201701175. [Google Scholar] [CrossRef]
13. Hu, W., Li, M., Qiu, Y. Y., Wang, R., Ding, Q. et al. (2021). Hirsutine ameliorates hepatic and cardiac insulin resistance in high-fat diet-induced diabetic mice and in vitro models. Pharmacological Research, 6, 105917. DOI 10.1016/j.phrs.2021.105917. [Google Scholar] [CrossRef]
14. He, X. F., Chen, J. J., Li, T. Z., Zhang, X. K., Guo, Y. Q. et al. (2021). Diarylheptanoid-flavanone hybrids as multiple-target antidiabetic agents from Alpinia katsumadai. Chinese Journal of Chemistry, 39, 3051–3063. DOI 10.1002/cjoc.202100469. [Google Scholar] [CrossRef]
15. Zhang, C. C., Geng, C. A., Huang, X. Y., Zhang, X. M., Chen, J. J. (2019). Antidiabetic stilbenes from peony seeds with PTP1B, α-glucosidase, and DPPIV inhibitory activities. Journal of Agricultural and Food Chemistry, 67, 6765–6772. DOI 10.1021/acs.jafc.9b01193. [Google Scholar] [CrossRef]
16. Aquino, R. (1990). New polyhydroxylated triterpenes from Uncaria tomentosa. Journal of Natural Products, 53, 559–564. DOI 10.1021/np50069a004. [Google Scholar] [CrossRef]
17. Kemp, M. S., Holloway, P. J., Burden, R. S. (1985). 3β,19α-Dihydroxy-2-oxours-12-en-28-oic acid: A pentacyclic triterpene induced in the wood of Malus pumila Mill. infected with Chondrostereum purpureum (Pers. ex Fr.) Pouzar., and a constituent of the cuticular wax of apple fruits. Journal of Chemical Research, 5, 154–155. [Google Scholar]
18. Wang, Z. (2013). α-Glucosidase inhibitory triterpenoids from the stem barks of Uncaria laevigata. Fitoterapia, 90, 30–37. DOI 10.1016/j.fitote.2013.07.005. [Google Scholar] [CrossRef]
19. Wang, F. (2006). Triterpenoids from the resin of Styrax tonkinensis and their antiproliferative and differentiation effects in human leukemia HL-60 cells. Journal of Natural Products, 69, 807–810. DOI 10.1021/np050371z. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |